Abstract
In this review, we compare and contrast the three different forms of vertebrate lens regeneration: Wolffian lens regeneration, cornea-lens regeneration, and lens regeneration from lens epithelial cells. An examination of the diverse cellular origins of these lenses, their unique phylogenetic distribution, and the underlying molecular mechanisms, suggests that these different forms of lens regeneration evolved independently and utilize neither conserved nor convergent mechanisms to regulate these processes.
Keywords: lens regeneration, signaling pathways, evolution, vertebrates
Examples of Lens Regeneration: Historical Perspective and Basic Descriptions
Some animals exhibit a remarkable capacity to regenerate the lens (Henry 2003; Tsonis et al. 2004; Gwon 2006; Henry et al. 2008; Henry and Tsonis 2010; Barbosa-Sabanero et al. 2012), and this phenomenon has fascinated investigators for over 200 years. Three principal types of lens regeneration have been reported. The first to be described was that of Wolffian lens regeneration, which occurs mainly in urodeles (newts and salamanders, table 1 and figs. 1–3). This type of lens regeneration was recorded by Bonnet (1781) and Bloomenbach (1787), and subsequently by Philippaux (1880), Collucci (1891), and Wolff (1894, 1895, 1901, 1904), with the later individuals having recognized that the new lens originated from pigmented epithelial cells located along the dorsal rim of the iris (fig. 1A–F). In contrast, lenses do not normally regenerate from the ventral rim of the iris. Based on the loss of pigment in the responding cells, as well as other histological and molecular changes, Wolffian regeneration provides a clear example of transdifferentiation, whereby one differentiated cell type undergoes dedifferentiation followed by redifferentiation along a different developmental trajectory. In general, Wolffian lens regeneration can occur during both larval and adult phases and experiments reveal that factors provided by the neural retina trigger the process of Wolffian lens regeneration (Zalokar 1944; Stone and Steinitz 1953; Stone 1958a, 1958b; Reyer 1966, 1971; Powell and Powers 1973; Inoue et al. 2012).
Table 1.
Examples for Each of Three Different Types of Lens Regeneration.
| Class/Order/Suborder/Family | Genus Species | +/− Regeneration | References |
|---|---|---|---|
| Wolffian Lens Regeneration | |||
| Class: Actinopterygii (ray-finned fishes) | |||
| Order: Cypriniformes | |||
| Suborder Cobitinae | |||
| Family: Cobitidae | Misgurnus anguillicaudatus | + | Sato 1961; Mitashov 1966 |
| Suborder: Danioninae | |||
| Family: Cyprinidae | Danio rerio | − | Suetsugu-Maki et al. 2012 |
| Suborder: Cyprinodontoidei | |||
| Family: Fundulidae | Fundulus heteroclitus | − | Stone and Sapir 1940 |
| Class: Amphibia | |||
| Order: Caudata (tailed amphibians) | |||
| Suborder: Salamandroidae | |||
| Family: Ambystomatidae | Ambystoma mexicanum | (+) | Stone 1967; Suetsugu-Maki et al. 2012 |
| Ambystoma punctatum | − | Stone 1967 | |
| Ambystoma tigrinum | − | Stone 1967 | |
| Ambystoma opacum | − | Stone 1967 | |
| Family: Salamandridae | Notopthalmus viridescens | + | Wachs 1914; Stone and Sapir 1940; Stone and Chace 1941; Reyer 1948; Stone 1952; Stone and Steintz 1953 |
| Notopthalmus peristriatus | + | Stone 1967 | |
| Taricha granulosa | + | Stone 1967 | |
| Taricha sierra | + | Stone 1967 | |
| Taricha rivularis | + | Stone 1967 | |
| Taricha terosa | + | Dinnean 1942; Stone 1967 | |
| Triturus taeniatus | + | Wachs 1914; Woerdeman 1922; Sato 1930, 1940; Monroy 1937; Stone 1967 | |
| Triturus cristatus | + | Wachs 1914; Zolakar 1944; Stone 1967 | |
| Triturus helveticus | + | Stone 1967 | |
| Triturus alpestris | + | Monroy 1937; Stone 1967 | |
| Triturus marmoratus | + | Stone 1967 | |
| Cynops pyrrhogaster | + | Ogawa 1921; Nakamura 1935; Ikeda 1936a; Sato 1940; Mikami 1941; Stone 1967 | |
| Cynops ensicuada | + | Kojima 1939 | |
| Salamandra salamandra | + | Fischel 1900, 1903, 1921; Reyer and Stone 1951, 1955 | |
| Salamandra perspicullata | + | Wachs 1914 | |
| Pleurodeles waltii | + | Vigh 1960 | |
| Family: Plethodontidae | Typhlotriton spelaeus | + | Stone 1964 |
| Eurycea lucifuga | + | Stone 1967 | |
| Eurycea bislineata | + | Berardi and McDevitt 1982 | |
| Eurycea longicauda | − | Stone 1967 | |
| Batrachoseps attenuatus | − | Stone 1967 | |
| Demognathus fuscus | − | Stone 1967 | |
| Order: Galliformes (birds) | |||
| Family: Phaianidae | Gallus gallus | (+?) | van Deth 1940; McKeehan 1961; Genis-Galvez 1962; Niazi 1967; Wedlock and McCallion 1968 |
| Cornea-Lens Regeneration | |||
| Class: Amphibia | |||
| Order: Caudata (tailed amphibians) | |||
| Suborder: Cryptobranchoidea | |||
| Family: Hynobiidae | Hynobius unnangso | + | Ikeda 1936a, 1936b, 1939 |
| Order: Anura (frogs) | |||
| Suborder: Archaeobatrachia | |||
| Family: Discoglossidae (Alytidae) | |||
| Discoglossus pictus | − | Filoni et al. 1977a; Bosco et al. 1991; Bosco, Sciacovelli, et al. 1993 | |
| Suborder: Mesobatrachia | |||
| Fam,ily: Pipidae | Xenopus laevis | + | Freeman 1963; Bosco et al. 1981; Bosco 1988b |
| Xenous borealis | (+) | Filoni et al. 2006 | |
| Xenopus tropicalis | (+) | Henry and Elkins 2001 | |
| Suborder: Neobatrachia | |||
| Family: Bufonidae | Bufo viridis | − | Bosco, Filoni, Cioni, et al. 1983 |
| Family: Hylidae | Hyla arborea | − | Bosco, Filoni, and Cioni 1983; Bosco, Gentili, et al. 1993 |
| Family: Ranoidae | Rana temporaria | − | Bosco 1988a |
| Rana esculenta | − | Filoni et al. 1976, 1979; Filoni 1980; Cioni et al. 1983 | |
| Rana dalmatina | − | Cioni et al. 1979 | |
| Rana latastei | − | Bosco 1988a | |
| Rana greaca | − | Bosco et al. 1987 | |
| Rana italica | − | Bosco et al. 1993 | |
| Rana sylvatica | − | Stone and Sapir 1940 | |
| Rana pipiens | − | Stone and Sapir 1940 | |
| Rana clamitans | − | Stone and Sapir 1940 | |
| Lens Epithelial Cell Regeneration | |||
| Class: Actinopterygii (ray-finned fishes) | |||
| Order: Cyprinodontiformes | |||
| Suborder: Cyprinodontoidei | |||
| Famly: Fundulidae | Fundulus heteroclitus | − | Stone and Sapir 1940 |
| Class: Amphibia | |||
| Order: Caudata (tailed amphibians) | |||
| Suborder: Salamandroidae | |||
| Family: Ambystomatidae | Ambystoma puntatum | − | Stone and Sapir 1940 |
| Ambystoma tigrinum | + | Stone and Sapir 1940 | |
| Ambystoma maculatum | + | Reyer 1974, 1977a, 1977b | |
| Family: Salamandridae | Notopthalmus viridescens | − | Stone and Sapir 1940 |
| Cynops pyrrhogaster | − | Ikeda and Amatatu 1941 | |
| Order: Anura (frogs) | |||
| Suborder: Archaeobatrachia | |||
| Family: Discoglossidae (Alytidae) | |||
| Discoglossus pictus | + | Reyer 1954 | |
| Suborder: Mesobatrachia | |||
| Family: Pelobatidae | |||
| Pelobates fuscus | + | Reyer 1954 | |
| Fam,ily: Pipidae | Xenopus laevis | + | Brahma and van Doorenmaalen 1968; Bosco and Willems 1994; Bosco, Testa, et al. 1997 |
| Suborder: Neobatrachia | |||
| Family: Bufonidae | Bufo viridis | + | Reyer 1954 |
| Bufo bufo | + | Reyer 1954 | |
| Family: Ranoidae | Rana clamitans | + | Stone and Sapir 1940 |
| Rana pipiens | + | Stone and Sapir 1940 | |
| Rana Sylvatica | + | Stone and Sapir 1940; Reyer 1954 | |
| Rana esculenta | + | Reyer 1954; Filoni et al. 1977b | |
| Rana arvalis | + | Reyer 1954 | |
| Rana catesbiana | + | Reyer 1954 | |
| Rana temporaria | + | Reyer 1954 | |
| Pelophylax ridibundus | + | Reyer 1954 | |
| Class: Mammalia | |||
| Order: Lagomorpha | |||
| Family: Leporidae | Oryctolagus cuniculus | + | Cocteau and D’Etoille 1827; Stewart and Espinasse 1959; Stewert 1960; Pettit 1963; Agarwal et al. 1964; Gwon et al. 1989, 1990, 1992; Gwon, Gruber, and Mantras 1993; Gwon, Gruber, Mantras, et al. 1993; Gwon 2006; Lin et al. 2016 |
| Order: Carnivora | |||
| Suborder: Feliformia | |||
| Family: Felidae | Felis catus | + | Miliot 1872; Gwon, Gruber, and Mantras 1993 |
| Suborder: Caniformia | |||
| Family: Canidae | Canis familiaris | (+?) | de Landau 1838 (see Randolph 1900); Miliot 1872 |
| Order: Rodentia | |||
| Suborder: Myomorpha | |||
| Family: Muridae | Mus musculus | + | Shekhawat et al. 2001; Call et al. 2004; Lois et al. 2005; Medvedovicx et al. 2006 |
| Rattus norvegicus | + | Lois et al. 2003 | |
| Suborder: Hystricomorpha | |||
| Family: Caviidae | Cavia porcellus | (+?) | Miliot 1872 |
| Order: Primates | |||
| Suborder: Haplorhini | |||
| Family: Hominidae | Homo sapiens | + | Gunn 1888; Becker 1900 (see Randolph 1900); Lin et al. 2016 |
| Family: Cercopithecidae | Macaca fascicularis | + | Lin et al. 2016 |
| Macaca mulatta | + | Agarwal et al. 1964 | |
| Order: Artiodactyla | |||
| Suborder: Ruminantia | |||
| Family: Bovidae | |||
| Bos taurus | (+?) | de Landau 1838 (see Randolph 1900) | |
| Ovis aries | (+?) | Miliot 1872 | |
| Sus scrofa domesticus | (+) | Jangir et al. 2005 | |
Note.—Some references are provided for each example, but this table is not intended to be inclusive of all studies pertaining to these different species.
+, indicates species is able to regenerate the lens; (+), indicates species is able to regenerate the lens under special circumstances; −, indicates species is unable to regenerate the lens; (+?), indicates reported ability to regenerate the lens may be questionable.
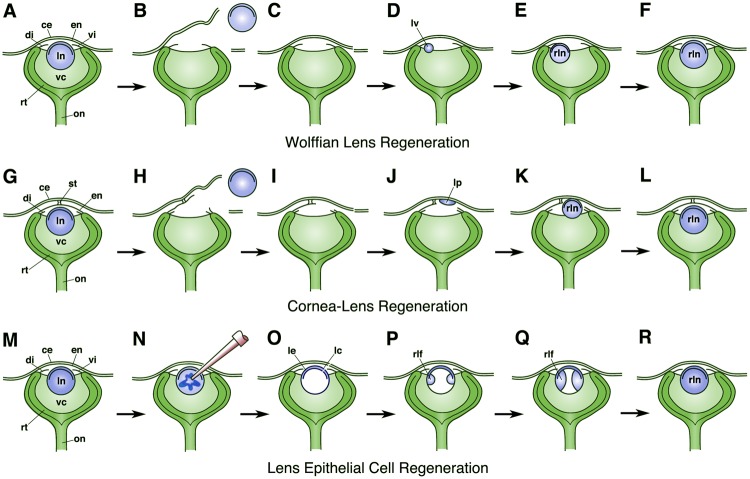
Fig. 1.
Diagrams illustrating the process of Wolffian lens regeneration (A–F), cornea-lens regeneration (G–L), and lens epithelial cell regeneration (M–R). In (B) and (H), simple lentectomy is performed to remove the intact lens along with its lens capsule. (N) Shows the process of phacoemulsification to remove the lens fiber cells while mainly leaving the lens epithelium and lens capsule intact (as seen in O). (A–F) and (M–R) show adult eyes. Unlike the case in the adult eye, notice that the Xenopus larval cornea epithelium is initially attached to the deeper cornea endothelium by only a small central stalk (as shown in G). This connection enlarges, and the collagenous stroma is deposited during later stages when the larva approaches the time of metamorphosis. Eye structures are labeled as: ce, cornea epithelium; di, dorsal iris; en, cornea endothelium; lc, lens capsule; le, lens epithelium; ln, lens; lp, lens placode; lv, lens vesicle; on, optic nerve; rlf, regenerated lens fiber cells; rln, regenerated lens; rt, retina, st, central stalk; vc, vitreous chamber; vi, ventral iris.
The second form of lens regeneration is that of cornea-lens regeneration, first described by Freeman (1963). Cornea-lens regeneration has only been observed in frogs within the genus Xenopus and one urodele, the Tohoku salamander, Hynobius lichenatus (formerly H. unnangso, see table 1 and figs. 1–3, Ikeda 1936b, 1937, 1939). In these cases, the new lens arises from the basal layer of the cornea epithelium, during larval stages, before the development of the substantia propria (fig. 1G–L). Like Wolffian lens regeneration, factors provided by the neural retina trigger cornea-lens regeneration (Freeman 1963; Henry and Mittleman 1995; Bosco, Testa, et al. 1997). On the other hand, this form of lens regeneration lacks a clear dedifferentiation step and is not likely an example of transdifferentiation, as originally suggested (Freeman 1963; see Henry 2003; Henry et al. 2008). Rather, evidence suggests that these lenses arise from undifferentiated stem cells and/or transit amplifying cells, which are located within the basal layer of the cornea epithelium (Perry et al. 2013; Hamilton and Henry 2016). Interestingly, experiments reveal that cells within the basal layer of the postmetamorphic frog cornea epithelium remain capable of expressing lens proteins, if the mature cornea is implanted directly into the vitreous chamber (Filoni et al. 1997; Hamilton and Henry 2016). Unlike the case in larval frogs, however, these cells do not become organized into normal lenses.
Wolffian lens regeneration and cornea-lens regeneration are both examples of de novo regeneration, where the lens arises from nonlens tissues in the absence of other lens cells. However, there is a third form of lens regeneration whereby missing parts of the lens are replaced by preexisting lens epithelial cells (fig. 1M–R). Though some have referred to this as mammalian lens regeneration, here we refer to this as lens epithelial cell (LEC) regeneration. This form of lens regeneration has been reported for several different mammals, including one example in rabbits dating back nearly 200 years (Cocteau 1827, see table 1 and fig. 2). Since that time, the rabbit has served as the primary model for studying LEC regeneration (Mayer 1832; Middlemore 1832; Valentin 1844; Miliot 1872; Randolph 1900; Sikhardldze 1956; Stewart and Espinasse 1959; Stewert 1960; Gwon et al. 1989, 1990, 1992; Gwon, Gruber, and Mantras 1993; Gwon, Gruber, Mantras, et al. 1993; Gwon 2006, 2008, 2009; Lin et al. 2016).
Fig. 2.
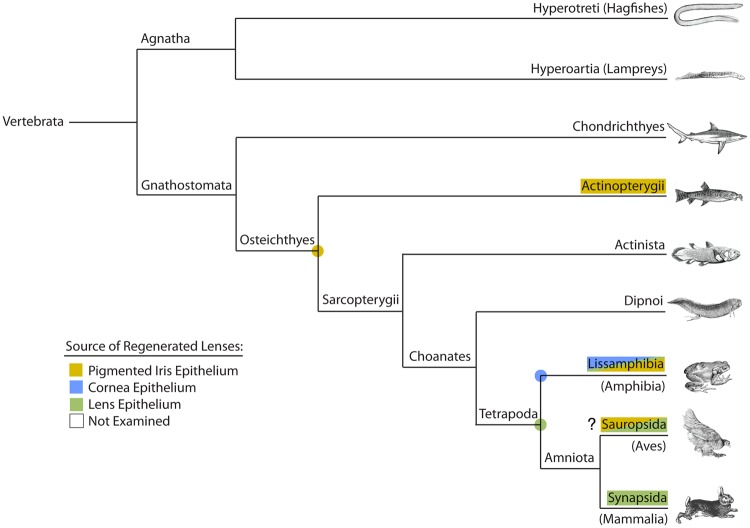
Phylogram showing major vertebrate clades and occurrence of examples that can regenerate the lens. The type of lens regeneration as indicated by different colors, as shown in the key. “?” indicates that reported examples of Wolffian lens regeneration in members of the Sauropsida (i.e., the chicken Gallus gallus) are questionable. Examples from several subphyla or classes, including the more basal groups, have either not yet been examined or reported in the literature. Colored dots represent possible presence of that matching character in the common ancestor for those particular nodes or branches. See text and table 1 for further details. Phylogenetic relationships are based on Meyer and Zardoya (2009).
Several investigators have also noted that lenses can reform from lens fragments that are either inadvertently or intentionally left inside the eye following attempts to remove the lens in fish and various amphibians (Okada 1939, 1943a, 1943b; Stone and Sapir 1940; Stone 1967; Brahma and van Doorenmaalen 1968; Reyer 1974, 1977a, 1977b; Filoni et al. 1977b; Filoni 1980, see table 1 and fig. 2). These fragments possess lens epithelial stem cells that continue to proliferate and ultimately give rise to differentiated lens fiber cells. More typically, however, this form of regeneration does not give rise to a normal lens without the presence of the lens capsule (reviewed by Gwon 2006; Tsonis 2006). These observations mirror the abnormal proliferation of lens epithelial cells that can lead to the formation of so-called secondary cataracts (or posterior capsule opacification) in human patients, when the lens cells are not completely removed from the lens capsule during cataract surgery (Gwon 2008). Recently, the clinical power of LEC regeneration has been demonstrated by one group that had better regenerative success using a minimally invasive capsulorhexis technique to remove the lens fibers in pediatric patients with congenital cataracts (Lin et al. 2016, fig. 1M–R). Through the preservation of LECs, patients were able to reform lenses with fairly normal refractive properties. Similar results were also obtained in rabbits and macaques (Lin et al. 2016).
Examples of Lens Regeneration: Phylogenetic Distribution
A survey of the literature uncovers that many animals are able to regenerate the lens (table 1). These cases were mapped onto phylogenetic trees that plot the relationships between various vertebrate clades (figs. 2 and 3). Cases that regenerate lenses via Wolffian lens regeneration are mainly restricted to members of the Subclass Lissamphibia (in the Class Amphibia), and the only other concrete examples are found in the more basal ray-finned fishes (Class Actinopterygii, Family Cobitidae, such as the Chinese Weather Loach Missgurnous anguilicaudatus). In contrast, the Mummichog (Fundulus heteroclitus) and Zebrafish (Danio rerio) are unable to regenerate the lens (Stone and Sapir 1940; Suetsugu-Maki et al. 2012). Although there are some reports of lens regeneration occurring from the iris in the chicken (Gallus gallus), those reports have been refuted (see review by Henry 2003). The widespread occurrence of Wolffian lens regeneration in newts and salamanders may suggest that the last common ancestor of the Salamandroidea possessed the capacity for Wolffian lens regeneration (fig. 3). Although deeper taxon sampling is required, it is possible that the last common ancestor of the Osteichthyes may have also possessed this ability.
Fig. 3.
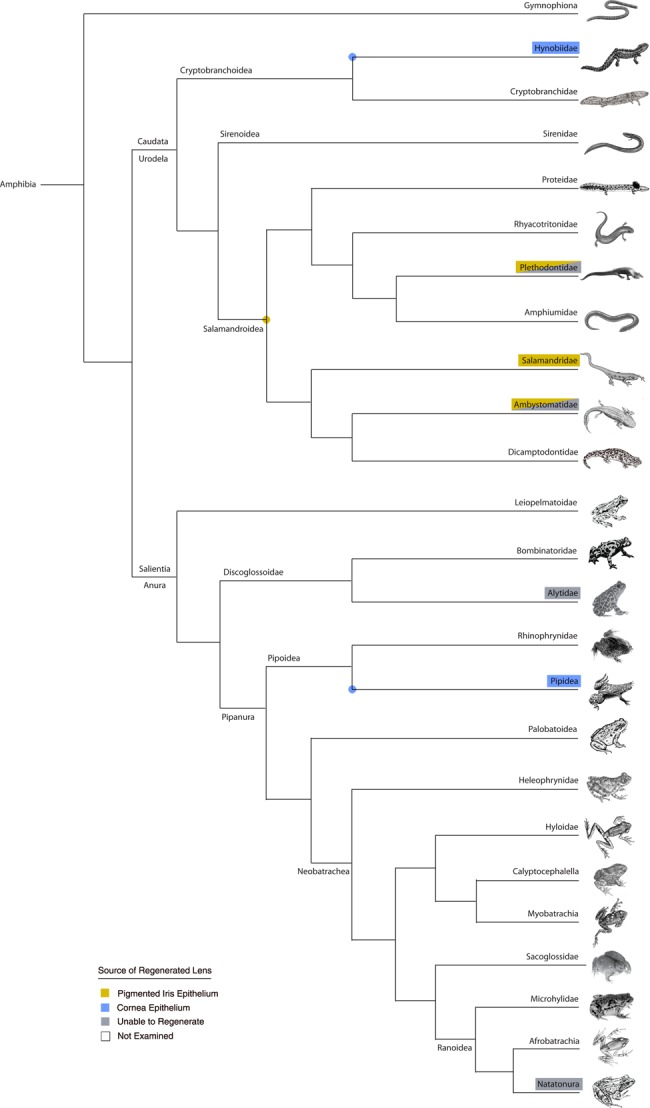
Phylogram showing major amphibian clades and occurrence of verified examples that can and cannot regenerate the lens. The type of lens regeneration is indicated by different colors, as shown in the key. Examples of lens epithelial cell (LEC) regeneration are not mapped onto this particular tree (however, see table 1). Representatives from several families, including the more basal groups, have not yet been examined. Colored dots represent possible presence of that matching character in the common ancestor for those particular nodes or branches. See text and table 1 for further details. Phylogenetic relationships based on Germain and Laurin 2009; Pyron and Wiens 2011; and Feng et al. 2017.
Examples of cornea-lens regeneration are more tightly restricted to frogs in the genus Xenopus, with one other example being the Japanese newt, Hynobious unnanangso, a basal representative within the Cryptobranchoidea (Hynobiidae, Ikeda 1936b, 1939, fig. 3). Anurans represent one of the most diverse groups of tetrapods, comprising over 6700 species distributed among at least 55 different families (Feng et al. 2017). The capacity to regenerate the lens has been specifically examined in some other frog species (including representatives of the Alytidae and Natatonura), yet there is no evidence that those frogs can regenerate a lens (either via cornea-lens regeneration or Wolffian lens regeneration, see table 1 and fig. 3). Of course, the number and range of species examined is relatively small, but it is possible that the capacity for cornea-lens regeneration is highly restricted and may have arisen independently in some members of the Pipidae and the Hynobiidae.
Examples of the third form of lens regeneration that occurs from LECs are found in the Tetrapoda, and include some mammals (e.g., rabbits, pigs, and humans, Gwon 2006, 2008, and see others listed in table 1) and amphibians (both urodeles and anurans, see table 1 and fig. 2). The ability to regenerate a lens from lens epithelial cells, whether that be of a normal or an abnormal form, may be widespread among vertebrates.
Though further investigation is clearly needed, examples of lens regeneration appear to be diverse, sporadically distributed and appear to have arisen independently throughout the vertebrates. On the other hand, given that the result is the same in all these cases, one can ask whether these different processes share a common or convergent set of underlying molecular, regulatory mechanisms?
Examples of Lens Regeneration: Diverse Signaling Mechanisms
Relatively little is known about the molecular pathways that regulate lens regeneration from lens epithelial cells in the animals mentioned earlier and listed in table 1. However, we do understand the roles that certain signaling pathways play during both Wolffian lens regeneration and cornea-lens regeneration (see Henry et al. 2013). Below, updated information is presented from studies undertaken on Wolffian and cornea-lens regeneration, and the deployment of these signaling pathways is compared (no corresponding data yet exists for these signaling pathways in cases of LEC regeneration).
FGF Signaling
FGF signaling plays a number of important roles in lens development (Chamberlain and McAvoy 1987; Donner et al. 2006; Robinson 2006; Garcia et al. 2011; Gunhaga 2011), and also appears to be important during lens regeneration. For instance, treatments with the FGFR inhibitor SU5402 were found to inhibit Wolffian lens regeneration (Del Rio-Tsonis et al. 1998; see also Hayashi et al. 2002). A similar result was obtained by McDevitt et al. (1997) when they injected a synthetic FGF mitotoxin into the eye following lentectomy (FGF-2 coupled with saporin). McDevitt et al. (1997) also showed that there is an asymmetric distribution of FGFR3 receptor protein expression in dorsal versus ventral irides, which appears to be reversed at later stages of lens regeneration. Likewise, Del-Rio Tsonis et al. (1998) showed that FGFR1 protein is expressed in dorsal but not ventral irides during Wolffian lens regeneration. Furthermore, injections of a soluble recombinant, competitive FGFR2 IIIc isoform (FGFR2/Fc), but not a different isoform FGFR2 (IIIb), inhibited lens regeneration (Hayashi et al. 2004).
Similarly, treatments with SU5402 also inhibited cornea-lens regeneration in vitro in cultured eyes from which the original lens had been removed (Fukui and Henry 2011). Furthermore, Arresta et al. (2005) showed that expression of FGFR2 IIIc (the bek isoform) is elevated in the lentogenic cornea epithelium in Xenopus. FGF2R IIIc expression also becomes elevated in head ectoderm, but not flank ectoderm, when those tissues are subjected to the inductive influences of ectopically implanted eyes, which had been inserted at earlier stages of larval development. Flank ectoderm is normally unable to respond to the inductive influences of the neural retina to reform a lens (Freeman 1963; Bosco and Filoni 1992; Cannata et al. 2003; Arresta et al. 2005). Correspondingly, head ectoderm exposed to an ectopic eye, but not the flank ectoderm, gains an increased ability to regenerate a lens when it is subsequently transplanted into the vitreous chamber. Based on these studies, Arresta et al. (2005) argued that elevated FGFR2 IIIc expression is an indicator of activated FGF signaling and confers lens-forming competence in anterior tissues to respond to the retinal signals that trigger lens regeneration.
Although those studies suggest that FGFR activation is necessary during both Wolffian lens regeneration and cornea-lens regeneration, the ligands responsible for activating those receptors appear to be different. FGF signaling appears to be sufficient to trigger transdifferentiation of pigmented epithelial cells from the dorsal iris during Wolffian lens regeneration (Cuny et al. 1986; Hyuga et al. 1993; Kodama and Eguchi 1994, 1995; Del Rio-Tsonis et al. 1997, 1998; McDevitt et al. 1997; Hayashi et al. 2002, 2004). Hyuga et al. (1993) showed that Basic FGF (FGF2) is essential for lens regeneration. Hayashi et al. (2002) also showed that FGF2 or 4 triggered lens development in cultures of dissociated dorsal pigmented iris epithelial cells, but FGF8 and 10 had no effect. Subsequently, Hayashi et al. (2004) verified that FGF2 was required to trigger Wolffian lens regeneration. In the newt, the levels of fgf2 mRNA increases in iris tissues following removal of the lens (Hayashi et al. 2004).
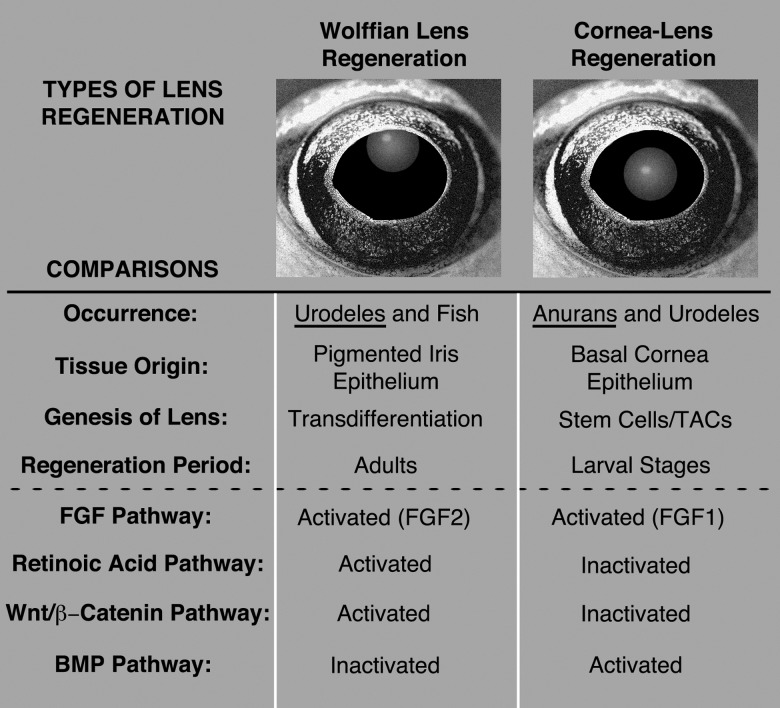
In contrast, FGF1, but no other FGFs tested (including FGF2, 8, and 9), was shown to trigger lens cell differentiation in primary cultures of Xenopus cornea epithelia (Bosco et al. 1994; Bosco, Venturini, et al. 1997; Moore 2015; Moore L and Henry JJ, unpublished data). Therefore, for both Wolffian lens regeneration and cornea-lens regeneration, FGF receptor activation and possibly the key receptor FGFR2 (IIIc) may be similar, but the ligand appears to be different (FGF2 vs. FGF1, see fig. 4).
Fig. 4.
Summary comparing features of Wolffian lens regeneration with cornea-lens regeneration. See text for further explanation. TACs, transit amplifying cells; BMP, bone morphogenetic protein; FGF, fibroblast growth factor; Wnt, Wingless-related integration site.
Wnt Signaling
Wnt signaling is another important signaling pathway involved in the development of the lens (reviewed in Fuhrmann 2008; Fujimura 2016). Several Wnt ligands, receptors, coreceptors, as well as some antagonists, are expressed during lens development, and Wnt signaling is thought to play important roles in the formation of the lens epithelium, as well as in regulating lens fiber cell differentiation in mammals (Stump et al. 2003; Ang et al. 2004; Chen et al. 2004, 2006; Fuhrmann 2008). Although the noncanonical Wnt/Planar Cell Polarity (PCP) pathway is important in regulating the downstream organization of lens fiber development (Chen et al. 2006, 2008; Sugiyama et al. 2011), the canonical Wnt/β-catenin signaling plays a much different role. Active Wnt/β-catenin signaling prevents the surface ectoderm from differentiating toward the lens fate, and it must be suppressed for lens development to occur (Smith et al. 2005; Kreslova et al. 2007; Machon et al. 2010). However, later during lens development this pathway becomes necessary for proper differentiation of the lens epithelium and lens fiber cells (Stump et al. 2003; Chen et al. 2004, 2008).
Several ligands and receptors of the Wnt signaling pathway are expressed in the iris during Wolffian lens regeneration (wnt2b, wnt5a, fz2, and fz4; Hayashi et al. 2006). Hayashi et al. (2006) also treated cultured dorsal irides with the Wnt antagonists DKK1 or SFRP1, which resulted in a significant reduction of successfully regenerated lenses. On the other hand, stimulation of canonical Wnt signaling by the addition of WNT3A not only resulted in larger lenses from dorsal irides but initiated several cases of regeneration from ventral irides, which are typically not capable of regenerating (Hayashi et al. 2006). Together, these results demonstrated that active Wnt signaling in the iris is necessary in order for Wolffian lens regeneration to occur.
The potential involvement of Wnt signaling during the process of cornea-lens regeneration was implicated by the identification of several Wnt signaling components from two independent screens for genes that are expressed during the early events of cornea-lens regeneration (Malloch et al. 2009; Day and Beck 2011). These studies identified several ligands (wnt2, wnt3, wnt5b, wnt6, and wnt7b), receptors (fz7 and fz8), downstream components (axin1, ck2α, dvl2, lrp6, tcf3, tcf7, and tcf7l2), as well as some antagonists (sfrp2, sfrp3, and sfrp5) of the Wnt signaling pathway (Malloch et al. 2009; Day and Beck 2011). Recent functional studies have revealed that, like the initial development of the lens, Wnt/β-catenin signaling must be suppressed in order for cornea-lens regeneration to occur (Hamilton et al. 2016). Using small molecule inhibitors (BIO and 1-azakenpaullone) of glycogen synthase kinase 3, Wnt/β-catenin signaling was held in a state of active signaling that resulted in a significant reduction in the cases of successful lens regeneration. Conversely, suppressing Wnt/β-catenin signaling using the small molecule inhibitor IWR-1, recombinant human DKK1, or heat-shock inducible transgenic expression of Xenopus DKK1, had no effect on the ability of the cornea to regenerate a lens (Hamilton et al. 2016). Consistent with this result, a decrease in active Wnt/β-catenin signaling occurs within cornea epithelial tissue 24 h postlentectomy, which recovers by 48 h (Hamilton et al. 2016). Of particular interest are the Wnt antagonists in the secreted frizzled-related protein family (sfrp2, sfrp3, and sfrp5) that were identified to be up-regulated during the early events of cornea-lens regeneration (Malloch et al. 2009; Day and Beck 2011). It is clear from these observations, that while Wnt/β-catenin signaling is important for both Wolffian and cornea-lens regeneration, the Wnt signaling strategies employed during these two processes are very different (see fig. 4).
Retinoic Acid Signaling
Retinoic acid (RA) signaling is known to play key roles in regulating the development of eye tissues, including the retina, lens, and cornea (Kastner et al. 1994; Enwright and Grainger 2000; Wagner et al. 2000; see review by Cvekl and Wang 2009). Normal morphogenesis of the eye also depends on RA signaling (Hyatt et al. 1996; Molotkov et al. 2006). Furthermore, RA signaling has been shown to induce lens crystallin expression (Gopal-Srivastava et al. 1998).
Retinoic acid signaling has been shown to be required for Wolffian lens regeneration (Tsonis et al. 2000, 2002). Retinoic acid receptors, such as RAR-alpha, are significantly up-regulated in the regenerating lens, particularly at later stages, during fiber cell differentiation. Although the application of exogenous retinoids (including all-trans retinoic acid, 9-cis-retinoic acid, or retinol palmitate) via implanted beads had no significant effect on lens regeneration, inhibition of retinoic acid receptors via application of AGN 193109 (Allergan, which blocks RAR-alpha, beta, and gamma) or AGN 194301 (which inhibits RAR-alpha) was found to inhibit Wolffian lens regeneration. Likewise, drugs that inhibit the enzymes involved in RA synthesis (e.g., disulfiram that inhibits retinal dehydrogenase) also inhibit Wolffian lens regeneration.
Components for retinoic acid metabolism are expressed in the frog cornea, including enzymes involved in retinoic acid synthesis (e.g., aldh1a1, aldh1a2, aldh1a3), as well as P450 cytochrome oxidases that metabolize retinoic acid (i.e., cyp26a1 and cyp26b, Thomas and Henry 2014). Therefore, it was interesting that the application of inhibitors of retinoic acid signaling did not inhibit lens regeneration, when applied to Xenopus eye cultures (including citral, an inhibitor of both retinol and retinal dehydrogenases, and LE-135, an inhibitor of RAR-alpha and beta, Thomas and Henry 2014). Rather, the activation of RA signaling inhibited cornea-lens regeneration. This was verified using several different reagents, including the application of exogenous retinoids (all-trans-retinoic acid or TTNPB, a synthetic retinoid that cannot be degraded by Cyp26), or liarizole, a potent inhibitor of retinoic acid metabolism by Cyp26. Therefore, unlike the case in Wolffian lens regeneration, retinoic acid signaling needs to be inhibited to permit cornea-lens regeneration (fig. 4). Significantly, the application of the pan-RAR antagonist, AGN193109 resulted in some remarkable cases of ectopic lens formation within the cornea in the newt Notopthalmus viridescens (Tsonis et al. 2000).
BMP Signaling
BMP signaling plays many roles during lens development, which includes the establishment of lens-forming competence in the head ectoderm, the process of lens induction via the eyecup, and regulates lens placode formation and lens fiber cell differentiation (Luo et al. 1995; Furuta and Hogan 1998; Wawersik et al. 1999; Belecky-Adams et al. 2002; Faber et al. 2002). A number of BMP and TGF-beta pathway members were found to be expressed in the dorsal iris during the process of Wolffian lens regeneration (Maki et al. 2010). However, Grogg et al. (2005) showed that treatments of explanted newt dorsal irides with either BMP4 or BMP7 reduced the capacity of this tissue to undergo transdifferentiation to form a lens when they were subsequently implanted inside the vitreous chamber. On the other hand, treatments with either chordin or a soluble BMP inhibitor, BMPR-IA, had no effect on lens regeneration in dorsal irides. In another set of experiments, they were able to trigger lens regeneration within some implanted fragments of ventral iris tissue by inhibiting BMP signaling using either chordin or a soluble competitor, BMPR-IA. This is a remarkable finding given that the ventral iris is not normally capable of supporting lens regeneration. Considering the known role of BMP signaling in establishing ventral identity (DeRobertis and Kuroda 2004), Grogg et al. (2005) argued that BMP signaling may act to ventralize iris tissue, which is somehow incompatible with lens regenerative capacity. Therefore, BMP signaling must be inhibited to enable Wolffian lens regeneration (Grogg et al. 2005, fig. 4).
In contrast, Day and Beck (2011) found that BMP signaling is required for cornea-lens regeneration in Xenopus (fig. 4). These investigators used a heat-shock activatable line of transgenic frogs to express noggin, a potent inhibitor of BMP signaling. They found that prolonged expression of noggin inhibits cornea-lens regeneration. Day and Beck (2011) also found that the gene Nipsnap1 is up-regulated during cornea-lens regeneration. Nipsnap1 is a known target of BMP signaling that is expressed in the embryonic eye (Peiffer et al. 2005). BMP5, as well as the gene encoding a protein known to inhibit BMP signaling, Sclerostin domain-containing protein 1 (SOSTDC1), are also up-regulated during Xenopus cornea-lens regeneration (Henry et al. 2002; Malloch et al. 2009). Therefore, the deployment of BMP signaling pathways is also different between Wolffian lens regeneration and cornea-lens regeneration.
Perspectives
It is not difficult to find examples of even closely related organisms that differ in their ability to regenerate a specific organ or tissue, and this raises interesting questions as to whether regenerative processes have evolved independently, or whether they have been lost from a common ancestor over time (Brockes and Kumar 2008; Bely 2010; Bely and Nyberg 2010). To better understand the evolutionary history of lens regeneration, much more work is needed to understand the prevalence of lens regeneration across various taxonomic groups. Particularly, examples of LEC regeneration seem to be widespread, as they can be found in both Mammalia and Amphibia, but LEC regeneration in other major Classes remains largely unstudied (Reptilia, Aves, Osteichthyes). It would be interesting and informative to search for various types of lens regeneration throughout the vertebrates. In addition, one should also examine whether lens regeneration occurs in invertebrates with camera eyes, such as the Cephalopoda (octopus, squids, and cuttlefish) or the Cubozoa (Box jellyfish).
From the information summarized in figure 4, it is apparent that there are substantial differences between Wolffian lens regeneration and cornea-lens regeneration. Based on these observations, we argue that these different types of lens regeneration likely arose independently in different animal lineages, as they appear to use neither conserved nor convergent mechanisms to regulate the process of lens formation. Other examples for independent evolutionary innovations within visual systems include camera eyes in invertebrates and vertebrates, and the recruitment of various proteins for lens crystallins (Wistow and Piatigorsky 1988; Piatigorsky and Wistow 1989; Tomarev et al. 1991; Piatigorsky 1993, 1998; Vopalensky and Kozmik 2009).
Another interesting question is how closely the molecular mechanisms used to regenerate the lens recapitulate those employed during its initial, embryonic development. It is clear that Wolffian lens regeneration uses a unique mechanism, which may make sense in light of the fact that the lens regenerates via transdifferentiation of the iris, which has a different developmental lineage than that of the cornea or lens. On the other hand, cornea-lens regeneration seems to more closely follow the broad cell signaling strategies employed in the surface ectoderm during the initial development of the lens. Both the cornea and lens have a common embryonic origin from head ectoderm that overlies the eyecup.
The findings discussed in this review have significant implications in terms of future attempts to activate lens regeneration in other animals, such as humans. No single system may inform us as to how to trigger this process, as a means to repair or replace damaged lenses, and further studies are needed in a variety of animal models to understand the full range of mechanisms that regulate lens regeneration. In particular, one should look for examples of lens regeneration in more basal vertebrates, such as the chondricthes and members of the Agnatha (Hagfishes and Lamprays), or basal amphibians, like Gymnophiona (Caecilians). Given the importance of good vision and the tremendous significance of this work for the field of regenerative biology and medicine, it seems surprising that relatively few labs are presently studying lens regeneration. In fact, the field has recently lost one of its leading pioneers, Dr Panagiotis (“Takis”) A. Tsonis. We hope this review will encourage more researchers to examine these fascinating and informative phenomena.
Acknowledgments
This work was supported by the National Eye Institute at the National Institutes of Health (grant number EY023979 to J.J.H.). The authors dedicate this review in honor and memory of Dr Panagiotis (“Takis”) A. Tsonis (1953–2016), a leader in the field of lens regeneration.
References
- Agarwal LP, Angra SK, Knosla PK, Tandon HD.. 1964. Lens regeneration in mammals: II Monkeys. Orient Arch Opthalmol. 2:47–59. [Google Scholar]
- Ang SJ, Stump RJ, Lovicu FJ, McAvoy JW.. 2004. Spatial and temporal expression of Wnt and Dickkopf genes during murine lens development. Gene Expr Patterns 43:289–295. [DOI] [PubMed] [Google Scholar]
- Arresta E, Bernardini S, Gargioli C, Filoni S, Cannata SM.. 2005. Lens-forming competence in the epidermis of Xenopus laevis during development. J Exp Zool. 303A1:1–12. [DOI] [PubMed] [Google Scholar]
- Barbosa-Sabanero K, Hoffmann A, Judge C, Lightcap N, Tsonis PA, Del Rio-Tsonis K.. 2012. Lens and retina regeneration: new perspectives from model organisms. Biochem J. 4473:321–334. [DOI] [PubMed] [Google Scholar]
- Belecky-Adams TL, Adler R, Beebe DC.. 2002. Bone morphogenetic protein signaling and the initiation of lens fiber cell differentiation. Development 12916:3795–3802. [DOI] [PubMed] [Google Scholar]
- Bely AE. 2010. Evolutionary loss of animal regeneration: pattern and process. Integr Comp Biol. 50:515–527. [DOI] [PubMed] [Google Scholar]
- Bely AE, Nyberg KG.. 2010. Evolution of animal regeneration: re-emergence of a field. Trends Ecol Evol. 253:161–170. [DOI] [PubMed] [Google Scholar]
- Berardi CA, McDevitt DS.. 1982. Lens regeneration from the dorsal iris in Eurycea bislineata, the two-lined salamander. Experientia 38:851–852. [DOI] [PubMed] [Google Scholar]
- Bloomenbach W. 1787. Speciman physiologiae ccomparatae inter animatea caldi et frigidi sanguinis. Comment Soc Regg Sci Göttingen 8:95. [Google Scholar]
- Bonnet C. 1781. Sur les reproductions des salamanders. Oeuvres Hist Natur Philos. 11:175–179. [Google Scholar]
- Bosco L. 1988a. The problem of lens regeneration in anuran amphibian tadpoles. Acta Embryol Morphol Expr. 9:25–38. [PubMed] [Google Scholar]
- Bosco L. 1988b. Transdifferentiation of ocular tissues in larval Xenopus laevis. Differentiation 39:4–15. [DOI] [PubMed] [Google Scholar]
- Bosco L, Filoni S.. 1992. Relationship between presence of the eye cup and maintenance of lens-forming capacity in larval Xenopus laevis. Dev Growth Differ. 346:619–625. [DOI] [PubMed] [Google Scholar]
- Bosco L, Filoni S, Cioni C.. 1983. Il problema della rigenerazione del cristallino degli anfibi anuri negli stadi post-embryionali. II. Esperinze di asportazione del cristallino in larve di Hyla arborea. Rend Ac Naz Lincei (Ser. VIII) LXXV:92–96. [Google Scholar]
- Bosco L, Filoni S, Cioni C, Bernardini S.. 1987. The problem of lens regeneration in anuran amphibians at postembryonic stages. VI. Lens removal experiments in Rana Graeca tadpoles. Atti Acc Lincei Rend Fis. 8:73–76. LXXXI: [Google Scholar]
- Bosco L, Filoni S, Cioni C, Palmieri O.. 1983. The problem of lens regeneration in anuran amphibians at the post-embryonic stage. V. Lens removal experiments in Bufo viridis tadpoles. Acta Embryol Morphol Exp. 4:149–156. [PubMed] [Google Scholar]
- Bosco L, Filoni S, Paglioni S.. 1981. Experimental analysis of the lens-forming competence of the cornea, iris, and retina in Xenopus laevis tadpoles. J Exp Zool. 216:267–276. [DOI] [PubMed] [Google Scholar]
- Bosco L, Gentili G, Willems D.. 1993. In vivo experimental analysis of lens transdifferentiation in larval Rana italica, Hyla arborea and Xenopus laevis. Anim Biol. 2:3–10. [Google Scholar]
- Bosco L, Sciacovelli L, Valle C.. 1991. Experimental analysis of the lens transdifferentiation process in anuran amphibian tadpoles. Acta Embryol Morphol Exp. 12:83–84. [Google Scholar]
- Bosco L, Sciacovelli L, Willems D.. 1993. Experimental analysis of the lens transdifferentiation process in larval anura. Mem Accad Naz Lincei Ser 9 2:67–85. [Google Scholar]
- Bosco L, Testa O, Venturini G, Willems D.. 1997. Lens fibre transdifferentiation in cultured larval Xenopus laevis outer cornea under the influence of neural retina-conditioned medium. Cell Mol Life Sci. 53:921–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco L, Venturini G, Willems D.. 1994. First evidence of lens-transdifferentiation of larval Xenopus laevis induced by brain-derived acidic FGF. Rendiconti Lincei 53:261–268. [Google Scholar]
- Bosco L, Venturini G, Willems D.. 1997. In vitro lens transdifferentiation of Xenopus laevis outer cornea induced by Fibroblast Growth Factor (FGF). Development 124:421–428. [DOI] [PubMed] [Google Scholar]
- Bosco L, Willems D.. 1994. Differentiation of transplanted lens epithelium of larval Xenopus laevis. Rend Fis Acc Lincei 5:63–69. [Google Scholar]
- Brahma SA, Van Doorenmaalen WJ.. 1968. Studies on lens regeneration in Xenopus laevis. Experientia 24:519–521. [DOI] [PubMed] [Google Scholar]
- Brockes JP, Kumar A.. 2008. Comparative aspects of animal regeneration. Annu Rev Cell Dev Biol. 24:525–549. [DOI] [PubMed] [Google Scholar]
- Call MK, Grogg MW, Del Rio Tsonis K, Tsonis PA.. 2004. Lens regeneration in mice: implications in cataracts. Exp Eye Res. 782:297–299. [DOI] [PubMed] [Google Scholar]
- Cannata SM, Arresta E, Bernardini S, Gargioli C, Filoni S.. 2003. Tissue interactions and lens-forming competence in the outer cornea of larval Xenopus laevis. J Exp Zool A Comp Exp Biol. 299A2:161–171. [DOI] [PubMed] [Google Scholar]
- Chamberlain CG, McAvoy JW.. 1987. Evidence that fibroblast growth factor promotes lens fibre differentiation. Curr Eye Res. 69:1165–1168. [DOI] [PubMed] [Google Scholar]
- Chen Y, Stump RJ, Lovicu FJ, McAvoy JW.. 2004. Expression of Frizzleds and secreted frizzled-related proteins (Sfrps) during mammalian lens development. Int J Dev Biol. 48(8–9):867–877. [DOI] [PubMed] [Google Scholar]
- Chen Y, Stump R, Lovicu F, McAvoy J.. 2006. A role for Wnt/Planar Cell Polarity signaling during lens fiber cell differentiation? Semin Cell Dev Biol. 176:712–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Stump R, Lovicu F, Shimono A, McAvoy J.. 2008. Wnt signaling is required for organization of the lens fiber cell cytoskeleton and development of lens three-dimensional architecture. Dev Biol. 3241:161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioni C, Filoni S, Bosco L.. 1979. The problem of lens regeneration in anuran amphibians at the post-embryonic stage. III. Lens removal experiments in Rana dalmatina tadpoles. Acta Embryol Exp. 3:247–253. [PubMed] [Google Scholar]
- Cioni C, Filoni S, Bosco L, Renzi A.. 1983. Experimental analysis of lens-forming competence of cornea, iris, and retina of Rana esculenta larvae. Acta Embryol Morphol Exp. 4:35–45. [PubMed] [Google Scholar]
- Cocteau LL, D’Etoille L.. 1827. Reproduction du crystallin. J Physiol Exp Pathol. 1:30. [Google Scholar]
- Collucci V. 1891. Sulla rigenerazione parziale deell’occhio nei tritoni: isogenesi e svilluppo-Studio seprimentale. Mem Accad Sci Istt Bologna Ser 5 1:593–629. [Google Scholar]
- Cuny R, Jeanny JC, Courtois Y.. 1986. Lens regeneration from cultured newt irises stimulated by retina-derived growth factors (EDGFs). Differentiation 323:221–229. [DOI] [PubMed] [Google Scholar]
- Cvekl A, Wang WL.. 2009. Retinoic acid signaling in mammalian eye development. Exp Eye Res. 89:280–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day RC, Beck CW.. 2011. Transdifferentiation from cornea to lens in Xenopus laevis depends on BMP signaling and involves upregulation of Wnt signaling. BMC Dev Biol. 11:54.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio-Tsonis K, Jung JC, Chiu I-M, Tsonis PA.. 1997. Conservation of fibroblast growth factor function in lens regeneration. Proc Natl Acad Sci U S A. 9425:13701–13706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio-Tsonis K, Trombley MT, McMahon G, Tsonis PA.. 1998. Regulation of lens regeneration by fibroblast growth factor receptor 1. Dev Dynamics 2131:140–146. [DOI] [PubMed] [Google Scholar]
- DeRobertis EM, Kuroda H.. 2004. Dorsal–ventral patterning and neural induction in Xenopus embryos. Annu Rev Cell Dev Biol. 20:285–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinnean FL. 1942. Lens regeneration form iris and its inhibition by lens reimplantation in Triturus torosus larvae. J Exp Zool. 903:461–478. [Google Scholar]
- Donner AL, Lachke SA, Maas RL.. 2006. Lens induction in vertebrates: variations on a conserved theme of signaling events. Semin Cell Dev Biol. 176:676–685. [DOI] [PubMed] [Google Scholar]
- Enwright JF, Grainger RM.. 2000. Altered retinoid signaling in the heads of small eye mouse embryos. Dev Biol. 2211:10–22. [DOI] [PubMed] [Google Scholar]
- Faber SC, Robinson ML, Makarenkova HP, Lang RA.. 2002. Bmp signaling is required for development of primary lens fiber cells. Development 12915:3727–3737. [DOI] [PubMed] [Google Scholar]
- Feng Y-J, Blackburn DC, Liang D, Hillis DM, Wake DB, Cannatella DC, Zhang P.. 2017. Phylogenomics reveals rapid, simultaneous diversification of three major clades of Gondwanan frogs at the Cretaceous–Paleogene boundary. Proc Natl Acad Sci US A. 11429:E5864–E5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filoni S. 1980. Regeneration capacity of the anuran eye. Boll Zool. 47(3–4):281–301. [Google Scholar]
- Filoni S, Bernardini S, Cannata SM.. 2006. Experimental analysis of lens-forming capacity in Xenopus borealis larvae. Jez-A 3057:538–550. [DOI] [PubMed] [Google Scholar]
- Filoni S, Bernardini S, Cannata SM, D’Alessio A.. 1997. Lens regeneration in larval Xenopus laevis: experimental analysis of the decline in regeneration capacity during development. Dev Biol. 187:13–24. [DOI] [PubMed] [Google Scholar]
- Filoni S, Bosco L, Cioni C.. 1976. Il problema della rigenerazione del cristallino degli anfibi anuri negli stadi post-embryionali. Esperinze di asportazione del cristallino in larve di Rana exculenta e Xenopus laevis. Acta Embryol Exp. 3:319–334. [PubMed] [Google Scholar]
- Filoni S, Bosco L, Cioni C.. 1977a. Il problema della rigenerazione del cristallino degli anfibi anuri negli stadi post-embryionali. II. Esperinze di asportazione del cristallino in larve di Discoglossus pictus. Acta Embryol Exp. 2:155–162. [PubMed] [Google Scholar]
- Filoni S, Bosco L, Cioni C.. 1977b. Ricostituzione del cristallino de frammenti epitheliocapsulari in larve de Rana esculenta. Acta Embryol Exp (Palermo) 1:41–50. [PubMed] [Google Scholar]
- Filoni S, Bosco L, Cioni C.. 1979. Experimental analysis of lens regeneration in Xenopus laevis and Rana esculenta tadpoles by means of xenoplastic grafts. J Exp Zool. 2072:201–216. [Google Scholar]
- Fischel A. 1900. Über die Regeneration der Lainse. Anaqt Hefte 14:1–256. [Google Scholar]
- Fischel A. 1903. Weitere Mittheilungen uuber die Regeeneration der Linse. Arch Entwicklungsmech Org. 15:1–138. [Google Scholar]
- Fischel A. 1921. Über normale und abnorme Entwicklung des Auges. I. Über Art und Ort der ersten Augenanlage sowie über die formale und kausale Genese der Cyklopie. II. Zur Entwicklungsmechanik der Linse. Wilhelm Roux’ Arch EntwMech Org. 49(3–4):383–462. [Google Scholar]
- Freeman G. 1963. Lens regeneration from the cornea in Xenopus laevis. J Exp Zool. 154:39–66. [DOI] [PubMed] [Google Scholar]
- Fuhrmann S. 2008. Wnt signaling in eye organogenesis. Organogenesis 4:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura N. 2016. WNT/β-catenin signaling in vertebrate eye development. Front Cell Dev Biol. 4:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui L, Henry JJ.. 2011. FGF signaling is required for lens regeneration in Xenopus laevis. Biol Bull. 221:137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Hogan BLM.. 1998. BMP4 is essential for lens induction in the mouse embryo. Genes Dev. 1223:3764–3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia CM, Huang J, Madakashira BP, Liu Y, Rajagopal R, Dattilo L, Robinson ML, Beebe DC.. 2011. The function of FGF signaling in the lens placode. Dev Biol. 3511:176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geniz-Galvez JM. 1962. The results of total and partial removal of the lens primordium in the chick embryo. Contribution to the study of lens regenerartin. Anales Del Desarrollo 10:249–267. [Google Scholar]
- Germain D, Laurin M.. 2009. Evolution of ossification sequences in salamanders and urodele origins assessed through event-pairing and new methods. Evol Dev. 112:170–190. [DOI] [PubMed] [Google Scholar]
- Gopal-Srivastava R, Cvekl A, Piatigorsky J.. 1998. Involvement of retinoic acid/retinoid receptors in the regulation of murine alphaB-crystallin/small heat shock protein gene expression in the lens. J Biol Chem. 27328:17954–17961. [DOI] [PubMed] [Google Scholar]
- Grogg MW, Call MK, Okamoto M, Vergara MN, Del Rio-Tsonis K, Tsonis PA.. 2005. BMP inhibition-driven regulation of six-3 underlies induction of newt lens regeneration. Nature 4387069:858–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunhaga L. 2011. The Lens: a classical model of embryonic induction providing new insights into cell determination in early development. Philos Trans R Soc B 3661568:1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn M. 1888. Growth of new lens fibers after spontaneous absorption of traumatic cataract. Tran Opthalmol Soc UK8:126. [Google Scholar]
- Gwon A. 2006. Lens regeneration in mammals: a review. Surv Opthalmol. 51:51–62. [DOI] [PubMed] [Google Scholar]
- Gwon A. 2008. Ch 13. The rabbit in cataract/IOL surgery In: Tsonis PA, editor. Animal models for eye research. San Diego, CA: Academic Press; p. 184–204. [Google Scholar]
- Gwon A. 2009. Ch 9. Tissue engineering of the lens: fundamentals In: Chirila T, Harkin D editors. Biomaterials and regenerative medicine in opthalmology. 1st ed Cambridge (United Kingdom: ): Woodhead Publishing Ltd; p. 243–262. [Google Scholar]
- Gwon A, Enomoto H, Horowitz J, Garner MH.. 1989. Induction of de novo synthesis of crystalline lenses in aphakic rabbits. Exp Eye Res. 496:913–926. [DOI] [PubMed] [Google Scholar]
- Gwon A, Gruber LJ, Mantras C.. 1993. Restoring lens capsule integrity enhances lens regeneration in New Zealand albino rabbits and cats. J Cataract Refract Surg. 19:735–746. [DOI] [PubMed] [Google Scholar]
- Gwon A, Gruber L, Mantras C, Cunanan C.. 1993. Lens regeneration in New Zealand albino rabbits after endocapsular cataract extraction. Invest Ophthalmol Vis Sci. 34:2124–2129. [PubMed] [Google Scholar]
- Gwon AE, Gruber LJ, Mundwiler KE.. 1990. A histologic study of lens regeneration in aphakic rabbits. Invest Ophthalmol Vis Sci. 313:540–547. [PubMed] [Google Scholar]
- Gwon AE, Jones RL, Gruber LJ, Mantras C.. 1992. Lens regeneration in juvenile and adult rabbits measured by image analysis. Invest Ophthalmol Vis Sci. 337:2279–2283. [PubMed] [Google Scholar]
- Hamilton PW, Henry JJ.. 2016. The lens regenerative competency of limbal vs. central regions of mature Xenopus cornea epithelium. Exp Eye Res. 152:94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton PW, Sun Y, Henry JJ.. 2016. Lens regeneration in Xenopus laevis requires suppression of Wnt/β-catenin signaling. Exp. Eye Res. 145:206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Mizuno N, Owaribe K, Kuroiwa A, Okamoto M.. 2002. Regulated lens regeneration from isolated pigmented epithelial cells of newt iris in culture in response to FGF2/4. Differentiation 70(2–3):101–108. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Mizuno N, Takada R, Takada S, Kondoh H.. 2006. Determinative role of Wnt signals in dorsal iris-derived lens regeneration in newt eye. Mech Dev. 12311:793–800. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Mizuno N, Ueda Y, Okamoto M, Kondoh H.. 2004. FGF2 triggers iris-derived lens regeneration in newt eye. Mech Dev. 1216:519–526. [DOI] [PubMed] [Google Scholar]
- Henry JJ. 2003. The cellular and molecular biology of lens regeneration. Int Rev Cytol. 228:195–264. [DOI] [PubMed] [Google Scholar]
- Henry JJ, Carinato ME, Schaefer JJ, Wolfe AD, Walter BE, Perry KJ, Elbl TN.. 2002. Characterizing gene expression during lens formation in Xenopus laevis: evaluating the model for embryonic lens induction. Dev Dynamics 224:168–185. [DOI] [PubMed] [Google Scholar]
- Henry JJ, Elkins ME.. 2001. Cornea-lens transdifferentiation in Xenopus tropicalis. Dev Genes Evol. 211(8–9):377–387. [DOI] [PubMed] [Google Scholar]
- Henry JJ, Mittleman J.. 1995. The matured eye of Xenopus laevis tadpoles produces factors that elicit a lens-forming response in embryonic ectoderm. Dev Biol. 1711:39–50. [DOI] [PubMed] [Google Scholar]
- Henry JJ, Thomas AG, Hamilton PW, Moore L, Perry KJ.. 2013. Cell signaling pathways in vertebrate lens regeneration In: Heber-Katz E, Stocum DL, editors. New perspectives in regeneration. Germany: Springer-Verlag. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JJ, Tsonis PA.. 2010. Molecular and cellular aspects of amphibian lens regeneration. Prog Retin Eye Res. 296:543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JJ, Wever JA, Veragara MN, Fukui L.. 2008. Ch. 6. Xenopus, an ideal vertebrate system for studies of eye development and regeneration In: Tsonis PA, editor. Animal models for eye research. San Diego, CA: Academic Press; p. 57–92. [Google Scholar]
- Hyatt GA, Schmitt EA, MarshArmstrong N, McCaffery P, Drager UC, Dowling JE.. 1996. Retinoic acid establishes ventral retinal characteristics. Development 1221:195–204. [DOI] [PubMed] [Google Scholar]
- Hyuga M, Kodama R, Eguchi G.. 1993. Basic fibroblast growth factor as one of the essential factors regulating lens transdifferentiation of pigmented epithelial cells. Int J Dev Biol. 372:319–326. [PubMed] [Google Scholar]
- Ikeda Y. 1936a. Neue Versuche zur Analyse de Wolffschen Linsregeneration. Arb Anat Inst Kais Jpn Univ Sendai 18:1–16. [Google Scholar]
- Ikeda Y. 1936b. Beiträge zur Frage der Fähigkeit zur Linsenregeneration bei einer Art von Hynobius (Hynobius unnangso Tago). Arb Anat Inst Kais Jpn Univ Sendai 18:17–50. [Google Scholar]
- Ikeda Y. 1937. Uber die Linsenbildung aus dem embryonalen Augenbecher des Hynobius unnangso, Tago, untersucht auf Grund der Transplantation der Augnebecheranlage in verschiedenen Entwicklungastadien. Arb Anat Inst Kais Jpn Univ Sendai 20:17–51. [Google Scholar]
- Ikeda Y. 1939. Zur Frage der Lensenpotenz der Hornhaut in spätembryonalen und larvvalen Stadien bei ener Art von Hynobius (Hynobius unnangso Tago). Arb Anat Inst Kais Jpn Univ Sendai 22:27–52. [Google Scholar]
- Ikeda Y, Amatatu H.. 1941. Über den Unterschied der Erhaltungsmöglichkeit der Linse bei zwei Urodelenarten (Triturus pyrrhogaster und Hynobius nebulosus), die sich bezüglich der Fähigkeit zurr Wolffschen Linsenregeneration voneinander wesentilich verschieden verhalten. Jpn J Med Sci. 8:205–226. [Google Scholar]
- Inoue T, Inoue R, Tsutsumi R, Tada K, Urata Y, Michibayashi C, Takemura S, Agata K.. 2012. Lens regenerates by means of similar processes and timeline in adults and larvae of the newt Cynops pyrrhogaster. Dev Dyn. 24110:1575–1583. [DOI] [PubMed] [Google Scholar]
- Jangir OP, Modi D, Manshi S.. 2005. Effect of vitamin A on lens regeneration in pigs. Indian J Exp Biol. 43:679–685. [PubMed] [Google Scholar]
- Kastner P, Grondona JM, Mark M, Gansmuller A, LeMeur M, Decimo D, Vonesch JL, Dollé P, Chambon P.. 1994. Genetic analysis of RXR alpha developmental function: convergence of RXR and RAR signaling pathways in heart and eye morphogenesis. Cell 786:987–1003. [DOI] [PubMed] [Google Scholar]
- Kodama R, Eguchi G.. 1994. Gene regulation and differentiation in vertebrate ocular tissues. Curr Opin Genet Dev. 45:703–708. [DOI] [PubMed] [Google Scholar]
- Kodama R, Eguchi G.. 1995. From lens regeneration in the newt to in-vitro transdifferentiation of vertebrate pigmented epithelial cells. Semin Cell Biol. 63:143–149. [DOI] [PubMed] [Google Scholar]
- Kojima T. 1939. Beiträge zur Kenntais über die Regeneration der Linse und der Kornea. Acta Med Morphol Nagasaki 1:186–189. [Google Scholar]
- Kreslova J, Machon O, Ruzickova J, Lachova J, Wawrousek EF, Kemler R, Krauss S, Piatigorsky J, Kozmik Z.. 2007. Abnormal lens morphogenesis and ectopic lens formation in the absence of beta-catenin function. Genesis 454:157–168. [DOI] [PubMed] [Google Scholar]
- Lin H, Ouyang H, Zhu J, Huang S, Liu Z, Chen S, Cao G, Li G, Signer RA, Xu Y et al. , . 2016. Lens regeneration using endogenous stem cells with gain of visual function. Nature 531:323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois N, Dawson R, McKinnon AD, Forrester JV.. 2003. A new model of posterior capsule opacification in rodents. Invest Ophthalmol Vis Sci. 448:3450–3457. [DOI] [PubMed] [Google Scholar]
- Lois N, Taylor J, McKinnon AD, Forrester JV.. 2005. Posterior capsule opacification in mice. Arch Ophthalmol. 1231:71–77. [DOI] [PubMed] [Google Scholar]
- Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G.. 1995. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev. 922:2808–220c. [DOI] [PubMed] [Google Scholar]
- Machon O, Kreslova J, Ruzickova J, Vacik T, Klimova L, Fujimura N, Lachova J, Kozmik Z.. 2010. Lens morphogenesis is dependent on Pax6-mediated inhibition of the canonical Wnt/beta-catenin signaling in the lens surface ectoderm. Genesis 48:86–95. [DOI] [PubMed] [Google Scholar]
- Maki N, Martinson J, Nishimura O, Tarui H, Meller J, Tsonis PA, Agata K.. 2010. Expression profiles during dedifferentiation in newt lens regeneration revealed by expressed sequence tags. Mol Vis. 16:72–87. [PMC free article] [PubMed] [Google Scholar]
- Malloch EL, Perry KJ, Fukui L, Johnson V, Wever J, Beck CW, King MW, Henry JJ.. 2009. Gene Expression Profiles of Lens Regeneration and Development in Xenopus laevis. Dev Dyn. 2389:2340–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. 1832. Uber die reproduktion der krystallinse. Berlin: von Graefe und Walther. J Chirurgie Augenheilkunde 17:524. [Google Scholar]
- McDevitt DS, Brahma SK, Courtois Y, Jeanny JC.. 1997. Fibroblast growth factor receptors and regeneration of the eye lens. Dev Dyn. 2082:220–226. [DOI] [PubMed] [Google Scholar]
- McKeehan MS. 1961. The capacity for lens regeneration in the chick embryo. Anat Rec. 141:227–230. [Google Scholar]
- Medvedovicx M, Tomlinson CR, Call MK, Grogg M, Tsonis PA.. 2006. Gene expression and discovery during lens regeneration in mouse: regulation of epithelial to mesenchymal transition and lens differentation. Mol Vis. 12:422–440. [PubMed] [Google Scholar]
- Meyer A, Zardoya R.. 2009. Recent advances in the (molecular) phylogeny of vertebrates. Annu Rev Ecol Evol Syst. 34:311–338. [Google Scholar]
- Middlemore R. 1832. On the reproduction fo the crystalline lens. Lond Med Gaz. 10:344–348. [Google Scholar]
- Mikami Y. 1941. Experimental analysis of the Wolffian lens-regeneration in adult newt, Triturus pyrrhogaster. Jpn J Zool. 9:269–302. [Google Scholar]
- Miliot B. 1872. De la regeneration du crystallin chez quelques mammiferes. J L’Anat Physiol. 8:1. [Google Scholar]
- Mitashov VI. 1966. Comparitive study of lens regeneration in Copitid fishes (Misgurnus fossilis, Nemachilus barbatulus, Namachilus dorsalis) Dokl. Akad Nauk Ser Biol. 3:298–318. [PubMed] [Google Scholar]
- Molotkov A, Molotkova N, Duester G.. 2006. Retinoic acid guides eye morphogenetic movements via paracrine signaling but is unnecessary for retinal dorsoventral patterning. Development 13310:1901–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroy A. 1937. Über die Linsenregeneration bei Urodelen verschiedenen Alters, unter besonderer Berticksichtigung der Metamorphose. Wilhelm Roux’ Arch EntwMech Org. 137:25–33. [DOI] [PubMed] [Google Scholar]
- Moore L. 2015. FGF signaling in Xenpus laevis lens regeneration. Ph.D. Thesis, University of Illinois-Urbana-Champaign.
- Nakamura O. 1935. Temperature influence on lens regeneration in urodels, Triturus pyrrhogaster (Boie). Proc Imp Acad Jpn. 11:121–124. [Google Scholar]
- Niazi IA. 1967. A contribution to the study of lens regeneration capacity in chick embryos. Experientia 2311:970–972. [DOI] [PubMed] [Google Scholar]
- Ogawa C. 1921. Experiments on the regeneration of the lens in Diemyctylus. J Exp Zool. 332:394–407. [Google Scholar]
- Okada TS. 1939. Studies on lens-formation in anuran amphibia. Preliminary observation and experiments. Mem Cell Sci Kyoto 15:139–166. [Google Scholar]
- Okada TS. 1943a. Studies on lens-regeneration in anuran amphibia II. Has the iris of anuran amphibia the power to regenerate lens? Jpn J Med Sci. 11:101–108. [Google Scholar]
- Okada TS. 1943b. Studies on lens-regeneration in anuran amphibia. III. Fate of the corneal transplant in the eye cavity, with particular reference to the lens-transformations obtained by Popov et al., (1936–’39) and Schottee and Hummel (1939) from tissue transplanted to the eye cavity. Jpn J Med Sci. 11:109–126. [Google Scholar]
- Peiffer DA, von Bubnoff A, Shin Y, Kitayama A, Mochii M, Ueno N, Cho KWY.. 2005. A Xenopus DNA microarray approach to identify novel direct BMP target genes involved in early embryonic development. Dev Dyn. 232:445–456. [DOI] [PubMed] [Google Scholar]
- Perry KJ, Thomas A, Henry JJ.. 2013. Expression of pluripotency factors in the larval epithelia of the frog Xenopus: evidence for the presence of cornea epithelial stem cells. Dev Biol. 380:281–294. (Erratum in Dev Biol 2013 Aug 15; 380(2): 363–364 for Figure 7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit T. 1963. A study of lens regeneration in the rabbit. Invest Opthalmol Vis Sci. 2:243–251. [PubMed] [Google Scholar]
- Philippaux JM. 1880. Note sur la production de l’oeil chez la salamander aquatique. Gaz Med Paris 51:453–457. [Google Scholar]
- Piatigorsky J. 1993. Puzzle of crystallin diversity in eye lenses. Dev Dyn. 1964:267–272. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J, Wistow GJ.. 1989. Enzyme/crystallins: gene sharing as an evolutionary strategy. Cell 572:197–199. [DOI] [PubMed] [Google Scholar]
- Powell JA, Powers C.. 1973. Effect on lens regeneration of implantation of spinal ganglia into eyes of the newt, Notophthalmus. J Exp Zool. 183:95–114. [DOI] [PubMed] [Google Scholar]
- Pyron RA, Wiens JJ.. 2011. A large-scale phylogeny of Amphibia including over 2800 species, and a revised classification of extant frogs, salamanders, and caecilians. Mol Phylogenet Evol. 61:543–583. [DOI] [PubMed] [Google Scholar]
- Randolph RL. 1900. The regeneration of the crystalline lens: an experimental study. Johns Hopkins Hosp Rep. 9:237–263. [Google Scholar]
- Reyer RW. 1948. An experimental study of lens regeneration in Triturus viridescens viridescens. I. Regeneration of a lens after lens extirpation in embryos and larvae of different ages. J Exp Zool. 1072:217–267. [DOI] [PubMed] [Google Scholar]
- Reyer RW. 1954. Regeneration of the lens in the amphibian eye. Q Rev Biol. 29:1–46. [DOI] [PubMed] [Google Scholar]
- Reyer RW. 1966. The influence of neural retina and lens on lens regeneration from dorsal iris implants in Triturus viridescens larvae. Dev Biol. 142:214–245. [DOI] [PubMed] [Google Scholar]
- Reyer RW. 1971. DNA synthesis and the incorporation of labeled iris cells into the lens during lens regeneration in adult newts. Dev Biol. 244:533–558. [DOI] [PubMed] [Google Scholar]
- Reyer RW. 1974. Differentiation of lens fibers from lens epithelium in Ambystoma maculatum larvae and the repolarization of reversed, regenerating lenses in adult Notophthalmu viridescens. Am Zool. 14:1302. [Google Scholar]
- Reyer RW. 1977a. The Amphibian Eye: development and Regeneration In: Crescitelli F, editor. Handbook of sensory physiology: vII/5, the visual system in vertebrates. Berlin, Heidelberg, New York: Springer-Verlag; p. 309–390. [Google Scholar]
- Reyer RW. 1977b. Morphological evidence for lens differentiation from intra-ocular implants of lens epithelium in Ambystoma maculatum. Exp Eye Res. 24:511–522. [DOI] [PubMed] [Google Scholar]
- Reyer RW, Stone LS.. 1951. Lens regeneration in Salamandra salamandra salamandra. Anat Rec. 109:341–342. [Google Scholar]
- Reyer RW, Stone LS.. 1955. A reinvestigation of lens regeneration in Salamandra salamandra salamandra. J Exp Zool. 129:257–290. [Google Scholar]
- Robinson ML. 2006. An essential role for FGF receptor signaling in lens development. Semin Cell Dev Biol. 176:726–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T. 1930. Beiträge zur Analyse der Wolffschen Linsenregeration. I. Wilhelm Roux’ Arch EntwMech Org. 122:451–493. [DOI] [PubMed] [Google Scholar]
- Sato T. 1940. Vergleichende Studien uuber die Geschwindigkeit der Wolffschen Linsenregeneration bei Triton taeniatus und Deimycylus pyrrhogaster. Wilhelm Roux’ Arch EntwMech Org. 140:570–613. [Google Scholar]
- Sato T. 1961. Über bei der Cobitiden-Fischen. I. Misgurnus anguillicandatus (Cantor). Embryologia 6:251–290. [Google Scholar]
- Shekhawat DS, Jangir OP, Prakash A, Pawan S.. 2001. Lens regeneration in mice under the influence of vitamin A. J Biosci. 265:571–576. [DOI] [PubMed] [Google Scholar]
- Sikhardldze TA. 1956. Exchange of crystallin lens in rabbits by embryonic skin ectoderm. Bull Acad. Sci Georg. 14:337. [Google Scholar]
- Smith AN, Miller LA, Song N, Taketo MM, Lang RA.. 2005. The duality of beta-catenin function: a requirement in lens morphogenesis and signaling supression of lens fate in periocular ectoderm. Dev Biol. 2852:477–489. [DOI] [PubMed] [Google Scholar]
- Stewert DS. 1960. Further observations on regerented crystalline lenses in rabbits, with special refefence to their refractive qualities. Trans Opthalmol Soc UK 80:357–372. [Google Scholar]
- Stewart DS, Espinasse PG.. 1959. Regenration of the lens of the rabbit. Nature 183:1815.. [DOI] [PubMed] [Google Scholar]
- Stone LS. 1952. An experimental study of the inhibition and release of lens regeneration in adult eyes of Triturus viridescens viridescens. J Exp Zool. 1211:181–223. [Google Scholar]
- Stone LS. 1958a. Inhibition of lens regeneration in newt eyes by isolating the dorsal iris from the neural retina. Anat Rec. 1312:151–172. [DOI] [PubMed] [Google Scholar]
- Stone LS. 1958b. Lens regeneration in adult eyes related to retina pigment cells and the neural retina factor. J Exp Zool. 139:69–84. [DOI] [PubMed] [Google Scholar]
- Stone LS. 1964. Lens regeneration in the cave salamander. J Exp Zool. 155:171–178. [DOI] [PubMed] [Google Scholar]
- Stone LS. 1967. An investigation recording all salamanders which can and cannot regenerate a lens from the dorsal iris. J Exp Zool. 1641:87–103. [DOI] [PubMed] [Google Scholar]
- Stone LS, Chace RR.. 1941. Experimentaal studies on the regenerating leens and the eye in adult Triturus viridesceens. Anat Rec. 793:333–348. [Google Scholar]
- Stone LS, Sapir P.. 1940. Experimental studies on the regeneration of the lens in the eye of anurans, urodeles and fishes. J Exp Zool. 85:71–101. [Google Scholar]
- Stone LS, Steinitz H.. 1953. The regeneration of lenses in eyes ith intact and regenerating retina in adult Triturus viridescens viridecens. J Exp Zool. 124:435–468. [Google Scholar]
- Stump RJW, Ang S, Chen Y, von Bahr T, Lovicu FJ, Pinson K, de Iongh RU, Yamaguchi TP, Sassoon DA, McAvoy JW.. 2003. A role for Wnt/β-catenin signaling in lens epithelial differentiation. Dev Biol. 2591:48–61. [DOI] [PubMed] [Google Scholar]
- Suetsugu-Maki R, Maki N, Nakamura K, Sumanas S, Zhu J, Del Rio Tsonis K, Tsoni PA.. 2012. Lens regeneration in axolotl: new evidence of developmental plasticity. BMC Biol. 10:103.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama Y, Lovicu F, McAvoy J.. 2011. Planar cell polarity in the mammalian eye lens. Organogenesis 73:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AG, Henry JJ.. 2014. Retinoic acid regulation by CYP26 in vertebrate lens regeneration. Dev Biol. 3862:291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomarev SI, Zinovieva RD, Piatigorsky J.. 1991. Crystallins of the octopus lens. Recruitment from detoxification enzymes. J Biol Chem. 266:24226–24231. [PubMed] [Google Scholar]
- Tsonis PA. 2006. How to build and rebuild a lens. J Anat. 2094:433–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsonis PA, Madhavan M, Tancous EE, Del Rio-Tsonis K.. 2004. A newt’s eye view of lens regeneration. Int J Dev Biol. 48(8–9):975–980. [DOI] [PubMed] [Google Scholar]
- Tsonis PA, Trombley MT, Rowland T, Chandraratna RAS, Del Rio-Tsonis K.. 2000. Role of retinoic acid in lens regeneration. Dev Dyn. 219:588–559. [DOI] [PubMed] [Google Scholar]
- Tsonis PA, Tsavaris M, Call MK, Chandraratna RAS, Del Rio-Tsonis K.. 2002. Expression and role of retinoic acid receptor alpha in lens regeneration. Dev Growth Differ. 445:391–394. [DOI] [PubMed] [Google Scholar]
- Valentin G. 1844. Mikroskopische Uuntersuchung zweier wiedererzeugter krystallinsen des kaninchens. Zeitschrift f Ration Medicin Bd 1:227–237. [Google Scholar]
- van Deth JH. 1940. Induction et regeneration du crystallin chez l’embryon de la poulle. Acta Ner Morph Norm Path 3:151–169. [Google Scholar]
- Vigh B. 1960. Über die regeneration der augenlinse des rippenmolches (Pleurodeles waltii). Acta Biol Sci Hungaricae 11:25–33. [Google Scholar]
- Vopalensky P, Kozmik Z.. 2009. Eye evolution: common use and independent recruitment of genetic components. Philos Trans R Soc Lond B Biol Sci. 3641531:2819–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachs H. 1914. Neue Versuche zur Wolffschen Linsenregeneration. Wilhelm Roux’ Arch EntwMech Org. 39(2–3):384–451. [Google Scholar]
- Wagner E, McCaffery P, Dräger UC.. 2000. Retinoic acid in the formation of the dorsoventral retina and its central projections. Dev Biol. 2222:460–470. [DOI] [PubMed] [Google Scholar]
- Wawersik S, Purcell P, Rauchman M, Dudley AT, Robertson EJ, Maas R.. 1999. BMP7 acts in murine lens placode development. Dev Biol. 2071:176–188. [DOI] [PubMed] [Google Scholar]
- Wedlock DE, McCallion DJ.. 1968. The question of lens regeneration form parts of the optic vesicle in the chick embryo. Experientia 246:620–621. [DOI] [PubMed] [Google Scholar]
- Wistow GJ, Piatigorsky J.. 1988. Lens crystallins: the evolution and expression of proteins for a highly specialized tissue. Annu Rev Biochem. 57:479–504. [DOI] [PubMed] [Google Scholar]
- Woerdeman MW. 1922. Über Linseenexstirpation bei Grasfrosclarven. Wilhelm Roux’ Arch EntwMech Org. 511:625–627. [Google Scholar]
- Wolff G. 1894. Bemerkungen zum Darwinismus mit einen experimentellen Beitrag zur Physiologie der Entwicklung. Biol Zbl. 14:609–620. [Google Scholar]
- Wolff G. 1895. Entwicklungsphyiologische Studien. I. Die Regeneration der Urodelenlinse. Wilhelm Roux’ Arch EntwMech Org. 13:380–390. [Google Scholar]
- Wolff G. 1901. Entwicklungsphyiologische Studien. II. Weitere Mittheilungen zur Regeneration der Urodelenlinse. Wilhelm Roux’ Arch EntwMech Org. 12:307–351. [Google Scholar]
- Wolff G. 1901. Entwicklungsphyiologische Studien. III. Zur Analyse Der Entwicklungspotenzen Des Irisepithels Bei Triton Arch Mikr Anat. 63:1–9. [Google Scholar]
- Zalokar M. 1944. Contribution à l’étude de la régénération du crystallin chez le Triton. Rev Suisse Zool. 51:444–521. [Google Scholar]