Abstract
Cell surface carbohydrates have been proven optimal targets for vaccine development. Conjugation of polysaccharides to a carrier protein triggers a T-cell-dependent immune response to the glycan moiety. Licensed glycoconjugate vaccines are produced by chemical conjugation of capsular polysaccharides to prevent meningitis caused by meningococcus, pneumococcus and Haemophilus influenzae type b. However, other classes of carbohydrates (O-antigens, exopolysaccharides, wall/teichoic acids) represent attractive targets for developing vaccines. Recent analysis from WHO/CHO underpins alarming concern toward antibiotic-resistant bacteria, such as the so called ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter spp.) and additional pathogens such as Clostridium difficile and Group A Streptococcus. Fungal infections are also becoming increasingly invasive for immunocompromised patients or hospitalized individuals. Other emergencies could derive from bacteria which spread during environmental calamities (Vibrio cholerae) or with potential as bioterrorism weapons (Burkholderia pseudomallei and mallei, Francisella tularensis). Vaccination could aid reducing the use of broad-spectrum antibiotics and provide protection by herd immunity also to individuals who are not vaccinated.
This review analyzes structural and functional differences of the polysaccharides exposed on the surface of emerging pathogenic bacteria, combined with medical need and technological feasibility of corresponding glycoconjugate vaccines.
Keywords: carbohydrates, glycoconjugates, vaccines, glycoengineering, antimicrobial resistance
This review is intended to analyze structural and functional differences of surface-exposed polysaccharides from emerging pathogenic bacteria and, combined with epidemiological, medical need and technological considerations, identify potential targets for glycoconjugate vaccines in the near future. How advances in the field of glycoconjugate vaccine production (conjugation of natural polysaccharides, chemo-enzymatic approaches, glycoengineering) can support the development of efficacious novel well-defined vaccines is also discussed.
INTRODUCTION
Surface carbohydrates, particularly capsular polysaccharides (CPS), have been proven optimal targets for bacterial vaccines development. Polysaccharide-based vaccines against meningococcus, pneumococcus and Haemophilus influenzae type b were licensed between the 1970s and the early 1980s. Due to their T-cell independent character, they are efficacious in adults, but fail to elicit adequate protection in high-risk groups, such as infants and children under 2 years of age (Peltola et al.1977a,b).
This limitation of polysaccharide vaccines can be overcome by conjugation to a carrier protein, which triggers a T-cell-dependent immune response to the carbohydrate moiety (Costantino, Rappuoli and Berti 2011) and assures efficacious vaccination of children and elderly.
Glycoconjugate vaccines have been used to control a variety of bacterial infections in recent years, and more vaccines are either under development at preclinical level or in clinical trials (Costantino, Rappuoli and Berti 2011).
The glycoconjugate vaccines licensed so far are obtained from CPS or derived fragments (Table 1). However, pathogenic bacteria also display other classes of carbohydrates that might represent good candidates for vaccine development, especially when the pathogen does not produce a capsule (e.g. most of the Shigella species or Vibrio cholerae) or the capsule mimics self-structures (e.g. α-(2→8) polysialic acid capsule of Neisseria meningitidis serogroup B, and the polyhyaluronic acid capsule of Group A Streptococcus) or the pathogen has a high number of strains with different CPS, making vaccine formulation development very complicated.
Table 1.
Examples of glycoconjugate vaccines in the market or in development.
| Type of glycan | Organism (WHO/CDC category of AMR top T high H medium M) | Manufacturer (licensed :L) (clinical:C) (discovery: D) | Saccharide | Approacha | Carrier | Ref |
|---|---|---|---|---|---|---|
| Hib (M) | GSK (L) | PS | SS | TT | CDC (2016) | |
| Sanofi (L) | PS | SS | TT | Zou and Jennings (2009) | ||
| GSK (L) | Oligo | SS | CRM197 | Costantino, Rappuoli and Berti (2011) | ||
| Merck (L) | Size reduced PS | SS | OMPC | Marburg et al. (1986) | ||
| Pfizer(L) | Oligo | SS | CRM197 | Anderson et al. (1986) | ||
| SIIL (L) | PS | SS | TT | Sharma et al. (2012) | ||
| CIGB (L) | Oligo | ST | TT | Verez-Bencomo et al. (2004) | ||
| Hilleman Lab (D) | Size reduced PS | SS | TT | Laferriere et al. (2011; Rana et al.2015; Schneerson et al.1980) | ||
| Bionet-Asia | ||||||
| Hia | NRC Canada (D) | Size reduced PS | SS | CRM197 and Protein D | Cox et al. (2017) | |
| Meningococcus | GSK (L) | Oligo MenC | SS | CRM197 | Costantino, Rappuoli and Berti (2011) | |
| Pfizer (Nuron) (L) | MenC size reduced PS | SS | CRM197 | Ravenscroft, Wheeler and Jones (2010) | ||
| Baxter (L) | MenC PS De-OAc Size reduced | SS | TT | Ravenscroft, Wheeler and Jones (2010) | ||
| Hilleman Lab (D) | MenX | ST | TT | Harale et al. (2015) | ||
| SIIL (L) | MenA Size reduced PS | SS | TT | Ravenscroft, Wheeler and Jones (2010) | ||
| GSK (D) | MenX Ps size reduced | SS | CRM197 | Fiebig et al. (2017); Micoli et al. (2013) | ||
| GSK (L) | MenACWY Oligos | SS | CRM197 | Broker et al. (2009) | ||
| Capsular polysaccharide | Pfizer (L) formerly GSK | MenACWY size reduced PS | SS | TT | Broker, Berti and Costantino (2016) | |
| Sanofi (L) | MenACWY size reduced PS | SS | DT | Ravenscroft, Wheeler and Jones (2010) | ||
| Sanofi (C) | MenACWY | SS | TT | McVernon et al. (2012) | ||
| SIIL (C) | MenACWYX PS | SS | TT, CRM197 | LaForce (2017) | ||
| Pneumococcus (M/H) | Pfizer (L) | 4, 6B, 9V, 14, 18C, 19F, 23F, PS except 18C size reduced | SS | CRM197 | Ravenscroft et al. (2015) | |
| Pfizer (L) | 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 23F PS except 18C size reduced | SS | CRM197 | Ravenscroft et al. (2015) | ||
| GSK (L) | 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, 23F PS except 23F size reduced | SS | Protein D, TT(18C), DT (19F) | Dhillon and Pace (2017) | ||
| Limmatech Biologics (D) | Multivalent | B | rEPA | Ravenscroft et al. (2017) | ||
| Merck (C) | 15 valent | SS | CRM197 | McFetridge et al. (2015) | ||
| CIGB (C) | 1, 5, 6B, 14, 18C, 19F, 23F | NA | TT | Linares-Perez et al. (2017) | ||
| GBS (na/M) | GSK (C ) | Ia, Ib, III PS | SS | CRM197 | Madhi et al. (2013) | |
| GSK (D) | Ia, Ib, II, III, V PS | SS | CRM197 | Kobayashi et al. (2016) | ||
| Various (D or C) | Ia, Ib, II, III, IV, V, VI, VII and VIII Ps | SS | TT and CRM197 | Heath (2016) | ||
| Pfizer (C ) | Multivalent | Platform developed for pneumo conjugates | CRM197 | Kobayashi et al. (2016) | ||
| Staphylococcus aureus (H) | GSK (C) | Type 5 and 8 PS | SS | TT | Levy et al. (2015) | |
| Pfizer (C ) | Type 5 and 8 PS | SS | CRM197 | Nissen et al. (2015); Frenck et al. (2017) | ||
| GlycoVaxyn ( now Limmatech Biologics) (D) | Type 5 and 8 PS | B | rEPA | Wacker et al. (2014) | ||
| Salmonella Typhi (H/M) | NIH (C), GVGH/Biological E (C), Biomed (L), Barath Biotech (L) | Vi PS and Fragments | SS | CRM197, TT, DT, rEPA | MacLennan, Martin and Micoli (2014) | |
| Burkholderia pseudomallei | DSTL (D) | Oligo | ST | TetHc | Scott et al. (2016) | |
| Klebsiella pneumoniae (T/T) | Max Plank Institute (D) | CPS repeating unit | ST | CRM197 | Seeberger et al. (2017) | |
| Shigella (M/H) | Limmatech Biologics (C ) | Sh. dysenteriae type 1 PS | B | rEPA | Hatz et al. (2015); Riddle et al. (2016) | |
| Sh. flexneri 2a PS | ||||||
| NICHHD (C ) | S. sonnei and Sh. flexneri 2a PS | SS | rEPA | Ashkenazi et al. (1999) | ||
| Institute Pasteur (C ) | Sh. flexneri 2a oligo | ST | TT | van der Put et al. (2016) | ||
| O-Antigen | Escherichia coli | Limmatech Biologics/J&J (C) | O1, O2, O6, O25 Expec | B | rEPA | van den Dobbelsteen et al. (2016) |
| Salmonella Paratyphi A and non-typhoidal Salmonella (H/M) | NVGH (D), NIH (C ), IVI (D) | O2 S. Parathyphi A, O9 S. Enteritidis, O4,5 S. Typhimurium | SS | TT, CRM197, DT | MacLennan, Martin and Micoli (2014) | |
| Pseudomonas aeruginosa (T/H) | SSVI/WRAIR (C ) program stopped | O1,2,3,4,5,6,11,12 | SS | EPA | Cryz et al. (1987, 1989); Lang et al. (2004); Schaad et al. (1991) | |
| Klebsiella pneumoniae (T/T) | University Maryland (D) | O1, O2a, O2a,c, O3, O4, O5, O7, O8, O12 | SS | PA flagellin | Simon, Cross and Tennant (2016) | |
| Vibrio cholerae | NIH, Institut Pasteur (D) | O1 (Inaba and Ogawa), O139 | SS; ST | BSA, rEPA, TThc | Gupta et al. (1998); Boutonnier et al. (2001); Chernyak et al. (2002); Wade et al. (2006); Rollenhagen et al. (2009); Alam et al. (2014); Sayeed et al. (2015); Soliman and Kovac (2016) | |
| Francisella tularensis | CCRC-NRCC and DSTL (D) | O-Ag | ST; B | KLH; rEPA | Boltje et al. (2012); Cuccui et al. (2013) | |
| Burkholderia pseudomallei | Academic (D) | OPSII | B; ST | AcrA; | Garcia-Quintanilla et al. (2014); Kenfack et al. (2017) | |
| Moraxella catarrhalis | NRC Canada (D) | Truncated LPS | SS | CRM197 | Cox et al. (2011) | |
| NDCD/NIH (D) | Detox LPS serotype A, B and C | SS | TT, NTHi HMP, UspA, CD, CRM197 | Gu et al. (1998); Hu et al. (2004); Yu and Gu (2005, 2007) | ||
| Teichoic acids | Enterococcus faecalis (H/H) | UML/Leiden University (D) | LTA | ST | BSA | Laverde et al. (2014) |
| PNAG | Acinetobacter baumannii (T/H) and other pathogens | Harvard Medical School, Alopexx (D) | β-(1→6)-oligo glucosamine | ST | TT | Cywes-Bentley et al. (2013); Gening et al. (2009) |
| ExoPS | Pseudomonas aeruginosa (T/H) | Harvard Medical School (C and D) | Polymannuronic acid; alginate | ST | ExoA, Flagellin; TT, KLH, OMV, synthetic peptides | Campodonico et al. (2011); Doring and Pier (2008); Farjah et al. (2015); Farjah et al. (2014); Kashef et al. (2006); Theilacker et al. (2003) |
| Clostridium difficile | Guelph University, Max Planck Institute (D) | PS-I | ST | CRM197 | Broecker et al. (2016a); Martin et al. (2013b) | |
| GSK, Guelph University, Max Planck Institute (D) | PS-II | ST; SS | CRM197, Clostridiumdifficile rToxins, | Adamo et al. (2012); Bertolo et al. (2012); Romano et al. (2014) | ||
| Max Planck Institute (D) | PS-III | ST | CRM197 | Broecker et al. (2016b); Cox et al. (2013); Martin et al. (2013a) | ||
| Cell Wall PS | Group A Streptococcus (GAS) (M/M) | GSK (D) | GAC fragments | ST | CRM197 | Kabanova et al. (2010) |
| Rockefeller University | PS | ST | TT | Sabharwal et al. (2006) | ||
| Various Academic Institutions (D) | GlcNAc deficient PS | ST | Sp0435 | van Sorge et al. (2014) | ||
| Aspergillus fumigatus | Zelinsky Inst. Org. Chem./Institute Pasteur (D) | α-(1→3)-glucans | ST | BSA | Komarova et al. (2015) | |
| Candida albicans (na/M) | GSK, CCRC (D) | β-(1→3)/β-(1→6)-glucans | SS; ST | CRM197 | Adamo et al. (2011, 2014); Bromuro et al. (2010); Liao et al. (2015, 2016); Torosantucci et al. (2005) | |
| Fungal glycans | Alberta University/ Theracarb/Novadigm (D) | β-(1→2)-mannotriose | ST | TT, Candida peptides | Johnson and Bundle (2013); Xin et al. (2008) | |
| Cryptococcus neoformans | Dublin University/J. Hopkins Bloomberg SPH (D) | GXM PS and oligosaccharides | SS; ST | HSA | Casadevall et al. (1992); Devi (1996); Guazzelli, McCabe and Oscarson (2016); Nakouzi et al. (2009) | |
| Mycobacterial glycans | Mycobacterium tubercolosis (H) | Uppsala University/Eurocine AB (D) | AM | SS | Ag85B, TT | Hamasur et al. (2003); Kallenius, Pawlowski and Hamasur (2008) |
Semisynthetic conjugates from natural carbohydrates: SS; Conjugates synthetic carbohydrates: ST; Bioconjugates: B; not available: na.
In these cases, other glycans, such as the O-antigen portion of lipopolysaccharide (LPS) molecules in Gram-negative or cell wall-associated glycans in Gram-positive bacteria, can be sufficiently accessible to the immune system to be taken into consideration as vaccine candidates. Notable examples are V. cholerae (Sayeed et al.2015), Shigella species (Mani, Wierzba and Walker 2016) and Escherichia coli (Huttner et al.2017).
The emerging of antimicrobial resistance (AMR) for some pathogens including, among others, Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella pneumoniae, Acinetobacter baumannii and Clostridium difficile, which are currently not treated by vaccination, is rendering the identification of future candidates more urgent (Garcia-Quintanilla et al.2016). In fact, vaccination could aid reducing the use of broad spectrum antibiotics and provide protection (herd immunity) also for individuals who are not vaccinated (Lipsitch and Siber 2016).
This review is intended to analyze structural and functional differences of surface-exposed polysaccharides from emerging pathogenic bacteria and, combined with epidemiological, medical need and technological considerations, identify potential targets for glycoconjugate vaccines in the near future. How advances in the field of glycoconjugate vaccine production (conjugation of natural polysaccharides, chemo-enzymatic approaches, glycoengineering) can support the development of efficacious novel well-defined vaccines is also discussed.
THE SURFACE CARBOHYDRATE STRUCTURES OF BACTERIA AND FUNGI
The bacterial cell envelope is surrounded by a dense layer of fibrous polysaccharides and glycoproteins, named glycocalyx. This structure helps bacteria to survive in unpredictable and often hostile environment, while it allows the selective passage of nutrients from the outside and waste products from the inside.
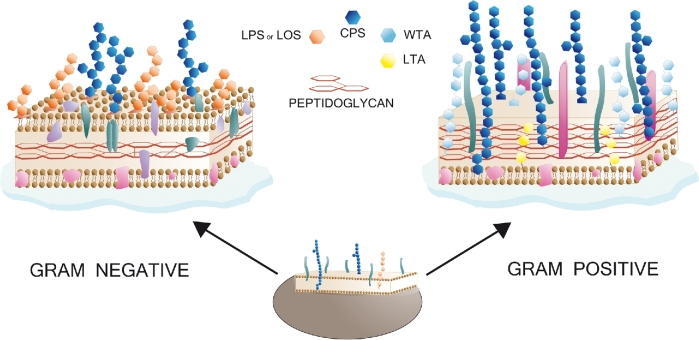
Generally, the capsule is the outermost surface polysaccharide of both Gram-negative and Gram-positive bacteria (Hendrickx et al.2011; Brown et al.2015; Filloux and Whitfield 2016). A given bacterial species might have strains with structurally different CPS resulting in different serogroups or serotypes. Immediately below the capsule, Gram-negative bacteria are characterized by an outer membrane (OM) from which anchored LPS, CPS and membrane proteins protrude. A thin peptidoglycan cell wall is sandwiched between OM and the inner cytoplasmic cell membrane. In contrast, Gram-positive bacteria lack an OM, and are surrounded by a much thicker layer of peptidoglycans compared to Gram-negatives (Fig. 1). Carbohydrates, like β-glucans, mannans, and others, are predominant components of the surface of fungal species (Gow, Latge and Munro 2017). Furthermore, glycosylphosphatidylinositol molecules, which are present on the surface of virtually all eukaryotic cells serving as surface protein anchors, occur at relatively high levels and with specific structures in parasitic protozoa, such as Plasmodium falciparum (Gowda, Gupta and Davidson 1997). The use of parasite carbohydrates as potential vaccine antigens has been recently reviewed and will not be in the scope of the present work (Jaurigue and Seeberger 2017).
Figure 1.
Structures of the cell walls of Gram-negative and Gram-positive bacteria. Both classes of bacteria can produce a capsule (CPS). Gram-negative bacteria express lipopolysaccharide (LPS) or lipooligosaccharide (LOS). Unlike Gram-negative bacteria which possess an outer membrane with an outmost layer rich of phospholipids and LPS molecules, Gram-positive bacteria lack of the outer membrane and possess lipoteichoic acids (LTA) and the more exposed wall teichoic acids (WTA).
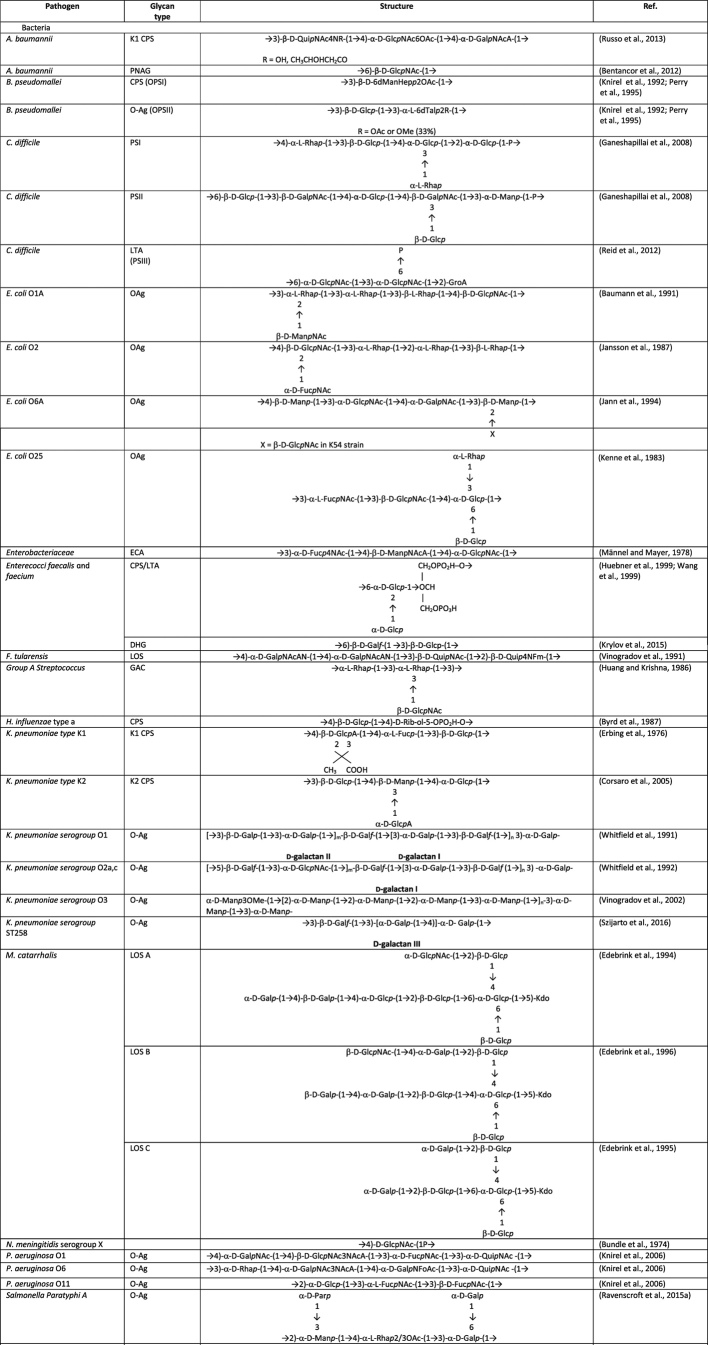
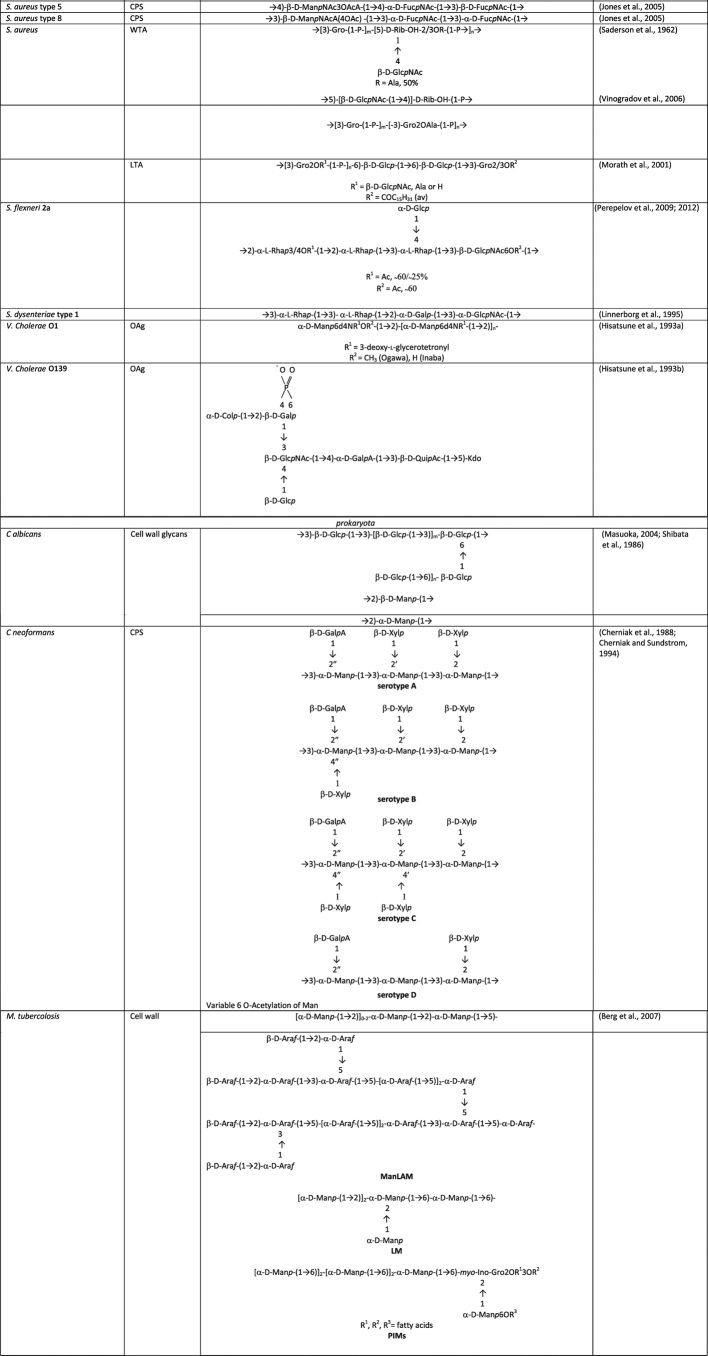
A general description of the different glycans present in bacteria and fungi is given below, and structures of glycans with potential of being used to extend the coverage of licensed glycoconjugate vaccines or for the development of future vaccines are given in Table 2.
Table 2.
Structures of some surface microbial carbohydrates tested as vaccine antigens in preclinical studies or in early clinical phase.
Polysaccharide capsule
CPS are typically made of negatively charged and highly hydrophilic long-chain polysaccharides, firmly anchored to the cell membrane.
CPS are well-established virulence factors, and they can interfere with innate immunity preventing the activation of the alternate complement pathway. Their hydrophilic character protects microorganisms from desiccation, thus facilitating host-to-host transmission, and their chemical structure sometimes mimics molecules produced by human cells so that the pathogen is not recognized as foreign by the immune system.
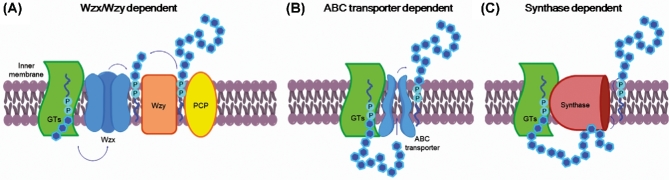
A paradigm of the remarkable structural diversity in CPS is embodied by almost 80 capsular serotypes in E. coli, more than 90 in Streptococcus pneumoniae and about 70 capsular serotypes in K. pneumoniae (Willis and Whitfield 2013). The most studied system for the biosynthesis of CPS is E. coli (Whitfield 2006). Its CPS are classified into four groups, numbered from 1 to 4 on the basis of genetic and biosynthetic criteria. In terms of biosynthesis three main pathways have been identified (Fig. 2): Wzx/Wzy, ATP-binding cassette (ABC) transporter and synthase dependent (Cuthbertson, Kos and Whitfield 2010; Willis and Whitfield 2013). The Wzx/Wzy- and ABC transporter-dependent pathways share some similarities: the polysaccharide is built on a lipid acceptor, usually undecaprenol diphosphate (UndPP), starting from activated building blocks, that are typically cytosolic sugar nucleotides (Fig. 2A). Glycosyltransferase reactions transfer sugars to UndPP, at the cytoplasmic face of the membrane. The final lipid-linked polysaccharide is located outside the cytoplasmic membrane. In the Wzx/Wzy-dependent pathway, the individual repeating units are first assembled and then exported across the membrane by a flippase Wzx protein. The exported UndPP-linked glycans are finally polymerized by a Wzy polymerase, which extends the growing chain one repeat unit at a time at the periplasmic face of the cytoplasmic membrane. A polysaccharide copolymerase controls the polymerization process. Groups 1 and 4 CPS are synthesized according to Wzy-dependent processes. These types of CPS are found in isolates causing intestinal infections, including enteropathogenic, enterotoxigenic and enterohemorrhagic E. coli, and other relevant pathogens, comprising St. pneumoniae and S. aureus (Yother 2011).
Figure 2.
Mechanisms for polysaccharide biosynthesis in bacteria. (A) In the Wzx/Wzy-dependent pathway, the polysaccharide is built on a undecaprenol diphosphate (UndPP) acceptor, on which cytosolic sugar nucleotides are attached by glycosyltransferase (GT) catalyzed reactions an then exported across the membrane by a flippase Wzx protein for final polymerization by a Wzy polymerase, under the control of a polysaccharide copolymerase (PCP). (B) In the ABC transport-dependent pathway, the polysaccharide is built up at the cytoplasmic face of the inner membrane by GTs, and then exported by the ABC transporter. (C) In the synthase-dependent pathway, the polysaccharide is assembled at the cytoplasmic face of the inner membrane by a synthase that is also involved in its transportation across the membrane.
In the ABC transport-dependent pathway, the polysaccharide is completed at the cytoplasmic face of the inner membrane (IM), and then exported by the ABC transporter (Fig. 2B). Group 2 and 3 capsules, generally found in isolates causing extra intestinal infections, are both assembled via this pathway. Their structural features vary extensively and seem reminiscent of N. meningitidis and H. influenzae CPS (Whitfield 2006).
In the synthase-dependent pathway (Fig. 2C), which is the less known of the three mechanisms, a polymerizing glycosyltransferase (the synthase) assembles the polysaccharide at the cytoplasmic face of the IM and is also believed to be involved in its export across the IM (Willis and Whitfield 2013). Serotypes 3 and 37 of St. pneumoniae are known to follow the synthase-dependent pathway for their CPS biosynthesis (Yother 2011).
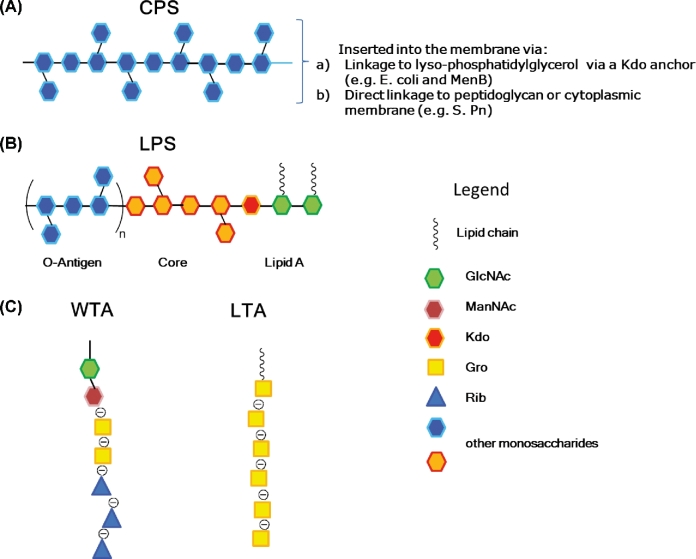
Unlike the CPS biosynthesis, structure and biochemical pathway for the anchor to the cell membrane is less known. In E. coli, N. meningitidis, H. influenzae and other Gram-negative pathogens, this anchor is made of (lyso)phoshatidylglycerol moiety to which CPS is attached via an oligosaccharide of five to nine β-linked 3-deoxy-D-manno-oct-2-ulosonic acid (Kdo) residues (Fig. 3A) (Willis et al.2013). The CPS of Salmonella Typhi (Vi antigen) has a unique lipid terminus composed of a reducing terminal HexNAc residue modified with two β-hydroxy fatty acids that resembles one half of lipid A structure (Liston, Ovchinnikova and Whitfield 2016). Some bacteria, in addition to the LPS molecules carrying the serological O-antigen, produce a CPS linked to a lipid A core, and therefore termed KLPS or K antigen (Whitfield 2006).
Figure 3.
General structures of bacterial surface polysaccharides. (A) Capsules are homopolymeric or heteropolymeric carbohydrate chains inserted into the membrane. (B) LPS is made of three components: lipid A, core-oligosaccharide and O-polysaccharide or O-antigen. LPS lacking of the O-antigen is termed LOS. (C) Teichoic acids are differentiated into lipoteichoic acids (LTA) and wall teichoic acids (WTA).
In most of capsule-forming Gram-positive bacteria, the majority of the polymers is covalently linked to the peptidoglycans or to membrane components, although some may be released from the cell (Yother 2011). There are exceptions, such as St. pneumoniae type 3, where the CPS is bound to the membrane through a phosphatidylglycerol anchor (Cartee, Forsee and Yother 2005).
Glycans associated to Gram-negative bacteria OM
The OM is a distinguishing feature of Gram-negative bacteria. Unlike most biological membranes, the OM is an asymmetrical lipid bilayer. Typically, the inner leaflet is composed predominantly of phospholipids and the outer leaflet of LPS (Raetz and Whitfield 2002; Filloux and Whitfield 2016).
The human innate immune system is sensitized to LPS which is generally an indicator of infection. LPS is responsible for the endotoxic shock associated with the septicemia caused by Gram-negative organisms (Raetz and Whitfield 2002). LPS is made of three components (Fig. 3B): lipid A, core-oligosaccharide and O-polysaccharide (O-PS) or O-antigen (O-Ag). The OM generally contains a complex mixture of LPS molecules, including molecules with only the lipid A and core-oligosaccharide (rough LPS), as well as molecules capped with O-PS (smooth LPS) (Knirel et al.2001).
The typical lipid A structure consists of a glucosamine disaccharide, substituted with fatty acids (Raetz and Whitfield 2002). The acyl chains are largely saturated and facilitate tight packing of OM, playing a critical role in the barrier function of the OM. The core-oligosaccharide is divided into two regions: the inner core consisting of Kdo and L-glycero-D-manno-heptose residues that is highly conserved, and the outer core, which displays limited structural diversity and consists mainly of hexose sugars. The O-Ag domain is made up of repeating units of one or more sugar residues and exhibits remarkable structural diversity. Variations in its composition are often the basis for serotyping classification by serological methods. Although not essential for growth in laboratory culture, O-Ag helps the bacterium to resist certain antimicrobial molecules, the complement system, and environmental stresses in its natural environment (Raetz and Whitfield 2002; Greenfield and Whitfield 2012).
Some Gram-negative bacteria, such as Neisseria spp., Haemophilus spp. and Bordetella pertussis, and in general mucosal pathogens are unable to synthesize O-Ag and produce instead a LPS form called lipooligosaccharide (LOS) that contains the inner core from which one or more mono- or oligosaccharide branches (which determine serological specificity) extend. Pseudomonas aeruginosa can produce a rough LPS, once colonization has been established (Knirel et al.2001).
Biosynthesis of the lipid A is a highly conserved process among Gram-negative species, which occurs partly in the cytoplasm, and partly at the inner leaflet of the IM (Greenfield and Whitfield 2012). The O-Ag is assembled following the same pathways than the CPS, except that the ABC transporter-dependent mechanism of O-Ag biosynthesis seems the most widespread (Fig. 2). The completed O-Ag is transferred from the UndPP linked intermediate and ligated to the lipid A-core in the periplasmic face of the IM. Very seldom the synthase-dependent mechanism is involved.
Glycans associated to Gram-positive bacterial cell wall
Peptidoglycan
Peptidoglycan (PG) is made up of repeating units of the disaccharide N-acetyl glucosamine-N-acetyl muramic acid, which are cross-linked by pentapeptide side chains (Vollmer, Blanot and de Pedro 2008). The PG sacculus is a very large polymer that, because of its rigidity, determines cell shape. The PG layer is much thicker in Gram-positive than in Gram-negative bacteria.
Teichoic and lipoteichoic acids
In Gram-positive bacteria, threading through the layer of peptidoglycans, there are teichoic acids (TA), zwitterionic glycopolymers containing phosphodiester-linked polyol repeat units (Armstrong et al.1958). TA play crucial roles in cell shape determination, regulation of cell division and other fundamental aspects of Gram-positive bacterial physiology. They are divided into lipoteichoic acids (LTA), which are anchored in the bacterial membrane via a glycolipid, and wall teichoic acids (WTA), which are covalently attached to peptidoglycans (Fig. 1) (Brown, Santa Maria and Walker 2013; Sewell and Brown 2014).
Fully extended membrane-linked LTA may not be able to completely penetrate the PG layer and only reach the bacterial surface once released from the membrane (Reichmann and Grundling 2011). WTA extend through and beyond the cell surface more than LTA do (Silhavy, Kahne and Walker 2010), as confirmed by cryo-EM images for S. aureus. WTA are highly abundant modifications of Gram-positive cell walls (Brown, Santa Maria and Walker 2013): in Bacillus subtilis and S. aureus, for instance, they represent up to 60% of the cell wall (Xia, Kohler and Peschel 2010). WTA are made by two components (Fig. 3C): a disaccharide unit that is highly conserved across bacterial species and a main chain polymer composed of phosphodiester-linked polyol repeating units, generally composed of 1,5-D-ribitol-phosphate (RibP) or (1→3)-L-α-glycerol-phosphate (GroP) (Endl et al.1983; Neuhaus and Baddiley 2003).
Structural diversity of WTA can derive from the presence or absence of substituents attached to the backbone (Fig. 2), including cationic D-alanine esters and a variety of mono- or oligosaccharides, commonly Glc or GlcNAc (Collins et al.2002).
LTA has a simpler and more conserved structure that typically consists of a polyglycerolphosphate (PGP) chain (Fischer, Koch and Haas 1983; Fischer et al.1993) (Fig. 3C). Similarly to WTA, the PGP backbone chain of LTA is modified with D-alanine residues, and in many bacteria with additional glycosyl groups.
Other glycans
Another polysaccharide structure found in many species is the poly-β-D-(1→6)-N-acetylglucosamine (PNAG), a polymer with partial N-deacetylation and O-succinyl substituents which is one of the major component of biofilms in S. epidermidis and S. aureus (Joyce et al.2003). Besides cell-to-cell adherence, PNAG also acts as an important virulence factor and protects bacteria against innate host defenses (Little et al.2014).
Synthesis of PNAG in Staphylococci is controlled by an operon, icaADBC, codifying for four proteins responsible of biosynthesis and transport across the IM. A similar operon, pgaABCD, has been found in A. baumannii as well as in the genomes of a number of Gram-negative bacteria, including Yersinia pestis, Y. enterocolitica, E. coli, B. pertussis, B. parapertussis, B. bronchiseptica, Burkholderia cepacia, P. fluorescens, Actinobacillus pleuropneumoniae and Aggregatibacter actinomycetemcomitans (Wang, Preston and Romeo 2004; Tiwary et al.2016), indicating that PNAG is ubiquitous in a number of species.
Mycobacteria and fungal glycans
The architecture of Gram-positive bacteria shares some similarities with mycobacteria and fungi, since they all possess a thick wall outside of their cellular membrane. The cell walls of mycobacteria consist of thin internal layers of peptidoglycans and arabinogalactans, surrounded by a thick layer of micolic acids, glycolipids and cell membrane anchored lipoarabinomannans protruding on the surface. The cell wall of fungi is instead dominated by polysaccharides like mannans (in the form of mannoproteins) and β-(1→3) and β-(1→6) glucans, while a chitin layer is located below (Masuoka 2004; Brown et al.2015; Gow, Latge and Munro 2017).
A particular case is encountered in Cryptococcus neoformans, an important cause of meningitis in Africa especially in those categories who are immunocompromised as a consequence of underlying disease like AIDS. Cryptococcus neoformans displays a polysaccharide capsule which is essential for its virulence and is composed primarily of glucuronoxylomannan and galactoxylomannan (McFadden, De Jesus and Casadevall 2006).
APPROACHES FOR PRODUCTION OF GLYCOCONJUGATE VACCINES
There are a number of methods to prepare glycococonjugate vaccines: some of them are well established and used in licensed products, others are emerging and increasingly applied to vaccines under development. They are mainly based on covalent linkage between CPS and carrier protein; however, it is worth to mention strategies for non-covalent interaction based on CPS biotinylation followed by association to carrier proteins fused with avidin like peptides (Zhang et al.2013), CPS entrapped in cross-linked protein (Thanawastien et al.2015), and liposomal encapsulation of CPS and proteins (Jones et al.2017). Historical attempts to develop glycoconjugate vaccines based on non-covalent association of CPS with proteins, although promising in animal models, have failed in humans (Anderson et al.1985), thus anticipating an intense validation effort for these new attempts. Below the main approaches for glycoconjugate vaccines are discussed, and some examples are schematically reported in Fig. 4.
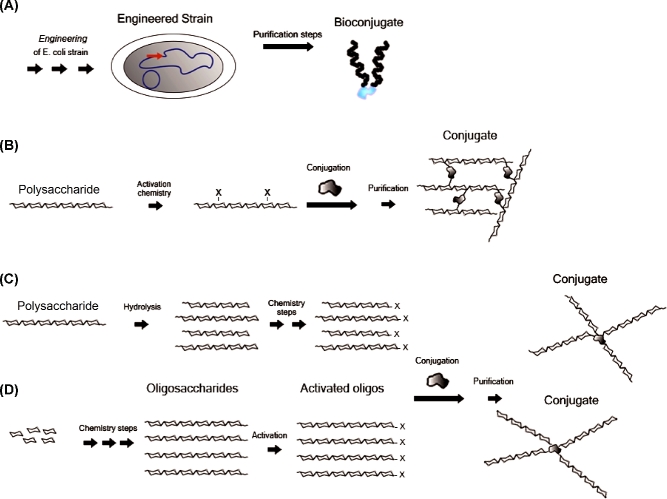
Figure 4.
Approaches for the production of glycoconjugate vaccines include (A) engineering of E. coli for expression of carbohydrate, carrier protein and in vivo conjugation, resulting in glycans radially oriented relative to protein; (B) polysaccharides activation of sugar residues along the chain and conjugation to the carrier protein, resulting in cross-linked structures; (C) polysaccharide fragmentation (hydrolysis or other methods discussed in the text), sizing and conjugation via end terminal residues, resulting in glycans radially oriented relative to protein; (D) construction of the oligosaccharide from appropriate building blocks with an in-built linker for conjugation, also resulting in glycans radially oriented relative to protein.
Semisynthesis: extraction of polysaccharide and carrier protein moieties from bacteria
The classical approach used for glycoconjugate vaccines is based on polysaccharide extraction from bacterial fermentation, subsequent purification and chemical modification to install a covalent linkage between the saccharide and the carrier protein (Costantino, Rappuoli and Berti 2011; Ravenscroft et al.2015; Khatun et al.2017). The carrier protein is also derived from bacteria by fermentation and subsequent purification and, depending on the chemistry used, it can be conjugated via its functional groups or alternatively derivatized before polysaccharide linkage. Adequate linkers are often used to facilitate the conjugation, reducing steric hindrance between protein and saccharide.
In some cases specific chemical moieties of the polysaccharide, such as the carboxyl group of sialic acid residues, are randomly derivatized and subsequently conjugated to the carrier protein. In some other cases, cis-diols present on the saccharide chain can be modified by NaIO4 oxidation in order to generate aldehydes (Fig. 4B). These chemical groups can be directly linked to the ε-amine of the protein lysine residues by reductive amination, or further derivatized before linkage to the protein (Anderson et al.1986; Marburg et al.1986; Zou and Jennings 2009; CDC 2016). Alternatively, the polysaccharides can be fragmented, for example, by acid treatment, and subsequently sized, by means of chromatography or ultrafiltration, to obtain more defined oligosaccharide populations for protein conjugation (Costantino et al.1999; Broker, Berti and Costantino 2016). In this case the protein coupling is generally carried out using the end reducing sugar which can be modified with a spacer bearing appropriate functional group reacting with the protein (Fig. 4C).
Depending on the polysaccharide structure (e.g. H. influenzae type b CPS), NaIO4 oxidation results in simultaneous fragmentation and generation of terminal aldehyde groups available for conjugation. Similarly, treatment of polysialic acids (e.g. N. meningitidis serogroup C) with hydrogen peroxide results in depolymerization and concomitant activation through introduction of carbonyl groups (Ryall 2003; Neyra, Paladino and Le Borgne 2015). Hydrogen peroxide has been used also for depolymerization of Salmonella Typhi (Vi CPS) (Arcuri et al.2017).
Many licensed glycoconjugate vaccines to prevent St. pneumoniae, N. meningitidis and H. influenzae caused infections are prepared based on these approaches. Five carrier proteins have been used in these vaccines: tetanus toxoid (TT), diptheria toxoid (DT), CRM197, the outer membrane protein complex of meningococcus B (OMPC) and Protein D from H. influenzae. For a more technical discussion on this topic, we refer the reader to other reviews (Broker et al.2011, 2017; Costantino, Rappuoli and Berti 2011).
Often the polysaccharides are attached to multiple points of the carrier proteins in a random fashion (Adamo et al.2013). However, recently the possibility to chemically conjugate the glycan at predetermined sites of the carrier protein is emerging (Nilo et al.2015; Stefanetti et al.2015). Also, by means of recombinant techniques, unnatural amino acids with side chains suitable for specific conjugation to the polysaccharide can be inserted in specific regions of the protein sequence (Hu, Berti and Adamo 2016; Kapoor et al.2018). These approaches render the conjugation procedures more selective, allowing for a more rational design of the vaccine and preservation of the key protein epitopes.
Recently, an alternative glycoconjugation method based on dry glycation following periodate oxidation of pneumococcal CPS has been reported (Turner et al.2017).
Synthetic approach: production of the saccharide moiety by means of organic synthesis
The immense progress in the chemical synthesis of carbohydrates seen over the last decades has led to developing protocols for the preparation of a variety of complex bacterial oligosaccharides (Smoot and Demchenko 2009; Morelli, Poletti and Lay 2011) (Fig. 4D).
The climax of this approach has been the development and introduction in Cuban routine vaccination schedule of a conjugate vaccine against H. influenzae type b, where the carbohydrate moiety has been obtained by large-scale synthesis of the capsular oligosaccharide (Verez-Bencomo et al.2004). A glycoconjugate based on synthetic carbohydrate of Sh. flexneri type 2a has recently entered clinical trials (van der Put et al.2016). Synthetic oligosaccharides are generally prepared with a built-in spacer for conjugation at the downstream end, and offer advantages including the avoidance of handling pathogens, lack of bacterial impurities, minimal batch-to-batch variability and higher quality control standards during process manufacturing (Adamo 2017).
The introduction of methods for solid phase automated oligosaccharides synthesis (Seeberger 2015; Hahm et al.2017) and automated polymer supported by HPLC-assisted flow chemistry (Ganesh et al.2012) could simplify and accelerate large-scale production of oligosaccharides. A study of the database ‘glycoscience.de’ showed that a minimal set of 36 monosaccharide building blocks would be sufficient to construct 75% of the catalogued 3299 mammalian oligosaccharides (Werz et al.2007). Despite bacterial glycans are generally characterized by more complex structures than mammalian ones, with numerous branchings and substituents, like acetyl or phosphate groups, this approach has been proven applicable for the fast production of a variety of carbohydrates (Schumann et al.2017).
In addition, other methods, including one-pot protocols (Huang et al.2004; Wu et al.2017) and chemo-enymatic approaches (Wang et al.2013; Fiebig et al.2016; Li and Wang 2016), are accelerating the production of synthetic saccharides rendering their use increasingly attractive, particularly when the polysaccharide is difficult to be purified at high yields, or for the production of more stable sugar mimics (Gao et al.2013).
Bioconjugation approach: engineering E. coli for in vivo glycoconjugate production
The in vivo production of glycoproteins has recently found application in the delivery of a series of glycoconjugate vaccine candidates (Wacker et al.2002). Escherichia coli is engineered with genome integrated pathogen glycan clusters, with an oligosaccharyl transferase (PglB) from Campylobacter jejuni, integrated in the genome or plasmid encoded, and with a plasmid encoding for the carrier protein with N-glycosylation consensus sequences Asp/Glu-Asn-X-Ser/Thr (where X can be any amino acid except proline) in selected sites (Feldman et al.2005; Kowarik et al.2006; Wacker et al.2006) (Fig. 4A). In detail, pathogen glycans repeating units are expressed in the cytoplasm, assembled onto the E. coli lipid carrier UndPP and then flipped across the cytoplasmic membrane. The repeats are polymerized by a polymerase in the periplasmic space, where PglB enables the transfer of the resulting lipid linked oligosaccharides to asparagine residues of the N-glycosylation consensus sequences of the carrier protein.
Biosynthesis of glycoproteins from a number of bacterial polysaccharides, including Salmonella enterica, Shigella spp, E. coli LPS and S. aureus serotype 5 or 8 CPS and St. pneumoniae CPS has been achieved through this technology (Wetter et al.2012, 2013; Wacker et al.2014; van den Dobbelsteen et al.2016; Ravenscroft et al.2017). Selective glycosylation allows for a better exploitation of proteins with the dual carrier/antigen role, as demonstrated for S. aureus α toxin Hla used as carrier for type 5 and 8 CPS (Wacker et al.2014). Phase-1 trials of monovalent vaccines against Shigella dysenteriae O1 and Sh. flexneri 2a (Hatz et al.2015), and a tetravalent anti-extra intestinal pathogenic E. coli (ExPEC) vaccine have been successfully completed (Huttner et al.2017).
Recently, glycoengineering of outer membrane vescicles (geOMVs) for expression of heterologous polysaccharides attached to O-Ag negative lipid A core has been proposed as a novel platform for polysaccharide vaccines (Chen et al.2016; Price et al.2016; Valguarnera and Feldman 2017). OMVs combine antigen presentation with optimal size for immune stimulation and proper adjuvant properties for the presence of Toll-like receptors (TLR) 2 and 4. Price et al. (2016) showed the efficacy of geOMVs as vaccines against St. pneumoniae in mice, and Ca. jejuni in chicken. In another study, Francisella tularensis O-Ag expressed on E. coli OMVs provided protection against F. tularensis (Chen et al.2016).
Cell surface glycans as target for glycoconjugate vaccines
The currently licensed glycoconjugate vaccines (Table 1) were developed based on the epidemiology of bacterial infectious diseases, which registered in the past decades a high incidence of bacterial meningitis caused by N. meningitidis, H. influenzae type b and St. pneumoniae (Pace and Pollard 2007; Pace 2013; Vella and Pace 2015). A number of new glycoconjugate vaccines are being advanced building up on the success of this first generation. Some of them address new pathogens, while others can be considered an extension of current ones to cover additional emerging serotypes.
For example, with the evolving of the pneumococcal epidemiology, non-vaccine serotypes are emerging. Therefore, extension from the current 10- and 13-valent conjugate vaccines (Geno et al.2015; Delgleize et al.2016) up to a 15-valent using CRM197 as carrier is under development (McFetridge et al.2015) (Table 1). The increasing medical need in elderly population and the additional emergence of non-vaccine-serotype disease is expected to driving the development of even higher valence vaccines.
Recent outbreaks in Africa (Boisier et al.2007) have highlighted the need for an anti serogroup X meningococcal vaccine (Xie et al.2013), in addition to the already available A, C, W and Y. Conjugates of CPS X (Table 2) (Bundle, Smith and Jennings 1974) were immunogenic and induced bactericidal antibodies in mice (Micoli et al.2013a). Oligomers of various lengths have also been produced by enzymatic and synthetic methods (Morelli et al.2014; Harale et al.2015; Fiebig et al.2016).
Among the novel targets, a recent analysis from WHO (WHO 2017) and CDC (CDC 2013a) highlights bacteria that are increasingly developing resistance to current antimicrobial therapies and are considered an emerging and serious threat for the public health (Garcia-Quintanilla et al.2016). Most of the resistant bacteria belong to the category of nosocomial pathogens, but there are also examples in the community acquired infectious diseases.
In addition to the CPS, other cell surface glycans are being taken into consideration in the development of novel conjugate vaccines. Moreover, along with the classical semisynthetic chemistry, other approaches, including the use of synthetic carbohydrates or E. coli glycoprotein expression, are increasingly taking place.
Table 1 reports a list of glycoconjugate vaccines which are at different stage: licensed (L), in clinical trials (C) or discovery (D). Proposed surface glycan antigens and utilized approaches are also included.
Below we describe the more relevant pathogens for which a medical need has been identified and the different classes of microbial glycans (CPS, O-Ag and other surface carbohydrates) that have been targeted for vaccine development.
Capsular polysaccharides
Acinetobacter baumannii
The Gram-negative bacterium A. baumannii is the major cause of nosocomial infections and it has frequently been reported in times of war and natural disasters (Fournier and Richet 2006). It affects different human organs, particularly the lungs, causing ventilator-associated pneumonia (VAP) which usually develops to septicemia in intensive care unit residents. Patients at risk are immunocompromised, elderly, premature neonates and patients undergoing surgeries, and its danger has increased due to emerging antibiotic resistance (Peleg, Seifert and Paterson 2008). Recently, a monoclonal antibody against A. baumannii K1 capsule was produced and shown protective in a rat challenge model (Russo et al.2013). The K1 CPS (Table 2) was also demonstrated as a potential vaccine antigen via passive immunization. However, an anti K1 CPS monoclonal antibody (mAb) recognized only 13% of the tested strains, and there is little information on the prevalence in clinical isolates (antigen epidemiology) of the nearly 40 serovars identified so far, which complicate the development of a CPS-based vaccine (Chen 2015).
Burkholderia pseudomallei and mallei
Burkholderia pseudomallei (Bp) is a Gram-negative saprophyte that causes melioidosis. It is highly resistant to harsh environmental pressures, and it is classified as a potential class B bioterrorism weapon due to its high infectivity when aerosolized (Silva and Dow 2013; Peacock et al.2012). The intrinsically high resistance of Bp to several different classes of antibiotics increases the potential danger of this organism. Melioidosis is acquired by skin inoculation, inhalation and ingestion, with pneumonia being the most common clinical presentation. This disease is prevalent in South-East Asia and Northern Australia, and persons with open skin wounds and those with diabetes or chronic renal disease are at increased risk for this infection, particularly among individuals performing agricultural work without health care standard protections.
Bp possesses a CPS (named O-polysaccharide I, OPSI) which was originally identified as an LPS (Knirel et al.1992). OPSI is composed of linear (1→3)-linked 2-O-acetyl-6-deoxy-β-D-manno-heptopyranose residues with O-acetylation at position 2 (Table 2), and it is required for serum resistance and virulence (Perry et al.1995). Similarly to Bp, B. mallei (Bm), the causative agent of glanders, expresses only this single serotype of capsule (DeShazer, Brett and Woods 1998; DeShazer et al.2001; Nelson et al.2004). Therefore, a CPS-based vaccine would potentially offer cross-protection against both pathogens (Scott et al.2014). CPS-BSA conjugates were immunogenic in mice and elicited antibodies protective against infection and opsonic (Nelson et al.2004). A synthetic hexasaccharide of Bp capsule coupled to the Hc domain of TT elicited anti-CPS antibodies in mice that were protective against Bp infection (Scott et al.2016).
Enterococcus faecalis and faecium
Until the late 1970s, Gram-positive enterococci were considered a relatively inoffensive group of pathogens against which effective antibiotics were readily available. Emergence of antibiotic resistance and increased isolation from hospitalized patients, where they account for 11% of nosocomial bloodstream isolates, have pointed out the relevance of Enterococci as nosocomial pathogens (Theilacker et al.2004), overtaken in terms of epidemiology only by S. aureus. It is estimated that in the USA about 66 000 enterococcal infections occur each year, and about 20 000 of these are due to multiple-drug-resistant strains, with about 1300 deaths per year (Reyes, Bardossy and Zervos 2016).
Initially, a CPS with an LTA-like structure composed of α-D-Glcp-(1→2)-α-D-Glcp-(1→2)-Gro-3P (Table 2) was isolated in En. faecalis (Huebner et al.1999; Wang et al.1999). A different surface-exposed polysaccharide composed of glucose, galactose, glycerol and phosphate in a 4:1:1:2 ratio was also reported (Hancock and Gilmore 2002). Based on the analysis of the biosynthetic CPS locus, four serotypes A-D were later described (Hufnagel et al.2004). A recent analysis of clinical En. faecalis isolates indicated that most pathogenic strains belong to serotype C. This suggested that a limited number of En. faecalis capsular serotypes would be needed for developing a broadly active immunotherapeutic agent. However, in another study, a collection of 157 isolates was examined, and only half of them could be typed to any of these four serotypes indicating that serotype diversity might be larger (Hufnagel et al.2006).
Early studies showed that purified CPS depleted the opsonic killing activity of immune rabbit sera, and elicited in rabbit high titers of antibodies mediating opsonic killing of bacteria (Huebner et al.1999, 2000). Approximately one-third of a sample of 15 En. faecalis strains and 7 vancomycin-resistant En. faecium strains were shown to possess shared CPS, target of opsonophagocytic antibodies. CPS also elicited protective antibodies in a mouse model of systemic enterococcal infection (Huebner et al.2000). Anti-CPS antibodies made in rabbits passively protected mice against serologically related enterococcal strains. Furthermore, capsule producing strains of serotype C and D resulted more resistant to complement-mediated opsonophagocytosis than unencapsulated strains (Thurlow et al.2009), supporting the role of the CPS as virulence factor and possible vaccine antigen.
Group B Streptococcus
Group B Streptococcus (Streptococcus agalactiae; GBS) is an encapsulated Gram-positive β-hemolytic pathogen, leading cause of neonatal sepsis and meningitis (Le Doare and Heath 2013). Risk factors for developing invasive GBS include maternal GBS vaginal-colonization, prematurity, prolonged rupture of membranes (>18 h), chorioamnionitis, young maternal age, black race and having a previous infant with invasive GBS disease. Current strategy for prevention of GBS infection in newborns is centered on maternal vaccination with CPS conjugates (Heath 2016). Since, based on the structure of the CPS structure, 10 serotypes can be differentiated, 5 of which are responsible for the majority of the epidemiology, multiple polysaccharides are used for the development of conjugate vaccines. GSK has sponsored phase-1 and -2 trials of an investigational trivalent (Ia, Ib, III) CPS-CRM197 conjugate vaccine, and is currently pursuing pre-clinical studies of a pentavalent (Ia, Ib, II, III, V) CPS-CRM197 vaccine (Kobayashi et al.2016). Pfizer has recently announced to enter clinical trials with a multivalent formulation (Kobayashi et al.2016).
Haemophilus influenzae type a
Among the six different capsulated strains (a–f) of the Gram-negative H. influenzae, b and a are the most infective ones. Hib was the first pathogen against which a conjugated CPS vaccine was developed and introduced in vaccination schedules, with consequent significant decrease of incidence. In recent years, increasing rates of invasive infection due to Hia have been reported in Canada, Alaska, Aboriginal populations in southwestern USA and Australia, and in Brazil (Ribeiro et al.2003; Boisvert and Moore 2015). However, due to lack of comprehensive surveillance programs in many countries, the epidemiological data of Hia-associated diseases are neither complete nor accurately recorded, potentially underestimating the impact of Hia infection worldwide (Ulanova and Tsang 2014). Cases of non-Hib disease have been reported to exhibit AMR to commonly used therapeutic agents making treatment more challenging (Skaare et al.2014). Hia and Hib share a similar CPS structure (Table 2), but no cross-protection is afforded to type a by immunization with Hib conjugate vaccines (Jin et al.2007). Considering the similarity of the two CPS, a conjugate vaccine against Hia is likely to be effective and the development of a vaccine comparable to the current Hib conjugate appears reasonable (Boisvert and Moore 2015). This hypothesis was corroborated by recent conjugation of sized and activated Hia polysaccharide to CRM197 and protein D as carriers (Cox et al.2017). The glycoconjugates were immunogenic in rabbits and elicited bactericidal antibodies.
Klebsiella pneumoniae
Klebsiella pneumoniae is a Gram-negative pathogen belonging to the family of Enterobacteriaceae, implicated in severe infections and outbreaks with high mortality especially for multidrug-resistant infections (Brady et al.2016). In 2013, it was reported among the top five causes of hospital-acquired infection in EU (Suetens et al.2013) and the second leading cause of Gram-negative blood stream infection. Depending on the type of infection and the mode of infectivity, cells of Klebsiella spp. may adhere and attack upper respiratory tract epithelial cells, cells in gastrointestinal tract, endothelial cells or uroepithelial cells, followed by colonization of mucosal membranes. Common underlying conditions include alcoholism, diabetes mellitus, chronic liver disease (cirrhosis), chronic renal failure, cancer, transplants, burns and/or use of catheters. Klebsiella spp. can be transmitted through skin contact with environmentally contaminated surfaces and/or objects, and less frequently by fecal transmission (Janda and Abbott 2006). Seventy-eight capsular antigens (K antigens), leading to different serogroups, have been identified, although about 24/25 were reported to cover about 70% of epidemiology (Podschun and Ullmann 1998). The role of the capsule as virulent factor was demonstrated by pioneering studies by Cryz et al. (Cryz 1983; Cryz, Furer and Germanier 1984). Strains with capsular serotypes K1 and K2 (Table 2) have been identified as the predominant virulent strains, and their virulence has been confirmed in mouse models (Struve et al.2015).
The capsules of K. pneumoniae are complex acidic polysaccharides (CPS) consisting of repeating units composed of four to six sugars, one of which is often an uronic acid (Corsaro et al.2005). The synthetic hexasaccharide repeating unit of the capsule from carbapenem-resistant strains belonging to the sequence type 258 (ST258), found in some isolates in the USA and Israel (Diago-Navarro et al.2014), has recently been demonstrated to bind a specific mAb and to promote, after conjugation, the production of phagocytic antibodies (Seeberger et al.2017).
A 24-valent CPS-based vaccine passed phase 1 in human trials, but the maximum protection coverage never exceeded 70% of the K. pneumoniae strains (Ahmad et al.2012a). Consequently, attention has been addressed to other surface polysaccharides, particularly the O-Ag, which will be discussed later.
Salmonella species
Salmonella enterica serovar Typhi (S. Typhi), which causes the so-called typhoid fever, is still a major problem in low-income countries, such as South and South-East Asia, affecting millions of people each year (Mogasale et al.2014). Vi CPS (Table 2) is currently licensed as a vaccine against typhoid fever (MacLennan, Martin and Micoli 2014). However, being an T-independent antigen, Vi is not immunogenic in infants and is only licensed for children over 2 years of age (Lebacq 2001). While a phase 3 study was reported more than 15 years ago demonstrating high protective efficacy of Vi CPS conjugated to rEPA (Lin et al.2001), only recently Vi-TT and Vi-rEPA conjugate vaccines were licensed in India and China (MacLennan, Martin and Micoli 2014). CPS-based glycoconjugate vaccines against S. Typhi are currently under development by a number of manufacturers (Table 1).
Staphylococcus aureus
Among the Gram-positive bacteria, staphylococci account for a large proportion of hospital-acquired infections (Theilacker et al.2004). High rates are observed for methicillin-resistant S. aureus infections (MRSA), which cause mostly pneumonia, skin-, wound-, bloodstream- and surgical site infections (Theilacker et al.2004). In the USA, the annual incidence of S. aureus bacteremia is of 15–17 cases per 100 000 population, of which nearly half are due to MRSA (Hidron et al.2008), justifying the need for vaccination. Although there are at least 12 capsular types, CPS5 and 8 (Jones 2005) (Table 2) comprise ∼85% of blood infections, and their use for vaccine development was explored (Robbins et al.2004). A single unadjuvanted dose of the bivalent vaccine composed of S. aureus CPS5 and 8, bound to rEPA, showed a trend of efficacy over the first 40 weeks postvaccination (Fattom et al.2004a,b). However, the same vaccine did not show benefit compared to placebo when tested in further trials in end-stage renal disease patients as target population (Fattom et al.2015). CPS5 and 8 conjugated to TT, in combination with mutated detoxified alpha-toxin and clumping factor A (ClfA), with and without the adjuvant AS03B, were safe and induced a strong humoral response in a phase-1 clinical trial with healthy adults conducted by GSK (Levy et al.2015). Also CRM197 conjugates of CPS5 and 8 in mixture with ClfA and manganese transporter C, without adjuvant, were well tolerated and immunogenic in a phase-1 clinical trial conducted by Pfizer (Nissen et al.2015; Frenck et al.2017).
O-Antigens
O-Ag components of LPS molecules have been recognized as virulence factors and suggested as potential vaccine candidates for different pathogens.
Burkhoderia pseudomallei and mallei
LPS from Bp, generally referred as OPSII, is genetically related and structurally similar to the one from Bm. The O-Ag structure consists of a linear heteropolymer of a disaccharide composed of β-D-glucopyranose (1→3)-linked to 6-deoxy-α-L-talopyranose (Table 2). While some studies have focused on the OPSI capsule as unique antigen for a vaccine, others indicate OPSII to be required for serum resistance and virulence (DeShazer, Brett and Woods 1998; DeShazer et al.2001). LPS-specific monoclonal antibodies were proven passively protective in animal models of infection (Treviño 2006; AuCoin 2012). Interspecies variations within the O-Ag lie in the different substitutions of the 6-deoxytalose residues, particularly O-acetylation at both C4 and C2 and O-methylation at C2 (Brett, Burtnick and Woods 2003). O-Acetylation at the C4 position has been detected in significant amounts in Bp, whereas it is absent in Bm strains (Heiss et al.2013). In a recent study, among a panel of seven disaccharides variably substituted, the disaccharide with a 2-O-acetylated-3-O-methylated 6d-Tal unit showed the best binding to a LPS-specific mAb, known to be passively protective in mouse models of melioidosis and glanders, and gave the highest anti-LPS immune response after conjugation to CRM197 (Kenfack et al.2017). A bioconjugate of OPSII with a Campylobacter protein AcrA was shown to be immunogenic in mice and moderately increased protection of mice after intranasal challenge (Garcia-Quintanilla et al.2014).
Escherichia coli
Escherichia coli is a Gram-negative bacterium that can be broadly classified as either diarrheagenic or extra-intestinal pathogenic E. coli (Croxen and Finlay 2009). ExPEC cause a broad variety of infections including urinary tract infections (UTI) and bacteremia, an increasing problem in the aging population (Russo and Johnson 2003). The emergence of antibiotic-resistant strains has resulted in increased numbers of hospitalizations for UTI, high risk of death in patients with bacteremia and intensified treatment costs (Zilberberg and Shorr 2013). O-Ag specific antibodies confer protection against E. coli infections (Sarkar et al.2014). The immunogenicity and safety of a tetravalent ExPEC vaccine produced by the process of bioconjugation, composed of the O1, O2, O6 and O25b antigens (Table 2) linked to rEPA, showed good immunogenicity in animal models (van den Dobbelsteen et al.2016). This bioconjugate vaccine candidate, co-developed by Limmatech Biologics AG and Janssen Pharmaceuticals Inc., was well tolerated and elicited functional antibody responses against all vaccine serotypes in women with a history of recurrent UTI (Huttner et al.2017).
Francisella tularensis
Francisella tularensis is a highly-infectious Gram-negative bacterium that causes the rapid, and often lethal disease, tularemia (Rowe and Huntley 2015). It has been classified by the Center for Disease Control and Prevention as a category A bioweapon (Dennis et al.2001). Humans can acquire this infection through several routes including a bite from an infected tick, deerfly or mosquito, contact with an infected animal or its dead body, drinking contaminated water and breathing contaminated dirt or aerosol. Clinical manifestation of the disease is dependent on the biotype, inoculum and port of entry (Ulu-Kilic and Doganay 2014).
A key factor in the biology of this bacterium is LPS, which poorly activates proinflammatory responses due to its lack of interaction with TLR4. LPS molecules can be modified by various carbohydrates, including Glc, Man and GalNAc, affecting various aspects of virulence. Mutants devoid of O-Ag (Table 2) show reduced intracellular survival and mouse virulence. The inability of the LPS to alarm the immune system coupled with its frequent modification/alteration likely aid the success of this pathogen during human infection (Gunn and Ernst 2007). An OAg-rEPA bioconjugate was successfully produced (Cuccui et al.2013) and resulted able to boost IgG levels and significantly increase the time to death upon subsequent challenge with F. tularensis. The inner core region of the LPS of F. tularensis was synthesized and proved to be recognized by antiserum against LPS and a live vaccine strain, supporting to further explore this compound as a vaccine candidate (Boltje et al.2012). O-Ag displayed on OMVs from a hyperblebbing E. coli strain induced high levels of specific IgG titers, as well as vaginal and bronchoalveolar IgA antibodies, and provided protection against challenge with F. tularensis strain (Chen et al.2016).
Klebsiella pneumoniae
In contrast to the large number of capsular serotypes, K. pneumoniae has only nine LPS O groups, and in a recent study serotypes O1, O2 and O3 accounted for 80% of infections (Follador et al.2016). The O-Ag is accessible to antibodies in encapsulated strains (Rukavina et al.1997; Ahmad et al.2012a). However, it is unclear whether this is true for most of clinical isolates, since in some K serogroups O-Ag appears masked by CPS (Tomás et al.1991).
Conjugate vaccine of the O-Ag from K. pneumoniae M 10 and iron-regulated cell surface proteins of the same organism was found immunogenic and protective against challenge in a rat lobar pneumonia model (Chhibber and Bajaj 1995). Immunization of rats with an O-Ag TT conjugate decreased bacterial colonization in lungs, and resulted in activation of alveolar macrophages capable of bacterial phagocytosis in vitro (Chhibber, Rani and Vanashree 2005).
The O1 O-Ag chemically linked to Klebsiella OM proteins elicited immunoglobulins against different Klebsiella infections, which were transferred via placenta to the offspring of the vaccinated rabbits (Ahmad et al.2012b). A non-toxic and immunogenic form of K. pneumoniae LPS was obtained by incorporation of the native preparation into liposomes (Chhibber, Wadhwa and Yadav 2004).
O1 and O2 O-Ag share a similar polygalactose structure, termed D-galactan-I, except that O1 is shielded by the outer repeating units (D-galactan-II, Table 2) (Whitfield et al.1991,1992; Vinogradov et al.2002).
Recently, it was found that the vast majority of ST258 isolates, a globally disseminated drug-resistant nosocomial strain, express a modified D-galactan-I O-Ag, termed D-galactan-III (Szijarto et al.2016). Since 83% of the more than 200 ST258 draft genome sequences currently available carry the corresponding operon, these isolates are predicted to express D-galactan-III antigens. Accordingly, a D-galactan-III specific mAb was produced, showing to bind to extracted LPS from a panel of ST258 isolates, irrespective of the distinct capsular antigens expressed. Based on these data, the D-galactan-III antigen may represent an attractive target for immunization approaches against K. pneumoniae ST258.
Conjugates of polysaccharides from different serovars (Table 1) to P. aeruginosa flagellin have been proven to induce protective antibodies (Simon, Cross and Tennant 2016). Attempts to target common motif in bacterial LPS such as the core tetrasaccharide Hep2Kdo2 have been recently made. The structure was synthesized and covalently attached to DT. Rabbit serum elicited against the conjugate was reactive to N. meningitidis strains as well as E. coli strain St1052 (W3110) and P. aeruginosa serotype O6 reference strain (St4017). The serum enabled N. meningitidis bacterial killing, when combined to an inhibitor of CPS transport (Kong et al.2016).
Moraxella catarrhalis
Moraxella catarrhalis, also known as Branhamella catarrhalis, and previously known as Neisseria catarrhalis or Micrococcus catarrhalis, is a Gram-negative, aerobic diplococcus, frequently found as a commensal of the upper respiratory tract, particularly in children.
Besides being recognized as the primary cause of acute otitis media after St. pneumoniae and H. influenzae (Enright and McKenzie 1997), M. catarrhalis is also implicated as a pathogen in bronchitis, sinusitis and laryngitis in adults and children and is a major cause of bronchopneumonia and exacerbation of existing chronic obstructive pulmonary disease (COPD) in elderly patients and long-term heavy smokers with chronic pulmonary disease (Sethi and Murphy 2001). It can also cause nosocomial infection, particularly in respiratory, pediatric and intensive-care units (Richards et al.1993). The clinical management of patients infected with M. catarrhalis relies predominantly on antimicrobial agents, and growing global emergence of β-lactamase-producing strains (Verduin et al.2002) has highlighted the need for vaccination (Kaieda et al.2005).
The LPS from M. catarrhalis lacks of a full-length O chain, and therefore the LOS is a possible virulence factor in the pathogenesis of human infections (Fomsgaard et al.1991). Serological studies have identified three M. catarrhalis LOS types (Table 2): A, B and C, representing 61%, 29% and 5%, respectively, of the 95% of the total 302 isolates tested from different geographic locations (Vaneechoutte et al.1990). The inner core is conserved among the three serotypes, while the difference lies in the diversity of their oligosaccharide branches (Edebrink et al.1994, 1995, 1996). Therefore, this LOS might be a good candidate for a vaccine antigen (Verduin et al.2002). Sera from lower respiratory tract-infected patients recognized M. catarrhalis LOS (Rahman et al.1995 ), and convalescent sera from COPD patients possessed IgA antibodies against this molecule (Murphy et al.2005).
Recent studies identified a mAb recognizing a common LOS epitope and facilitating complement killing of M. catarrhalis strains from all three major serotypes (Gergova et al.2007). Immunization with detoxified LOS A, B and C coupled to carrier proteins produced in mouse and rabbit models sera inducing complement-mediated bactericidal activity against homologous and some heterologous strains (Gu et al.1998; Yu and Gu 2005, 2007). Mouse antisera elicited by detoxified LOS conjugated to OMP CD or to UspA proteins showed high titers of specific anti-LOS antibodies, with complement-dependent bactericidal activity toward M. catarrhalis. In addition, mice immunized with both conjugates showed a significant enhancement of the clearance of M. catarrhalis from lungs compared with control mice (Hu et al.2000, 2004).
Neisseria gonorrhoeae
Neisseria gonorrhoeae is the causative agent of the sexually transmitted disease gonorrhea. WHO reports an estimated global incidence of over 106 million cases per year, with a 21% increase in incidence having occurred between 2005 and 2008 (WHO 2012; Edwards et al.2016). A 11% increase in the number of cases has been reported in the USA from 2009 to 2013 (CDC 2013b) and a 90% increase in Australia from 2009 to 2014 (NNDSS 2015). Incidence is likely underestimated due to inadequate surveillance and diagnostics methods in many regions, as well as the high number of asymptomatic cases. Gonococcus has developed resistance to all classes of antibiotics used to treat it over the past seven decades, including the sulphonamides, penicillins, tetracyclines, macrolides and quinolones (Unemo 2015). As other Neisseria species, gonococcus biosynthesizes a core LPS pentasaccharide (Table 2), of which extensions from the LOS core heptoses (HepI and HepII) are phase variable. The mAb 2C7 which attenuates gonococcal burden in the mouse vaginal colonization model is directed to LOS. Sugar motifs responsible for total, partial or no complement-dependent killing by mAb 2C7 have been identified (Yamasaki et al.2010; Chakraborti et al.2016). Heptose-monophosphate (HMP) found in N. gonorrhoeae core LOS was found as the link between gonorrhea and HIV, since it activates CD4 + T cells to invoke an NF-κB–dependent transcriptional response that drives HIV-1 expression and viral production (Malott et al.2013). The 2C7 epitope is a conserved oligosaccharide (OS) structure expressed by 94% of gonococci that reside in the human genital tract and by 95% of first passaged isolates (Gulati et al.1996).
A peptide mimic (called PEP1) as an immunological surrogate of the 2C7-OS epitope and reconfigured into a multi-antigenic peptide (MAP1) was investigated. Mice immunized with MAP1 developed a Th1-biased anti-LOS IgG antibody response that was also bactericidal, resulting in reduction of the carriage length (Gulati et al.2013).
Non-typeable Haemophilus influenzae
Non-typeable Haemophilus influenzae (NHTi) strains lack the polysaccharide capsule, and their virulence is associated with multiple factors, including LOS. So far, the only known natural habitat of H. influenzae is the human respiratory tract. The hallmark of NTHi is heterogeneity, and this has been the major obstacle for developing a successful vaccine. H. influenzae cause a wide spectrum of diseases ranging from respiratory tract infections to severe invasive disease, such as meningitis, sepsis, bacteraemic pneumonia and epiglottitis (Cerquetti and Giufre 2016). After introduction of Hib vaccination, a marked change in the predominant invasive serotype from Hib to NTHi has taken place. Invasive NTHi disease occurs across all age groups and account for 77% of all notified invasive H. influenzae cases in Europe. NTHi is also the most frequently isolated bacterial pathogen in otitis media and sinusitis in children (Murphy, Bakaletz and Smeesters 2009). Acute exacerbations in COPD in adults are almost exclusively associated with NTHi isolates (Soriano and Lamprecht 2012). NTHi colonization/infection is also quite common in young children with cystic fibrosis (CF). In developing countries, NTHi is the major cause of hearing loss, affecting an estimated 65 million to 300 million people globally (Murphy 2015).
Vaccines to prevent otitis media and COPD will have a broad impact in reducing antimicrobial use and resistance (Murphy 2015).
NTHi LOS is structurally and antigenically heterogeneous. To date, 10 serotypes have been identified (Campagnari et al.1987; Patrick et al.1987). Detoxified LOS (dLOS) conjugated to proteins resulted immunogenic in mice and rabbits and conferred T-cell-dependent immunological protection against otitis media in chinchillas (Gu et al.1996, 1997; Sun et al.2000). Intranasal immunization with a dLOS-TT conjugate elicited LOS-specific mucosal and systemic immunity, which enhanced not only the homologous but also heterologous bacterial clearance in mouse nasopharynx (Hirano et al.2003). NTHi OM protein P6 was evaluated as carrier for dLOS, due to its conservation and potential to elicit bactericidal antibodies (Wu et al.2005). Animal studies revealed that P6 could serve as an effective carrier for dLOS.
Peptides that mimic NTHi LOS, conjugated to KLH, were able to induce anti-LOS antibodies in rabbits (Hou and Gu 2003; Balakrishnan 2017). Passive immunization with the anti-LOS sera resulted in enhanced pulmonary bacterial clearance in a mouse model that could be eliminated after pre-absorption of the sera with LOS.
Pseudomonas aeruginosa
Pseudomonas aeruginosa (PA) is a Gram-negative, ubiquitous bacterium, capable of both aerobic and anaerobic growth that can survive on minimal nutritional requirements and tolerate harsh physical conditions, persisting in both community and hospital settings. Serious infections with PA are predominantly hospital acquired (Sharma, Krause and Worgall 2011) and PA has highest mortality rate (37%) of nosocomial infections (Klevens, Edwards and Gaynes 2008; Lister, Wolter and Hanson 2009).
PA may cause fulminant and acute VAP (Klompas et al.2011; Sandiumenge and Rello 2012), be a colonizer in COPD or cause a chronic infection in CF patients (Sharma, Krause and Worgall 2011), with slowly progressive deterioration of pulmonary function as well as non-CF bronchiectasis and COPD patients (Kraemer et al.2005; Veesenmeyer et al.2009).
Antibiotic resistance of PA is a major concern. Antibiotics can alter the bacterial flora in the upper respiratory tract, favoring colonization of resistant nosocomial pathogens and subsequent pneumonia. Treatment of infections can be very challenging, since most PA are resistant to at least one of the classes of antibiotics, and a few PA are resistant even to all of the antibiotics available (Talbot et al.2006; Pier 2007).
At least 20 different O-Ag structures can be distinguished, although only about 11 of these are expressed in the majority of clinical PA isolates. The lipid A shows variation from CF to bronchiectasis patients in the acylation pattern. Also O-Ag shows variability, as transition to chronic infections is correlated with changes in LPS from smooth (with O-Ag) to rough (with no O-Ag). The core structure seems to remain identical. The most commonly isolated serotypes in acute infection are O1, 6 and 11 (Table 2), yet there is a quote of isolates not expressing O-Ag (Jennings et al.2015).
A heptavalent vaccine prepared from LPS of seven different serotypes, called Pseudogen, showed efficacy in preventing fatal PA infections in non-randomized trials among adult cancer and burn patients, but these vaccines were limited by toxicity and showed no benefit moreover in studies with leukemia and CF patients (Alexander, Fisher and MacMillan 1971; Haghbin, Armstrong and Murphy 1973; Young, Meyer and Armstrong 1973; Pennington et al.1975; Hortobagyi et al.1978). When Pseudogen was tested in CF patients already infected with PA, the patients did clinically worse compared with non-vaccinated controls, perhaps due to exacerbation of inflammation engendered by vaccination (MacIntyre, McVeigh and Owen 1986). An improved LPS-based polyvalent vaccine (16 strains) was investigated in naïve CF patients. The vaccine failed to reduce the rate of PA colonization when compared with the non-vaccinated control group. The same vaccine was also tested in burn patients with inconclusive results (Jones, Roe and Gupta 1978; Langford and Hiller 1984). Possibly, the vaccine did not protect against a sufficient range of PA LPS serotypes (Doring and Pier 2008; Priebe and Goldberg 2014). Aerugen, an 8-valent vaccine from O-Ag conjugated to exotoxin A (EPA), was initially demonstrated safe and immunogenic in plasma donors, bone marrow transplant and non-colonized CF patients (Cryz et al.1987, 1989; Schaad et al.1991; Lang et al.2004). In a small open study involving 30 non-colonized CF, the vaccine was confirmed well tolerated and induced antibodies to the O-Ag promoting the opsonophagocytic killing which were maintained up to 3 years (Cryz et al.1994). In a cohort of 25 CF patients, yearly vaccinations over 10 years induced IgG levels lower than infection-induced IgG titers, but affinity and epitope specificity rather than the quantity of the antibodies was shown to mediate protection (Zuercher et al.2006). However, in a larger trial in European CF patients, Aerugen showed good safety but no significant differences from the placebo (http://www.biospace.com/News/crucell-n-v-announces-suspension-of-aerugenr/24447). In bronchiectasis patients, high titers of IgG2 specific for the O-Ag resulted in impaired PA serum-mediated killing (Wells et al.2014), which could explain the inconsistent results of LPS-based approaches.
Salmonella species
Due to the lack of CPS, the development of O-Ag based vaccines is in progress for S. Paratyphi A, and non-typhoid Salmonella (NTS). In Asia, a significant proportion of enteric fever is caused by S. Paratyphi A, while NTS, mainly S. Typhimurium and S. Enteritidis, is major cause of bloodstream infection in sub-Saharan Africa (MacLennan, Martin and Micoli 2014). O-Ag from three of the principal invasive serovars (namely, O:2 for S. Paratyphi A, and O:4,5 for S. Typhimurium and O:9 for S. Enteritidis) have been conjugated to carrier proteins and tested in animal models (MacLennan, Martin and Micoli 2014). Conjugation of S. Typhi O-Ag to rEPA has also been carried out to cover Vi-negative strains (Salman et al.2017). However, no vaccine is yet available against these diseases.
Shigella species
Shigella species have recently been reviewed (Mani, Wierzba and Walker 2016). Shigellosis is caused by the ingestion of bacteria of the genus Shigella, of which three species are responsible for the majority of infections. Shigella flexneri is the most frequently isolated species worldwide, accounting for most cases in the least-developed countries; Sh. sonnei is more common in low- and middle-income countries; and Sh. dysenteriae has historically caused epidemics of dysentery, particularly in confined populations such as refugee camps (Mani, Wierzba and Walker 2016). Immunity to Shigella appears to be strain-specific, so an O-Ag-based vaccine covering the most commonly detected strains (i.e. Sh. flexneri 2a, 3a, 6 and Sh. sonnei) is desirable.
A conjugate vaccine composed of Sh. sonnei O-Ag bound to rEPA conferred type-specific protection against Sh. sonnei shigellosis when tested in Israeli army recruits (Cohen et al.1997). Shigella dysenteriae type 1 and Sh. flexneri 2a bioconjugates (of which the glycan component is depicted in Table 2) elicited significant LPS specific humoral responses in phase-1 studies (Hatz et al.2015; Riddle et al.2016). These promising studies support the use of O-Ag-based conjugates for human vaccination. Efforts have also been addressed to the synthesis of Sh. flexneri 2a and 3a O-Ag through rational investigation of minimal structural epitopes and impact of O-acetylation pattern in the structures (Boutet and Mulard 2008; Vulliez-Le Normand et al.2008; Phalipon et al.2009; Gauthier et al.2014). The conjugate of a synthetic pentadecasaccharide composed of three consecutive repeating units of Sh. flexneri 2a O-Ag, developed at the Pasteur Institute in France, has been recently tested in a phase-1 study (van der Put et al.2016).
Vibrio cholerae
Cholera is a severe dehydrating diarrheal disease caused by toxigenic strains of Gram-negative V. cholerae. It represents a major international health concern: ∼3–5 million cases of cholera and 100 000–130 000 deaths due to cholera occur each year globally (WHO 2010). Children, especially younger than 5 years of age, are at particular risk in endemic areas.
There are more than 200 serogroups of V. cholerae, classified based on the O-Ag specificity. Among these serogroups, cholera is mainly caused by V. cholerae serogroup O1 and less commonly by serogroup O139. O1 can be classified into two serotypes, Ogawa and Inaba, whose O-Ags are linear homopolymers of α-(1→2)-linked 3-deoxy-glycero-tetronamido-D-perosamine, differing for the non-reducing end terminal perosamine unit that is 2-O-methylated only in the Ogawa serotype (Table 2) (Chatterjee and Chaudhuri 2003).
Killed oral cholera vaccines are increasingly becoming a standard cholera prevention and control tool. However, their use has been hampered by the requirement of two or three priming doses, relatively short-term protection and responses of lower magnitude and shorter duration in young children (Balakrishnan 2017).
Conjugates from detoxified Inaba LPS linked to cholera toxin (CT) variants CT-1 and CT-2 were shown safe in adult volunteers and induced anti-LPS vibrocidal antibodies (Gupta et al.1998). Recently, O1 Ogawa and Inaba O-Ags conjugated to recombinant tetanus toxoid heavy chain fragment (TThc) induced carbohydrate specific immune responses and resulted respectively in 95% and 55% protective efficacy in a mouse survival cholera challenge model (Alam et al.2014; Sayeed et al.2015). Likewise, O139 linked to TT induced specific antibodies that were vibriocidal and protective in the neonatal mouse model of cholera infection (Boutonnier et al.2001). To avoid LPS toxicity, synthetic oligosaccharides deriving from O1 and O139 O-Ag have also been produced for conjugation to carrier proteins (Saksena et al.2006; Soliman and Kovac 2016). While a Ogawa type hexsaccharide bound to BSA induced antibodies that were protective in a challenge assay (Chernyak et al.2002; Rollenhagen et al.2009), the Inaba counterpart linked to rEPA induced specific antibodies which were neither vibriocidal nor protective in the infant mouse cholera model (Wade et al.2006). The elicited antibodies, regardless of serotype, were cross-reactive to heterologous LPS; however, anti-Ogawa serum did not kill Inaba bacteria, suggesting that immunodominant LPS epitopes differ between Ogawa and Inaba LPS.
Other surface carbohydrates
In addition to CPS or O-Ag, other carbohydrates decorating the surface of microbial pathogens have been targeted for the development of glycoconjugate vaccines.
A typical example is given by Gram-positive Group A Streptococcus (GAS), which is a major cause of pharyngitis in children across the world, with a high frequency of severe sequelae in low- and middle-income countries including acute rheumatic fever, rheumatic heart disease and post streptococcal glomerulo nephritis (Mitchell 2003). Native GAS capsule has the same structure as mammalian hyaluronic acid; thus, it is not a suitable target for a polysaccharide-based vaccine (MacLennan 1956). In contrast, the Lancefield group A carbohydrate (GAC), comprising a polyrhamnose backbone with an immunodominant GlcNAc side chain (Table 2), is a virulence factor, and conjugated to TT carrier protein elicited a protective immune response against systemic or nasal challenge with GAS (Sabharwal et al.2006). An inverse relationship between high Ab titers against GAS PS and the presence of GAS in the throat of Mexican children was observed (Sabharwal et al.2006). The epitope recognized by human antisera was identified in the hexamer composed by two repeating units (Michon et al.2005). Based on this observation, synthetic GAS oligosaccharides (2 or 4 repeats) conjugated to CRM197 were demonstrated as efficacious as the conjugated PS in protecting mice from challenge with the pathogen (Kabanova et al.2010). Concerns about GlcNAc-containing vaccines have been raised due to a possible role of anti-GAS-PS antibodies in the development of GAS infection sequelae, like acute rheumatic fever or Sydenham's chorea (Shikhman, Greenspan and Cunningham 1993; Malkiel et al.2000; Kirvan et al.2006). GlcNAc-deficient GAS PS conjugated to recombinant pneumococcal protein SP0435 was also able to induce Abs promoting opsonophagocytic killing of multiple GAS serotypes and able to protect against GAS challenge after passive immunization (van Sorge et al.2014).
Polyrhamnan, besides being a component of GAS PS backbone, is constantly expressed as cell wall polysaccharide in St. pyogenes and St. agalactiae (van Sorge et al.2014).
The ubiquitous exo-polysaccharide PNAG (Table 2) appears to play an important role in biofilm formation, immune evasion and pathogenesis in a variety of bacterial species including S. aureus (Cerca et al.2007), S. epidermidis (Mack et al.2004), Actinobacillus species (Kaplan and Mulks 2005) and E. coli (Wang, Preston and Romeo 2004; Cerca and Jefferson 2008).
When native PNAG from S. aureus (90% O-acetylated) was chemically treated to reduce acetylation to 15%, the resulting de-acetylated PNAG glycoform (dPNAG) elicited opsonic and protective antibodies against S. aureus (Maira-Litran et al.2002, 2005). In contrast, antibodies specific to the acetylated form were poorly opsonic and not protective (Maira-Litran et al.2004). Notably, most humans (95%) have high titers of natural antibody directed to the acetylated epitopes of native PNAG, and this antibody is poorly opsonic and not protective in animal models (Perez et al.2009). A synthetic dPNAG composed of nine repeating units conjugated to staphylococcal non-toxic mutant of alpha-hemolysin (Hla H35L) induced carbohydrate specific antibodies that reduced the bacterial burdens in animal models of S. aureus (skin abscesses, pneumonia and nasal colonization) (Pozzi et al.2012). Carrier-protein specific immunity was also shown to be effective in reducing bacterial levels in infected lungs and in nasal colonization. Rabbit antibodies induced against the synthetic oligosaccharide conjugated to TT induced complement mediated killing of A. baumannii S1, a high-PNAG-producing strain, but not its PNAG-negative mutant (Bentancor et al.2012). Immunization significantly reduced post infection levels of A. baumannii in the lungs or blood, compared to control groups, demonstrating that the PNAG conjugate could prevent pneumonia and pathogen caused bacteremia.
Another ubiquitous glycan is represented by the enterobacterial common antigen (ECA) (Table 2) (Männel and Mayer 1978). Monomer and dimer of this trisaccharide repeating unit have been synthesized and conjugated to BSA for the development of a specific mAb (SM250–1 A5), which recognized a variety of species, such as K. pneumoniae, Sh. sonnei, Sh. flexneri, Citrobacter freundii, E. coli, Y. enterocolitica, Enterobacter aerogenes, S. Typhimurium, while it was not reactive to other Gram-negative bacteria (V. cholerae) and Gram-positive bacteria (Listeria monocytogenes) (Liu et al.2015) .
Alginate, a polysaccharide made by variable ratios of mannuronic to guluronic acids partially O-acetylated, was found in mucoid strains of PA (Pier et al.1994; Pier 2005; Sharma, Krause and Worgall 2011). Conjugated to a variety of carrier proteins, including EPA, TT, KLH, OMV (from N. meningitidis serogroup B) or synthetic peptide containing T- and B-cell epitopes, alginate was successful in inducing protective immunity in mice, mediated by opsonophagocityc antibodies at preclinical level, but has never been tested in human (Theilacker et al.2003; Kashef et al.2006; Doring and Pier 2008; Farjah et al.2014, 2015). The structurally similar polymannuronic acid conjugated to type a flagellin also exhibited protective efficacy in a mouse lung infection model (Campodonico et al.2011).
Pel and Psl are other exopolysaccharides produced by PA (Ma et al.2006, 2007; Colvin et al.2012; Jennings et al.2015). Psl is found in 76% of analyzed clinical isolates, and its expression not only occurs in the primary infecting strains, but has also been implicated in establishing a persistent infection. Psl is responsible for the formation and maintenance of biofilms and an anti-Psl mAb exhibited opsonophagocytic killing of a number of strains and conferred significant protection in multiple animal models (DiGiandomenico et al.2012).
TAs have been explored as potential vaccine candidates for Gram-positive bacteria accounting for a large proportion of hospital-acquired antibiotic-resistant infections, such as staphylococci and enterococci (Theilacker et al.2004). Immunization of mice with (poly)glycerolphosphate backbone induced in mice antibodies which mediated opsonophagocytic killing in vitro of S. epidermidis and S. aureus (Table 2) and, upon passive transfer, reduced mortality in a murine S. aureus peritonitis model (Theilacker et al.2006). Although LTA has been reported to be an TLR2 agonist, this activity might derive from contaminating lipoproteins/lipopeptides (Zahringer et al.2008). After conjugation to TT, a synthetic version of the (poly)glycerolphosphate backbone was also able to elicit murine-specific IgG enhancing opsonophagocytic killing of live S. aureus in vitro. Mice actively immunized with the (poly)glycerolphosphate conjugate vaccine showed a rapid clearance of staphylococcal bacteremia in vivo and, in contrast to purified LTA, did not exhibit detectable inflammatory activity (Chen et al.2013).
LTA substituted or not with alanine have been isolated and recognized by opsonic antibodies against enterococci (Theilacker et al.2006; Sava et al.2010). The kojibiose–TA structure of one type of LTA (Table 2) isolated from En. faecalis strains was very similar to the CPS (Wang et al.1999). To circumvent the polymer heterogeneity, various syntheses for the preparation of defined LTA antigens have been described, including automated solid-phase synthesis (Hogendorf et al.2010, 2011, 2012).
A short LTA hexamer with a single disaccharide substituent conjugated to BSA elicited the production of antibodies in rabbit that induced opsonic killing of enterococci and S. aureus strain MW2 (Laverde et al.2014). The LTA from E. faecalis strain 12030 was found identical to the CPS, except for the substitution at position C-2 with D-alanine (Theilacker et al.2006). Osponic serum recognized this polysaccharide similarly to LTA lacking of alanine (Sava et al.2010), indicating that anti-LTA antibodies are cross reactive irrespective of the different polysaccharide decorations.
In addition to TA, other classes of heteroglycans have been isolated from enterocci, including a heteroglycan composed of rhamnose, glucose, galactose, mannosamine and glucosamine (Hsu et al.2006). Two polysaccharides containing altruronic acid and legionaminic acid, respectively, and the fructose homopolymer levan have been isolated from En. faecium, in addition to a glucosylated LTA (Kodali et al.2015). Immunization of rabbits with CRM197 conjugates of these polysaccharides showed that while antibodies raised against levan failed to mediate opsonophagocytic killing, the other two conjugated polysaccharides elicited antibodies with opsonic activity, which were also capable of reducing bacterial load in mouse liver or kidney tissue. Fragments of the capsule-like diheteroglycan (DHG) (Table 2) were recently synthesized and characterized as a promising vaccine candidate (Krylov et al.2015).
Clostridium difficile is a Gram-positive anaerobic spore-forming bacterium, the incidence and severity of which infection appears increased in the last decades, particularly in the USA and Canada (Warny et al.2005). An hypervirulent strain (NAP1/027/BI) with particularly severe antibiotic resistance was identified as the cause of these outbreaks.
Three surface polysaccharide structures, PSI, PSII and PSIII (Table 2), have been isolated (Ganeshapillai et al.2008). PSII was found the more abundant in the hypervirulent strain NAP1/027/BI and many other clinical isolates (Danieli et al.2011; Oberli et al.2011). Conjugates of the polysaccharide or the synthetic repeating unit with CRM197, recombinant bacterial toxins or ETEC proteins elicited carbohydrate specific antibodies (Adamo et al.2012; Bertolo et al.2012; Romano et al.2014). By microarray screening of synthetic structures, higher levels of IgA antibodies against PSI were found in patients with low or high severity of disease compared to asymptomatic control (Martin, Weishaupt and Seeberger 2011; Martin et al.2013b). Synthetic glycans selected by epitope mapping studies and displayed on a synthetic scaffold were shown to be immunogenic as larger fragment, demonstrating the candidacy of small structures for vaccine development (Broecker et al.2016a). PSIII is an LTA-like polymer, and anti-PSIII antibodies have also been detected in the blood of infected patients (Martin et al.2013a). Immunizations with conjugates of intact or de-O-acylated PSIII fractions raised IgG antibodies recognizing the polymer on C. difficile cellular surface (Cox et al.2013).
Serum, but not fecal, anti-PSIII IgG were found in hospitalized individuals, possibly due to pre-exposure to the pathogen (Broecker et al.2016b). A synthetic LTA fragment conjugated to CRM197 elicited in mice antibodies that bound to the surface of a series of C. difficile strains. The alum-adjuvanted CRM197 conjugate reduced the bacterial colonization in mice challenged with live C. difficile cells.
Mycobacterial and fungal surface carbohydrates
Although an anti-tuberculosis vaccine (BCG) is available, the overall effective rate of prevention is inferior to 50%. The emergence of antibiotic-resistant strains, particularly in HIV-infected individuals, is highlighting the need of improvement of the current preventive therapy. Lipoarabinomannan (LAM, Table 2) is a major lipoglycan component of the outer cell wall of all mycobacterial species. LAM is an important immunomodulating compound, contributing to the pathogenesis of mycobacterial tubercolosis (MBT) infections. The derived AM, obtained by removal of the lipid in order to avoid any immunosuppressive effect, was covalently linked to the mycobacterial protein Ag85B or TT showing protective efficacy in mice and guinea pigs in terms of prolonged survival and reduced pathology, when administered with L3 adjuvant using a subcutaneous priming-intranasal boost regime (Hamasur et al.2003; Kallenius, Pawlowski and Hamasur 2008). Attempts to use conjugates of synthetic mannans (Leelayuwapan et al.2017), phosphatidylinositol mannosides (Boonyarattanakalin et al.2008) or rhamnans (Vignal et al.2003; Meng et al.2017) are in progress, although cross reactivity against MBT has not been so far elucidated.
Candida species have become the fourth most common nosocomial bloodstream isolate in the USA and in most European countries, and a vaccine would be beneficial particularly for patients at high risk like those in intensive care units (Cutler et al.2007; Cassone 2008). Resistance to therapeutic treatment is increasing among Candida species and particular concern is being raised by the emergence of Candida auris in health care settings because this fungus is very resistant to drugs and can be easily spread from person to person. Branched β-(1→3)-(1→6)-glucans have been considered to develop vaccines against infections caused by Candida species. β-Glucans are component of most, if not all, fungi and therefore are attractive targets for fungal vaccines development. Conjugates based on β-glucans and mannans (Table 2), either extracted from a natural source or chemically synthesized, have been proposed as possible vaccine candidates (Torosantucci et al.2005; Bromuro et al.2010; Adamo et al.2011, 2014; Hu et al.2013; Johnson and Bundle 2013; Lipinski et al.2013; Paulovicova et al.2013; Liao et al.2015, 2016). β-Glucans are immunostimulator molecules through Dectin-1 activation (Donadei et al.2015); thus, bicomponent β-glucan and β-mannan conjugate has also been made (Lipinski et al.2013), showing enhanced immunoresponse against the latter antigen.
More recently, other species are emerging as targets for vaccination, such as Cr. neoformans and Aspergillus. Cryptococcus neoformans is an opportunistic encapsulated yeast that causes cryptococcal meningoencephalitis (cryptococcosis) in immunocompromised individuals, including AIDS patients and organ transplant recipients (Cogliati 2013). Invasive aspergillosis (IA), caused by Aspergillus, is the second most common cause of nosocomial, invasive fungal infections, with an incidence of approximately 5 per 100 000 population in the USA (Wilson et al.2002).
Similarly to Candida, these species are surrounded by β-(1→3)-glucans (Maubon et al.2006), and capacity of anti β-(1→3)-glucan antibodies to inhibit the cellular growth of acapsular Cryptococcus strains has been proven (Rachini et al.2007).
Both Cryptococcus and Aspergilli present at their surface α-(1→3)-glucans (Table 2). In the first case, they anchor the polysaccharide capsule to the cell wall. In Aspergillus fumigatus (Fontaine et al.2010), α-(1→3)-glucans induce the aggregation of germinating fungal conidia. Antibodies elicited in mice by conjugated synthetic α-glucans recognize the cell wall of Aspergillus (Komarova et al.2015).
Cryptococcus neoformans also exhibits a peculiar CPS. Four serotypes, A, B, C and D (Table 2), are distinguished and they are composed for a large extent of mannose trimers with glucopyranosyluronate and xylopyranosyl substituents (GXM) creating the so called ‘triads’ (Cherniak, Jones and Reiss 1988). Strains of serotype A and D are the most frequent cause of cryptococcosis in humans and thus the serotypes of primary interest for a human vaccine (Cherniak and Sundstrom 1994).
A glycoconjugate vaccine composed of unfractionated GXM polysaccharide conjugated to bovine gamma globulin was initially seen highly immunogenic, but protection from infection was not achieved (Goren and Middlebrook 1967). Fractionated GXM polysaccharide conjugated to TT was again highly immunogenic (Devi et al.1991), and both active and passive immunization of mice conferred protection against experimental cryptococcosis (Casadevall et al.1992; Devi 1996). However, using a library of mAbs it was later observed that the GXM-TT vaccine elicit protective, non-protective and even deleterious (disease-enhancing) antibodies (Mukherjee, Scharff and Casadevall 1992; Mukherjee et al.1995). The free unconjugated GXM polysaccharide would also have potent immunosuppressive properties (Vecchiarelli 2000).
These early findings led to the hypothesis that conjugated GXM could contain protective and non-protective epitopes, and a non-protective antibody response would prevent the action of formed protective antibodies (Nakouzi et al.2009). Efforts are currently ongoing to identify the protective and the non-protective epitopes present in the GXM through a library of synthetic oligosaccharides followed by conjugation to protein carrier and testing in mice (Oscarson et al.2005; Guazzelli, Ulc and Oscarson 2015; Guazzelli et al.2015; Guazzelli, McCabe and Oscarson 2016).
CONCLUSIONS
Glycans coating the bacterial surface are key antigens for the development of vaccines against bacterial and fungal diseases. Over the last decades, glycoconjugate vaccines licensed to combat N. meningitidis, H. influenzae and St. pneumoniae have proven to be safe, efficacious and cost effective (Sharma et al.2012; Delgleize et al.2016; Zarei, Almehdar and Redwan 2016; Delea et al.2017; Linares-Perez et al.2017).
Nevertheless, the continuously evolving epidemiology requires additional efforts to extend the coverage, as in the case of St. pneumoniae, while other pathogens request to be incessantly monitored to be prepared in case inclusion of emerging serotypes in the vaccine composition would be needed (i.e. N. meningitidis serogroup X or H. influenzae type a) (Micoli et al. 2013b; Cox et al.2017; LaForce 2017b)
Many diseases still remain to be controlled. Over the last years, alarming concern is emerging toward antibiotic-resistant bacteria, including, among others, the so-called ESKAPE pathogens, which are leading causes of nosocomial infections through the world (Boucher et al.2009; Garcia-Quintanilla et al.2016; Bloom, Black and Rappuoli 2017). Fungal infections are also becoming increasingly invasive for immunocompromised patients, such those with immune systems impaired by cancer chemotherapy, or hospitalized individuals (Spellberg 2011). Recent analyses have highlighted the need of novel therapeutic approaches to tackle these pathogens, and WHO and CDC have ranked and prioritized the relevance of these bacterial targets (CDC 2013; WHO 2017). Other emergencies could derive from bacteria which spread during environmental calamities (V. cholerae), particularly impacting poor countries, or which could be used as bioterrorism weapons (B. pseudomallei and mallei, F. tularensis).
Additional factors which will increase the need of preventative therapies are the changes in the age population composition, shifting toward a larger presence of elderly as consequence of the increased life expectation in the developed countries; the increased numbers of travelers, as well as the migration flows from the south to the north of the world; and even climatic changes which could play a role in selecting dangerous pathogens (Rappuoli et al.2011).
Nowadays different tools are available for the manufacturing of glycoconjugates, which could accelerate the development of novel carbohydrate-based vaccines. Semisynthetic conjugation is a well-established approach which delivered to patients efficacious vaccines against deadly pathogens. It requires a complex multistep manufacturing and quality control flow; however, it will continue to represent a reference approach, particularly for those structures difficult to express by glycoengineering methods and not promptly accessible by chemical synthesis.
The synthetic approach has been considered time demanding and expensive for vaccine manufacture compared to the use of bacterial-derived carbohydrates. Nonetheless, clear proofs of feasibility have been provided by the marketed synthetic Hib vaccine (Verez-Bencomo et al.2004) and the pentadecasaccharide Shigella conjugate recently entered phase-1 clinical trial (van der Put et al.2016). Additionally, synthesis of a number of challenging pneumococcal serotypes conjugates has been recently achieved (Geissner et al.2016; Parameswarappa et al.2016; Emmadi et al.2017; Lisboa et al.2017; Schumann et al.2017).
Synthetic carbohydrate chemistry can aid identification of the sugar target epitope, optimal carbohydrate density and attachment site to the carrier protein, supporting the rational design of vaccines with improved immunogenicity and preserved key protective protein epitopes.
Bioconjugation will play a pivotal role for faster and cheaper production of multicomponent vaccines. Bioconjugation has already provided candidates against Sh. dysenteriae O1 and Sh. flexneri 2a (Hatz et al.2015), and against ExPEC (Huttner et al.2017) tested in phase-1 clinical studies. Escherichia coli N-glycosylation so far applied is limited to the use of PglB for the transfer of the nascent polysaccharide chain with an end-terminal NAc hexosamine to the carrier protein. Directed enzyme evolution is applicable to increase the transferase promiscuity toward other sugar acceptors (Ihssen et al.2015). PglL, the O-transferase from N. meningitidis (Musumeci et al.2013), and NCT from Ac. pleuropneumoniae (Cuccui et al.2017) could also give access to a broader range of hexose ending polysaccharides.
Examples so far achieved of bioconjugate vaccines are related to polysaccharides assembled through group 1 and 4 CPS biochemical pathways. Other groups are more challenging and represent an opportunity to further progress this technology. The use of glycoengineered OMVs appears as a flexible but at a very early stage approach. Synthetic oligosaccharides and bioconjugation will aid the development of vaccines with higher standards in terms of product manufacturing and characterization compared to traditional conjugate vaccines.
The present analysis underscores how carbohydrate antigens will be key for the development of future vaccines against emerging pathogens, primarily the ESKAPE bacteria A. baumannii, En. faecium, K. pneumoniae, P. aeruginosa, classified and critical targets by WHO and CDC, as well as C. difficile and GAS, categorized as serious threats. Vaccination for prevention of relevant targets, such as S. aureus and GBS infections, is progressing at clinical level. Fungal infections (Ca. albicans, Cr. neoformans) could also benefit of carbohydrate-based vaccination. Among the pathogens discussed in this review there are examples where a simple vaccine formulation with one or two antigens has the potential to ensure large coverage (e.g. F. tularensis, B. pseudomallei and mallei, V. cholerae, Haemophilus type a), and the development of a glycoconjugate vaccine appears feasible. For M. catarrhalis, N. gonorrhoeae and NTHi LOS, alone or in combination with protein antigens, could be key targets for vaccine design. In most of the other cases, a combination of multiple sugar components would be required. This is the case of Klebsiella, for which O-Ag could ensure a better coverage with a smaller number of antigens as compared to the CPS, or E. coli. In other cases, such as PA, the complexity of the bacterial mechanism of infection with transition from acute to chronic phase might render more challenging the identification of the optimal antigens. Use of pathogen-derived proteins as carrier for the glycan haptens could reduce the number of vaccine components, by broadening across strain protection and/or by tackling the pathogen on different virulence factors, provided that the key protective epitopes of the protein carrier are identified and preserved during conjugation (Broker et al.2017).
Recent advances in the field of glycoconjugate vaccines, synergy of the different technologies currently available and their appropriate selection will enable the tailored design of glycoconjugates of novel targets, expanding the number of available vaccines and tackling currently unmet medical needs.
Conflict of interest. FM, PC and RA are employees of GSK group of companies.
REFERENCES
- Adamo R. Advancing homogeneous antimicrobial glycoconjugate vaccines. Acc Chem Res 2017;50:1270–9. [DOI] [PubMed] [Google Scholar]
- Adamo R, Hu Q-Y, Torosantucci A et al. Deciphering the structure–immunogenicity relationship of anti-Candida glycoconjugate vaccines. Chem Sci 2014;5:4302–11. [Google Scholar]
- Adamo R, Nilo A, Castagner B et al. Synthetically defined glycoprotein vaccines: current status and future directions. Chem Sci 2013;4:2995–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamo R, Romano MR, Berti F et al. Phosphorylation of the synthetic hexasaccharide repeating unit is essential for the induction of antibodies to Clostridium difficile PSII cell wall polysaccharide. ACS Chem Biol 2012;7:1420–8. [DOI] [PubMed] [Google Scholar]
- Adamo R, Tontini M, Brogioni G et al. Synthesis of laminarin fragments and evaluation of a β-(1,3) glucan hexasaccharide-CRM 197 conjugate as vaccine candidate against Candida albicans. J Carbohydr Chem 2011;30:249–80. [Google Scholar]
- Ahmad TA, El-Sayed LH, Haroun M et al. Development of immunization trials against Klebsiella pneumoniae. Vaccine 2012a;30:2411–20. [DOI] [PubMed] [Google Scholar]
- Ahmad TA, Haroun M, Hussein AA et al. Development of a new trend conjugate vaccine for the prevention of Klebsiella pneumoniae. Infect Dis Rep 2012b;4:e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam MM, Bufano MK, Xu P et al. Evaluation in mice of a conjugate vaccine for cholera made from Vibrio cholerae O1 (Ogawa) O-specific polysaccharide. PLoS Neglect Trop D 2014;8:e2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JW, Fisher MW, MacMillan BG. Immunological control of Pseudomonas infection in burn patients: A clinical evaluation. Arch Surg 1971;102:31–5. [DOI] [PubMed] [Google Scholar]
- Anderson PW, Pichichero ME, Insel RA et al. Capsular antigens noncovalently or covalently associated with protein as vaccines to Haemophilus influenzae type b: comparison in two-year-old children. J Infect Dis 1985;152:634–6. [DOI] [PubMed] [Google Scholar]
- Anderson PW, Pichichero ME, Insel RA et al. Vaccines consisting of periodate-cleaved oligosaccharides from the capsule of Haemophilus influenzae type b coupled to protein carrier: structural and temporal a requirements for priming in the human infant. J Immunol 1986;137:1181–6. [PubMed] [Google Scholar]
- Arcuri M, Di Benedetto R, Cunningham AF et al. The influence of conjugation variables on the design and immunogenicity of a glycoconjugate vaccine against Salmonella Typhi. PLoS ONE 2017;12:e0189100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong JJ, Baddiley J, Buchanan JG et al. 882. Isolation and structure of ribitol phosphate derivatives (teichoic acids) from bacterial cell walls. J Chem Soc 1958;4344–54. [Google Scholar]
- Ashkenazi S, Passwell JH, Harlev E et al. Safety and immunogenicity of Shigella sonnei and shigella flexneri 2a O-Specific polysaccharide conjugates in children. J Infect Dis 1999;179:1565–8. [DOI] [PubMed] [Google Scholar]
- AuCoin DP, Reed DE, Marlenee NL et al. Polysaccharide specific monoclonal antibodies provide passive protection against intranasal challenge with Burkholderia pseudomallei. PLoS ONE 2012;7:e35386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan VS. Cholera in Yemen. Lancet Infect Dis 2017;17:700–1. [DOI] [PubMed] [Google Scholar]
- Baumann H, Jansson P-E, Kenne L et al. Structural studies of the Escherichia coli O1A O-polysaccharide, using the computer program CASPER. Carbohydr Res 1991;211:183–90. [DOI] [PubMed] [Google Scholar]
- Bentancor LV, O’Malley JM, Bozkurt-Guzel C et al. Poly- N -acetyl-beta-(1-6)-Glucosamine is a target for protective immunity against Acinetobacter baumannii infections. Infect Immun 2012;80:651–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg S, Kaur D, Jackson M et al. The glycosyltransferases of Mycobacterium tuberculosis-roles in the synthesis of arabinogalactan, lipoarabinomannan, and other glycoconjugates. Glycobiology 2007;17:35R–56R. [DOI] [PubMed] [Google Scholar]
- Bertolo L, Boncheff AG, Ma Z et al. Clostridium difficile carbohydrates: glucan in spores, PSII common antigen in cells, immunogenicity of PSII in swine and synthesis of a dual C. difficile-ETEC conjugate vaccine. Carbohydr Res 2012;354:79–86. [DOI] [PubMed] [Google Scholar]
- Bloom DE, Black S, Rappuoli R. Emerging infectious diseases: A proactive approach. Proc Natl Acad Sci USA 2017;114:4055–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisier P, Nicolas P, Djibo S et al. Meningococcal meningitis: unprecedented incidence of serogroup X-related cases in 2006 in Niger. Clinical Infect Dis 2007;44:657–63. [DOI] [PubMed] [Google Scholar]
- Boisvert A-A, Moore DP. Invasive disease due to haemophilus influenzae type a in children in Canada's north: A priority for prevention. Can J Infect Dis Med 2015;26:291–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boltje TJ, Zhong W, Park J et al. Chemical synthesis and immunological evaluation of the inner core oligosaccharide of Francisella tularensis. J Am Chem Soc 2012;134:14255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonyarattanakalin S, Liu X, Michieletti M et al. Chemical synthesis of all phosphatidylinositol mannoside (PIM) glycans from Mycobacterium tuberculosis. J Am Chem Soc 2008;130:16791–9. [DOI] [PubMed] [Google Scholar]
- Boucher HW, Talbot GH, Bradley JS et al. Bad bugs, no drugs: no ESKAPE! An update from the infectious diseases society of America. Clin Infect Dis 2009;48:1–12. [DOI] [PubMed] [Google Scholar]
- Boutet J, Mulard LA. Synthesis of two Tetra- and four pentasaccharide fragments of Shigella flexneri serotypes 3a and X O-antigens from a common tetrasaccharide intermediate. Eur J Org Chem 2008;2008:5526–42. [Google Scholar]
- Boutonnier A, Villeneuve S, Nato F et al. Preparation, immunogenicity, and protective efficacy, in a murine model, of a conjugate vaccine composed of the polysaccharide moiety of the lipopolysaccharide of vibrio cholerae o139 bound to tetanus toxoid. Infect Immun 2001;69:3488–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady M, Cunney R, Murchan S et al. Klebsiella pneumoniae bloodstream infection, antimicrobial resistance and consumption trends in Ireland: 2008 to 2013. Eur J Clin Microbiol 2016;35:1777–85. [DOI] [PubMed] [Google Scholar]
- Brett PJ, Burtnick MN, Woods DE. The wbiA locus is required for the 2-O-acetylation of lipopolysaccharides expressed by Burkholderia pseudomallei and Burkholderia thailandensis. FEMS Microbiol Lett 2003;218:323–8. [DOI] [PubMed] [Google Scholar]
- Broecker F, Hanske J, Martin CE et al. Multivalent display of minimal Clostridium difficile glycan epitopes mimics antigenic properties of larger glycans. Nat Commun 2016a;7:11224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broecker F, Martin CE, Wegner E et al. Synthetic lipoteichoic acid glycans are potential vaccine candidates to protect from Clostridium difficile infections. Cell Chem Biol 2016b;23:1014–22. [DOI] [PubMed] [Google Scholar]
- Broker M, Berti F, Costantino P. Factors contributing to the immunogenicity of meningococcal conjugate vaccines. Hum Vacc Immunother 2016;12:1808–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broker M, Berti F, Schneider J et al. Polysaccharide conjugate vaccine protein carriers as a “neglected valency”—potential and limitations. Vaccine 2017;35:3286–94. [DOI] [PubMed] [Google Scholar]
- Broker M, Costantino P, DeTora L et al. Biochemical and biological characteristics of cross-reacting material 197 (CRM197), a non-toxic mutant of diphtheria toxin: use as a conjugation protein in vaccines and other potential clinical applications. Biologicals 2011;39:195–204. [DOI] [PubMed] [Google Scholar]
- Broker M, Dull PM, Rappuoli R et al. Chemistry of a new investigational quadrivalent meningococcal conjugate vaccine that is immunogenic at all ages. Vaccine 2009;27:5574–80. [DOI] [PubMed] [Google Scholar]
- Bromuro C, Romano MR, Chiani P et al. Beta-glucan-CRM197 conjugates as candidates antifungal vaccines. Vaccine 2010;28:2615–23. [DOI] [PubMed] [Google Scholar]
- Brown L, Wolf JM, Prados-Rosales R et al. Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat Rev Microbiol 2015;13:620–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, Santa Maria JP Jr, Walker S. Wall teichoic acids of gram-positive bacteria. Annu Rev Microbiol 2013;67:313–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundle DR, Smith ICP, Jennings HJ. Determination of the structure and conformation of bacterial polysaccharides by Carbon 13 Nuclear Magnetic Resonance. J Biol Chem 1974;249:2275–81. [PubMed] [Google Scholar]
- Byrd RA, Egan W, Summers MF et al. New n.m.r.-spectroscopic approaches for structural studies of polysaccharides: application to the Haemophilus influenzae type a capsular polysaccharide. Carbohydr Res 1987;166:47–58. [DOI] [PubMed] [Google Scholar]
- Campagnari AA, Gupta MR, Dudas KC et al. Antigenic diversity of lipooligosaccharides of nontypable Haemophilus influenzae. Infect Immun 1987;55:882–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campodonico VL, Llosa NJ, Bentancor LV et al. Efficacy of a conjugate vaccine containing polymannuronic acid and flagellin against experimental Pseudomonas aeruginosa lung infection in mice. Infect Immun 2011;79:3455–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartee RT, Forsee WT, Yother J. Initiation and synthesis of the Streptococcus pneumoniae type 3 capsule on a phosphatidylglycerol membrane anchor. J Bacteriol 2005;187:4470–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A, Mukherjee J, Devi SJ et al. Antibodies elicited by a Cryptococcus neoformans-Tetanus toxoid conjugate vaccine have the same specificity as those elicited in infection. J Infect Dis 1992;165:1086–93. [DOI] [PubMed] [Google Scholar]
- Cassone A. Fungal vaccines: real progress from real challenges. Lancet Infect Dis 2008;8:114–24. [DOI] [PubMed] [Google Scholar]
- CDC. Antibiotic Resistance Threats in the United States, 2013a. https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-2508.pdf.
- CDC. Sexually Transmitted Diseases Surveillance. 2013b. http://www.cdc.gov/std/stats13/default.htm [last accessed 7 Mar 2014].
- CDC. Food and drug administration approval for use of hiberix as a 3-Dose primary haemophilus influenzae Type b (Hib) vaccination series. MMWR Morb Mortal Wkly Rep 2016;65:418–9. [DOI] [PubMed] [Google Scholar]
- Cerca N, Jefferson KK. Effect of growth conditions on poly-N-acetylglucosamine expression and biofilm formation in Escherichia coli. FEMS Microbiol Lett 2008;283:36–41. [DOI] [PubMed] [Google Scholar]
- Cerca N, Maira-Litran T, Jefferson KK et al. Protection against Escherichia coli infection by antibody to the Staphylococcus aureus poly-N-acetylglucosamine surface polysaccharide. Proc Natl Acad Sci USA 2007;104:7528–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerquetti M, Giufre M. Why we need a vaccine for non-typeable Haemophilus influenzae. Hum Vacc Immunother 2016;12:2357–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborti S, Lewis LA, Cox AD et al. Phase-variable heptose I glycan extensions modulate efficacy of 2C7 vaccine antibody directed against neisseria gonorrhoeae lipooligosaccharide. J Immunol 2016;196:4576–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee SN, Chaudhuri K. Lipopolysaccharides of Vibrio cholerae. BBA- Mol Basis Dis 2003;1639:65–79. [DOI] [PubMed] [Google Scholar]
- Chen L, Valentine JL, Huang C-J et al. Outer membrane vesicles displaying engineered glycotopes elicit protective antibodies. Proc Natl Acad Sci USA 2016;113:E3609–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Dintaman J, Lees A et al. Novel synthetic (poly)glycerolphosphate-based antistaphylococcal conjugate vaccine. Infect Immun 2013;81:2554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. Current advances and challenges in the development of Acinetobacter vaccines. Hum Vacc Immunother 2015;11:2495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherniak R, Jones RG, Reiss E. Structure determination of Cryptococcus neoformans serotype A-variant glucuronoxylomannan by 13C-n.m.r. spectroscopy. Carbohydr Res 1988;172:113–38. [DOI] [PubMed] [Google Scholar]
- Cherniak R, Sundstrom JB. Polysaccharide antigens of the capsule of Cryptococcus neoformans. Infect Immun 1994;62:1507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernyak A, Kondo S, Wade TK et al. Induction of protective immunity by synthetic Vibrio cholerae hexasaccharide derived from V. cholerae O1 Ogawa lipopolysaccharide bound to a protein carrier. J Infect Dis 2002;185:950–62. [DOI] [PubMed] [Google Scholar]
- Chhibber S, Bajaj J. Polysaccharide-iron-regulated cell surface protein conjugate vaccine: its role in protection against Klebsiella pneumoniae-induced lobar pneumonia. Vaccine 1995;13:179–84. [DOI] [PubMed] [Google Scholar]
- Chhibber S, Rani M, Vanashree Y. Immunoprotective potential of polysaccharide-tetanus toxoid conjugate in Klebsiella pneumoniae induced lobar pneumonia in rats. Indian J Exp Biol 2005;43:40–5. [PubMed] [Google Scholar]
- Chhibber S, Wadhwa S, Yadav V. Protective role of liposome incorporated lipopolysaccharide antigen of Klebsiella pneumoniae in a rat model of lobar pneumonia. Jpn J Infect Dis 2004;57:150–55. [PubMed] [Google Scholar]
- Cogliati M. Global molecular epidemiology of Cryptococcus neoformans and Cryptococcus gattii: an analysis of the molecular types. Scientifica (Cairo) 2013;2013:675213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D, Ashkenazi S, Green MS et al. Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet 1997;349:155–9. [DOI] [PubMed] [Google Scholar]
- Collins LV, Kristian SA, Weidenmaier C et al. Staphylococcus aureus strains lacking D-Alanine modifications of teichoic acids are highly susceptible to human neutrophil killing and are virulence attenuated in mice. J Infect Dis 2002;186:214–9. [DOI] [PubMed] [Google Scholar]
- Colvin KM, Irie Y, Tart CS et al. The Pel and Psl polysaccharides provide Pseudomonas aeruginosa structural redundancy within the biofilm matrix. Environ Microbiol 2012;14:1913–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsaro MM, De Castro C, Naldi T et al. 1H and 13C NMR characterization and secondary structure of the K2 polysaccharide of Klebsiella pneumoniae strain 52145. Carbohydr Res 2005;340:2212–7. [DOI] [PubMed] [Google Scholar]
- Costantino P, Norelli F, Giannozzi A et al. Size fractionation of bacterial capsular polysaccharides for their use in conjugate vaccines. Vaccine 1999;17:1251–63. [DOI] [PubMed] [Google Scholar]
- Costantino P, Rappuoli R, Berti F. The design of semi-synthetic and synthetic glycoconjugate vaccines. Expert Opin Drug Dis 2011;6:1045–66. [DOI] [PubMed] [Google Scholar]
- Cox AD, St Michael F, Aubry A et al. Investigating the candidacy of a lipoteichoic acid-based glycoconjugate as a vaccine to combat Clostridium difficile infection. Glycoconj J 2013;30:843–55. [DOI] [PubMed] [Google Scholar]
- Cox AD, St Michael F, Cairns CM et al. Investigating the potential of conserved inner core oligosaccharide regions of Moraxella catarrhalis lipopolysaccharide as vaccine antigens: accessibility and functional activity of monoclonal antibodies and glycoconjugate derived sera. Glycoconj j 2011;28:165–82. [DOI] [PubMed] [Google Scholar]
- Cox AD, Williams D, Cairns C et al. Investigating the candidacy of a capsular polysaccharide-based glycoconjugate as a vaccine to combat Haemophilus influenzae type a disease: a solution for an unmet public health need. Vaccine 2017;35:6129–36. [DOI] [PubMed] [Google Scholar]
- Croxen MA, Finlay BB. Molecular mechanisms of Escherichia coli pathogenicity. Nat Rev Microbiol 2009;9:26–38. [DOI] [PubMed] [Google Scholar]
- Cryz SJ. Progress in immunization against Klebsiella infections. Eur J Clin Microbiol 1983;2:523–8. [DOI] [PubMed] [Google Scholar]
- Cryz SJ, Furer E, Cross AS et al. Safety and immunogenicity of a Pseudomonas aeruginosa O-polysaccharide toxin A conjugate vaccine in humans. J Clin Invest 1987;80:51–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryz SJ, Furer F, Germanier R. Experimental Klebsiella pneumoniae burn wound sepsis: role of capsular polysaccharide. Infect Immun 1984;43:440–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryz SJ, Sadojr JC, Cross AS et al. Octavalent pseudomonas aeruginosa O-polysaccharide-toxin a conjugate vaccine. Microb Pathogenesis 1989;6:75–80. [DOI] [PubMed] [Google Scholar]
- Cryz SJ, Wedgwood J, Lang AB et al. Immunization of noncolonized cystic fibrosis patients against Pseudomonas aeruginosa. J Infect Dis 1994;169:1159–62. [DOI] [PubMed] [Google Scholar]
- Cuccui J, Terra VS, Bosse JT et al. The N -linking glycosylation system from Actinobacillus pleuropneumoniae is required for adhesion and has potential use in glycoengineering. Open Biol 2017;7:160212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuccui J, Thomas RM, Moule MG et al. Exploitation of bacterial N-linked glycosylation to develop a novel recombinant glycoconjugate vaccine against Francisella tularensis. Open Biol 2013;3:130002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbertson L, Kos V, Whitfield C. ABC transporters involved in export of cell surface glycoconjugates. Microbiol Mol Biol R 2010;74:341–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler JE, Deepe GS Jr, Klein BS. Advances in combating fungal diseases: vaccines on the threshold. Nat Rev Microbiol 2007;5:13–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cywes-Bentley C, Skurnik D, Zaidi T et al. Antibody to a conserved antigenic target is protective against diverse prokaryotic and eukaryotic pathogens. Proc Natl Acad Sci USA 2013;110, E2209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danieli E, Lay L, Proietti D et al. First Synthesis of C. difficile PS-II cell wall polysaccharide repeating unit. Org Lett 2011;13:378–81. [DOI] [PubMed] [Google Scholar]
- Delea TE, Weycker D, Atwood M et al. Cost-effectiveness of alternate strategies for childhood immunization against meningococcal disease with monovalent and quadrivalent conjugate vaccines in Canada. PLoS ONE 2017;12:e0175721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgleize E, Leeuwenkamp O, Theodorou E et al. Cost-effectiveness analysis of routine pneumococcal vaccination in the UK: a comparison of the PHiD-CV vaccine and the PCV-13 vaccine using a Markov model. BMJ Open 2016;6:e010776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis DT, Inglesby TV, Henderson DA et al. Tularemia as a biological weapon. JAMA 2001;285:2763–73. [DOI] [PubMed] [Google Scholar]
- DeShazer D, Brett PJ, Woods DE. The type II O-antigenic polysaccharide moiety of Burkholderia pseudomallei lipopolysaccharide is required for serum resistance and virulence. Mol Microbiol 1998;30:1081–100. [DOI] [PubMed] [Google Scholar]
- DeShazer D, Waag DM, Fritz DL et al. Identification of a Burkholderia mallei polysaccharide gene cluster by subtractive hybridization and demonstration that the encoded capsule is an essential virulence determinant. Microb Pathogenesis 2001;30:253–69. [DOI] [PubMed] [Google Scholar]
- Devi SJ. Preclinical efficacy of a Glucuronoxylomannan-Tetanus toxoid conjugate vaccine of Cryptococcus neoformans in a murine model. Vaccine 1996;14:841–4. [DOI] [PubMed] [Google Scholar]
- Devi SJ, Schneerson R, Egan W et al. Cryptococcus neoformans serotype a glucuronoxylomannan-protein conjugate vaccines: synthesis, characterization, and immunogenicity. Infect Immun 1991;59:3700–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon S, Pace D. Meningococcal quadrivalent tetanus toxoid conjugate vaccine (MenACWY-TT; Nimenrix®): a review. Drugs 2017;77:1881–96. [DOI] [PubMed] [Google Scholar]
- Diago-Navarro E, Chen L, Passet V et al. Carbapenem-resistant Klebsiella pneumoniae exhibit variability in capsular polysaccharide and capsule associated virulence traits. J Infect Dis 2014;210:803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGiandomenico A, Warrener P, Hamilton M et al. Identification of broadly protective human antibodies to Pseudomonas aeruginosa exopolysaccharide Psl by phenotypic screening. J Exp Med 2012;209:1273–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donadei A, Gallorini S, Berti F et al. Rational design of adjuvant for skin delivery: conjugation of synthetic beta-Glucan Dectin-1 agonist to protein antigen. Mol Pharmaceutics 2015;12:1662–72. [DOI] [PubMed] [Google Scholar]
- Doring G, Pier GB. Vaccines and immunotherapy against Pseudomonas aeruginosa. Vaccine 2008;26:1011–24. [DOI] [PubMed] [Google Scholar]
- Edebrink P, Jansson P-E, Rahman M et al. Structural studies of the O-polysaccharide from the lipopolysaccharide of Moraxella (Branhamella) catarrhalis serotype A (strain ATCC 25238). Carbohydr Res 1994;257:269–84. [DOI] [PubMed] [Google Scholar]
- Edebrink P, Jansson P-E, Rahman MM et al. Structural studies of the O-antigen oligosaccharides from two strains of Moraxella catarrhalis serotype C. Carbohydr Res 1995;266:237–61. [DOI] [PubMed] [Google Scholar]
- Edebrink P, Jansson P-E, Widmalm G et al. The structures of oligosaccharides isolated from the lipopolysaccharide of Moraxella catarrhalis serotype B, strain CCUG 3292. Carbohydr Res 1996;295:127–46. [DOI] [PubMed] [Google Scholar]
- Edwards JL, Jennings MP, Apicella MA et al. Is gonococcal disease preventable? The importance of understanding immunity and pathogenesis in vaccine development. Crit Rev Microbiol 2016;42:928–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmadi M, Khan N, Lykke L et al. A Streptococcus pneumoniae Type 2 oligosaccharide glycoconjugate elicits opsonic antibodies and is protective in an animal model of invasive pneumococcal disease. J Am Chem Soc 2017;139:14783–91. [DOI] [PubMed] [Google Scholar]
- Endl J, Seidl HP, Fiedler F et al. Chemical composition and structure of cell wall teichoic acids of staphylococci. Arch Microbiol 1983;135:215–23. [DOI] [PubMed] [Google Scholar]
- Enright MC, McKenzie H. Moraxella (Branhamella) catarrhalis—clinical and molecular aspects of a rediscovered pathogen. J Med Microbiol 1997;46:360–71. [DOI] [PubMed] [Google Scholar]
- Erbing C, Kenne L, Lindberg B et al. Structural studies of the capsular polysaccharide from Klebsiella Type 1. Carbohydr Res 1976;50:115–20. [DOI] [PubMed] [Google Scholar]
- Farjah A, Owlia P, Siadat SD et al. Conjugation of alginate to a synthetic peptide containing T- and B-cell epitopes as an induction for protective immunity against Pseudomonas aeruginosa. J Biotechnol 2014;192:240–7. [DOI] [PubMed] [Google Scholar]
- Farjah A, Owlia P, Siadat SD et al. Immunological evaluation of an alginate-based conjugate as a vaccine candidate against Pseudomonas aeruginosa. APMIS 2015;123:175–83. [DOI] [PubMed] [Google Scholar]
- Fattom A, Fuller S, Propst M et al. Safety and immunogenicity of a booster dose of Staphylococcus aureus types 5 and 8 capsular polysaccharide conjugate vaccine (StaphVAX®) in hemodialysis patients. Vaccine 2004a;23:656–63. [DOI] [PubMed] [Google Scholar]
- Fattom AI, Horwith G, Fuller S et al. Development of StaphVAX, a polysaccharide conjugate vaccine against S. aureus infection: from the lab bench to phase III clinical trials. Vaccine 2004b;22:880–7. [DOI] [PubMed] [Google Scholar]
- Fattom A, Matalon A, Buerkert J et al. Efficacy profile of a bivalent Staphylococcus aureus glycoconjugated vaccine in adults on hemodialysis: Phase III randomized study. Hum Vacc Immunother 2015;11:632–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman MF, Wacker M, Hernandez M et al. Engineering N-linked protein glycosylation with diverse O antigen lipopolysaccharide structures in Escherichia coli. Proc P Natl Acad Sci USA 2005;102:3016–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebig T, Litschko C, Freiberger F et al. Efficient solid-phase synthesis of meningococcal capsular oligosaccharides enables simple and fast chemoenzymatic vaccine production. J Biol Chem 2018;293:953–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebig T, Romano MR, Oldrini D et al. An efficient cell free enzyme-based total synthesis of a meningococcal vaccine candidate. NPJ Vaccines 2016;1:16017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filloux A, Whitfield C. Editorial: The many wonders of the bacterial cell surface. FEMS Microbiol Rev 2016;40:161–3. [DOI] [PubMed] [Google Scholar]
- Fischer W, Behr T, Hartma R et al. Teichoic acid and lipoteichoic acid of Streptococcus pneumoniae possess identical chain structures. A reinvestigation of teichoid acid (C polysaccharide). Eur J Biochem 1993;215:851–7. [DOI] [PubMed] [Google Scholar]
- Fischer W, Koch HU, Haas R. Improved preparation of lipoteichoic acids. Eur J Biochem 1983;133:523–30. [DOI] [PubMed] [Google Scholar]
- Follador R, Heinz E, Wyres KL et al. The diversity of Klebsiella pneumoniae surface polysaccharides. Microb Genom 2016;2:e000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fomsgaard JS, Fomsgaard A, Høiby N et al. Comparative immunochemistry of lipopolysaccharides from Branhamella catarrhalis strains. Infect Immun 1991;59:3346–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine T, Beauvais A, Loussert C et al. Cell wall alpha1-3glucans induce the aggregation of germinating conidia of Aspergillus fumigatus. Fungal Genet Biol 2010;47:707–12. [DOI] [PubMed] [Google Scholar]
- Fournier PE, Richet H. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin Infect Dis 2006;42:692–9. [DOI] [PubMed] [Google Scholar]
- Frenck RW Jr, Creech CB, Sheldon EA et al. Safety, tolerability, and immunogenicity of a 4-antigen Staphylococcus aureus vaccine (SA4Ag): results from a first-in-human randomised, placebo-controlled phase 1/2 study. Vaccine 2017;35:375–84. [DOI] [PubMed] [Google Scholar]
- Ganesh NV, Fujikawa K, Tan YH et al. HPLC-assisted automated oligosaccharide synthesis. Org Lett 2012;14:3036–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganeshapillai J, Vinogradov E, Rousseau J et al. Clostridium difficile cell-surface polysaccharides composed of pentaglycosyl and hexaglycosyl phosphate repeating units. Carbohydr Res 2008;343:703–10. [DOI] [PubMed] [Google Scholar]
- Gao Q, Tontini M, Brogioni G et al. Immunoactivity of protein conjugates of carba analogues from Neisseria meningitidis a capsular polysaccharide. ACS Chem Biol 2013;8:2561–7. [DOI] [PubMed] [Google Scholar]
- Garcia-Quintanilla F, Iwashkiw JA, Price NL et al. Production of a recombinant vaccine candidate against Burkholderia pseudomallei exploiting the bacterial N-glycosylation machinery. Front Microbiol 2014;5:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Quintanilla M, Pulido MR, Carretero-Ledesma M et al. Vaccines for antibiotic-resistant bacteria: possibility or pipe dream? Trends Pharmacol Sci 2016;37:143–52. [DOI] [PubMed] [Google Scholar]
- Gauthier C, Chassagne P, Theillet FX et al. Non-stoichiometric O-acetylation of Shigella flexneri 2a O-specific polysaccharide: synthesis and antigenicity. Org Biomol Chem 2014;12:4218–32. [DOI] [PubMed] [Google Scholar]
- Geissner A, Pereira CL, Leddermann M et al. Deciphering antigenic determinants of Streptococcus pneumoniae serotype 4 capsular polysaccharide using synthetic oligosaccharides. ACS Chem Biol 2016;11:335–44. [DOI] [PubMed] [Google Scholar]
- Gening ML, Maira-Litran T, Kropec A et al. Synthetic b-(1->6)-linked N-acetylated and nonacetylated oligoglucosamines used to produce conjugate vaccines for bacterial pathogens. Infect Immun 2009;78:764–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geno KA, Gilbert GL, Song JY et al. Pneumococcal capsules and their types: past, present, and future. Clin Microbiol Rev 2015;28:871–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergova RT, Iankov ID, Haralambieva IH et al. Bactericidal monoclonal antibody against Moraxella catarrhalis lipooligosaccharide cross-reacts with Haemophilus spp. Curr Microbiol 2007;54:85–90. [DOI] [PubMed] [Google Scholar]
- Goren MB, Middlebrook GM. Protein conjugates of polysaccharide from Cryptococcus neoformans. J Immunol 1967;98:901–13. [PubMed] [Google Scholar]
- Gow NAR, Latge JP, Munro CA. The fungal cell wall: structure, biosynthesis, and function. Microbiol Spectr 2017;5, doi: 10.1128/microbiolspec.FUNK-0035-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowda DC, Gupta P, Davidson EA. Glycosylphosphatidylinositol anchors represent the major carbohydrate modification in proteins of intraerythrocytic stage Plasmodium falciparum. J Biol Chem 1997;272:6428–39. [DOI] [PubMed] [Google Scholar]
- Greenfield LK, Whitfield C. Synthesis of lipopolysaccharide O-antigens by ABC transporter-dependent pathways. Carbohydr Res 2012;356:12–24. [DOI] [PubMed] [Google Scholar]
- Gu X-X, Chao-Ming T, Ueyama T et al. Synthesis, characterization, and immunologic properties of detoxified lipooligosaccharide from Nontypeable Haemophilus influenzae conjugated to proteins. Infect Immun 1996;64:4047–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X-X, Chen J, Barenkamp SJ et al. Synthesis and characterization of lipooligosaccharide-based conjugates as vaccine candidates for Moraxella (Branhamella) catarrhalis. Infect Immun 1998;66:1891–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu XX, Sun J, Jin S et al. Detoxified lipooligosaccharide from Nontypeable Haemophilus influenzae conjugated to proteins confers protection against otitis media in chinchillas. Infect Immun 1997;65:4488–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guazzelli L, McCabe O, Oscarson S. Synthesis of part structures of Cryptococcus neoformans serotype C capsular polysaccharide. Carbohydr Res 2016;433:5–13. [DOI] [PubMed] [Google Scholar]
- Guazzelli L, Ulc R, Oscarson S. Synthesis of a glucuronic acid-containing thioglycoside trisaccharide building block and its use in the assembly of Cryptococcus neoformans capsular polysaccharide fragments. ChemistryOpen 2015;4:729–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guazzelli L, Ulc R, Rydner L et al. A synthetic strategy to xylose-containing thioglycoside tri- and tetrasaccharide building blocks corresponding to Cryptococcus neoformans capsular polysaccharide structures. Org Biomol Chem 2015;13:6598–610. [DOI] [PubMed] [Google Scholar]
- Gulati S, McQuillen DP, Mandrell RE et al. Immunogenicity of Neisseria gonorrhoeae lipooligosaccharide epitope 2C7, widely expressed in vivo with no immunochemical similarity to human glycosphingolipids. J Infect Dis 1996;174:1223–37. [DOI] [PubMed] [Google Scholar]
- Gulati S, Zheng B, Reed GW et al. Immunization against a saccharide epitope accelerates clearance of experimental gonococcal infection. PLoS Pathog 2013;9:e1003559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn JS, Ernst RK. The structure and function of Francisella lipopolysaccharide. Ann NY Acad Sci 2007;1105:202–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK, Taylor DN, Bryla DA et al. Phase 1 evaluation of Vibrio cholerae O1, serotype inaba, polysaccharide-cholera toxin conjugates in adult volunteers. Infect Immun 1998;66:3095–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghbin M, Armstrong D, Murphy ML. Controlled prospective trial of Pseudomonas aeruginosa vaccine in children with acute leukemia. Cancer 1973;32:761–6. [DOI] [PubMed] [Google Scholar]
- Hahm HS, Schlegel MK, Hurevich M et al. Automated glycan assembly using the Glyconeer 2.1 synthesizer. Proc Natl Acad Sci USA 2017;114:E3385–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamasur B, Haile M, Pawlowski A et al. Mycobacterium tuberculosis arabinomannan-protein conjugates protect against tuberculosis. Vaccine 2003;21:4081–93. [DOI] [PubMed] [Google Scholar]
- Hancock LE, Gilmore MS. The capsular polysaccharide of Enterococcus faecalis and its relationship to other polysaccharides in the cell wall. Proc Natl Acad Sci USA 2002;99:1574–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harale KR, Dumare NB, Singh D et al. Synthesis of a tetrasaccharide and its glycoconjugate corresponding to the capsular polysaccharide of Neisseria meningitidis serogroup X and its immunochemical studies. RSC Adv 2015;5:41332–40. [Google Scholar]
- Hatz CF, Bally B, Rohrer S et al. Safety and immunogenicity of a candidate bioconjugate vaccine against Shigella dysenteriae type 1 administered to healthy adults: a single blind, partially randomized Phase I study. Vaccine 2015;33:4594–601. [DOI] [PubMed] [Google Scholar]
- Heath PT. Status of vaccine research and development of vaccines for GBS. Vaccine 2016;34:2876–9. [DOI] [PubMed] [Google Scholar]
- Heiss C, Burtnick MN, Roberts RA et al. Revised structures for the predominant O-polysaccharides expressed by Burkholderia pseudomallei and Burkholderia mallei. Carbohydr Res 2013;381:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickx AP, Budzik JM, Oh SY et al. Architects at the bacterial surface - sortases and the assembly of pili with isopeptide bonds. Nat Rev Microbiol 2011;9:166–76. [DOI] [PubMed] [Google Scholar]
- Hidron A, Edwards J, Patel J et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol 2008;29:996–1011. [DOI] [PubMed] [Google Scholar]
- Hirano T, Hou Y, Jiao X et al. Intranasal immunization with a lipooligosaccharide-based conjugate vaccine from nontypeable Haemophilus influenzae enhances bacterial clearance in mouse nasopharynx. FEMS Immunol Med Micr 2003;35:1–10. [DOI] [PubMed] [Google Scholar]
- Hisatsune K, Kondo S, Isshiki Y et al. Occurrence of 2-O-methyl-N-(3-deoxy-L-glycero-tetronyl)-D-perosamine (4-amino-4,6-dideoxy-D-manno-pyranose) in lipopolysaccharide from Ogawa but not from Inaba O forms of O1 Vibrio cholerae. Biochem Biophys Res Co 1993a;190:302–7. [DOI] [PubMed] [Google Scholar]
- Hisatsune K, Kondo S, Isshiki Y et al. O-antigenic lipopolysaccharide of Vibrio cholerae O139 Bengal, a new epidemic strain for recent cholera in the Indian subcontinent. Biochem Bioph Res Co 1993b;196:1309–15. [DOI] [PubMed] [Google Scholar]
- Hogendorf WF, Bos LJ, Overkleeft HS et al. Synthesis of an alpha-kojibiosyl substituted glycerol teichoic acid hexamer. Bioorg Med Chem 2010;18:3668–78. [DOI] [PubMed] [Google Scholar]
- Hogendorf WF, Kropec A, Filippov DV et al. Light fluorous synthesis of glucosylated glycerol teichoic acids. Carbohydr Res 2012;356:142–51. [DOI] [PubMed] [Google Scholar]
- Hogendorf WF, Meeuwenoord N, Overkleeft HS et al. Automated solid phase synthesis of teichoic acids. Chem Commun 2011;47:8961–3. [DOI] [PubMed] [Google Scholar]
- Hortobagyi GN, Gutterman JU, Snyder RD et al. Pseudomonas vaccine: a phase I evaluation for cancer research. Cancer Immunol Immunother 1978;4:201–7. [Google Scholar]
- Hou Y, Gu XX. Development of peptide mimotopes of lipooligosaccharide from Nontypeable Haemophilus influenzae as vaccine candidates. J Immunol 2003;170:4373–9. [DOI] [PubMed] [Google Scholar]
- Hsu CT, Ganong AL, Reinap B et al. Immunochemical characterization of polysaccharide antigens from six clinical strains of Enterococci. BMC Microbiol 2006;6:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q-Y, Allan M, Adamo R et al. Synthesis of a well-defined glycoconjugate vaccine by a tyrosine-selective conjugation strategy. Chem Sci 2013;4:3827–32. [Google Scholar]
- Hu Q-Y, Berti F, Adamo R. Towards the next generation of biomedicines by site-selective conjugation. Chem Soc Rev 2016;45:1691–719. [DOI] [PubMed] [Google Scholar]
- Hu WG, Berry J, Chen J et al. Exploration of Moraxella catarrhalis outer membrane proteins, CD and UspA, as new carriers for lipooligosaccharide-based conjugates. FEMS Immunol Med Micr 2004;41:109–15. [DOI] [PubMed] [Google Scholar]
- Hu WG, Chen J, Battey JF et al. Enhancement of clearance of bacteria from murine lungs by immunization with detoxified lipooligosaccharide from Moraxella catarrhalis conjugated to proteins. Infect Immun 2000;68:4980–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Huang L, Wang H et al. Iterative one-pot synthesis of oligosaccharides. Angew Chem Int Ed 2004;43:5221–4. [DOI] [PubMed] [Google Scholar]
- Huang DH, Krishna NR. Characterization of the group A streptococcal polysaccharide by two dimensional 1H-nuclear-magnetic-resonance spectroscopy. Carbohydr Res 1986;155:193–9. [DOI] [PubMed] [Google Scholar]
- Huebner J, Quaas A, Krueger WA et al. Prophylactic and therapeutic efficacy of antibodies to a capsular polysaccharide shared among vancomycin-sensitive and -resistant Enterococci. Infect Immun 2000;68:4631–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner J, Wang J, Krueger WA et al. Isolation and chemical characterization of a capsular polysaccharide antigen shared by clinical isolates of Enterococcus faecalis and vancomycin-resistant Enterococcus faecium. Infect Immun 1999;67:1213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hufnagel M, Carey VJ, Baldassarri L et al. Distribution of four capsular serotypes of Enterococcus faecalis among clinical isolates from different geographical origins and infection sites. Infection 2006;34:22–25. [DOI] [PubMed] [Google Scholar]
- Hufnagel M, Hancock LE, Koch S et al. Serological and genetic diversity of capsular polysaccharides in Enterococcus faecalis. J Clin Microbiol 2004;42:2548–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner A, Hatz C, van den Dobbelsteen G et al. Safety, immunogenicity, and preliminary clinical efficacy of a vaccine against extraintestinal pathogenic Escherichia coli in women with a history of recurrent urinary tract infection: a randomised, single-blind, placebo-controlled phase 1b trial. Lancet Infect Dis 2017;17:528–37. [DOI] [PubMed] [Google Scholar]
- Ihssen J, Haas J, Kowarik M et al. Increased efficiency of Campylobacter jejuni N-oligosaccharyltransferase PglB by structure-guided engineering. Open Biol 2015;5:140227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda JM, Abbott SL. The Genera Klebsiella and Raoultella. The Enterobacteria, 2nd edn, Washington, USA: ASM Press; 2006, pp. 115–29. [Google Scholar]
- Jann B, Shashkov AA, Kochanowski H et al. Structural comparison of the O6 specific polysaccharides from E. coli O6:K2:H1, E. coli O6:K13:H1, and E. coli O6:K54:H10. Carbohydr Res 1994;263:217–25. [DOI] [PubMed] [Google Scholar]
- Jansson PE, Lennholm H, Lindberg B et al. Structural studies of the O-specific side-chains of the Escherichia coli O2 lipopolysaccharide. Carbohydr Res 1987;161:273–9. [DOI] [PubMed] [Google Scholar]
- Jaurigue JA, Seeberger PH. Parasite carbohydrate vaccines. Front Cell Infect Microbiol 2017;7:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings LK, Storek KM, Ledvina HE et al. Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proc Natl Acad Sci USA 2015;112:11353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z, Romero-Steiner S, Carlone GM et al. Haemophilus influenzae type a infection and its prevention. Infect Immun 2007;75:2650–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MA, Bundle DR. Designing a new antifungal glycoconjugate vaccine. Chem Soc Rev 2013;42:4327–44. [DOI] [PubMed] [Google Scholar]
- Jones C. Revised structures for the capsular polysaccharides from Staphylococcus aureus Types 5 and 8, components of novel glycoconjugate vaccines. Carbohydr Res 2005;340:1097–106. [DOI] [PubMed] [Google Scholar]
- Jones RJ, Roe EA, Gupta JL. Low mortality in burned patients in a Pseudomonas vaccine trial. Lancet 1978:401–3. [DOI] [PubMed] [Google Scholar]
- Jones CH, Zhang G, Nayerhoda R et al. Comprehensive vaccine design for commensal disease progression. Sci Adv 2017;3:e1701797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce JG, Abeygunawardana C, Xu Q et al. Isolation, structural characterization, and immunological evaluation of a high-molecular-weight exopolysaccharide from Staphylococcus aureus. Carbohydr Res 2003;338:903–22. [DOI] [PubMed] [Google Scholar]
- Kabanova A, Margarit I, Berti F et al. Evaluation of a Group A Streptococcus synthetic oligosaccharide as vaccine candidate. Vaccine 2010;29:104–14. [DOI] [PubMed] [Google Scholar]
- Kaieda S, Yano H, Okitsu N et al. In vitro investigation of the indirect pathogenicity of beta-lactamase-producing microorganisms in the nasopharyngeal microflora. Int J Pediat Otorhi 2005;69:479–85. [DOI] [PubMed] [Google Scholar]
- Kallenius G, Pawlowski A, Hamasur B. Mycobacterial glycoconjugates as vaccine candidates against tuberculosis. Trends Microbiol 2008;16:456–62. [DOI] [PubMed] [Google Scholar]
- Kaplan JB, Mulks MH. Biofilm formation is prevalent among field isolates of Actinobacillus pleuropneumoniae. Vet Microbiol 2005;108:89–94. [DOI] [PubMed] [Google Scholar]
- Kapoor N, Vanjak I, Rozzelle J et al. Malaria derived glycosylphosphatidylinositol anchor enhances anti-Pfs25 functional antibodies that block malaria transmission. Biochemistry 2018;57:516–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashef N, Behzadian-Nejad Q, Najar-Peerayeh S et al. Synthesis and characterization of Pseudomonas aeruginosa alginate-tetanus toxoid conjugate. J Med Microbiol 2006;55:1441–6. [DOI] [PubMed] [Google Scholar]
- Kenfack MT, Mazur M, Nualnoi T et al. Deciphering minimal antigenic epitopes associated with Burkholderia pseudomallei and Burkholderia mallei lipopolysaccharide O-antigens. Nat Commun 2017;8:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenne L, Lindberg B, Madden JK et al. Structural studies of the Escherichia coli O-antigen 25. Carbohydr Res 1983;122:249–56. [DOI] [PubMed] [Google Scholar]
- Khatun F, Stephenson RJ, Toth I. An overview of structural features of antibacterial glycoconjugate vaccines that influence their immunogenicity. Chem Eur J 2017;23:4233–54. [DOI] [PubMed] [Google Scholar]
- Kirvan CA, Swedo SE, Snider LA et al. Antibody-mediated neuronal cell signaling in behavior and movement disorders. J Neuroimmunol 2006;179:173–9. [DOI] [PubMed] [Google Scholar]
- Klevens RM, Edwards JR, Gaynes RP. The impact of antimicrobial-resistant, health care–associated infections on mortality in the United States. Clin Infect Dis 2008;47:927–30. [DOI] [PubMed] [Google Scholar]
- Klompas M, Khan Y, Kleinman K et al. Multicenter evaluation of a novel surveillance paradigm for complications of mechanical ventilation. PLoS One 2011;6:e18062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knirel YA, Bystrova OV, Kocharova NA et al. Conserved and variable structural features in the lipopolysaccharide of Pseudomonas aeruginosa. J Endotoxin Res 2006;12:324–36. [DOI] [PubMed] [Google Scholar]
- Knirel YA, Bystrova OV, Shashkov AS et al. Structural analysis of the lipopolysaccharide core of a rough, cystic fibrosis isolate of Pseudomonas aeruginosa. Eur J Biochem 2001;268:4708–19. [DOI] [PubMed] [Google Scholar]
- Knirel YA, Paramonov NA, Shashkov AS et al. Structure of the polysaccharide chains of Pseudomonas pseudomallei lipopolysaccharides. Carbohydr Res 1992;233:185–93. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Schrag SJ, Alderson MR et al. WHO consultation on group B Streptococcus vaccine development: Report from a meeting held on 27-28 April 2016. Vaccine 2016, doi: 10.1016/j.vaccine.2016.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodali S, Vinogradov E, Lin F, Khoury N et al. A vaccine approach for the prevention of infections by multidrug-resistant Enterococcus faecium. J Biol Chem 2015;290:19512–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarova BS, Orekhova MV, Tsvetkov YE et al. Synthesis of a pentasaccharide and neoglycoconjugates related to fungal alpha-(1→3)-Glucan and their use in the generation of antibodies to trace Aspergillus fumigatus cell wall. Chem Eur J 2015;21:1029–35. [DOI] [PubMed] [Google Scholar]
- Kong L, Vijayakrishnan B, Kowarik M et al. An antibacterial vaccination strategy based on a glycoconjugate containing the core lipopolysaccharide tetrasaccharide Hep2Kdo2. Nat Chem 2016;8:242–9. [DOI] [PubMed] [Google Scholar]
- Kowarik M, Young NM, Numao S et al. Definition of the bacterial N-glycosylation site consensus sequence. EMBO J 2006;25:1957–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer R, Blum A, Schibler A et al. Ventilation inhomogeneities in relation to standard lung function in patients with cystic fibrosis. Am J Resp Crit Care 2005;171:371–8. [DOI] [PubMed] [Google Scholar]
- Krylov VB, Gerbst AG, Argunov DA et al. Definitive structural assessment of enterococcal diheteroglycan. Chem Eur J 2015;21:1749–54. [DOI] [PubMed] [Google Scholar]
- Laferriere C, Ravenscroft N, Wilson S et al. Experimental design to optimize an Haemophilus influenzae type b conjugate vaccine made with hydrazide-derivatized tetanus toxoid. Glycoconjugate J 2011;28:463–72. [DOI] [PubMed] [Google Scholar]
- LaForce FM. Progress on MenACWYX vaccine: phase 1 trial results. Meningitis Research Foundation Conference 2017, https://www.meningitis.org/mrf-conference-2017 (26 March 2018, date last accessed). [Google Scholar]
- Lang AB, Rüdeberg A, Schöni MH et al. Vaccination of cystic fibrosis patients against Pseudomonas aeruginosa reduces the proportion of patients infected and delays time to infection. Pediatr Infect Dis J 2004;23:504–10. [DOI] [PubMed] [Google Scholar]
- Langford DT, Hiller J. Prospective, controlled study of a polyvalent pseudomonas vaccine in cystic fibrosis–three year results. Arch Dis Child 1984;59:1131–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laverde D, Wobser D, Romero-Saavedra F et al. Synthetic teichoic acid conjugate vaccine against nosocomial Gram-positive bacteria. PLoS ONE 2014;9:e110953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Doare K, Heath PT. An overview of global GBS epidemiology. Vaccine 2013;31:D7–12. [DOI] [PubMed] [Google Scholar]
- Lebacq E. Comparative tolerability and immunogenicity of Typherix or Typhim Vi in healthy adults. BioDrugs 2001;15:5–12. [DOI] [PubMed] [Google Scholar]
- Leelayuwapan H, Kangwanrangsan N, Chawengkirttikul R et al. Synthesis and immunological studies of the lipomannan backbone glycans found on the surface of Mycobacterium tuberculosis. J Org Chem 2017;82:7190–9. [DOI] [PubMed] [Google Scholar]
- Levy J, Licini L, Haelterman E, Moris P et al. Safety and immunogenicity of an investigational 4-component Staphylococcus aureus vaccine with or without AS03 B adjuvant: results of a randomized phase I trial. Hum Vacc Immunother 2015;11:620–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Wang LX. Endoglycosidases for the synthesis of polysaccharides and glycoconjugates. Adv Carbohydr Chem Biochem 2016;73:73–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao G, Zhou Z, Burgula S et al. Synthesis and immunological studies of linear oligosaccharides of beta-Glucan as antigens for antifungal vaccine development. Bioconjugate Chem 2015;26:466–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao G, Zhou Z, Liao J et al. 6-O-Branched Oligo-beta-Glucan-Based antifungal glycoconjugate vaccines. ACS Infect Dis 2016;2:123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FY, Ho VA, Khiem HB et al. The efficacy of a Salmonella typhi Vi conjugate vaccine in two-to-five-year-old children. N Engl J Med 2001;344:1263–9. [DOI] [PubMed] [Google Scholar]
- Linares-Perez N, Toledo-Romani ME, Santana-Mederos D et al. From individual to herd protection with pneumococcal vaccines: the contribution of the Cuban pneumococcal conjugate vaccine implementation strategy. Int J Infect Dis 2017;60:98–102. [DOI] [PubMed] [Google Scholar]
- Linnerborg M, Widmalm G, Weintraub A et al. Structural elucidation of the O-Antigen lipopolysaccharide from two strains of plesiomonas shigelloides that share a type-specific antigen with Shigella flexneri 6, and the common group 1 antigen with Shigella flexneri spp and Shigella dysenteriae 1. Eur J Biochem 1995;231:839–44. [PubMed] [Google Scholar]
- Lipinski T, Fitieh A, St Pierre J et al. Enhanced immunogenicity of a tricomponent mannan tetanus toxoid conjugate vaccine targeted to dendritic cells via Dectin-1 by incorporating beta-Glucan. J Immunol 2013; 190:4116–28. [DOI] [PubMed] [Google Scholar]
- Lipsitch M, Siber GR. How can vaccines contribute to solving the antimicrobial resistance problem? mBio 2016;7:e00428–00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisboa MP, Khan N, Martin C et al. Semisynthetic glycoconjugate vaccine candidate against Streptococcus pneumoniae serotype 5. Proc Natl Acad Sci USA 2017;114:11063–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister PD, Wolter DJ, Hanson ND. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin Microbiol Rev 2009;22:582–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston SD, Ovchinnikova OG, Whitfield C. Unique lipid anchor attaches Vi antigen capsule to the surface of Salmonella enterica serovar Typhi. Proc Natl Acad Sci USA 2016;113:6719–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little DJ, Li G, Ing C et al. Modification and periplasmic translocation of the biofilm exopolysaccharide poly-beta-1,6-N-acetyl-D-glucosamine. Proc Natl Acad Sci USA 2014;111:11013–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zha J, DiGiandomenico A et al. Synthetic enterobacterial common antigen (ECA) for the development of a universal immunotherapy for drug-resistant enterobacteriaceae. Angew Chem Int Ed 2015;54:10953–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Jackson KD, Landry RM et al. Analysis of Pseudomonas aeruginosa conditional psl variants reveals roles for the psl polysaccharide in adhesion and maintaining biofilm structure postattachment. J Bacteriol 2006;188:8213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Lu H, Sprinkle A et al. Pseudomonas aeruginosa Psl is a galactose- and mannose-rich exopolysaccharide. J Bacteriol 2007;189:8353–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden DC, De Jesus M, Casadevall A. The physical properties of the capsular polysaccharides from Cryptococcus neoformans suggest features for capsule construction. J Biol Chem 2006;281:1868–75. [DOI] [PubMed] [Google Scholar]
- McFetridge R, Meulen AS, Folkerth SD et al. Safety, tolerability, and immunogenicity of 15-valent pneumococcal conjugate vaccine in healthy adults. Vaccine 2015;33:2793–9. [DOI] [PubMed] [Google Scholar]
- MacIntyre S, McVeigh T, Owen P. Immunochemical and biochemical analysis of the polyvalent Pseudomonas aeruginosa vaccine PEV. Infect Immun 1986;51:675–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack D, Becker P, Chatterjee I et al. Mechanisms of biofilm formation in Staphylococcus epidermidis and Staphylococcus aureus: functional molecules, regulatory circuits, and adaptive responses. Int J Med Microbiol 2004;294:203–12. [DOI] [PubMed] [Google Scholar]
- MacLennan AP. The production of capsules, hyaluronic acid and hyaluronidase by Group A and Group C Streptococci. J Gen Microbiol 1956;14:134–42. [DOI] [PubMed] [Google Scholar]
- MacLennan CA, Martin LB, Micoli F. Vaccines against invasive salmonella disease. Hum Vacc Immunother 2014;10:1478–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVernon J, Nolan T, Richmond P et al. A randomized trial to assess safety and immunogenicity of alternative formulations of a quadrivalent meningococcal (A, C, Y, and W-135) tetanus protein conjugate vaccine in toddlers. Pediatr Infect Dis J 2012;31:e15–23. [DOI] [PubMed] [Google Scholar]
- Madhi SA, Dangor Z, Heath PT et al. Considerations for a phase-III trial to evaluate a group B Streptococcus polysaccharide-protein conjugate vaccine in pregnant women for the prevention of early- and late-onset invasive disease in young-infants. Vaccine 2013;31:D52–7. [DOI] [PubMed] [Google Scholar]
- Maira-Litran T, Kropec A, Abeygunawardana C et al. Immunochemical properties of the staphylococcal Poly-N-Acetylglucosamine surface polysaccharide. Infect Immun 2002;70:4433–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maira-Litran T, Kropec A, Goldmann D et al. Biologic properties and vaccine potential of the staphylococcal poly-N-acetyl glucosamine surface polysaccharide. Vaccine 2004;22:872–9. [DOI] [PubMed] [Google Scholar]
- Maira-Litran T, Kropec A, Goldmann DA et al. Comparative opsonic and protective activities of Staphylococcus aureus conjugate vaccines containing native or deacetylated staphylococcal Poly-N-Acetyl-beta-(1-6)-Glucosamine. Infect Immun 2005;73:6752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkiel S, Liao L, Cunningham MW et al. T-Cell-dependent antibody response to the dominant epitope of streptococcal polysaccharide, N-Acetyl-Glucosamine, is cross-reactive with cardiac myosin. Infect Immun 2000;68:5803–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malott RJ, Keller BO, Gaudet RG et al. Neisseria gonorrhoeae-derived heptose elicits an innate immune response and drives HIV-1 expression. Proc Natl Acad Sci USA 2013;110:10234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani S, Wierzba T, Walker RI. Status of vaccine research and development for shigella. Vaccine 2016;34:2887–94. [DOI] [PubMed] [Google Scholar]
- Männel D, Mayer H. Isolation and chemical characterization of the enterobacterial common antigen. Eur J Biochem 1978;86:361–70. [DOI] [PubMed] [Google Scholar]
- Marburg S, Jom D, Tolman RL et al. Bimolecular chemistry of macromolecules: synthesis of bacterial polysaccharide conjugates with Neisseria meningitidis membrane protein. J Am Chem Soc 1986;108:5282–7. [Google Scholar]
- Martin CE, Broecker F, Eller S et al. Glycan arrays containing synthetic Clostridium difficile lipoteichoic acid oligomers as tools toward a carbohydrate vaccine. Chem Commun 2013a;49:7159–61. [DOI] [PubMed] [Google Scholar]
- Martin CE, Broecker F, Oberli MA et al. Immunological evaluation of a synthetic Clostridium difficile oligosaccharide conjugate vaccine candidate and identification of a minimal epitope. J Am Chem Soc 2013b;135:9713–22. [DOI] [PubMed] [Google Scholar]
- Martin CE, Weishaupt MW, Seeberger PH. Progress toward developing a carbohydrate-conjugate vaccine against Clostridium difficile ribotype 027: synthesis of the cell-surface polysaccharide PS-I repeating unit. Chem Commun 2011;47:10260. [DOI] [PubMed] [Google Scholar]
- Masuoka J. Surface glycans of Candida albicans and other pathogenic fungi: physiological roles, clinical uses, and experimental challenges. Clin Microbiol Rev 2004;17:281–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maubon D, Park S, Tanguy M et al. AGS3, an alpha(1-3)glucan synthase gene family member of Aspergillus fumigatus, modulates mycelium growth in the lung of experimentally infected mice. Fungal Genet Biol 2006;43:366–75. [DOI] [PubMed] [Google Scholar]
- Meng X, Ji C, Su C et al. Synthesis and immunogenicity of PG-tb1 monovalent glycoconjugate. Eur J Med Chem 2017;134:140–6. [DOI] [PubMed] [Google Scholar]
- Michon F, Moore SL, Kim J et al. Doubly branched hexasaccharide epitope on the cell wall polysaccharide of group A streptococci recognized by human and rabbit antisera. Infect Immun 2005;73:6383–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micoli F, Adamo R, Proietti D et al. Meningococcal X polysaccharide quantification by high-performance anion-exchange chromatography using synthetic N-acetylglucosamine-4-phosphate as standard. Anal Biochem 2013a;442:259–61. [DOI] [PubMed] [Google Scholar]
- Micoli F, Romano MR, Tontini M et al. Development of a glycoconjugate vaccine to prevent meningitis in Africa caused by meningococcal serogroup X. Proc Natl Acad Sci USA 2013b;110:19077–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell TJ. The pathogenesis of streptococcal infections: from tooth decay to meningitis. Nat Rev Microbiol 2003;1:219–30. [DOI] [PubMed] [Google Scholar]
- Mogasale V, Maskery B, Ochiai RL et al. Burden of typhoid fever in low-income and middle-income countries: a systematic, literature-based update with risk-factor adjustment. Lancet Gob Health 2014;2:e570–80. [DOI] [PubMed] [Google Scholar]
- Morath S, Geye A, Hartung T. Structure-function relationship of cytokine induction by lipoteichoic acid from Staphylococcus aureus. J Exp Med 2001;193:393–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli L, Cancogni D, Tontini M et al. Synthesis and immunological evaluation of protein conjugates of Neisseria meningitidis X capsular polysaccharide fragments. Beilstein J Org Chem 2014;10:2367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli L, Poletti L, Lay L. Carbohydrates and immunology: synthetic oligosaccharide antigens for vaccine formulation. Eur J Org Chem 2011;29:5723–77. [Google Scholar]
- Mukherjee J, Nussbaum G, Scharff MD et al. Protective and nonprotective monoclonal antibodies to Cryptococcus neoformans originating from one B Cell. J Exp Med 1995;181:405–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee J, Scharff MD, Casadevall A. Protective murine monoclonal antibodies to Cryptococcus neoformans. Infect Immun 1992;60:4534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TF. Vaccines for Nontypeable Haemophilus influenzae: the future is now. Clin Vaccine Immunol 2015;22:459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TF, Bakaletz LO, Smeesters PR. Microbial interactions in the respiratory tract. Pediatr Infect Dis J 2009;28:S121–6. [DOI] [PubMed] [Google Scholar]
- Murphy TF, Brauer AL, Aebi C et al. Antigenic specificity of the mucosal antibody response to Moraxella catarrhalis in chronic obstructive pulmonary disease. Infect Immun 2005;73:8161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musumeci MA, Hug I, Scott NE et al. In vitro activity of neisseria meningitidis PglL O -Oligosaccharyltransferase with diverse synthetic lipid donors and a UDP-activated sugar. J Biol Chem 2013;288:10578–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakouzi A, Zhang T, Oscarson S et al. The common Cryptococcus neoformans glucuronoxylomannan M2 motif elicits non-protective antibodies. Vaccine 2009;27:3513–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M, Prior JL, Lever MS et al. Evaluation of lipopolysaccharide and capsular polysaccharide as subunit vaccines against experimental melioidosis. J Med Microbiol 2004;53:1177–82. [DOI] [PubMed] [Google Scholar]
- Neuhaus FC, Baddiley J. A continuum of anionic charge: structures and functions of D-alanyl-teichoic acids in Gram-Positive bacteria. Microbiol Mol Biol R 2003;67:686–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyra C, Paladino J, Le Borgne M. Mechanisms of depolymerization and activation of a polysialic acid and its tetramer by hydrogen peroxide. Carbohydr Polym 2015;115:494–501. [DOI] [PubMed] [Google Scholar]
- Nilo A, Morelli L, Passalacqua I et al. Anti-Group B streptococcus glycan-conjugate vaccines using pilus protein GBS80 As carrier and antigen: comparing lysine and tyrosine-directed conjugation. ACS Chem Biol 2015;10:1737–46. [DOI] [PubMed] [Google Scholar]
- Nissen M, Marshall H, Richmond P et al. A randomized phase I study of the safety and immunogenicity of three ascending dose levels of a 3-antigen Staphylococcus aureus vaccine (SA3Ag) in healthy adults. Vaccine 2015;33:1846–54. [DOI] [PubMed] [Google Scholar]
- NNDSS. National Notifiable Diseases Surveillance System. Australian Government Department of Health. 2015. http://www9.health.gov.au/cda/source/rpt_3_sel.cfm (last accessed 1 October 2015). [Google Scholar]
- Oberli MA, Hecht ML, Bindschadler P et al. A possible oligosaccharide-conjugate vaccine candidate for Clostridium difficile is antigenic and immunogenic. Chem Biol 2011;18:580–8. [DOI] [PubMed] [Google Scholar]
- Oscarson S, Alpe M, Svahnberg P et al. Synthesis and immunological studies of glycoconjugates of Cryptococcus neoformans capsular glucuronoxylomannan oligosaccharide structures. Vaccine 2005;23:3961–72. [DOI] [PubMed] [Google Scholar]
- Pace D. Glycoconjugate vaccines. Expert Opin Biol Ther 2013;13:11–33. [DOI] [PubMed] [Google Scholar]
- Pace D, Pollard AJ. Meningococcal A, C, Y and W-135 polysaccharide-protein conjugate vaccines. Arch Dis Child 2007;92:909–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parameswarappa SG, Reppe K, Geissner A et al. A semi-synthetic oligosaccharide conjugate Vaccine candidate confers protection against Streptococcus pneumoniae serotype 3 infection. Cell Chem Biol 2016;23:1407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CC, Kimura A, Jackson MA et al. Antigenic characterization of the oligosaccharide portion of the lipooligosaccharide of nontypable Haemophilus influenzae. Infect Immun 1987;55:2902–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulovicova E, Paulovicova L, Pilisiova R et al. Synthetically prepared glycooligosaccharides mimicking Candida albicans cell wall glycan antigens - novel tools to study host-pathogen interactions. FEMS Yeast Res 2013;13:659–73. [DOI] [PubMed] [Google Scholar]
- Peacock SJ, Limmathurotsakul D, Lubell Y et al. Melioidosis vaccines: a systematic review and appraisal of the potential to exploit biodefense vaccines for public health purposes. PLoS Neglect Trop D 2012; 6:e1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 2008;21:538–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltola H, Käythy H, Sivonen A et al. Haemophilus influenzae Type b capsular polysaccharide vaccine in children: A double-blind field study of 100,000 vaccinees 3 months to 5 years of age in Finland. Pediatrics 1977a;60:727–32. [PubMed] [Google Scholar]
- Peltola H, Mäkelä H, Käyhty H et al. Clinical efficacy of meningococcus group A capsular polysaccharide vaccine in children three months to five years of age. N Engl J Med 1977b;297:686–91. [DOI] [PubMed] [Google Scholar]
- Pennington JE, Reynolds HY, Wood RE et al. Use of a Pseudomonas aeruginosa vaccine in patients with acute leukemia and cystic fibrosis. Am J Med 1975;58:629–36. [DOI] [PubMed] [Google Scholar]
- Perepelov AV, Shekht ME, Liu B et al. Shigella flexneri O-antigens revisited: final elucidation of the O-acetylation profiles and a survey of the O-antigen structure diversity. FEMS Immunol Med Micr 2012;66:201–10. [DOI] [PubMed] [Google Scholar]
- Perez MM, Prenafeta A, Valle J et al. Protection from Staphylococcus aureus mastitis associated with poly-N-acetyl beta-1,6 glucosamine specific antibody production using biofilm-embedded bacteria. Vaccine 2009;27:2379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry MB, Maclean LL, Schollaardt T et al. Structural characterization of the lipopolysaccharide O-antigens of Burkholderia pseudomallei. Infect Immun 1995;63:3348–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phalipon A, Tanguy M, Grandjean C et al. A synthetic carbohydrate-protein conjugate vaccine candidate against Shigella flexneri 2a infection. J Immunol 2009;182:2241–7. [DOI] [PubMed] [Google Scholar]
- Pier DB. Application of vaccine technology to prevention of Pseudomonas aeruginosa infections. Expert Rev Vaccines 2005;4:645–56. [DOI] [PubMed] [Google Scholar]
- Pier GB. Pseudomonas aeruginosa lipopolysaccharide: a major virulence factor, initiator of inflammation and target for effective immunity. Int J Med Microbiol 2007;297:277–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier GB, DesJardin D, Grout M et al. Human immune response to Pseudomonas aeruginosa mucoid exopolysaccharide (alginate) vaccine. Infect Immun 1994;62:3972–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev 1998;11:589–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi C, Wilk K, Lee JC et al. Opsonic and protective properties of antibodies raised to conjugate vaccines targeting six Staphylococcus aureus antigens. PLoS ONE 2012;7:e46648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price NL, Goyette-Desjardins G, Nothaft H et al. Glycoengineered outer membrane vesicles: a novel platform for bacterial vaccines. Sci Rep 2016;6:24931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priebe GP, Goldberg JB. Vaccines for Pseudomonas aeruginosa: a long and winding road. Expert Rev Vaccines 2014;13:507–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachini A, Pietrella D, Lupo P et al. An Anti-beta-Glucan monoclonal antibody inhibits growth and capsule formation of Cryptococcus neoformans in vitro and exerts therapeutic, anticryptococcal activity in vivo. Infect Immun 2007;75:5085–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem 2002;71:635–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M, Holme T, Jönsson I et al. Lack of serotype-specific antibody response to lipopolysaccharide antigens of Moraxella catarrhalis during lower respiratory tract infection. Eur J Clin Microbiol Infect Dis 1995;14:297–304. [DOI] [PubMed] [Google Scholar]
- Rana R, Dalal J, Singh D et al. Development and characterization of Haemophilus influenzae type b conjugate vaccine prepared using different polysaccharide chain lengths. Vaccine 2015;33:2646–54. [DOI] [PubMed] [Google Scholar]
- Rappuoli R, Mandl CW, Black S et al. Vaccines for the twenty-first century society. Nat Rev Immunol 2011; 11:865–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravenscroft N, Costantino P, Talaga P et al. Glycoconjugate Vaccines. From Vaccine Analysis: Strategies, Principles, and Control. Berlin Heidelberg: Springer, 2015:301–81. [Google Scholar]
- Ravenscroft N, Richardson N, Serventi F et al. NMR characterization of pneumococcal bioconjugate vaccines. Abstract from 19th European Carbohydrate Symposium 2017;697. [Google Scholar]
- Ravenscroft N, Wheeler JX, Jones C. Bioanalysis of meningococcal vaccines. Bioanalysis 2010;2:343–61. [DOI] [PubMed] [Google Scholar]
- Reichmann NT, Grundling A. Location, synthesis and function of glycolipids and polyglycerolphosphate lipoteichoic acid in Gram-positive bacteria of the phylum Firmicutes. FEMS Microbiol Lett 2011;319:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid CW, Vinogradov E, Li J et al. Structural characterization of surface glycans from Clostridium difficile. Carbohydr Res 2012;354:65–73. [DOI] [PubMed] [Google Scholar]
- Reyes K, Bardossy AC, Zervos M. Vancomycin-resistant enterococci. Infect Dis Clin N Am 2016;30:953–65. [DOI] [PubMed] [Google Scholar]
- Ribeiro GS, Reis JN, Cordeiro SM et al. Prevention of Haemophilus influenzae Type b (Hib) meningitis and emergence of serotype replacement with type a strains after introduction of hib immunization in Brazil. J Infect Dis 2003;187:109–16. [DOI] [PubMed] [Google Scholar]
- Richards SJ, Greening AP, Enright MC et al. Outbreak of Moraxella catarrhalis in a respiratory unit. Thorax 1993;48:91–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle MS, Kaminski RW, Di Paolo C et al. Safety and immunogenicity of a candidate bioconjugate vaccine against Shigella flexneri 2a administered to healthy adults: A single-blind, randomized Phase I Study. Clin Vaccine Immunol 2016;23:908–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins JB, Schneerson R, Horwith G et al. Staphylococcus aureus types 5 and 8 capsular polysaccharide-protein conjugate vaccines. Am Heart J 2004;147:593–8. [DOI] [PubMed] [Google Scholar]
- Rollenhagen JE, Kalsy A, Saksena R et al. Transcutaneous immunization with a synthetic hexasaccharide-protein conjugate induces anti-Vibrio cholerae lipopolysaccharide responses in mice. Vaccine 2009;27:4917–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano MR, Leuzzi R, Cappelletti E et al. Recombinant Clostridium difficile toxin fragments as carrier protein for PSII surface polysaccharide preserve their neutralizing activity. Toxins 2014;6:1385–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe HM, Huntley JF. From the outside-in: the Francisella tularensis envelope and virulence. Front Cell Infect Microbiol 2015;5:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rukavina T, Tícac B, Susa M et al. Protective effect of antilipopolysaccharide monoclonal antibody in experimental Klebsiella infection. Infect Immun 1997;65:1754–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo TA, Beanan JM, Olson R et al. The K1 Capsular polysaccharide from acinetobacter baumannii is a potential therapeutic target via passive immunization. Infect Immun 2013;81:915–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo TA, Johnson JR. Medical and economic impact of extraintestinal infections due to Escherichia coli: focus on an increasingly important endemic problem. Microbes Infect 2003;5:449–56. [DOI] [PubMed] [Google Scholar]
- Ryall RP. Multivalent meningococcal polysaccharide-proteinconjugate vaccine. European Patent 2003, 2003707551. [Google Scholar]
- Sabharwal H, Michon F, Nelson D et al. Group A Streptococcus (GAS) carbohydrate as an immunogen for protection against GAS infection. J Infect Dis 2006;193:129–35. [DOI] [PubMed] [Google Scholar]
- Saderson AR, Strominger JL, Nathenson SG. Chemical structure of teichoic acid from Staphylococcus aureus, strain copenhagen. J Chem Biol 1962;237:3603–13. [PubMed] [Google Scholar]
- Salman M, St. Michael F, Ali A et al. First characterization of immunogenic conjugates of Vi negative Salmonella Typhi O-specific polysaccharides with rEPA protein for vaccine development. J Immunol Methods 2017;450:27–33. [DOI] [PubMed] [Google Scholar]
- Sandiumenge A, Rello J. Ventilator-associated pneumonia caused by ESKAPE organisms. Curr Opin Pulm Med 2012;18:187–93. [DOI] [PubMed] [Google Scholar]
- Saksena R, Ma X, Wade TK et al. Length of the linker and the interval between immunizations influences the efficacy of Vibrio cholerae O1, Ogawa hexasaccharide neoglycoconjugates. 2006;47:116–28. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Ulett GC, Totsika M et al. Role of capsule and O antigen in the virulence of uropathogenic Escherichia coli. PLoS One 2014;9:e94786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sava IG, Heikens E, Kropec A et al. Enterococcal surface protein contributes to persistence in the host but is not a target of opsonic and protective antibodies in Enterococcus faecium infection. J Med Microbiol 2010;59:1001–4. [DOI] [PubMed] [Google Scholar]
- Sayeed MA, Bufano MK, Xu P et al. A cholera conjugate vaccine containing o-specific polysaccharide (OSP) of V. cholerae O1 inaba and recombinant fragment of tetanus toxin heavy chain (OSP:rTTHc) induces serum, memory and lamina proprial responses against osp and is protective in mice. PLoS Neglect Trop D 2015;9:e0003881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaad UB, Lang AB, Ruedeberg A et al. Safety and immunogenicity of Pseudomonas aeruginosa conjugate A vaccine in cystic fibrosis. Lancet 1991;338:1236–7. [DOI] [PubMed] [Google Scholar]
- Schneerson R, Barrera O, Sutton A et al. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J Exp Med 1980;152:361–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann B, Hahm HS, Parameswarappa S et al. A semisynthetic Streptococcus pneumoniae serotype 8 glycoconjugate vaccine. Sci Transl Med 2017;9:eaaf5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott AE, Burtnick MN, Stokes MGM et al. Burkholderia pseudomallei capsular polysaccharide conjugates provide protection against acute melioidosis. Infect Immun 2014;82:3206–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott AE, Christ WJ, George AJ et al. Protection against experimental melioidosis with a synthetic manno -Heptopyranose hexasaccharide glycoconjugate. Bioconjugate Chem 2016;27:1435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeberger PH. The logic of automated glycan assembly. Acc Chem Res 2015;48:1450–63. [DOI] [PubMed] [Google Scholar]
- Seeberger PH, Pereira CL, Khan N et al. A semi-synthetic glycoconjugate vaccine candidate for carbapenem-resistant Klebsiella pneumoniae. Angew Chem Int Ed 2017;56:13973–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi S, Murphy TF. Bacterial infection in chronic obstructive pulmonary disease in 2000: a state-of-the-art review. Clin Microbiol Rev 2001;14:336–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell EW, Brown ED. Taking aim at wall teichoic acid synthesis: new biology and new leads for antibiotics. J Antibiot 2014;67:43–51. [DOI] [PubMed] [Google Scholar]
- Sharma A, Krause A, Worgall S. Recent developments for Pseudomonas vaccines. Hum Vaccines 2011;7:999–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma HJ, Patil VD, Lalwani SK et al. Assessment of safety and immunogenicity of two different lots of diphtheria, tetanus, pertussis, hepatitis B and Haemophilus influenzae type b vaccine manufactured using small and large scale manufacturing process. Vaccine 2012;30:510–6. [DOI] [PubMed] [Google Scholar]
- Shibata N, Kobayashi H, Tojo M et al. Characterization of phosphomannan-protein complexes isolated from viable cells of yeast and mycelial forms of Candida albicans NIH B-792 strain by the action of Zymolyase-100T. Arch Biochem Biophys 1986;251:697–708. [DOI] [PubMed] [Google Scholar]
- Shikhman AR, Greenspan NS, Cunningham MW. A subset of mouse monoclonal antibodies cross-reactive with cytoskeletal proteins and group A streptococcal M proteins recognizes N-acetyl-beta-D-glucosamine. J Immunol 1993;153:3902–13. [PubMed] [Google Scholar]
- Silhavy TJ, Kahne D, Walker S. The bacterial cell envelope. Cold Spring Harb Persp Biol 2010;2:a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva EB, Dow SW. Development of Burkholderia mallei and pseudomallei vaccines. Front Cell Infect Microbiol 2013;3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R, Cross A, Tennant S. Broad spectrum conjugate vaccine to prevent Klebsiella pneumoniae and Pseudomonas aeruginosa infections. 2016WO 2016044773 A1.
- Skaare D, Anthonisen IL, Kahlmeter G et al. Emergence of clonally related multidrug resistant Haemophilus influenzae with penicillin-binding protein 3-mediated resistance to extended-spectrum cephalosporins, Norway, 2006 to 2013. Euro Surveill 2014;18:20986. [DOI] [PubMed] [Google Scholar]
- Smoot JT, Demchenko AV. Chapter 5 Oligosaccharide synthesis: from conventional methods to modern expeditious strategies. Adv Carbohyd Chem Bi 2009;62:161–250. [DOI] [PubMed] [Google Scholar]
- Soliman SE, Kovac P. Total synthesis of the complete protective antigen of Vibrio cholerae O139. Angew Chem Int Ed 2016;55:12850–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano JB, Lamprecht B. Chronic obstructive pulmonary disease. Med Clin N Am 2012;96:671–80. [DOI] [PubMed] [Google Scholar]
- Spellberg B. Vaccines for invasive fungal infections. F1000 Med Rep 2011;3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanetti G, Hu Q-Y, Usera A et al. Sugar-protein connectivity impacts on the immunogenicity of site-selective salmonella O-Antigen glycoconjugate vaccines. Angew Chem Int Ed 2015;54:13198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struve C, Roe CC, Stegger M et al. Mapping the evolution of hypervirulent Klebsiella pneumoniae. mBio 2015;6:e00630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetens C, Hopkins S, Kolman J et al. Point prevalence survey of healthcare associated infections and antimicrobial use in European acute care hospitals. EDCPC 2013, doi: 10.2900/86011. [DOI] [Google Scholar]
- Sun J, Chen J, Cheng Z et al. Biological activities of antibodies elicited by lipooligosaccharide based-conjugate vaccines of nontypeable Haemophilus influenzae in an otitis media model. Vaccine 2000;18:1264–72. [DOI] [PubMed] [Google Scholar]
- Szijártó V, Guachalla LM, Hartl K et al. Both clades of the epidemic KPC-producing Klebsiella pneumoniae clone ST258 share a modified galactan O-antigen type. Int J Med Microbiol 2016;306:89–98. [DOI] [PubMed] [Google Scholar]
- Talbot GH, Bradley J, Edwards JEJ et al. Bad bugs need drugs: an update on the development pipeline from the antimicrobial availability task force of the infectious diseases society of America. Clin Infect Dis 2006;42:657–68. [DOI] [PubMed] [Google Scholar]
- Thanawastien A, Cartee RT, Griffin TJIV et al. Conjugate-like immunogens produced as protein capsular matrix vaccines. Proc Natl Acad Sci USA 2015;112:E1143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theilacker C, Coleman FT, Mueschenborn S et al. Construction and characterization of a Pseudomonas aeruginosa mucoid exopolysaccharide-alginate conjugate vaccine. Infect Immun 2003;71:3875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theilacker C, Kaczynski Z, Kropec A et al. Opsonic antibodies to Enterococcus faecalis strain 12030 are directed against lipoteichoic acid. Infect Immun 2006;74:5703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theilacker C, Krueger WA, Kropec A et al. Rationale for the development of immunotherapy regimens against enterococcal infections. Vaccine 2004;22:S31–8. [DOI] [PubMed] [Google Scholar]
- Thurlow LR, Thomas VC, Fleming SD et al. Enterococcus faecalis capsular polysaccharide serotypes C and D and their contributions to host innate immune evasion. Infect Immun 2009;77:5551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwary BK, Kumar A, Pathak RK et al. The Locus PgaABCD of Acinetobacter junii putatively responsible for poly-(1,6)-acetylglucosamine biosynthesis might be related to biofilm formation: a computational analysis. AiM 2016;6:222–32. [Google Scholar]
- Tomás JM, Camprubi S, Merino S et al. Surface exposure of O1 serotype lipopolysaccharide in Klebsiella pneumoniae strains expressing different K antigens. Infect Immun 1991;59:2006–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torosantucci A, Bromuro C, Chiani P et al. A novel glyco-conjugate vaccine against fungal pathogens. J Exp Med 2005;202:597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treviño SR, Permenter AR, England MJ et al. Monoclonal antibodies passively protect BALB/c mice against Burkholderia mallei aerosol challenge. Infect Immun 2006;74:1958–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner AEB, Gerson JE, So HY et al. Novel polysaccharide-protein conjugates provide an immunogenic 13-valent pneumococcal conjugate vaccine for S. pneumoniae. Synth Syst Biotechnol 2017;2:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulanova M, Tsang RSW. Haemophilus influenzae serotype a as a cause of serious invasive infections. Lancet Infect Dis 2014;14:70–82. [DOI] [PubMed] [Google Scholar]
- Ulu-Kilic A, Doganay M. An overview: tularemia and travel medicine. Travel Med Infect Dis 2014;12:609–16. [DOI] [PubMed] [Google Scholar]
- Unemo M. Current and future antimicrobial treatment of gonorrhoea - the rapidly evolving Neisseria gonorrhoeae continues to challenge. BMC Infect Dis 2015;15:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valguarnera E, Feldman MF. Glycoengineered outer membrane vesicles as a platform for vaccine development. Methods Enzymol 2017;597:285–310. [DOI] [PubMed] [Google Scholar]
- van den Dobbelsteen GP, Fae KC et al. Immunogenicity and safety of a tetravalent E. coli O-antigen bioconjugate vaccine in animal models. Vaccine 2016;34:4152–60. [DOI] [PubMed] [Google Scholar]
- van der Put RM, Kim TH, Guerreiro C et al. A synthetic carbohydrate conjugate vaccine candidate against Shigellosis: improved bioconjugation and impact of Alum on immunogenicity. Bioconjugate Chem 2016;27:883–92. [DOI] [PubMed] [Google Scholar]
- van Sorge NM, Cole JN, Kuipers K et al. The classical Lancefield antigen of group a Streptococcus is a virulence determinant with implications for vaccine design. Cell Host Microbe 2014;15:729–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaneechoutte M, Verschraegen G, Claeys G et al. Serological typing of Branhamella catarrhalis strains on the basis of lipopolysaccharide antigens. J Clin Microbiol 1990;28:182–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchiarelli A. Immunoregulation by capsular components of Cryptococcus neoformans. Med Mycol 2000;38:407–17. [DOI] [PubMed] [Google Scholar]
- Veesenmeyer JL, Hauser AR, Lisboa T et al. Pseudomonas aeruginosa virulence and therapy: evolving translational strategies. Crit Care Med 2009;37:1777–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella M, Pace D. Glycoconjugate vaccines: an update. Expert Opin Biol Th 2015;15:529–46. [DOI] [PubMed] [Google Scholar]
- Verduin CM, Hol C, Fleer A et al. Moraxella catarrhalis: from emerging to established pathogen. Clin Microbiol Rev 2002;15:125–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verez-Bencomo V, Fernandez-Santana V, Hardy E et al. A synthetic conjugate polysaccharide vaccine against Haemophilus influenzae type b. Science 2004;305:522–5. [DOI] [PubMed] [Google Scholar]
- Vignal C, Guerardel Y, Kremer L et al. Lipomannans, but not lipoarabinomannans, purified from Mycobacterium chelonae and Mycobacterium kansasii induce TNF- and IL-8 secretion by a CD14-toll-like receptor 2-dependent mechanism. J Immunol 2003;171:2014–23. [DOI] [PubMed] [Google Scholar]
- Vinogradov E, Frirdich E, MacLean LL et al. Structures of lipopolysaccharides from Klebsiella pneumoniae. J Biol Chem 2002;277:25070–81. [DOI] [PubMed] [Google Scholar]
- Vinogradov E, Sadovskaya I, Li J et al. Structural elucidation of the extracellular and cell-wall teichoic acids of Staphylococcus aureus MN8m, a biofilm forming strain. Carbohydr Res 2006;341:738–43. [DOI] [PubMed] [Google Scholar]
- Vinogradov EV, Shashkov AS, Knirel YA et al. Structure of the O-antigen of Francisella tularensis strain 15. Carbohydr Res 1991;214:289–97. [DOI] [PubMed] [Google Scholar]
- Vollmer W, Blanot D, de Pedro MA. Peptidoglycan structure and architecture. FEMS Microbiol Rev 2008;32:149–67. [DOI] [PubMed] [Google Scholar]
- Vulliez-Le Normand B, Saul FA, Phalipon A et al. Structures of synthetic O-antigen fragments from serotype 2a Shigella flexneri in complex with a protective monoclonal antibody. Proc Natl Acad Sci USA 2008;105:9976–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker M, Feldman MF, Callewaert N et al. Substrate specificity of bacterial oligosaccharyltransferase suggests a common transfer mechanism for the bacterial and eukaryotic systems. Proc Natl Acad Sci USA 2006;103:7088–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker M, Linton D, Hitchen PG et al. N-linked glycosylation in Campylobacter jejuni and its functional transfer into E. coli. Science 2002;298:1790–3. [DOI] [PubMed] [Google Scholar]
- Wacker M, Wang L, Kowarik M et al. Prevention of Staphylococcus aureus infections by glycoprotein vaccines synthesized in Escherichia coli. J Infect Dis 2014;209:1551–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade TK, Saksena R, Shiloach J et al. Immunogenicity of synthetic saccharide fragments of Vibrio cholerae O1 (Ogawa and Inaba) bound to Exotoxin A. FEMS Immunol Med Micr 2006;48:237–51. [DOI] [PubMed] [Google Scholar]
- Wang X, Preston JF, Romeo T. The pgaABCD locus of Escherichia coli promotes the synthesis of a polysaccharide adhesin required for biofilm formation. J Bacteriol 2004;186:2724–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Huebner J, Tzianabos AO et al. Structure of an antigenic teichoic acid shared by clinical isolates of Enterococcus faecalis and vancomycin-resistant Enterococcus faecium. Carbohydr Res 1999;316:155–60. [DOI] [PubMed] [Google Scholar]
- Wang Z, Chinoy ZS, Ambre SG et al. A general strategy for the chemoenzymatic synthesis of asymmetrically branched N-glycans. Science 2013;341:379–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warny M, Pepin J, Fang A et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet 2005;366:1079–84. [DOI] [PubMed] [Google Scholar]
- Wells TJ, Whitters D, Sevastsyanovich YR et al. Increased severity of respiratory infections associated with elevated anti-LPS IgG2 which inhibits serum bactericidal killing. J Exp Med 2014;211:1893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werz DB, Ranzinger R, Herget S et al. Exploring the structural diversity of mammalian carbohydrates (“Glycospace”) by statistical databank analysis. ACS Chem Biol 2007;2:685–91. [DOI] [PubMed] [Google Scholar]
- Wetter M, Goulding D, Pickard D et al. Molecular characterization of the viaB locus encoding the biosynthetic machinery for Vi capsule formation in Salmonella Typhi. PLoS One 2012;7:e45609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetter M, Kowarik M, Steffen M et al. Engineering, conjugation, and immunogenicity assessment of Escherichia coli O121 O antigen for its potential use as a typhoid vaccine component. Glycoconj J 2013;30:511–22. [DOI] [PubMed] [Google Scholar]
- Whitfield C. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu Rev Biochem 2006;75:39–68. [DOI] [PubMed] [Google Scholar]
- Whitfield C, Perry MB, Maclean LL et al. Structural analysis of the O-antigen side chain polysaccharides in the lipopolysaccharides of Klebsiella serotypes O2(2a), O2(2a,2b), and O2(2a,2c). Infect Immun 1992;174:4913–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield C, Richards JC, Perry MB et al. Expression of two structurally distinct D-galactan O antigens in the lipopolysaccharide of Klebsiella pneumoniae serotype O1. Infect Immun 1991;173:1420–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Cholera vaccines: WHO position paper. Weekly epidemiological record. Relevé épidémiologique hebdomadaire 2010;85:117–28. [PubMed] [Google Scholar]
- WHO. Global Action Plan to Control the Spread and Impact of Antimicrobial Resistance in Neisseria Gonorrhoeae, on World Health Organization (WHO), 2012. http://www.who.int/reproductivehealth/publications/rtis/9789241503501/en/ (last accessed 7 March 2014). [Google Scholar]
- WHO Global Priority List of Antibiotic-Resistant Bacteria to Giude Research, Dicovery, and Development of New Antibiotics. 2017. http://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf (26 March 2018, date last accessed).
- Willis LM, Stupak J, Richards M et al. Conserved glycolipid termini in capsular polysaccharides synthesized by ATP-binding cassette transporter-dependent pathways in Gram-negative pathogens. Proc Natl Acad Sci USA 2013;110:7868–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis LM, Whitfield C. Structure, biosynthesis, and function of bacterial capsular polysaccharides synthesized by ABC transporter-dependent pathways. Carbohydr Res 2013;378:35–44. [DOI] [PubMed] [Google Scholar]
- Wilson LS, Reyes CM, Stolpman M et al. The direct cost and incidence of systemic fungal infections. Value Health 2002;5:26–34. [DOI] [PubMed] [Google Scholar]
- Wu T, Chen J, Murphy TF et al. Investigation of nontypeable Haemophilus influenzae outer membrane protein P6 as a new carrier for lipooligosaccharide conjugate vaccines. Vaccine 2005;23:5177–85. [DOI] [PubMed] [Google Scholar]
- Wu Y, Xiong DC, Chen SC et al. Total synthesis of mycobacterial arabinogalactan containing 92 monosaccharide units. Nat Commun 2017;8:14851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia G, Kohler T, Peschel A. The wall teichoic acid and lipoteichoic acid polymers of Staphylococcus aureus. Int J Med Microbiol 2010;300:148–54. [DOI] [PubMed] [Google Scholar]
- Xie O, Pollard AJ, Mueller JE et al. Emergence of serogroup X meningococcal disease in Africa: need for a vaccine. Vaccine 2013;31:2852–61. [DOI] [PubMed] [Google Scholar]
- Xin H, Dziadek S, Bundle DR et al. Synthetic glycopeptide vaccines combining beta-mannan and peptide epitopes induce protection against candidiasis. Proc Natl Acad Sci USA 2008;105:13526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki R, Yabe U, Kataoka C et al. The oligosaccharide of gonococcal lipooligosaccharide contains several epitopes that are recognized by human antibodies. Infect Immun 2010;78:3247–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yother J. Capsules of Streptococcus pneumoniae and other bacteria: paradigms for polysaccharide biosynthesis and regulation. Annu Rev Microbiol 2011;65:563–81. [DOI] [PubMed] [Google Scholar]
- Young LS, Meyer RD, Armstrong D. Pseudomonas aeruginosa vaccine in cancer patients. Ann Intern Med 1973;79:518–27. [DOI] [PubMed] [Google Scholar]
- Yu S, Gu XX. Synthesis and characterization of lipooligosaccharide-based conjugate vaccines for serotype B Moraxella catarrhalis. Infect Immun 2005;73:2790–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Gu XX. Biological and immunological characteristics of lipooligosaccharide-based conjugate vaccines for serotype C Moraxella catarrhalis. Infect Immun 2007;75:2974–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahringer U, Lindner B, Inamura S et al. TLR2 - promiscuous or specific? A critical re-evaluation of a receptor expressing apparent broad specificity. Immunobiology 2008;213:205–24. [DOI] [PubMed] [Google Scholar]
- Zarei AE, Almehdar HA, Redwan EM. Hib vaccines: past, present, and future perspectives. J Immunol Res 2016;2016;1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Lu Y-J, Malley R. Multiple antigen-presenting system (MAPS) to induce comprehensive B- and T-cell immunity. Proc Natl Acad Sci USA 2013;113:13564–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberberg MD, Shorr AF. Secular trends in Gram-Negative resistance among urinary tract infection hospitalizations in the United States, 2000–2009. Infect Control Hosp Epidemiol 2013;34:940–6. [DOI] [PubMed] [Google Scholar]
- Zou W, Jennings HJ. Preparation of glycoconjugate vaccines. In: Carbohydrate-Based Vaccines and Immunotherapies. Guo Z, Boons G-J (eds.) Hoboken, NJ: John Wiley & Sons, Inc, 2009, 55–88. [Google Scholar]
- Zuercher AW, Horn MP, Que JU et al. Antibody responses induced by long-term vaccination with an octovalent conjugate Pseudomonas aeruginosa vaccine in children with cystic fibrosis. FEMS Immunol Med Micr 2006;47:302–8. [DOI] [PubMed] [Google Scholar]