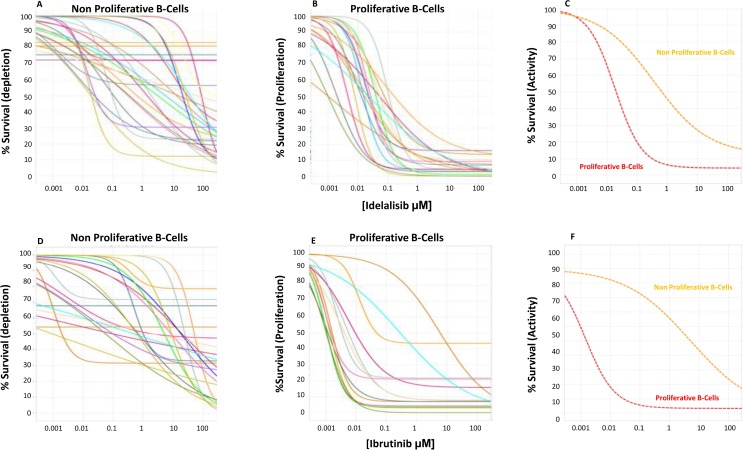
Figure 3. Idelalisib dose exposure evaluation.
Twenty-nine progressive CLL frozen samples (represented with differently colored lines) were tested at 96h with the CpG+IL2+HS+CLL NE media for dose response of idelalisib (Panels A–C) and ibrutinib (Panels D–F) in both the non-proliferative (panel A, D) and proliferative (panel B, E) fractions, measuring the % of live leukemic cells at each concentration shown as % Survival. We found little effect on the non-proliferative CLL fraction, suggesting a limited pro-apoptotic depletion activity of the drugs. In contrast, potent inhibition of proliferation with median potency (EC50) of 28 nM for idelalilisib and 550 nM for ibrutinib was observed. The efficacy was nearly complete leaving a median of 5% and 8% resistant CLL cells that proliferated at the highest doses of idelalisib or ibrutinib. (Panel C) (idelalisib) and (Panel F) (ibrutinib) represents the media of the effect in the non-proliferative CLL cells (orange line) and the proliferative B-fraction (red line) showing a predominant antiproliferative activity of both drugs.