Summary
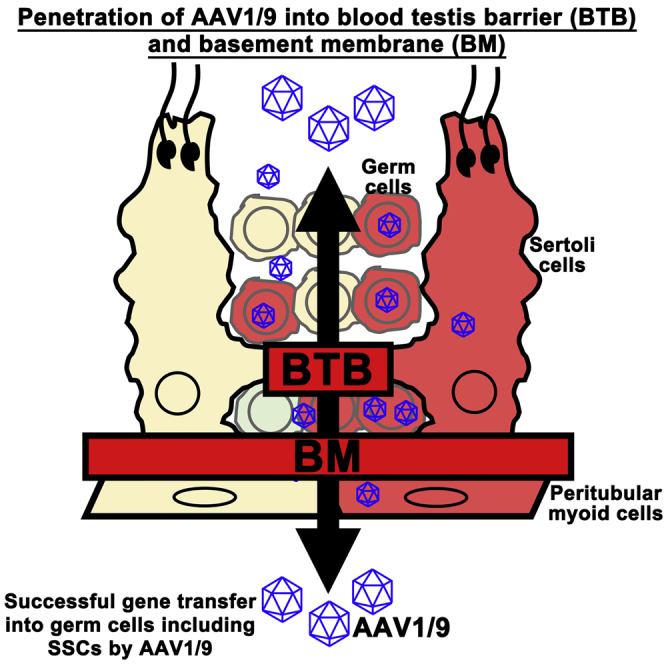
Adeno-associated virus (AAV) penetrates the blood-brain barrier, but it is unknown whether AAV penetrates other tight junctions. Genetic manipulation of testis has been hampered by the basement membrane of seminiferous tubules and the blood-testis barrier (BTB), which forms between Sertoli cells and divides the tubules into basal and adluminal compartments. Here, we demonstrate in vivo genetic manipulation of spermatogonial stem cells (SSCs) and their microenvironment via AAV1/9. AAV1/9 microinjected into the seminiferous tubules penetrated both the basement membrane and BTB, thereby transducing not only Sertoli cells and SSCs but also peritubular cells and Leydig cells. Moreover, when congenitally infertile KitlSl/KitlSl-d mouse testes with defective Sertoli cells received Kitl-expressing AAVs, spermatogenesis regenerated and offspring were produced. None of the offspring contained the AAV genome. Thus, AAV1/9 allows efficient germline and niche manipulation by penetrating the BTB and basement membrane, providing a promising strategy for the development of gene therapies for reproductive defects.
Keywords: adeno-associated virus, blood-testis barrier, Sertoli cells, spermatogonia, spermatogenesis
Graphical Abstract
Highlights
-
•
AAVs penetrate the blood-testis barrier and infect SSCs and their environment
-
•
AAVs also penetrate the basement membrane of the seminiferous tubules
-
•
AAVs could rescue spermatogenesis in congenitally infertile KitlSl/KitlSl-d mice
-
•
Offspring did not carry transgenes after microinsemination
In this article, Shinohara and colleagues show the feasibility of adeno-associated virus (AAV) to penetrate the blood-testis barrier and the basal membrane of the seminiferous tubules of mouse testes. AAVs infected not only germ cells but also their microenvironment. The technique was used to rescue a mouse model of infertility with Sertoli cell defect.
Introduction
Spermatogonial stem cells (SSCs) provide the foundation of spermatogenesis (de Rooij and Russell, 2000, Meistrich and van Beek, 1993). SSCs constantly undergo self-renewal division on the basement membrane of the seminiferous tubules in the germline niche. Although the exact cell composition of the germline niche is unknown, Sertoli cells, which form a simple epithelial monolayer in the seminiferous tubules, provide self-renewing factors and nutritional support for spermatogenic cells, which make them the major regulator of SSC fate (França et al., 2016). Sertoli cells form the blood-testis barrier (BTB), which is composed of several claudin proteins (Dym and Fawcett, 1970, Mital et al., 2011, Cheng and Mruk, 2012). The BTB divides the seminiferous tubules into the basal and adluminal compartments. Spermatogonia in the basal compartment divide and differentiate into preleptotene spermatocytes, which then transmigrate through the BTB into the adluminal compartment.
Because Sertoli cells regulate the biology of SSCs, developing methods to introduce genes to Sertoli cells would be useful not only for the basic study of SSCs but also for clinical application for male infertility treatment. In previous studies, transduction of genes into Sertoli cells has been achieved by microinjection of lentiviruses or adenoviruses into the seminiferous tubules (Ikawa et al., 2002, Kanatsu-Shinohara et al., 2002). Lentivirus or adenovirus expressing Kitl was able to restore spermatogenesis in adult KitlSl/KitlSl-d male mice and normal offspring were obtained. Although no offspring were reported, electroporation of Kitl gene also induced spermatogenesis in KitlSl/KitlSl-d mice (Yomogida et al., 2002). Because normal offspring without the transgene integration were born using virus vectors, these results raised the possibility of gene therapy for animal and human infertility.
However, limitations of these approaches became evident in later studies. For example, adenovirus causes inflammatory reaction and decline of long-term gene expression of adenovirus was also reported (Blanchard and Boekelheide, 1997, Kojima et al., 2003). Moreover, electroporation and lentivirus can transduce genes to germ cells, including SSCs (Huang et al., 2000, Nagano et al., 2002). The lack of gene transduction in SSCs in the original studies was probably due to the BTB. This was supported by our previous observation that transgenic mice were born after microinjection of retrovirus into immature testes before BTB development (Kanatsu-Shinohara et al., 2004). Thus, there is clearly a need to develop safer and more efficient approaches for transducing Sertoli cells in vivo.
One candidate technique for overcoming these problems is to use adeno-associated viruses (AAVs) (Daya and Berns, 2008). Unlike retroviruses and lentiviruses, AAVs are non-pathogenic and infect both dividing and non-dividing cells without apparent cytotoxicity or insertional mutagenesis. AAVs are also unique in that they are highly selective regarding target cell infection. To date, 12 primate serotypes have been described (AAV1–12), and more than 110 distinct AAV capsid sequences have been isolated. More importantly, AAVs have a unique ability to penetrate the blood-brain barrier (BBB) (Foust et al., 2009). Injection of AAV9-GFP into veins of newborn mice led to widespread transduction of neurons and astrocytes throughout the brain. On the basis of these observations, we hypothesized that AAVs may also penetrate the BTB, which is another type of tight junction.
In this study, we detail the utility of AAVs in transducing testis cells in situ. In our attempt to determine the target cell populations of AAVs in testes, we found that AAVs can transduce many cell types. In particular, AAV1/9 can penetrate not only the BTB but also the basement membrane of the seminiferous tubules. We used this strategy to transduce both SSCs and Sertoli cells with no harmful reactions, and produced offspring without AAV integration. This new strategy of gene transduction via AAVs may be useful for studying the mechanisms of spermatogenesis, and has potential applications for fertility restoration in animals and humans.
Results
Screening of AAV Serotypes by In Vivo Microinjection
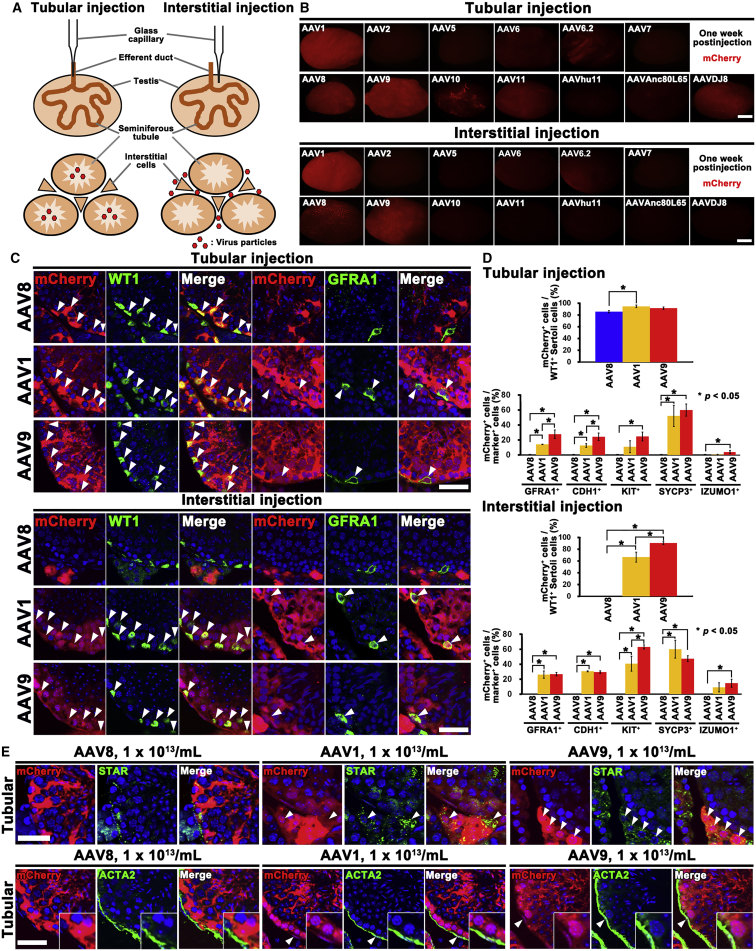
We screened 13 types of AAVs with different capsids (AAV1, 2, 5, 6, 6.2, 7, 8, 9, 10, 11, hu11, Anc80L65, and DJ8), all of which express mCherry. Virus particles were microinjected into the seminiferous tubules or interstitial tissues. Of the 13 tested serotypes, mCherry fluorescence was observed when AAV1, 8, 9, 11, and DJ8 were microinjected into the seminiferous tubules (Figures 1A and 1B). Focal, but consistent, infection was found for AAV6.2 and AAV10 despite repeated attempts. We also noted relatively strong signals in testes that received interstitial injections of AAV1, AAV8, and AAV9, but no fluorescence was observed following interstitial injection with AAVDJ8. We tried three different titers of virus (0.2 × 1012/mL, 1 × 1012/mL, and 5 × 1012/mL), but mCherry expression pattern was essentially the same by macroscopic appearance (Figure S1).
Figure 1.
Screening of AAV Serotypes
(A) Route of injection.
(B) Macroscopic appearance of wild-type mouse testes 1 week after microinjection with mCherry-expressing AAVs.
(C) Immunostaining of AAV1, AAV8, or AAV9-mCherry-injected testes for undifferentiated spermatogonia (GFRA1) and Sertoli cell (WT1) markers 1 week after microinjection with AAV-mCherry. Arrowheads indicate target cells infected with AAVs.
(D) Quantification of immunostaining. Three tubules from three different testes were counted for tubular and interstitial injection.
(E) Immunostaining of AAV1 or AAV9-mCherry-injected testes for STAR or ACTA2 1 week after microinjection with AAV-mCherry. Arrowheads indicate target cells infected with AAVs.
Error bars represent standard errors. Scale bars, 1 mm (B) and 40 μm (C and E). Counterstain, Hoechst 33342 (C and E). See also Figures S1–S6 and Table S1.
Double immunohistochemistry of testes that received tubular injection revealed that AAV1 and AAV9 infected both Sertoli cells and germ cells, while AAV8 selectively transduced Sertoli cells (Figures 1C and S2). Microinjection of AAV6, AAV6.2, AAV10, AAV11, and AAVDJ8 into the seminiferous tubules also transduced Sertoli cells, but the infection efficiency was very low compared with AAV8 (Figure S3). No germ cell infection was found with these viruses.
AAV1 and AAV9 transduced not only haploid cells in the adluminal compartment but also spermatogonia on the basement membrane. Quantification of cells expressing GFRA1 (a marker for Asingle [As], Apaired [Apr] spermatogonia, and some Aaligned [Aal] spermatogonia) or CDH1 (a marker for undifferentiated spermatogonia) showed that AAV9 is superior to AAV1 at transducing these cell types (Figure 1D). There were no significant differences in transduction efficiency for KIT+ (a marker for differentiating spermatogonia) or SYCP3+ (a spermatocyte marker) cells, but IZUMO1+ (a marker for spermatids developing into sperm) cells were rarely transduced by these viruses. Cells expressing WT1 (a Sertoli cell marker) were infected with comparable efficiency. Spermatogonia and Sertoli cell transduction was also confirmed in testes 3 months after microinjection (Figures S4A–S4C). However, the superiority of AAV9 was not evident at this point. The infection of primitive spermatogonia populations suggests that AAV1 and AAV9 that were introduced into the adluminal compartment penetrated the BTB and infected undifferentiated spermatogonia in the basal compartment.
Although the results of three different doses were essentially the same for most AAVs (0.2 × 1012/mL, 1 × 1012/mL, and 5 × 1012/mL), we noted that AAV1 and AAV9 could infect cells expressing STAR (a Leydig cell marker) at the highest dose (5 × 1012/mL). These signals became stronger when we confirmed the results by further increasing the AAV concentration (1 × 1013/mL) (Figure 1E). Cells expressing ACTA2 (a marker for peritubular cells) also showed mCherry expression at this dose, suggesting that they are not as efficiently transduced as Leydig cells. Nevertheless, these results suggested that AAV1 and AAV9 can penetrate not only the BTB but also the basement membrane. In contrast, AAV8 could not infect germ cells even at the highest dose (1 × 1013/mL).
Surprisingly, immunohistochemistry of testes that received AAV1 and AAV9 interstitial injections showed very similar mCherry expression patterns. In addition to infection of Leydig cells and peritubular cells (Figure S2), a significant number of germ cells in both the basal and adluminal compartments were infected (Figures 1C, 1D, S2, and S4A–S4C). Signals in the germ cell compartment ranged from GFRA1+ undifferentiated spermatogonia to the IZUMO1+ spermatid-sperm stage. Although KIT+ cells were constantly infected with higher efficiency by AAV9, this was not evident at 3 months. GFRA1+ and CDH1+ spermatogonia were infected with comparable efficiency at both stages. Sertoli cells were also efficiently transduced by AAV1 and AAV9 interstitial injection at both time points. In contrast to tubular injection, no infected Sertoli cells were found following interstitial injection of AAV8 even at the highest dose (1 × 1013/mL). Although AAV8 infected Leydig cells, peritubular cells also did not show fluorescence. These patterns of gene transduction were not observed with lentiviral or adenoviral injections (Figure S5). These results suggest that AAV8 does not penetrate the basement membrane and has distinct properties from AAV1 and AAV9.
Because of the unique transduction patterns of AAV1 and AAV9, we examined their receptor distribution. AAV9 binds to terminal N-linked galactose, while a2,3/a2,6 N-linked sialic acid is the receptor for AAV1 (Bell et al., 2011). However, these molecules were widespread in the seminiferous tubules and did not show specific distribution patterns (Figure S6).
Kinetics of AAV Transduction
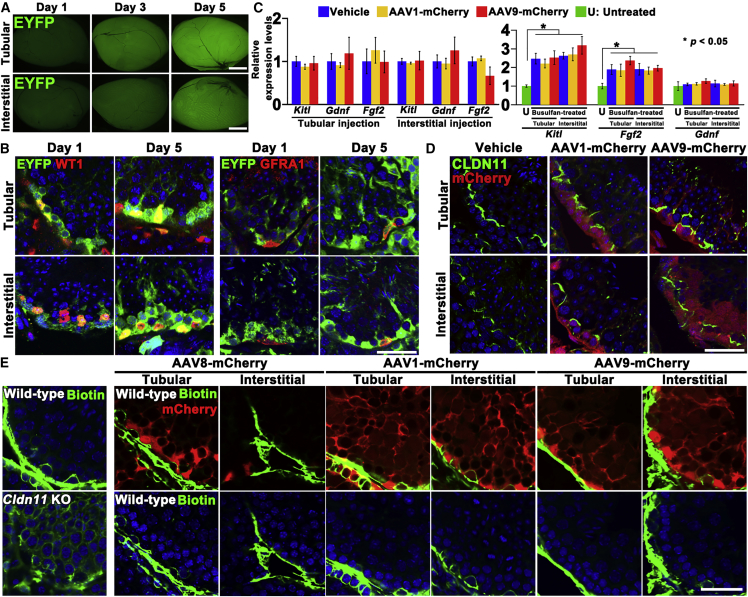
To study transduction kinetics, we used R26R-Eyfp mice, which allowed for more sensitive identification of infected cells (Watanabe et al., 2017). We infected these mice by Cre-expressing AAV9. AAV9-Cre infection initiated expression of EYFP, which was somewhat stronger by tubular injection (Figure 2A). Immunostaining showed that WT1+ Sertoli cells were infected as early as 1 day after microinjection, whereas GFRA1+ spermatogonia did not show fluorescence (Figure 2B), suggesting that Sertoli cell infection is a very rapid process.
Figure 2.
Transduction Kinetics Analyzed by Microinjection of AAV9-Cre into R26R-Eyfp Mice
(A) Macroscopic appearance of R26R-Eyfp mouse testes at indicated time points after AAV9-Cre microinjection.
(B) Immunostaining of R26R-Eyfp mouse testes for undifferentiated spermatogonia (GFRA1) and Sertoli cell (WT1) markers.
(C) Real-time PCR analysis of Gdnf, Fgf2, and Kitl expression in wild-type untreated and busulfan-treated testes 2 days after microinjection. Three testes from three different animals were used (n = 3).
(D) Immunostaining of CLDN11 1 week after microinjection with AAV1- or AAV9-mCherry.
(E) Functional assessment of the BTB. Wild-type and Cldn11 KO mouse testes transduced with AAV8-, AAV1-, or AAV9-mCherry were injected interstitially with biotin (green). Samples were recovered 30 min after microinjection (n= 3).
Error bars represent standard errors. Scale bars, 1 mm (A) and 40 μm (B, D, and E). Counterstain, Hoechst 33342 (B, D, and E). See also Tables S1 and S2.
Infection with AAV did not appear to significantly influence the SSC microenvironment, as real-time PCR showed that neither AAV1 nor AAV9 transduction influenced the expression levels of cytokines involved in spermatogonia self-renewal and differentiation (Figure 2C). Because the proportion of Sertoli cells is very small (∼3%; Bellvé, 1993), we also analyzed the expression in busulfan-treated testes that contain enriched Sertoli cells due to loss of germ cells. Although busulfan treatment increased Kitl and Fgf2 expression, we were not able to find significant impact of AAV infection even in busulfan-treated testes.
Because AAV1 and AAV9 penetrated the BTB, we also examined the distribution of CLDN11, a major component of the BTB. However, CLDN11 expression was not observably altered by AAV1 or AAV9 infection (Figure 2D). The integrity of the BTB was further confirmed in a functional manner by tracer experiments. Three days after tubular or interstitial injection of AAV1- or AAV9-mCherry, we microinjected biotin (557 D) into the interstitium of adult testes. Cldn11 knockout (KO) mouse testes, which lack the BTB, were used as a positive control. Although leakage into the tubule lumen was readily observed in Cldn11 KO mice (Figure 2E), AAV injection did not induce apparent biotin leakage into the adluminal compartment. These results suggested that both AAV1 and AAV9 penetrated the basement membrane as well as the BTB and infected both Sertoli cells and germ cells.
In Vivo Transduction of SSCs by AAV1/9
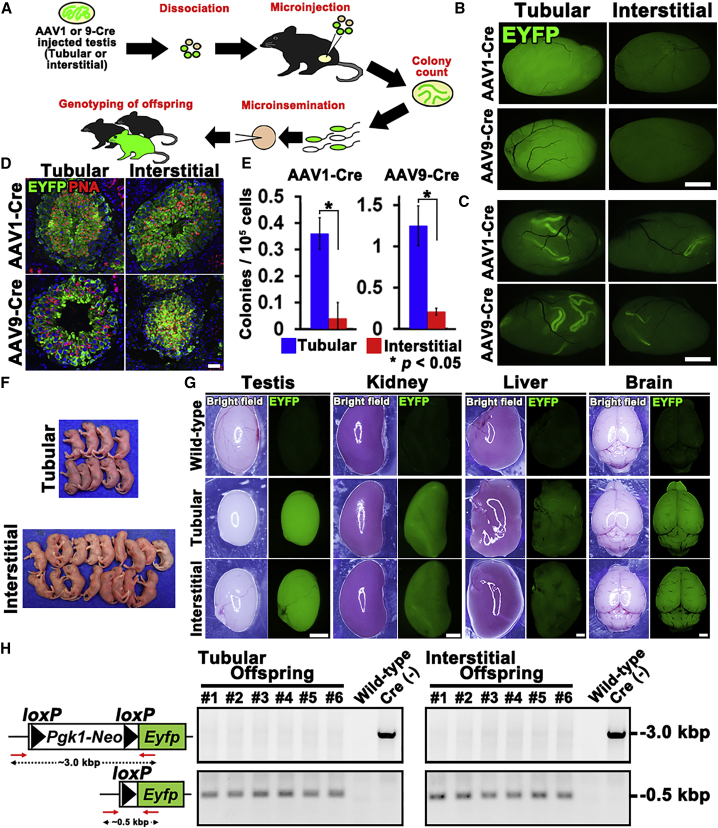
To confirm the transduction of SSCs, we used spermatogonial transplantation (Brinster and Zimmermann, 1994) (Figure 3A). In this experiment, Cre-expressing AAV1 or AAV9 was microinjected into the seminiferous tubules of R26R-Eyfp mice. Between 1 and 2 weeks after microinjection, their testes were recovered and used for transplantation. Testes of R26R-Eyfp mice that received tubular injection showed stronger fluorescence under UV light than those with interstitial injection (Figure 3B).
Figure 3.
Functional Analysis of SSC Transduction by AAVs
(A) Experimental schematic.
(B) Macroscopic appearance of R26R-Eyfp mouse testes at the time of donor cell collection.
(C) Macroscopic appearance of recipient testes.
(D) Lectin staining of recipient testes by a haploid (peanut agglutinin, PNA) cell marker showing the normal appearance of donor-derived spermatogenesis.
(E) Colony counts (n = 18–19). Nine to ten recipients were analyzed.
(F) Offspring born after microinsemination using AAV9-infected R26R-Eyfp donor testis cells.
(G) EYFP fluorescence in the offspring.
(H) PCR analysis of Cre-mediated deletion. Tail DNA samples from offspring produced after tubular or interstitial injection were analyzed using indicated primers (red arrows).
Error bars represent standard errors. Scale bars, 1 mm (B, C, and G) and 40 μm (D). Counterstain, Hoechst 33342 (D). See also Table S1.
Donor testes were then microinjected into the seminiferous tubules of busulfan-treated mice. When recipient testes were analyzed, all donor types produced germ cell colonies, demonstrating that SSCs are transduced by AAV1/9 regardless of injection route (Figure 3C). Histological analyses showed normal-appearing spermatogenesis in the recipient testes (Figure 3D). However, the frequency of colonization was dependent on virus type and injection route. R26R-Eyfp testis cells that received AAV1 and AAV9 via tubular injection produced more colonies than those that received interstitial injection (Figure 3E), suggesting that SSCs are transduced more efficiently by tubular injection.
To confirm the function of Cre-transfected SSCs, we carried out in vitro microinsemination. We focused on AAV9 because of its efficient transduction. Elongated spermatids from two types of recipients were microinjected into oocytes (Table 1). Both types of cells produced normal offspring with EYFP fluorescence under UV light (Figures 3F and 3G), and deletion of loxP was confirmed (Figure 3H). These results showed that SSCs that were transduced by AAV9 in vivo are functionally normal.
Table 1.
In Vitro Microinsemination with Spermatozoa Recovered after In Vivo Genetic Manipulation by AAV
| Type of Experiment | Site of Injection | No. of Embryos Cultured | No. of Embryos Transferred (%) | No. of Embryos Implanted (%) | No. of Pups (%) |
|---|---|---|---|---|---|
| AAV9-Crea | tubule | 57 | 30 (73.7) | 12 (21.0) | 10 (17.5) |
| interstitial | 58 | 43 (74.1) | 26 (44.8) | 18 (31.0) | |
| AAV9-Kitlb | tubule | 135 | 98 (72.6) | 56 (41.5) | 31 (23.0) |
| interstitial | 118 | 100 (84.7) | 51 (43.2) | 27 (22.9) |
Embryos were cultured for 24 hr and transferred at the 2-cell stage.
Elongated spermatids were collected from recipient mouse testes that had received transplantation of R26R-Eyfp mouse testis cells.
Elongated and round spermatids were collected from KitlSl/KitlSl-d testes that had received AAV9-Kitl microinjection.
Improvement of Transfection of AAV9 by Neuraminidase
Although both tubular and interstitial injection successfully transduced germ and Sertoli cells, efficiency was still limited. Because of its better transduction efficiency, we focused on AAV9 in the following experiments. To improve the efficiency of AAV-mediated gene transduction, we first used an AAV9 mutant capsid, which is known to delay ubiquitination and has been used to improve transfection efficiency (Dalkara et al., 2012). However, we did not observe any apparent improvement (Figure S7A).
We then used neuraminidase to improve infection efficiency. AAV9 uptake in lungs was greatly increased when terminal sialic acids were removed by neuraminidase (Bell et al., 2011). We co-injected AAV9-mCherry with neuraminidase and the mice were euthanized 1 week after the injection. Testes that received co-injection of neuraminidase showed significantly enhanced mCherry expression regardless of the route of injection (Figure S7B). Double immunostaining showed that neuraminidase significantly increased the transduction efficiency of GFRA1+ undifferentiated spermatogonia by ∼2.2- and ∼1.5-fold by tubule and interstitial injection, respectively (Figures S7C and S7D). However, we did not find any significant differences in Sertoli cell transduction, possibly because these cells were already infected with high efficiency even without any treatment. These results showed that neuraminidase treatment can improve AAV9 transduction efficiency in undifferentiated spermatogonia.
Rescue of Infertility in KitlSl/KitlSl-d Mice by AAV9 Transduction of Sertoli Cells
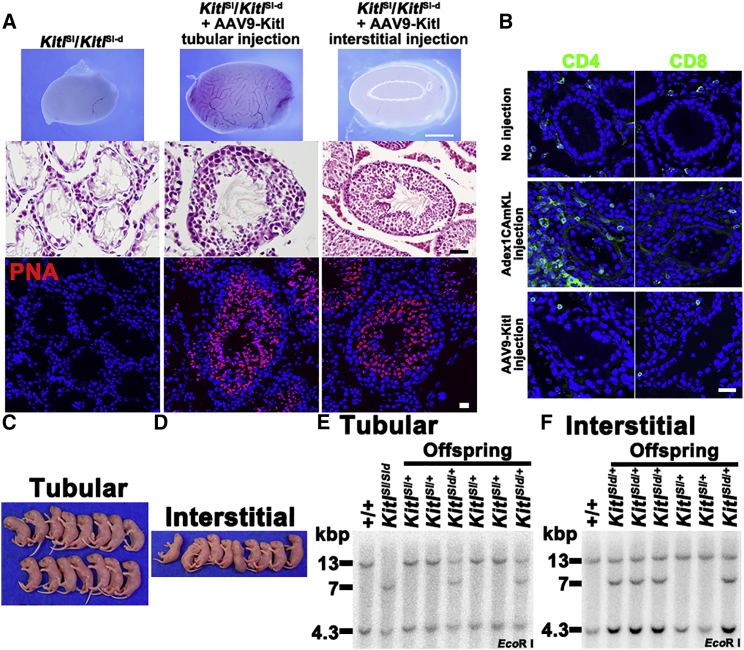
In the final set of experiments, we tested the utility and safety of AAV by correcting infertility in KitlSl/KitlSl-d male mice (Figure 4A). These mice are congenitally infertile because they lack expression of membrane-bound Kitl in Sertoli cells. However, these testes contain a small number of SSCs (∼5% of the wild-type number) (Ogawa et al., 2000, Shinohara et al., 2000). Although we previously used adenovirus and restored spermatogenesis, adenovirus infection causes inflammatory reactions (Blanchard and Boekelheide, 1997, Kojima et al., 2003) and CD4+, but not CD8+ cells, were found in the testes as early as 1 week after microinjection into the seminiferous tubules (Figure 4B). Based on these observations, we microinjected AAV9-Kitl into the seminiferous tubules and into the interstitial tissue of KitlSl/KitlSl-d male mice. Neuraminidase was also added in some injections to improve transduction efficiency. Immunostaining confirmed lack of infiltrating CD4+ cells.
Figure 4.
Rescue of Infertility in KitlSl/KitlSl-d Mice by AAV9-Kitl Infection
(A) Macroscopic, histological, and lectin-histochemical appearance of KitlSl/KitlSl-d mouse testes after microinjection with AAV9-Kitl.
(B) Immunostaining of KitlSl/KitlSl-d mouse testes by CD4 and CD8 antibodies 1 week after microinjection of Adex1CAmKL or AAV9-Kitl into the seminiferous tubules.
(C and D) Offspring born after microinsemination using sperm generated after tubular (C) or interstitial (D) injection of AAV9-Kitl.
(E and F) Southern blot analysis of DNA samples using a Kitl cDNA probe. DNA samples were collected from representative offspring born after microinsemination using sperm generated after tubular (E) or interstitial (F) injection of Kitl-expressing AAV9. DNA was digested with the indicated restriction enzymes. The wild-type locus produced hybridization bands at 4.3 and 13 kb, the KitlSl locus produced no bands, and the KitlSl-d locus produced bands at 4.3 and 7 kb.
Scale bars, 1 mm (A, upper panels) and 40 μm (A, middle and lower panels; B). Counterstain, H&E (A, middle) and Hoechst 33342 (A, bottom; B). See also Figure S7 and Table S1.
We euthanized the animals 3 months after microinjection to examine the levels of spermatogenesis. Although KitlSl/KitlSl-d mouse testes are small (∼11 mg), testes that received AAV9-Kitl injections grew larger regardless of injection route (Figure 4A), suggesting regeneration of spermatogenesis. Histological analyses of the injected testes revealed spermatogenesis in all of the samples. The success of the restoration of spermatogenesis varied greatly among the samples, but comparable numbers of seminiferous tubules exhibited spermatogenesis with either injection type (Table 2). Unlike adenovirus-mediated gene transduction, no apparent inflammatory reaction was found by histological analysis (Figure 4A). These results suggested that Sertoli cells can be transduced by AAV vectors to restore spermatogenesis regardless of administration route.
Table 2.
Spermatogenesis Following Microinjection of AAV9-Kitl into KitlSl/KitlSl-d Mouse Testes
| Type of Experiment | Sample Number | Virus Titer (/mL) | Days after Injection | Testis Weight (mg)a | Tubule with Spermatogenesis (%)b |
|---|---|---|---|---|---|
| Tubular | 1 | 1.0 × 1011 | 92 | 21.5 | 43.2 (48/111) |
| 2 | 1.0 × 1011 | 92 | 29.8 | 77.1 (91/118) | |
| 3 | 1.0 × 1011 | 92 | 22.1 | 28.7 (56/195) | |
| 4 | 5.0 × 1012 | 126 | 30.0 | 33.9 (43/127) | |
| 5 | 5.0 × 1012 | 126 | 36.8 | 50.4 (67/133) | |
| Interstitial | 1 | 5.0 × 1012 | 91 | 21.7 | 21.5 (31/144) |
| 2 | 5.0 × 1012 | 91 | 28.5 | 64.1 (66/103) | |
| 3c | 5.0 × 1012 | 91 | 27.6 | 53.8 (64/119) | |
| 4c | 5.0 × 1012 | 91 | 24.0 | 24.0 (42/175) | |
| 5c | 5.0 × 1012 | 94 | 23.5 | 38.9 (44/113) |
Average weight of untreated KitlSl/KitlSl-d mouse testes is 11.0 mg (n = 4).
Total number of tubule cross-sections containing spermatogenesis/total number of cross-sections examined is indicated in parentheses.
Neuraminidase was co-injected with AAV9-Kitl.
To test the fertility of regenerated germ cells, we performed microinsemination. Thirty-one and 27 offspring were born, respectively, from KitlSl/KitlSl-d mice that had received tubular and interstitial injection, although one of the offspring died soon after birth in each experiment (Figures 4C and 4D). Because KitlSl/KitlSl-d mice can produce sperm bearing KitlSl or KitlSl-d, microinsemination using these sperm will result in producing two types of offspring. To confirm the genotype of the offspring, we carried out Southern blot analysis, which showed that each offspring carried KitlSl or KitlSl-d mutant alleles and 15 KitlSl/+ and 15 KitlSl-d/+ offspring were born by tubular injection (Figures 4E and 4F). Likewise, 9 KitlSl/+ and 17 KitlSl-d/+ offspring were born by interstitial injection.
Lack of AAV9 Transduction in the Offspring
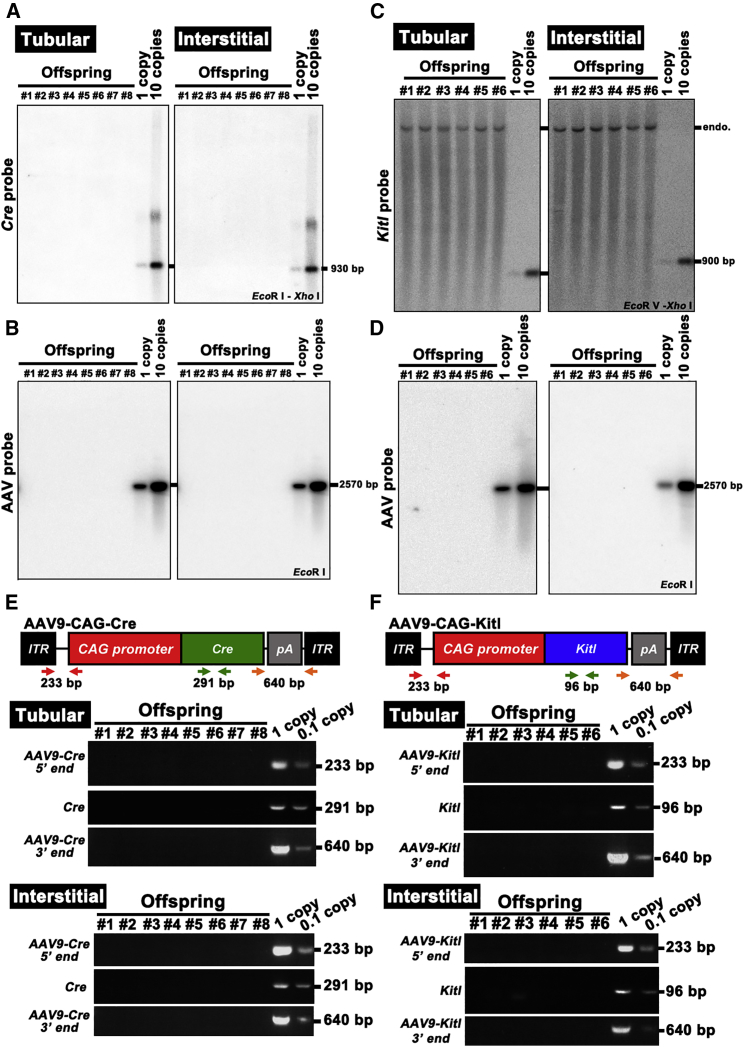
To test whether the offspring carried AAV9 genome, we first carried out Southern blotting. We analyzed tail DNA from all offspring born from SSC or Sertoli cell transduction experiments. Southern blot analysis showed lack of apparent signals in any of the offspring (Figures 5A–5D). We also carried out PCR using primers specific to AAV as well as insert DNA (i.e., Cre and Kitl), but failed to detect AAV9 integration (Figures 5E and 5F). These results indicate that AAV9 transduced both SSCs and Sertoli cells without stable integration into the genome.
Figure 5.
Analysis of AAV9 Integration in the Offspring
(A–D) Southern blot analysis of F1 DNA samples from R26R-Eyfp (A and B) or KitlSl/KitlSl-d (C and D) mice hybridized with Cre (A), Kitl (C), or empty AAV vector (B and D). Controls represent viral DNA in amounts equivalent to 1 and ten copies of viral DNA per diploid genome. DNA was digested with the indicated restriction enzymes. Note endogenous (endo.) Kitl locus produced bands in all samples in KitlSl/KitlSl-d mouse experiment.
(E) PCR analysis of AAV9-Cre integration in the tail DNA. AAV9-specific 5′- or 3′-end-specific primers as well as Cre-specific primers were used. Samples were collected from offspring produced after tubular or interstitial injection with AAV9-Cre.
(F) PCR analysis of AAV9-Kitl integration in the tail DNA. AAV9-specific 5′- or 3′-end-specific primers as well as Kitl-specific primers were used. Samples were collected from offspring produced after tubular or interstitial injection with AAV9-Kitl. Controls containing 0.1 mg of normal mouse DNA were spiked with viral DNA representing 0.1 and 1 copies of the viral genome.
See also Table S2.
Discussion
Previous studies have shown infection of germ cells by AAVs in several animal models. For example, AAV2 was shown to appear in semen after intravenous injection in rabbits (Favaro et al., 2009, Schuettrumpf et al., 2006). Moreover, AAV2 was found to integrate into the genomes of SSCs not only in mice but also in pigs by in vitro infection (Honaramooz et al., 2008, Zeng et al., 2013). These experiments suggested that AAV-mediated gene therapy is potentially dangerous. However, we recently reported that AAV1, but not AAV2, can infect SSCs but do not integrate into the genome, and offspring without AAV integration were born after in vitro infection and spermatogonial transplantation (Watanabe et al., 2017). Although it is very difficult to exclude the possibility of germline integration completely, our in vitro experiment raised a possibility that at least some types of AAVs may be used to transduce SSCs in vivo without germline integration. Compared with other routes of AAV administration, direct injection of AAVs into testes would provide a more direct approach to address the potential of germ cell infection, which has important implications in future applications.
We tested 13 types of AAV capsids, examining their ability to infect germ cells and Sertoli cells by intratubular injection. AAV8 transduced Sertoli cells, whereas AAV1 and AAV9 efficiently transduced both Sertoli cells and germ cells. The pattern of AAV1/9 infection was striking because these viruses infected not only spermatocytes in the adluminal compartment but also spermatogonia in the basal compartment. These results suggested that AAV1/9 particles passed through the BTB, unlike lentivirus or adenovirus that cannot penetrate the BTB. Because GFRA1 is an SSC marker (Kanatsu-Shinohara and Shinohara, 2013), this observation raised the possibility that SSCs are also transduced by AAV1/9. We tested this hypothesis by spermatogonial transplantation. Colony formation by R26R-Eyfp mice after AAV9-Cre transduction clearly demonstrated that SSCs were indeed transduced by AAV1/9 before transplantation. These experiments confirmed that AAVs transduced SSCs in situ.
Although AAV1 and AAV9 transduction patterns appeared to be quite similar in the infected testis, they had distinct characteristics. For example, the unique ability of AAV9 to penetrate tight junctions was previously reported in BBB (Foust et al., 2009). However, the BBB differs from the BTB in that the former is found between the endothelial cell junctions in the capillary walls. Moreover, the molecular composition is also different: the BBB is composed of CLDN1, CLDN3, CLDN5, and CLDN12 (Schrade et al., 2012), but a different set of claudins, including CLDN3, CLDN5, and CLDN11, contribute to the BTB (Gow et al., 1999, Meng et al., 2005, Morrow et al., 2009). Therefore, AAV9 penetrates tight junctions composed of different subsets of claudins. AAV1, in contrast, has not been reported to penetrate tight junctions. AAV1 is also distinct from AAV9 in that the former can infect germline stem cells in vitro (Watanabe et al., 2017). In this sense, the infection of SSCs by AAV9 injection was unexpected, and suggests that SSCs may show distinct properties in vivo versus in vitro, confirming our previous observations on the phenotypic plasticity of SSCs (Morimoto et al., 2009).
Although we currently do not know how AAV1/9 penetrated the BTB, the most straightforward explanation is disturbance of the tight junction proteins. However, we did not find any changes in the BTB with biotin tracer experiments. Moreover, receptors for these viruses were expressed widely in the testis, and do not explain the unique properties of these viruses. In this context, a more likely explanation is virus transcytosis (Pasquale and Chiorini, 2006). Transcytosis is a type of transcellular transport whereby various macromolecules are transported across the interior of a cell. In vitro experiments showed that AAV5 penetrated reconstituted endothelial cells and epithelia, which was blocked by AAV5-specific antisera or tannic acid (Pasquale and Chiorini, 2006). Although transcytosis has not been reported in Sertoli cells to our knowledge, we speculate that AAV1/9 migration through the BTB is regulated by a similar process. Further investigation will be required to address this point.
Another unexpected feature of AAVs was the penetration through the basement membrane. The basement membrane has been a critical impediment for AAV transduction (Dalkara et al., 2009). However, our results showed that AAVs can penetrate the basement membrane by both tubular and interstitial injections. However, the efficiency of transduction was more dramatic by interstitial injection. There are at least two possible explanations for this result. One possibility is AAV inactivation. Sertoli cells produce enormous amounts of seminiferous tubule fluid and proteases (Monsees et al., 1997, Rato et al., 2010). The ionic and protein composition of this fluid is strikingly different from that of interstitial fluid, which is derived from blood as a capillary filtrate (Waites and Gladwell, 1982). This difference may affect the stability or turnover of AAVs. Alternatively, the unidirectional infection could be caused by the differences in the virus titers. Considering the number of target cells, the concentration of viruses to target cells may be much lower when they penetrate the basement membrane from the adluminal side. This could reduce the virus transduction efficiency from the luminal side.
The most striking result in this study was the generation of offspring from congenitally infertile KitlSl/KitlSl-d mice. This result demonstrated proof of principle that AAVs can be used to treat male infertility resulting from environmental defect. Although Sertoli cells may be infected by other types of viruses (Ikawa et al., 2002, Kanatsu-Shinohara et al., 2002), adenoviruses induced inflammation and gradually lost expression, and lentiviruses have the potential to integrate into the germline. Given the apparent infection of SSCs by AAV9 in previous experiments, SSCs in KitlSl/KitlSl-d testes must have been infected by Kitl-expressing AAV9. However, none of the offspring showed evidence of germline AAV integration. Although we cannot exclude the possibility of extrachromosomal/episomal virus before germline transmission, we were not able to detect viral DNA in offspring, suggesting that AAVs did not stably integrate into the genome after germline transmission. Because no apparent abnormalities were found in spermatogenesis or testes following AAV9 infection, AAVs are apparently superior to previous approaches based on lentiviruses or adenoviruses and offer a promising strategy for treating animal or human infertility.
Our results show that AAVs represent a unique tool for manipulating both germ cells and Sertoli cells. Targeting Sertoli cells and germ cells with interstitial injection is attractive for genetic manipulation of these cell types in many animal species for which tubular microinjection is difficult or impossible due to differences in anatomical structures. Currently, an ultrasound-guided technique is being used for large animal species and primates for microinjection into the seminiferous tubules (Hermann et al., 2012), but their genetic manipulation has not been possible. Because spermatogonial transplantation requires sterilizing treatments of the recipients, this also potentially damages the testicular microenvironment. In this context, in vivo genetic manipulation using AAVs is attractive because neither sterilizing treatments nor ultrasound-guided techniques are required. AAV-mediated gene therapy may also be applicable to treating spermatogenic defects in humans. Although more extensive studies to examine off-target effects of AAVs and inadvertent germline integration need to be carried out (Büning et al., 2015, Honaramooz et al., 2008), our study provides a new experimental strategy for genetic manipulation of germ cells and their microenvironment, which may be applied to cure human infertility with extreme caution.
Experimental Procedures
Virus Production
AAV production was previously described (Watanabe et al., 2017). In brief, an AAV vector plasmid (pAAV-CAG-mCherry, pAAV-CAG-Kitl or pAAV-CAG-Cre), an adenovirus helper plasmid (pHelper; Agilent Technologies, Santa Clara, CA), and an AAV helper plasmid (pAAV-RC, Agilent Technologies; pAAV1, pXR5, pAAV6, pAAV6.2, pAAV7, pAAV8, pAAV9, pAAV10, pAAVhu11, gifts from Penn Vector Core [University of Pennsylvania, PA], pAAV11, gift from Dr. T. Kanda [National Institute of Infectious Diseases, Tokyo, Japan]; Anc80L65, gift from Dr. L. Vandenberghe [Harvard Medical School, MA]; pAAV-DJ, pAAV-DJ8 [Cell Biolabs, San Diego, CA]) were transiently transfected into AAV-293 cells. Viral titers were determined by real-time PCR using FastStart Universal SYBR Green Master Mix (Roche Diagnostic, Penzberg, Germany) and specific primers, as described previously (Watanabe et al., 2017). A lentivirus expressing Cre (CSII-EF-Cre-IRES2-Puro) and its production by transient transfection in 293T cells were previously described (Morimoto et al., 2015). The viral culture supernatant was concentrated by ultracentrifugation at 19,400 × g for 2 hr. The viral titer was determined using a qPCR Lentivirus Titration Kit (Applied Biological Materials, Richmond, Canada). An adenovirus expressing Cre (AxCANCre) or Kitl (Adex1CAmKL; both from RIKEN BRC, Tsukuba, Japan) was produced in 293 cells and prepared using CsCl centrifugation, and the viral titer was determined as described elsewhere (Takehashi et al., 2007). The viral titer was 1 × 1012/mL unless otherwise indicated.
Animals and Transplantation
For in vivo screening and tracer experiments, we used 4- to 5-week-old C57BL/6 (B6) × DBA/2 F1 (BDF1) mice. Cldn11 KO mice were used as a positive control for tracer experiments (a gift from Dr. S. Tsukita, Osaka University, Osaka, Japan) (Kitajiri et al., 2004). In some experiments, we used 4- to 8-week-old R26R-Eyfp mice (a gift from Dr. F. Costantini, Columbia University Medical Center, NY) (Srinivas et al., 2001). For fertility restoration experiments, 4- to 5-week-old KitlSl/KitlSl-d mice were used (Japan SLC, Shizuoka, Japan). We also used 4-week-old Prkdcscid mice (CLEA Japan, Tokyo, Japan) for CLDN11 immunostaining.
Microinjection of Virus Particles and Spermatogonial Transplantation
For the tubular injections, virus particles were introduced into the seminiferous tubules via the efferent duct (Ogawa et al., 1997). Approximately 10 or 4 μL were administered to testes of BDF1 or KitlSl/KitlSl-d mice, respectively, because the latter were smaller. The same amount of virus was used for interstitial injection. Where indicated, we used neuraminidase (Sigma, St. Louis, MO) to enhance infectivity, as described previously (Bell et al., 2011).
Spermatogonial transplantation was carried out as previously described (Ogawa et al., 1997). Donor testis cells were dissociated into single cells with a two-step enzymatic procedure using collagenase and trypsin (both from Sigma). Cells were microinjected into the efferent ducts of BDF1 mice that had been treated with busulfan (44 mg/kg, Sigma) at 4 weeks of age. Each injection filled 75%–85% of the seminiferous tubules. All busulfan-treated recipient mice were used 4–8 weeks after busulfan treatment. The Institutional Animal Care and Use Committee of Kyoto University approved all of the animal experimentation protocols.
Tracer Experiment
A tracer experiment was carried out as described previously (Takashima et al., 2011). In brief, Sulfo-NHS-LC-biotin (7.5 mg/mL; 557 D; Thermo Fisher Scientific, Waltham, MA) was dissolved in PBS. Approximately 20 μL of freshly prepared solution was microinjected into the testis interstitium. After 30 min, the animals were euthanized and their testes immediately removed and fixed overnight in 3.7% neutral-buffered formalin at 4°C. The samples were embedded in Tissue-Tek OCT compound (Sakura Finetek, Tokyo, Japan), and processed for cryosectioning. Sulfo-NHS-LC-biotin was detected by incubation with Alexa Fluor 488-conjugated streptavidin (BD Biosciences, San Jose, CA) before counterstaining.
Analysis of Recipient Testes
For the assessment of SSC activity, recipients were euthanized 2 months after transplantation and donor cell colonization was examined under UV light. Germ cell clusters were defined as colonies when the entire basal surface of the tubule was occupied and the cell clusters were at least 0.1 mm in length. To evaluate donor cell colonization by histological analysis, we viewed sections at 400× magnification to determine the extent of colonization in the testis, and collected images of the sections under an inverted microscope equipped with a CCD camera (DP70, Olympus, Tokyo, Japan) using Photoshop software (Adobe Systems, San Jose, CA). All sections were stained with Hoechst 33342.
Immunohistochemistry and Lectin Immunostaining
For immunohistochemistry, testis samples were fixed in 4% paraformaldehyde for 2 hr and embedded in Tissue-Tek OCT compound for cryosectioning. Immunostaining of cryosections was carried out by treating the samples with 0.1% Triton X-100 in PBS. After immersion in blocking buffer (0.1% Tween 20, 1% BSA, and 1% donkey or goat serum in PBS) for >1 hr, samples were incubated with the indicated primary antibodies at 4°C overnight. After three washes with PBS, samples were incubated with the secondary antibody. The antibodies and lectins used in the study are listed in Table S1.
Analysis of Gene Expression
Total RNA was isolated using TRIzol (Invitrogen, Carlsbad, CA), and first-strand cDNA was synthesized using the Verso cDNA Synthesis Kit (Thermo Fisher Scientific) and used for RT-PCR. For real-time PCR, the StepOnePlus Real-Time PCR system and FastStart Universal SYBR Green PCR Master Mix (Roche, Basel, Switzerland) were used according to the manufacturer's protocol (Applied Biosystems, Warrington, UK). Transcript levels were normalized relative to those of Hprt. PCR conditions were 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Each reaction was performed in duplicate. PCR primer sequences are listed in Table S2.
DNA Analysis
Genomic DNA was isolated from the offspring by the standard procedure using phenol/chloroform extraction and ethanol precipitation. Deletion of the floxed allele and detection of virus integration were estimated by PCR. PCR primer sequences are listed in Table S2.
For detecting viral DNA by Southern blotting, we digested 20 μg of DNA with indicated restriction enzymes and separated the fragments by electrophoresis on a 1.0% agarose gel. DNA was transferred and blotted onto a nylon membrane (Hybond-N+; Amersham Biosciences, Buckinghamshire, UK). Hybridization was performed according to a standard protocol using entire AAV virus, a 930-bp EcoRI-XhoI fragment of the full-length Cre cDNA, or a 434-bp NciI-BglI fragment of the full-length Kitl cDNA as probes. The membrane was hybridized for 16 hr at 65°C with a 32P-labeled probe.
Microinsemination
Testes that had been injected with AAV were refrigerated overnight and were used for microinsemination on the day after collection, as described previously (Ogonuki et al., 2006). The seminiferous tubules of recipient testes were dissected and then dissociated by repeatedly pipetting the tubule fragments. In experiments using R26R-Eyfp mouse testes, testis tubule fragments containing EYFP-expressing donor cells were dissociated under UV illumination. Microinsemination was performed by intracytoplasmic injection into BDF1 oocytes. After in vitro culture, two-cell-stage embryos were transferred into the oviducts of day-1 ICR female mice (CLEA Japan). Offspring were born by cesarean section on day 19.5.
Statistical Analyses
The results are presented as means ± SEM. Significant differences between means for single comparisons were determined by Student's t tests. Multiple comparison analyses were carried out using ANOVA followed by Tukey's honestly significant difference test.
Author Contributions
S.W. and T.S. conceived the project. T.S. did in vivo experiments, and S.W. analyzed the samples. N.O., S.M., and A.O. carried out microinsemination. S.W., M.K.-S., and T.S. wrote the manuscript.
Acknowledgments
We thank Dr. T. Furukawa (Osaka University) for providing us pAAV-CAG-mCherry constructs, Drs. D. Watanabe and R. Matsui (Kyoto University) for AAV production and infection protocols, and Ms. Y. Ogata for technical assistance. Financial support for this research was provided by the Uehara Memorial Foundation, the Takeda Science Foundation, the Naito Foundation, the Japan Science and Technology Agency (PRESTO), AMED (17933225), and grants-in-aid for Scientific Research on Innovative Areas “Epigenome Dynamics and Regulation in Germ Cells”(25112003) and grant-in-aid for Young Scientists (B) (16K18400) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Published: April 5, 2018
Footnotes
Supplemental Information includes seven figures and two tables and can be found with this article online at https://doi.org/10.1016/j.stemcr.2018.03.005.
Supplemental Information
References
- Bell C.L., Vandenberghe L.H., Bell P., Limberis M.P., Gao G.P., Van Vliet K., Agbandje-McKenna M., Wilson J.M. The AAV9 receptor and its modification to improve in vivo lung gene transfer in mice. J. Clin. Invest. 2011;121:2427–2435. doi: 10.1172/JCI57367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellvé A.R. Purification, culture, and fractionation of spermatogenic cells. Methods Enzymol. 1993;225:84–113. doi: 10.1016/0076-6879(93)25009-q. [DOI] [PubMed] [Google Scholar]
- Blanchard K.T., Boekelheide K. Adenovirus-mediated gene transfer to rat testis in vivo. Biol. Reprod. 1997;56:495–500. doi: 10.1095/biolreprod56.2.495. [DOI] [PubMed] [Google Scholar]
- Brinster R.L., Zimmermann J.W. Spermatogenesis following male germ-cell transplantation. Proc. Natl. Acad. Sci. USA. 1994;91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büning H., Huber A., Zhang L., Meumann N., Hacker U. Engineering the AAV capsid to optimize vector-host-interactions. Curr. Opin. Pharmacol. 2015;24:94–104. doi: 10.1016/j.coph.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Cheng C.Y., Mruk D.D. The blood-testis barrier and its implications for male contraception. Pharmacol. Rev. 2012;64:16–64. doi: 10.1124/pr.110.002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalkara D., Kolstad K.D., Caporale N., Visel M., Klimczak R.R., Schaffer D.V., Flannery J.G. Inner limiting membrane barriers to AAV-mediated retinal transduction from the vitreous. Mol. Ther. 2009;17:2096–2102. doi: 10.1038/mt.2009.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalkara D., Byrne L.C., Lee T., Hoffmann N.V., Schaffer D.V., Flannery J.G. Enhanced gene delivery to the neonatal retina through systemic administration of tyrosine-mutated AAV9. Gene Ther. 2012;19:176–181. doi: 10.1038/gt.2011.163. [DOI] [PubMed] [Google Scholar]
- Daya S., Berns K.I. Gene therapy using adeno-associated virus vectors. Clin. Microbiol. Rev. 2008;21:583–593. doi: 10.1128/CMR.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij D.G., Russell L.D. All you wanted to know about spermatogonia but were afraid to ask. J. Androl. 2000;21:776–798. [PubMed] [Google Scholar]
- Dym M., Fawcett D.W. The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol. Reprod. 1970;3:308–326. doi: 10.1093/biolreprod/3.3.308. [DOI] [PubMed] [Google Scholar]
- Favaro P., Downey H.D., Zhou J.S., Wright J.F., Hauck B., Mingozzi F., High K.A., Arruda V.R. Host and vector-dependent effects on the risk of germline transmission of AAV vectors. Mol. Ther. 2009;17:1022–1030. doi: 10.1038/mt.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foust K.D., Nurre E., Montgomery C.L., Hernandez A., Chan C.M., Kaspar B.K. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat. Biotechnol. 2009;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- França L.R., Hess R.A., Dufour J.M., Hofmann M.C., Griswold M.D. The Sertoli cell: one hundred fifty years of beauty and plasticity. Andrology. 2016;4:189–212. doi: 10.1111/andr.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow A., Southwood C.M., Li J.S., Pariali M., Riordan G.P., Brodie S.E., Danias J., Bronstein J.M., Kachar B., Lazzarini R.A. CNS protein and Sertoli cell tight junction strands are absent in Osp/claudin-11 null mice. Cell. 1999;99:649–659. doi: 10.1016/s0092-8674(00)81553-6. [DOI] [PubMed] [Google Scholar]
- Hermann B.P., Sukhwani M., Winkler F., Pascarella J.N., Peters K.A., Sheng Y., Valli H., Rodriguez M., Ezzelarab M., Dargo G. Spermatogonial stem cell transplantation into Rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell. 2012;11:715–726. doi: 10.1016/j.stem.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honaramooz A., Megee S., Zeng W., Destrempes M.M., Overton S.A., Luo J., Galantino-Homer H., Modelski M., Chen F., Blash S. Adeno-associated virus (AAV)-mediated transduction of male germ line stem cells results in transgene transmission after germ cell transplantation. FASEB. J. 2008;22:374–382. doi: 10.1096/fj.07-8935com. [DOI] [PubMed] [Google Scholar]
- Huang Z., Tamura M., Sakurai T., Chuma S., Saito T., Nakatsuji N. In vivo transfection of testicular germ cells and transgenesis by using the mitochondrially localized jellyfish fluorescent protein gene. FEBS Lett. 2000;487:248–251. doi: 10.1016/s0014-5793(00)02271-7. [DOI] [PubMed] [Google Scholar]
- Ikawa M., Tergaonkar V., Ogura A., Ogonuki N., Inoue K., Verma I.M. Restoration of spermatogenesis by lentiviral gene transfer: offspring from infertile mice. Proc. Natl. Acad. Sci. USA. 2002;99:7524–7529. doi: 10.1073/pnas.072207299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajiri S., Miyamoto T., Mineharu A., Sonoda N., Furuse K., Hata M., Sasaki H., Mori Y., Kubota T., Ito J. Compartmentalization established by claudin-11-based tight junctions in stria vascularis is required for hearing through generation of endocochlear potential. J. Cell Sci. 2004;117:5087–5096. doi: 10.1242/jcs.01393. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M., Ogura A., Ikegawa M., Inoue K., Ogonuki N., Tashiro K., Toyokuni S., Honjo T., Shinohara T. Adenovirus-mediated gene delivery and in vitro microinsemination produce offspring from infertile male mice. Proc. Natl. Acad. Sci. USA. 2002;99:1383–1388. doi: 10.1073/pnas.022646399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M., Toyokuni S., Shinohara T. Transgenic mice produced by retroviral transduction of male germ line stem cells in vivo. Biol. Reprod. 2004;71:1202–1207. doi: 10.1095/biolreprod.104.031294. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M., Shinohara T. Spermatogonial stem cell self-renewal and development. Annu. Rev. Cell Dev. Biol. 2013;29:163–187. doi: 10.1146/annurev-cellbio-101512-122353. [DOI] [PubMed] [Google Scholar]
- Kojima Y., Sasaki S., Uemoto Y., Hashimoto Y., Hayashi Y., Kohri K. Effects of adenovirus mediated gene transfer to mouse testis in vivo on spermatogenesis and next generation. J. Urol. 2003;170:2109–2114. doi: 10.1097/01.ju.0000092898.91658.08. [DOI] [PubMed] [Google Scholar]
- Meistrich M.L., van Beek M.E.A.B. Spermatogonial stem cells. In: Desjardins C.C., Ewing L.L., editors. Cell and Molecular Biology of the Testis. Oxford University Press; 1993. pp. 266–295. [Google Scholar]
- Meng J., Holdcraft R.W., Shima J.E., Griswold M.D., Braun R.E. Androgens regulate the permeability of the blood-testis barrier. Proc. Natl. Acad. Sci. USA. 2005;102:16696–16700. doi: 10.1073/pnas.0506084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mital P., Hinton B.T., Dufour J.M. The blood-testis and blood-epididymis barriers are more than just their tight junctions. Biol. Reprod. 2011;84:851–858. doi: 10.1095/biolreprod.110.087452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsees T.K., Schill W.B., Miska W. Protease-protease inhibitor interactions in Sertoli cell-germ cell crosstalk. Adv. Exp. Med. Biol. 1997;424:111–123. doi: 10.1007/978-1-4615-5913-9_20. [DOI] [PubMed] [Google Scholar]
- Morimoto H., Kanatsu-Shinohara M., Takashima S., Chuma S., Nakatsuji N., Takehashi M., Shinohara T. Phenotypic plasticity of mouse spermatogonial stem cells. PLoS One. 2009;4:e7909. doi: 10.1371/journal.pone.0007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto H., Kanatsu-Shinohara M., Shinohara T. ROS-generating oxidase Nox3 regulates the self-renewal of mouse spermatogonial stem cells. Biol. Reprod. 2015;92:1–10. doi: 10.1095/biolreprod.114.127647. [DOI] [PubMed] [Google Scholar]
- Morrow C.M., Tyagi G., Simon L., Carnes K., Murphy K.M., Cooke P.S., Hofmann M.C., Hess R.A. Claudin 5 expression in mouse seminiferous epithelium is dependent upon the transcription factor ets variant 5 and contributes to blood-testis barrier function. Biol. Reprod. 2009;81:871–879. doi: 10.1095/biolreprod.109.077040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano M., Watson D.J., Ryu B.Y., Wolfe J.H., Brinster R.L. Lentiviral vector transduction of male germ line stem cells in mice. FEBS Lett. 2002;524:111–115. doi: 10.1016/s0014-5793(02)03010-7. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Aréchaga J.M., Avarbock M.R., Brinster R.L. Transplantation of testis germinal cells into mouse seminiferous tubules. Int. J. Dev. Biol. 1997;41:111–122. [PubMed] [Google Scholar]
- Ogawa T., Dobrinski I., Avarbock M.R., Brinster R.L. Transplantation of male germ line stem cells restore fertility in infertile mice. Nat. Med. 2000;6:29–34. doi: 10.1038/71496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogonuki N., Mochida K., Miki H., Inoue K., Fray M., Iwaki T., Moriwaki K., Obata Y., Morozumi K., Yanagimachi R. Spermatozoa and spermatids retrieved from frozen reproductive organs of frozen whole bodies of male mice can produce normal offspring. Proc. Natl. Acad. Sci. USA. 2006;103:13098–13103. doi: 10.1073/pnas.0605755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquale G.D., Chiorini J.A. AAV transcytosis through barrier epithelia and endothelium. Mol. Ther. 2006;13:506–516. doi: 10.1016/j.ymthe.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Rato L., Socorro S., Cavaco J.E., Oliveira P.F. Tubular fluid secretion in the seminiferous epithelium: ion transporters and aquaporins in Sertoli cells. J. Membr. Biol. 2010;236:215–224. doi: 10.1007/s00232-010-9294-x. [DOI] [PubMed] [Google Scholar]
- Schrade A., Sade H., Couraud P.O., Romero I.A., Weksler B.B., Niewoehner J. Expression and localization of claudins-3 and -12 in transformed human brain endothelium. Fluids Barriers CNS. 2012;9:6. doi: 10.1186/2045-8118-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuettrumpf J., Liu J.H., Couto L.B., Addya K., Leonard D.G., Zhen Z., Sommer J., Amuda V.R. Inadvertent germline transmission of AAV2 vector:findings in a rabbit model correlate with those in a human clinical trial. Mol. Ther. 2006;13:1064–1073. doi: 10.1016/j.ymthe.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Shinohara T., Avarbock M.R., Brinster R.L. Functional analysis of spermatogonial stem cells in Steel and cryptorchid infertile mouse models. Dev. Biol. 2000;220:401–411. doi: 10.1006/dbio.2000.9655. [DOI] [PubMed] [Google Scholar]
- Srinivas S., Watanabe T., Lin C.S., William C.M., Tanabe Y., Jessell T.M., Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC. Dev. Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima S., Kanatsu-Shinohara M., Tanaka T., Takehashi M., Morimoto H., Shinohara T. Rac mediates mouse spermatogonial stem cell homing to germline niche by regulating transmigration through the blood-testis barrier. Cell Stem Cell. 2011;9:463–475. doi: 10.1016/j.stem.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Takehashi M., Kanatsu-Shinohara M., Inoue K., Ogonuki N., Miki H., Toyokuni S., Ogura A., Shinohara T. Adenovirus-mediated gene delivery into mouse spermatogonial stem cells. Proc. Natl. Acad. Sci. USA. 2007;104:2596–2601. doi: 10.1073/pnas.0609282104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites G.M., Gladwell R.T. Physiological significance of fluid secretion in the testis and blood-testis barrier. Physio. Rev. 1982;62:624–671. doi: 10.1152/physrev.1982.62.2.624. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Kanatsu-Shinohara M., Ogonuki N., Matoba S., Ogura A., Shinohara T. Adeno-associated virus-mediated delivery of genes to mouse spermatogonial stem cells. Biol. Reprod. 2017;96:221–231. doi: 10.1095/biolreprod.116.143495. [DOI] [PubMed] [Google Scholar]
- Yomogida K., Yagura Y., Nishimune Y. Electroporated transgene-rescued spermatogenesis in infertile mutant mice with a Sertoli cell defect. Biol. Reprod. 2002;67:712–717. doi: 10.1095/biolreprod.101.001743. [DOI] [PubMed] [Google Scholar]
- Zeng W., Tang L., Bondareva A., Honaramooz A., Tanco V., Dores C., Modelski M., Rodriguez-Soza J.R., Paczkowski M., Silva E. Viral transduction of male germline stem cells results in transgene transmission after germ cell transplantation in pigs. Biol. Reprod. 2013;88:27. doi: 10.1095/biolreprod.112.104422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.