Abstract
Objectives
The objectives of this study were to detect those characteristics that were specifically associated with infection or colonization by Acinetobacter baumannii, describe the clinical manifestations of those patients in whom the infection was detected in intensive care unit (ICU) or non-ICU wards, and analyze the prognosis-associated factors in patients from whom A. baumannii was isolated.
Patients and methods
A sample of 122 patients from whom A. baumannii was recovered during an endemic period in a teaching hospital was included. Only those cases in which A. baumannii was recovered as the unique microbe were considered. Demographic data; ward of admission; intrinsic and extrinsic risk factors for infection or colonization; chronic underlying condition severity, as evaluated by the McCabe classification or Charlson index and Acute Physiology and Chronic Health Evaluation (APACHE) II score; and clinical manifestations were analyzed to differentiate specific characteristics of colonized or infected patients. Factors independently associated with the mortality at 30 days were also analyzed by Cox regression.
Results
A total of 73 (60%) patients were colonized and 49 (40%) individuals were infected with A. baumannii. A non-fatal McCabe class (when compared to ultimately and rapidly fatal), days of hospitalization prior to isolation of A. baumannii, and present ICU admission were associated with the diagnosis of infection. The more frequent clinical picture was respiratory infection (tracheobronchitis, 16 [33%] cases; pneumonia, 27 [55%] cases). Mortality at 30 days was 24% (n=29). A non-fatal McCabe class (Exp[B] 2.44, 95% confidence interval [CI] 1.05–5.66, p=0.039) and the absence of infection (Exp[B] 2.75, 95% CI 1.18–6.38, p=0.019) were independently associated with survival.
Conclusion
Parameters associated with infection by A. baumannii in an endemic situation are the admission at ICU and the number of days of hospitalization. Mortality of patients from whom A. baumannii was isolated was independently influenced by the chronic underlying basal state and the presence of infection by A. baumannii.
Keywords: Acinetobacter baumannii, colonization, infection, mortality, tracheobronchitis, pneumonia, care unit
Introduction
Acinetobacter baumannii is one of the most significant bacteria causing nosocomial infections.1 Two characteristics have been implicated in the importance of A. baumannii as a human pathogen: first, its ability to survive on animate and unanimate surfaces for long periods with the risk of a prolonged endemic2 and second, its resistance to multiple antibiotics, including carbapenems, which complicates treatment.3,4 Although infections are typically acquired in intensive care units (ICUs), they are also becoming increasingly common in non-ICU wards and long-term care facilities. The differential characteristics between infections acquired in ICU or non-ICU wards have not been clearly delineated.5
Owing to characteristics of the patients from whom A. baumannii is isolated, it is often difficult to differentiate colonization from infection. Thus, up to half of the cases in which A. baumannii is isolated represent colonization.5–7 Moreover, up to 36% of bacteremias in which A. baumannii is isolated are polymicrobial (with additional isolation of other microbes, such as coagulase-negative Staphylococcus, Enterococcus spp. or Pseudomonas spp.), suggesting that some of the isolates are contaminants from skin or the environment. Thus, it is important to clinically differentiate between infection and pseudoinfection.8,9 This situation needs to be considered to avoid diagnostic errors and unnecessary antibiotic therapy.
Risk factors for the acquisition of A. baumannii include previous use of antibiotics, major surgery, severe burns, immune depression, and invasive procedures.10 Clinical manifestations related to A. baumannii infection include septic shock, mechanical ventilation-associated pneumonia, tracheobronchitis, bacteremia, surgery-associated infection, skin and soft tissue infection, and urinary tract infection.11
The present work describes our experience with an outbreak of A. baumannii during an endemic period (defined by a significant increase in patients colonized/infected by this pathogen). The analysis of this was performed to detect those characteristics that were specifically associated with infection (with a careful analysis to differentiate from colonization), describe the clinical manifestations of those patients in whom the infection was detected in ICU or non-ICU wards, and determine the prognosis-associated factors in patients from whom A. baumannii was isolated.
Patients and methods
Patients older than 14 years from whom A. baumannii was isolated from any clinical sample, hospitalized in a university hospital (732 beds), during an endemic period (2009–2011) were included. Active surveillance samples (such as samples performed to detect colonization for the purpose of infection control) were not considered. The microbiological characteristics of this outbreak have been published previously.12 The population size was 124 patients.
Definitions
Infection by A. baumannii was defined when, aside from the isolation of the bacteria as the unique microbe in a clinical sample, clinical signs or abnormalities in the complementary studies supported the diagnosis of infection and no other clinical focus could justify the clinical manifestations, according to the criteria defined by the Centers for Disease Control and Prevention (CDC)13 If these criteria were not fulfilled, the case was considered colonization. In addition, colonization was considered when both clinical and analytical parameters of infection improved after an empiric treatment to which the isolate of A. baumannii was resistant.
In those patients from whom A. baumannii was recovered in several evolutive moments, the possibility of infection or colonization was considered in each of these moments. If it was considered colonization in all of them, the case was included only the first time, analyzing the parameters associated with the first positive culture. If one isolate corresponded to infection, the case was considered infection and the variables associated in that moment were analyzed.
Sterile samples, including blood and pleural, cerebrospinal, or ascitic fluid, were accepted. Non-sterile samples had to fulfill the standard criteria for quality, and quantitative cultures were used.
A primary bacteremia was diagnosed if A. baumannii grew in blood cultures in a patient with fever >38°C in the absence of other clinical foci. If there was a defined clinical focus, the bacteremia was considered as secondary. More than 15 colonies (semiquantitative method) were required in the intravascular catheter tip to consider a catheter-associated bloodstream infection. For the diagnosis of pneumonia or tracheobronchitis, only cultures of tracheal aspiration with >106 colony forming units (cfu)/mL or cultures of bronchial aspirate or bronchoalveolar lavage with a growth of ≥104 cfu/mL were accepted. Sputum was accepted in cases without orotracheal intubation or tracheostomy if the culture was pure and the sample was representative of lower airways (absence of epithelial cells and ≥25 polymorphonuclear neutrophils/microscopic field).
The diagnosis of pneumonia required systemic signs (temperature >38°C and/or leukocytosis >12000/mm3 or leukopenia <4000/mm3) and radiologic (a new lung infiltrate or consolidation in chest X-ray or computed tomography [CT]) and pulmonary criteria, including at least two of the following: 1) an increase in respiratory secretions or purulent secretions; 2) the appearance or increase in dyspnea, cough, or tachypnea; 3) rhonchi or crepitations at lung auscultation; or 4) a decrease in the hemoglobin saturation of oxygen or an increase in the requirement for oxygen or mechanical ventilation. If all abovementioned characteristics were fulfilled with the exception of lung infiltrate, the diagnosis of tracheobronchitis was made.
A urinary infection was diagnosed if in the urine culture, ≥105 cfu/mL from spontaneous micturition or ≥103 cfu/mL from urinary catheter were obtained and the patient had urinary infection symptoms (dysuria, suprapubic pain).
The diagnosis of skin and soft tissue or surgical infections was made in patients using local inflammatory data (±systemic signs of infection) and a positive culture obtained by biopsy.
Study schedule
After the culture from human samples, only those samples in which A. baumannii was isolated as the unique microbe were selected. When A. baumannii was recovered from two or more sources in the same patient, only one isolate obtained was considered for analysis and the patient was included only once (in cases where susceptibility results were different, isolation with the highest level of resistance was included). After the isolation of A. baumannii, the following variables were obtained from the patient’s chart: age, sex, hospitalization ward (classified as ICUs or non-ICU wards), previous stay at the ICU, acquisition of A. baumannii as community, and health care related (including patients living in elderly residences or long-stay care facilities such as health care facilities) or nosocomial (according to conventional CDC definitions).13,14
Risk factors for the infection were classified as intrinsic (decompensated diabetes mellitus [glycated hemoglobin >7.5% or associated microvascular or macrovascular complications], liver disease [liver insufficiency or portal hypertension-derived complications], heart failure, respiratory insufficiency, kidney failure [previous creatinine clearance <60 mL/min/1.73 m2 according to Chronic Kidney Disease Epidemiology Collaboration equation or dialysis], immune depression [chemotherapy, radiotherapy, immunosuppressive disease, and/or immunosuppressive drugs], uncontrolled cancer [active treatment with chemotherapy or radiotherapy or under palliative care], central nervous system disease [dementia, sequela of neurosurgical or vascular lesion], obesity, or malnutrition) or extrinsic (previous treatment with antibiotic, if any; venous or urinary catheter; nasogastric intubation; enteral or parenteral nutrition; mechanical ventilation; tracheostomy; surgical procedures in the previous 90 days; and digestive or respiratory endoscopy in the previous 7 days).
The severity of chronic underlying conditions was measured by the McCabe classification15 and Charlson index16 in all patients and by the Acute Physiology and Chronic Health Evaluation (APACHE) II score17 in those admitted to the ICU.
Lengths of hospital stay before and after isolation of A. baumannii and clinical and therapeutic data were obtained. Inflammatory response was classified as sepsis, severe sepsis, and septic shock.18 Medical treatments were those chosen by physicians in charge of the patients. Only directed antibiotic therapy (after known data about susceptibility of the isolate) was considered.
All patients were followed for 30 days after isolation of the A. baumannii or until death.
Microbiological studies
All isolates, presumptively identified as Acinetobacter species using conventional phenotypic and/or biochemical methods, were definitively identified using matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF) analyses (Microflex LT; Bruker Daltonik GmbH, Leipzig, Germany). Only patients whose isolates were definitively identified as A. baumannii were included. Microdilution susceptibility testing was performed, according to the recommendations of the Clinical and Laboratory Standards Institute.19 For sulbactam, tigecycline, and rifampin, isolates with a minimum inhibitory concentration ≤8, 1, and 4 mg/L, respectively, were considered as susceptible.20
The isolates of A. baumannii were classified based on their resistance to antibiotics, according to International Consensus.21 The classifications were as follows: 1) multidrug resistant, where resistance to antibiotics in three or more classes was observed; 2) extensively resistant, where resistance to at least one agent in all but two or fewer antibiotic classes, was observed; and 3) pandrug resistant, where resistance to all possible antibiotics was observed.
Outcome of the infection
Mortality was analyzed after 30 days of isolation of A. baumannii. Death was judged to be related to A. baumannii infection when persistent signs or symptoms of infection were present at the time of death and/or when death occurred within 1 week of initiation of antibiotic therapy without any other clear explanation.22 Three authors (AM-A, FG-S, FG-C) analyzed the clinical charts of the patients to attribute deaths to A. baumannii infections or to other concomitant processes.
Statistical analysis
Descriptive data were expressed as the mean ± standard deviation or as an absolute number (percentage). Qualitative variables were compared by the chi-square test or Fisher’s exact test when necessary. Quantitative variables were compared using the Mann–Whitney U test or analysis of variance (ANOVA) when necessary. The Pearson’s correlation test analyzed the association between quantitative variables.
To analyze the differences between colonization and infection, factors with significant differences in the bivariate analysis were included in a logistic regression model. Variables with a univariate p value of <0.1 were included and selected using a stepwise backward procedure.
To analyze the factors influencing mortality at 30 days after the isolation of A. baumannii (dependent variable), the following independent variables were considered: age, sex, infection vs colonization, hospital ward, McCabe classification, Charlson index, APACHE II score, and severe sepsis/septic shock. Cumulated incidence of death was evaluated from the Kaplan–Meier curves. The association between this variable and the abovementioned independent factors was analyzed by the log-rank test. The multivariate analysis of survival was performed using Cox regression.
A p value of <0.05 was considered as statistically significant. The statistical analysis was carried out using the SPSS, version 18.0, statistical software package (SPSS Inc., Chicago, IL, USA).
Ethical aspects
This study was performed according to the Declaration of Helsinki. The project was approved by the local ethical research committee of the Hospital Puerta del Mar, Cádiz. The need to obtain informed consent was waived due to the observational nature of the study, and patient data accessed were de-identified.
Results
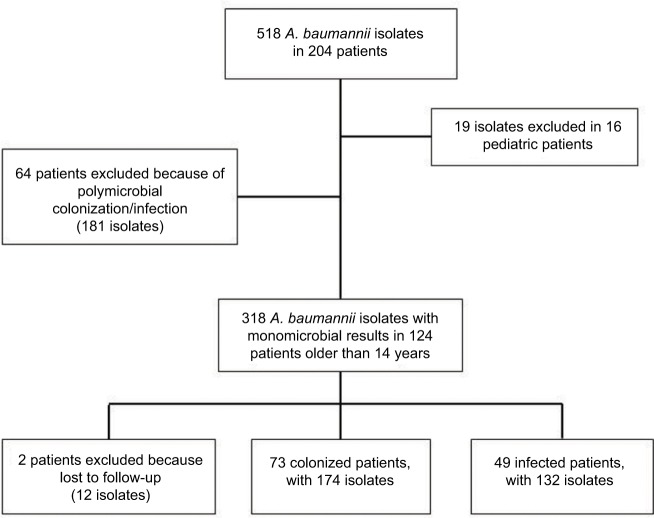
During the period of study, 124 patients presented with colonization or infection by A. baumannii. Two of them were excluded because they were transferred to another hospital at 8 and 10 days, respectively, from isolation of A. baumannii (Figure 1). Thus, the population of study was composed of 122 patients (age, 61±17 years; male sex, 83 [68%] individuals).
Figure 1.
Diagram showing excluded and included patients in the study.
Note: As one patient usually had more than one culture and isolation, total number of isolates in each group is shown.
Abbreviation: A. baumannii, Acinetobacter baumannii.
The incidence density rate of colonization/infection was 0.71 cases/1000 patient-days. A total of 59 (48%) cases presented to the ICU (incidence density rate, 8.22 cases/1000 patient-days), while 63 cases presented to non-ICU wards (incidence density rate, 0.38 cases/1000 patient-days).
Colonization/infection was considered as nosocomial in 119 (97.5%) cases and health care facilities associated in the other three (2.5%) cases. In all, 37 (30%) patients had not ever been admitted to an ICU.
Antibiotic susceptibility testing demonstrated that more than 90% of A. baumannii isolates were susceptible to colistin, minocycline, and tigecycline. In contrast, only 15–20% of the isolates were susceptible to cefepime, carbapenems, or ampicillin/sulbactam.
Differences between colonized and infected patients
In all, 73 (60%) out of 122 patients were considered colonized, whereas infection by A. baumannii was diagnosed in 49 (40%) individuals. The differential characteristics between both groups are shown in Table 1.
Table 1.
Differential characteristics between colonized and infected individuals, by A. baumannii (n=122)
| Parameter | Colonized patients (n=73) | Infected patients (n=49) | p univariant | Multivariant analysis
|
|
|---|---|---|---|---|---|
| Exp(B) (95% CI) | p | ||||
| Age (years), mean ± standard deviation | 65±15 | 55±18 | 0.002 | ||
| Sex (male), n (%) | 44 (60) | 39 (80) | 0.030 | ||
| Present admission at ICU, n (%) | 27 (37) | 32 (65) | 0.002 | 4.55 (1.79–11.53) | 0.001 |
| Previous admission at ICU, n (%) | 42 (57) | 43 (88) | <0.001 | ||
| Length of stay previous to isolation of A. baumannii (days) | 22±19 | 31±36 | 0.049 | 1.02 (1.01–1.04) | 0.012 |
| Presence of intrinsic risk factors (chronic underlying condition), n (%)a | 65 (89) | 30 (61) | 0.001 | ||
| McCabe classification, n (%) | 0.001 | ||||
| Non-fatal | 31 (43) | 37 (76) | 2.94 (1.26–2.89) | 0.013 | |
| Ultimately or rapidly fatal | 42 (58) | 12 (24) | |||
| Charlson index, mean ± standard deviation | 2.95±2.34 | 1.31±2.05 | <0.001 | ||
| APACHE II scoreb, mean ± standard deviation | 22±8 | 23±9 | 0.309 | ||
| Presence of extrinsic risk factors, n (%) | |||||
| Central venous catheter | 50 (69) | 40 (82) | 0.142 | ||
| Urinary catheter | 55 (75) | 43 (88) | 0.108 | ||
| Parenteral nutrition | 7 (10) | 2 (4) | 0.312 | ||
| Enteral nutrition | 27 (37.0) | 34 (69.4) | 0.001 | ||
| Mechanical ventilation | 25 (34.2) | 26 (53.1) | 0.042 | ||
| Tracheostomy | 6 (8.2) | 13 (26.5) | 0.010 | ||
| Major surgical procedure | 26 (35.6) | 13 (26.5) | 0.327 | ||
| Cerebrospinal fluid derivation | 5 (7) | 11 (22) | 0.402 | ||
| Digestive endoscopy in previous week | 0 | 2 (4) | 0.159 | ||
| Antibiotic therapy in the previous 90 days | 70 (96) | 46 (94) | 0.683 | ||
| Treatment with carbapenems in the previous 90 days | 29 (40) | 20 (41) | 1.000 | ||
| Isolation sites for A. baumannii, n (%) | <0.001 | ||||
| Sputum | 6 (8) | 7 (14) | |||
| Tracheal aspirate | 26 (36) | 36 (74) | |||
| Blood | 0 (0) | 3 (6) | |||
| Urine | 10 (14) | 0 (0) | |||
| Ascitic fluid | 2 (3) | 1 (2) | |||
| Surgical wound | 18 (25) | 1 (2) | |||
| Others | 11 (15) | 1 (2) | |||
| Resistance pattern, n (%) | 0.081 | ||||
| Susceptible | 3 (4) | 0 | |||
| Multidrug resistant | 18 (25) | 6 (12) | |||
| Extensively resistant | 52 (71) | 42 (86) | |||
| Pandrug resistant | 0 (0) | 1 (2) | |||
Notes:
The presence of cardiac (colonized, 30 [41%] patients; infected, seven [14%] cases; p=0.002) and respiratory (colonized, 21 [29%] patients; infected, four [8%] cases; p=0.006) chronic underlying conditions was significantly more frequent in colonized individuals. The rest of the intrinsic risk factors did not show significant differences between both groups.
APACHE II was measured only in patients in ICU (n=59; colonized, 27 cases; infected, 32 cases).
Abbreviations: A. baumannii, Acinetobacter baumannii; CI, confidence interval; ICU, intensive care unit; APACHE, Acute Physiology and Chronic Health Evaluation.
Parameters independently associated with the presence of infection were assessed by linear regression analysis. A non-fatal McCabe index (when compared to conjoined ultimately and rapidly fatal), days of hospitalization previous to isolation of A. baumannii, and present ICU admission were associated with the diagnosis of infection (Table 1).
Differentiation between colonization/infection by A. baumannii in ICU and non-ICU wards
Because one of the independent factors associated with infection was the stay at ICU and 52% of the cases (n=63) were diagnosed in non-ICU wards, a differential analysis of factors associated with infection in both hospitalization wards was made.
When compared with colonized individuals (n=46), infected patients (n=17) in non-ICU wards more frequently had previous stays in the ICU (colonized, 15 [33%] cases; infected, 11 [65%] cases; p=0.042), longer lengths of stays from admission to isolation of A. baumannii (colonized, 26±21 days; infected, 57±47 days; p=0.001), more frequent tracheostomies (colonized, three [7%]; infected, 10 [59%]; p<0.001), less frequent isolation of A. baumannii from urine (colonized, nine [20%]; infected, zero [0%]; p=0.048) or surgical wounds (colonized, 16 [35%]; infected, one [6%]; p=0.022), and a lower Charlson index (colonized, 3.37±2.49; infected, 2.18±2.65; p=0.047). The rest of the analyzed parameters (age, sex, chronic underlying conditions, extrinsic risk factors other than tracheostomy, McCabe class, and resistance pattern) were similar between colonized and infected individuals. Logistic regression analyses demonstrated that tracheostomy was the unique factor independently associated with the presence of infection (Exp[B] 20.00, 95% confidence interval [CI] 4.38–91.28, p<0.001)
Compared with colonized individuals (n=27), patients (n=32) admitted to the ICU with infection by A. baumannii presented with lower frequencies of chronic underlying conditions (colonized, 24 [89%]; infected, 14 [44%]; p<0.001), including the presence of cardiovascular disease (colonized, 12 [44%]; infected, four [13%]; p=0.008), a lower percentage in the McCabe non-fatal class (colonized, 12 [44%]; infected, 28 [88%]; p=0.001), and a lower Charlson index (colonized, 2.22±1.91; infected, 0.84±1.51; p=0.003). The rest of the analyzed parameters (age, sex, extrinsic risk factors, source of isolates, and resistance pattern) were similar between colonized and infected individuals. Logistic regression analyses demonstrated that the absence of chronic underlying conditions (Exp[B] 5.40, 95% CI 1.18–24.65, p=0.029) and a non-fatal McCabe class (Exp[B] 4.17, 95% CI 1.01–17.31, p=0.049) were the independent factors associated with infection.
Characteristics of patients infected by A. baumannii
The more frequent clinical picture was respiratory infections (tracheobronchitis, 16 [33%] cases; pneumonia, 27 [55%] cases). Primary bacteremia was detected in three (6%) patients and osteoarticular, surgery-derived intra-abdominal or soft tissue infection in one (2%) case each. The frequency in which patients presented with sepsis or severe sepsis/septic shock was 76% (n=37) and 14% (n=12), respectively.
Differences in the acquisition of infection in ICU or non-ICU wards are shown in Table 2. Patients admitted to the ICU showed a lower frequency of chronic underlying diseases. Non-fatal McCabe class and a lower Charlson index were also more frequent in patients admitted to the ICU. Moreover, ICU patients showed pneumonia as the main respiratory infection, whereas those hospitalized in non-ICU wards predominantly showed tracheobronchitis. A similar mortality at 30 days was observed in both groups of patients.
Table 2.
Characteristics of patients infected by A. baumannii (n=49) according to the ward of hospitalization (ICU vs non-ICU)
| Parameter | ICU (n=32) | Non-ICU (n=17) | p |
|---|---|---|---|
| Age (years), mean ± standard deviation | 54±16 | 56±22 | 0.377 |
| Sex (male), n (%) | 23 (72) | 16 (94) | 0.066 |
| Presence of intrinsic risk factors (chronic underlying conditions), n (%) | 14 (44) | 16 (94) | 0.001 |
| McCabe classification, n (%) | 0.007 | ||
| Non-fatal | 28 (88) | 9 (53) | |
| Ultimately or rapidly fatal | 4 (12) | 8 (47) | |
| Charlson index, mean ± standard deviation | 0.84±1.51 | 2.18±2.65 | 0.012 |
| Sites of infection, n (%) | 0.001 | ||
| Pneumonia | 25 (78) | 2 (12) | |
| Tracheobronchitis | 6 (19) | 10 (59) | |
| Primary bacteremia | 1 (3) | 2 (12) | |
| Surgery-related intra-abdominal | 0 | 1 (6) | |
| Osteoarticular | 0 | 1 (6) | |
| Soft tissue | 0 | 1 (6) | |
| Secondary bacteremia | 5 (16) | 1 (6) | |
| Severity of infection, n (%) | 0.131 | ||
| Sepsis | 22 (69) | 15 (92) | |
| Severe sepsis/septic shock | 10 (31) | 2 (12) | |
| Combination antibiotherapy, n (%) | 16 (50) | 12 (71) | 0.229 |
| Mortality at 30 days, n (%) | 7 (22) | 6 (35) | 0.331 |
Abbreviations: A. baumannii, Acinetobacter baumannii; ICU, intensive care unit.
Directed therapy was derived from antibiotic sensitivity. The most frequently used agents were colistin, tigecycline, and meropenem. Monotherapy was used in 21 (43%) patients, of whom 33% presented with severe sepsis/septic shock. Combination therapies were administered in 28 patients, 18% of whom had severe sepsis/septic shock.
Mortality and differences in the function of the presence of infection or colonization
Mortality at 30 days was 24% (n=29). Crude mortality at 30 days was 22% (n=16) among colonized patients and 27% (n=13) among infected patients (p=0.665). However, a near significant reduction in time to death was observed among infected individuals compared to colonized patients (6±11 days vs 8±7 days, p=0.051). The attributable mortality to infection by A. baumannii was considered in nine out of 29 (31%) patients.
With the objective of assessing these factors associated with survival, a bivariant analysis was performed. A lower age, the absence of chronic underlying conditions, a McCabe classification as non-fatal, a lower Charlson index, and an APACHE II score were significantly associated with survival. The resistance pattern of isolated A. baumannii approached statistical significance. Multivariate analysis (Cox regression) included these characteristics, as well as the diagnosis of infection vs colonization. It was demonstrated that a non-fatal McCabe class (Exp[B] 2.44, 95% CI 1.05–5.66, p=0.039) and the absence of infection (Exp[B] 2.75, 95% CI 1.18–6.38, p=0.019) were independently associated with survival (Table 3). Kaplan–Meier curves for the survival of patients based on the McCabe classification and the presence of infection or colonization are shown in Figure 2.
Table 3.
Factors associated with survival in a series of hospitalized patients colonized or infected by A. baumannii (n=122)
| Parameter | Survivors (n=93) | Non-survivors (n=29) | p univariant | Multivariant analysis
|
|
|---|---|---|---|---|---|
| Exp(B) (95% CI) | p | ||||
| Age (years), mean ± standard deviation | 59±18 | 66±12 | 0.035 | ||
| Sex (male), n (%) | 62 (66.7) | 21 (72.4) | 0.652 | ||
| Present admission at ICU, n (%) | 45 (48) | 14 (48) | 1.000 | ||
| Length of stay previous to isolation of A. baumannii (days), | 28±30 | 19±15 | 0.127 | ||
| mean ± standard deviation | |||||
| Presence of intrinsic risk factors (chronic underlying conditions), n (%)a | 67 (72) | 28 (97) | 0.004 | ||
| McCabe classification, n (%) | <0.001 | ||||
| Non-fatal | 60 (65) | 8 (28) | 2.44 (1.05–5.66) | 0.039 | |
| Ultimately or rapidly fatal | 33 (35) | 21 (72) | |||
| Charlson index, mean ± standard deviation | 1.87±2.28 | 3.62±2.87 | <0.001 | ||
| APACHE II scoreb, mean ± standard deviation | 21±7 | 26±6 | 0.013 | ||
| Presence of extrinsic risk factors, n (%) | |||||
| Central venous catheter | 68 (73) | 22 (76) | 1.000 | ||
| Urinary catheter | 76 (82) | 22 (76) | 0.593 | ||
| Parenteral nutrition | 6 (7) | 3 (10) | 0.442 | ||
| Enteral nutrition | 45 (48) | 16 (55) | 0.671 | ||
| Mechanical ventilation | 37 (40) | 14 (48) | 0.518 | ||
| Tracheostomy | 15 (16) | 4 (14) | 1.000 | ||
| Major surgical procedure | 33 (36) | 6 (21) | 0.173 | ||
| Cerebrospinal fluid derivation | 12 (13) | 4 (14) | 0.753 | ||
| Digestive endoscopy in the previous week | 1 (1) | 1 (3) | 0.420 | ||
| Antibiotic therapy in the previous 90 days | 88 (95) | 28 (97) | 1.000 | ||
| Treatment with carbapenems in the previous 90 days | 39 (43) | 10 (35) | 0.517 | ||
| Isolation sites for A. baumannii, n (%) | 0.171 | ||||
| Sputum | 8 (9) | 5 (17) | |||
| Tracheal aspirate | 46 (50) | 16 (55) | |||
| Blood | 2 (2) | 1 (3) | |||
| Urine | 7 (8) | 3 (10) | |||
| Ascitic fluid | 1 (1) | 2 (7) | |||
| Surgical wound | 18 (19) | 1 (3) | |||
| Others | 10 (11) | 1 (3) | |||
| Resistance pattern, n (%) | 0.074 | ||||
| Susceptible | 2 (2) | 1 (3) | |||
| Multidrug resistant | 23 (25) | 1 (3) | |||
| Extensively resistant | 66 (72) | 27 (93) | |||
| Pandrug resistant | 1 (1) | 0 | |||
| Infected patients, n (%) | 36 (39) | 13 (45) | 0.665 | 2.75 (1.18–6.38) | 0.019 |
Notes:
The presence of liver disease (survivors, four (4%) patients; non-survivors, five (17%) cases; p=0.034), cardiac failure (survivors, 23 (25%) patients; non-survivors, 14 (48%) cases; p=0.021), and immune depression (survivors, 11 (12%) patients; non-survivors, 12 (41%) cases; p=0.001) was significantly less frequent in survivors. The rest of the intrinsic risk factors did not show significant differences between both groups.
APACHE II was measured only in patients in ICU (n=59).
Abbreviations: A. baumannii, Acinetobacter baumannii; CI, confidence interval; ICU, intensive care unit; APACHE, Acute Physiology and Chronic Health Evaluation.
Figure 2.
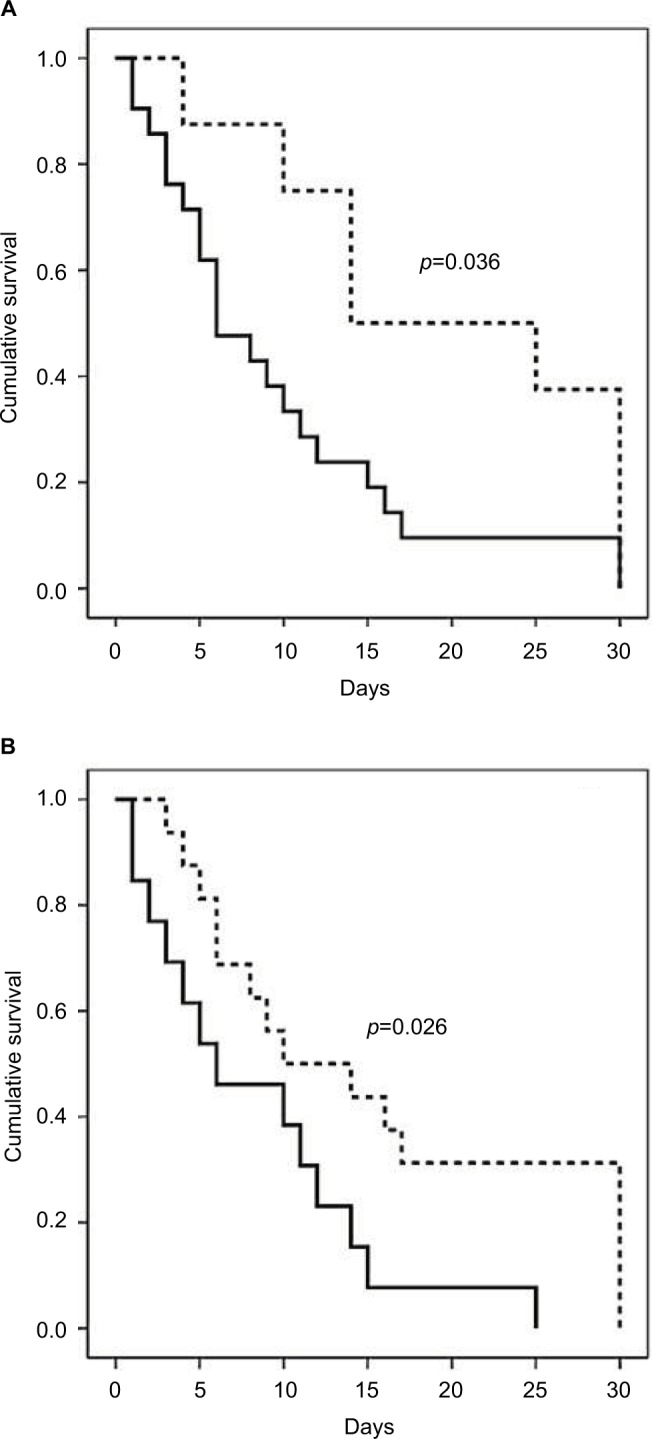
Kaplan–Meier curves plotting the survival of patients colonized or infected with A. baumannii (n=122), based on (A) McCabe classification: non-fatal class (dashed line) or rapidly or ultimately fatal (continuous line) and (B) colonization (dashed line) or infection (continuous line) by A. baumannii.
Abbreviation: A. baumannii, Acinetobacter baumannii.
When analyzing only patients infected by A. baumannii (n=49), survival was more frequent in younger individuals lacking chronic underlying conditions and who had a non-fatal McCabe class, lower Charlson index and APACHE II scores. The absence of severe sepsis/septic shock also approached significance. The presence of each external risk factor (data not shown), infection site (respiratory infection vs others), the pattern of resistance to antibiotics, or the use of combination antibiotic therapy was similar in survivors and non-survivors (Table 4).
Table 4.
Factors associated with survival in a series of hospitalized patients infected by A. baumannii (n=49)
| Parameter | Survivors (n=36) | Non-survivors (n=13) | p univariant |
|---|---|---|---|
| Age (years), mean ± standard deviation | 50±18 | 69±10 | <0.001 |
| Sex (male), n (%) | 29 (81) | 10 (77) | 1.000 |
| Present admission at ICU, n (%) | 25 (70) | 7 (54) | 0.331 |
| Length of hospital stay previous to isolation of A. baumannii (days), mean ± standard deviation | 35±40 | 21±18 | 0.209 |
| Presence of intrinsic risk factors (basal disease), n (%) | 18 (50) | 12 (92) | 0.008 |
| McCabe classification, n (%) | <0.001 | ||
| Non-fatal | 33 (92) | 4 (31) | |
| Ultimately or rapidly fatal | 3 (8) | 9 (69) | |
| Charlson index, mean ± standard deviation | 0.56±0.81 | 3.38±2.93 | <0.001 |
| APACHE II scorea, mean ± standard deviation | 21±8 | 31±10 | 0.029 |
| Respiratory infection vs others, n (%) | 32 (89) | 11 (86) | 0.687 |
| Severe sepsis/septic shock, n (%) | 6 (17) | 6 (46) | 0.058 |
| Resistance pattern, n (%) | 0.687 | ||
| Multidrug resistant | 5 (14) | 1 (8) | |
| Extensively resistant | 30 (83) | 12 (92) | |
| Pandrug resistant | 1 (3) | 0 (0) | |
| Combination antibiotherapy, n (%) | 22 (61) | 6 (46) | 0.514 |
Note:
APACHE II was measured only in patients in ICU (n=32).
Abbreviations: A. baumannii, Acinetobacter baumannii; ICU, intensive care unit; APACHE, Acute Physiology and Chronic Health Evaluation.
Discussion
This work analyzed the differential characteristics of infection vs colonization by A. baumannii. In particular, clinical or microbiological data were analyzed, depending on whether the infection occurred in patients admitted to ICU or non-ICU wards. The parameters associated with mortality were also assessed. To interpret the data obtained, it is necessary to remark that this study proceeded from an endemic outbreak in a unique university hospital.
A remarkable aspect in the appearance of infections by multidrug resistant microbes, such as A. baumannii, is the inadequate use of antibiotics; thus, it is a priority to avoid their use in situations in which they are not indicated, including colonization.23 In this study, a high proportion of patients (60%) was colonized by A. baumannii. This high proportion was due to the endemic outbreak. Both prospective and retrospective24–26 studies have observed that the probability of colonization by A. baumannii increases as the incidence density rate increases. In our series, the incidence density rate both in ICU (8.22 cases/1000 patient-days) and non-ICU hospitalizations (0.83 cases/1000 patient-days) was high, even higher than in a recent Spanish multicenter study.5
Differential characteristics between infected and colonized patients have been previously assessed. However, the heterogeneity of cohorts, variability of studied parameters, and/or the scarce number of patients have limited the conclusions. A recently published prospective study of patients hospitalized in the ICU revealed that the main factors associated with infection by multidrug-resistant microbes were the use of carbapenems in the previous 6 months and the duration of hospitalization in the ICU.27 Our study included a larger number of patients and extended the analysis to patients hospitalized in wards other than the ICU.
In the 90 days prior to admission to ICU and non-ICU wards, the extended use of antibiotics (>90% of patients were treated with them), including carbapenems (they had been administered in 40% of patients), was observed. There was no significant difference in the pattern of resistance to antibiotics among colonized and infected patients and this was probably due to the endemic, which this outbreak was described as.7
In the multivariate analysis, the admission to the ICU was one of the factors associated with infection. The risk of infection increased by 2% per day of hospital stay. The presence of chronic underlying conditions, classified as non-fatal according to the McCabe classification, was also associated with a higher probability of infection.
In those patients in whom the diagnosis was made in non-ICU wards, it was also evident that the previous admission to the ICU favored the presence of infection, as did the occurrence of a previous tracheostomy (in fact, the unique factor independently associated with infection in the multivariate analysis), supporting that the ICU admission and invasive procedures were factors clearly implicated in the risk of infection. A remarkable finding was the length of hospital stay in colonized individuals. The length of stay was significantly lower than that of infected patients, suggesting that colonization preceded the infection, although this issue has not been specifically addressed in this article. Another remarkable feature was the lower Charlson index in infected patients, including those admitted to non-ICU wards. These data suggested that the number of invasive procedures was lower in patients with a poor baseline status and a poor prognosis, thus reducing the risk of invasion.
Consistent with previous studies,5,28,29 the clinical picture of infection was predominantly respiratory. However, there were differences between ICU and non-ICU patients. In non-ICU areas, tracheobronchitis was the more frequent, whereas in ICUs, pneumonia predominated. In non-ICU wards, sampling of patients with tracheostomy after minimal signs of infection was common, allowing a diagnosis of respiratory infection and directed treatment before the development of lung infiltrate.30 In each case, recent publications from ICUs concluded that severity and treatment are similar in tracheobronchitis and pneumonia.31,32 Moreover, prognosis in some studies, as could be detected in ours, is also similar,31,32 although controversial data have been published in studies with high mortalities in cases of pneumonia.33,34
Mortality in our study was 24%, lower than that communicated by the majority of authors35,36 and similar to two multicenter Spanish studies.5,37 Differences in mortality were dependent on the clinical characteristics of the patients, the hospitalization sites (ICU or non-ICU), and the presence of other co-pathogens (in up to 44% of patients). However, in our study, even the ICU admission failed to influence the mortality rate (22% vs 35% in patients hospitalized in ICU vs non-ICU wards, respectively).
One of the objectives of the study was the analysis of the influence of colonization vs infection on the mortality of patients in whom A. baumannii was isolated. A revision of case–control studies has observed that mortality oscillates from 34 to 50% in colonized patients and 31 to 58% in infected patients.36 The heterogeneity of the samples was considered a factor that compromised obtaining statistical differences between colonized and infected cases. In our study, the mortality of colonized individuals (22%) was similar to that of infected patients (26%); however, the time to death was nearly significantly lower in infected patients. After Cox regression analyses, the two parameters independently associated with mortality were the presence of a rapidly or ultimately fatal disease, according to the classification of McCabe, and the presence of infection. Non-survivors had a chronic underlying condition classified as ultimately or rapidly fatal, according to the McCabe classification, more frequently than survivors (72% vs 33%) and a higher Charlson index (3.62 vs 1.87). There was no significant difference in the frequency of the extrinsic risk factors (ie, central venous catheter or mechanical ventilation) or in the antibiotic resistance pattern. In infected patients, the presence of respiratory infections, a factor associated in other studies to mortality,38,39 did not influence survival.
In our study, nine out of 29 (31%) deaths were directly attributable to infection, according to the evaluation performed independently by the three authors. Other studies revealed that the mortality attributable to infection by A. baumannii represented 8–43% of the total.22,36,40 However, even in infected patients, our results, demonstrating that the presence of a chronic disease influences their mortality, emphasized the importance of the underlying state of the patient (measured either by McCabe classification or Charlson index). The presence of severe sepsis or septic shock was a factor approaching statistical significance in our study. Notably, this factor has also been indicated as an independent factor of mortality in other studies.22,40
Resistance to carbapenems has been associated with increased mortality. It is difficult to attribute an independent influence to this factor, because it was generally associated with a more severe disease and inadequate empiric treatment.35 In our study, 80% of A. baumannii isolates were resistant to carbapenems. Consequently, this factor was ruled out in the analysis of mortality.
One of the strengths of this work was the fact that it included only those cases in which A. baumannii was the only recovered microbe. Other studies included the presence of polymicrobial infections, even in >60% of the cases,22,37 limiting the ability to discriminate A. baumannii as a colonizer vs the infectious agent when mortality occurred.
As limitations of the study, errors in the diagnosis of colonization or infection would have occurred. However, the majority of cases of infection were respiratory, with clearly established criteria to define infection, and in contrast, a great percentage of samples in the group of colonization cases were from urine or the surgical wound, without data of focal infection or systemic repercussion in each of the patients evaluated. A second limitation could be attributable to the empirical treatment, which was not included as a factor for survival. Although this factor could influence the mortality at 7 or 14 days, we believe that this is not a factor for mortality after 30 days.
Conclusion
Parameters with the ability to differentiate patients colonized or infected with A. baumannii in an endemic situation were admission to the ICU, the presence of non-fatal McCabe class diseases, and the number of days of hospitalization. The predominant clinical picture of infection was respiratory, tracheostomy-favored tracheobronchitis in non-ICU hospitalization, and pneumonia in ICU patients. Mortality was independently influenced by the chronic underlying basal state and the presence of infection by A. baumannii.
Acknowledgments
This study was supported by a local grant from Instituto para la Investigación e Innovación en Ciencias Biomédicas de Cádiz (INiBICA), Spain. The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Author contributions
Andrés Martín-Aspas designed the protocol. Andrés Martín-Aspas, Francisca M Guerrero-Sánchez, Francisco García-Colchero, and Sebastián Rodríguez-Roca were involved in the clinical follow-up of patients. Andrés Martín-Aspas and José-Antonio Girón-González analyzed the data. Andrés Martín-Aspas and José-Antonio Girón-González wrote the draft. All authors contributed to conception of the study, data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Dijkshoorn L, Nemec A, Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol. 2007;5(12):939–951. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- 2.Rodríguez-Baño J, García L, Ramírez E, et al. Long-term control of hospital-wide, endemic multidrug-resistant Acinetobacter baumannii through a comprehensive “bundle” approach. Am J Infect Control. 2009;37(9):715–722. doi: 10.1016/j.ajic.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kempf M, Rolain JM. Emergence of resistance to carbapenems in Acinetobacter baumannii in Europe: clinical impact and therapeutic options. Int J Antimicrob Agents. 2012;39(2):105–114. doi: 10.1016/j.ijantimicag.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Qureshi ZA, Hittle LE, O’Hara JA, et al. Colistin-resistant Acinetobacter baumannii: beyond carbapenem resistance. Clin Infect Dis. 2015;60(9):1295–1303. doi: 10.1093/cid/civ048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villar M, Cano ME, Gato E, et al. GEIH/GEMARA/REIPI-Ab20101 Group Epidemiologic and clinical impact of Acinetobacter baumannii colonization and infection: a reappraisal. Medicine (Baltimore) 2014;93(5):202–210. doi: 10.1097/MD.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodríguez-Baño J, Cisneros JM, Fernández-Cuenca F, et al. Grupo de Estudio de Infección Hospitalaria (GEIH) Clinical features and epidemiology of Acinetobacter baumannii colonization and infection in Spanish hospitals. Infect Control Hosp Epidemiol. 2004;25(10):819–824. doi: 10.1086/502302. [DOI] [PubMed] [Google Scholar]
- 7.Tacconelli E, Cataldo MA, De Pascale G, et al. Prediction models to identify hospitalized patients at risk of being colonized or infected with multidrug-resistant Acinetobacter baumannii calcoaceticus complex. J Antimicrob Chemother. 2008;62(5):1130–1137. doi: 10.1093/jac/dkn289. [DOI] [PubMed] [Google Scholar]
- 8.Wisplinghoff H, Edmond MB, Pfaller MA, Jones RN, Wenzel RP, Seifert H. Nosocomial bloodstream infections caused by Acinetobacter species in United States hospitals: clinical features, molecular epidemiology, and antimicrobial susceptibility. Clin Infect Dis. 2000;31(3):690–697. doi: 10.1086/314040. [DOI] [PubMed] [Google Scholar]
- 9.Cisneros JM, Rodríguez-Baño J. Nosocomial bacteremia due to Acinetobacter baumannii: epidemiology, clinical features and treatment. Clin Microbiol Infect. 2002;8(11):687–693. doi: 10.1046/j.1469-0691.2002.00487.x. [DOI] [PubMed] [Google Scholar]
- 10.Fournier PE, Richet H. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin Infect Dis. 2006;42(5):692–699. doi: 10.1086/500202. [DOI] [PubMed] [Google Scholar]
- 11.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21(3):538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guerrero-Lozano I, Fernández-Cuenca F, Galán-Sánchez F, Egea P, Rodríguez-Iglesias M, Pascual A. Description of the OXA-23 β-lactamase gene located within Tn2007 in a clinical isolate of Acinetobacter baumannii from Spain. Microb Drug Resist. 2015;21(2):215–217. doi: 10.1089/mdr.2014.0155. [DOI] [PubMed] [Google Scholar]
- 13.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Friedman ND, Kaye KS, Stout JE, et al. Health care-associated blood-stream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137(10):791–797. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 15.McCabe WR, Jackson GG. Gram-negative bacteremia I. Etiology and ecology. Arch Intern Med. 1962;110(6):847–855. [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 18.Levy MM, Fink MP, Marshall JC, et al. SCCM/ESICM/ACCP/ATS/SIS 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med. 2003;31(4):1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 19.Clinical and CLSI, Laboratory Standards Institute . Approved Standard M100–S20. Wayne, PA: NCCLS; 2010. Performance Standards for Antimicrobial Susceptibility Testing–20th Informational Supplement. [Google Scholar]
- 20.Fernandez Cuenca F, Tomas Carmona M, Caballero Moyano F, et al. Actividad de 18 agentes antimicrobianos frente a aislados clinicos de Acinetobacter baumannii: segundo estudio nacional multicentrico (proyecto GEIH-REIPI-Ab 2010) Enferm Infecc Microbiol Clin. 2013;31(1):4–9. doi: 10.1016/j.eimc.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an inter-national expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 22.Livermore DM, Hill RL, Thomson H, et al. C-MRAB Study Group Antimicrobial treatment and clinical outcome for infections with carbapenem- and multiply-resistant Acinetobacter baumannii around London. Int J Antimicrob Agents. 2010;35(1):19–24. doi: 10.1016/j.ijantimicag.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 23.Kollef MH, Golan Y, Micek ST, Shorr AF, Restrepo MI. Appraising contemporary strategies to combat multidrug resistant Gram-negative bacterial infections-proceedings and data from the Gram-negative resistance summit. Clin Infect Dis. 2011;53(suppl 2):S33–S55. doi: 10.1093/cid/cir475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corbella X, Montero A, Pujol M, et al. Emergence and rapid spread of carbapenem resistance during a large and sustained hospital outbreak of multiresistant Acinetobacter baumannii. J Clin Microbiol. 2000;38(11):4086–4095. doi: 10.1128/jcm.38.11.4086-4095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Playford EG, Craig JC, Iredell JR. Carbapenem-resistant Acinetobacter baumannii in intensive care unit patients: risk factors for acquisition, infection and their consequences. J Hosp Infect. 2007;65(3):204–211. doi: 10.1016/j.jhin.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Arvaniti K, Lathyris D, Ruimy R, et al. The importance of colonization pressure in multiresistant Acinetobacter baumannii acquisition in a Greek intensive care unit. Crit Care. 2012;16(3):R102. doi: 10.1186/cc11383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vasudevan A, Mukhopadhyay A, Li J, Yuen EG, Tambyah PA. A prediction tool for nosocomial multi-drug resistant Gram-negative bacilli infections in critically ill patients – prospective observational study. BMC Infect Dis. 2014;14:615. doi: 10.1186/s12879-014-0615-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernández-Torres A, García-Vázquez E, Gómez J, et al. Colonización/infección por Acinetobacter baumannii multirresistente y resistente a carbapenémicos: epidemiología y factores predictivos de infección. Med Clin (Barc) 2010;135(9):389–396. doi: 10.1016/j.medcli.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 29.Munoz-Price LS, Arheart K, Nordmann P, et al. Eighteen years of experience with Acinetobacter baumannii in a tertiary care hospital. Crit Care Med. 2013;41(12):2733–2742. doi: 10.1097/CCM.0b013e318298a541. [DOI] [PubMed] [Google Scholar]
- 30.Martin-Loeches I, Povoa P, Rodríguez A, et al. TAVeM Study Incidence and prognosis of ventilator-associated tracheobronchitis (TAVeM): a multicenter, prospective, observational study. Lancet Respir Med. 2015;3(11):859–868. doi: 10.1016/S2213-2600(15)00326-4. [DOI] [PubMed] [Google Scholar]
- 31.Dallas J, Skrupky L, Abebe N, Boyle WA, Kollef MH. Ventilator-associated tracheobronchitis in a mixed surgical and medical ICU population. Chest. 2011;139(3):513–518. doi: 10.1378/chest.10-1336. [DOI] [PubMed] [Google Scholar]
- 32.Craven DE, Lei Y, Ruthazer R, Sarwar A, Hudcova J. Incidence and outcomes of ventilator-associated tracheobronchitis and pneumonia. Am J Med. 2013;126(6):542–549. doi: 10.1016/j.amjmed.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 33.Bouza E, Pérez A, Muñoz P, et al. Cardiovascular Infection Study Group Ventilator-associated pneumonia after heart surgery: a prospective analysis and the value of surveillance. Crit Care Med. 2003;31(7):1964–1970. doi: 10.1097/01.ccm.0000084807.15352.93. [DOI] [PubMed] [Google Scholar]
- 34.Craven DE, Chroneou A, Zias N, Hjalmarson KI. Ventilator-associated tracheobronchitis: the impact of targeted antibiotic therapy on patient outcomes. Chest. 2009;135(2):521–528. doi: 10.1378/chest.08-1617. [DOI] [PubMed] [Google Scholar]
- 35.Lemos EV, de la Hoz FP, Einarson TR, et al. Carbapenem resistance and mortality in patients with Acinetobacter baumannii infection: systematic review and meta-analysis. Clin Microbiol Infect. 2014;20(5):416–423. doi: 10.1111/1469-0691.12363. [DOI] [PubMed] [Google Scholar]
- 36.Falagas ME, Rafailidis PI. Attributable mortality of Acinetobacter baumannii: no longer a controversial issue. Crit Care. 2007;11(3):134. doi: 10.1186/cc5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.López-Cortés LE, Cisneros JM, Fernández-Cuenca F, et al. GEIH/REIPI-Ab2010 Group Monotherapy versus combination therapy for sepsis due to multidrug-resistant Acinetobacter baumannii: analysis of a multicentre prospective cohort. J Antimicrob Chemother. 2014;69(11):3119–3126. doi: 10.1093/jac/dku233. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Z, Duan J. Nosocomial pneumonia in non-invasive ventilation patients: incidence, characteristics, and outcomes. J Hosp Infect. 2015;91(2):153–157. doi: 10.1016/j.jhin.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 39.Yang YS, Lee YT, Huang TW, et al. Acinetobacter baumannii nosocomial pneumonia: is the outcome more favorable in non-ventilated than ventilated patients? BMC Infect Dis. 2013;13:142. doi: 10.1186/1471-2334-13-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.del Mar Tomas M, Cartelle M, Pertega S, et al. Hospital outbreak caused by a carbapenem-resistant strain of Acinetobacter baumannii: patient prognosis and risk-factors for colonisation and infection. Clin Microbiol Infect. 2005;11(7):540–546. doi: 10.1111/j.1469-0691.2005.01184.x. [DOI] [PubMed] [Google Scholar]