Abstract
Background
Elevated vitamin B12 is a sign for liver damage, but its significance in chronic stable heart failure (HF) is less known. The present study investigated the clinical correlates and prognostic significance of vitamin B12 levels in stable systolic HF.
Methods
A total of 129 consecutive patients with HF and 50 control subjects were enrolled. Data regarding demographics, clinical signs, therapeutic and conventional echocardiographic measurements were recorded for all patients. Right-sided HF was defined as the presence of at least one of the typical symptoms (ankle swelling) or specific signs (jugular venous distention or abdominojugular reflux) of right HF. Cox proportional hazards regression analyses were performed to determine the independent prognostic determinants of mortality.
Results
Baseline B12 levels in HF patients (n=129) with and without right sided HF were significantly higher compared to healthy controls (n=50): Median 311 pg/mL and 235 pg/mL vs 198 pg/mL, respectively (P=0.005). Folic acid levels were similar between the study groups. Age, ejection fraction, left atrial size, estimated glomerular filtration rate, and direct and indirect bilirubin levels were significantly correlated to serum B12 level in univariate analysis. In multivariate analysis, independent correlates of B12 were direct bilirubin (R=0.51, P<0.001) and age (R=0.19, P=0.028). Patients with HF were followed-up for a median period of 32 months. Median B12 levels were significantly higher in patients who subsequently died (n=35) compared to survivors, but folic acid was not different between the two groups. ROC analysis showed that B12 values ≥270 pg/mL had 80% sensitivity and 58% specificity for predicting all-cause mortality (area under the curve=0.672, 95% CI=0.562−0.781; P=0.003). However, in Cox regression analysis, only left atrial diameter, level of direct bilirubin, and the presence of abdominojugular reflux were independent predictors of death.
Conclusion
Increased B12 in stable HF patients is associated with increased direct bilirubin due to right HF, indicating a cardiohepatic syndrome, but neither B12 nor folic acid are independently associated with mortality.
Keywords: heart failure, vitamin B12, bilirubin, prognosis
Introduction
Anemia is a common co-morbidity in heart failure (HF), with a prevalence varying from 4% to 61%.1 It is more common in advanced HF, the elderly, and in patients with renal impairment, and is associated with advanced myocardial remodeling, inflammation, and volume overload.2,3 Anemia is an important prognostic indicator for greater risk of HF hospitalization and reduced survival. The most common form of anemia in HF is iron deficiency anemia, which – either with or without anemia – also has an independent detrimental impact on clinical outcome.4,5
Other nutritional deficiencies, like B12 and folate deficiencies, are less well studied entities in HF. Theoretically, due to the increased frequency of nutritional deficiencies, a decrease in B12 and folic acid may be expected. However, studies on the prevalence of B12 and folic acid deficiency revealed opposite findings, and showed a prevalence of only 1.3%–8%.6–8 Furthermore, higher concentrations of serum B12 were associated with the severity of HF, and showed a positive correlation with New York Heart Association (NYHA) functional class and NT-proBNP level. In contrast to iron deficiency, the prognostic value of B12 and folic acid was not significant.8
Increased levels of serum cobalamin are associated with malignancies, autoimmune diseases, and renal and liver failure.9,10 It is a marker for liver-cell damage, due to release of the vitamin from damaged liver cells.11 Higher vitamin B12 levels in acute HF have been attributed to liver congestion and liver function abnormalities.12,13 However, the status in chronic stable HF is less clear. Similar to acute decompensated HF patients, B12 levels in chronic stable HF patients may be associated with right-sided HF and liver congestion/damage; on the contrary, an increase of vitamin B12 may be a prognostic indicator for impaired survival in HF patients. In the present study, we investigated clinical correlates and prognostic value of vitamin B12 and folic acid levels in chronic systolic HF patients, with a special focus on the relation to right-sided HF.
Methods
Study population
The study group consisted of consecutive HF patients admitted to the Department of Cardiology, School of Medicine, Kocaeli University between January 2013 and December 2013. Inclusion criteria were chronic stable HF with echocardiographic findings of reduced left ventricular ejection fraction (≤45%), and symptoms with a functional class of NYHA II–IV. Exclusion criteria included HF with preserved ejection fraction, acute decompensated HF, prior myocardial infarction within the last 6 months, myocarditis, uncontrolled hypertension, active infection, hypertrophic and restrictive cardiomyopathy, clinically significant heart valve disease, renal failure requiring dialysis, malignancies, connective tissue-inflammatory and autoimmune diseases, pregnancy, and treatment for anemia within the last 12 months.
Data regarding demographics, clinical signs, and therapeutic and conventional echocardiographic measurements were recorded for all patients. Right-sided HF was defined as the presence of at least one of the typical symptoms (ankle swelling) or specific signs (jugular venous distention or abdominojugular reflux) of right HF.
A gender matched healthy group served as controls for comparison of B12 and folic acid levels.
Laboratory measurements
Fasting venous blood samples were drawn from the study participants and analyzed on the same day. Renal function was assessed using the estimated glomerular filtration rate (eGFR), calculated with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula. Whole blood counts were determined using an automated blood cell counter. Anemia was defined as a hemoglobin level <12 g/dL in women and <13 g/dL in men.14 Ferritin, vitamin B12, folic acid, and NT-proBNP levels were estimated by chemiluminescence using the Beckman Coulter Access Immunoassay Systems. Iron and iron binding capacity were measured with Roche Cobas C 702 System. Iron deficiency was defined as a serum ferritin level <100 ng/mL for absolute deficiency, and 100–299 ng/mL with a transferrin saturation <20% for functional iron deficiency.4,5 The reference interval for vitamin B12 by the manufacturer was 180–914 pg/mL. Vitamin B12 deficiency was defined as <200 pg/mL, and folic acid deficiency was defined as <4.0 ng/mL.15,16
An endpoint of the study was all-cause mortality. Patients were followed-up until April 2017 by outpatient visits or by phone contact if they were not able to come for visits.
The study was approved by the institutional review board of the Kocaeli University and was in accordance with the principles of the Helsinki Declaration. Written informed consent was obtained from each patient before data collection.
Statistical analysis
All analyses were performed using SPSS 20.0 statistical software package (IBM Corporation, Armonk, NY, USA). Continuous variables are presented as mean and standard deviation when normally distributed, and as median and 25th–75th percentiles when non-normally distributed. Categorical variables are given as percentages. Patient groups and controls were compared by Student’s t-test or Mann–Whitney U-test when appropriate. The chi-square test was used for the comparison of categorical variables. Significant correlates of vitamin B12 were assessed with the univariate linear regression model using all clinical, echocardiographic, and laboratory variables as covariates. Variables with a significant univariable correlation (P<0.10) were entered in the multivariable linear regression model. Receiver operating characteristic curve (ROC) analysis was performed to assess the cut-off value of vitamin B12 for predicting all-cause mortality. The Kaplan–Meier method was used to determine cumulative probability of all-cause mortality according to serum vitamin B12 level, and the survival curves were compared by the log-rank test. Cox proportional hazard regression model, adjusted for age, gender, presence of right HF, coronary artery disease, angiotensin converting enzyme (ACE)-inhibitor/angiotensin receptor blocker, beta-blocker, mineralocorticoid receptor antagonist usage, atrial fibrillation, systolic blood pressure, sodium, eGFR, NT-proBNP, hs-CRP, direct bilirubin, serum B12, ejection fraction, left ventricular end-diastolic diameter, left atrial diameter, right ventricular diameter, and pulmonary artery systolic pressure, was used to determine the independent prognostic determinants of mortality. A two-sided P-value <0.05 was accepted as statistically significant.
All authors had full access to all the data in the study, and took responsibility for the integrity of data and accuracy of data analysis.
Results
The study group consisted of 129 chronic stable HF patients and 50 healthy subjects. Baseline characteristics of the HF patients and controls are shown in Table 1. Mean NYHA functional class of the heart failure with reduced ejection fraction (HFrEF) patients was 2.7±0.4, and 71 of them (55%) had symptoms or signs of right HF. Anemia was present in 64 (49.6%) of the patients and in six (12%) of the controls (P<0.001). Most of the HFrEF patients had iron deficiency (108; 83.7%). Serum vitamin B12 deficiency was significantly less frequent in HFrEF patients compared to controls (35 patients [27%] vs 24 controls [48%] respectively; P=0.006), and folic acid deficiency was similar between the two groups (10 patients [8%] vs 1 control [2%]; P=0.184). A total of 43 patients (33%) had received an implantable cardioverter defibrillator and/or cardiac resynchronization therapy. Median B12 levels were not different between those who had and had not received a device therapy (245 pg/mL vs 272 pg/mL, respectively; P=0.680). On the contrary, B12 levels in HFrEF patients with and without right HF were significantly higher compared to controls (HFrEF with right HF: median 311 pg/mL [220–449], HFrEF without right HF: median 235 pg/mL [178–392]; P=0.022), and all five cases with a B12 level higher than 800 pg/mL were in the HFrEF group (Figure 1).
Table 1.
Baseline characteristics of the HFrEF patients and control group
| Variables | HFrEF (n=129) | Control group (n=50) | P-value |
|---|---|---|---|
| Demographic and clinical characteristics | |||
| Age (years) | 65±12 | 49±12 | <0.001 |
| Male | 73 (57%) | 22 (44%) | 0.130 |
| Body mass index (kg/m2) | 26.8 (23.9–29.0) | 26.5 (24.2–28.1) | 0.799 |
| Right-sided heart failure | 71 (55%) | 0% | <0.001 |
| Hypertension | 98 (76%) | 0% | <0.001 |
| Diabetes mellitus | 53 (41%) | 0% | <0.001 |
| Ischemic etiology | 70 (54%) | 0% | <0.001 |
| Atrial fibrillation | 61 (47%) | 0% | <0.001 |
| Echocardiographic characteristics | |||
| Ejection fraction (%) | 25 (20–40) | 70 (65–76) | <0.001 |
| Left ventricular end-diastolic diameter (mm) | 58±10 | 46±5 | <0.001 |
| Left atrium diameter (mm) | 48 (42–54) | 36 (34–38) | <0.001 |
| Right ventricle outflow tract diameter (mm) | 27 (25–30) | 24 (22–25) | <0.001 |
| Pulmonary artery systolic pressure (mmHg) | 40 (25–50) | 20 (20–25) | <0.001 |
| Medications | |||
| ACE-I/ARB | 75 (58%) | 0 (0%) | <0.001 |
| Beta-blockers | 101 (78%) | 0 (0%) | <0.001 |
| MRAs | 21 (16%) | 0 (0%) | 0.002 |
| Statins | 42 (33%) | 0 (0%) | <0.001 |
| Digoxin | 21 (16%) | 0 (0%) | 0.002 |
| Loop diuretics | 104 (81%) | 0 (0%) | <0.001 |
| Warfarin | 50 (39%) | 0 (0%) | <0.001 |
| ICD/CRT | 43 (33%) | 0 (0%) | <0.001 |
| Laboratory analysis | |||
| NT-proBNP (pg/mL) | 1,390 (443–4,290) | 61 (25–105) | <0.001 |
| eGFR (mL/min) | 65 (43–89) | 101 (88–107) | <0.001 |
| Uric acid (mg/dL) | 7.4±2.7 | 5.3±1.5 | <0.001 |
| Sodium (mEq/L) | 136 (134–138) | 138 (138–139) | <0.001 |
| Hs-CRP (mg/dL) | 0.90 (0.27–2.93) | 0.29 (0.09–0.55) | <0.001 |
| AST (U/L) | 19 (15–27) | 18 (15–22) | 0.321 |
| ALT (U/L) | 16 (12–26) | 19 (14–29) | 0.247 |
| Direct bilirubin (mg/dL) | 0.4 (0.2–0.6) | 0.2 (0.2–0.3) | <0.001 |
| Indirect bilirubin (mg/dL) | 0.3 (0.2–0.5) | 0.3 (0.2–0.4) | 0.073 |
| INR | 1.14 (1.05–1.31) | 0.99 (0.96–1.03) | <0.001 |
| Albumin (mg/dL) | 3.6±0.6 | 4.3±0.3 | <0.001 |
| Total cholesterol (mg/dL) | 158±45 | 199±34 | <0.001 |
| Hematological and hematinic parameters | |||
| Hemoglobin (g/dL) | 12.6±2.0 | 13.6±1.3 | 0.003 |
| MCV (10 fL) | 86 (82–93) | 90 (85–93) | 0.031 |
| Platelets (103 µL) | 235 (200–290) | 253 (217–321) | 0.099 |
| Ferritin (µg/L) | 48 (31–85) | 29 (13–62) | <0.001 |
| Transferrin saturation (%) | 15 (10–23) | 21 (13–30) | 0.022 |
| Folic acid (ng/mL) | 7.7 (5.8–10.1) | 8.5 (6.4–10.5) | 0.305 |
| B12 (pg/mL) | 271 (188–415) | 198 (140–321) | 0.005 |
Abbreviations: ACE-I/ARB, angiotensin converting enzyme inhibitors/angiotensin receptor blockers; ALT, alanine aminotransferase; AST, aspartate aminotransferase; eGFR, estimated glomerular filtration rate; HFrEF, heart failure with reduced ejection fraction; Hs-CRP, high sensitive C-reactive protein; ICD/CRT, implantable cardioverter defibrillator/cardiac resynchronization therapy; INR, international normalized ratio; MCV, mean corpuscular volume; MRAs, mineralocorticoid receptor antagonists; NT-proBNP, N-terminal prohormone of brain natiuretic peptide.
Figure 1.
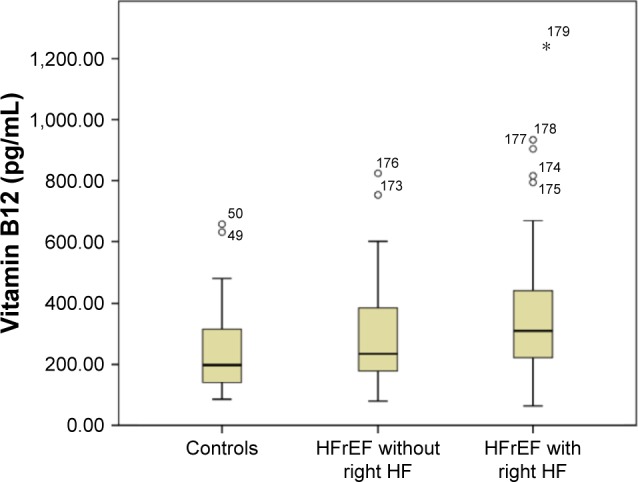
Serum vitamin B12 level in controls and in HFrEF patients with and without right-sided HF.
Abbreviations: HFrEF, heart failure with reduced ejection fraction; HF, heart failure.
Significant univariate and multivariate clinical, echocardiographic, and laboratory correlates with serum vitamin B12 in HFrEF patients are presented in Table 2. Age, ejection fraction, left atrial size, eGFR, and direct and indirect bilirubin levels were significantly correlated to B12 level in univariate analysis. In multivariate analysis, direct bilirubin and age appeared as the two independent correlates with the B12 level in HFrEF patients (R=0.51, P<0.001 and R=0.19, P=0.028, respectively). Right HF, per se, showed a modest association with B12 level (R=0.205, P=0.033) in univariate analysis, but lost its statistical significance in multivariate analysis. Similarly, direct bilirubin – the main correlate of serum B12 level – was significantly associated with right HF (R=0.21, P=0.020) in univariate analysis, but in multivariate analysis, transaminases, total cholesterol, and – among the individual signs of right HF – the presence of abdominojugular reflux emerged as the independent correlates of direct bilirubin (R=0.744; P<0.001).
Table 2.
Significant univariate and multivariate correlates of vitamin B12 in HFrEF patients
| Variables | Univariate regression coefficient (95% CI) | P-value | Multivariate regression coefficient (95% CI) | P-value |
|---|---|---|---|---|
| Age | 5.6 (1.5 to 9.7) | 0.008 | 2.9 (0.32–5.53) | 0.028 |
| Ejection fraction | 5.1 (−0.61 to 10.7) | 0.079 | ||
| Left atrium diameter | 5.5 (−0.25 to 11.3) | 0.060 | ||
| eGFR | 1.69 (0.07 to 3.31) | 0.041 | ||
| Direct bilirubin | 223 (139 to 305) | <0.001 | 138 (96–180) | <0.001 |
| Indirect bilirubin | −165 (−340 to 10.4) | 0.065 |
Abbreviations: HFrEF, heart failure with reduced ejection fraction; CI, confidence interval; eGFR, estimated glomerular filtration rate.
The HFrEF group was followed-up for a median duration of 32 (17−44) months. Mortality data were available for all patients, and death occurred in 35 patients (27%). Serum vitamin B12 levels were significantly higher in who subsequently died compared to survivors (373 [274−477] pg/mL vs 247 [182−388] pg/mL; P=0.003) (Figure 2), but folic acid was not different between the two groups (7.1 [5.1−9.7] pg/mL vs 7.9 [5.9−10.6] pg/mL; P=0.379). Highest serum B12 levels were observed in deceased patients with right HF. ROC curve analysis showed that values of serum B12 ≥270 pg/mL had 80% sensitivity and 58% specificity for predicting all-cause mortality (area under the curve [AUC]=0.672, 95% CI=0.562−0.781; P=0.003). In Kaplan–Meier analysis, event-free survival was significantly lower in patients with a serum B12 ≥270 pg/mL than in patients with serum B12 levels <270 pg/mL (mortality-free survival rate 53% vs 85%, P<0.001) (Figure 3). In Cox regression analysis, independent determinants of death were left atrial diameter (exp(B)=1.111, 95% CI=1.058−1.167; P<0.001), level of direct bilirubin (exp(B)=1.869, 95% CI=1.282−2.724; P=0.001), and presence of abdominojugular reflux (exp(B)=3.502, 95% CI=1.672−7.334; P=0.001), whereas B12 level did not show a significant independent association to all-cause mortality.
Figure 2.
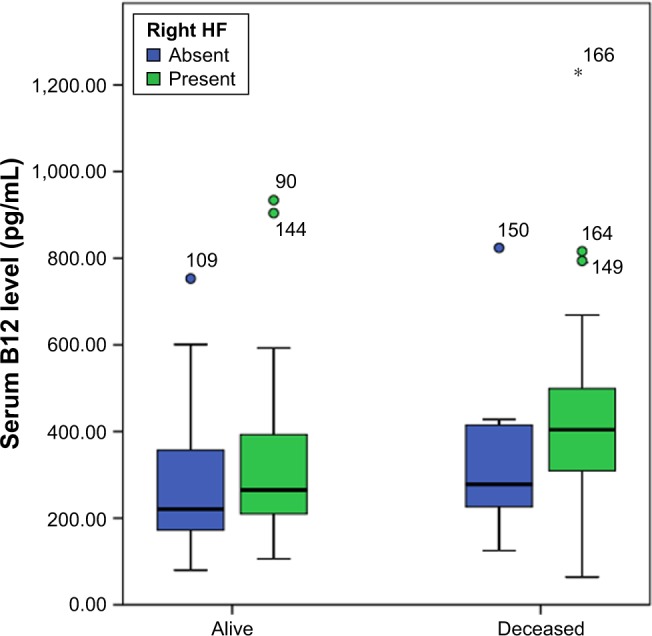
B12 levels in patients who subsequently died and survived HFrEF patients.
Abbreviations: HFrEF, heart failure with reduced ejection fraction; HF, heart failure.
Figure 3.
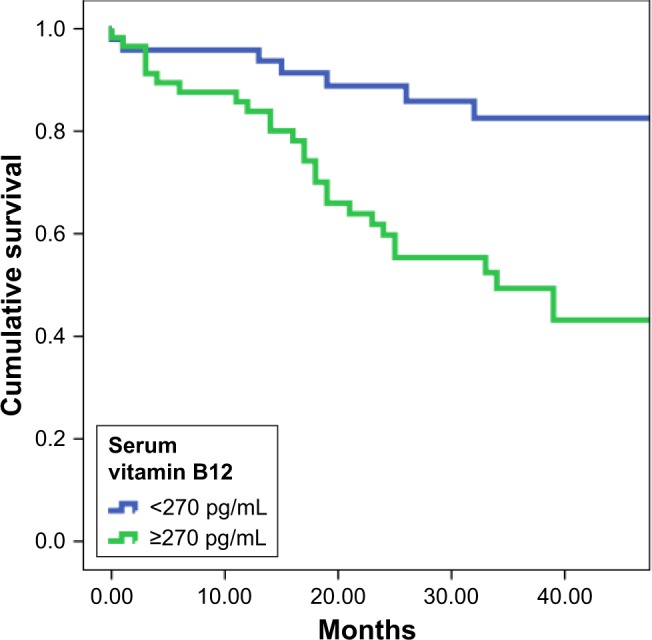
Kaplan–Meier survival curves for the HFrEF patients with high and low serum B12 levels.
Abbreviation: HFrEF, heart failure with reduced ejection fraction.
Discussion
Findings of this study show that vitamin B12 and folate deficiency are infrequent (27% and 8%, respectively) in chronic HFrEF patients, and, in fact, increased B12 levels are more prevalent in patients with specifically right HF. The main correlate of serum vitamin B12 level was direct bilirubin, which was significantly associated with the presence of abdominojugular reflux and other metabolic findings of liver dysfunction (ie, elevation in transaminases and decrease in total cholesterol). Vitamin B12 and folic acid were not independent determinants of long-term mortality, but B12 levels were significantly increased in deceased HFrEF patients, particularly in the presence of right HF.
These findings confirm previous studies that had reported a positive correlation between serum B12 level and severity of HF,8,17 and further show a direct relationship between elevated B12 levels, signs of right HF, and liver function abnormalities (eg, direct bilirubin levels). The initial report on the serum cyanocobalamin as an index of hepatic damage in severe HF was published by Rachmilewitz et al12 in 1959. Their study consisted of 28 decompensated HF patients with marked hepatomegaly, and their serum cyanocobalamin levels – ranging from 500–3,500 mcg/mL before treatment – decreased significantly after diuresis. During the following years, most of the attention was paid to hyperhomocysteinemia and anemia studies, and the report of Rachmilewitz et al12 was followed by a few studies that showed similar associations with B12 and HF severity.8,13,17 Two of these three studies assessed the relationship of B12 level to liver function tests,13,17 and showed significant relationships to transaminases, gamma-glutamyl transferase levels, and to total bilirubin level in decompensated patients with biventricular failure. As acute HF is associated with an increase in these parameters, their changes after treatment and their effect on B12 levels would be important. Unfortunately, none of the studies reported changes after stabilization of the patients.
The patients included in this study were stable outpatient HFrEF patients with either no, or mild, signs of clinical right HF. Therefore, we had to define right-sided HF as the presence of at least one of the typical (ankle swelling) or specific signs (jugular venous distention or abdominojugular reflux) of right HF. Likewise, total bilirubin of the patient group was either normal or only slightly exceeding the normal range. Interestingly, the median direct bilirubin level of HFrEF patients was significantly higher than the controls, despite being only slightly more elevated than the normal upper limit. Direct hyperbilirubinemia is increasingly recognized as an indirect marker of right heart dynamics and a prognostic indicator for HF.18,19 It is observed in patients with clinical signs of increased right-sided pressure, and is commonly accompanied by other parameters of HF severity, like increased plasma renin activity and decreased total cholesterol. The lack of an independent association between B12 level and clinical right HF, but a significant relationship to mildly increased direct bilirubin levels, indicate that a possible ongoing occult liver injury due to subclinical hepatic congestion is the main determinant of B12 elevation in these patients.20
Serum B12 level did not show an independent association with all-cause mortality, and its significant univariate association with the prognosis seems to be secondary to metabolic abnormalities due to subclinical liver dysfunction in HFrEF patients. In ROC analysis, a B12 level ≥270 pg/mL could successfully predict all-cause mortality. These findings were similar to the results of van der Wal et al’s8 study, which also had not been able to detect an independent relationship of serum B12 to mortality. However, our cut-off value for predicting mortality is much lower than the value (>600 pg/mL) identified in their study. Interestingly, despite being higher than in the control group, the median B12 level in our HFrEF patients is in the range of the lowest quartile of their study, suggesting a possible dietary differences between the study groups.
Elevated vitamin B12 is a frequent and understated abnormality, observed mainly in hepatocellular carcinomas and metastatic colon, breast, and pancreatic cancer. Its clinical presentation may be paradoxical with clinical signs of B12 deficiency, but lack a decrease in serum B12 levels.21,22 The underlying pathophysiological mechanisms include excess production of inactive transcobalamines in inflammation, hepatic release in liver damage, and defects in clearance, like in renal failure. Significant elevation in serum B12 level (>800 pg/mL) is accepted as an early sign for an underlying severe condition. In this study, significant B12 elevation was observed in only five patients, three of whom died in the follow-up period. Most of the other patients showed only mild, clinically inconspicuous elevations compared to the control group, precluding usage of B12 as a marker. Nevertheless, an accompanying mild increase in direct bilirubin should alert clinicians, even in the presence of normal clinical signs in HF, and these patients may require a closer follow-up.
Limitations
The study has several limitations. The total number of the patients and the controls included in the study is relatively low. As we wanted to evaluate thoroughly healthy individuals, we recruited a relatively younger control group from an outpatient clinic. The true incidence of B12 deficiency in the general population is unknown, but it seems to increase with advancing age. In one study, 15% of adults older than 65 years had vitamin B12 deficiency.23 We did not measure homocysteine level, and could not assess its relationship to B12 level. The sensitivity of homocysteine levels for identifying vitamin B12 deficiency is greater than 95%, and accepting a B12 level <200 pg/mL without measuring homocyteine level may be misleading to diagnose true B12 deficiency. Nevertheless, previous studies on the level of homocysteine in patients with HF showed a consistent progressive increase in homocysteine levels with worsening functional capacity and poor survival. Data on the B12 level was rather vague, and, considering the older age of the patients and the increase in homocysteine level, a decrease in B12 level would be more rational. Unexpectedly, studies on HF and findings of our study did not confirm this hypothesis. Homocysteine and B12 in chronic HF were evaluated in the study of Herrmann et al,17 and they showed total homocysteine, but not folate and B12, is related to the severity of HF. Similar to our findings, they also observed an increase in B12 with NYHA class, and a negative correlation with EF. Lack of liver imaging is another limitation to demonstrate a significant association with B12 elevation, subclinical hepatic congestion, and impaired mortality.
Conclusion
In patients with chronic stable HF, B12 and folic acid deficiencies are uncommon diseases. Mild elevation in serum vitamin B12 levels is associated with clinical signs of right HF and slightly increased direct bilirubin levels, indicating a subclinical cardiohepatic syndrome, but it is not an independent predictor for increased all-cause mortality.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Tang YD, Katz SD. Anemia in chronic heart failure: prevalence, etiology, clinical correlates, and treatment options. Circulation. 2006;113(20):2454–2461. doi: 10.1161/CIRCULATIONAHA.105.583666. [DOI] [PubMed] [Google Scholar]
- 2.O’Meara E, Rouleau JL, White M, et al. Heart failure with anemia: novel findings on the roles of renal disease, interleukins, and specific left ventricular remodeling processes. Circ Heart Fail. 2014;7:773–781. doi: 10.1161/CIRCHEARTFAILURE.114.001100. [DOI] [PubMed] [Google Scholar]
- 3.Nanas JN, Matsouka C, Karageorgopoulos D, et al. Etiology of anemia in patients with advanced heart failure. J Am Coll Cardiol. 2006;48(12):2485–2489. doi: 10.1016/j.jacc.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 4.Ponikowski P, van Veldhuisen DJ, Comin-Colet J, et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J. 2015;36(11):657–668. doi: 10.1093/eurheartj/ehu385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18(8):891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 6.Tereshchenko SN, Uskach TM, Kochetov AG. Analysis of causes of development of anemia in patients with chronic heart failure. Kardiologiia. 2011;51(5):20–26. [PubMed] [Google Scholar]
- 7.Witte KK, Desilva R, Chattopadhyay S, Ghosh J, Cleland JG, Clark AL. Are hematinic deficiencies the cause of anemia in chronic heart failure? Am Heart J. 2004;147(5):924–930. doi: 10.1016/j.ahj.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 8.van der Wal HH, Comin-Colet J, Klip IT, et al. Vitamin B12 and folate deficiency in chronic heart failure. Heart. 2015;101(4):302–310. doi: 10.1136/heartjnl-2014-306022. [DOI] [PubMed] [Google Scholar]
- 9.Zulfiqar AA, Sebaux A, Dramé M, Pennaforte JL, Novella JL, Andrès E. Hypervitaminemia B12 in elderly patients: Frequency and nature of the associated or linked conditions. Preliminary results of a study in 190 patients. Eur J Intern Med. 2015;26(10):e63–e64. doi: 10.1016/j.ejim.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Arendt JF, Nexo E. Unexpected high plasma cobalamin: proposal for a diagnostic strategy. Clin Chem Lab Med. 2013;51(3):489–496. doi: 10.1515/cclm-2012-0545. [DOI] [PubMed] [Google Scholar]
- 11.Mechie NC, Goralzcyk AD, Reinhardt L, Mihm S, Amanzada A. Association of serum vitamin B12 levels with stage of liver fibrosis and treatment outcome in patients with chronic hepatitis C virus genotype 1 infection: a retrospective study. BMC Res Notes. 2015;8:260. doi: 10.1186/s13104-015-1248-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rachmilewitz M, Stein Y, Aronovitch J, Grossowicz N. Serum cyanocobalamin (vitamin B12) as an index of hepatic damage in chronic congestive heart failure. Arch Intern Med. 1959;104:406–410. doi: 10.1001/archinte.1959.00270090060010. [DOI] [PubMed] [Google Scholar]
- 13.Zafarullah H, Shahbaz AU, Alturkmani R, et al. Elevated serum cobalamin in patients with decompensated biventricular failure. Am J Med Sci. 2008;336(5):383–388. doi: 10.1097/01.MAJ.0000310651.34229.73. [DOI] [PubMed] [Google Scholar]
- 14.Nutritional anemias. Report of a WHO scientific group. World Health Organ Tech Rep Ser. 1968;405:5–37. [PubMed] [Google Scholar]
- 15.Stabler SP. Clinical practice. Vitamin B12 deficiency. N Engl J Med. 2013;368(2):149–160. doi: 10.1056/NEJMcp1113996. [DOI] [PubMed] [Google Scholar]
- 16.de Benoist B. Conclusions of a WHO Technical Consultation on folate and vitamin B12 deficiencies. Food Nutr Bull. 2008;29(2 Suppl):S238–S244. doi: 10.1177/15648265080292S129. [DOI] [PubMed] [Google Scholar]
- 17.Herrmann M, Müller S, Kindermann I, et al. Plasma B vitamins and their relation to the severity of chronic heart failure. Am J Clin Nutr. 2007;85(1):117–123. doi: 10.1093/ajcn/85.1.117. [DOI] [PubMed] [Google Scholar]
- 18.Okada A, Sugano Y, Nagai T, et al. Usefulness of the direct and/or total bilirubin to predict adverse outcomes in patients with acute decompensated heart failure. Am J Cardiol. 2017;119(12):2035–2041. doi: 10.1016/j.amjcard.2017.03.033. [DOI] [PubMed] [Google Scholar]
- 19.Philip J, Samraj RS, Lopez-Colon D, Gonzalez-Peralta R, Chandran A, Bleiwies MS. Severe direct hyperbilirubinemia as a consequence of right heart failure in congenital heart disease. World J Pediatr Congenit Heart Surg. 2016 doi: 10.1177/2150135116640786. pii: 2150135116640786. [DOI] [PubMed] [Google Scholar]
- 20.Witte KK, Nikitin NP, Parker AC, et al. The effect of micronutrient supplementation on quality-of-life and left ventricular function in elderly patients with chronic heart failure. Eur Heart J. 2005;26(21):2238–2244. doi: 10.1093/eurheartj/ehi442. [DOI] [PubMed] [Google Scholar]
- 21.Kanazawa S, Herbert V. Total corrinoid, cobalamin (vitamin B12), and cobalamin analogue levels may be normal in serum despite cobalamin in liver depletion in patients with alcoholism. Lab Invest. 1985;53(1):108–110. [PubMed] [Google Scholar]
- 22.Andrès E, Serraj K, Zhu J, Vermorken AJ. The pathophysiology of elevated vitamin B12 in clinical practice. QJM. 2013;106(6):505–515. doi: 10.1093/qjmed/hct051. [DOI] [PubMed] [Google Scholar]
- 23.Pennypacker LC, Allen RH, Kelly JP, et al. High prevalence of cobalamin deficiency in elderly outpatients. J Am Geriatr Soc. 1992;40:1197–1204. [PubMed] [Google Scholar]


