Abstract
Background
To investigate the efficacy and safety of a combined oral contraceptive containing estradiol valerate and dienogest (EV/DNG) in healthy Asian women.
Methods
In this multicenter Phase III study, women received oral EV/DNG in a 28-day regimen for 13 cycles. The primary efficacy endpoint was the number of unintended pregnancies, measured by the Pearl Index (PI); secondary efficacy endpoints included bleeding pattern and cycle control parameters. Adverse events were monitored during the study and overall satisfaction with treatment was determined on completion of the study.
Results
A total of 954 Asian women (97.7% of subjects assigned to study medication; mean age 33.4 years) were treated. Five pregnancies were reported during EV/DNG treatment over 796.34 relevant woman-years of exposure, giving an unadjusted PI of 0.63 and a cumulative failure rate of 0.0049; 3 pregnancies during EV/DNG treatment over 760.35 relevant woman-years of exposure gave an adjusted PI of 0.39. The bleeding pattern improved during the reporting periods within the study. The proportion of women who experienced withdrawal bleeding decreased with treatment (84.9% of women during Cycle 1 vs 79.3% in Cycle 13), and the mean length of withdrawal bleeding decreased with treatment (4.2 vs 3.4 days). The number and maximum length of intracyclic bleeding/spotting episodes also decreased with EV/DNG. EV/DNG was well tolerated, and 92% of women included in the study were very satisfied or somewhat satisfied with EV/DNG.
Conclusion
EV/DNG showed high contraceptive efficacy, was well tolerated in Asian women, and may be effectively used in this population.
Clinical trials registry
ClinicalTrials.gov identifier: NCT01638910.
Keywords: Asian, bleeding pattern, combined oral contraceptive, cycle control, estradiol valerate/dienogest, women
Introduction
Since their introduction, combined oral contraceptives (COCs) have been a popular choice of contraception among women of reproductive age. COCs are generally a combination of an estrogen and a progestin. Earlier, COCs containing ethinyl estradiol (EE) were not tolerated well, and efforts to improve tolerability through a dose reduction in EE resulted in increased risk of bleeding disturbances.1,2 Another attempt to address tolerability was made by replacing EE with 17β-estradiol; however, 17β-estradiol-containing COCs were associated with reports of bleeding irregularities, leading to treatment discontinuation.3,4 Tolerability is an important factor for a COC and newer COCs have been developed to overcome this challenge.
Qlaira® (Bayer Healthcare Pharmaceuticals) is a unique, 4-phasic COC, containing a combination of estradiol valerate (EV), a natural estrogen that is metabolized to estradiol, and dienogest (DNG), a progestin that is also effective when used as monotherapy for endometriosis.5–7 It has a dynamic dosing regimen where EV/DNG is administered using an estrogen step-down and a progestin step-up approach over 28-day cycles, comprising 26 days of active treatment followed by 2 hormone-free days. It is approved as an oral contraceptive in many countries around the world, including the USA,8 Europe,9 Canada,10 Australia,11 Singapore,12 and a number of Latin American countries (such as Argentina, Chile, Colombia, Ecuador, and Peru). A randomized, multicenter, double-blind, double-dummy study reported that Caucasian women receiving EV/DNG showed fewer bleeding/spotting days, good cycle control, and contraceptive efficacy compared with EE/levonorgestrel treatment.13 The present study aimed to assess the contraceptive efficacy and safety of EV/DNG in healthy Asian women and determine the cycle control, bleeding pattern, and overall satisfaction with the use of EV/DNG in this population.
Methods
This multicenter, open-label, uncontrolled, Phase III study included women from different Asian countries between June 2012 and November 2014 (ClinicalTrial.gov identifier: NCT01638910). The study protocol was reviewed and approved by each study site’s Independent Ethics Committee/Institutional Review Board (see Supplementary material for the list of institutional review boards) before the start of the study, and the study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and the International Conference on Harmonisation guideline E6: Good Clinical Practice. All women included in the study provided written informed consent.
The main study inclusion criteria were as follows: healthy women (aged 18–50 years) requesting contraception, including smokers (aged <35 years at study entry), history of regular menstrual cycle without the use of hormonal contraceptives, and clinically insignificant cervical smear not requiring further follow-up. Women were excluded from the study if they were pregnant or lactating, had a body mass index >32 kg/m2, or a disease condition that could interfere with the conduct of the study or worsen with hormonal treatment.
Study design
Women received daily oral tablets of EV/DNG for 13 cycles, with the following dosing regimen per 28-day cycle: 2 days of 3.0 mg EV, 5 days of 2.0 mg EV+2.0 mg DNG, 17 days of 2.0 mg EV+3.0 mg DNG, and 2 days of 1.0 mg EV, followed by 2 days of placebo. The primary efficacy endpoint was the number of unintended pregnancies, as measured by the Pearl Index (PI)14 and a life table analysis. Secondary efficacy endpoints included bleeding pattern and cycle control parameters. Global assessment of satisfaction with EV/DNG therapy was performed at the final visit using a questionnaire.
The PI was defined as the number of pregnancies while taking EV/DNG, including up to 2 days after the last dose, over 100 woman-years. The cumulative treatment failure rate was defined as the probability of getting pregnant when on EV/DNG treatment. Treatment compliance was defined as the total number of tablets taken by a woman divided by the woman’s exposure days. Bleeding episodes were described using the reference period method recommended by the World Health Organization (reference periods 1–4), where the length of the reference periods was 90 days each and the first reference period started on the first day of study medication.15 Intracyclic bleeding/spotting was defined as any unexpected bleeding episodes between menstrual cycles.
Safety was assessed by monitoring the adverse events (AEs), pregnancy tests, vital signs/body weight, general physical and gynecological examination, and clinical laboratory parameters during the study.
Statistical analysis
Statistical analysis was performed using the software package SAS release 9.2 or higher (SAS Institute Inc., Cary, NC, USA) and all variables were analyzed using descriptive statistical methods. The primary and secondary efficacy endpoints were analyzed using the full analysis set (FAS), which included all women in the study who took at least 1 tablet of study medication and for whom at least 1 observation after admission to treatment was available. Adjusted and unadjusted PI was calculated for the number of pregnancies and its 95% CIs were calculated. The cumulative EV/DNG failure rate and the corresponding 2-sided 95% CI were calculated using the Kaplan–Meier estimator, where the estimated day of conception was used for calculation of the Kaplan–Meier product limit estimator. The safety analysis set (SAF) consisted of all women who took at least 1 dose of the study medication. AEs were coded in accordance with the Medical Dictionary for Regulatory Activities (MedDRA 17.1).
Results
The study was conducted at 33 centers in China, Hong Kong, Thailand, India, and Taiwan, and enrolled 1,200 women, of whom 976 were assigned to study medication (Figure 1). A total of 954 women (97.7% of subjects assigned to study medication; mean age 33.4±5.9 years) were treated (Table 1), and comprised the FAS and SAF.
Figure 1.
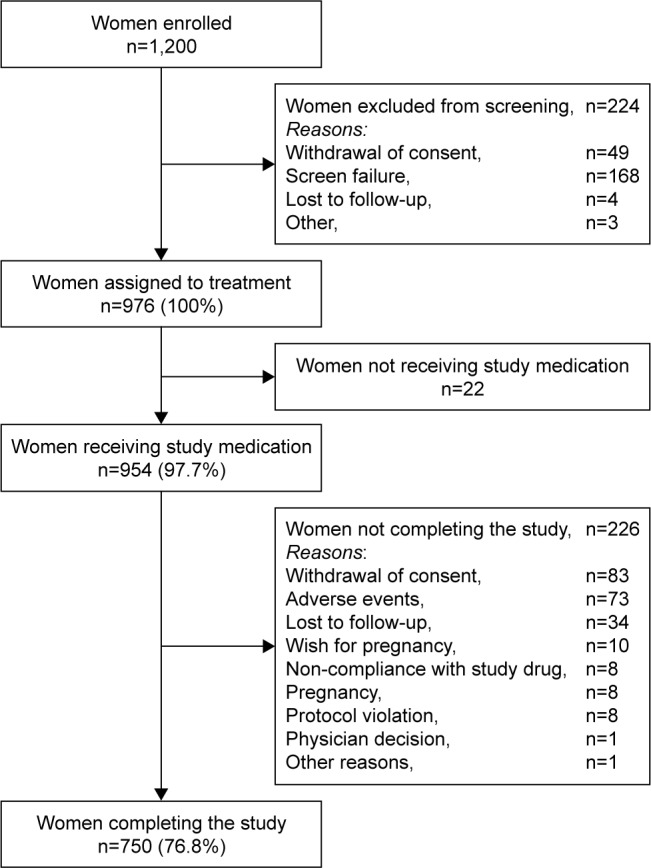
Disposition of the study population.
Table 1.
Demographic and baseline characteristics of women included in the study
| Baseline characteristics | n=954 |
|---|---|
| Age, years | 33.4±5.9 |
| Age group, n (%) | |
| 18–35 years | 624 (65.4) |
| 36–50 years | 330 (34.6) |
| Weight, kg | 56.55±8.04 |
| Height, cm | 160.40±4.88 |
| BMI, kg/m2 | 21.91±2.86 |
| Country/region, n (%) | |
| China | 686 (71.9) |
| Hong Kong | 36 (3.8) |
| India | 16 (1.7) |
| Thailand | 92 (9.6) |
| Taiwan | 124 (13.0) |
| Smoking history, n (%) | |
| Never | 929 (97.4) |
| Former | 6 (0.6) |
| Current | 19 (2.0) |
| Alcohol use, n (%) | |
| Abstinent | 853 (89.4) |
| Light | 99 (10.4) |
| Moderate | 2 (0.2) |
| Medical history, n (%) | |
| Surgical and medical procedures | 376 (39.4) |
| Reproductive system and breast disorders | 120 (12.6) |
| Pregnancy, puerperium, and perinatal conditions | 113 (11.8) |
| Infections and infestations | 85 (8.9) |
| Neoplasms benign, malignant and unspecified (incl. cysts and polyps) | 52 (5.5) |
| Gastrointestinal disorders | 30 (3.1) |
| Immune system disorders | 29 (3.0) |
| Others | 149 (15.7) |
| Gynecological history | |
| Age at menarche, years | 13.4±1.5 |
| Average duration of menstrual bleeding, days | 5.3±1.4 |
| Average length of cycle, days | 29.4±2.9 |
| Number of abortions | 1.2±1.5 |
| Amenorrhea during last 6 months, n (%) | 5 (0.5) |
| Intracyclic bleeding during last 6 months, n (%) | 18 (1.9) |
| Dysmenorrhea during last 6 months, n (%) | 155 (16.2) |
| Prior/concomitant therapy, n (%) | |
| Genitourinary system and sex hormones | 225 (23.6) |
| Alimentary tract and metabolism | 44 (4.6) |
| Anti-infectives for systemic use | 44 (4.6) |
| Dermatological | 35 (3.7) |
| Respiratory system | 28 (2.9) |
| Sensory organs | 27 (2.8) |
| Nervous system | 26 (2.7) |
| Others | 528 (55.1) |
Notes: All values are presented as mean±SD unless otherwise stated.
Abbreviations: BMI, body mass index; incl., including.
Efficacy
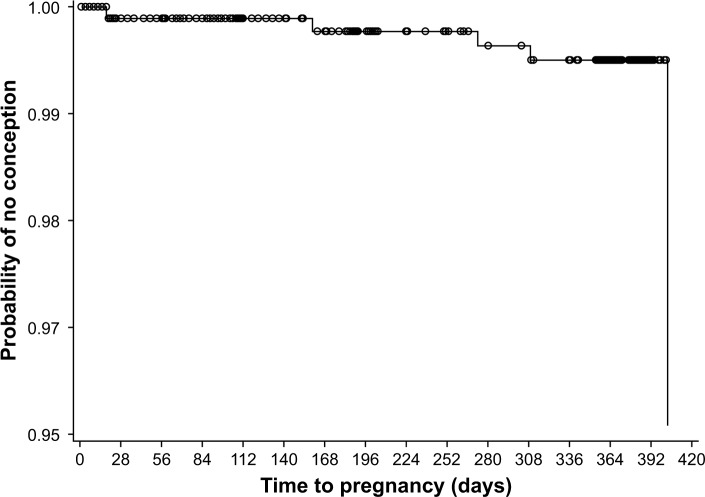
Overall, EV/DNG demonstrated a high contraceptive efficacy. A total of 14 pregnancies were reported during the study, 5 of which were considered to have occurred during EV/DNG treatment; all 5 women discontinued treatment due to pregnancy. During the study, the unadjusted PI (based on 5 pregnancies and 796.34 relevant woman-years of exposure) was 0.63, and the adjusted PI (based on 3 pregnancies considered to be due to EV/DNG method failure and 760.35 relevant woman-years of exposure) was 0.39 (Table 2). The cumulative treatment failure rate at the end of the study using the Kaplan–Meier estimate was 0.0049 and the failure rate for each study cycle was <0.005 (Figure 2).
Table 2.
Pearl Index and the exposure time during estradiol valerate/dienogest treatment in the full analysis set (n=954)
| Age group, years | PI type | Total exposure, days | Relevant exposure, WY | Number of pregnancies | PI | 95% CI |
|---|---|---|---|---|---|---|
| Overall | Adjusted PI | 299,752 | 760.35 | 3 | 0.39 | 0.08–1.15 |
| 18–35 | 197,503 | 500.36 | 3 | 0.60 | 0.12–1.75 | |
| 36–50 | 102,249 | 259.99 | 0 | 0.00 | 0.00–1.42 | |
| Overall | Unadjusted PI | 299,752 | 796.34 | 5 | 0.63 | 0.20–1.47 |
| 18–35 | 197,503 | 524.38 | 4 | 0.76 | 0.21–1.95 | |
| 36–50 | 102,249 | 271.97 | 1 | 0.37 | 0.01–2.05 |
Abbreviations: PI, Pearl Index; WY, woman-year.
Figure 2.
Kaplan–Meier plot of time to pregnancy during the 13 cycles of estradiol valerate/dienogest treatment in the full analysis set (n=954).
Bleeding pattern showed improvement with EV/DNG treatment. During reference periods 2–4, fewer women experienced ≥1 bleeding/spotting days and ≥1 spotting day compared with reference period 1 (Table 3). Similarly, the number, mean length, maximum length, and range of length of bleeding/spotting episodes and spotting only episodes also generally decreased during reference periods 2–4 compared with reference period 1 (Table 3).
Table 3.
Bleeding pattern parameters in the full analysis set (n=954)
| Bleeding pattern parameters | RP 1 (n=874) | RP 2 (n=811) | RP 3 (n=762) | RP 4 (n=746) |
|---|---|---|---|---|
| Women with ≥1 bleeding/spotting day, n (%) | 874 (100) | 798 (98.4) | 747 (98.0) | 729 (97.7) |
| Number of bleeding/spotting days | 20.3±9.4 | 14.3±6.9 | 13.5±6.7 | 13.6±6.7 |
| Women with ≥1 spotting day, n (%) | 802 (91.8) | 740 (91.2) | 681 (89.4) | 671 (89.9) |
| Number of spotting days | 7.6±6.7 | 5.9±5.2 | 6.0±4.9 | 6.1±5.0 |
| Number of bleeding/spotting episodes | 3.6±1.1 | 3.0±0.9 | 3.0±0.9 | 3.0±0.8 |
| Length of bleeding/spotting episodes, daysa | 5.2±3.2 | 4.5±1.9 | 4.4±2.2 | 4.4±1.9 |
| Maximum length of bleeding/spotting episodes, daysb | 7.3±5.9 | 5.7±3.3 | 5.5±3.8 | 5.3±3.4 |
| Range of length of bleeding/spotting episodes, daysc | 3.8±5.4 (n=867) | 2.1±3.1 (n=805) | 2.0±3.4 (n=757) | 1.8±3.0 (n=743) |
| Number of spotting-only episodes | 0.5±0.9 | 0.4±0.8 | 0.5±0.9 | 0.4±0.9 |
| Length of spotting-only episodes, daysa | 1.0±3.4 | 0.7±1.7 | 0.8±1.7 | 0.8±1.8 |
| Maximum length of spotting-only episodes, daysb | 1.1±3.6 | 0.8±2.0 | 0.9±1.9 | 0.9±2.0 |
| Range of length of spotting-only episodes, daysc | 0.3±1.2 (n=867) | 0.2±1.0 (n=805) | 0.2±0.7 (n=757) | 0.2±0.8 (n=743) |
Notes: All values are presented as mean±SD unless otherwise stated.
Per patient, calculated by dividing the total length of all episodes by the number of episodes.
Per patient, the maximum length of a single episode.
Per patient, the maximum length of single episode minus the minimum length of a single episode.
Abbreviation: RP, reference period.
Cycle control showed satisfactory stability in terms of both monthly withdrawal bleeding and intracyclic bleeding episodes. Withdrawal bleeding was seen in 84.9% of women in Cycle 1 and 79.3% of women in Cycle 13, and a decrease in mean length of withdrawal bleeding was observed (4.2±3.0 days vs 3.4±2.3 days). The maximum intensity of withdrawal bleeding episodes was light to normal (68.8% of women in Cycle 1 and 66.2% of women in Cycle 13) and the proportion of women who reported heavy bleeding was reduced with EV/DNG treatment (5.8% vs 2.9%, respectively).
Intracyclic bleeding/spotting occurred in 14.7% of women during Cycle 1 and 4.9% of women in Cycle 13 and the number of intracyclic bleeding/spotting episodes decreased from 0.1±0.3 to 0.0±0.2. Similarly, the maximum length of intracyclic bleeding/spotting episodes decreased from 1.1±3.6 days in Cycle 1 to 0.2±1.6 days in Cycle 13, and the number of intracyclic bleeding/spotting days also decreased from 1.1±3.7 days to 0.2±1.6 days. The maximum intensity for the majority of intracyclic bleeding episodes was spotting (6.1% of women in Cycle 1 and 1.9% of women in Cycle 13) or light (4.8% vs 1.4% of women). A total of 173 women (19.2%) and 254 women (28.2%) had at least 1 intracyclic bleeding episode during Cycles 2–6 and 2–13, respectively. The mean EV/DNG compliance during the study was 0.99±0.07 tablets/day.
Safety
Treatment-emergent AEs (TEAEs) were reported in 457 women (47.9%), and categorized as mild (n=296; 31.0%), moderate (n=145; 15.2%), or severe (n=16; 1.7%). The most commonly reported TEAEs were upper respiratory tract infection (n=82; 8.6%), nasopharyngitis (n=44; 4.6%), and vulvovaginal candidiasis (n=31; 3.2%; Table 4).
Table 4.
TEAEs occurring in ≥1% of women, study drug-related TEAEs occurring in ≥0.5% of women and TE-SAEs reported during the study by primary system organ class and preferred term in the safety analysis set (n=954)
| TEAEs, n (%) | Study drug-related TEAEs, n (%) | TE-SAEs, n (%) | |
|---|---|---|---|
| Total | 457 (47.9) | 155 (16.2) | 13 (1.4) |
| Gastrointestinal disorders | |||
| Diarrhea | 10 (1.0) | – | – |
| Infections and infestations | |||
| Nasopharyngitis | 44 (4.6) | – | – |
| Pharyngitis | 14 (1.5) | – | – |
| Upper respiratory tract infection | 82 (8.6) | – | – |
| Vaginal infection | 17 (1.8) | – | – |
| Vulvovaginal candidiasis | 31 (3.2) | 5 (0.5) | – |
| Investigations–increases in | |||
| Alanine aminotransferase | 12 (1.3) | 8 (0.8) | – |
| Fibrin D dimera | 11 (1.2) | – | – |
| Aspartate aminotransferase | – | 5 (0.5) | – |
| Bodyweight | – | 6 (0.6) | – |
| Nervous system disorders | |||
| Dizziness | 15 (1.6) | 6 (0.6) | – |
| Headache | 27 (2.8) | 9 (0.9) | – |
| Hypoesthesia | – | – | 1 (0.1) |
| Reproductive system and breast disorders | |||
| Amenorrhea | 10 (1.0) | 10 (1.0) | – |
| Breast hyperplasia | 19 (2.0) | 11 (1.2) | – |
| Breast pain | 14 (1.5) | 12 (1.3) | – |
| Cervical dysplasia | 19 (2.0) | 5 (0.5) | – |
| Vaginal hemorrhage | 11 (1.2) | 10 (1.0) | 1 (0.1) |
| Breast engorgement | – | 5 (0.5) | – |
| Skin and subcutaneous tissue disorders | |||
| Acne | 17 (1.8) | 10 (1.0) | – |
| Psychiatric disorders | |||
| Libido decreased | – | 5 (0.5) | – |
| Ear and labyrinth disorders | |||
| Deafness unilateral | – | – | 1 (0.1) |
| Hepatobiliary disorders | |||
| Cholecystitis | – | – | 1 (0.1) |
| Hepatitis cholestatic | – | – | 1 (0.1) |
| Injury, poisoning and procedural complications | |||
| Ankle fracture | – | – | 1 (0.1) |
| Neoplasms benign, malignant and unspecified | |||
| Brain neoplasm benign | – | – | 1 (0.1) |
| Fibroadenoma of breast | – | – | 1 (0.1) |
| Papillary thyroid cancer | – | – | 1 (0.1) |
| Tubular breast carcinoma | – | – | 1 (0.1) |
| Pregnancy, puerperium and perinatal conditions | |||
| Imminent abortion | – | – | 1 (0.1) |
| Renal and urinary disorders | |||
| Calculus ureteric | – | – | 1 (0.1) |
| Respiratory, thoracic and mediastinal disorders | |||
| Vocal cord polyp | – | – | 1 (0.1) |
Note:
Measured in women from mainland China and Hong Kong only.
Abbreviations: TEAEs, treatment-emergent adverse events; TE-SAEs, treatment-emergent serious adverse events.
Study drug-related TEAEs were reported in 155 women (16.2%; Table 4). At least 1 treatment-emergent serious AE (SAE) was reported in 13 women during the study (1.4%; Table 4). Study drug-related SAEs were reported in 4 women (0.4%); and included cholestatic hepatitis, cholecystitis, unilateral deafness, and hypoesthesia (n=1; 0.1% each; Table 5). TEAEs leading to treatment discontinuation were reported in 67 women (7.0%) and included reproductive system and breast disorders (n=20; 2.1%), psychiatric disorders (n=10; 1.0%), nervous system disorders (n=8; 0.8%), and vaginal hemorrhage (n=5; 0.5%). Study drug-related TEAEs led to treatment discontinuation in 52 women (5.5%; Table S1). No deaths were reported during this study, and laboratory safety parameters and vital signs showed no safety concerns.
Table 5.
Serious adverse events and treatment-emergent SAEs reported during the study by primary system organ class and preferred term in the safety analysis set (n=954)
| SAEs, n (%) | Study drug-related SAEs, n (%) | |
|---|---|---|
| Ear and labyrinth disorders | ||
| Deafness unilateral | 1 (0.1) | 1 (0.1) |
| Hepatobiliary disorders | ||
| Cholecystitis | 1 (0.1) | 1 (0.1) |
| Hepatitis cholestatic | 1 (0.1) | 1 (0.1) |
| Injury, poisoning and procedural complications | ||
| Ankle fracture | 1 (0.1) | 0 |
| Neoplasms benign, malignant and unspecified (incl. cysts/polyps) | ||
| Brain neoplasm benign | 1 (0.1) | 0 |
| Fibroadenoma of breast | 1 (0.1) | 0 |
| Papillary thyroid cancer | 1 (0.1) | 0 |
| Tubular breast carcinoma | 1 (0.1) | 0 |
| Nervous system disorders | ||
| Hypesthesia | 1 (0.1) | 1 (0.1) |
| Pregnancy, puerperium and perinatal conditions | ||
| Imminent abortion | 1 (0.1) | 0 |
| Renal and urinary disorders | ||
| Calculus ureteric | 1 (0.1) | 0 |
| Reproductive system and breast disorders | ||
| Cervical dysplasia | 1 (0.1) | 0 |
| Respiratory, thoracic and mediastinal disorders | ||
| Vocal cord polyp | 1 (0.1) | 0 |
Abbreviation: SAEs, serious adverse events.
Satisfaction with EV/DNG
Almost all women included in the study (92%) were very satisfied or somewhat satisfied with EV/DNG. Moreover, 44% of women thought their overall physical well-being was much better or somewhat better throughout the study, while 40.1% of women thought their overall emotional well-being was much better or somewhat better throughout the study. Overall, 55.5% of women would continue with the study contraceptive when given the choice.
Discussion
Most of the available mono- and multi-phasic COCs are formulated with EE; however, EE-containing COCs are often associated with poor tolerability.13 EV/DNG, which has been available since 2010 in the USA and Europe,8,9 was developed to address the tolerability concerns of EE-containing COCs and provide alternative contraceptive options for women.
While contraceptive use in Asian countries is increasing,16 COC treatment among Asian women is complicated by cultural differences in their attitude to contraceptive use.17
In this multicenter, open-label, uncontrolled Phase III study in healthy Asian women, EV/DNG treatment was associated with good contraceptive efficacy. EV/DNG had low treatment failure rates and improved bleeding patterns, with the length of bleeding/spotting episodes, the bleeding intensity, and the proportion of women with heavy bleeding decreasing over the treatment period. Treatment also displayed good cycle control and reduced the number of intracyclic bleeding episodes. EV/DNG was well tolerated and no deaths were reported during the study. The incidence of treatment satisfaction was high and, when given a choice, more than half of the study population would continue with the use of EV/DNG for contraception.
The efficacy and tolerability of EV/DNG in healthy Asian women in the current study is comparable with that observed in previous studies of EV/DNG in Caucasian women.13,18,19 A multicenter, randomized Phase III study conducted in Caucasian women reported that EV/DNG showed results comparable with a monophasic COC containing EE and levonorgestrel with regard to improving the bleeding pattern and cycle control, with fewer bleeding/spotting episodes and less bleeding.13 Another Phase III study reported good contraceptive efficacy and safety of EV/DNG, with lower discontinuation rates in Caucasian women.20 Similarly, a multicenter study conducted in the USA and Canada reported effective contraception with EV/DNG in women aged 18–50 years.19 EV/DNG was also reported to be associated with lower hormone withdrawal-associated symptoms.18 In the present study, EV/DNG showed high contraceptive efficacy when used in Asian women aged 18–50 years over 13 cycles, as illustrated by the low pregnancy rate reported during the study. Furthermore, treatment also improved bleeding pattern and cycle control and reduced the number of bleeding and spotting episodes. Thus, EV/DNG may be effectively used as an oral contraceptive in this population.
The safety outcomes of this study indicated that contraception with EV/DNG was well tolerated. Laboratory parameters and vital signs showed no safety concerns, and no deaths were reported during the study. Study drug-related TEAEs were similar to those reported in other studies with EV/DNG,19,21 and were reported in a limited number of the study population (16.2%). This minor proportion of drug-related TEAEs over a relatively long EV/DNG treatment period during this study is suggestive of a good risk-benefit ratio that is comparable with that reported for other marketed oral contraceptives.22–25
The majority of women included in the study were very satisfied with EV/DNG treatment. More than 40% of women thought that EV/DNG improved their overall physical and emotional well-being, and more than half of the study population would continue with the study contraceptive when given the choice. These results are in line with previous reports on EV/DNG, which demonstrated improved quality of life and sexual function,26–28 and similar treatment satisfaction with EV/DNG.20,29
The main limitation of the study was the absence of an active comparator group, which allowed only indirect comparison of the results with other similar clinical studies. Also, since the study population was carefully chosen based on specific selection criteria and closely monitored throughout the trial, treatment compliance reported in this study may not be an accurate reflection of the real-world clinical practice. In conclusion, EV/DNG showed high contraceptive efficacy in healthy Asian women. Over 13 cycles, EV/DNG was associated with good cycle control, stable bleeding pattern, and high levels of overall satisfaction. The treatment was well tolerated and may be an effective option for use as an oral contraceptive in this population.
Supplementary materials
List of institutional review boards
China
The Second Hospital of Tianjin Medical University No 23, Ping Jiang Road, He Xi District, 300211 Tianjin
Peking Union Medical College Hospital Clinical Trial Office, No 41 Damucang Hutong, Xicheng District, 100730 Beijing
1st Affiliate Hosp., Dalian Med Univ. EC office No 222 Zhongshan Road, 116011 Dalian
The First Affiliated Hospital of Third Military Medical University, PLA 30, Gaotanyan Street, Shapingba District, 400038 Chongqing
Peking University People’s Hospital, No 11 South Avenue, Xizhimen, Xicheng District, 100044 Beijing
Renji Hospital, Shanghai Jiao Tong University, School of Medicine, No 1630 Dongfang Road, 200127 Shanghai
1st Affiliated Hospital, Guangzhou University, TCM No 16, Ji Chang Road, San Yuan Li, 510405 Guangzhou
Obstetrics & Gynecology Hospital of Fudan University, No 419 Fangxie Road, 200011 Shanghai
The Third Xiangya Hospital of Central South University Ethics Committee, No 138, Tongzipo Road, 410013 Changsha
Affiliated Ruijin Hospital Shanghai Jiao Tong University Med School Ethics Committee, No 197 Ruijin Er Road, 200025 Shanghai
People’s Hospital of Hebei Province Ethics Committee, No 348, West Peace Street, 050051 Shijiazhuang
The Third Affiliated Hospital of Third Military Medical University, No 10, Daping Changjiang Branch Road, Yuzhong District, 400042 Chongqing
The First Affiliated Hospital of Nanhua University, No 69, Chuanshan Road, 421001 Hengyang
The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical Hospital, No 306, Hualongqiao Road, Lucheng District, 325027 Wenzhou, Zhejiang Province. The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, 325027 Wenzhou
International Peace Maternity and Child Health Hospital, No 910, Hengshan Road, 200030 Shanghai
West China Second University Hospital, No 17, 3rd Section of Renmin South Road, 610041 Chengdu
Family Planning Research Institute, Tongji Medical College, Huazhong University, Hangkong Road 13#, 430030 Wuhan
Qilu Hospital, Shandong University, No 107, Wenhua West Road, 250012 Jinan
Union Hospital of Tongji Medical College, Huazhong University, No 13, Hangkong Road, Wuhan 430030, Hubei
The Affiliated Zhongda Hospital of Southeast University, No 87, Dingjiaqiao Road, 210009 Nanjing
Hong Kong
Queen Mary Hospital HKU/HA HKW Institutional Review Board Room 901, Administration Block, Queen Mary Hospital, 102, Pokfulam Road, Hong Kong
India
Ruby Hall Clinic Poona Medical Research Foundation Medical Director, 40 Sasson Road, 411001 Pune
Bharti Research Institute of Diabetes and Endocrinology CLINICOM, “Sushruta”, #1/1, lst Temple Road, 15th Cross, Malleashwaram, 560003 Banglore
Sunshine Hospital Institutional Ethics Committee, Sunshine Hospitals, PG Road, Paradise, Secunderabad 500003
Thailand
King Chulalongkorn Memorial Hospital Institutional Review Board, Faculty of Medicine, Chulalongkorn University, 3rd Floor, Anuntamahidol Building, 1873 Rama IV Road, Pathumwan, 10330 Bangkok
Maharaj Nakorn Chiang Mai Hospital Research Ethics Committee, Faculty of Medicine, Chiang Mai University, 110 Intravaroros Road, Amphoe Muang, 50200 Chiang Mai
Siriraj Hospital, Mahidol Siriraj Institutional Review Board, Faculty of Medicine Siriraj Hospital, Mahidol University, 2 Prannok Road, Bangkoknoi, 10700 Bangkok
Taiwan
Chang Gung Memorial Hospital at Linkou Institutional Review Board, Chang Gung Memorial Hospital (IRB/CGMH), No 123, Dinghu Road, Gueishan Township, 333 Taoyuan
Taichung Veterans General Hospital, The Institutional Review Board 160, Section 3, Tai-Chung-Kang Road, 40705 Tai-Chung
Kaohsiung Medical University Chung-Ho Memorial Hospital Clinical Pharmacy, No 100, Tzyou 1st Road, Kaohsiung
National Taiwan University Hospital, No 7, Chung-Shan South Road, 10016 Taipei
Taipei Veterans General Hospital, No 201, Section 2, Shih Pai Road, 11217 Taipei
Taipei Municipal Wanfang Hospital, Taipei Medical University–Joint Institutional Review Board, No 1, Aly. 59, Ln. 220, Wuxing Street, Xinyi District, 110 Taipei
Table S1.
Study drug-related TEAEs leading to treatment discontinuation in the safety analysis set (n=954)
| Study drug-related TEAEs | n (%) |
|---|---|
| Ear and labyrinth disorders | |
| Deafness unilateral | 1 (0.1) |
| Vertigo | 1 (0.1) |
| Eye disorders | |
| Ocular hypertension | 1 (0.1) |
| Gastrointestinal disorders | |
| Abdominal discomfort | 1 (0.1) |
| Abdominal pain | 1 (0.1) |
| Nausea | 2 (0.2) |
| Hepatobiliary disorders | |
| Cholecystitis | 1 (0.1) |
| Hepatitis cholestatic | 1 (0.1) |
| Infections and infestations | |
| Bacterial vaginosis | 1 (0.1) |
| Investigations | |
| Alanine aminotransferase increased | 1 (0.1) |
| Aspartate aminotransferase increased | 1 (0.1) |
| Blood pressure increased | 2 (0.2) |
| Blood triglycerides increased | 1 (0.1) |
| Fibrin D dimer increaseda | 1 (0.1) |
| Gamma-glutamyl transferase increased | 1 (0.1) |
| Weight increased | 3 (0.3) |
| Neoplasms benign, malignant and unspecified (including cysts and polyps) | |
| Uterine leiomyoma | 1 (0.1) |
| Nervous system disorders | |
| Dizziness | 2 (0.2) |
| Headache | 3 (0.3) |
| Hypesthesia | 1 (0.1) |
| Migraine without aura | 1 (0.1) |
| Psychiatric disorders | |
| Anger | 1 (0.1) |
| Depression | 3 (0.3) |
| Irritability | 1 (0.1) |
| Libido decreased | 2 (0.2) |
| Major depression | 1 (0.1) |
| Mood swings | 1 (0.1) |
| Somatoform disorder cardiovascular | 1 (0.1) |
| Reproductive system and breast disorders | |
| Amenorrhea | 3 (0.3) |
| Breast hyperplasia | 3 (0.3) |
| Breast mass | 2 (0.2) |
| Breast pain | 2 (0.2) |
| Breast swelling | 1 (0.1) |
| Hypomenorrhea | 2 (0.2) |
| Metrorrhagia | 1 (0.1) |
| Nipple pain | 1 (0.1) |
| Ovarian cyst | 1 (0.1) |
| Vaginal hemorrhage | 5 (0.5) |
| Skin and subcutaneous tissue disorders | |
| Acne | 2 (0.2) |
| Chloasma | 1 (0.1) |
| Eczema | 1 (0.1) |
Note:
Measured in women from mainland China and Hong Kong only.
Abbreviation: TEAEs, treatment-emergent adverse events.
Acknowledgments
The authors would like to thank Nishad Parkar of inScience Communications, Springer Healthcare, for writing the outline and the first draft of this manuscript, and Sarah Greig of inScience Communications, Springer Healthcare, for post-submission revisions. This medical writing assistance was funded by Bayer Healthcare Pharmaceuticals. The authors would also like to thank all the study site investigators and patients who participated in the study. This study was funded by Bayer Healthcare Pharmaceuticals.
Footnotes
Disclosure
ZZ is an employee of Bayer Healthcare. SP is an employee of Bayer Pharmaceuticals. The authors report no other conflicts of interest in this work.
References
- 1.Gallo MF, Nanda K, Grimes DA, Schulz KF. 20 mcg versus >20 mcg estrogen combined oral contraceptives for contraception. Cochrane Database Syst Rev. 2005;(2):CD003989. doi: 10.1002/14651858.CD003989.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Gerstman BB, Piper JM, Tomita DK, Ferguson WJ, Stadel BV, Lundin FE. Oral contraceptive estrogen dose and the risk of deep venous throm-boembolic disease. Am J Epidemiol. 1991;133(1):32–37. doi: 10.1093/oxfordjournals.aje.a115799. [DOI] [PubMed] [Google Scholar]
- 3.Serup J, Bostofte E, Larsen S, Westergaard J. Effectivity and acceptability of oral contraceptives containing natural and artificial estrogens in combination with a gestagen. A controlled double-blind investigation. Acta Obstet Gynecol Scand. 1981;60(2):203–206. [PubMed] [Google Scholar]
- 4.Wenzl R, Bennink HC, van Beek A, Spona J, Huber J. Ovulation inhibition with a combined oral contraceptive containing 1 mg micronized 17 beta-estradiol. Fertil Steril. 1993;60(4):616–619. doi: 10.1016/s0015-0282(16)56210-4. [DOI] [PubMed] [Google Scholar]
- 5.Caruso S, Iraci M, Cianci S, Casella E, Fava V, Cianci A. Quality of life and sexual function of women affected by endometriosis-associated pelvic pain when treated with dienogest. J Endocrinol Invest. 2015;38(11):1211–1218. doi: 10.1007/s40618-015-0383-7. [DOI] [PubMed] [Google Scholar]
- 6.Lagana AS, Vitale SG, Granese R, et al. Clinical dynamics of dienogest for the treatment of endometriosis: from bench to bedside. Expert Opin Drug Metab Toxicol. 2017;13(6):593–596. doi: 10.1080/17425255.2017.1297421. [DOI] [PubMed] [Google Scholar]
- 7.Lagana AS, Vitale SG, Muscia V, et al. Endometrial preparation with dienogest before hysteroscopic surgery: a systematic review. Arch Gynecol Obstet. 2017;295(3):661–667. doi: 10.1007/s00404-016-4244-1. [DOI] [PubMed] [Google Scholar]
- 8.Bayer HealthCare Pharmaceuticals Inc Natazia®: prescribing information. 2010. [Accessed 25 October 2016]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022252s002lbl.pdf.
- 9.Bayer Pharma AG. Qlaira®: summary of product characteristics. 2010. [Accessed 25 October 2016]. Available from: http://qlaira.com/static/assets/SPC_nlh-1230-001.pdf.
- 10.Government of Canada Natazia®: product monograph. 2015. [Accessed 25 October 2016]. Available from: https://health-products.canada.ca/dpd-bdpp/dispatch-repartition.do;jsessionid=8BDAB463F6A0BAFD94293937593EB9BF.
- 11.Bayer Australia Limited Qlaira® (oestradiol valerate/dienogest): product information. 2009. [Accessed 25 October 2016]. Available from: http://www.bayerresources.com.au/resources/uploads/PI/file9422.pdf.
- 12.Singapore Government Health Sciences Authority Qlaira film coated tablet. 2011. [Accessed 25 October 2016]. Available from: http://www.hsa.gov.sg/content/hsa/en/Health_Products_Regulation/Western_Medicines/New_Drug_Approvals/2011/September.html#Qlaira.
- 13.Ahrendt HJ, Makalova D, Parke S, Mellinger U, Mansour D. Bleeding pattern and cycle control with an estradiol-based oral contraceptive: a seven-cycle, randomized comparative trial of estradiol valerate/dienogest and ethinyl estradiol/levonorgestrel. Contraception. 2009;80(5):436–444. doi: 10.1016/j.contraception.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 14.Gerlinger C, Endrikat J, van der Meulen EA, Dieben TO, Dusterberg B. Recommendation for confidence interval and sample size calculation for the Pearl Index. Eur J Contracept Reprod Health Care. 2003;8(2):87–92. [PubMed] [Google Scholar]
- 15.Belsey EM, Machin D, d’Arcangues C. The analysis of vaginal bleeding patterns induced by fertility regulating methods. World Health Organization Special Programme of Research, Development and Research Training in Human Reproduction. Contraception. 1986;34(3):253–260. doi: 10.1016/0010-7824(86)90006-5. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organisation Family planning/contraception: fact sheet no 351. 2015. [Accessed June 15, 2016]. Available from: http://www.who.int/mediacentre/factsheets/fs351/en.
- 17.Najafi-Sharjabad F, Zainiyah Syed Yahya S, Abdul Rahman H, Hanafiah Juni M, Abdul Manaf R. Barriers of modern contraceptive practices among Asian women: a mini literature review. Glob J Health Sci. 2013;5(5):181–192. doi: 10.5539/gjhs.v5n5p181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen JT, Parke S, Mellinger U, Serrani M, Mabey RG., Jr Hormone withdrawal-associated symptoms: comparison of oestradiol valerate/dienogest versus ethinylestradiol/norgestimate. Eur J Contracept Reprod Health Care. 2013;18(4):274–283. doi: 10.3109/13625187.2013.785516. [DOI] [PubMed] [Google Scholar]
- 19.Nelson A, Parke S, Mellinger U, Zampaglione E, Schmidt A. Efficacy and safety of a combined oral contraceptive containing estradiol valerate/dienogest: results from a clinical study conducted in North America. J Womens Health (Larchmt) 2014;23(3):204–210. doi: 10.1089/jwh.2013.4320. [DOI] [PubMed] [Google Scholar]
- 20.Palacios S, Wildt L, Parke S, Machlitt A, Romer T, Bitzer J. Efficacy and safety of a novel oral contraceptive based on oestradiol (oestradiol valerate/dienogest): a Phase III trial. Eur J Obstet Gynecol Reprod Biol. 2010;149(1):57–62. doi: 10.1016/j.ejogrb.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Endrikat J, Parke S, Trummer D, Schmidt W, Duijkers I, Klipping C. Ovulation inhibition with four variations of a four-phasic estradiol valerate/dienogest combined oral contraceptive: results of two prospective, randomized, open-label studies. Contraception. 2008;78(3):218–225. doi: 10.1016/j.contraception.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Teichmann A, Apter D, Emerich J, et al. Continuous, daily levonorgestrel/ethinyl estradiol vs. 21-day, cyclic levonorgestrel/ethinyl estradiol: efficacy, safety and bleeding in a randomized, open-label trial. Contraception. 2009;80(6):504–511. doi: 10.1016/j.contraception.2009.05.128. [DOI] [PubMed] [Google Scholar]
- 23.Anderson FD, Gibbons W, Portman D. Safety and efficacy of an extended-regimen oral contraceptive utilizing continuous low-dose ethinyl estradiol. Contraception. 2006;73(3):229–234. doi: 10.1016/j.contraception.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Nakajima ST, Archer DF, Ellman H. Efficacy and safety of a new 24-day oral contraceptive regimen of norethindrone acetate 1 mg/ethinyl estradiol 20 micro g (Loestrin 24 Fe) Contraception. 2007;75(1):16–22. doi: 10.1016/j.contraception.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Mircette® Study Group An open-label, multicenter, noncomparative safety and efficacy study of Mircette™, a low-dose estrogen-progestin oral contraceptive. Am J Obstet Gynecol. 1998;179(1):S2–S8. doi: 10.1016/s0002-9378(98)70290-x. [DOI] [PubMed] [Google Scholar]
- 26.Di Carlo C, Gargano V, De Rosa N, Tommaselli GA, Sparice S, Nappi C. Effects of estradiol valerate and dienogest on quality of life and sexual function according to age. Gynecol Endocrinol. 2014;30(12):925–928. doi: 10.3109/09513590.2014.975688. [DOI] [PubMed] [Google Scholar]
- 27.Caruso S, Agnello C, Romano M, et al. Preliminary study on the effect of four-phasic estradiol valerate and dienogest (E2V/DNG) oral contraceptive on the quality of sexual life. J Sex Med. 2011;8(10):2841–2850. doi: 10.1111/j.1743-6109.2011.02409.x. [DOI] [PubMed] [Google Scholar]
- 28.Grandi G, Xholli A, Napolitano A, Palma F, Cagnacci A. Pelvic pain and quality of life of women with endometriosis during quadriphasic estradiol valerate/dienogest oral contraceptive: a patient-preference prospective 24-week pilot study. Reprod Sci. 2015;22(5):626–632. doi: 10.1177/1933719114556488. [DOI] [PubMed] [Google Scholar]
- 29.Graziottin A. Contraception containing estradiol valerate and dienogest–advantages, adherence and user satisfaction. Minerva Ginecol. 2014;66(5):479–495. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Study drug-related TEAEs leading to treatment discontinuation in the safety analysis set (n=954)
| Study drug-related TEAEs | n (%) |
|---|---|
| Ear and labyrinth disorders | |
| Deafness unilateral | 1 (0.1) |
| Vertigo | 1 (0.1) |
| Eye disorders | |
| Ocular hypertension | 1 (0.1) |
| Gastrointestinal disorders | |
| Abdominal discomfort | 1 (0.1) |
| Abdominal pain | 1 (0.1) |
| Nausea | 2 (0.2) |
| Hepatobiliary disorders | |
| Cholecystitis | 1 (0.1) |
| Hepatitis cholestatic | 1 (0.1) |
| Infections and infestations | |
| Bacterial vaginosis | 1 (0.1) |
| Investigations | |
| Alanine aminotransferase increased | 1 (0.1) |
| Aspartate aminotransferase increased | 1 (0.1) |
| Blood pressure increased | 2 (0.2) |
| Blood triglycerides increased | 1 (0.1) |
| Fibrin D dimer increaseda | 1 (0.1) |
| Gamma-glutamyl transferase increased | 1 (0.1) |
| Weight increased | 3 (0.3) |
| Neoplasms benign, malignant and unspecified (including cysts and polyps) | |
| Uterine leiomyoma | 1 (0.1) |
| Nervous system disorders | |
| Dizziness | 2 (0.2) |
| Headache | 3 (0.3) |
| Hypesthesia | 1 (0.1) |
| Migraine without aura | 1 (0.1) |
| Psychiatric disorders | |
| Anger | 1 (0.1) |
| Depression | 3 (0.3) |
| Irritability | 1 (0.1) |
| Libido decreased | 2 (0.2) |
| Major depression | 1 (0.1) |
| Mood swings | 1 (0.1) |
| Somatoform disorder cardiovascular | 1 (0.1) |
| Reproductive system and breast disorders | |
| Amenorrhea | 3 (0.3) |
| Breast hyperplasia | 3 (0.3) |
| Breast mass | 2 (0.2) |
| Breast pain | 2 (0.2) |
| Breast swelling | 1 (0.1) |
| Hypomenorrhea | 2 (0.2) |
| Metrorrhagia | 1 (0.1) |
| Nipple pain | 1 (0.1) |
| Ovarian cyst | 1 (0.1) |
| Vaginal hemorrhage | 5 (0.5) |
| Skin and subcutaneous tissue disorders | |
| Acne | 2 (0.2) |
| Chloasma | 1 (0.1) |
| Eczema | 1 (0.1) |
Note:
Measured in women from mainland China and Hong Kong only.
Abbreviation: TEAEs, treatment-emergent adverse events.