Abstract
The obesity paradox is a term coined for human conditions in which obesity is associated with a lower mortality than being underweight (i.e., body below normal or healthy body weight). Smoking is often cited as a key cofounder of obesity paradox but how smoking causes obesity paradox is unknown. Here we highlight that the obesity paradox can be attributed to insulin resistance and aberrant lipolysis in cardiovascular diseases.
Keywords: Obesity paradox, insulin resistance, lipolysis, nicotine
Obesity Paradox in Cardiovascular Diseases
Obesity is an independent risk factor for the development of diseases associated with increased mortality in the general population such as cardiovascular diseases, for which obesity increases the risk by approximately two-fold. However, for the elderly population or patients with specific conditions, such as cancer cachexia,1 chronic obstructive lung disease, end-stage renal disease, or advanced chronic kidney disease (who are receiving hemodialysis therapy), a higher body mass index (BMI) is often found to be associated with reduced mortality whereas lower body mass index is related to higher mortality in these patients. To describe these human conditions, a new term called “obesity paradox” is coined. The obesity paradox has also been reported in many patients with cardiovascular events; For example, Class I obesity (BMI: 30–35 kg/m2) patients that experience heart failure, have lower short- and intermediate-term mortality than their leaner counterparts. Similarly, overall in-hospital mortality after acute myocardial infarction (AMI) is dramatically lower in elderly obese patients (70 years and older) of all classes compared to AMI patients without obesity. The obesity paradox has also been reported in cardiovascular therapy; for example, elderly patients that are obese or overweight have lower mortality with implantable cardioverter defibrillators than normal BMI patients.2 Since the data to support obesity paradox are exclusively obtained from clinical observations, there are intense debates on whether or not obesity paradox is real or a bias/an artifact derived from observational studies.
Cigarette Smoking May Underlie the Obesity Paradox in Cardiovascular Diseases
Smoking is a critical independent risk factor for cardiovascular mortality and cardiovascular diseases, including peripheral arterial disease, stroke, coronary heart disease, and heart failure.3 In addition, smoking cessation dramatically decreases the relative risk of cardiovascular mortality and coronary heart disease when compared with active smokers, although the risk of stroke is not lowered.3 As smokers, who tend to be leaner, are subjected to higher mortality rates whereas smoking cessation is often linked to improved CVD conditions with body weight gain, several studies have unveiled that obesity paradox is the potential bias resulting from the confounding effects of illness-induced weight loss and smoking.4–5 Obesity paradox is absent in cardiovascular events among never-smokers. This outcome is not that surprising, as failure to properly control for smoking cigarettes when in studies of the relationship between obesity and cardiovascular mortality is a classic example of confounding. Generally, smokers have lower body weights and smoking is inversely correlated with BMI. In addition, patients who quit smoking (i.e. former smokers) will frequently and substantially gain body weight. Hence, failure to adequately control for on-going smoking is likely to result in artificially elevated mortality among the lean subjects, and failure to entirely control for smoking cessation or former smokers may lead to decreased measurements of mortality among the obese or overweight subjects. Overall, these epidemiological studies support that smoking is a mystery of statistical confounder for obesity paradox in cardiovascular diseases.
Lipolysis, Insulin Resistance and Obesity Paradox in Smokers
Fat is a metabolically active and dynamic organ. Fat accumulation is determined by the balance between fat synthesis (lipogenesis) and fat breakdown (lipolysis/fatty acid oxidation). Lipogenesis is very responsive to changes in the diet and is partly regulated by hormones (insulin, leptins, etc.,). Similarly, lipolysis is directly activated by sympathetic nervous system activation, glucagon, or growth hormone, etc.
Insulin is a key regulator for both lipogenesis and lipolysis. Insulin inhibits lipolysis and slows the breakdown of adipose tissue caused by lipolytic signals such as sympathetic nervous system activation, glucagon, or growth hormone, etc. The impact of insulin on lipolysis is largely determined by the intensities of lipolytic signals. In insulin resistance (IR) state, insulin’s inhibition on lipolysis is diminished. As a result, IR in hypertrophic adipocytes leads to increased lipolysis and the release of free fatty acid (FFA) into circulation. In return, FFA elevation has been postulated to play a critical role in the development of IR. Recent studies in both humans and mice indicate that high levels of FFA, which is associated with low body weight, impairs insulin sensitivity, whereas genetic inhibition of adipose tissue lipolysis by deletion of adipose triglyceride lipase, results in improved insulin sensitivity but with gain in body weights and fat levels (paradox).6
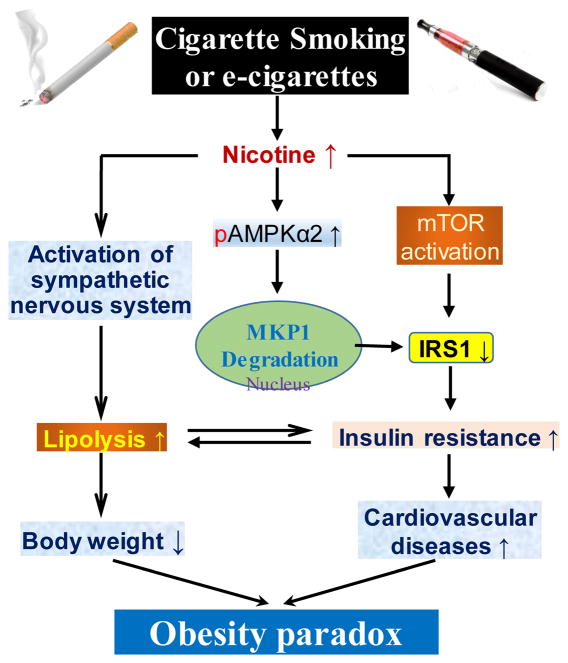
Cigarette smokers often have IR and compensatory hyperinsulinemia. In addition, long-term nicotine replacement therapy (nicotine gum or e-cigarettes), when used as a method of weight control, was reported to induce IR and other metabolic disorders.7 Several mechanisms by which cigarette smoke or e-cigarettes cause whole-body IR have been reported. First, nicotine is the major bioactive component of cigarette smoke and e-cigarettes and activates sympathetic nervous system leading to increased lipolysis in white adipose tissue8 and resultantly elevated FFA, which contributes to IR and weight loss (Figure). Second, nicotine acts through the nicotinic acetylcholine receptor subunit α7 (α7nAchR) and causes increased levels of reactive oxygen and nitrogen species and selectively activates 5′ adenosine monophosphate-activated protein kinase alpha2 (AMPKα2) in white adipocytes. Consequently, AMPKα2 phosphorylates mitogen-activated protein kinase phosphatase-1 (MKP1) at serine 334,9 initiating its proteasome-dependent degradation. The nicotine-mediated reduction of MKP1 induces aberrant activation of both p38 mitogen-activated protein kinase and c-Jun N-terminal kinase, leading to increased phosphorylation of insulin receptor substrate 1 (IRS1) at serine 307, its degradation, and resultant IR (Figure). Third, nicotine induces IR through activation of mammalian target of rapamycin (mTOR) in skeletal muscle (Figure). Cigarette exposure-induced IR is highly associated with an increased risk of cardiovascular diseases, such as ischemic heart disease and coronary heart disease. Moreover, elevated FFA caused by IR contributes to myocardial dysfunction, which is associated with heart failure and acute coronary syndromes.
Figure.
Schematic representation of the role of cigarette smoking-induced IR in the obesity paradox. First, nicotine-enhanced lipolysis by sympathetic nervous system activation contributes to IR and body weight loss. Second, nicotine acts via adipocyte α7nAchR to induce ROS and activate AMPKα2. This subsequently enhances MKP1 degradation, resulting in IRS1 reduction and IR. Third, nicotine induces IR via mTOR activation in the skeletal muscle. Hence, nicotine promotes both body weight loss and IR-induced cardiovascular diseases, which may underlie the so-called obesity paradox. →, induce or act on; ↑, increase; ↓, decrease. Other abbreviations are defined in the main text.
Nicotine acts through numerous mechanisms to promote weight loss. For example, recent evidence has demonstrated that nicotine functions in the central nervous system by binding to the α3β4 nicotinic acetylcholine receptor and activating proopiomelanocortin (POMC) neurons in the arcuate nucleus of the hypothalamus to decrease food intake, thereby contributing to weight loss. Moreover, nicotine inhibits AMPK in the ventromedial nucleus of the hypothalamus to increase brown adipose tissue thermogenesis through the sympathetic nervous system, which also causes weight loss. Finally, nicotine-triggered IR exacerbates lipolysis in white adipose tissue, further enhancing weight loss. Taken together, nicotine replicates a condition in which low body weight is linked to high CVD.
On the other hand, smoking cessation causes obesity paradox as when people quit smoking, they undergo substantial weight gain10, improved insulin sensitivity, and decreased CVD. The fact that smoking cessation improves IR with body weight gains suggests that smoking’s impacts on both cardiovascular health and insulin sensitivity are likely greater than body weight alone. Thus, obese smokers or former smokers, after smoking cessation, might have better insulin sensitivity and lower CVD risks than obese nonsmokers. That is to say, a former smoker that gain weights might have weaker IR than the obese general publics do. Thus, it is highly likely that removing CVD-reduced obese smokers or former smokers would epidemiologically increase CVD risk in the remaining nonsmoking obesity population, a typical phenomenon described as obesity paradox.
Insulin Resistance, Aberrant Lipolysis, and Obesity Paradox in Non-smokers
The obesity paradox also has been reported in non-smokers with cancer cachexia or cardiac cachexia,11 a wasting syndrome primarily characterized by weight loss, including adipose tissues or/and skeletal muscle wasting. Although central nervous system- and inflammation-related anorexia regulates cancer cachexia, emerging data indicate that IR-accelerated lipolysis is a contributor.12 For example, tumor-derived inflammatory cytokines such as tumor necrosis factor and interleukin-6 induce IR, decrease lipogenesis, and increase lipolysis. Moreover, the sympathetic nervous system is also responsible for part of the visceral fat lipolysis in the fasting stage or anorexia. In addition, AMPKα1 reduction in cancer or stromal fibroblast cells13 contributes to enhanced lipolysis in adipocytes and subsequent cancer cachexia.14 Interestingly, the insulin sensitizer rosiglitazone attenuates adipose depletion, the consequent weight loss, and cancer cachexia.15 Taken together, these findings indicate that IR is a key contributor to cachexia and suggest that IR is a potential therapeutic target.
Conclusions
Aberrant lipolysis accounts for the obesity paradox in smoking and non-smoking-related conditions. IR is a critical pathological factor for aberrant lipolysis. Therefore, insulin sensitizing drugs that attenuate IR might be a promising strategy to reduce CVD and related mortality in the patients with low- and moderate body weights.
Acknowledgments
Sources of Funding
Dr. Ming-Hui Zou’s laboratory was supported by the grants from the following sources: National Institutes of Health RO1 (HL079584, HL089920, HL080499, HL110488, HL128014, HL132500, HL137371, HL142287, HL140954, AG047776, and CA213022). Dr. Ping Song is supported in part by HL140954. Dr. Xi-Yong Yu is supported by National Natural Science Foundation of China (No. 81330007 and No. U1601227).
References
- 1.Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nature reviews Clinical oncology. 2013;10:90–9. doi: 10.1038/nrclinonc.2012.209. [DOI] [PubMed] [Google Scholar]
- 2.Jahangir A, Mirza M, Shahreyar M, Mengesha T, Shearer R, Sultan S, Jahangir A, Choudhuri I, Nangia V, Dhala A, Bhatia A, Niazi I, Sra J, Tajik AJ. Presence of obesity is associated with lower mortality in elderly patients with implantable cardioverter defibrillator. Int J Obes (Lond) 2018;42:169–174. doi: 10.1038/ijo.2017.211. [DOI] [PubMed] [Google Scholar]
- 3.Pan A, Wang Y, Talaei M, Hu FB. Relation of Smoking With Total Mortality and Cardiovascular Events Among Patients With Diabetes Mellitus: A Meta-Analysis and Systematic Review. Circulation. 2015;132:1795–804. doi: 10.1161/CIRCULATIONAHA.115.017926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global BMIMC, Di Angelantonio E, Bhupathiraju Sh N, Wormser D, Gao P, Kaptoge S, Berrington de Gonzalez A, Cairns BJ, Huxley R, Jackson Ch L, Joshy G, Lewington S, Manson JE, Murphy N, Patel AV, Samet JM, Woodward M, Zheng W, Zhou M, Bansal N, Barricarte A, Carter B, Cerhan JR, Smith GD, Fang X, Franco OH, Green J, Halsey J, Hildebrand JS, Jung KJ, Korda RJ, McLerran DF, Moore SC, O’Keeffe LM, Paige E, Ramond A, Reeves GK, Rolland B, Sacerdote C, Sattar N, Sofianopoulou E, Stevens J, Thun M, Ueshima H, Yang L, Yun YD, Willeit P, Banks E, Beral V, Chen Z, Gapstur SM, Gunter MJ, Hartge P, Jee SH, Lam TH, Peto R, Potter JD, Willett WC, Thompson SG, Danesh J, Hu FB. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388:776–86. doi: 10.1016/S0140-6736(16)30175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, Moore SC, Tobias GS, Anton-Culver H, Freeman LB, Beeson WL, Clipp SL, English DR, Folsom AR, Freedman DM, Giles G, Hakansson N, Henderson KD, Hoffman-Bolton J, Hoppin JA, Koenig KL, Lee IM, Linet MS, Park Y, Pocobelli G, Schatzkin A, Sesso HD, Weiderpass E, Willcox BJ, Wolk A, Zeleniuch-Jacquotte A, Willett WC, Thun MJ. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–9. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmadian M, Abbott MJ, Tang T, Hudak CS, Kim Y, Bruss M, Hellerstein MK, Lee HY, Samuel VT, Shulman GI, Wang Y, Duncan RE, Kang C, Sul HS. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab. 2011;13:739–48. doi: 10.1016/j.cmet.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eliasson B, Taskinen MR, Smith U. Long-term use of nicotine gum is associated with hyperinsulinemia and insulin resistance. Circulation. 1996;94:878–81. doi: 10.1161/01.cir.94.5.878. [DOI] [PubMed] [Google Scholar]
- 8.Middlekauff HR, Park J, Moheimani RS. Adverse effects of cigarette and noncigarette smoke exposure on the autonomic nervous system: mechanisms and implications for cardiovascular risk. J Am Coll Cardiol. 2014;64:1740–50. doi: 10.1016/j.jacc.2014.06.1201. [DOI] [PubMed] [Google Scholar]
- 9.Wu Y, Song P, Zhang W, Liu J, Dai X, Liu Z, Lu Q, Ouyang C, Xie Z, Zhao Z, Zhuo X, Viollet B, Foretz M, Wu J, Yuan Z, Zou MH. Activation of AMPKalpha2 in adipocytes is essential for nicotine-induced insulin resistance in vivo. Nat Med. 2015;21:373–82. doi: 10.1038/nm.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flegal KM, Troiano RP, Pamuk ER, Kuczmarski RJ, Campbell SM. The influence of smoking cessation on the prevalence of overweight in the United States. N Engl J Med. 1995;333:1165–70. doi: 10.1056/NEJM199511023331801. [DOI] [PubMed] [Google Scholar]
- 11.von Haehling S, Lainscak M, Springer J, Anker SD. Cardiac cachexia: a systematic overview. Pharmacol Ther. 2009;121:227–52. doi: 10.1016/j.pharmthera.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Asp ML, Tian M, Wendel AA, Belury MA. Evidence for the contribution of insulin resistance to the development of cachexia in tumor-bearing mice. Int J Cancer. 2010;126:756–63. doi: 10.1002/ijc.24784. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Y, Xu H, Ding Y, Lu Q, Zou MH, Song P. AMPKalpha1 deletion in fibroblasts promotes tumorigenesis in athymic nude mice by p52-mediated elevation of erythropoietin and CDK2. Oncotarget. 2016;7:53654–53667. doi: 10.18632/oncotarget.10687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rohm M, Schafer M, Laurent V, Ustunel BE, Niopek K, Algire C, Hautzinger O, Sijmonsma TP, Zota A, Medrikova D, Pellegata NS, Ryden M, Kulyte A, Dahlman I, Arner P, Petrovic N, Cannon B, Amri EZ, Kemp BE, Steinberg GR, Janovska P, Kopecky J, Wolfrum C, Bluher M, Berriel Diaz M, Herzig S. An AMP-activated protein kinase-stabilizing peptide ameliorates adipose tissue wasting in cancer cachexia in mice. Nat Med. 2016;22:1120–1130. doi: 10.1038/nm.4171. [DOI] [PubMed] [Google Scholar]
- 15.Asp ML, Tian M, Kliewer KL, Belury MA. Rosiglitazone delayed weight loss and anorexia while attenuating adipose depletion in mice with cancer cachexia. Cancer biology & therapy. 2011;12:957–65. doi: 10.4161/cbt.12.11.18134. [DOI] [PMC free article] [PubMed] [Google Scholar]