Abstract
Importance
Racial disparities in survival after trauma are well-described for patients less than 65 years of age. Similar information among older patients is lacking as existing trauma databases do not include important patient co-morbidity information.
Objective
To determine whether disparities in trauma survival for Blacks versus Whites persist in patients aged ≥65 years using an approach that adjusts for both patient injury severity and co-morbidity information in data analysis.
Setting
Nationwide Inpatient Sample (NIS, 2003–2010)
Participants
Trauma patients were extracted from the NIS using ICD-9-CM diagnosis codes. Injury severity was ascertained by applying the Trauma Mortality Prediction Model, and patient co-morbidities were quantified using the Charlson Co-Morbidity Index (CCI).
Main Outcomes and Measures
The odds of death after trauma for Blacks versus Whites for younger (age 16–64 years) and older (age ≥65 years) patients were compared using 3 different statistical methods: multivariable logistic regression with and without clustering for hospital effects and coarsened exact matching. Model covariates included age, gender, insurance status, mechanism of injury, overall injury severity, head injury severity, and co-morbid conditions.
Results
1,073,195 patients were included (502,167 patients aged 16–64 years; 571,028 patients aged ≥65 years). The majority of older patients were White (95.9%), female (71.1%), insured (99.4%), with CCI scores ≥ 1 (56.7%). The unadjusted odds of death for Blacks versus Whites was 1.35 (95% CI 1.28–1.42) for patients aged 16–64 years, and 1.00 (95% CI 0.93–1.08) for patients aged ≥65 years. After risk-adjustment, racial disparities in survival persisted in the younger Black age group [1.21 (95% CI 1.13–1.30)] but were reversed in the older age group [0.83 (95% CI 0.76–0.90)]. This finding was consistent across all three statistical methods.
Conclusions and Relevance
In this study that risk-adjusts for both patient-specific co-morbidity data and injury severity information, differential racial disparities exist between White patients and Black patients depending on their age group. While younger White patients have better outcomes after trauma than younger Black patients, older White patients have a higher odds of death than older Black patients. Exploration of this paradoxical finding may help us better understand mechanisms that lead to disparities in trauma outcomes.
Keywords: Outcomes, trauma, racial disparities, elderly
Introduction
Disparities in survival after traumatic injury among minorities and the uninsured have been well described for patients under 65 years of age1–7. In a review of patients aged 18–64 years registered in the National Trauma Data Bank (NTDB), Black patients had significantly higher unadjusted mortality rates and an increased adjusted odds ratio (OR) of death compared with White patients8. Recent meta-analyses of research investigating racial disparities in both trauma care and surgical outcomes further support the finding that Black patients tend to have higher adjusted mortality rates compared to similar White patients9, 10. Similar health care inequities have been reported in the pediatric (aged 0–17 years) trauma population1, and in populations with a wide range of injury mechanisms2–7.
Despite the recent demonstration of racial disparities after trauma among younger patients, information regarding the effect of race on trauma outcomes among older trauma patients is lacking. Most authors choose to exclude older patients from analysis due to the lack of important co-morbidity data in existing trauma databases. Co-morbid conditions have been shown to significantly impact trauma outcomes. In a study of over 3000 patients, a single point increase in Charlson comorbidity index was independently associated with a 1.24 odds ratio of 1-year mortality after trauma11. Data from the Healthcare Cost and Utilization Project has demonstrated that patient comorbities may have an interaction with race and socioeconomic status in post-traumatic mortality as well12. Unfortunately, trauma-specific databases such as the National Trauma Data Bank are unable to collect adequate measures of patients’ pre-injury health status, so the aforementioned analyses comparing racial disparities after trauma1–10 have limited applicability to older patients who commonly have significant comorbidities.
The objective of the current study was to determine whether the previously described racial disparities in outcomes after trauma continue to persist among older trauma patients. Using an approach that allows for the incorporation of patient co-morbidity information with traumatic injury severity information, we assessed in-hospital mortality in White patients versus Black patients after trauma using three different statistical methodologies. We hypothesized that racial disparities may not be present in patients 65 years of age and older due to better access to pre-injury medical care (i.e. Medicare).
Methods
After receiving expedited IRB approval from the Johns Hopkins Medicine Institutional Review Board, trauma patients were extracted from the Nationwide Inpatient Sample (NIS) for the years 2003–2010 using International Classification of Diseases 9th Edition (ICD-9-CM) diagnosis codes 800 through 959 inclusive. Patients with late effects of injury (905–909), superficial injuries (910–924), and foreign bodies (930–939) were excluded in attempt to mimic the National Trauma Data Bank’s definition of a trauma admission as closely as possible13. The NIS is a national US dataset that represents a 20% sample of patients discharged from community hospitals in participating states14, but its use in studying trauma has been limited due to lack of recorded injury severity indexes. The recently validated ICD Programs for Injury Categorization (ICDPIC) program15 was used to generate both the Injury Severity Score (ISS)16 and Trauma Mortality Prediction Model (TMPM) score17 as measures of injury severity for each trauma patient in the NIS. White and Black patients aged 16 years or older with blunt or penetrating injuries were included. Patients of other races were excluded because accounts of racial disparities in these groups are conflicting and less consistently described than for Blacks versus Whites10. Patients from the 12 states that do not reliably report race data (≥40% missing) were excluded (i.e. NC, NV, OR, KY, IL, GA, NE, WA, MT, WV, OH and MN). Patients who were transferred in from or out to another acute care facility in the NIS were also excluded (n = 50,847), as their ultimate in-hospital survival could not be accurately ascertained. For the final sample, missing data was less than 0.5% overall.
Demographic data including race, age, race, gender, insurance status, mechanism of injury, intent of injury and head injury severity was abstracted from the records. Race, age, gender, insurance status, mechanism of injury (blunt vs. penetrating), and severe head injury (Abbreviated Injury Scale ≥3)18 were coded as binary variables. Intent of injury was classified according to the CDC external cause of injury intent categorization (unintentional, self-inflicted, assault, undetermined and others)19. Age was categorized into six groups: 16–25, 26–35, 36–45, 46–55, 56–64, 75–75, 76–85, and ≥86 years. ISS was divided into 4 groups: <9, 9–15, 16–24, 25–75. TMPM scores were treated as a continuous variable. To risk-adjust patients based on their co-morbid conditions, we employed an available STATA module20 to generate the Charlson Co-Morbidity Index (CCI)21 from diagnosis codes specific to each patient within the dataset. The total scores derived from the STATA CCI module were divided into the following groups: 0, 1, 2 and ≥3 for descriptive purposes (Table 1), but treated as a continuous variable for statistical analyses.
Table 1.
Patient Characteristics
| Age < 65 years (n = 502,167) |
Age ≥ 65 years (n = 571,028) |
||
|---|---|---|---|
| Age (years) 16–25 | 1028,101 (21.5%) | . | |
| 26–35 | 81,860 (16.3%) | . | |
| 36–45 | 93,695 (18.7%) | . | |
| 46–55 | 117,357 (23.4%) | . | |
| 56–64 | 101,154 (20.1%) | . | |
| 65–75 | . | 139,392 (24.4%) | |
| 76–85 | . | 237,247 (41.2%) | |
| ≥86 | . | 194,389 (34.0%) | |
| Gender Male | 337,195 (65.2%) | 164,833 (28.9%) | |
| Females | 174,886 (34.8%) | 406,158 (71.1%) | |
| Missing | 86 (0.02%) | 37 (0.01%) | |
| Race White | 413,072 (82.3%) | 547,325 (95.9%) | |
| Black | 89,095 (17.7%) | 23,703 (4.2%) | |
| Insured Yes | 413,650 (82.4%) | 567,361 (99.4%) | |
| No | 86,221 (17.2%) | 3,075 (0.5%) | |
| Missing | 2,296 (0.5%) | 592 (0.1%) | |
| Intent Unintentional | 442,770 (88.2%) | 569,324 (99.7%) | |
| Self-inflicted | 6,288 (1.3%) | 594 (0.1%) | |
| Assault | 49,879 (9.9%) | 1,010 (0.18%) | |
| Undetermined | 2,488 (0.5%) | 70 (0.01%) | |
| Other | 742 (0.2%) | 30 (0.01%) | |
| Type of injury Blunt | 450,820 (89.8%) | 568,485 (99.6%) | |
| Penetrating | 51,347 (10.2%) | 2,543 (0.5%) | |
| Injury Severity Score <9 | 275,019 (54.8%) | 218,819 (38.3%) | |
| 9–15 | 158,443 (31.6%) | 307,850 (53.9%) | |
| 16–24 | 51,171 (10.2%) | 39,224 (6.9%) | |
| 25–75 | 17,137 (3.4%) | 5,077 (0.9%) | |
| Severe head/neck injury (AIS>=3) Yes | 69,566 (13.9%) | 57,705 (10.1%) | |
| No | 432,601 (86.2%) | 513,323 (89.9%) | |
| Charlson Comorbidity Index 0 | 400,434 (79.7%) | 247,287 (43.3%) | |
| 1 | 71,068 (14.2%) | 172,299 (30.2%) | |
| 2 | 17,925 (3.57%) | 83,881 (14.7%) | |
| ≥3 | 12,740 (2.5%) | 67,561 (11.8%) | |
| Mortality Yes | 8,837 (1.8%) | 19,697 (3.5%) | |
| No | 493,263 (98.2%) | 551,331 (96.6%) | |
Statistical Methods
Three different techniques were employed to determine the independent impact of race on the main outcome measure of in-hospital mortality after trauma among a) younger (16–64 years of age) and b) older (≥ 65 years of age) patients. We first used the standard methods of univariable logistic regression and multivariable logistic regression [adjusting for age, gender, insurance status, type of injury (blunt vs. penetrating), intent of injury, injury severity (TMPM), head injury severity, and CCI] with and without clustering for hospital effects. Covariates were chosen based on the 5 minimum covariates that are considered to be essential when performing a risk-adjusted analysis of trauma mortality outcomes22. Regression diagnostics for the multivariable logistic regression model without clustering demonstrated areas under the curve and Homer-Lemeshow statistics of 0.93 and 84.3 for patients age < 65 years and 0.80 and 224.5 for patients age ≥ 65 years, respectively. Clustering patients by hospitals produces more reliable confidence intervals by taking into account the inter-facility correlation of patient outcomes (i.e. patient outcomes are more likely to be similar within rather than across hospitals)23, 24. Model performance was not affected by clustering as clustering mainly affects how standard errors and confidence intervals are handled between facilities.
We then performed Coarsened Exact Matching (CEM) to match Black patients to White patients on age, gender, insurance status, type of injury, intent of injury, overall injury severity, head injury severity, and co-morbid conditions (Figure 1). CEM is a statistical means of matching patients that aims to reduce the imbalance in covariates between two groups using monotonic imbalance bounding25. Unlike traditional patient matching, CEM employs the use of broader categorical bins that allows for matching based on the reasonable assumption that patients within those bins will behave similarly. This technique has been shown to efficiently match patients even in large datasets26, 27, and allows for a greater overall number of successfully matched patients while still bounding the degree of model dependence and the average treatment estimation error25. We report the L1 distance between Black and White patients in each age group before and after CEM in order to demonstrate the change in group differences following implementation of the CEM technique. The L1 statistic is a multivariate measure of imbalance ranging from 1 (complete separation) to 0 (perfect global match) that is calculated based on the differences of all model covariates for the case vs. control groups28. Following CEM, conditional logistic regression was employed to generate the odds of death for Blacks versus Whites for younger (16–64 years of age) and older (≥ 65 years of age) patients.
Figure 1. Example of Coarsened Exact Matching on Age.
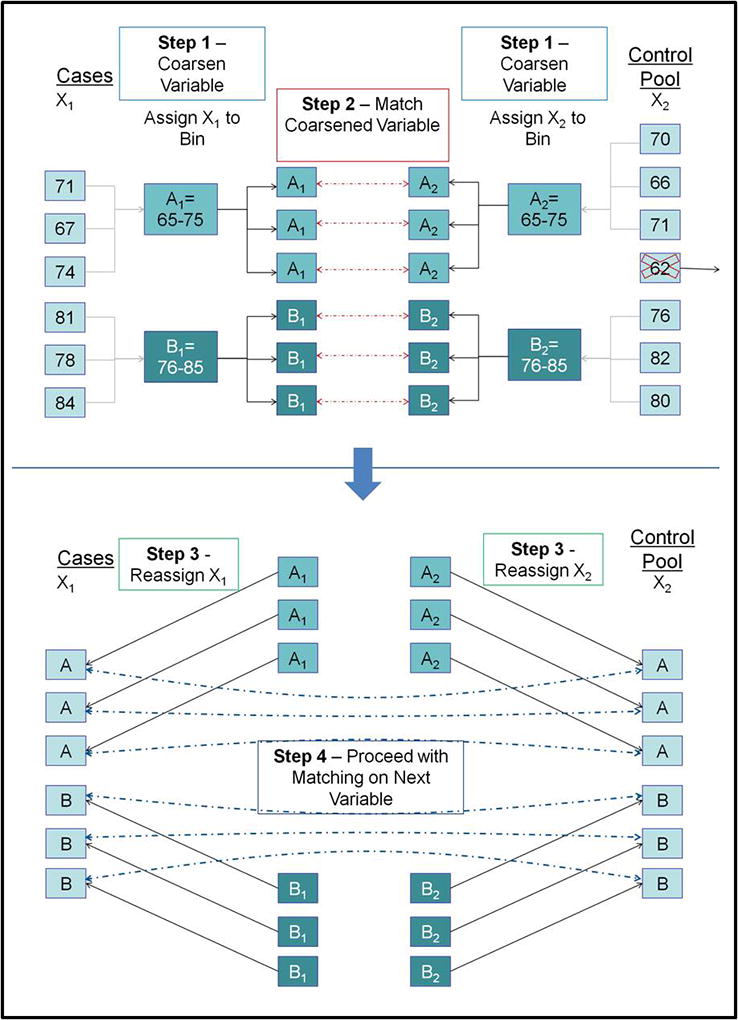
CEM is a statistical means of matching patients based on categorical bins that allows for a greater overall number of successfully matched patients compared to traditional matching while still bounding the degree of model dependence and the average treatment estimation error. The technique involves 4 main steps: 1) The variable of interest (e.g. age) is “coarsened”, meaning that it is assigned categorical bins that are chosen on the reasonable assumption that patients within those bins will behave similarly; 2) Patients from the case (e.g. Black) and Control (e.g. White) groups are matched on the coarsened variable; 3) The patients are assigned back into their group (i.e. Case or Control); and 4) The patients are matched on the next variable.
All statistics were performed using STATA software using STATA/MP Version 11.0 (StataCorp, College Station, Texas USA). Statistical significance was defined as p ≤ 0.05.
Results
Over the eight years studied, 1,073,195 patients met inclusion criteria (502,167 patients aged 16–64 years; 571,028 patients aged ≥ 65 years) (Table 1). The majority of older patients were White (95.9%), female (71.1%), and had insurance (99.4%). CCI scores were ≥ 1 in 56.7% of older patients. Most of these patients sustained blunt (99.6%) accidental (99.7%) trauma resulting in injury severity scores ≥ 9 (61.7%).
Notable findings in the demographics of the older vs. younger patients included a lower proportion of males (28.9% vs. 65.2 %), higher incidence of insurance (99.4% vs. 82.4%), and higher mortality (3.5% vs. 1.8%) in the older population. Younger patients also tended to be much healthier, with the majority having no reported comorbid conditions (79.7% vs. 43.3% in the older population).
Coarsened exact matching was successfully utilized in both the younger and older patient cohorts. In both age groups, the multivariate LI distance decreased substantially after matching (0.17 vs. 0.39 and 0.18 vs. 0.28 for younger and older patients, respectively). There were also very few unmatched patients in each group (Table 2).
Table 2.
Results of Coarsened Exact Matching
| Age < 65 years (n = 502,167) |
Age ≥ 65 years (n = 571,028 |
|||
|---|---|---|---|---|
| Blacks | Whites | Blacks | Whites | |
| All | 89,095 | 413,072 | 23,703 | 547,325 |
| Matched | 87,485 | 407,944 | 23,530 | 541,946 |
| Unmatched | 1,610 | 5,128 | 173 | 5,379 |
| Unmatched Multivariate L1 distance | 0.39 | 0.28 | ||
| Matched Multivariate L1 distance | 0.17 | 0.18 | ||
The unadjusted odds of death for Blacks versus Whites was 1.35 (95% CI 1.28–1.42) for patients < 65 years of age, and 1.00 (95% CI 0.93–1.08) for patients ≥ 65 years of age. After coarsened exact matching, racial disparities in survival persisted in the younger Black age group [1.21 (95% CI 1.13–1.30)] but were reversed in the older age group [0.83 (95% CI 0.76–0.90)]. These findings were consistent with multivariable regression analysis [OR 1.21 (95% CI 1.13–1.29) vs. OR 0.83 (95% CI 0.77–0.90)] and with multivariable regression analysis controlled for clustering [OR 1.21 (95% CI 1.13–1.29) vs. OR 0.83 (95% CI 0.77–0.90)] (Figure 2).
Figure 2. Odds Ratio for Mortality: Black vs. White by Age Category.
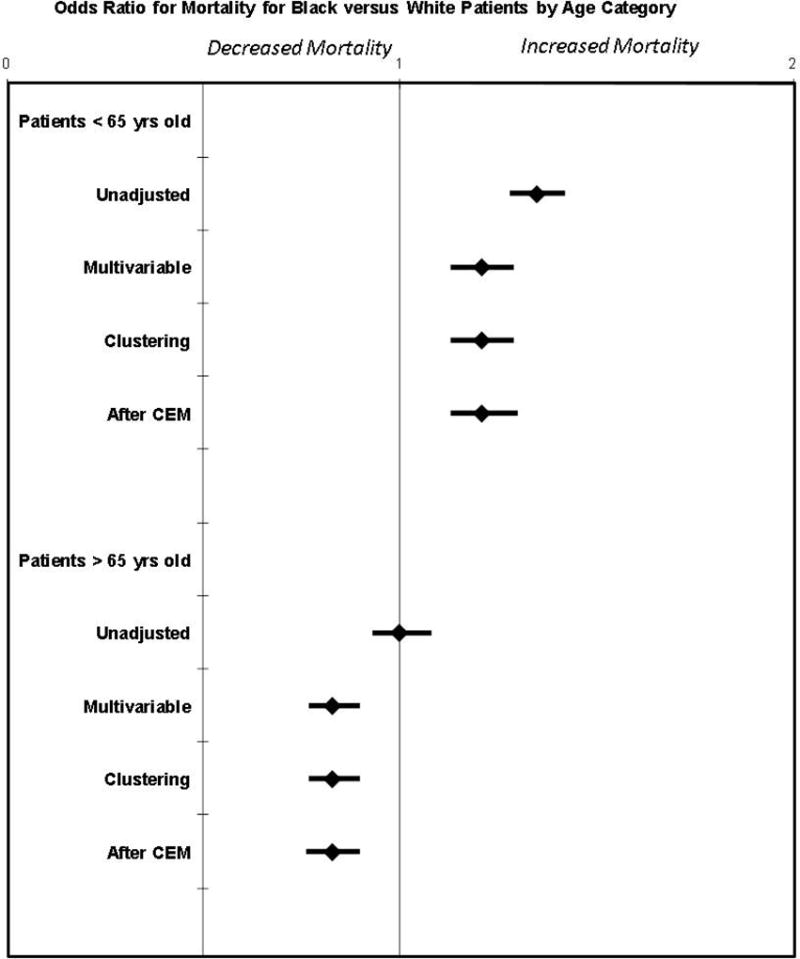
There was a higher unadjusted odds of death for Blacks versus Whites for patients < 65 years of age, but not for patients ≥ 65 years of age. After coarsened exact matching (CEM), racial disparities in survival persisted in the younger Black age group, but were reversed in the older age group. These findings were consistent across three different analysis techniques.
Discussion
In this study that risk-adjusts for both patient-specific co-morbidity data and injury severity information, we found that differential racial disparities exist between White patients and Black patients depending on their age group. For patients under the age of 65, White patients have better outcomes after trauma than Black patients. However, among older patients, Whites have a higher odds of death than similarly injured, matched Black patients. Based on these data, it appears that different racial disparities exist between white patients and Black patients depending on their age group.
The paradox of the racial disparity findings that we report was initially surprising. However, previous literature reporting outcomes associated with race and age have reported similarly paradoxical findings in non-trauma populations. In an analysis of over 1 million dialysis patients black patients were found to have a lower risk of death than white patients, but only in older adults; for patients less than 50 years of age, black patients actually had a higher incidence of mortality than white patients29. One commonly posited reason for these age-dependent racial disparities is the availability of Medicare, and hence better access to pre-stressor care, in the older population. Insurance status has been independently associated with better outcomes after trauma in younger patients8 and children1. Similar disparities are known to exist in cancer and medical patient populations as well30, 31. It is possible that, for older trauma patients who have access to Medicare, the differences in outcomes as attributable to insurance status are minimized and even reversed as previously uninsured patients establish routine health care. This phenomenon has been recently reported in a study of 541,471 trauma patients from the NTDB by Singer et al.32; the authors demonstrated that older trauma patients are four times more likely to be insured than young patients, and that insurance- and race-related disparities in mortality after blunt trauma are reduced in the age ≥65 years population. That study’s findings are limited by its inclusion of blunt trauma patients only, as well as its inability to account for the potential confounding effects of medical comorbidities. Nonetheless, the findings are consistent with those previously reported in other fields, including the Veterans Affairs system where racial disparities after surgical procedures that are well described in the general population are not present in a population with ubiquitous insurance coverage33. Thus improved access to health care may lead to better overall health status and a reduction in race-based disparities for patients of all ages.
It is also possible that the differences in trauma outcomes with respect to race are different in younger compared to older patients partially because there is reduction in treatment biases. Among patients less than 65 years of age, adjusted post-traumatic mortality has been demonstrated to be worse for patients treated at hospitals with higher proportions of minority patients34. Although the explanation for this finding is unclear, it may be a reflection of unconscious provider bias. In a study of 287 providers examining the effects of unconscious racial biases on clinical decision-making, physicians had significantly greater unconscious preferences for White patients over Black patients, and by extension a greater likelihood of recommending certain interventions for the former, despite reporting no differences in conscious racial biases35. However, reported perceptions of racial biases within the healthcare system are much greater among patients who are less than 65 years of age36. In addition, the mortality effect of discrimination is actually more pronounced in White patients compared to Black patients in an older population37. It is hypothesized that, because discrimination is more normative throughout their lives, older black patients may have developed coping and other adaptive strategies that enable them to withstand the effects of discrimination38. Therefore it is possible that treatment biases against Black patients compared to White patients may have less of an overall impact on mortality in the older population.
An additional mechanism that may contribute to the different racial disparities that we report in younger versus older patients could be the presence of a “healthy survivor bias”. There are well-documented disparities in access to care for younger Black patients39. Therefore it is possible that Black patients who make it to age 65 potentially have gotten there using minimal health care or without the benefit of care, and thus are less frail than their White counterparts of similar age. This theory is somewhat counterintuitive, in that it appears to refute the concept of the Weathering Hypothesis proposed by Geronimus et al. that states that because Black patients tend to be exposed to a greater allostatic load with repeated stressors and required adaptation, they have tendency for earlier health deterioration40. However, it is possible that the Weathering Hypothesis is either not applicable to trauma, which usually occurs as a single, isolated event rather than a series of stressors; or that by the time patients reach 65 years of age, those with the greatest allostatic loads have already succumbed to the stresses of life, leaving behind only the heartiest of the original population. One potential way to assess the latter hypothesis would be compare outcomes after trauma in older patients matched by age with respect to life expectancy, since the life expectancy of US Blacks is nearly 5 years less than that of US Whites41. There are also various measures of frailty that have been developed in non-trauma populations that could potentially be useful for evaluating our observed outcomes in this context42, 43.
Of note, the differences in outcomes observed between Black patients and White patients in the older trauma population were only demonstrable after patient co-morbidities and other covariates were accounted for; on univariate analysis, there were no reportable differences in mortality within the older patients. Previously published research on the subject of racial disparities after trauma are limited by their lack of co-morbidity information1, 5–8, 32. As demonstrated by the low prevalence of co-morbidities in the younger population in our study (only 20% of patients under age 65 had CCI scores greater than 0), the inclusion of this variable may not be important in these studies; young Black patients had higher odds of mortality compared to White patients in both our unadjusted and adjusted analyses (Figure 2). However, co-morbidity information appears to be much more relevant in the analysis of outcomes for the older age group; more than 50% of patients ≥ 65 years have at least one co-morbidity in our study, and more than 25% have more than one. Whether older patients experience mortality after trauma specifically as a result of their traumatic injuries, their co-morbidities, or a combination of the two remains to be determined, but clearly co-morbidity information is an important consideration in the interpretation of outcomes within an older population.
To this end, we also demonstrate the feasibility of using the NIS database – which includes co-morbidity information - for trauma-based analyses by extracting injury severity measures using the ICDPIC program15. Three different well-known statistical analysis techniques, including multivariable logistic regression, multivariable logistic regression with clustering for hospital effects, and coarsened exact matching produced consistent, reproducible study results. This study incorporates both trauma severity information and patient co-morbidity data into a single analysis, and we hope that our methods and statistical techniques will provide the basis for more extensive investigations involving the use of previously underutilized data in administrative datasets pertaining to trauma.
The limitations of our study deserve discussion. The basis of our data is the NIS, which is an administrative database. Although the NIS is a well-respected national database, as previously mentioned its use in trauma is infrequent because of a lack of traumatic injury scoring. Clark et al. developed the ICDPIC program15 that enabled us to overcome this shortcoming, and which has been previously validated to perform just as well as ISS scores at predicting mortality16. However, as with any administrative database, there is the potential for incorrect coding, and a number of assumptions must be made regarding the accuracy and reliability of the data. In addition, all retrospective analyses are limited by data availability, although we minimized the amount of missing data in our study by excluding patients from states that do not reliably report race data. It is also always possible that we did not consider an important variable in our analysis that may better explain the racial differences in mortality after trauma that we report. We chose the covariates for our regression modeling (age, gender, insurance status, blunt vs. penetrating injury, injury severity, head injury severity, and CCI) based on model parsimony and their ubiquitous use in previous outcomes-based trauma studies. In addition, the L1 distance we report after CEM was excellent, indicating effective matching of the Black and White patient groups. However, one could argue that among older trauma patients, other factors such as hospital length-of-stay, pre-retirement income bracket, etc. may also be important. In addition, we assessed the effect of race on in-hospital mortality after trauma, which in the older population may not actually translate to trauma-related death. Finally, we restricted our analysis to White and Black patients only. We chose to exclude Hispanic patients and other minority populations because there are lower numbers in the older age group and a tendency for more heterogeneity in patients within these populations that can lead to disparate findings depending on the outcomes studied9. Future studies addressing the effects of age on racial disparities in outcomes after trauma in Hispanic, Asian, and other minority groups will be of interest.
In conclusion, the results of the present study suggest that different racial disparities exist between White patients and Black patients depending on their age group. We also demonstrate the feasibility of using the NIS database for trauma-based analyses by extracting injury severity measures using the ICDPIC and STATA CCI programs. Further exploration of the racial disparities within different populations, including analysis of the effect of insurance status on outcomes in the ≥ 65 years population, may help us better understand mechanisms that lead to disparities in trauma outcomes. In addition, future studies incorporating the use of frailty indexes, surrogate measures of morbidity (i.e. hospital length-of-stay), and trauma-specific mortality will further elucidate the true effects of race on outcomes after trauma in the older population. The ICDPIC program may assist in this endeavor by allowing for comparisons between national databases such as the NIS and the National Trauma Data Bank.
Acknowledgments
Source of Funding:
The authors warrant that the article is original, does not infringe upon any copyright or other proprietary right of any third party, is not under consideration by another journal, and has not been published previously.
Financial support for this work was provided by: Financial support for this work was provided by: National Institutes of Health/NIGMS K23GM093112-01 and American College of Surgeons C. James Carrico Fellowship for the study of Trauma and Critical Care (Dr. Haider).
Footnotes
Presented at the 2012 American College of Surgeons Maryland Committee on Trauma Resident Trauma Papers Competition in Baltimore, MD (Oct. 26, 2012) and the 2012 American College of Surgeons Region III Committee on Trauma Resident Trauma Papers Competition in Newark, DE (Dec. 1, 2012).
Conflicts of Interest:
The authors have no conflicts of interest to disclose.
References
- 1.Hakmeh W, Barker J, Szpunar SM, Fox JM, Irvin CB. Effect of race and insurance on outcome of pediatric trauma. Acad Emerg Med. 2010;17(8):809–812. doi: 10.1111/j.1553-2712.2010.00819.x. [DOI] [PubMed] [Google Scholar]
- 2.Haskins AE, Clark DE, Travis LL. Racial disparities in survival among injured drivers. Am J Epidemiol. 2013;177(5):380–387. doi: 10.1093/aje/kws242. 10.1093/aje/kws242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris AR, Fisher GA, Thomas SH. Homicide as a medical outcome: Racial disparity in deaths from assault in US level I and II trauma centers. J Trauma Acute Care Surg. 2012;72(3):773–782. doi: 10.1097/TA.0b013e318226eb39. 10.1097/TA.0b013e318226eb39. [DOI] [PubMed] [Google Scholar]
- 4.Schoenfeld AJ, Belmont PJ, Jr, See AA, Bader JO, Bono CM. Patient demographics, insurance status, race, and ethnicity as predictors of morbidity and mortality after spine trauma: A study using the national trauma data bank. Spine J. 2013 doi: 10.1016/j.spinee.2013.03.024. 10.1016/j.spinee.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 5.Maybury RS, Bolorunduro OB, Villegas C, et al. Pedestrians struck by motor vehicles further worsen race- and insurance-based disparities in trauma outcomes: The case for inner-city pedestrian injury prevention programs. Surgery. 2010;148(2):202–208. doi: 10.1016/j.surg.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Crompton JG, Pollack KM, Oyetunji T, et al. Racial disparities in motorcycle-related mortality: An analysis of the national trauma data bank. Am J Surg. 2010;200(2):191–196. doi: 10.1016/j.amjsurg.2009.07.047. [DOI] [PubMed] [Google Scholar]
- 7.Haider AH, Efron DT, Haut ER, DiRusso SM, Sullivan T, Cornwell EE., 3rd Black children experience worse clinical and functional outcomes after traumatic brain injury: An analysis of the national pediatric trauma registry. J Trauma. 2007;62(5):1259–62. doi: 10.1097/TA.0b013e31803c760e. discussion 1262-3. [DOI] [PubMed] [Google Scholar]
- 8.Haider AH, Chang DC, Efron DT, Haut ER, Crandall M, Cornwell EE., 3rd Race and insurance status as risk factors for trauma mortality. Arch Surg. 2008;143(10):945–949. doi: 10.1001/archsurg.143.10.945. [DOI] [PubMed] [Google Scholar]
- 9.Haider AH, Scott VK, Rehman KA, et al. Racial disparities in surgical care and outcomes in the united states: A comprehensive review of patient, provider, and systemic factors. J Am Coll Surg. 2013;216(3):482–92.e12. doi: 10.1016/j.jamcollsurg.2012.11.014. 10.1016/j.jamcollsurg.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haider AH, Weygandt PL, Bentley JM, et al. Disparities in trauma care and outcomes in the united states: A systematic review and meta-analysis. J Trauma Acute Care Surg. 2013;74(5):1195–1205. doi: 10.1097/TA.0b013e31828c331d. 10.1097/TA.0b013e31828c331d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niven DJ, Kirkpatrick AW, Ball CG, Laupland KB. Effect of comorbid illness on the long-term outcome of adults suffering major traumatic injury: A population-based cohort study. Am J Surg. 2012;204(2):151–156. doi: 10.1016/j.amjsurg.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Arthur M, Hedges JR, Newgard CD, Diggs BS, Mullins RJ. Racial disparities in mortality among adults hospitalized after injury. Med Care. 2008;46(2):192–199. doi: 10.1097/MLR.0b013e31815b9d8e. [DOI] [PubMed] [Google Scholar]
- 13.The National Trauma Data Standard of the NTDB. NTDS - national trauma data standard. [Accessed 06/05, 2013]; http://www.ntdsdictionary.org/
- 14.Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project (HCUP) Introduction to the HCUP nationwide inpatient sample (NIS) [Accessed 12/02/2012];2010 http://www.hcup-us.ahrq.gov/db/nation/nis/NIS_Introduction_2010.jsp.
- 15.Clark DE, Osler TM, Hahn DR. ICDPIC: Stata module to provide methods for translating international classification of diseases (ninth revision) diagnosis codes into standard injury categories and/or scores. [Accessed 2013, 04/27];2013 http://ideas.repec.org/c/boc/bocode/s457028.html#biblio.
- 16.Rutledge R, Hoyt DB, Eastman AB, et al. Comparison of the injury severity score and ICD-9 diagnosis codes as predictors of outcome in injury: Analysis of 44,032 patients. J Trauma. 1997;42(3):477–87. doi: 10.1097/00005373-199703000-00016. discussion 487-9. [DOI] [PubMed] [Google Scholar]
- 17.Glance LG, Osler TM, Mukamel DB, Meredith W, Wagner J, Dick AW. TMPM-ICD9: A trauma mortality prediction model based on ICD-9-CM codes. Ann Surg. 2009;249(6):1032–1039. doi: 10.1097/SLA.0b013e3181a38f28. [DOI] [PubMed] [Google Scholar]
- 18.Baker SP, O'Neill B, Haddon W, Jr, Long WB. The injury severity score: A method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14(3):187–196. [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC) Table II. external cause-of-injury mortality matrix based on ICD–9 external cause of injury codes. [Accessed 06/04, 2013]; http://www.cdc.gov/nchs/data/injury/icd9_external.pdf.
- 20.Stagg V. CHARLSON: Stata module to calculate charlson index of comorbidity. [Accessed 06/18, 2013]; http://ideas.repec.org/c/boc/bocode/s456719.html#author.
- 21.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 22.Haider AH, Saleem T, Leow JJ, et al. Influence of the national trauma data bank on the study of trauma outcomes: Is it time to set research best practices to further enhance its impact? J Am Coll Surg. 2012;214(5):756–768. doi: 10.1016/j.jamcollsurg.2011.12.013. 10.1016/j.jamcollsurg.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Localio AR, Berlin JA, Ten Have TR, Kimmel SE. Adjustments for center in multicenter studies: An overview. Ann Intern Med. 2001;135(2):112–123. doi: 10.7326/0003-4819-135-2-200107170-00012. [DOI] [PubMed] [Google Scholar]
- 24.Roudsari B, Field C, Caetano R. Clustered and missing data in the US national trauma data bank: Implications for analysis. Inj Prev. 2008;14(2):96–100. doi: 10.1136/ip.2007.017129. 10.1136/ip.2007.017129. [DOI] [PubMed] [Google Scholar]
- 25.Blackwell M, King G, Iacus S, Porro G. Cem: Coarsened exact matching in STATA. The Stata Journal. 2010;9(4):524–546. [Google Scholar]
- 26.Stevens GA, King G, Shibuya K. Deaths from heart failure: Using coarsened exact matching to correct cause-of-death statistics. Popul Health Metr. 2010;8:6. doi: 10.1186/1478-7954-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haider AH, David JS, Zafar SN, et al. Comparative effectiveness of inhospital trauma resuscitation at a french trauma center and matched patients treated in the united states. Ann Surg. 2013 doi: 10.1097/SLA.0b013e31828226b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iacus S, King G, Porro G. CEM: Software for coarsened exact matching. [Accessed 06/24, 2013]; http://gking.harvard.edu/files/cem.pdf.
- 29.Kucirka LM, Grams ME, Lessler J, et al. Association of race and age with survival among patients undergoing dialysis. JAMA. 2011;306(6):620–626. doi: 10.1001/jama.2011.1127. 10.1001/jama.2011.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roetzheim RG, Pal N, Tennant C, et al. Effects of health insurance and race on early detection of cancer. J Natl Cancer Inst. 1999;91(16):1409–1415. doi: 10.1093/jnci/91.16.1409. [DOI] [PubMed] [Google Scholar]
- 31.Harlan LC, Greene AL, Clegg LX, Mooney M, Stevens JL, Brown ML. Insurance status and the use of guideline therapy in the treatment of selected cancers. J Clin Oncol. 2005;23(36):9079–9088. doi: 10.1200/JCO.2004.00.1297. [DOI] [PubMed] [Google Scholar]
- 32.Singer MB, Liou DZ, Clond MA, et al. Insurance-and race-related disparities decrease in elderly trauma patients. J Trauma Acute Care Surg. 2013;74(1):312–316. doi: 10.1097/TA.0b013e31826fc899. 10.1097/TA.0b013e31826fc899. [DOI] [PubMed] [Google Scholar]
- 33.Hofmann LJ, Lee S, Waddell B, Davis KG. Effect of race on colon cancer treatment and outcomes in the department of defense healthcare system. Dis Colon Rectum. 2010;53(1):9–15. doi: 10.1007/DCR.0b013e3181bdcdb2. [DOI] [PubMed] [Google Scholar]
- 34.Haider AH, Ong'uti S, Efron DT, et al. Association between hospitals caring for a disproportionately high percentage of minority trauma patients and increased mortality: A nationwide analysis of 434 hospitals. Arch Surg. 2012;147(1):63–70. doi: 10.1001/archsurg.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Green AR, Carney DR, Pallin DJ, et al. Implicit bias among physicians and its prediction of thrombolysis decisions for black and white patients. J Gen Intern Med. 2007;22(9):1231–1238. doi: 10.1007/s11606-007-0258-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shavers VL, Fagan P, Jones D, et al. The state of research on racial/ethnic discrimination in the receipt of health care. Am J Public Health. 2012;102(5):953–966. doi: 10.2105/AJPH.2012.300773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barnes LL, de Leon CF, Lewis TT, Bienias JL, Wilson RS, Evans DA. Perceived discrimination and mortality in a population-based study of older adults. Am J Public Health. 2008;98(7):1241–1247. doi: 10.2105/AJPH.2007.114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lincoln KD, Chatters LM, Taylor RJ. Psychological distress among black and white americans: Differential effects of social support, negative interaction and personal control. J Health Soc Behav. 2003;44(3):390–407. [PMC free article] [PubMed] [Google Scholar]
- 39.Williams DR, Mohammed SA, Leavell J, Collins C. Race, socioeconomic status, and health: Complexities, ongoing challenges, and research opportunities. Ann N Y Acad Sci. 2010;1186:69–101. doi: 10.1111/j.1749-6632.2009.05339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geronimus AT. The weathering hypothesis and the health of african-american women and infants: Evidence and speculations. Ethn Dis. 1992;2(3):207–221. [PubMed] [Google Scholar]
- 41.Minino AM, Murphy SL, Xu J, Kochanek KD. Deaths: Final data for 2008. Natl Vital Stat Rep. 2011;59(10):1–126. [PubMed] [Google Scholar]
- 42.Jones DM, Song X, Rockwood K. Operationalizing a frailty index from a standardized comprehensive geriatric assessment. J Am Geriatr Soc. 2004;52(11):1929–1933. doi: 10.1111/j.1532-5415.2004.52521.x. [DOI] [PubMed] [Google Scholar]
- 43.Rubenstein LZ, Stuck AE, Siu AL, Wieland D. Impacts of geriatric evaluation and management programs on defined outcomes: Overview of the evidence. J Am Geriatr Soc. 1991;39(9 Pt 2):8S–16S. doi: 10.1111/j.1532-5415.1991.tb05927.x. discussion 17S–18S. [DOI] [PubMed] [Google Scholar]


