Abstract
Marijuana is one of the most abused drugs among pregnant women leading to maternal and fetal abnormalities. Cannabinoids are the active ingredients of marijuana, which interact with cannabinoid receptors such as CNR1 and CNR2 to activate cellular signaling pathways. Human endometrium and placenta are known to express CNR1 and CNR2 and can respond to cannabinoid signaling. In this study, we show that marijuana use significantly increases mRNA or protein expression of CNR1 and CNR2 in human endometrium from the first and early second trimester pregnancies, with minor effects on placental expression of CNRs.
Keywords: Marijuana, cannabinoid, cannabinoid receptor, placenta, uterine endometrium
Introduction
Marijuana is extracted from plants of Cannabis sp. [1]. Over the last decade, marijuana use by pregnant women has increased by ~65% in the US particularly among young women of low socio-economic status [2, 3]. Metadata analysis indicates that marijuana use prior to or during pregnancy could contribute to low fetal birth weight, preterm delivery, congenital malformation and neonatal complications [2, 4, 5].
Marijuana is a mixture of >60 plant cannabinoids (CBs) which are highly lipophilic with high protein binding capacity, and can accumulate in a variety of tissues and release slowly over extended periods [6, 7]. The endocannabinoids (eCBs), like N-arachidonoyl- ethanolamine and 2-arachidonoylglycerol, are active endogenous cannabinoid-like molecules generated via fatty acid metabolism in higher vertebrates [8, 9]. Both CBs and eCBs interact with cannabinoid receptors (CNRs) such as CNR1 and CNR2 to activate intracellular signaling cascades, which plays important roles in various physiological and pathophysiological processes, including cell death, inflammation and neuroprotection [10, 11].
The endocannabinoid system is expressed in reproductive organs and regulates diverse reproductive events [12, 13]. Higher endocannabinoid signaling can lead to early pregnancy failures [14]. There is very limited knowledge about how marijuana use either alone or in combination with confounding factors such as tobacco, alcohol and opioid abuse might influence CNR expression in reproductive tissues critical for a successful pregnancy, such as the uterine endometrium (decidua) and placenta. Since CNRs are necessary for cannabinoid signaling, in the current study, we evaluate CNR1 and CNR2 mRNA and protein expression in early gestation human endometrial and placental tissues obtained from non-users, marijuana users and tobacco/alcohol users.
Materials and Methods
See “Supplemental Materials”.
Results and Discussion
We categorized endometrial and placental tissues of early gestation into non-users, tobacco/alcohol users and marijuana users (Table S1) as described in “Supplemental Materials”. In some cases, such as subject 368, only placental tissue was obtained and analyzed. In other instances, we analyzed either mRNA or protein due to limited amounts of tissues available, and hence placental tissues from subjects 367, 372, 419, 422, 424 and endometrial sample from subject 405 were analyzed for mRNA expression only.
CNR1 and CNR2 could be readily detected in all endometrium and placenta samples analyzed; however, there were considerable inter-individual variations in mRNA (Fig. 1A and Fig. 2A) and protein (Fig. 1C, Fig. 1D and Fig. 2C, Fig. 2D) expression pattern. While the mRNA levels of CNR1 and CNR2 in the endometrium of marijuana users were significantly higher compared to those of non-users and tobacco/alcohol users combined (Fig. 1B), only the levels of CNR1 mRNA in tobacco/alcohol users were higher than those in non-users (Fig. 1B). In contrast, the protein levels of CNR2, but not of CNR1, in endometrium of marijuana users were significantly higher than those of non-users and tobacco/alcohol users combined (Fig. 1E). There were no significant differences in protein expression of CNR1 and CNR2 between nonusers and tobacco/alcohol users (Fig. 1E). We did not observe significant differences in the levels of mRNA or protein expression of both CNR1 and CNR2 in the placentas between nonusers and marijuana users or tobacco/alcohol users (Fig. 2B and Fig. 2E). The protein levels of CNR2 in the placentas of marijuana users were significantly lower than those of tobacco/alcohol users (Fig. 2E). Our data suggest that marijuana use during early gestation increases mRNA and protein expression of CNR1 or CNR2 in the endometrium, with little effects on CNR expression in the placenta.
Figure 1. Endometrial expression of CNRs.
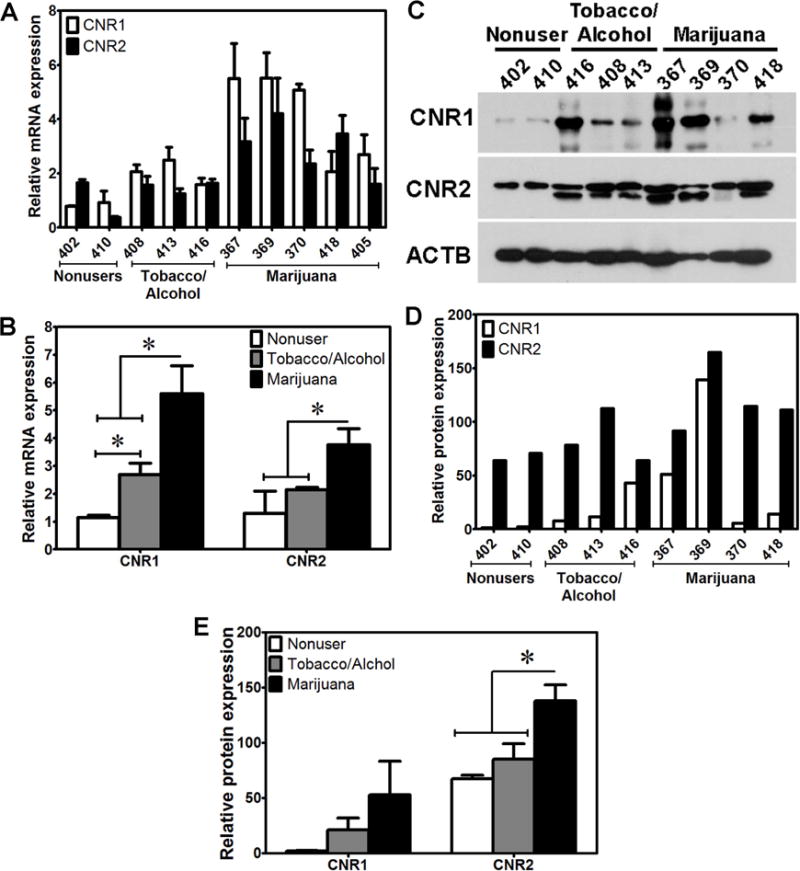
A) The relative levels of mRNA expression of CNR1 and CNR2 in endometrium of individual donors. B) The relative levels of mRNA expression of CNR1 and CNR2 in endometrium among the three groups of non-users, tobacco/alcohol users and marijuana users. C) Western blot detecting CNR1 and CNR2 protein expression in endometrium of individual donors. D) The relative levels of protein expression of CNR1 and CNR2 in endometrium of individual donors. E) The relative levels of protein expression of CNR1 and CNR2 in endometrium among the three groups of non-users, tobacco/alcohol users and marijuana users. *indicates significant differences (p < 0.05) in mRNA or protein levels of CNR1 or CNR2 between marijuana users and marijuana non-users.
Figure 2. Placental expression of CNRs.
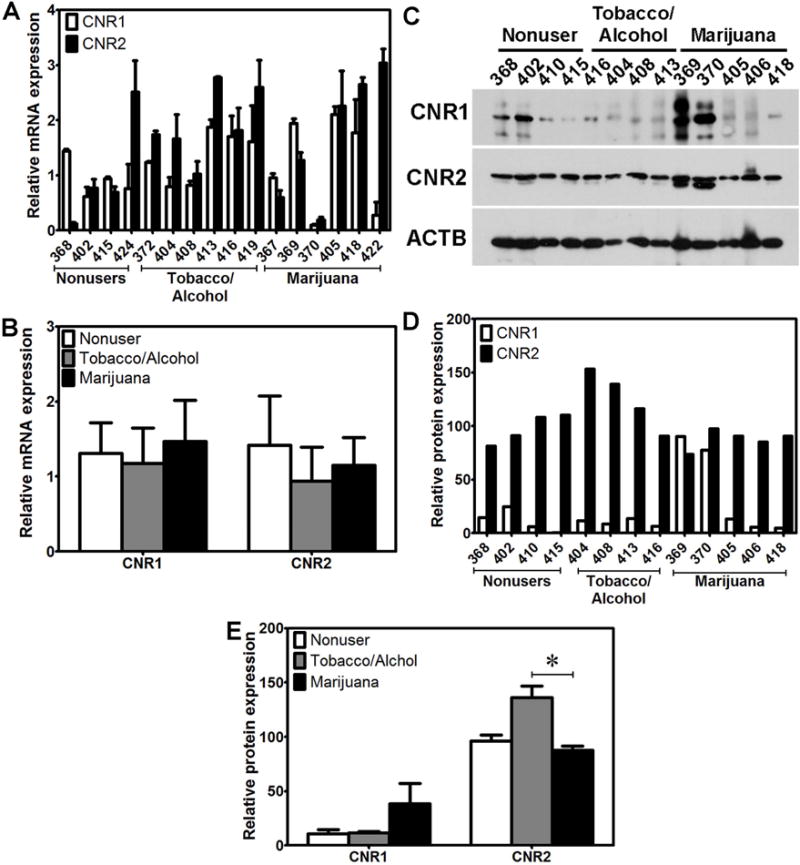
A) The relative levels of mRNA expression of CNR1 and CNR2 in placentas of individual donors. B) The relative levels of mRNA expression of CNR1 and CNR2 in placentas among the three groups of non-users, tobacco/alcohol users and marijuana users. C) Western blot detecting CNR1 and CNR2 protein expression in placentas of individual donors. D) The relative levels of protein expression of CNR1 and CNR2 in placentas of individual donors. E) The relative levels of protein expression of CNR1 and CNR2 in placentas among the three groups of non-users, tobacco/alcohol users and marijuana users. *indicates significant differences (p < 0.05) in protein levels of CNR2 between marijuana users and only tobacco/alcohol users.
Successful pregnancy requires fruitful reciprocal interactions between a competent embryo and a receptive endometrium, and interference in this process can compromise pregnancy [15]. In this study, we confirmed previous reports that CNR1 and CNR2 were detectable at mRNA and/or protein levels in human endometrium/decidua [16] and placenta [17]. This study is the first to evaluate decidual CNR expression in early pregnancy and demonstrates the potential impact of marijuana use by pregnant women during early gestation on CNR expression in the endometrium and placenta. We showed that, while tobacco/alcohol use generally did not seem to alter CNR expression, marijuana use significantly increased mRNA or protein expression of CNR1 and CNR2 in the endometrium, suggesting possible autoinduction/autoregulation of CNR expression. Optimal cannabinoid signaling is critical for pregnancy and parturition, and sustained CNR signaling may compromise endometrial function and pregnancy [18]. Our finding suggests that marijuana use could affect decidual cannabinoid signaling potentially by altering CNR expression, thus negatively affecting pregnancy. On the other hand, this study suggests that, unlike in the endometrium, the system that is responsible for autoinduction/autoregulation of CNR expression potentially does not exist in the placenta.
We recognize the limitations of this study. While the mean values of the CNR1 protein levels in the endometrium or placenta of marijuana users were 3 – 5-fold higher than those of non-users and/or tobacco/alcohol users (Fig. 1E and Fig. 2E), the differences were not statistically significant. Hence, the effects of marijuana use on CNR1 protein expression in these tissues could possibly have gone unnoticed with the small sample size of this study. Further, categorization was done on self-reporting of marijuana use with no information about dosage and frequency, which may not truly reflect the levels of CB exposure in these tissues. Finally, race, genetic makeup, capacity of metabolism and elimination could also affect CNR expression and these factors were not considered in this study. Further studies with larger sample sizes and more detailed CB exposure and genetic information should be done to confirm our findings and generate a comprehensive understanding.
Supplementary Material
Expression of cannabinoid receptors in early trimester endometrium
Expression of cannabinoid receptors in early trimester placenta
Effects of marijuana use in early pregnancy on cannabinoid receptor expression
Acknowledgments
Funding: This work was supported in part by the National Institutes of Health [grant number DA032507] to QM, and a pilot grant from the University of Washington National Institute on Drug Abuse sponsored Alcohol and Drug Abuse Institute to NKN.
Abbreviations
- CNR1
cannabinoid receptor 1
- CNR2
cannabinoid receptor 2
- CBs
cannabinoids
- eCBs
endocannabinoids
- ACTB
β-actin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: The authors have no conflict of interest.
References
- 1.Striegel H, Rossner D, Simon P, Niess AM. The World Anti-Doping Code 2003–consequences for physicians associated with elite athletes. International journal of sports medicine. 2005;26(3):238–43. doi: 10.1055/s-2004-830545. [DOI] [PubMed] [Google Scholar]
- 2.Gynecologists ACoOa. Marijuana use during pregnancy and lactation. Obstet Gynecol: American College of Obstetricians and Gynecologists 2015:234–8. [Google Scholar]
- 3.Volkow ND, Compton WM, Wargo EM. The risks of marijuana use during pregnancy. JAMA. 2017;317(2):129–30. doi: 10.1001/jama.2016.18612. [DOI] [PubMed] [Google Scholar]
- 4.Hayatbakhsh MR, Flenady VJ, Gibbons KS, Kingsbury AM, Hurrion E, Mamun AA, Najman JM. Birth outcomes associated with cannabis use before and during pregnancy. Pediatr Res. 2012;71(2):215–9. doi: 10.1038/pr.2011.25. [DOI] [PubMed] [Google Scholar]
- 5.Gunn JK, Rosales CB, Center KE, Nunez A, Gibson SJ, Christ C, Ehiri JE. Prenatal exposure to cannabis and maternal and child health outcomes: a systematic review and meta-analysis. BMJ open. 2016;6(4):e009986. doi: 10.1136/bmjopen-2015-009986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huestis MA, Henningfield JE, Cone EJ. Blood cannabinoids. II. Models for the prediction of time of marijuana exposure from plasma concentrations of delta 9-tetrahydrocannabinol (THC) and 11-nor-9-carboxy-delta 9-tetrahydrocannabinol (THCCOOH) Journal of analytical toxicology. 1992;16(5):283–90. doi: 10.1093/jat/16.5.283. [DOI] [PubMed] [Google Scholar]
- 7.Hunault CC, Mensinga TT, de Vries I, Kelholt-Dijkman HH, Hoek J, Kruidenier M, Leenders ME, Meulenbelt J. Delta-9-tetrahydrocannabinol (THC) serum concentrations and pharmacological effects in males after smoking a combination of tobacco and cannabis containing up to 69 mg THC. Psychopharmacology. 2008;201(2):171–81. doi: 10.1007/s00213-008-1260-2. [DOI] [PubMed] [Google Scholar]
- 8.Di Marzo V, De Petrocellis L, Bisogno T, Maurelli S. The endogenous cannabimimetic eicosanoid, anandamide, induces arachidonate release in J774 mouse macrophages. Advances in experimental medicine and biology. 1997;407:341–6. doi: 10.1007/978-1-4899-1813-0_51. [DOI] [PubMed] [Google Scholar]
- 9.Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372(6507):686–91. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- 10.Salazar M, Carracedo A, Salanueva ÍJ, Hernández-Tiedra S, Lorente M, Egia A, Vázquez P, Blázquez C, Torres S, García S, Nowak J, Fimia GM, Piacentini M, Cecconi F, Pandolfi PP, González-Feria L, Iovanna JL, Guzmán M, Boya P, Velasco G. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. The Journal of Clinical Investigation. 2009;119(5):1359–72. doi: 10.1172/JCI37948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun Y, Alexander SP, Garle MJ, Gibson CL, Hewitt K, Murphy SP, Kendall DA, Bennett AJ. Cannabinoid activation of PPAR alpha; a novel neuroprotective mechanism. British journal of pharmacology. 2007;152(5):734–43. doi: 10.1038/sj.bjp.0707478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park B, McPartland JM, Glass M. Cannabis, cannabinoids and reproduction. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2004;70(2):189–97. doi: 10.1016/j.plefa.2003.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Costa MA. The endocannabinoid system: A novel player in human placentation. Reproductive Toxicology. 2016;61:58–67. doi: 10.1016/j.reprotox.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Taylor AH, Finney M, Lam PM, Konje JC. Modulation of the endocannabinoid system in viable and non-viable first trimester pregnancies by pregnancy-related hormones. Reprod Biol Endocrinol. 2011;9 doi: 10.1186/1477-7827-9-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teklenburg G, Salker M, Molokhia M, Lavery S, Trew G, Aojanepong T, Mardon HJ, Lokugamage AU, Rai R, Landles C, Roelen BAJ, Quenby S, Kuijk EW, Kavelaars A, Heijnen CJ, Regan L, Brosens JJ, Macklon NS. Natural Selection of Human Embryos: Decidualizing Endometrial Stromal Cells Serve as Sensors of Embryo Quality upon Implantation. PloS one. 2010;5(4):e10258. doi: 10.1371/journal.pone.0010258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Habayeb OM, Taylor AH, Bell SC, Taylor DJ, Konje JC. Expression of the endocannabinoid system in human first trimester placenta and its role in trophoblast proliferation. Endocrinology. 2008;149 doi: 10.1210/en.2007-1799. [DOI] [PubMed] [Google Scholar]
- 17.Taylor AH, Abbas MS, Habiba MA, Konje JC. Histomorphometric evaluation of cannabinoid receptor and anandamide modulating enzyme expression in the human endometrium through the menstrual cycle. Histochemistry and cell biology. 2010;133(5):557–65. doi: 10.1007/s00418-010-0695-9. [DOI] [PubMed] [Google Scholar]
- 18.Sun X, Deng W, Li Y, Tang S, Leishman E, Bradshaw HB, Dey SK. Sustained Endocannabinoid Signaling Compromises Decidual Function and Promotes Inflammation-induced Preterm Birth. The Journal of Biological Chemistry. 2016;291(15):8231–40. doi: 10.1074/jbc.M115.707836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


