Abstract
Objective
To test the effects of doxazosin, an α1 antagonist, on cognitive functioning during tobacco withdrawal in smokers.
Methods
Participants (n = 35) were randomly assigned to receive placebo, 4 mg/day, or 8 mg/day doxazosin. They completed a continuous performance task (CPT) and self-reported their withdrawal symptoms at baseline and twice following a medication titration period: once in a tobacco deprived state and again in a non-deprived state. Ability to resist smoking was assessed using a laboratory smoking lapse paradigm.
Results
Participants showed poorer cognitive performance on most measures taken from the CPT when tobacco deprived. Eight mg/day doxazosin improved inhibitory control during the non-deprivation session but did not affect sustained attention or reaction time. Participants receiving doxazosin reported fewer withdrawal symptoms during deprivation than those on placebo. Those showing the greatest improvement of inhibitory control under doxazosin were better able to resist smoking (i.e., latency to smoke) during a smoking lapse task. Self-reported withdrawal symptoms also were negatively associated with time to smoking.
Conclusions
Doxazosin reduced symptoms of tobacco withdrawal according to self-report and cognitive assessment and improved inhibitory control above pre-drug levels. This research identifies potential mechanisms by which doxazosin might improve smoking outcomes.
Keywords: doxazosin, α1 antagonist, tobacco withdrawal, inhibitory control, smoking cessation
1. Introduction
Noradrenergic pathways in the prefrontal cortex play a critical role in supporting higher-order cognitive functions (Berridge & Spencer, 2016). There are three classes of noradrenergic receptors, including α1, α2, and β receptors. A sizeable body of work has explored the effects of drugs targeting α2 and β on cognitive performance in humans (Greenblatt, Scavone, Harmatz, Engelhardt, & Shader, 1993; Jakala et al., 1999); however, less is known about potential cognitive effects of drugs that target α1 receptors. Preclinical studies indicate that α1 receptors are selectively engaged when high concentrations of norepinephrine are released (e.g., stress, withdrawal), and activation of α1 receptors impairs cognitive performance (Ramos & Arnsten, 2007). Administration of an α1 antagonist blocked any disruptive effects of stress on cognitive performance in rats, suggesting that therapeutic action at α1 may be beneficial to cognitive functioning (Birnbaum, Gobeske, Auerbach, Taylor, & Arnsten, 1999). Other preclinical studies have examined which aspects of cognitive performance are affected by α1 signaling, finding that activation of α1 receptors is associated with impairment of some cognitive functions (e.g., working memory) but may improve others (e.g., sustained attention; Berridge & Spencer, 2016).
These preclinical findings highlight a complex role for α1 receptors in cognition, and less is known about the cognitive effects of drugs targeting α1 receptors in humans. One study found that prazosin, a short duration α1 antagonist, can improve cognitive performance in healthy human participants (Winder-Rhodes et al., 2010). Such cognitive enhancing effects of α1 antagonists may explain why these drugs can reduce rates of substance use. Indeed, several clinical trials along these lines have already been conducted for substance use disorders. Doxazosin was effective at reducing cocaine use in those with cocaine use disorder (Shorter, Lindsay, & Kosten, 2013), and prazosin reduced both cue-induced craving and alcohol consumption among groups of patients with alcohol use disorder (Fox et al., 2012).
That α1 antagonists can improve cognitive functioning suggests that these drugs may have efficacy as smoking cessation aids. Current FDA-approved medications aid smokers attempting to quit by reducing withdrawal symptoms and blocking tobacco-related reinforcement (Ashare & Schmidt, 2014). Even with these smoking cessation medications, the majority of smokers attempting to quit are unsuccessful in the long term (Fiore et al., 2008), highlighting the importance of developing more effective treatments for smoking. Recent work has identified withdrawal-related cognitive deficits as a pharmacological target for tobacco use disorder (Ashare & Schmidt, 2014). Smokers attempting to quit who show cognitive impairment during withdrawal are at increased risk for relapse (Patterson et al., 2010), likely because they must rely on executive cognitive functions to maintain abstinence as they experience cravings and other symptoms of withdrawal.
This manuscript reports data taken from a larger preliminary human laboratory study (Verplaetse et al., 2017) screening doxazosin (placebo; 4 mg/day; 8 mg/day) as a treatment for tobacco use disorder in a group of non-treatment seeking daily smokers. The current study was the first to examine the effects of an α1 antagonist on cognitive performance during tobacco withdrawal in humans. A continuous performance task (CPT; Conners, 2000) was used to measure different aspects of cognitive performance, including inhibitory control (commission errors), sustained attention (reaction time variability), and reaction time (RT). Participants completed the CPT during three separate sessions. First, they completed a medication-free baseline assessment. Following a medication titration period, they completed the CPT and self-reported symptoms of tobacco withdrawal during separate tobacco deprived and non-tobacco deprived laboratory sessions. During the deprivation session, participants also completed a smoking lapse task to measure their ability to resist the urge to smoke following a period of tobacco deprivation (McKee, 2009).
For the present set of analyses, we hypothesized that doxazosin would improve performance on the CPT as evidenced by improvement from baseline to the non-deprived session. We predicted that participants would perform worse on all measures on the CPT during tobacco deprivation compared to their baseline assessment. However, we hypothesized that participants receiving doxazosin would show less impairment when tobacco deprived than those receiving placebo. We also predicted that participants receiving doxazosin would report fewer withdrawal symptoms during tobacco deprivation than those on placebo. We hypothesized that individual differences in doxazosin effects on cognitive functioning and withdrawal would be associated with smoking behavior on the smoking lapse task. Finally, we hypothesized that self-reported withdrawal symptoms would be associated with cognitive impairment during deprivation. This prediction was based on research suggesting that cognitive impairment is a core symptom of tobacco withdrawal that should be expected to covary with the intensity of the broader withdrawal syndrome (Ashare, Falcone, & Lerman, 2014).
2. Method
2. 1 Participants
Eligible participants were non-treatment seeking adult smokers (18–60 years old) who smoked ≥ 10 cigarettes per day for the past year, had baseline carbon monoxide (CO) levels ≥ 10 ppm, had urine cotinine levels ≥ than 150 ng/ml, and were normotensive with normal EKGs. Participants were excluded for medical conditions that contraindicated smoking or doxazosin use, other DSM-IV Axis I diagnosis except alcohol abuse or tobacco dependence, and illicit drug use except occasional marijuana use. Thirty-five individuals (4 mg/day doxazosin, n = 11; 8 mg/day doxazosin, n = 13; placebo, n = 11) enrolled in and completed the study. As seen in Table 1, groups were well-matched on most baseline variables, although there were significant group differences in FTND scores (Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991). The study was approved by the Yale Human Investigations Committee. All participants provided written informed consent and were compensated for participation. Additional participant details are reported in the parent publication (Verplaetse et al., 2017).
Table 1.
Baseline characteristics by medication condition
| Placebo (n = 11) | 4 mg/day doxazosin (n = 11) | 8 mg/day doxazosin (n = 13) | |
|---|---|---|---|
| Age | 37.36 (9.29) | 34.82 (10.85) | 34.23 (9.83) |
| Gender (male) | 8 (72.7%) | 7 (63.6%) | 9 (69.2%) |
| Race (white) | 6 (54.5%) | 6 (54.5%) | 4 (30.8%) |
| Education (≥ high school | 9 (81.8%) | 4 (36.4%) | 9 (69.2%) |
| Cigarettes per day | 18.55 (6.77) | 13.00 (6.07) | 16.23 (12.52) |
| FTND* | 6.00 (2.10) | 3.36 (2.20) | 4.85 (1.82) |
| Carbon monoxide (ppm) | 32.09 (29.41) | 29.36 (22.62) | 32.46 (30.15) |
FTND is the Fagerstrom Test of Nicotine Dependence. Values report are means (standard deviations) or count (percentage%).
One way ANOVAs found that all baseline comparisons across medication conditions were not significant (p > 0.05), except FTND (*p = 0.02).
2.2 Study Design
This experiment used a double-blind and placebo-controlled design to examine the effects of doxazosin on cognitive functioning and tobacco withdrawal. Doxazosin was administered once daily and titrated to steady-state levels over 18 days (for 4 mg/day doxazosin: 1 mg/daily for Days 1–4, 2 mg daily for days 5–9, 4 mg daily on and after Day 10; for 8 mg/day doxazosin 1 mg/daily for Days 1–4, 2 mg daily for Days 5–9, 5 mg daily for Days 10–13, 6 mg daily for Days 14–17, 8 mg daily on and after Day 18). Placebos were matched in appearance and were taken on the same schedule as the active medication. Medication compliance was monitored by pill counts and riboflavin marker. Following completion of the laboratory sessions, participants were tapered from medication over a four-day period. Participants attended a premedication baseline assessment session and two laboratory testing sessions, including a non-deprivation session and a deprivation session. Adverse event and other methodological details not pertinent to the current report are described in the parent publication (Verplaetse et al., 2017).
2.3 Material and Measures
2.3.1 Conners’ Continuous Performance Test (CPT)
The CPT is a computerized assessment tool that measures cognitive performance (Conners, 2000). Participants viewed a series of letters on a computer monitor for 14 minutes. They were instructed to respond as quickly as possible to target stimuli (all letters but “X”) and to refrain from responding to the infrequent non-target stimuli (“X”). Criterion variables were the percentage of non-target (X) trials that participants made a response (% commission errors), reaction time (RTgo), and reaction time variability (RTvar). Omission errors occurred infrequently (< 5% of trials) and are not reported.
2.3.2 Smoking lapse task
The smoking lapse task is a validated model of smoking relapse (McKee, Krishnan-Sarin, Shi, Mase, & O’Malley, 2006). Prior to completing the smoking lapse task, participants were exposed to neutral imagery using personalized guided-imagery in order to induce a neutral mood (McKee et al., 2011). This mood induction was included as part of a larger manipulation described in the parent study. The smoking lapse task consists of two phases. During the delay phase, participants were presented with a tray containing 8 cigarettes of their preferred brand, a lighter, and an ashtray. They were told that they could commence smoking at any point during the next 50 minutes; however, for each 5 minutes that they delayed smoking they would earn US $1 for a maximum of US $10 during the delay period. They were informed that the delay session would end after 1 hour regardless of whether they chose to smoke. The second phase was a free access period that started after participants decided to end the delay phase by smoking or waiting for 50 minutes. Participants were provided with 8 cigarettes of their preferred brand.
Criterion variables included smoking delay (i.e., time between the beginning of the delay phase and the participant choosing to smoke) smoking latency (i.e., time between the beginning of the free access smoking phase and the participant taking his or her first puff), and total cigarettes (i.e., number of cigarettes smoked during the free access phase).
2.3.3 Minnesota Nicotine Withdrawal Scale (MNWS; Hughes & Hatsukami, 1986)
The MNWS is an eight-item questionnaire used to assess current withdrawal symptoms. Participants rate the intensity of their current symptoms (e.g., anxiety, difficulty concentrating, craving for cigarettes) on a five-point scale ranging from 0 (none) to 4 (severe) with scores ranging from 0 to 32.
2.4 Procedure
2.4.1 Premedication baseline assessment
This baseline assessment session began at 9:00 am on day 1 and participants were instructed not to smoke after 10:00 pm on the previous night. Abstinence was confirmed with a carbon monoxide (CO) reading. Baseline assessments of breath CO and breath alcohol were conducted, a urine sample was collected for drug and pregnancy screen, and past-month self-reported smoking was assessed. Participants were given cigarette breaks as needed after biological specimens were collected. They completed the CPT within one hour of smoking to ensure that the baseline cognitive assessment was completed in a satiated state.
2.4.2 Laboratory sessions
Participants attended two laboratory sessions including a non-deprivation and tobacco deprived laboratory session.
2.4.2.1 Non-deprivation session
The non-deprivation session occurred between 17 and 21 days following the premedication baseline assessment. This session began between 9:00 am and 2:00 pm and required approximately 3 hours to complete. Although participants were instructed to remain abstinent for at least 11 hours preceding the session, they were allowed to take smoke breaks as needed after providing urine and blood samples. They completed the CPT within 1 hour of smoking a cigarette.
2.4.2.2 Deprivation session
Participants attended a second laboratory session where they completed the same procedures in a tobacco deprived state. This session occurred approximately 24 days (with allowance for variability based on participants’ schedules) following the premedication baseline assessment. Participants were instructed to abstain from smoking for at least 11 hours preceding the session, and they were not allowed to smoke until the free access period of the smoking lapse task. The session began at 8:00 am. Smoking abstinence was biochemically confirmed with carbon monoxide readings (less than 50% of their CO level at intake) (Kahler et al., 2012) and later with serum nicotine levels (less than 2 ng/ml). Participants completed the CPT at 9:15 am. They began the smoking delay period at 1:10 pm.
2.5 Statistical Analyses
Performance data from the CPT were analyzed using a 3 dose (placebo, 4 mg/day, 8 mg/day) X 3 session (baseline session, non-deprivation session, deprivation session) mixed-design analysis of variance (ANOVA). A 2 session (non-deprived versus deprived) X 3 (dose) mixed design analysis of covariance (ANCOVA) that included MNWS score during baseline assessment as a covariate was used to examine the effects of medication on withdrawal symptoms. FTND scores also were included as a covariate but did not change the pattern of results. Final models are presented without FTND scores included as a covariate.
Any significant interaction was probed using one tailed a priori t tests comparing each session to baseline within each dose. Because the study was preliminary, one tailed tests were used to reduce the risk of type II error. We expected that performance during the non-deprivation session would improve compared to baseline and performance during the deprivation session was expected to decline compared to baseline (comparison was between non-deprived and deprived laboratory sessions for MNWS scores). When these ANOVAs or ANCOVAs identified a significant main effect of session but no significant interaction, we collapsed across medication conditions and used a priori t-tests to compare performance during tobacco deprivation to baseline performance. Similar a priori t tests on MNWS scores were used to detect any increases in scores during the deprivation session relative to the non-deprivation session. Correlation analyses were used to test whether MNWS scores during the deprivation session were associated with changes in cognitive performance during that same session.
A second set of analyses tested whether any medication-related changes in withdrawal symptoms or cognitive functioning were associated with smoking behavior on the smoking lapse task. Medication effect scores were calculated as the difference in performance between the baseline and non-deprivation medication session and deprivation scores as the difference between the baseline session and deprivation session. We only calculated these scores on CPT variables that showed a significant session X medication interaction effect to reduce the number of comparisons being made. Correlational analyses determined whether medication-induced changes in cognitive functioning were associated with criterion variables from the smoking lapse task. For these analyses, we collapsed the 4 mg/day and 8 mg/day doxazosin groups and conducted correlational analyses separately for those receiving active medication and those receiving placebo. A similar set of analyses was conducted to test the relation between withdrawal symptoms during deprivation and smoking behavior. Withdrawal was defined as the difference between MNWS scores at baseline and during the deprivation session.
3. Results
3.1 CPT Performance
3.1.1 Commission errors
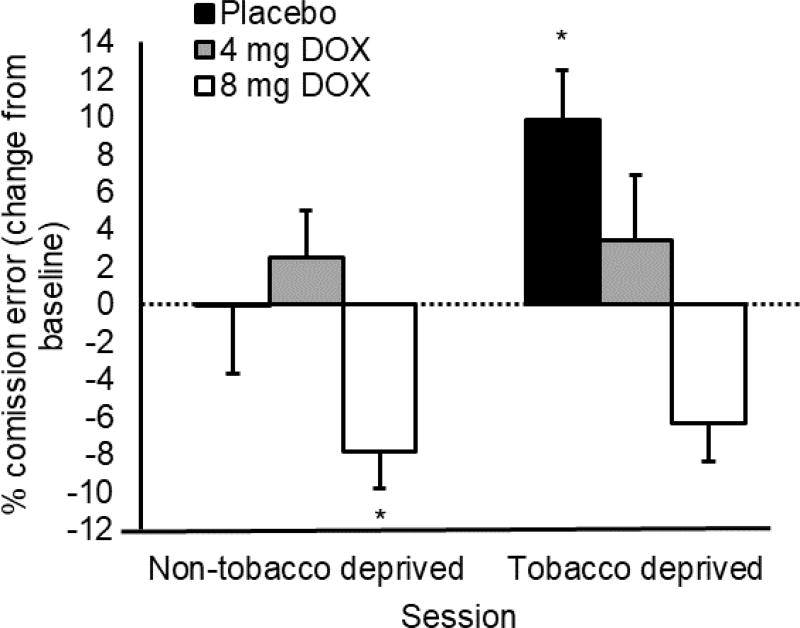
Percentage of commission errors are charted in Figure 1. The ANOVA found no main effect of medication, F (1, 32) = 0.76, p = 0.48, or session, F (2, 64) = 0.55, p = 0.46. There was, however, a significant medication X session interaction, F (4, 64) = 3.08, p = 0.02. Only the 8 mg/day group decreased errors from the pre-medication baseline to the non-tobacco deprived assessment, t (12) = 2.00, p = 0.04, whereas the placebo and 4mg group demonstrated no significant change, ps ≥ 0.29. When comparing the pre-medication baseline to the tobacco deprived assessment, the placebo group made more commission errors, t (10) = 2.69, p = 0.01, but neither the 4 mg/day group, t (10) = 0.65, p = 0.26, nor the 8 mg/day group, t (12) = −2.10, p = 0.97, showed a similar increase.
Figure 1.
Effect of doxazosin and tobacco deprivation on commission errors on the CPT. Capped vertical bars represent SEM. Broken line crossing Y axis at 0 represents pre-medication baseline performance. Non-tobacco deprived sessions were conducted within 1 hour of smoking. Tobacco deprived sessions were conducted following at least 11 hours of abstinence. * above bar shows significant difference from premedication baseline, p < 0.05.
Further examination of Figure 1 shows that, contrary to our hypothesis, participants receiving 8 mg/day doxazosin made fewer commission errors during the tobacco deprivation session compared to premedication baseline. Our initial analytic strategy did not allow us to test the significance of this reduction, so an exploratory t test was conducted to confirm that this improvement was significant, t (12) = 2.10, p = 0.03, suggesting that participants receiving 8 mg/day doxazosin retained their improvements of inhibitory control compared to premedication baseline even when tobacco deprived.
3.1.2 Reaction time to go targets
Two participants were identified as outliers (RTgo > 600 ms) and removed from analyses of RTgo and RTvar. RTgo is charted in Supplementary Figure 1. There was no main effect of medication, F (2, 28) = 0.02, p = 0.98, or medication X session interaction, F (4, 56) = 0.63, p = 0.65. The main effect of session was significant, F (2, 56) = 10.20, p < 0.01. Because there was no significant interaction effect, we collapsed across medication groups for our a priori tests of session effects. RTgo was no faster during the non-deprivation session compared to the premedication baseline assessment, t (32) = 0.21, p = 0.42. They did, however, show slower RTgo during the deprivation session compared to their premedication baseline, t (32) = 3.62, p < 0.01.
3.1.3 Reaction time variability
RTvar is plotted in Supplementary Figure 2. There was a significant main effect of session, F (2, 56) = 4.01, p = 0.02. The main effect of medication was not significant, F (2, 28) = 0.24, p = 0.79, nor was the session X medication interaction, F (4, 56) = 0.78, p = 0.55. We collapsed across medication group and used a priori t tests to probe the main effect of session. RTvar was not reduced during the non-deprivation session compared to the premedication baseline assessment, t (32) = 0.86, p = 0.24. During the deprivation session, RTvar increased compared to the medication-free baseline assessment, t (32) = 3.28, p = 0.01.
3.2 Self-Reported Withdrawal Symptoms
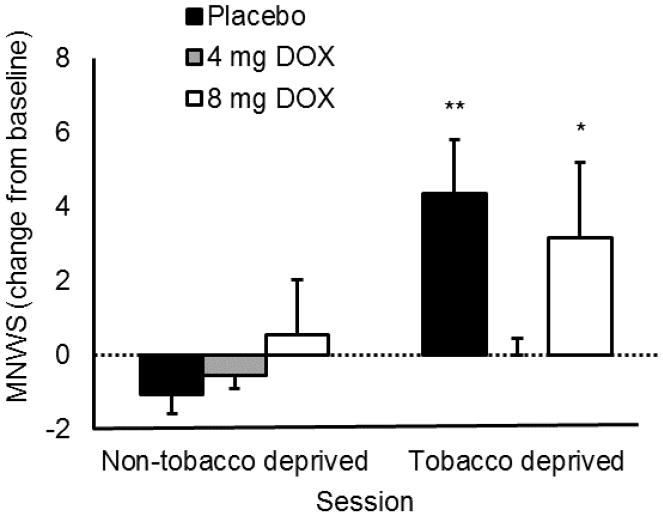
MNWS scores are reported in Figure 2. The ANCOVA found a significant main effect of session, F (1, 31) = 8.05, p = 0.01, confirming that participants reported greater withdrawal symptoms during the deprivation session than the non-deprived session. There was a significant session X medication interaction, F (2, 31) = 3.97, p = 0.03. In the placebo group, participants reported significantly greater withdrawal symptoms during the deprived session than during the non-deprived session, t (10) = 3.90, p < 0.01. There was no significant difference in MNWS scores between sessions among those receiving 4 mg/day doxazosin, t (10) = 0.92, p = 0.19. Those receiving 8 mg/day doxazosin showed a significant increase in withdrawal symptoms during the deprivation session relative to the non-deprived session, t (12) = 2.01, p = 0.03.
Figure 2.
Effects of doxazosin and tobacco deprivation on withdrawal symptoms on the Minnesota Nicotine Withdrawal Symptoms Scale. Capped bars represent SEM. Broken line crossing Y axis at 0 represents pre-medication baseline performance. Non-tobacco deprived sessions were conducted within 1 hour of smoking. Tobacco deprivation sessions were conducted following at least 11 hours of abstinence. Symbol above bar shows significant increase from premedication baseline within that treatment condition, *p < 0.05, **p < 0.01.
Correlational analyses found an association between MNWS scores and increased commission errors during the deprivation session in the placebo group, r (9) = 0.595, p = 0.05, such that individuals reporting more withdrawal symptoms showed larger increases in commission errors compared to baseline. This association was not significant among those receiving active doxazosin, r (22) = −.257, p = 0.23.
3.3 Medication and Deprivation Effects Predicting Smoking Behavior
Among participants receiving active medication, the average reduction in percentage commission errors during the steady state medication assessment was 2.65% (SD = 16.13%). The average reduction during the deprivation session was 2.29% (SD = 13.46). In the placebo group, there was little change from the baseline assessment to the non-deprivation session (M change = 0.04%, SD = 8.93%). The average increase in percentage commission errors during the deprivation session was 9.83% (SD = 12.13%).
The relation between medication and deprivation effects on commission errors on the CPT and smoking variables are reported in Table 2. There were no significant relations among smoking variables and medication/deprivation effects in the placebo group. In the active medication conditions, however, participants who showed the largest improvement on commission errors also delayed smoking the longest.
Table 2.
Relation among medication and deprivation effects on inhibitory control and smoking variables
| Placebo (n = 11) | Delay | Latency | Cigarettes Smoked | |||
|---|---|---|---|---|---|---|
|
| ||||||
| r | p | r | p | r | p | |
|
|
|
|||||
| Commission errors (non-deprived) | 0.19 | 0.58 | −0.19 | 0.58 | 0.56 | 0.08 |
| Commission errors (deprived) | −0.17 | 0.62 | −0.12 | 0.73 | 0.43 | 0.18 |
| Withdrawal symptoms | −0.22 | 0.51 | −0.30 | 0.36 | 0.65 | 0.03* |
| DOX (n = 24) | ||||||
|
|
||||||
| Medication Effect | 0.10 | 0.65 | 0.43 | 0.04* | 0.20 | 0.35 |
| Deprivation Effect | 0.01 | 0.95 | −0.23 | 0.29 | 0.05 | 0.81 |
| Withdrawal Symptoms | −0.43 | 0.04* | −0.41 | 0.05* | 0.48 | 0.02* |
Note. DOX = active doxazosin conditions (i.e., 4 mg/day; 8 mg/day). For doxazosin correlations, df = 22. For placebo correlations, df = 9. Commission errors (non-deprived) is the difference in % commission errors between pre-medication baseline assessment and non-deprived laboratory session. Commission errors (deprived) is the difference in % commission errors between pre-medication baseline assessment and deprived laboratory session. Withdrawal symptoms are the difference in MNWS scores between pre-medication baseline assessment and deprived laboratory session.
p < 0.05
3.4 MNWS Scores during Deprivation Predicting Smoking Behavior
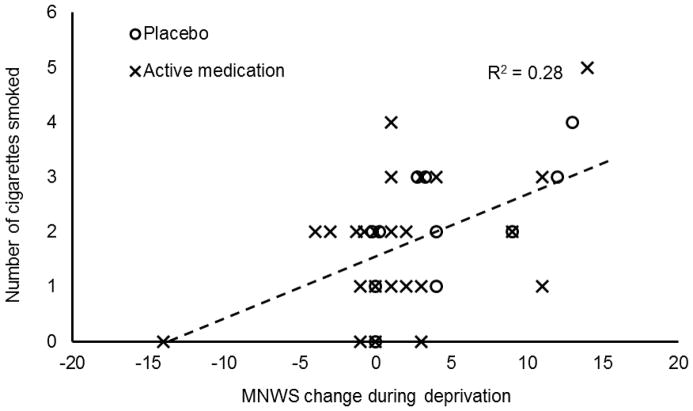
Compared to baseline, scores on the MNWS during the deprivation session increased by 4.36 (SD = 4.84) in the placebo group and 1.71 (SD = 5.63) in the active medication groups. Relations among deprivation-induced increases in withdrawal symptoms and smoking behavior are described in Table 1. In the active medication group, those who reported the largest increase in withdrawal symptoms during deprivation had the briefest delay to smoking during both phases of the smoking lapse task (i.e., smoking delay, smoking latency) and smoked more cigarettes during the free access phase. This relation is illustrated in Figure 3. Participants who received active doxazosin self-reported fewer withdrawal symptoms during tobacco deprivation, and having fewer withdrawal symptoms was associated in turn with smoking fewer cigarettes when given ad libitum access.
Figure 3.
Relation between degree of increase in Minnesota Withdrawal Scale score during deprivation session relative to baseline and number of cigarettes smoked during the free access phase of the smoking lapse task. Dashed line is least squares linear regression line for combined sample. The association is significant at p < 0.05.
3.5 Medication Effects on Smoking Behavior
There were no significant treatment emergent symptoms reported by participants in this study. Direct effects of medication condition on smoking behavior are reported in the primary manuscript from the parent study (Verplaetse et al., 2017).
4. Discussion
This study tested the effects of doxazosin on cognitive functioning and withdrawal symptoms in a group of smokers with tobacco use disorder in non-tobacco deprived and deprived states. Doxazosin improved inhibitory control during the non-deprivation medication session above pre-drug levels. Consistent with prior research, participants receiving placebo had poorer cognitive performance under tobacco deprivation (Shiffman, Paty, Gnys, Kassel, & Elash, 1995). Doxazosin blocked the deprivation-induced impairment of inhibitory control and self-reported withdrawal symptoms. Given the importance of inhibitory control for maintaining goals in the presence of motivational conflict (e.g., remaining abstinent during craving; Fillmore, 2003), these findings support the use of doxazosin as a smoking cessation aid. Smokers may use their improved inhibitory control to stop themselves from smoking when they may otherwise relapse. Indeed, the improvements to inhibitory control during the non-deprivation session under doxazosin were associated with a behavioral indicator of smoking risk during the smoking lapse task, suggesting that these improvements in inhibitory control could help support quit attempts.
In addition to demonstrating doxazosin’s ability to attenuate some aspects of cognitive impairment during withdrawal, we also found that the drug reduced self-reported withdrawal symptoms per the MNWS. This effect was most evident among participants receiving 4 mg/day doxazosin— those receiving 8 mg/day reported an increase in withdrawal symptoms during deprivation. As reported in the parent study and in prior investigations (Stoschitzky et al., 2003; Verplaetse et al., 2017), doxazosin increases heartrate, suggesting that the drug may paradoxically have sympathomimetic effects under certain circumstances. These effects may have strengthened at higher doses, potentially offsetting the beneficial effects of the drug on withdrawal symptoms. Another possibility relates to the U-shaped association between noradrenergic tone and stress response (Arnsten, 2009). The 8 mg/day doxazosin dose may have altered noradrenergic tone below optimal levels, effectively increasing susceptibility to stressors such as tobacco withdrawal. Additional research will be necessary to determine the mechanisms by which noradrenergic medications can attenuate tobacco withdrawal and to better characterize the dose-response curve. The lack of association between self-reported withdrawal symptoms and behavioral disinhibition among those receiving active medication suggests that doxazosin attenuated the expression of disinhibition as a symptom within the broader tobacco withdrawal syndrome (Shiffman et al., 1995).
Participants receiving active doxazosin reported fewer withdrawal symptoms; those with fewer withdrawal symptoms could resist smoking for longer and smoked less when given free access to cigarettes. This finding is consistent with prior preclinical research demonstrating that prazosin administered during tobacco withdrawal attenuated the withdrawal-induced reduction in brain reward threshold, which may explain its effects on withdrawal symptoms (Bruijnzeel et al., 2010). Although some extant smoking cessation medications block withdrawal symptoms by acting as nicotinic acetylcholine receptor (nAChR) agonists, this strategy can lead to increased expression of nAChR which may prolong withdrawal (Hussmann et al., 2012). Doxazosin may reduce withdrawal-related symptoms without targeting nAChRs, potentially allowing for quicker downregulation of these receptors while providing relief from withdrawal.
This study was among the first to examine the effects of an α1 antagonist on cognitive functioning in humans and provides insight into the role of α1 receptors in cognitive functioning. Prior research in humans found that the α2 agonist guanfacine can improve inhibitory control (Fox, Sofuoglu, & Sinha, 2015), suggesting that increasing activity at presynaptic α2 receptors can improve cognitive functioning, likely by reducing synaptic concentrations of norepinephrine (Arnsten, 2011). Results of the current study suggests that α1 antagonism also can improve inhibitory control, presumably through a similar mechanism. A future direction for this line of work may be examining gender differences in responses to noradrenergic medications such a doxazosin, because drugs targeting the noradrenergic system may attenuate smoking through gender-specific mechanisms (i.e., stress reactivity in women, tobacco reinforcement in men; Verplaetse et al., 2015).
These findings identify a potential mechanism by which doxazosin improves outcomes across several psychiatric and neurological conditions. Rationale for prior clinical trials using noradrenergic drugs to reduce substance use has focused on its ability to block stress responses to minimize stress-induced relapse (Kenna et al., 2016; Verplaetse et al., 2015) or block drug reinforcment (Drouin et al., 2002; Ventura, Morrone, & Puglisi-Allegra, 2007). However, we found that doxazosin may enhance inhibitory control beyond medication free baseline levels, which may explain its ameliorative effects on a range of disorders characterized by behavioral disinhibition (e.g., alcohol and cocaine use disorder; Kenna et al., 2016; Shorter et al., 2013).
This study provides important information regarding the cognitive effects of an α1 adrenergic antagonist in daily smokers; however, these findings should be interpreted in light of some limitations. First, this was a small preliminary study. Analyses were intended to be hypothesis generating. The sample size was underpowered to detect small or medium effects, particularly for correlation analyses related to the smoking lapse task. Only one of the critical correlations involving task performance was significant. It will be important to replicate these findings in a larger sample. Second, participants in this study were not seeking treatment to reduce tobacco use. Although the smoking lapse task models motivation to remain abstinent by compensating participants for resisting smoking, it is possible that treatment seekers may be more motivated to use their improved inhibitory control to avoid smoking. Finally, the order of the deprived and non-deprived laboratory sessions was not counterbalanced. It is possible that order effects may have influenced our findings.
4.1 Conclusion
In conclusion, doxazosin improved inhibitory control in smokers and may reduce withdrawal symptoms during tobacco deprivation. This research identifies a potential pathway by which doxazosin might improve outcomes for smokers as well as people with other disorders characterized by cognitive impairment. Additional research will be necessary to replicate and extend these findings.
Supplementary Material
Acknowledgments
Role of funding sources: This research was supported by NIH grants R01AA017976 R21DA033597, P50DA033945, T32DA007238, R01MH077681 and TR001863. The sponsors had no involvement in study design or collection, analysis, or interpretation of data, nor in the writing or submission of this report.
Footnotes
Conflict of Interest: Sherry A. McKee has consulted to Cerecor and Embera, has received research support for investigator-initiated studies from Pfizer and Cerecor, and has ownership in Lumme. All other authors declare that there is no conflict of interest.
References
- Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews: Neuroscience. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT. Catecholamine influences on dorsolateral prefrontal cortical networks. Biological Psychiatry. 2011;69:89–99. doi: 10.1016/j.biopsych.2011.01.027. doi:0.1016/j.biopsych.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare RL, Falcone M, Lerman C. Cognitive function during nicotine withdrawal: Implications for nicotine dependence treatment. Neuropharmacology. 2014;76:581–591. doi: 10.1016/j.neuropharm.2013.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare RL, Schmidt HD. Optimizing treatments for nicotine dependence by increasing cognitive performance during withdrawal. Expert Opinion in Drug Discovery. 2014;9:579–594. doi: 10.1517/17460441.2014.908180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Spencer RC. Differential cognitive actions of norepinephrine α2 and α1 receptor signaling in the prefrontal cortex. Brain Reearch. 2016;1641:189–196. doi: 10.1016/j.brainres.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum S, Gobeske KT, Auerbach J, Taylor JR, Arnsten AF. A role for norepinephrine in stress-induced cognitive deficits: alpha-1-adrenoceptor mediation in the prefrontal cortex. Biological Psychiatry. 1999;46:1266–1274. doi: 10.1016/s0006-3223(99)00138-9. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Bishnoi M, van Tuijl IA, Keijzers KFM, Yavarovich KR, Pasek TM, … Yamada H. Effects of prazosin, clonidine, and propranolol on the elevations in brain reward thresholds and somatic signs associated with nicotine withdrawal in rats. Psychopharmacology (Berl) 2010;212:485–499. doi: 10.1007/s00213-010-1970-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK. The Conners Continuous Performance Test--Second Edition. Toronto, Canada: Multi-Health Systems; 2000. [Google Scholar]
- Drouin C, Darracq L, Trovero F, Blanc G, Glowinski J, Cotecchia S, Tassin JP. Alpha1b-adrenergic receptors control locomotor and rewarding effects of psychostimulants and opiates. Journal of Neuroscience. 2002;22:2873–2884. doi: 10.1523/JNEUROSCI.22-07-02873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT. Drug abuse as a problem of impaired control: current approaches and findings. Behavior and Cognitive Neuroscience Reviews. 2003;2:179–197. doi: 10.1177/1534582303257007. [DOI] [PubMed] [Google Scholar]
- Fiore M, Jaen CR, Baker T, Bailey W, Benowitz N, Curry Se, … Healton C. Treating tobacco use and dependence: 2008 update. Rockville, MD: US Department of Health and Human Services; 2008. [Google Scholar]
- Fox H, Sofuoglu M, Sinha R. Guanfacine enhances inhibitory control and attentional shifting in early abstinent cocaine-dependent individuals. Journal of Psychopharmacology. 2015;29:312–323. doi: 10.1177/0269881114562464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Anderson GM, Tuit K, Hansen J, Kimmerling A, Siedlarz KM, … Sinha R. Prazosin effects on stress- and cue-induced craving and stress response in alcohol-dependent individuals: preliminary findings. Alcoholism: Clinical and Experimental Research. 2012;36:351–360. doi: 10.1111/j.1530-0277.2011.01628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt DJ, Scavone JM, Harmatz JS, Engelhardt N, Shader RI. Cognitive effects of beta-adrenergic antagonists after single doses: pharmacokinetics and pharmacodynamics of propranolol, atenolol, lorazepam, and placebo. Clinical Pharmacology & Therapeutics. 1993;53:577–584. doi: 10.1038/clpt.1993.73. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hussmann GP, Turner JR, Lomazzo E, Venkatesh R, Cousins V, Xiao YX, … Kellar KJ. Chronic sazetidine-A at behaviorally active doses does not increase nicotinic cholinergic receptors in rodent brain. Journal of Pharmacology and Experimental Therapeutics. 2012;343:441–450. doi: 10.1124/jpet.112.198085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakala P, Riekkinen M, Sirvio J, Koivisto E, Kejonen K, Vanhanen M, Riekkinen P., Jr Guanfacine, but not clonidine, improves planning and working memory performance in humans. Neuropsychopharmacology. 1999;20:460–470. doi: 10.1016/S0893-133X(98)00127-4. [DOI] [PubMed] [Google Scholar]
- Kahler CW, Metrik J, Spillane NS, Leventhal AM, McKee SA, Tidey JW, … Rohsenow DJ. Sex differences in stimulus expectancy and pharmacologic effects of a moderate dose of alcohol on smoking lapse risk in a laboratory analogue study. Psychopharmacology. 2012;222:71–80. doi: 10.1007/s00213-011-2624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenna GA, Haass-Koffler CL, Zywiak WH, Edwards SM, Brickley MB, Swift RM, Leggio L. Role of the alpha1 blocker doxazosin in alcoholism: A proof-of-concept randomized controlled trial. Addiction Biology. 2016;21:904–914. doi: 10.1111/adb.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA. Developing human laboratory models of smoking lapse behavior for medication screening. Addiction Biology. 2009;14:99–107. doi: 10.1111/j.1369-1600.2008.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Krishnan-Sarin S, Shi J, Mase T, O’Malley SS. Modeling the effect of alcohol on smoking lapse behavior. Psychopharmacology (Berl) 2006;189:201–210. doi: 10.1007/s00213-006-0551-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Sinha R, Weinberger AH, Sofuoglu M, Harrison EL, Lavery M, Wanzer J. Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. Journal of Psychopharmacology. 2011;25:490–502. doi: 10.1177/0269881110376694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Loughead J, Perkins K, Strasser AA, Siegel S, … Lerman C. Working memory deficits predict short-term smoking resumption following brief abstinence. Drug and Alcohol Dependence. 2010;106:61–64. doi: 10.1016/j.drugalcdep.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos BP, Arnsten AF. Adrenergic pharmacology and cognition: Focus on the prefrontal cortex. Pharmacology & Therapeutics. 2007;113:523–536. doi: 10.1016/j.pharmthera.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JD, Elash C. Nicotine withdrawal in chippers and regular smokers: Subjective and cognitive effects. Health Psychology. 1995;14:301–309. doi: 10.1037//0278-6133.14.4.301. [DOI] [PubMed] [Google Scholar]
- Shorter D, Lindsay JA, Kosten TR. The alpha-1 adrenergic antagonist doxazosin for treatment of cocaine dependence: A pilot study. Drug and Alcohol Dependence. 2013;131:66–70. doi: 10.1016/j.drugalcdep.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoschitzky K, Koshucharova G, Zweiker R, Lercher P, Maier R, Watzinger N, … Donnerer J. Different effects of propranolol, bisoprolol, carvedilol and doxazosin on heart rate, blood pressure, and plasma concentrations of epinephrine and norepinephrine. Journal of Clinical Basic Cardiology. 2003;6:69–72. [Google Scholar]
- Ventura R, Morrone C, Puglisi-Allegra S. Prefrontal/accumbal catecholamine system determines motivational salience attribution to both reward- and aversion-related stimuli. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:5181–5186. doi: 10.1073/pnas.0610178104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verplaetse TL, Weinberger AH, Oberleitner LM, Smith KM, Pittman BP, Shi JM, … McKee SA. Effect of doxazosin on stress reactivity and the ability to resist smoking. Journal of Psychopharmacology. 2017;31:830–840. doi: 10.1177/0269881117699603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verplaetse TL, Weinberger AH, Smith PH, Cosgrove KP, Mineur YS, Picciotto MR, … McKee SA. Targeting the noradrenergic system for gender-sensitive medication development for tobacco dependence. Nicotine & Tobacco Research. 2015;17:486–495. doi: 10.1093/ntr/ntu280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder-Rhodes SE, Chamberlain SR, Idris MI, Robbins TW, Sahakian BJ, Muller U. Effects of modafinil and prazosin on cognitive and physiological functions in healthy volunteers. Journal of Psychopharmacology. 2010;24:1649–1657. doi: 10.1177/0269881109105899. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.