Abstract
Introduction
Triple negative breast cancer (TNBC) is a heterogeneous disease associated with a high risk of recurrence, and therapeutic options are currently limited to cytotoxic therapy. Germ-line mutations may occur in up to 20% of unselected patients with TNBC, which may serve as a biomarker identifying which patients may have tumors that are particularly sensitive to platinums and/or inhibitors of poly(ADP-ribose)polymerase. A substantial proportion of patients with TNBCs not associated with germ-line BRCA mutations may have tumors that are ‘BRCA-like’, rendering those individuals potential candidates for similar strategies.
Areas covered
The purpose of this review is to highlight the current standard and experimental treatment strategies.
Expert opinion
Recent research that has illuminated the molecular heterogeneity of the disease rationalizes its diverse biological behavior and differential response to chemotherapy. Modern technology platforms provide molecular signatures that can be mined for therapeatic interventions. Target pathways that are commonly dysregulated in cancer cells control cellular processes such as apoptosis, proliferation, angiogenesis, DNA repair, cell cycle progression, immune modulation and invasion, and metastasis. Novel trial design and re-defined endpoints as surrogates to clinical outcome have been introduced to expedite the development of breakthrough therapies to treat high-risk early-stage breast cancer.
Keywords: basal-like breast cancer, BRCA mutations, poly(ADP-ribose)polymerase inhibitor, phosphatidylinositide 3-kinase pathway, platinums, triple negative breast cancer
1. Introduction
Triple negative breast cancer (TNBC), defined as tumors lacking the expression of estrogen receptor (ER) and progesterone receptor (PR) by immunohistochemistry (IHC) and overexpression of human epidermal growth factor receptor 2 (HER2/neu) protein or HER2/neu gene amplification, comprises ~ 15 – 20% of all breast cancers in the United States [1]. TNBC has been variably defined in the literature due primarily to the variability of ER and PR expression, with some definitions including up to 10% ER or PR expression by IHC. The American Society of Clinical Oncology (ASCO) and College of American College of Pathology have recently defined ER/PR-negative disease by immunohistochemical (IHC) analysis as exhibiting < 1% expression of tumor cell nuclei immunoreactive for ER or PR, which provides the most stringent definition of TNBC [2]. The clinical and molecular characteristics of the disease are summarized in Table 1, and described herein. TNBC occurs more commonly in younger women and women of black race or Hispanic ethnicity [3–5]. Although sensitive to chemotherapy, TNBC has an intrinsic aggressive clinical course associated with a higher risk of distant recurrence, high rates of visceral and central nervous metastases, earlier time to recurrence, and worse prognosis after recurrence than hormone receptor-positive subtypes [6–8]. Among breast cancer patients with a hereditary BRCA1 mutation, > 80% are TNBC [9]. When occurring sporadically without a germ-line mutation, TNBC shares many clinical and molecular features with BRCA1-associated cancers, including defective DNA repair, which may be due to methylation-induced silencing of BRCA or mutations in other genes-encoding proteins involved in DNA repair [10,11]. In addition, it has recently been shown that TNBC is associated with activation of unfolded protein and/or endoplasmic reticulum stress response that drives tumorigenicity by facilitating assembly of transcriptional complexes with HIF1-α, providing a rationale for targeting these pathways [12].
Table 1.
Summary of clinical and molecular characteristics of triple negative breast cancer.
| Clinical |
| Accounts for ~ 15% of all breast cancers in USA |
| More common in women of black race and/or Hispanic ethnicity |
| Younger age at presentation |
| Higher risk of visceral metastases, including brain metastasis |
| Associated with germ-line BRCA mutations in ~ 50% of patients with a strong family history of breast and/or ovarian cancer, and up to 20% of unselected patients |
| Molecular |
| Basal-like subtype and claudin-low subtypes are the most common ‘intrinsic subtypes’ by gene expression |
| When present, BRCA mutations are associated with defective DNA repair and sensitivity to DNA damaging agents and poly(ADP-ribose) polymerase inhibitors |
| Sporadic cancers not associated with BRCA mutations are often BRCA-like due to methylation-induced silencing of BRCA1 and/or loss of other DNA repair proteins |
| Commonly associated with somatic p53 mutations, but ‘clinically actionable’ aberrations occur in < 20% (BRAF V600E, high-level EGFR amplifications, and ERBB2 and ERBB3 mutations) and may not be driver aberrations |
| PI3K pathway activation, despite the low PI3K mutation rate, due to PTEN and INPP4B loss and/or amplification of PIK3CA |
PI3K: Phosphatidylinositide 3-kinase.
1.1 Data sources and literature searches
MEDLINE and EMBRACE (for at least the last decade) were searched. Abstracts published in the proceedings of annual meetings of the ASCO, European Society of Medical Oncology, and the San Antonio Breast Cancer Symposium were reviewed. We also considered all relevant ongoing clinical trials registered in the ClinicalTrials.gov.
2. Molecular classification of breast cancer
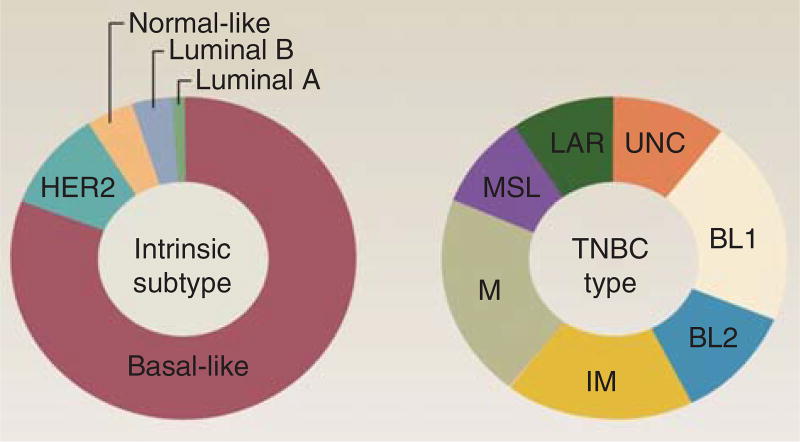
Several groups have made substantial progress in unraveling the biologic diversity of TNBC and relating gene expression patterns to molecular or genotypic subtype [13–15]. Initial molecular classifications of breast cancer using the PAM50 by gene expression analysis to segregate them into the four ‘intrinsic’ subtypes placed most TNBCs into the basal-like (BL) group, with the remainder divided between the luminal and HER2-enriched groups (Figure 1) [16]. BL breast cancers are highly proliferative and about one-third of them exhibit loss of function of BRCA 1 or BRCA 2. As BRCA1 and BRCA2 are involved in homologous recombination (HR), mutation carriers have demonstrated a high sensitivity to drugs that induce DNA double-strand breaks (DSBs) including alkylating agents or inhibitors of poly(ADP-ribose) polymerase (PARP) [17,18], and not to spindle poisons, such as taxanes [19]. Identification of a fifth intrinsic subtype, the claudin-low subtype as less proliferative than BL tumors but enriched for expression of mesenchymal genes and genes associated with tumor-initiating capacity, provided a further refinement [20,21]. Lehmann et al. evaluated gene expression data from 21 publicly available data sets and identified 587 TNBC cases [13]. It is important to note that as the majority of these tumors lacked sufficient molecular analysis of ER, PR, and HER2, they filtered each data set for ER, PR, and HER2 mRNA expression to identify triple-negative status, which is a different and more stringent definition of TNBC commonly use in clinical practice. This transcriptome-based dissection has enabled a re-classification of TNBC disease such that approximately seven distinct molecular subtypes displaying Figure 1 unique gene expression and ontologies have been identified. Based on identification of cell lines corresponding to each subtype, they demonstrated that each subtype may be responsive to different targeted therapies based upon in vitro and in vivo evaluation of these cell lines (Table 2). The subtypes identified included BL1, BL2, mesenchymal (M), mesenchymal-stem cell-like (MSL), immunomodulary (IM), luminal androgen receptor/luminal-like (LAR) and unclassified subtype [13]. The MSL subtype corresponds to the previously described claudin low subtype [14]. More subtle transcriptional differences among TNBCs were uncovered including the subtypes of the BL group and separation of androgen receptor (AR) expressing tumors from the luminal and HER2-enriched subsets. The improved understanding of the heterogeneity of TNBC by this analysis offers the opportunity to develop new therapeutic strategies.
Figure 1. PAM50 intrinsic subtype and TNBCtype.
Most TNBC cases are classified as basal-like by PAM50, whereas TNBCtype identifies seven classes of TNBC.
BL: Basal-like; LAR: Luminal androgen receptor/luminal-like; M: Mesenchymal; MSL: Mesenchymal-stem cell-like; TNBC: Triple negative breast cancer.
Table 2.
Targeting ‘driver’ signaling pathways identified in GSE-A of triple negative breast cancer subtypes.
| Subtypes | ‘Driver’ pathways | Representative cell lines | Agents |
|---|---|---|---|
| BL1 | Cell cycle, DNA damage response | HCC2157*, HCC1599‡, HCC1937*, HCC1143, HCC3153*, MDA-MB-468, HCC38 | Cisplatin, Poly(ADP-ribose)polymerase inhibitors (veliparib, olaparib) |
| BL2 | Cell cycle, DNA damage response, growth factor signaling | SUM149PT*, CAL-851, HCC70, HCC1806, HDQ-P1 | |
| Immunomodulary | Immune signaling, cytokine signaling, antigen presentation | HCC1187, DU4475 | |
| Mesenchymal | Cell motility, ECM receptor interactions, cell differentiation | BT-549, CAL-51§, CAL-120 | Abl/Src inhibitor (dasatinib), PI3K/mTOR inhibitor (NVP-BEZ235) |
| Mesenchymal-stem cell-like | Cell motility, cell differentiation, growth factor signaling | Hs578T, MDA-MB-157, SUM159PT§, MDA-MB-436*, MDA-MB-231 | |
| Luminal androgen receptor | Steroid synthesis and metabolism | MDA-MB-453§, SUM185PE§, CAL-148§, MFM-223§ | Bicalutamide, Hsp90 inhibitor (17-DMAG), PI3K/mTOR inhibitor (NVP-BEZ235) |
BRCA2 mutant,
BRCA1 mutant,
PIK3CA mutant.
Data taken from [13].
BL: Basal-like; ECM: Extracellular matrix; GSE-A: Gene set enrichment analysis; mTOR: Mammalian target of rapamycin; PI3K: Phosphatidylinositide 3-kinase.
Using next-generation sequencing, other reports focused on identifying somatic mutations in breast cancer, including the Cancer Genome Atlas (TCGA) [22], which identified mutations in genes previously implicated in breast cancer (PIK3CA, PTEN, AKT1, TP53, GATA3, CDH1, RB1, MLL2, MAP3K1, CDKN1B), plus a number of novel genes recently identified in other studies (TXB1, RUNX1, CBFB, AFF2, PIK3R1, PTPN22, PTPRD, NF1, SF3B1, CCND3). The overall mutation rate was lowest in the luminal A subtype and highest in the BL and HER2-enriched intrinsic subtypes. The distribution of mutations varied by subtype, with PIK3CA mutations occurring more commonly in luminal A/B and HER2-enriched compared with basal subtypes (45/29 and 39 vs 9%), and TP53 mutations dominating in basal (80%) and HER2-enriched subtype (72%) compared with luminal B (29%) and luminal A (12%) subtypes. The types of mutations also differed by intrinsic subtypes, including differences in TP53 mutations between BL (nonsense and frame shift) and luminal tumors (missense). Approximately 9% of 507 cases evaluated revealed germ-line predisposing variants (e.g., ATM, BRCA1, BRCA2, BRIP1, CHEK2, NBN, PTEN, RAD51C, and TP53). Similar to other reports, copy number changes correlated with some intrinsic subtypes, including loss of 5q and gain of 10p in BL cancers, and gain of 1q and/or 16q loss in luminal tumors. The basal subtype showed a high degree of TP53 mutations and high phosphatidylinositide 3-kinase (PI3K) pathway activity, despite a low PI3K mutation rate (due to PTEN and INPP4B loss and/or amplification of PIK3CA). Similar to serous ovarian carcinoma, DNA repair deficits (ATM mutations, BRCA1 and 2 inactivation, RB1 loss, and cycle E activation), genomic instability, and increased activity of the HIF1-α/ARNT, MYC and FOXM1 pathways was also common. In another analysis Shah et al. described an analysis of 104 TNBC subjected to RNA-seq and deep resequencing measurements of allelic abundance for > 2400 somatic mutations [23]. About 20% of tumors had potentially ‘clinically actionable’ somatic aberrations, including BRAF V600E, high-level EGFR amplifications, and ERBB2 and ERBB3 mutations. The distribution of somatic mutation abundance varied in a continuous distribution and was unrelated to copy number abnormality or tumor cellularity. In another report, Banerji et al. identified a recurrent MAGI3-AKT3 fusion in TNBC that led to constitutive activation of AKT kinase that was abolished by a competitive AKT small-molecule inhibitor [24]. Initial attempts to match targeted therapies with genomic aberrations in breast cancer has produced clinical benefit in < 5% of screened patients [25], although other studies are now in progress utilizing more advanced genomic sequence techniques and a more expansive portfolio of targeted agents (e.g., MATCH trial).
3. Cytotoxic therapy
Cytotoxic chemotherapy remains the mainstay of treatment for operable and advanced breast cancer. A number of agents have activity in localized and advanced disease, including antibutulins (e.g., paclitaxel, nab-paclitaxel docetaxel, eribulin, vinorelbine, Ixabepilone), anthracyclines (doxorubicin, epiribucin), alkylating agents (e.g., cyclophosphamide), antimetabolites (e.g., methotrexate, capecitabine, gemcitabine), and platinums (e.g., carboplatin, cisplatin) [26,27]. Standard adjuvant and neoadjuvant regimens typically include an anthracycline doxorubicin or epirubicin) plus an alkylating agent (cyclophosphamide) given either concurrently with a taxane (docetaxel) or sequentially before or after a taxane (docetaxel or paclitaxel), and have resulted in the highest pathologic complete response (pCR) rates when used in the neoadjuvant setting and lowest recurrence rates when used in the adjuvant setting [28–33]. Few studies have been designed specifically for evaluating novel treatment approaches in TNBC, and those that have been reported, or are in progress, are described below.
3.1 Antitublin therapy
Several trials have evaluated the role of antitublin agents in TNBC by performing retrospective analysis or prespecified subgroup analysis. For example, a retrospective analysis including 399 patients with measurable disease included in two randomized Phase III studies comparing ixabepilone plus capecitabine with capecitabine alone, showed an improvement in progression-free survival (median 4.1 vs 1.7 months, HR 0.63, p < 0.001) and response rate (RR) (28 vs 14%), but not improvement in overall survival (OS) in the 443 patients with measurable and non-measurable disease (median 10.3 vs 9.0 months, HR 0.87, p = 0.18) [34]. The 301 study failed to demonstrate an improvement in OS in 1102 patients with anthracycline- and taxane-pretreated metastatic breast cancer (MBC) when eribulin was compared with capecitabine in patients who had progressive disease after up to two prior chemotherapy regimens for metastatic disease (median OS 15.9 vs 14.5 months, HR 0.88, = 0.056), although the 284 patients with TNBC exhibited improved OS with eribulin in a prespecified subgroup analysis (median 14.4 vs 9.4 months; HR 0.70, 95% CI: 0.56, 0.91) [35].
3.2 Platinums
Interest in evaluating platinum agents in TNBC has been renewed based on the greater susceptibility of some subclasses of triple negative and BRCA1/2 mutant tumors to DNA-damaging chemotherapy agents [13], and recent studies have focused on the role of platinums when used as a component of neoadjuvant chemotherapy (NAC). Higher pCR are consistently observed in TNBC compared with non-TNBC, and pCR of invasive carcinoma in the breast and axillary nodes has been shown to be associated with improved long-term outcomes for TNBC [36]. In addition, higher pCR rates are observed in BRCA mutation carriers compared with non-mutation carriers treated with such regimens [37,38]. The US FDA issued draft guidance recognizing pCR as an acceptable surrogate end point that supported accelerated approval, but required improved event-free survival (EFS) as a condition for full approval [39]. This was based on a recent meta-analysis of 12 trials that included 11,955 patients. Cortazar et al. found a strong correlation between pCR and EFS and OS in both HER2/neu-positive disease and TNBC although there was little association in the trial-level data analysis between increases in frequency of pCR and EFS (coefficient of determination [R2] = 0.03; 95% CI, 0.00 – 0.25) and OS (R2 = 0.24; 95% CI, 0.00 – 0.70) [36]. This guidance was used as a basis for providing accelerated approval for pertuzmab in combination with trastuzumab and cytotoxic chemotherapy as NAC for locally advanced HER2-posititve breast cancer. In addition, the I-SPY2 program uses pCR as an endpoint to identify promising agents in Phase II trials, which may be ‘graduated’ to more definitive evaluation in Phase III trials; among first successful graduates was the PARP inhibitor veliparib in TNBC [40].
Several trials have evaluated platinum therapy, including specifically BRCA mutation carriers and patients not selected by the mutation status (Table 3). A small proof-of-concept neoadjuvant study of 25 BRCA1 mutation carriers showed a pCR rate of 72% with single agent neoadjuvant cisplatin [41], which compared favorably to a 21% pCR rate in 28 unselected patients with TNBC, 2 of whom had BRCA mutations [42]. Telli reported a pCR rate of 36% in 80 patients with TNBC treated with the carboplatin-gemcitabine combination, including 47% in patients with germ-line BRCA mutations [43]. In addition, two randomized Phase II trials demonstrated increases in pCR rates with the addition of carboplatin to taxane-containing therapy in patients with sporadic breast cancer not selected by the mutation status. The GeparSixto trial demonstrated that pCR increased by ~ 20% (59 vs 38%; p < 0.05) when carboplatin was added to neoadjuvant taxane/anthracycline plus bevacizumab in 293 patients with TNBC. However, about one-half of patients receiving weekly carboplatin (AUC 1.5 – 2.0) discontinued treatment because of significant toxicity, and breast conservation rates were not significantly impacted [44]. In the Cancer and Leukemia Group B 40603 randomized Phase II trial, 493 patients with stage II/III sporadic TNBC were randomly assigned to standard weekly paclitaxel for 12 courses with or without the addition of carboplatin (AUC 6 every 3 weeks for 4 cycles), bevacizumab, or the combination. All patients also received dose dense doxorubicin-cyclophosphamide for four courses after paclitaxel and before surgery. The addition of carboplatin significantly increased the pCR rate (54 vs 41% p = 0.0029); similar benefits were observed in the absence (49 vs 39%) and presence (60 vs 43%) of bevacizumab, and no interaction was observed (p = 0.52), indicating a lack of a synergistic effect [45]. Although numerically more patients were deemed potential candidates for breast-conserving surgery when carboplatin was used (57 vs 44%), the difference was not statistically significant, and the actual rates were not reported. Neither trial was adequately powered to determine carboplatin reduced recurrence, nor were patients followed long enough to observe such a difference, if it existed. Platinum-containing agents are not regarded as a standard for neoadjuvant therapy of TNBBC, at least for now for several reasons: first, given that the addition of platinum results in added toxicity, the clinical benefits should be clear, which is not yet evident in either trial with regard to long-term or short-term outcomes. Second, it is possible that the improvements in pCR rates may be a result of down-staging of low-volume residual disease, which would not be predicted to translate into fewer recurrences. Third, pCR may not be associated with improved outcomes in BRCA1/2 mutation carriers, suggesting the inconsistency of its prognostic effect. Fourth, the trial-level data analysis by Cortazar et al. called into question whether pCR is a sufficiently robust short-term surrogate endpoint for predicting long-term outcomes in patient populations.
Table 3.
Platinum trials in the neoadjuvant setting in triple negative breast cancer and BRCA-associated breast cancer.
| Phase | N | BRCA status | Regimen | pCR Rate | Ref. |
|---|---|---|---|---|---|
| II | 28 | Unselected | Cisplatin | 21% | [42] |
| II | 25 | Mutant | Cisplatin | 72% | [41] |
| Randomized phase II | 454 | Unselected | Weekly paclitaxel, dose-dense AC, +bevacizumab, +carboplatin, or +carboplatin/bevacizumab | 60% (+carboplatin) | [45] |
| 46% (−carboplatin) | |||||
| Randomized phase II | 315 | Unselected | Anthracycline/taxane ± carboplatin | 59% (+carboplatin) | [44] |
| 38% (−carboplatin) |
4. Drug resistance and response to therapy as a pharmacodynamic biomarker
Tumor heterogeneity has been recognized as a factor contributing to acquired resistance and a major barrier to curative therapy. Although sensitive populations of tumor cells may be eradicated, there may be selective enrichment of residual tumor cells that are often genetically and histologically distinct from sensitive cells [21,46]. There is also evidence that some cytotoxic agents may promote epithelial-to-mesenchymal transition (EMT), and/or enrich for tumor cells with tumor initiating and promote metastasis [21], whereas other agents may reverse EMT and thereby suppresses metastasis [47,48]. Resistance to kinase inhibitors is often mediated by feedback loops that are hard-wired to adapt to changes in activity within a signaling network [49].
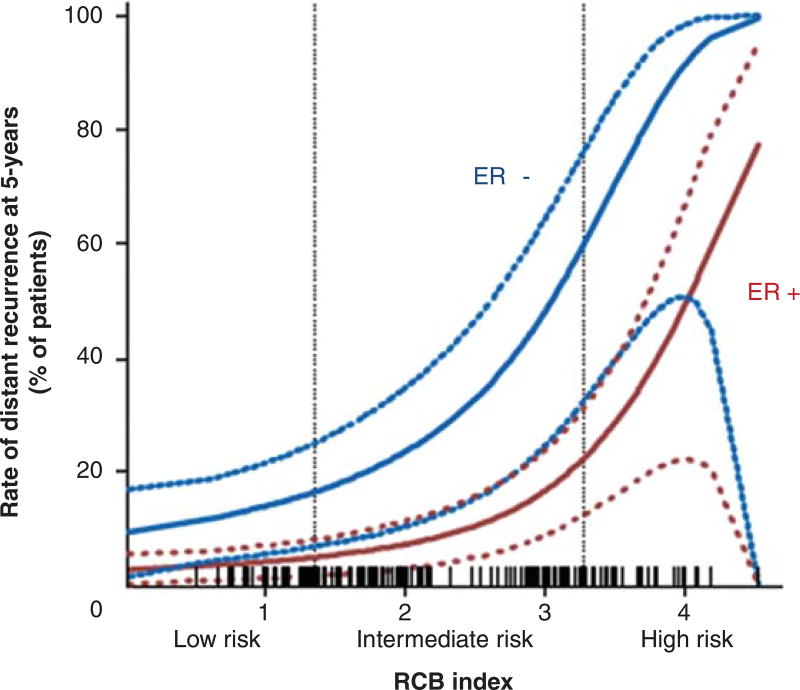
Although achieving a pCR after NAC is associated with a favorable prognosis, the prognosis for patients with residual cancer is variable and differs by molecular subtypes [50,51]. The 5-year recurrence rate is significantly higher for patients with extensive residual disease compared with patients with no or minimal residual disease after NAC, especially in ER-negative disease (Figure 2) [52]. There is no proven role for continuing systemic therapy for patients with extensive residual TNBC who remain at high risk for recurrence despite receiving a course of taxane and anthracycline-containing NAC. There may be opportunities to evaluate the characteristics of residual disease in order to tailor specific therapies for patients who remain at high risk. For example, Balko et al. [46] identified diverse molecular lesions and pathway activation in drug-resistant tumor cells that were potentially treatable with a currently available targeted therapy in the majority of cases; providing a foundation for further evaluation of this strategy, as exemplified by a recent report evaluating cisplatin alone or in combination with the PARP inhibitor rucaparib [53].
Figure 2. RCB index, a measure of the residual tumor burden after neoadjuvant chemotherapy, predicts risk of distant relapse at 5 years.
For a given RCB score, ER patients have a higher risk of developing distant metastases than ER+ patients.
Courtesy of W.F. Symmans, MD.
ER: Estrogen receptor; RCB: Residual cancer burden.
5. Targeted strategies
A variety of targeted therapies have been previously tested are currently in development for TNBC, as summarized in Table 4, and discussed in greater detail herein, including some that are being evaluated in randomized Phase II and III trials as summarized in Table 5. Successful implementation of a strategy for some agents may require the identification of predictive gene expression profiles, specific driver mutations, or other assays. For example, BL1 and BL2 subtypes have higher expression of cell cycle and DNA damage response genes and preferentially respond to cisplatin. M and MSL subtypes are enriched in EMT and growth factor pathways and are sensitive to inhibitors of PI3K/Akt/mammalian target of rapamycin (mTOR) pathway and abl/src inhibitor), and the LAR subtype is sensitive to anti-androgens (Table 2) [54].
Table 4.
Novel agents in clinical development for treatment of triple negative breast cancer.
| Therapeutic target | Agents | Phase of study |
|---|---|---|
| DNA repair pathway: poly(ADP-ribose)polymerase | ABT-888 (veliparib), AZD2281 (olaparib), BMN763 | I/II/III |
| Epidermal growth factor receptor | Erlotinib, cetuximab, panitumumab | II |
| Angiogenesis | Bevacizumab | III |
| Sunitinib, pazopanib | II | |
| Histone deacetylase | Vorinostat, entinostat | II |
| Hsp90 | Ganetespib | II |
| Androgen receptor | Bicalutamide, enzalutamide | II |
| Phosphatidylinositide 3-kinase/AKT/mammalian target of rapamycin | GDC-0941, BKM120 ipatasertib (GDC-0068), GSK2141795, everolimus, temsirolimus | II |
| MAPK/extracellular signal regulated kinase | Trametinib (GSK1120212) | Presurgical/II |
| Sarcoma | Dasatinib | II |
| Stem cell pathways: | R04929097, MK-0752 | |
| Notch | I | |
| Wnt | LGK974 | I |
| CXCR1/2 | Reparixin | Presurgical/Ib |
| Cyclin dependent kinases | P276-00, dinaciclib | I/II |
| c-Met | Onartuzumab, ARQ197, foretinib | II |
| Aurora kinase | ENMD-2076 | II |
| DNA repair pathway: | PF-00477736, CHIR-124 | Preclinical |
| Checkpoint kinase 1 | SAR-020106, AZD7762 | |
| Death receptors | Piperine | Preclinical |
| tigatuzumab | II | |
| Inhibitors of apoptosis | YM155 | I |
| LCL161 | II | |
| Immune | MUC-1 vaccine, dendritic cell - cytokine-induced killer cell | 0/I |
| CSF1 receptor, c-kit | PLX 3397 | Ib/II |
| Targeted Cytotoxic | Glembatumumab vedotin (CDX-011) | II |
Table 5.
Selected active clinical trials for triple negative breast cancer or BRCA-associated breast cancer.
| Clinical trial title | Phase | Cancer.gov |
|---|---|---|
| Platinum containing regimens | ||
| Carboplatin and paclitaxel albumin-stabilized nanoparticle formulation before surgery in treating patients with locally advanced or inflammatory triple negative breast cancer | II | NCT01525966 |
| Evaluate risk/benefit of Nab paclitaxel in combination with gemcitabine and carboplatin compared to gemcitabine and carboplatin in triple negative metastatic breast cancer (or metastatic triple negative breast cancer) (tnAcity) | II/III | NCT01881230 |
| A randomized Phase III postoperative trial of cisplatin versus observation in patients with residual basal-like triple negative breast cancer following neoadjuvant chemotherapy. (E1113) | III | In development |
| Poly(ADP-ribose)polymerase inhibitors | ||
| Olaparib as adjuvant treatment in patients with germline BRCA mutated high-risk HER2 negative primary breast cancer (OlympiA) | III | NCT02032823 |
| Assessment of the efficacy and safety of olaparib monotherapy versus physicians choice chemotherapy in the treatment of metastatic breast cancer patients with germ-line BRCA1/2 mutations. (OlympiAD) | III | NCT02000622 |
| A study evaluating safety and efficacy of the addition of ABT-888 plus carboplatin versus the addition of carboplatin to standard chemotherapy versus standard chemotherapy in subjects with early-stage triple negative breast cancer (Brightness) | III | NCT02032277 |
| An adaptive, randomized Phase II trial to determine pathologic complete response with the addition of carboplatin with and without veliparib to standard chemotherapy in the neoadjuvant treatment of triple-negative breast cancer | II | NCT01818063 |
| A Phase III randomized, placebo-controlled trial of carboplatin and paclitaxel with or without veliparib (ABT-8888) in Her2-negative metastatic or locally advanced unresectable BRCA-associated breast cancer (AbbVie) | III | NCT02163694 |
| PI3K/AKT/mammalian target of rapamycin inhibitors | ||
| GDC-0941 and cisplatin in treating patients with androgen receptor-negative triple negative metastatic breast cancer | I/II | NCT01918306 |
| Trametinib and Akt inhibitor GSK2141795 in treating patients with metastatic triple-negative breast cancer | II | NCT01964924 |
| A trial of BKM120 (a PI3K Inhibitor) in patients with triple negative metastatic breast cancer (SOLTI) | II | NCT01629615 |
| Temsirolimus plus neratinib for patients with metastatic HER2-amplified or triple negative breast cancer Other targeted agents | I/II | NCT01111825 |
| Study of glembatumumab vedotin (CDX-011) in patients with metastatic, gpNMB over-expressing, triple negative breast cancer (METRIC) | II | NCT01997333 |
| Safety and efficacy study of enzalutamide in patients with advanced, androgen receptor-positive, triple negative breast cancer | II | NCT01889238 |
| Study of pazopanib, paclitaxel, and carboplatin in patients with advanced solid tumors Immunotherapy | I | NCT01407562 |
| Targeted T cells after neoadjuvant chemotherapy in treating women with stage II or stage III breast cancer undergoing surgery | II | NCT01147016 |
PI3K: Phosphatidylinositide 3-kinase.
5.1 EGFR inhibitors
The majority of TNBC tumors overexpress EGFR [4,55]. Preclinical studies suggest that basal breast cancers depend on EGFR for proliferation and may be candidates for anti-EGFR therapies. Notably, despite the known expression of this receptor tyrosine kinase (RTK), only limited benefit has been demonstrated in clinical trials with some occasional responses using anti-EGFR agents such as cetuximab. Three randomized Phase II trials have evaluated the effectiveness of a platinum–cetuximab combination. The Translational Breast Cancer Research Consortium TBCRC 001 randomized Phase II trial evaluated the combination of cetuximab and carboplatin compared with cetuximab alone, followed by the combination after progression on cetuximab monontherapy in 102 patients with pretreated metastatic TNBC. The combination arm was associated with a marginally improved objective RR (17 vs 6%) and median time to progression (2.1 vs 1.4 months), but was very low for both arms, and 16% who progressed on cetuximab monotherapy responded when cross over to the combination [56]. Another randomized Phase II study evaluating the combination of irinotecan and carboplatin (90 mg/m2 and AUC 2, respectively, on days 1 and 8 every 3 weeks) with or without cetuximab (400 mg/m2 IV, then 250 mg/m2 weekly) in 72 evaluable patients with metastatic TNBC with up to one prior chemotherapy regimen reported higher overall response rate (ORR) (49 vs 30%) for the cetuximab arm, but similar median progression-free survival (PFS) (4.7 vs 5.1 months). Toxicity was higher among patients who received cetuximab [57]. A third randomized Phase II randomized compared cisplatin (75 mg/m2 every 3 weeks × 6 cycles) alone or in combination with cetuximab (400 mg/m2 IV, then 250 mg/m2) in 173 patients with metastatic TNBC allowing patients receiving cisplatin alone who had not more than one prior chemotherapy regimen for metastatic disease. The combination was associated with a non-significant improvement ORR (20 vs 10%, odds ratio, 2.13; 95% CI, 0.81 – 5.59; p =. 11) and longer median PFS (median, 3.7 vs 1.5 months; hazard ratio [HR], 0.67; 95% CI, 0.47 – 0.97; p = 0.032). Median OS was similar in the two arms (median 12.9 vs 9.4 months ([HR], 0.82; 95% CI, 0.56 – 1.20; p = 0.31), and the addition of cetuximab was associated with acneform rash [58]. A few selected studies have also evaluated the efficacy of small molecule EGFR inhibitors such as erlotinib and gefinitinib. Erlotinib had minimal activity in unselected previously treated women with MBC [59]. Modest activity has also been reported with gefitinib [60].
5.2 Antiangiogenic agents
Bevacizumab, a monoclonal antibody, is an anti-angiogenic agent targeting all forms of VEGF-A and is active in a variety of solid tumors including breast cancer. The landmark E2100 trial, an open-label, randomized, Phase III trial, demonstrated a significant improvement in PFS (11.8 vs 5.9 months, p < 0.001) and ORR (36.9 vs 21.2%, p < 0.001) but not OS (26.7 vs 25.2 months; [HR], 0.88; p = 0.16) with paclitaxel plus bevacizumab compared with paclitaxel alone as initial chemotherapy for patients with HER2-negative MBC. HRs favored combined therapy in all clinically relevant subgroups. The treatment effect persisted in ER/PR–negative patients ([HR] = 0.53) in this largely HER2-negative patient population [61]. The most prevalent toxicities associated with bevacizumab included hypertention, proteinuria, headaches, cerebrovascular ischemia and infection. Based on this data, the US FDA initially granted accelerated approval for the use of bevacizumab as first-line therapy in the treatment of HER2-negative MBC, then revoked this approval in November 2011 following the report of two other Phase III studies in the first-line setting, which demonstrated smaller absolute improvements in median PFS than the E2100 trial, similar adverse event profile, and no improvement in OS [62,63]. A meta-analysis of these trials including 2447 patients demonstrated an improved median PFS (9.2 vs 6.7 months, HR 0.64, 95% CI 0.57 – 0.71) and ORR (49 vs 32%), but no difference in OS (median 26.7 vs 26.4 months) [64]. Similar improvements were noted in 621 patients with TNBC for median PFS (8.1 vs 5.4 months, HR 0.63, 95% CI 0.52 – 0.76, p < 0.001) and ORR (42 vs 21%, p < 0.001), whereas median OS (18.9 vs 17.5 months) and 1-year OS rates were similar (71 vs 65%). Although bevacizumab is not approved in the US for Her2-negative metastatic breast cancer, it remains approved in Europe and other continents for this indication in combination with weekly paclitaxel. In addition, the results of the ongoing MERiDiAN trial are awaited to address whether a predictive biomarker can be used to identify patients with metastatic breast cancer who are more likely to benefit from bevacizumab. Similar to the E2100 trial, the MERiDiAN trial will investigate paclitaxel with or without bevacizumab in patients with HER2-negative, chemotherapy naive locally recurrent or MBC, stratifying for low or high VEGF-A status, adjuvant therapy and ER status. Additionally, small-molecule inhibitors of the VEGF pathway appear to have activity in the subset of pretreated TNBC [65]; although in the definitive Phase III study the addition of sunitinib to capecitabine does not improve the clinical outcome of patients with MBC pretreated with anthracyclines and taxanes [66].
Adding bevacizumab to NAC significantly increased the rate of pathological complete response among patients with early-stage HER2-negative breast cancer, with the most notable and pronounced improvement seen in the subgroup of patients with triple-negative disease in the GeparQuinto trial [67], in contrast to the findings in the NSABP-40 in which the greatest benefit from adding an antiangiogenic agent was seen in patients with hormone-receptor-positive tumors [68]. A number of differences including inclusion criteria and study design might have contributed to the divergent results. Furthermore, bevacizumab is not supported as adjuvant treatment in unselected patients with TNBC based on the results of a large international randomized Phase III (BEATRICE) [69].
5.3 PARP inhibitors
PARP is an essential nuclear enzyme that plays a role in the recognition of DNA damage and subsequent repair [70]; therefore, inhibition of PARP is hypothesized to potentiate the cytotoxicity of DNA-damaging agents. PARP nuclear enzymes are activated by DNA single or DSBs, resulting in the poly(ADP-ribosyl)ation of other nuclear DNA-binding proteins involved in efficient DNA repair and survival [70–73]. Several studies have documented enrichment of germ-line BRCA mutations in TNBC [22]. The TCGA reported that BRCA1/2 mutations are harbored in ~ 20% of BL breast tumors and that two-thirds are germ-line [22]. Cells with deficient HR (BRCA1 or BRCA2 mutant) are exquisitely sensitive to PARP1 inhibition; hypothesized to be due to ‘synthetic lethality’, that is, shutdown of the predominant DNA repair pathways that confers augmented cell death/apoptosis [72]. This hypothesis has been substantiated by preclinical and clinical data such that PARP has emerged as a promising therapeutic target for metastatic TNBC. Several drugs targeting PARP enzymes are currently in clinical development (Table 5).
Iniparib was purported to be a PARP inhibitor that initially showed promising results in randomized Phase II trials in patients with TNBC [74]. In a randomized Phase II trial the combination of iniparib with gemcitabine/carboplatin tripled the clinical benefit rate (CBR), which was the primary endpoint, and significantly improved the median PFS (6.9 vs 3.3 months; [HR] = 0.34 [95% CI 0.20 – 0.58] p < 0.0001) with no significant additional toxicity compared with chemotherapy alone. The chemotherapy backbone was selected due to previous demonstrated activity in the MBC setting and preclinical evidence of synergy between the agents resulting in double-strand DNA breaks and intrastrand DNA cross-links, which rely on BRCA1/BRCA2 for repair. The positive findings of this investigation were the basis of a subsequent Phase III clinical trial in 519 patients with TBNC, which failed to meet the primary endpoint [75]. The negative results reduced enthusiasm for iniparib. Recently, data from cell-based experiments suggest that iniparib is not only structurally distinct from other described PARP inhibitors, but is also a poor inhibitor of PARP activity. Additional studies suggest that iniparib exerts its cytotoxic effects via alterations in the metabolism of reactive oxygen species in cancer cells [76,77]. Thus, the concept of targeting PARP to induce ‘synthetic lethality’ is still considered worthy of additional exploration/clinical development; albeit with a drug that is a potent in vivo inhibitor of PARP.
Early-phase clinical trials demonstrated efficacy and safety of Olaparib, an oral PARP inhibitor. In a Phase I trial enriched with carriers of a BRCA1/2 mutation olaparib was well tolerated, and moreover exhibited PARP inhibition and antitumor activity in cancer associated with the BRCA1/2 mutations [17]. The results of a multicenter Phase II sequential cohort study enrolling 54 patients with impaired BRCA1/2 provide a positive proof of concept and show a favorable therapeutic index for a novel targeted treatment strategy in patients with tumors that have genetic loss of function of BRCA1/2-associated DNA repair. The ORR was 41% whereas the median PFS was 5.7 months for the cohort assigned to the optimal dose of 400 mg twice daily with no significant toxicity [78]. No confirmed objective responses were reported in patients with sporadic advanced TNBC in a Canadian Phase II non-randomized study [79]. Ongoing Phase III trials are testing Olaparib in adjuvant and metastatic setting chemotherapy in breast cancer patients with germ-line BRCA1/2 mutations (Table 5).
Veliparib (ABT-888) is an orally available, small molecule inhibitor of PARP1 and 2 and has been studied in early-phase clinical trials that included patients with MBC. Veliparib was administered as a single dose, ranging from 10 to 50 mg, to 13 subjects enrolled in an exploratory investigational new drug study conducted by the National Cancer Institutes as an initial study in the phase 0 program [80]. Veliparib demonstrated good oral bioavailability and was well tolerated. Statistically significant inhibition of PARP levels was observed in tumor biopsies and peripheral blood mononuclear cells at the 25 and 50-mg dose levels. This novel statistical approach has provided the pivotal biochemical and pharmacokinetic data that have guided the design of subsequent Phase I trials of veliparib in combination with chemotherapy drugs as well as monotherapy. In addition to accelerating the development of veliparib, the rapid conclusion of this trial demonstrated the feasibility of conducting proof-of-principle phase 0 trials as part of an alternative paradigm for early drug development in oncology. Pharmacokinetic results from a Phase I study of veliparib in combination with the alkylating agent temozolomide were consistent with those seen in the Phase 0 study. Safety and efficacy results of this combination treatment are available from a study of 41 patients with heavily pretreated MBC including 8 BRCA1/2 mutant patients and 23 with TNBC. Whereas the CBR and PFS was 17% and 1.9 months respectively across the entire patient population, among the BRCA mutation carriers the CBR was 62% and the median PFS 5.5 months, highlighting the impact of the dysfunctional HR and synthetic lethality with PARP inhibition [81]. This combination was subsequently tested in an expansion cohort of 21 patients, all BRCA mutation carriers, and was associated with a CBR of 43% and median PFS of ~ 3.5 months. Veliparib is currently being studied in neoadjuvant and metastatic setting in several clinical trials (Table 5).
5.4 Androgen receptor antagonists
Interest in targeting the AR was based on gene expression profiling, which identified a subset of TNBCs with an active hormonally regulated transcriptional program and AR expression [82]. These observations provided the basis of a Phase II single-arm trial of the non-steroidal anti-androgen bicalutamide in patients TNBC that was AR positive by IHC; of > 450 patients screened, 10% had AR expression, and the 6 month CBR was 19% with bicalutamide [83]. It is debatable where this study provides the proof of principle and the modest activity could well be due to the indolent nature of luminal disease.
5.5 Histone deacetylase inhibition
Histone deacetylases (HDACs) are a family of enzymes that regulate chromatin remodeling and gene transcription via the dynamic process of acetylation and deacetylation of core histones. HDAC inhibitors have been shown to exhibit activity in TNBC preclinical models [84,85]. Despite these promising preclinical data, the addition of vorinostat to neoadjuvant carboplatin and nabpaclitaxel was not associated with improved pCR rates in a randomized Phase II study of 62 patients with TNBC [86]. It is recognized that HDAC inhibitors cause genome-wide effects, for example they may permit re-expression of ER, or BRCA1/2, this may be accompanied by silencing of other genes that have tumor suppressive functions; thus masking a potential anti-tumor benefit. Efforts are underway to identify which classes of HDACs regulate tumor promoting classes of genes, and developing therapeutic agents to these specific classes.
5.6 PI3K-AKT-mTOR pathway inhibitors
Inhibition of the PI3K and downstream AKT and mTOR have been recognized as a promising therapeutic targets, due to their known hyperactivation and participation in different tumorigenic processes in numerous malignancies. The TCGA identified activation of the PI3K pathway, either directly via PI3KCA mutations or indirectly via PTEN loss and/or INPP4B loss, as common in TNBC BL breast cancer [22]. Preclinical studies demonstrated that inhibition of the PI3K pathway results in transient quiescence in TNBC [87]. Preclinical data also supports effective inhibition of the PI3K/mTOR pathway in the M and MSL subsets of TNBC [13]. The BL subtype of TNBC has also been shown to be sensitive to mTORC1 inhibitors, in both in vitro and in vivo studies, again resulting in quiescence [87,88]. The combination of a PI3K inhibitor with a PARP inhibitor was shown to provide in vivo synergy for treatment of an endogenous mouse model for BRCA1-related breast cancers [89]. PI3K blockade results in HR impairment and sensitization to PARP inhibition in TNBCs without BRCA mutations, providing a rationale for an ongoing clinical trial combining the PI3K inhibitor BKM120 plus the PARP inihibitor olaparib in metastatic TNBC [90].
Although the mTOR inhibitor everolimus prolongs PFS when added to exemestane in ER-positive, aromatase inhibitor-resistant MBC and is approved for this indication [91], there are very limited clinical data on TNBC treated with either rapalogs or PI3K/mTOR inhibitors. Combination of weekly paclitaxel with evelolimus followed by anthracycline in a neoadjuvant clinical trial of 50 women with TNBC showed no statistically significant increase in pCR (30 vs 26% p = 0.76) [92]. A small study of everolimus plus carboplatin in 25 women with advanced TNBC reported a CBR of 36% and median PFS was 3.3 months, with thrombocytopenia the most common dose limiting toxicity requiring carboplatin dose reduction [93]. Other clinical trials are ongoing (Table 5) [94,95].
5.7 MAPK kinase/MEK
Although Ras and Raf are not mutated at a high frequency in TNBC, there is evidence of MAPK pathway activation in the disease, thought to be caused by multiple mechanisms including activation of upstream RTKs, and/or activating mutations in proteins upstream. Suppression of MEK induces a compensatory feedback effect that activates a range of upstream RTKs in TNBC [96] although this phenomenon is widespread among signaling inhibitors and also occurs in other malignancies. A novel design preoperative clinical study compared the kinomes of TNBC tumors pre- and post-treatment with the MEK1/2 inhibitor GSK1120212 (trametinib) and demonstrated that MEK1/2 inhibition induced differential RTK activation in basal versus mesenchymal TNBC, indicating that the pattern of feedback is dictated by molecular phenotype. Importantly, a subset of kinases were shared between both subtypes affording identification of potential pathways of acute resistance and enabling rational combinations with MEK inhibitors to be proposed [97,98].
One of the pathways known to be activated in response to MEK inhibition is PI3K/Akt [99], which has led to numerous pre-clinical studies evaluating the efficacy of MEK inhibitors in combination with PI3K/mTOR pathway inhibitors in TNBC [100] and other malignancies [101,102]. Ongoing work will more precisely characterize the anti-tumor effects of different combinations of MEK with PI3K pathway inhibitors in the BL and mesenchymal subtypes of TNBC [103].
5.8 Checkpoint kinase 1 inhibition
Checkpoint kinase 1 (Chk-1) inhibitors have become an attractive potential target for the treatment of TNBC-harboring p53 mutations. In addition to TCGA several studies have identified high rates of p53 mutations in TNBC. In this scenario cells in need of DNA damage repair rely on Chk1 to arrest the cell cycle and push potentially defective cells toward apoptosis. Also, p53-deficient mouse models of breast cancer were shown sensitive to Chk-1 inhibition [104,105].
5.9 Immune modulation
Results from recent studies suggest a potential value of immune modulation in treating breast cancer patients with an aggressive biology. Tumor-infiltrating lymphocytes (TIL) were reported to be prognostic and also predictive in TNBC. With regard to prognosis, Loi et al. [106] showed using primary tumor samples from the BIG 02–98 study that every 10% increase in intramural: i) and stromal TILs (sTILs) was associated with 17 and 15% reduced risk of relapse (p = 0.10 and p =. 0025), respectively, and 27 and 17% reduced risk of death (p = 0.035 and p = 0.023), respectively, in patients with TNBC. Similar results were reported from the combined analysis of adjuvant trial E2197 and E1199. sTIL was positively correlated with distant recurrence-free survival and OS [107]. With regard to prediction, a prospective analysis in the GeparSixto trial showed that 60% of 142 patients with lymphocyte predominant breast cancer achieved pCR when compared with 40% of all the women in the study and 34% of women with low levels of infiltrating lymphocytes (p < 0.0005). The predictive effect for response to NAC was particularly high in patients treated with carboplatin. In fact a 74% pCR was reported for lymphocyte predominant TNBC patients treated with carboplatin plus paclitaxel/doxorubicin [108]. In PreCOG 0105, both sTILs and iTILs were shown to be predictive of response to platinum-based neoadjuvant therapy and were associated with the IM subtype [109].
Several different immunotherapy strategies, aiming at blocking or activating specific targets, are currently under study including antagonists against inhibitory up-regulated receptors on antitumor T cells, such as programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte antigen 4 (CTLA-4) [110]. Recent evidence for specific tumor antigens, such as MUC-1 and NY-ESO-1, led to the development targetable vaccine antigens. Early-phase trials targeting the T-cell inhibitory molecule PD-L1 have shown clinical efficacy in cancer [111]. The TCGA RNA sequencing data showed significantly greater expression of the PD-L1 gene in TNBC compared to non-TNBC, and a recent report showed an association of biomarkers involved in immune evasion including PD-L1 with other biological pathways. Results suggest that subsets of TNBC such as AR-negative might derive benefit from PD-L1 and CTLA-4 targeted therapy. The positive correlation of PIK3CA and PD-L1 also indicates that combination therapy targeting both pathways may be beneficial [112]. In addition, the inverse correlation of BRCA1 with PD-L1 suggests a potential role for platinum-based therapy in combination with anti-PD-L1. Further prospective validation of these findings is ongoing [113].
6. Ongoing randomized Phase II–III trials
Table 5 summarizes selected active or planned randomized Phase II–III clinical trials in TNBC. Of particular interest is the EA1131 Phase III trial coordinated by the ECOG-ACRIN Research Group that will be conducted by the U.S. National Clinical Trials Network. This trial will determine whether platinum monotherapy improves EFS in patients with residual BL TNBC when given after completing a taxane-containing neoadjuvant regimen and local therapy. The lack of achieving a pCR will be used as a pharmacodynamic biomarker to enrich for individual patients with BL breast cancer who have a high risk of recurrence and disease that is more sensitive to platinums [46], and also spare the 30 – 40% of patients who achieve a pCR with standard therapy and would otherwise have an excellent prognosis. The successful completion of this trial and additional analysis of the Alliance and GeparSixto trials will add another important chapter in this evolving story and improve our ability to predict the future for individual patients.
7. Conclusion
At the present time, available treatment options for TNBC remain limited; however a significant amount of ongoing research is focusing on the identification and characterization of ‘drugable’ targets and pathways that contribute to the aggressive biology. Continued, concerted efforts in this area will ensure the evolution of novel strategies in the management of TNBC, with the ultimate long-term goal of replacing generic standard of care therapy with rationally derived treatment regimens.
Studies of BRCA1 mutated breast cancers suggest sensitivity to DNA damaging chemotherapeutics such as cisplatin or carboplatin, and to PARP inhibitors used alone or in combination with DNA-damaging agents.
Inhibitors of the PI3K/AKT/mTOR pathway are also being evaluated because this pathway is commonly dysregulated in TNBC.
Lymphocytic infiltration is associated with improved response to NAC and better prognosis in TNBC, raising the possibility of using immune checkpoint blockage as a therapeutic strategy.
8. Expert opinion
TNBC is a molecular diverse clinical entity that has poor prognosis, for which chemotherapy remains the cornerstone of standard treatment. Recent delineation of the molecular heterogeneity of the disease rationalizes its diverse biological behavior and differential response to chemotherapy. Although still at the drawing board, the feeling in the clinical and translational community is that we have traversed a barrier in our understanding of TNBC that now needs to be translated into clinical practice.
Breast cancer was one of the first malignancies for which personalized approaches to therapeutics was pioneered decades ago, with the successful implementation of Her2- and ER-targeting strategies. Presently, we are still evolving a stratified medicine approach for diagnosis and treatment, as exemplified by the now routine genotyping of patients with familial disease for BRCA1/2 mutations, and the recent call for larger genomic screening of all TNBC patients under 60 including those with sporadic disease. We have been successful in assigning BRCA1/2 mutant patients largely to the TNBC cohort, and concerted efforts are underway to enroll these patients into clinical trials of novels therapies with the advent of PARP inhibitors. Coupled with the rapid development of functional assays and cheaper technologies to survey genomic alterations relevant to disease biology, the revolution is well and truly upon us.
Efforts to define molecular ‘dependencies’ upon which tumors are inherently reliant for survival, such as delineation of the AR pathway participation in the LAR subtype of TNBC and dependency of the DNA damage repair pathway in BRCA-defective tumors and BL1 subtypes, have resulted in the evaluation of promising new systemic therapies that are essentially new indications for ‘older’ FDA-approved drugs, such as the use of platinums and anti-AR therapies for the treatment of BL1 and AR/luminal-like subtypes, respectively.
Intertwined with these successes, we have gained a deeper appreciation of the therapeutic challenges that accompany the clinical management of other TNBC subtypes, such as mesenchymal and mesenchymal stem-like tumors that are intrinsically ‘hard-wired’ to migrate and invade, and BL2 subtypes that are inherently chemoresistant. The clinical management of these recalcitrant tumors requires the focus and persistence of innovative pre-clinical research, coupled with commitment to translate novel findings into novel clinical trials, such as window-of-opportunity designs. There is no doubt that emerging research gives the field real hope. Novel targets are being elucidated, some of which have great potential for therapeutic development, including PI3K/mTOR pathway alterations frequently found in some TNBCs. The biggest challenge we face as a community is acceptance and commitment to changing current clinical practice. Standard NAC has served a proportion of TNBC patients well; however the time is upon us to move toward implementation of molecular testing at diagnosis to define the genetic ‘fingerprint’ and accompanying dependencies of the tumors we seek to eliminate. We also need to evolve the mindset of how we manage TNBC for the long-term, such that we treat it as a chronic disease. Successful implementation of this will only be realized by a commitment to define and re-define the molecular signature of a given tumor at multiple points along its evolutionary lineage so that therapy is tailored to a changing tumor microenvironment. This can be achieved through novel tissue collection protocols and window-of-opportunity clinical trial designs whereby oncologists, surgeons and scientists synergistically collaborate.
highlights.
Triple negative breast cancer (TNBC) is a heterogeneous disease associated with higher risk of recurrence than ER-positive or HER2/neu-positive disease, for which chemotherapy is the only treatment option.
Recent research has illuminated the biologic heterogeneity of the disease and provided insight into identifying other potentially effective systemic therapies, including cytotoxic agents and other therapies that exploit cancers defective in DNA damage repair.
Evaluation for germ-line BRCA mutations is recommend as a standard of care for all women with TNBC younger than 60 years of age, providing a foundation for using this as a biomarker to identify subjects who have tumors that may be particularly sensitive to DNA-damaging agents and/or PARP inhibitors.
Molecular biomarker-based patient selection in early-phase trial has the potential to accelerate anticancer drug development.
Acting upon the molecular heterogeneity of the disease. Re-defined endpoints of clinical benefit (Disease-free survival and overall survival) in patients with high-risk biology:pCR
Next-generation oncology trials: presurgical and neoadjuvant setting as a testing platform. A major aspect of this innovative direction is the impact of modern technology platforms.
This box summarizes key points contained in the article.
Acknowledgments
HM McDaid and JA Sparano receive research funding from the Breast Cancer Research Foundation. This work was supported by National Cancer Institute Grant CA077263 (HMD). JA Sparano is supported by Genentech, Roche, Eisai, Pfizer, Celgene, Celldex Therapeutics and Eli Lilly.
Footnotes
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- 1.Bauer KR, Brown M, Cress RD, et al. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109(9):1721–8. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 2.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version) Arch Pathol Lab Med. 2010;134(7):e48–72. doi: 10.5858/134.7.e48. [DOI] [PubMed] [Google Scholar]
- 3.Brown M, Tsodikov A, Bauer KR, et al. The role of human epidermal growth factor receptor 2 in the survival of women with estrogen and progesterone receptor-negative, invasive breast cancer: the California Cancer Registry, 1999–2004. Cancer. 2008;112(4):737–47. doi: 10.1002/cncr.23243. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen TO, Hsu FD, Jensen K, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10(16):5367–74. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 5.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 6.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429–34. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 7.Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26(8):1275–81. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 8.Smid M, Wang Y, Zhang Y, et al. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008;68(9):3108–14. doi: 10.1158/0008-5472.CAN-07-5644. [DOI] [PubMed] [Google Scholar]
- 9.Lakhani SR, Van De Vijver MJ, Jacquemier J, et al. The pathology of familial breast cancer: predictive value of immunohistochemical markers estrogen receptor, progesterone receptor, HER-2, and p53 in patients with mutations in BRCA1 and BRCA2. J Clin Oncol. 2002;20(9):2310–18. doi: 10.1200/JCO.2002.09.023. [DOI] [PubMed] [Google Scholar]
- 10.Turner NC, Reis-Filho JS, Russell AM, et al. BRCA1 dysfunction in sporadic basal-like breast cancer. Oncogene. 2007;26(14):2126–32. doi: 10.1038/sj.onc.1210014. [DOI] [PubMed] [Google Scholar]
- 11.Turner N, Tutt A, Ashworth A. Hallmarks of ’BRCAness’ in sporadic cancers. Nat Rev Cancer. 2004;4(10):814–19. doi: 10.1038/nrc1457. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Iliopoulos D, Zhang Q, et al. XBP1 promotes triple-negative breast cancer by controlling the HIF1alpha pathway. Nature. 2014;508(7494):103–7. doi: 10.1038/nature13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121(7):2750–67. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prat A, Perou CM. Deconstructing the molecular portraits of breast cancer. Mol Oncol. 2011;5(1):5–23. doi: 10.1016/j.molonc.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98(19):10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parker JS, Mullins M, Cheang MC, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27(8):1160–7. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361(2):123–34. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 18.Kriege M, Seynaeve C, Meijers-Heijboer H, et al. Sensitivity to first-line chemotherapy for metastatic breast cancer in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2009;27(23):3764–71. doi: 10.1200/JCO.2008.19.9067. [DOI] [PubMed] [Google Scholar]
- 19.Kriege M, Jager A, Hooning MJ, et al. The efficacy of taxane chemotherapy for metastatic breast cancer in BRCA1 and BRCA2 mutation carriers. Cancer. 2012;118(4):899–907. doi: 10.1002/cncr.26351. [DOI] [PubMed] [Google Scholar]
- 20.Prat A, Parker JS, Karginova O, et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010;12(5):R68. doi: 10.1186/bcr2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Creighton CJ, Li X, Landis M, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci USA. 2009;106(33):13820–5. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cancer Genome Atlas N. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah SP, Roth A, Goya R, et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 2012;486(7403):395–9. doi: 10.1038/nature10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banerji S, Cibulskis K, Rangel-Escareno C, et al. Sequence analysis of mutations and translocations across breast cancer subtypes. Nature. 2012;486(7403):405–9. doi: 10.1038/nature11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andre F, Bachelot T, Commo F, et al. Comparative genomic hybridisation array and DNA sequencing to direct treatment of metastatic breast cancer: a multicentre, prospective trial (SAFIR01/UNICANCER) Lancet Oncol. 2014;15(3):267–74. doi: 10.1016/S1470-2045(13)70611-9. [DOI] [PubMed] [Google Scholar]
- 26.Dawood S, Broglio K, Gonzalez-Angulo AM, et al. Trends in survival over the past two decades among white and black patients with newly diagnosed stage IV breast cancer. J Clin Oncol. 2008;26(30):4891–8. doi: 10.1200/JCO.2007.14.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Availablr from: http://www.nccn.org
- 28.Gianni L, Norton L, Wolmark N, et al. Role of anthracyclines in the treatment of early breast cancer. J Clin Oncol. 2009;27(28):4798–808. doi: 10.1200/JCO.2008.21.4791. [DOI] [PubMed] [Google Scholar]
- 29.Hayes DF, Thor AD, Dressler LG, et al. HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med. 2007;357(15):1496–506. doi: 10.1056/NEJMoa071167. [DOI] [PubMed] [Google Scholar]
- 30.R P The worldwide overview: provisional results of updated (2005–2006) meta-analyses of trials. Proceeding from the San Antonio meeting, Eleni. Breast Cancer Res Treat. 2007;106:S1. [Google Scholar]
- 31.Hugh J, Hanson J, Cheang MC, et al. Breast cancer subtypes and response to docetaxel in node-positive breast cancer: use of an immunohistochemical definition in the BCIRG 001 trial. J Clin Oncol. 2009;27(8):1168–76. doi: 10.1200/JCO.2008.18.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones S, Holmes FA, O’Shaughnessy J, et al. Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US oncology research trial 9735. J Clin Oncol. 2009;27(8):1177–83. doi: 10.1200/JCO.2008.18.4028. [DOI] [PubMed] [Google Scholar]
- 33.Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358(16):1663–71. doi: 10.1056/NEJMoa0707056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas ES, Gomez HL, Li RK, et al. Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol. 2007;25(33):5210–17. doi: 10.1200/JCO.2007.12.6557. [DOI] [PubMed] [Google Scholar]
- 35.Twelves C, Cortes J, Vahdat LT, et al. Phase III trials of eribulin mesylate (E7389) in extensively pretreated patients with locally recurrent or metastatic breast cancer. Clin Breast Cancer. 2010;10(2):160–3. doi: 10.3816/CBC.2010.n.023. [DOI] [PubMed] [Google Scholar]
- 36.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–72. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez-Angulo AM, Timms KM, Liu S, et al. Incidence and outcome of BRCA mutations in unselected patients with triple receptor-negative breast cancer. Clin Cancer Res. 2011;17(5):1082–9. doi: 10.1158/1078-0432.CCR-10-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hurley J, Reis IM, Rodgers SE, et al. The use of neoadjuvant platinum-based chemotherapy in locally advanced breast cancer that is triple negative: retrospective analysis of 144 patients. Breast Cancer Res Treat. 2013;138(3):783–94. doi: 10.1007/s10549-013-2497-y. [DOI] [PubMed] [Google Scholar]
- 39.Prowell TM, Pazdur R. Pathological complete response and accelerated drug approval in early breast cancer. N Engl J Med. 2012;366(26):2438–41. doi: 10.1056/NEJMp1205737. [DOI] [PubMed] [Google Scholar]
- 40.Rugo HS, Olopade O, DeMichele A, et al. Veliparib/carboplatin plus standard neoadjuvant therapy for high-risk breast cancer: First efficacy results from the I-SPY 2 TRIAL[abstract S5-02]; Presented at: the 36th Annual San Antonio Breast Cancer Symposium; 10 – 14 December; San Antonio, TX. [Google Scholar]
- 41.Gronwald J, Byrski T, Huzarski T, et al. Neoadjuvant therapy with cisplatin in BRCA1-positive breast cancer patients. J Clin Oncol (Meeting Abstracts) 2009;27(15 Suppl) doi: 10.1007/s10549-008-0128-9. abstract502. [DOI] [PubMed] [Google Scholar]
- 42.Silver DP, Richardson AL, Eklund AC, et al. Efficacy of neoadjuvant cisplatin in triple-negative breast cancer. J Clin Oncol. 2010;28(7):1145–53. doi: 10.1200/JCO.2009.22.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Telli M. Optimizing chemotherapy in triple-negative breast cancer: the role of platinum. Am Soc Clin Oncol Educ Book. 2014:e37–42. doi: 10.14694/EdBook_AM.2014.34.e37. [DOI] [PubMed] [Google Scholar]
- 44.von Minckwitz G, Schneeweiss A, Loibl S, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 2014;15(7):747–56. doi: 10.1016/S1470-2045(14)70160-3. [DOI] [PubMed] [Google Scholar]
- 45.Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of carboplatin (Cb) and/or bevacizumab (B) to neoadjuvant weekly paclitaxel (P) followed by dose-dense AC on pathologic complete response (pCR) rates in triple-negative breast cancer (TNBC): CALGB 40603 (Alliance) [abstract S5-01]; San Antonio Breast Cancer Symposium; 10 – 14 December 2013. [Google Scholar]
- 46.Balko JM, Cook RS, Vaught DB, et al. Profiling of residual breast cancers after neoadjuvant chemotherapy identifies DUSP4 deficiency as a mechanism of drug resistance. Nat Med. 2012;18(7):1052–9. doi: 10.1038/nm.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshida T, Ozawa Y, Kimura T, et al. Eribulin mesilate suppresses experimental metastasis of breast cancer cells by reversing phenotype from epithelial-mesenchymal transition (EMT) to mesenchymal-epithelial transition (MET) states. Br J Cancer. 2014;110(6):1497–505. doi: 10.1038/bjc.2014.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu J, Horwitz SB, McDaid HM. Abstract B165: the antitumor efficacy of eribulin is mediated via suppression of invasion and profound induction of cell death in triple negative breast cancer. Mol Cancer Ther. 2013;12(11 Suppl):B165. [Google Scholar]
- 49.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 50.von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30(15):1796–804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 51.Buchholz TA, Hill BS, Tucker SL, et al. Factors predictive of outcome in patients with breast cancer refractory to neoadjuvant chemotherapy. Cancer J. 2001;7(5):413–20. [PubMed] [Google Scholar]
- 52.Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25(28):4414–22. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 53.Dwadasi STY, Walsh T, Danso MA, et al. Cisplatin with or without rucaparib after preoperative chemotherapy in patients with triple-negative breast cancer (TNBC): Hoosier Oncology Group BRE09-146. J Clin Oncol. 2014;32(5 Suppl) abstract1019^. [Google Scholar]
- 54.Chen X, Li J, Gray WH, et al. TNBCtype: a subtyping tool for triple-negative breast cancer. Cancer Inform. 2012;11:147–56. doi: 10.4137/CIN.S9983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Livasy CA, Karaca G, Nanda R, et al. Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Mod Pathol. 2006;19(2):264–71. doi: 10.1038/modpathol.3800528. [DOI] [PubMed] [Google Scholar]
- 56.Carey LA, Rugo HS, Marcom PK, et al. TBCRC 001: randomized phase II study of cetuximab in combination with carboplatin in stage IV triple-negative breast cancer. J Clin Oncol. 2012;30(21):2615–23. doi: 10.1200/JCO.2010.34.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Shaughnessy JWD, Vukelja SJ, McIntyre K, et al. Preliminary results of a randomized phase II study of weekly irinotecan/carboplatin with or without cetuximab in patients with metastatic breast cancer [abstract 308] Breast Cancer Res Treat. 2007;106(Suppl 1):S32. [Google Scholar]
- 58.Baselga J, Gomez P, Greil R, et al. Randomized phase II study of the anti-epidermal growth factor receptor monoclonal antibody cetuximab with cisplatin versus cisplatin alone in patients with metastatic triple-negative breast cancer. J Clin Oncol. 2013;31(20):2586–92. doi: 10.1200/JCO.2012.46.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dickler MN, Cobleigh MA, Miller KD, et al. Efficacy and safety of erlotinib in patients with locally advanced or metastatic breast cancer. Breast Cancer Res Treat. 2009;115(1):115–21. doi: 10.1007/s10549-008-0055-9. [DOI] [PubMed] [Google Scholar]
- 60.Gutteridge E, Agrawal A, Nicholson R, et al. The effects of gefitinib in tamoxifen-resistant and hormone-insensitive breast cancer: a phase II study. Int J Cancer. 2010;126(8):1806–16. doi: 10.1002/ijc.24884. [DOI] [PubMed] [Google Scholar]
- 61.Miller K, Wang M, Gralow J, et al. Paclitaxel plus Bevacizumab versus Paclitaxel Alone for Metastatic Breast Cancer. N Engl J Med. 2007;357(26):2666–76. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 62.Miles DW, Chan A, Dirix LY, et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28(20):3239–47. doi: 10.1200/JCO.2008.21.6457. [DOI] [PubMed] [Google Scholar]
- 63.Robert NJ, Dieras V, Glaspy J, et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol. 2011;29(10):1252–60. doi: 10.1200/JCO.2010.28.0982. [DOI] [PubMed] [Google Scholar]
- 64.Miles DW, Dieras V, Cortes J, et al. First-line bevacizumab in combination with chemotherapy for HER2-negative metastatic breast cancer: pooled and subgroup analyses of data from 2447 patients. Ann Oncol. 2013;24(11):2773–80. doi: 10.1093/annonc/mdt276. [DOI] [PubMed] [Google Scholar]
- 65.Burstein HJ, Elias AD, Rugo HS, et al. Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2008;26(11):1810–16. doi: 10.1200/JCO.2007.14.5375. [DOI] [PubMed] [Google Scholar]
- 66.Crown JP, Dieras V, Staroslawska E, et al. Phase III trial of sunitinib in combination with capecitabine versus capecitabine monotherapy for the treatment of patients with pretreated metastatic breast cancer. J Clin Oncol. 2013;31(23):2870–8. doi: 10.1200/JCO.2012.43.3391. [DOI] [PubMed] [Google Scholar]
- 67.von Minckwitz G, Eidtmann H, Rezai M, et al. Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer. N Engl J Med. 2012;366(4):299–309. doi: 10.1056/NEJMoa1111065. [DOI] [PubMed] [Google Scholar]
- 68.Bear HD, Tang G, Rastogi P, et al. Bevacizumab added to neoadjuvant chemotherapy for breast cancer. N Engl J Med. 2012;366(4):310–20. doi: 10.1056/NEJMoa1111097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cameron D, Brown J, Dent R, et al. Adjuvant bevacizumab-containing therapy in triple-negative breast cancer (BEATRICE): primary results of a randomised, phase 3 trial. Lancet Oncol. 2013;14(10):933–42. doi: 10.1016/S1470-2045(13)70335-8. [DOI] [PubMed] [Google Scholar]
- 70.Ame JC, Spenlehauer C, de Murcia G. The PARP superfamily. BioEssays. 2004;26(8):882–93. doi: 10.1002/bies.20085. [DOI] [PubMed] [Google Scholar]
- 71.Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434(7035):913–17. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 72.Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917–21. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 73.Ratnam K, Low JA. Current development of clinical inhibitors of poly (ADP-ribose) polymerase in oncology. Clin Cancer Res. 2007;13(5):1383–8. doi: 10.1158/1078-0432.CCR-06-2260. [DOI] [PubMed] [Google Scholar]
- 74.O’Shaughnessy J, Osborne C, Pippen JE, et al. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med. 2011;364(3):205–14. doi: 10.1056/NEJMoa1011418. [DOI] [PubMed] [Google Scholar]
- 75.O’Shaughnessy JSL, Danso MA. A randomized phase III study of iniparib (BSI-201) in combination with gemcitabine/carboplatin (G/C) in metastatic triple-negative breast cancer (TNBC) J Clin Oncol. 2011;29(Suppl) abstract 1007. [Google Scholar]
- 76.Liu X, Shi Y, Maag DX, et al. Iniparib nonselectively modifies cysteine-containing proteins in tumor cells and is not a bona fide PARP inhibitor. Clin Cancer Res. 2012;18(2):510–23. doi: 10.1158/1078-0432.CCR-11-1973. [DOI] [PubMed] [Google Scholar]
- 77.Patel AG, De Lorenzo SB, Flatten KS, et al. Failure of iniparib to inhibit poly (ADP-Ribose) polymerase in vitro. Clin Cancer Res. 2012;18(6):1655–62. doi: 10.1158/1078-0432.CCR-11-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376(9737):235–44. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 79.Gelmon KA, Tischkowitz M, Mackay H, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011;12(9):852–61. doi: 10.1016/S1470-2045(11)70214-5. [DOI] [PubMed] [Google Scholar]
- 80.Kinders R, Parchment RE, Ji J, et al. Phase 0 clinical trials in cancer drug development: from FDA guidance to clinical practice. Mol Interv. 2007;7(6):325–34. doi: 10.1124/mi.7.6.9. [DOI] [PubMed] [Google Scholar]
- 81.Isakoff SJOB, Tung NM, Gelman RM, et al. A phase II trial of the PARP inhibitor veliparib (ABT888) and temozolomide for metastatic breast cancer. J Clin Oncol. 2010;28(15 Suppl) abstract 1019. [Google Scholar]
- 82.Doane AS, Danso M, Lal P, et al. An estrogen receptor-negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene. 2006;25(28):3994–4008. doi: 10.1038/sj.onc.1209415. [DOI] [PubMed] [Google Scholar]
- 83.Gucalp A, Tolaney S, Isakoff SJ, et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic Breast Cancer. Clin Cancer Res. 2013;19(19):5505–12. doi: 10.1158/1078-0432.CCR-12-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Keen JC, Yan L, Mack KM, et al. A novel histone deacetylase inhibitor, scriptaid, enhances expression of functional estrogen receptor alpha (ER) in ER negative human breast cancer cells in combination with 5-aza 2’-deoxycytidine. Breast Cancer Res Treat. 2003;81(3):177–86. doi: 10.1023/A:1026146524737. [DOI] [PubMed] [Google Scholar]
- 85.Tate CR, Rhodes LV, Segar HC, et al. Targeting triple-negative breast cancer cells with the histone deacetylase inhibitor panobinostat. Breast Cancer Res. 2012;14(3):R79. doi: 10.1186/bcr3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Connolly RM, Jeter S, Zorzi J, et al. A multi-institutional double-blind phase II study evaluating response and surrogate biomarkers to carboplatin and nab-paclitaxel (CP) with or without vorinostat as preoperative systemic therapy (PST) in HER2-negative primary operable breast cancer (TBCRC008) J Clin Oncol. 2010;28(15 Suppl) abstractTPS111. [Google Scholar]
- 87.Marty B, Maire V, Gravier E, et al. Frequent PTEN genomic alterations and activated phosphatidylinositol 3-kinase pathway in basal-like breast cancer cells. Breast Cancer Res. 2008;10(6):R101. doi: 10.1186/bcr2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yunokawa M, Koizumi F, Kitamura Y, et al. Efficacy of everolimus, a novel mTOR inhibitor, against basal-like triple-negative breast cancer cells. Cancer Sci. 2012;103(9):1665–71. doi: 10.1111/j.1349-7006.2012.02359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Juvekar A, Burga LN, Hu H, et al. Combining a PI3K inhibitor with a PARP inhibitor provides an effective therapy for BRCA1-related breast cancer. Cancer Discov. 2012;2(11):1048–63. doi: 10.1158/2159-8290.CD-11-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ibrahim YH, Garcia-Garcia C, Serra V, et al. PI3K inhibition impairs BRCA1/2 expression and sensitizes BRCA-proficient triple-negative breast cancer to PARP inhibition. Cancer Discov. 2012;2(11):1036–47. doi: 10.1158/2159-8290.CD-11-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366(6):520–9. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gonzalez-Angulo AM, Akcakanat A, Liu S, et al. Open-label randomized clinical trial of standard neoadjuvant chemotherapy with paclitaxel followed by FEC versus the combination of paclitaxel and everolimus followed by FEC in women with triple receptor-negative breast cancer dagger. Ann Oncol. 2014;25(6):1122–7. doi: 10.1093/annonc/mdu124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Singh JC, Stein S, Volm M, et al. RAD001-carboplatin combination in triple-negative metastatic breast cancer (TNMBC): A phase II trial. J Clin Oncol. 2013;31(Suppl) abstract1042. [Google Scholar]
- 94.NCT01629615 SAToBaPKIiPWTNMBCCgi.
- 95.NCT01300962 LSoBoBaCiPWMBCCgi.
- 96.Hoeflich KP, O’Brien C, Boyd Z, et al. In vivo antitumor activity of MEK and phosphatidylinositol 3-kinase inhibitors in basal-like breast cancer models. Clin Cancer Res. 2009;15(14):4649–64. doi: 10.1158/1078-0432.CCR-09-0317. [DOI] [PubMed] [Google Scholar]
- 97.Johnson LGAK, Duncan JS, Whittle M, et al. Kinome reprogramming response to MEK inhibition: a window-of-opportunity trial in triple-negative breast cancer (TNBC) J Clin Oncol. 2013;31(Suppl) abstract 512. [Google Scholar]
- 98.Duncan JS, Whittle MC, Nakamura K, et al. Dynamic reprogramming of the kinome in response to targeted MEK inhibition in triple-negative breast cancer. Cell. 2012;149(2):307–21. doi: 10.1016/j.cell.2012.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mirzoeva OK, Das D, Heiser LM, et al. Basal subtype and MAPK/ERK kinase (MEK)-phosphoinositide 3-kinase feedback signaling determine susceptibility of breast cancer cells to MEK inhibition. Cancer Res. 2009;69(2):565–72. doi: 10.1158/0008-5472.CAN-08-3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Roberts PJ, Usary JE, Darr DB, et al. Combined PI3K/mTOR and MEK inhibition provides broad antitumor activity in faithful murine cancer models. Clin Cancer Res. 2012;18(19):5290–303. doi: 10.1158/1078-0432.CCR-12-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Engelman JA, Chen L, Tan X, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14(12):1351–6. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Legrier ME, Yang CP, Yan HG, et al. Targeting protein translation in human non small cell lung cancer via combined MEK and mammalian target of rapamycin suppression. Cancer Res. 2007;67(23):11300–8. doi: 10.1158/0008-5472.CAN-07-0702. [DOI] [PubMed] [Google Scholar]
- 103.Schweber SJ, Rodriguez-LaRocca AG, Calvert V, et al. Protein pathway activation mapping guided biomarker development to identify optimal combinations of MEK inhibitor with PI3K/mTOR pathway inhibitors for the treatment of triple-negative breast cancer. J Clin Oncol. 2013;31(Suppl) abstract 2612. [Google Scholar]
- 104.Chen MS, Ryan CE, Piwnica-Worms H. Chk1 kinase negatively regulates mitotic function of Cdc25A phosphatase through 14-3-3 binding. Mol Cell Biol. 2003;23(21):7488–97. doi: 10.1128/MCB.23.21.7488-7497.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ma CX, Cai S, Li S, et al. Targeting Chk1 in p53-deficient triple-negative breast cancer is therapeutically beneficial in human-in-mouse tumor models. J Clin Invest. 2012;122(4):1541–52. doi: 10.1172/JCI58765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31(7):860–7. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 107.Adams S, Gray RJ, Demaria S, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32(27):2959–66. doi: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Denkert CLS, Salat C, et al. Increased tumor-associated lymphocytes predicts benefit from addition of carboplatin to neoadjuvant therapy for triple-negative and HER2-positive early breast cancer in the GeparSixto trial (GBG 66/AGO-B) [abstract S1-06]; San Antonio Breast Cancer Symposium; Presented; 11 December 2013.2013. [Google Scholar]
- 109.Vinayak SGR, Adams S, Jensen KC, et al. Association of increased tumor-infiltrating lymphocytes (TILs) with immunomodulatory (IM) triple-negative breast cancer (TNBC) subtype and response to neoadjuvant platinum-based therapy in PrECOG0105. J Clin Oncol. 2014;32(5 Suppl) abstract 1000^. [Google Scholar]
- 110.Stagg J, Allard B. Immunotherapeutic approaches in triple-negative breast cancer: latest research and clinical prospects. Ther Adv Med Oncol. 2013;5(3):169–81. doi: 10.1177/1758834012475152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Soliman H, Khalil F, Antonia S. PD-L1 expression is increased in a subset of basal type breast cancer cells. PLoS One. 2014;9(2):e88557. doi: 10.1371/journal.pone.0088557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Basu GD, Ghazalpour A, Gatalica Z, et al. Expression of novel immunotherapeutic targets in triple-negative breast cancer. J Clin Oncol. 2014;32(5 Suppl) abstract 1001. [Google Scholar]