Abstract
Objective
To determine if minority trauma patients are more commonly treated at trauma centers (TCs) with worse observed-to-expected survival.
Background
Racial disparities in survival after traumatic injury have been described. However, the mechanisms that lead to these inequities are not well understood.
Methods
Analysis of Level I/II TCs included in the National Trauma Data Bank 2007–2010. White, Black and Hispanic patients ≥16 years sustaining blunt/penetrating injuries with an Injury Severity Score ≥9 were included. TC’s with ≥ 50% Hispanic or Black patients classified as predominantly-minority TCs. Multivariate logistic regression adjusting for several patient/injury characteristics was used to predict the expected number of deaths for each TC. Observed-to-expected (O/E) mortality ratios were then generated and used to rank individual TCs as low (O/E <1), intermediate or high mortality (O/E>1).
Results
556,720 patients from 181 TCs were analyzed; 86(48%) TCs were classified as low mortality, 6(3%) intermediate and 89(49%) as high mortality. More of the predominantly-minority TCs {(82%(22/27) versus 44%(67/154)}were high mortality (p<0.001). Approximately 64%(55,673/87,575) of Black patients were treated at high mortality TCs compared to 54%(32,677/60,761) Hispanics and 41%(165,494/408,384) Whites(p<0.001).
Conclusions
Minority trauma patients are clustered at hospitals with significantly higher-than-expected mortality. Black and Hispanic patients treated at low mortality hospitals have a significantly lower odds of death compared to similar patients treated at high mortality hospitals. Differences in trauma center outcomes and quality of care may partially explain trauma outcomes disparities.
Introduction
Racial disparities in outcomes have been well described for a host of surgical conditions, including traumatic injury in the United States (US) (1–10). A recent meta-analysis suggests that Black and uninsured patients are more likely to die after trauma, even after controlling for factors such as patient demographics, severity of injury and pre-injury co-morbidities (11). Trauma is the leading cause of death for ages 1–44 in America, and is now estimated as the third largest contributor to healthcare disparities in the US, making it an urgent national priority (12,13). Ascertaining the exact mechanisms that lead to the disparities in trauma outcomes is a critical prerequisite in designing and implementing interventions aimed at reducing racial inequities in health care.
Empirical evidence from other areas in health care and prior trauma literature cite poor access to care, discrepant health care utilization, pre-existing medical conditions and potential provider biases as some of the reasons for these disparate outcomes (11,14). Along with these patient and provider factors, increasingly, institutional and health system associated parameters have also been described as significant contributors to racial disparities (15,16). Hospitals serving a disproportionately higher number of minority patients, often located in underprivileged urban neighborhoods, have been shown to perform poorly on multiple patient outcomes for a variety of conditions (16,17). For example, using national Medicare data, Lucas et al demonstrated that hospitals treating a large proportion of Black patients had a substantially higher rate of post-operative mortality following 8 cardiovascular and cancer procedures including coronary artery bypass, abdominal aortic aneurysm repair and esophagectomy (18). Similarly, patients treated at high Black-serving hospitals have been shown to have a significantly greater risk-adjusted mortality rate following acute myocardial infarction (19). Institutional level findings have also been reported for trauma outcomes. Using data from the National Trauma Data Bank (NTDB), it has been demonstrated that moderate to severely injured patients treated at predominantly-minority hospitals (where >50% of patients were minorities) had a 37% higher risk of mortality compared to those treated at predominantly-majority hospitals, after adjusting for known confounders (20). Together, these reports from multiple areas of health-care, including trauma, suggest that factors related to quality of care contribute significantly to racial disparities in the United States.
Improving quality of care provides an intuitive avenue towards reducing morbidity, mortality and costs for all patients, including minorities and others, who are at risk for disparate outcomes(21). Over the past several years, organizations such as the American Association for the Surgery of Trauma (AAST) and the American College of Surgeons (ACS) Committee on Trauma (COT) have paid significant attention towards performance improvement (PI) and the quality of trauma care by developing a systematic approach that focuses on outcomes assessment (22–27). A central method for this has been to compare trauma center performance using risk adjusted analyses so that centers can evaluate themselves in relation to their peers and strive to improve or maintain excellent outcomes (28,29). Such an approach is extremely useful in the identification of institutional factors that lead to better or varying outcomes and can help in designing, implementing and modifying hospital-based PI initiatives aimed at improving quality of care (QoC).
Previous patient level analyses have demonstrated that individuals treated at trauma centers with a high proportion of minority patients have increased mortality (20). However, the relationship between trauma center performance and racial disparities in trauma mortality is not known. In this current analysis, we attempt to bring together a hospital level analysis of trauma centers and a patient level analysis of survival among individuals of different races. We hypothesize that predominantly-minority hospitals are likely to also be high mortality hospitals, and this clustering of minority patients at these high mortality centers may be a driver for racial disparities seen at the national level. The primary objective of this study is to determine if minority trauma patients are more commonly treated at trauma centers with worse observed-to-expected survival. The secondary objective is to determine if a minority patient is more likely to survive an injury if he or she is treated at a facility with an overall low mortality.
Methods
For this study data from the National Trauma Data Bank (NTDB) was used. The NTDB is maintained by the ACS and now comprises over 2.5 million patient records contributed by over 900 centers from across the United States (30). In 2007, the NTDB adopted the National Trauma Data Standard which has significantly standardized and improved the quality of data (31). Therefore only data from the years 2007–2010 were analyzed.
White, Black and Hispanic adults (≥16 years) from level I/II trauma centers (TCs) with blunt/penetrating injuries and an Injury Severity Score ≥9 were included. Patients who were dead-on-arrival or had unknown hospital discharge information were excluded. TCs reporting >20% missing data for race or any of the variables used in risk adjustment were excluded, as were facilities with <100 eligible patients. TCs treating ≥50% Black or Hispanic patients were classified as predominantly-minority TCs and those with >50% White patients classified as predominantly majority. For additional analyses, hospitals were also classified by tertiles of percent penetrating trauma and uninsured patients treated.
Observed-to-expected (O/E) mortality ratios were used to categorize trauma centers as: high, intermediate or low mortality centers (Figure 1). To generate, O/E ratios we first performed multivariate logistic regression analysis, with mortality as the outcome, to predict the expected number of deaths at each TC. Patient level co-variates included in this model were: age, gender, type of injury (blunt versus penetrating), presence of hypotension at admission (systolic blood pressure <90 mmHg), pulse rate at admission, total Glasgow Coma Scale, Injury Severity Score (ISS), presence of severe head injury [Abbreviated Injury Scale (AIS) ≥3] and need for ventilator use. ISS derived from facility reported AIS version 1998 was used for 97.3% of patients for whom it was available. This ISS (ISSAIS) was chosen since it provided the best discriminative ability, both alone and in conjunction with other model covariates, than all other ISS types reported in the dataset. For the few cases for which this was not available, ISS was derived from AIS scores calculated using the ICD/AIS map, ICDMAP-90, 1995 update (computer program: ICODERI.DLL, Windows version. Johns Hopkins University, 1997). Multiple imputation using previously described techniques was used to impute any further missing data (32,33). Model discrimination and calibration was assessed using area under the receiver operator characteristic curve (AuROC) and calibration curves, respectively. Calibration curves were preferred over Hosmer-Lemeshow goodness-of-fit test since the latter has been demonstrated to be overly sensitive to even the slightest departure from model fit (34). Using this model, the individual patient probabilities of mortality were estimated and then summed for each TC to generate the total number of “expected deaths” (E). The observed (O), or actual, number of deaths were then divided by E to generate an O/E mortality ratio for each TC and with 95% confidence intervals (95% CI). This 95% CI was calculated using the normal approximation method with the following formula; O/E±zα/2√(variance of O/E), where zα/2=1.96 for 95% CI (35). Based on these O/E ratios, each center was classified as: low mortality (O/E <1), intermediate mortality (O/E 95% CI overlapping 1) or high mortality (O/E >1).
Figure 1.
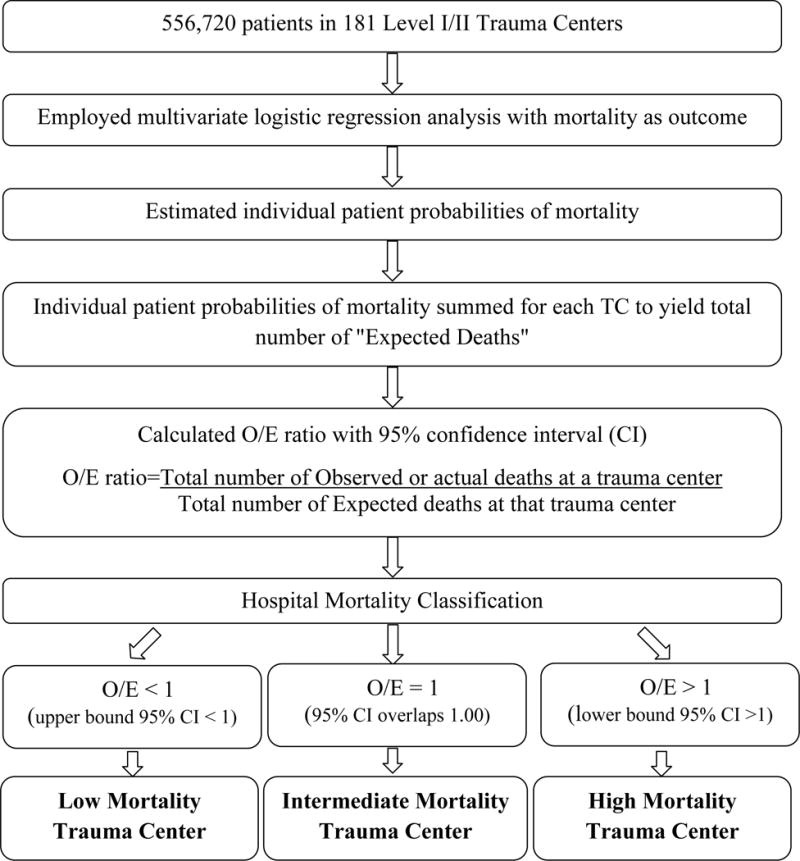
Schema for generating hospital mortality classification using observed-to-expected (O/E) mortality ratios.
The association between hospital mortality classification and race was analyzed in several ways. Baseline patient demographics, including race and insurance status, and injury severity characteristics were compared across varying levels of hospital mortality. Univariate analysis was performed to investigate hospital and patient level clustering of minority patients at high mortality hospitals. We further explored this by assessing the proportions of hospitals in the highest tertiles of percent uninsured and penetrating trauma patients treated by hospital mortality and minority classification. Additionally, using multivariate logistic regression, we estimated the adjusted odds of survival for patients who were treated at high mortality TCs compared to similarly injured patients of the same race/ethnicity treated at a low mortality hospital. Patient level co-variates used in this model were the same as above and clustering by unique hospital ID was performed to account for patient correlation within TCs.
A sensitivity analysis was performed to compare our results to the methodology for hospital benchmarking reported by the ACS Trauma Quality Improvement Program (TQIP) (20). The TQIP program focuses on identifying outliers. Accordingly, only a few hospitals are classified as low or high performers, with most facilities deemed to be an average performer. For this sensitivity analysis, trauma centers were ranked using risk-adjusted O/E mortality ratios as described above and the 95% CIs were generated using Byar’s approximation of exact Poisson distributed observed deaths [lower bound CI=O/E[1− (1/9O) − zα/2/(3√O)]3 and upper bound CI=O+1/E[1− (1/9(O+1)+ zα/2/(3√(O+1))]3] (36). Based on these O/E ratios, each center was classified using the TQIP terminology as: high performing (upper bound 95% CI less than 1), average performing (95% CI overlapping 1) or low performing (lower bound 95% CI greater than 1).
All analyses were performed using Stata12/MP statistical software package (StataCorp, College Station, TX).
Results
The NTDB 2007–2010 contained a total of 2,539,818 patients from 773 hospitals. After excluding hospitals and patients as described in Figure 2, a total of 556,720 patients from 181 ACS/State verified level I/II trauma centers were available for analysis. Table 1 describes the baseline patient demographics and injury severity characteristics by hospital mortality classification. Compared to patients at low and average mortality centers, patients at high mortality centers were younger and sustained more penetrating injuries. Approximately one third of the patients at high mortality centers were Black or Hispanic compared to one fifth at low mortality centers.
Figure 2.
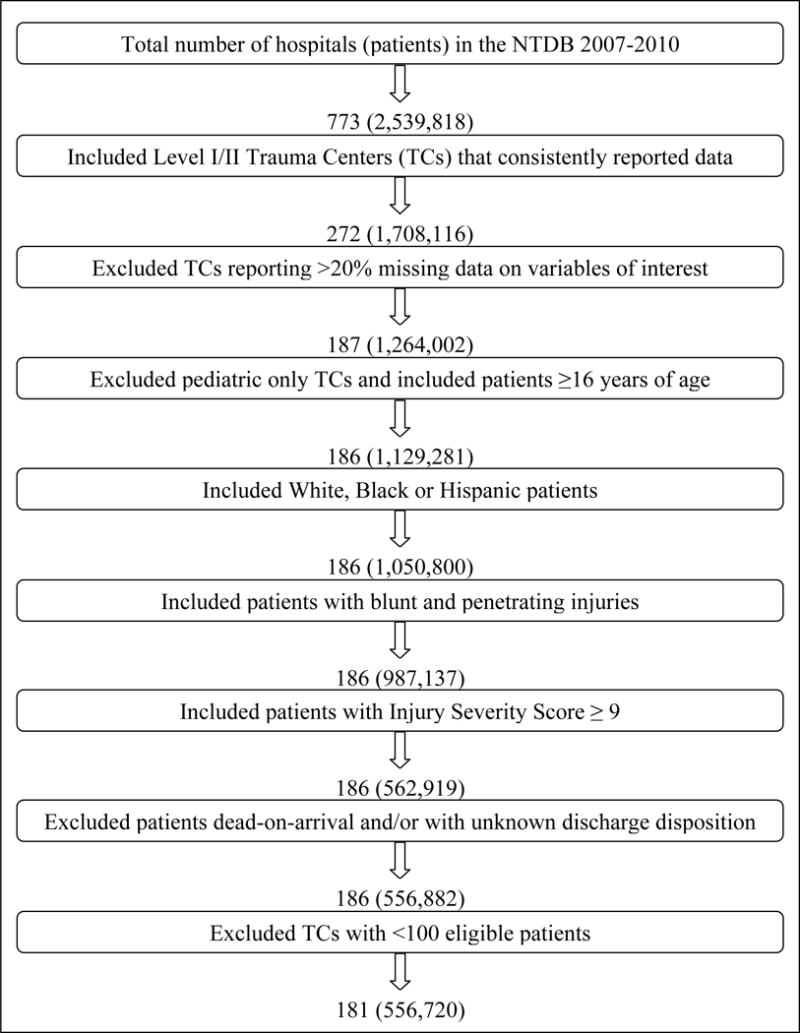
Flowchart describing hospital and patient selection.
Table 1.
Baseline patient demographics and injury severity characteristics by hospital mortality classification.
| Characteristic | Low Mortality 283,625 patients 86 TCs |
Intermediate Mortality 19,251 patients 6 TCs |
High Mortality 253,844 patients 89 TCs |
p value |
|---|---|---|---|---|
| All patients, % | 51.0 | 3.5 | 45.6 | |
| Percent mortality | 6.4 | 7.8 | 8.5 | <0.001 |
| Mean age in years | 51 | 52 | 47 | <0.001 |
| Percent males | 63.2 | 61.5 | 69.0 | <0.001 |
| Race | ||||
| White | 80.8 | 71.4 | 65.2 | <0.001 |
| Black | 9.9 | 20.7 | 21.9 | |
| Hispanic | 9.4 | 8.0 | 12.9 | |
| Type of injury | ||||
| Blunt | 92.8 | 91.0 | 86.2 | <0.001 |
| Penetrating | 7.2 | 9.0 | 13.8 | |
| Hypotensive on arrival (systolic blood pressure <90 mmHg) | 4.6 | 4.5 | 6.3 | <0.001 |
| Total Glasgow Coma Scale | ||||
| 3 | 9.9 | 11.4 | 9.5 | <0.001 |
| 4–8 | 2.3 | 2.0 | 3.5 | |
| 9–12 | 2.7 | 2.5 | 3.5 | |
| 13–15 | 85.2 | 84.0 | 83.5 | |
| Injury Severity Score | ||||
| 9–15 | 56.6 | 59.5 | 57.5 | <0.001 |
| 16–24 | 25.5 | 24.9 | 25.3 | |
| 25–75 | 17.9 | 15.6 | 17.3 | |
| Head Abbreviated Injury Scale ≥3 | 32.7 | 29.8 | 32.2 | <0.001 |
Note: p value is for comparing, both low mortality to intermediate and then low mortality to high mortality facilities for each group.
Figure 3 demonstrates the risk-adjusted mortality based O/E ranking of ACS/State verified level I/II trauma centers. The multivariate model demonstrated excellent discrimination (AuROC = 0.94) and adequate calibration (assessed using calibration curves). 86(48%) TCs were ranked as low mortality, 6(3%) as intermediate and 89(49%) as high mortality. Approximately 15%(27/181) centers were classified as predominantly-minority. A nearly two-fold difference was observed in the proportions of predominantly-minority TCs classified as high mortality versus predominantly-majority TCs classified as high mortality [81.5%(22/27) versus 43.5%(67/154), respectively (p<0.05)] (Table 2). Similarly, Figure 4 demonstrates the patient level clustering of racial minorities at high mortality TCs. A greater proportion of Black and Hispanic patients were treated at high mortality facilities compared to White patients.
Figure 3.
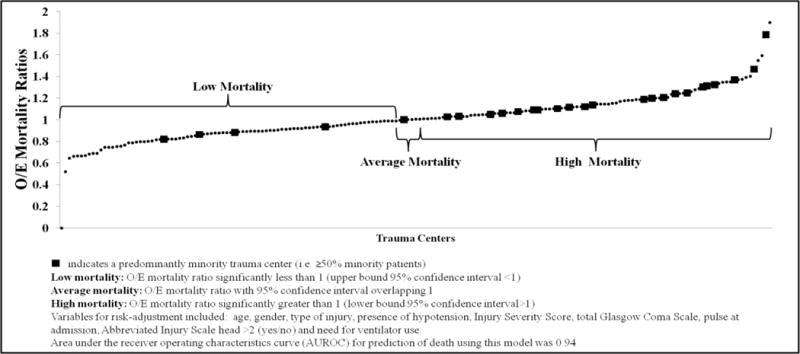
Risk-adjusted observed-to-expected (O/E) mortality ranking of ACS/State verified Level I/II Trauma Centers.
Table 2.
Percentage of trauma centers (TCs) classified as high mortality by proportion of minorities treated at the hospital, (comparison significant at p<0.05).
| Trauma Centers | High Mortality | Low/Intermediate Mortality |
|---|---|---|
| Predominantly-minority, %(n) | 81.5 (22/27) | 18.5 (5/27) |
| Predominantly-majority, %(n) | 43.5 (67/154) | 56.5 (87/154) |
Figure 4.
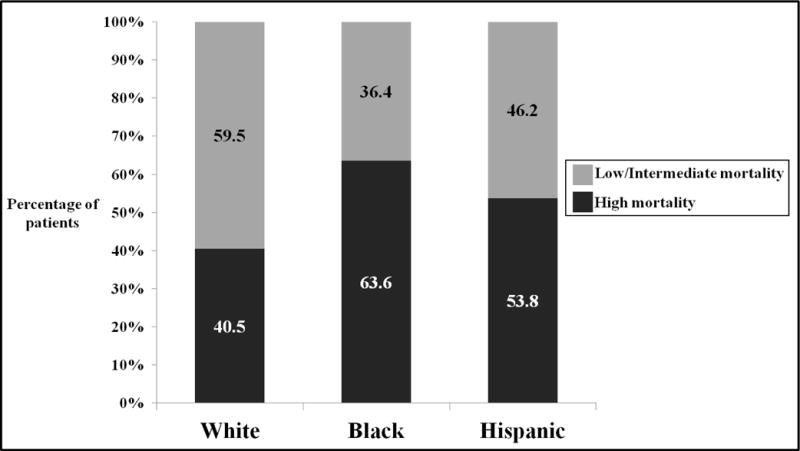
Proportion of each race/ethnicity patients treated at low/intermediate and high mortality trauma centers (all comparisons significant at p<0.05).
Table 3 describes the distribution of uninsured and penetrating trauma patients by trauma center mortality and minority classification. A greater than 2-fold difference was observed between the proportions of low versus high mortality TCs classified among the highest tertile of percentage uninsured patients treated. Similarly, a greater proportion of high mortality TCs were classified among the highest tertile of percentage penetrating trauma patients treated versus low mortality TCs. When examined by hospital minority classification, a greater proportion of predominantly-minority TCs were found to be centers also classified among the highest tertile of uninsured and penetrating trauma patients treated.
Table 3.
Proportions of low, intermediate and high mortality trauma centers, and predominantly-minority and predominantly-majority trauma centers classified among the highest tertile of percent uninsured and percent penetrating trauma patients treated
| Proportion of Trauma Centers with highest tertile of | ||||
|---|---|---|---|---|
| Uninsured Patients | Penetrating Trauma Patients | |||
| By hospital mortality classification | Percentage % | p value | Percentage% | p value |
| Low mortality | 20.9 | <0.05 | 14.0 | <0.05 |
| Intermediate mortality | 33.3 | 16.7 | ||
| High mortality | 44.9 | 52.8 | ||
| By hospital minority classification | ||||
| Predominantly-minority Trauma Centers | 51.9 | <0.05 | 88.9 | <0.05 |
| Predominantly-majority Trauma Centers | 29.9 | 23.4 | ||
Figure 5 demonstrates the adjusted odds ratios of mortality for patients from each race/ethnicity when treated at a low mortality TC compared to a high mortality TC (reference group). White, Black and Hispanic trauma patients treated at a low mortality TCs each had a greater than 40% survival advantage compared to patients of similar race/ethnicity and equivalent injuries treated at a high mortality hospital.
Figure 5.
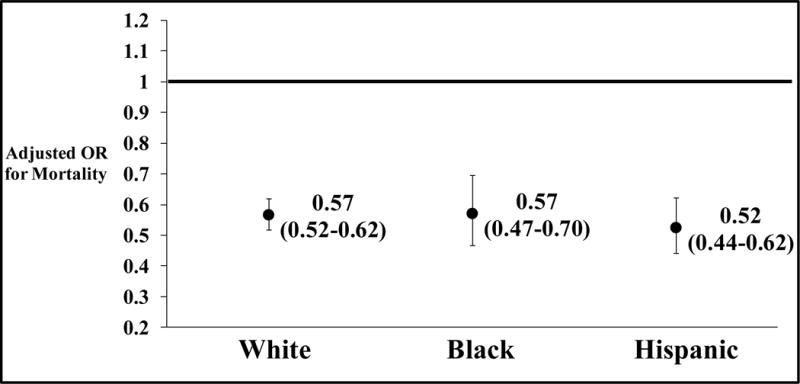
Adjusted odds ratios of mortality for patients, by race/ethnicity, treated at a low mortality trauma center compared to patients of the same race/ethnicity treated at a high mortality trauma center.
Co-variates used to risk-adjust were: age, gender, type of injury (blunt versus penetrating), presence of hypotension at admission (systolic blood pressure <90 mmHg), pulse rate at admission, total Glasgow Coma Scale, Injury Severity Score, presence of severe head injury [Abbreviated Injury Scale (AIS) ≥3] and need for ventilator use. The black line represents the OR for the reference group i.e. patients of the same race/ethnicity treated at high mortality trauma centers.
Figure 6 describes the results of the sensitivity analyses using the ACS TQIP-adapted methodology. Using this methodology, 34(19%) TCs were found to be high performing, 114 (63%) average and 33(18%) low performing. Although the absolute proportions of predominantly-minority and majority TCs with worse outcomes differed compared to the main analysis, the results were qualitatively the same. A similar two-fold difference between the proportions of predominantly-minority TCs classified as high mortality versus predominantly-majority TCs classified as high mortality [33.3% (9/27) versus 15.6% (24/154), respectively (p<0.05)] was observed.
Figure 6.
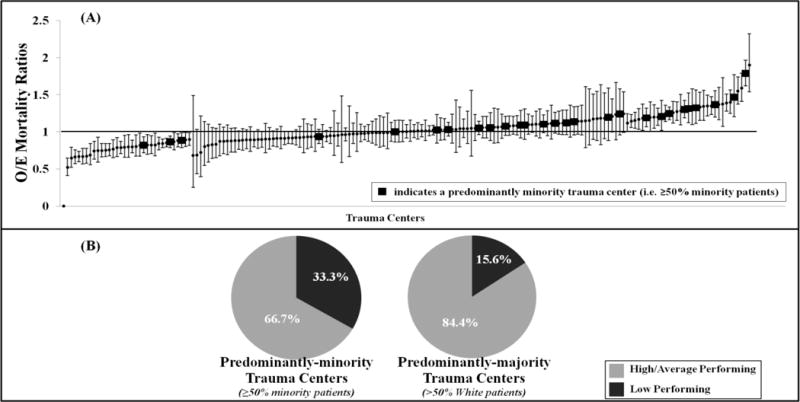
Sensitivity analyses using American College of Surgeons Trauma Quality Improvement Program (ACS TQIP) trauma center performance evaluation: A)Trauma centers ranked using observed-to-expected (O/E) mortality ratios with 95% confidence intervals; B)Percentage of trauma centers (TCs) classified as low performing by proportions of minorities treated at the hospital (comparison significant at p<0.05).
Discussion
This study describes the association between trauma center outcomes and racial disparities in mortality after injury. The results demonstrate that nearly 80% of predominantly minority serving trauma centers can also be classified as high mortality trauma centers, as they have a high observed to expected in-hospital mortality ratio. These high mortality centers also revealed a clustering of uninsured and penetrating trauma patients. Additionally, patients from all race/ethnic groups studied (Black, Hispanic and White) appear to be 40% less likely to die, if they are treated at a low mortality trauma center, compared to patients of the same race/ethnicity with equivalent injuries treated at a high mortality center.
Disparities in trauma outcomes have always been somewhat surprising, given its perceived universal access and the highly protocolized nature of trauma management plans. Several previous studies have demonstrated significant differences in risk adjusted survival between trauma treating institutions (11,20). These are similar to other areas of healthcare that demonstrate marked variations in hospital quality of care (QoC) for both surgical and non-surgical conditions (17–19). For example, Breslin et al, in their recent risk-adjusted evaluation of 5-year survival following surgery for breast and colon cancer reported that hospital factors, including quality of care, explained up to 54% of the excess Black mortality compared to White patients (37). In agreement with this previous body of literature, the results from the present study suggest that trauma center quality of care contributes significantly towards racial disparities in trauma mortality in the United States.
Our findings present an excellent opportunity to mitigate racial disparities in trauma outcomes by improving QoC. Systematic improvements in QoC have been shown to increase patient survival and reduce morbidity and costs for both traumatic and non-traumatic conditions (38–41). Moreover, evidence from outside of trauma suggests that, in addition to improving outcomes for all patients, quality improvement (QI) initiatives may help mitigate racial disparities (42,43). For example, Trivedi et al, while evaluating outcomes for Medicare beneficiaries in managed-care plans, describe that substantial improvements in QoC were paralleled by a significant decrease in racial disparities for most QoC measures assessed (44). Similarly, Cohen and colleagues determined that in hospitals participating in a national quality improvement program, evidence-based acute myocardial infarction care improved progressively over time and racial disparities in care were either reduced or eliminated entirely (45). More recently, Parsons et al, have reported that minority cancer patients treated at hospitals participating in the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) had similar 30-day post-operative outcomes as their White counterparts (46). Although, similar formal evaluations for trauma outcomes need to be undertaken, these and other studies demonstrate that trauma QI initiatives have great potential for ameliorating racial disparities.
A successful model aimed at improving the quality of trauma care is the ACS COT Trauma Quality Improvement Program (TQIP), which was initiated in 2006 to “improve quality of trauma care through robust risk-adjusted benchmarking of trauma centers (27).” Such benchmarking enables direct comparison of a participating hospital’s annual performance with that of its peers, which incentivizes improvements in QoC. Additionally, TQIP conducts site visits to study institutional factors directly affecting trauma center performance. Structural and procedural factors at high performing centers can then be applied to all centers in an attempt to improve patient outcomes. While these concerted efforts will certainly help in improving outcomes for all patients and may reduce racial disparities following trauma, there is also an acute need to study why some predominantly-minority institutions have a lower mortality than their high mortality counterparts with similar patient populations. Identifying potential institutional differences, for example in processes of care, expertise or availability of adjunct paramedical human resources, will constitute a critical step towards designing interventions aimed at reducing between-hospitals racial disparities.
An important consideration in improving trauma QoC is providing hospitals with adequate resources to undertake QI initiatives. Our results show that centers with higher mortality were also the centers treating the highest proportions of uninsured patients. Since the Donabedian model suggests that QoC is a summation of structure, process and outcome, we tend to equate high mortality with low QoC (47). However, the disproportionate number of uninsured patients treated at centers with higher mortality (Table 3) suggests that intense resource utilizing amenities such as: high ICU nurse:patient ratio, high blood bank transfusion capacities, and 24-hour pharmacist presence as part of the trauma critical care team may be less likely at these centers. Furthermore, the clustering of penetrating trauma patients, who more frequently require immediate surgical interventions, transfusions and ICU care, are an additional financial burden on these high mortality centers.
There is also concern that recently introduced, pay-for-performance programs will inappropriately curtail financially intensive QI initiatives at poor performing, under-resourced, predominantly-minority hospitals caring for uninsured patients and further worsen outcomes (17). Conversely though, failing to incentivize positive processes and outcomes may undermine the performance of similarly resourced, high-performing, predominantly-minority hospitals and inappropriately discourage their QI undertakings. In order to further clarify this tension between allocation of resources and accountability, a formal assessment of hospital efficiency using methods such as stochastic frontier analyses may be warranted (48).
Recent work has demonstrated that systemic undertakings that focus on improving overall efficiency are superior to simple patient or provider centered approaches (49–55). Such initiatives need to be rooted in multi-disciplinary efforts drawing upon experiences in clinical medicine, systems biology, sociology, industrial psychology, human factor engineering, health information technology, economics, epidemiology and bioinformatics (56). This approach has been effective, and has brought success in multiple other areas of health-care and services delivery (57–62). Hence, an application of this approach to improve trauma QI efficiency is warranted and may improve QoC and reduce disparities.
Our results indicate that low performing trauma centers also cluster a greater proportion of penetrating trauma patients. This may lead to the common perception that the high penetrating trauma volume at these institutions puts them at a selective disadvantage compared to their peers. However, the previously described O/E mortality-based techniques used in our analyses employs risk-adjustment models to predict the expected number of deaths at a center, given their particular patient case-mix and compares it to observed or actual number of deaths (27). The O/E mortality ratio, in essence, normalizes the hospital performance, and Shafi et al, have demonstrated that despite differences in type of injury between centers, this system provides a fair comparison (63).
Our results are at odds with a long held, but not directly proven assumption that racial disparities in multiple health-care areas could solely be explained by patient factors, particularly comorbidities. This less controversial view distracts from important underlying provider effects, including differential hospital QoC. In fact, recent studies suggests that although patient factors such as comorbidities and socioeconomic status, contribute significantly towards racial disparities, these do not offer a complete explanation of disparate outcomes (64,65). Therefore, it is critically important to maintain a holistic view and include considerations of QoC while exploring reasons for previously documented racial disparities.
In order to understand the relationship between trauma center mortality outcomes and race, in this analysis we purposely chose a statistical methodology which provides a narrow 95% confidence interval around the calculation of an individual trauma center’s O/E mortality ratio. This makes it easier to categorize centers as either high mortality or low mortality centers with very few centers classified as intermediate. On the other hand the ACS TQIP program uses a methodology that has wider confidence intervals for the same that classifies the majority of trauma centers as average performers. This is done as TQIP is interested in performance measures that identify outliers and this technique ensures that only outliers are judged to be high or low performing. This is different from our approach of identifying high mortality and low mortality centers. While the two methodologies differ in their construction of confidence intervals, they are both validated techniques used by state authorities and the ACS, respectively (27,35,36). However, in an effort to ensure that our analyses can be compared to the TQIP practices we also conducted a sensitivity analysis replicating the TQIP approach. As expected, the number of average/intermediate performing trauma centers significantly increased and the number of high mortality and low mortality trauma centers decreased accordingly. However, qualitatively the results did not change as nearly twice the proportion of predominantly minority serving trauma centers were judged to be outliers and classified as poor performers (33%) compared with predominantly majority facilities (only 16% classified as poor performers, p<0.05)[compared with 81.5% of the predominantly-minority centers versus 43.5% of the predominantly-majority centers classified as high mortality, (p<0.05) using the methods described for the main analysis]. The inclusion of both these methods enabled us to confirm our hypothesis using a spectrum of corroborated results, thus obviating overreliance on a singular metric.
There are several important limitations of this work. We used data from the NTDB, which is largely a convenience sample of voluntarily submitted trauma data from participating institutions. Some previously reported limitations of this dataset include: 1)patients who die at the scene or are discharged from the emergency room without a hospital admission are not recorded; 2)lack of depth on clinically important variables (e.g. amount of blood transfusions) and inconsistent charting may create a potential for residual and/or unknown confounding; 3)inconsistent reporting of data regarding diagnostics, interventions, comorbidities and potential patient safety events, including complications; 4)only discharge level data is collected, hence post-discharge outcomes cannot be tracked; 5)substantial amounts of missing data on important variables like Glasgow Coma Scale; 6)limited in its ability to assess for outcomes other than mortality. More specifically, comorbidity information is not completely reported in the NTDB and therefore was not considered when performing risk adjustment. We attempted to minimize each of these limitations in several different ways. We used data from the NTDB for the year 2007 onwards, as following implementation of the National Trauma Data Standard, the quality of data has improved substantially due to an institution of guidelines facilitating consistent data collection and reporting procedures. We restricted our analysis to level I/II centers as data reporting from these centers is much more reliable. Additionally, level I centers are now required by the ACS to submit data to the NTDB as part of their verification process and up to 95% of level I centers now submit their data. We used multiple imputation to handle missing data using previously validated procedures (32,33). We used standardized techniques to build our risk-adjustment model to predict the expected number of deaths for each center. Prior NTDB-based evaluations suggest that trauma center rankings are affected by few important patient characteristics only and are not influenced by patient comorbidities (28,66). This present analysis accounted for most of these important characteristics (such as ISS, age, gender, systolic blood pressure, head AIS). Therefore, despite the absence of some important predictors of mortality, such as comorbidities, our risk-adjustment model demonstrated excellent discrimination (as assessed by AuROC) and calibration (as assessed using calibration curves). These model performance statistics equaled those reported for the TQIP methodology to classify trauma center performance as high, average or low using widely accepted O/E mortality ratios (AuROC=0.94 versus TQIP model AuROC=0.93). For this present study, we restricted our analysis to mortality outcomes only since this is the most widely used measure to benchmark trauma center performance and most of the racial disparities in trauma outcomes have been reported using this outcome. However, in the course of future research, we hope to focus on other trauma outcomes such as complications, patient safety events and/or failure to rescue.
In conclusion, this large database evaluation of level I/II trauma centers in the U.S. demonstrates that differences in trauma center outcomes, at least partially, explain racial disparities in trauma mortality. Additionally, Black, White and Hispanic patients treated at low mortality centers appear to enjoy the same survival advantage compared to patients of the same race/ethnicity race with similar injuries treated at a high mortality center. This research suggests that improving quality of care at trauma centers with higher than expected mortality may afford an excellent opportunity to mitigate racial disparities in trauma.
Mini Abstract.
Mechanisms leading to racial disparities in trauma outcomes remain ill-characterized. We describe that minority trauma patients are clustered at hospitals with significantly higher-than-expected mortality. Differences in trauma center outcomes and quality of care may partially explain inequalities in survival after injury.
Acknowledgments
Conflicts of Interest and Source of Funding: National Institutes of Health/NIGMS K23GM093112-01 (Dr Haider)
American College of Surgeons C. James Carrico Fellowship for the study of Trauma and Critical Care (Dr Haider)
National Institute of Health/NHLBI grants K24HL083113 and P50HL0105187 (Dr Cooper)
Footnotes
For Presentation at the American Surgical Association’s 133rd annual meeting, April 5, 2013
Author Contributions
This paper was conducted by authors from multiple institutions and represents the input of an interdisciplinary team of surgeons, (Haider, Zafar, Efron, Haut, Cornwell) biostatisticians, (Hui, Schneider) disparity experts (Cooper, Hashmi) and a Health Policy expert (MacKenzie), which is why we needed to have 10 authors in total.
Adil Haider: Study Design, Data Analysis, Data Interpretation, and Writing of Manuscript
Zain Hashmi: Study Design, Data Analysis, Data Interpretation, and Writing of Manuscript
Syed Nabeel Zafar: Study Design, Data Analysis, Data Interpretation, and Writing of Manuscript
Xuan Hui: Study Design, Data Analysis and Data Interpretation
Eric Schneider: Study Design, Data Analysis and Data Interpretation
David Efron: Study Design, Data Interpretation and Critical Review
Elliott Haut: Study Design, Data Interpretation and Critical Review
Lisa Cooper: Study Design, Data Interpretation and Critical Review
Ellen MacKenzie: Study Design, Data Interpretation and Critical Review
Edward Cornwell: Study Design, Data Interpretation and Critical Review
References
- 1.Ricciardi R, Selker HP, Baxter NN, et al. Disparate use of minimally invasive surgery in benign surgical conditions. Surgical endoscopy. 2008 Sep;22(9):1977–86. doi: 10.1007/s00464-008-0003-0. [DOI] [PubMed] [Google Scholar]
- 2.Kim DH, Daskalakis C, Lee AN, et al. Racial disparity in the relationship between hospital volume and mortality among patients undergoing coronary artery bypass grafting. Annals of surgery. 2008 Nov;248(5):886–92. doi: 10.1097/SLA.0b013e318189b1bc. [DOI] [PubMed] [Google Scholar]
- 3.Kim J, Artinyan A, Mailey B, et al. An interaction of race and ethnicity with socioeconomic status in rectal cancer outcomes. Annals of surgery. 2011 Apr;253(4):647–54. doi: 10.1097/SLA.0b013e3182111102. [DOI] [PubMed] [Google Scholar]
- 4.Morris AM, Wei Y, Birkmeyer NJO, et al. Racial disparities in late survival after rectal cancer surgery. Journal of the American College of Surgeons. 2006 Dec;203(6):787–94. doi: 10.1016/j.jamcollsurg.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Greenstein AJ, Litle VR, Swanson SJ, et al. Racial disparities in esophageal cancer treatment and outcomes. Annals of surgical oncology. 2008 Mar;15(3):881–8. doi: 10.1245/s10434-007-9664-5. [DOI] [PubMed] [Google Scholar]
- 6.Curry WT, Carter BS, Barker FG. Racial, ethnic, and socioeconomic disparities in patient outcomes after craniotomy for tumor in adult patients in the United States, 1988–2004. Neurosurgery. 2010 Mar;66(3):427–37. doi: 10.1227/01.NEU.0000365265.10141.8E. discussion 437–8. [DOI] [PubMed] [Google Scholar]
- 7.Rosen H, Saleh F, Lipsitz S, et al. Downwardly mobile: the accidental cost of being uninsured. Archives of surgery (Chicago, Ill: 1960) 2009 Nov;144(11):1006–11. doi: 10.1001/archsurg.2009.195. [DOI] [PubMed] [Google Scholar]
- 8.Arthur M, Hedges JR, Newgard CD, et al. Racial disparities in mortality among adults hospitalized after injury. Medical care. 2008 Feb;46(2):192–9. doi: 10.1097/MLR.0b013e31815b9d8e. [DOI] [PubMed] [Google Scholar]
- 9.Bowman SM, Martin DP, Sharar SR, et al. Racial disparities in outcomes of persons with moderate to severe traumatic brain injury. Medical care. 2007 Jul;45(7):686–90. doi: 10.1097/MLR.0b013e31803dcdf3. [DOI] [PubMed] [Google Scholar]
- 10.Glance LG, Osler TM, Mukamel DB, et al. Trends in Racial Disparities for Injured Patients Admitted to Trauma Centers. Health services research. 2013 May 13; doi: 10.1111/1475-6773.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haider AH, Weygandt PL, Bentley JM, et al. Disparities in trauma care and outcomes in the United States: A systematic review and meta-analysis. The journal of trauma and acute care surgery. 2013 May;74(5):1195–205. doi: 10.1097/TA.0b013e31828c331d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. Web-based Injury Statistics Query and Reporting System (WISQARS) [Internet] [cited 2013 Mar 30]. Available from: http://www.cdc.gov/injury/wisqars.
- 13.Wong MD, Shapiro MF, Boscardin WJ, et al. Contribution of major diseases to disparities in mortality. The New England journal of medicine. 2002 Nov 14;347(20):1585–92. doi: 10.1056/NEJMsa012979. [DOI] [PubMed] [Google Scholar]
- 14.Haider AH, Scott VK, Rehman KA, et al. Racial disparities in surgical care and outcomes in the United States: a comprehensive review of patient, provider, and systemic factors. Journal of the American College of Surgeons. 2013 Mar;216(3):482–92.e12. doi: 10.1016/j.jamcollsurg.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasnain-Wynia R, Baker DW, Nerenz D, et al. Disparities in health care are driven by where minority patients seek care: examination of the hospital quality alliance measures. Archives of internal medicine. 2007 Jun 25;167(12):1233–9. doi: 10.1001/archinte.167.12.1233. [DOI] [PubMed] [Google Scholar]
- 16.Baicker K, Chandra A, Skinner JS. Geographic variation in health care and the problem of measuring racial disparities. Perspectives in biology and medicine. 2005 Jan;48(1 Suppl):S42–53. [PubMed] [Google Scholar]
- 17.Ly DP, Lopez L, Isaac T, et al. How do black-serving hospitals perform on patient safety indicators? Implications for national public reporting and pay-for-performance. Medical care. 2010 Dec;48(12):1133–7. doi: 10.1097/MLR.0b013e3181f81c7e. [DOI] [PubMed] [Google Scholar]
- 18.Lucas FL, Stukel TA, Morris AM, et al. Race and surgical mortality in the United States. Annals of surgery. 2006 Mar;243(2):281–6. doi: 10.1097/01.sla.0000197560.92456.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skinner J, Chandra A, Staiger D, et al. Mortality after acute myocardial infarction in hospitals that disproportionately treat black patients. Circulation. 2005 Oct 25;112(17):2634–41. doi: 10.1161/CIRCULATIONAHA.105.543231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haider AH, Ong’uti S, Efron DT, et al. Association between hospitals caring for a disproportionately high percentage of minority trauma patients and increased mortality: a nationwide analysis of 434 hospitals. Archives of surgery (Chicago, Ill: 1960) 2012 Jan;147(1):63–70. doi: 10.1001/archsurg.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiscella K, Franks P, Gold MR, et al. Inequality in quality: addressing socioeconomic, racial, and ethnic disparities in health care. JAMA: the journal of the American Medical Association. 2000 May 17;283(19):2579–84. doi: 10.1001/jama.283.19.2579. [DOI] [PubMed] [Google Scholar]
- 22.Nathens AB, Cryer HG, Fildes J. The American College of Surgeons Trauma Quality Improvement Program. The Surgical clinics of North America. 2012 Apr;92(2):441–54. x–xi. doi: 10.1016/j.suc.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Asensio JA, Chahwan S, Forno W, et al. Penetrating esophageal injuries: multicenter study of the American Association for the Surgery of Trauma. The Journal of trauma. 2001 Mar;50(2):289–96. doi: 10.1097/00005373-200102000-00015. [DOI] [PubMed] [Google Scholar]
- 24.Demetriades D, Velmahos GC, Scalea TM, et al. Blunt traumatic thoracic aortic injuries: early or delayed repair–results of an American Association for the Surgery of Trauma prospective study. The Journal of trauma. 2009 Apr;66(4):967–73. doi: 10.1097/TA.0b013e31817dc483. [DOI] [PubMed] [Google Scholar]
- 25.Kuan JK, Wright JL, Nathens AB, et al. American Association for the Surgery of Trauma Organ Injury Scale for kidney injuries predicts nephrectomy, dialysis, and death in patients with blunt injury and nephrectomy for penetrating injuries. The Journal of trauma. 2006 Mar;60(2):351–6. doi: 10.1097/01.ta.0000202509.32188.72. [DOI] [PubMed] [Google Scholar]
- 26.Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. The Journal of trauma. 2003 Jun;54(6 Suppl):S235–310. doi: 10.1007/s00068-003-1288-2. [DOI] [PubMed] [Google Scholar]
- 27.Shafi S, Nathens AB, Cryer HG, et al. The Trauma Quality Improvement Program of the American College of Surgeons Committee on Trauma. Journal of the American College of Surgeons. 2009 Oct;209(4):521–530.e1. doi: 10.1016/j.jamcollsurg.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Hemmila MR, Nathens AB, Shafi S, et al. The Trauma Quality Improvement Program: pilot study and initial demonstration of feasibility. The Journal of trauma. 2010 Mar;68(2):253–62. doi: 10.1097/TA.0b013e3181cfc8e6. [DOI] [PubMed] [Google Scholar]
- 29.Newgard CD, Fildes JJ, Wu L, et al. Methodology and analytic rationale for the American College of Surgeons Trauma Quality Improvement Program. Journal of the American College of Surgeons. 2013 Jan;216(1):147–57. doi: 10.1016/j.jamcollsurg.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 30.American College of Surgeons. National Trauma Data Bank: NTDB Research Data Set Admission Year 2010, Annual Report. Chicago, IL: American College of Surgeons; 2010. [Google Scholar]
- 31.American College of Surgeons. National Trauma Data Standard [Internet] Available from: http://www.ntdsdictionary.org/index.html.
- 32.Glance LG, Osler TM, Mukamel DB, et al. Impact of statistical approaches for handling missing data on trauma center quality. Annals of surgery. 2009 Jan;249(1):143–8. doi: 10.1097/SLA.0b013e31818e544b. [DOI] [PubMed] [Google Scholar]
- 33.Oyetunji TA, Crompton JG, Ehanire ID, et al. Multiple imputation in trauma disparity research. The Journal of surgical research. 2011 Jan;165(1):e37–41. doi: 10.1016/j.jss.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 34.Kramer AA, Zimmerman JE. Assessing the calibration of mortality benchmarks in critical care: The Hosmer-Lemeshow test revisited. Critical care medicine. 2007 Sep;35(9):2052–6. doi: 10.1097/01.CCM.0000275267.64078.B0. [DOI] [PubMed] [Google Scholar]
- 35.Washington State Department of Health. Guidelines for Using Confidence Intervals for Public Health Assessment [Internet] 2012 Available from: http://www.doh.wa.gov/Portals/1/Documents/5500/ConfIntGuide.pdf.
- 36.Breslow NE, Day NE. Statistical Methods in Cancer Research, Volume II: The Design and Analysis of Cohort Studies. New York: Oxford University Press; 1987. [PubMed] [Google Scholar]
- 37.Breslin TM, Morris AM, Gu N, et al. Hospital factors and racial disparities in mortality after surgery for breast and colon cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009 Aug 20;27(24):3945–50. doi: 10.1200/JCO.2008.20.8546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacKenzie EJ, Rivara FP, Jurkovich GJ, et al. A national evaluation of the effect of trauma-center care on mortality. The New England journal of medicine. 2006 Jan 26;354(4):366–78. doi: 10.1056/NEJMsa052049. [DOI] [PubMed] [Google Scholar]
- 39.Marciniak TA, Ellerbeck EF, Radford MJ, et al. Improving the quality of care for Medicare patients with acute myocardial infarction: results from the Cooperative Cardiovascular Project. JAMA: the journal of the American Medical Association. 1998 May 6;279(17):1351–7. doi: 10.1001/jama.279.17.1351. [DOI] [PubMed] [Google Scholar]
- 40.MacKenzie EJ, Weir S, Rivara FP, et al. The value of trauma center care. The Journal of trauma. 2010 Jul;69(1):1–10. doi: 10.1097/TA.0b013e3181e03a21. [DOI] [PubMed] [Google Scholar]
- 41.Khuri SF, Daley J, Henderson WG. The comparative assessment and improvement of quality of surgical care in the Department of Veterans Affairs. Archives of surgery (Chicago, Ill: 1960) 2002 Jan;137(1):20–7. doi: 10.1001/archsurg.137.1.20. [DOI] [PubMed] [Google Scholar]
- 42.Betancourt JR, Duong JV, Bondaryk MR. Strategies to reduce diabetes disparities: an update. Current diabetes reports. 2012 Dec;12(6):762–8. doi: 10.1007/s11892-012-0324-1. [DOI] [PubMed] [Google Scholar]
- 43.Weech-Maldonado R, Elliott M, Pradhan R, et al. Can hospital cultural competency reduce disparities in patient experiences with care? Medical care. 2012 Nov;50(Suppl):S48–55. doi: 10.1097/MLR.0b013e3182610ad1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trivedi AN, Zaslavsky AM, Schneider EC, et al. Trends in the quality of care and racial disparities in Medicare managed care. The New England journal of medicine. 2005 Aug 18;353(7):692–700. doi: 10.1056/NEJMsa051207. [DOI] [PubMed] [Google Scholar]
- 45.Cohen MG, Fonarow GC, Peterson ED, et al. Racial and ethnic differences in the treatment of acute myocardial infarction: findings from the Get With the Guidelines-Coronary Artery Disease program. Circulation. 2010 Jun 1;121(21):2294–301. doi: 10.1161/CIRCULATIONAHA.109.922286. [DOI] [PubMed] [Google Scholar]
- 46.Parsons HM, Habermann EB, Stain SC, et al. What happens to racial and ethnic minorities after cancer surgery at American College of Surgeons National Surgical Quality Improvement Program hospitals? Journal of the American College of Surgeons. 2012 Apr;214(4):539–47. doi: 10.1016/j.jamcollsurg.2011.12.024. discussion 547–9. [DOI] [PubMed] [Google Scholar]
- 47.Donabedian A. Evaluating the quality of medical care. 1966. The Milbank quarterly. 2005 Jan;83(4):691–729. doi: 10.1111/j.1468-0009.2005.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zuckerman S, Hadley J, Iezzoni L. Measuring hospital efficiency with frontier cost functions. Journal of health economics. 1994 Oct;13(3):255–80. doi: 10.1016/0167-6296(94)90027-2. discussion 335–40. [DOI] [PubMed] [Google Scholar]
- 49.Donchin Y, Gopher D, Olin M, et al. A look into the nature and causes of human errors in the intensive care unit. Critical care medicine. 1995 Feb;23(2):294–300. doi: 10.1097/00003246-199502000-00015. [DOI] [PubMed] [Google Scholar]
- 50.Tzeng H-M, Yin CY. Innovation in patient safety: a new task design in reducing patient falls. Journal of nursing care quality. 23(1):34–42. doi: 10.1097/01.NCQ.0000303803.07457.e5. [DOI] [PubMed] [Google Scholar]
- 51.Hudson DW, Holzmueller CG, Pronovost PJ, et al. Toward improving patient safety through voluntary peer-to-peer assessment. American journal of medical quality: the official journal of the American College of Medical Quality. 27(3):201–9. doi: 10.1177/1062860611421981. [DOI] [PubMed] [Google Scholar]
- 52.Gurses AP, Kim G, Martinez EA, et al. Identifying and categorising patient safety hazards in cardiovascular operating rooms using an interdisciplinary approach: a multisite study. BMJ quality & safety. 2012 Oct;21(10):810–8. doi: 10.1136/bmjqs-2011-000625. [DOI] [PubMed] [Google Scholar]
- 53.Gurses AP, Seidl KL, Vaidya V, et al. Systems ambiguity and guideline compliance: a qualitative study of how intensive care units follow evidence-based guidelines to reduce healthcare-associated infections. Quality & safety in health care. 2008 Oct;17(5):351–9. doi: 10.1136/qshc.2006.021709. [DOI] [PubMed] [Google Scholar]
- 54.Carayon P, Schoofs Hundt A, Karsh B-T, et al. Work system design for patient safety: the SEIPS model. Quality & safety in health care. 2006 Dec;15(Suppl 1):i50–8. doi: 10.1136/qshc.2005.015842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haider AH, Pronovost PJ. Health information technology and the collection of race, ethnicity, and language data to reduce disparities in quality of care. Joint Commission journal on quality and patient safety/Joint Commission Resources. 2011 Oct;37(10):435–6. doi: 10.1016/s1553-7250(11)37054-7. [DOI] [PubMed] [Google Scholar]
- 56.Gurses AP, Marsteller JA, Ozok AA, et al. Using an interdisciplinary approach to identify factors that affect clinicians’ compliance with evidence-based guidelines. Critical care medicine. 2010 Aug;38(8 Suppl):S282–91. doi: 10.1097/CCM.0b013e3181e69e02. [DOI] [PubMed] [Google Scholar]
- 57.Pennathur PR, Thompson D, Abernathy JH, et al. Technologies in the wild (TiW): human factors implications for patient safety in the cardiovascular operating room. Ergonomics. 2013 Mar;56(2):205–19. doi: 10.1080/00140139.2012.757655. [DOI] [PubMed] [Google Scholar]
- 58.Martinez EA, Shore A, Colantuoni E, et al. Cardiac surgery errors: results from the UK National Reporting and Learning System. International journal for quality in health care: journal of the International Society for Quality in Health Care/ISQua. 2011 Apr;23(2):151–8. doi: 10.1093/intqhc/mzq084. [DOI] [PubMed] [Google Scholar]
- 59.Ulrich RS, Zimring C, Zhu X, et al. A review of the research literature on evidence-based healthcare design. HERD. 2008 Jan;1(3):61–125. doi: 10.1177/193758670800100306. [DOI] [PubMed] [Google Scholar]
- 60.James KL, Barlow D, McArtney R, et al. Incidence, type and causes of dispensing errors: a review of the literature. The International journal of pharmacy practice. 2009 Mar;17(1):9–30. [PubMed] [Google Scholar]
- 61.Becker F. Nursing unit design and communication patterns: what is “real” work? HERD. 2007 Jan;1(1):58–62. doi: 10.1177/193758670700100115. [DOI] [PubMed] [Google Scholar]
- 62.Smith TJ, Schoenbeck K, Clayton S. Staff perceptions of work quality of a neonatal intensive care unit before and after transition from an open bay to a private room design. Work (Reading, Mass) 2009 Jan;33(2):211–27. doi: 10.3233/WOR-2009-0868. [DOI] [PubMed] [Google Scholar]
- 63.Shafi S, Nathens AB, Parks J, et al. Trauma quality improvement using risk-adjusted outcomes. The Journal of trauma. 2008 Mar;64(3):599–604. doi: 10.1097/TA.0b013e31816533f9. discussion 604–6. [DOI] [PubMed] [Google Scholar]
- 64.Tammemagi CM, Nerenz D, Neslund-Dudas C, et al. Comorbidity and survival disparities among black and white patients with breast cancer. JAMA: the journal of the American Medical Association. 2005 Oct 12;294(14):1765–72. doi: 10.1001/jama.294.14.1765. [DOI] [PubMed] [Google Scholar]
- 65.White A, Vernon SW, Franzini L, et al. Racial disparities in colorectal cancer survival: to what extent are racial disparities explained by differences in treatment, tumor characteristics, or hospital characteristics? Cancer. 2010 Oct 1;116(19):4622–31. doi: 10.1002/cncr.25395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nathens AB, Xiong W, Shafi S. Ranking of trauma center performance: the bare essentials. The Journal of trauma. 2008 Sep;65(3):628–35. doi: 10.1097/TA.0b013e3181837994. [DOI] [PubMed] [Google Scholar]


