Summary
It is well known that there are significant racial disparities in health care outcomes, including surgery. However, the mechanisms that lead to these disparities are still not fully understood. In this comprehensive review of the currently published surgical disparity literature in the United States, we assess racial disparities in outcomes after surgical procedures, focusing on patient, provider, and systemic factors. The PubMed, EMBASE, and Cochrane Library electronic databases were searched with the keywords: healthcare disparities AND surgery AND outcome AND US. Only primary research articles published between April 1990 and December 2011 were included in the study. Studies analyzing surgical patients of all ages and assessing the endpoints of mortality, morbidity, or the likelihood of receiving surgical therapy were included. A total of 88 articles met the inclusion criteria. This evidence-based review was compiled in a systematic manner, relying on retrospective, cross-sectional, case-control, and prospective studies in the absence of Class I studies. The review found that patient factors such as insurance status and socioeconomic status (SES) need to be further explored, as studies indicated only a premature understanding of the relationship between racial disparities and SES. Provider factors such as differences in surgery rates and treatment by low volume or low quality surgeons also appear to play a role in minority outcome disparities. Finally, systemic factors such as access to care, hospital volume, and hospital patient population have been shown to contribute to disparities, with research consistently demonstrating that equal access to care mitigates outcome disparities.
Introduction
Racial disparities in healthcare outcomes have been uncovered in most areas of medicine, and surgery is no exception. Despite awareness, an incomplete understanding of the underlying mechanisms that lead to these disparities has impaired their improvement. The Institute of Medicine’s report Unequal Treatment found that such racial disparities have multi-factorial roots, including patient, provider, and systemic factors.1 A significant body of research has accumulated in the field of surgical outcome disparities, focused on the interplay between race, insurance status, gender, income, and comorbidities. This has led some to believe that racial disparities may be a mere reflection of pre-existing inequities based on insurance and socioeconomic status. A 2010 review on disparities in surgical oncology outcomes has largely debunked such views,2 but an examination of the broader surgery field has yet to be presented. This, as well as the recent addition of several pertinent studies, warrants an in-depth review of the currently published surgical disparity literature.
In this PRISMA-registered review, we assess racial disparities in outcomes after surgical procedures in the United States. We first evaluate the extent of surgical disparities by race. We then attempt to elucidate the proposed mechanisms that lead to such inequities in the context of patient, provider, and systemic factors. Specifically, the underlying mechanisms that are thought to lead to disparities in surgical oncology are reflected upon as they relate to the broader field of surgery. In closing, research and policy proposals are presented to aid in creating effective countermeasures. Trauma studies are purposely not included in this review as the mechanisms that lead to inequities in that field may be very different.
Methods
We searched the electronic databases PubMed, EMBASE, and the Cochrane library. The final search criteria included the following keywords: healthcare disparities AND surgery AND outcome AND US. Only primary research articles published between April 1990 and December 2011 were included in the study. A total of 88 articles met the inclusion criteria for this evidence-based review as shown in Table 1. Studies analyzing surgical patients of all ages were included, assessing the endpoints of mortality, morbidity, and the likelihood of receiving surgical therapy. The review was registered with PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses, which is a minimum set of items to report for a systematic review (registration number CRD42011001837). In the absence of randomized controlled trials, this review has relied on retrospective, cross-sectional, case-control, and prospective studies. This evidence-based, comprehensive review was compiled in a systematic manner, providing a valuable outline and summary of the academic work in the field of surgical outcome disparities.
Table 1.
Details and Characteristics of Included Studies
| Lead Author |
Year | Title | Study Design | Population and Health Outcomes |
|---|---|---|---|---|
| Al-Refaie60 | 2012 | Who receives their complex cancer surgery at low-volume hospitals? | Retrospective Cross-sectional | Examining 59,841 patients from the NIS, non-white race, non-private insurance and increased comorbidities were predictive of receipt of lung, esophageal, and pancreatic cancer surgeries at low-volume hospitals (p<.05). Black, Hispanic and Asian patients were each significantly more likely than white patients to be treated at low-volume hospitals for lung and pancreatic surgeries |
| Alderman70 | 2009 | Racial and ethnic disparities in the use of postmastectomy breast reconstruction: results from a population- based study | Retrospective Cross-sectional using survey data | Post-mastectomy raw reconstructive surgery rates were 40.9% of whites, 33.5% of blacks, 41.2% of high-acculturated Latinas and 13.5% low-acculturated Latinas. Controlling for demographic and clinical factors, less acculturated Latinas compared to whites: AOR 0.35, 95% CI 0.15–0.78. Used SEER data from two cities. |
| Alosh3 | 2009 | Insurance status, geography, race, and ethnicity as predictors of anterior cervical spine surgery rates and in-hospital mortality: An examination of US trends from 1992 to 2005 | Retrospective Cross-sectional | Blacks and Hispanics had significantly lower anterior cervical spine procedure rates than whites (AOR 0.52, CI 0.516–0.525; AOR 0.315, CI 0.311–0.319 respectively). Black in-hospital mortality rates were higher than whites (AOR 1.57, CI 1.16–2.12), as were Medicare and Medicaid patients compared to the privately insured (AOR 4.76, 3.2 respectively, p<0.001 each). NIS data. |
| Anger71 | 2007 | Racial disparities in the surgical management of stress incontinence among female Medicare beneficiaries | Retrospective Cross-sectional | Of 27,120 stress urinary incontinence (SUI) Medicare patients, white and Hispanic women diagnosed with SUI were more likely to have surgery than black and Asian women (p<0.01). Non-white women were twice as likely to have complications one-year post-op. |
| Aranda85 | 2008 | Do racial/ethnic disparities exist in the utilization of high-volume surgeons for women with ovarian cancer? | Retrospective Cross-sectional | Of 13,186 ovarian cancer patients, Black (RR: .70, p<0.05) and Hispanic (RR: .75, p<0.05) women were significantly less-likely to be treated by a high-volume surgeon (HVS) and Hispanic women were more likely to be treated by a low-volume surgeon (RR 1.1, p<0.05). |
| Artinyan23 | 2010 | Race, ethnicity, and socioeconomic status influence the survival of patients with hepatocellular carcinoma in the United States | Retrospective Cross-sectional | Analysis of 4 735 HCC patients found that following transplant, Black patients had higher mortality rates than White patients (AOR: 1.15, CI: 1.09–1.22), while Asian patients had lower rates (AOR:0.87, 95% CI:0.83–0.91). SEER database 1973–2004, UNOS 1987–2008. |
| Axelrod48 | 2010 | The interplay of socioeconomic status, distance to center, and interdonor service area travel on kidney transplant access and outcomes | Retrospective Cohort | Of 203,267 kidney transplant patients, top quartile SES patients had greater transplant access compared to the lowest quartile (AOR 1.76, 95% CI 1.7–1.83). Waitlist death was significantly lower in the top SES cohort (AOR 0.86, 95% CI 0.84–0.89). OPTN/UNOS data. |
| Banez89 | 2009 | Race and time from diagnosis to radical prostatectomy: does equal access mean equal timely access to the operating room?--Results from the SEARCH database | Retrospective Cross-sectional | In four VA medical centers, there was no significant difference by race (Black and White patient cohorts) in time from biopsy to surgery for 1,532 men treated for prostate cancer with radical prostatectomy. SEARCH database, 1988–2007. |
| Bang73 | 2010 | Total hip and total knee arthroplasties: trends and disparities revisited | Retrospective Cross-sectional | A nationwide NIS sample of primary and revision hip and knee arthroplasties between 1996 and 2005 revealed both racial and income disparities in surgery rates, though racial disparities were more significant than income disparities. |
| Bennett46 | 2010 | Patient socioeconomic status is an independent predictor of operative mortality | Retrospective Cross-sectional | Surgical records for 13 different cardiovascular and oncologic procedures revealed SES as a strong predictor of operative mortality. An increase of a single tier in SES resulted in a decrease in operative mortality risk by 7.1%. NIS data. |
| Birkmeyer47 | 2008 | Socioeconomic status and surgical mortality in the elderly | Retrospective Cross-sectional | Adjusting for patient factors, lowest income quintile had higher mortality than highest income quintile patients for each of six common, high-risk procedures. Adjusting for hospital factors, disparities reduced for all surgery types and were significant for only three types. MEDPAR database. |
| Bowman50 | 2010 | Impact of race and socioeconomic status on presentation and management of ventral hernias | Retrospective Cohort | A single-institution univariate analysis of 321 insured ventral hernia repair patients showed higher rates of acute hernia complications on presentation for black patients than whites (11% vs 4%, p<.01). |
| Breslin4 | 2009 | Hospital factors and racial disparities in mortality after surgery for breast and colon cancer | Retrospective Cross-sectional | 5-year mortality rates for breast cancer (AOR 1.25, CI 1.16–1.34) and colon cancer (AOR 1.13, CI 1.07–1.19) were higher for blacks than whites, controlling for patient and clinical factors. Hospitals with large minority populations had higher mortality rates regardless of race. Hospital factors explained 36% of breast and 54% of colon cancer outcomes disparities. SEER data for >47,000 patients. |
| Brookfield5 | 2009 | Disparities in survival among women with invasive cervical cancer: a problem of access to care | Retrospective Cross-sectional | In a study of 5367 cervical cancer patients, Black women were significantly less-likely to receive surgery than white patients. Insurance status was an independent predictor of outcomes. While race and SES outcomes were disparate, they were not independent predictors of outcomes. |
| Bryant59 | 2009 | Racial disparities in survival among patients with germ cell tumors of the ovary- United States | Retrospective cohort | Complete surgical staging was more frequent for white (49%) than Black (38%) patients (p=0.001). Black patients were more likely to present with advanced stage tumors (24% vs 18%, p>0.05). Race was not an independent predictor of survival when controlling for stage and severity. |
| Castellanos84 | 2009 | Racial and ethnic disparities in access to higher and lower quality cardiac surgeons for coronary artery bypass grafting | Retrospective Cross-sectional | Using risk-standardized outcomes for 56 surgeons, a statewide analysis of CABG patients showed Hispanics were more likely to be treated by bottom- than top-decile surgeons (AOR 2.85, CI 1.82–4.47), and half as likely to be treated by top decile surgeons than whites (AOR 0.51, CI 0.35–0.75). Whites had more top- than bottom- decile surgeons (AOR 1.37, CI 1.07–1.76), and did not differ from blacks. |
| Chang42 | 2002 | Female sex as a risk factor for in-hospital mortality among children undergoing cardiac surgery | Retrospective Cross-sectional | There was no significant difference in mortality by race or median income among 6, 593 pediatric cardiac surgery patients from the CA OSHPD database. Compared to managed health plan patients, the privately insured had higher mortality rates (AOR: 1.64, CI 1.03–2.61), and publicly-insured patient outcomes did not differ. |
| Cheung49 | 2010 | Are patients of low socioeconomic status receiving suboptimal management for pancreatic adenocarcinoma? | Retrospective Cross-sectional | An analysis of 16, 104 pancreatic adenocarcinoma patients found that low SES patients were less likely to receive surgery (16.5% vs 19.8%, p<.001), and that SES was an independent predictor of mortality. |
| Chew24 | 2005 | Comparative analysis of autogenous infrainguinal bypass grafts in African Americans and Caucasians: The association of race with graft function and limb salvage | Retrospective Cross-sectional | Black race was a negative predictor of 5-year graft patency in a single institution study of 1, 459 autogenous infrainguinal bypasses. Compared to a white cohort, blacks were younger, with higher rates of comorbidities and gangrene. |
| Curry6 | 2010 | Racial, ethnic and socioeconomic disparities in patient outcomes after craniotomy for tumor in adult patients in the United States, 1988–2004 | Retrospective Cross-sectional | In an large analysis of craniotomies, hospital case volume was lower for Black and Hispanic patients. Black patients had higher pooled odds of hospital mortality than whites (OR: 1.64, CI 1.32–2.03). |
| Du32 | 2010 | Racial/Ethnic disparities in socioeconomic status, diagnosis, treatment and survival among medicare-insured men and women with head and neck cancer | Retrospective Cross-sectional | Low SES significantly reduced the likelihood of receiving head or neck cancer surgeries. After adjusting for treatment type, mortality did not differ by SES. Controlling for SES and other factors, black patients were less likely to receive surgery than whites (AOR 0.59, CI 0.47–0.73), and had a higher risk of all cause mortality (HR 1.19, CI 1.07–1.33). Asians had lower mortality, and Hispanic mortality did not differ. SEER data. |
| Dunlop68 | 2008 | Age and Racial/Ethnic Disparities in Arthritis-Related Hip and Knee Surgeries | Retrospective Cross-sectional Survey | Longitudinal Health and Retirement survey data showed blacks >65yo had lower rates of arthritis-related hip and knee surgery than whites (AOR 0.40, 95% CI 0.19–0.58). Hispanics and blacks 51–64yo did not differ from whites. Low income patients >64yo had significantly lower rates of surgery (AOR 0.58, 95% CI 0.40–0.77). |
| Durham51 | 2010 | The impact of socioeconomic factors on outcome and hospital costs associated with femoropopliteal revascularization | Retrospective Cross-sectional | A study of 187 cases from a femoropopliteal revascularization database showed that income above 100% of the federal poverty line to be protective against limb loss (AOR 0.06, 95% CI 0.01–.051, p<0.001). Income level was correlated to advanced presentation and age. |
| Fairfield55 | 2010 | Regional variation in cancer-directed surgery and mortality among women with epithelial ovarian cancer in the Medicare population | Retrospective Cross-sectional | A study of 4 589 ovarian cancer patients found white race to be an independent predictor of receiving surgery compared to non-white women (AOR 1.41, 95% CI 1.10–1.82). Median income for census tract (stratified into three groups) was not a significant predictor for receipt of surgery. SEER data 1998–2002. |
| Farjah58 | 2009 | Racial disparities among patients with lung cancer who were recommended operative therapy | Retrospective Cross-sectional | Among early stage cancer patients, blacks were less likely than whites to have recommended lung resection (69% vs 83%, 14% difference, CI 11–18%), even after adjusting for patient characteristics (AOR 0.47, 95% CI 0.38–0.57) and cancer characteristics (AOR 0.45, 95% CI 0.37–0.56). Despite therapy differences, mortality was not significantly different by race. SEER database. |
| Feyssa25 | 2009 | Racial/ethnic disparity in kidney transplantation outcomes: influence of donor and recipient characteristics | Retrospective Cohort | In a single-institution study of 2 130 kidney transplants, black patients had lower graft survival rates than whites (p<0.001). |
| Gelber38 | 2006 | Ethnic disparities in breast cancer management among Asian Americans and Pacific Islanders | Retrospective Cross-sectional | In an analysis of 2,030 Japanese, Chinese, Filipino, Hawaiian and White women with early breast cancer, Japanese (OR 0.62, 95% CI 0.48–0.80) and Filipino (OR 0.47, 95% CI 0.33–0.66) women were significantly less likely than White women to have breast conserving surgery. |
| Greenstein7 | 2008 | Racial disparities in esophageal cancer treatment outcomes | Retrospective Cross-sectional | An analysis of 1522 esophageal cancer patients showed that Black patients had worse 5-year mortality rates than Whites (37 vs 60%, p<0.0001), and had lower surgery rates. After controlling for severity and therapy, race was no longer a predictor of survival. |
| Guagliardo52 | 2003 | Racial and ethnic disparities in pediatric appendicitis rupture rate | Observational Cross-sectional | In California, Hispanic pediatric patients had higher odds of rupture than whites (AOR 1.30, 95% CI 1.14–1.48). In New York, Asian and black patients had higher rupture rates (AOR 1.44, 95% CI 1.07–1.95; AOR 2.09, 95% CI 1.36–3.21 respectively). Disparities roughtly paralleled state proportions of immigrant children. Public, self-pay insurance, and low SES had higher perforation rates in CA but not NY. |
| Gooden86 | 2008 | The effect of hospital and surgeon volume on racial differences in recurrence-free survival after radical prostatectomy | Retrospective Cohort | Controlling for patient and cancer characteristics, there were racial disparities in post-surgery prostate cancer recurrence rates in medium and high hospital volume cohorts (AOR 1.36, 1.45 respectively; each p<0.05). Recurrence disparities also existed within medium and high surgeon volume cohorts, as black patients were more likely to have recurrence than white patients (AOR 1.37, 1.59 respectively; each p<0.05). SEER data. |
| Gundle43 | 2010 | Effect of insurance status on the rate of surgery following a meniscal tear | Retrospective Cohort | A single institution study of 1,127 meniscal tears and 446 repairs found the self-pay cohort had a lower surgery rate than the privately insured (AOR: 0.33, 95% CI: 0.14–0.75), though Medicaid (AOR: 1.63, 95% CI: 1.09–2.42) and Workers' Compensation cohorts (AOR:1.93, 95% CI: 1.05–3.55) had higher surgery rates. |
| Halm80 | 2009 | Racial and ethnic disparities in outcomes and appropriateness of carotid endarterectomy: impact of patient and provider factors | Retrospective Cohort | In a statewide study of Medicare patients, minorities (blacks and Hispanics) were more likely to be receive carotid endarterectomies (CEA) with low volume hospitals and surgeons (p<0.001). Black and white outcomes did not differ after adjusting for patient, surgeon and hospital factors, though Hispanic patients continued to have higher 30-day stroke or mortality (AOR 1.87, 95% CI 1.09–3.19). Minorities had higher rates of inappropriate surgery, largely due to comorbidities. (Hispanics 17.6%, blacks 13%, white 7.9%, p<0.0001). |
| Hanchate54 | 2008 | Exploring the determinants of racial and ethnic disparities in total knee arthroplasty: health insurance, income, and assets | Retrospective Cohort | An analysis of US longitudinal Health and Retirement survey found Hispanic males were less likely than white females to have TKAs (AOR: 0.56, 95% CI: 0.33–0.95), though in an arthritis subsample, there was no significant difference by race (black, white and Hispanic cohorts). Insurance and SES were also important factors. |
| Haas77 | 2011 | Association of area sociodemographic characteristics and capacity for treatment with disparities in colorectal cancer care and mortality | Retrospective Cross-sectional | Black and Hispanic patients were less likely than whites to undergo colorectal cancer resection (OR .57, 95% CI .52–.63; OR .82, 95% CI .70–.95). Individuals living in areas with highest-tertile surgeon capacity were more likely to undergo resection than low-surgeon capacity areas. Surgery use declined as percentage of Blacks in the area increased. Adjusting for area capacity modestly mediated racial disparities, with a decrease of 5.3%. |
| Hofmann88 | 2010 | Effect of race on colon cancer treatment and outcomes in the Department of Defense healthcare system | Retrospective Cohort | In the Department of Defense Healthcare System, black and white colon cancer patien cohorts had no difference in surgical resection rates and 5-year survival, adjusting for age, gender, tumor grade and stage. Concluded that in a setting with equal access to healthcare, race is not associated with mortality. DoD ACTUR data. |
| Holman28 | 2011 | Racial disparities in the use of revascularization before leg amputation in Medicare patients | Retrospective Cross-sectional | Black amputees were significantly less-likely than whites to have revascularization, limb-related admissions, toe amputation, or wound debridement prior to amputation. These disparities in care may contribute to higher amputation rates for Black patients. |
| Kamath21 | 2010 | Ethnic and gender differences in the functional disparities after primary total knee arthroplasty. | Prognostic study | A single-institution study of total knee arthroplasties (TKA) (90 black and 112 non-black) found black patients had longer time to presentation. At two years post-op, blacks had worse range of motion (ROM), but similar gains in ROM, after controlling for patient factors. |
| Kelz41 | 2004 | Morbidity and mortality of colorectal carcinoma surgery differs by insurance status | Retrospective Cohort | A study of 13,415 colorectal carcinoma patients (age 40–64) showed that Medicaid patients were 22% more likely than the privately insured to have in-hospital complications (AOR:1.22, CI:1.06–1.4) and 57% higher mortality (AOR: 1.57, CI:1.01–2.42), controlling for patient and hospital characteristics. NIS data. |
| Kennedy31 | 2007 | Elective and isolated carotid endarterectomy: health disparities in utilization and outcomes, but not readmission | Retrospective Cross-sectional | A statewide analysis of elective carotid endarterectomies (CEAs) found that crude non-white mortality was higher until controlling for patient and hospital factors. Non-whites had more in-hospital strokes (AOR: 1.7, CI: 1.1–2.7). |
| Kim8 | 2008 | Racial disparity in the relationship between hospital volume and mortality among patients undergoing coronary artery bypass grafting | Retrospective Cross-sectional | An analysis of 71,949 CABG patients found a significant interaction variables for race by volume (p=0.033), indicating that the benefit of higher volume hospital treatment was stronger for black than white patients. Racial disparities were highest in lowest volume hospitals. Universal Health System Consortium Data. |
| Kim9 | 2011 | An interaction of race and ethnicity with socioeconomic status in rectal cancer outcomes | Retrospective Cross-sectional | A county-wide analysis of rectal adenocarcinoma patients found that race and SES were independent predictors of survival. Asian patients had the best overall survival rates followed by Hispanics, whites, and then blacks. |
| Konety10 | 2005 | Patient and hospital differences underlying racial variation in outcomes after coronary artery bypass graft surgery | Retrospective Cohort | A large study of Medicare CABG patients showed no racial disparity in mortality at 30 and 90 days post-op, controlling for patient and hospital factors, though black mortality higher at 365 days (AOR 1.17, 95% CI 1.12–1.22). Black patients were more likely than whites to have surgery in hospitals with the highest mortality (56% vs 47%) and in the lowest volume quintile (24% vs 20%). |
| Kruper74 | 2011 | Disparities in reconstruction rates after mastectomy: patterns of care and factors associated with the use of breast reconstruction in Southern California | Retrospective Cross-sectional | In a statewide study of post-mastectomy reconstructive surgeries, Black (OR .58, CI .46–.73) and Asian (OR .37, 95% CI .29–.47) women were less-likely than white women to receive surgery. |
| Lapar39 | 2011 | Primary payer status affects outcomes for cardiac valve operations | Retrospective Cross-sectional | Among cardiac valve operative patients, Uninsured and Medicaid patients had significantly higher risk-adjusted in-hospital mortality and morbidity than those with Medicare and private insurance. |
| Lathan56 | 2006 | The effect of race on invasive staging and surgery in non-small-cell lung cancer | Retrospective Cross-sectional | Black lung cancer patients were less likely than whites to undergo cancer staging (AOR 0.75, CI 0.67–0.83), to have surgery after staging (AOR 0.55, 95% CI 0.47–0.64), and to be recommendation for surgery when not contraindicated (p<.05). Black patients were also more likely to decline surgery (3.4% vs 2%, p<0.05). Controlling for treatment type, race did not impact survival. SEER database. |
| Lee53 | 2010 | Perforated appendicitis in children: equal access to care eliminates racial and socioeconomic disparities | Retrospective Cross-sectional | A study of 7 247 pediatric patients in the Kaiser Permanente health system found no significant difference by race or income in appendiceal perforation (AP) rates, (compared Blacks, Hispanics, Asians each to White patients). |
| Lee81 | 2011 | Equal access to healthcare does not eliminate disparities in the management of adults with appendicitis | Retrospective Cross-sectional | In a single-provider system with equal healthcare access, disparate use of laparascopic appendectomy by race, age, gender, and income was observed. |
| Lemaire11 | 2008 | The impact of race and insurance type on the outcome of endovascular abdominal aortic aneurysm (AAA) repair | Retrospective Cross-sectional | A bivariate analysis of 8,367 infrarenal EVARs found white patients had lower perioperative mortality and postoperative complications than black and Hispanic patients. Self-pay patients had higher adverse outcomes then private insurance patients. NIS data. |
| Liu20 | 2011 | Racial disparities in survival after lung transplantation | Retrospective Cohort | Race was not a significant predictor of lung transplant outcomes, controlling for patient factors including medical condition, mechanical ventilator, SES, insurance, procedure type, and comorbidities (Compared Black, Hispanic, and White patient groups). >16,000 records from UNOS data 1987–2009. |
| Luan26 | 2010 | Influence of recipient race on the outcome of simultaneous pancreas and kidney transplantation | Retrospective Cross-sectional | An analysis of 6,585 silmultaneous Pancreas and Kidney transplant patients reported no disparity in early graft failure or long-term mortality between black and non-black cohorts. Blacks were more likely to have late graft loss (AOR: 1.38, 95% CI 1.10–1.73). OPTN data. |
| Lucas96 | 2006 | Race and surgical mortality in the United States | Retrospective Cross-sectional | Controlling for patient factors, Blacks had higher crude mortality than Whites for 7 out of 8 surgical procedures. Significant racial differences persisted for only 2 procedures after controlling for hospital. Hospitals that treated high proportions of black patients had higher mortality for all procedures. Medicare data 1994–1999. |
| Mahle27 | 2005 | Disparities in outcome for black patients after pediatric heart transplantation | Retrospective Cross-sectional | A study of 4 227 pediatric heart transplant receipients showed that black race is significantly associated with graft failure (AOR 1.67, 95% CI 1.47,1.87, p<.001), despite controlling for SES. UNOS data. |
| Mathur33 | 2010 | Racial/ethnic disparities in access to care and survival for patients with early-stage hepatocellular carcinoma | Retrospective Cohort | Analysis of 13,244 early stage (I/II) HCC patients revealed lower survival for black patients (AOR:1.11, 95% CI:1.03–1.20), adjusting for stage and therapy type. After adjustment, Asians had lower mortality than whites (AOR .84, 95% CI:0.79–0.89), and Hispanic mortality did not differ. SEER data 1995–2006. |
| Morris82 | 2004 | Racial disparities in rectal cancer treatment: a population-based analysis | Retrospective Cross-sectional cohort | Black rectal cancer patients were more likely to have sphincter-ablating procedures (AOR 1.42, 95% CI 1.23–1.65) than whites, controlling for tumor characteristics and therapy type. Sphincter sparing surgery is associated with higher quality of life. Blacks were diagnosed with more advanced disease at younger age, implying a need for more aggressive screening. SEER 1988–1999. |
| Morris12 | 2006 | Racial disparities in late survival after rectal cancer surgery | Retrospective Cohort | Controlling for demographic and clinical factors, black rectal cancer surgery patients had higher mortality rates than whites (AOR 1.13, 95% CI 1.01–1.26). Blacks were more likely to be treated by low volume surgeons (p<0.0001). After adjusting for provider variables, race was not a statistically significant factor. SEER data. |
| Morrissey79 | 2007 | Disparities in the treatment and outcomes of vascular disease in Hispanic patients | Retrospective Cross-sectional | In an analysis of New York and Florida discharges, Hispanic patients had significantly lower rates of lower extremity revascularizations, carotid revascularizations, and AAA repairs compared to whites. |
| Mukherjee92 | 2010 | Disparities in Access to Neuro-oncologic Care in the United States | Retrospective Cross-sectional | In a representative sample of craniotomy patients (for tumor biopsy or resection) in all hospitals from 37 states, Hispanic (OR 0.49) and Black (OR 0.62) patients were less likely than whites to be admitted to high volume hospitals. |
| Murphy34 | 2010 | Effects of ethnicity and insurance status on outcomes after thoracic endoluminal aortic aneurysm repair (TEVAR) | Retrospective Cross-sectional | A study of 875 thoracic aortic endoluminal repair (TEVAR) patients found no significant difference in mortality or complications attributable to race (Black, White, Hispanic) or insurance status. Broad confidence intervals due to relatively small sample. NIS data. |
| Murphy78 | 2009 | Racial differences in cancer specialist consultation, treatment, and outcomes for locoregional pancreatic adenocarcinoma | Retrospective Cross-sectional | Analysis of pancreatic adenocarcinomas showed black race was a negative predictor of medical oncologist consult (AOR: 0.74, p<0.01), radiation oncologist consult (AOR: 0.75, p<0.01), and surgeon consult (AOR: 0.75, p<0.01). Controlling for consultation, Black patients were less likely to have surgical resection (AOR 0.79, p=0.05). Crude survival rate was lower for black patients, but did not differ from whites after adjusting for therapy type. SEER 1991–2002. |
| Murphy57 | 2009 | Pancreatic resection: a key component to reducing racial disparities in pancreatic adenocarcinoma | Retrospective Cross-sectional | Though referred for resection at different rates, Black pancreatic adenocarcinoma patients were significantly less-likely than whites to undergo surgery (AOR .69, 95% CI 0.57–0.84). Though Black survival was significantly lower overall, survival of resection patients did not significantly differ by race. |
| Nathan35 | 2008 | Racial disparity in surgical mortality after major hepatectomy | Retrospective Cohort | A study of 3 552 major hepatectomys found black patients had higher surgical mortality (AOR 2.15, 95% CI 1.28–3.57) than whites, adjusting for insurance status, clinical and hospital factors. Hispanic and Asian outcomes did not differ from whites. Uninsured status was a negative predictor of mortality (AOR 2.90, 95% CI 1.46–5.73), but income was not. NIS data. |
| Neighbors91 | 2007 | Ethnic/racial disparities in hospital procedure volume for lung resection for lung cancer | Retrospective Cross-sectional | Black (AOR .45, 95% CI 0.34–0.58) and Latino patients (AOR 0.44, 95% CI 0.32–0.63) were less likely to have lung resections performed high-volume hospitals than whites, as were Medicaid and uninsured patients compared to the privately insured. After controlling for hospital volume, race was not a risk factor for in-hospital mortality. NIS data. |
| Nguyen29 | 2009 | Disparity in outcomes of surgical revascularization for limb salvage: race and gender are synergistic determinants of vein graft failure and limb loss | Retrospective Cross-sectional with PSM | Using propensity score matching, an analysis of 1,404 peripheral vascular surgeries found no significant difference by race for 1-year primary patency, 30-day or 1-year survival, and 1-year amputation free survival. Black men had higher early graft failure than white men (AOR: 2.83, 95% CI: 1.39–5.76). PREVENT III data. |
| Osborne99 | 2010 | Understanding the racial disparity in the receipt of endovascular abdominal aortic aneurysm repair | Retrospective Cross-sectional | Review of 160,785 open or endovascular AAA repairs (EVAR) showed blacks were 33% less likely than whites to have EVAR (AOR: 0.67, 95% CI:0.63–0.71), especially in hospitals that treat a higher proportion of black patients for AAA: (31% of blacks had EVAR at high- vs 39.6% at low-proportion hospitals). Medicare data. |
| Osborne17 | 2009 | Explaining racial disparities in mortality after abdominal aortic aneurysm repair | Retrospective Cross-sectional | Black Medicare patients had a higher risk-adjusted mortality following AAA repair (AOR: 1.33, 95% CI: 1.18–1.50), until adjusting for location of care. An estimated 25% of racial disparity was due to hospitals factors. Other contributing factors included SES, comorbidities and repair type. |
| Oster13 | 2011 | Racial and ethnic disparities in post-operative mortality following congenital heart surgery | Retrospective Cross-sectional | Adjusting for patient characteristics and severity, RR of in-hospital post-op death in congenital heart disease pediatric patients was 1.32 (95% CI 1.14–1.52) for non-Hispanic blacks and 1.21 (1.07–1.37) for Hispanics compared to Non-Hispanic whites. This did not change significantly after controlling for insurance type and hospital. |
| Paquette67 | 2010 | Patient and hospital factors associated with use of sphincter-sparing surgery for rectal cancer | Retrospective Cohort | Analysis of 47,713 rectal cancer patients showed that, adjusting for hospital and patient factors, whites were more likely to have sphincter-sparing surgery than minorities (OR 1.08, p<0.001). Also significant were high procedural volume (OR 1.55, 95% CI 1.33–1.79) and urban location (OR 1.26, 95% CI 1.33–1.79). NIS data 1988–2006. |
| Parsons93 | 2012 | What happens to racial and ethnic minorities after cancer surgery at American College of Surgeons National Surgical Quality Improvement Program hospitals? | Retrospective Cross-sectional | In a study of 38,926 patients undergoing thoracic, abdominal, or pelvic cancer surgery between 2005–2008 at American College of Surgeons National Surgical Quality Improvement Program hospitals, racial and ethnic minorities had short-term operative outcomes similar to white patients. However, black, Hispanic and American Indian/Alaska Native patients were more likely to have a prolonged hospital stay (black vs. white patients: OR 1.33, p<0.001). NSQIP data. |
| Perryman76 | 2007 | The effects of location and race on the performance of cardiac procedures | Retrospective Cross-sectional | In a statewide analysis, race was significantly (p<0.0001) associated with receipt of cardiovascular procedures (coronary arteriography, percutaneous transluminal coronary andioplasty, and coronary artery bypass graft procedures). |
| Pollack87 | 2011 | Racial disparities in changing to a high-volume urologist among men with localized prostate cancer | Retrospective Cross-sectional | In study of 26,058 Black and white prostatectomy patients, linking each patient to the diagnosing and treating physician, Black men were significantly less likely to be treated by a high-volume urologist (OR .76, 95% CI .67–.87) and significantly less likely to change from a low volume diagnosing urologist to a high-volume treating urologist (OR .61, 95% CI .47–.79). |
| Press37 | 2005 | Race/ethnicity, poverty status, and renal transplant outcomes | Retrospective Cohort | Following 10-year outcomes of 4 471 renal transplants in 1990, African-American and Hispanic race/ethnicity were independently predictive of graft failure (AOR 1.8, 95% CI 1.6–1.9; AOR 1.3, 95% CI 1.2–1.6, respectively). Poverty status was not significant. UNOS. |
| Rhoads98 | 2008 | Quality of colon cancer outcomes in hospitals with a high percentage of medicaid patients | Retrospective Cohort | A statewide study of 17,835 colon cancer patients showed that high Medicaid hospitals (HMH), (>40% Medicaid patients), had higher mortality at 30-day after colon procedures (1% versus 0.6%, p=0.04) and 1-year (3.4 versus 2.4%, p=0.001), but not 5-years. Adjusting for surgical volume eliminated the effect of HMH at 30 days but not 1 year (3.4 versus 2.5%; p <0.01). |
| Ricciardi18 | 2008 | Disparate use of minimally invasive surgery in benign surgical conditions | Retrospective Cohort | In a study of appendectomies, gastric fundoplications, and gastric bypasses, black patients had higher mortality than whites (AOR 2.01, 95% CI 1.1–3.69), though Hispanic and Asian cohorts did not differ from whites. Blacks and Hispanics were less likely to undergo Minimally invasive surgery (MIS) than whites (AOR 0.77, 95% CI 0.72–0.82; AOR .93, 95% CI 0.88–0.98), controlling for patient and hospital characteristics including surgical volume. NIS data. |
| Robinson90 | 2010 | Ethnic disparities are reduced in VA colon cancer patients | Retrospective Cohort | A study of 214 colon cancer patients at one VA institution showed no significant difference between White and Black patient cohorts in survival rates, stage of disease at presentation, and in mean time from diagnosis to surgical resection. With presumed equal access to care there was no apparent disparity in outcome. |
| Robinson36 | 2009 | Inferior outcomes of autogenous infrainguinal bypass in Hispanics: an analysis of ethnicity, graft function, and limb salvage | Prospective Cohort | A single institution study of autogenous infrainguinal bypasses from prospective registry data found similar perioperative mortality and morbidity for black, Hispanic, and white cohorts, though 5-year graft patency was significantly lower for blacks and Hispanics. Controlled for severity but not insurance status or SES. |
| Rogers75 | 2004 | A population-based study of survival among elderly persons diagnosed with colorectal cancer: does race matter if all are insured? (United States) | Retrospective Cohort | Among Tennessee colorectal cancer patients with both Medicare and Medicaid, Black patients were significantly less likely to receive surgery (86% vs 91%, p=0.02). |
| Rucker-Whitaker30 | 2003 | Explaining racial variation in lower extremity amputation: a 5-year retrospective claims data and medical record review at an urban teaching hospital | Retrospective Case-control | A single-institution two-step case control study of 1,127 LE arterial bypass grafts, angioplasties, and major amputations found that, controlling for patient factors, blacks were equally likely to undergo primary amputation, but repeat amputees were 2.5 times more likely to be black. Blacks were younger (p<0.05) compared to Whites. |
| Singh22 | 2011 | Improved survival in heart transplant recipients in the United States: racial differences in era effect | Retrospective Cross-sectional | A study of 36,748 heart transplants revealed that higher black than white mortality and retransplation within 6 months (AOR:1.15, 95% CI:1.05–1.26). Though early survival improved over time (1987–2008) for all groups (Black, White and Hispanic), long-term survival improved only for whites (AOR: 0.95, 95% CI: 0.92–0.97). OPTN data. |
| Sosa14 | 2007 | Racial disparities in clinical and economic outcomes from thyroidectomy | Retrospective Cohort | Black thyroidectomy patients had higher in-hospital mortality (0.4% vs 0.1%) and complications (4.9% vs 3.8%)than non-blacks, despite adjusting for surgeon volume. The majority of Hispanic (55%), and black (52%) patients were treated by low volume surgeons, compared to 44% of whites. NIS data. |
| Steyerberg72 | 2005 | Racial differences in surgical evaluation, treatment, and outcome of locoregional esophageal cancer: a population-based analysis of elderly patients | Retrospective Cross-sectional | Black esophageal cancer patients were less likely to receive surgical evaluation (AOR 0.62, 95% CI 0.47–0.82) and surgery following surgical evaluation (AOR 0.62, 95% CI 0.46–0.85). Black patients had higher mortality when adjusting for patient factors (AOR 1.16, 95% CI 1.03–1.32), but when adjusting for treatment type there was no significant difference by race. SEER data 1991–1999. |
| Terplan15 | 2012 | Have racial disparities in ovarian cancer increased over time? An analysis of SEER data | Retrospective Cross-sectional | African American diagnosed with ovarian cancer have worse survival rates than whites (HR 1.10, 95% CI 1.06–1.15), with a trend widening over the time period of 1973 to 2007 (p<0.01). Disparities remained after controlling for receipt of surgery. |
| Trinh83 | 2011 | Improvement of racial disparities with respect to the utilization of minimally invasive radical prostatectomy in the United States | Retrospective Cross-sectional | In an analysis of 65,148 radical prostatectomies, Black patients were 14% less likely to have MIS compared to whites (p=0.01). Analysis over time suggests improvement; while Blacks were 22% less likely to have MIS between 2001 and 2005 (p=0.007), in 2006 to 2007, they were only 11% less likely (p=0.1). |
| Trivedi16 | 2006 | Impact of Hospital Volume on Racial Disparities in Cardiovascular Procedure Mortality | Retrospective Cross-sectional | Black and Hispanic patients were more likely than whites to receive cardiovascular procedures in low-volume hospitals. Black patients, but not Hispanics, had significantly higher risk-adjusted mortality than whites following elective AAA repair, CABG, and CEA, but not PTCA. Adjusting for hospital volume did not change mortality risks. |
| Vouyouka19 | 2010 | Lessons learned from the analysis of gender effect on risk factors and procedural outcomes of lower extremity arterial disease. | Retrospective Cross-sectional | Black race was a mortality risk factor in peripheral artery disease surgeries after adjustment for baseline comorbidities and procedure type (AOR 1.20, 95% CI 1.11–1.30, p<.0001). NY, NJ, FL state inpatient discharge databases >372,000 records. |
| Wilson69 | 2008 | Racial disparities in abdominal aortic aneurysm repair among male Medicare beneficiaries | Retrospective Cross-sectional | Found the screen-detected disease prevalence of AAA for black men was less than half that of white men. Adjusting for prevalence, found that black patients were still less likely to receive elective AAA repair (AOR 0.77, 95% CI 0.68–0.77) and more likely to receive urgent repair (AOR 1.30, 95% CI 1.21–1.41), possibly due to lower frequency of elective procedures. |
| Yang40 | 2010 | Do racial or socioeconomic disparities exist in lung cancer treatment? | Retrospective Cross-sectional | Blacks with early stage lung cancer were 15% less likely to receive surgical treatment than their white counterparts. Controlling for treatment type received, race was not a significant predictor of outcomes. Independent predictors of negative outcomes included Medicaid insurance, uninsured status, and patients from communities with >15% poverty. FL cancer registry. |
| Vogel44 | 2008 | AAA Repair: Sociodemographic Disparities in Management and Outcomes | Retrospective Cross-sectional | A statewide analysis of 6,227 open or endovascular AAA repairs (EVAR) found Hispanic patients were less likely to receive EVAR than whites (AOR 0.67, 95% CI 0.52–0.86), as were the uninsured compared to the insured (AOR 0.54, 95% CI 0.30–0.97). Black mortality was significantly higher than for whites (AOR 2.59, 95% CI 1.47–4.54), though EVAR likelihood did not differ. |
| Zak45 | 2011 | Predictors of surgical intervention for hepatocellular carcinoma: race, socioeconomic status, and hospital type | Retrospective Cohort | In 12,148 HCC cases, there were racial disparities in treatment type which decreased after adjusting for insurance and SES. Patients with low SES and no private insurance were less likely to receive surgery. |
Disparities in Surgical Outcomes for Different Race and Ethnic Groups
The vast majority of research on race and surgical outcomes indicates that black patients experience higher crude mortality rates than white patients.3–17 Ricciardi et al’s nationwide study of appendectomy, gastric fundoplication, and gastric bypass surgeries found that black patients were significantly more likely to die in-hospital than white patients (2.6 per 1000 patients versus 1.6 per 1000 patients, p< 0.05).18 Race has also been shown to interact with other risk factors for surgical mortality to influence outcomes. For instance, in an analysis of the effect of gender on outcomes of lower extremity arterial disease, Vouyouka et al. showed that female gender increased the odds of mortality among black patients specifically (OR 1.20, 95% CI 1.11–1.30).19 Larger disparities are noted in higher risk surgeries; an analysis of the Nationwide Inpatient Survey showed that mortality among anterior cervical spine surgery patients was 1.57 times higher for black patients than for white patients, despite adjusting for age, insurance status, and geographic region.3 One retrospective cohort study on 16,875 adults receiving primary lung transplants from 1987 to 2009 did find that while five year survival rates were lower for nonwhites than whites from 1987–2000, there was no significant difference in lung transplant survival from 2001 to 2009.20 Research also demonstrates that black patients have higher morbidity,6,21,22 with lower rates of graft survival23–27 and higher rates of limb-loss.28,29 Across several types of surgery, blacks also appear to suffer higher rates of in-hospital complications and/or disease recurrence.22,30,31
Hispanic and white surgical mortality rates were compared in fifteen studies.3,5,9,11,13,14,16,18,22,23,32– 36 Thirteen of these studies found that Hispanic mortality outcomes were as good or better than that of white patients.3,5, 9,14,16,18,22,23,32–36 In a study of invasive cervical cancer patients in Florida, median survival was significantly longer for Hispanics compared to non-Hispanic patients (52.8 months vs. 41.6 months, respectively).5 There was conflicting evidence on differences in surgical graft survival rates between Hispanic and white patients. Press et al. found that Hispanic patients had 1.3 times higher rates of renal transplant failure (95% CI 1.2–1.6).37 However, Artinyan et al. found that among patients undergoing transplantation for hepatocellular carcinoma, Hispanic patients had significantly better mean graft survival than white patients (82.4 months vs. 63.8 months, respectively).23
Asian surgical outcomes appear to be as favorable or better than whites. In the five studies that compared mortality rates9,18,23,32,33, four studies found that Asian patients had significantly better outcomes.9,23,32,33 However, understanding surgical disparities among the Asian population likely warrants additional careful research. An analysis of Japanese, Chinese, Filipino, Hawaiian, and white early breast cancer patients found that Japanese and Filipino women were significantly less likely to receive breast-conserving surgery than white women.38 Such findings warrant further research focused on possible disparities suffered by subsets within the heterogeneous Asian population.
Factors Contributing to Racial Surgical Disparities: Patient, Provider, and Systemic Levels
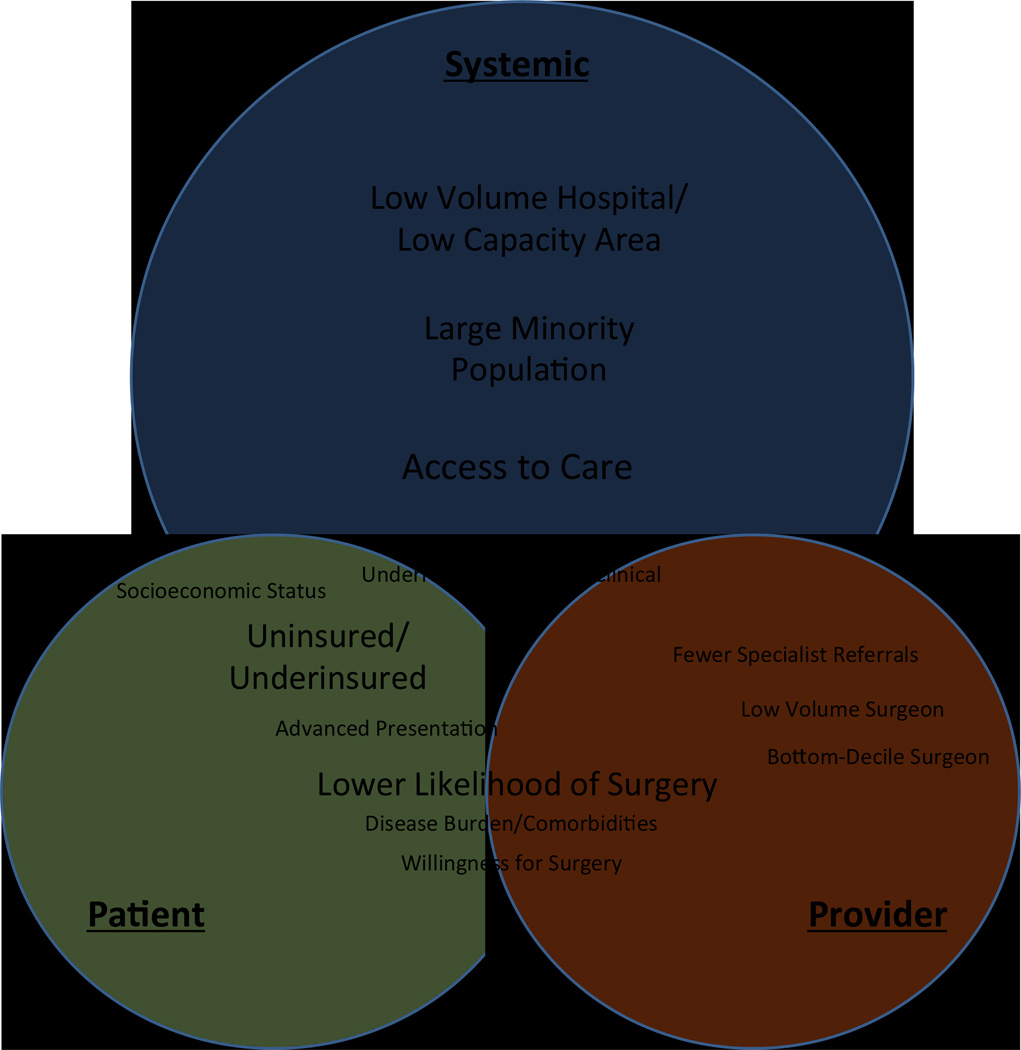
This review of the literature exposed three main, overlapping levels of factors that contribute to racial disparities in surgical care and outcomes: patient, provider, and systemic factors. As depicted in Figure 1, large-scale systemic factors influence and overlap with patient and provider factors to affect surgical outcomes. Below we have presented an in-depth review of our findings on each of these levels of contributing factors.
Figure 1. Factors Contributing to Health Care Disparity.
Factors contributing to disparities. Size of font reflects perceived relative importance of factor.
Underlying Mechanisms: Patient factors
Complex ties exist between race, socioeconomic status, and insurance coverage in the United States. Currently, uninsured/underinsured status, underlying comorbidities, and low-income status predict inadequate access to optimal and timely surgical care and poorer surgical outcomes. All of these factors are typically found in a higher percentage of non-white persons, especially African-Americans, Hispanics and Native Americans. It is postulated that this hinders a growing population in the U.S. from receiving optimal surgical care. Here we highlight the negative impact of patient-related factors on overall surgical care in the U.S.
Insurance
Insurance status appears to significantly influence surgical mortality outcomes. Uninsured and publicly insured patients typically have higher morbidity and mortality than privately insured patients,3,5,6,11,18,39–41 with a few exceptions.34,42 Insurance status may also influence the likelihood of receiving elective surgery and the type of surgery received (e.g. open versus minimally-invasive).18,43–45 However, while there is a correlation between uninsured or publicly insured status and minority race, this does not fully explain observed disparities. Racial outcome inequities continue to be observed when controlling for insurance status.35 For instance, in one study of patients undergoing major hepatectomy, black patients had 2.15 times higher odds than white patients of death after surgery, despite controlling for insurance status, clinical and hospital factors (95% CI 1.28–3.57).35 Even so, the correlation between race and insurance status may suggest that broader access to adequate insurance can reduce overall racial inequities.
Socioeconomic Status
Socioeconomic status is a complex surrogate of affluence in the US, as it depends on interrelated factors including annual household income, level of education, and residence location. Furthermore, in many administrative datasets use of SES is highly dependent on zip code location income, rather the actual household income. As such, in the current body of literature, SES may not always accurately reflect an individual’s wealth status. Multiple studies have found SES to be an independent predictor of surgical mortality.9,32,40,46–49 In a recent study using the Nationwide Inpatient Sample (NIS), Bennett et al. found that SES was a strong independent predictor of operative mortality in thirteen cardiovascular and oncologic procedures.46 In fact, an increase of a single tier in SES resulted in a decrease in operative mortality risk by 7.1 percent. However, substantial conflicting evidence also exists.5,35,50 Using Medicare data from 1999 to 2003 and adjusting for patient factors, Birkmeyer et al. found that the lowest income quintile patients had higher mortality than the highest income quintile for each of six common, high-risk procedures (colectomy, gastrectomy, coronary artery bypass grafting (CABG), aortic valve replacement, mitral valve replacement and lung resection).47 Yet after adjusting for hospital factors, the disparities were reduced for each surgery and only remained significant for three surgery types. Annual income was not a significant factor in a large, nationwide study of mortality after appendectomy, gastric fundoplication and gastric bypass.18
The evidence on SES and morbidity is also somewhat conflicting. Income above 100 percent of the federal poverty line was protective against limb loss in a small study of femoropopliteal revascularization patients (AOR 0.06, 95% CI 0.01–0.51, p<0.001).51 Guagliardo et al found that SES was a significant predictor of appendiceal perforation (AP) for California pediatric patients, but not for New Yorkers.52 While this area will certainly benefit from further study, Lee et al. note that this potential inequality may be the manifestation of an access barrier.53 In a study of Kaiser Permanente patients, a closed system of roughly equal access to members, income was not a significant factor in AP rates.53
While the relationship between SES and surgical mortality and morbidity remains unclear,50 SES does appear to influence care received. Several studies in this review concluded that SES is an important factor in receiving surgical treatment,45,48–49,54 while two found no significant relationship.55,56 Using survey data, Hanchate et al. found that Total Knee Arthroplasties (TKAs) were less common in cohorts with household incomes below $20,000 or assets below $5,000.54 Comparable are the results of an analysis of Organ Procurement and Transplant Network data (OPTN), which showed that top quartile SES patients had greater access to kidney transplant compared to low SES patients (AOR 1.76, 95% CI 1.7–1.83).48
Willingness to Undergo Surgery
There is some evidence to suggest that fewer black patients choose surgical treatment compared to white patients.56–57 In a study of early-stage lung cancer patients who were recommended for surgery, black patients were significantly less likely to undergo lung resection compared with white patients (69% vs. 83%, 95% CI 11–18%).58 This remained true even after adjusting for patient characteristics (AOR 0.47, 95% CI 0.38–0.57) and cancer characteristics (AOR 0.45, 95% CI 0.37–0.56). Study participants were Medicare beneficiaries and the model controlled for income. However, there was no control for supplementary insurance, which may have played an important role in treatment decisions. Another analysis of the SEER database showed similar findings: among non-small cell lung cancer patients, black patients were more likely than white patients to decline recommended surgery (3.4% vs 2%, p< 0.05).56 These findings suggest a possible difference in the biases and expectations of patient and provider, including issues related to time spent face-to-face and operative consent.
Advanced Presentation
Research indicates that for some surgical conditions, black patients may present later in disease than white patients.24,59 In a single-institution analysis of autogenous infrainguinal bypasses, black patients had a significantly higher frequency of gangrene at presentation than white patients.24 While late presentation is often considered a product of both patient factors (e.g. mistrust, under-/uninsured status) and systemic factors (e.g. accepting poor payer mix patients), limited evidence indicating differences in patient behavior exists. To the contrary, where there is presumed equal access to care, racial differences in late presentation seem to dissipate. A study of 7,247 pediatric Kaiser patients comparing black, Hispanic, and Asian cohorts to white patients found no significant difference by race in appendiceal perforation (AP) rates when controlling for parental income and education level.53 Promoting equitable access to care may mitigate excess morbidity and mortality due to late presentation.
Disease Burden and Comorbidities
Incidence and prevalence of a variety of medical conditions can complicate the study of health care disparities, but should not be overlooked as they are essential to any discussion of health disparities. Beyond this review, there is substantial evidence of racial disparities in disease burden, which are undoubtedly multi-factorial in origin.60–66 For example, African-Americans have significantly higher rates of cardiovascular disease, particularly among young and middle-aged adults.62 Adjusting for demographics such as SES, blacks have 2.74 times higher odds of hypertension than whites (95% CI 2.32–3.25).63 Racial and ethnic disparities have been well-documented in the incidence and prevalence of chronic kidney disease (CKD), as well as end-stage renal disease (ESRD).64 The range of health disparities uncovered is continuously expanding.
We acknowledge the complex ties among comorbidities, race, and socioeconomic status and the additional difficulties in assessing their influence on the quality of surgical care and survival outcomes. It is possible that the additional magnitude of comorbidities among minority patients contributes to racial disparities throughout the continuum of surgical care, especially for issues regarding the interplay of various treatment decisions. Underlying comorbidities that may be more prevalent among racial and ethnic minorities will likely impact surgical decision-making in many regards. For example, in a setting with a high burden of cardiovascular disease and a lack of access to a limb claudication management program, patients may be more likely to present with a need for limb amputation. It is likely that the presence of co-existing comorbidities serves as a hazardous effect modifier on the overall outcome of racial/ethnic minorities.
Underlying Mechanisms: Provider Factors
Receipt and Type of Surgery
Disparities in the likelihood of receiving surgery appear to play a role in surgical outcome inequalities, particularly for black patients. Twenty studies reported on racial differences in the likelihood receiving surgery,3,5,7,32,40, 44,54,55,57,67–77 with all but one of these studies finding that minority patients were less likely than whites to receive a variety of surgeries.44 For example, black men over 65 years of age less frequently received abdominal aortic aneurysm (AAA) repair compared to white men despite higher disease prevalence.69 In a nationwide study of pancreatic adenocarcinoma patients, black patients were less likely to have a medical oncologist consultation (AOR: 0.74, p< 0.01), a radiation oncologist consultation (AOR: 0.75, p< 0.01), or a surgery consultation (AOR 0.71, p< 0.01).78 Even after controlling for consultation, black patients were still less likely to receive surgical resection (AOR 0.79, p=0.05). While black patients in this study had lower survival rates than white patients, there was no significant mortality difference after adjusting for resection and adjuvant therapy. Racial differences in survival were also shown to disappear after controlling for type of therapy among pancreatic adenocarcinoma patients57 and esophageal cancer patients.72
There is some evidence of disparities in surgery rates between white and Hispanic patients.3,70,77,79 In a study of 3,252 patients with breast cancer in the Los Angeles and Detroit Surveillance, Epidemiology, and End results registries, Alderman et al. found significant differences in the receipt of postmastectomy breast reconstruction according to patient race. While the proportion of “highly acculturated” Latinas receiving reconstructive surgery was similar to white patients (41.2% and 40.9%, respectively), only 13.5% of “less acculturated” Latinas received surgery (p<0.001). Such differences demonstrate the importance of considering heterogeneity within the Hispanic community and show that analysis of racial and ethnic subgroups can be beneficial in disparities research.
There is evidence of racial differences in the type and appropriateness of surgery received as well.18,70,67,80–83 Ricciardi et al.’s cohort analysis of all patients who underwent appendectomy, gastric fundoplication, and gastric bypass in the Nationwide Inpatient Sample found that black and Hispanic patients were less likely than whites to be treated with minimally invasive surgery (AOR 0.77, 95% CI 0.72–0.82; AOR 0.93, 95% CI 0.88–0.98, respectively).18 In contrast, a retrospective review of 321 patients undergoing ventral hernia repair at a single institution found no racial difference in the use of laparoscopic vs open operative technique, despite a significant disparity in patient presentation with complicated ventral hernia.50 Interestingly, while most studies focus on the underutilization of surgery in minority patients, Halm et al found that among Medicare beneficiaries in New York who received carotid endarterectomies, minority patients had higher rates of inappropriate surgery according to clinical indication, largely due to higher comorbidity (Hispanics 17.6%, blacks 13%, whites 7.9%, p<0.0001).80
Surgeon Volume and Quality
Surgeon volume is an under-investigated area of research in the field of surgical care disparities. One of the reasons behind this scarcity of research is that surgeon volume indicators may not fully reflect a surgeon’s actual operative volume, as many surgeons tend to operate across multiple hospitals, with their case load varying at each hospital. Nevertheless, current research demonstrates that differences in surgeon volume contribute to racial outcome disparities.12,14,80,84 In a retrospective study of ovarian cancer surgeries in the California Cancer Registry (CCR), black and Hispanic patients were significantly less likely (RR 0.70, p<0.05 and RR 0.75, p<0.5, respectively) to be treated by a high-volume surgeon than white patients.85 Black patients with rectal cancer were also more likely than white patients to receive care by very low-volume surgeons in a study using Surveillance, Epidemiology, and End Results (SEER)-Medicare data from 1992 to 2003 (26.8% versus 19.0%).12 While black mortality in this study was higher overall, race was not a statistically significant predictor of mortality after adjusting for provider variables. In another study using SEER data on men who received surgery for prostate cancer, black patients were more likely than white men to be treated by a low volume surgeon.86 Pollack et al conducted a study linking prostatectomy patients to both the diagnosing and the treating urologist and found that black men were significantly less likely than white men to change from a low-volume diagnosing urologist to a high-volume treating urologist (OR 0.61, 95% CI 0.47–0.79), potentially exacerbating disparities related to hospital clustering.87
In addition to surgeon volume, racial differences may also exist in the quality of surgeon as measured by risk-standardized outcomes. White patients were more likely to be treated by higher quality, top-decile surgeons than by lower quality, bottom-decile surgeons in a statewide study of CABG patients and cardiac surgeons (AOR 1.37, 95% CI 1.07–1.76).84 Though black and white patient groups did not differ in receipt of treatment by top or bottom-decile surgeons, Hispanic patients were half as likely as whites to be treated by top-decile surgeons (AOR 0.51, 95% CI 0.35–0.75).
Underlying Mechanism: Systemic Factors
Access
Where access to care is unequal, healthcare disparities seem to follow. Hofmann et al.’s analysis of colon cancer in the Department of Defense Healthcare System showed no difference in surgical resection rates or five year survival rates between black and white patient cohorts, adjusting for age, gender, tumor grade and stage.88 Given these results, the authors concluded that in settings of equal access to healthcare, race may not be associated with increased mortality. Banez et al. found that access is a significant factor in racial disparities. In their small study of one Veterans Affairs hospital, the authors found that there were no significant differences in white and black patient cohort survival rates, stage of disease at presentation, or mean time from diagnosis to surgical resection.89 Robinson et al. also showed that there were no significant racial differences in survival rates, stage of disease at presentation, or mean time from diagnosis to surgical resection among colon cancer patients at a single Veterans Affairs hospital.90
Hospital volume
Hospital volume has been extensively shown to predict surgical outcomes, with high hospital volume leading to favorable surgical outcomes. Indeed, some scholars propose using “hospital volume” as a proxy of quality of care. However, the geographic location where minority patients reside or seek their surgical care tends to be in urban settings. On the one hand, limited access to high-volume hospitals (as a result of under- or no insurance) among minorities is one likely reason behind outcome disparities.6,8 Patients treated at high-volume hospitals typically have lower mortality rates,35 and studies consistently show racial differences in whether care is provided at high or low volume hospitals.10,16,35,80,91–92 For example, Neighbors et al showed that while black and Latino patients were less likely than white patients to be treated at a high-volume hospital (AOR 0.45, 95% CI 0.34–0.58; AOR 0.44, 95% CI 0.32–0.63, respectively) for lung resection for lung cancer, race was not an independent predictor of mortality after controlling for hospital volume.91 A study of prostate cancer outcomes also showed that mortality inequalities decreased when controlling for hospital volume.86 However, academic hospitals also tend to care for disproportionately minority populations with suboptimal insurance, thus providing access to high-volume hospitals. Furthermore, racial disparities may dissipate within these high performing hospitals. Given the dichotomized nature of surgical care for minorities living in urban areas, further research on the impact of hospital volume on racial disparities is warranted. A recently conducted study of cancer surgery outcomes showed that although non-white patients were more likely to have more comorbidities and undergo more complex surgeries, minorities who received treatment at quality-seeking hospitals (American College of Surgeons National Quality Improvement Program (ACS NSQIP) hospitals) had similar short-term mortality and complication outcomes compared to whites, although they did remain hospitalized for longer.93 Access to quality-seeking facilities may reduce racial disparities regardless of underlying comorbidities or patient population mix.
Hospital volume may be a particularly important factor for complex and high-risk surgeries. Al-Refaie et al. found that non-white race and increased comorbidities contributed to receipt of complex cancer surgeries at low-volume hospitals.60 Over the past several years, significant research has shown that complex cancer outcomes are better at high-volume hospitals.94–95 This knowledge has fueled a centralization trend of referring complex cancer surgeries to high-volume hospitals. Such practices may also be wise for all complex surgeries.60 In a fascinating nationwide analysis of CABG patients, Kim et. al examined outcome inequalities, controlling for patient risks, geographic region, hospital volume, and proportion of black patients treated at a hospital.8 Their model revealed a significant interaction variables for race by volume (p=0.033) and region by volume (p=0.033), indicating that the benefit of higher-volume hospital treatment was stronger for blacks than whites. They concluded that racial disparities were most prominent in the lowest volume hospitals.
Area capacity may serve as an additional contributing factor in the racial/ethnic minorities surgical disparities relationship. In an analysis of colorectal cancer resections, individuals living in highest-tertile surgeon capacity areas were more likely to undergo resection than in lowest-capacity areas.77 Although black patients in this study were significantly less likely than whites to undergo resection, there was a modest decrease in disparities after adjusting for area capacity. A thorough review of oncology outcome disparities shared these findings; the most significant racial inequalities were observed where treatment had a significant impact on cancer outcomes.2
Hospital patient population
There is evidence that the racial distribution of patients within a hospital is linked to surgical outcomes.4,96 In a study of breast and colon cancer patients, hospitals with large minority populations had higher mortality rates regardless of race.4 An outcomes study of eight types of vascular and oncologic surgeries confirmed these findings, revealing that hospitals treating a large proportion of black patients had higher mortality rates for all procedures, for both white and black patients.96 Though racial mortality outcomes differed for each of eight procedures, disparities in only two procedures persisted after controlling for these hospital factors. Haider et al. also recently found that trauma patients seen at hospitals serving higher proportions of minority patients had significantly higher mortality rates.97 A statewide study of colon cancer patients found that high Medicaid hospitals (>40% Medicaid patients) had higher mortality at 30 days after colon procedures (1% vs. 0.6%, p=0.04) and at one year (3.4% vs. 2.4%, p=0.001), although not at five years post-surgery.98
Thus far the message is undisputed: hospital population matters. Analyzing nationwide data of outcome disparities in AAA repairs, Osborne et al. estimate that 25 percent of racial disparities are attributable to black patients receiving care in “lower-quality” hospitals.17 “Lower-quality” may be more accurately described as a reflection of hospital resources than as an indicator of performance. In the present tight economic environment existing reimbursement models to hospitals and their providers may not be fiscally sustainable when caring for a large cohort of surgical patients with higher prevalence of comorbidities in the setting of under/uninsured health care coverage. If so, strengthening these under-resourced hospitals could potentially improve current inequalities in outcomes.
Ethnic/racial minorities and clinical trials
As previously mentioned, racial and ethnic minorities face the additional barriers of co-existing comorbidities and lack of adequate health insurance coverage. These barriers restrict minority patients’ access to guideline-recommended care and eligibility to participate in clinical trials. Research in cancer outcomes has shown that patients who are racial and ethnic minorities, under/uninsured, or have comorbidities typically receive cancer care that deviates from recommended care. Al-Refaie et al. found that non-white race/ethnicity and sub-optimal insurance status are significant predictors of non-adherence to guideline-recommended cancer care and under-enrollment in cancer clinical trials.61 This may be due in part to current stringent inclusion criteria in the development of most cancer trials, as persons who are older, have comorbidities, or suboptimal health insurance are frequently excluded from such trials. The close relationship between non-white race, suboptimal health insurance and comorbidities results in a tendency to exclude minority patients from cancer trials. While more than one-third of the U.S population are minorities, minority groups represent less than one percent of adult cancer patients enrolled in clinical trials.61 Unless minority patients and patients with comorbidities are better represented in these trials, surgeons will continue to question the applicability of resulting guidelines in their practices.61
Conclusion
While Class I data on surgical outcomes disparities is rare, substantive research in the field exists. Our findings echo the Institute of Medicine’s report that the majority of disparities research is focused on differences between African-American and white patient groups. We agree with their recommendation that an increase in attention on Hispanic and Asian patient groups, and particularly sub-groups of these populations, is needed, as health care interactions may be complicated by linguistic and cultural differences, immigration status, and other access-related issues.
Significant methodological challenges exist in surgical health disparities research. The vast majority of the studies in this review utilized retrospective data. Retrospective data and claims data may have been collected for other purposes, such as billing, rather than to study disparities and thus may be missing key data on patient race. Advanced statistical techniques such as multiple imputation can be utilized if race data is missing at random. However, if race data is systematically missing, as with states that do not report race data to the Nationwide Inpatient Sample, those patients must be excluded from the analysis. An additional challenge presented in the analysis of race-related data is variation in the reporting of race data, with some databases using patient self-report and others using demographics collected by a third party.
Despite these challenges, investigating patient, provider, and systemic characteristics contributing to disparities has revealed important avenues for research and policy. While uninsured and Medicaid patients appear to have higher morbidity and mortality than privately insured patients, racial outcome disparities persist even after controlling for insurance status. The correlation between race and insurance status suggests that broader access to adequate insurance may reduce racial inequities. By contrast, the conflicting research on the relationship of SES to surgical outcomes gives only a premature understanding of the dependence of racial disparities on SES. Prospective studies are needed to better understand patient and provider decision-making, to assess care management at different points along the continuum of care, and to assess the impact of these interactions on diagnosis and treatment. This research should include the patient-doctor interactions that precede surgery and have a qualitative focus on understanding possible racial preferences for surgical therapy. The IOM recommends that such research consider the range of influences on patients’ and providers’ attitudes and expectations in the clinical encounter, and the influence of race on clinical decision-making processes. The nature and quality of communication between patients and providers should also be considered, particularly as it occurs across cultural or linguistic barriers.
Provider factors such as differences in surgery rates and status as low volume or low quality surgeons appear to play a role in outcome disparities for minority groups. Amending provider differences in surgery rates, volumes, and quality may be useful targets for improving racial outcome disparities. Reducing inequity in receipt of surgical therapy has been shown to reduce disparities. Hospitals could potentially play a role in monitoring and reducing these differences. The observation of potential differences in surgical quality also warrants further research, including research on the measure of surgeon quality used. For instance, is risk-standardization an appropriate measure of quality, or does this metric potentially mask the struggle of good surgeons in strained settings?
Systemic factors such as access to care, hospital volume, and hospital patient population are shown to contribute to disparities. Further research is needed on the impact of hospital volume and patient mix on the quality of care disparities experienced by minorities. Broadening access to high-quality surgical care is likely to play a significant role in reducing disparities. Research consistently shows that where access to care is equal, outcome disparities become indiscernible. Elucidating and ameliorating the structural mechanisms that limit access to care among minority populations will likely have a positive impact on reducing racial disparities.
Acknowledgments
Financial support for this work was provided by: National Institutes of Health/NIGMS K23GM093112-01 and American College of Surgeons C. James Carrico Fellowship for the study of Trauma and Critical Care (Dr. Haider).
References
- 1.Smedley B, Stith A, A Nelson. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Institute of Medicine; 2003. [PubMed] [Google Scholar]
- 2.Morris AM, Rhoads KF, Stain SC, Birkmeyer JD. Understanding racial disparities in cancer treatment and outcomes. Journal of the American College of Surgeons. 2010 Jul;211(1):105–113. doi: 10.1016/j.jamcollsurg.2010.02.051. [DOI] [PubMed] [Google Scholar]
- 3.Alosh H, Riley LH, 3rd, Skolasky RL. Insurance status, geography, race, and ethnicity as predictors of anterior cervical spine surgery rates and in-hospital mortality: an examination of United States trends from 1992 to 2005. Spine. 2009 Aug 15;34(18):1956–1962. doi: 10.1097/BRS.0b013e3181ab930e. [DOI] [PubMed] [Google Scholar]
- 4.Breslin TM, Morris AM, Gu N, et al. Hospital factors and racial disparities in mortality after surgery for breast and colon cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009 Aug 20;27(24):3945–3950. doi: 10.1200/JCO.2008.20.8546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brookfield KF, Cheung MC, Lucci J, Fleming LE, Koniaris LG. Disparities in survival among women with invasive cervical cancer: a problem of access to care. Cancer. 2009 Jan 1;115(1):166–178. doi: 10.1002/cncr.24007. [DOI] [PubMed] [Google Scholar]
- 6.Curry WT, Jr, Carter BS, Barker FG., 2nd Racial, ethnic, and socioeconomic disparities in patient outcomes after craniotomy for tumor in adult patients in the United States, 1988–2004. Neurosurgery. 2010 Mar;66(3):427–437. doi: 10.1227/01.NEU.0000365265.10141.8E. discussion 437-428. [DOI] [PubMed] [Google Scholar]
- 7.Greenstein AJ, Litle VR, Swanson SJ, et al. Racial disparities in esophageal cancer treatment and outcomes. Annals of surgical oncology. 2008 Mar;15(3):881–888. doi: 10.1245/s10434-007-9664-5. [DOI] [PubMed] [Google Scholar]
- 8.Kim DH, Daskalakis C, Lee AN, et al. Racial disparity in the relationship between hospital volume and mortality among patients undergoing coronary artery bypass grafting. Annals of surgery. 2008 Nov;248(5):886–892. doi: 10.1097/SLA.0b013e318189b1bc. [DOI] [PubMed] [Google Scholar]
- 9.Kim J, Artinyan A, Mailey B, et al. An interaction of race and ethnicity with socioeconomic status in rectal cancer outcomes. Annals of surgery. 2011 Apr;253(4):647–654. doi: 10.1097/SLA.0b013e3182111102. [DOI] [PubMed] [Google Scholar]
- 10.Konety SH, Vaughan Sarrazin MS, Rosenthal GE. Patient and hospital differences underlying racial variation in outcomes after coronary artery bypass graft surgery. Circulation. 2005 Mar 15;111(10):1210–1216. doi: 10.1161/01.CIR.0000157728.49918.9F. [DOI] [PubMed] [Google Scholar]
- 11.Lemaire A, Cook C, Tackett S, Mendes DM, Shortell CK. The impact of race and insurance type on the outcome of endovascular abdominal aortic aneurysm (AAA) repair. Journal of vascular surgery. 2008 Jun;47(6):1172–1180. doi: 10.1016/j.jvs.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 12.Morris AM, Wei Y, Birkmeyer NJ, Birkmeyer JD. Racial disparities in late survival after rectal cancer surgery. Journal of the American College of Surgeons. 2006 Dec;203(6):787–794. doi: 10.1016/j.jamcollsurg.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Oster ME, Strickland MJ, Mahle WT. Racial and ethnic disparities in post-operative mortality following congenital heart surgery. The Journal of pediatrics. 2011 Aug;159(2):222–226. doi: 10.1016/j.jpeds.2011.01.060. [DOI] [PubMed] [Google Scholar]
- 14.Sosa JA, Mehta PJ, Wang TS, Yeo HL, Roman SA. Racial disparities in clinical and economic outcomes from thyroidectomy. Annals of surgery. 2007 Dec;246(6):1083–1091. doi: 10.1097/SLA.0b013e31812eecc4. [DOI] [PubMed] [Google Scholar]
- 15.Terplan M, Schluterman N, McNamara EJ, Tracy JK, Temkin SM. Have racial disparities in ovarian cancer increased over time? An analysis of SEER data. Gynecologic oncology. 2012 Apr;125(1):19–24. doi: 10.1016/j.ygyno.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 16.Trivedi AN, Sequist TD, Ayanian JZ. Impact of hospital volume on racial disparities in cardiovascular procedure mortality. Journal of the American College of Cardiology. 2006 Jan 17;47(2):417–424. doi: 10.1016/j.jacc.2005.08.068. [DOI] [PubMed] [Google Scholar]
- 17.Osborne NH, Upchurch GR, Jr, Mathur AK, Dimick JB. Explaining racial disparities in mortality after abdominal aortic aneurysm repair. Journal of vascular surgery. 2009 Oct;50(4):709–713. doi: 10.1016/j.jvs.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 18.Ricciardi R, Selker HP, Baxter NN, Marcello PW, Roberts PL, Virnig BA. Disparate use of minimally invasive surgery in benign surgical conditions. Surgical endoscopy. 2008 Sep;22(9):1977–1986. doi: 10.1007/s00464-008-0003-0. [DOI] [PubMed] [Google Scholar]
- 19.Vouyouka AG, Egorova NN, Salloum A, et al. Lessons learned from the analysis of gender effect on risk factors and procedural outcomes of lower extremity arterial disease. Journal of vascular surgery. 2010 Nov;52(5):1196–1202. doi: 10.1016/j.jvs.2010.05.106. [DOI] [PubMed] [Google Scholar]
- 20.Liu V, Weill D, Bhattacharya J. Racial disparities in survival after lung transplantation. Arch Surg. 2011 Mar;146(3):286–293. doi: 10.1001/archsurg.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamath AF, Horneff JG, Gaffney V, Israelite CL, Nelson CL. Ethnic and gender differences in the functional disparities after primary total knee arthroplasty. Clinical orthopaedics and related research. 2010 Dec;468(12):3355–3361. doi: 10.1007/s11999-010-1461-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh TP, Almond C, Givertz MM, Piercey G, Gauvreau K. Improved survival in heart transplant recipients in the United States: racial differences in era effect. Circulation. Heart failure. 2011 Mar;4(2):153–160. doi: 10.1161/CIRCHEARTFAILURE.110.957829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Artinyan A, Mailey B, Sanchez-Luege N, et al. Race, ethnicity, and socioeconomic status influence the survival of patients with hepatocellular carcinoma in the United States. Cancer. 2010 Mar 1;116(5):1367–1377. doi: 10.1002/cncr.24817. [DOI] [PubMed] [Google Scholar]
- 24.Chew DK, Nguyen LL, Owens CD, et al. Comparative analysis of autogenous infrainguinal bypass grafts in African Americans and Caucasians: the association of race with graft function and limb salvage. Journal of vascular surgery. 2005 Oct;42(4):695–701. doi: 10.1016/j.jvs.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 25.Feyssa E, Jones-Burton C, Ellison G, Philosophe B, Howell C. Racial/ethnic disparity in kidney transplantation outcomes: influence of donor and recipient characteristics. Journal of the National Medical Association. 2009 Feb;101(2):111–115. doi: 10.1016/s0027-9684(15)30822-1. [DOI] [PubMed] [Google Scholar]
- 26.Luan FL, Kommareddi M, Cibrik DM, Samaniego M, Ojo AO. Influence of recipient race on the outcome of simultaneous pancreas and kidney transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010 Sep;10(9):2074–2081. doi: 10.1111/j.1600-6143.2010.03211.x. [DOI] [PubMed] [Google Scholar]
- 27.Mahle WT, Kanter KR, Vincent RN. Disparities in outcome for black patients after pediatric heart transplantation. The Journal of pediatrics. 2005 Dec;147(6):739–743. doi: 10.1016/j.jpeds.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 28.Holman KH, Henke PK, Dimick JB, Birkmeyer JD. Racial disparities in the use of revascularization before leg amputation in Medicare patients. Journal of vascular surgery. 2011 Aug;54(2):420–426. 426 e421. doi: 10.1016/j.jvs.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen LL, Hevelone N, Rogers SO, et al. Disparity in outcomes of surgical revascularization for limb salvage: race and gender are synergistic determinants of vein graft failure and limb loss. Circulation. 2009 Jan 6;119(1):123–130. doi: 10.1161/CIRCULATIONAHA.108.810341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rucker-Whitaker C, Feinglass J, Pearce WH. Explaining racial variation in lower extremity amputation: a 5-year retrospective claims data and medical record review at an urban teaching hospital. Arch Surg. 2003 Dec;138(12):1347–1351. doi: 10.1001/archsurg.138.12.1347. [DOI] [PubMed] [Google Scholar]
- 31.Kennedy BS, Fortmann SP, Stafford RS. Elective and isolated carotid endarterectomy: health disparities in utilization and outcomes, but not readmission. Journal of the National Medical Association. 2007 May;99(5):480–488. [PMC free article] [PubMed] [Google Scholar]
- 32.Du XL, Liu CC. Racial/Ethnic disparities in socioeconomic status, diagnosis, treatment and survival among medicare-insured men and women with head and neck cancer. Journal of health care for the poor and underserved. 2010 Aug;21(3):913–930. doi: 10.1353/hpu.0.0331. [DOI] [PubMed] [Google Scholar]
- 33.Mathur AK, Osborne NH, Lynch RJ, Ghaferi AA, Dimick JB, Sonnenday CJ. Racial/ethnic disparities in access to care and survival for patients with early-stage hepatocellular carcinoma. Arch Surg. 2010 Dec;145(12):1158–1163. doi: 10.1001/archsurg.2010.272. [DOI] [PubMed] [Google Scholar]
- 34.Murphy EH, Davis CM, Modrall JG, Clagett GP, Arko FR. Effects of ethnicity and insurance status on outcomes after thoracic endoluminal aortic aneurysm repair (TEVAR) Journal of vascular surgery. 2010 Apr;51(4 Suppl):14S–20S. doi: 10.1016/j.jvs.2009.11.079. [DOI] [PubMed] [Google Scholar]
- 35.Nathan H, Frederick W, Choti MA, Schulick RD, Pawlik TM. Racial disparity in surgical mortality after major hepatectomy. Journal of the American College of Surgeons. 2008 Sep;207(3):312–319. doi: 10.1016/j.jamcollsurg.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson WP, 3rd, Owens CD, Nguyen LL, Chong TT, Conte MS, Belkin M. Inferior outcomes of autogenous infrainguinal bypass in Hispanics: an analysis of ethnicity, graft function, and limb salvage. Journal of vascular surgery. 2009 Jun;49(6):1416–1425. doi: 10.1016/j.jvs.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 37.Press R, Carrasquillo O, Nickolas T, Radhakrishnan J, Shea S, Barr RG. Race/ethnicity, poverty status, and renal transplant outcomes. Transplantation. 2005 Oct 15;80(7):917–924. doi: 10.1097/01.tp.0000173379.53347.31. [DOI] [PubMed] [Google Scholar]
- 38.Gelber RP, McCarthy EP, Davis JW, Seto TB. Ethnic disparities in breast cancer management among Asian Americans and Pacific Islanders. Annals of surgical oncology. 2006 Jul;13(7):977–984. doi: 10.1245/ASO.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 39.Lapar DJ, Bhamidipati CM, Walters DM, et al. Primary payer status affects outcomes for cardiac valve operations. Journal of the American College of Surgeons. 2011 May;212(5):759–767. doi: 10.1016/j.jamcollsurg.2010.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang R, Cheung MC, Byrne MM, et al. Do racial or socioeconomic disparities exist in lung cancer treatment? Cancer. 2010 May 15;116(10):2437–2447. doi: 10.1002/cncr.24986. [DOI] [PubMed] [Google Scholar]
- 41.Kelz RR, Gimotty PA, Polsky D, Norman S, Fraker D, DeMichele A. Morbidity and mortality of colorectal carcinoma surgery differs by insurance status. Cancer. 2004 Nov 15;101(10):2187–2194. doi: 10.1002/cncr.20624. [DOI] [PubMed] [Google Scholar]
- 42.Chang RK, Chen AY, Klitzner TS. Female sex as a risk factor for in-hospital mortality among children undergoing cardiac surgery. Circulation. 2002 Sep 17;106(12):1514–1522. doi: 10.1161/01.cir.0000029104.94858.6f. [DOI] [PubMed] [Google Scholar]
- 43.Gundle KR, McGlaston TJ, Ramappa AJ. Effect of insurance status on the rate of surgery following a meniscal tear. The Journal of bone and joint surgery. American volume. 2010 Oct 20;92(14):2452–2456. doi: 10.2106/JBJS.I.01369. [DOI] [PubMed] [Google Scholar]
- 44.Vogel TR, Cantor JC, Dombrovskiy VY, Haser PB, Graham AM. AAA repair: sociodemographic disparities in management and outcomes. Vascular and endovascular surgery. 2008 Jan;42(6):555–560. doi: 10.1177/1538574408321786. Dec-2009. [DOI] [PubMed] [Google Scholar]
- 45.Zak Y, Rhoads KF, Visser BC. Predictors of surgical intervention for hepatocellular carcinoma: race, socioeconomic status, and hospital type. Arch Surg. 2011 Jul;146(7):778–784. doi: 10.1001/archsurg.2011.37. [DOI] [PubMed] [Google Scholar]
- 46.Bennett KM, Scarborough JE, Pappas TN, Kepler TB. Patient socioeconomic status is an independent predictor of operative mortality. Annals of surgery. 2010 Sep;252(3):552–557. doi: 10.1097/SLA.0b013e3181f2ac64. discussion 557–558. [DOI] [PubMed] [Google Scholar]
- 47.Birkmeyer NJ, Gu N, Baser O, Morris AM, Birkmeyer JD. Socioeconomic status and surgical mortality in the elderly. Medical care. 2008 Sep;46(9):893–899. doi: 10.1097/MLR.0b013e31817925b0. [DOI] [PubMed] [Google Scholar]
- 48.Axelrod DA, Dzebisashvili N, Schnitzler MA, et al. The interplay of socioeconomic status, distance to center, and interdonor service area travel on kidney transplant access and outcomes. Clinical journal of the American Society of Nephrology : CJASN. 2010 Dec;5(12):2276–2288. doi: 10.2215/CJN.04940610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheung MC, Yang R, Byrne MM, Solorzano CC, Nakeeb A, Koniaris LG. Are patients of low socioeconomic status receiving suboptimal management for pancreatic adenocarcinoma? Cancer. 2010 Feb 1;116(3):723–733. doi: 10.1002/cncr.24758. [DOI] [PubMed] [Google Scholar]
- 50.Bowman K, Telem DA, Hernandez-Rosa J, Stein N, Williams R, Divino CM. Impact of race and socioeconomic status on presentation and management of ventral hernias. Arch Surg. 2010 Aug;145(8):776–780. doi: 10.1001/archsurg.2010.141. [DOI] [PubMed] [Google Scholar]
- 51.Durham CA, Mohr MC, Parker FM, Bogey WM, Powell CS, Stoner MC. The impact of socioeconomic factors on outcome and hospital costs associated with femoropopliteal revascularization. Journal of vascular surgery. 2010 Sep;52(3):600–606. doi: 10.1016/j.jvs.2010.04.011. discussion 606–607. [DOI] [PubMed] [Google Scholar]
- 52.Guagliardo MF, Teach SJ, Huang ZJ, Chamberlain JM, Joseph JG. Racial and ethnic disparities in pediatric appendicitis rupture rate. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 2003 Nov;10(11):1218–1227. doi: 10.1111/j.1553-2712.2003.tb00606.x. [DOI] [PubMed] [Google Scholar]
- 53.Lee SL, Shekherdimian S, Chiu VY, Sydorak RM. Perforated appendicitis in children: equal access to care eliminates racial and socioeconomic disparities. Journal of pediatric surgery. 2010 Jun;45(6):1203–1207. doi: 10.1016/j.jpedsurg.2010.02.089. [DOI] [PubMed] [Google Scholar]
- 54.Hanchate AD, Zhang Y, Felson DT, Ash AS. Exploring the determinants of racial and ethnic disparities in total knee arthroplasty: health insurance, income, and assets. Medical care. 2008 May;46(5):481–488. doi: 10.1097/MLR.0b013e3181621e9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fairfield KM, Lucas FL, Earle CC, Small L, Trimble EL, Warren JL. Regional variation in cancer-directed surgery and mortality among women with epithelial ovarian cancer in the Medicare population. Cancer. 2010 Oct 15;116(20):4840–4848. doi: 10.1002/cncr.25242. [DOI] [PubMed] [Google Scholar]
- 56.Lathan CS, Neville BA, Earle CC. The effect of race on invasive staging and surgery in non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006 Jan;24(3):413–418. doi: 10.1200/JCO.2005.02.1758. [DOI] [PubMed] [Google Scholar]
- 57.Murphy MM, Simons JP, Hill JS, et al. Pancreatic resection: a key component to reducing racial disparities in pancreatic adenocarcinoma. Cancer. 2009 Sep 1;115(17):3979–3990. doi: 10.1002/cncr.24433. [DOI] [PubMed] [Google Scholar]
- 58.Farjah F, Wood DE, Yanez ND, 3rd, et al. Racial disparities among patients with lung cancer who were recommended operative therapy. Arch Surg. 2009 Jan;144(1):14–18. doi: 10.1001/archsurg.2008.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bryant CS, Kumar S, Shah JP, et al. Racial disparities in survival among patients with germ cell tumors of the ovary--United States. Gynecologic oncology. 2009 Sep;114(3):437–441. doi: 10.1016/j.ygyno.2009.05.039. [DOI] [PubMed] [Google Scholar]
- 60.Al-Refaie WB, Muluneh B, Zhong W, et al. Who receives their complex cancer surgery at low-volume hospitals? Journal of the American College of Surgeons. 2012 Jan;214(1):81–87. doi: 10.1016/j.jamcollsurg.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 61.Al-Refaie WB VS. Cameron JL, editor. Are Cancer Trials Valid and Useful for the General Surgeon and Surgical Oncologist? Advances in Surgery. Vol 462012:269–281. doi: 10.1016/j.yasu.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 62.Jolly S, Vittinghoff E, Chattopadhyay A, Bibbins-Domingo K. Higher cardiovascular disease prevalence and mortality among younger blacks compared to whites. The American journal of medicine. 2010 Sep;123(9):811–818. doi: 10.1016/j.amjmed.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 63.Kershaw KN, Diez Roux AV, Burgard SA, Lisabeth LD, Mujahid MS, Schulz AJ. Metropolitan-level racial residential segregation and black-white disparities in hypertension. American journal of epidemiology. 2011 Sep 1;174(5):537–545. doi: 10.1093/aje/kwr116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Norris KC, Agodoa LY. Unraveling the racial disparities associated with kidney disease. Kidney international. 2005 Sep;68(3):914–924. doi: 10.1111/j.1523-1755.2005.00485.x. [DOI] [PubMed] [Google Scholar]
- 65.Waxman AJ, Mink PJ, Devesa SS, et al. Racial disparities in incidence and outcome in multiple myeloma: a population-based study. Blood. 2010 Dec 16;116(25):5501–5506. doi: 10.1182/blood-2010-07-298760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chin AL, Negash S, Hamilton R. Diversity and disparity in dementia: the impact of ethnoracial differences in Alzheimer disease. Alzheimer disease and associated disorders. 2011 Jul-Sep;25(3):187–195. doi: 10.1097/WAD.0b013e318211c6c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paquette IM, Kemp JA, Finlayson SR. Patient and hospital factors associated with use of sphincter-sparing surgery for rectal cancer. Diseases of the colon and rectum. 2010 Feb;53(2):115–120. doi: 10.1007/DCR.0b013e3181bc98a1. [DOI] [PubMed] [Google Scholar]
- 68.Dunlop DD, Manheim LM, Song J, et al. Age and racial/ethnic disparities in arthritis-related hip and knee surgeries. Medical care. 2008 Feb;46(2):200–208. doi: 10.1097/MLR.0b013e31815cecd8. [DOI] [PubMed] [Google Scholar]
- 69.Wilson CT, Fisher E, Welch HG. Racial disparities in abdominal aortic aneurysm repair among male Medicare beneficiaries. Arch Surg. 2008 May;143(5):506–510. doi: 10.1001/archsurg.143.5.506. [DOI] [PubMed] [Google Scholar]
- 70.Alderman AK, Hawley ST, Janz NK, et al. Racial and ethnic disparities in the use of postmastectomy breast reconstruction: results from a population- based study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009 Nov 10;27(32):5325–5330. doi: 10.1200/JCO.2009.22.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anger JT, Rodriguez LV, Wang Q, Chen E, Pashos CL, Litwin MS. Racial disparities in the surgical management of stress incontinence among female Medicare beneficiaries. The Journal of urology. 2007 May;177(5):1846–1850. doi: 10.1016/j.juro.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 72.Steyerberg EW, Earle CC, Neville BA, Weeks JC. Racial differences in surgical evaluation, treatment, and outcome of locoregional esophageal cancer: a population-based analysis of elderly patients. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005 Jan 20;23(3):510–517. doi: 10.1200/JCO.2005.05.169. [DOI] [PubMed] [Google Scholar]
- 73.Bang H, Chiu YL, Memtsoudis SG, et al. Total hip and total knee arthroplasties: trends and disparities revisited. Am J Orthop (Belle Mead NJ) 2010 Sep;39(9):E95–E102. [PubMed] [Google Scholar]
- 74.Kruper L, Holt A, Xu XX, et al. Disparities in reconstruction rates after mastectomy: patterns of care and factors associated with the use of breast reconstruction in Southern California. Annals of surgical oncology. 2011 Aug;18(8):2158–2165. doi: 10.1245/s10434-011-1580-z. [DOI] [PubMed] [Google Scholar]
- 75.Rogers SO, Ray WA, Smalley WE. A population-based study of survival among elderly persons diagnosed with colorectal cancer: does race matter if all are insured? (United States) Cancer causes & control : CCC. 2004 Mar;15(2):193–199. doi: 10.1023/B:CACO.0000019511.67989.09. [DOI] [PubMed] [Google Scholar]
- 76.Perryman M, Rivers PA. The effects of location and race on the performance of cardiac procedures. Journal of health care finance. 2007 Summer;33(4):79–85. [PubMed] [Google Scholar]
- 77.Haas JS, Brawarsky P, Iyer A, Fitzmaurice GM, Neville BA, Earle C. Association of area sociodemographic characteristics and capacity for treatment with disparities in colorectal cancer care and mortality. Cancer. 2011 Sep 15;117(18):4267–4276. doi: 10.1002/cncr.26034. [DOI] [PubMed] [Google Scholar]
- 78.Murphy MM, Simons JP, Ng SC, et al. Racial differences in cancer specialist consultation, treatment, and outcomes for locoregional pancreatic adenocarcinoma. Annals of surgical oncology. 2009 Nov;16(11):2968–2977. doi: 10.1245/s10434-009-0656-5. [DOI] [PubMed] [Google Scholar]
- 79.Morrissey NJ, Giacovelli J, Egorova N, et al. Disparities in the treatment and outcomes of vascular disease in Hispanic patients. Journal of vascular surgery. 2007 Nov;46(5):971–978. doi: 10.1016/j.jvs.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Halm EA, Tuhrim S, Wang JJ, et al. Racial and ethnic disparities in outcomes and appropriateness of carotid endarterectomy: impact of patient and provider factors. Stroke; a journal of cerebral circulation. 2009 Jul;40(7):2493–2501. doi: 10.1161/STROKEAHA.108.544866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee SL, Yaghoubian A, Stark R, Shekherdimian S. Equal access to healthcare does not eliminate disparities in the management of adults with appendicitis. The Journal of surgical research. 2011 Oct;170(2):209–213. doi: 10.1016/j.jss.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 82.Morris AM, Billingsley KG, Baxter NN, Baldwin LM. Racial disparities in rectal cancer treatment: a population-based analysis. Arch Surg. 2004 Feb;139(2):151–155. doi: 10.1001/archsurg.139.2.151. discussion 156. [DOI] [PubMed] [Google Scholar]
- 83.Trinh QD, Schmitges J, Sun M, et al. Improvement of racial disparities with respect to the utilization of minimally invasive radical prostatectomy in the United States. Cancer. 2012 Apr 1;118(7):1894–1900. doi: 10.1002/cncr.26527. [DOI] [PubMed] [Google Scholar]
- 84.Castellanos LR, Normand SL, Ayanian JZ. Racial and ethnic disparities in access to higher and lower quality cardiac surgeons for coronary artery bypass grafting. The American journal of cardiology. 2009 Jun 15;103(12):1682–1686. doi: 10.1016/j.amjcard.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 85.Aranda MA, McGory M, Sekeris E, Maggard M, Ko C, Zingmond DS. Do racial/ethnic disparities exist in the utilization of high-volume surgeons for women with ovarian cancer? Gynecologic oncology. 2008 Nov;111(2):166–172. doi: 10.1016/j.ygyno.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gooden KM, Howard DL, Carpenter WR, et al. The effect of hospital and surgeon volume on racial differences in recurrence-free survival after radical prostatectomy. Medical care. 2008 Nov;46(11):1170–1176. doi: 10.1097/MLR.0b013e31817d696d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pollack CE, Bekelman JE, Epstein AJ, Liao K, Wong YN, Armstrong K. Racial disparities in changing to a high-volume urologist among men with localized prostate cancer. Medical care. 2011 Nov;49(11):999–1006. doi: 10.1097/MLR.0b013e3182364019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hofmann LJ, Lee S, Waddell B, Davis KG. Effect of race on colon cancer treatment and outcomes in the Department of Defense healthcare system. Diseases of the colon and rectum. 2010 Jan;53(1):9–15. doi: 10.1007/DCR.0b013e3181bdcdb2. [DOI] [PubMed] [Google Scholar]
- 89.Banez LL, Terris MK, Aronson WJ, et al. Race and time from diagnosis to radical prostatectomy: does equal access mean equal timely access to the operating room?-- Results from the SEARCH database. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009 Apr;18(4):1208–1212. doi: 10.1158/1055-9965.EPI-08-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Robinson CN, Balentine CJ, Marshall CL, et al. Ethnic disparities are reduced in VA colon cancer patients. American journal of surgery. 2010 Nov;200(5):636–639. doi: 10.1016/j.amjsurg.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 91.Neighbors CJ, Rogers ML, Shenassa ED, Sciamanna CN, Clark MA, Novak SP. Ethnic/racial disparities in hospital procedure volume for lung resection for lung cancer. Medical care. 2007 Jul;45(7):655–663. doi: 10.1097/MLR.0b013e3180326110. [DOI] [PubMed] [Google Scholar]
- 92.Mukherjee D, Zaidi HA, Kosztowski T, et al. Disparities in access to neuro-oncologic care in the United States. Arch Surg. 2010 Mar;145(3):247–253. doi: 10.1001/archsurg.2009.288. [DOI] [PubMed] [Google Scholar]
- 93.Parsons HMHE, Stain SC, Vickers SM, Al-Refaie WB. What happens to racial and ethnic minorities after cancer surgery at American College of Surgeons National Surgical Quality Improvement Program hospitals? Journal of the American College of Surgeons. 2012;214(4):539–547. doi: 10.1016/j.jamcollsurg.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 94.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. The New England journal of medicine. 2002 Apr 11;346(15):1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 95.Bach PB, Cramer LD, Schrag D, Downey RJ, Gelfand SE, Begg CB. The influence of hospital volume on survival after resection for lung cancer. The New England journal of medicine. 2001 Jul 19;345(3):181–188. doi: 10.1056/NEJM200107193450306. [DOI] [PubMed] [Google Scholar]
- 96.Lucas FL, Stukel TA, Morris AM, Siewers AE, Birkmeyer JD. Race and surgical mortality in the United States. Annals of surgery. 2006 Feb;243(2):281–286. doi: 10.1097/01.sla.0000197560.92456.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Haider AHOuS, Efron DT, Oyetunji TA, Crandall ML, Scott VK, Haut ER, Schneider EB, Powe NR, Cooper LA, Cornwell EE., 3rd Association between hospitals caring for a disproportionately high percentage of minority trauma patients and increased mortality: a nationwide analysis of 434 hospitals. Arch Surg. 2012;147(1):63–70. doi: 10.1001/archsurg.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rhoads KF, Ackerson LK, Jha AK, Dudley RA. Quality of colon cancer outcomes in hospitals with a high percentage of Medicaid patients. Journal of the American College of Surgeons. 2008 Aug;207(2):197–204. doi: 10.1016/j.jamcollsurg.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 99.Osborne NH, Mathur AK, Upchurch GR, Jr, Dimick JB. Understanding the racial disparity in the receipt of endovascular abdominal aortic aneurysm repair. Arch Surg. 2010 Nov;145(11):1105–1108. doi: 10.1001/archsurg.2010.213. [DOI] [PubMed] [Google Scholar]