Abstract
Aim
To evaluate the efficacy and tolerability of tetracycline vs. high-dose amoxicillin in bismuth-based quadruple therapy for Helicobacter pylori(H. pylori) eradication.
Methods
This randomized, open-label clinical trial included 228 patients with H.pylori infection and duodenal ulcer without a history of H.pylori treatment. Patients were randomly divided into two groups. The amoxicillin group received metronidazole 500mg, bismuth subcitrate 240mg, and amoxicillin 1000mg, all three times a day, plus omeprazole 20 mg twice a day, for 14 days. The tetracycline group received metronidazole 500mg three times a day; bismuth subcitrate240mg and tetracycline HCl 500mg, both four times a day; and omeprazole 20 mg twice a day, for 14 days. Evaluation for compliance and drug-relatedadverse effects were evaluated at the end of two weeks. Eight weeks after the end of treatment, the rate of H.pylori eradication was assessed by the C13urease breath test.
Results
There were no significant demographic differences between the two groups. Eradication rate was higher with the amoxicillin-containing regimen than the tetracycline-containing regimen: 105/110 (95.51%; 95% confidence interval, 91.5%–99.3%) vs. 88/105 (83.8%; 95%CI, 76.7%–90.8%) by per-protocol analysis (p = 0.005) and 92.9% (95%CI, 88.1%–97.6%) vs. 76.5% (95%CI, 68.7%–84.2%) by intention-to-treat analysis (ITT, p = 0.001). Adverse effects were significant higher in the tetracycline groupthan in the amoxicillin group (65.2% vs. 43.4%; p = 0.001).
Conclusion
Bismuth-based quadruple therapy including high-dose amoxicillin and metronidazole achieved an acceptable rate of H.pylori infection eradication with good tolerance in patients with duodenal ulcer. This regimen can overcome treatment resistance in areas with high prevalence of metronidazole and clarithromycin resistance.
Trial registration
The Thai Clinical Trial Registry (TCTR) 20170623004
Introduction
Helicobacter pylori(H. pylori) is a gram-negative bacterium that infects approximately 50% of people in industrialized nations and up to 80% in less-developed countries[1]. H. pyloricauses peptic ulcer disease, chronic gastritis, gastric adenocarcinoma, and mucosa-associated lymphoid tissue lymphoma[2]. Although the bacterium is susceptible to most antimicrobial agentsinvitro, successful treatment of H.pyloriremains a challenge[3].Factors contributing to success inH. pylori treatment are drug efficacy, host compliance, and bacterial resistance[1, 2]. Resistance to commonly used drugs such as metronidazole and clarithromycin, is the most important reason for treatment failure of current regimens[1]. Emerging evidence shows high resistance to clarithromycin in countries with high consumption of macrolidederivatives[4], whereas regimen containing clarithromycin was reported to achieve 80% eradication rate[5]. Previous studies from our region (Iran)revealed that more than 20% of H. pylori isolates were resistant to clarithromycin and that over 50% of the H. pylori isolates were resistant to metronidazole [6–8].
Due to high resistance to metronidazole and clarithromycin, regimens containing these antibiotics are not efficient[9]. In some studies on patients with metronidazole- and clarithromycin-resistantH. pylori, bismuth-based quadruple therapy was reported as a preferable regimen for eradication of H. pylori[10, 11]. This bismuth-based quadruple therapy includesbismuth, a proton pump inhibitor (PPI), and tetracycline together with metronidazole or tinidazole, with proper doses and duration. Quadruple regimen with suboptimal metronidazole doses (<1500 mg/day) was reported to achieve an overall eradication rate of 70% [3,4]. Increasing the dose of metronidazole in bismuth-based quadruple therapy was the first step in increasing eradication rates to acceptable levels, and bismuth compound was reported to be necessary for such response [12, 13]. However, low compliance,as well as increased side effects, are major issues that arise if full-dose metronidazole and tetracycline are used. There is also some evidence suggesting an increase in H.pylori resistance to tetracycline [14–16].
Outcomes with substitution of tetracycline with amoxicillin in bismuth-based quadruple therapy have not been widely studied, especially in countries with high H.pylori resistance to metronidazole and clarithromycin such as Iran. Our pilot study revealed that a very good H.pylori eradication rate could be achieved with a modified bismuth-based quadruple therapy containing high-dose amoxicillin (3 g/day), adequate-dose metronidazole (1.5 g/day), and a PPI.Therefore, this open-label, randomized clinical trial was designed to compare the classic bismuth-based quadruple regimen containing metronidazole (1500mg/day) with a modified bismuth-based quadruple therapy containing high-dose amoxicillin (3 g/day), metronidazole (1.5 g/day),and a PPI, with the aim to compare eradication rates, adverse effects, and patient compliance.
Materials and methods
Patient population
This was a prospective, randomized, open-label clinical trial study conducted at ShahidSadoughi University (SSU) of Medical Center, a tertiary care hospital located in Yazd, Iran. Criteria for inclusion in this study were as follows: no history of treatment for H.pylori eradication, age above 18 years, endoscopically confirmed diagnosis of duodenal ulcer, and positive rapidureasetest. Patients with a history of previous gastric surgery, allergy to antibiotics, those who were treated with antibiotics in the preceding eight weeks,those with major systemic disease, and those who were pregnantor lactating were excluded from the study. The primary endpoint was H.pylori eradication ratebyintention-to-treat (ITT) analysis. The secondary endpoint was frequency of adverse effects and treatment compliance.
Registration
Ethics approval for this study was obtained from Yazd University Medical Sciences ethics committee on September 20, 2014, prior to patient enrollment. The clinical trial registration was completed retrospectively several months after patient enrollment was started, due to a miscommunication with the institution. We emphasize that our study begins and progress with complete consideration of ethicalrules. The authors confirm that all ongoing and related trials related to this study are registered.
Intervention
Patients were enrolled between October 20, 2014 and July 15, 2016. After the patients provided consent to participate in this trial, they were randomly assigned at a 1:1 ratio to receive one of the following two treatment regimens. The amoxicillin group (group I) received metronidazole 500mg, amoxicillin 1000mg, bismuth subcitrate 240mg, all three times a day, plus omeprazole 20mg twice a day, for 14 days. The tetracycline group (group II) received omeprazole 20mg twice a day; bismuth 240 mg, and tetracycline HCl500 mg, both four times a day;and metronidazole 500 mg three times a day, for 14 days. Omeprazole and bismuth were taken before meals, and antibiotics were used after meals. All patients were instructed on potential adverse effects and kept under observation during treatment for evaluation of adverse effects and compliance. All patients were requested to record any adverse effects that occurred during therapy, including bad taste, diarrhea, dizziness, weakness, nausea, loss of appetite, vomiting, fatigue, fever, and skin rash. Severe adverse effects were defined as those that would be considered to disrupt daily activities that required treatment discontinuation by the patient.
Outcomes
For evaluation for the primary outcome of H. pylori eradication rate, patients were asked to stop the PPI or the H2 blocker for at least four weeks before follow-up evaluation. Eight weeks after conclusion of the two-week study treatment, patients were assessed by the C13urease breath test by personnel who were blinded to the treatment, and a value of less than 4% was defined as successfulH. pylori eradication. For evaluation of secondary outcomes, data on adverse effects were collected through a standard sideeffect questionnaire, and good compliance was defined as ingesting more than 80% of the total number of doses included in the regimen.
Sampling and blinding
Sample size for the trial was calculated based on the following assumptions. Average rate of successfully achieved eradication of H.pyloriwith the standard quadruple therapy was 80% [17]. Based onourpilot study results showing an H.pylori eradication rate of 94% with the modified bismuth-based quadruple therapy containing high-dose amoxicillin (3 g/day),adequate-dose metronidazole dose (1.5 g/day) and omeprazole, we chose a two-sided alpha value of 0.05, and a power of 80%.Based on these assumptions at least 208 participates (104 subjects in each groups) would be required. In order to accommodate a 10% rate of lost to follow-up, we enrolled 228 patients.
Statistical methods
All registered data were analyzed using SPSS software version 22 for Windows (SPSS, Chicago, IL). Data were presented as means with standard deviation (SD), frequencies, and percentages. The chi-square and Fisher’s exact tests were used for comparison of categorical data between the two groups. P values of less than 0.05 were considered significant for all analyses. ITT and per-protocol (PP)analyses were performed to calculate eradication rates. The ITT analysis included all randomized patients. Individuals who did not take at least 80% of the drugs andthosewith unknown post-treatment H. pylori status were excluded from the PP analysis. Odds ratios with 95% confidence intervals (CIs) were calculated where appropriate.
Ethical consideration
This study was approved by the Ethics Committee of ShahidSadoughiUniversityof Medical Sciences in Yazd, Iran and registered with the protocol number ˮIr.ssu.rec.1394.13712”on September 20, 2014. Participants provided written informed consent and were included in the study after they were provided information on treatment methods. This trial was also registered with Thai Clinical Trial Registry (Number: TCTR20170623004). The Consort 2010 checklist, the study protocol and the Ethic approval of study are given S1 Checklist, S1 File and S2 File.
Results
Patient characteristics and compliance
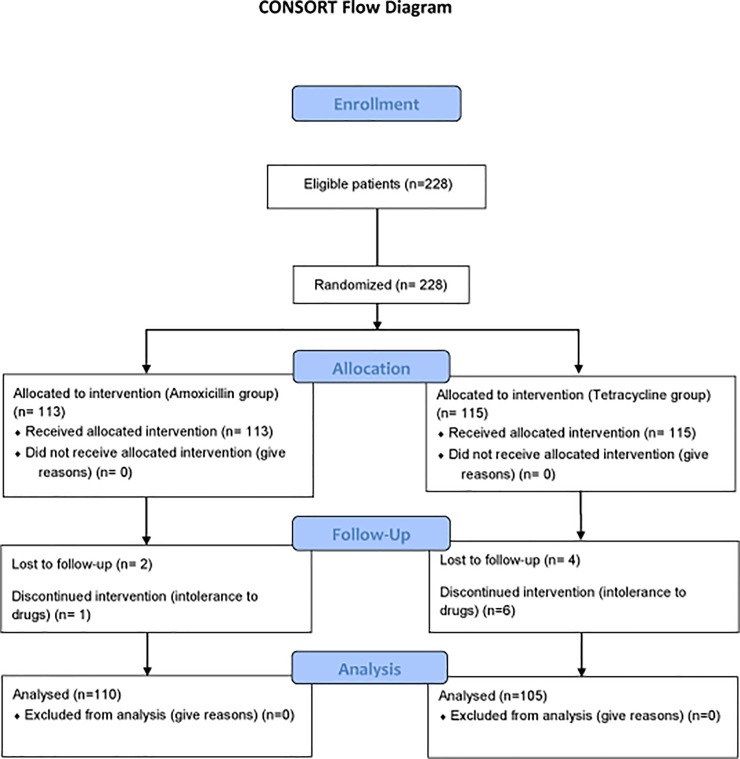
A total of 228 patients who were eligible were recruited from the SSU Gastroenterology Department. There were no significant differences in baseline characteristics including age and sex between the two treatment groups (Table 1). All patients except those who were lost to follow-up and those who could not tolerate the regimen were assessed by the C13urease breath test after the conclusion of treatments. The CONSORTflow diagram is depicted in Fig 1.
Table 1. Baseline characteristics of patients randomized in the two groups.
| Variables | Amoxicillin group | Tetracycline group | P value | |
|---|---|---|---|---|
| Number of subjects | 113 | 115 | ||
| Age (mean±SD) and range | 41.8±13.96 (19–72) |
39.84±14.36 (18–74) |
0.29* | |
| Gender | Male | 61 | 56 | 0.42** |
| Female | 52 | 59 | ||
| Lost to follow up | 2 | 4 | ||
| Cannot tolerate drugs | 1 | 6 | ||
| Compliance (taken 80% of tablets) |
(110/113)98.23% | (105/115)91.30% | ||
* Independent Sample T Test;
**Pearson Chi Square Test
Fig 1. CONSORT flow diagram.
H. pylori eradication rates
Overall, the eradication rate by per-protocol analysis was 105/110 (95.51%; 95%CI, 91.5%–99.3%) in group I and 88/105 (83.8%; 95%CI, 76.7%–90.8%) in group II, which was significantly different between the two treatment groups (p = 0.005). As presented in Table 2, the ITT analysis also revealed that the eradication rates were significantly different between the amoxicillin and tetracycline groups (92.9%; 95%CI, 88.1%–97.6% and 76.5%; 95%CI, 68.7%–84.2%, respectively; p<0.001). These results clearly showed that the H. pylori eradication rate was significantly higher in the amoxicillin group than in the tetracycline group.
Table 2. H.pylorieradication rates in intention-to-treat and per-protocol analyses.
| Analysis | Amoxicillin group | Tetracycline group | 95% CI for difference between amoxicillin and tetracycline group | Mean difference | p value |
|---|---|---|---|---|---|
| ITT | 92.9% (105/113) | 76.5% (88/115) | 7.3% to 25.4% | 16.3% | 0.001* |
| 95% CI | 88.1% to 97.6% | 68.7% to 84.2% | |||
| PP | 95.5% (105/110) | 83.8% (88/105) | 3.5% to 19.6% | 11.6% | 0.005* |
| 95% CI | 91.5% to 99.3% | 76.7% to 90.8% |
*Pearson Chi Square;
ITT: intention-to-treat; PP: per protocol; CI: confidence interval
Adverse effects and compliance
Overall, 4 patients in the tetracycline group and 2 patients in the amoxicillin group were lost to follow-up, and no outcome data were available for these patients. Additionally, 6 patients in the tetracycline group and 1 patient in the amoxicillin group could not tolerate the medication, i.e.,failed to take at least 80% of the prescribed medication, due to severe adverse effects that started a few days after initiation of the treatment. Adverse effects included tolerable diarrhea, bad taste, dizziness, weakness, nausea, skin rash, fever, fatigue, and vomiting, which were reported by 43.4% (49 of 113) of the patients in the amoxicillin group and 65.2% (75 of 115) of the patients in the tetracycline group, and adverse effects were significantly more frequent in the tetracycline group (p = 0.001). Bad taste was the most common side effect in both groups, whereas the frequency of vomiting was significantly higher in the tetracycline treatment group (4 vs. 0 in tetracycline vs.amoxicillin groups; p = 0.04).A summary of adverse effects in this study is presented in Table 3.
Table 3. Side effects of treatment in groups.
| Variables | Amoxicillin group | Tetracycline group | P Value* |
|---|---|---|---|
| Tolerable diarrhea | 6 (5.3%) | 8 (7.0%) | 0.60 |
| Bad taste | 19 (16.8%) | 22 (19.1%) | 0.64 |
| Dizziness | 3 (2.7%) | 4 (3.5%) | 0.71 |
| Weakness | 4 (3.5%) | 6 (5.2%) | 0.61 |
| Nausea | 9 (8.0%) | 17 (14.8%) | 0.10 |
| Skin rash | 4 (3.5%) | 4(3.5%) | 0.98 |
| Fever | 2 (1.8%) | 3 (2.6%) | 0.66 |
| Fatigue | 2 (1.8%) | 7 (6.1%) | 0.09 |
| Vomiting | 0 (0%) | 4 (3.5%) | 0.04 |
| Total side effects % (n) | 49 (43.4%) | 75 (65.2%) | 0.001 |
*Pearson Chi Square Test
Discussion
Results of this open-label randomized study revealed that substitution of tetracycline with high-dose amoxicillin in classic bismuth-based quadruple therapy can increase H. pylori eradication rate and compliance in patients with duodenal ulcer. In patients who received high-dose amoxicillin in bismuth-based quadruple therapy, the eradication rates were 95.6% and 92.9% by the PP and ITT analyses, respectively. Conversely, the eradication rates in patients who received classic bismuth-based quadruple therapy were 83.3% and 75% by PP and ITT analyses, respectively. These findings indicatedthat substitution of tetracycline with high-dose amoxicillin significantly increased the eradication rate not only by the ITT analysis but also the PP analysis.As more than 50% of H. pylori strains in Iran are resistant to metronidazole[6–8], amoxicillin might achieve better synergistic effects with metronidazole than tetracycline for eradication of metronidazole-resistantH. pylori strains. Additionally, patients had better tolerance to bismuth-based quadruple therapy containing amoxicillin instead of tetracycline.
Generally, management of disorders associated with H. pylori infection requires treatment regimens that have eradication rates of more than 90% to 95%, if possible [18]. Use of similar effective first-line treatments is important not only for disease cure but also for prevention of secondary antibiotic resistance [19].
A recent review of various regimens used as primary treatment in West Asia suggested that bismuth-furazolidone-metronidazole treatment for 10 days, clarithromycin-based hybrid treatment for 14 days, and classic bismuth-based quadruple therapy including PPIs, bismuth, tetracycline, and metronidazole for 14 days,wereequally effective [20]. Since the furazolidone-based regimen has many side effects, the regimen containing adequate-dose furazolidone (400mg/day) is not recommended [21, 22]. Due to high resistance to clarithromycin, clarithromycin-containing regimen isnot acceptable in our treatment course [23]. Potentially high resistance to levofloxacin also limits its use as front-line treatment [9, 23]. H. pylori culture and antibiogram are expensive, and lack of information on pretreatment susceptibility to antibiotics is the main reason for bismuth-based quadruple therapy as a primary regimen for eradication of H. pylori in our region, as recommended by the Toronto Consensus [24]. Malfertheiner and colleagues showed that in regions with high resistance to clarithromycin, quadruple treatment containing bismuth, metronidazole, tetracycline, and a PPI was preferable as the primary therapy for H. pylori eradication. Quadruple therapy achieves superior eradication with safety and tolerability comparable to those obtained with standard triple therapy [25]. In contrast to metronidazole, increase in clarithromycin dose was reported not to increase the eradication rate of clarithromycin-resistant H. pylori[26].
Liang et al. concluded that four different bismuth-based quadruple treatments exhibited eradication ratesof>90% against H. pylori in subjects who were unresponsive to prior therapies, including those showing resistance to clarithromycin and metronidazole [27].Therefore, the two-week bismuth-based quadruple therapy containing high-dose metronidazole, a PPI, and tetracycline is a proper first-line treatment regimen in our patients with duodenal ulcer [28–30]. This regimen requires modifications with respect to drugs, doses, and duration to reach excellent H. pylori eradication rates. Bismuth and metronidazole are necessaryadditionsin this regimen. Increase in H. pylorieradication rate with an increased metronidazole dose is dependent on the presence of bismuth. Bismuth also improves the elimination ofH. pylori by triple therapy regimen [31]. In a systematic review and meta-analysis of 35 randomized clinical trials and 4763 subjects, the safety profile of bismuth was assessed, which revealed insignificant side effects in patients undergoing bismuth-based treatment [32].Emerging evidence of increasing resistance to tetracycline and reports demonstrating that adequate-dose metronidazole in combination with tetracycline results in more side effects raise concerns regarding substitution of tetracycline with another antibiotic.
To achieve increased compliance to classic bismuth-based quadruple therapy, tetracycline was replaced with high-dose amoxicillin, based on several lines of evidence:In a recent study, high-dose amoxicillin (750mg four times a day) in combination with a potent PPI achieved a very good eradication rate, suggesting that amoxicillin is a unique anti-H. pylori antibiotic that has an acceptable eradication rate when used as part of a dual therapy containing a PPI [33, 34].The resistance rates to amoxicillin, metronidazole, and clarithromycin were about 2%, 44%, and 29% in America; 0.7%, 35%, and 18% in Europe; and 2%, 38%, and 21% in Asia, respectively [35]. H. pyloriwas found to rapidly acquire antibiotic resistance to clarithromycin, metronidazole, and levofloxacin but not to amoxicillin after a single course of anti-H. pylori therapy [36]. One potential explanation for the difference in acquired antibiotic resistance is that a single-point mutation can lead to resistance to clarithromycin, metronidazole, and levofloxacin, whereas multiple-site mutations are necessary to confer amoxicillin resistance [37–39]. A recent study among US male patients revealed increased resistance to clarithromycin and levofloxacin but no resistance to amoxicillin [40]. In Iran, 1.6%, 16.7%, and 57.5% ofH. pylori strainsshowed resistance to amoxicillin, clarithromycin, and metronidazole, respectively; however, no tetracycline resistance was reported [41]. However, this sensitivity pattern was found to have changed five years later, and there is an increasing resistance to tetracycline [42]. A recent study in Yazd, Iran revealed that H.pylori is more resistant to tetracycline than amoxicillin[43]. In a study in China, amoxicillin, in combination with metronidazole, bismuth, and lansoprazole, eradicated metronidazole-resistantH. pylori. In their study, Zhang et al. used 2g amoxicillin per day in combination with lansoprazole, metronidazole, and bismuth [44]. Lansoprazole, albeit more potent, is more expensive than omeprazole in Iran; therefore, high-dose amoxicillin with omeprazole was preferred over 2g amoxicillin with lansoprazole.
The ITT analysis revealed an eradication rate of 92.92% in the amoxicillin group compared with the rate of 76.5% in the tetracycline group, indicating that bismuth-based quadruple therapy containing tetracycline with adequate-dose metronidazole was associated with more adverse effects and intolerance. Unfortunately, severe adverse effects that lead to intolerance in the tetracycline group occurred 4–5 days after treatment initiation. In contrast, there were no severe drug-associated complications with the high-dose amoxicillin regimen, and the patient compliance was good.
Inability to evaluate the H. pyloriantimicrobial resistance patterns was one of the main limitations of this study; howevera recent regional study indicated thatH. pylori resistance to tetracycline was more common than that to amoxicillin, which might explain the 16% failure rate in the tetracycline groupcompared to the 5% failure rate in the amoxicillin group based on the PP analysis[43].
Conclusion
The results of this randomized, open-label clinical trial revealed that a two-week bismuth-based quadruple therapy including high-dose amoxicillin and metronidazole had an acceptable eradication rate with good tolerance in patients with duodenal ulcer and H. pylori infection. This therapy might overcome treatment resistance in areas with high prevalence of H. pylori resistance to metronidazole and clarithromycin.
Supporting information
(DOC)
(DOC)
(PDF)
Acknowledgments
We would like to thank the following staff members of the Gastroenterology Ward at ShahidSadoughi Hospital for their distinguished services: Mr. Dehmoubed, Mr. Ahmadi, Mrs. Ameri, and Mrs. Farhangfar.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Go M. Natural history and epidemiology of Helicobacter pylori infection. Alimentary pharmacology & therapeutics. 2002;16(s1):3–15. [DOI] [PubMed] [Google Scholar]
- 2.Khademi F, Poursina F, Hosseini E, Akbari M, Safaei HG. Helicobacter pylori in Iran: A systematic review on the antibiotic resistance. Iranian journal of basic medical sciences. 2015;18(1):2 [PMC free article] [PubMed] [Google Scholar]
- 3.Fischbach L, Evans E. Meta‐analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple first‐line therapies for Helicobacter pylori. Alimentary pharmacology & therapeutics. 2007;26(3):343–57. [DOI] [PubMed] [Google Scholar]
- 4.Lu H, Zhang W, Graham DY. Bismuth-containing quadruple therapy for Helicobacter pylori: lessons from China. European journal of gastroenterology & hepatology. 2013;25(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59(8):1143–53. doi: 10.1136/gut.2009.192757 [DOI] [PubMed] [Google Scholar]
- 6.Shokrzadeh L, Alebouyeh M, Mirzaei T, Farzi N, Zali MR. Prevalence of multiple drug-resistant Helicobacter pylori strains among patients with different gastric disorders in Iran. Microbial Drug Resistance. 2015;21(1):105–10. doi: 10.1089/mdr.2014.0081 [DOI] [PubMed] [Google Scholar]
- 7.KeshavarzAziziRaftar S, Moniri R, Saffari M, RazaviZadeh M, Arj A, Mousavi SGA, et al. The helicobacter pylori resistance rate to clarithromycin in Iran. Microbial Drug Resistance. 2015;21(1):69–73. doi: 10.1089/mdr.2014.0104 [DOI] [PubMed] [Google Scholar]
- 8.Farshad S, Alborzi A, Japoni A, Ranjbar R, Asl KH, Badiee P, et al. Antimicrobial susceptibility of Helicobacter pylori strains isolated from patients in Shiraz, Southern Iran. World Journal of Gastroenterology: WJG. 2010;16(45):5746 doi: 10.3748/wjg.v16.i45.5746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham DY, Lee YC, Wu MS. Rational Helicobacter pylori therapy: evidence-based medicine rather than medicine-based evidence. Clinical Gastroenterology and Hepatology. 2014;12(2):177–86. e3. doi: 10.1016/j.cgh.2013.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischbach L, Zanten S, Dickason J. Meta‐analysis: the efficacy, adverse events, and adherence related to first‐line anti‐Helicobacter pylori quadruple therapies. Alimentary pharmacology & therapeutics. 2004;20(10):1071–82. [DOI] [PubMed] [Google Scholar]
- 11.Calvet X, Ducons J, Guardiola J, Tito L, Andreu V, Bory F, et al. One‐week triple vs. quadruple therapy for Helicobacter pylori infection—a randomized trial. Alimentary pharmacology & therapeutics. 2002;16(7):1261–7. [DOI] [PubMed] [Google Scholar]
- 12.Goodwin C, Marshall B, Blincow E, Wilson D, Blackbourn S, Phillips M. Prevention of nitroimidazole resistance in Campylobacter pylori by coadministration of colloidal bismuth subcitrate: clinical and in vitro studies. Journal of clinical pathology. 1988;41(2):207–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laine L, Hunt R, El-Zimaity H, Nguyen B, Osato M, Spénard J. Bismuth-based quadruple therapy using a single capsule of bismuth biskalcitrate, metronidazole, and tetracycline given with omeprazole versus omeprazole, amoxicillin, and clarithromycin for eradication of Helicobacter pylori in duodenal ulcer patients: a prospective, randomized, multicenter, North American trial. The American journal of gastroenterology. 2003;98(3):562–7. [DOI] [PubMed] [Google Scholar]
- 14.Abadi AT, Taghvaei T, Mobarez AM, Carpenter BM, Merrell DS. Frequency of antibiotic resistance in Helicobacter pylori strains isolated from the northern population of Iran. Journal of microbiology (Seoul, Korea). 2011;49(6):987–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JJ, Reddy R, Lee M, Kim JG, El-Zaatari FA, Osato MS, et al. Analysis of metronidazole, clarithromycin and tetracycline resistance of Helicobacter pylori isolates from Korea. The Journal of antimicrobial chemotherapy. 2001;47(4):459–61. [DOI] [PubMed] [Google Scholar]
- 16.Mendonca S, Ecclissato C, Sartori MS, Godoy AP, Guerzoni RA, Degger M, et al. Prevalence of Helicobacter pylori resistance to metronidazole, clarithromycin, amoxicillin, tetracycline, and furazolidone in Brazil. Helicobacter. 2000;5(2):79–83 [DOI] [PubMed] [Google Scholar]
- 17.A Fischbach L, Evans EL Meta-analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple first-line therapies for Helicobacter pylori. Aliment PharmacolTher. 2007. August 1; 26(3):343–57. [DOI] [PubMed] [Google Scholar]
- 18.Graham DY. Efficient identification and evaluation of effective Helicobacter pylori therapies. Clinical Gastroenterology and Hepatology. 2009;7(2):145–8. doi: 10.1016/j.cgh.2008.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang J, Hunt R. Treatment after failure: the problem of “non-responders”. Gut. 1999;45(suppl 1):I40–I4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fakheri H, Bari Z, Aarabi M, Malekzadeh R. Helicobacter pylori eradication in West Asia: a review. World Journal of Gastroenterology: WJG. 2014;20(30):10355 doi: 10.3748/wjg.v20.i30.10355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roghani HS, Massarrat S, Shirekhoda M, Butorab Z. Effect of different doses of furazolidone with amoxicillin and omeprazole on eradication of Helicobacter pylori. Journal of gastroenterology and hepatology. 2003;18(7):778–82. [DOI] [PubMed] [Google Scholar]
- 22.Sadeghifard N, Seidnazari T, Ghafourian S, Soleimani M, Maleki A, Qomi MA, et al. Survey in Iran of clarithromycin resistance in Helicobacter pylori isolates by PCR-RFLP. Southeast Asian Journal of Tropical Medicine and Public Health. 2013;44(1):89 [PubMed] [Google Scholar]
- 23.De Francesco V, Giorgio F, Hassan C, Manes G, Vannella L, Panella C, et al. Worldwide H. pylori antibiotic resistance: a systematic review. Journal of Gastrointestinal & Liver Diseases. 2010;19(4). [PubMed] [Google Scholar]
- 24.Fallone CA, Chiba N, van Zanten SV, Fischbach L, Gisbert JP, Hunt RH, et al. The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology. 2016;151(1):51–69. e14. doi: 10.1053/j.gastro.2016.04.006 [DOI] [PubMed] [Google Scholar]
- 25.Delchier J, Malfertheiner P, Thieroff‐Ekerdt R. Use of a combination formulation of bismuth, metronidazole and tetracycline with omeprazole as a rescue therapy for eradication of Helicobacter pylori. Alimentary pharmacology & therapeutics. 2014;40(2):171–7. [DOI] [PubMed] [Google Scholar]
- 26.Murakami K, Sato R, Okimoto T, Nasu M, Fujioka T, Kodama M, et al. Eradication rates of clarithromycin‐resistant Helicobacter pylori using either rabeprazole or lansoprazole plus amoxicillin and clarithromycin. Alimentary pharmacology & therapeutics. 2002;16(11):1933–8. [DOI] [PubMed] [Google Scholar]
- 27.Liang X, Xu X, Zheng Q, Zhang W, Sun Q, Liu W, et al. Efficacy of bismuth-containing quadruple therapies for clarithromycin-, metronidazole-, and fluoroquinolone-resistant Helicobacter pylori infections in a prospective study. Clinical Gastroenterology and Hepatology. 2013;11(7):802–7. e1. doi: 10.1016/j.cgh.2013.01.008 [DOI] [PubMed] [Google Scholar]
- 28.Salazar CO, Cardenas VM, Reddy RK, Dominguez DC, Snyder LK, Graham DY. Greater than 95% success with 14‐day bismuth quadruple anti‐helicobacter pylori therapy: a pilot study in US Hispanics. Helicobacter. 2012;17(5):382–90. doi: 10.1111/j.1523-5378.2012.00962.x [DOI] [PubMed] [Google Scholar]
- 29.Roghani HS, Massarrat S, Pahlewanzadeh M, Dashti M. Effect of two different doses of metronidazole and tetracycline in bismuth triple therapy on eradication of Helicobacter pylori and Its resistant strains. European journal of gastroenterology & hepatology. 1999;11(7):709–12. [DOI] [PubMed] [Google Scholar]
- 30.Graham D, Osato M, Hoffman J, Opekun A, Anderson S, Kwon D, et al. Metronidazole containing quadruple therapy for infection with metronidazole resistant Helicobacter pylori: a prospective study. Alimentary Pharmacology and Therapeutics. 2000;14(6):745–50. [DOI] [PubMed] [Google Scholar]
- 31.Dore MP, Lu H, Graham DY. Role of bismuth in improving Helicobacter pylori eradication with triple therapy. Gut. 2016;65(5):870–8. doi: 10.1136/gutjnl-2015-311019 [DOI] [PubMed] [Google Scholar]
- 32.Ford AC, Malfertheiner P, Giguère M, Santana J, Khan M, Moayyedi P. Adverse events with bismuth salts for Helicobacter pylori eradication: systematic review and meta-analysis. World journal of gastr [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang J-C, Lin C-J, Wang H-L, Chen J-D, Kao JY, Shun C-T, et al. High-dose dual therapy is superior to standard first-line or rescue therapy for Helicobacter pylori infection. Clinical Gastroenterology and Hepatology. 2015;13(5):895–905. e5. doi: 10.1016/j.cgh.2014.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zullo A, Ridola L, De Francesco V, Gatta L, Hassan C, Alvaro D, et al. High-dose esomeprazole and amoxicillin dual therapy for first-line Helicobacter pylori eradication: a proof of concept study. Annals of gastroenterology: quarterly publication of the Hellenic Society of Gastroenterology. 2015;28(4):448. [PMC free article] [PubMed] [Google Scholar]
- 35.Sun Q-J, Liang X, Zheng Q, Gu W-Q, Liu W-Z, Xiao S-D, et al. Resistance of Helicobacter pylori to antibiotics from 2000 to 2009 in Shanghai. World journal of gastroenterology: WJG. 2010;16(40):5118 doi: 10.3748/wjg.v16.i40.5118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berry V, Jennings K, Woodnutt G. Bactericidal and morphological effects of amoxicillin on Helicobacter pylori. Antimicrobial agents and chemotherapy. 1995;39(8):1859–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rimbara E, Noguchi N, Kawai T, Sasatsu M. Mutations in penicillin-binding proteins 1, 2 and 3 are responsible for amoxicillin resistance in Helicobacter pylori. Journal of antimicrobial chemotherapy. 2008;61(5):995–8. doi: 10.1093/jac/dkn051 [DOI] [PubMed] [Google Scholar]
- 38.Yang J-C, Lu C-W, Lin C-J. Treatment of Helicobacter pylori infection: current status and future concepts. World Journal of Gastroenterology: WJG. 2014;20(18):5283 doi: 10.3748/wjg.v20.i18.5283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Megraud F. Helicobacter pylori and antibiotic resistance. Gut. 2007;56(11):1502–. doi: 10.1136/gut.2007.132514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shiota S, Reddy R, Alsarraj A, El-Serag HB, Graham DY. Antibiotic resistance of Helicobacter pylori among male United States veterans. Clinical Gastroenterology and Hepatology. 2015;13(9):1616–24. doi: 10.1016/j.cgh.2015.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohammadi M, Doroud D, Mohajerani N, Massarrat S. Helicobacter pylori antibiotic resistance in Iran. World Journal of Gastroenterology: WJG. 2005;11(38):6009 doi: 10.3748/wjg.v11.i38.6009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Massarrat S, Sheykholeslami A. Increase in resistance rates of H. pylori isolates to metronidazole and tetracycline-comparison of three 3-year studies. Archives of Iranian medicine. 2010;13(3):177 [PubMed] [Google Scholar]
- 43.Navidifar T, Eslami, Akhondi M, Baghbanian M, Zadeh HF, Zandi H. Antibacterial resistance patterns of helicobacter pyloriclinical isolates from gastric biopsy of patients in yazd. Int J Enteric Pathog. 2014;2(2):e17791. [Google Scholar]
- 44.Zhang W, Chen Q, Liang X, Liu W, Xiao S, Graham DY, et al. Bismuth, lansoprazole, amoxicillin and metronidazole or clarithromycin as first-line Helicobacter pylori therapy. Gut. 2015:gutjnl-2015-30990 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.