Abstract
Background
There is limited literature about the clinicopathological characteristics and outcomes of rare histologic variants of gallbladder cancer (GBC).
Methods
Using SEER database, surgically managed GBC patients with microscopically confirmed adenocarcinoma, adenosquamous/squamous cell carcinoma and papillary carcinoma were identified from 1988 to 2009. Patients with second primary cancer and distant metastasis at presentation were excluded. The effect of clinicopathological variables on overall survival (OS) and disease specific survival (DSS) were analyzed using univariate and multivariate proportional hazards modeling. All associations were considered statistically significant at an alpha error of 0.01.
Results
Out of 4738 cases, 217 adenosquamous/squamous (4.6%), 367 papillary (7.7%), and 4154 adenocarcinomas (87.7%) were identified. Median age was 72 years. Higher tumor grade (grade 2, 3, 4 versus grade 1), higher T stage (T2, T3, T4 versus T1), lymph node positivity (N1 versus N0) and adenosquamous/squamous histology (versus adenocarcinoma) had worse OS and DSS (p < .001). Papillary GBC had better OS and DSS than adenocarcinoma (HR = 0.7; p < .001). Radical surgery (versus simple cholecystectomy) had better OS (HR = 0.83, p = 0.002) in multivariate analysis. OS rates at 3 and 5 years were 0.56 and 0.44 for papillary, 0.3 and 0.22 for adenocarcinoma, and 0.14 and 0.12 for adenosquamous/squamous histology, while DSS rates at 3 and 5 years were 0.67 and 0.61 for papillary, 0.38 and 0.31 for adenocarcinoma, and 0.17 and 0.16 for adenosquamous/squamous subtypes respectively.
Conclusion
Papillary GBC had better survival outcomes while adenosquamous/squamous GBC had worse survival outcomes compared to gallbladder adenocarcinoma.
Introduction
Gall bladder cancer (GBC) is the most common biliary tract malignancy and the fifth most common gastrointestinal cancer [1, 2]. GBC and nearby large bile duct cancers accounted for an estimated 11,420 new cases and 3710 deaths in the United States in 2016. GBC has a dismal prognosis and majority of the cases are asymptomatic and are incidentally diagnosed during gall stone exploration or after cholecystectomy performed for a non-malignant indication[3]. Therefore, the index surgical procedure is often a simple resection of the gallbladder and a revision surgery is planned based on the staging results [4]. According to the National Comprehensive Cancer Network (NCCN) guidelines [2], a simple cholecystectomy is an adequate treatment for T1a tumors. While there is some controversy over T1b tumors [2], a complete surgical resection consisting of cholecystectomy with a limited hepatic resection and portal lymphadenectomy is the only curative treatment for T2 or greater tumors. It is performed either as an index procedure or as a revised procedure at a later date.
Adenocarcinoma is the most common histologic subtype in GBC, representing approximately 76–90% of cases (5). Among other GBC histologic subtypes, papillary tumors constitute 5–6%, while squamous and adenosquamous constitute 2–10% of cases [5, 6]. Due to the rarity of these histologic subtypes, current literature on the behavior and clinical outcomes of papillary and adenosquamous/squamous gall bladder cancer is limited to case reports or single institution studies [6–10]. The aim of this study was to identify the effects of tumor characteristics, clinicopathological variables and surgery on survival outcomes for these rare histologic variants in comparison to adenocarcinoma.
Materials and methods
Data source
Surveillance, Epidemiology, and End Results (SEER) Program database was used to identify the cohort of patients for this retrospective analysis. SEER database contains cancer specific data of approximately 26% of the Unites States population from 18 cancer registries and 14 geographically distinct regions. Specific de-identified data pertaining to demographics, tumor stage (TNM) and histologic grade, cancer directed surgery and radiation treatment is captured in the SEER database.
Study population
The study cohort included patients diagnosed with gall bladder cancer from 1988 to 2009. Cases diagnosed prior to 1988 were not included as the type of cancer directed surgery is not specified in SEER database. The site specific ICD code of C.23.9 was used to identify gallbladder cancer patients. The histologic subtype of the tumor was identified using specific coding data inside the SEER database i.e. code 8140 represents adenocarcinoma, codes 8070, 8071, 8075, and 8560 represent all or predominant squamous histology (squamous and adenosquamous), and codes 8050, and 8260 represent all or predominant papillary histology (papillary carcinoma and papillary adenocarcinoma). All tumor stages except premalignant lesions (e.g. carcinoma in situ) and distant metastases were included in the study. Patients with a second primary cancer diagnosis and patients with cancer diagnosed at autopsy were excluded from the study. Patients who underwent a cancer directed surgery (index procedure) were identified in the study sample by comparing the diagnosis codes with surgery/procedure codes. The cancer directed surgery is either cholecystectomy (simple removal of gall bladder with or without regional lymph node dissection) or a radical surgical resection (removal of the gallbladder with partial or complete removal of surrounding structures, i.e. partial or total hepatic lobectomy with or without bile duct resection).
Statistical analysis
Univariate associations between covariates and histology (adenocarcinoma, papillary carcinoma and adenosquamous/squamous carcinoma) were examined with the Kruskal-Wallis test for ordinal variables and the Pearson Chi-square test for categorical variables. Covariates included age, sex, race, tumor characteristics (T, N status, histologic grade, and tumor size,), type of surgery and radiation treatment. Univariate and multivariate Cox proportional hazards modeling results were used to assess the effect of histology and covariates on survival. Relative prognosis was summarized using estimates and 95% confidence limits for the hazard ratio (HR). Overall survival (OS), defined as the time (in months) from diagnosis to death from any cause, was the primary endpoint. Disease specific survival (DSS), defined as the time (in months) from diagnosis to death specifically from cancer, was the secondary endpoint. Patients dying from other causes were censored at date of death, and those alive were censored at the date of last follow up. Kaplan-Meier method was used to derive the OS and DSS. All associations were considered statistically significant at an alpha error of 0.01 (P value 0.01). All statistical analyses were performed using the SAS software version 9.2.
Results
14,349 patients with GBC were identified between 1988-and 2009. Of these, 6004 patients belonged to the three histological subtypes of interest; adenocarcinoma (n = 5321; 88.6%), adenosquamous/squamous (n = 284; 4.7%) and papillary (n = 399; 6.6%). Among the adenosquamous/squamous GBC patients, 157 (55%) were adenosquamous, while most of the papillary GBC group (n = 382; 96%) included papillary adenocarcinoma.
Of the total 6004 cases of interest, 1266 (21.1%) patients had distant metastatic disease who were excluded from further analysis. Out of 4738 cases, there were 217 adenosquamous/squamous (4.6%), 367 papillary (7.7%), and 4154 adenocarcinoma (87.7%). Overall, 80% of the GBC population was white and 9% was black. Male to female ratio was 1:3. The mean age at diagnosis was 72 years. Table 1 shows the baseline characteristics of the three histologic subtypes. T3 and T4 status were more prevalent in adenosquamous/squamous GBC (52.5% and 11.5% respectively) compared to papillary (15.8% and 1.6%) and adenocarcinoma (39.5% and 5%) patients (p< 0.001). Also, adenosquamous/squamous GBC patients had worse histologic grade of tumor at presentation; grade 3 and grade 4 cancers in adenosquamous/squamous group constituted 38.7% and 1.8%, papillary group were 9.5% and 0% and adenocarcinoma group were 34.2% and 1.3% respectively (p<0.001). Regional and metastatic spread of disease was statistically more prevalent in adenosquamous/squamous GBC patients at the time of diagnosis. Lymph node involvement was lower in the papillary group (9.5% N1) compared to the adenosquamous/squamous (9.8% N1) and adenocarcinoma (21% N1) group of patients (p<0.001).
Table 1. Descriptive statistics by histology.
| Characteristics | Papillary N = 367 (7.7%) | Adenosquamous/Squamous N = 217(4.6%) | Adenocarcinoma N = 4154 (87.7%) | Total N = 4738 (100%) | p value | |
|---|---|---|---|---|---|---|
| Sex | Female | 271 (73.8%) | 154 (71.0%) | 3,053 (73.5%) | 3,478 (73.4%) | 0.7 |
| Male | 96 (26.2%) | 63 (29%) | 1,101 (26.5%) | 1,260 (26.6%) | ||
| Age, median | 70 years | 70 years | 73 years | 72 years | 0.001 | |
| Race | White | 265 (72.2%) | 176 (81.1%) | 3,325 (80.0%) | 3,766 (79.5%) | 0.007 |
| Black | 39 (10.6%) | 17 (7.8%) | 354 (8.5%) | 410 (8.7%) | ||
| Others | 63 (17.2%) | 24 (11.1%) | 475 (11.4%) | 562 (11.9%) | ||
| T status | T1 | 200 (54.5%) | 39 (18.0%) | 1,052 (25.3%) | 1,291 (27.2%) | <0.001 |
| T2 | 103 (28.1%) | 39 (18.0%) | 1,254 (30.2%) | 1,396 (29.5%) | ||
| T3 | 58 (15.8%) | 114 (52.5%) | 1,640 (39.5%) | 1,812 (38.2%) | ||
| T4 | 6 (1.6%) | 25 (11.5%) | 208 (5.0%) | 239 (5.0%) | ||
| Node status | N0 | 275 (74.9%) | 123 (56.7%) | 2,443 (58.8%) | 2,841 (60.0%) | <0.001 |
| N1 | 35 (9.5%) | 43 (19.8%) | 874 (21.0%) | 952 (20.1%) | ||
| NX | 57 (15.5%) | 51 (23.5%) | 837 (20.1%) | 945 (19.9%) | ||
| SEER historic staging (excluding metstatic) | Localized | 301 (82%) | 91 (41.9%) | 2,407 (57.9%) | 2,799 (59.1%) | <0.001 |
| Regional | 66 (18%) | 126 (58.1%) | 1,747 (42.1%) | 1,939 (40.9%) | ||
| Histologic grade | 1 | 119 (32.4%) | 8 (3.7%) | 647 (15.6%) | 774 (16.3%) | <0.001 |
| 2 | 148 (40.3%) | 83 (38.32%) | 1,688 (40.6%) | 1,919 (40.5%) | ||
| 3 | 35 (9.5%) | 84 (38.7%) | 1,420 (34.62%) | 1,539 (32.5%) | ||
| 4 | 0 (0%) | 4 (1.8%) | 53 (1.3%) | 57 (1.2%) | ||
| Surgery | Simple resection | 332 (90.5%) | 178 (82.0%) | 3,743 (90.1%) | 4,253 (89.8%) | <0.001 |
| Radical surgical resection | 35 (9.5%) | 39 (18.0%) | 411 (9.9%) | 485 (10.2%) | ||
| Radiation treatment | Yes | 55 (15.0%) | 51 (23.5%) | 795 (19.1%) | 901 (19.1%) | 0.021 |
| No | 307 (83.7%) | 158 (72.8%) | 3,280 (79.0%) | 3,745 (79.0%) | ||
| Unknown | 5 (1.4%) | 8 (3.7%) | 79 (1.9%) | 92 (1.9%) |
Univariate and multivariate modeling for OS and DSS were performed to determine predictors of outcome with regards to various clinicopathological variables. The variables included in the model were T-status (T3, T2, T4 versus T1), N status (N0 versus N1), histologic grade of tumor (2, 3, 4 versus 1), size of tumor (>5cm versus <5cm), histology of the tumor (papillary, adenosquamous/squamous versus adenocarcinoma), index surgery (radical surgical resection versus simple resection) and radiation treatment (treatment versus no radiation treatment). Advanced T and N status, higher histologic grade, adenosquamous/squamous histology, and size >5cm were predictors of poor survival (see Tables 2 and 3) in both univariate and multivariate analysis. Papillary histology had better survival outcomes compared to adenosquamous/squamous and adenocarcinoma in both univariate and multivariate analysis for OS and DSS.
Table 2. Univariate and multivariate analysis model for overall survival.
| Independent variables | Reference | Univariate model | Multivariate model | ||||
|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | p-value | Hazard ratio | 95% CI | p-value | ||
| T status | |||||||
| T2 | T1 | 1.19 | (1.08, 1.31) | < .001 | 1.16 | (1.05, 1.27) | 0.004 |
| T3 | 2.47 | (2.26, 2.69) | < .001 | 2.2 | (2.01, 2.41) | < .001 | |
| T4 | 4.04 | (3.48, 4.69) | < .001 | 3.53 | (3.01, 4.14) | < .001 | |
| Histologic grade | |||||||
| 2 | Grade 1 | 1.39 | (1.25, 1.54) | < .001 | 1.26 | (1.14, 1.40) | < .001 |
| 3 | 2.29 | (2.06, 2.54) | < .001 | 1.81 | (1.62, 2.02) | < .001 | |
| 4 | 2.19 | (1.64, 2.92) | < .001 | 1.86 | (1.39, 2.49) | < .001 | |
| Size | |||||||
| > = 5cm | Size < 5cm | 1.4 | (1.26, 1.56) | < .001 | 1.18 | (1.06, 1.32) | 0.003 |
| Unknown | 1.57 | (1.42, 1.74) | < .001 | 1.37 | (1.24, 1.52) | < .001 | |
| Node status | |||||||
| N1 | N0 | 1.53 | (1.40, 1.66) | < .001 | 1.28 | (1.17, 1.40) | < .001 |
| Nx | 1.48 | (1.36, 1.61) | < .001 | 1.26 | (1.16, 1.37) | < .001 | |
| Histology | |||||||
| Papillary | Adenocarcinoma | 0.52 | (0.45, 0.60) | < .001 | 0.74 | (0.64, 0.86) | < .001 |
| Adenosquamous/Squamous | 1.58 | (1.36, 1.84) | < .001 | 1.29 | (1.11, 1.50) | 0.001 | |
| Surgery | |||||||
| Radical surgical resection | |||||||
| Simple resection | 1.07 | (0.96, 1.19) | 0.246 | 0.83 | (0.74, 0.93) | 0.002 | |
| Radiation | |||||||
| No radiation | Yes | 1.12 | (1.03, 1.22) | 0.006 | 1.49 | (1.36, 1.62) | < .001 |
| Unknown | 1.61 | (1.26, 2.04) | < .001 | 1.93 | (1.52, 2.46) | < .001 | |
Table 3. Univariate and multivariate analysis model for disease specific survival.
| Independent variables | Reference | Univariate model | Multivariate model | ||||
|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | p-value | Hazard ratio | 95% CI | p-value | ||
| T status | |||||||
| T2 | T1 | 1.37 | (1.22, 1.54) | < .001 | 1.29 | (1.14, 1.46) | < .001 |
| T3 | 3.25 | (2.93, 3.61) | < .001 | 2.72 | (2.43, 3.04) | < .001 | |
| T4 | 5.62 | (4.76, 6.64) | < .001 | 4.45 | (3.73, 5.31) | < .001 | |
| Histologic grade | |||||||
| 2 | Grade 1 | 1.61 | (1.42, 1.83) | < .001 | 1.38 | (1.17, 1.66) | < .001 |
| 3 | 2.84 | (2.50, 3.23) | < .001 | 2.04 | (1.79, 2.33) | < .001 | |
| 4 | 2.89 | (2.11, 3.95) | < .001 | 2.22 | (1.62, 3.05) | < .001 | |
| Size | |||||||
| > = 5cm | Size < 5cm | 1.53 | (1.35, 1.73) | < .001 | 1.23 | (1.08, 1.39) | 0.002 |
| Unknown | 1.7 | (1.51, 1.91) | < .001 | 1.45 | (1.28, 1.63) | < .001 | |
| Node status | |||||||
| N1 | N0 | 1.78 | (1.62, 1.95) | < .001 | 1.36 | (1.23, 1.50) | < .001 |
| Nx | 1.63 | (1.48, 1.79) | < .001 | 1.34 | (1.22 1.48) | < .001 | |
| Histology | |||||||
| Papillary | Adenocarcinoma | 0.4 | (0.33, 0.48) | < .001 | 0.63 | (0.52, 0.76) | < .001 |
| Adenosquamous/Squamous | 1.83 | (1.56, 2.15) | < .001 | 1.43 | (1.21, 1.68) | < .001 | |
| Surgery | |||||||
| Radical surgical resection | |||||||
| Simple resection | 1.19 | (1.06, 1.34) | 0.005 | 0.87 | (0.76, 0.98) | 0.025 | |
| Radiation | |||||||
| No radiation | Yes | 1.16 | (1.07, 1.26) | < .001 | 1.47 | (1.35, 1.61) | < .001 |
| Unknown | 1.5 | (1.19, 1.90) | < .001 | 1.76 | (1.39, 2.23) | < .001 | |
Radical surgical resection as index surgery predicted poor outcome compared to simple resection in univariate analysis for DSS (HR 1.19; 95% CI 1.06, 1.34, p = 0.005) but not OS (HR 1.07; 95% CI 0.96, 1.19, p = 0.25). However, after adjustment for tumor variables in the multivariate model, radical surgical resection predicted better survival compared to simple resection among the three histologies; HR for OS of 0.83, 95% CI 0.74–0.93, p = 0.002) and HR for DSS of 0.87, 95% CI 0.76–0.98, p = 0.025). Patients who did not receive radiation had poor survival outcomes compared to those who received radiation, with a HR for OS of 1.49 (95% CI 1.36, 1.62. p <0.001) and HR for DSS of 1.47 (95% CI 1.35, 1.61, p<0.001). The interaction between histologic type and radiation was not a significant predictor of survival.
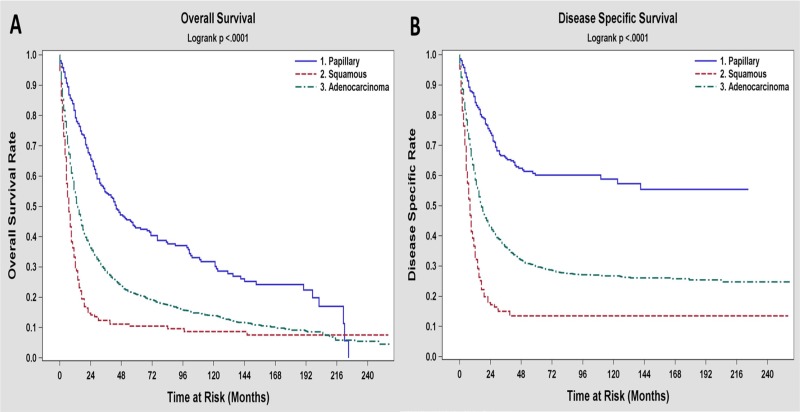
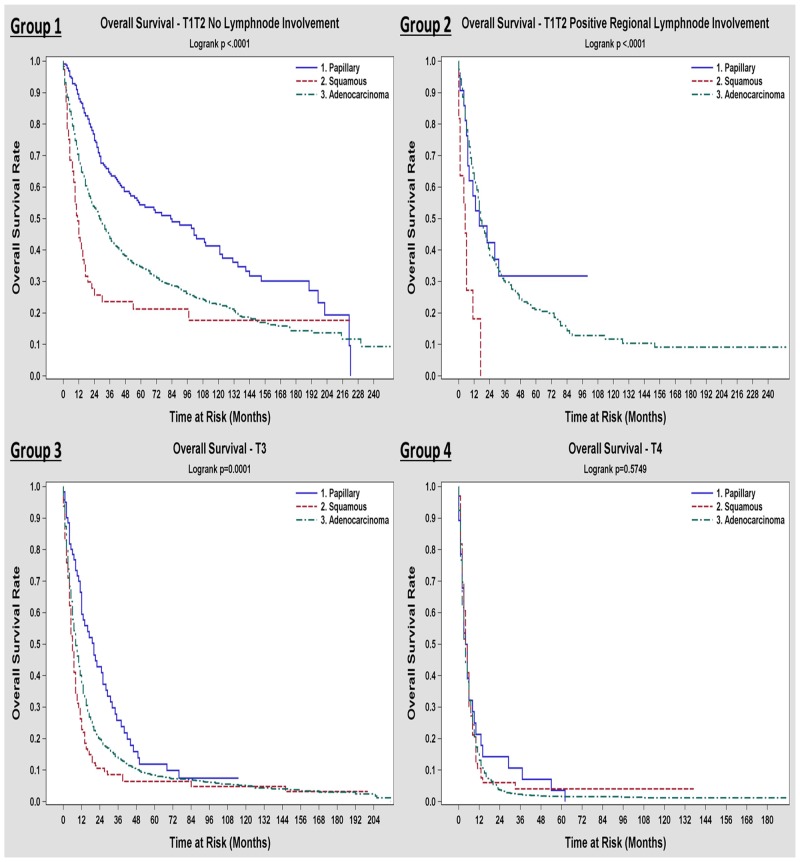
The 3 and 5-year survival rates for the three different histologic types depicted in Table 4, suggesting better outcomes for papillary and worse outcomes for adenosquamous/squamous subtypes compared to adenocarcinoma. At the time of the analysis, 25.9% of the patients were alive at a median follow up time of 81 months. Unadjusted median overall survival was 15 months. Papillary GBC patients had better median OS (44 months), while the adenosquamous/squamous GBC patients had the worst outcome, with a median survival of 7 months and 5-year survival rate of 12%. Figs 1 and 2 show the Kaplan-Meier plots of OS and DSS for all the patients based on the three histologic types respectively. In our study, we did not see different outcomes across histology for T4 tumors. However when we looked at patients with earlier T-status with or without LN involvement, papillary histology had a better survival compared to adenocarcinoma and adenosquamous/squamous histology had the worst outcome (Fig 2). 3-year and 5-year survival as well as median survival data for different T and N-status across histology, is presented in S1 Table.
Table 4. Overall survival and disease-specific survival rates.
| Survival | Papillary | Adenosqouamous/Squamous | Adenocarcinoma |
|---|---|---|---|
| Overall Survival | |||
| 3-year survival rate | 0.56 | 0.14 | 0.30 |
| 5-year survival rate | 0.44 | 0.12 | 0.22 |
| Median overall survival (months) | 44 | 7 | 15 |
| Disease specific survival | |||
| 3-year survival rate | 0.67 | 0.17 | 0.38 |
| 5-year survival rate | 0.61 | 0.16 | 0.31 |
Fig 1. Kaplan-Meier curves for overall survival (A) and disease specific survival (B) based on the three histologic types of gallbladder carcinoma.
Fig 2. Kaplan-Meier overall survival curves among the three histologic types of gallbladder carcinoma based on the American Joint Committee on cancer staging groups [31].
Discussion
The rarity of adenosquamous/squamous and papillary histologic types of GBC in the general population precludes the description of their clinicopathological characteristics, survival outcomes and treatment responses [10, 11]. A national cancer registry like SEER database samples tumor data from approximately 26 percent of the US population and is an ideal tool for quality and outcome studies on rare cancer histology. To the best of our knowledge, our study is the largest in showing the clinicopathological characteristics and survival outcomes of the papillary, adenosquamous/squamous variants of GBC and comparing them with pure adenocarcinoma of gallbladder.
Squamous histology in gallbladder cancer could arise from pre-existing squamous metaplasia or squamous differentiation of the adenocarcinoma cells [11, 12]. The clinical outcomes of adenosquamous/squamous GBC were compared with those of gallbladder adenocarcinoma in prior small retrospective studies with discordant results, however, majority of them favored poor prognosis of adenosquamous/squamous GBC. Chan et al from Taiwan compared 14 adenosquamous/squamous GBC cases with adenocarcinoma controls and reported slightly better 3-year and 5-year survival rates for adenosquamous/squamous GBC [7]. Another retrospective study from Korea showed significant poorer 1-year survival in 16 adenosquamous/squamous GBC cases compared to adenocarcinoma controls (18.8% vs. 87.3%, P < 0.001)[13]. Two retrospective studies from Chile and China compared 34 cases of adenosquamous/squamous GBC each with adenocarcinoma [10, 11]; these studies identified a poor median OS for adenosquamous/squamous histology compared to adenocarcinoma that was statistically significant (p<0.05). Our study with a larger cohort of patients (n = 4738) confirmed poor survival outcomes for adenosquamous/squamous GBC compared to adenocarcinoma (median OS of 7 months versus 15 months; p < 0.001). Higher T resulting in poor outcomes for adenosquamous/squamous GBC patients and these features were consistent in prior smaller studies [10, 14]. Thus, adenosquamous/squamous GBC tend to have localized bulky growth with a propensity to infiltrate adjacent organs. In addition, several studies reported higher proliferative rate and lower lymphatic spread with adenosquamous/squamous GBC [14–16]. The risk of N1 disease was lower for adenosquamous/squamous GBC in our study compared to adenocarcinoma (19.8% versus 21%, p < 0.001).
Despite lower lymphatic spread of adenosquamous/squamous GBC, the features of higher T status at presentation and the tendency to infiltrate adjacent organs can pose a substantial challenge for the surgeons in obtaining tumor free margins at resection (R0), attaining which was shown to improve the prognosis and outcomes [13,17].
Pure papillary GBC is extremely rare with only 17 patients identified in our study. Majority of the papillary group of patients had predominant papillary differentiation within adenocarcinoma (papillary adenocarcinoma). The hypothesis that papillary adenocarcinomas originate from papillary adenomas might not be true, as some studies proved that this pathway plays only a minor role in GBC as opposed to the dysplasia-carcinoma pathway [18, 19]. A recent retrospective study by Cariati et al identified 100% association of pancreatobiliary reflux and C-Ki-ras point mutations with papillary adenocarcinoma of gallbladder [20]. In regards to prognosis, earlier studies suggested better survival outcomes with papillary GBC. Albores-Saavedra et al studied survival estimates of invasive papillary GBC retrospectively using SEER database and reported better survival rates (52% versus <10%) for those confined to the gallbladder wall, compared to those spread to the lymph nodes [21]. Patients with papillary histology in our study had significantly better survival outcomes (median OS of 44 months; 5 -year survival rate of 44%) compared to both adenocarcinoma and adenosquamous/squamous GBC. Papillary GBC tend to be of low histologic grade (approximately 73% with grade 1 and 2 disease in our study), but present as bigger 40% were ≥ 5cm) exophytic growths. Their delayed invasion of gallbladder wall results in much earlier stages at presentation (approximately 82% with T1/T2, 8% only with N1 in our study) compared to adenosquamous/squamous and adenocarcinomas. Recently, Wan et al from China reported similar clinicopathological features and improved 1-year, 3-year and 5- year survival rates for papillary adenocarcinomas as opposed to adenocarcinoma [22].
Surgery is considered the primary modality of treatment for patients diagnosed with localized GBC. Apart from tumor stage and biology, the extent of surgical resection has strong correlation with survival, as evident from several studies showing greater survival with radical resection in early stage GBC [23, 24]. The current NCCN guidelines and expert consensus recommend simple cholecystectomy for T1a disease, while extended surgery is associated with improved survival in T2 or greater tumors [2, 25]. In our study, compared to simple cholecystectomy, radical surgical resection as the index procedure showed improved OS and DSS in multivariate analysis, after adjustment for tumor characteristics and histology. While approximately 73% of our study population had ≥ T2 disease, only 10.2% had radical resection as the index surgery. A study by Mayo et al analyzed SEER data of surgically managed gallbladder adenocarcinoma patients over 15 years [26]. They reported a slightly higher rate of radical resection (13%) than our study as they linked the SEER data with Medicare claims data, resulting in more accurate capture of all surgical procedures. These figures suggest dramatic underutilization of radical surgery for early stage GBC. However, it should be acknowledged that SEER data lists only the index surgical procedure and it is very likely that a proportion of patients who underwent simple resection as index procedure could have had a more extensive revision surgery later on.
Certain other limitations of our study include absence of data on chemotherapy and performance status of the patients at diagnosis. Changes in clinical outcomes attributable to these factors are therefore not available. Attaining microscopic negative margins (R0 resection), aside from the extent of surgery, is the key determinant of surgical outcomes in early GBC [17, 27–30]. Information on the surgical margin status was not available from the SEER data in our study. Although radical/extended surgery showed better survival outcomes on multivariate analysis compared to simple cholecystectomy in the whole cohort, the survival analysis comparing these two surgical procedures among the different T and N status of papillary and adenosquamous/squamous GBC was not possible due to the very low number of patients reported to have extended surgery in each of these subgroups.
In summary, GBC is an aggressive malignancy with dismal outcome. Papillary and adenosquamous/squamous histologic variants of GBC are rare and differ from gallbladder adenocarcinoma in their clinicopathological characteristics. Most of the earlier studies in the literature reporting the outcomes of these rare histologies are very small in sample size and represented Asian population. Our study is unique in that it is the largest study to review and compare the clinicopathological features and survival outcomes of these rare histological variants of GBC with gallbladder adenocarcinoma in US population over a period of 21 years. We found that papillary GBC has the best survival outcomes following surgery, while adenosquamous/squamous GBC had the worst outcome in our large population based analysis. Differences in tissue invasion and lymph node spread among these different histologic types of GBC are important to consider while planning curative surgery. Based on our data, one may take a cautious approach to radical surgery in GB cancer with squamous/adenosquamous histology, especially in elderly or frail patients since the outcome is poor. In this era of precision medicine, studies are needed to analyze molecular and genetic mechanisms contributing to the differences in the behavior of these histologic variants, which could identify potential biomarkers predicting response to adjuvant therapies. Finally, clinicians should focus on enrolling GB cancer patients with rare histology in clinical trials that use novel therapies until new data becomes available.
Supporting information
(XLSX)
Acknowledgments
This work was supported by National Cancer Institute (NCI) grant P30CA016056 involving the use of Roswell Park Cancer Institute’s Biostatistics Shared Resource.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by National Cancer Institute (NCI) grant P30CA016056 involving the use of Roswell Park Cancer Institute’s Biostatistics Shared Resource. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians. 2016; 66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Benson AB 3rd, D’Angelica MI, Abbott DE, Abrams TA, Alberts SR, Saenz DA et al. NCCN Guidelines Insights: Hepatobiliary Cancers, Version 1.2017. J Natl Compr Canc Netw. 2017. May; 15(5):563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausania F, Tsirlis T, White SA, French JJ, Jaques BC, Charnley RM et al. Incidental pT2-T3 gallbladder cancer after a cholecystectomy: outcome of staging at 3 months prior to a radical resection. HPB (Oxford). 2013. August;15(8):633–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belli G, Cioffi L, D’Agostino A, Limongelli P, Belli A, Russo G et al. Revision surgery for incidentally detected early gallbladder cancer in laparoscopic era. J Laparoendosc Adv Surg Tech A. 2011. Jul-Aug;21(6):531–4. doi: 10.1089/lap.2011.0078 [DOI] [PubMed] [Google Scholar]
- 5.Henson DE, Albores-Saavedra J, Corle D. Carcinoma of the gallbladder. Histologic types, stage of disease, grade, and survival rates. Cancer. 1992;70(6):1493–1497. [DOI] [PubMed] [Google Scholar]
- 6.Yang Z, Yang Z, Zou Q, Yuan Y, Li J, Li D et al. A comparative study of clinicopathological significance, FGFBP1, and WISP-2 expression between squamous cell/adenosquamous carcinomas and adenocarcinoma of the gallbladder. Int J Clin Oncol. 2014. April;19(2):325–35. doi: 10.1007/s10147-013-0550-9 [DOI] [PubMed] [Google Scholar]
- 7.Chan KM, Yu MC, Lee WC, Jan YY, Chen MF. Adenosquamous/squamous cell carcinoma of the gallbladder. Journal of surgical oncology. 2007;95(2):129–134. doi: 10.1002/jso.20576 [DOI] [PubMed] [Google Scholar]
- 8.Onuma M, Miura M, Fujisaka Y, Zuguchi M, Asonuma S, Umemura T et al. [A case of huge papillary adenocarcinoma of the gallbladder with marked necrosis]. Nihon Shokakibyo Gakkai Zasshi. 2013. January;110(1):95–103. [PubMed] [Google Scholar]
- 9.Adsay V, Jang KT, Roa JC, Dursun N, Ohike N, Bagci P et al. Intracholecystic papillary-tubular neoplasms (ICPN) of the gallbladder (neoplastic polyps, adenomas, and papillary neoplasms that are ≥1.0 cm): clinicopathologic and immunohistochemical analysis of 123 cases. Am J Surg Pathol. 2012. September;36(9):1279–301. doi: 10.1097/PAS.0b013e318262787c [DOI] [PubMed] [Google Scholar]
- 10.Song HW, Chen C, Shen HX, Ma L, Zhao YL, Zhang GJ et al. Squamous/adenosquamous carcinoma of the gallbladder: Analysis of 34 cases and comparison of clinicopathologic features and surgical outcomes with adenocarcinoma. J Surg Oncol. 2015. November;112(6):677–80. doi: 10.1002/jso.24065 [DOI] [PubMed] [Google Scholar]
- 11.Roa JC, Tapia O, Cakir A, Basturk O, Dursun N, Akdemir D et al. Squamous cell and adenosquamous carcinomas of the gallbladder: clinicopathological analysis of 34 cases identified in 606 carcinomas. Mod Pathol. 2011. August;24(8):1069–78. doi: 10.1038/modpathol.2011.68 [DOI] [PubMed] [Google Scholar]
- 12.Roppongi T, Takeyoshi I, Ohwada S, Sato Y, Fujii T, Honma M et al. Minute squamous cell carcinoma of the gallbladder: a case report. Jpn J Clin Oncol. 2000. January;30(1):43–5. [DOI] [PubMed] [Google Scholar]
- 13.Kim WS, Jang KT, Choi DW, Choi SH, Heo JS, You DD et al. Clinicopathologic analysis of adenosquamous/squamous cell carcinoma of the gallbladder. J Surg Oncol. 2011. March 1;103(3):239–42. doi: 10.1002/jso.21813 [DOI] [PubMed] [Google Scholar]
- 14.Kalayarasan R, Javed A, Sakhuja P, Agarwal AK. Squamous variant of gallbladder cancer: is it different from adenocarcinoma? American journal of surgery. 2013;206(3):380–385. doi: 10.1016/j.amjsurg.2012.12.012 [DOI] [PubMed] [Google Scholar]
- 15.Kondo M, Dono K, Sakon M, Shimizu J, Nagano H, Nakamori S et al. Adenosquamous carcinoma of the gallbladder. Hepatogastroenterology. 2002. Sep-Oct;49(47):1230–4. [PubMed] [Google Scholar]
- 16.Emura F, Kamma H, Ghosh M, Koike N, Kawamoto T, Saijo K et al. Establishment and characterization of novel xenograft models of human biliary tract carcinomas. Int J Oncol. 2003. November;23(5):1293–300. [PubMed] [Google Scholar]
- 17.Oohashi Y, Shirai Y, Wakai T, Nagakura S, Watanabe H, Hatakeyama K. Adenosquamous carcinoma of the gallbladder warrants resection only if curative resection is feasible. Cancer. 2002;94(11):3000–3005. doi: 10.1002/cncr.10578 [DOI] [PubMed] [Google Scholar]
- 18.Albores-Saavedra J, Chable-Montero F, Gonzalez-Romo MA, Ramirez Jaramillo M, Henson DE. Adenomas of the gallbladder. Morphologic features, expression of gastric and intestinal mucins, and incidence of high-grade dysplasia/carcinoma in situ and invasive carcinoma. Human pathology. 2012;43(9):1506–1513. doi: 10.1016/j.humpath.2011.11.011 [DOI] [PubMed] [Google Scholar]
- 19.Roa I, de Aretxabala X, Araya JC, Roa J. Preneoplastic lesions in gallbladder cancer. Journal of surgical oncology. 2006;93(8):615–623. doi: 10.1002/jso.20527 [DOI] [PubMed] [Google Scholar]
- 20.Cariati A, Piromalli E, Cetta F. Gallbladder cancers: associated conditions, histological types, prognosis, and prevention. European journal of gastroenterology & hepatology. 2014;26(5):562–569. [DOI] [PubMed] [Google Scholar]
- 21.Albores-Saavedra J, Tuck M, McLaren BK, Carrick KS, Henson DE. Papillary carcinomas of the gallbladder: analysis of noninvasive and invasive types. Archives of pathology & laboratory medicine. 2005;129(7):905–909. [DOI] [PubMed] [Google Scholar]
- 22.Wan X, Zhang H, Chen C, Yang X, Wang A, Zhu C et al. Clinicopathological features of gallbladder papillary adenocarcinoma. Medicine (Baltimore). 2014. December;93(27):e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen EH, Abraham A, Habermann EB, Al-Refaie WB, Vickers SM, Virnig BA et al. A critical analysis of the surgical management of early-stage gallbladder cancer in the United States. J Gastrointest Surg. 2009. April;13(4):722–7. doi: 10.1007/s11605-008-0772-8 [DOI] [PubMed] [Google Scholar]
- 24.Coburn NG, Cleary SP, Tan JC, Law CH. Surgery for gallbladder cancer: a population-based analysis. Journal of the American College of Surgeons. 2008;207(3):371–382. doi: 10.1016/j.jamcollsurg.2008.02.031 [DOI] [PubMed] [Google Scholar]
- 25.Aloia TA, Járufe N, Javle M, Maithel SK, Roa JC, Adsay V et al. Gallbladder cancer: expert consensus statement. HPB (Oxford). 2015. August;17(8):681–90. doi: 10.1111/hpb.12444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayo SC, Shore AD, Nathan H, Edil B, Wolfgang CL, Hirose K et al. National trends in the management and survival of surgically managed gallbladder adenocarcinoma over 15 years: a population-based analysis. J Gastrointest Surg. 2010. October;14(10):1578–91. doi: 10.1007/s11605-010-1335-3 [DOI] [PubMed] [Google Scholar]
- 27.Pawlik TM, Gleisner AL, Vigano L, Kooby DA, Bauer TW, Frilling A et al. Incidence of finding residual disease for incidental gallbladder carcinoma: implications for re-resection. J Gastrointest Surg. 2007. November;11(11):1478–86 doi: 10.1007/s11605-007-0309-6 [DOI] [PubMed] [Google Scholar]
- 28.D’Hondt M, Lapointe R, Benamira Z, Pottel H, Plasse M, Letourneau R et al. Carcinoma of the gallbladder: patterns of presentation, prognostic factors and survival rate. An 11-year single centre experience. Eur J Surg Oncol. 2013. June;39(6):548–53. doi: 10.1016/j.ejso.2013.02.010 [DOI] [PubMed] [Google Scholar]
- 29.Butte JM, Waugh E, Meneses M, Parada H, De La Fuente HA. Incidental gallbladder cancer: analysis of surgical findings and survival. Journal of surgical oncology. 2010;102(6):620–625. doi: 10.1002/jso.21681 [DOI] [PubMed] [Google Scholar]
- 30.Dixon E, Vollmer CM Jr, Sahajpal A, Cattral M, Grant D, Doig C et al. An aggressive surgical approach leads to improved survival in patients with gallbladder cancer: a 12-year study at a North American Center. Ann Surg. 2005. March;241(3):385–94. doi: 10.1097/01.sla.0000154118.07704.ef [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.B Edge S, American Joint Committee on Cancer, American Cancer Society. AJCC cancer staging handbook: from the AJCC cancer staging manual. 7th ed New York: Springer, 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper.