Abstract
Low temperature is one of the important limiting factors for growing season and geographical distribution of plants. Zoysiagrass (Zoysia Willd) is one of the widely used warm-season turfgrass that is distribute in many parts of the world. Zoysaigrass native to high latitude may have evolved higher cold tolerance than the ones native to low latitude. The objective of this study was to investigate the cold stress response in zoysiagrass native to diverse latitude at phenotypic, physiological and metabolic levels. Two zoysiagrass (Z. japonica) genotypes, Latitude-40 (higher latitude) and Latitude-22 (lower latitude) were subjected to four temperature treatments (optimum, 30/25°C, day/night; suboptimum, 18/12°C; chilling, 8/2°C; freezing, 2/-4°C) progressively in growth chambers. Low temperature (chilling and freezing) increased leaf electrolyte leakage (EL) and reduced plant growth, turf quality, chlorophyll (Chl) content, photochemical efficiency (Fv/Fm) and photosynthesis (Pn, net photosynthetic rate; gs, stomatal conductance; intercellular CO2; Tr, transpiration rate) in two genotypes, with more rapid changes in Latitude-22. Leaf carbohydrates content (glucose, fructose, sucrose, trehalose, fructan, starch) increased with the decreasing of temperature, to a great extend in Latitude-40. Leaf abscisic acid (ABA), salicylic acid (SA) and jasmonic acid (JA) content increased, while indole-3-acetic acid (IAA), gibberellic acid (GA3) and trans-zeatin ribside (t-ZR) content decreased with the reduction of temperature, with higher content in Latitude-40 than in Latitude-22. Chilling and freezing induced the up-regulation of C-repeat binding factor (ZjCBF), late embryogenesis abundant (ZjLEA3) and dehydration-responsive element binding (ZjDREB1) transcription factors in two genotypes, whereas those genes exhibited higher expression levels in Latitude-40, particularly under freezing temperature. These results suggested that zoysiagrass native to higher latitude exhibited higher freezing tolerance may attribute to the higher carbohydrates serving as energy reserves and stress protectants that stabilize cellular membranes. The phytohormones may serve signals in regulating plant growth, development and adaptation to low temperature as well as inducing the up-regulated ZjCBF, ZjLEA3 and ZjDREB1 expressions thus result in a higher cold tolerance.
Introduction
Low temperature is the primary determining factor limiting geographical distribution and growing season of plants [1]. The injury symptoms are often exhibited at temperatures below 12°C for plant species native to tropical and subtropical areas [2]. However, the injuries caused by low temperature is generally categorized into chilling stress (temperatures above 0°C) and freezing stress (temperatures below 0°C) [3]. Chilling stress is often leading to growth and photosynthesis reduction, leaf wilting and chlorosis or even necrosis, cellular membrane damages as well as oxidative stress in plants [4]. In addition, temperatures declined below 0°C caused freezing stress, which result in the formation of ice crystals within the cell, mechanical damages as well as metabolic dysfunction in plants [5]. However, plants have evolved complex mechanisms to tolerate chilling and freezing stresses, such as accumulation of carbohydrates and proteins, resulting in large quantities of soluble sugars, amino acids and cold induced stress-related proteins [6], as well as hormone homeostasis inducing the gene expression that function to stabilize membranes against freezing-induced injury [7–8].
The acquisition of freezing tolerance called cold acclimation, which is generally initiated after a period of decreases in above 0°C temperatures and photoperiod [9]. The growth inhibition resulting from low temperature reduced the capacity for energy utilization that lead to feedback inhibition of photosynthesis [3]. Differences in the capacity to minimize photoinhibition and recover photosynthesis during cold acclimation have been shown to contribute to intra- and inter-specific differences in freezing tolerance [10]. Carbohydrates are synthesized from a complex series of reactions involving photosynthesis in plants [3]. Changes in the three major types of water-soluble carbohydrates monosaccharides (glucose and fructose), disaccharides (sucrose, trehalose) and fructans have often been reported in plants subjected to low temperature [11]. Those sugars may served as a typical osmoprotectant, cellular membrane stabilizer, scavengers of reactive oxygen species and signaling molecules in plants to tolerant chilling and freezing stress [11]. Many studies have showed that higher accumulation of sugars, or individual sugar fractions such as starch [12], fructans [13] and sucrose [12] during cold acclimation are associated with great cold tolerance in turfgrass [14–15]. Sucrose consists of the monosaccharides glucose and fructose, and its intimate involvement in growth, development, storage, signaling and stress acclimation [16]. Leaf starch potentially contributed carbon to carbohydrate accumulation in response to low temperature [17]. Fructans often accumulated during cold acclimation in cereals and grasses, which serve to stabilize cell membranes [18]. Furthermore, trehalose, a non-reducing disaccharide of glucose, has been implicated in modulating levels of freezing tolerance and may also be involved in starch accumulation [19].
Plant hormones may serve as signals in regulating plant growth, development and adaptation to environmental stress [20]. For abscisic acid (ABA), they can affect the diverse processes such as leaf senescence, seed dormancy and germination as well as cell division and elongation [21]. In addition, it has been also reported to be involved in the responses to abiotic stresses including chilling and freezing in plants [21]. Cytokinins (such as tZR) are essentially involved in various plant developmental processes including cell division and enlargement, chloroplast biogenesis, nutrient mobilization and leaf senescence [22], as well as facilitate the responses to delay both stomatal closure and leaf senescence under abiotic stresses [22]. Auxin such as IAA can promote root initiation and also delay plant senescence [23]. Bioactive GAs such as GA3 is involved in plant growth and development such as leaf expansion, stem elongation and flowering [22]. Jasmonic acid (JA) is a critical signaling molecule for diverse developmental processes and defense responses in plants [24], and has been shown to induce a response similar to that of ABA for alleviating chilling injury [25]. Salicylic acid (SA) has also been demonstrated to be involved in the responses of plants to a broad range of abiotic stresses, including low temperature [26].
Cold acclimation and the acquisition of freezing tolerance in plants is highly dependent upon the induction of stress-related genes, such as the dehydration-responsive elements or C-repeat binding factor genes (CBF/DREB1) as well as the cold-induced stress proteins such as late-embryogenesis abundant (LEA) proteins [7, 27]. Low temperature up-regulated the expression of CBF/DREB1 which in turn induce the expression of COLD-REGULATED (COR) genes that to enhance freezing tolerance [28]. The accumulation of LEA functions in direct protection from freezing-induced cellular dehydration and mechanical damage [11].
Zoysiagrass (Zoysia spp.) is one of the most widely used warm-season turfgrasses in home lawns, athletic fields and parks because of its good performance to high temperature, water deficit, traffic tolerance and low maintenance [23, 29]. The optimum temperature for the growth and development of zoysiagrass is from 25°C to 30°C. Thus, low temperature stress is one of the essential environmental imiting factors for the geographic distribution and growing season of zoysiagrass in transitional and temperate regions [30]. The native distribution of the recognized species in this genus extends from New Zealand to the island of Hokkaido in Japan, and from French Polynesia through Malaysia west to Mauritius [31]. In eastern China, their natural distribution covers from latitude 19°03′ N to 41°02′ N and from longitude 109°03′ E to124°04′ E [32]. Among all the warm-season turfgrasses, however, zoysiagrass is the most tolerant species in freezing tolerance than other ones, but the cold injuries during the winter season vary widely among zoysiagrass species and genotypes [29]. Our preliminary investigation in a field study showed that zoysiagrass native to high latitude exhibit great freezing tolerance than the ones native to low latitude. The physiological, biochemical and molecular basis for those differences has only partially been explored. The objective of this study was to investigate the cold stress response in zoysiagrass native to diverse latitude at phenotypic, physiological, metabolic and molecular levels under environment-controlled conditions.
Materials and methods
Plant materials and growth conditions
Two zoysiagrass (Zoysia japonica Steud.) genotypes collected from diverse latitudes (Table 1) were propagated vegetatively in the fields at the Turfgrass Research Farm of Hunan Agricultural University, Changsha, China. Mature zoysiagrass sod plugs were taken from the field plots and transplanted to the pots (18 cm diameter, 20 cm deep) filled with a mixture of top soil and sand (4:1, w/w) in a temperature-controlled greenhouse with the temperature of 30/25°C (day/night) and a photosynthetic active radiation (PAR) at 400 μmol.m–2.s–1 with 12-h photoperiod. Plants were watered 3 times per week to keep the growth medium at field capacity, and fertilized biweekly with compound fertilizers with the N:P:K = 15:15:10 at a total amount of 57 kg N ha-1. Turf was cut 3 times per week at about 4 cm height. Plants were kept in the above mentioned conditions for about two month to allow the full establishment of turf canopies and root systems.
Table 1. Origin of the collected zoysiagrass genotypes.
| Genotype | Latitude | Longitude | Altitude | Location |
|---|---|---|---|---|
| Latitude-40 | N 40°18.457 | E 123°34.602 | 80 m | Dayingzhi Town, Xiuyan County, Liaoning Province |
| Latitude-22 | N 22°53.435 | E 112°16.945 | 18 m | Yaogu Town, Yunfu City, Guandong Province |
Treatments and experimental design
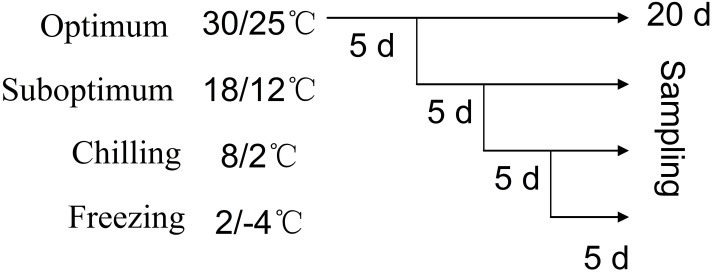
The experiment consisted of four treatments with four replicates in each treatment and a schematic diagram of the low temperature treatment as show in Fig 1: optimum (30/25°C, day/night), suboptimum (18/12°C), chilling (8/2°C) and freezing (2/-4°C). Two months after transplanting, pots were placed in four growth chambers and subjected to temperature treatment with a PAR of 400 μmol·m–2·s–1 with 12-h photoperiod for a period of 20 d. The low temperature treatment were progressive decreased from 30/25°C, 18/12°C, 8/2°C and 2/-4°C. The irrigation was provided only in need to make sure no moisture stress occurred. No fertilization was provided during 20 d of cold treatment.
Fig 1. A schematic diagram of the low temperature treatment.
Measurements
Turf quality and canopy height
Turf quality was evaluated visually based on the turfgrass color, plant density and uniformity on a 1–9 scale according to Hu et al. (2016) [33]described. Turf canopy height were determined before and after treatment using a ruler according to the method described by Hu et al. (2016) [33].
Leaf electrolyte leakage (EL) and LT50
Leaf electrolyte leakage (EL) was determined with about 0.1 g fresh leaf segments collected from each pot and incubated in 15 mL of distilled deionized water on a shaker for 24 h. The initial conductance (Ci) was determined using a conductance meter (YSI-3100; Guangzhou, China). Leaf tissues in the incubation solution were then autoclaved at 120°C for 30 min. The maximum conductance (Cmax) of the incubation solution with killed tissues was determined after the solution cooled to room temperature. Relative EL was calculated as (Ci/Cmax)×100.
The lethal temperature for 50% loss of electrolytes (LT50) was performed on the two genotypes after cold acclimation (before starting freezing treatment) to determine their level of freeze tolerance according to Xuan et al. (2013) [34]. Leaves were cut from the plants (10 leaves for each freezing temperature of 0, -2, -6, -10 and -14°C) and maintained in a programmed freezer (Polyscience 9610, Polysciences, Inc., U.S. Corporate Headquarters, Pennsylvania, USA) with a temperature error of ± 0.1°C. The freezing temperature was decreased at a rate of 2°C h-1 and then held at each freezing temperature for 1.5 h. The samples were then removed from the freezer and thawed at 2°C overnight. The EL of the leaves collected after freezing was determined by measuring the electrical conductivity of tissues as described above. The LT50 values were estimated from a linear regression fitted to the central (linear) part of the sigmoid relationship between the freezing temperature and electrolyte leakage at the five temperatures [34].
Leaf chlorophyll content
Leaf chlorophyll content was determined by immersing the fresh leaves (about 0.15 g) in 15 mL of dimethyl sulfoxid and kept in the dark for about 72 h according to Hiscox and Israelstam (1979) [35]. The OD663 and OD645 of supernatants were determined using a spectrophotometer (UV-2600, UNICO (Shanghai) Instruments Co., Ltd., Shanghai, China) after centrifuged at 5000 g for 10 min at room temperature. The concentration of chlorophyll (mg·g–1 fresh weight) was calculated using Arnon (1949) [36] equations.
Leaf photochemical efficiency and photosynthetic gas exchange
Leaf photochemical efficiency (Fv/Fm) was determined with a chlorophyll fluorometer (OS1-FL, Opti-Sciences, Hudson, NH) on the intact leaves after plants adapted in darkness for 30 min.
Photosynthetic gas exchange measurements (net photosynthetic rate, Pn; stomatal conductance, gs; intercellular CO2 concentration, Ci; transpiration rate, Tr) were measured with the infrared gas analyzer (Li-6400XT, LICOR, Inc., Lincoln, NE). The measurements environment were set at 400 μmol mol–1 CO2, 500 μmol s–1 flow rate and 500 μmol m–2s–1 light intensity at 25°C with three subsamples in each pot and totally twelve records in each treatment.
Leaf carbohydrates determination
Leaf carbohydrates (glucose, fructose, sucrose, fructan, trehalose, starch) were extracted according to Zhang et al. (2012) [37]. Briefly, 0.1 g of leaf sample was ground in liquid nitrogen and then the tubes were shaken vigorously for 10 min after added 1 mL ethanol (92%, v/v). The extracts were centrifuged at 20,000 × g for 10 min at room temperature. The contents in total soluble carbohydrates were determined using a spectrophotometer (UV-2600, UNICO (Shanghai) Instruments Co., Ltd., Shanghai, China) by mixing 3 mL anthrone reagent (150 mg anthrone and 100 mL of 72% sulfuric acid) with 100 μL supernatant under 625 nm after boiling for 10 min. The remaining supernatant was evaporated to dryness at 40°C in a concentrator, and resolubilized in 300 μL deionized water for extraction of glucose, fructose, sucrose and trehalose. 0.5 mL of deionized water was added and the tubes were heated at 100°C for 10 min. The intermixture was enzymolyzed with 0.1 mL α-amylase (400 U/mL) and 0.1 mL starch transglucosylase (2U/mL) in 0.4 mL acetic buffer (200 mM, pH 5.1) at 55°C for 20 h and centrifuged at 20,000 x g for 10 min. The supernatant was kept boiled in acid environment (0.5 M sulfuric acid) for 15 min. The cooled mixture was neutralized with equal amount of sodium hydroxide (1 M) for the analysis of fructan and starch.
Carbohydrates were analyzed by HPLC (Waters, Massachusetts, USA). Sucrose, fructose, glucose and trehalose were separated on a crest amino column (4.6 × 250 mm, 5 μm) and eluted isocratically at 40°C with buffer [50% (v/v) acetonitrile, 50% (v/v) H2O] at a flow rate of 1.0 mL/min. Online detection was performed using a Waters 410 differential refractometer detector, and the data were analyzed by Empower® software. Sucrose, glucose and fructose were used as the standards.
Leaf hormone extraction and fractionation for HPLC-MS analysis
Abscisic acid (ABA), trans-zeatin riboside (tZR), indole actic acid (IAA), gibbrelic acid (GA3), jasmonic acid (JA) and salicylic acid (SA) were extracted and purified according to the method describe by Dobrev and Kaminek (2002) [38]. The extracted and purified elute was evaporated in a vacuum concentrator at 40°C (MiniVac Beta, LABOGENE, Danmark) and then reconstituted with 2 mL of 1 M formic acid. After washing with 1 M formic acid, ABA and auxins were eluted with 2 mL of methanol, and cytokinins were eluted with 2 mL of 0.35 M ammonia in 60% (v/v) methanol from an Oasis MCX 96-Well Plate as indicated by Dobrev and Kaminek (2002) [38]. Each fraction was evaporated to dryness in a vacuum concentrator and the residues were dissolved in a 500 μl of water/methanol (70/30, v/v) mixture. Prior to injection, dissolved fraction was filtered through 13-mm-diameter Millex filters with a 0.22-μm-pore nylon membrane (Millipore, Bedford, MA, USA) and the samples were transferred to 2-mL HPLC vials.
Hormones analysis were carried out on a high-performance liquid chromatography (HPLC)-mass spectrometry (MS) system (Accela; Thermo Fisher Scientific, San José, CA, USA) according to Ghanem et al. (2008) [39]. For each sample, 10 μL was injected into a Zorbax SB-C18 HPLC column (3.5 μm, 150×2.1 mm, Agilent Technologies) maintained at 35°C and eluted at a flow rate of 200 μL min-1. The level of plant hormones tZR, ABA, IAA, GA3, SA, JA) in the samples were determined based on retention times and ion products and standards of each compound.
Gene expression analysis
Total RNA extraction, cDNA synthesis and qRT-PCRs were performed according to Hu et al. (2016) [33] described. Briefly, total RNA was extracted from fresh tissues using Trizol reagent (Invitrogen, Carlsbad, CA). The first strand cDNA fragments were synthesized from 2 μg of total RNA using oligo(dT)12-18 primer using cDNA synthesis kit (Fermentas, Burlington, Ontario, Canada) after RNA quality and integrity was checked by Nanodrop 2000 and 0.8% agarose gel. Gene-specific primers (Table 2) were designed based on the target gene sequences using Primer 5 software. The qRT-PCRs were performed with ABI7500 in a final volume of 20 μL, with each containing 2 μl of cDNA, 10 μL of 2×SYBR Green qPCR Mix (Takara, Otsu, Shiga, Japan) and 2 μM of the forward and reverse primers. Three independent biological replicates of each sample and two technical replicates of each biological replicate were used for real-time PCR analysis. The thermal cycling conditions were as follows: 40 cycles of 95°C denaturation for 5 s, and 52~55°C annealing and extension for 20 s. To determine relative fold differences for each sample, the CT value for each gene was normalized to the CT value for the reference gene and was calculated relative to a calibrator using the DDCT method as described by Livak and Schmittgen (2001) [40].
Table 2. Genes and primers used in this study.
| Gene | Primer sequence | |
|---|---|---|
| ZjCBF | F | 5'-CGC ATA GCA CTGATC GTC ACC CG-3' |
| R | 5'-CCT CAC CGC CGT CAT CAT CGT C-3' | |
| ZjLEA3 | F | 5'-TGGGTAGTCAGCCTGGTAGACG-3' |
| R | 5'-TGGGTAGTCAGCCTGGTAGACG-3' | |
| ZjDREB1 | F | 5'-TTGGAGGCTGCTCATGCATA-3' |
| R | 5'-TTCAACGCATGCACCTCAGT-3' | |
| Actin | F | 5'-TGT GCT CAG CGG TGG TTC AA-3' |
| R | 5'-TGC TGG GCC AGA CTC GTC AT-3' |
Statistical analysis
All data were subjected to two-way analysis of variance using SAS software (v. 9.3 for Window; SAS Institute, Cary, NC, USA, 2010). The treatment means were separated using Ducan’s multiple range test at the P < 0.05 probability level.
Results
Freezing tolerance (LT50 and EL)
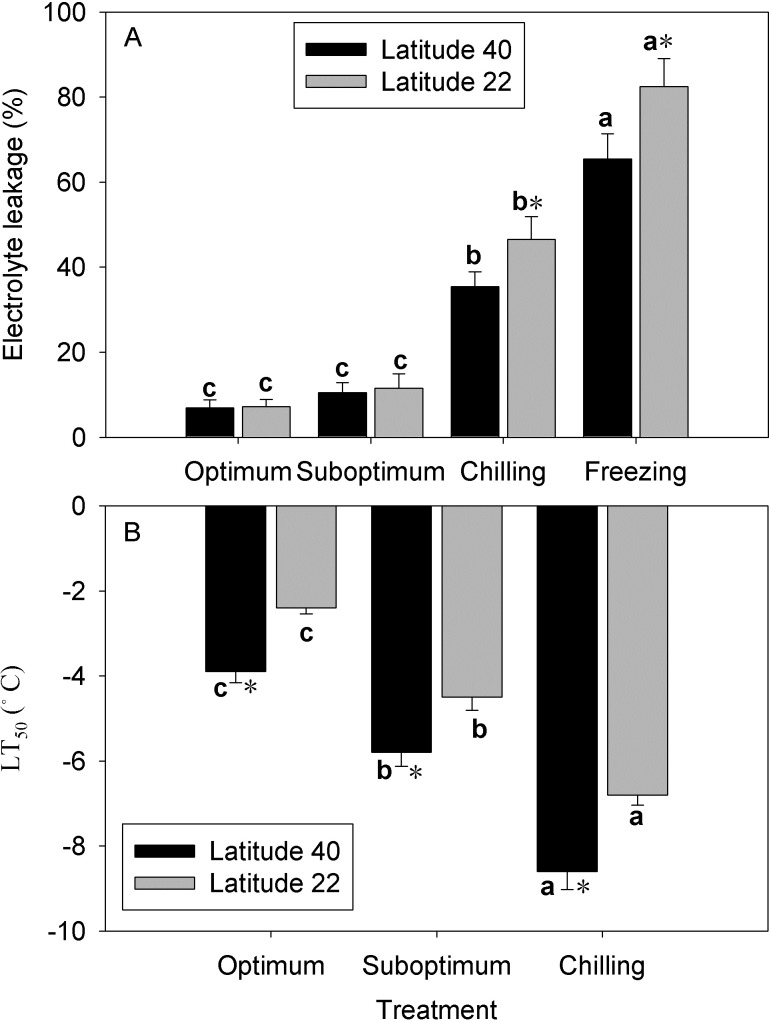
Suboptimum temperature (18/12°C, day/night) has no significant effect on the leaf EL for two zoysiagrass genotypes (Fig 2A). However, chilling and freezing temperature significantly increased leaf EL for both genotypes, with more increasing in Latitude-22 than in Latitude-40, which had 11.1% and 17.1% higher in Latitude-22 under chilling and freezing temperature, respectively.
Fig 2. Effect of low temperature on the leaf electrolyte leakage (EL, A) and lethal temperature for 50% loss of electrolytes (LT50, B) in two zoysiagrass genotypes native to diverse latitude.
Vertical bars on the top indicate standard deviation, and bars with the same letter indicate no significant difference at P < 0.05 for the comparison of differential temperature treatment at a given genotype, and * on the top indicate significant difference at P < 0.05 between two genotypes at a given temperature treatment.
Under optimum temperature conditions, the freezing tolerance (LT50) of Latitude-40 and Latitude-22 were -3.9 and -2.4°C (Fig 2B). After exposure to cold acclimating conditions, the leaves freezing tolerance (LT50) of the two zoysiagrass genotypes decreased from -2.4 to -4.5°C and -6.8°C for Latitude-22, and decreased from -3.9 to 5.8°C and -8.6°C for Latitude-40 under suboptimum and chilling temperature, respectively.
Turf quality and canopy height
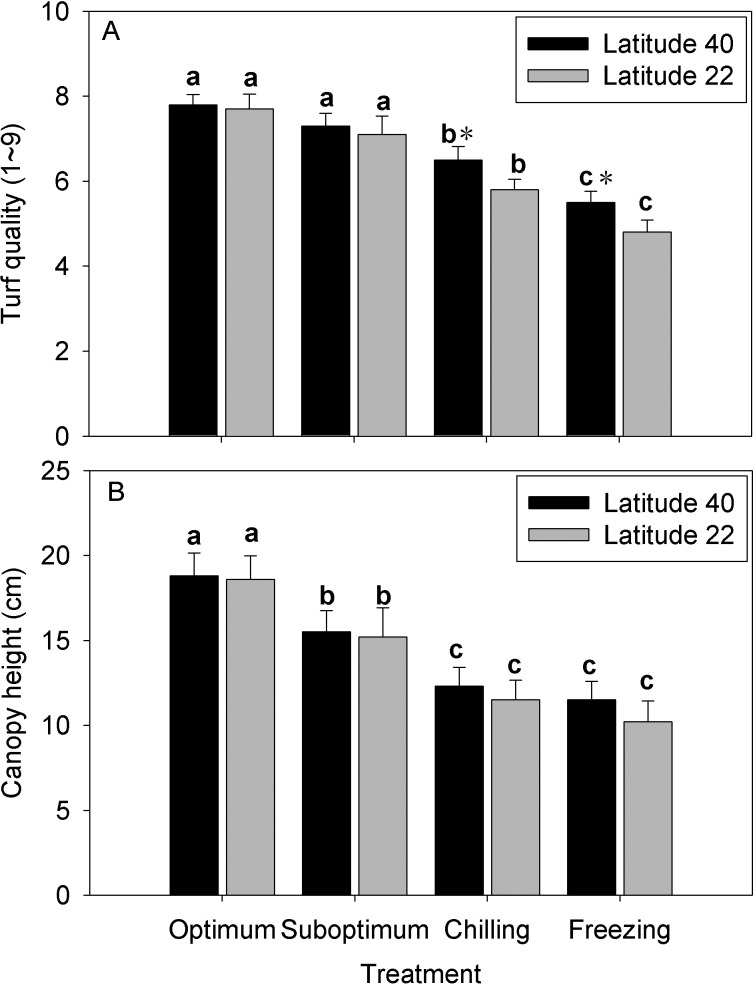
There was no significant reduction in turf quality for both genotypes under suboptimum temperature (Fig 3A). Chilling and freezing temperature caused significantly decrease in turf quality for both genotypes, to a great extent for Latitude-22. Decreasing temperature reduced plant growth as indicated by the canopy height, but no significant difference was observed between the two genotypes at each temperature (Fig 3B).
Fig 3. Effect of low temperature on the turf quality (A) and canopy height (B) in two zoysiagrass genotypes native to diverse latitude.
Vertical bars on the top indicate standard deviation, and bars with the same letter indicate no significant difference at P < 0.05 for the comparison of differential temperature treatment at a given genotype, and * on the top indicate significant difference at P < 0.05 between two genotypes at a given temperature treatment.
Leaf Chl content and photochemical efficiency (Fv/Fm)
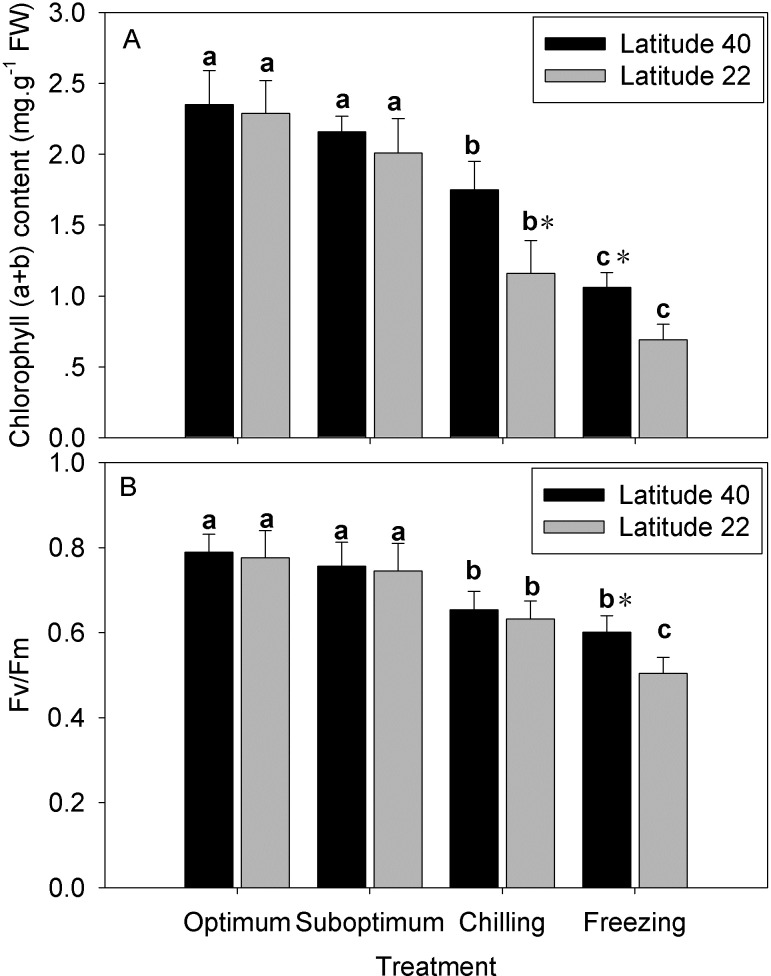
No significant reduction in chlorophyll content (Chl, Fig 4A) and Fv/Fm (Fig 4B) for both genotypes under suboptimum temperature. Chilling and freezing temperature caused significantly decrease in Chl and Fv/Fm for both genotypes, to a great extent for Latitude-22. Chlorophyll content decreased to 92%, 74% and 45% of the optimum temperature levels for Latitude-40, and decreased to 88%, 51% and 30% of the optimum temperature levels for Latitude-22 at suboptimum, chilling and freezing temperature, respectively (Fig 4A). Fv/Fm decreased to 83% and 76% of the optimum temperature levels for Latitude-40, and decreased to 81% and 67% of the optimum temperature levels for Latitude-22 at chilling and freezing temperature, respectively (Fig 4B).
Fig 4. Effect of low temperature on the total chlorophyll content in two zoysiagrass genotypes native to diverse latitude.
Vertical bars on the top indicate standard deviation, and bars with the same letter indicate no significant difference at P < 0.05 for the comparison of differential temperature treatment at a given genotype, and * on the top indicate significant difference at P < 0.05 between two genotypes at a given temperature treatment.
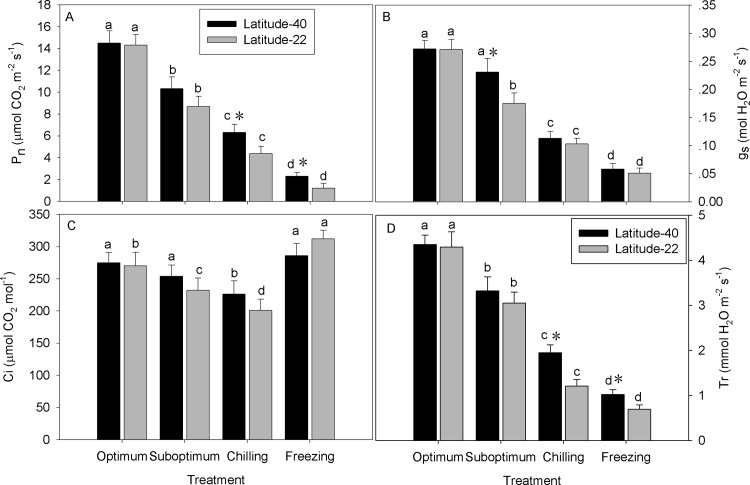
Leaf photosynthetic gas exchange
Leaf Pn declined with the temperature decreasing in both genotypes, to a great extend in Latitude-22 (Fig 5A). Leaf Pn decreased to 71%, 43% and 16% of the optimum temperature levels for Latitide-40, and decreased up to 60%, 30% and 8% of the optimum temperature levels for Latitide-22 at suboptimum, chilling and freezing temperature, respectively (Fig 5A). Suboptimum temperature decreased gs in Latitude-22 but not in Latitude-40, whereas chilling and freezing temperature decreased leaf gs in both genotypes (Fig 5B). Suboptimum temperature decreased leaf Ci in Latitude-22 but not in Latitude-40, whereas chilling decreased leaf gs in both genotypes. However, leaf gs increased to the optimum temperature level in Latitude-40, even higher than the optimum temperature level in Latitude-22, and no significant difference was observed between the two genotypes (Fig 5C). Leaf Tr declined with the reduction of temperature in both genotypes, with more rapid decrease in Latitude-22 than Latitude-40 (Fig 5D). Leaf Tr decreased to 76%, 45% and 23% of the optimum temperature level for Latitude-40, while decreased up to 71, 28 and 16% of optimum temperature level for Latitude-40 under suboptimum, chilling and freezing temperature (Fig 5D).
Fig 5. Effect of low temperature on the total chlorophyll content in two zoysiagrass genotypes native to diverse latitude.
Vertical bars on the top indicate standard deviation, and bars with the same letter indicate no significant difference at P < 0.05 for the comparison of differential temperature treatment at a given genotype, and * on the top indicate significant difference at P < 0.05 between two genotypes at a given temperature treatment.
Leaf carbohydrates content
Leaf carbohydrates content generally increased with the reduction of temperature in two genotypes (Table 3). Sucrose and fructose content remarkably increased in both genotypes under low temperature, with more rapid increase in Latitude-40 than in Latitude-22. Sucrose levels increased by 3.1-, 7.1- and 7.9-fold in Latitude-40, and increased by only 1.8-, 3.8- and 5.3-fold in Latitude-22 at suboptimum, chilling and freezing temperature, respectively. Fructose content increased by to 1.6-, 3.3- and 4.5-fold for Latitude-40, and increased by 1.1-, 1.9- and 2.8-fold for Latitude-22 at suboptimum, chilling and freezing temperature, respectively.
Table 3. Effect of low temperature on carbohydrate content in leaves of two zoysiagrass genotypes.
| Treatments | Genotypes | Carbohydrates content (mg/g DW) | ||||||
|---|---|---|---|---|---|---|---|---|
| Sucrose | Glucose | Fructose | Trehalose | Fructan | Starch | TSS | ||
| Optimum | Latitude 40 | 8.3±0.56 a | 23.5±0.34 a | 7.6±0.86 a | 3.5±0.43 a | 45.3±3.75 a | 72.5±5.64 a | 52.3±4.31 a |
| Latitude 22 | 8.5±0.76 a | 22.4±0.29 a | 7.9±0.49 a | 2.9±0.38 a | 42.3±3.02 a | 73.7±6.35 a | 50.5±3.45 a | |
| Suboptimum | Latitude 40 | 25.6±3.21 a | 28.5±3.21 a | 12.5±1.58 a | 16.5±1.97 a | 68.5±5.34 a | 93.5±6.34 a | 95.1±5.64 a |
| Latitude 22 | 15.6±2.14 b | 25.3±2.12 a | 8.6±1.34 b | 6.5±1.12 b | 47.6±3.78 b | 79.3±5.31 b | 68.3±4.31 b | |
| Chilling | Latitude 40 | 58.6±5.31 a | 38.6±2.98 a | 25.3±1.78 a | 25.7±1.71 a | 94.5±6.78 a | 113.4±8.64 a | 153.1±8.64 a |
| Latitude 22 | 32.5±4.12 b | 28.5±3.45 b | 15.4±1.13 b | 12.5±1.35 b | 68.3±4.58 b | 92.1±9.31 b | 98.3±7.61 b | |
| Freezing | Latitude 40 | 65.4±5.34 a | 42.3±3.42 a | 34.1±2.12 a | 52.1±3.97 a | 110.5±7.34 a | 62.5±4.36 b | 210.2±9.31 a |
| Latitude 22 | 45.3±3.98 b | 32.1±2.97 b | 22.1±1.97 b | 21.2±4.31 b | 88.4±4.31 b | 93.5±6.84 a | 143.5±9.84 b | |
Note: Means followed by the same lower-case letters in a column within a temperature treatment indicated no significant difference between two genotypes at P<0.05.
There were no significant difference in glucose content between two genotypes under optimum and suboptimum temperature, and the glucose levels were 26 and 24% higher in Latitude-40 than in Latitude-22 under chilling and freezing temperature respectively (Table 3).
Trehalose, fructan and total soluble sugar (TSS) levels considerably enhanced as the temperature decline, to a great extend for Latitude-40 (Table 3). Trehalose content increased by 2.2-, 4.3- and 7.3-fold in Latitude-22, whereas increased by up to 4.7-, 7.3- and 14.9-fold in Latitude-40 at suboptimum, chilling and freezing temperature, respectively. Starch content significantly increased at suboptimum and chilling temperature and then decline at freezing temperature for Latitude-40. For Latitude-22, however, starch levels increased at chilling and freezing temperature, and no significant change at suboptimum temperature.
Leaf hormone content
Leaf ABA, SA and JA levels generally increased with the decrease of temperature in both genotypes, with more rapid increase in Latitude-40, whereas IAA, tZR and GA3 levels remarkably declined with the reduction of temperature in two genotypes, to a great extend in Latitude-22 (Table 4). Leaf ABA content increased by 1.5-, 2.9- and 3.1-fold for Latitude-40, and increased by 1.2-, 2.3- and 2.4-fold for Latitude-22 at suboptimum, chilling and freezing temperature. There were no significant difference in leaf IAA and tZR levels between two genotypes under suboptimum temperature, but maintained a relatively higher levels in Latitude-40 than in Latitude-22 under chilling and freezing temperature (Table 4). Leaf GA3 content significantly declined under suboptimum, chilling and freezing temperature for both genotypes, and no significant difference between the two genotypes.
Table 4. Effect of low temperature on hormones content in leaves of two zoysiagrass genotypes.
| Treatments | Genotypes | Hormones content (ng/g FW) | |||||
|---|---|---|---|---|---|---|---|
| ABA | IAA | tZR | GA3 | SA | JA | ||
| Optimum | Latitude 40 | 23.7±1.94 a | 76.5±5.34 a | 45.6±3.45 a | 8.3±0.65 a | 10.3±1.24 a | 9.3±1.10 a |
| Latitude 22 | 24.7±2.02 a | 72.3±4.34 a | 47.3±2.98 b | 7.9±0.59 a | 9.8±1.17 a | 8.8±0.97 a | |
| Suboptimum | Latitude 40 | 35.5±2.97 a | 62.3±4.34 a | 39.3±2.64 a | 6.5±0.49 a | 11.5±1.09 a | 14.3±1.54 a |
| Latitude 22 | 30.5±2.01 b | 58.5±3.97 a | 36.7±3.01 a | 6.3±0.67 a | 12.5±1.35 a | 8.9±0.94 b | |
| Chilling | Latitude 40 | 68.6±4.35 a | 50.4±3.94 a | 30.4±2.81 a | 4.3±0.52 a | 18.4±1.12 a | 19.3±2.14 a |
| Latitude 22 | 56.4±3.97 b | 35.9±2.78 b | 21.5±1.97 b | 5.0±0.39 a | 14.5±1.71 b | 12.3±1.57 b | |
| Freezing | Latitude 40 | 72.3±5.34 a | 34.2±2.04 a | 19.4±1.12 b | 1.9±0.15 a | 23.8±1.47 a | 21.3±2.23 a |
| Latitude 22 | 58.3±4.64 b | 21.4±3.12 b | 10.5±1.08 b | 1.6±0.25 a | 18.6±1.68 b | 15.8±1.84 b | |
Note: Means followed by the same lower-case letters in a column within a temperature treatment indicated no significant difference between two genotypes at P<0.05.
Leaf SA levels remarkably increased with the temperature decline, with more rapid increase in Latitude-40 than in Latitude-22 (Table 4). Leaf SA levels increased by 1.3-, 1.5- and 1.9- fold for Latitude-22, whereas increased by up to 1.1-, 1.8- and 2.3-fold for Latitude-40 at suboptimum, chilling and freezing temperature, respectively. Leaf JA content increased by 1.5-, 2.1- and 2.3-fold in Latitude-40, and increased by only 1.0-, 1.4- and 1.8-fold in Latitude-22 at suboptimum, chilling and freezing temperature (Table 4).
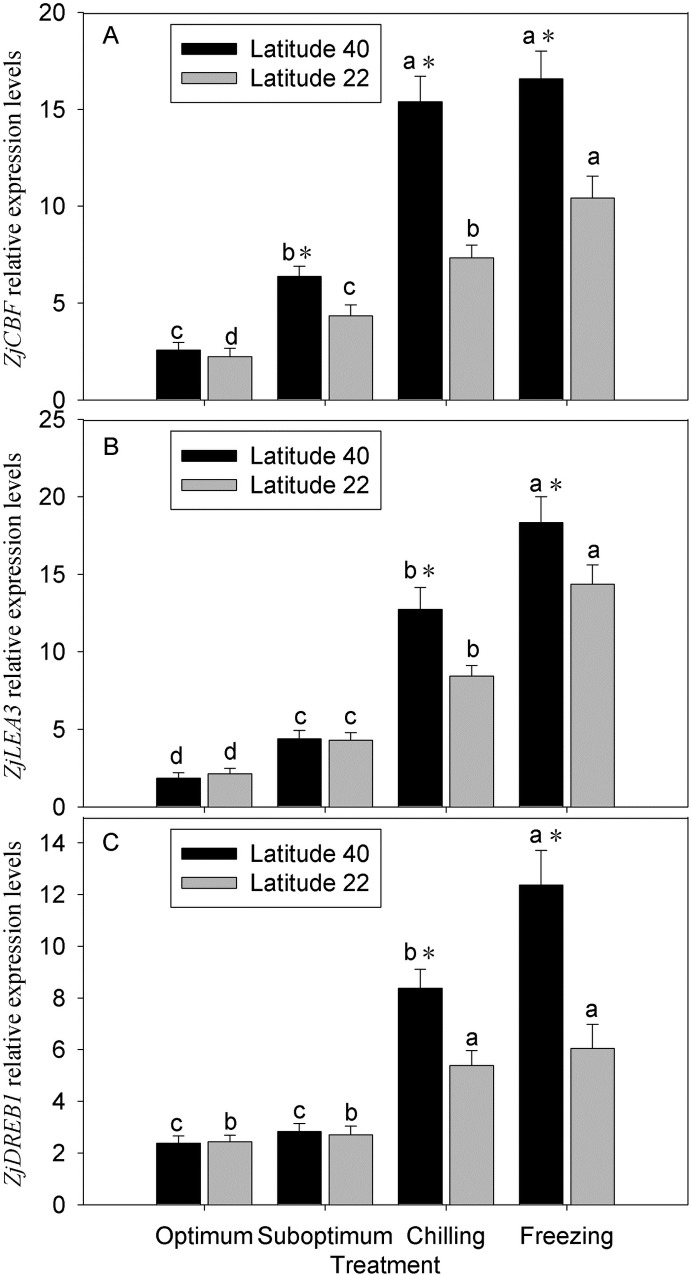
Cold stress related gene expression
Temperature reduction induced up-regulation of ZjCBF and ZjLEA3 expressions in leaves of two zoysiagrass genotypes, with more increasing in Latitude-40 than in Latitude-22 (Fig 6A and 6B). The expression levels of ZjCBF gene increased by 2.8-, 5.9- and 6.4-fold for Latitude-40, whereas increased by only 1.9-, 3.3- and 4.7-fold for Latitude-22 at suboptimum, chilling and freezing temperature, respectively (Fig 6A). The ZjLEA3 gene expression levels increased by 2.0-, 3.9- and 6.7-fold in Latitude-22, while increased by up to 2.4-, 6.9- and 9.9-fold in Latitude-40 (Fig 6B). The ZjDREB1 gene expression level was not induced by suboptimum temperature, and considerably induced by chilling and freezing temperature in leaves of two zoysiagrass genotypes (Fig 6C). The ZjDREB1 gene expression levels increased by 2.2- and 2.5-fold in Latitude-22, whereas increased by up to 3.5- and 5.2-fold in Latitude-40 (Fig 6C).
Fig 6. Effects of low temperature on the cold induced gene expressions in leaves of two zoysiagrass genotypes native to diverse latitude.
Vertical bars on the top indicate standard deviation, and bars with the same letter indicate no significant difference at P < 0.05 for the comparison of differential temperature treatment at a given genotype, and * on the top indicate significant difference at P < 0.05 between two genotypes at a given temperature treatment.
Discussion
Temperature may be the main environmental factor determining the natural latitudinal and altitudinal distribution of plants. The native distribution of zoysiagrass in China extends diverse latitude covers from 19°03′ N to 41°02′ N [32], which showed greater freezing tolerance than other warm-season turfgrasses and vary cold tolerance among zoysiagrass species and genotypes [29]. Two representative genotypes were chosen for this study based on our preliminary investigation in a field study, which demonstrated that the genotype native to higher latitude exhibit higher freezing tolerance than the ones native to warmer latitude. In this study, we further confirmed the result in a controlled environment that genotypes native to higher latitude showed higher freezing tolerance as indicated by the EL and LT50 under both cold-acclimated and non-acclimated conditions. It has been suggested that the enhanced freeze tolerance in plants partially attribute to the cold acclimation [31]. The leaf LT50 was from -3.9°C to -8.6°C for the higher latitude genotype and from -2.4°C to -6.8°C for the lower latitude genotype under optimum to chilling temperature, suggesting that cold acclimation improved freeze tolerance for zoysiagrass regardless of their latitude origins.
Low temperature caused reduction in photosystem performance and photosynthetic apparatus activities have been associated with photo-inhibition arised from the reduced capacity for energy utilization [3]. In our study, leaf Pn, gs and Tr remarkably declined with the reduction of temperature, along with a great decrease in plant canopy height, suggesting that the cessation of growth resulting from cold stress reduces the capacity for energy utilization, causing feedback inhibition of photosynthesis during the initial phase of chilling stress [41]. Differences in the capacity to minimize photo-inhibition of photosynthesis during low temperature have been implied to contribute to the intra- and inter-specific differences in freezing tolerance [10]. In this study, genotype Latitude-40 exhibited higher photosynthesis under low temperature compared with Latitude-22, suggesting that genotypes native to higher latitude may have a higher capacity to minimize the photo-inhibition of photosynthesis that leading to the improved freezing tolerance.
A decrease in Fv/Fm indicated photosynthesis reduction or photo-inhibition under environmental stress [42]. The results of this study showed that Fv/Fm did not decline under suboptimum temperature until chilling and freezing temperature. These results indicated that photochemical efficiency in zoysiagrass was relatively less sensitive to temperature decline than the gs and Ci. Thus, the early inhibition of photosynthesis by low temperature in zoysiagrass could not be associated with the damage of PSII at this stage. A similar results in Fv/Fm was also observed in cool-season turfgrass creeping bentgrass and annual bluegrass subjected to chilling stress or during cold acclimation [43].
Nonstructural carbohydrates (starch, hexose, sucrose) are essentially involved in the recovery from dormancy, re-growth and recuperative capacity from abiotic stress, particular under low temperature [44]. Changes in the accumulation of water soluble sugars (WSC) during low temperature have been reported in many warm- and cool-season turfgrass [12–15, 45]. Soluble carbohydrates have been associated with freeze tolerance and could leading to inter- and intra-specific differences in plant freezing tolerance in various warm-season turfgrasses [12–13, 45]. In our study, total WSC (TSS) significantly increased in leaves of both genotypes under low temperature. The genotype Latitude-40 exhibited significantly higher TSS compared to Latitude-22. This result suggests that higher accumulations of TSS in leaves of cold tolerant genotype may account for the improved membrane stability at low temperatures and contributed to the better freezing tolerance.
Sucrose content increased from optimum to freezing temperature for both genotypes. Previous works on cool- and warm-season turfgrasses also showed an increase in sucrose content under low temperature [13, 45]. However, there have been various reports on the associations between the accumulation of sucrose and freezing tolerance in turfgrass species. There were high correlations between surose content and freezing tolerance in warm-season grass [12, 14, 46], whereas no correlations were found between sucrose levels and freezing tolerance in cool-season annual bluegrass (Poa annua) [13]. In this study, the genotype native to higher latitude (Latitude-40) exhibited higher sucrose accumulation along with a lower LT50 than the ones native to lower latitude (Latitude-22) with the temperature decline, suggesting a positively correlation between the sucrose accumulation and the freezing tolerance in zoysiagrass.
An enhancement in soluble carbohydrates levels during cold acclimation generally along with a reduction in starch levels [9]; however, studies on starch content in stolons and rhizomes of warm-season grasses vary during cold-acclimation. Starch concentration in rhizomes and stolons of bermudagrass and centipedegrass decreased during cold acclimation [14], whereas the starch concentration of carpetgrass (Axonopus affinis) and zoysiagrass has been reported to increase during cold acclimation [47–48]. The results of this study are in agreement with the reports that starch content significant increased in zoysiagrass under suboptimum and chilling temperature [47–48]. However, starch content decreased for the genotype native higher latitude and increased for the lower latitude genotype under freezing temperature. These results suggested that cold-acclimated zoysiagrass contain less starch after cold acclimation potentially contributed to carbohydrate accumulation in response to cold leading to more freeze tolerance [17, 49].
Soluble sugars trehalose and fructans are documented to protect plants from freeze-induced dehydration and reduce ice formation by increasing the intracellular solute concentration [18]. In the current study, trehalose and fructan increased with the temperature decline in two genotypes, with higher content in the genotype native to higher latitude along with a lower LT50, suggesting those two soluble sugars are important factors in the cold acclimation process and involved in the freezing tolerance in zoysiagrass, which has also been implicated in perennial ryegrass that increased fructan synthesis was positively associated with improved freezing tolerance [50].
ABA has been considered as signaling molecules for inducing plant antioxidant defense systems against abiotic stresses [51]. Cytokinins are reported to promote shoot initiation, lateral bud growth, leaf expansion, nutrient mobilization, chloroplast differentiation, activation of shoot meristems and delay senescence [52]. The results of this study showed that low temperature lead to an increase in ABA and decline in t-ZR content in leaves of zoysiagrass. This is consistent with previous studies in warm-season turfgrass species [23, 53]. It is reported that ABA plays a crucial role in promoting plant tolerance to cold and exogenous application of ABA promotes freezing tolerance in plants [54]. The higher ABA content were observed in the genotype native to higher latitude under low temperature, suggesting that ABA accumulation during cold acclimation is associated with freezing tolerance in zoysiagrass [54].
Endogenous GA content has been reported to declined with the reduction of temperature, which is correlated with the shoot growth inhibition in carrot (Daucus carota) [55] and sunflower (Helianthus annuus) [28]. In our study, GA3 levels significantly reduced in both zoysiagrass genotype. Concomitantly, the cold-stressed plants exhibited lower canopy height and no difference were observed between the two zoysiagrass genotypes. These results suggested that the growth reduction under low temperature may partially attribute to the lowered GA levels in zoysiagrass. It was reported that cold acclimation involves a decrease in endogenous IAA levels in Arabidopsis [56]. However, an increased endogenous IAA concentration was observed in the seedlings of in rice (Oryza sativa) seedlings under low temperature stress [57]. In wheat, a significant increase in IAA concentrations was found in crown tissues but not in leaves [58]. In addition, whereas IAA levels were not affected by cold stress in spring wheat and significant increases occurred in winter wheat after cold exposure [59]. In our study, IAA content decreased in leaves of zoysiagrass under low temperature, suggesting that cold stress appears to affect IAA levels with differences depending on plant species, developmental context and organs.
Salicylic acid (SA) has also been reported to be associated with the low temperature stress tolerance in plants [60]. Salicylic acid pretreatment obviously ameliorated the damages of cold stress in several plant species such as banana (Musa acuminate) [61], corn (Zea mays), cucumber (Cucumis sativus) and rice [62] and tomato (Lycopersicon esculentum) plants [63]. In our study, subjected to low temperatures increased the endogenous SA levels in zoysiagrass, with higher levels in the genotype native to higher latitude. This result suggested that SA is involved in the cold stress tolerance in zoysiagrass, which may partially attribute to the SA induced CBFs following cold treatment and promoted freezing tolerance, because jasmonate function is a critical upstream signal in ICE1-CBFs pathway to positively regulate low temperature tolerance [25, 64]. In addition to GAs, SA is another hormone that appears to contribute to the low temperature-induced growth retardation of plants. Cold-induced increases in SA levels were reported for both chilling-sensitive and freezing-tolerant plant species [58]. In this study, great growth reduction was observed along with a significant decline in GA3 levels but a slight increase in SA levels under suboptimum temperature, whereas a great increase in SA content at chilling to freezing temperature when GA3 reduced to a very low levels. These results suggested that SA may act to regulate plant growth inhibition in the later stages of cold treatment or under the severe cold stress, when the role of GA becomes less pronounced.
Stress tolerance in plants is highly dependent upon the induction of specific stress related signaling pathway and genes [64]. The best understood cold signaling pathway is mediated by ICE1/CBF/COR transcriptional cascade. In this sense, the C-repeat (CRT)-binding factors (CBFs)/dehydration-responsive elements (DREBs) are induced by cold, leading to the induction of cold responsive (COR) gene expression associated with freezing tolerance [8]. The current study measured changes in the expression of three cold responsive genes including the CBF, DREB1 and LEA3, and those genes significantly up-regulated with the decreasing of temperature in zoysiagrass, whereas the expression levels was higher in the genotype native to higher latitude along with a lower LT50. These results suggested that a higher expression of these TFs and genes in zoysiagrass were associated with an increased tolerance to chilling and freezing temperatures, which in agreement with previous studies in Arabidopsis [65], Citrus species [66].
Key components of the ABA and JA signaling pathways have been shown to modulate CBFs under low temperature [67]. Moreover, certain CBF genes are induced by exogenous application of ABA, contributing to sustain cold tolerance [68]. JA has been shown to induce a response similar to that of ABA for alleviating chilling and freezing injury in plants [25]. The results of this study indicated that low temperature induced up-regulation of ZjCBF, ZjDREB1 and ZjLEA genes in leaves of zoysiagrass with the concomitant increase in ABA and JA content, suggesting ABA and JA contribute to the cold tolerance in zoysiagrass served as positive regulators during the signaling pathways under low temperature.
Conclusions
Zoysiagrass native to higher latitude exhibited higher freezing tolerance, and the higher freezing tolerance may attribute to the higher carbohydrates content serving as energy reserves and stress protectants that stabilize cellular membranes. The higher levels of phytohormones may serve signals in regulating plant growth, development and adaptation to low temperature as well as involved in the signaling pathways that induced the up-regulated ZjCBF, ZjLEA3 and ZjDREB1 expressions thus result in a higher cold tolerance.
Acknowledgments
This work was financially supported by Natural Science Foundation of Hunan Province (2018JJ3223) to Longxing Hu and Science Foundation for The Excellent Youth of Hunan Provincial Education Department to Longxing Hu and Yong Yang (17B120 and 16B149)
Data Availability
All relevant data are within the paper.
Funding Statement
This work was financially supported by Natural Science Foundation of Hunan Province (2018JJ3223) to Longxing Hu and Science Foundation for The Excellent Youth of Hunan Provincial Education Department (17B120 and 16B149) to Longxing Hu and Yong Yang.
References
- 1.Anderson JA, Taliaferro CM. Freeze tolerance of seed-producing turf bermudagrasses. Crop Sci. 2000; 42:190–192. doi: 10.2135/cropsci2002.1900 [DOI] [PubMed] [Google Scholar]
- 2.Esmaili S, Salehi H. Effects of temperature and photoperiod on postponing bermudagrass (Cynodon dactylon [L.] Pers.) turf dormancy. J Plant Physiol. 2012; 169:851–858. doi: 10.1016/j.jplph.2012.01.022 [DOI] [PubMed] [Google Scholar]
- 3.Huang BR, DaCosta M, Jiang YW. Research advances in mechanisms of turfgrass tolerance to abiotic stresses: from physiology to molecular biology. Critical Rev Plant Sci. 2014; 33:2–3, 141–189. doi: 10.1080/07352689.2014.870411 [Google Scholar]
- 4.Suzuki N, Mittler R. Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiol Plant. 2006; 126:45–51. doi: 10.1111/j.0031-9317.2005.00582.x [Google Scholar]
- 5.Xin Z, Browse J. Cold comfort farm: the acclimation of plants to freezing temperatures. Plant Cell Environ. 2000; 23:893–902. doi: 10.1046/j.1365-3040.2000.00611.x [Google Scholar]
- 6.Trischuk RG, Schilling BS, Low NH, Gray GR, Gusta LV. Cold acclimation, de-acclimation and re-acclimation of spring canola, winter canola and winter wheat: The role of carbohydrates, cold-induced stress proteins and vernalization. Environ Exp Bot. 2014; 106:156–163. doi: 10.1016/j.envexpbot.2014.02.013 [Google Scholar]
- 7.Thomashow MF. Plant Cold Acclimation: Freezing tolerance genes and regulatory mechanisms. Ann Rev Plant Physiol Plant Mol Biol. 1999; 50:571–599. doi: 10.1146/annurev.arplant.50.1.571 [DOI] [PubMed] [Google Scholar]
- 8.Fowler S, Thomashow MF. Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. Plant Cell. 2002; 14:1675–1690. doi: 10.1105/tpc.003483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levitt J. Responses of Plants to Environmental Stresses, Vol 1: Chilling, Freezing and High Temperature Stresses, Ed 2 Academic Press, New York, 1980. [Google Scholar]
- 10.Pocock TH, Hurry VM, Savitch LV, Huner NPA. Susceptibility to low-temperature photoinhibition and the acquisition of freezing tolerance in winter and spring wheat: the role of growth temperature and irradiance. Physiol Plant. 2001; 113:499–506. [Google Scholar]
- 11.Theocharis A, Clément C, Barka EA. Physiological and molecular changes in plants grown at low temperatures. Planta. 2012; 235:1091–1105. doi: 10.1007/s00425-012-1641-y [DOI] [PubMed] [Google Scholar]
- 12.Zhang XZ, Ervin EH, LaBranche AJ. Metabolic defense responses of seeded bermudagrass during acclimation to freezing stress. Crop Sci. 2006; 46:2598–2605. doi: 10.2135/cropsci2006.02.0108 [Google Scholar]
- 13.Dionne J, Castonguay Y, Nadeau P, Desjardins Y. Freezing tolerance and carbohydrate changes during cold acclimation of green-type annual bluegrass (Poa annua L.) ecotypes. Crop Sci. 2001; 41:443–451. doi: 10.2135/cropsci2001.412443x [Google Scholar]
- 14.Cai Q, Wang S, Cui Z, Sun J, Ishii Y. Changes in freezing tolerance and its relationship with the contents of carbohydrates and proline in overwintering centipedegrass (Eremochloa ophiuroides (Munro) Hack.). Plant Production Sci. 2004; 7:421–426. doi: 10.1626/pps.7.421 [Google Scholar]
- 15.Hoffman L, DaCosta M, Ebdon JS, Watkins E. Physiological changes during cold acclimation of perennial ryegrass accessions differing in freeze tolerance. Crop Sci. 2010; 50:1037–1047. doi: 10.2135/cropsci2009.06.0293 [Google Scholar]
- 16.Janska A, Marsik P, Zelenkova S, Ovesna J. Cold stress and acclimation-what is important for metabolic adjustment? Plant Biol. 2010; 12:395–405. doi: 10.1111/j.1438-8677.2009.00299.x [DOI] [PubMed] [Google Scholar]
- 17.Sicher R. Carbon partitioning and the impact of starch deficiency on the initial response of Arabidopsis to chilling temperatures. Plant Sci. 2011; 181:167–176. doi: 10.1016/j.plantsci.2011.05.005 [DOI] [PubMed] [Google Scholar]
- 18.Livingston HDIII, Heyer A. Fructan and its relationship to abiotic stress tolerance in plants. Cell Mol Life Sci. 2009; 66:2007–2023. doi: 10.1007/s00018-009-0002-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Theocharis A, Bordiec S, Fernandez O, Paquis S, Dhondt-Cordelier S, Baillieul F, et al. Burkholderia phytofirmans PsJN primes Vitis vinifera L. and confers a better tolerance to low nonfreezing temperatures. Mol Plant Microbe Interact. 2012; 25:241–249. doi: 10.1094/MPMI-05-11-0124 [DOI] [PubMed] [Google Scholar]
- 20.Strivastava LM. 2002. Plant growth and development: hormones and environment. Academic Press, San Diego, CA. [Google Scholar]
- 21.Finkelstein RR, Gibson SI. ABA and sugar interactions regulating development: cross-talk or voices in a crowd? Cur Opinion Plant Biol. 2002; 5:26–32. doi: 10.1016/S1369-5266(01)00225-4 [DOI] [PubMed] [Google Scholar]
- 22.Ryu H, Cho YG. Plant hormones in salt stress tolerance. J Plant Biol. 2015; 58:147–155. doi: 10.1007/s12374-015-0103-z [Google Scholar]
- 23.Zhang XZ, Ervin EH, Evanylo GK, Haering KC. Impact of biosolids on hormone metabolism in drought-stresses tall fescue. Crop Sci. 2009; 49:364–370. doi: 10.2135/cropsci2008.09.0521 [Google Scholar]
- 24.Llanes A, Andrade A, Alenabo S, Luna V. Alterations of endogenous hormonal levels in plants under drought and salinity. Amer J Plant Sci. 2016; 7:1357–1371. doi: 10.4236/ajps.2016.79129 [Google Scholar]
- 25.Hu Y, Jiang L, Wang F, Yu D. (2013) Jasmonate regulates the INDUCER OF CBF EXPRESSION-C-REPEAT BINDING FACTOR/DRE BINDING FACTOR1 cascade and freezing tolerance in Arabidopsis. Plant Cell 2013; 25:2907–292. doi: 10.1105/tpc.113.112631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan MI, Fatma M, Per TS, Anjum NA, Khan NA. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front Plant Sci. 2015; 6:462 doi: 10.3389/fpls.2015.00462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hundertmark M, Hincha DK. LEA (late embryogenesis abundant) proteins and their encoding genes in Arabidopsis thaliana. BMC Genomics. 2008; 9:118 doi: 10.1186/1471-2164-9-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurepin LV, Walton LJ, Pharis RP, Emery RJN, Reid DM. Interactions of temperature and light quality on phytohormone-mediated elongation of Helianthus annuus hypocotyls. Plant Growth Regul. 2011; 64:147–154. doi: 10.1007/s10725-010-9549-7 [Google Scholar]
- 29.Patton AJ, Reicher ZJ. Zoysiagrass species and genotypes differ in their winter injury and freeze tolerance. Crop Sci. 2007; 47:1619–1627. doi: 10.2135/cropsci2006.11.0737 [Google Scholar]
- 30.Pompeiano A, Caturegli L, Grossi N, Volterrani M, Guglielminetti L. Carbohydrate metabolism during wintering period in four Zoysiagrass genotypes. Plant Production Sci. 2015; 18:43–51. doi: 10.1626/pps.18.43 [Google Scholar]
- 31.Anderson, S. (2000). Taxonomy of Zoysia (Poaceae): Morphological and Molecular Variation. Ph.D. Thesis. Texas A&M University.
- 32.Xuan JP, Liu JX, Gao H, Hu HG, Cheng XL. Evaluation of low-temperature tolerance of zoysia grass. Tropical Grasslands 2009; 43:118–124. [Google Scholar]
- 33.Hu LX, Zhang ZF, Xiang ZX, Yang ZJ (2016) Exogenous application of citric acid ameliorates the adverse effect of heat stress in tall fescue (Lolium arundinaceum). Front Plant Sci 7:179 doi: 10.3389/fpls.2016.00179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xuan JP, Song YF, Zhang HX, Liu JX, Hua YL. Comparative proteomic analysis of the stolon cold stress response between the C4 perennial grass species Zoysia japonica and Zoysia metrella. PLoS ONE. 2013; 8:e75705 doi: 10.1371/journal.pone.0075705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hiscox J.D., and Israesltam G.F. (1979). A method for extration of chlorophyll from leaf without maceration. Can J. Bot. 57, 1332–1334. [Google Scholar]
- 36.Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol.1949; 24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang PP, Fu JM, Hu LX. Effects of alkali stress on growth, free amino acids and carbohydrates metabolism in Kentucky bluegrass (Poa pratensis). Ecotoxicology. 2012; 21:1911–1918. doi: 10.1007/s10646-012-0924-11 [DOI] [PubMed] [Google Scholar]
- 38.Dobrev PI, Kamínek M. Fast and efficient separation of cytokinins from auxin and abscisic acid and their purification using mixed-mode solid-phase extraction. J Chromatogra A. 2002; 950:21–219. doi: 10.1016/S0021-9673(02)00024-9 [DOI] [PubMed] [Google Scholar]
- 39.Ghanem ME, Albacete A, Martínez-Andújar C, Acosta M, Romero-Aranda R. Dodd IC, et al. Hormonal changes during salinity-induced leaf senescence in tomato (Solanum lycopersicum L.). J Exp Bot. 2008; 5:3039–3050. doi: 10.1093/jxb/ern153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Livaka KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 −ΔΔCT method. Methods. 2001; 25:402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 41.Ruelland E, Zachowski A. How plants sense temperature. Environ Exp Bot. 2010; 69:225–232. doi: 10.1016/j.envexpbot.2010.05.011 [Google Scholar]
- 42.Souza RP, Machado EC, Silva JAB, Lagoa AMMA, Silveira JAG. Photosynthetic gas exchange, chlorophyll fluorescence and some associated metabolic changes in cowpea (Vigna unguiculata) during water stress and recovery. Environ Exp Bot. 2004; 51:45–56. doi: 10.1016/S0098-8472(03)00059-5 [Google Scholar]
- 43.Sarkar D, Bhowmik PC, Kwon YI, Shetty K. Cold acclimation responses of three cool-season turfgrasses and the role of proline-associated pentose phosphate pathway. J Amer Soc Horticul Sci. 2009; 134:210–220. [Google Scholar]
- 44.Munshaw GC, Ervin EH, Shang C, Askew SD, Zhang XZ, Lemus RW. Influence of late-season iron, nitrogen, and seaweed extract on fall color retention and cold tolerance of four bermudagrass cultivars. Crop Sci. 2005; 46:273–283. doi: 10.2135/cropsci2005.0078 [Google Scholar]
- 45.Patton AJ, Cunningham SM, Volenec JJ, Reicher ZJ. Differences in freeze tolerance of Zoysiagrasses: II. Carbohydrate and proline accumulation. Crop Sci. 2007; 47:2170–2181. doi: 10.2135/cropsci2006.12.0784 [Google Scholar]
- 46.Ball S, Qian YL, Stushnoff C. Soluble carbohydrates in two Buffalograss cultivars with contrasting freezing tolerance. J Amer Soc Horticul Sci. 2002; 127:1002–1005. [Google Scholar]
- 47.Bush E, Wilson P, Shepard D, McCrimmon J. Freezing tolerance and nonstructural carbohydrate composition of carpetgrass (Axonopus affinis Chase). HortScience. 2000; 35:187–189. [Google Scholar]
- 48.Rogers RA, Dunn JH, Nelson CJ. Cold hardening and carbohydrate composition of Meyer zoysia. Agron J. 1975; 67:836–838. doi: 10.2134/agronj1975.00021962006700060029x [Google Scholar]
- 49.Macolino S, Serena M, Leinauer B, Ziliotto U. Preliminary findings on the correlation between water soluble carbohydrate content in stolons and first year green-up of seeded bermudagrass cultivars. Horttechnology. 2010; 20:758–776. [Google Scholar]
- 50.Hisano H, Kanazawa A, Kawakami A, Yoshida M, Shimamoto Y, Yamada T. Transgenic perennial ryegrass plants expressing wheat fructosyltransferase genes accumulate increased amounts of fructan and acquire increased tolerance on a cellular level to freezing. Plant Sci. 2004; 167:861–868. doi: 10.1016/j.plantsci.2004.05.037 [Google Scholar]
- 51.Xiong L, Schumaker KS, Zhu JK. Cell signaling during cold, drought, and salt stress. Plant Cell. 2002;14:S165–S183. doi: 10.1105/tpc.000596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davies PJ. Plant Hormones: Biosynthesis, Signal Transduction, Action, 3rd Edn New York, NY: Springer, 2010. [Google Scholar]
- 53.Zhang XZ, Wang KH, Ervin EH, Waltz C, Murphy T. Metabolic changes during cold acclimation and deacclimation in five Bermudagrass varieties. I. Proline, total amino acid, protein, and dehydrin expression. Crop Sci. 2011; 51:838 doi: 10.2135/cropsci2010.06.0345 [Google Scholar]
- 54.Zhang XZ, Wang KH, Ervin EH. Bermudagrass freezing tolerance associated with abscisic acid metabolism and dehydrin expression during cold acclimation. J Amer Soc Horticul Sci. 2008; 133:542–550. [Google Scholar]
- 55.Hiller LK, Kelly WC, Powell LE. Temperature interactions with growth regulators and endogenous gibberellin-like activity during seeds talk elongation in carrots. Plant Physiol. 1979; 163:1055–1061. doi: 10.1104/pp.63.6.1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shibasaki K, Uemura M, Tsurumi S, Rahman A. Auxin response in Arabidopsis under cold stress: underlying molecular mechanisms. Plant Cell. 2009; 21:3823–3838. doi: 10.1105/tpc.109.069906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Du H, Liu H, Xiong L. Endogenous auxin and jasmonic acid levels are differentially modulated by abiotic stresses in rice. Front Plant Sci. 2013a; 4:397 doi: 10.3389/fpls.2013.00397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kosová K, Prášil IT, Vítámvás P, Dobrev P, Motyka V, Floková K, et al. Complex phytohormone responses during the cold acclimation of two wheat cultivars differing in cold tolerance, winter Samanta and spring Sandra. J Plant Physiol. 2012; 169:567–576. doi: 10.1016/j.jplph.2011.12.013 [DOI] [PubMed] [Google Scholar]
- 59.Majláth I., Szalai G., Soós V., Sebestyén E., Balázs E., Vanková R., Dobrev P.I., Tari I., Tandori J., and Janda T. (2012). Effect of light on the gene expression and hormonal status of winter and spring wheat plants during cold hardening. Physiol. Plant. 145, 296–314. doi: 10.1111/j.1399-3054.2012.01579.x [DOI] [PubMed] [Google Scholar]
- 60.Horváth E, Szalai G, Janda T. Induction of abiotic stress tolerance by salicylic acid signaling. J. Plant Growth Regul. 2007; 26:290–300. doi: 10.1007/s00344-007-9017-4 [Google Scholar]
- 61.Kang GZ, Wang CH, Sun GC, Wang ZX. Salicylic acid changes activities of H2O2-metabolizing enzymes and increases the chilling-tolerance of banana seedlings. Environ Exp Bot. 2003; 50:9–15. doi: 10.1016/S0098-8472(02)00109-0 [Google Scholar]
- 62.Kang HM, Saltveit ME. Chilling tolerance of maize, cucumber and rice seedling leaves and roots are differentially affected by salicylic acid. Physiol Plant. 2002; 115:571–576. doi: 10.1034/j.1399-3054.2002.1150411.x [DOI] [PubMed] [Google Scholar]
- 63.Ding CK, Wang CY, Gross KC, Smith DL. Jasmonate and salicylate induce the expression of pathogenesis-related-protein genes and increase resistance to chilling injury in tomato fruit. Planta. 2002; 214:895–901. doi: 10.1007/s00425-001-0698-9 [DOI] [PubMed] [Google Scholar]
- 64.Cook D, Fowler S, Fiehn O, Thomashow MF. A prominent role for the CBF cold response pathway in configuring the low-temperature metabolome of Arabidopsis. Proc Natl Acad Sci USA. 2004; 101:15243–15248. doi: 10.1073/pnas.0406069101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chinnusamy V, Zhu J, Zhu JK. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007; 12:444–451. doi: 10.1016/j.tplants.2007.07.002 [DOI] [PubMed] [Google Scholar]
- 66.Champ KI, Febres VJ, Moore BD. The role of CBF transcriptional activators in two Citrus species (Poncirus and Citrus) with contrasting levels of freezing tolerance. Physiol Plant. 2007; 129:529–541. [Google Scholar]
- 67.Achard P, Gong F, Cheminant S, Alioua M, Hedden P, Genschik P. The cold-inducible CBF1 factor-dependent signaling pathway modulates the accumulation of the growth-repressing DELLA proteins via its effect on gibberellin metabolism. Plant Cell. 2008; 20:2117–2129. doi: 10.1105/tpc.108.058941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Knight H, Zarka DG, Okamoto H, Thomashow MF, Knight MR. Abscisic acid induces CBF gene transcription and subsequent induction of cold-regulated genes via the CRT promoter element. Plant Physiol. 2004; 135:1710–1717. doi: 10.1104/pp.104.043562 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.