Abstract
Background
The plant-derived omega-3 fatty acid alpha-linolenic acid (ALA) may reduce the risk of cardiovascular disease.
Objective
We have investigated associations between the content of ALA in adipose tissue and the risk of ischemic stroke and its subtypes.
Methods
Incident cases of ischemic stroke among participants enrolled into the Danish Diet, Cancer and Health cohort (n = 57,053) were identified by linkage with the Danish National Patient Register. Subsequently, all potential cases were validated and classified into ischemic stroke subtypes. The fatty acid composition of adipose tissue was determined by gas chromatography in cases and in a randomly drawn sub-cohort (n = 3500). Statistical analyses were performed using weighted Cox regression.
Results
During a median of 13.4 years of follow-up, 1735 cases of total ischemic stroke were identified including 297 cases of large artery atherosclerosis, 772 cases of small-vessel occlusion, 99 cases of cardio-embolism, 91 cases with stroke of other etiology and 476 cases with stroke of undetermined etiology. The median content of ALA in adipose tissue within the sub-cohort was 0.84% (95% central range: 0.53–1.19%). Multivariable analyses showed a U-shaped association between adipose tissue content of ALA and the rate of total ischemic stroke, but this association was not statistically significant (p = 0.172). In analyses of ischemic stroke subtypes, we observed a statistically significant U-shaped association between ALA and the rate of ischemic stroke due to large artery atherosclerosis (p = 0.017), whereas no appreciable association was observed between ALA and the rate of small-vessel occlusion (p = 0.427). A positive but statistically non-significant association was observed between ALA and the rate of ischemic stroke due to cardio-embolism (p = 0.162).
Conclusions
The content of ALA in adipose tissue was statistically non-significantly U-shaped associated with risk of total ischemic stroke. For ischemic stroke subtypes a statistically significant, U-shaped association with large artery atherosclerosis was observed.
Introduction
Alpha-linolenic acid (ALA) is an essential fatty acid which is found mainly in plant oils, seeds and walnuts, but it can also be found in varying concentrations in other foods such as green leafy vegetables, whole grain-cereals, margarines, mayonnaises, potatoes, dairy-products and meat [1–3].
ALA has been associated with several beneficial effects important for development of cardiovascular disease including reduced vascular inflammation [4,5], impaired platelet aggregability [6] and a reduced atherosclerotic plaque burden [7,8]. However, controversy remains whether ALA is associated with a lower risk of ischemic stroke.
ALA has been suggested to be an important nutrient, possibly explaining the protective effect on coronary heart disease (CHD) provided by a Mediterranean diet [9]. However, while some observational studies [2,10–13] have suggested that ALA intake may be associated with a lower risk of CHD, other observational studies have not confirmed these findings [1,14–18]. Previous observational studies investigating the association between ALA intake and the risk of ischemic stroke are sparse and have given inconsistent results [14–16,19,20]. One explanation may be that most previous studies did not distinguish between subtypes of ischemic stroke. This is important because ALA could play different roles in ischemic stroke subtypes due to differences in underlying etiologies. In addition, studies based on dietary intake may be prone to measurement error due to self-reported food intakes and inaccurate food composition tables which may lead to underestimation of potential associations. Furthermore, ALA intake may be difficult to quantify in food questionnaires because sources such a plant oils and margarines often are included in convenience foods [21]. In contrast, biomarker studies may provide objective measures of ALA exposure, and adipose tissue is considered the best biomarker of long-term intake of many fatty acids including ALA [22,23].
The objective of this study was to investigate the associations between the content of ALA in adipose tissue and the risk of ischemic stroke and ischemic stroke subtypes. We hypothesized that adipose tissue content of ALA would be inversely associated with development of ischemic stroke and ischemic stroke subtypes.
Patients and methods
Study population and study design
This case-cohort study was based on data from the Diet, Cancer and Health cohort, which previously has been described in detail [24]. Briefly, the cohort was established between 1993 and 1997 by inviting 160,725 men and women to participate. All eligible participants were native Danish citizens without a previous diagnosis of cancer, aged 50–64 years, and living in the urban areas of Copenhagen and Aarhus. Potential participants were retrieved through the Danish Civil Registration System [24]. A sub-cohort of 3500 participants was drawn randomly from the cohort.
We excluded participants with a diagnosis of cancer before baseline that was not yet registered in the Danish Cancer Registry at the time of invitation. Also, participants registered with a diagnosis of stroke before enrollment as well as participants for whom information was missing on exposure or other covariates were excluded.
The study complied with the Declaration of Helsinki. The Diet, Cancer and Health cohort has been approved by the Health Research Ethics, Capital Region of Denmark, and the Danish Data Protection Agency. All participants gave written informed consent at inclusion [24].
Measurement of adipose tissue content of ALA
An biopsy of subcutaneous adipose tissue from the buttock was taken at baseline from all participants using a Luer-lock system (Terumo, Terumo Corp, Tokyo, Japan) consisting of a needle, a venoject multi-sample Luer adaptor and an evacuated blood tube, according to the method of Beynen and Katan [25]. Samples were stored in liquid nitrogen vapour until analysis. The adipose tissue content of ALA was quantified among all cases and the sub-cohort participants with the use of gas chromatography at a specialized lipid laboratory as described previously [1,26]. Before analysis, the biopsies were thawed and tissue was removed to a glass and prewarmed at 50 oC for ten min and subsequently dissolved in heptane at 50 oC and transesterified by 2mol/L potassium hydroxide in methanol at 50 oC for two min according to the IUPAC standard methods for analysis of oils, fats and derivates. The fatty acid composition was measured using a Varian 3900 gas chromatograph with a CP-8400 auto sampler (Varian, Middleburg, The Netherlands) equipped with a flame ionization detector. Split injecting mode, a CP-sil 88 60m x 0.25mm capillary column and temperature programming (90 oC to 210 oC) were used. Helium was used as carrier gas. Peak retention times and area percentages of fatty acids were identified using commercially available standards (Nu-check-Prep, Inc., US) [26]. Adipose tissue content of ALA was expressed as the weight percentage of total fatty acids. The inter-assay coefficient of variation for the assessment of ALA in adipose tissue was 1.9%.
Identification of cases
Incident cases of ischemic stroke were identified through the Danish National Patient Register that was established in 1977 and includes information on discharge diagnoses from all hospitals in Denmark [27].
Potential stroke cases included participants registered with either a primary or secondary discharge diagnosis of stroke according to the International Classification of Diseases (ICD) (ICD-8: 430, 431, 433, 434, 436.01, or 436.90 and ICD-10: I60, I61, I63 or I64). Stroke was defined as a disease with rapid onset of focal or global neurologic deficit of vascular origin persisting beyond 24 hours or leading to death. However, patients with focal neurological deficits of shorter duration were also considered as stroke cases if CT/MR imaging showed a recent stroke [28]. All potential stroke cases were validated by a physician with neurological experience and classified according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST)-classification [29] based on assumed etiology [28]. The TOAST-classification separates cerebral infarctions into five groups: large artery atherosclerosis, small-vessel occlusion, cardio-embolism, stroke of other etiology and stroke of undetermined etiology based on clinical findings, brain imaging, imaging of extra cranial arteries, laboratory tests, electrocardiograms, and echocardiography.
Participants were followed from baseline until the first registration of stroke, death, emigration, or end of follow-up in November 2009.
Covariates
At baseline, participants completed a detailed questionnaire on health status, social factors and lifestyle such as length of schooling, smoking habits, physical activity, history of hypercholesterolemia and/or use of lipid-lowering medication, history of hypertension and/or use of anti-hypertensive medication and a history of diabetes mellitus [24]. Information on alcohol intake and diet was obtained from a validated 192-item semi-quantitative food frequency questionnaire filled in at baseline [30,31]. The questionnaires were checked for reading errors and missing information by technicians who also performed anthropometric measurements including height, weight and waist circumference of the participants [24].
Information on a history of atrial fibrillation/flutter before baseline was obtained by record linkage with the National Patient Register (ICD-8: 42793, 42794 & ICD-10: I48). All potential ischemic stroke risk factors were selected a priori to data analysis.
Statistical analyses
The associations between adipose tissue content of ALA and the rate of ischemic stroke and ischemic stroke subtypes were investigated using hazard ratios (HRs). HRs and 95% confidence intervals (CIs) were calculated using weighted Cox proportional hazard regression allowing for separate baseline hazards between men and women and with age as underlying time axis. All cases were assigned a weight equal to one, whereas all non-cases in the sub-cohort were assigned a weight calculated as the ratio between the number of non-cases in the cohort after exclusions divided by the number of non-cases in the sub-cohort [32]. Individual weights were calculated for cases of total ischemic stroke and for each ischemic stroke subtype. A robust variance estimator was used for estimating standard errors. This weighting scheme has been shown to perform well in a simulation study [33].
The adipose tissue content of ALA was included as a continuous variable using restricted cubic splines with three knots. The knots were placed at the 10th, 50th and 90th percentile as recommended by Harrell [34]. Splines were plotted to visually assess the shape of the associations with the median as reference and the spline curves were formally tested against a horizontal line using Wald tests. In further analyses, the adipose tissue content of ALA was included as a categorical variable in quintiles with the lowest quintile as reference.
We examined the association between adipose tissue content of ALA and the risk of ischemic stroke in three different models. In model 1A baseline age (years, continuous) was included in order to ensure comparison of participants for whom the exposure was of the same age. In model 1B, baseline information on the following established ischemic stroke risk factors was added: length of schooling (≤7, 8–10, or >10 years), smoking (never, former, current 1–14, 15–24, or ≥24 g tobacco/d), physical activity (inactive, moderately inactive, moderately active, or active), waist circumference adjusted for body mass index (cm, continuous) and alcohol intake (g/d, continuous). In model 2, we adjusted for all the covariates of model 1B and in addition for the following clinical characteristics, which may both be considered potential confounders and potential intermediate variables: self-reported history of hypercholesterolemia or use of lipid-lowering medication (yes, no, or unknown), self-reported history of hypertension or use of anti-hypertensive medication (yes, no, or unknown), self-reported history of diabetes mellitus (yes, no, or unknown), and a diagnosis of atrial fibrillation/flutter recorded in the Danish National Patient Register (yes, no). Adjustments for continuous variables were performed using restricted cubic splines with three knots.
The spline plots were shown for the 95% central range of adipose tissue content of ALA and presented with 95% confidence bonds. In sensitivity analyses, we plotted the whole exposure range of adipose tissue content of ALA and modified the number and placement of the knots.
The proportional hazard assumption was evaluated by plotting scaled Schoenfeld residuals.
Potential differences in the underlying dietary pattern related to adipose tissue content of ALA were assessed graphically in a radar plot comparing the median intake of selected foods and beverages among participants in the highest and lowest quintile of adipose tissue content of ALA.
Data were analyzed using Stata statistical software (version 14; StataCorp LP), and a p-value <0.05 was considered statistically significant.
Results
A total of 57,053 men and women accepted to participate in the Diet, Cancer and Health cohort study. We excluded 2355 participants because they either had a diagnosis of cancer (n = 569) or stroke (n = 597) before entry, or had missing baseline information on the primary exposure (n = 350) or covariates (n = 961).
During a median of 13.4 years (95% central range: 3.9–15.2) of follow-up, 1735 validated cases of total ischemic stroke with complete information on covariates were identified (S1 Fig). The incidence rate of total ischemic stroke was 2.46 cases per 1000 person years. Total ischemic stroke cases included 297 cases of large artery atherosclerosis, 772 cases of small-vessel occlusion, 99 cases of cardio-embolism, 91 cases with stroke of other etiology and 476 cases with stroke of undetermined etiology. The incidence rate was 0.42 per 1000 person years for large artery atherosclerosis, 1.10 per 1000 person years for small-vessel occlusion and 0.14 per 1000 person years for cardio-embolism.
Baseline characteristics of the participants in the sub-cohort and among total ischemic stroke cases are shown in Table 1, while baseline characteristics among cases of ischemic stroke subtypes are given in S1 Table. Known ischemic stroke risk factors were more prevalent among participants who became cases compared with participants within the sub-cohort. Accordingly, we observed a larger proportion of men and participants with higher age, a shorter duration of schooling, larger waist circumference and a higher alcohol intake and a larger proportion of physically inactive and current smokers among cases. A history of hypercholesterolemia, hypertension and diabetes mellitus was also more prevalent in subsequent cases. Atrial fibrillation/flutter was more prevalent among cases of total ischemic stroke and cases classified with cardio-embolism, ischemic stroke of other etiology and stroke of undetermined etiology compared with the sub-cohort.
Table 1. Baseline characteristics among cases and sub-cohort participants.
| Sub-cohort (n = 3185) |
Total ischemic stroke cases (n = 1735) | ||
|---|---|---|---|
| Gender, % | |||
| Males | 54.1 | 61.8 | |
| Females | 45.9 | 38.2 | |
| Age at enrollment, years | 56.3 | 58.8 | |
| Duration of schooling, % | |||
| ≤7 years | 32.7 | 40.7 | |
| 8–10 years | 45.0 | 42.7 | |
| >10 years | 22.3 | 16.6 | |
| Smoking, % | |||
| Never | 34.8 | 24.6 | |
| Former | 29.3 | 25.8 | |
| Current <15 g/d | 13.5 | 15.5 | |
| Current 15–25 g/d | 15.7 | 23.8 | |
| Current ≥25 g/d | 6.8 | 10.3 | |
| Physical activity, % | |||
| Inactive | 11.0 | 14.7 | |
| Moderately inactive | 30.4 | 30.2 | |
| Moderately active | 23.7 | 21.4 | |
| Active | 35.0 | 33.7 | |
| Waist circumference, cm a, b | 91.1 (73.9, 104.5) | 93.6 (74.9, 105.8) | |
| Alcohol intake, g/da | 13.9 (0.2, 85.0) | 14.5 (0.0, 93.6) | |
| Clinical characteristics, % | |||
| Hypercholesterolemia | 7.8 | 10.7 | |
| Hypertension | 15.6 | 28.4 | |
| Diabetes mellitus | 2.0 | 4.2 | |
| Atrial fibrillation/flutter | 0.9 | 1.4 | |
a Median; 2.5th–97.5th percentiles in parentheses
b Adjusted for body mass index
Association between ALA and ischemic stroke and ischemic stroke subtypes
The median content of ALA in adipose tissue within the sub-cohort was 0.84% (95% central range: 0.53–1.19%).
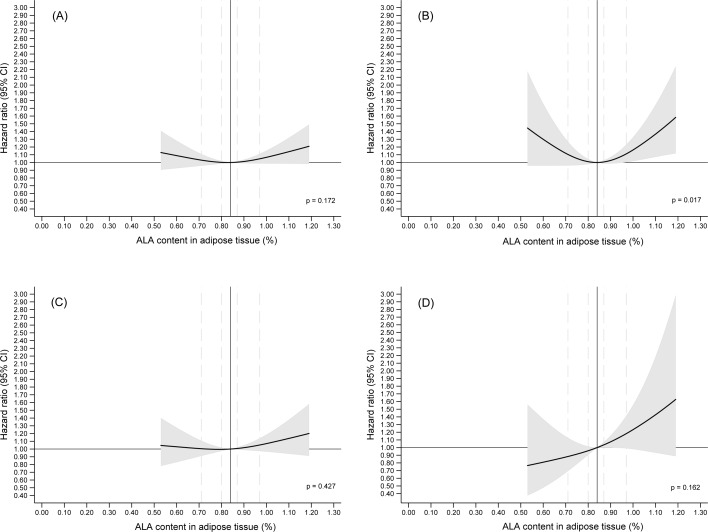
Multivariable analyses of adipose tissue content of ALA modeled as a restricted cubic spline and adjusted for established ischemic stroke risk factors (model 1B) showed a U-shaped association between ALA in adipose tissue and the rate of total ischemic stroke, but this association was not statistically significantly different from a horizontal line (p = 0.172) (Fig 1). In multivariable analyses (model 1B) of ischemic stroke subtypes, we observed a U-shaped association between adipose tissue content of ALA and the rate of large artery atherosclerosis and this association was statistically significantly different from a horizontal line (p = 0.017) (Fig 1). In analyses of the association between ALA in adipose tissue and the rate of small-vessel occlusion, we observed a weak positive association above the median ALA content, however this was not statistically significant (p = 0.427). Analyses of the association between ALA in adipose tissue and rate of cardio-embolism showed a positive association, which was not statistically significant (p = 0.162) (Fig 1). Analyses of associations between adipose tissue content of ALA and the rate of ischemic stroke of other etiology and rate of ischemic stroke of undetermined etiology are given in S2 Fig.
Fig 1. The content of alpha-linolenic acid in adipose tissue and the risk of incident total ischemic stroke and ischemic stroke subtypes.
The multivariable analyses were adjusted for established risk factors (model 1B) with median adipose tissue content as reference (solid vertical line). The 20th, 40th, 60th, and 80th percentiles of adipose tissue content of ALA are marked by dashed lines. Only 2.5th–97.5th percentiles of ALA are shown.
Analyses of the adipose tissue content of ALA in quintiles and risk of ischemic stroke and ischemic stroke subtypes are shown in Table 2 and S2 Table.
Table 2. Quintiles of adipose tissue content of ALA and hazard ratios for total ischemic stroke and ischemic stroke subtypes.
| Cases | Model 1Aa | Model 1Bb | Model 2c | |||||
|---|---|---|---|---|---|---|---|---|
| (n) | HR | 95% CI | HR | 95% CI | HR | 95% CI | ||
| Total ischemic stroke | ||||||||
| 0.31–0.71% | 361 | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| 0.71–0.80% | 338 | 0.94 | 0.77, 1.13 | 0.95 | 0.78, 1.16 | 0.93 | 0.76, 1.15 | |
| 0.80–0.87% | 295 | 0.80 | 0.66, 0.98 | 0.86 | 0.70, 1.06 | 0.89 | 0.72, 1.09 | |
| 0.87–0.97% | 357 | 0.93 | 0.77, 1.12 | 0.93 | 0.76, 1.14 | 0.92 | 0.75, 1.13 | |
| 0.97–1.69% | 384 | 1.02 | 0.85, 1.24 | 1.01 | 0.82, 1.23 | 1.03 | 0.84, 1.27 | |
| Large artery atherosclerosis | ||||||||
| 0.31–0.71% | 67 | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| 0.71–0.80% | 50 | 0.75 | 0.51, 1.11 | 0.72 | 0.48, 1.08 | 0.72 | 0.48, 1.08 | |
| 0.80–0.87% | 43 | 0.63 | 0.42, 0.94 | 0.63 | 0.41, 0.96 | 0.66 | 0.43, 1.01 | |
| 0.87–0.97% | 64 | 0.91 | 0.63, 1.30 | 0.83 | 0.56, 1.22 | 0.85 | 0.58, 1.26 | |
| 0.97–1.69% | 73 | 1.07 | 0.75, 1.53 | 0.95 | 0.65, 1.40 | 0.99 | 0.68, 1.46 | |
| Small-vessel occlusion | ||||||||
| 0.31–0.71% | 158 | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| 0.71–0.80% | 150 | 0.96 | 0.75, 1.24 | 1.00 | 0.77, 1.31 | 0.98 | 0.75, 1.28 | |
| 0.80–0.87% | 138 | 0.86 | 0.66, 1.11 | 0.96 | 0.73, 1.25 | 0.98 | 0.74, 1.28 | |
| 0.87–0.97% | 162 | 0.97 | 0.76, 1.25 | 1.02 | 0.78, 1.33 | 1.01 | 0.77, 1.33 | |
| 0.97–1.69% | 164 | 1.02 | 0.79, 1.30 | 1.05 | 0.80, 1.38 | 1.08 | 0.82, 1.42 | |
| Cardio-embolism | ||||||||
| 0.31–0.71% | 19 | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| 0.71–0.80% | 17 | 0.91 | 0.47, 1.78 | 1.05 | 0.53, 2.06 | 1.04 | 0.50, 2.14 | |
| 0.80–0.87% | 18 | 0.93 | 0.48, 1.79 | 1.16 | 0.59, 2.27 | 1.18 | 0.58, 2.42 | |
| 0.87–0.97% | 15 | 0.73 | 0.36, 1.48 | 0.90 | 0.43, 1.87 | 0.87 | 0.41, 1.85 | |
| 0.97–1.69% | 30 | 1.50 | 0.83, 2.72 | 1.91 | 0.98, 3.70 | 2.02 | 1.01, 4.03 | |
ALA, alpha-linolenic acid; HR, Hazard ratio; CI, Confidence interval.
Hazard ratios with 95% CI intervals were calculated using weighted Cox regression. All models are adjusted for gender by allowing for separate baseline hazards.
a Model 1A included baseline age
b Model 1B included the variables of model 1A and the following risk factors for ischemic stroke: duration of schooling, smoking, physical activity, waist circumference adjusted for body mass index and alcohol intake.
c Model 2 included the variables of model 1B and the following potential intermediate variables: self-reported history hypercholesterolemia and/or use of lipid-lowering medication, hypertension and/or use of antihypertensive medication, diabetes mellitus, and history of atrial fibrillation/ flutter recorded in the Danish National Patient Register.
In analyses including adjustment for established risk factors (model 1B), a U-shaped pattern of association was observed between quintiles of adipose tissue content of ALA and the risk of total ischemic stroke and large artery atherosclerosis, but the hazards in the second to fifth quintiles were not statistically significantly different from the reference except for the third quintile in analysis of large artery atherosclerosis (HR: 0.63, 95% CI: 0.41–0.96). Additional adjustment for a history of hypercholesterolemia, hypertension, diabetes mellitus and atrial fibrillation/flutter (model 2) also showed a U-shaped pattern of associations, but the individual hazards in second to fifth quintiles were also not statistically significantly different from the reference.
No appreciable or consistent associations were observed between quintiles of adipose tissue content of ALA and the risk of ischemic stroke due to small-vessel occlusion nor the risk of ischemic stroke due to cardio-embolism in either of the models. However, a higher rate of stroke due to cardio-embolism was observed in the highest quintile of adipose tissue content of ALA although this was only statistically significant after adjustment for established ischemic stroke risk factors and potential intermediate variables (model 2).
Sensitivity analyses showed that the models using restricted cubic splines were robust when the location and number of knots were modified. No evidence of deviation from the proportionality assumption was observed in either of the models.
A radar plot of the background diet within the sub-cohort revealed several differences in the median intake of selected foods and beverages among participants in different quintiles of adipose tissue content of ALA (S3 Fig). Participants in the highest quintile of adipose tissue content of ALA had higher intakes of margarines, vegetable oils and mayonnaises, refined cereals, processed meat and fish compared with subjects in the lowest quintile of adipose tissue content of ALA within the sub-cohort, and lower intakes of alcohol, dairy products, peanuts, snacks and fatty potatoes, soft drinks and juices, fruit and vegetables.
Discussion
In this large case-cohort study, we observed a statistically non-significant U-shaped association between adipose tissue content of ALA and the rate of total ischemic stroke and a statistically significant U-shaped association between adipose tissue content of ALA and the rate of ischemic stroke due to large artery atherosclerosis, whereas no appreciable and no statistically significant association was observed between ALA and the rate of ischemic stroke due to small-vessel occlusion. A positive association was observed between ALA and the risk of ischemic stroke due to cardio-embolism, but this was not statistically significant.
Some strengths and limitations should be mentioned. This study holds the advantage of a prospective design with nearly complete follow-up and case ascertainment in a nationwide register independent of the baseline ALA measurement limiting the potential of selection and information bias. A major strength of this study was the use of adipose tissue samples for determination of ALA exposure, which is considered the gold standard as it may reflect long-term fatty acid intake and metabolism [22]. The use of an objective biomarker also limits the concern of random measurement error. However, repeated measurements of the content of ALA in adipose tissue would have been preferable because changes in dietary habits might have occurred during follow-up. Furthermore, the content of ALA in adipose tissue may be influenced by a combination of several factors such as gender, genetics and background diet [22,23]. We therefore consider the ALA content in adipose tissue a marker of endogenous exposure to ALA. Another major strength of this study was that all cases of ischemic stroke were validated and classified into ischemic stroke subtypes. However, this approach did not allow for gender-specific analyses and overfitting may be a concern in the rare subtypes of ischemic strokes due to a limited number of cases. Therefore, the observed positive association between adipose tissue content of ALA and the risk of ischemic stroke caused by cardio-embolism should be interpreted with caution.
Detailed information on ischemic stroke risk factors was included in the analyses, but residual confounding from known or unknown risk factors may still be of importance for the observed associations. Adjustment for established ischemic stroke risk factors (model 1B) somewhat weakened the observed associations although not consistently. Additional adjustment for a history of hypercholesterolemia, hypertension, diabetes mellitus and atrial fibrillation/flutter (model 2) showed similar patterns of association. However, the interpretation of this model is complicated because these clinical characteristics may represent intermediate steps in the causal pathways between ALA exposure and the risk of ischemic stroke and adjustment for these variables could introduce collider stratification bias. Therefore, we consider model 1B to be the most appropriate model for interpretation. We did not include adjustments for dietary factors in the analyses because the content of fatty acids in adipose tissue was expressed as a percentage of total fatty acids and the content of any individual fatty acids in adipose tissue thus depends on the content of other fatty acids. Furthermore, adjustments for dietary factors would introduce restrictions in the underlying dietary pattern. Therefore, measures of associations including adjustment for dietary factors would have been without a clear interpretation in this study.
To our knowledge, no previous cohort studies have investigated the association between adipose tissue content of ALA and the risk of ischemic stroke or ischemic stroke subtypes. However, few shorter-term biomarker studies have investigated the association between the content of ALA in blood fractions and the risk of ischemic stroke. A case-control study nested within a US cohort of postmenopausal women thus reported a modest inverse statistically non-significant association between serum concentrations of ALA and the risk of total ischemic stroke [35]. A follow-up study from Finland showed a U-shaped pattern of association across quartiles of serum concentrations of ALA and the risk of total ischemic stroke among men [36]. Other previous shorter-term biomarker studies have reported no clear associations between the content of ALA in blood fractions and the risk of ischemic stroke [15,37–40]. A potential explanation for the observed inconsistencies between previous biomarker studies could possibly be differences in the background diet. We used a radar plot to illustrate the content of ALA in adipose tissue as an indicator of the background diet and to evaluate potential confounding from the diet. We observed several differences, but the radar plot did not indicate that a high content of ALA overall reflected a healthy dietary pattern. The intake of ALA in our cohort was derived from a variety of foods with margarines, mayonnaises, whole-grain cereals, butter, potatoes, red meat, vegetable oils, fruit, fatty dairy products and processed meat being the major contributors [1], which might be different from ALA sources elsewhere. In the Nurses’ Health study from the US, the major intake of ALA was also derived from several foods with mayonnaises, oils and vinegar and other salad dressings, margarines, meat, dairy products and green leafy vegetables being the largest contributors [2]. Importantly, the intake of marine omega-3 fatty acids in our study was markedly higher than compared with previous cohort studies that have reported inverse associations between ALA intake and the risk of cardiovascular disease [2,10–13].
This may be important given that a previous study has suggested that ALA in particular may reduce CHD risk when the intake of marine omega-3 fatty acids is low [11].
To our knowledge, no clinical trials have investigated the role of ALA supplementation on the risk of ischemic stroke and limited evidence exists from clinical trials on other cardiovascular outcomes. The Lyon Diet Heart Study reported a significantly lower risk of recurrent myocardial infarction (MI) and cardiac death among participants randomized to a Mediterranean diet high in ALA compared to a prudent Western diet [41,42], but given the nature of the intervention the observed effects could not necessarily be attributed to ALA. Finally, the Alpha-Omega Trial reported a modest statistically non-significant lower risk of major cardiovascular events among participants with prior MI randomized to ALA, compared to marine omega-3 fatty acid supplementation or placebo [43]. It must be emphasized that our study did not evaluate the effect of a Mediterranean diet on ischemic stroke, but a possible association between ALA in adipose tissue and ischemic stroke and subtypes of ischemic stroke. The suggested beneficial effects of a Mediterranean diet on cardiovascular disease may be attributable to the sum of several nutrients rather than a single component.
In conclusion, adipose tissue content of ALA was statistically non-significantly U-shaped associated with risk of total ischemic stroke. For ischemic stroke subtypes a statistically significant, U-shaped association with ischemic stroke due to large artery atherosclerosis was observed.
Supporting information
(PDF)
The content of adipose tissue content of ALA and the risk of stroke of other etiology (A) and stroke of undetermined etiology (B). The multivariate models are adjusted for ischemic stroke risk factors (model 1B) and presented with the median adipose tissue content of ALA as reference (solid vertical line). The 20th, 40th, 60th, and 80th percentiles of adipose tissue content of ALA are marked by dashed lines. Shaded grey areas show 95% confidence intervals of hazard ratios of ischemic stroke subtypes (curves). Only the 2.5th–97.5th percentiles of ALA are shown.
(PDF)
The content of ALA in adipose tissue was indexed according to the overall median intake of the selected food groups (grey solid line) within the sub-cohort (n = 3185). The dots represent percentages-wise differences relative to the overall median intake.
(PDF)
(PDF)
(PDF)
Data Availability
Data are available from the Diet, Cancer and Health Institutional Data Access (https://www.cancer.dk/research/diet-genes-environment/dgedch/). To access data, an application must be approved by the Scientific Board. Furthermore, as data contain potentially identifying or sensitive information, access to data has to be registered and approved by The Danish Data Protection Agency (https://www.datatilsynet.dk/english/the-danish-data-protection-agency) and/or a Health Research Ethics Committee (http://www.nvk.dk/english).”
Funding Statement
This work was supported by The Danish Heart Foundation, grant number 17-R115-A7415-22060 (https://hjerteforeningen.dk/forskning/). The grant was given to CSB. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bork C, Jakobsen M, Lundbye-Christensen S, Tjønneland A, Schmidt E, Overvad K. Dietary intake and adipose tissue content of alpha-linolenic acid and risk of myocardial infarction: a Danish cohort study. Am J Clin Nutr. 2016;104: 41–48. doi: 10.3945/ajcn.115.127019 [DOI] [PubMed] [Google Scholar]
- 2.Hu F, Stampfer M, Manson J, Rimm E, Wolk A, Colditz G, et al. Dietary intake of alpha-linolenic acid and risk of fatal ischemic heart disease among women. Am J Clin Nutr. 1999;69: 890–897. doi: 10.1093/ajcn/69.5.890 [DOI] [PubMed] [Google Scholar]
- 3.Gebauer S, Psota T, Harris W, Kris-Etherton P. n-3 Fatty acid dietary recommendations and food sources to achieve essentiality and cardiovascular benefits. Am J Clin Nutr. 2006;83: 1526–1535. [DOI] [PubMed] [Google Scholar]
- 4.Rallidis L, Paschos G, Liakos G, Velissaridou A, Anastasiadis G, Zampelas A. Dietary alpha-linolenic acid decreases C-reactive protein, serum amyloid A and interleukin-6 in dyslipidaemic patients. Atherosclerosis. 2003;167: 237–242. [DOI] [PubMed] [Google Scholar]
- 5.Lopez-Garcia E, Schulze M, Manson J, Meigs J, Albert C, Rifai N, et al. Consumption of (n-3) fatty acids is related to plasma biomarkers of inflammation and endothelial activation in women. J Nutr. 2004;134: 1806–1811. doi: 10.1093/jn/134.7.1806 [DOI] [PubMed] [Google Scholar]
- 6.Owren P, Hellem A, Odegaard A. Linolenic acid for the prevention of thrombosis and myocardial infarction. Lancet. 1964;2: 975–980. [DOI] [PubMed] [Google Scholar]
- 7.Sala-Vila A, Cofán M, Pérez-Heras A, Nunez I, Gilabert R, Junyent M, et al. Fatty acids in serum phospholipids and carotid intima-media thickness in Spanish subjects with primary dyslipidemia. Am J Clin Nutr. 2010;92: 186–193. doi: 10.3945/ajcn.2009.28807 [DOI] [PubMed] [Google Scholar]
- 8.Sala-Vila A, Cofán M, Núñez I, Gilabert R, Junyent M, Ros E. Carotid and femoral plaque burden is inversely associated with the alpha-linolenic acid proportion of serum phospholipids in Spanish subjects with primary dyslipidemia. Atherosclerosis. 2011;214: 209–214. doi: 10.1016/j.atherosclerosis.2010.10.026 [DOI] [PubMed] [Google Scholar]
- 9.de Lorgeril M, Salen P. Mediterranean diet and n-3 fatty acids in the prevention and treatment of cardiovascular disease. J Cardiovasc Med. 2007;8 Suppl 1: 38–41. [DOI] [PubMed] [Google Scholar]
- 10.Dolecek T. Epidemiological evidence of relationships between dietary polyunsaturated fatty acids and mortality in the multiple risk factor intervention trial. Proc Soc Exp Biol Med. 1992;200: 177–182. [DOI] [PubMed] [Google Scholar]
- 11.Mozaffarian D, Ascherio A, Hu F, Stampfer M, Willett W, Siscovick D, et al. Interplay between different polyunsaturated fatty acids and risk of coronary heart disease in men. Circulation. 2005;111: 157–164. doi: 10.1161/01.CIR.0000152099.87287.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koh A, Pan A, Wang R, Odegaard A, Pereira M, Yuan J, et al. The association between dietary omega-3 fatty acids and cardiovascular death: the Singapore Chinese Health Study. Eur J Prev Cardiol. 2015;22: 364–372. doi: 10.1177/2047487313517576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vedtofte M, Jakobsen M, Lauritzen L, O’Reilly E, Virtamo J, Knekt P, et al. Association between the intake of alpha-linolenic acid and the risk of CHD. Br J Nutr. 2014;112: 735–743. doi: 10.1017/S000711451400138X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Goede J, Verschuren W, Boer J, Kromhout D, Geleijnse J. Alpha-linolenic acid intake and 10-year incidence of coronary heart disease and stroke in 20,000 middle-aged men and women in the Netherlands. PLoS One. 2011;6: e17967 doi: 10.1371/journal.pone.0017967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fretts A, Mozaffarian D, Siscovick D, Sitlani C, Psaty B, Rimm E, et al. Plasma phospholipid and dietary α-linolenic acid, mortality, CHD and stroke: the Cardiovascular Health Study. Br J Nutr. 2014;112: 1206–2013. doi: 10.1017/S0007114514001925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhee J, Kim E, Buring J, Kurth T. Fish Consumption, Omega-3 Fatty Acids, and Risk of Cardiovascular Disease. Am J Prev Med. Elsevier; 2017;52: 10–19. doi: 10.1016/j.amepre.2016.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oomen C, Ocké M, Feskens E, Kok F, Kromhout D. Alpha-Linolenic acid intake is not beneficially associated with 10-y risk of coronary artery disease incidence: the Zutphen Elderly Study. Am J Clin Nutr. 2001;74: 457–463. doi: 10.1093/ajcn/74.4.457 [DOI] [PubMed] [Google Scholar]
- 18.Albert C, Oh K, Whang W, Manson J, Chae C, Stampfer M, et al. Dietary alpha-linolenic acid intake and risk of sudden cardiac death and coronary heart disease. Circulation. 2005;112: 3232–3238. doi: 10.1161/CIRCULATIONAHA.105.572008 [DOI] [PubMed] [Google Scholar]
- 19.Larsson S, Virtamo J, Wolk A. Dietary fats and dietary cholesterol and risk of stroke in women. Atherosclerosis. Elsevier Ireland Ltd; 2012;221: 282–286. doi: 10.1016/j.atherosclerosis.2011.12.043 [DOI] [PubMed] [Google Scholar]
- 20.He K, Rimm E, Merchant A, Rosner B, Stampfer M, Willett W, et al. Fish Consumption and Risk of Stroke in Men. JAMA. 2002;288: 3130–3136. [DOI] [PubMed] [Google Scholar]
- 21.Rajaram S. Health benefits of plant-derived alpha-linolenic acid. Am J Clin Nutr. 2014;100: 443–448. [DOI] [PubMed] [Google Scholar]
- 22.Hodson L, Skeaff C, Fielding B. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res. 2008;47: 348–380. doi: 10.1016/j.plipres.2008.03.003 [DOI] [PubMed] [Google Scholar]
- 23.Arab L, Akbar J. Biomarkers and the measurement of fatty acids. Public Health Nutr. 2002;5: 865–871. [DOI] [PubMed] [Google Scholar]
- 24.Tjønneland A, Olsen A, Boll K, Stripp C, Christensen J, Engholm G, et al. Study design, exposure variables, and socioeconomic determinants of participation in Diet, Cancer and Health: a population-based prospective cohort study of 57,053 men and women in Denmark. Scand J Public Health. 2007;35: 432–441. doi: 10.1080/14034940601047986 [DOI] [PubMed] [Google Scholar]
- 25.Beynen A, Katan M. Rapid sampling and long-term storage of subcutaneous adipose-tissue biopsies for determination of fatty acid composition. Am J Clin Nutr. 1985;42: 317–322. doi: 10.1093/ajcn/42.2.317 [DOI] [PubMed] [Google Scholar]
- 26.Joensen A, Overvad K, Dethlefsen C, Johnsen S, Tjønneland A, Rasmussen L, et al. Marine n-3 polyunsaturated fatty acids in adipose tissue and the risk of acute coronary syndrome. Circulation. 2011;124: 1232–1238. doi: 10.1161/CIRCULATIONAHA.110.987057 [DOI] [PubMed] [Google Scholar]
- 27.Andersen T, Madsen M, Jørgensen J, Mellemkjær L, Olsen J. The Danish National Hospital Register. A valuable source of data for modern health sciences. Dan Med Bull. 1999;46: 263–268. [PubMed] [Google Scholar]
- 28.Lühdorf P, Overvad K, Schmidt E, Johnsen S, Bach F. Predictive value of stroke discharge diagnoses in the Danish National Patient Register. Scand J Public Heal. 2017;45: 630–636. [DOI] [PubMed] [Google Scholar]
- 29.Adams H, Bendixen B, Kappelle L, Biller J, Love B, Gordon D, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24: 35–41. [DOI] [PubMed] [Google Scholar]
- 30.Overvad K, Tjønneland A, Haraldsdóttir J, Ewertz M, Jensen O. Development of a semiquantitative food frequency questionnaire to assess food, energy and nutrient intake in Denmark. Int J Epidemiol. 1991;20: 900–905. [DOI] [PubMed] [Google Scholar]
- 31.Tjønneland A, Overvad K, Haraldsdóttir J, Bang S, Ewerts M, Jensen O. Validations of a semiquantative food frequency questionnaire developed in Denmark. Int J Epidemiol. 1991;20: 906–912. [DOI] [PubMed] [Google Scholar]
- 32.Kalbfleisch J, Lawless J. Likelihood analysis of multi-state models for disease incidence and mortality. Stat Med. 1988;7: 149–160. [DOI] [PubMed] [Google Scholar]
- 33.Petersen L, Sørensen T, Andersen P. Comparison of case-cohort estimators based on data on premature death of adult adoptees. Stat Med. 2003;22: 3795–3803. doi: 10.1002/sim.1672 [DOI] [PubMed] [Google Scholar]
- 34.Harrell FE. Regression Modeling Strategies With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. Second Edi New York: Springer; 2015. [Google Scholar]
- 35.Yaemsiri S, Sen S, Tinker L, Robinson W, Evans R, Rosamond W, et al. Serum fatty acids and incidence of ischemic stroke among postmenopausal women. Stroke. 2013;44: 2710–2717. doi: 10.1161/STROKEAHA.111.000834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daneshmand R, Kurl S, Tuomainen T, Virtanen J. Associations of serum n-3 and n-6 PUFA and hair mercury with the risk of incident stroke in men: the Kuopio Ischaemic Heart Disease Risk Factor Study (KIHD). Br J Nutr. 2016;115: 1851–1859. doi: 10.1017/S0007114516000982 [DOI] [PubMed] [Google Scholar]
- 37.Yamagishi K, Folsom A, Steffen L. Plasma fatty acid composition and incident ischemic stroke in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Cerebrovasc Dis. 2013;36: 38–46. doi: 10.1159/000351205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiberg B, Sundström J, Árnlöv J, Terént A, Vessby B, Zethelius B, et al. Metabolic Risk Factors for Stroke and Transient Ischemic Attacks in Middle-Aged Men: a community-based study with long-term follow-up. Stroke. 2006;37: 2898–2903. doi: 10.1161/01.STR.0000249056.24657.8b [DOI] [PubMed] [Google Scholar]
- 39.De Goede J, Verschuren WMM, Boer JMA, Kromhout D, Geleijnse JM. N-6 and n-3 fatty acid cholesteryl esters in relation to incident stroke in a Dutch adult population: A nested case-control study. Nutr Metab Cardiovasc Dis; 2013;23: 737–743. doi: 10.1016/j.numecd.2012.03.001 [DOI] [PubMed] [Google Scholar]
- 40.Iso H, Sato S, Umemura U, Kudo M, Koike K, Kitamura A, et al. Linoleic acid, other fatty acids, and the risk of stroke. Stroke. 2002;33: 2086–2093. [DOI] [PubMed] [Google Scholar]
- 41.de Lorgeril M, Renaud S, Mamelle N, Salen P, Martin J, Monjaud I, et al. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet. 1994;343: 1454–1459. [DOI] [PubMed] [Google Scholar]
- 42.de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999;99: 779–785. [DOI] [PubMed] [Google Scholar]
- 43.Kromhout D, Giltay E, Geleijnse J, Alpha Omega Trial G. N-3 Fatty Acids and Cardiovascular Events After Myocardial Infarction. N Engl J Med. 2010;363: 2015–2026. doi: 10.1056/NEJMoa1003603 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
The content of adipose tissue content of ALA and the risk of stroke of other etiology (A) and stroke of undetermined etiology (B). The multivariate models are adjusted for ischemic stroke risk factors (model 1B) and presented with the median adipose tissue content of ALA as reference (solid vertical line). The 20th, 40th, 60th, and 80th percentiles of adipose tissue content of ALA are marked by dashed lines. Shaded grey areas show 95% confidence intervals of hazard ratios of ischemic stroke subtypes (curves). Only the 2.5th–97.5th percentiles of ALA are shown.
(PDF)
The content of ALA in adipose tissue was indexed according to the overall median intake of the selected food groups (grey solid line) within the sub-cohort (n = 3185). The dots represent percentages-wise differences relative to the overall median intake.
(PDF)
(PDF)
(PDF)
Data Availability Statement
Data are available from the Diet, Cancer and Health Institutional Data Access (https://www.cancer.dk/research/diet-genes-environment/dgedch/). To access data, an application must be approved by the Scientific Board. Furthermore, as data contain potentially identifying or sensitive information, access to data has to be registered and approved by The Danish Data Protection Agency (https://www.datatilsynet.dk/english/the-danish-data-protection-agency) and/or a Health Research Ethics Committee (http://www.nvk.dk/english).”