Abstract
Synopsis
Transitional toric intraocular lens (IOL) was developed to improve refractive outcomes in cataract surgery. We report refractive, vectorial outcomes, and stability of spherical equivalent over 12 months after implantation of this IOL.
Purpose
To evaluate visual and refractive outcomes of a transitional conic toric intraocular lens (IOL) (Precizon®) for the correction of corneal astigmatism in patients undergoing cataract surgery.
Setting
The Ocular Microsurgery Institute (IMO), a private practice in Barcelona, Spain.
Design
This is a retrospective, non-randomized study.
Methods
Retrospective chart review of 156 patients with preoperative regular corneal astigmatism >0.75 diopters (D) who underwent consecutive phacoemulsification and Precizon toric IOL implantation between January 2014 and December 2015 was performed. Two groups were divided according to attempted residual refraction: group 1 with emmetropia and group 2 with mild myopia for monovision. Uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA), and manifest refraction were analyzed preoperatively and 3, 6, and 12 months postoperatively.
Results
Precizon toric IOL was implanted in 97 eyes of 61 patients. Six months postoperatively, none of the eyes lost any line of CDVA. In all, 98% of the eyes were within ±1.00 D of attempted spherical correction. The mean preoperative keratometric cylinder was 1.92 ± 1.04 D (range 0.75–6.78), and the mean postoperative refractive cylinder was 0.77 ± 0.50 D (range 0–2.25), with 81% of the eyes with ≤1.00 D of residual cylinder. Two IOLs required realignment due to intra-operative positioning error. Eleven eyes required enhancement with corneal refractive surgery.
Conclusion
Preexisting regular corneal astigmatism was effectively and safely corrected by the implantation of the transitional conic toric IOL in patients undergoing cataract surgery.
Keywords: corneal astigmatism, refractive astigmatism, keratometry, cataract surgery, toric intraocular lens, biometry, phacoemulsification
Video abstract
Introduction
Modern cataract surgery is a refractive procedure that aims to reduce or eliminate refractive errors to improve visual function and to give patients as much independence of glasses as possible at the same time. Important factors precluding emmetropia include remaining corneal astigmatism and biometry prediction errors in astigmatic and ametropic eyes.1
Preoperative corneal astigmatism is observed in 87% of patients, with 36% presenting astigmatism >1.25 diopters (D).2,3 Leaving astigmatism uncorrected may cause a significant decrease in visual function, especially in low-contrast settings.4 Although accurate correction of astigmatism <0.3 D does not seem to improve visual acuity (VA) in most cases, refractive and cataract surgery procedures should aim to leave uncorrected small amounts of natural astigmatism, typically <0.5 D, to obtain optimal visual outcomes.5
Previous reports on toric intraocular lens (IOL) implantation in patients with corneal astigmatism undergoing cataract surgery have shown excellent visual and refractive outcomes. Compared to non-toric IOLs associated with limbal relaxing incisions, toric IOLs provide better uncorrected distance visual acuity (UDVA), greater spectacle independence, and lower amounts of residual astigmatism.6,7 Because misalignment of a toric IOL results in an effective loss of the cylinder power (3.3% of effective loss of cylinder power per degree), accurate intraoperative alignment, IOL rotational stability, and tolerance to misalignment are key to achieve the best potential outcomes.6,7 The Precizon toric IOL (Ophtec BV, Groningen, the Netherlands) has a transitional conic surface that has been shown to provide superior image quality despite pupil size changes and the presence of decentration, as well as maximum rotation tolerance compared with the other IOLs.8 These characteristics may result in excellent visual outcomes, predictability of refractive results, rotational stability, and good optical performance.6,7 The aim of this study was to evaluate the visual and refractive outcomes and the rotational stability of the Precizon toric IOL in a series of 97 eyes, which, to our knowledge, is the largest series to date.
Patients and methods
We performed a retrospective chart review of 156 patients who underwent consecutive phacoemulsification and Precizon toric IOL implantation between January 2014 and December 2016. All patients were operated on by the same surgeon (JLG) at the Instituto de Microcirugia Ocular (IMO, Barcelona, Spain).
All patients were fully informed about the details and the potential risks of the procedure. Written informed consent for the surgical procedure was obtained. Additionally, permission for the use of patients’ data for research, analysis, and publication purposes was also obtained. The study was conducted in accordance with the institution’s Good Clinical Practices and the Declaration of Helsinki. Institutional review board (IRB) approval was obtained from Ophthalmologic Microsurgery Institute (IMO) Ethical Committee.
The inclusion criteria for outcome analysis were as follows: 1) preoperative corneal astigmatism >0.75; 2) uncomplicated cataract surgery; 3) phacoemulsification and Precizon toric IOL implantation; and 4) minimum follow-up of 3 months. Both eyes of the same subject were included when applicable. Micro-monovision was performed with a target of −0.50 and −1.25 D in the non-dominant eye to improve spectacle independency according to each patient’s referred needs. The neutral asphericity of this implant helps to maintain the corneal natural positive spherical aberration, which combines natural depth of focus with monovision.9
Exclusion criteria were as follows: irregular astigmatism or abnormal corneal topography, previous corneal or intraocular surgery, low VA caused by preexisting ocular pathology that impeded manifest refraction, and complicated cataract surgery.
Preoperative examination and follow-up
Preoperative assessment included manifest refraction, corrected distance visual acuity (CDVA), and UDVA using a standardized Snellen chart and light-box system at 20 feet; slit-lamp examination; eye dominance checked by our resident optometrists with “hole-in-the-card” technique; Goldmann applanation tonometry; biometry using the IOLMaster 500 (Carl Zeiss Meditec AG, Jena, Germany); corneal topography using Orbscan II (Bausch & Lomb Incorporated, Bridgewater, NJ, USA); and posterior segment evaluation.
Follow-up postoperative visits were held 1 day, and 1, 3, 6, and 12 months after surgery. Manifest refraction, UDVA, and CDVA were repeated at each follow-up visit. Additionally, IOL orientation was checked at the slit-lamp examination with dilated pupil.
Surgical technique
Toric IOL calculation
Precizon toric IOL presents a cylinder correction ranging from 1.0 to 10.0 D in 0.5 steps, which correlates to astigmatism correction on the corneal plane from 0.68 to 6.85 D in an average eye. The spherical and cylindrical correction of the IOL was calculated using the data obtained using the IOLMaster 500 with the Haigis biometry formula of optimized constants and the Ophtec toric IOL calculation software (http://calculator.ophtec.com). There was an overall coincidence of biometry and topography readings, for cases in which there was a divergence between keratometric readings and axis; values for calculation were picked from the IOLMaster 500. The dominant eye was targeted to emmetropia, and the non-dominant eye was targeted between −0.50 and −1.25 D, depending on the case.
Surgical technique and IOL orientation
Preoperative marking was performed using the RoboMarker (Surgilūm, Wilmington, NC, USA) with the patient in a seated position. The vast majority of patients received topical anesthesia. If retrobulbar anesthesia was required, the horizontal axis was marked before the retrobulbar injection.
Desired IOL orientation was marked using a Wallace Mendez Degree Gauge (Storz; Bausch & Lomb Incorporated) and a surgical marking pen. Phacoemulsification was performed using the Centurion® Vision System (Alcon Laboratories, Inc., Fort Worth, TX, USA) using either a divide and conquer approach or the phaco rolling technique described by one of the authors in 2004.10 A bimanual irrigation–aspiration technique was used. ArtiVisc (Ophtec BV) and OcuCoat (Bausch & Lomb Incorporated) were used as ophthalmic viscosurgical devices (OVDs).
Intraoperative IOL alignment was performed before OVD aspiration and was rechecked at the end of surgery.
Postoperative treatment consisted of topical tobramycin 0.3% and dexamethasone 0.1% (Tobradex; Alcon Cusi, El Masnou, Barcelona, Spain) four times daily, timolol 0.5% (Cusimolol; Alcon Cusi) two times daily, and dexamethasone 0.05% and chloramphenicol 1% ointment (Deicol; Alcon Cusi) at bedtime for 3 weeks and then was stopped in absence of any inflammatory signs or signs of rejection.
Outcome analysis
Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) was used for data collection and to perform vector analysis. Standard outcomes analysis was performed in accordance with the Standard Graphs for Reporting IOL Based Refractive Surgery.11 Outcome measures were UDVA, CDVA, manifest refraction, IOL rotation, and complications. VA measurements were converted from decimal to LogMar in order to facilitate statistical analysis and to Snellen to build the graphics. All data were analyzed preoperatively and 1, 3, 6, and 12 months postoperatively.
Continuous variables were described by mean, standard deviation (SD), and range. Accuracy of refractive correction (percentage of eyes within ±1 and ±0.5 D of attempted spherical equivalent [SEQ] and cylinder correction) was calculated. Safety was assessed by loss of CDVA. Intraoperative and postoperative complications were registered.
The results were analyzed using Prism software version 6.0 (GraphPad Software, Inc., La Jolla, CA, USA). Normality of data was assessed with the Shapiro–Wilk test. Comparison between preoperative and postoperative data was performed using the paired t-test or the Wilcoxon signed-rank test, depending on normality. p-values <0.05 were considered as statistically significant.
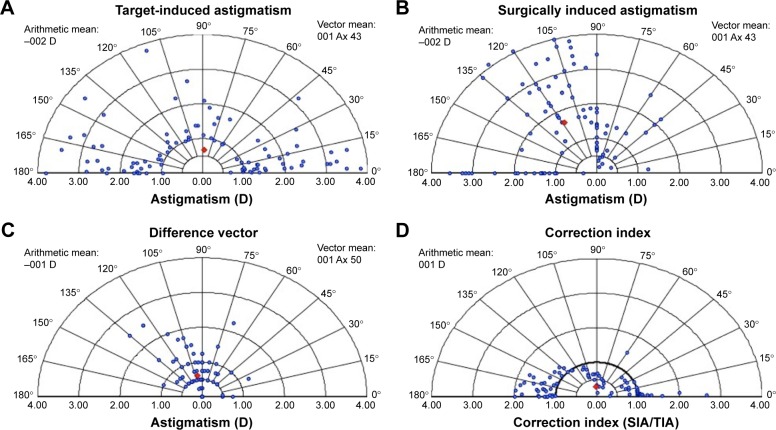
Vector analysis is displayed in double-angle plot graphics for intended cylindrical correction, error vector (EV), normalized EV, and treatment EV (Figure 1).
Figure 1.
Cylinder vector analysis.
Notes: (A) TIA vector. (B) EV. (C) NEV. (D) TEV.
Abbreviations: TIA, target-induced astigmatism; EV, error vector; NEV, normalized error vector; TEV, treatment error vector; D, diopters; SIA, surgically induced astigmatism.
Preoperative keratometric astigmatism and postoperative refractive astigmatism were analyzed 3 months post-operatively by vector analysis using the Alpins method.12,13 The Alpins method assesses changes in both magnitude and axis of astigmatism taking into account three vectors: target-induced astigmatism (TIA) vector, which refers to desired change in astigmatic magnitude and axis that the surgery was intended to induce; surgically induced astigmatism (SIA) vector, which is the actual amount and axis of astigmatic change that the surgery induced; and the EV, which is the astigmatic change by magnitude and axis that would enable the initial surgery to achieve its intended target.13,14 It is an absolute measure of success and is preferably 0.
Relationships between these three fundamental vectors were also calculated. The correction index (CI) is the ratio of SIA to TIA and is preferably 1.0. The magnitude of error (ME) is the arithmetic difference between the magnitudes of SIA and TIA. The angle of error (AE) represents the angle between the vectors of SIA and TIA. If the achieved correction is counterclockwise away from the intended axis, the AE value will be positive; if the achieved correction is deviated clockwise, this value will be negative. The index of success (IS) is calculated by dividing the EV by the TIA. It represents a relative measure of success and is preferably 0.14,15
Results
Baseline characteristics
Of a total of 156 charts reviewed, 97 eyes of 61 patients complied with the inclusion criteria. The reasons for exclusion of the analysis were abnormal corneal topography (n = 47), Fuchs’ dystrophy with clinical corneal edema (n = 2), retinal diseases (n = 1), previous corneal transplantation (n = 2), previous refractive surgery (n = 4), previous retinal surgery (n = 1), and previous iris-claw intraocular phakic lens implantation (n = 2). Emmetropia with toric transitional lens implantation after cataract surgery was planned for 29 eyes of 29 patients and in both eyes of two patients. Sixty-four eyes of 32 patients underwent toric transitional lens implantation with myopic target in one eye and emmetropia in the fellow eye. Mean follow-up time was 11.61 ± 7.56 months (range 3–28 months).
Table 1 summarizes baseline characteristics for all patients. For evaluation purposes, visual and SEQ outcomes were analyzed separately for eyes targeted to emmetropia (group 1) and mild myopia (group 2).
Table 1.
Demographic data
| Demographic data | |
|---|---|
| Age (years) | 59.16 ± 12.35 (42–87) |
| Follow-up (months) | 11.61 ± 7.65 (2.5–28) |
| Axial length (mm) | 23.29 ± 1.53 (20.23–27.60) |
| Anterior chamber depth (mm) | 3.12 ± 0.49 (2.06–4.10) |
| UDVA (LogMar) | 0.32 ± 0.30 (0–1) |
| CDVA (LogMar) | 0.14 ± 0.18 (0–0.7) |
| SE (D) | −0.01 ± 3.24 (−8.75 to 6.38) |
| Cylinder (D) | −1.92 ± 1.04 (−6.79 to −0.68) |
Note: Results are displayed as mean ± SD (range).
Abbreviations: UDVA, uncorrected distance visual acuity; CDVA, corrected distance visual acuity; SE, spherical equivalent; D, diopters; SD, standard deviation.
Visual outcomes, efficacy, and safety
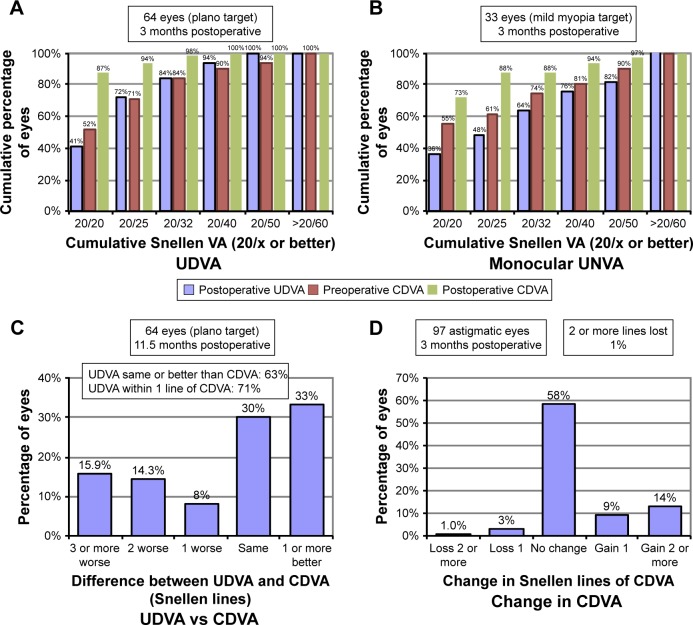
A significant improvement in LogMar UDVA and LogMar CDVA was observed in both groups. In all, 64% of the patients achieved an uncorrected near visual acuity (UNVA) of 20/40 or better. Figure 2A shows 3-month postoperative cumulative UDVA and CDVA for eyes targeted to emmetropia (group 1). Figure 2B shows 3-month postoperative UNVA and CDVA for eyes targeted to mild myopia (group 2). Figure 2C portraits the efficacy of UDVA correction compared to preoperative CDVA for eyes targeted to emmetropia. Figure 2D shows percentage of lines of CDVA gained/lost 3 months postoperatively in all patients. Percentage of eyes that gained one or more lines was 23% (25 eyes in group 1 and 11 eyes in group 2). Four percent of eyes lost one or more lines (four eyes in group 1 and zero eyes in group 2). Postoperatively, UDVA and CDVA remained stable throughout the follow-up period.
Figure 2.
Three months postoperative cumulative UNVA/UDVA and CDVA.
Notes: (A) Preoperative CDVA and postoperative UDVA and CDVA for group 1 (emmetropia). (B) Preoperative CDVA and postoperative UNVA and CDVA for group 2 (mild myopia for monovision). (C) Difference between UDVA and CDVA for group 1 (emmetropia). (D) Change in CDVA for all 97 eyes.
Abbreviations: UNVA, uncorrected near visual acuity; UDVA, uncorrected distance visual acuity; CDVA, corrected distance visual acuity.
Manifest refraction and accuracy of refractive correction
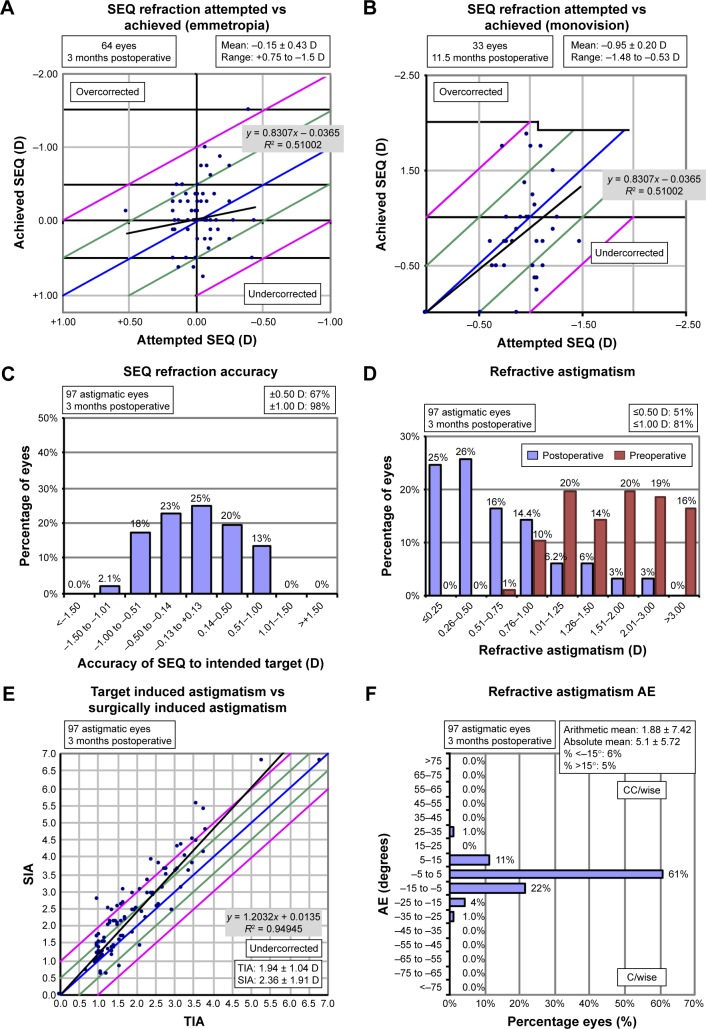
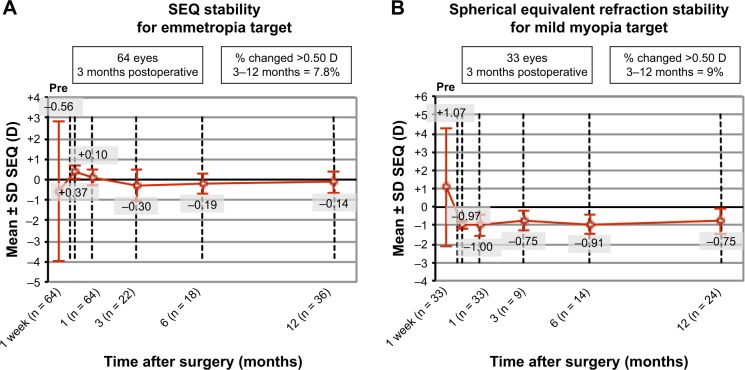
Mean SEQ in groups 1 and 2 was reduced from −0.56 ± 3.42 to −0.15 ± 0.42 (p = 0.35) and from 1.07 ± 3.2 to −0.97 ± 0.48 (p = 0.0032), respectively. Mean refractive cylinder was significantly reduced compared to previous keratometric cylinder in both groups, from 1.98 ± 1.1 to 0.66 ± 0.54 (p < 0.0001) and from 1.84 ± 0.88 to 0.79 ± 0.63 (p < 0.0001), respectively (Tables 2 and 3). Figure 3 shows refractive outcomes and accuracy graphs. Ninety-eight percent of the patients presented residual standard error (SE) within ±1 D of the attempted SE and 81% presented residual astigmatism ≤1 D. Figure 4A and B shows the evolution of SE during the follow-up period for groups 1 and 2, respectively. Postoperatively, SE and cylinder remained stable throughout the follow-up in both the groups.
Table 2.
Pre- versus 3-month postoperative data for group 1
| Preoperative | 3 months post-surgery | p-value | |
|---|---|---|---|
| Emmetropia (group 1) | |||
| UDVA (LogMar) | 0.41 ± 0.48 | 0.12 ± 0.12 | 0.88 |
| CDVA (LogMar) | 0.13 ± 0.17 | 0.026 ± 0.06 | <0.0001 |
| Cylinder (D) | −1.98 ± 1.11 (−6.79 to −0.93) | −0.66 ± 0.54 (−2.25 to 0) | <0.0001 |
| SE (D) | −0.57 ± 3.43 (−8.75 to +6.38) | −0.15 ± 0.43 (−1.5 to +0.75) | 0.35 |
Note: Results are displayed as mean ± SD (range).
Abbreviations: UDVA, uncorrected distance visual acuity; CDVA, corrected distance visual acuity; D, diopters; SE, standard error; SD, standard deviation.
Table 3.
Pre- versus 3-month postoperative data for group 2
| Preoperative | 3 months post-surgery | p-value | |
|---|---|---|---|
| Mild myopia (group 2) | |||
| UNVA (LogMar) | – | 0.16 | – |
| UDVA (LogMar) | 0.13 ± 0.19 (0–0.7) | 0.34 ± 0.25 (0–1) | 0.0003 |
| CDVA (LogMar) | 0.17 ± 0.20 (0–0.7) | 0.07 ± 0.15 (0–0.7) | 0.02 |
| Cylinder (D) | −1.84 ± 0.88 (−3.79 to −0.68) | −0.79 ± 0.63 (−1.88 to 0) | <0.0001 |
| SE (D) | 1.07 ± 3.20 (−6.5 to +6.38) | −0.87 ± 0.48 (−2.5 to 0) | 0.0032 |
Note: Results are displayed as mean ± SD (range).
Abbreviations: UNVA, uncorrected near visual acuity; UDVA, uncorrected distance visual acuity; CDVA, corrected distance visual acuity; D, diopters; SE, standard error; SD, standard deviation.
Figure 3.
Accuracy of refractive correction.
Notes: (A) Attempted versus achieved SEQ: group 1. (B) Attempted versus achieved SEQ refraction: group 2. (C) SEQ accuracy (all patients). (D) Pre- versus postoperative astigmatism. (E) TIA versus SIA. (F) Refractive astigmatism AE. The blue lines show attempted correction = achieved correction; the green lines show +0.5 and −0.5 from attempted correction; the pink lines show +1 and −1 from attempted correction; the black lines correspond to the linear regression analysis; the linear regression equations are on the gray boxes in the graphs.
Abbreviations: SEQ, spherical equivalent; TIA, target-induced astigmatism; SIA, surgically induced astigmatism; AE, angle of error; D, diopters.
Figure 4.
Refractive stability of SEQ.
Notes: (A) Refractive stability of SEQ for emmetropia target (group 1). (B) Refractive stability of SEQ for mild myopia target (group 2).
Abbreviations: SEQ, spherical equivalent; SD, standard deviation; D, diopters.
Vector analysis
Vector analysis was performed at the 6-week follow-up visit with results from both groups together. Results of the vector analysis with the Alpins method are shown in Table 3 and Figure 1. The SIA was significantly different from the TIA (p < 0.05) with a trend to overcorrection. The EV was also significantly different from zero at 1.5–3 months (−0.72 D at 58°; p < 0.05). Mean values for ME, absolute and arithmetic AE, EI, CI, and IS are displayed in Table 4.
Table 4.
Cylinder vector analysis
| Average | |
|---|---|
| TIA vector | 1.93 ± 1.04 |
| SIA vector | 2.06 ± 1.21 |
| Error of magnitude | 0.13 ± 0.64 |
| Arithmetic error of axis | 5.18 ± 14.00 |
| Absolute error of axis | 9.36 ± 11.60 |
| Axis shift | −30.71 ± 27.67 |
| Error ratio | 0.37 ± 0.18 |
| Correction ratio | 1.07 ± 0.31 |
| IS | 0.37 ± 0.36 |
Note: Results are displayed as mean ± SD.
Abbreviations: TIA, target-induced astigmatism; SIA, surgically induced astigmatism; IS, index of success.
Safety: complications
On day after surgery, two eyes of two patients underwent surgical repositioning of the IOL because they were more than 30° away from the planned axis; five patients presented the IOL 3°–6° away from the planned axis but did not need further intervention because the refractive result was satisfactory; all other patients presented the IOL 3° within the intended axis. Only two patients presented divergent axis between topography with Orbscan and IOLMaster 500 keratometry; in these cases, the keratometric values from the IOLMaster were preferred over the Orbscan. One patient presented zonular damage and needed implantation of a capsular tension ring to stabilize the capsular bag, and the lens remained on the planned axis during all the follow-up time. No other intraoperative complications were observed in either group.
Eleven patients in group 1 underwent further refractive surgery enhancement due to residual cylinder and ametropia (nine LASIK and two photorefractive keratotomy). Three patients in group 2 were subjected to refractive surgery enhancement in one eye to improve uncorrected near VA. None of the IOLs had to be explanted. Three patients presented one or 2 lines decrease in CDVA. The causes for loss of lines in CDVA were as follows: (1 and 2) 61-year-old patient who experienced cystoid macular edema in both eyes after surgery and regained previous CDVA after clinical treatment; (3) 76-year-old patient with stable Fuchs’ dystrophy who presented with a one-line decrease in CDVA in both eyes due to subclinical corneal edema that resolved within 4 months, regaining 20/20 vision after 6 months; and (4) 48-year-old patient with microcornea, a very shallow anterior chamber and high hyperopia with 20.56 mm axial length. She experienced postoperative anterior displacement of the IOL with a change in the effective lens position, which was successfully treated with peripheral iridotomy and LASIK to correct residual ametropia and achieved UDVA 20/25.
Discussion
Precizon toric transitional IOLs have been successfully implanted in eyes with astigmatism during cataract surgery to correct a wide range of refractive errors with satisfactory visual and refractive results. Visual outcomes were excellent and in agreement with previous publications, with 72% of patients in group 1 achieving 20/25 UDVA or better and 84% of patients achieving 20/32 UDVA after surgery.6 Patients in group 2 also benefited from the new IOL implantation, with the majority of patients being able to read and perform routine activities free of glasses for either near or far distance. The procedure can also be regarded as safe; only 4% of all eyes presented loss of two lines or less of VA; these cases had transient decrease of VA, which was resolved with clinical treatment. In all, 11% of the eyes were submitted to further refractive enhancement due to dissatisfaction with the residual cylinder (n = 9) and to improve near vision (n = 2). All of them achieved the desired outcome after the procedure.
Refractive outcomes were considered satisfactory with 67% of all eyes achieving an SE within 0.5 D of the target refraction and 98% of the eyes within 1.0 D. Our findings are comparable to previous reported results;6 we achieved 51% of the eyes with a residual cylinder of #0.5 D and 81% of the eyes with ≤1.0 D.
Even though our clinical and refractive results might be considered satisfactory, the vector analysis showed a mean EV of −0.72 at 59° with an IS of 0.37, which is relatively far from 0 and an undesirable result. However, this is in line with previous toric IOL vector results, which ranged from 0.12 to 0.42.16–20 There was an overall tendency to overcorrection, with a mean error of magnitude of −0.13 ± 0.64 and a mean arithmetic error of angle of 5.18 ± 14.00, which is also comparable to previous reports (range from 0.63 to 9.16).16–20
We considered a refractive surprise >0.75 D of residual refractive cylinder or <50% of refractive astigmatic correction; 41% of our patients met these criteria. Interestingly, these cases presented particular features in common as follows: 51% high or very low astigmatism (<1.5 or >2.5 D), 44% very long or short eyes (<22 or >24.5 mm), 51% very shallow or deep anterior chamber depth (ACD; <3 or >3.5 mm), 15% oblique astigmatism, 2% against-the-rule astigmatism, and 2% dry eye. Of note, the manufacturer IOL calculator does not take into consideration the ACD; it presumes the same effective lens position to all patients. According to previous publications, effective lens position can affect the effective cylindrical power of the IOL in both deep and shallow eyes.21 We have already contacted the developers of the software about this issue; meanwhile, we are conducting a study comparing algorithms for the calculation that include the posterior cornea and total corneal power. Our results differ from previous published data on Precizon toric transitional IOL. This may be due to the fact that our practice is a cornea and refractive surgery reference center, and many of our patients have high astigmatism or are hypermetropic and come to us to have early cataract surgery. The other authors did not mention the influence of ACD, axial length, or orientation of astigmatism in their outcomes.19,22
One of the drawbacks of our study is that we could not take into account the effect of posterior corneal astigmatism in IOL calculations, which might have led to both axis and power minor miscalculations. This might be the reason why we found a tendency for hypercorrection in patients who presented with-the-rule astigmatism and could also be a source of bias when interpreting the rotation tolerance of the lens that the design of our study could not entirely clarify. The disagreement between the vector analysis and clinical/refractive results could be explained because of the conical design of the cylindrical correction, which mimics the anatomic curvature of the eye; hence, it might be able to overcome slight axis misalignment due to either misposition or miscalculation of the cylindrical correction of the lens.8
Conclusion
We showed that Precizon toric transitional IOL is a suitable and safe alternative for astigmatic correction during cataract surgery, with adequate refractive and visual outcomes being our toric IOL of choice in our daily practice. However, we believe that results with this lens can be improved with the incorporation of the effective lens position into its calculator and using the total corneal astigmatism for toric IOL calculation.
Acknowledgments
This study was presented in part as a poster at the XXXIV Congress of the European Society of Cataract and Refractive Surgeons (ESCRS), Copenhagen, Denmark, September 10–14, 2016.
Footnotes
Disclosure
Dr Güell is a consultant for Ophtec and Carl Zeiss Meditec AG and reports no other conflicts of interest in this work. The rest of the authors have no financial or proprietary interest in any material or method mentioned, and report no conflicts of interest in this work.
References
- 1.Behndig A, Montan P, Stenevi U, Kugelberg M, Zetterström C, Lundström M. Aiming for emmetropia after cataract surgery: Swedish National Cataract Register study. J Cataract Refract Surg. 2012;38(7):1181–1186. doi: 10.1016/j.jcrs.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 2.Ferrer-Blasco T, Montés-Micó R, Peixoto-de-Matos SC, González-Méijome JM, Cerviño A. Prevalence of corneal astigmatism before cataract surgery. J Cataract Refract Surg. 2009;35(1):70–75. doi: 10.1016/j.jcrs.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 3.Hoffmann PC, Abraham M, Hirnschall N, Findl O. Prediction of residual astigmatism after cataract surgery using swept source Fourier domain optical coherence tomography. Curr Eye Res. 2014;39(12):1178–1186. doi: 10.3109/02713683.2014.898376. [DOI] [PubMed] [Google Scholar]
- 4.Wolffsohn JS, Bhogal G, Shah S. Effect of uncorrected astigmatism on vision. J Cataract Refract Surg. 2011;37(3):454–460. doi: 10.1016/j.jcrs.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 5.Villegas EA, Alcon E, Artal P. Minimum amount of astigmatism that should be corrected. J Cataract Refract Surg. 2014;40(1):13–19. doi: 10.1016/j.jcrs.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Kessel L, Andresen J, Tendal B, Erngaard D, Flesner P, Hjortdal J. Toric intraocular lenses in the correction of astigmatism during cataract surgery: a systematic review and meta-analysis. Ophthalmology. 2016;123(2):275–286. doi: 10.1016/j.ophtha.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Visser N, Bauer NJ, Nuijts RM. Toric intraocular lenses: historical overview, patient selection, IOL calculation, surgical techniques, clinical outcomes, and complications. J Cataract Refract Surg. 2013;39(4):624–637. doi: 10.1016/j.jcrs.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 8.Kim MJ, Yoo YS, Joo CK, Yoon G. Evaluation of optical performance of 4 aspheric toric intraocular lenses using an optical bench system: Influence of pupil size, decentration, and rotation. J Cataract Refract Surg. 2015;41(10):2274–2282. doi: 10.1016/j.jcrs.2015.10.059. [DOI] [PubMed] [Google Scholar]
- 9.Zheleznyak L, Sabesan R, Oh JS, MacRae S, Yoon G. Modified monovision with spherical aberration to improve presbyopic through-focus visual performance. Invest Ophthalmol Vis Sci. 2013;54(5):3157–3165. doi: 10.1167/iovs.12-11050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Güell JL, Vázquez M, Lucena J, Velasco F, Manero F. Phaco rolling technique. J Cataract Refract Surg. 2004;30(10):2043–2045. doi: 10.1016/j.jcrs.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Reinstein DZ, Archer TJ, Srinivasan S, et al. Standard for reporting refractive outcomes of intraocular lens-based refractive surgery. J Refract Surg. 2017;33(4):218–222. doi: 10.3928/1081597X-20170302-01. [DOI] [PubMed] [Google Scholar]
- 12.Alpins N, Stamatelatos G. Vector analysis applications to photorefractive surgery. Int Ophthalmol Clin. 2003;43(3):1–27. doi: 10.1097/00004397-200343030-00003. [DOI] [PubMed] [Google Scholar]
- 13.Alpins NA. Vector analysis of astigmatism changes by flattening, steepening, and torque. J Cataract Refract Surg. 1997;23(10):1503–1514. doi: 10.1016/s0886-3350(97)80021-1. [DOI] [PubMed] [Google Scholar]
- 14.Goggin M. Vector analysis terminology. J Cataract Refract Surg. 2013;39(10):1626–1627. doi: 10.1016/j.jcrs.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Reinstein DZ, Archer TJ, Srinivasan S, et al. Standard for reporting refractive outcomes of intraocular lens-based refractive surgery. J Cataract Refract Surg. 2017;43(4):435–439. doi: 10.1016/j.jcrs.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Alió JL, Piñero DP, Tomás J, Plaza AB. Vector analysis of astigmatic changes after cataract surgery with implantation of a new toric multifocal intraocular lens. J Cataract Refract Surg. 2011;37(7):1217–1229. doi: 10.1016/j.jcrs.2010.12.064. [DOI] [PubMed] [Google Scholar]
- 17.Emesz M, Dexl AK, Krall EM, et al. Randomized controlled clinical trial to evaluate different intraocular lenses for the surgical compensation of low to moderate-to-high regular corneal astigmatism during cataract surgery. J Cataract Refract Surg. 2015;41(12):2683–2694. doi: 10.1016/j.jcrs.2015.07.036. [DOI] [PubMed] [Google Scholar]
- 18.Kawahara A, Takayanagi Y. Vector analysis investigation of toric intraocular lens with no deviation from the intended axis. Clin Ophthalmol. 2016;10:2199–2203. doi: 10.2147/OPTH.S119755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vale C, Menezes C, Firmino-Machado J, et al. Astigmatism management in cataract surgery with Precizon((R)) toric intraocular lens: a prospective study. Clin Ophthalmol. 2016;10:151–159. doi: 10.2147/OPTH.S91298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang JS, Zhao JY, Sun Q, Ma LW. Distance vision after bilateral implantation of AcrySof toric intraocular lenses: a randomized, controlled, prospective trial. Int J Ophthalmol. 2011;4(2):175–178. doi: 10.3980/j.issn.2222-3959.2011.02.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eom Y, Kang SY, Song JS, Kim YY, Kim HM. Effect of effective lens position on cylinder power of toric intraocular lenses. Can J Ophthalmol. 2015;50(1):26–32. doi: 10.1016/j.jcjo.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Ferreira TB, Berendschot TT, Ribeiro FJ. Clinical outcomes after cataract surgery with a new transitional toric intraocular lens. J Refract Surg. 2016;32(7):452–459. doi: 10.3928/1081597X-20160428-07. [DOI] [PubMed] [Google Scholar]