Abstract
Objective
Low-level high-sensitivity cardiac troponin T (hs-cTnT) increases in elderly population. In this study, the relationship between hs-cTnT level and all-cause death of elderly inpatients with non-acute coronary syndrome (non-ACS) after discharge from the hospital was investigated.
Materials and methods
Non-ACS patients aged >65 years admitted in the General Practice Wards and Department of Geriatrics of Fuxing Hospital Affiliated to Capital Medical University were enrolled in the study. The patients were grouped according to the tertiles of hs-cTnT levels. Biochemical markers, hs-cTnT, and amino-terminal pro-brain natriuretic peptide were measured. The median follow-up period was 47 months, and all-cause deaths of the patients were observed.
Results
A total of 722 patients, including 473 males and 249 females, aged 65–98 (82.43±5.98) years were enrolled in the study. The level of hs-cTnT was found to be higher in males, and increased with age and comorbidities (P<0.01). Compared with low-level group, NT-proBNP level of patients in high-level group was higher, while hemoglobin (Hb), estimated glomerular filtration rate (eGFR), and left ventricular ejection fraction (LVEF) levels were lower (P<0.001). The mortality rate increased significantly with increased hs-cTnT levels (P<0.001). The total number of deaths was 136 (18.8%), and of these, 108 (79.4%) were noncardiac deaths. Risk of all-cause deaths in the highest hs-cTnT level group was 7.3 times higher than that of the lowest hs-cTnT level group (95% CI: 4.29–12.51, P<0.001). After adjusting for gender, age, comorbidities, NT-proBNP, Hb, eGFR, and LVEF, hs-cTnT level still affected the patient’s survival time (HR: 3.01, 95% CI: 1.67–5.43, P<0.001).
Conclusion
These findings suggest that low-level hs-cTnT was increased in elderly inpatients without ACS. They further highlight that baseline hs-cTnT level was associated with increased risk of all-cause deaths among patients after their discharge, and most deaths were from non-cardiovascular diseases.
Keywords: high-sensitivity cardiac troponin T, elderly inpatients, non-acute coronary syndrome, prognosis
Introduction
Cardiac troponin T (cTnT) is the preferred indicator for diagnosing myocardial infarction due to its super-high specificity and high sensitivity.1 High-sensitivity cTnT (hs-cTnT)2 assay can detect cTnT levels 10 times lower than those detected by traditional methods. This plays an important role in the early diagnosis and prognosis of acute coronary syndrome (ACS). Due to its high sensitivity, hs-cTnT can be detected in apparently healthy people and patients without myocardial infarction. Some studies have also found increased hs-cTnT levels in the elderly population.3 Higher proportions of hs-cTnT levels were found to be greater than the cutoff point in elderly population. A study conducted by Chew et al4 demonstrated increased hs-cTnT levels in non-coronary events, and this was similar to the correlation of long-term mortality with unstable plaque-induced spontaneous myocardial infarction. Gore et al3 showed that >10% of men, aged 65–74 years, with no cardiovascular disease had cTnT values above the current myocardial infarction threshold. Elderly inpatients usually have more comorbidities and functional disorders; however, it is unclear whether the increased values of hs-cTnT are correlated with increased risk of death in elderly inpatients after discharge from hospital. Hence, a follow-up cohort study was performed for elderly inpatients without ACS to investigate the relation-ship between baseline hs-cTnT level and death risk after discharge from hospital.
Materials and methods
Study subjects
A total of 722 patients admitted in the General Practice Wards and Department of Geriatrics of Fuxing Hospital Affiliated to Capital Medical University were enrolled in June 2010. Inclusion criteria were as follows: patients should be aged ≥65 years and willing to sign informed consent. Patients with ACS, acute stroke, acute or chronic cardiac failure, malignancies, bedridden status, poor general condition, disturbance of consciousness, and who received hemodialysis were excluded. This study was approved by the Ethics Committee of Fuxing Hospital Affiliated to Capital Medical University.
Methods
General data of the patients were collected, including age, gender, height, weight, body mass index (weight/height squared), and heart rate, as well as comorbidities such as stable coronary artery disease (CAD), hypertension, diabetes mellitus (DM), cerebral infarction, COPD, chronic kidney disease (CKD), and atrial fibrillation (AF).
Biochemistry
The patients were asked to fast for more than 12 h, following which their venous blood samples were collected. hs-cTnT was measured using a kit from Roche Diagnostics. hs-cTnT level >14 ng/L was considered as the upper limit of the 99th percentile in diagnosing acute myocardial infarction. The patients were grouped based on the tertiles of hs-cTnT level. Serum creatinine, fasting blood glucose (FBG), and amino-terminal pro-brain natriuretic peptide (NT-proBNP) were determined. Estimated glomerular filtration rate (eGFR) was calculated based on China’s simplified Modification of Diet in Renal Disease formula, which was as follows: eGFR=175× (serum creatinine/88.4)−1.234× (age)−0.169(female×0.79);5 eGFR <60 mL/min·1.73 m2 was considered as decreased renal function.
Ultrasonic cardiogram
Ultrasonic cardiogram was performed using Philips IE33 with the probe of S5-1 and a spectrum of 3.0–3.5 MHz. The patients were placed on the left lateral position and had to have stable breathing. Standardized two-dimensional echocardiography was performed based on the recommendations from the American Society of Echocardiography. Quality control was performed to evaluate the accuracy, sensitivity, and stability of the instrument before the examination. Interventricular septal depth, left ventricular post-wall depth, and left ventricular end-diastolic diameter were measured in the long-axis view of the parasternal left ventricle. The left ventricular ejection fraction (LVEF) was measured using improved single-plane Simpson’s method under standard apex four-chamber and two-chamber views. Height, weight, and body surface area of the patients were calculated. The left ventricular mass was calculated using body surface area index, and left ventricular mass index (LVMI) (g/m2) was calculated using The Devereux Method.6
Accompanying diseases
Patients were considered to have CAD if they had a prior history of chronic stable angina, unstable angina, or acute myocardial infarction and had undergone previous surgical or percutaneous coronary revascularization. Hypertension was defined as a mean systolic blood pressure of 140 mmHg, and/or a mean diastolic blood pressure of 90 mmHg, and/or use of antihypertensive medication.7 DM was defined as A1c of 6.5%, FBG of 7.0 mmol/L, and 2-h plasma glucose of 11.1 mmol/L. Oral glucose tolerance test was used for patients with classic symptoms of hyperglycemia or hyperglycemic crisis, a random plasma glucose of 11.1 mmol/L, or receiving antihyperglycemic medication.8 CKD was evaluated by eGFR.5 COPD was defined as the presence of a post-bronchodilator FEV1/FVC <0.70 by spirometry.9 Cerebral infarction was defined as having chronic ischemic cerebral infarction. AF was characterized by the replacement of consistent P waves by rapid oscillations or fibrillatory waves that varied in amplitude, shape, and timing by electrocardiograph.10
Observation end point
Follow-up of patients after discharge was performed through phone or outpatient visits, which lasted for 12–75 months (medium follow-up was 47 months). The observation end point was all-cause death.
Statistical analysis
All statistical tests were performed using SPSS software program version 18.0. Continuous variables were expressed as mean±SD or median (interquartile range). Statistical comparison of the groups was performed by one-way ANOVA, Student’s t-test, or nonparametric Kruskal–Wallis tests. Chi-square analysis was performed for categorical variables which were reported as percent of the total. The survival curve was established by Kaplan–Meier method, and Cox proportional hazard regression model was used to analyze the prognosis. A P-value <0.05 was considered as statistically significant.
Results
Baseline characteristics and distribution of hs-cTnT
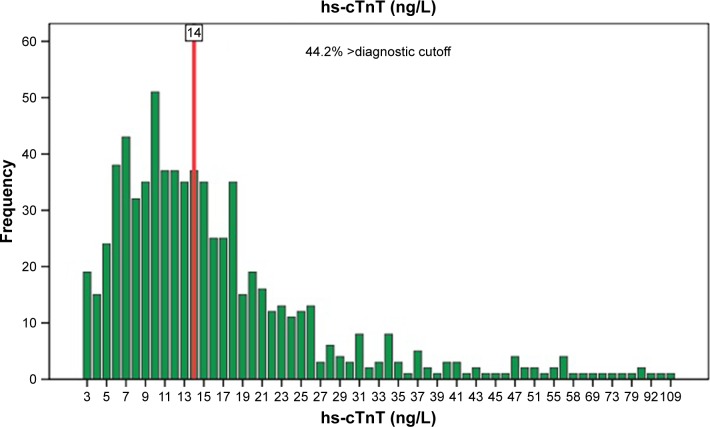
Of the 722 patients, 473 were males (65.5%), with an age range of 65–98 (82.43±5.98) years. The hs-cTnT levels were 3–109 ng/L. There were 257 cases in the low-level group (3–10 ng/L), 231 in the medium-level group (11–16 ng/L), and 234 in the high-level group (≥17 ng/L). hs-cTnT was detected in 703 patients (97.4%). Among them, hs-cTnT value of 319 patients (44.2%) was greater than the cutoff point (14 ng/L, 99th percentile upper limit [URL]; Figure 1).
Figure 1.
Distribution of hs-cTnT.
Abbreviation: hs-cTnT, high-sensitivity cardiac troponin T.
A total of 233 (32.3%) patients suffered from CAD. The number of comorbidities was higher, and the proportion of patients with four or more comorbidities showed significant increase in hs-cTnT level (P<0.001). hs-cTnT level was higher in males and increased with age. Compared with the low-level group, the NT-proBNP and LVMI levels were significantly higher in the high-level group (P<0.05), while hemoglobin (Hb), eGFR, and LVEF were significantly lower (P<0.001; Table 1).
Table 1.
Characteristics of the participants
| Characteristics of the participants | Tertile 1, n=257, 3–10 ng/L | Tertile 2, n=231, 11–16 ng/L | Tertile 3, n=234, ≥17 ng/L | P-value |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age (years) | 79.77±6.13 | 83.04±5.24 | 84.73±5.34 | <0.001 |
| Male gender | 140 (54.5) | 152 (65.8) | 181 (77.4) | <0.001 |
| Body mass index (kg/m2) | 23.39±3.30 | 23.62±3.30 | 23.80±3.29 | 0.376 |
| Heart rate (beats/min) | 72.31±12.08 | 74.23±13.22 | 75.23±13.51 | 0.058 |
| Comorbidities | ||||
| CAD | 64 (24.9) | 78 (33.8) | 91 (38.9) | 0.004 |
| Hypertension | 194 (75.5) | 182 (78.8) | 203 (86.8) | 0.006 |
| DM | 77 (30) | 97 (42) | 99 (42.3) | 0.005 |
| Cerebral infarction | 145 (56.4) | 136 (58.9) | 154 (65.8) | 0.092 |
| COPD | 37 (14.4) | 42 (18.2) | 59 (25.2) | 0.009 |
| CKD | 43 (16.7) | 49 (21.2) | 84 (35.9) | <0.001 |
| AF | 30 (11.7) | 45 (19.5) | 31 (13.2) | 0.039 |
| Number of comorbidities | ||||
| ≤1 | 78 (30.4) | 46 (19.9) | 31 (13.2) | <0.001 |
| 2–3 | 137 (53.3) | 119 (51.5) | 112 (47.9) | 0.258 |
| ≥4 | 42 (16.3) | 66 (28.6) | 91 (38.9) | <0.001 |
| Biochemistry | ||||
| NT-proBNP (pg/mL) | 171 (80.06, 402.90) | 297 (147.10, 606.50) | 562.1 (225.40, 1,174.00) | <0.001 |
| eGFR (mL/min·1.73 m2) | 78.73±22.69 | 76.48±23.59 | 67.86±27.54 | <0.001 |
| Hb (g/L) | 131.35±15.53 | 128.84±19.39 | 123.44±21.92 | <0.001 |
| FBG (mmol/L) | 5.41±1.30 | 5.66±1.96 | 5.83±2.24 | 0.041 |
| Echocardiography | ||||
| LVEF (%) | 59.97±3.40 | 59.33±4.00 | 57.89±3.34 | <0.001 |
| LVMI (g/m2) | 117.90±31.90 | 123.73±37.40 | 128.42±40.64 | 0.008 |
Notes: Data are presented as number (%) or mean±SD or median (interquartile range). P-value is for comparison by ANOVA or Kruskal–Wallis tests.
Abbreviations: CAD, coronary artery disease; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; AF, atrial fibrillation; NT-proBNP, amino-terminal pro-brain natriuretic peptide; eGFR, estimated glomerular filtration rate; Hb, hemoglobin; FBG, fasting blood glucose; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index.
Relationship between hs-cTnT level and death risk
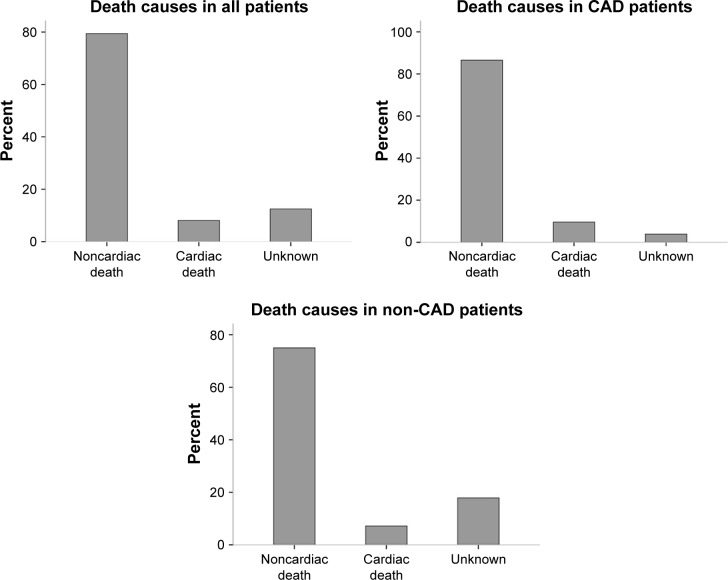
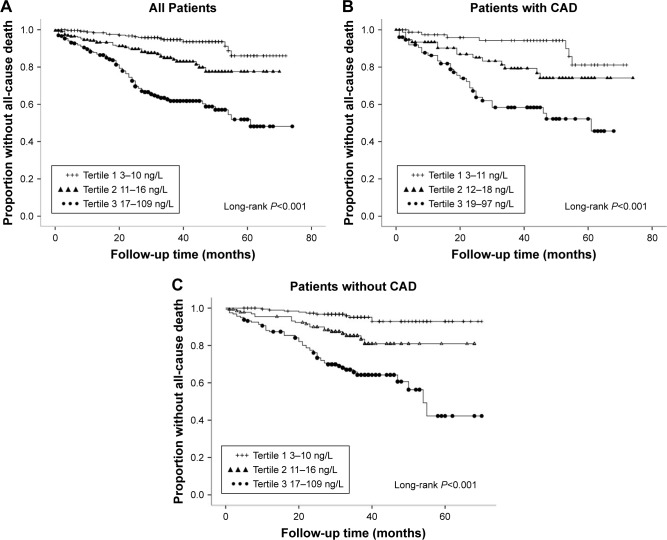
The patients were followed up for an average of 47 months. Of the 722 patients, 45 cases (6.2%) died within 1 year. The total number of deaths was 136 (18.8%), and 108 deaths (79.4%) were noncardiac. Moreover, there were 84 deaths (17.2%) in population without CAD, and 52 (22.2%) in CAD population (Figure 2). The mortality rate of the three groups was compared using Kaplan–Meier curve which showed differences among the groups: low-level group, 6.2%; medium-level group, 15.2%, and high-level group, 36.3% (Figure 3A). The mortality rate was also found to be increased (8.8%, 19.2%, and 39.5%, respectively) with increased hs-cTnT level in CAD population (Figure 3B). The same was also observed for the population without CAD, with differences in mortality rates among the three groups (4.7%, 14.8%, and 34.4%, respectively) (Figure 3C, P<0.001).
Figure 2.
Distribution of death causes.
Abbreviation: CAD, coronary artery disease.
Figure 3.
Kaplan–Meier curves for the estimation of risk on all-cause mortality (A–C).
Abbreviation: CAD, coronary artery disease.
The disease prognosis was analyzed using Cox regression model. In the total population, the risk of all-cause death in the highest hs-cTnT level group was 7.3 times higher than that of the lowest hs-cTnT level group. After adjusting for population factor, comorbidity, NT-proBNP, Hb, eGFR, LVEF, etc., hs-cTnT level was still an indicator of death risk (HR: 3.01, 95% CI: 1.67–5.43, P<0.001). Cox regression analysis was performed for population with and without CAD. Results suggested that hs-cTnT level was still an independent predictor of all-cause death of the patients, regardless of adjusting the risk factors or major comorbidities in the population without CAD (P<0.001), and the risk of mortality was higher than that of the population with CAD (Table 2).
Table 2.
Cox regression analysis to examine the prognostic value of hs-cTnT for mortality when adjusted for significant risk factors and important comorbidities
| Ln (hs-cTnT) | All patients
|
Patients with CAD
|
Patients without CAD
|
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Univariable | 7.33 (4.29–12.51) | <0.001 | 4.16 (1.84–9.43) | 0.001 | 10.02 (4.93–20.36) | <0.001 |
| Multivariable Model 1 | 4.85 (2.79–8.43) | <0.001 | 3.31 (1.44–7.59) | 0.005 | 5.98 (2.85–12.55) | <0.001 |
| Multivariable Model 2 | 4.85 (2.79–8.43) | <0.001 | 3.31 (1.44–7.59) | 0.005 | 6.15 (2.93–12.89) | <0.001 |
| Multivariable Model 3 | 3.01 (1.67–5.43) | <0.001 | 2.06 (0.86–4.96) | 0.11 | 3.50 (1.53–8.02) | <0.001 |
Notes: Model 1, adjusted for age and gender. Model 2, adjusted for age, gender, hypertension, DM, COPD, AF, and CKD. Model 3, adjusted for age, gender, hypertension, DM, COPD, AF, CKD, NT-proBNP, Hb, eGFR, LVMI, and LVEF.
Abbreviations: hs-cTnT, high-sensitivity cardiac troponin T; CAD, coronary artery disease; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease; AF, atrial fibrillation; CKD, chronic kidney disease; NT-proBNP, amino-terminal pro-brain natriuretic peptide; Hb, hemoglobin; eGFR, estimated glomerular filtration rate; LVMI, left ventricular mass index; LVEF, left ventricular ejection fraction.
Discussion
In this study, the elderly inpatients without ACS and with hs-cTnT >14 ng/L (99th percentile URL) comprised 44.2% of the population. A relation between increased hs-cTnT level and older age, male gender, hypertension, diabetes, CAD, COPD, the number of comorbidities, decreased renal function, anemia, and cardiac structural abnormality as well as decreased LVEF was observed. Cardinaels et al11 performed a study in 495 nursing home residents with an average age of 82 years. The results revealed that about 80% of patients had hs-cTnT level greater than the 99th percentile URL, while almost all the patients suffered from one accompanying disease. Wassef et al12 carried out a study in patients with an average age of 63 years, admitted in the Department of Cardiology due to chest pain. Findings suggested that hs-cTnT >75 ng/L had a higher accuracy in diagnosing acute myocardial infarction in high-risk patients with cardiac disease. Results of our study were consistent with the previous studies, suggesting slightly increased hs-cTnT levels in the elderly inpatients.
Results of the cohort study showed that baseline hs-cTnT level in elderly patients without ACS was associated with all-cause death after discharge, and the mortality rate increased with increased hs-cTnT level. The mortality of patients in high-level group was 7.3 times higher than that of the low-level group. After considering the risk factors and major comorbidities and adjusting NT-proBNP, Hb, eGFR, and LVEF factors, the mortality in the high-level group was 3.4 times higher than that of the low-level group. de Lemos et al13 performed a 6.4-year follow-up study of apparently healthy people in their community and found an increased rate of all-cause mortality with increased hs-cTnT level, which was 1.9% in the lowest hs-cTnT level group and as high as 28.4% in the highest hs-cTnT level group. hs-cTnT level was still an independent risk factor for all-cause death after factors such as traditional cardiovascular risk factors, C-reactive protein level, CKD, and brain natriuretic peptide level were adjusted. Baron et al14 found that the cTnT elevations were commonly observed in noncardiac critically ill patients, but often at levels undetectable by traditional assays. Elevation in hs-cTnT level predicted a more complex clinical course and an increased risk of death. Cardinaels et al11 conducted a 1-year follow-up study among nursing home residents, and found that all-cause mortality in high-level group was 3.6 times higher than that of the low-level group. Our findings were consistent with the results of Cardinaels et al11 and de Lemos et al13 studies conducted on elderly inpatients without ACS; all-cause death risk increased with increase in the levels of baseline hs-cTnT.
A subgroup analysis of the patients with and without CAD demonstrated that hs-cTnT level was a predictor of all-cause death risk regardless of adjusting the risk factors and major comorbidities in the population without CAD. Moreover, the risk of mortality was higher than that in the population with CAD, suggesting increased risk of all-cause death with increased hs-cTnT levels in elderly patients whether or not diagnosed with CAD. cTnT is the biomarker of cardiac injury, and any factor leading to necrosis of myocardial cells, such as heart failure, renal failure, myocarditis, arrhythmias, pulmonary embolism, or otherwise uneventful percutaneous or surgical coronary procedures, resulted in increased cTnT levels. These patients should not be labeled as having myocardial infarction, but rather as having myocardial injury.1 Geriatric patients always suffer from comorbidities, while noncardiac diseases are more common among geriatric inpatients. Nonmyocardial ischemic factors and even non-cardiac factors might lead to increased hs-cTnT levels in patients with or without CAD. We found that among the total deaths, almost 80% were noncardiac, suggesting noncardiac factors as main causes of death in this study. Iversen et al15 performed a study among 1,176 elderly inpatients who were grouped based on the tertiles of hs-cTnT level and found that the mortality rate in the highest hs-cTnT level group was 7 times more than that in the lowest hs-cTnT level group in patients without ischemic heart disease. This suggests that hs-cTnT level was the strongest predictor of all-cause death risk after discharge regardless of the existence of CAD. Our study results were consistent with the findings of this study.
Current studies have demonstrated that non-coronary factors, such as age, male gender, diabetes, renal functions, cardiac failure, and cardiac structural abnormality, lead to increased hs-cTnT levels.13,16–19 Except for myocardial ischemia, the causes for the increased low-level hs-cTnT include the following: 1) Increased age, cardiac aging, increase in cell metabolism and apoptosis, different degrees of myocardial vascular endothelium damage, and “physiological reconstruction” processes due to the release of cardiac troponin from cardiomyocytes of elderly individuals in turn result in the loss of myocytes, histological changes characterized by hypertrophy of the residual myocytes, and myocardial structural calcification, and ultimately leads to increase in cardiac troponin released in cardiomyocytes.20 2) Glomerular filtration rate gradually declines with increase in age, causing decreased cardiac troponin metabolism.18 3) With growing age, a variety of cardiac structural and functional abnormalities occur, resulting in the damage of cardiac contraction and filling, and increase in the ventricular wall tension and intraventricular pressure, as well as increase in the hs-cTnT release.21 The reasons for the correlation of high hs-cTnT level with all-cause deaths included the following: First, the patients with increased hs-cTnT levels were usually of older age, and their life expectancy was relatively short.3 Second, almost all the patients had at least one accompanying disease, such as CAD, diabetes, hypertension, CKD, or COPD. Complications of the above diseases, such as cardiac failure, hypoglycemia, hyperglycemic coma, hypotension, deterioration of renal function, or acute infection, were considered as high-risk factors that lead to patient’s death.22–24 Last, patients with high hs-cTnT levels usually had cardiac structural abnormality, increased NT-proBNP level and decreased Hb level, and so on.13,12 More-over, they were associated with potential myocardial ischemia and hypoxia risk and were more prone to secondary cardiovascular events, and thus associated with increased death risk.
Limitations
Our study has few limitations. First, we focused only on collecting the baseline hs-cTnT levels associated with the risk factors of cardiovascular disease and comorbidities, but did not study the follow-up measures of hs-cTnT levels. Second, the diagnosis of CAD was mainly based on the disease history, but not on the findings of coronary angiography or coronary CT examination. In addition, symptoms of myocardial ischemia in elderly people were not typical; thus, ACS patients cannot be completely excluded. Third, the end point events during the follow-up period were relatively homogeneous, and rehospitalization, cardiovascular events, and so on were not recorded.
Conclusion
A slightly increased level of hs-cTnT was observed in the elderly inpatients without ACS, and about half of the hs-cTnT levels were higher than the 99th percentile URL. Baseline hs-cTnT level was associated with increased all-cause death risks after discharge, and most of the deaths occurred from non-cardiovascular diseases. The prognostic value of hs-cTnT among the elderly inpatients with or without CAD was strongly associated with the mortality rate. Moreover, hs-cTnT measurement in elderly inpatients without ACS helped to assess the risk of all-cause deaths.
Footnotes
Disclosure
All authors declare that no support, financial or otherwise, has been received from any organization that may have an interest in the submitted work and there are no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Thygesen K, Alpert JS, Jaffe AS, et al. ESC Committee for Practice Guidelines (CPG) Third universal definition of myocardial infarction. Eur Heart J. 2012;33(20):2551–2567. doi: 10.1093/eurheartj/ehs184. [DOI] [PubMed] [Google Scholar]
- 2.Mingels A, Jacobs L, Michielsen E, Swaanenburg J, Wodzig W, van Dieijen-Visser M. Reference population and marathon runner sera assessed by highly sensitive cardiac troponin T and commercial cardiac troponin T and I assays. Clin Chem. 2009;55(1):101–108. doi: 10.1373/clinchem.2008.106427. [DOI] [PubMed] [Google Scholar]
- 3.Gore MO, Seliger SL, Defilippi CR, et al. Age and sex dependent upper reference limits for the high sensitivity cardiac troponin T assay. J Am Coll Cardiol. 2014;63(14):1441–1448. doi: 10.1016/j.jacc.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chew DP, Briffa TG, Alhammad NJ, et al. High sensitivity-troponin elevation secondary to non-coronary diagnoses and death and recurrent myocardial infarction: an examination against criteria of causality. Eur Heart J Acute Cardiovasc Care. 2015;4(5):419–428. doi: 10.1177/2048872614564083. [DOI] [PubMed] [Google Scholar]
- 5.Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17(10):2937–2944. doi: 10.1681/ASN.2006040368. [DOI] [PubMed] [Google Scholar]
- 6.Devereux RB, Reiehek N. Echocardiographic determination of left ventricular mass in man. Circulation. 1977;55(4):613–618. doi: 10.1161/01.cir.55.4.613. [DOI] [PubMed] [Google Scholar]
- 7.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34(28):2159–2219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association Standards of medical care in diabetes-2013. Diabetes Care. 2013;36(Suppl 1):S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Global Initiative for Chronic Obstructive Lung Disease. 2010. [Accessed May 5, 2018]. Available from: http://goldcopd.org/
- 10.Fuster V, Rydén LE, Cannom DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol. 2011;57(11):e101–e198. doi: 10.1016/j.jacc.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Cardinaels EPM, Daamen MAMJ, Bekers O, et al. Clinical interpretation of elevated concentrations of cardiac troponin T, but not troponin I, in nursing home residents. J Am Med Dir Assoc. 2015;16(10):884–891. doi: 10.1016/j.jamda.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 12.Wassef AF, Hiebert B, Saeed MF, et al. Novel high-sensitivity troponin assay requires higher cut-off value to separate acute myocardial infarction from non-acute myocardial infarction in a high-risk population. Can J Physiol Pharmacol. 2015;93(10):873–877. doi: 10.1139/cjpp-2014-0473. [DOI] [PubMed] [Google Scholar]
- 13.de Lemos JA, Drazner MH, Omland T, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304(22):2503–2512. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baron JM, Lewandrowski EL, Januzzi JL, Bajwa EK, Thompson BT, Lewandrowski KB. Measurement of high-sensitivity troponin T in noncardiac medical intensive care unit patients. Am J Clin Pathol. 2014;141(4):488–493. doi: 10.1309/AJCPLVQQY35XTFVN. [DOI] [PubMed] [Google Scholar]
- 15.Iversen K, Køber L, Gøtze JP, et al. Troponin T is a strong marker of mortality in hospitalized patients. Int J Cardiol. 2013;168(2):818–824. doi: 10.1016/j.ijcard.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Ndrepepa G, Braun S, Mehilli J, et al. Prognostic value of sensitive troponin T in patients with stable and unstable angina and undetectable conventional troponin. Am Heart J. 2011;161(1):68–75. doi: 10.1016/j.ahj.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 17.Rubin J, Matsushita K, Ballantyne CM, Hoogeveen R, Coresh J, Selvin E. Chronic hyperglycemia and subclinical myocardial injury. J Am Coll Cardiol. 2012;59(5):484–489. doi: 10.1016/j.jacc.2011.10.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubin RF, Li Y, He J, et al. CRIC Study Investigators Predictors of high sensitivity cardiac troponin T in chronic kidney disease patients: a cross sectional study in the chronic renal in sufficiency cohort (CRIC) BMC Nephrol. 2013;14:229. doi: 10.1186/1471-2369-14-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kusumoto A, Miyata M, Kubozono T, et al. Highly sensitive cardiac troponin T in heart failure: comparison with echocardiographic parameters and natriuretic peptides. J Cardiol. 2012;59(2):202–208. doi: 10.1016/j.jjcc.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 20.Eggers KM, Lind L, Ahlstrom H, et al. Prevalence and pathophysiological mechanisms of elevated cardiac troponin I levels in a population-based sample of elderly subjects. Eur Heart J. 2008;29(18):2252–2258. doi: 10.1093/eurheartj/ehn327. [DOI] [PubMed] [Google Scholar]
- 21.Otsuka T, Kawada T, Ibuki C, Seino Y. Association between high-sensitivity cardiac troponin T levels and the predicted cardiovascular risk in middle-aged men without overt cardiovascular disease. Am Heart J. 2010;159(6):972–978. doi: 10.1016/j.ahj.2010.02.036. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura Y, Yoshihisa A, Takiguchi M, et al. High-sensitivity cardiac troponin T predicts non-cardiac mortality in heart failure. Circ J. 2014;78(4):890–895. doi: 10.1253/circj.cj-13-1372. [DOI] [PubMed] [Google Scholar]
- 23.Hitsumoto T. Factors associated with high-sensitivity cardiac troponin T in patients with type 2 diabetes mellitus. J Nippon Med Sch. 2015;82(6):274–280. doi: 10.1272/jnms.82.274. [DOI] [PubMed] [Google Scholar]
- 24.Neukamm AM, Høiseth AD, Hagve TA, Søyseth V, Omland T. High-sensitivity cardiac troponin T levels are increased in stable COPD. Heart. 2013;99(6):382–387. doi: 10.1136/heartjnl-2012-303429. [DOI] [PMC free article] [PubMed] [Google Scholar]