Abstract
Objectives
Based on the functionality of AQP-5 characterized in various physiological processes, our study aimed to investigate the effect of AQP-5 silencing by siRNA interference on chemosensitivity of breast cancer cells.
Materials and methods
The expression levels of AQP-5 mRNA in different experimental groups were measured by reverse transcription PCR. The chemosensitivity of the cells to adriamycin (ADR) was detected by a CCK-8 kit. Cell invasion, migration, and apoptosis were assessed. Western blot was used to detect the expression levels of AQP-5, drug resistance-related protein, and apoptosis-related protein.
Results
The expression level of AQP-5 in MCF-7/ADR cells was significantly reduced by AQP-5 siRNA transfection. The invasion and migration were significantly reduced in MCF-7/ADR after AQP-5 siRNA interference. AQP-5 silencing significantly increased the chemosensitivity of MCF-7/ADR cells to ADR and activated caspase-dependent apoptosis in MCF-7/ADR cells. AQP-5 silencing also decreased the expression levels of drug resistance-related proteins (P-gp and GST-π).
Conclusion
The inhibition of AQP-5 expression may reverse the drug resistance and enhance the chemosensitivity of breast cancer cells.
Keywords: aquaporin 5, breast cancer, ADR, chemosensitivity, apoptosis
Introduction
Chemotherapy is widely used in the treatment of different types of cancers. However, the efficiency of chemotherapy is usually limited by the resistance of cancer cells to the drugs used in the treatment.1 Drug resistance occurs due to the tolerance of a disease to drug treatments, which leads to poor treatment efficiency.2 Two main types of drug resistance in chemotherapy have been identified: intrinsic and acquired. The intrinsic resistance, which is caused by preexisting resistance factors, exists before the drug treatment, while the acquired resistance develops during the treatment due to the mutations induced by the drug or the activation of compensatory signaling pathways.3 Breast cancer, which affects millions of women worldwide, is the most common cancer type in women.4 Studies have proved that about half of the patients with breast cancer failed to respond to initial drug treatments possibly due to the intrinsic resistance.5 Even worse, most of the breast cancer patients develop a resistance after the drug treatment, leading to the development of aggressive malignancies.6,7 In view of the negative effects of drug resistance on the treatment of various human diseases including breast cancer,8–10 it will be of great clinical value to identify new molecular targets to overcome drug resistance, so as to enhance the treatment efficiency of chemotherapy.
It has been well accepted that the growth, development, invasion, and metastasis of a tumor are closely related to the microenvironment and metabolism.11 Previous studies have shown that water molecules play pivotal roles in the development of malignant epithelial tumors,12 providing a new strategy for the treatment of cancer. Aquaporins (AQPs) are water channel proteins responsible for the transportation of water molecules.13 The expression levels of AQPs have been shown to be altered in various tumor tissues. One of the AQPs, AQP-5, was shown to be overexpressed in pancreatic cancer tissue and is believed to be involved in the proliferation of tumor cells.14 AQP-5 knockout was found to be able to reduce the volume of liquid secreted from different tissues, so as to increase the protein and salt concentration,15 indicating that AQP-5 can change the microenvironment and metabolism of the tumor cells. In addition, it has been reported that AQP-5 silencing can improve drug resistance in colon cancer cells by suppressing p38 MAPK signaling.16 So, it is reasonable to hypothesize that AQP-5 may also play pivotal roles in drug resistance in breast cancer cells.
In our study, the effects of AQP-5 on drug resistance in breast cancer cells were investigated through the establishment of adriamycin (ADR)-resistant breast cancer cell line MCF-7 (MCF-7/ADR) and the downregulation of AQP-5 by siRNA interference in both MCF-7 and MCF-7/ADR. The original MCF-7 cells were used as drug-sensitive cell line (MCF-7/S). We found that the expression level of AQP-5 was increased in MCF-7/ADR cells compared with that in MCF-7/S cells. In addition, AQP-5 silencing significantly inhibited the proliferation and induced the apoptosis of MCF-7/ADR cells. So, we believe that inhibition of AQP-5 expression may reverse the drug resistance and increase the chemosensitivity of breast cancer cells to ADR.
Materials and methods
Cell culture
MCF-7 cells (ATCC, Manassas, VA, USA) were cultured in DMEM containing 10% FBS and 0.1% penicillin-streptomycin (Gibco®; Thermo Fisher Scientific, Waltham, MA, USA). The cell culture was performed in an incubator (37°C, 5% CO2). The ADR-resistant cell line MCF-7/ADR was established by gradually increasing the concentration of ADR (Shenzhen Zhijun Pharmaceutical Co., Ltd., Shenzhen, People’s Republic of China). Then, the concentration of ADR was maintained at 1 μg/mL. ADR treatment was stopped 2 weeks before the experiment. The original MCF-7 cells were used as drug-sensitive cell line (MCF-7/S).
MTT assay
The experimental groups were as follows: MCF-7/ADR cells, MCF-7/S cells, MCF-7/ADR cells treated with different concentrations of ADR, and MCF-7/S cells treated with different concentrations of ADR.
The resistance of MCF-7/ADR and MCF-7/S cells was determined by the conventional MTT reduction assay. The cells were collected at logarithmic growth phase after digestion with 0.25% trypsin (Thermo Fisher Scientific) and resuspended, and the cell density was adjusted to 1 × 104 cells/mL. Then, 100 μL cells/well were inoculated into a 96-well plate (Corning, Corning, NY, USA). After 24 h, ADR at different concentrations (0.1, 0.2, 0.3, 0.4, and 0.5 mg/mL) was added, and the cells were cultured at 37°C with 5% CO2. After 48 h, cells were co-incubated with 20 μL MTT (5 mg/mL) for 4 h. The supernatant was discarded, and 200 μL DMSO (Sigma-Aldrich, St. Louis, MO, USA) was added. Absorbance was measured at 490 nm to calculate the half maximal inhibitory concentration (IC50):
AQP-5 siRNA transfection
The experimental groups were as follows: MCF-7/ADR control cells, MCF-7/ADR cells transfected with negative control (NC)-siRNA, and MCF-7/ADR cells transfected with AQP-5 siRNA.
Sense primer 5′-AAAACTCTGCGAACACGGCCCCTGTCTC-3′ and antisense primer 5′-AAGGCCGTGTTCGCAGAGTTCCTGTCTC-3′ were used for AQP-5 siRNA transfection to knock down the expression of AQP-5. The primer for NC-siRNA was 5′-GGUCUCACUCCCCAUAGAGTT-3′. MCF-7 cells (4 × 105/well) were inoculated into six-well plates 24 h before transfection. Cells were washed with serum-free and antibiotic-free DMEM before transfection. AQP-5 siRNA and control NC-siRNA were diluted with serum-free and antibiotic-free DMEM and mixed with transfection reagent Lipofectamine™ 2000 (Thermo Fisher Scientific). The transfection was performed by Western blot and reverse transcription PCR (RT-PCR), respectively.
RT-PCR
The expression of AQP-5 mRNA was measured by RT-PCR. Total RNA was extracted using Trizol kit (Thermo Fisher Scientific), according to the instructions. The RNA samples were quantified by UV spectrophotometry, and reverse transcription was performed according to the instructions. The following primers were used for PCR: 5′-TGAACGGGAAGCTCACTGG-3′ (sense) and 5′-GCTTCACCACCTTCTTGATGTC-3′ (anti-sense) for GAPDH; 5′-TGTGCTCCCTTGCC TTCT-3′ (sense) and 5′-AGTAATGGCTGGATTGATGTG-3′ (anti-sense) for AQP-5. PCR was performed as follows: 95°C pre-denaturation for 10 min, followed by 35 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s, and then 10 min at 72°C. After PCR, 10 μL of the product was subjected to agarose gel electrophoresis, and Bio-Rad Imager (Bio-Rad, Hercules, CA, USA) was used to take photos and analyze data. The AQP-5 mRNA expression level was measured by the ratio of value of AQP-5 to that of GAPDH.
Detection of the chemosensitivity of the cells to ADR
The experimental groups were as follows: MCF-7/ADR cells, MCF-7/ADR cells transfected with NC-siRNA, and MCF-7/ADR cells transfected with AQP-5 siRNA.
Forty-eight hours after transfection, the transfected and untransfected cells were plated and cultured overnight, and the original medium was removed. The cells were mixed with medium containing same concentrations of ADR in 96-well plates (three repeats for each group), followed by incubation for another 48 h. The absorbance at 450 nm was then measured with a CCK-8 kit (TOYOBO, Osaka, Japan) and by an enzyme-labeled meter (PerkinElmer, Waltham, MA, USA). The cell growth inhibition rate and IC50 of ADR were calculated.
Cell invasion
The experimental groups were as follows: normal MCF-7/ADR cells, MCF-7/ADR cells transfected with AQP-5 siRNA, MCF-7/ADR cells treated with ADR, and MCF-7/ADR cells transfected with AQP-5 siRNA and treated with ADR.
Cell invasion experiments were performed using Corning™ BioCoat™ Matrigel™ Invasion Chamber (Corning). For this, 500 μL serum-free DMEM preheated at 37°C was added to the invasion chamber. The cells were collected 24 h after transfection. After digestion, the cells were resuspended in serum-free DMEM. The cell density was adjusted to 2.5 × 105 cells/mL, and 200 μL cell suspension was added to the chamber. DMEM (600 μL) containing 10% FBS was added to the 24-well plate in the lower part of the chamber. After 24 h of routine culture, cotton swabs were used to remove the cells that failed to pass through the membrane. The membrane was fixed in 4% paraformaldehyde for 15 min, followed by 0.1% crystal violet staining for 15 min. After washing, the membrane was air-dried and observed under an inverted microscope (Olympus, Tokyo, Japan). Five high-power fields (×100) were randomly selected, and the invasion ability was measured by the number of the cells passed through the membrane.
Cell migration
The experimental groups were as follows: normal MCF-7/ADR cells, MCF-7/ADR cells transfected with AQP-5 siRNA, MCF-7/ADR cells treated with ADR, and MCF-7/ADR cells transfected with AQP-5 siRNA and treated with ADR.
Cell migration was measured by wound healing assay. The cells were collected at logarithmic growth phase after digestion with 0.25% trypsin (Thermo Fisher Scientific) and resuspended, and the cell density was adjusted to 3 × 105 cells/mL. The cells were inoculated into a six-well plate (Corning). After the cell layer reached confluence, a 100 μL tip was used to scratch the cells. The cell culture medium was removed, and the cells were washed with PBS. The cell migration was recorded at 0 and 24 h after scratching. Image-ProPlus 6.0 software was used to measure the width of the wound to calculate cell migration rate.
Apoptosis detection by flow cytometry
The experimental groups were as follows: normal MCF-7/ADR cells, MCF-7/ADR cells transfected with AQP-5 siRNA, MCF-7/ADR cells treated with ADR, and MCF-7/ADR cells transfected with AQP-5 siRNA and treated with ADR.
Apoptosis was detected by Annexin V-PI apoptosis detection kit (BD Pharmingen™; BD, Franklin Lakes, NJ, USA). The treated cells were collected in a centrifuge tube and washed with precooled PBS. After centrifugation at 1,500 rpm for 5 min, the cells were resuspended with 200 μL PBS. After incubation with Annexin V for 5 min, PI was added followed by incubation for another 5 min, and then the flow cytometry was performed to detect the apoptosis.
Western blot
Protein was extracted from the cells of each group, and protein concentration was measured by BCA method. The protein samples were subjected to SDS-PAGE and transferred to PVDF membrane (EMD Millipore, Billerica, MA, USA) for 2 h. After washing, TBST solution containing 5% skim milk powder was used to block the membrane for 1 h. The primary antibodies of AQP-5 (Cell Signaling, Danvers, MA, USA), P-gp (Cell Signaling), GST-π (Cell Signaling), caspase-3 (Cell Signaling), caspase-9 (Cell Signaling), cleaved PARP (Cell Signaling), and β-actin (Cell Signaling) were diluted to appropriate concentration and then were incubated with the membrane overnight at 4°C. Then, the membrane was washed, and secondary antibody (Santa Cruz, Dallas, TX, USA) was added and incubated for 1 h at room temperature. After washing, the ECL luminescent substrate was added, and the results were analyzed using gel electrophoresis image analyzer (Bio-Rad).
Statistical analysis
SPSS 18.0 (SPSS Inc., Chicago, IL, USA) was used to analyze the data. All the data were expressed as mean ± SD and analyzed by one-way ANOVA followed by LSD post test. A p-value less than 0.05 was considered to be statistically significant.
Results
AQP-5 expression was higher in MCF-7/ADR than MCF-7/S cells
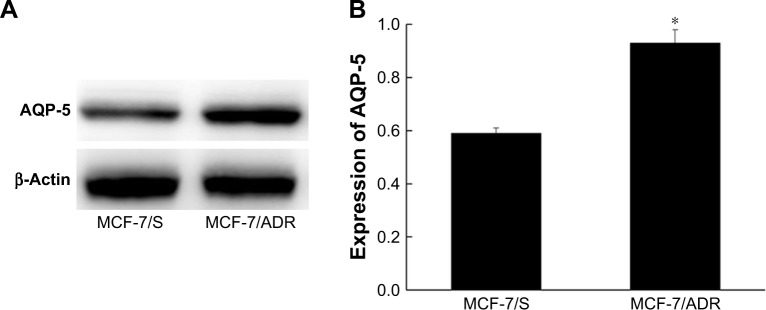
The expression levels of AQP-5 protein in MCF-7/S and MCF-7/ADR cells were detected by Western blot. The results showed that the expression level of AQP-5 in MCF-7/ADR cells was significantly higher than that in MCF-7/S (p < 0.05; Figure 1).
Figure 1.
The expression of AQP-5 protein in MCF-7/ADR and MCF-7/S cells. (A) Western blot. (B) The expression of AQP-5 protein. *p < 0.05 vs MCF-7/S group.
Abbreviation: ADR, adriamycin.
Resistance of MCF-7/S and MCF-7/ADR
Figure 2 shows the proliferation of MCF-7/S and MCF-7/ADR after treatment with different concentrations of ADR. ADR had a significant inhibitory effect on the proliferation of MCF-7/S (p < 0.05), while no significant effect on the proliferation of MCF-7/ADR was observed (p > 0.05). The RI was calculated to be 3.30. These results indicated that MCF-7/ADR was resistant to ADR.
Figure 2.
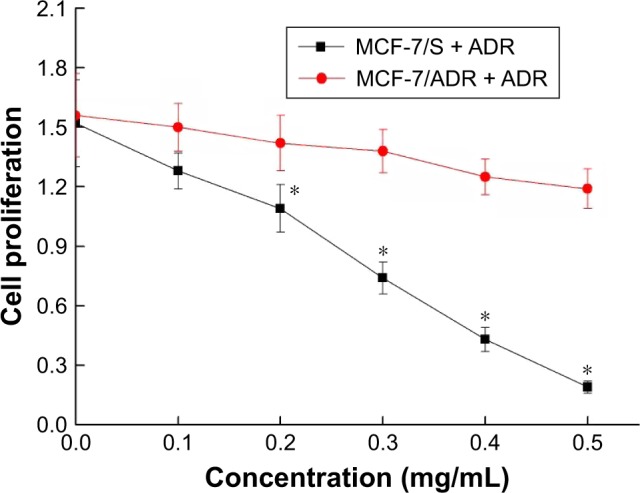
Resistance of MCF-7/S and MCF-7/ADR. *p < 0.05 vs MCF-7/ADR group.
Abbreviation: ADR, adriamycin.
Effects of siRNA transfection on the expression of AQP-5
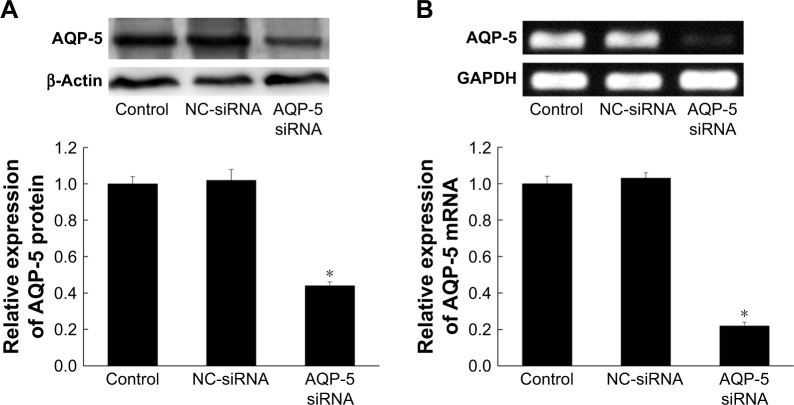
SiRNA was transfected into MCF-7/ADR cells, and the expression of AQP-5 was detected by Western blot and PCR. The results showed that the expression of AQP-5 in MCF-7/ADR cells transfected with AQP-5 siRNA was significantly lower than that in MCF-7/ADR cells transfected with NC-siRNA at both protein and mRNA levels (p < 0.05; Figure 3). No significant difference was found in the expression of AQP-5 between the MCF-7/ADR cells transfected with NC-siRNA and the control cells without transfection at both protein and mRNA levels (p > 0.05; Figure 3).
Figure 3.
Effect of siRNA interference on the expression of AQP-5 in MCF-7/ADR cells. (A) The expression of AQP-5 protein detected by Western blot. (B) The expression of AQP-5 mRNA detected by RT-PCR. *p < 0.05 vs NC-siRNA group.
Abbreviations: NC, negative control; RT-PCR, reverse transcription PCR.
Effects of AQP-5 silencing by siRNA interference on the chemosensitivity of MCF-7/ADR
CCK-8 kit was used to detect the response of cells to different concentrations of ADR to reflect the chemosensitivity. As shown in Figure 4, the inhibitory rate was significantly higher in the AQP-5 siRNA group than in the MCF-7/ADR control group when the same concentration of ADR was applied to the cells, and the NC-siRNA did not notably affect the chemosensitivity of the MCF-7/ADR cells. The IC50 value of the resistant cell line (MCF-7/ADR) against ADR decreased from 65.6 to 19.9 μg/mL after AQP-5 siRNA interference (p < 0.05).
Figure 4.
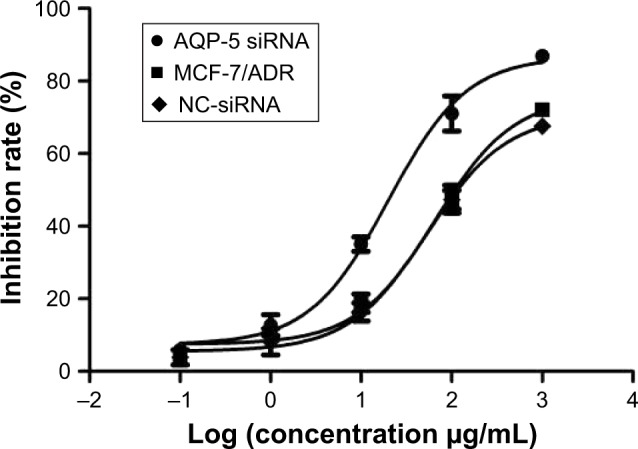
Expression of drug resistance-related proteins in MCF-7/ADR after AQP-5 silencing.
Abbreviation: NC, negative control.
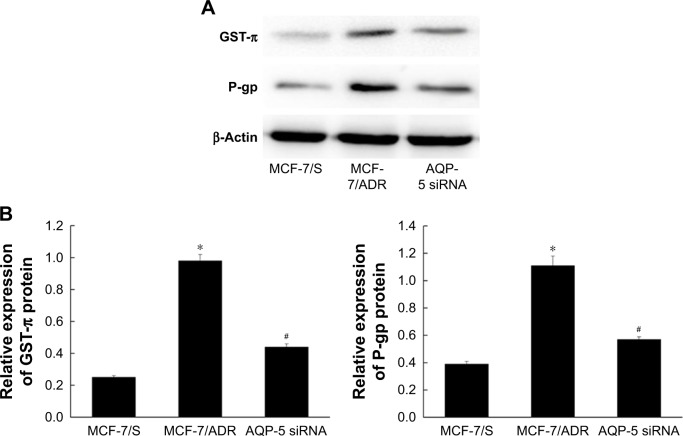
The results of chemosensitivity test confirmed that inhibition of AQP-5 expression can reverse the resistance of breast cancer cells to ADR and reduce the IC50 value of MCF-7/ADR to ADR. Therefore, we used Western blot to detect the expression levels of drug resistance-related protein in MCF-7/ADR after AQP-5 silencing. As shown in Figure 5, the expression levels of P-gp and GST-π were significantly increased in MCF-7/ADR compared with those in MCF-7/S (p < 0.05; Figure 5). However, the expression levels of P-gp and GST-π were significantly reduced in MCF-7/ADR after AQP-5 silencing (p < 0.05; Figure 5).
Figure 5.
Effects of AQP-5 silencing on the expression of drug resistance-related genes. (A) Western blot. (B) The expression of P-gp and GST-π proteins. *p < 0.05 vs MCF-7/S group; #p < 0.05 vs MCF-7/ADR group.
Effects of AQP-5 silencing by siRNAon invasion
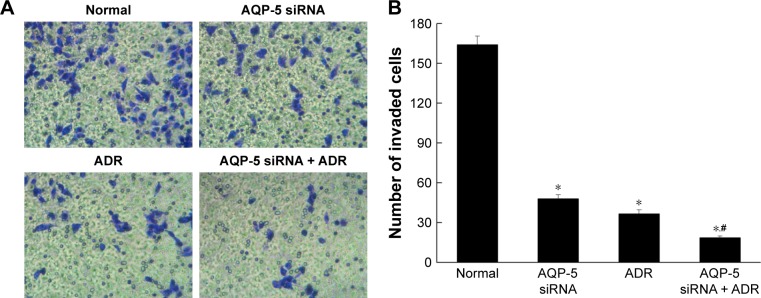
As shown in Figure 6, compared with MCF-7/ADR cells (164.00 ± 6.48), the cell invasion ability of MCF-7/ADR cells transfected with AQP-5 siRNA (48.00 ± 2.94, p < 0.05) and MCF-7/ADR cells treated with ADR (36.67 ± 2.87, p < 0.05) was significantly decreased. Compared with MCF-7/ADR cells treated with ADR (36.67 ± 2.87), the cell invasion ability of MCF-7/ADR cells transfected with AQP-5 siRNA and treated with ADR was significantly decreased (18.67 ± 1.25, p < 0.05). These results indicated that transfection with AQP-5 siRNA can inhibit the invasion of MCF-7/ADR cells.
Figure 6.
Effects of AQP-5 silencing by siRNA on invasion. (A) Invasion diagram. (B) Number of invaded cells in different groups. *p < 0.05 vs normal group; #p < 0.05 vs AQP-5 siRNA group. The magnification is ×400.
Abbreviation: ADR, adriamycin.
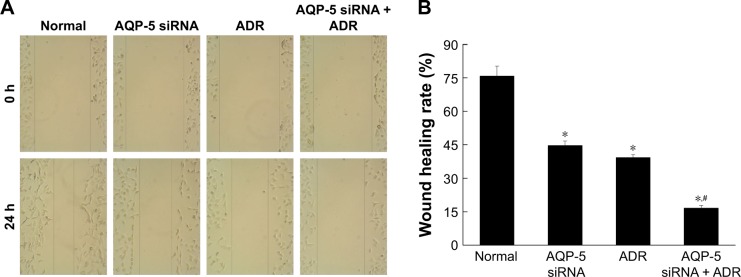
Effects of AQP-5 silencing by siRNA on migration
As shown in Figure 7, compared with MCF-7/ADR cells (75.78 ± 4.42), the cell migration ability of MCF-7/ADR cells transfected with AQP-5 siRNA (44.67% ± 2.05%, p < 0.05) and MCF-7/ADR cells treated with ADR (39.33% ± 1.25%, p < 0.05) was significantly decreased. Compared with MCF-7/ADR cells treated with ADR (39.33% ± 1.25%), the cell migration ability of MCF-7/ADR cells transfected with AQP-5 siRNA and treated with ADR was significantly decreased (16.67% ± 1.25%, p < 0.05). These data indicated that transfection with AQP-5 siRNA can inhibit the migration of MCF-7/ADR cells.
Figure 7.
Effects of AQP-5 silencing by siRNA on migration. (A) Migration diagram. (B) Wound healing rate of different groups. *p < 0.05 vs normal group; #p < 0.05 vs AQP-5 siRNA group.
Abbreviation: ADR, adriamycin.
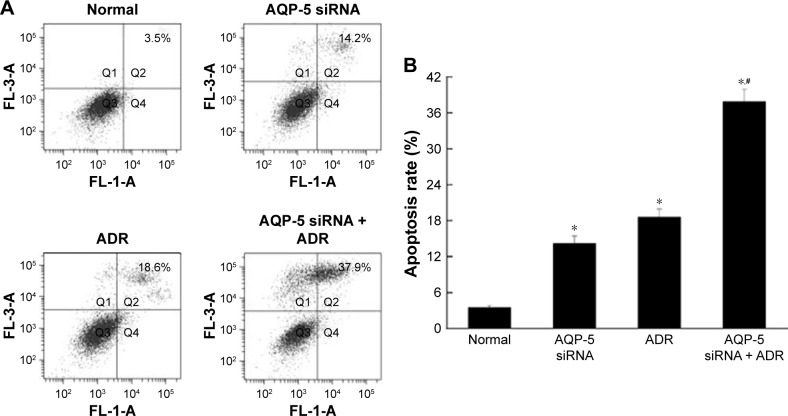
Effects of AQP-5 silencing by siRNA on ADR-induced apoptosis
The results of flow cytometry showed that the apoptosis rate of normal MCF-7/ADR cells was 3.5%, which was significantly lower than that of MCF-7/ADR cells transfected with AQP-5 siRNA (14.2%, p < 0.05; Figure 8). The apoptosis rate of MCF-7/ADR cells after ADR treatment was 18.6%, while after downregulating AQP-5 expression by siRNA transfection, the apoptosis rate induced by ADR in MCF-7/ADR cells increased to 37.9% (p < 0.05 vs MCF-7/ADR group; Figure 8).
Figure 8.
Effects of AQP-5 silencing by siRNA and ADR treatment on the apoptosis detected by flow cytometry. (A) Flow cytometry diagram. (B) Apoptosis rate of different groups. *p < 0.05 vs normal group; #p < 0.05 vs AQP-5 siRNA group.
Abbreviations: ADR, adriamycin; FL, fluorescence.
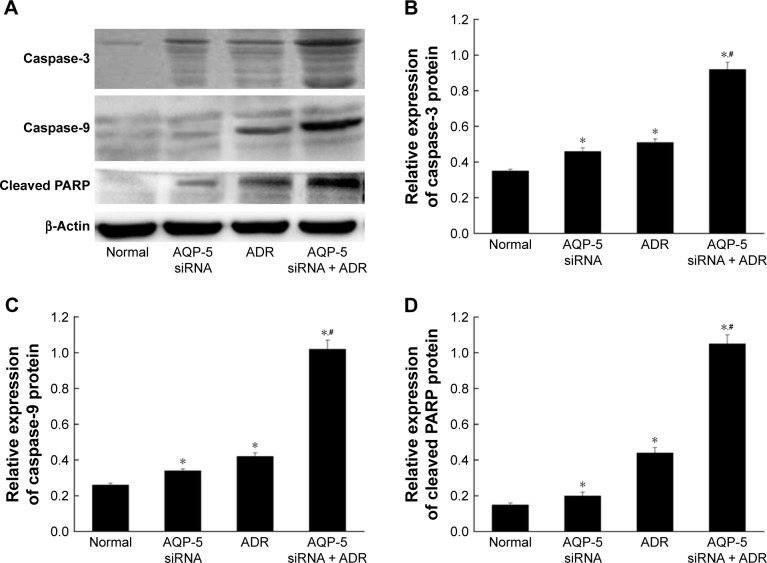
Inhibition of AQP-5 expression activates caspase-dependent apoptosis in MCF-7/ADR cells
The results of flow cytometry showed that the ADR-induced apoptosis was significantly increased by AQP-5 silencing. So, we detected the expression of apoptosis-related proteins by Western blot. As shown in Figure 9, the protein levels of caspase-3, caspase-9, and cleaved PARP were all significantly higher in the MCF-7/ADR cells transfected with AQP-5 siRNA than those in MCF-7/ADR cells (p < 0.05). The differences in the protein levels of caspase-3, caspase-9, and cleaved PARP were also found to be higher in the MCF-7/ADR cells transfected with AQP-5 siRNA and treated with ADR (p < 0.05; Figure 9).
Figure 9.
Effects of AQP-5 silencing by siRNA and ADR treatment on the expression of apoptosis-related proteins in MCF-7/ADR cells. (A) Western blot. (B) The expression of caspase-3 protein. (C) The expression of caspase-9 protein. (D) The expression of cleaved PARP protein. *p < 0.05 vs normal group; #p < 0.05 vs AQP-5 siRNA group.
Abbreviation: ADR, adriamycin.
Discussion
It has been reported the expression of AQP-5 is usually altered in tumor tissue and the altered expression of AQP-5 plays pivotal roles in the proliferation and differentiation of cancer cells, indicating that AQP-5 may be important for cancer development.17–19 For example, increased expression level of AQP-5 was found in lung cancer tissues as compared with normal tissues, and the increased AQP-5 expression was closely related to poor prognosis.20 In our study, the expression level of AQP-5 in ADR-resistant breast cancer cell line MCF-7 was significantly higher than that in normal MCF-7 cells (p < 0.05; Figure 1), and MCF-7/ADR cells were resistant to ADR (Figure 2). In addition, with the successfully established AQP-5 siRNA interference line (Figure 3), the inhibitory effect of ADR treatment on different groups of cells with different expression levels of AQP-5 was detected. We found that the inhibition rate was significantly higher in the MCF-7/ADR cells transfected with AQP-5 siRNA than in the MCF-7/ADR cells when the same concentration of ADR was applied to the cells, and the IC50 value of the resistant cell line (MCF-7/ADR) against ADR decreased from 65.6 to 19.9 μg/mL after AQP-5 silencing (Figure 4). Our data suggested that AQP-5 is not only correlated with the development of cancer but also positively related to the development of drug resistance.
P-gp and GST-π are drug resistance-related proteins. P-gp protein, which is encoded by MDR1, can target structurally unrelated chemotherapeutic agents such as Etopo-side, ADR, and Taxol.21 As a member of glutathione-S-transferase family, GST-π can inactivate cytotoxic drugs.22 In our study, the expression levels of P-gp and GST-π in MCF-7/S cells, MCF-7/ADR cells, and MCF-7/ADR cells transfected with AQP-5 siRNA were detected by Western blot. We found that the expression levels of GST-π and P-gp were significantly increased in MCF-7/ADR than in MCF-7/S (p < 0.05; Figure 5), indicating the important function of P-gp and GST-π in the resistance of MCF-7 to ADR. However, the expression levels of P-gp and GST-π were significantly decreased in MCF-7/ADR cells with AQP-5 silencing compared with those in normal MCF-7/ADR cells (p < 0.05; Figure 5). Our data suggested that AQP-5 can be involved in the development of drug resistance by regulating the expression of drug resistance-related genes.
Transfection with AQP-5 siRNA can inhibit the invasion and migration of MCF-7/ADR cells (Figures 6 and 7). Previous studies have shown that AQP-5 plays pivotal roles in the apoptosis of various cell types.23,24 It has been reported that AQP-5 was associated with antiapoptosis in the development of tumor.25 Similar results were found in our study – the apoptosis rate of normal MCF-7/ADR cells was 3.5%, which was significantly lower than that of MCF-7/ADR cells transfected with AQP-5 siRNA (14.2%, p < 0.05; Figure 8). After treatment with ADR, the apoptosis rate of MCF-7/ADR cells was 18.6%, while after downregulating AQP-5 expression by siRNA transfection, the apoptosis rate induced by ADR in MCF-7/ADR cells increased to 37.9% (p < 0.05 vs MCF-7/ADR group; Figure 8). These data suggested that AQP-5 can inhibit the apoptosis of breast cancer cells, thus improving the development of tumor.
Caspase-3 and caspase-9 play pivotal roles in apoptosis. Caspase-3, which can be activated by caspase-9, is a key executor of apoptosis.26 PARP is responsible for DNA repair and programmed cell death.27 PARP can be cleaved by caspase-3 to form the inactivated form of PARP, cleaved PARP, which is a marker for apoptosis. In our study, the protein levels of caspase-3, caspase-9, and cleaved PARP were detected in MCF-7/ADR cells and MCF-7/ADR cells transfected with AQP-5 siRNA with and without ADR treatment. We found that the levels of caspase-3, caspase-9, and cleaved PARP were all significantly higher in MCF-7/ADR cells transfected with AQP-5 siRNA and treated with ADR than those in MCF-7/ADR cells (p < 0.05; Figure 9). The levels of the three abovementioned proteins were also found to be higher in the cells with AQP-5 knockout than those in MCF-7/ADR cells after ADR treatment (p < 0.05; Figure 9). In addition, the apoptosis rate of MCF-7/ADR cells transfected with AQP-5 siRNA and treated with ADR was significantly higher than that of the MCF-7/ADR cells with each treatment alone (p < 0.05; Figure 9). Our data suggested that AQP-5 siRNA can activate caspase-dependent apoptosis in MCF-7/ADR cells to promote apoptosis.
Conclusion
The expression level of AQP-5 in MCF-7/ADR cells was significantly higher than that in MCF-7/S cells. AQP-5 silencing by siRNA interference can significantly inhibit the proliferation and induce the apoptosis of MCF-7/ADR cells and can reverse the drug resistance of MCF-7/ADR cells to ADR, thus increasing the chemosensitivity. So, we believe that inhibition of AQP-5 expression may reverse the drug resistance and enhance the chemosensitivity of breast cancer cells.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13(10):714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 2.Housman G, Byler S, Heerboth S, et al. Drug resistance in cancer: an overview. Cancers (Basel) 2014;6(3):1769–1792. doi: 10.3390/cancers6031769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Longley DB, Johnston PG. Molecular mechanisms of drug resistance. J Pathol. 2005;205(2):275–292. doi: 10.1002/path.1706. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 5.O’Driscoll L, Clynes M. Biomarkers and multiple drug resistance in breast cancer. Curr Cancer Drug Targets. 2006;6(5):365–384. doi: 10.2174/156800906777723958. [DOI] [PubMed] [Google Scholar]
- 6.Ellis LM, Hicklin D. Resistance to targeted therapies: refining anticancer therapy in the era of molecular oncology. Clin Cancer Res. 2009;15(24):7471–7478. doi: 10.1158/1078-0432.CCR-09-1070. [DOI] [PubMed] [Google Scholar]
- 7.Sorrentino A, Liu CG, Addario A, Peschle C, Scambi G, Ferlini C. Role of microRNAs in drug resistant ovarian cancer cells. Gynecol Oncol. 2008;111(3):478–486. doi: 10.1016/j.ygyno.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Shah NS, Richardson J, Moodley P, et al. Increasing drug resistance in extensively drug-resistant tuberculosis, South Africa. Emerg Infect Dis. 2011;17(3):510–513. doi: 10.3201/eid1703.101363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turner NC, Reis-Filho JS. Genetic heterogeneity and cancer drug resistance. Lancet Oncol. 2012;13(4):e178–e185. doi: 10.1016/S1470-2045(11)70335-7. [DOI] [PubMed] [Google Scholar]
- 10.Meng H, Mai WX, Zhang H, et al. Codelivery of an optimal drug/siRNA combination using mesoporous silica nanoparticles to overcome drug resistance in breast cancer in vitro and in vivo. ACS Nano. 2013;7(2):994–1005. doi: 10.1021/nn3044066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koontongkaew S. The tumor microenvironment contribution to development, growth, invasion and metastasis of head and neck squamous cell carcinomas. J Cancer. 2013;4(1):66–83. doi: 10.7150/jca.5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sekine S, Shimada Y, Nagata T, et al. Prognostic significance of aquaporins in human biliary tract carcinoma. Oncol Rep. 2012;27(6):1741–1747. doi: 10.3892/or.2012.1747. [DOI] [PubMed] [Google Scholar]
- 13.Moon C, Soria JC, Jang SJ, et al. Involvement of aquaporins in colorectal carcinogenesis. Oncogene. 2003;22(43):6699–6703. doi: 10.1038/sj.onc.1206762. [DOI] [PubMed] [Google Scholar]
- 14.Burghardt B, Elkaer ML, Kwon TH, et al. Distribution of aquaporin water channels AQP1 and AQP5 in the ductal system of the human pancreas. Gut. 2003;52(7):1008–1016. doi: 10.1136/gut.52.7.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen Y, Wang Y, Chen Z, et al. Role of aquaporin 5 in antigen-induced airway inflammation and mucous hyperproduction in mice. J Cell Mol Med. 2011;15(6):1355–1363. doi: 10.1111/j.1582-4934.2010.01103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi X, Wu S, Yang Y, et al. AQP5 silencing suppresses p38 MAPK signaling and improves drug resistance in colon cancer cells. Tumour Biol. 2014;35(7):7035–7045. doi: 10.1007/s13277-014-1956-3. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z, Chen Z, Song Y, Zhang P, Hu J, Bai C. Expression of aquaporin 5 increases proliferation and metastasis potential of lung cancer. J Pathol. 2010;221(2):210–220. doi: 10.1002/path.2702. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe T, Fujii T, Oya T, et al. Involvement of aquaporin-5 in differentiation of human gastric cancer cells. J Physiol Sci. 2009;59(2):113–122. doi: 10.1007/s12576-008-0017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang JH, Shi YF, Cheng Q, Deng L. Expression and localization of aquaporin-5 in the epithelial ovarian tumors. Gynecol Oncol. 2006;100(2):294–299. doi: 10.1016/j.ygyno.2005.08.054. [DOI] [PubMed] [Google Scholar]
- 20.Shen L, Zhu Z, Huang Y, et al. Expression profile of multiple aquaporins in human gastric carcinoma and its clinical significance. Biomed Pharmacother. 2010;64(5):313–318. doi: 10.1016/j.biopha.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Sun H, Dai H, Shaik N, Elmquist WF. Drug efflux transporters in the CNS. Adv Drug Deliv Rev. 2003;55(1):83–105. doi: 10.1016/s0169-409x(02)00172-2. [DOI] [PubMed] [Google Scholar]
- 22.Ali-Osman F, Caughlan J, Gray GS. Decreased DNA interstrand cross-linking and cytotoxicity induced in human brain tumor cells by 1,3-bis(2-chloroethyl)-1-nitrosourea after in vitro reaction with glutathione. Cancer Res. 1989;49(21):5954–5958. [PubMed] [Google Scholar]
- 23.Lakner AM, Walling TL, McKillop IH, Schrum LW. Altered aquaporin expression and role in apoptosis during hepatic stellate cell activation. Liver Int. 2011;31(1):42–51. doi: 10.1111/j.1478-3231.2010.02356.x. [DOI] [PubMed] [Google Scholar]
- 24.Lin X, Shaw PC, Sze SC, Tong Y, Zhang Y. Dendrobium officinale polysaccharides ameliorate the abnormality of aquaporin 5, pro-inflammatory cytokines and inhibit apoptosis in the experimental Sjögren’s syndrome mice. Int Immunopharmacol. 2011;11(12):2025–2032. doi: 10.1016/j.intimp.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 25.Sekine S, Shimada Y, Nagata T, et al. Role of aquaporin-5 in gallbladder carcinoma. Eur Surg Res. 2013;51(3–4):108–117. doi: 10.1159/000355675. [DOI] [PubMed] [Google Scholar]
- 26.Kavanagh E, Rodhe J, Burguillos MA, Venero JL, Joseph B. Regulation of caspase-3 processing by cIAP2 controls the switch between pro-inflammatory activation and cell death in microglia. Cell Death Dis. 2014;5:e1565. doi: 10.1038/cddis.2014.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sosna J, Voigt S, Mathieu S, et al. TNF-induced necroptosis and PARP-1-mediated necrosis represent distinct routes to programmed necrotic cell death. Cell Mol Life Sci. 2014;71(2):331–348. doi: 10.1007/s00018-013-1381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]