Abstract
Background
The purpose of this study is to assess dental antibiotic prescribing trends over time, quantify the number and types of antibiotics dentists prescribe inappropriately, and estimate the excess healthcare costs of inappropriate antibiotic prescribing using a large cohort of general dentists in the United States (U.S.).
Methods
We used a quasi-Poisson regression model to analyze antibiotic prescriptions trends by general dentists between 1/1/2013 and 12/31/2015 using data from Express Scripts, a large pharmacy benefits manager. We evaluated antibiotic duration and appropriateness for general dentists. Appropriateness was evaluated by reviewing the antibiotic prescribed and the duration of the prescription.
Results
Overall, the number and rate of antibiotic prescriptions prescribed by general dentists remained stable in our cohort. Over the three year study period, approximately 14% of antibiotic prescriptions were deemed inappropriate based on the antibiotic prescribed, antibiotic treatment duration, or both indicators. The quasi-Poisson regression model, which adjusted for number of beneficiaries covered, revealed a small but statistically significant decrease in the monthly rate of inappropriate antibiotic prescriptions by 0.32% (95% CI: 0.14%–0.50%; p=0.001).
Conclusions
Overall antibiotic prescribing practices among general dentists in this cohort remained stable over time. The rate of inappropriate antibiotic prescriptions by general dentists decreased slightly over time.
Practical Implications
Based on these authors’ definition of appropriate antibiotic prescription choice and duration, inappropriate antibiotic prescriptions are common (14% of all antibiotic prescriptions) among general dentists. Further analyses using chart review, administrative datasets, or other approaches are needed to better evaluate antibiotic prescribing practices among dentists.
Background
Since the first use of penicillin in 1942,1 antibiotics have become an essential tool in modern healthcare. Antibiotics have reduced morbidity and mortality from infections and have facilitated the advancement of surgical treatments, cancer care, transplantation and treatment of many other diseases. The Centers for Disease Control and Prevention (CDC) estimates that over 262 million antibiotics are prescribed in United States (US) outpatient settings annually.2 However, approximately 30–50% of antibiotic prescriptions are unnecessary.1,3 While antibiotics have been indispensable in treating bacterial infections, the misuse and overuse of antibiotics has serious negative consequences. Increased antibiotic use is associated with development of increasing antibiotic resistance,4,5 Clostridium difficile infections,6,7 adverse drug events, and additional healthcare costs.8 Antibiotic resistant infections account for 23,000 deaths and billions of dollars in excess spending in the US annually.1
Several organizations have made efforts to improve antibiotic prescribing. In the mid-1990s, the CDC spearheaded efforts to better characterize, monitor, and reduce the inappropriate use of antibiotics and antibiotic resistance. Specifically, the CDC’s Get Smart about Antibiotics campaign aims to reduce unnecessary antibiotic use in the outpatient and hospital settings,9 and provides clinical guidelines for antibiotic stewardship programs in multiple healthcare settings.10–12 Other healthcare stakeholders, such as The Pew Charitable Trusts and The Joint Commission (TJC), a US hospital accreditation agency, have also provided public commitments to improve antibiotic prescribing practices.13,14 The Centers for Medicare & Medicaid Services (CMS) are also currently developing a Condition of Participation policy requiring antimicrobial stewardship programs in hospitals in line with the new standards of TJC.14 Although these initiatives represent large efforts by the CDC and other organizations, most of the work has been aimed at improving antimicrobial stewardship programs directed towards physicians’ antibiotic prescribing patterns.
Prescriptions by general dentists account for 10% of all antibiotic prescriptions in the US;2,15 however, compared to the literature on physicians, fewer publications have evaluated antibiotic prescribing practices amongdentists.16–20 Some of the available dental studies suggest that inappropriate (non-guideline adherent) antibiotic prescribing among dentists is present.16–18, 20 For example, Roberts et al. reported that dentists prescribed several antibiotic agents that have no dental indications.16 In addition, in a survey of dentists regarding the American Heart Association recommendations for antibiotic prophylaxis prior to dental procedures, approximately 70% of dentists reported prescribing antibiotics for prophylaxis outside the American Heart Association guidelines.17 Furthermore, antibiotic prescribing may be increasing among dentists, according to one Canadian study.18 This is in contrast to some studies demonstrating declines in antibiotic prescribing rates among US physicians.21–23
This study aims to evaluate longitudinal antibiotic prescribing trends among dentists, quantify the number of potentially inappropriate antibiotic prescriptions, and estimate the healthcare costs of inappropriate antibiotic prescriptions among a large cohort of patients prescribed antibiotics by dentists in the United States.
Methods
We analyzed data on outpatient antibiotic prescriptions from January 1, 2013 through December 31, 2015. The data was obtained from Express Scripts Holding Company (ESHC), the largest independent prescription benefits manager in the United States. ESHC holds detailed prescription data for over 80 million American beneficiaries. We extracted data on provider specialty, name of antibiotic, dose, and treatment duration (days’ supply) for individuals in this large cohort in the United States (US). We defined the US as all 50 states and Washington, DC. Other non-state US territiories were excluded from our analysis. Provider specialty was obtained from the ESHC database which uses designations from the Centers for Medicare and Medicaid Services, in addition to a proprietary source. We also obtained data on total costs for antibiotics and the number of beneficiaries in the database during the study period. Costs examined in this study were calculated by adding the prescription drug plan costs and out-of-pocket patient costs. Plan costs included ingredient costs, taxes, dispensing fees and administrative fees and were not adjusted to exclude rebates. Prescriptions with missing claims and/or provider information were excluded. Topical antibiotics, systemic or topical antifungals, antiparasitics, and antivirals were also excluded. Antibiotics with the same active ingredient, but a different formulation (e.g., extended release tablets) were combined. Antimicrobials with antibacterial properties (e.g., methenamine) were included.
We analyzed the count and cost of antibiotics prescribed by general dentists to individuals equal to or greater than 18 years of age. Individuals who were less than 18 years old were specifically excluded to ensure an accurate estimation of antibiotic prescription duration (defined as days’ supply). This was done to prevent possible errors in antibiotic prescription duration estimates generated from pediatric weight-based antibiotic dosing. We then used antibiotic duration to categorize prescriptions into 3 separate purpose categories: “prophylaxis”, “indeterminate”, and “treatment”. Antibiotic prescriptions for 1 day or less were defined as “prophylaxis”; antibiotic prescriptions for 2 to 4 days were defined as “indeterminate”; and antibiotic prescriptions for 5 days or more was defined as “treatment”. Antibiotic purpose classified as “prophylaxis” or “treatment” was defined as “appropriate”, whereas those classified as “indeterminate” were defined as “inappropriate”. This designation was made by expert opinion, American Dental Association (ADA) website materials on antibiotic stewardship,24 and consensus of coauthors. Of note, with the exception of erythromycin (which our group thought was inappropriate), our definition of appropriate antibiotics was broader (included more antibiotics) than the one listed on the ADA website. We also evaluated appropriateness of antibiotic use based on antibiotic prescribed. Antibiotics without common, clear dental indications were deemed “inappropriate” by consensus of coauthors (Supplemental Table).
We summed the number of prescriptions and total drug costs by each purpose and appropriateness category. We also calculated the number of prescriptions and drug costs per 1,000 eligible beneficiaries for each category.
To investigate antibiotic prescribing trends over time, a quasi-Poisson regression model using calendar month as the independent variable was used; p-values of <0.05 were considered statistically significant. We evaluated trends of inappropriate antibiotic prescribing by evaluating antibiotic prescribed (e.g., ciprofloxacin), antibiotic treatment duration (e.g., 2–4 days supply), and inappropriate prescriptions that met either criterion.
The number of prescriptions for “treatment”, “indeterminate” and “prophylaxis” were summed to generate the “overall” category. All analyses were performed using the statistical software package R (v3.3.1). This study was approved by the Washington University in St. Louis Human Research Protection Office.
Results
Antibiotic uses and costs overall
Approximately 6.2 million antibiotic prescriptions were identified over the three year study period (Table 1). While the overall number of antibiotic prescriptions remained stable each year, the overall antibiotic costs declined during our study period from around 19 million dollars in 2013 to a little more than 15 million dollars in 2015. After adjusting for the number of beneficiaries, the overall cost of antibiotics also declined from 614 dollars per 1,000 beneficiaries in 2013 to roughly 512 dollars per 1,000 beneficiaries in 2015.
Table 1.
Assessment of general dentists’ antibiotic prescribing by treatment duration, within the Express Scripts database, January 2013 to December 2015.
| Year | Duration | No. of Prescriptions (millions) | Prescriptions/1,000 beneficiaries (%) | Total Drug Costs ($) | Costs per 1,000 beneficiaries ($) |
|---|---|---|---|---|---|
| 2013 | Overall | 2.08 | 68.10 | 18,762,360 | 613.71 |
| Treatment | 1.71 | 55.86 (82) | 17,350,229 | 567.52 | |
| Indeterminate (Inappropriate) | 0.26 | 8.53 (13) | 1,119,868 | 36.63 | |
| Prophylaxis | 0.11 | 3.71 (5) | 292,263 | 9.56 | |
| 2014 | Overall | 2.08 | 66.50 | 16,595,615 | 530.24 |
| Treatment | 1.72 | 54.92 (83) | 15,345,196 | 490.29 | |
| Indeterminate (Inappropriate) | 0.26 | 8.21 (12) | 996,651 | 31.84 | |
| Prophylaxis | 0.11 | 3.38 (5) | 253,768 | 8.11 | |
| 2015 | Overall | 2.07 | 69.160 | 15,294,704 | 512.09 |
| Treatment | 1.73 | 57.74 (83) | 14,166,070 | 474.30 | |
| Indeterminate (Inappropriate) | 0.24 | 8.14 (12) | 912,755 | 30.56 | |
| Prophylaxis | 0.10 | 3.28 (5) | 215,879 | 7.23 |
Note. “Prophylaxis” was defined as 1 days’ supply of antibiotics or fewer. “Indeterminate” was defined as 2 to 4 days’ supply of antibiotics. “Treatment” was defined as 5 or more days’ supply of antibiotics. Antibiotic prescriptions with an “Indeterminate” were defined as inappropriate because they could not be categorized as prophylaxis or treatment. Costs examined in this study were calculated by adding the prescription drug plan costs and out-of-pocket patient costs. Plan costs included ingredient costs, taxes, dispensing fees and administrative fees and were not adjusted to exclude rebates.
Inappropriate antibiotic use and costs by antibiotic treatment duration (prescription durations that could not be categorized into either prophylaxis or treatment of dental infections based on prescribed duration)
Over 12% of antibiotic prescriptions and 6% of total antibiotic costs were inappropriate due to being prescribed for an indeterminate duration (2–4 days; Table 1). This duration of antibiotics is too long for prophylaxis and shorter than appropriate for most treatment of dental infections. Over the three-year study period, this represents over 3 million dollars in inappropriate prescriptions based on duration alone.
Inappropriate antibiotic use and costs by antibiotic prescribed (agents that should not be routinely used in general dentistry)
Over the three year study period, there were approximately 100,000 (1.63%) inappropriate antibiotic prescriptions based on antibiotic prescribed out of 6,228,948 total prescriptions in this cohort (Table 2). The overall number of inappropriate antibiotics based on agent prescribed decreased from 38,424 (1.84% of total) prescriptions in 2013 to 28,516 prescriptions in 2015 (1.38% of total) (Table 3). In total, inappropriate antibiotic prescriptions by agent prescribed accounted for approximately 5 million dollars (10.36% of total) in drug costs during the three year study period. Interestingly, despite the decreasing number of inappropriate antibiotic prescriptions by agent prescribed annually, the annual inappropriate antibiotic costs by agent prescribed increased by 24.71% from 1.5 million dollars in 2013 to 2.0 million dollars in 2015.
Table 2.
Antibiotic prescriptions among general dentists stratified by appropriateness of antibiotic prescribed (if the antibiotic is routinely used in general dentistry), within the Express Scripts database, January 2013 to December 2015.
| Year | Appropriateness | No. of Prescriptions (millions) | Prescriptions/1,000 beneficiaries (%) | Total Drug Costs ($) | Costs per 1,000 beneficiaries ($) |
|---|---|---|---|---|---|
| 2013 | Appropriate | 2.04 | 66.84 (98) | 17,245,993 | 564.11 |
| Inappropriate | 0.04 | 1.25 (2) | 1,516,367 | 49.60 | |
| 2014 | Appropriate | 2.05 | 65.44 (98) | 14,878,372 | 475.38 |
| Inappropriate | 0.03 | 1.06 (2) | 1,717,242 | 54.87 | |
| 2015 | Appropriate | 2.04 | 68.20 (98) | 13,280,603 | 444.66 |
| Inappropriate | 0.03 | 0.95 (2) | 2,014,101 | 67.44 | |
| Total | Appropriate | 6.13 | 66.83 (98) | 45,404,968 | 494.72 |
| Inappropriate | 0.10 | 1.09 (2) | 5,247,710 | 57.30 |
Note. Detailed appropriateness designations are available in supplemental table. Costs examined in this study were calculated by adding the prescription drug plan costs and out-of-pocket patient costs. Plan costs included ingredient costs, taxes, dispensing fees and administrative fees and were not adjusted to exclude rebates.
Table 3.
Antibiotic prescribing by general dentists stratified by appropriateness of antibiotic prescribed (if agent should be routinely used in general dentistry) and treatment duration (prescription duration is for either prophylaxis or treatment of an infection), within the Express Scripts database, January 2013-December 2015.
| Year | Appropriateness | No. of Prescriptions (millions) | Prescriptions/1,000 beneficiaries (%) | Total Drug Costs ($) | Costs per 1,000 beneficiaries ($) |
|---|---|---|---|---|---|
| 2013 | Appropriate | 1.79 | 58.41 (86) | 16,192,050 | 529.64 |
| Inappropriate | 0.30 | 9.69 (14) | 2,570,309 | 84.07 | |
| 2014 | Appropriate | 1.79 | 57.31 (86) | 13,957,298 | 445.95 |
| Inappropriate | 0.29 | 9.19 (14) | 2,638,316 | 84.30 | |
| 2015 | Appropriate | 1.80 | 60.14 (87) | 12,472,193 | 417.59 |
| Inappropriate | 0.27 | 9.02 (13) | 2,822,511 | 94.50 | |
| Total | Appropriate | 5.38 | 58.62 (86) | 42,621,541 | 464.39 |
| Inappropriate | 0.85 | 9.30 (14) | 8,031,137 | 87.62 |
Note. Costs examined in this study were calculated by adding the prescription drug plan costs and out-of-pocket patient costs. Plan costs included ingredient costs, taxes, dispensing fees and administrative fees and were not adjusted to exclude rebates. Appropriate antibiotic duration as defined as ≤1 day of antibiotic treatment or ≥5 days of antibiotics. A list of inappropriate antibiotics based on agent prescribed can be found in the supplemental materials.
Total burden of inappropriate antibiotic prescriptions
Taking into account inappropriate prescriptions based on both treatment duration and agent prescribed, Over 850,000 (13.70%) antibiotics were prescribed inappropriately during the three year study period. The total number of inappropriate antibiotics prescribed decreased over time from 296,329 (14.23%) prescriptions in 2013 to 269,419 (13.04%) prescriptions in 2015 (Table 3). In total, these inappropriate antibiotic prescriptions represent over 8 million dollars (15.86%) in unnecessary healthcare expenditures within this cohort. The annual inappropriate antibiotic spending increased from 2.6 million dollars in 2013 to 2.8 million dollars in 2015.
Trends in antibiotic use
From January 2013 to December 2015, the quasi-Poisson regression model demonstrated that overall rate of antibiotics prescribed per 1,000 beneficiaries remained stable (p=0.701; Figure 1). Similar results were observed for antibiotics used for treatment purposes (p=0.210; Figure 1). However, the rate of antibiotics prescribed for an indeterminate duration decreased by 0.21% per month (95% CI: 0.03%–0.40%; p=0.033); the rate of antibiotics prescribed for prophylaxis decreased by 0.50% per month (95% CI: 0.28%–0.72%; p< 0.001) (Figure 1).
Figure 1.
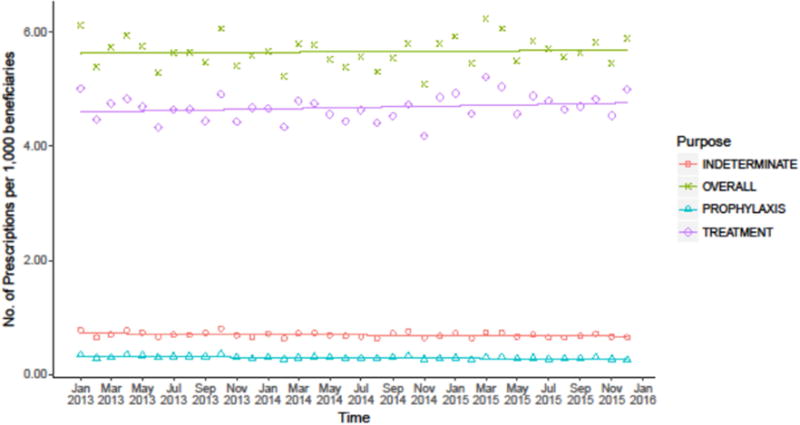
Trend in antibiotic prescriptions by treatment duration per 1,000 beneficiaries, within the Express Scripts database, January 2013 to December 2015.
Note. “Prophylaxis” was defined as 1 days’ supply of antibiotics or fewer. “Indeterminate” was defined as 2 to 4 days’ supply of antibiotics. “Treatment” was defined as 5 or more days’ supply of antibiotics. Antibiotic prescriptions with an “Indeterminate” were defined as inappropriate because they could not be categorized as prophylaxis or treatment. Trend lines were generated using a quasi-Poisson regression model.
When using a quasi-Poisson regression model to evaluate the rate of inappropriate antibiotic prescriptions per 1,000 beneficiaries by antibiotic agent prescribed, there were additional noteworthy trends. Appropriate antibiotic use overall remained stable over time (p=0.526; data not shown). However, the rate of inappropriate antibiotic prescribing (based on agent prescribed) declined by 1.14% per month (95% CI: 0.98–1.31%; p<0.001; Figure 2).
Figure 2.
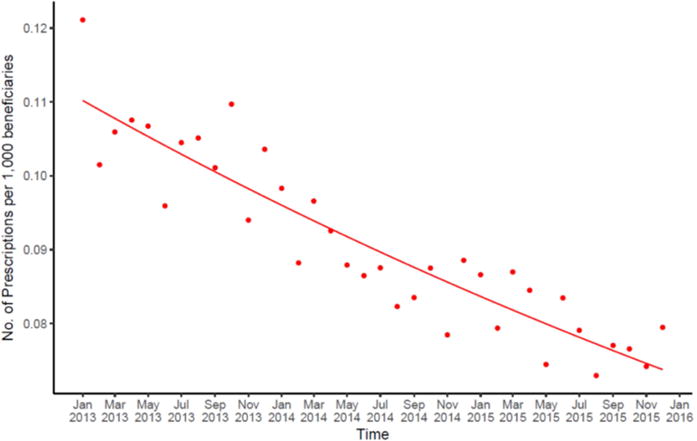
Trend in inappropriate antibiotic prescriptions by antibiotic prescribed per 1,000 beneficiaries, within the Express Scripts database, January 2013 to December 2015.
Note. Detailed appropriateness designations are available in supplemental table. Trend lines were created using a quasi-Poisson regression model.
When a quasi-Poisson regression model was used to evaluate antibiotic prescriptions by both treatment duration and agent prescribed, the rate of appropriate antibiotic prescribing remained stable over time (p= 0.278; data not shown). However, general dentists prescribed significantly fewer inappropriate drugs over time by 0.31% per month (95% CI: 0.14%–0.49%; p=0.002; Figure 3).
Figure 3.
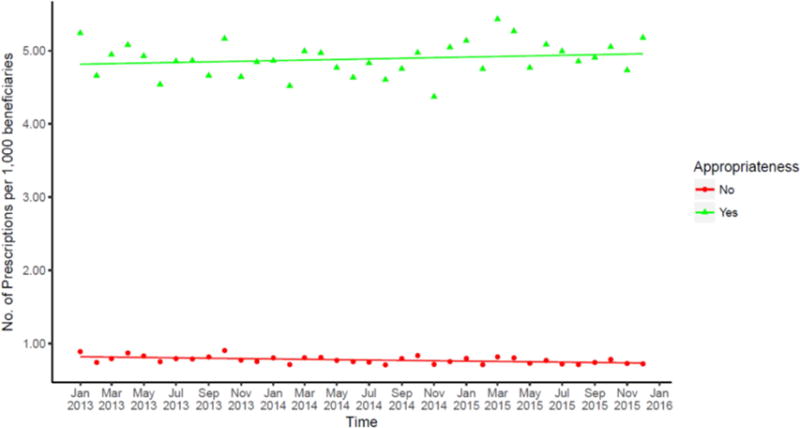
Trend in inappropriate antibiotic prescriptions by antibiotic prescribed (if agent should not be routinely used in general dentistry) and treatment duration (prescription duration is not consistent with prophylaxis or treatment of dental infection) per 1,000 beneficiaries, within the Express Scripts database, January 2013 to December 2015.
Note: Antibiotic prescriptions with an “indeterminate” 2–4 day treatment duration were defined as inappropriate because they could not be categorized as prophylaxis or treatment. Detailed appropriateness designations are available in supplemental table. Trend lines were generated using quasi-Poisson regression model.
Discussion
To our knowledge, this study is the first and largest longitudinal evaluation of antibiotic prescribing practices of general dentists in a large cohort in the United States. This study is the first to quantify the amount of inappropriate antibiotics prescribed by general dentists in the US and to estimate the cost of inappropriately prescribed antibiotics. Although the rate of antibiotic prescribing in this cohort was stable over time, there was a slight decrease in antibiotics used for indeterminate and prophylaxis purposes. Antibiotic expeditures did decrease over time. However, this was likely related to cost containment strageties (e.g effective purchase contracts and more utilization of generics) from the pharmacy benefits manager. Inappropriate antibiotic use based on agent prescribed and duration was common and contributed to millions of dollars in unnecessary prescription expenditures annually.
The results of this study are in contrast to findings from some other countries. Marra et al. reported a concerning trend of increasing antibiotic prescribing by Canadian dentists from 1996–2013,19 and longitudinal studies of dental prescribing practices in Australia25 and the Czech Republic26 also showed increasing trends in dental antibiotic prescribing.
Reports from dentists cite numerous reasons for prescribing antibiotic. Themes identified in a survey of Canadian dentists include antibiotic use when surgery is indicated, slow adoption of new prophylaxis guidelines, and an aging patient population, among others.19 A British study that investigated patient behaviors and dental visit characteristics associated with antibiotic prescriptions found that patients requesting antibiotics and refusing surgery and dentists’ desire to save time led to more antibiotic prescriptions.27 Another study in the United Kingdom noted that dentists’ tendencies to prescribe antibiotics cannot be predicted by their gender, postgraduate qualification status, or years since qualification, indicating that antibiotic over-prescribing is likely a widespread problem across dental providers.28
While dental prescribing rates have remained unchanged overall, dentists appear to be prescribing fewer antibiotics for prophylaxis. Some of this improvement may be related to the 2007 American Heart Association (AHA) guidelines, which restricted the number of appropriate categories for endocarditis prophylaxis.29 However, more recent guidelines may also be playing a role. In 2014, the ADA Council on Scientific Affairs altered previous guidelines by recommending that antibiotic prophylaxis not be used prior to dental procedures for patients with prosthetic joints, although it is entirely unclear to what extent this has impacted the use of antibiotics for this purpose.30 There are likely many opportunities for continued improvement.
Other than guidelines on the use of antibiotic prophylaxis for patients with prosthetic joints or at risk of infective endocarditis, there are ADA guidelines that comprehensively cover antibiotic usage. One publication in 2004 from the ADA Council on Scientific Affairs31 provides general recommendations, but these do not explicitly endorse the use of specific antibiotics for certain types of infections. There are no recommendations for duration of treatment in any of these guidelines. The American Academy of Pediatric Dentistry, however, has more specific guidelines for pediatric dental patients that include recommendations for a variety of orofacial conditions.32,33
Other countries have established dental guidelines for antibiotic use. In 2016, The Scottish Dental Clinical Effectiveness Programme (SDCEP) published its third iteration of prescription guidelines for dentists.34 This publication contains guidelines for a variety of dental and oral infections and specifies first-line versus second-line antibiotic medication choices and appropriate doses and durations.34 The Canadian Collaboration on Clinical Practice Guidelines in Dentistry has also published guidelines for treating conditions like acute apical periodontitis and abscess.35 Clearly, detailed treatment guidelines for US dentists are necessary to improve and standardize antibiotic prescribing.
Identifying evidence-based guideline recommendations may be challenging. The Cochrane Oral Health Group has attempted to develop dental antibiotic recommendations based on systematic review/meta-analysis for irreversible pulpitis, apical periodontitis and acute apical abscess.36,37 The lack of randomized clinical trials investigating antibiotic use in the scope of these conditions prevented the authors from drawing definitive conclusions.36,37
Several antimicrobial stewardship interventions have demonstrated success in improving antibiotic utilization among dentists. In a 2001 study of 175 general dentists in the United Kingdom, researchers demonstrated a 42.5% decrease in antibiotic prescriptions after an educational intervention in which guidelines were issued.18 In a follow-up qualitative study describing the experiences of dentists who took part in the audit, over 97% of participating dentists stated the audit was useful and almost 70% of dentists reported changing their prescribing practices as a result of the audit.38 Other investigators have studied the audit and feedback intervention with similarly encouraging results.20,39 However, further investigation is needed to determine if these initial positive results are maintained long-term.
Further research is needed to more completely characterize dental antibiotic prescribing trends in the US. Specifically, qualitative investigations on dentists’ rationale for antibiotic selection and prescription duration are needed to truly evaluate their appropriateness. Such insights could result in the successful development and implementation of more effectively targeted interventions. Additionally, longer study periods are needed to further evaluate trends over time and more completely assess the effects of updated prophylaxis guidelines.
Our study has limitations. Individuals analyzed in our study were limited to patients within the ESHC database, so our results may not be generalizable to the entire US population. Additionally, the ESHC database only includes prescriptions which were paid for by a patient’s insurance plan. Cheaper prescriptions, which may be less likely to prompt patients to use insurance benefits, may result in an underestimation of antibiotic prescription rates. Our classifications for appropriateness were fairly narrow and based on expert opinion and limited available US-based guidelines. Although we believe that the antibiotic agents prescribed and treatment durations we classified as inappropriate represent inappropriate antibiotics, this was an assumption in our calculations. Some of the indeterminate antibiotic prescriptions may be appropriately prescribed. For example, providers may elect to treat a patient with antibiotics until they could be seen by a dentist for an extraction or other another definitive procedure. Similarly, dentists may prescribe a two day supply of antibiotics for individuals who may require several dental procedures in the near future. In addition, our prescriptions were classified by treatment duration based on data from ESHC, so some atypical doses or instructions on the prescription bottle could have caused some antibiotic prescriptions to be misclassified. Unfortunately since the ESHC database did not contain indications for the antibiotic prescriptions, we were unable to fully assess the magnitude of inappropriate antibiotic prescribing among US general dentists in our cohort.
Conclusions
We demonstrated that the overall rate of antibiotic prescriptions among general dentists has remained stable over time. In contrast to studies from other countries, we observed decreasing rates of inappropriate antibiotic prescriptions based on agent prescribed and treatment duration. Nonetheless, inappropriate antibiotic prescriptions based on antibiotic type and treatment duration were common (14%) in our study and represented millions of dollars in inappropriate pharmaceutical healthcare expenditures annually. Further interventions are necessary to improve antibiotic prescribing practices among general dentists. Antimicrobial stewardship programs specifically targeted to dentists should be implemented and evaluated.
Supplementary Material
Acknowledgments
This study was funded by the CDC Epicenters U54CK000482 (MJD, KS, YHS QF, SRJ, and VJF).
Research reported in this publication was also supported by the Washington University Institute of Clinical and Translational Sciences grant UL1TR000448, sub-award KL2TR000450, from the National Center for Advancing Translational Sciences (NCATS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosure. Dr. Durkin has received funds from Medscape to create continuing medical education materials regarding the management of acute bacterial skin and skin structure infections. These funds were provided to Medscape through an unrestricted educational grant from Merck. Dr. Munshi states that outside of the submitted work, he is an employee of Express Scripts and have received company stock/stock options as part of employee compensation. Dr. Frasier has grants from NIH, CDC and the Foundation for Barnes-Jewish Hospital. In addition, her spouse is Senior Vice President and Chief Medical Officer for Express Scripts.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Statements:
Michael J. Durkin contributed to all aspects of the project.
Qianxi Feng and Kyle Warren performed data analysis, manuscript preparation, and interpretation of results.
Kiraat Munshi and Rochelle Henderson assisted design of the study, acquisition of the data, analysis of the results, and revising the manuscript.
Kevin Hsueh contributed to the design of the study, interpretation of the results, and drafting the manuscript
Peter Lockhart and Martin Thornhill served as content experts; they contributed to the design of the work, analysis and interpretation of the results, and critically revising the manuscript.
Victoria Fraser contributed to the design of the study, interpretation of the results, and critically revising the manuscript.
References
- 1.Antibiotic resistance threats in the United States. 2013 Available at: http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed May 15,2017. [PubMed]
- 2.Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clinical Infectious Diseases. 2015 May 1;60(9):1308–16. doi: 10.1093/cid/civ076. [DOI] [PubMed] [Google Scholar]
- 3.Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. Journal of the American Medical Association. 2016;315(17):1864–73. doi: 10.1001/jama.2016.4151. [DOI] [PubMed] [Google Scholar]
- 4.Goossens H, Ferech M, Vander Stichele R, Elseviers M, Group EP Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. The Lancet. 2005;365(9459):579–87. doi: 10.1016/S0140-6736(05)17907-0. [DOI] [PubMed] [Google Scholar]
- 5.Van De Sande-Bruinsma N, Grundmann H, Verloo D, et al. Antimicrobial drug use and resistance in Europe. Emerging Infectious Diseases. 2008;14(11):1722. doi: 10.3201/eid1411.070467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leffler DA, Lamont JT. Clostridium difficile infection. New England Journal of Medicine. 2015;372(16):1539–48. doi: 10.1056/NEJMra1403772. [DOI] [PubMed] [Google Scholar]
- 7.Bartlett JG, Chang TW, Gurwith M, Gorbach SL, Onderdonk AB. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. New England Journal of Medicine. 1978;298(10):531–34. doi: 10.1056/NEJM197803092981003. [DOI] [PubMed] [Google Scholar]
- 8.Suda KJ, Hicks LA, Roberts RM, Hunkler RJ, Danziger LH. A national evaluation of antibiotic expenditures by healthcare setting in the United States, 2009. Journal of Antimicrobial Chemotherapy. 2013;68(3):715–18. doi: 10.1093/jac/dks445. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention get smart about antibiotics. Available at: https://www.cdc.gov/getsmart/community/index.html. Accessed 8/25/2017.
- 10.Centers for Disease Control and Prevention core elements of hospital antibiotic stewardship programs. doi: 10.1093/cid/ciu542. Available at: https://www.cdc.gov/getsmart/healthcare/pdfs/core-elements.pdf. Accessed May 15,2017. [DOI] [PMC free article] [PubMed]
- 11.Centers for Disease Control and Prevention core elements of antibiotic stewardship for nursing homes. Available at: https://www.cdc.gov/longtermcare/pdfs/core-elements-antibiotic-stewardship.pdf. Accessed May 14, 2017.
- 12.Centers for Disease Control and Prevention core elements of outpatient antibiotic stewardship. Available at: https://www.cdc.gov/getsmart/community/improving-prescribing/core-elements/core-outpatient-stewardship.html. Accessed May 15,2017.
- 13.Joint statement on importance of outpatient antibiotic stewardship from 12 national health organizations. Available at: https://www.cdc.gov/getsmart/community/pdfs/Joint-Statement-on-Importance-of-Outpatient-Antibiotic-Stewardship-with-logos.pdf. Accessed August 25, 2017.
- 14.The Joint Commission new antimicrobial stewardship standard. Available at: https://www.jointcommission.org/assets/1/6/New_Antimicrobial_Stewardship_Standard.pdf. Accessed May, 15 2017.
- 15.Durkin MJHK, Sallah YA, Hsueh K. An examination of dental antibiotic prescribing in the United States. Journal of the American Dental Association. doi: 10.1016/j.adaj.2017.07.019. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts RM, Bartoces M, Thompson SE, Hicks LA. Antibiotic prescribing by general dentists in the United States, 2013. The Journal of the American Dental Association. 2017;148(3):172–78. doi: 10.1016/j.adaj.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lockhart PB, Hanson NB, Ristic H, Menezes AR, Baddour L. Acceptance among and impact on dental practitioners and patients of American Heart Association recommendations for antibiotic prophylaxis. The Journal of the American Dental Association. 2013;144(9):1030–35. doi: 10.14219/jada.archive.2013.0230. [DOI] [PubMed] [Google Scholar]
- 18.Palmer N, Dailey Y, Martin M. Pharmacology: Can audit improve antibiotic prescribing in general dental practice? British Dental Journal. 2001;191(5):253–55. doi: 10.1038/sj.bdj.4801156a. [DOI] [PubMed] [Google Scholar]
- 19.Marra F, George D, Chong M, Sutherland S, Patrick DM. Antibiotic prescribing by dentists has increased: Why? The Journal of the American Dental Association. 2016;147(5):320–27. doi: 10.1016/j.adaj.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 20.Chate R, White S, Hale L, et al. The impact of clinical audit on antibiotic prescribing in general dental practice. British Dental Journal. 2006;201(10):635–41. doi: 10.1038/sj.bdj.4814261. [DOI] [PubMed] [Google Scholar]
- 21.Steinman MA, Gonzales R, Linder JA, Landefeld CS. Changing use of antibiotics in community-based outpatient practice, 1991–1999. Annals of Internal Medicine. 2003;138(7):525–33. doi: 10.7326/0003-4819-138-7-200304010-00008. [DOI] [PubMed] [Google Scholar]
- 22.Hicks LA, Chien Y-W, Taylor TH, Haber M, Klugman KP. Outpatient antibiotic prescribing and nonsusceptible Streptococcus pneumoniae in the United States, 1996–2003. Clinical infectious Diseases. 2011;53(7):631–39. doi: 10.1093/cid/cir443. [DOI] [PubMed] [Google Scholar]
- 23.Suda KJ, Hicks LA, Roberts RM, Hunkler RJ, Taylor TH. Trends and seasonal variation in outpatient antibiotic prescription rates in the United States, 2006 to 2010. Antimicrobial Agents and Chemotherapy. 2014;58(5):2763–66. doi: 10.1128/AAC.02239-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Dental Association Oral Health Topics, Anibiotic Stewardship. Available at: http://www.ada.org/en/member-center/oral-health-topics/antibiotic-stewardship. Accessed October 4, 2017.
- 25.Ford P, Saladine C, Zhang K, Hollingworth S. Prescribing patterns of dental practitioners in Australia from 2001 to 2012. Antimicrobials Australian Dental Journal. 2017;62(1):52–57. doi: 10.1111/adj.12427. [DOI] [PubMed] [Google Scholar]
- 26.Pipalova R, Vlcek J, Slezak R. The trends in antibiotic use by general dental practitioners in the Czech Republic (2006–2012) International Dental Journal. 2014;64(3):138–43. doi: 10.1111/idj.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cope AL, Francis NA, Wood F, Chestnutt IG. Antibiotic prescribing in UK general dental practice: a cross-sectional study. Community Dentistry and Oral Epidemiology. 2016;44(2):145–53. doi: 10.1111/cdoe.12199. [DOI] [PubMed] [Google Scholar]
- 28.Seager J, Howell-Jones R, Dunstan F, et al. A randomised controlled trial of clinical outreach education to rationalise antibiotic prescribing for acute dental pain in the primary care setting. British Dental Journal. 2006;201(4):217–22. doi: 10.1038/sj.bdj.4813879. [DOI] [PubMed] [Google Scholar]
- 29.Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. The Journal of the American Dental Association. 2008;139(Suppl):3s–24s. doi: 10.14219/jada.archive.2008.0346. [DOI] [PubMed] [Google Scholar]
- 30.Sollecito TP, Abt E, Lockhart PB, et al. The use of prophylactic antibiotics prior to dental procedures in patients with prosthetic joints: evidence-based clinical practice guideline for dental practitioners—a report of the American Dental Association Council on Scientific Affairs. The Journal of the American Dental Association. 2015;146(1):11–16. doi: 10.1016/j.adaj.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 31.American Dental Association Council on Scientific Affairs. Combating antibiotic resistance. The Journal of the American Dental Association. 2004;135(4):484–87. doi: 10.14219/jada.archive.2004.0214. [DOI] [PubMed] [Google Scholar]
- 32.Guideline on use of antibiotic therapy for pediatric dental patients. Pediatric Dentistry. 2014;37(6):325–327. [PubMed] [Google Scholar]
- 33.Guideline on antibiotic prophylaxis for dental patients at risk for infection. Pediatric Dentistry 2008–2009. 30(7 Suppl):215–8. [PubMed] [Google Scholar]
- 34.Drug prescribing for dentistry: dental clinical guidance. 3rd. Dundee: Scottish dental clinical effectiveness programme; Available at: http://www.sdcep.org.uk/wp-content/uploads/2016/03/SDCEP-Drug-Prescribing-for-Dentistry-3rd-edition.pdf. Accessed 8/25/2017. [Google Scholar]
- 35.Glenny A-M, Simpson T. Clinical practice guideline on emergency management of acute apical periodontitis (AAP) in adults. Evidence-Based Dentistry. 2004;5(1):7. doi: 10.1038/sj.ebd.6400233. [DOI] [PubMed] [Google Scholar]
- 36.Cope A, Francis N, Wood F, Mann MK, Chestnutt IG. Systemic antibiotics for symptomatic apical periodontitis and acute apical abscess in adults. Cochrane Database Systematic Reviews. 2014 Jun;26(6):CD010136. doi: 10.1002/14651858.CD010136.pub2. [DOI] [PubMed] [Google Scholar]
- 37.Agnihotry A, Fedorowicz Z, van Zuuren EJ, Farman AG, Al-Langawi JH. Antibiotic use for irreversible pulpitis. Cochrane Database Systematic Reviews. 2016 Feb 17;2:CD004969. doi: 10.1002/14651858.CD004969.pub4. [DOI] [PubMed] [Google Scholar]
- 38.Palmer N, Dailey Y. General dental practitioners’ experiences of a collaborative clinical audit on antibiotic prescribing: a qualitative study. British Dental Journal. 2002;193(1):46–49. doi: 10.1038/sj.bdj.4801480. [DOI] [PubMed] [Google Scholar]
- 39.Elouafkaoui P, Young L, Newlands R, et al. An audit and feedback intervention for reducing antibiotic prescribing in general dental practice: the RAPiD cluster randomised controlled trial. Public Library of Science Medicine. 2016;13(8):e1002115. doi: 10.1371/journal.pmed.1002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


