Abstract
Objective and design
This study aimed to evaluate the effect of all-trans retinoic acid (atRA) on suppressing the inflammatory response and promoting the osteoblastic differentiation of bone marrow stromal cells (BMSCs) on titanium in a lipopolysaccharide (LPS)-induced microenvironment.
Methods
BMSCs were divided into four groups and treated with LPS (1 μg/mL), atRA (1 nmol/L), LPS + atRA, or left untreated. Cells were then cultured on titanium surfaces and cell function compared. BMSC proliferation and osteoblastic differentiation were assessed using the MTT assay, alkaline phosphatase (ALP) activity, alizarin red staining, and quantitative real-time polymerase chain reaction (RT-PCR). Expression levels of inflammatory factors were measured by quantitative RT-PCR and enzyme-linked immunosorbent assay.
Results
Increased mineralized nodule formation, ALP activity, osteocalcin, and osteopontin expression levels were detected in LPS + atRA-treated BMSCs after osteogenic induction, when compared with LPS-treated cells. In addition, the high levels of tumor necrosis factor-α, interleukin-1β, and receptor activator of nuclear factor-κ B ligand (RANKL) expression induced by LPS were inhibited after treatment with atRA.
Conclusions
Our results showed the effects of atRA on suppressing inflammatory responses and promoting osteoblastic differentiation of BMSCs on titanium in an LPS-induced microenvironment. This indicates the potential therapeutic value of atRA for treating peri-implants inflammatory disease.
Keywords: Retinoic acid, Lipopolysaccharide, BMSCs, Titanium, Inflammation, Osteogenic
Introduction
Periodontal tissues exposed to the oral microbial environment are easily infected by bacteria and undergo excessive alveolar bone resorption and attached soft-tissue destruction, regularly seen in periodontitis and peri-implantitis [1, 2]. Porphyromonas gingivalis (P. g) [3] is considered the major pathogen of periodontitis and peri-implantitis; therefore, many studies have been conducted to identify the pathogenic mechanism in these diseases [4, 5]. Lipopolysaccharide (LPS) is a highly bioactive molecule produced by Gram-negative bacterium, including P. g [6]. LPS functions predominantly to promote bone-absorbing cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and receptor activator of nuclear factor-κ B ligand (RANKL). LPS, therefore, plays an important role in the development of periodontal soft-tissue destruction and alveolar bone resorption [7, 8].
In peri-implantitis patients, LPS initiates peri-implant inflammation and subsequently affects implant success rate [9]. Soluble LPS can adhere to the titanium surface and become resistant to removal by sterilization, even when treated with antibiotics [10, 11]. Furthermore, another study found that LPS adhered to titanium surfaces did not affect cell attachment but inhibited osteogenic differentiation [12]. To reduce LPS-induced inflammation, several pharmaceutical approaches have been applied, including endotoxin-specific antibodies, cytokine receptor antagonists, and anti-inflammatory cytokines [10, 13].
Administration of vitamin A [14] has been identified to suppress inflammatory responses induced by pathogens [15, 16]. However, neither the anti-inflammatory effect nor osteogenic potential of vitamin A in the P. g-LPS-induced environment is fully understood. Bone marrow stromal cells (BMSCs) are a population of multipotent cells with potential therapeutic value. Owing to their osteogenic capability, BMSCs have been applied for treatment of periodontal bony defects and to facilitate osseointegration [17, 18]. In this study, we aimed at investigating the regulatory effects of all-trans retinoic acid (atRA), a biologically active metabolite of vitamin A, on LPS-stimulated BMSCs cultured on titanium surfaces.
Materials and methods
Isolation and identification of BMSCs
BMSCs were obtained as previously described [19, 20]. Briefly, under aseptic conditions, both femur and tibia bones were excised from 8-week-old female Sprague–Dawley rats, followed by carefully removing the attached muscles. Both ends of each bone were then cut and the bone marrow was flushed out by injecting alpha Minimal Essential Medium Eagle (Gibco-BRL, Grand Island, NY, USA) containing 20 % fetal bovine serum (Gibco-BRL) and 1 % penicillin/streptomycin. The cell suspension was plated and cultured at 37 °C with 5 % CO2. Non-adherent cells were removed by changing the medium after 72 h. Cells at passages 2–4 were used in this study. This study was approved by the ethics committee of Wuhan University, China. BMSCs were obtained as previously described [19, 20].
Single-cell BMSC (at passages 2–4) suspensions were obtained and flow cytometry was performed using a FACSCalibur flow cytometer (San Jose, CA, USA) to detect CD29, CD90, and CD45 cell surface markers. The following mAbs were used: FITC-labeled anti-CD29, PE-labeled anti-CD45, anti-CD90, and their isotype controls. All antibodies were purchased from BD Biosciences and R&D Systems.
Titanium disk preparation and BMSC seeding
Samples of commercial pure titanium, 15 mm in diameter and 1 mm thick, were purchased from the Nonferrous Metal Industry Co. (Baoji, China). In accordance with previous reports, samples were first polished using sandpaper, then were washed in acetone, followed by absolute ethyl alcohol and double-distilled water (twice), prior to air drying [21, 22]. Disks were individually placed into wells of a 24-well plate with the polished surface upwards.
BMSCs were detached from culture surfaces by treatment with 0.025 % trypsin/0.05 % EDTA and were reseeded onto the titanium disks in 50 μL of suspension to cover the disk surface, at 5 × 104 cells/cm2. An equivalent amount of cells was seeded into wells of a 24-well plate as a control. Cells were incubated at 37 °C with 5 % CO2 for 4 h, prior to adding 1 mL/well media. Cell medium was changed every 3 days.
MTT assay
Cultures were maintained for 24 h in medium supplemented with 10 % FBS. Different concentrations of LPS (10, 1, 0.1, and 0.01 μg/mL) were used to stimulate BMSCs. At a concentration of 1 μg/mL, LPS did not affect BMSC proliferation but significantly decreased mineralization; therefore, this concentration was used for the study. In accordance with previous studies, a dose of 1 nmol/L atRA was selected for our study [15, 16]. BMSCs were divided into four groups as follows: (1) stimulation with LPS (1 μg/mL); (2) stimulation with atRA (1 nmol/L); stimulation with LPS (1 μg/mL), and atRA (1 nmol/L); (4) untreated control. BMSC proliferation was assessed using the MTT assay [23]. Briefly, cells were seeded as described above and were exposed to MTT for 4 h. After removal of the medium and addition of dimethyl sulfoxide, the absorbance was measured at 490 nm.
Mineralization assay
After BMSC adhesion and expansion on titanium, the cells were cultivated in osteogenic medium as previously described [24, 25]. Identical concentrations of P. g-LPS and atRA were added into the medium, with medium changes every 3 days thereafter. At day 14, BMSCs on the titanium were fixed with 4 % cold paraformaldehyde for 20 min at room temperature, and incubated with Alizarin Red for 30 min. Excess stain was removed by washing three times with phosphate-buffered saline (PBS). Hexa-decylpyridinium chloride was used to dissolve the mineralized nodules, and obtain quantitative data by measuring OD values at 562 nm.
Alkaline phosphatase activity
At days 7 and 14, BMSCs cultured in osteogenic medium were treated with triton for 4 h. The supernatants were then added into wells of a 96-well microplate. Enzyme assay solution (200 μL) was added to each well and the plate was incubated for 30 min in a 37 °C incubator. The enzyme reaction was terminated by the addition of 50 μL of 0.1 mol/L NaOH. The ALP reaction product was determined by measuring the absorbance at 405 nm using a microtiter plate reader.
Enzyme-linked immunosorbent assay (ELISA) for cytokine determination
The presence of the cytokines, TNF-α and IL-1β, in the supernatants was evaluated using ELISA kits (Elabscience Technology Co., Wuhan, China) according to the manufacturer’s instructions.
Quantitative real-time polymerase chain reaction (RT-PCR)
BMSCs on titanium surfaces were washed with PBS and total cellular RNA was extracted using an HP RNA Mini kit (Omega, Norcross, GA, USA). Total RNA was reverse transcribed in accordance with the manufacturer’s instructions (Takara, Tokyo, Japan). After reverse transcription, the 20-μL reaction mixture was diluted to 200 μL so that 5 μL of the RT reaction, when added to a PCR, would be equivalent to 100 ng of total RNA.
cDNAs were synthesized from 1 μg of total RNA from the cultured cells. PCR amplification was performed in a real-time PCR system with specific primers for TNF-α, IL-1β, osteocalcin (OCN), osteopontin (OPN), and RANKL, and β-actin was used as an internal control. The reaction conditions for PCR were 40 cycles of denaturation at 95 °C for 15 s, annealing at 55 °C for 34 s, and extension at 72 °C for 1 min. Primer sequences for differentiation markers are detailed in Table 1.
Table 1.
The primers used for real-time RT-PCR (β-actin was used as a housekeeping gene)
| Primer | Sequences 5′–3′ |
|---|---|
| OCN | Forward: CTGACAAAGCCTTCATGTCCA |
| Reverse: CACATGCCCTAAACGGTGGT | |
| OPN | Forward: ACGAATCTCACCATTCCGAT |
| Reverse: AGGTCCTCATCTGTGGCATC | |
| RANKL | Forward: TTCATGTTCCTGGCGCTCCT |
| Reverse: TGCTTCTGTGTCTTCGCTCTCC | |
| IL-1β | Forward: TGTGAAATAGCAGCTTTCGAC |
| Reverse: ACAGCCACAATGAGTGACA | |
| TNF-α | Forward: TACTGAACTTCGGGGTGATTGGTCC |
| Reverse: CAGCCTTGTCCCTTGAAGAGAACC | |
| β-actin | Forward: ATTGAACACGGCATTGTCAC |
| Reverse: AGGCATACAGGGACAACACA |
Statistical analysis
All values were expressed as mean ± standard deviations of three independent experiments. Statistical significance of the differences between each group was assessed by one-way analysis of variance with Tukey’s post-test and P < 0.05 was considered significant.
Results
Molecular phenotype of primary BMSCs
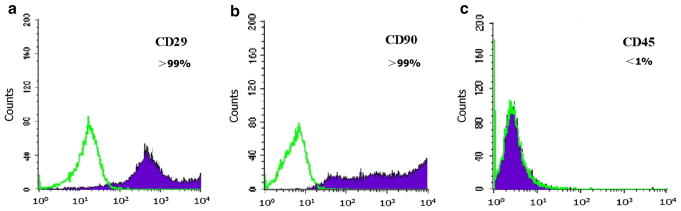
Flow cytometry using single-cell BMSC suspensions (at passages 2–4) showed that expression of CD29 and CD90 was greater than 95 %, while expression of CD45 was less than 5 %. This indicated that the BMSC population used in our experiments was uniform with high purity (Fig. 1).
Fig. 1.
FACS assay showing the expression of CD29 and CD90 was more than 95 %, but CD45 less than 5 %; auto fluorescence is marked as a green filled histograms (color figure online)
BMSCs proliferation on titanium disks after stimulation
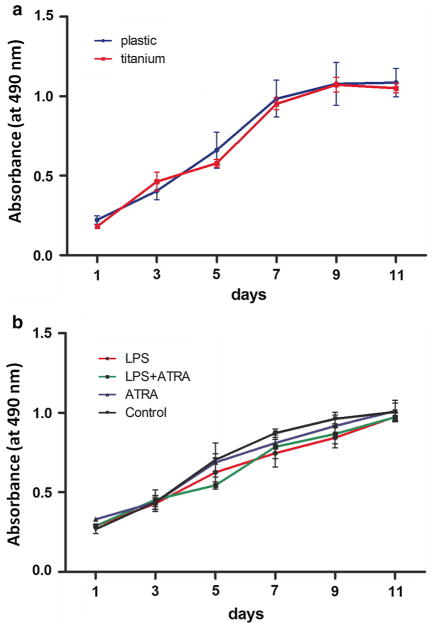
There was no statistically significant difference in BMSC proliferation between titanium disk and tissue culture plastic during 11 days culture in vitro (Fig. 2a).
Fig. 2.
Cell viability of BMSCs cultured on titanium surface up to 11 days, and cells grown on plastic surface were used as control (a); BMSCs cultured on titanium surface after stimulation with LPS (1 μg/mL), atRA (1 nmol/L), LPS + atRA, or left untreated. Data were expressed as mean ± SD from three independent cultures with triplicates
Similarly, there was no significant difference in BMSC proliferation between P. g-LPS and atRA-stimulated cells compared with the untreated control group (Fig. 2b). This result suggested that neither P. g-LPS nor atRA influenced BMSC proliferation on titanium disks.
Alizarin Red staining and ALP activity
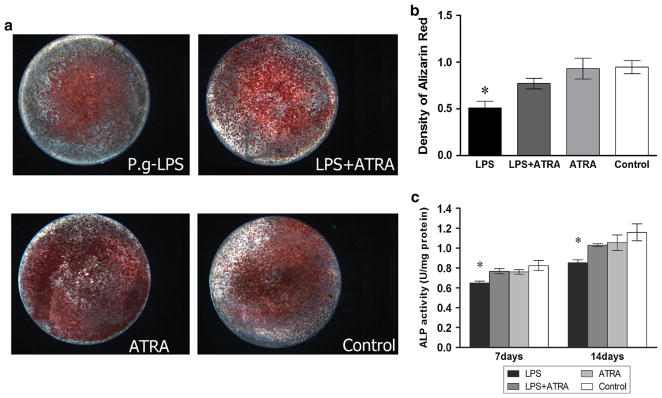
Figure 3a shows images of Alizarin Red-stained cells from each group following culture in osteogenic medium. Fewer mineralized nodules formed when P. g-LPS was present in the medium, whilst more mineralized nodules formed when atRA was added simultaneously. Quantitative assessment of Alizarin Red confirmed the results (Fig. 3b).
Fig. 3.
a Representative Alizarin Red staining showing the osteoblastic differentiation of BMSCs cultured on titanium surface for 2 weeks after stimulation (×10). b OD values of the destained solution of Alizarin Red from above cells staining. c Quantitative results of ALP assay of BMSCs in osteogenic medium. Data were expressed as mean ± SD from three independent cultures with triplicates, *P < 0.05 indicated the difference compared to control group
Alkaline phosphatase (ALP) activity at day 14 is shown in Fig. 3c. Compared with the control, P. g-LPS treatment decreased ALP activity but levels were recovered after addition of atRA.
Osteogenic gene expression in BMSCs
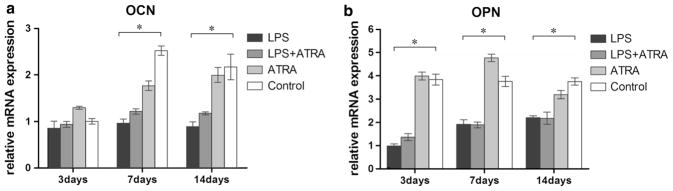
Quantitative real-time RT-PCR results for osteogenic-related marker mRNA expression in each group, relative to the control, are shown in Fig. 4. P. g-LPS markedly decreased mRNA expression of OCN and OPN in BMSCs at almost every time point. However, in the presence of atRA, there was no obvious effect on expression of OPN or OCN.
Fig. 4.
Real-time PCR analysis of osteogenic genes in BMSCs cultured on titanium disks after treatments: OCN and OPN expression at 3, 7, 14 days (a, b). The results were represented as relative ratio to LPS (LPS = 1) at the first time point. Data were expressed as mean ± SD from three independent cultures with triplicates, * P < 0.05
Inflammatory cytokine production and gene expression in BMSCs
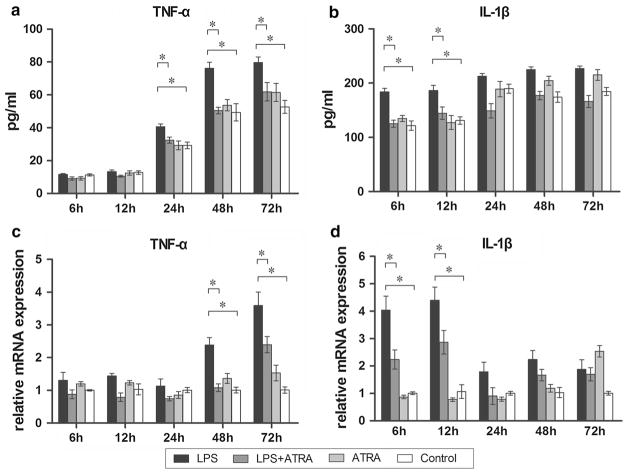
Changes in TNF-α and IL-1β in the culture medium of the BMSC groups were evaluated by ELISA (Fig. 5a, b). The level of IL-1β in the P. g-LPS-treated group increased significantly at 6 and 12 h, while the level of TNF-α increased significantly at 24, 48, and 72 h compared with the control (P < 0.05). Interestingly, levels of IL-1β and TNF-α returned to normal at identical time points when atRA was given in combination with P. g-LPS.
Fig. 5.
ELISA analysis of TNF-α and IL-1β produced by BMSCs cultured on titanium surface after 6, 12, 24, 48 and 72 h treatments (a, b). Real-time PCR analysis of inflammatory genes TNF-α and IL-1β in BMSCs cultured on titanium disks after 6, 12, 24, 48 and 72 h treatments (c, d). The results were represented as relative ratio to control (control = 1) at the first time point. Data were expressed as mean ± SD from three independent cultures with triplicates, *P < 0.05
Gene expression of TNF-α and IL-1β was also examined (Fig. 5c, d). Addition of P. g-LPS alone to the cell culture medium produced an increase in both IL-1β and TNF-α mRNA. The expression level of IL-1β increased almost fourfold at early time points (6 and 12 h, P < 0.01), and the level went down significantly when atRA was added (P < 0.05). With respect to TNF-α, increased expression occurred at later time points (48 and 72 h, P < 0.05). Similarly, addition of atRA had an inhibition effect on TNF-α expression (P < 0.05).
Discussion
Inflammatory responses caused by over-production of inflammatory mediators involved in the pathogenesis of periodontal diseases. Therefore, controlling these inflammatory responses is one major approach to treat periodontitis and peri-implantitis. Previous studies reported that vitamin A is involved in the inflammation reaction and has anti-inflammatory properties [15, 16]. In this study, we confirmed that atRA attenuated the inflammatory response induced in LPS-treated BMSCs.
P. g-LPS plays an important role in the destruction of periodontal tissue by stimulating production of bone-absorbing inflammatory cytokines and inhibiting osteoblastic differentiation of pre-osteoblasts [7, 26]. BMSCs were cultivated on titanium disks and P. g-LPS was added to imitate an inflammatory microenvironment. Both gene expression and protein level of IL-1β showed an obvious rise at early time points, whilst TNF-α increase occurred later (24, 48, and 72 h). These time-dependent changes in inflammatory factors were similar with previous findings [15]. Kato et al. and Herath et al. [5, 8] reported the promoting effect of P. g-LPS on the production of TNF-α and IL-1β, proving an integral role of P. g-LPS as a potent stimulator of inflammatory cytokines.
When atRA was added simultaneously with P. g-LPS, the evoking expression levels of TNF-α and IL-1β went down obviously. However, no significant differences were found at time points with no increased expression of inflammatory cytokines. Another interesting phenomenon is that atRA did not reduce TNF-α and IL-1β expression when added alone to BMSCs. This might explain why the anti-inflammatory effect of atRA occurred when there was a significant increase in inflammatory factors caused by the presence of P. g-LPS. These results are consistent with some previous reports. Wang et al. [16] investigated the effect of atRA on P. g-LPS-induced inflammatory responses in vitro, and Gu et al. [15] found that atRA attenuates the LPS-induced inflammatory response.
When bone tissue is confronted by bacterium and their products, the balance between bone formation and resorption is broken with suppressed osteoblastic activity and enhanced osteoclastic activity. In the alveolar bone area, bacterium and LPS significantly affect bone resorption and regeneration, and excessive bone resorption could arise causing periodontitis and peri-implantitis, when faced with such inflammations [27]. In our study, investigation was performed to determine the effect of atRA on the osteogenesis of BMSCs in an inflammatory environment on titanium surfaces. P. g-LPS had no inhibitory effect on the adhesion and proliferation of BMSCs. However, during osteogenic differentiation, mineralized nodule formation and ALP activity decreased significantly when P. g-LPS was added, indicating inhibition of osteogenesis. Although atRA is widely reported as an anti-inflammatory, reports of its effect on the osteoblastic differentiation of BMSCs under an inflammatory environment remain limited. In our study, atRA was observed to inhibit the P. g-LPS-induced osteoblastic-inhibition effect. BMSCs in the P. g-LPS + atRA-treated group exhibited more mineralized nodule formation and a higher ALP activity compared with the P. g-LPS-treated group. These results indicate that atRA might work effectively on the osteogenesis of BMSCs in an inflammatory environment. OCN and OPN are markers of osteoblastic differentiation [28]. Levels of these markers were significantly reduced at the majority of time points in the control group compared with the P. g-LPS-treated group. This decreased expression of OCN and OPN also provided evidence for the inhibition effect of P. g-LPS on the osteoblastic differentiation of BMSCs. However, when atRA was added in combination with P. g-LPS, ALP activity was higher, with no significant increase in OCN and OPN compared with the P. g-LPS-treated group. Furthermore, there was no difference between the atRA-treated group and control with respect to OCN and OPN expression, mineralized nodules, and ALP activity. It is possible that atRA regulates ALP expression during BMSCs osteoblastogenesis, which is related to early stages of osteoblast differentiation, but not in late stages when OCN and OPN are involved. Results from Hisada et al. [29] suggest that atRA is a specific regulator of ALP expression during osteoblastogenesis and it does not increase expression of OCN or other osteoblastogenic genes.
In addition, Hu et al. [30] report that atRA inhibits RANK-stimulated osteoclast differentiation by suppressing RANK in vitro. We also found that the inhibition effect of atRA on RANKL was more apparent when P. g-LPS stimulation occurred (results not shown). TNF-α and IL-1β are proinflammatory cytokines expressed in BMSCs, which inhibit osteoblast differentiation. These cytokines inhibit ALP activity and expression of osteoblast differentiation markers [31, 32]. Considering these findings, the present data indicate that atRA might help to promote osteogenesis of BMSCs on titanium through attenuating P. g-LPS-induced inflammatory and osteoclastic responses.
Within this study, the titanium surface was partially hydrophilic and, therefore, able to offer initial adhesion and differentiation of BMSCs [33]. An identical proportion of cells was seen to adhere to the titanium and plastic surfaces after 4 h. Furthermore, subsequent cell expansion was unchanged, which coincides with the results of studies by Nebe et al. and Wall et al. [34, 35]. The P. g-LPS-induced inflammatory condition and inhibiting role of atRA on titanium are discussed, to indicate a potential effect of atRA on titanium surfaces in vivo [36]. However, effects of atRA on osteoblastogenesis have been shown to both promote and inhibit osteoblast function [29, 37]. These studies were carried out under standard osteoblastic conditions, and until now no studies have explored the effects of atRA under a P. g-LPS-induced inflammatory environment. Further studies are necessary to clarify the molecular mechanisms for investigating the detailed effects of atRA and P. g-LPS on bone formation.
In conclusion, this in vitro study has demonstrated for the first time that atRA inhibits the proinflammatory and osteoclastic impact effect of P. g-LPS, promoting osteogenic differentiation of BMSCs on titanium surfaces. This suggests that atRA might represent a potential therapeutic factor for treating peri-implant inflammatory diseases.
Acknowledgments
This study was supported by the National Nature Science Foundation of China (No. 81170992). The authors declare that they have no conflict of interest.
References
- 1.Esposito M, Hirsch JM, Lekholm U, Thomsen P. Biological factors contributing to failures of osseointegrated oral implants. (I). Success criteria and epidemiology. Eur J Oral Sci. 1998;106:527–51. doi: 10.1046/j.0909-8836..t01-2-.x. [DOI] [PubMed] [Google Scholar]
- 2.Koyanagi T, Sakamoto M, Takeuchi Y, Maruyama N, Ohkuma M, Izumi Y. Comprehensive microbiological findings in peri-implantitis and periodontitis. J Clin Periodontol. 2013;40:218–26. doi: 10.1111/jcpe.12047. [DOI] [PubMed] [Google Scholar]
- 3.Lamont RJ, Jenkinson HF. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol Mol Biol Rev. 1998;62:1244–63. doi: 10.1128/mmbr.62.4.1244-1263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu C, Yang J, Sun J, Shi J, Gou J, Li A. Induction of immune response and prevention of alveolar bone loss with recombinant Porphyromonas gingivalis peptidylarginine deiminase. Arch Oral Biol. 2013;58:1777–83. doi: 10.1016/j.archoralbio.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Kato H, Taguchi Y, Tominaga K, Umeda M, Tanaka A. Por-phyromonas gingivalis LPS inhibits osteoblastic differentiation and promotes pro-inflammatory cytokine production in human periodontal ligament stem cells. Arch Oral Biol. 2014;59:167–75. doi: 10.1016/j.archoralbio.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Bandow K, Maeda A, Kakimoto K, Kusuyama J, Shamoto M, Ohnishi T, et al. Molecular mechanisms of the inhibitory effect of lipopolysaccharide (LPS) on osteoblast differentiation. Biochem Biophys Res Commun. 2010;402:755–61. doi: 10.1016/j.bbrc.2010.10.103. [DOI] [PubMed] [Google Scholar]
- 7.Xing Q, Ye Q, Fan M, Zhou Y, Xu Q, Sandham A. Porphyromonas gingivalis lipopolysaccharide inhibits the osteoblastic differentiation of preosteoblasts by activating Notch1 signaling. J Cell Physiol. 2010;225:106–14. doi: 10.1002/jcp.22201. [DOI] [PubMed] [Google Scholar]
- 8.Herath TD, Wang Y, Seneviratne CJ, Lu Q, Darveau RP, Wang CY, et al. Porphyromonas gingivalis lipopolysaccharide lipid A heterogeneity differentially modulates the expression of IL-6 and IL-8 in human gingival fibroblasts. J Clin Periodontol. 2011;38:694–701. doi: 10.1111/j.1600-051X.2011.01741.x. [DOI] [PubMed] [Google Scholar]
- 9.Nouneh RA, Wataha JC, Hanes PJ, Lockwood PE. Effect of lipopolysaccharide contamination on the attachment of osteoblast-like cells to titanium and titanium alloy in vitro. J Oral Implantol. 2001;27:174–9. doi: 10.1563/1548-1336(2001)027<0174:EOLCOT>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 10.Walters SM, Dubey VS, Jeffrey NR, Dixon DR. Antibiotic-induced Porphyromonas gingivalis LPS release and inhibition of LPS-stimulated cytokines by antimicrobial peptides. Peptides. 2010;31:1649–53. doi: 10.1016/j.peptides.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Ragab AA, Van De Motter R, Lavish SA, Goldberg VM, Ninomiya JT, Carlin CR, et al. Measurement and removal of adherent endotoxin from titanium particles and implant surfaces. J Orthop Res. 1999;17:803–9. doi: 10.1002/jor.1100170603. [DOI] [PubMed] [Google Scholar]
- 12.Bonsignore LA, Anderson JR, Lee Z, Goldberg VM, Greenfield EM. Adherent lipopolysaccharide inhibits the osseointegration of orthopedic implants by impairing osteoblast differentiation. Bone. 2013;52:93–101. doi: 10.1016/j.bone.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamano E, Miyauchi M, Furusyo H, Kawazoe A, Ishikado A, Makino T, et al. Inhibitory effects of orally administrated liposomal bovine lactoferrin on the LPS-induced osteoclastogenesis. Lab Invest. 2010;90:1236–46. doi: 10.1038/labinvest.2010.80. [DOI] [PubMed] [Google Scholar]
- 14.Semba RD. Vitamin A as ‘‘anti-infective’’ therapy, 1920–1940. J Nutr. 1999;129:783–91. doi: 10.1093/jn/129.4.783. [DOI] [PubMed] [Google Scholar]
- 15.Gu B, Miao J, Fa Y, Lu J, Zou S. Retinoic acid attenuates lipo-polysaccharide-induced inflammatory responses by suppressing TLR4/NF-kappaB expression in rat mammary tissue. Int Immunopharmacol. 2010;10:799–805. doi: 10.1016/j.intimp.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Allen C, Ballow M. Retinoic acid enhances the production of IL-10 while reducing the synthesis of IL-12 and TNF-alpha from LPS-stimulated monocytes/macrophages. J Clin Immunol. 2007;27:193–200. doi: 10.1007/s10875-006-9068-5. [DOI] [PubMed] [Google Scholar]
- 17.Weng Y, Wang M, Liu W, Hu X, Chai G, Yan Q, et al. Repair of experimental alveolar bone defects by tissue-engineered bone. Tissue Eng. 2006;12:1503–13. doi: 10.1089/ten.2006.12.1503. [DOI] [PubMed] [Google Scholar]
- 18.Chen KY, Chung CM, Chen YS, Bau DT, Yao CH. Rat bone marrow stromal cells-seeded porous gelatin/tricalcium phosphate/oligomeric proanthocyanidins composite scaffold for bone repair. J Tissue Eng Regen Med. 2013;7:708–19. doi: 10.1002/term.1461. [DOI] [PubMed] [Google Scholar]
- 19.Boeloni JN, Ocarino NM, Goes AM, Serakides R. Comparative study of osteogenic differentiation potential of mesenchymal stem cells derived from bone marrow and adipose tissue of osteoporotic female rats. Connect Tissue Res. 2013;55:103–14. doi: 10.3109/03008207.2013.860970. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Zhang Y, Qi G. Evaluation of isolation methods and culture conditions for rat bone marrow mesenchymal stem cells. Cytotechnology. 2013;65:323–34. doi: 10.1007/s10616-012-9497-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amoroso PF, Adams RJ, Waters MG, Williams DW. Titanium surface modification and its effect on the adherence of Porphyromonas gingivalis: an in vitro study. Clin Oral Implants Res. 2006;17:633–7. doi: 10.1111/j.1600-0501.2006.01274.x. [DOI] [PubMed] [Google Scholar]
- 22.Colombo JS, Carley A, Fleming GJ, Crean SJ, Sloan AJ, Waddington RJ. Osteogenic potential of bone marrow stromal cells on smooth, roughened, and tricalcium phosphate-modified titanium alloy surfaces. Int J Oral Maxillofac Implants. 2012;27:1029–42. [PubMed] [Google Scholar]
- 23.Li Y, Li J, Zhu S, Luo E, Feng G, Chen Q, et al. Effects of strontium on proliferation and differentiation of rat bone marrow mesenchymal stem cells. Biochem Biophys Res Commun. 2012;418:725–30. doi: 10.1016/j.bbrc.2012.01.088. [DOI] [PubMed] [Google Scholar]
- 24.Maggini J, Mirkin G, Bognanni I, Holmberg J, Piazzon IM, Nepomnaschy I, et al. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One. 2010;5:e9252. doi: 10.1371/journal.pone.0009252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ocarino NM, Boeloni JN, Goes AM, Silva JF, Marubayashi U, Serakides R. Osteogenic differentiation of mesenchymal stem cells from osteopenic rats subjected to physical activity with and without nitric oxide synthase inhibition. Nitric Oxide. 2008;19:320–5. doi: 10.1016/j.niox.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Tang Y, Sun F, Li X, Zhou Y, Yin S, Zhou X. Porphyromonas endodontalis lipopolysaccharides induce RANKL by mouse osteoblast in a way different from that of Escherichia coli lipo-polysaccharide. J Endod. 2011;37:1653–8. doi: 10.1016/j.joen.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 27.Lee SK, Chung JH, Choi SC, Auh QS, Lee YM, Lee SI, et al. Sodium hydrogen sulfide inhibits nicotine and lipopolysaccharide-induced osteoclastic differentiation and reversed osteoblastic differentiation in human periodontal ligament cells. J Cell Biochem. 2013;114:1183–93. doi: 10.1002/jcb.24461. [DOI] [PubMed] [Google Scholar]
- 28.Weinreb M, Shinar D, Rodan GA. Different pattern of alkaline phosphatase, osteopontin, and osteocalcin expression in developing rat bone visualized by in situ hybridization. J Bone Miner Res. 1990;5:831–42. doi: 10.1002/jbmr.5650050806. [DOI] [PubMed] [Google Scholar]
- 29.Hisada K, Hata K, Ichida F, Matsubara T, Orimo H, Nakano T, et al. Retinoic acid regulates commitment of undifferentiated mesenchymal stem cells into osteoblasts and adipocytes. J Bone Miner Metab. 2013;31:53–63. doi: 10.1007/s00774-012-0385-x. [DOI] [PubMed] [Google Scholar]
- 30.Hu L, Lind T, Sundqvist A, Jacobson A, Melhus H. Retinoic acid increases proliferation of human osteoclast progenitors and inhibits RANKL-stimulated osteoclast differentiation by suppressing RANK. PLoS One. 2010;5:e13305. doi: 10.1371/journal.pone.0013305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abbas S, Zhang YH, Clohisy JC, Abu-Amer Y. Tumor necrosis factor-alpha inhibits pre-osteoblast differentiation through its type-1 receptor. Cytokine. 2003;22:33–41. doi: 10.1016/s1043-4666(03)00106-6. [DOI] [PubMed] [Google Scholar]
- 32.Lacey DC, Simmons PJ, Graves SE, Hamilton JA. Proinflammatory cytokines inhibit osteogenic differentiation from stem cells: implications for bone repair during inflammation. Osteoarthritis Cartilage. 2009;17:735–42. doi: 10.1016/j.joca.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 33.Zhao G, Schwartz Z, Wieland M, Rupp F, Geis-Gerstorfer J, Cochran DL, et al. High surface energy enhances cell response to titanium substrate microstructure. J Biomed Mater Res A. 2005;74:49–58. doi: 10.1002/jbm.a.30320. [DOI] [PubMed] [Google Scholar]
- 34.Nebe JG, Luethen F, Lange R, Beck U. Interface interactions of osteoblasts with structured titanium and the correlation between physicochemical characteristics and cell biological parameters. Macromol Biosci. 2007;7:567–78. doi: 10.1002/mabi.200600293. [DOI] [PubMed] [Google Scholar]
- 35.Wall I, Donos N, Carlqvist K, Jones F, Brett P. Modified titanium surfaces promote accelerated osteogenic differentiation of mesenchymal stromal cells in vitro. Bone. 2009;45:17–26. doi: 10.1016/j.bone.2009.03.662. [DOI] [PubMed] [Google Scholar]
- 36.Martire-Greco D, Landoni VI, Chiarella P, Rodriguez-Rodrigues N, Schierloh P, Rearte B, et al. all-trans-Retinoic acid improves immunocompetence in a murine model of lipopolysaccharide-induced immunosuppression. Clin Sci (Lond) 2014;126:355–65. doi: 10.1042/CS20130236. [DOI] [PubMed] [Google Scholar]
- 37.Lind T, Sundqvist A, Hu L, Pejler G, Andersson G, Jacobson A, et al. Vitamin a is a negative regulator of osteoblast mineralization. PLoS One. 2013;8:e82388. doi: 10.1371/journal.pone.0082388. [DOI] [PMC free article] [PubMed] [Google Scholar]