Abstract
DNA supercoiling plays critical roles in several essential DNA metabolic pathways, such as replication, transcription and recombination. Typically plasmid DNA molecules are used to measure DNA supercoiling status inside bacterial cells. In this chapter, we describe how to isolate plasmid DNA molecules from E. coli cells and determine DNA supercoiling density by 1% agarose gel electrophoresis containing chloroquine using plasmid pACYC184 as an example.
Keywords: agarose gel electrophoresis, DNA supercoiling, DNA topoisomers, supercoiling density
1. Introduction
DNA supercoiling plays a key role in many essential DNA metabolic pathways including DNA replication, transcription, and recombination [1–6]. Many naturally occurring DNA molecules, such as plasmids, bacterial chromosomes, mitochondrial and chloroplast DNA, exist as closed circular DNA [7] and are usually negatively supercoiled, which is equivalent to unwinding of the right-handed DNA double helix, although those isolated from thermophilic archaea are positively supercoiled [8]. In E. coli or other bacteria, the supercoiling status of DNA is a result of counteractions of two topoisomerases, DNA topoisomerase I and gyrase [1–4].
As pointed out by James White [5, 6], DNA supercoiling can be described by one topological parameter, the linking number (Lk), and two geometric parameters, writhe (Wr) and Twist (Tw) [6–8]:
| (1) |
For definitions of Lk, Wr, and Tw, please refer to previous publications by White and colleagues for details [6]. If Lk0 is the linking number for relaxed DNA, the linking number difference or linking difference (ΔLk) can be used to describe the negatively supercoiled DNA in solution:
| (2) |
Since ΔLk is dependent on the size of the DNA molecule, the specific linking difference or supercoiling (superhelical) density (σ), is used to describe DNA supercoiling (ref):
| (3) |
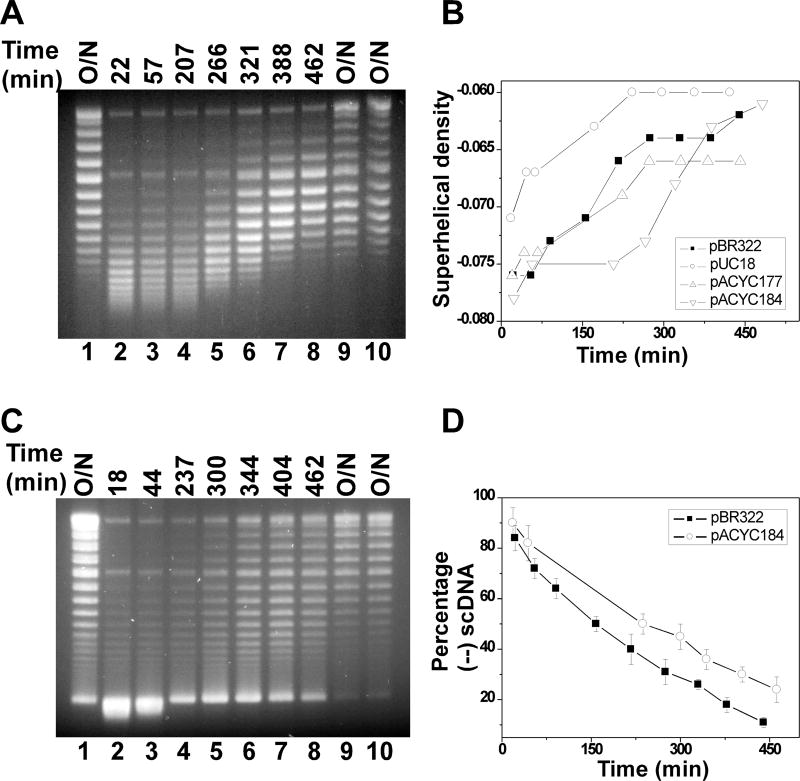
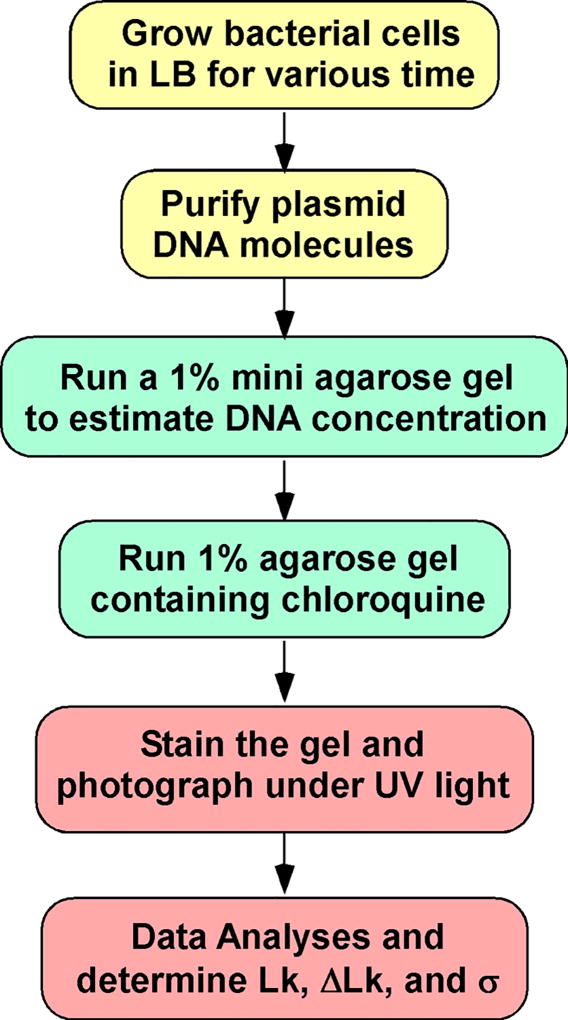
Lk, ΔLk, and σ can be determined experimentally. For instance, to study DNA supercoiling in bacteria, we usually reply on the analysis of small circular DNA molecules. Fig. 1 shows the time course of DNA supercoiling density of different plasmids in E. coli. In this chapter, we provide a detailed protocol to determine supercoiling density of plasmid DNA molecules in E. coli. Fig. 2 shows an experimental procedure for this determination.
Figure 1.
Time courses of DNA supercoiling status in MG1655 (A, B) and VS111 (C, D). Overnight cultures of E.coli cells carrying plasmids pBR322, pUC18, pACYC177, or pACYC184 were diluted 100-fold in LB and grown to the time points indicated. The DNA molecules were isolated using the alkaline lysis assays using the QIAprep Spin Miniprep Kit. The DNA samples were subjected to 1% agarose gel electrophoresis in the presence of 5 µg/mL of chloroquine. The DNA supercoiling densities were determined as detailed in Method 3.3 and 3.4. The symbol of (-) represents the hypernegatively supercoiled DNA.
Figure 2.
An experimental procedure to determine the supercoiling density (σ) of plasmid DNA molecules isolated from bacterial cells.
2. Materials
2.1. Purify plasmid DNA molecules from bacterial cells
E. coli strain FL#474 (MG1655/pACYC184).
Luria-Bertani (LB) broth: dissolve 10 g of Bacto tryptone, 5 g of Bacto yeast extract, and 10 g of NaCl into 1 L of DI H2O. Adjust pH to 7.0 with 5 M NaOH and autoclave. (The approximate volume of 5 M NaOH is 200 µL).
Tetracycline solution (10 mg/mL): dissolve 100 mg tetracycline into 10 mL DI H2O.
Chloramphenicol solution (25 mg/mL): dissolve 250 mg Chloramphenicol into 10 mL DI H2O.
A 37°C air shaker.
A spectrophotometer (Cary 50 UV-Visible Spectrophotometer) and cuvettes.
QIAprep Spin Miniprep Kit or GeneJET Plasmid Miniprep Kit.
50× TAE Buffer: dissolve 121 g of Tris base into 250 mL DI H2O, and then add 28.6 mL of acetic acid and 50 mL of 0.5 M EDTA (pH 8.0) into the Tris base solution. Finally bring the total volume to 500 mL in a graduated cylinder with DI H2O.
A microwave oven.
A UV-transparent gel tray (7 cm (W)×10 cm (L)×0.5 cm (H)).
50 mL of 1% agarose gel: add 0.5 g of agarose into 50 mL 1×TAE in a conical flask. Heat the solution in a microwave oven for ~1 min to completely dissolve the agarose. Let the agarose solution cool down to ~55°C and then pour the warm agarose solution into the UV-transparent gel tray mounted in a plastic mold supplied with the electrophoresis apparatus. Make sure the appropriate comb has been inserted into the tray. Allow the gel to solidify at room temperature.
Basic power supply for gel electrophoresis.
Ethidium bromide solution (10 mg/mL): dissolve 100 mg of ethidium bromide into 10 mL of DI H2O with stirring overnight. Store at room temperature using a dark brown bottle to blocking light.
A UV transilluminator.
A gel documentation system (Kodak DEAS 290 gel imaging system) with a digital camera.
6×gel loading dye: dissolve 25 mg of xylene cyanol, 25 mg of bromophenol blue, and 4 g of sucrose into 10 mL of DI H2O. Store at 4°C.
DNA marker: λ DNA HindIII digest.
2.2. Resolve DNA topoisomers by 1% agarose gel electrophoresis in 1×TAE containing chloroquine
A CBS Scientific Co. SGU-040T-02 horizontal system for agarose gel electrophoresis.
A UV-transparent gel tray (18.5 cm (W)×40 cm (L)×0.5 cm (H)).
Chloroquine stock solution (10 mg/mL): dissolve 100 mg of chloroquine into 10 mL of DI H2O, protect from light with aluminum foil. Store at 4°C for 2 weeks.
900 mL of 1% agarose gel containing 5 µg/mL Chloroquine: add 9 g of agarose into 900 mL 1× TAE in a conical flask. Heat the solution in a microwave oven for ~9 min to completely dissolve the agarose. Let the agarose solution cool down to ~55°C and then add 450 µL of chloroquine stock solution into the agarose solution. Pour the warm agarose solution into the UV-transparent gel tray as described in section 3.2 and Fig. 3. Allow the gel to solidify at room temperature.
Plastic film (Sarah wrap)
SYBR Gold or SYBR Green Nucleic Acid Gel Stain.
Figure 3.
Assembling of the non-submarine gel system and casting of the 1% agarose gel. (A) Assembling the modified gel system. (B) Making the agarose bridges between the gel and the TAE buffer reservoirs. (C) Pouring 1% agarose into the gel tray. (D) Adding 1TAE on the gel surface to preventing it from drying. (E) Adding 1TAE into reservoirs. (F) Running the gel. A layer of plastic membrane was used to cover the gel surface during gel electrophoresis.
2.3. Determine the DNA supercoiling density
900 mL of 1% agarose gel containing 0, 0.5, 1, and 2.5 µg/mL Chloroquine, respectively: add 9 g of agarose into 900 mL 1× TAE in a conical flask. Heat the solution in a microwave oven for ~9 min to completely dissolve the agarose. Let the agarose solution cool down to ~55°C and then add 0, 45, 90, and 225 µL of chloroquine stock solution into the agarose solution, respectively. Pour the warm agarose solution into the UV-transparent gel tray as described in section 3.2 and Fig. 3. Allow the gel to solidify at room temperature.
BSA solution (100 µg/mL). Dissolve 1mg of BSA in 10 mL of DI-H2O.
10×topoisomerase I buffer: 200 mM Tris-acetate (pH 7.9), 500 mM KAc, 100 mM Mg(Ac)2, 10 mM DTT and 100 µg/mL BSA,
E. coli topoisomerase I (20 µM)
Phenol equilibrated with 10 mM Tris HCl, pH 8.0, 1 mM EDTA.
Ethanol and 70% ethanol.
TE Buffer: 10 mM Tri-HCl, pH 8.0 and 1 mM EDTA.
10 mM Tris-HCl, pH 8.5.
KODAK 1D Image Analysis Software.
Origin 7 professional software.
3. Methods
3.1. Purify plasmid DNA molecules from bacterial cells
Inoculate 5 mL of LB containing appropriate antibiotics, for example 25 µg/mL of chloramphenicol and 10 µg/mL tetracycline, with a bacterial strain carrying a plasmid, such as E. coli strain FL#474 (MG1655/pACYC184), and grow the bacterial cells in an air shaker at 37°C with vigorous agitation (250–300 rpm) overnight.
Inoculate 200 mL of LB containing 25 µg/mL of chloramphenicol and 10 µg/mL tetracycline with the overnight cell culture at 1:100 dilution. Grow the culture at 37°C with vigorous agitation (250 rpm) to different time points, i.e., 30, 60, 120, 180, 240 min, and overnight. Determine the concentration of cells by measuring OD600 with a spectrophotometer, such as Cary 50 UV-Visible Spectrophotometer, with the assumption of that 0.1 OD is equivalent to 1×108 cells/mL.
Purify plasmid DNA molecules by alkaline lysis assay using a commercially available plasmid purification kit, such as QIAprep Spin Miniprep Kit or GeneJET Plasmid Miniprep Kit. For dilute cell samples, a floor centrifuge may be required to pellet bacteria cells. Otherwise, a desktop personal centrifuge is sufficient. Please follow the steps provided by manufacturers to purify plasmid DNA molecules. We recommend using the same amount of bacterial cells for all samples for DNA purification. In the final step, use 50 µL of elution buffer (50 mM Tris-HCl, pH 8.5) to elute plasmid DNA. The eluted DNA samples can be used immediately or store at −80°C for long-term storage (see Note 1).
To make a 1% agarose mini-gel (7 cm (W)×10 cm (L)×0.5 cm (H)), add 0.5 g of agarose into 50 mL of 1×TAE Buffer in a 200 mL conical flask. Heat the mixture in a microwave oven for about 1 min until the agarose is completely dissolved. Let agarose solution cool down to about 50°C, usually ~5 min. Pour the warm gel solution into a UV-transparent gel tray (7×10 cm) with an inserted comb and let it sit at room temperature until it has completely solidify (~30 min).
Run the purified DNA samples on the 1% agarose gel in 1×TAE buffer at 100 V for 90 min. After this step, the gel is stained by ethidium bromide for 45 to 60 min and photographed using a Kodak DEAS 290 gel imaging system. The DNA concentration of each sample is estimated by comparing their band density with those of a DNA standard on the same gel, such as λ DNA HindIII digest. If necessary, adjust all DNA samples to the same concentration using elution buffer (see Note 2).
3.2. Resolve DNA topoisomers by 1% agarose gel electrophoresis in 1×TAE containing chloroquine
A CBS Scientific Co. SGU-040T-02 horizontal system for agarose gel electrophoresis is used. Gel bed dimension is 18.5 cm (W)×40 cm (L)×0.5 cm (H). To make a 1% gel containing 5 µg/mL of chloroquine, 9 grams of agarose is added into 900 mL of 1×TAE in a 3,800 mL of conical flask. Heat the mixture in a microwave oven until the agarose is completely dissolved (~8 min). Cool the gel down to 55°C in a 55°C water bath (5 to 10 min), then add 450 µL of 10 mg/mL Chloroquine into the gel and mix well. The gel system was slightly modified to run non-submarine gels to increase the separation resolution (Fig. 3). After two agarose bridges (Fig. 3B) are made first between buffer reservoirs and the gel tray, the rest of gel solution is poured into the gel tray with a comb inserted (Fig. 3C) and solidified at room temperature. Remove the plates for the agarose gel bridges and the comb when the gel becomes solidified and ready to use (see Note 3).
Prepare 1,500 mL of 1×TAE containing 5 µg/mL of chloroquine. Add 1×TAE onto gel surface to prevent the gel surface becoming too dry (Fig. 3D). Fill sample wells with 1×TAE. Add the rest of 1×TAE into the two reservoirs of the gel system (Fig. 3E).
Prepare DNA samples in 1×DNA loading dye. For instance, add 4 µL of 6×DNA loading dye into 20 µL of DNA sample and mix well. Load 20 to 30 µL of DNA samples in 1×DNA loading dye into each well.
Run the gel for 30 min at 140 V to allow the DNA samples entering the gel. Then place a piece of plastic film (Sarah wrap is used in our lab) on the gel to prevent the gel surface from getting too dry (Fig. 3F). After this step, run the gel for an additional 15–16 hours min at 110 V, usually overnight.
Stain the gel in 0.5 µg/mL Ethidium Bromide for ~3–4 hours.
Visualize DNA bands under a UV light and cut the gel to a smaller size.
Destain the gel in de-ionized water. Please change de-ionized water frequently, for example once or twice per hour. If necessary, leave the gel in de-ionized water for overnight.
If necessary, stain the gel in SYBR Gold or SYBR Green for 1 to 3 hours.
After the staining step, the gel is photographed using a gel documentation system, such as a Kodak DEAS 290 gel imaging system. The gel images may be analyzed by image analysis software, such as KODAK 1D Image Analysis Software.
3.3. Determine the supercoiling density (σ) of a plasmid DNA molecule
In order to determine the supercoiling density of a plasmid DNA molecule, it is required to generate a series of DNA samples with different supercoiling densities and resolve DNA topoisomers using 1% agarose gels containing various concentrations of chloroquine as described in section 3.2. Here we use plasmid pACYC184 as an example.
1.5 µg of supercoiled pACYC184 was relaxed at 37°C for 1 hour by E. coli topoisomerase I in 100 µL of topoisomerase I buffer (20 mM Tris –acetate (pH 7.9), 50 mM KAc, 10 mM Mg(Ac)2, 1 mM DTT and 100 µg/mL BSA) in the presence of 0, 0.5, 1, 1.5, 2, 2.5, 5, and 7.5 µM of ethidium bromide (see Note 4).
Add an equal volume of phenol (100 µL) to stop the relaxation reactions. The DNA samples were centrifuged for 3 min at 13,000 rpm at room temperature. The aqueous layers were transferred into new eppendorf centrifuge tubes. After adding 11 µL of 3 M NaAC, pH 5.3, the DNA samples were precipitated with 2 volumes of ethanol and centrifuged at 13,000 rpm at 4°C for 15 min. The pellets were washed with 500 µL of 70% ethanol once. After removing ethanol, the DNA samples were air dried for 10 min and dissolved into 85 µL of TE or 10 mM Tris-HCl, pH 8.5.
The topological status of each DNA sample was analyzed by electrophoresis in a 1% agarose gel in 1×TAE buffer in the presence of different concentrations of chloroquine (0, 0.5, 1, and 2.5 µg/mL).
Run the gel at 120 V for 16 h, usually overnight.
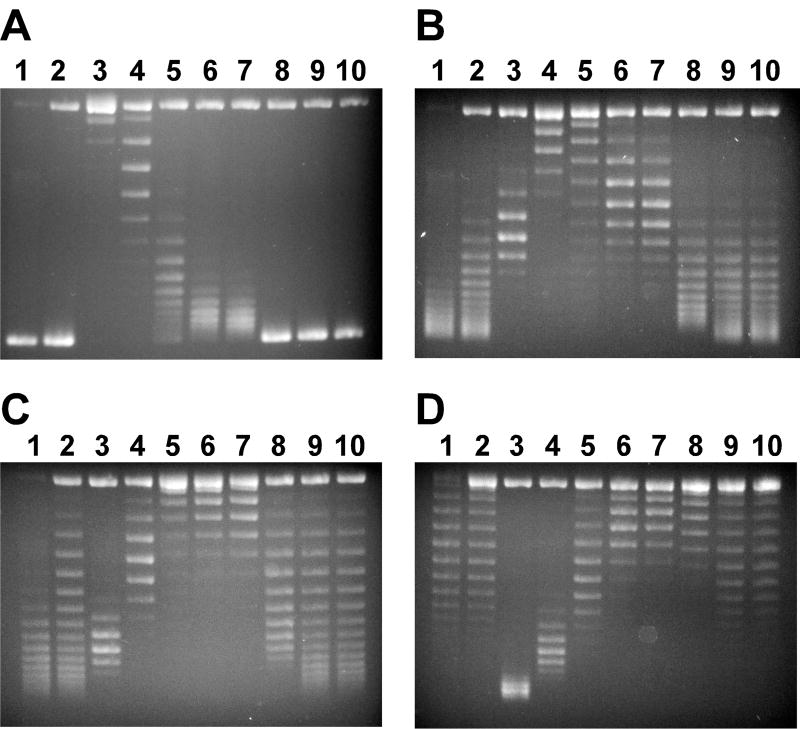
The gel was stained and photographed as described in section 3.2. Fig. 4 shows the gel images that were used to calculate σ of pACYC184.
The linking number (Lk0) for relaxed pACYC184 was calculated to be 404 with an assumption of 10.5 bp per turn of DNA helix for relaxed B-form DNA [7]: Lk0 = 4245 bp/10.5 bp = 404 (the linking number is an integer).
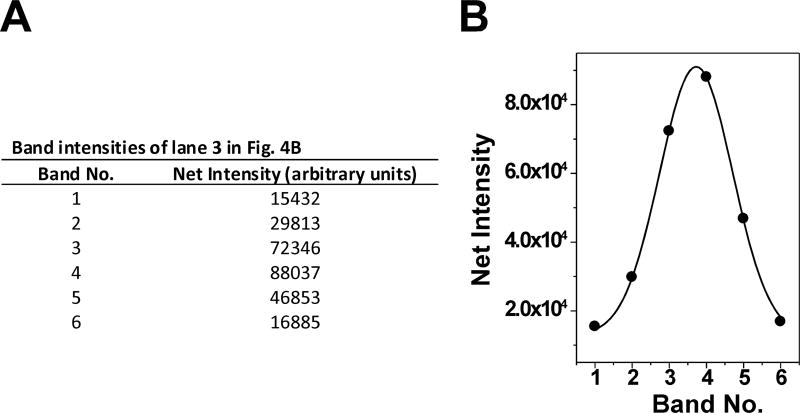
Figs. 1 and 4 were used to calculate the supercoiling density (σ) of pACYC184 isolated from E. coli strain FL#474. First, the band of lane 3 of Fig. 4B that represents Lk0 was determined by analyzing the intensity of the different DNA topoisomers. The intensity of DNA topoisomers were calculated by using KODAK 1D Image Analysis Software and then fit to Gaussian distribution in Origin Data Analysis and Graphing Software (Fig. 5). The DNA linking number (Lk) was determined by band counting. σ was calculated by equations 2 and 3.
Figure 4.
Analysis of DNA topoisomers by using 1% agarose gel containing different concentrations of chloroquine. Briefly, negatively supercoiled plasmid pACYC184 DNA samples were relaxed by E. coli DNA topoisomerase I in the presence of different concentrations of ethidium bromide (please see section 3.3 for details). Panels (A), (B), (C) and (D) are 1% agarose gel in 1TAE buffer containing 0, 0.5, 1 and 2.5 µg/mL of chloroquine. Lanes 1 and 2 are negatively supercoiled pACYC184. Lanes 3 to 10 contain DNA samples relaxed by E. coli DNA topoisomerase I in the presence of 0, 0.5, 1, 1.5, 2, 2.5, 5, and 7.5 µM of ethidium bromide, respectively.
Figure 5.
Gaussian distribution of DNA topoisomers. (A) Band intensities of lane 3 in Fig. 4B (the relaxed pACYC184) were calculated by using KODAK 1D Image Analysis Software and then (B) fit to Gaussian distribution in Origin Data Analysis and Graphing Software.
Footnotes
It is critical to isolate plasmid DNA molecules as soon as the bacterial cell growth reaches the time point. The topological status of DNA molecules inside bacterial cells is always changing and therefore it is not recommended to store the cells at room temperature or on ice for plasmid purification later. Since freeze and thaw will result in breaking plasmid DNA molecules, DNA samples should be stored at 4 °C for short-term storage.
It is always recommended to run a 1% mini gel to check the DNA concentrations for plasmid DNA molecules. Alternatively, DNA concentration can be determined by using a UV spectrophotometer.
In our hands, non-submarine gels always have higher separation resolution for DNA topoisomers. Submarine gels can also be used to resolve DNA topoisomers.
Although E. coli DNA topoisomerase I was used here, eukaryotic DNA topoisomerase I, such as human topoisomerase I, may be used to relax DNA molecules. E. coli DNA topoisomerase I only relaxes negatively supercoiled DNA molecules.
References
- 1.Champoux JJ. DNA topoisomerases: structure, function, and mechanism. Annu. Rev. Biochem. 2001;70:369–413. doi: 10.1146/annurev.biochem.70.1.369. [DOI] [PubMed] [Google Scholar]
- 2.Snoep JL, van der Weijden CC, Andersen HW, Westerhoff HV, Jensen PR. DNA supercoiling in Escherichia coli is under tight and subtle homeostatic control, involving gene-expression and metabolic regulation of both topoisomerase I and DNA gyrase. Eur. J. Biochem. 2002;269:1662–1669. doi: 10.1046/j.1432-1327.2002.02803.x. [DOI] [PubMed] [Google Scholar]
- 3.Wang JC. DNA topoisomerases. Annu. Rev. Biochem. 1996;65:635–692. doi: 10.1146/annurev.bi.65.070196.003223. [DOI] [PubMed] [Google Scholar]
- 4.Zechiedrich EL, Khodursky AB, Bachellier S, Schneider R, Chen D, Lilley DM, Cozzarelli NR. Roles of topoisomerases in maintaining steady-state DNA supercoiling in Escherichia coli. J. Biol. Chem. 2000;275:8103–8113. doi: 10.1074/jbc.275.11.8103. [DOI] [PubMed] [Google Scholar]
- 5.White JH, Bauer WR. Calculation of the twist and the writhe for representative models of DNA. J. Mol. Biol. 1986;189:329–341. doi: 10.1016/0022-2836(86)90513-9. [DOI] [PubMed] [Google Scholar]
- 6.Cozzarelli NR, Boles TC, White JH. A Primer on the Topology and Geometry of DNA Supercoiling. In: Cozzarelli NR, Wang JC, editors. DNA Topology and Its Biological Effects. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York 11724: 1990. pp. 139–184. [Google Scholar]
- 7.Bates AD, Maxwell A. DNA Topology. 2. Oxford University Press; Oxford, UK: 2005. [Google Scholar]
- 8.White JH, Cozzarelli NR, Bauer WR. Helical repeat and linking number of surface-wrapped DNA. Science. 1988;241:323–327. doi: 10.1126/science.3388041. [DOI] [PubMed] [Google Scholar]