Abstract
Society is on the cusp of harnessing recent advances in synthetic biology to discover new bio-based products and routes to their affordable and sustainable manufacture. This is no more evident than in the discovery and manufacture of Synthetic Biological Materials, where synthetic biology has the capacity to usher in a new Materials from Biology era that will revolutionise the discovery and manufacture of innovative synthetic biological materials. These will encompass novel, smart, functionalised and hybrid materials for diverse applications whose discovery and routes to bio-production will be stimulated by the fusion of new technologies positioned across physical, digital and biological spheres. This article, which developed from an international workshop held in Manchester, United Kingdom, in 2017 [1], sets out to identify opportunities in the new materials from biology era. It considers requirements, early understanding and foresight of the challenges faced in delivering a Discovery to Manufacturing Pipeline for synthetic biological materials using synthetic biology approaches. This challenge spans the complete production cycle from intelligent and predictive design, fabrication, evaluation and production of synthetic biological materials to new ways of bringing these products to market. Pathway opportunities are identified that will help foster expertise sharing and infrastructure development to accelerate the delivery of a new generation of synthetic biological materials and the leveraging of existing investments in synthetic biology and advanced materials research to achieve this goal.
Keywords: Synthetic biology, Materials, Biological materials, Biomaterials, Advanced materials
1. Introduction: the dawn of a new era for advanced materials
Strong synergies exist between materials and chemicals sciences and their allied technologies, which have driven understanding at the atomic and molecular levels of the complex relationships between the chemical compositions, structures and macroscopic properties of materials. This has been the predominant agenda behind the development of new Advanced Materials to modify properties and enhance performance. The market pull is defined in the main by societal grand challenges. These include The Digital Economy, Energy, Living with Environmental Change and Life-long Health and Well-being, each placing demands on overall performance to suit new applications. Advanced materials are at the core of Systems Engineering that relates to the design and management of complex systems over their complete life cycles, and its nexus with industrial engineering, manufacturing engineering, other branches of engineering and human-centered disciplines (e.g. project and risk management). Embedded in this is the need for Sustainable Materials Management and, increasingly, Sustainable Materials Manufacture, to reuse and sustain materials more productively, and affordably.
All this places great demand on the need to engineer new advanced materials. Whilst synthetic chemistry has, and continues to, advance core synthetic technologies to ‘build’ new materials through monomer provision, higher order polymerisation and functionalisation, synthetic biology is beginning to identify new ways of accessing chemical space [[2], [3], [4]]. This opens up possible new chemical connectivities not accessible to the synthetic chemist and the rapid exploration of diverse (bio)-molecular structures. The conflation of synthetic biology and (combinatorial) synthetic chemistry, and exploration of potential connections with contemporary manufacturing platforms such as Additive Manufacturing (3D printing), defines a new era in the exploration of new advanced materials extending from basal materials with new (desirable) properties to complex and well-defined 3D meso-structures (3D topologies). Supplement that with developments in Artificial Intelligence (e.g. Machine Learning) to learn and predictively design new advanced materials in rapidly implemented (automated) iterative Design, Build, Evaluate, Learn cycles, and one has a powerful series of technology platforms with which to navigate the new advanced materials landscape.
The unification of synthetic biology with other frontier sciences and technologies will usher in the new synthetic biological materials era. In the main, the definition of Biomaterials has been associated traditionally with healthcare applications, for example in the development of biocompatible scaffold materials (tissue regeneration), structural biocompatible materials (prosthetics) and new materials for drug delivery (biomedical devices) [5]. This can be classified as Materials for Biology. With synthetic biological materials the focus is more on Materials from Biology and the harnessing of new capability platforms (e.g. synthetic biology; additive manufacturing) in an integrated fashion with leading developments in more established fields (e.g. ‘Click’ chemistry; machine learning; automation; miniaturisation of materials evaluation platforms). By bringing deeper biological thinking to advanced materials societal grand challenges can be met. Biology will bring sustainable and affordable manufacture of complex new materials that will impact not only in Healthcare, but also in other sector challenge areas (e.g. Energy, Military, Advanced Manufacturing, Living with Environmental Change, Digital Economy etc.). This will give rise to a wide range of new advanced materials, especially – although not exclusively – in the realm of soft materials that can be functionalised, elaborated and assembled hierarchically, and validated rapidly, for target applications.
The opportunity: By harnessing the power of synthetic biology, existing materials discovery platforms and fabrication technologies would be augmented to widen the materials development space and define a new materials paradigm – defined as Synthetic Biological Materials. This would enable delivery of next generation advanced materials with new and extended functional properties to address a wide range of unmet needs. Realising this opportunity would also provide access to affordable and sustainable routes to the production of synthetic biological materials. This would be an ambitious, high-risk and high-gain proposition, dependent on the emerging science of synthetic biology, but one that would deliver a new landscape for advanced materials and in a way that is fundamentally different to more traditional materials discovery and fabrication platforms. Through the engineering of biology, the synthetic biological materials paradigm would give access to i) rapid expansion of materials diversity, ii) hierarchical assembly of new multi-component, multi-functional materials (e.g. by uniting synthetic biology and materials fabrication platforms including additive manufacturing, spinning, coating technologies), iii) affordable and/or sustainable routes to production (e.g. fermentation on waste feedstocks) and iv) lessened environmental impact in next generation materials manufacture. This defines a large landscape for synthetic biological materials through the ‘writing of DNA’, and assembly of these biological ‘parts’ with other (e.g. non-biological) components. Synthetic biological materials will therefore span soft and hard materials, composites and other more complex multi-component and multi-functional structures.
2. Synthetic biological materials: navigating a new landscape to advanced materials
There are diverse potential application areas for synthetic biological materials as a distinct, but major, contributor to the advanced materials landscape, impacting across multiple grand challenge themes – The Digital Economy, Energy, Living with Environmental Change, Life-long Health and Well-being. Using synthetic biology platforms the materials scientist can access a new design space not available with other platform technologies. That alone however is not sufficient. A requirement of any strategy for synthetic biological materials is identifying unmet application needs (i.e. new materials performance properties) and to deliver routes to new bio-sourced components, with appropriate chemistries and functionalities, that enable rapid assembly of new materials and the emergence of higher order functionality to satisfy those needs. Clearly, predictive design and rapid evaluation are at the core of any synthetic biology approach, alongside parallelised assembly of new materials through laboratory automation, high throughput characterisation and post production processing.
‘Industry Pull’ will identify ‘hard-to-make’ material targets and early wins for synthetic biology, but there is a need also to deliver a ‘Creative Push’ to generate new ways of working that lead to transformative application solutions. This ‘out-of-the-box’ mode of operating will define proof of concept applications that will challenge convention and deliver solutions for contemporary problems faced by industry.
Any investment in synthetic biological materials will be a relatively high-risk, high-gain venture. The substantial investment in synthetic biology made by the UK government [1] provides some impetus for synthetic biological materials but inertia remains, especially in uniting manufacturing and materials discovery communities to harness opportunities emerging from synthetic biology. Any strategy therefore must also provide for, and mobilise, a skilled workforce from discovery science through to application, as well as the infrastructure to support it. The unifying concepts are therefore: platform technologies to support the delivery of synthetic biological materials; a highly trained interdisciplinary workforce and academic/industry/government co-development that can implement and innovate these technologies; standardisation and interoperability of biological parts for new materials; sustainable materials manufacturing and management, and a common language and vision that places synthetic biology at the nexus of other disciplines, especially materials science, chemistry, computer science and engineering.
Clearly, a high-level strategy review document cannot provide comprehensive appraisal of application areas and unmet needs. But, consideration can be given to exemplar areas where investment in synthetic biological materials will facilitate step-change (see Table 1). For example, early challenge areas might include Corrosion (costing $1.1 trillion in the USA alone i.e. 6% GDP) [6], where next generation synthetic biological materials can harness protective, biologically compatible coatings, by capturing the power of biological functions (e.g. biocides) ‘out of context’.
Table 1.
Definitions and potential sector applications of synthetic biological materials.
| Definitions | |
|---|---|
| Synthetic Biology and Materials from Biology | The rapid and predictable engineering of biological micro-organisms to provide access to new materials requiring:
|
| Synthetic Biological Materials | New Advanced Materials that are genetically encoded and generated from biology by harnessing synthetic biology platforms to create new higher order materials and their components parts. Also, materials produced using synthetic biology platforms in conjunction with other capability platforms (e.g. additive manufacturing) to fabricate multi-component, multi-functional and novel composite materials. |
| Advanced Materials | Sector Relevance and Examples of Potential Application Areas |
| Corrosion | Protective and biocompatible coatings. |
| Bioelectronics | Healthcare for early detection/monitoring and rapid in vivo analysis for micro-bioelectronic systems. In situ monitoring of industrial manufacture systems. Forensics, security, manufacture and diagnostics; electro-active biopolymers. Electro-Genetic devices for bidirectional communication between electronic devices and biological systems. Self-assembly of nanowires for electronics; peptide scaffolds as templates from metalation. Microbial communication for biocomputing, bioenergy and biosensing. |
| Optical Materials | Novel hierarchical photonic structures for new micro-structures and fabrication of smart optical devices for optical sensors, light-energy conversion and optical materials. 3D photonic structures for application in multifunctional composite materials with applications in communications and specialised applications in the military. |
| Synthetic Biomimetic Materials | Bio-inspired materials with improved functionality with wide-ranging structures and properties for use as synthesis guides for controlled fabrication of enhanced material (e.g. added strength) and new composites with applications in defence (e.g. armour). |
| Smart Materials | Multiphase systems/membranes or functionalised materials that can self-assemble, repair or evolve as responsive/controllable materials with diverse applications e.g. in healthcare (personalised medicine) or responsive wearable devices. |
| Self-healing Materials | Materials able to repair following damage without external intervention used for e.g. the repair of industrial systems through to new touchscreen technologies. |
| Micro-Mechanical Devices | Bioengineering for biomolecular control, e.g. responsive structures and behavioural materials for biomolecular robotics or biomaterials-based actuators engineered as artificial muscle. |
| Multicomponent Responsive Materials | Responsive polymers and interface materials with therapeutic or diagnostic medical applications (e.g. drug delivery systems and intelligent therapeutics). |
In the field of Bioelectronics how signals can be effectively transduced (e.g. in coatings) to link capabilities (e.g. sensing) with other components, or modules, in complex materials is a major challenge. Here biology can provide new designs, genetically encoded in the form of redox polymers (proteins) or nanowires, for further elaboration to enable integration into new materials where signal transduction is an embedded function. Predicted examples of the impact of synthetic biological materials in Bioelectronics are found in Healthcare where early detection/monitoring, rapid in vivo analysis, biomolecule to biosystem monitoring/management, and the design of scaled intelligent micro-bioelectronic systems are set to have major impact (e.g. cancer diagnosis and type II diabetes cost ∼ $200 billion in direct medical costs in the US and heart disease afflicts 22 million US citizens costing $172 billion). Coupled with a wide range of industrial applications (in situ monitoring for industrial manufacture) this field is poised for exponential growth, and will impact in cross cutting ways, e.g. medicine, health and well-being, forensics, homeland security, manufacture, and parallel diagnostics/miniaturisation. Synthetic biological materials can make substantial and unique contributions to all these challenge areas [7] and electroactive biopolymers [8] are beginning to show great promise attributed to the high tune-ability of chemical and physical properties that can be tailored to application. Here fabrication to form blends, composites or hybrids in the form of coatings or porous materials with improved conductive properties can drive diverse applications to satisfy unmet needs e.g. development of battery electrodes for improved storage capacity and charge/discharge rates, or rechargeable batteries for mobile electronic devices. Synthetic biology can be used to generate Electro-genetic Devices to provide bidirectional communication between electronic devices and biological systems [9]. Self-assembly into well-ordered structures at the nanometer scale are sought by the electronics industry, whilst the fabrication of nanowires using peptide scaffolds could also provide templates for metalation [10]. Extracellular electron transfer pathways that allow bacteria to communicate electrically between their intracellular chemical energy stores and extracellular solids, also present potential applications in biocomputing, bioenergy and biosensing [11].
Optical Materials will undoubtedly emerge as important synthetic biological materials. Natural biological systems have developed novel hierarchical photonic structures able to manipulate light propagation (e.g. structural colour, anti-reflection, light focus and chirality) [12] that are inspiring the design of new micro-structures and fabrication of smart optical devices for optical sensors, light-energy conversion and optical plasmonic material. Bio-templating methods have demonstrated success, but are not suitable for mass production, and reproducibility is poor. Biomimetic methods using engineering fabrication (e.g. nano-imprinting, 3D lithography and laser writing) are also promising but time consuming and expensive. Cross-discipline challenges should therefore provide the impetus and know-how to find bio-based nanofabrication solutions to build 3D photonic structures for real-world applications, where additional multi-functional natural properties (e.g. self-cleaning, directional adhesion, flexibility and fluorescence) will allow fabrication of multi-functional composite materials. These should find widespread application, especially in The Digital Economy and Communications sectors and specialised applications in the Military.
Synthetic biological materials will lead also to next generation Synthetic Biomimetic Materials and Mineral Biomaterials. The natural world has evolved a bewildering range of materials of desirable strengths, flexibility, adhesion, transparency, and reflectivity and conductance properties [13]. The challenge now is to build on a growing understanding of these materials to feed the development of new bio-inspired materials with improved functionality. Interactions between inorganic phases (simple salts or oxides), ions and biomolecules (e.g. proteins, lipids, carbohydrates or polyamides) provide complex structures with physical and mechanical properties that can support or withstand stress. The resulting composites have wide-ranging structures and properties that can serve as synthesis guides for controlled fabrication of materials with enhanced mechanical or other properties. Bringing the power of synthetic biology to diversify these materials further will facilitate deeper exploration of their properties and the creation of new composite materials for a wide range of application. A notable example is natural nacre (mother of pearl), which has superior properties to artificial materials of similar composition. The challenge here is to fabricate synthetic nacre in which the tiny aragonite slabs are held together using synthetic structures that mimic the structural and mechanical characteristics of their natural counterparts. Early reports to make synthetic nacre by predesigned matrix-directed mineralisation are now beginning to emerge [14].
There is intense interest in developing Smart Materials (e.g. multiphase systems or membranes) with integrated biological functions, or the functionalisation of existing materials, that can self-assemble, self-repair or evolve to provide connective, responsive and controllable materials (e.g. expansion or motion). These materials will support diverse applications, especially in Healthcare (e.g. personalised medicines). The use of genetically tractable microbial cells to create multifunctional responsive interfaces (e.g. to moisture) where new functionality is added (e.g. fluorophores, colour or odours), or metabolically responsive to environmental cues towards wearable devices, would meet multiple unmet needs [15]. Here a synthetic biological materials approach is powerful and enables assembly of responsive materials in a relatively facile and sustainable way that simply is not possible using other contemporary approaches.
Self-healing Materials have a built-in ability to automatically repair following damage without external problem diagnosis or human intervention. These materials could solve time-consuming and expensive repair of wear, tear and spontaneous damage. Whilst polymers embedded with self-healing adhesives that work through polymerisation and microcapsules exist, they have major drawbacks, e.g. ‘single use’. The challenge is to create autonomous adaptive structures (e.g. thermoplastics) or self-healing polymer materials that utilise ion-dipole interactions. New materials are in development, which use stretchable polymer chains linked by ion-dipole interactions between polymer polar groups and ionic salts. The resulting materials can stretch up to 50 times their usual size, and after being torn in two, can self-repair over the course of one day [16]. The creation of self-repairing materials capable of conducting electricity would have wide ranging applications, e.g. for use in touchscreens. Again, synthetic biological materials platforms would facilitate rapid exploration of new components for Self-healing Materials and define routes to their affordable production through biomanufacturing.
Synthetic biological materials offers new opportunities for the development of Micro-mechanical Devices. For example, bioengineering for biomolecular control (utilising DNA, RNA and other biomolecules for motion) to generate responsive structures and behavioural materials with expansion or motion towards biomolecular programmable robots is underway. Whilst development of helical coils of orientated polymer fibres that generate large, reversible changes in length as a result of thermal expansion under geometric constraint (winding/unwinding of helical constructs) could lead to actuators engineered to act as artificial muscle [17]. This provides motion through processes associated with intrinsic changes in constituent materials and could provide a solid-state alternative to mechanical machines (i.e. materials-based actuators).
In a similar vein, synthetic biologicl materials platforms can support the discovery and development of Multicomponent Responsive Materials (e.g. turbulent drag reduction materials, responsive polymers and responsive interface materials) and 'Synbio-fy' the more traditional field of Biomaterials. The latter covers a spectrum of research effort for materials engineered to interact specifically with biological systems. This might be for therapeutic or diagnostic medical purposes, including advanced formulation and fabrication for drug delivery systems and intelligent therapeutics that work harmoniously with the body for targeted or responsive delivery [18]. It includes the design and fabrication of polymer-based hydrophilic materials, or hydrogels, that allow incorporation of functional groups. By tailoring responsive polymers to applications in tissue engineering the properties of engineered scaffolds can be made responsive to a variety of physiological stimuli (e.g. pH, temperature, and salt conc.).
The above are examples of what might be achieved in the emerging field of synthetic biological materials, and others will follow. The synthetic biological materials agenda will be supported by the ability to define affordable and/or sustainable routes to biopolymeric materials and small molecule monomers through the bioengineering of extended metabolic space. Feedstock engineering can provide affordable routes to relatively low value monomers and there is already precedent from earlier investments in the synthetic biology of chemicals production, where bio-based production of monomers for a wide range of materials is already under development. Early exemplars include routes to terpene-based monomers [19], terephthalic acid and other materials components. Synthetic biology also opens up engineering of molecular chirality [20] and order, or the inexpensive incorporation of stable isotopes (e.g. deuterium), which in solid materials can lead to enhancement of physical and functional properties. Harnessing and extending these capabilities will also augment the drive to expedite new routes to synthetic biological materials.
3. Synergy of disciplines: areas of focus for early investments
In order to synergise synthetic biology, materials sciences and related disciplines in this burgeoning field, it is important to identify early challenges and consider exemplar areas where strategic investment would bring about a step-change in the early delivery of next generation advanced materials. Unmet challenges are wide-ranging and include applications in anti-fouling, coatings, bioelectronics, electro-genetic devices, optical materials, biomimetic and mineral based biomaterials, smart materials with added functionality, self-healing and multicomponent responsive materials. Identification of ‘early wins’ through interconnected challenge projects, with the potential to impact across multiple application areas, is crucial. This will address urgent unmet needs and catalyse additional growth to generate further ‘co-development partnerships’ with industry and government. Focus areas uniquely accessible to synthetic biology that would provide early impact across multiple application areas are identified below. These challenge themes are complementary; combined they would provide powerful new approaches to deliver diverse materials enabled through synthetic biology. These challenge areas embrace the need to: 1) emphasise transformative fundamental research that will accelerate delivery of next generation biomaterial production methods; 2) drive molecule library development to produce large-scale repositories for immediate application; 3) develop small-scale rapid screening to predict and test performance and properties of individual molecules within the library; 4) ensure predictive key feature and functionality characterisation using deep learning applications in the discovery pipeline; 5) standardise data compilation and collection.
The following areas would be especially attractive for early investment in synthetic biological materials and would build on existing investments and infrastructures available for example in the UK. The themes below are interrelated and provide a framework for early delivery of new advanced materials
-
➢
Small molecule monomer libraries to produce a large-scale, shared repository of generic small molecule building blocks for multiple applications. This is needed to speed up delivery of parts (e.g. functional components) and polymeric building blocks for subsequent diversification and hierarchical assembly (polymeric scaffolds and decoration/tailoring). Here computational approaches will provide the ability to search extended metabolic spaces and facilitate iterative design, build, test and learn (DBTL) cycles to new monomeric building blocks using existing high throughput (HTP) automated metabolic engineering infrastructures available (e.g. in the United Kingdom at SYNBIOCHEM [21,22]). This would provide sustainable synthetic biology routes (metabolic modules) to a wide range of monomers used in diverse polymeric materials. These include: sustainable polymers derived from terpene monomers (e.g. pinene; limonene; menthol; α-methyl-γ-butyrolactone and others) to provide platform scaffolds for further (hierarchical) modification; terephthalic acid, diamines, furan dicarboxylic acid, lactides and succinic acid (base monomers for many currently used polymers); long chain monomer precursors (oleic acid, ricioleic acid and derivatives), epoxidised/functionalised fatty acids.
-
➢
Biopolymer building blocks with, for example, signal transduction and sensing capabilities for bioelectronics and optical properties engineering. These would be protein modules, elaborated to interface with other components (scaffolds, surfaces and other bespoke molecules) that, for example, transmit electronic signals from one part of a material to a distal region to facilitate signal transduction. Protein nanowires can be built through diversification of natural protein components (e.g. multi-heme proteins; metallo-protein domains) and functionalised genetically (conventional amino acid substitution and orthogonal incorporation of non-proteinogenic amino acids into biopolymers) using synthetic biology platforms. This would provide a repository of self-assembling components that can form nanowires functionalised to form hybrid/composite materials; components engineered to form linear/branched/networked structures; components functionalised to interface (e.g. through Click chemistry) with other materials components, biopolymers and surfaces; nanowire components that are readily interfaced with other components for capture and monitoring (e.g. electrical, optical, fluorescence signals). Similarly optically responding protein biopolymers (e.g. reflectins; phytochromes, to name but a few) can be likewise functionalised to generate higher order composite materials.
-
➢
Rapid higher order assembly and fabrication of materials from component scaffolds. Higher order fabrication of materials with novel properties will necessitate further development of instrumentation and characterisation platforms such as spinning techniques and interfacing with 2/3D printing (e.g. in layers), miniaturisation and high throughput (HTP) capabilities. Establishing new routes to spinning synthetic biological material polymers, assembling layered materials through additive manufacturing and self-assembling novel and diverse components (e.g. through Click chemistry to form novel coatings) are early targets to drive higher order assembly of new materials. New coatings could be rapidly assembled through use of laboratory automation and functionalised scaffolds/components building blocks. This would enable rapid integration of optical or sensing chromophore components (e.g. development of tunable, photon absorbing optical limiting materials to prevent laser dazzle for transparent sensor protective coatings, or camouflage coatings with reflectins). Protein based scaffolds could also provide the basis for nanowires for subsequent functionalisation and diversification. An early objective would therefore be to establish rapid and robust fabrication routes to such higher order materials.
-
➢
Robust production chassis for affordable production of synthetic biological materials. Despite exciting advances in the scale and scope of metabolic engineering, major limitations of laboratory (e.g. yeast, E. coli) and industrial strains (e.g. bacillus, yeast) have prevented affordable development of sustainable bio-based manufacture at scale. Materials components fall into the high volume/low cost category and robust production chassis are now urgently required to drive affordable production. Development of robust industrial production strains that can be grown in non-sterile environments, with reduced water demand, and reduced capital investment in establishing production facilities offers the potential to develop sustainable and affordable bio-production platforms and distributed manufacture in challenging environments for both small molecule and biopolymer-based materials. For example, some industrial strains have been adopted for the high volume commercial production of bioplastics but not adopted more widely as a production hosts for other materials. Early objectives would be to migrate production platforms into new robust strains for affordable and sustainable production at scale. This would require establishment of a comprehensive synthetic biology repository of tools and resources for production of materials in these new hosts.
The above describes interconnected early focus areas that would have the broadest impact and also guarantee early wins for synthetic biological materials, both in terms of new materials discovery that cuts across multiple application areas and also affordable and sustainable manufacture of the building blocks for these materials and broad range applications.
4. Integrated delivery platforms for advanced materials
To accelerate discovery and delivery of synthetic biological materials, integration of interdisciplinary and advanced platform technologies would be required to form a pipeline capability that is able to progress smoothly from early target building block design, through to material fabrication and scaled-up production. The early consideration of multiple technology components, described below, will be important. The field will draw on existing and emerging synthetic biology tools (e.g. design software for macromolecules; mapping of extended metabolic space; software to facilitate the rapid engineering of production organisms and/or cell lines), but will also require the development of new tools and platforms, especially in the area of materials evaluation, given the diversity of new structures that will emerge from automated materials DBTL platforms.
Embedding AI/machine learning at every stage of the DBTL cycle will facilitate accelerated delivery. Predictive design driven by machine learning using more limited experimental datasets will accelerate the delivery of new materials with desirable properties and needs to work alongside more established Design areas such as Multi-scale Modelling. Application of iterative rounds of intelligent Design, Build, Test and Re-design with the development and adoption of ‘higher-level’ language and abstraction towards Design will enable further understanding of complex systems. Leveraging frontier developments in AI, data analytics, machine learning and deep-deep learning will synergise this approach and will define new routes to in silico exploration of new materials, their structures and functional properties [23,24].
The assembly of binary (or higher order) materials, where properties only emerge, on mixing or assembly of components, is an essential consideration. Important drivers will be rapid generation of materials diversity, scalability of synthesis, synthesis of biological and chemical stability, modular synthesis and production of composites. Total integration of all synthetic methodology embracing chemical, biological and nanotechnology approaches, will be required for rapid design and assembly of new materials. Additive manufacturing is an exciting field covering the design, fabrication, assembly and measurement of bio-elements to deliver structures, devices, and systems, and their subsequent in vivo or in vitro integration into larger scale structures. Innovation in this space will be required to drive synthetic biological materials into the fabrication of more complex hybrid materials that will enable new properties and functions to emerge. Future infrastructure investment will therefore need flexible, adaptive manufacturing capability to design and manufacture a range of synthetic biological materials.
Rapid diversification of basal materials and layering to form composites and other higher order structures, exploiting additive manufacturing, will be enabled by genetic encoding of non-canonical amino acids in synthetic biology platforms which would support further diversification by efficient chemical tagging of small molecules, other biological components, surfaces or non-biological polymers/mineral surfaces. This would require expertise in the bioengineering of metabolic and regulatory parts, pathways and production systems, for example those for chemicals and proteins, and for feedstock engineering. The challenge here will be to drive existing capabilities for parts, pathways and chassis engineering towards more complex hierarchical assembly of new materials by interfacing with technology platforms established in other disciplines, including synthetic and materials chemistry, and engineering.
Accelerated discovery of natural biological materials would be required to explore the diversity of materials and provide access to new materials properties currently lacking. Recent advances in next generation sequencing are key here, and would considerably reduce the time constraints in identifying new biological materials. Research programs would need to embrace these new technologies to give access to the potential power of vast libraries of biological materials (natural and synthetic) and to deliver these new biopolymers into artificial systems through further chemical/biological elaboration, and/or through the assembly of new composites.
The required evaluation platforms present major challenges, because of the sheer diversity of new Synthetic Biological Materials that will emerge from ‘Design’, ‘Build’ and ‘Production’ platforms. Here we have an opportunity to harness leading national infrastructures (e.g. the Henry Royce Institute [26] and other materials Centre's throughout the UK), and to establish more generic parallelised and miniaturised approaches to the evaluation of general properties. The demands on evaluation of new advanced materials properties will be extensive, encompassing a variety of sub-themes including structural (size, strength, flexibility, shape etc.), functional (e.g. conductivity, programmable, light responsive, visco-/poro-elasticity etc.), compound properties through inventive combinations and arrangements of component materials (emergent properties), biocompatibility, biological and chemical stability, and the ability to self-assemble or nucleate hierarchical complexity and anisotropic behaviour. A comprehensive ‘Evaluation’ capability platform that harnesses state-of-the-art analytical tools for synthetic biological materials characterisation would be of great benefit. The platform technologies for evaluation of physical properties would typically include: HTP rheology, tensile testing, small/wide angle X-ray scattering, light scattering, thermal characterisation and structural analysis through a suite of imaging and spectroscopy approaches. Likewise surface analysis would require a suite of specialised methods.
There is a pressing need to establish new sensitive high throughput evaluation methods to support the production and characterisation of building blocks delivered through synthetic biology approaches. High content analysis through quantitative cell analysis (e.g. ‘Cellomics’) is needed to assess biocompatibility and toxicity of expressed building blocks designed and synthesised in the production platforms. This uses state-of-the-art bioimaging methods and informatics workflows comprising: image acquisition, image analysis, and data visualisation and management. These generally automated processes depend on sophisticated software for acquisition and management of both of qualitative and quantitative data. High content analysis would contribute to the active learning that runs throughout the pipeline, providing data on biocompatibility that can inform the design and synthesis of next generation building blocks. Critical to the evaluation and active learning programmes will be access to HTP sequencing, to map sequence-property relationships, which again through active learning can then be further optimised.
Similarly, miniaturisation of parallel evaluation methods would be required to assess and sort (filter) desirable properties for the diverse range of synthetic biological materials produced. This might include rapid read out of Infra-Red signatures to assess the folded nature of biopolymers, or optical/fluorescence properties of assembled materials components using automated screening/evaluation methods, or micro-rheology. Picodroplet technology would be especially powerful for rapid sorting of building blocks at the front end of the production platform. Here DNA writing to encode building blocks could include extended sequences to encode auto-fluorescent protein tags or peptide extensions that could be tagged with small molecule fluorophores. Picodroplet microfluidic technology processes (e.g. fuse, split and sort) and analyses (using fluorescent detection methods) would allow interrogation of tens of millions of strains per day (i.e. ultra-high throughput screening). This would allow generic screening and identification of ‘folded’ versus unfolded/unstable building blocks at a frequency >150 s−1. Each clone could be sequenced to identify components with desirable predicted properties that are then synthesised in the same way and tested in an iterative loop i.e. active learning. In this way, the facility would pioneer the rapid expansion and provision of stable bio-based modules for further combinatorial assembly.
Ultimately selected building blocks would be needed in high-yield to drive functional elaboration and application. At the laboratory scale stable building blocks could also be encoded with suitable tags (e.g. polyhistidine, biotin tags and similar) at the DNA writing stage. These would enable high throughput recovery from expression hosts in 96- or 384-well format using automated liquid handling platforms incorporating magnetic bead recovery of biosynthesised products. The design possibilities are endless – tags can be retained, cleaved and even positioned at various points in the encoding DNA sequence (N and C-terminal, or within exposed internal loops of a biopolymer). For medium scale production, methods could be transferred to automated affinity chromatography platforms where sequentially or in parallel production optimisation is scouted with minimal operator intervention.
In due course the optimised components would need to be manufactured at larger scale. This would be achieved through fermentation of host strains that enable scale-out/scale-up at laboratory scale (typically 10–50L scale) following scouting of optimised production conditions in automated fermentation or mammalian cell culture platforms. Production could be coupled to established downstream processing to generate building blocks (mg-g scale) providing material that will support hybrid synthesis and assembly of complex biomaterials. Production chassis might need to be engineered to use available, affordable or waste feedstocks (e.g. CO2, SynGas, lignocellulosic, glycerine and other wastes), and these feedstock pathways would need to be leveraged from existing capabilities, for example in Synthetic Research Biology Centres across the UK [22,25]. This is especially the case for higher volume/lower value products where the economics of production and recovery of new components would be important factors. Any investment infrastructure would need to innovate in the production area to lower such costs, for example by lowering barriers to capital investment to production. This might be achieved through expression in robust industrial hosts that are less dependent on high cost infrastructure for cell growth, or expensive media for cell propagation. In selected cases expression of building blocks could be achieved in mammalian or yeast cell lines, with new opportunities emerging in genome editing. This would allow targeted biological post-translational modification (e.g. glycosylation) of building blocks that could contribute to novel functional properties.
Finally innovation is needed for development of new production platforms. These might involve the production of biological layers/sheets for hard and soft materials, the spinning of polymer fibres using innovative spinning technologies and the production of materials in situ. This development of production and fabrication platforms for hybrid materials would require wide interdisciplinary collaboration. Whilst the generation of materials, rather than production of new precursor chemicals and biological modules, would require revolutionary approaches to cellular production. Challenges would be around Formulation of Materials and how to insert synthetic biology steps to improve and functionalise materials. Here activity will need to integrate expertise, for example, the 2D nano community has extensive knowledge in the area of graphene-layered hybrid materials, as does the electronics industry in related materials. Formulation of nanoparticles from atomic precursors, the organisation of molecules on the surface of nanoparticles, and materials built from nanoparticles, could provide uniformity and control of one length scale profoundly affecting material properties.
It is clear that to take advantage of this burgeoning interdisciplinary field and deliver a new paradigm of synthetic biological materials with wide-spread applications, will require convergence of expertise, co-development of novel platforms and interdisciplinary, international and inter-sector collaborations coordinated through an innovation pipeline. An innovation pipeline for synthetic biological materials should extend from the discovery, development, testing and fabrication of new advanced materials through to industrialisation and commercialisation. A supporting strategy should seek to establish and integrate existing expert capabilities, resources and infrastructures in order to achieve this vision. Many of the tools and infrastructures required to develop the synthetic biological materials vision are available and common to related Synthetic Biology application areas (e.g. bio-based chemicals production). The challenge now is to harness these resources and interface them with new fabrication and evaluation platforms that would enable rapid exploration of functional properties for the emergent synthetic biological materials.
5. Delivering the new paradigm of Synthetic Biological Materials
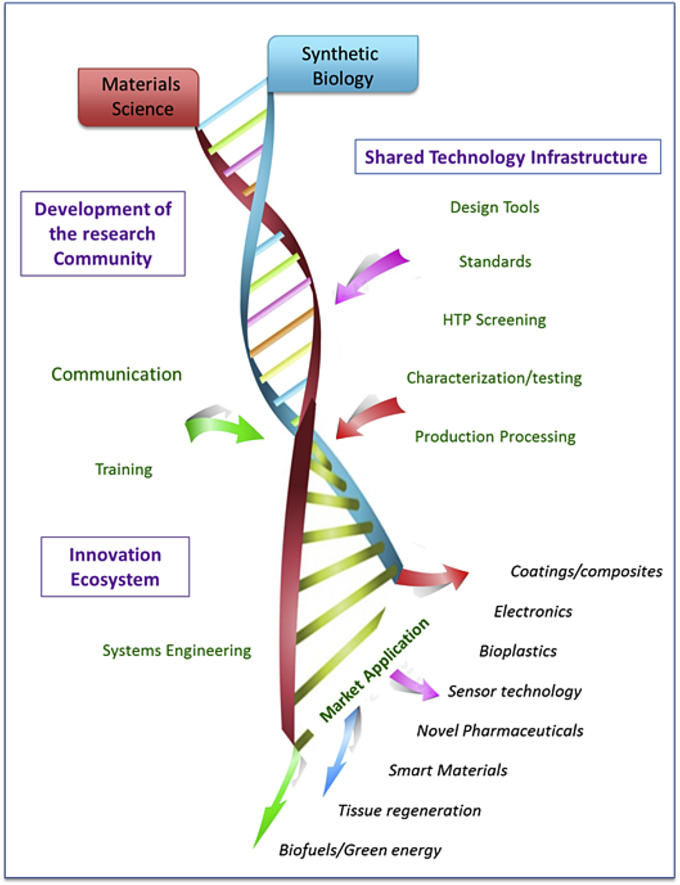
Driving intersectorial, interdisciplinary and international connectivity, and the leveraging of existing investments in synthetic biology, materials science, allied science and technology areas, are the major challenges in delivering the Materials from Biology vision (Fig. 1). This is alongside a need to establish early stage partnerships with industry to define unmet needs in advanced materials and to maintain continued engagement from early-stage discovery and development, through to manufacturing delivery and commercialisation. Unification of these fields will create major opportunities for new materials discovery, their sustainable and affordable manufacture and application to unmet needs for industry.
Fig. 1.
Developing an ecosystem to deliver a living foundry for Synthetic Biological Materials.
In conclusion, by harnessing the power of synthetic biology existing materials discovery platforms and fabrication technologies can be augmented to widen the materials development space and define a new materials paradigm – ‘Synthetic Biological Materials’. Realising this opportunity would deliver next generation advanced materials with new and extended functional properties that address a wide range of unmet needs, and provide access to affordable and sustainable routes to the production of synthetic biological materials.
Declarations of interest
None.
Acknowledgements
The authors are grateful to all colleagues who contributed to the discussions at the Manchester workshop in 2017 whose views were used to inform this Roadmap for Synthetic Biological Materials. The authors also acknowledge funders of the workshop including Office of Naval Research Global, Defence Science and Technology Laboratory and the University of Manchester's Centre for Synthetic Biology (SYNBIOCHEM grant BB/M017702/1). Disclaimer: Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the DSTL or ONRG.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Rosalind A. Le Feuvre, Email: R.a.le-feuvre@manchester.ac.uk.
Nigel S. Scrutton, Email: nigel.scrutton@manchester.ac.uk.
References
- 1.International workshop: foundry for Synthetic Biomaterials: scientific and technological challenges in defining a new paradigm for sustainable biomaterials. Manchester Institute of Biotechnology, 16–17/05/17.
- 2.Biodesign for the bioeconomy. UK synthetic biology strategic plan UK synthetic biology leadership council. 2016. https://connect.innovateuk.org/web/synthetic-biology-special-interest-group/2016-uk-synbio-strategic-plan
- 3.Industrialisation of biology: a roadmap to accelerate the advanced manufacturing of chemicals. National Academics Press; 2015. https://doi.org/10.17226/19001 [PubMed] [Google Scholar]
- 4.Smanski M.J., Zhou H., Claesen J., Shen B., Fischbach M.A., Voigt C.A. Synthetic biology to access and expand nature’s chemical diversity. Nat Rev Microbiol. 2016;14:135–149. doi: 10.1038/nrmicro.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stratton S., Shelkeab N.B., Hoshino K., Sangamesh R., Kumbar G. Bioactive polymeric scaffolds for tissue engineering. Bioactive Materials. 2016;1:93–108. doi: 10.1016/j.bioactmat.2016.11.001. https://doi.org/10.1016/j.bioactmat.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cost of Corrosion report: http://www.g2mtlabs.com/corrosion/cost-of-corrosion/. [Accessed 5 April 2018].
- 7.A framework for bioelectronics: discovery and innovation. National Institute of Standards and Technology; 2009. https://www.nist.gov/sites/default/files/documents/pml/div683/bioelectronics_report.pdf [Google Scholar]
- 8.Guarino V., Zuppolini S., Borriello A., Ambrosio L. Electro-active polymers (EAPs): a promising route to design bio-organic/bioinspired platforms with on demand functionalities. Polymers. 2016;8:185. doi: 10.3390/polym8050185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tschirhart T., Kim E., McKay R., Ueda H., Wu H.C., Pottash A.E., Zargar A., Negrete A., Shiloach J., Payne G.F., Bentley W.E. Electronic control of gene expression and cell behaviour in Escherichia coli through redox signalling. Nat Commun. 2017;8:14030. doi: 10.1038/ncomms14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan K.H., Lee W.H., Zhuo S., Ni M. Harnessing supramolecular peptide nanotechnology in biomedical applications. Int J Nanomed. 2017;12:1171–1182. doi: 10.2147/IJN.S126154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.TerAvest M.A., Ajo-Franklin C.M. Transforming exoelectrogens for biotechnology using synthetic biology. Biotechnol Bioeng. 2015;4:687–697. doi: 10.1002/bit.25723. [DOI] [PubMed] [Google Scholar]
- 12.Wu L., He J., Shang W., Deng T., Gu J., Su H., Liu Q., Zhang W., Zhang D. Optical functional materials inspired by biology. Adv. Optical Mater. 2016;4:195–224. [Google Scholar]
- 13.Wegst U.G.K., Bai H., Saiz E., Tomsia A.P., Ritchie R.O. Bioinspired structural materials. Nat Mater. 2014;14:23–36. doi: 10.1038/nmat4089. [DOI] [PubMed] [Google Scholar]
- 14.Mao L.-B., Gao H.-L., Yao H.-B., Cölfen H., Liu G., Chen S.-M., Li S.-K. Synthetic nacre by predesigned matrix-directed mineralization. Science. 2016;354:107–110. doi: 10.1126/science.aaf8991. [DOI] [PubMed] [Google Scholar]
- 15.Wang J., Sun L., Zou M., Gao W., Liu C., Shang L., Gu Z. Zhao. Bioinspired shape-memory graphene film with tunable wettability. Sci Adv Mater. 2017;3:e1700004. doi: 10.1126/sciadv.1700004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao Y., Morrissey T.G., Acome E., Allec S.L., Wong B.M., Keplinger C., Wang C. A transparent, self-healing, highly stretchable ionic conductor. Sci Adv Mater. 2017;29 doi: 10.1002/adma.201605099. [DOI] [PubMed] [Google Scholar]
- 17.Haines C.S., Li N., Spinks G.M., Aliev A.E., Di J., Baughman R.H. New twist on artificial muscles. Proc Natl Acad Sci. 2016;113:11709–11716. doi: 10.1073/pnas.1605273113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi Z., Gao X., Ullah M.W., Li S., Wang Q., Yang G. Electroconductive natural polymer-based hydrogels. Biomaterials. 2016;111:40–54. doi: 10.1016/j.biomaterials.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 19.Zebec Z., Wilkes J., Jervis A.J., Scrutton N.S., Takano E., Breitling R. Towards synthesis of monoterpenes and derivatives using synthetic biology. Curr Opin Chem Biol. 2016;34:37–43. doi: 10.1016/j.cbpa.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Challener C.A. Synthetic Biology: the Next Frontier in Chiral Chemistry for API Synthesis: the commercial availability of an increasing diversity of enzymes has led to the growing use of biocatalysts for API synthesis. Pharmaceut Technol. 2014;38(3) [Google Scholar]
- 21.SYNBIOCHEM – The Synthetic Biology Research Centre for fine and speciality chemicals at the University of Manchester. http://synbiochem.co.uk. [Accessed 5 April 2018].
- 22.Clarke L.J., Kitney R.I. Synthetic biology in the UK – an outline of plans and progress. Synthetic and Systems Biotechnology. 2016;1:243–257. doi: 10.1016/j.synbio.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y., Zhao T., Ju W., Shi S. Materials discovery and design using machine learning. J Materiomics. 2017;3:159–177. [Google Scholar]
- 24.Carbonell P., Currin A., Jervis A.J., Rattray N.J.W., Swainston N., Ya C., Takano E., Breitling R. Bioinformatics for the synthetic biology of natural products: integrating across the Design–Build– Test cycle. Nat Prod Rep. 2016;33:925–932. doi: 10.1039/c6np00018e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Humphreys C.M., Minton N.P. Advances in metabolic engineering in the microbial production of fuels and chemicals from C1 gas. Curr Opin Biotechnol. 2018;50:174–181. doi: 10.1016/j.copbio.2017.12.023. [DOI] [PubMed] [Google Scholar]
- 26.Henry Royce Institute http://www.royce.ac.uk/. [Accessed 5 April 2018].