Abstract
The nucleus pulposus (NP) of intervertebral discs experiences dynamic changes in tissue osmolarity because of diurnal loading of the spine. TonEBP/NFAT5 is a transcription factor that is critical in osmoregulation as well as survival of NP cells in the hyperosmotic milieu. The goal of this study was to investigate whether cyclooxygenase-2 (COX-2) expression is osmoresponsive and dependent on TonEBP, and whether it serves an osmoprotective role. NP cells up-regulated COX-2 expression in hyperosmotic media. The induction of COX-2 depended on elevation of intracellular calcium levels and p38 MAPK pathway, but independent of calcineurin signaling as well as MEK/ERK and JNK pathways. Under hyperosmotic conditions, both COX-2 mRNA stability and its proximal promoter activity were increased. The proximal COX-2 promoter (−1840/+123 bp) contained predicted binding sites for TonEBP, AP-1, NF-κB, and C/EBP-β. While COX-2 promoter activity was positively regulated by both AP-1 and NF-κB, AP-1 had no effect and NF-κB negatively regulated COX-2 protein levels under hyperosmotic conditions. On the other hand, TonEBP was necessary for both COX-2 promoter activity and protein up-regulation in response to hyperosmotic stimuli. Ex vivo disc organ culture studies using hypomorphic TonEBP+/− mice confirmed that TonEBP is required for hyperosmotic induction of COX-2. Importantly, the inhibition of COX-2 activity under hyperosmotic conditions resulted in decreased cell viability, suggesting that COX-2 plays a cytoprotective and homeostatic role in NP cells for their adaptation to dynamically loaded hyperosmotic niches.
Keywords: cyclooxygenase (COX), NFAT transcription factor, transcription factor, cell signaling, nucleus pulposus, intervertebral disc, TonEBP, COX-2, calcium, osmoregulation, cell biology
Introduction
The nucleus pulposus (NP)3 has a complex matrix that consists of primarily large aggregating proteoglycan aggrecan within the network of collagen II. High concentration of negatively charged sulfated glycosaminoglycans on aggrecan allows the NP to attract sodium ions within the tissue, resulting in physiologically elevated osmolarity compared with surrounding tissues. These cations also cause the NP tissue to imbibe water, providing high osmotic swelling pressure responsible for the ability of discs to resist compressive loads in the spine (1, 2). The increased matrix degradation by proteolysis, changes in matrix composition, and inflammatory signaling result in compromised mechanical function as well as decreased osmolarity during intervertebral disc degeneration (3–6). Moreover, the efflux and influx of water from discs caused by diurnal loading of the spine lead to dynamic changes of osmolarity in NP tissue. As a result, NP cells have evolved to adapt to the changes in extracellular osmolarity.
Tonicity-responsive enhancer-binding protein (TonEBP/nuclear factor of activated T-cells 5 (NFAT5)) is a Rel homology transcription factor, whose induction is coupled to hyperosmolarity. TonEBP promotes cell survival by regulating the expression of osmoprotective genes in a variety of tissues including NP (7, 8). Under osmotic stress, TonEBP prevents excess influx of sodium by controlling the intracellular levels of nonionic osmolites such as taurine, sodium/myoinositol, and betaine through regulation of their transporters and enzymes involved in their synthesis: taurine transporter (TauT), betaine-GABA transporter (BGT-1), sodium/myoinositol cotransporter (SMIT), and aldose reductase (AR) (8–12). Additionally, heat shock protein 70 (HSP 70), a chaperone involved in cellular trafficking, protein folding, and degradation of misfolded proteins, is regulated by TonEBP under hyperosmotic condition (13).
In Madin-Darby canine kidney cells, TonEBP has been shown to regulate cyclooxygenase-2 (COX-2), an enzyme necessary for prostaglandin (PG) synthesis (14). PGs are important for regulating renal salt and water balance as well as inflammation (15). Other members of the NFAT family also control COX-2 expression under various stimuli, including hyperosmolarity (16–18). Whereas in renal cells COX-2 is linked to cell survival and osmo-adaptation (19–21), its regulation and function in the setting of hyperosmolarity remain largely unexplored in the disc. The objective of this study was to elucidate how COX-2 expression is controlled in the hyperosmotic milieu of NP cells as well as its functional implications. We show that COX-2 expression is up-regulated under hyperosmotic conditions through intracellular calcium independent of calcineurin signaling. In addition, we determine that this induction is through TonEBP-mediated increase in COX-2 proximal promoter activity and its mRNA stability. Importantly, our study demonstrates that COX-2 plays cytoprotective and osmo-adaptive role in the hyperosmotic microenvironment of the intervertebral disc.
Results
Hyperosmolarity and increased intracellular calcium up-regulate COX-2 expression in NP cells
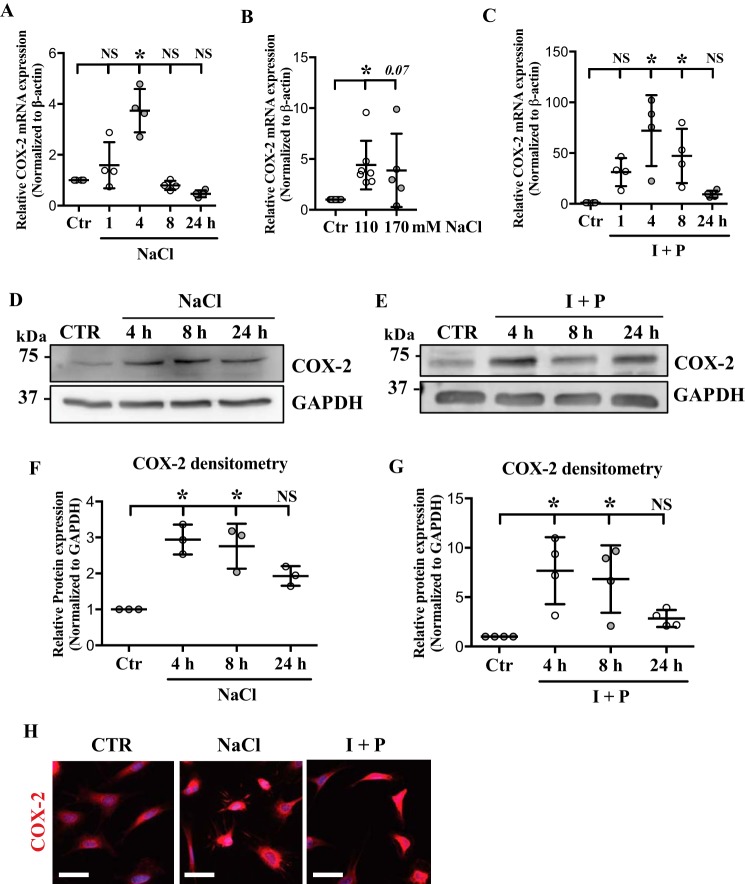
The effect of hyperosmolarity on COX-2 expression was examined by culturing NP cells under hyperosmotic conditions and measuring COX-2 mRNA and protein levels. COX-2 mRNA levels were significantly increased in response to NaCl treatment at 4 h, and decreased to below the baseline level by 24 h (Fig. 1A). Treating NP cells with NaCl up to 170 mm resulted in COX-2 mRNA up-regulation (Fig. 1B). All the remaining experiments in this study were done with 110 mm NaCl. Because extracellular hyperosmolarity leads to increased [Ca2+]i in NP cells (22, 23), we examined the effect of increased [Ca2+]i on COX-2 levels. Treatment with calcium ionophore, ionomycin, with phorbol 12-myristate 13-acetate (PMA) resulted in a significant induction of COX-2 mRNA at 4 and 8 h (Fig. 1C). Although there was a decrease in the levels of COX-2 at 24 h post ionomycin/PMA treatment, the levels remained higher than the baseline (Fig. 1C). Western blot analyses demonstrated induction of COX-2 protein levels in response to hyperosmolarity and ionomycin/PMA treatment (Fig. 1, D–G). COX-2 protein levels were significantly up-regulated at 4 and 8 h post hyperosmolarity and ionomycin treatments. Immunofluorescence staining supported the Western blot data showing increased COX-2 levels with NaCl or ionomycin/PMA treatment (Fig. 1H).
Figure 1.
COX-2 is up-regulated in response to hyperosmolarity and high intracellular calcium in NP cells. A and B, qRT-PCR analysis demonstrates (A) time- and (B) dose-dependent COX-2 mRNA increase under hyperosmotic conditions. C, COX-2 mRNA is induced by ionomycin/PMA (I + P) treatment. D and E, Western blots of COX-2 after NaCl or I + P time course treatments show significant induction of COX-2 expression. F and G, densitometry analyses of Western blots from D and E. H, immunofluorescence staining of COX-2 in NP cells confirms that its expression is increased with NaCl as well as I + P treatment. Scale bar: 50 μm. All the quantitative data are represented as mean ± S.D. from at least three independent experiments. *, p < 0.05.
Induction of COX-2 is independent of calcineurin pathway
Because calcineurin is an important mediator of calcium signaling, we investigated whether hyperosmolarity-induced COX-2 up-regulation in NP cells involved calcineurin pathway. Cells were treated with NaCl with or without BAPTA, a potent calcium chelator, or FK-506 and cyclosporin A (CsA), calcineurin inhibitors. Reducing intracellular calcium levels using BAPTA inhibited hyperosmotic induction of COX-2 mRNA and protein (Fig. 2, A–C). However, FK-506/CsA treatment did not alter the NaCl-induced COX-2 levels, indicating that COX-2 induction was independent of calcineurin signaling (Fig. 2, A–C). Similarly, ionomycin/PMA-dependent induction of COX-2 mRNA and protein levels were completely abolished by BAPTA, but not by FK-506/CsA, confirming that calcineurin did not play a role in this process (Fig. 2, D–F).
Figure 2.
Hyperosmolarity-mediated induction of COX-2 is through intracellular calcium but independent of calcineurin signaling. A–C, hyperosmotic induction of COX-2 mRNA (A), and protein (B and C) is suppressed by BAPTA, a calcium chelator, but is unaffected by FK-506/CsA (F/C), a calcineurin inhibitor. D–F, induction of COX-2 mRNA (D) and protein (E and F) in response to ionomycin/PMA (I + P) treatment is completely abolished with BAPTA, but not with FK/CsA treatment. All the quantitative data are represented as mean ± S.D. from at least three independent experiments (three biological replicates). NS: nonsignificant; *, p < 0.05.
p38 MAPK pathway mediates hyperosmotic induction of COX-2
Hyperosmolarity as well as calcium signaling is known to activate MAPK signaling pathways (24–28). Previous studies also showed that MAPK pathways regulate COX-2 expression in some cell types (29, 30). We therefore investigated if this pathway contributed to regulation of COX-2 expression in NP cells. We first determined the changes in activation status of p38 in NP cells under hyperosmotic condition. Phospho-p38 levels were rapidly increased as early as 15 min and stayed significantly up-regulated until 1 h following NaCl treatment (Fig. 3, A and B). Although there was a trend of increasing phospho-p38 at 5 min, 4 h, and 24 h, it did not reach statistical significance. We then treated NP cells with MAPK inhibitors including SB202190, p38 inhibitor; PD98059, MEK/ERK inhibitor; and SP600125, JNK inhibitor, under hyperosmotic conditions and measured COX-2 levels. Treating NP cells with p38 inhibitor resulted in blockage of hyperosmotic induction of COX-2 mRNA (Fig. 3C). However, inhibition of MEK/ERK or JNK pathways did not affect COX-2 induction (Fig. 3D). Similarly, COX-2 protein up-regulation in response to NaCl treatment was abolished with p38 inhibition, indicating that p38 MAPK is involved in hyperosmotic induction of COX-2. However, when NP cells were treated with ionomycin/PMA, p38 inhibition had no effect on COX-2 up-regulation (Fig. 3, E–G), suggesting that p38 MAPK is dispensable to ionomycin-induced COX-2 expression.
Figure 3.
Hyperosmotic induction of COX-2 is mediated by p38, but not by ERK or JNK. A and B, phospho-p38 (p-p38) levels significantly increase in response to hyperosmolarity. C and D, hyperosmotic increase of COX-2 mRNA is completely suppressed by p38 inhibitor, SB202190, but unaffected by JNK inhibitor, SP600125, and MEK/ERK inhibitor, PD98059. E, Western blot images showing that p38 inhibition prevents COX-2 induction in response to hyperosmolarity. F, Western blot images showing that ionomycin/PMA (I + P)-mediated COX-2 induction is not affected by p38 inhibition. G, densitometry analyses of Western blots shown in E and F. All the quantitative data are represented as mean ± S.D. from at least three independent experiments (three biological replicates). NS: nonsignificant; *, p < 0.05. PD: PD98059 (MEK/ERK inhibitor); SB90: SB202190 (p38 inhibitor); SP: SP600125 (JNK inhibitor).
Hyperosmolarity increases COX-2 transcription as well as its mRNA stability
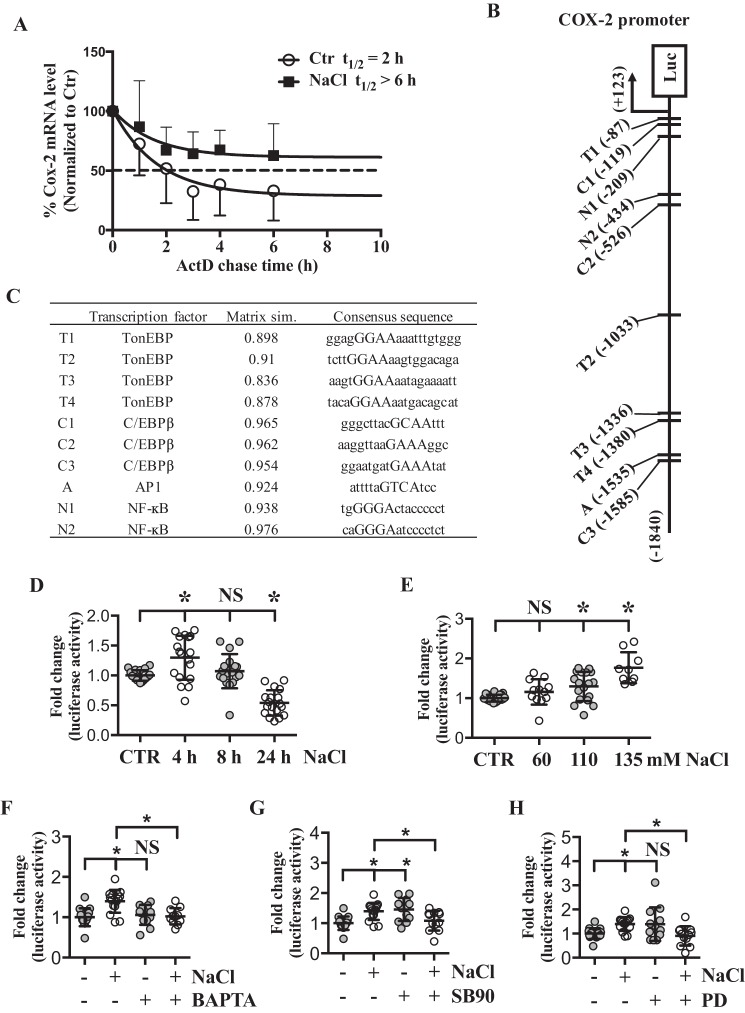
To further elucidate the mechanism of COX-2 induction by hyperosmolarity, we first investigated if hyperosmolarity affects COX-2 mRNA stability. Actinomycin D chase study showed that in NP cells, the half-life of COX-2 mRNA under isoosmotic condition was ∼2 h, whereas under hyperosmotic condition it was longer than 6 h (Fig. 4A), suggesting that increased COX-2 expression was in part due to elevated mRNA stability.
Figure 4.
Both increased mRNA stability and proximal promoter activity account for COX-2 up-regulation in response to hyperosmolarity. A, actinomycin D chase study demonstrates that estimated half-life of COX-2 mRNA is increased by hyperosmolarity, representing increased mRNA stability (n = 5). B, a schematic showing TonEBP, AP-1, NF-κB, and C/EBP-β–binding sites on ∼1.8 kb COX-2 proximal promoter region. C, list of putative binding sites and consensus sequence for each transcription factor. Four bp core consensus sequence used for query was marked as capital letters. Matrix similarity score based on the most conserved nucleotide at each position of the matrix is also shown. D and E, COX-2 promoter activity shows (D) time- and (E) dose-dependent increase under hyperosmotic conditions. F, hyperosmolarity-dependent increase of COX-2 promoter activity is inhibited with a calcium chelator, BAPTA. G and H, up-regulation of COX-2 promoter activity under hyperosmotic condition was inhibited by p38 inhibitor (SB90) and MEK/ERK inhibitor (PD). All the quantitative data are represented as mean ± S.D. from at least three independent experiments (three biological replicates). Promoter activity experiments were done with three technical replicates per independent experiment. NS: nonsignificant; *, p < 0.05. SB90: SB202190 (p38 inhibitor); PD: PD98059 (MEK/ERK inhibitor).
Because MAPK pathways are involved in activation of various transcription factors, we investigated if hyperosmotic induction of COX-2 was also transcriptionally regulated. Luciferase reporter assays were performed using COX-2 promoter fragment (−1840/+123 bp) that has shown transcriptional regulatory activity in other cell types (31). Genomatix MatInspector software was used to scan potential TonE- and other relevant transcription factor–binding sites within the COX-2 promoter (Fig. 4B). Putative binding sites and consensus sequence for each transcription factor with four core consensus bp in capital letters are listed in the table (Fig. 4C). Matrix similarity score based on the most conserved nucleotide at each position of the matrix is also shown. Four TonE sites as well as binding sites for AP-1, C/EBP-β, and NF-κB were identified. We assessed the sensitivity of the promoter to hyperosmotic stimuli; the promoter activity was significantly increased at 4 h, and then declined at 24 h (Fig. 4D). COX-2 promoter activity also showed dose-dependent induction in response to hyperosmolarity (Fig. 4E). In addition, hyperosmotic induction of COX-2 promoter activity was reduced to basal level when the cells were treated with BAPTA, supporting our earlier data on calcium-dependent induction of COX-2 mRNA and protein levels under hyperosmotic conditions (Fig. 4F). Similarly, when the cells were treated with p38 inhibitor under hyperosmotic condition, COX-2 promoter activity was decreased to that of baseline (Fig. 4G). Interestingly, inhibition of p38 under isoosmotic condition resulted in increased COX-2 promoter activity (Fig. 4G). Furthermore, although COX-2 mRNA levels were not affected, its promoter activity was significantly reduced by MEK/ERK inhibitor (Fig. 4H). These results indicated that hyperosmotic induction of COX-2 was because of increased mRNA stability as well as transcription.
AP-1, C/EBP-β, and NF-κB do not contribute to hyperosmotic induction of COX-2 expression
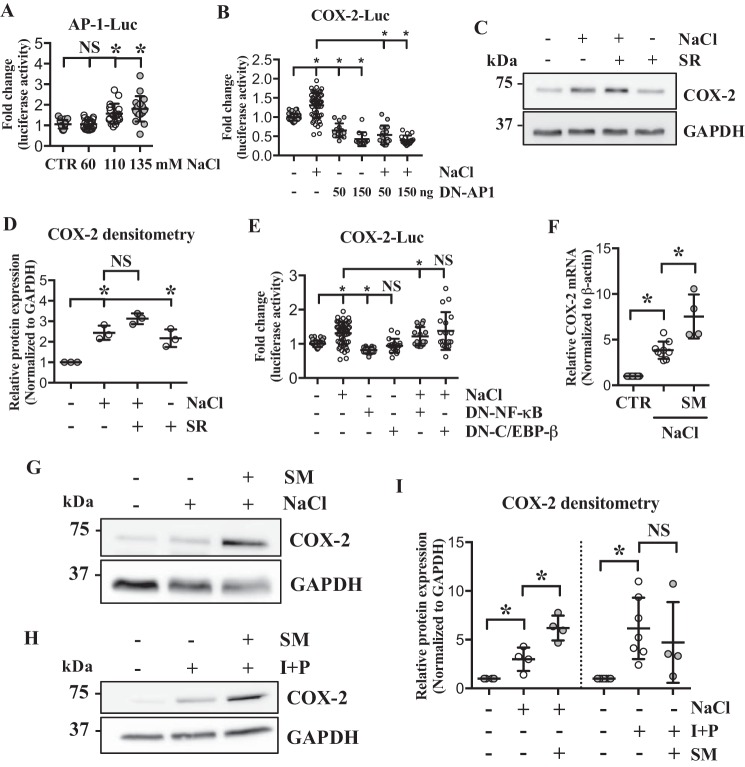
Because we identified several potential transcription factor–binding sites in the ∼1.8 kb proximal promoter region of COX-2, we determined whether these transcription factors were responsible for modulating COX-2 transcription. AP-1 has been shown to regulate COX-2 expression (32, 33), and we and others have shown that AP-1 and TonEBP coregulate expression of certain target genes (28, 34). Therefore, we first investigated if AP-1 activity was modified under hyperosmotic conditions. AP-1–responsive reporter activity showed significant increase in response to NaCl treatment (Fig. 5A). In addition, dominant negative (DN)–AP-1 (A-Fos) significantly suppressed COX-2 promoter activity under both isoosmotic and hyperosmotic conditions (Fig. 5B). Surprisingly however, COX-2 protein levels remained elevated when NP cells were treated with AP-1–specific inhibitor, SR11302, under hyperosmotic condition (Fig. 5, C and D). Inhibition of AP-1 under isoosmotic condition also resulted in elevated COX-2 levels, suggesting that AP-1 is a negative regulator of COX-2 in NP cells.
Figure 5.
AP-1, NF-κB, and C/EBP-β are not involved in hyperosmotic induction of COX-2. A, luciferase assay using AP-1 reporter shows that AP-1 activity is up-regulated by hyperosmolarity. B, COX-2 promoter activity under hyperosmotic condition is significantly decreased by DN–AP-1. C and D, AP-1 inhibition by a specific inhibitor, SR11302, does not block hyperosmotic induction of COX-2. E, COX-2 promoter activity is decreased by DN–NF-κB, but unaltered by DN–C/EBP-β. F, unlike the promoter activities, COX-2 mRNA levels are further up-regulated by NF-κB inhibitor, SM7368, under hyperosmotic condition. G–I, NF-κB inhibition resulted in further up-regulation of COX-2 protein levels under hyperosmotic condition, but had no effect when the cells were treated with ionomycin/PMA. All the quantitative data are represented as mean ± S.D. from at least three independent experiments (three biological replicates). Promoter activity experiments were done with three technical replicates per independent experiment. NS: nonsignificant; *, p < 0.05. SR: SR11302 (AP-1 inhibitor); SM: SM7368 (NF-κB inhibitor); I + P: ionomycin/PMA.
NF-κB has also been shown to control COX-2 expression in other types of cells under hyperosmotic conditions (35, 36). For example, renal medullary interstitial cells up-regulate COX-2 in response to hyperosmotic stimuli through co-activation of NF-κB and C/EBP-β (36). We investigated whether such regulatory mechanism exists in NP cells. Transfecting cells with DN–NF-κB resulted in slight but statistically significant decrease in COX-2 promoter activity under both isoosmotic and hyperosmotic conditions, whereas DN–C/EBP-β did not affect COX-2 promoter activity (Fig. 5E). However, in contrast to the decreased promoter activity, COX-2 mRNA as well as protein levels were further up-regulated by NF-κB inhibitor, SM7368 (Fig. 5, F, G, and I), suggesting that NF-κB was not responsible for hyperosmotic induction of COX-2 but rather served as a negative regulator in NP cells. Interestingly, when cells were treated with ionomycin/PMA, NF-κB inhibition had no significant effect on COX-2 expression (Fig. 5, H and I), suggesting that NF-κB is not involved in ionomycin-induced COX-2 expression.
TonEBP regulates COX-2 expression in response to hyperosmolarity
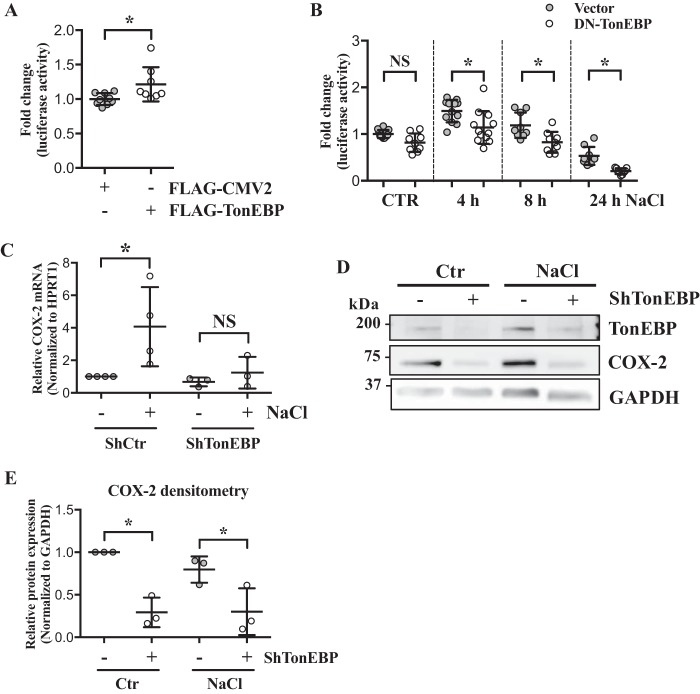
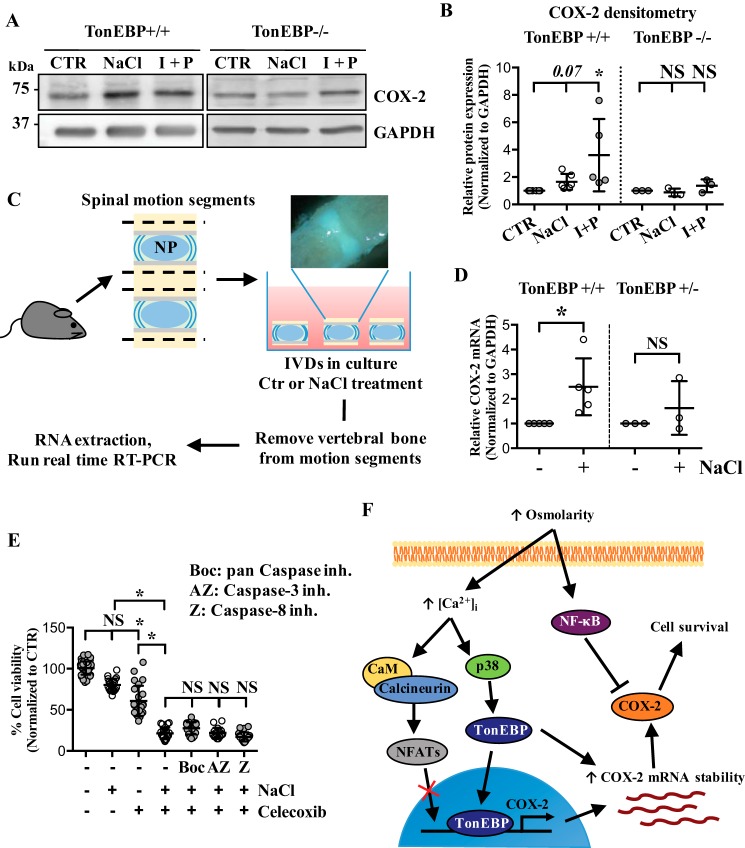
Because TonEBP/NFAT5 is an important mediator of osmotic response and known to be activated in a calcium-dependent but calcineurin-independent manner (22, 23), and because COX-2 proximal promoter had four predicted TonEBP-binding sites, we investigated whether hyperosmotic induction of COX-2 in NP cells was through TonEBP. To assess the role of TonEBP in regulation of COX-2 promoter, we performed gain- and loss-of-function experiments. TonEBP overexpression increased, whereas DN–TonEBP significantly decreased COX-2 promoter activity (Fig. 6, A and B). The involvement of TonEBP in COX-2 regulation was further tested through stably silencing TonEBP by transducing NP cells with lentivirus expressing either control or TonEBP-targeting shRNA. In the absence of TonEBP, hyperosmotic up-regulation of COX-2 mRNA was inhibited (Fig. 6C). Under both isoosmotic and hyperosmotic conditions, COX-2 protein levels were significantly decreased by TonEBP silencing (Fig. 6, D and E), indicating that hyperosmotic induction of COX-2 was TonEBP-dependent. To confirm these findings, we analyzed COX-2 expression in WT and TonEBP null mouse embryonic fibroblasts (MEFs) in response to hyperosmolarity and ionomycin/PMA treatments. Under both conditions, WT MEFs increased COX-2 expression. In contrast, TonEBP null MEFs failed to up-regulate COX-2 levels (Fig. 7, A and B). We also performed an ex vivo organ culture study using intervertebral discs from WT (TonEBP+/+) and haploinsufficient TonEBP heterozygous mice (TonEBP+/−) (Fig. 7C). Culturing under hyperosmotic condition resulted in COX-2 mRNA up-regulation in the WT discs, but not in the TonEBP+/− discs (Fig. 7D), further supporting the role of TonEBP in regulating COX-2 expression under hyperosmotic condition.
Figure 6.
TonEBP is necessary for hyperosmotic induction of COX-2 in NP cells. A, COX-2 promoter activity is significantly increased by TonEBP overexpression. B, transfection of cells with DN–TonEBP under hyperosmotic conditions resulted in significant decrease in COX-2 promoter activity. C, stable silencing of TonEBP results in inhibition of COX-2 mRNA induction in response to hyperosmolarity. D and E, Western blotting and densitometry analyses of COX-2 show that without TonEBP, NP cells are unable to up-regulate COX-2 under hyperosmotic condition. All the quantitative data are represented as mean ± S.D. from at least three independent experiments (three biological replicates). Promoter activity experiments were done with three technical replicates per independent experiment. NS: nonsignificant; *, p < 0.05.
Figure 7.
COX-2 activity under hyperosmotic condition promotes NP cell survival. A and B, TonEBP null MEFs, unlike WT MEFs, do not induce COX-2 in response to either hyperosmolarity or ionomycin/PMA treatment. C, a schematic describing ex vivo disc organ culture system. Briefly, mouse disc motion segments were dissected from WT or haploinsufficient TonEBP+/− mice and cultured in isoosmotic or hyperosmotic media, and then tissue RNA was extracted to perform qRT-PCR. Picture in the schematic shows single motion segment. D, TonEBP+/− mouse discs do not up-regulate COX-2 mRNA in response to hyperosmotic stimulus. E, MTT assay demonstrates a significant reduction in cell viability with celecoxib, a COX-2 inhibitor, under both isoosmotic and hyperosmotic conditions. This cell death cannot be rescued by various caspase inhibitors. F, increased extracellular osmolarity leads to increased intracellular calcium levels, which in turns controls various cellular pathways including p38 MAPK to activate TonEBP. Although intracellular calcium can activate calcineurin signaling pathway, calcineurin-NFAT pathway is not involved in hyperosmotic up-regulation of COX-2 in NP cells. TonEBP transcriptionally up-regulates COX-2 expression and increases COX-2 mRNA stability, eventually promoting NP cell survival under hyperosmotic condition. In contrast, NF-κB negatively regulates COX-2 protein expression under hyperosmotic condition. All the quantitative data are represented as mean ± S.D. from at least three independent experiments (three biological replicates). Cell viability experiments were performed with four technical replicates per independent experiment. NS: nonsignificant; *, p < 0.05. I + P: ionomycin/PMA; Boc: Boc-D-FMK (pan caspase inhibitor), AZ: AZ10417808 (caspase-3 inhibitor); Z: Z-IE(OMe)TD(OMe)-FMK (caspase-8 inhibitor).
COX-2 activity promotes NP cell survival
Because many of the TonEBP targets induced under osmotic stress promote cell survival, we investigated the functional importance of COX-2 under hyperosmotic condition. NP cell viability was measured following culture in isoosmotic or hyperosmotic media, with or without celecoxib, a COX-2 inhibitor. Celecoxib treatment reduced cell viability under isoosmotic condition (Fig. 7E). Moreover, under hyperosmotic condition, celecoxib treatment resulted in even further cell death, suggesting that COX-2 plays an important cytoprotective function in NP cells (Fig. 7E). To further delineate if COX-2–mediated cell survival was through inhibition of caspase activity, we treated cells with different caspase inhibitors in the presence of celecoxib. Interestingly, treatment with AZ10417808 (caspase-3 inhibitor), Z-IE(OMe)TD(OMe)–FMK (caspase-8 inhibitor), or Boc-D-FMK (pan caspase inhibitor) failed to block cell death caused by celecoxib (Fig. 7E), suggesting that COX-2 did not mediate its cytoprotective effects through inhibition of caspase activity.
Discussion
In this study, we elucidated the mechanism of COX-2 expression in NP cells in response to hyperosmotic stress. We demonstrated that COX-2 mRNA and protein were up-regulated by hyperosmolarity through calcium signaling activating p38 and TonEBP. Our data suggested that both COX-2 transcription and COX-2 mRNA stability were increased under hyperosmotic condition. Interestingly, both processes were dependent on TonEBP as shown by gain- and loss-of-function studies and TonEBP+/− mouse disc ex vivo organ culture study. The functional analysis showed that COX-2 activity was crucial for NP cell survival not only under isoosmotic condition, but more so under hyperosmotic stress. Taken together, our study showed that COX-2 is a TonEBP target in NP cells, and that it plays a cytoprotective role during acute osmotic challenge.
Several studies have shown that different stimuli, including high glucose levels, dehydration-caused hyperosmolarity, and inflammatory stimuli, can induce COX-2 expression (14, 16, 17, 35, 37). Our results showed that COX-2 was induced in NP cells in response to osmotic challenge as well as ionomycin/PMA treatment. Both stimuli resulted in the highest induction of COX-2 by 4 h and significant decrease at 24 h following hyperosmotic stimulus. This suggested that COX-2 induction is a relatively early response and that the temporal regulation of its expression is important. In various cell types, COX-2 expression is regulated by intracellular calcium through several pathways including calcineurin-NFAT as well as reactive oxygen species (ROS) and cAMP activation (18, 39–41). The data from our studies using calcium chelator BAPTA confirmed that hyperosmolarity- and ionomycin-mediated COX-2 expression is through changes in intracellular calcium levels. Interestingly, when NP cells were treated with calcineurin inhibitors FK-506/CsA, COX-2 induction by hyperosmolarity or ionomycin remained unaltered, indicating that COX-2 up-regulation in response to hyperosmolarity or ionomycin is independent of calcineurin-NFAT pathway.
In renal medullary interstitial cells, MAPK pathway, known to be downstream of calcium signaling, modulates COX-2 expression under hyperosmotic condition (29, 30). Our data clearly showed that MAPK, specifically p38, was involved in hyperosmotic induction of COX-2 in NP cells. On the other hand, COX-2 induction by ionomycin treatment was not responsive to p38 inhibition, indicating that in presence of excessively high levels of intracellular calcium, p38 activity is redundant in promoting COX-2.
MAPK pathways have been shown to regulate multiple transcription factors, including TonEBP, NFATs, AP-1, and NF-κB, that have been implicated in COX-2 regulation (14, 16, 17, 32, 33, 35, 36). Our analysis showed that COX-2 promoter has multiple predicted binding sites for TonEBP as well as other transcription factors. Induction of COX-2 promoter activity under hyperosmotic condition that mirrors the mRNA expression profile showed that the increase in COX-2 expression was in part because of increased transcription. Moreover, COX-2 promoter sensitivity to BAPTA treatment confirmed that this transcriptional induction was calcium-dependent. Importantly, actinomycin D chase experiment clearly demonstrated that COX-2 mRNA was significantly stabilized under hyperosmotic condition, indicating that up-regulation of COX-2 is because of combinatorial effect of transcriptional regulation and increased mRNA stability.
Both AP-1 and NF-κB have been implicated in COX-2 regulation in a cell type– and context-dependent manner (35, 36). Our promoter studies revealed that although both transcription factors modulated COX-2 promoter activity, under hyperosmotic conditions, AP-1 did not regulate COX-2 protein levels, whereas, NF-κB evidenced a negative relationship to COX-2 levels. This discordance suggests that AP-1 and NF-κB do not control COX-2 transcription through its proximal promoter in response to hyperosmotic stimuli, and that they are not the primary inducers of hyperosmolarity-mediated COX-2 expression in NP cells. In addition, despite the presence of C/EBP-β–binding sites, these sites did not contribute to hyperosmotic regulation of COX-2 promoter activity.
Our earlier work has shown that hyperosmolarity-dependent TonEBP activation in NP cells was through increased [Ca2+]i, yet did not involve calcineurin pathway (22, 23). Likewise, both PKA and ROS have been linked to TonEBP activation (43, 44). We therefore hypothesized that TonEBP may be involved in COX-2 regulation. There have been a few studies demonstrating association between TonEBP and COX-2 expression in different cell types (14, 45). However, none of the previous studies have investigated the role of TonEBP in controlling COX-2 proximal promoter activity. Our analysis predicted four TonE sites within the proximal 1.8 kb promoter region of COX-2. TonEBP gain- and loss-of-function experiments showed that COX-2 promoter activity was responsive to TonEBP. In contrast to AP-1 and NF-κB, stable silencing of TonEBP resulted in inhibition of hyperosmotic up-regulation of COX-2 in NP cells. Further support for TonEBP contribution to COX-2 regulation was evident from analyses of TonEBP null MEFs. COX-2 protein expression was responsive to neither hyperosmolarity nor high intracellular calcium in TonEBP null MEFs. Importantly, ex vivo disc organ culture studies using haploinsufficient TonEBP+/− mice firmly supported in vitro studies. In the intervertebral discs of TonEBP+/− mice, COX-2 gene expression was not induced by hyperosmolarity, confirming that TonEBP is necessary for COX-2 expression under hyperosmotic stress in NP cells. Although TonEBP has been associated with both AP-1 and NF-κB in regulating certain targets in NP cells under certain conditions (28, 45–47), this was not the case in controlling COX-2 expression, implying its target-specificity. Furthermore, complete inhibition of COX-2 up-regulation in the absence of TonEBP indicated that TonEBP not only transcriptionally regulates COX-2, but also indirectly stabilizes COX-2 mRNA under hyperosmotic condition. Interestingly, the magnitude of increase in COX-2 promoter activity in response to hyperosmolarity or TonEBP overexpression is subdued compared with the increase in half-life of COX-2 mRNA. This suggests that, unlike in other cell types, the main mode of TonEBP regulation of COX-2 in NP may be through mRNA stabilization.
The function of COX-2 as an osmoprotective factor has been shown in leukemia (48) and renal cells (19–21). NP cells, similar to renal cells, require tight osmoregulation to protect cells from deleterious effects of dynamic changes in osmolarity and mechanical loading. Therefore, we hypothesized that TonEBP-mediated dynamic COX-2 expression serves a cytoprotective and homeostatic role rather than an inflammatory function in NP cells. Cell viability assay following COX-2 activity inhibition clearly demonstrated that COX-2 activity contributed to NP cell survival regardless of extracellular osmolarity. It is noteworthy that inhibition of caspases did not rescue compromised cell viability caused by COX-2 inhibition, indicating that the observed cell death was caspase-independent. This was different from what has been seen in leukemia cells, where COX-2 promotes cell survival by activating anti-apoptotic proteins Bcl-2 and Bcl-xL, and preventing caspase-3, poly [ADP-ribose] polymerase (PARP), and lamin B cleavage through its target, PGE2 (48).
Taken together, our study demonstrated that calcium-MAPK-TonEBP axis plays a critical role in controlling COX-2 expression in NP cells under hyperosmotic condition, and that COX-2 serves a prosurvival function. It should be noted that the function of COX-2 is context-dependent as has been demonstrated in the cases of fracture healing and inflammatory diseases of bone and other tissues (49–51). Inhibition of COX-2 has been shown to be beneficial in treating inflammation of herniated discs as well (52). Our study reveals a novel function of COX-2 in maintaining intervertebral disc health, and has an important clinical implication as chronic use of NSAIDs for back pain or other musculoskeletal diseases may alleviate pain and reduce inflammation in pathological discs but can also inhibit COX-2 in other healthy discs, adversely affecting the NP health. Therefore, it is crucial to understand underlying mechanisms that distinguish physiological and homeostatic versus pathological roles of COX-2 in the disc. Our study provides a better understanding on the regulatory mechanism of a key cellular pathway necessary for NP cell survival and function.
Experimental procedures
Reagents and plasmids
Plasmids were kindly provided by Drs. Ben C. Ko, University of Hong Kong (FLAG–DN–TonEBP, FLAG–TonEBP, FLAG–CMV2 (53); DN–TonEBP contains amino acids 157 through 581 of human TonEBP (from clone KIAA0827)); Charles Vinson, NIH (DN–AP-1); Silvio Gutkind, NIH (AP-1 reporter); Dan Dixon, University of South Carolina (1.8 kb human COX-2 promoter luciferase reporter construct (31)). psPAX2 (no. 12260) and pMD2.G (no. 12259) developed by Dr. Didier Trono, and pCMV–FLAG–LIP (DN–C/EBP-β; no. 15737) by Dr. Joan Massague were obtained from Addgene. Lentiviral shTonEBP (TRCN0000020019) and control shRNA plasmids were purchased from Sigma. TonEBP/NFAT5 WT and null MEFs (from Dr. Steffan N. Ho) (42) were provided by Dr. Feng Chen, Washington University, St. Louis. TonEBP+/+ and haploinsufficient TonEBP+/− developed by Dr. Steffan Ho (42) were a kind gift from H. Moo Kwon, Ulsan National Institute of Science and Technology.
Cell culture and treatments
Rat NP cells were isolated using a method previously reported by Risbud et al. (38) and approved by Jefferson's Institutional Animal Care and Use Committee. Cells were maintained in DMEM with 10% FBS supplemented with antibiotics until confluent. For hyperosmolarity treatment, 110 or 170 mm NaCl was added to the culture media for NP cells, resulting in 550 and 670 mOsm kg−1 media, respectively, and 85 mm NaCl was added to the culture media for TonEBP WT and null MEFs, resulting in 500 mOsm kg−1 media. In some experiments, cells were treated with 1 μm ionomycin (Sigma) and 100 ng/ml PMA (Sigma) with or without pretreatment with either 10 μm BAPTA/AM (EMD Millipore) or FK-506 (10 ng/ml)/CsA (1 μg/ml) (EMD Millipore) for 1.5 h. In some experiments, cells were pretreated with 10 μm SB202190 (Tocris Bioscience), 10 μm PD98059 (Tocris), 10 μm SP600125 (Sigma), 10 μm SM7368 (Calbiochem), 10 μm SR11302 (Tocris), 30 μm AZ10417808 (Tocris), 20 μm Z-IE(OMe)TD(OMe)-FMK (Calbiochem), 100 μm Boc-D-FMK (Calbiochem), or 40 μm celecoxib (Sigma) for 1–1.5 h prior to NaCl or iononomycin/PMA treatment.
Real-time RT-PCR analysis
Total RNA was extracted from NP cells using RNeasy Mini columns (Qiagen). The purified, DNA-free RNA was converted to cDNA using RNA to cDNA EcoDryTM Premix (Clontech). Template cDNA and gene-specific primers were added to SYBR Green Master Mix (Applied Biosystems) and mRNA expression was quantified using the Step One Plus Real-Time PCR System (Applied Biosystems). β-actin was used to normalize gene expression. All the primers used were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA).
Bioinformatic analysis of promoter
Human COX-2 proximal promoter sequence was downloaded from the University of California, Santa Cruz Genome Table Browser. MatInspector (Genomatix) was used to identify predicted binding sites for TonEBP as well as AP-1, NF-κB, and C/EBP-β with a Matrix Similarity Score cutoff of 0.8.
Transfections and dual luciferase assay
Cells were plated on 48-well plates (2 × 104 cells/well) 1 day before transfection. Cells were transfected with 250 ng of COX-2 reporter plasmid and 250 ng of pRL-TK plasmid, or 150 ng of COX-2 reporter plasmid, 150 ng of various dominant-negative or overexpression plasmids, with or without appropriate backbone vector, and 200 ng of pRL-TK plasmid. For each transfection, plasmids were premixed with the transfection reagent Lipofectamine 2000 (Invitrogen). The treatments were done so that the cells were lysed 48 h after the transfection. Dual-LuciferaseTM reporter assay system (Promega) was used for sequential measurements of firefly and Renilla luciferase activities using TECAN Infinite200 Pro microplate reader (TECAN). At least three independent transfections were performed and all analyses were carried out in triplicate.
Protein extraction and Western blotting
Following treatment, cells were immediately placed on ice and washed with ice-cold PBS. All the wash buffers and the final cell lysis/resuspension buffers included 1× protease inhibitor mixture (Roche), NaF (5 mm), and Na3VO4 (200 μm). Proteins were resolved on 10% SDS-polyacrylamide gels and transferred by electroblotting to PVDF membranes (Bio-Rad). The membranes were blocked with 5% nonfat dry milk in TBS with Tween 20 and incubated overnight at 4 °C in 5% nonfat dry milk in TBS with Tween 20 with the antibodies against COX-2 (1:1000; no. 12282, rabbit, Cell Signaling Technology), phospho-p38 (1:1000; no. 8690, rabbit, Cell Signaling Technology), NFAT5 (1:1000; no. NB120–3446, rabbit, Novus), or GAPDH (1:3000; NB300–221, mouse, Novus), and then appropriate secondary antibodies for 1 h at room temperature. Immunolabeling was detected using the ECL reagent (Amersham Biosciences).
Immunofluorescence microscopy
NP cells were plated on glass coverslips. Following treatment cells were fixed and permeabilized with cold methanol at −20 °C for 15 min, blocked with 5% normal goat serum in PBS with 0.3% Triton X-100 for 1 h at room temperature. Cells were incubated with anti–COX-2 antibody (1:800; no. 12282, rabbit, Cell Signaling Technology) at 4 °C overnight, then incubated with Alexa Fluor 594 secondary antibody (1:800; Jackson ImmunoResearch Laboratories) at room temperature for 1 h. Cells were washed and were mounted with ProLongTM Gold Antifade Mountant with DAPI (Thermo Fisher). All mounted slides were visualized using a Zeiss Axio Imager A2 (Carl Zeiss).
Cell viability assay
To measure cell viability, the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was carried out as described previously (8). Briefly, cells were cultured in isotonic or hyperosmotic condition (120 mm NaCl) for 24 h with or without 1 h pretreatment with celecoxib (40 μm) or caspase inhibitors. After treatments, MTT diluted in PBS was added (0.5 mg/ml) and incubated for 3 h at 37 °C. Precipitated formazan crystals were solubilized in DMSO, and the absorbance was measured at 570 nm using Infinite 200 Pro microplate reader (TECAN).
Lentiviral particle production and viral transduction
HEK293T cells were plated in 10-cm plates (5 × 106 cells/plate) in DMEM with 10% heat-inactivated FBS 1 day before transfection. Cells were transfected with 9 μg of sh-Ctr or sh-TonEBP plasmids along with 6 μg psPAX2 and 3 μg pMD2.G using Lipofectamine 2000 (Invitrogen). After 6 h, transfection media were replaced with fresh DMEM with 10% FBS. Lentiviral media were harvested at 48 to 60 h post transfection, and virus was precipitated using 7% PEG 6000 solution. NP cells were transduced with fresh media containing viral particles along with 8 μg/ml Polybrene. Cells were harvested for protein extraction 4–5 days after transduction to ensure maximum knockdown efficiency.
Ex vivo disc organ culture
TonEBP+/+ and haploinsufficient TonEBP+/− mice on C57BL/6 background were socially housed under barrier conditions using aseptic techniques. Mice were provided with Lab Diet 5010 Laboratory Autoclavable Rodent Diet ad libitum. Both male and female mice at 4 months were sacrificed according to the guidelines by the Thomas Jefferson University's Institutional Animal Care and Use Committee. Whole spines were dissected from mice en bloc and muscle, tendon, and ligaments were carefully removed. Individual motion segments, including vertebral bone and the intervertebral disc were isolated and allowed to equilibrate overnight in DMEM with 10% FBS. For each experimental group, lumbar and caudal motion segments from a single mouse were pooled together. The next day, fresh media with or without 110 mm NaCl were added for 8 h. After treatment, vertebrae and bony endplates were removed from the intervertebral disc using a dissecting microscope (Zeiss Stemi 305), and discs were snap frozen in liquid nitrogen and pulverized using a BioSpec BioPulverizer before RNA isolation.
Statistical analysis
All measurements were performed in at least three independent experiments. No data were excluded. Data are presented as the mean ± S.D. Differences between groups were assessed by Student's t test and analysis of variance (ANOVA) with appropriate post-hoc analysis using Prism 6 (GraphPad Software). p < 0.05 was considered statistically significant.
Author contributions
H. C., W. C., A. C. D., Z. I. J., S. S. G., and Z. R. S. data curation; H. C., W. C., A. C. D., Z. I. J., S. S. G., and Z. R. S. formal analysis; H. C., W. C., A. C. D., Z. I. J., S. S. G., Z. R. S., I. M. S., and M. V. R. validation; H. C., W. C., A. C. D., Z. I. J., and I. M. S. investigation; H. C., A. C. D., Z. I. J., S. S. G., and Z. R. S. visualization; H. C., W. C., I. M. S., and M. V. R. methodology; H. C. writing-original draft; H. C., W. C., A. C. D., Z. I. J., S. S. G., Z. R. S., I. M. S., and M. V. R. writing-review and editing; I. M. S. and M. V. R. conceptualization; I. M. S. and M. V. R. resources; I. M. S. and M. V. R. supervision; I. M. S. and M. V. R. funding acquisition; I. M. S. and M. V. R. project administration.
This study was funded by NIAMS, National Institutes of Health Grants R01AR055655 and R01AR064733, and T32AR052273 (to Z. I. J. and A. C. D.), and F30AR066506 (to Z. R. S.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- NP
- nucleus pulposus
- PG
- prostaglandin
- PMA
- phorbol 12-myristate 13-acetate
- DN
- dominant negative
- MEF
- mouse embryonic fibroblast
- ROS
- reactive oxygen species
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
References
- 1. Johnson Z. I., Shapiro I. M., and Risbud M. (2014) Extracellular osmolarity regulates matrix homeostasis in the intervertebral disc and articular cartilage: Evolving role of TonEBP. Matrix Biol. 40, 10–16 10.1016/j.matbio.2014.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Silagi E. S., Shapiro I. M., and Risbud M. V. (2018) Glycosaminoglycan synthesis in the nucleus pulposus: Dysregulation and the pathogenesis of disc degeneration. Matrix Biol. 10.1016/j.matbio.2018.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Binch A. L. A., Shapiro I. M., and Risbud M. V. (2016) Syndecan-4 in intervertebral disc and cartilage: Saint or synner? Matrix Biol. 52-54, 355–362 10.1016/j.matbio.2016.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Risbud M. V., and Shapiro I. M. (2014) Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat. Rev. Rheumatol. 10, 44–56 10.1038/nrrheum.2013.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Choi H., Tessier S., Silagi E. S., Kyada R., Yousefi F., Pleshko N., Shapiro I. M., and Risbud M. V. (2018) A novel mouse model of intervertebral disc degeneration shows altered cell fate and matrix homeostasis. Matrix Biol. 10.1016/j.matbio.2018.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tian Y., Yuan W., Li J., Wang H., Hunt M. G., Liu C., Shapiro I. M., and Risbud M. V. (2016) TGFβ regulates galectin-3 expression through canonical Smad3 signaling pathway in nucleus pulposus cells: Implications in intervertebral disc degeneration. Matrix Biol. 50, 39–52 10.1016/j.matbio.2015.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Libert S., Willermain F., Weber C., Bryla A., Salik D., Gregoire F., Bolaky N., Caspers L., Perret J., and Delporte C. (2016) Involvement of TonEBP/NFAT5 in osmoadaptative response of human retinal pigmented epithelial cells to hyperosmolar stress. Mol. Vis. 22, 100–115 [PMC free article] [PubMed] [Google Scholar]
- 8. Tsai T.-T., Danielson K. G., Guttapalli A., Oguz E., Albert T. J., Shapiro I. M., and Risbud M. V (2006) TonEBP/OREBP is a regulator of nucleus pulposus cell function and survival in the intervertebral disc. J. Biol. Chem. 281, 25416–25424 10.1074/jbc.M601969200 [DOI] [PubMed] [Google Scholar]
- 9. Ko B. C., Ruepp B., Bohren K. M., Gabbay K. H., and Chung S. S. (1997) Identification and characterization of multiple osmotic response sequences in the human aldose reductase gene. J. Biol. Chem. 272, 16431–16437 10.1074/jbc.272.26.16431 [DOI] [PubMed] [Google Scholar]
- 10. Ito T., Fujio Y., Hirata M., Takatani T., Matsuda T., Muraoka S., Takahashi K., and Azuma J. (2004) Expression of taurine transporter is regulated through the TonE (tonicity-responsive element)/TonEBP (TonE-binding protein) pathway and contributes to cytoprotection in HepG2 cells. Biochem. J. 382, 177–182 10.1042/BJ20031838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miyakawa H., Woo S. K., Chen C. P., Dahl S. C., Handler J. S., and Kwon H. M. (1998) Cis- and trans-acting factors regulating transcription of the BGT1 gene in response to hypertonicity. Am. J. Physiol. 274, F753–F761 [DOI] [PubMed] [Google Scholar]
- 12. Rim J. S., Atta M. G., Dahl S. C., Berry G. T., Handler J. S., and Kwon H. M. (1998) Transcription of the sodium/myo-inositol cotransporter gene is regulated by multiple tonicity-responsive enhancers spread over 50 kilobase pairs in the 5′-flanking region. J. Biol. Chem. 273, 20615–20621 10.1074/jbc.273.32.20615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gogate S. S., Fujita N., Skubutyte R., Shapiro I. M., and Risbud M. (2012) Tonicity enhancer binding protein (TonEBP) and hypoxia-inducible factor (HIF) coordinate heat shock protein 70 (Hsp70) expression in hypoxic nucleus pulposus cells: Role of Hsp70 in HIF-1α degradation. J. Bone Miner. Res. 27, 1106–1117 10.1002/jbmr.1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Favale N. O., Casali C. I., Lepera L. G., Pescio L. G., and Fernández-Tome M. C. (2009) Hypertonic induction of COX2 expression requires TonEBP/NFAT5 in renal epithelial cells. Biochem. Biophys. Res. Commun. 381, 301–305 10.1016/j.bbrc.2008.12.189 [DOI] [PubMed] [Google Scholar]
- 15. Nørregaard R., Kwon T.-H., and Frøkiær J. (2015) Physiology and pathophysiology of cyclooxygenase-2 and prostaglandin E2 in the kidney. Kidney Res. Clin. Pract. 34, 194–200 10.1016/j.krcp.2015.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hernández G. L., Volpert O. V., Iñiguez M. A., Lorenzo E., Martínez-Martínez S., Grau R., Fresno M., and Redondo J. M. (2001) Selective inhibition of vascular endothelial growth factor-mediated angiogenesis by cyclosporin A: Roles of the nuclear factor of activated T cells and cyclooxygenase 2. J. Exp. Med. 193, 607–620 10.1084/jem.193.5.607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yiu G. K., and Toker A. (2006) NFAT induces breast cancer cell invasion by promoting the induction of cyclooxygenase-2. J. Biol. Chem. 281, 12210–12217 10.1074/jbc.M600184200 [DOI] [PubMed] [Google Scholar]
- 18. Yan Y., Li J., Ouyang W., Ma Q., Hu Y., Zhang D., Ding J., Qu Q., Subbaramaiah K., and Huang C. (2006) NFAT3 is specifically required for TNF-α-induced cyclooxygenase-2 (COX-2) expression and transformation of Cl41 cells. J. Cell Sci. 119, 2985–2994 10.1242/jcs.03014 [DOI] [PubMed] [Google Scholar]
- 19. Breyer M. D., and Harris R. C. (2001) Cyclooxygenase 2 and the kidney. Curr. Opin. Nephrol. Hypertens. 10, 89–98 10.1097/00041552-200101000-00014 [DOI] [PubMed] [Google Scholar]
- 20. Hao C. M., Kömhoff M., Guan Y., Redha R., and Breyer M. D. (1999) Selective targeting of cyclooxygenase-2 reveals its role in renal medullary interstitial cell survival. Am. J. Physiol. 277, F352–F359 [DOI] [PubMed] [Google Scholar]
- 21. Moeckel G. W., Zhang L., Fogo A. B., Hao C.-M., Pozzi A., and Breyer M. D. (2003) COX2 activity promotes organic osmolyte accumulation and adaptation of renal medullary interstitial cells to hypertonic stress. J. Biol. Chem. 278, 19352–19357 10.1074/jbc.M302209200 [DOI] [PubMed] [Google Scholar]
- 22. Gajghate S., Hiyama A., Shah M., Sakai D., Anderson D. G., Shapiro I. M., and Risbud M. V (2009) Osmolarity and intracellular calcium regulate aquaporin2 expression through TonEBP in nucleus pulposus cells of the intervertebral disc. J. Bone Miner. Res. 24, 992–1001 10.1359/jbmr.090103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hiyama A., Gajghate S., Sakai D., Mochida J., Shapiro I. M., and Risbud M. V (2009) Activation of TonEBP by calcium controls β1,3-glucuronosyltransferase-I expression, a key regulator of glycosaminoglycan synthesis in cells of the intervertebral disc. J. Biol. Chem. 284, 9824–9834 10.1074/jbc.M807081200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ko B. C. B., Lam A. K. M., Kapus A., Fan L., Chung S. K., and Chung S. S. M. (2002) Fyn and p38 signaling are both required for maximal hypertonic activation of the osmotic response element-binding protein/tonicity-responsive enhancer-binding protein (OREBP/TonEBP). J. Biol. Chem. 277, 46085–46092 10.1074/jbc.M208138200 [DOI] [PubMed] [Google Scholar]
- 25. Tsai T.-T., Guttapalli A., Agrawal A., Albert T. J., Shapiro I. M., and Risbud M. V. (2007) MEK/ERK signaling controls osmoregulation of nucleus pulposus cells of the intervertebral disc by transactivation of TonEBP/OREBP. J. Bone Miner. Res. 22, 965–974 10.1359/jbmr.070322 [DOI] [PubMed] [Google Scholar]
- 26. Wang H., Ferraris J. D., Klein J. D., Sands J. M., Burg M. B., and Zhou X. (2015) PKC-α contributes to high NaCl-induced activation of NFAT5 (TonEBP/OREBP) through MAPK ERK1/2. Am. J. Physiol. Renal Physiol. 308, F140–F148 10.1152/ajprenal.00471.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nahm O., Woo S. K., Handler J. S., and Kwon H. M. (2002) Involvement of multiple kinase pathways in stimulation of gene transcription by hypertonicity. Am. J. Physiol. Cell Physiol. 282, C49–C58 10.1152/ajpcell.00267.2001 [DOI] [PubMed] [Google Scholar]
- 28. Hiyama A., Gogate S. S., Gajghate S., Mochida J., Shapiro I. M., and Risbud M. V. (2010) BMP-2 and TGF-β stimulate expression of β1,3-glucuronosyl transferase 1 (GlcAT-1) in nucleus pulposus cells through AP1, TonEBP, and Sp1: Role of MAPKs. J. Bone Miner. Res. 25, 1179–1190 10.1359/jbmr.091202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cheng H. F., Wang J. L., Zhang M. Z., McKanna J. A., and Harris R. C. (2000) Role of p38 in the regulation of renal cortical cyclooxygenase-2 expression by extracellular chloride. J. Clin. Invest. 106, 681–688 10.1172/JCI10318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cheng H.-F., and Harris R. C. (2002) Cyclooxygenase-2 expression in cultured cortical thick ascending limb of Henle increases in response to decreased extracellular ionic content by both transcriptional and post-transcriptional mechanisms. Role of p38-mediated pathways. J. Biol. Chem. 277, 45638–45643 10.1074/jbc.M206040200 [DOI] [PubMed] [Google Scholar]
- 31. Dixon D. A., Tolley N. D., King P. H., Nabors L. B., McIntyre T. M., Zimmerman G. A., and Prescott S. M. (2001) Altered expression of the mRNA stability factor HuR promotes cyclooxygenase-2 expression in colon cancer cells. J. Clin. Invest. 108, 1657–1665 10.1172/JCI12973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang D., Li J., Song L., Ouyang W., Gao J., and Huang C. (2008) A JNK1/AP-1–dependent, COX-2 induction is implicated in 12-O-tetradecanoylphorbol-13-acetate–induced cell transformation through regulating cell cycle progression. Mol. Cancer Res. 6, 165–174 10.1158/1541-7786.MCR-07-0181 [DOI] [PubMed] [Google Scholar]
- 33. Kim S.-H., Oh J.-M., No J.-H., Bang Y.-J., Juhnn Y.-S., and Song Y.-S. (2009) Involvement of NF-κB and AP-1 in COX-2 upregulation by human papillomavirus 16 E5 oncoprotein. Carcinogenesis. 30, 753–757 10.1093/carcin/bgp066 [DOI] [PubMed] [Google Scholar]
- 34. Irarrazabal C. E., Williams C. K., Ely M. A., Birrer M. J., Garcia-Perez A., Burg M. B., and Ferraris J. D. (2008) Activator protein-1 contributes to high NaCL-induced increase in tonicity-responsive enhancer/osmotic response element-binding protein transactivating activity. J. Biol. Chem. 283, 2554–2563 10.1074/jbc.M703490200 [DOI] [PubMed] [Google Scholar]
- 35. Hao C. M., Yull F., Blackwell T., Kömhoff M., Davis L. S., and Breyer M. D. (2000) Dehydration activates an NF-κB-driven, COX2-dependent survival mechanism in renal medullary interstitial cells. J. Clin. Invest. 106, 973–982 10.1172/JCI9956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen J., Zhao M., Rao R., Inoue H., and Hao C.-M. (2005) C/EBPβ and its binding element are required for NFκB-induced COX2 expression following hypertonic stress. J. Biol. Chem. 280, 16354–16359 10.1074/jbc.M411134200 [DOI] [PubMed] [Google Scholar]
- 37. Madonna R., Giovannelli G., Confalone P., Renna F. V., Geng Y.-J., and De Caterina R. (2016) High glucose-induced hyperosmolarity contributes to COX-2 expression and angiogenesis: Implications for diabetic retinopathy. Cardiovasc. Diabetol. 15, 18 10.1186/s12933-016-0342-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Risbud M. V., Guttapalli A., Stokes D. G., Hawkins D., Danielson K. G., Schaer T. P., Albert T. J., and Shapiro I. M. (2006) Nucleus pulposus cells express HIF-1α under normoxic culture conditions: A metabolic adaptation to the intervertebral disc microenvironment. J. Cell. Biochem. 98, 152–159 10.1002/jcb.20765 [DOI] [PubMed] [Google Scholar]
- 39. Lee Y. S., Lee S. J., Seo K. W., Bae J. U., Park S. Y., and Kim C. D. (2013) Homocysteine induces COX-2 expression in macrophages through ROS generated by NMDA receptor-calcium signaling pathways. Free Radic. Res. 47, 422–431 10.3109/10715762.2013.784965 [DOI] [PubMed] [Google Scholar]
- 40. Ogata S., Kubota Y., Satoh S., Ito S., Takeuchi H., Ashizuka M., and Shirasuna K. (2006) Ca2+ stimulates COX-2 expression through calcium-sensing receptor in fibroblasts. Biochem. Biophys. Res. Commun. 351, 808–814 10.1016/j.bbrc.2006.10.098 [DOI] [PubMed] [Google Scholar]
- 41. Kusama K., Yoshie M., Tamura K., Imakawa K., Isaka K., and Tachikawa E. (2015) Regulatory action of calcium ion on cyclic AMP-enhanced expression of implantation-related factors in human endometrial cells. PLoS One 10, e0132017 10.1371/journal.pone.0132017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Go W. Y., Liu X., Roti M. A., Liu F., and Ho S. N. (2004) NFAT5/TonEBP mutant mice define osmotic stress as a critical feature of the lymphoid microenvironment. Proc. Natl. Acad. Sci. U.S.A. 101, 10673–10678 10.1073/pnas.0403139101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ferraris J. D., Persaud P., Williams C. K., Chen Y., and Burg M. B. (2002) cAMP-independent role of PKA in tonicity-induced transactivation of tonicity-responsive enhancer/osmotic response element-binding protein. Proc. Natl. Acad. Sci. U.S.A. 99, 16800–16805 10.1073/pnas.222659799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim N.-H., Hong B.-K., Choi S. Y., Moo Kwon H., Cho C.-S., Yi E. C., and Kim W.-U. (2013) Reactive oxygen species regulate context-dependent inhibition of NFAT5 target genes. Exp. Mol. Med. 45, e32 10.1038/emm.2013.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shin H. J., Kim H., Heo R. W., Kim H. J., Choi W. S., Kwon H. M., and Roh G. S. (2014) Tonicity-responsive enhancer binding protein haplodeficiency attenuates seizure severity and NF-κB-mediated neuroinflammation in kainic acid-induced seizures. Cell Death Differ. 21, 1095–1106 10.1038/cdd.2014.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Johnson Z. I., Shapiro I. M., and Risbud M. V. (2016) RNA sequencing reveals a role of TonEBP in regulation of pro-inflammatory genes in response to hyperosmolarity in healthy nucleus pulposus cells: A homeostatic response? J. Biol. Chem. 291, 26686–26697 10.1074/jbc.M116.757732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Johnson Z. I., Doolittle A. C., Snuggs J. W., Shapiro I. M., Le Maitre C. L., and Risbud M. V. (2017) TNF-α promotes nuclear enrichment of TonEBP/NFAT5 to selectively control inflammatory but not osmoregulatory responses in nucleus pulposus cells. J. Biol. Chem. 292, 17561–17575 10.1074/jbc.M117.790378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shehzad A., Lee J., and Lee Y. S. (2015) Autocrine prostaglandin E2 signaling promotes promonocytic leukemia cell survival via COX-2 expression and MAPK pathway. BMB Rep. 48, 109–114 10.5483/BMBRep.2015.48.2.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Peskar B. M. (2001) Role of cyclooxygenase isoforms in gastric mucosal defence. J. Physiol. Paris 95, 3–9 10.1016/S0928-4257(01)00003-1 [DOI] [PubMed] [Google Scholar]
- 50. Coon D., Gulati A., Cowan C., and He J. (2007) The role of cyclooxygenase-2 (COX-2) in inflammatory bone resorption. J. Endod. 33, 432–436 10.1016/j.joen.2006.12.001 [DOI] [PubMed] [Google Scholar]
- 51. Simon A. M., Manigrasso M. B., and O'Connor J. P. (2002) Cyclo-oxygenase 2 function is essential for bone fracture healing. J. Bone Miner. Res. 17, 963–976 10.1359/jbmr.2002.17.6.963 [DOI] [PubMed] [Google Scholar]
- 52. van Dijk B., Potier E., van Dijk M., Langelaan M., Papen-Botterhuis N., and Ito K. (2015) Reduced tonicity stimulates an inflammatory response in nucleus pulposus tissue that can be limited by a COX-2-specific inhibitor. J. Orthop. Res. 33, 1724–1731 10.1002/jor.22946 [DOI] [PubMed] [Google Scholar]
- 53. Lam A. K. M., Ko B. C. B., Tam S., Morris R., Yang J. Y., Chung S. K., and Chung S. S. M. (2004) Osmotic response element-binding protein (OREBP) is an essential regulator of the urine concentrating mechanism. J. Biol. Chem. 279, 48048–48054 10.1074/jbc.M407224200 [DOI] [PubMed] [Google Scholar]