Abstract
BACKGROUND
Bladder cancer is a disease of the elderly that is associated with high morbidity in those treated with radical cystectomy. In this observational study of patients with bladder cancer undergoing radical cystectomy, we analyzed and compared patient-reported outcomes from those treated with Enhanced Recovery After Surgery (ERAS) methods versus those who received traditional perioperative care.
METHODS
We enrolled patients who underwent radical cystectomy at a high-volume tertiary care referral center from November 2013 to December 2016, when the ERAS concept was being introduced into postoperative care at our institution. Patients reported symptom outcomes using the MD Anderson Symptom Inventory (MDASI) preoperatively and on postoperative days 1–5. Mann-Whitney U tests were used to compare symptom burden between the ERAS and traditional-care groups. General linear mixed-effects models were used for longitudinal data; linear regression models were used for multivariable analysis.
RESULTS
Patients (N=383) reported dry mouth, disturbed sleep, drowsiness, fatigue, pain, and lack of appetite as the most severe symptoms. Compared with the traditional-care group, the ERAS group had significantly less pain (est.=−0.98, P=0.005), drowsiness (est=−0.91, P=0.009), dry mouth (est.=−1.21, P=0.002), disturbed sleep (est.= −0.97, P=0.01) and interference with functioning (est.= −0.70, P=0.022) (adjusted for age, sex, surgical technique, and neoadjuvant chemotherapy status).
CONCLUSIONS
These results suggest that ERAS practice significantly reduced immediate postoperative symptom burden in bladder cancer patients recovering from radical cystectomy, supporting the use of patient-reported symptom burden as an outcome measure in perioperative care.
Keywords: Patient-reported outcomes (PROs), cystectomy, ERAS, bladder cancer
INTRODUCTION
Bladder cancer is the fourth-most-common cancer in men.1 Depending on stage, radical cystectomy with a urinary diversion may be recommended as treatment. However, intrusive treatment options for bladder cancer are often associated with morbidity, high postoperative symptom burden, and even readmission.
Increasingly, patient-reported outcomes (PROs) are being utilized to assess symptomatic effects across cancer types and treatments in patient care.2,3 Some PROs have become standard for evaluating both short-term and long-term health-related quality-of-life (HRQoL) outcomes, such as satisfaction with urinary diversion.4 They are also increasingly used for evaluation of laparoscopic surgery and bladder preservation procedures.5–7
Enhanced recovery after surgery (ERAS) programs move the traditional model of surgical care toward a patient-centered model. ERAS principles, including patient education, consistency of care, avoidance of gastrointestinal complications, early feeding, early ambulation, and earlier return to home,8 aim to return the patient to their previous state of health in a seamless fashion and in an expedient manner.9,10 ERAS programs for other surgical disciplines, such as colorectal surgery and hepatobiliary surgery, have been found to produce clinical benefit and an improvement in HRQoL.11–15 In patients undergoing radical cystectomy for bladder cancer, ERAS programs may also reduce several of the arduous symptoms associated with the procedure. Given the increased use of ERAS pathways across the perioperative period, routine monitoring of PROs for patients who undergo cystectomy for bladder cancer might provide evidence as to whether ERAS programs improve symptom burden trajectories and HRQoL for these patients8—and may also help to characterize the degree to which ERAS pathways affect postoperative PROs and whether there are any tradeoffs for these changes.9 However, because PROs are often not included in studies evaluating ERAS pathways,16 there is little evidence to demonstrate any significant differences.17
Appropriate tools are required to collect PROs that can reliably demonstrate symptom burden or improved functional ability and HRQoL after a major surgery such as radical cystectomy.18–22 Previous studies suggest that the choice of PRO tool matters.23,24 The widely used Brief Pain Inventory25 is an example of how the routine use of a 0–10 scale can be used easily and successfully to rate pain severity in perioperative patient care. The MD Anderson Symptom Inventory (MDASI) is a validated tool for capturing multiple PROs, including pain, among patients with cancer.26 The MDASI has been used in other surgical specialties, where it has been demonstrated to be a sensitive tool for detecting postoperative symptom burden and functioning recovery.27,28
In this observational clinical study, we tracked perioperative symptom burden in patients with bladder cancer undergoing radical cystectomy either under ERAS or not, using the MDASI to assess symptoms and interference before surgery and during the first 5 days of postsurgical inpatient care.
MATERIALS AND METHODS
Patients
We approached patients who were scheduled for radical cystectomy in the Genitourinary Center at The University of Texas MD Anderson Cancer Center between November 2013 and December 2016. Eligible patients could have bladder cancer of any stage and had to be able to speak and read English. Surgeons had designated which patients were on the ERAS pathway and which were receiving traditional care prior to our approaching them. Patients receiving cystectomy in our institution underwent either robotic or open surgery.
ERAS pathways are designed to decrease the physical and emotional stresses of surgery. The ERAS pathway used in this study included the following elements that were not in the traditional-care pathway: limited fasting, goal-directed fluid therapy, extended deep venous thrombosis prophylaxis, limited narcotic usage, μ-opioid blocker-alvimopan, local analgesia, early feeding, avoidance of nasogastric tubes left in postoperatively, early mobilization, and early discharge. These ERAS procedures were used for both open and robotic cystectomy.
The study was approved by the MD Anderson Institutional Review Board. All patients provided written informed consent to participate.
Study Design and Outcome Measures
Symptoms and functional status were measured with the MDASI, a brief, psychometrically validated measure of the severity of 13 cancer-related symptoms and 6 items of symptom interference in daily living experienced by the patient during the previous 24 hours. Each symptom is rated from 0 (not present) to 10 (as bad as can be imagined). Similarly, interference items are rated 0 (did not interfere) to 10 (interfered completely). Patients completed the MDASI with either paper and pencil or electronic entry via a handheld tablet computer. Patients were asked to complete the surveys in both the ambulatory and hospitalized setting, including preoperatively and on all inpatient postoperative days (POD) up to the fifth day (POD1 through POD5).
Demographic characteristics and clinical variables were collected by study staff from the patient’s medical record.
Statistical Analysis
Mann-Whitney U tests were used to compare symptom burden between the ERAS group and traditional-care group. The most severe symptoms were identified with all available PRO data for each symptom from the group of patients who completed at least two MDASI assessments (on POD1 and at least once more from POD2–POD5) in order to identify the most severe symptoms for this patient population. This subset of symptoms was further analyzed with longitudinal modeling. A composite score for all 6 symptom interference items was calculated, along with two subscale component scores: physical interference (WAW, the mean score for MDASI interference with work, general activity, and walking), and affective interference (REM, the mean score for MDASI interference with relations with others, enjoyment of life, and mood).
General linear mixed effects models were used to compare changes in symptom severity and interference from POD1 to POD5 between the ERAS and traditional-care patients.29 Days from surgery were included as the time variable in this model. All multivariate analysis models were adjusted for age, sex, surgical technique (open vs. robotic), and neoadjuvant chemotherapy status.
To confirm that data used in the longitudinal analysis was not subject to selection bias, we conducted multiple regression analyses of data from each day, from before surgery through POD5, in a cross-sectional approach. Results from the multiple regression analyses include those with a change in score of more than a half (0.5) point between ERAS and traditional care. The models were adjusted for age, sex, surgical technique (open vs. robotic), and neoadjuvant chemotherapy status.
All statistical analyses were done with SAS 9.1 for Windows (SAS Institute, Cary, NC). The significance level was set as 0.05 for all tests.
RESULTS
A total of 542 patients were approached preoperatively to participate in the study; of these, 383 (70.7%) agreed to be enrolled and completed at least one MDASI survey during the perioperative period. This resulted in 1423 observations (MDASI assessments) overall. Of the 383 patients enrolled consecutively, 245 (64.0%) were being treated on an ERAS pathway (844 observations) and 138 (36.0%) were being treated on a traditional-care pathway (579 observations). As ERAS practice became more widely adopted in the Genitourinary Center, the proportion of patients to be managed by ERAS principles increased. No traditional-care patients were switched to the ERAS pathway after surgery.
Demographic and clinical characteristics for the study participants are shown in Table 1. We observed no differences between patients under ERAS versus traditional care, except for a significantly shorter length of hospital stay for the ERAS group (P<0.01). Preoperatively, no differences were found between the ERAS and traditional-care patients for any MDASI symptom score.
Table 1.
Demographics (N=383)
| Traditional Care (n=138) | ERAS (n=245) | P | |
|---|---|---|---|
| Length of stay, days | |||
| Mean (SD) | 7.8 (5.70) | 6.5 (6.00) | <0.01 |
| Median (range) | 7 (3–51) | 5 (2–49) | |
| Age, years | |||
| Mean (SD) | 67.6 (9.90) | 68.4 (9.60) | 0.46 |
| Median (range) | 69 (39–88) | 68 (34–90) | |
| n (%) | n (%) | ||
| Sex | 0.91 | ||
| Male | 112 (81.2) | 200 (81.6) | |
| Female | 26 (18.8) | 45 (18.4) | |
| Surgical technique | 0.02 | ||
| Open | 108 (78.3) | 164 (66.9) | |
| Robotic | 30 (21.7) | 81 (33.1) | |
| Diversion | 0.48 | ||
| Ileal conduit | 115 (83.3) | 197 (80.4) | |
| All others | 23 (16.7) | 48 (19.6) | |
| Did the patient receive neoadjuvant chemotherapy before cystectomy? | 0.26 | ||
| No | 76 (55.1) | 125 (51.0) | |
| Yes | 62 (44.9) | 120 (49.0) |
Abbreviations: ERAS, Enhanced Recovery after Surgery; SD, standard deviation.
Longitudinal Analyses
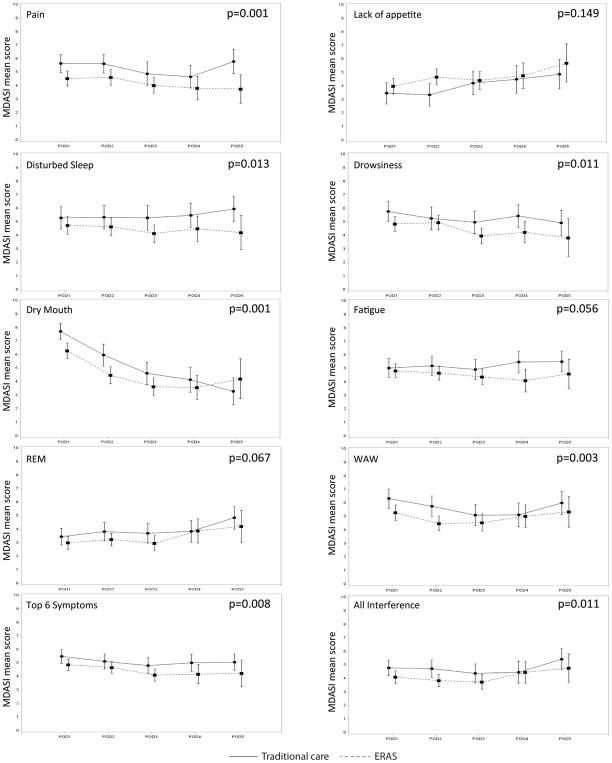
From POD1 to POD5, 721 observations were collected for each symptom from the 207 patients who completed at least two MDASI assessments, one of which occurred on POD1. The six most burdensome symptoms were dry mouth (mean=5.1), disturbed sleep (mean=4.93), drowsiness (mean=4.89), fatigue (mean=4.85), pain (mean=4.78), and lack of appetite (mean=4.23). Figure 1 presents the severity over time for these top six symptoms by ERAS status during the first 5 days of the postoperative period.
Figure 1.
Each of these graphs represents the mean symptom score and the confidence interval for each postoperative day (POD) 1 through 5 for the Enhanced Recovery after Surgery (ERAS) and traditional-care patient groups. PROs from the MD Anderson Symptom Inventory (MDASI) included the individual symptoms and component scores for the six most severe symptoms and for symptom interference.
We observed significant group-by-time interactions for individual symptoms and PRO component scores between the ERAS and traditional-care groups during the 5 days postsurgery (Table 2). For ERAS patients, dry mouth, disturbed sleep, drowsiness, and pain were significantly less-severe; progressing postoperatively, dry mouth, drowsiness, and pain decreased significantly. Taken together, the top six symptoms were found to be lower in ERAS patients and to decrease significantly over time. Physical functioning interference (MDASI WAW) was significantly less-severe for the ERAS group and decreased significantly over time.
Table 2.
Longitudinal Analysis of Major Symptom Burden during First 5 days after Radical Cystectomy: General Linear Mixed-Effects Models (N=207)*
| Symptoms | ERAS | Time | ||
|---|---|---|---|---|
| Estimate (SE) | P | Estimate (SE) | P | |
| Dry mouth | −1.21 (0.39) | 0.002 | −1.08 (0.07) | <0.001 |
| Disturbed sleep | −0.97 (0.37) | 0.010 | −0.06 (0.09) | 0.468 |
| Drowsiness | −0.91 (0.35) | 0.009 | −0.29 (0.08) | <0.001 |
| Pain | −0.98 (0.34) | 0.005 | −0.24 (0.07) | <0.001 |
| Fatigue | −0.63 (0.33) | 0.056 | −0.08 (0.07) | 0.221 |
| Lack of appetite | 0.41 (0.38) | 0.283 | 0.27 (0.08) | <0.001 |
| Top 6 symptoms | −0.73 (0.28) | 0.009 | −0.26 (0.05) | <0.001 |
| Interference | ||||
| WAW | −0.93 (0.35) | 0.008 | −0.22 (0.07) | 0.002 |
| REM | −0.53 (0.32) | 0.102 | 0.19 (0.06) | 0.002 |
Each model adjusted for age, sex, surgical technique, and neoadjuvant chemotherapy status.
A composite mean was calculated for all items where symptoms of recovery were causing interference in daily activities.
Abbreviations: ERAS, Enhanced Recovery after Surgery; REM, the average score of interference with relations with others, enjoyment of life, and mood; SE, standard error; WAW, the average score of interference with work, general activity, and walking.
Cross-Sectional Analyses
On POD1, multiple regression analysis revealed a significantly reduced symptoms of pain, drowsiness, dry mouth, and interference level for the ERAS group versus the traditional-care group (all P<0.05; see Table 3). In addition, these symptoms were likely to be more severe for younger patients than for older patients. We found no impact on PROs from sex, surgical technique, or neoadjuvant chemotherapy. On POD2, we observed significantly lower symptom burden in the ERAS group compared with the traditional-care group. The ERAS patients reported less-severe pain, lack of appetite, and dry mouth, along with less interference. Female patients reported higher pain and fatigue scores than did male patients (P=0.033 and P=0.046, respectively). Again, younger patients reported more pain than did older patients.
Table 3.
Cross-Sectional Analysis Multiple Regression Results [Estimate (Standard Error), p-value]
| Symptom and Time Point | ERAS | Age | Sex | Surgical Technique | Neoadjuvant Chemotherapy | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate (SE) | P | Estimate (SE) | P | Estimate (SE) | P | Estimate (SE) | P | Estimate (SE) | P | |
| POD1 (n=222) | ||||||||||
| Pain | −0.93 (0.42) | 0.028 | −0.08 (0.02) | <0.001 | 0.65 (0.54) | 0.233 | 0.39 (0.48) | 0.418 | −0.27 (0.42) | 0.518 |
| Drowsiness | −0.92 (0.45) | 0.039 | −0.10 (0.02) | <0.001 | 0.45 (0.57) | 0.430 | −0.12 (0.50) | 0.813 | 0.04 (0.44) | 0.921 |
| Dry Mouth | −1.34 (0.43) | 0.002 | −0.01 (0.02) | 0.537 | 1.10 (0.54) | 0.046 | −0.19 (0.48) | 0.702 | −0.24 (0.43) | 0.579 |
| Fatigue | −045 (042) | 0.285 | −0.09 (0.02) | <0.001 | 0.22 (0.54) | 0.680 | 0.97 (0.47) | 0.041 | −0.50 (0.42) | 0.230 |
| Lack of appetite | 0.24 (0.49) | 0.627 | −0.01 (0.03) | 0.968 | −0.39 (0.63) | 0.534 | 0.73 (0.55) | 0.187 | −0.46 (0.49) | 0.349 |
| Disturbed sleep | −0.70 (0.51) | 0.168 | −0.10 (0.03) | <0.001 | −0.65 (0.65) | 0.323 | 0.42 (0.57) | 0.467 | −0.17 (0.50) | 0.731 |
| Top 6 symptoms | −0.69 (0.31) | 0.028 | −0.06 (0.02) | <0.001 | 0.23 (0.40) | 0.574 | 0.36 (0.35) | 0.306 | −0.27 (0.31) | 0.389 |
| Total interference | −0.73 (0.36) | 0.043 | −0.07 (0.02) | <0.001 | 0.17 (0.46) | 0.716 | 0.20 (0.41) | 0.628 | 0.05 (0.36) | 0.895 |
| WAW | −0.96 (0.45) | 0.032 | −0.08 (0.02) | 0.001 | 0.11 (0.58) | 0.847 | 0.21 (0.50) | 0.674 | 0.23 (0.44) | 0.60 |
| REM | −0.61 (0.39) | 0.119 | −0.06 (0.02) | 0.005 | 0.17 (0.50) | 0.740 | 0.17 (0.44) | 0.705 | −0.09 (0.39) | 0.823 |
| POD2 (n=224) | ||||||||||
| Pain | −0.90 (0.42) | 0.035 | −0.04 (0.02) | 0.037 | 1.12 (0.52) | 0.033 | −0.24 (0.49) | 0.621 | −0.80 (0.42) | 0.056 |
| Dry Mouth | −1.35 (0.48) | 0.005 | 0.05 (0.02) | 0.036 | 0.62 (0.60) | 0.266 | −1.18 (0.55) | 0.033 | −0.30 (0.46) | 0.517 |
| Fatigue | −0.20 (0.41) | 0.616 | −0.03 (0.02) | 0.206 | 1.00 (0.50) | 0.046 | −0.31 (0.47) | 0.510 | −0.37 (0.40) | 0.349 |
| Lack of appetite | 1.01 (0.48) | 0.036 | 0.01 (0.02) | 0.730 | 0.26 (0.60) | 0.659 | 0.26 (0.55) | 0.632 | 0.15 (0.47) | 0.756 |
| Disturbed sleep | −0.46 (0.50) | 0.366 | −0.03 (0.03) | 0.248 | −0.82 (0.63) | 0.196 | −1.02 (0.58) | 0.082 | −0.16 (0.50) | 0.742 |
| Top 6 symptoms | −0.35 (0.33) | 0.290 | −0.01 (0.02) | 0.565 | 0.61 (0.41) | 0.138 | −0.49 (0.39) | 0.204 | −0.31 (0.33) | 0.344 |
| Total interference | −0.89 (0.35) | 0.011 | −0.03 (0.02) | 0.122 | 0.03 (0.44) | 0.954 | −0.50(0.41) | 0.220 | −0.06 (0.34) | 0.860 |
| WAW | −1.11 (0.42) | 0.009 | −0.05 (0.02) | 0.010 | −0.22 (0.53) | 0.677 | −0.73 (0.49) | 0.138 | 0.23 (0.41) | 0.580 |
| REM | −0.735 (0.36) | 0.044 | 0.01 (0.02) | 0.971 | 0.28 (0.46) | 0.539 | −0.31 (0.42) | 0.465 | −0.28 (0.36) | 0.443 |
Abbreviations: ERAS, Enhanced Recovery after Surgery; POD, postoperative day; REM, the average score of interference with relations with others, enjoyment of life, and mood; SE, standard error; WAW, the average score of interference with work, general activity, and walking.
By POD3, the only significant difference between ERAS and traditional care was interference in enjoyment of life. On POD4 there were no significant differences in any PRO. POD5 revealed a change, with an increase in symptoms. Disturbed sleep was 1.53 units higher for the traditional-care group (P=0.020). The distress symptom score was 1.26 units higher for the traditional-care group (P=0.013), 1.91 units higher for ERAS robotic-cystectomy group compared with the ERAS open-surgery group, and 1.09 points higher for patients who received neoadjuvant chemotherapy.
DISCUSSION
The purpose of an ERAS pathway in radical cystectomy is to reduce recovery-related symptoms throughout all phases of care. This observational study of patients who underwent radical cystectomy for bladder cancer demonstrates that procedure-associated symptom burden in the 5 days after surgery was lessened for an ERAS group versus a traditional-care group, as measured with patient-reported outcomes. We were able to collect MDASI PROs daily beginning presurgery, and we observed significant reductions over time in the severity of pain, disturbed sleep, dry mouth, drowsiness, and symptom interference with walking, activity, work, relations, enjoyment of life, and mood. Our study adds evidence for the effect of an ERAS pathway on patient outcomes after radical cystectomy, indicating the importance of PROs as an outcome measure for assessing perioperative care.30
Patient perceptions about the effectiveness of ERAS after surgery compared with traditional care is an important area to explore; therefore, it is essential to choose a PRO measure that is sensitive enough to detect differences in recovery pathways.31 Previous studies using disease-specific PRO tools (the Bladder Cancer Index and Functional Assessment of Cancer Therapy-Vanderbilt Cystectomy Index) have assessed patient-reported HRQoL in robotic or open cystectomy but have not been able to demonstrate an advantage from robotic cystectomy in terms of patient symptom burden.7,32,33 The Convalescence and Recovery Evaluation (CARE) survey has been used to evaluate robotic cystectomy recovery immediately after surgery, but no published reports have shown whether this can effectively detect ERAS pathway effects on PROs.34 Survivorship follow-up PRO-based surveys are often insensitive to immediate survivorship needs and recovery from radical cystectomy, making them less sensitive to the detection of symptom severity from ERAS compared with traditional care.6 Unfortunately, there is no data on what statistical difference is required for a PRO to translate into a clinically meaningful difference. Nonetheless, given that ERAS has been associated with clinically meaningful differences in objectively evaluated metrics, such as reduced length of stay and fewer gastrointestinal complications, any improvement in a PRO most likely confirms that the change in clinical care may be meaningful and that such PRO-based assessments could be useful for identifying high-risk patients and improving vigilance in follow-up.
The current study demonstrates that the MDASI multisymptom assessment tool can sensitively and effectively capture differences in PROs for symptom burden and functional status between an ERAS cystectomy pathway and a traditional-care pathway. The MDASI interference items successfully captured better improvement in functional recovery under the ERAS pathway, demonstrating that a standard PRO tool for use in perioperative care and clinical studies should include both symptom items and functioning items. Also, the two component scores of MDASI interference (WAW and REM, physical and affective functioning) could be used in a future study in this patient cohort to define the trajectory of ERAS-associated functional recovery after patients are discharged from the hospital.
This study had several limitations. Frist, the PRO data collection was limited by patient willingness to complete the MDASI. Some patients (non-response rate: 29.3%) did not contribute PROs at all, either because they did not wish to fill out the MDASI or because of administrative error. We also had a smaller sample for the longitudinal analysis (N=207) than we did for the cross-sectional analysis (N=383). Some patients were randomly missing MDASI assessments at baseline or postoperatively (especially over the weekend), or simply were discharged earlier than other patients (before POD5). However, the missing-data rate for the 207 patients was 23.1%, an acceptable rate for a longitudinal study. Second, the general (“core”) MDASI used for this study might not represent all procedure/disease-specific symptom items that are important in this patient population. To that end, we are developing and validating a perioperative MDASI module to measure PROs in patients with bladder cancer undergoing cystectomy, based on the best practices of qualitative study and psychometric research. Third, the study is focused only on the hospitalization period, such that readmissions, complications after discharge, and complication-related symptom burden are unknown; a longitudinal study during the weeks after hospital discharge is warranted. Finally, it is worth noting that the study reflected practice change (ERAS concept adoption) over time. When we were planning the study, it was impossible to design a randomized trial because the number of patients on the ERAS pathway was low and any future increase in this number could not be accurately predicted. Over time, ERAS was increasingly incorporated into practice and thus more patients managed by ERAS were recruited into our study.
CONCLUSION
PROs, such as those obtained from the MDASI, are helpful for establishing clinically meaningful evidence of the positive effects of an ERAS pathway for radical cystectomy, including significantly reduced symptom burden, quicker recovery of functioning, and improved HRQoL. PRO measures can be used in both routine patient care and clinical research to identify benefits/harms of new treatment methodologies and to inform treatment decisions, with the ultimate goal of improving the patient’s recovery experience.
Acknowledgments
The authors thank Jeanie F. Woodruff, BS, ELS, for editorial support.
FUNDING
This study was supported in part by a grant from the National Cancer Institute, R01 CA205146 to XSW. Additional funding was provided to JBS from the MD Anderson Clinical Innovator Award and a Career Development Award from the MD Anderson SPORE in Bladder Cancer.
Footnotes
Presented at the 17th Annual Meeting of the Society of Urologic Oncology, San Antonio TX, November 30–December 2, 2016, and at the American Society of Clinical Oncology 2017 Genitourinary Cancers Symposium, Orlando FL, February 16–18, 2017.
- Study concept and design: Jay B. Shah, Xin Shelley Wang
- Acquisition of data: Janet Baack Kukreja, Qiuling Shi, Courtney M. Chang, Mohamed A. Seif, Brandon M. Sterling, Kelly M. Creel, Jay B. Shah, Xin Shelley Wang
- Analysis and interpretation: Ting-Yu Chen, Jay B. Shah, Xin Shelley Wang, Qiuling Shi, Janet Baack Kukreja
- Study supervision: Ashish M. Kamat, Colin P. Dinney, Neema Navai, Jay B. Shah, Xin Shelley Wang
CONFLICTS OF INTEREST
The authors declare that there is no conflict of interest.
References
- 1.American Cancer Society. Cancer facts & figures 2017. 2017 https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf.
- 2.Jakobsson J, Idvall E, Wann-Hansson C. General health and state anxiety in patients recovering from colorectal cancer surgery. J Adv Nurs. 2016;72(2):328–338. doi: 10.1111/jan.12841. [DOI] [PubMed] [Google Scholar]
- 3.Shida D, Wakamatsu K, Tanaka Y, et al. The postoperative patient-reported quality of recovery in colorectal cancer patients under enhanced recovery after surgery using QoR-40. BMC Cancer. 2015;15:799. doi: 10.1186/s12885-015-1799-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldberg H, Baniel J, Mano R, Rotlevy G, Kedar D, Yossepowitch O. Orthotopic neobladder vs. ileal conduit urinary diversion: A long-term quality-of-life comparison. Urol Oncol. 2016;34(3):121, e121–127. doi: 10.1016/j.urolonc.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Miller C, Campain NJ, Dbeis R, et al. Introduction of robot-assisted radical cystectomy within an established enhanced recovery programme. BJU Int. 2017;120(2):265–272. doi: 10.1111/bju.13702. [DOI] [PubMed] [Google Scholar]
- 6.Mak KS, Smith AB, Eidelman A, et al. Quality of Life in Long-term Survivors of Muscle-Invasive Bladder Cancer. Int J Radiat Oncol Biol Phys. 2016;96(5):1028–1036. doi: 10.1016/j.ijrobp.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 7.Li AY, Filson CP, Hollingsworth JM, et al. Patient-Reported Convalescence and Quality of Life Recovery: A Comparison of Open and Robotic-Assisted Radical Cystectomy. Surg Innov. 2016;23(6):598–605. doi: 10.1177/1553350616656284. [DOI] [PubMed] [Google Scholar]
- 8.Feldman LS, Lee L, Fiore J., Jr What outcomes are important in the assessment of Enhanced Recovery After Surgery (ERAS) pathways? Can J Anaesth. 2015;62(2):120–130. doi: 10.1007/s12630-014-0263-1. [DOI] [PubMed] [Google Scholar]
- 9.Cerantola Y, Valerio M, Persson B, et al. Guidelines for perioperative care after radical cystectomy for bladder cancer: Enhanced Recovery After Surgery (ERAS((R))) society recommendations. Clin Nutr. 2013;32(6):879–887. doi: 10.1016/j.clnu.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy RH, Francis A, Dutton S, et al. EnROL: a multicentre randomised trial of conventional versus laparoscopic surgery for colorectal cancer within an enhanced recovery programme. BMC Cancer. 2012;12:181. doi: 10.1186/1471-2407-12-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song W, Wang K, Zhang RJ, Dai QX, Zou SB. The enhanced recovery after surgery (ERAS) program in liver surgery: a meta-analysis of randomized controlled trials. Springerplus. 2016;5:207. doi: 10.1186/s40064-016-1793-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bond-Smith G, Belgaumkar AP, Davidson BR, Gurusamy KS. Enhanced recovery protocols for major upper gastrointestinal, liver and pancreatic surgery. Cochrane Database Syst Rev. 2016;2:CD011382. doi: 10.1002/14651858.CD011382.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H, Zhu D, Liang L, et al. Short-term quality of life in patients undergoing colonic surgery using enhanced recovery after surgery program versus conventional perioperative management. Qual Life Res. 2015;24(11):2663–2670. doi: 10.1007/s11136-015-0996-5. [DOI] [PubMed] [Google Scholar]
- 14.He F, Lin X, Xie F, Huang Y, Yuan R. The effect of enhanced recovery program for patients undergoing partial laparoscopic hepatectomy of liver cancer. Clin Transl Oncol. 2015;17(9):694–701. doi: 10.1007/s12094-015-1296-9. [DOI] [PubMed] [Google Scholar]
- 15.Fearon KC, Ljungqvist O, Von Meyenfeldt M, et al. Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr. 2005;24(3):466–477. doi: 10.1016/j.clnu.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Neville A, Lee L, Antonescu I, et al. Systematic review of outcomes used to evaluate enhanced recovery after surgery. Br J Surg. 2014;101(3):159–170. doi: 10.1002/bjs.9324. [DOI] [PubMed] [Google Scholar]
- 17.Tsutsumi R, Kakuta N, Kadota T, et al. Effects of oral carbohydrate with amino acid solution on the metabolic status of patients in the preoperative period: a randomized, prospective clinical trial. J Anesth. 2016;30(5):842–849. doi: 10.1007/s00540-016-2217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller TE, Mythen M. Successful recovery after major surgery: moving beyond length of stay. Perioper Med (Lond) 2014;3:4. doi: 10.1186/2047-0525-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan SA, Ullah S, Ahmed J, et al. Influence of enhanced recovery after surgery pathways and laparoscopic surgery on health-related quality of life. Colorectal Dis. 2013;15(7):900–907. doi: 10.1111/codi.12191. [DOI] [PubMed] [Google Scholar]
- 20.O’Neill CB, Baxi SS, Atoria CL, et al. Treatment-related toxicities in older adults with head and neck cancer: A population-based analysis. Cancer. 2015;121(12):2083–2089. doi: 10.1002/cncr.29262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujikuni N, Tanabe K, Tokumoto N, et al. Enhanced recovery program is safe and improves postoperative insulin resistance in gastrectomy. World J Gastrointest Surg. 2016;8(5):382–388. doi: 10.4240/wjgs.v8.i5.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones EL, Wainwright TW, Foster JD, Smith JR, Middleton RG, Francis NK. A systematic review of patient reported outcomes and patient experience in enhanced recovery after orthopaedic surgery. Ann R Coll Surg Engl. 2014;96(2):89–94. doi: 10.1308/003588414X13824511649571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basch E, Abernethy AP, Mullins CD, et al. Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. J Clin Oncol. 2012;30(34):4249–4255. doi: 10.1200/JCO.2012.42.5967. [DOI] [PubMed] [Google Scholar]
- 24.Cleeland CS, Sloan JA, Cella D, et al. Recommendations for including multiple symptoms as endpoints in cancer clinical trials: a report from the ASCPRO (Assessing the Symptoms of Cancer Using Patient-Reported Outcomes) Multisymptom Task Force. Cancer. 2013;119(2):411–420. doi: 10.1002/cncr.27744. [DOI] [PubMed] [Google Scholar]
- 25.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. AnnAcadMedSingapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 26.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89(7):1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 27.Fagundes CP, Shi Q, Vaporciyan AA, et al. Symptom recovery after thoracic surgery: Measuring patient-reported outcomes with the MD Anderson Symptom Inventory. J Thorac Cardiovasc Surg. 2015;150(3):613–619. e612. doi: 10.1016/j.jtcvs.2015.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi Q, Mendoza TR, Wang XS, Cleeland CS. Using a symptom-specific instrument to measure patient-reported daily functioning in patients with cancer. Eur J Cancer. 2016;67:83–90. doi: 10.1016/j.ejca.2016.07.027. [DOI] [PubMed] [Google Scholar]
- 29.Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. Hoboken, N.J: Wiley-Interscience; 2004. [Google Scholar]
- 30.Stucky CC, Pockaj BA, Novotny PJ, et al. Long-term follow-up and individual item analysis of quality of life assessments related to laparoscopic-assisted colectomy in the COST trial 93-46-53 (INT 0146) Ann Surg Oncol. 2011;18(9):2422–2431. doi: 10.1245/s10434-011-1650-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stowers MD, Lemanu DP, Hill AG. Health economics in Enhanced Recovery After Surgery programs. Can J Anaesth. 2015;62(2):219–230. doi: 10.1007/s12630-014-0272-0. [DOI] [PubMed] [Google Scholar]
- 32.Aboumohamed AA, Raza SJ, Al-Daghmin A, et al. Health-related quality of life outcomes after robot-assisted and open radical cystectomy using a validated bladder-specific instrument: a multi-institutional study. Urology. 2014;83(6):1300–1308. doi: 10.1016/j.urology.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 33.Messer JC, Punnen S, Fitzgerald J, Svatek R, Parekh DJ. Health-related quality of life from a prospective randomised clinical trial of robot-assisted laparoscopic vs open radical cystectomy. BJU Int. 2014;114(6):896–902. doi: 10.1111/bju.12818. [DOI] [PubMed] [Google Scholar]
- 34.Stegemann A, Rehman S, Brewer K, et al. Short-term patient-reported quality of life after robot-assisted radical cystectomy using the Convalescence and Recovery Evaluation. Urology. 2012;79(6):1274–1279. doi: 10.1016/j.urology.2011.12.062. [DOI] [PubMed] [Google Scholar]