Abstract
Background
Previous studies suggest that low emotional resilience may correspond with increased or over-active amygdala function. Complementary studies suggest that emotional resilience increases with age; older adults tend to have decreased attentional bias to negative stimuli compared to younger adults. Amygdala nuclei and related brain circuits have been linked to negative affect, and depressed patients have been demonstrated to have abnormal amygdala function.
Methods
In the current study, we correlated psychological resilience measures with amygdala function measured with resting-state arterial spin-labelled (ASL) and blood-oxygenation-level-dependent (BOLD) functional magnetic resonance imaging (fMRI) in older adults with and without depression. Specifically, we targeted the basolateral, centromedial, and superficial nuclei groups of the amygdala, which have different functions and brain connections.
Results
High levels of psychological resilience correlated with lower basal levels of amygdala activity measured with ASL fMRI. High resilience also correlated with decreased connectivity between amygdala nuclei and the ventral default-mode network independent of depression status. Instead, lower depression symptoms were associated with higher connectivity between the amygdalae and dorsal frontal networks.
Limitations
Future multi-site studies with larger sample size and improved neuroimaging technologies are needed. Longitudinal studies that target resilience to naturalistic stressors will also be a powerful contribution to the field.
Conclusions
Our results suggest that resilience in older adults is more closely related to function in ventral amygdala networks, while late-life depression is related to reduced connectivity between the amygdala and dorsal frontal regions.
Keywords: resilience, late-life depression, aging, fMRI, amygdala
INTRODUCTION
In psychological terms, resilience refers to a person’s ability to resist the deleterious effects of life stress on mental and physical health (Lavretsky and Irwin 2007; Lavretsky 2014). Resilience is often described and studied in the context of depression and post-traumatic stress disorder; however, it is increasingly studied as a construct independent of any single mental health condition (Russo et al. 2012).
The neurobiology underlying resilience (or nonresilience) to negative events is thought to involve brain networks associated with stress response, negative affect, and emotional control (Russo et al. 2012). For example, resilience is associated with circuits underlying regulation of the stress response, including the hypothalamic-pituitary-adrenal (HPA) axis, limbic circuitry, and self-regulation networks (Russo et al. 2012; van der Werff et al. 2013). The amygdala and its associated networks are central to many neurobiological models of resilience, and recent studies have linked increased amygdala reactivity and amygdala-frontal connectivity to resilience to early life stress (Gee et al. 2013; Yamamoto et al. 2017).
These reported links between the amygdala and resilience correspond well with an existing literature associating amygdala function with negativity bias in healthy and depressed individuals (e.g., a greater tendency to attribute negative affect to neutral stimuli) (Dannlowski, Ohrmann, Bauer, Kugel, Arolt, Heindel, and Suslow 2007; Dannlowski, Ohrmann, Bauer, Kugel, Arolt, Heindel, Kersting, et al. 2007) as well as amygdala hyperactivity in depression (Sheline et al. 2001, 2010; Siegle et al. 2002). However, the extent to which the neurobiology underlying resilience and depressive symptoms overlap, or not, remains unclear. In many older adults, the volume of amygdala, medial temporal lobe, and some cortical structures may decrease over time (Good et al. 2001b; Allen et al. 2005), which would suggest a greater risk for depression and mental health problems. However, reports also indicate a decrease in negativity bias and a hypothesized “positivity bias” that increases with age (Charles et al. 2003; Reed and Carstensen 2012). The issue becomes further complicated when considering that memory can decline with age, which has been associated with medial temporal lobe atrophy (Good et al. 2001a; Duara et al. 2008) and, perhaps related to these neuroanatomical changes, a greater risk for depression (Ismail et al. 2017).
In the current study, we examined the relationship between resilience and amygdala function in older adults with and without depression. We targeted amygdala function measured with ASL and BOLD functional MRI measured “at rest” to capture basal levels of activity and connectivity in varied amygdala subnuclei and amygdala-prefrontal networks. Our main hypothesis was that resilience and depressive symptoms would have dissociable relationships with amygdala function. As such, we applied a dimensional analysis (Insel et al. 2010) to our fMRI data, including resilience (Connor-Davidson Resilience Scale; CD-RISC) and depression (Hamilton Depression Rating Scale; HAMD) scores within the same statistical model irrespective of depression diagnosis in order to examine their individual effects on different aspects of amygdala function. Recognizing the complexity of amygdala function and connectivity, we targeted three groups of amygdala nuclei, the basolateral, centromedial, and superficial nuclei groups as defined using the Jülich histological atlas (Amunts et al. 2005). In each nucleus, we measured basal function with ASL-fMRI and connectivity with prefrontal networks with BOLD-fMRI. Finally, when relationships between resilience and amygdala function were identified, post-hoc analyses also examined potential contributions of depression status to these effects. In this way, we hoped to determine how different aspects of amygdala function relate to resilience and depressive symptoms as independent (and as potentially interdependent) dimensional constructs.
METHODS
Subjects
Older adults (age ≥ 55 years, Table 1) were recruited via advertisements from UCLA outpatient clinics and UCLA Longevity Center Program from 2014 to 2015. All participants underwent IRB-approved informed consent procedures prior to enrolling in the study. Seventeen volunteers (n=17, NCT01902004) had major depression according to DSM-5 criteria as confirmed by the Hamilton Depression Rating Scale (HAMD 24 item; score ≥ 16); the remaining volunteers were not depressed (n=31).
Table 1.
Volunteer Characteristics
| Nondepressed | Depressed | Nondepressed vs. Depressed | |||
|---|---|---|---|---|---|
|
|
|||||
| Mean | SD | Mean | SD | Statistics | |
| Age | 67.48 | 8.87 | 67.71 | 6.58 | t(46) = 0.09, p = 0.93 |
| Sex | 12f,5m | 15f,14m | x^2(1) = 0.58, p = 0.29 | ||
| Hamilton Depression Rating Scale 21-item (HAMD21) | 3.32 | 3.51 | 18.00 | 2.94 | t(46) = 14.65, p < 0.0001 |
| Connor-Davidson Resilience Scale (RISC) | 75.26 | 11.44 | 57.59 | 15.39 | t(46) = 4.52, p < 0.0001 |
| Mini-Mental State Examination (MMSE) | 28.840 | 1.130 | 28.180 | 1.630 | t(46) = 1.66, p = 0.10 |
For depressed volunteers, inclusion criteria were as follows: (1) current episode of unipolar MDD according to DSM-5 criteria; (2) Hamilton Depression Rating Scale (HDRS-24) score≥16; (3) Mini-Mental State Exam (MMSE) score ≥24; and (4) subjective memory complaints. Exclusion criteria were: (1) history of any other psychiatric disorders (other than unipolar MDD); (2) severe or acute unstable medical illness; (3) acute suicidal, violent behavior or history of suicide attempt within the last year; or (4) any other central nervous system diseases. Subjects were free of psychotropic medications for at least two weeks before participating in the study.
For healthy volunteers, inclusion criteria were: (1) Mini-Mental State Exam (MMSE) score ≥24; (2) subjective memory complaints; (3) no current, or history of, depression. Exclusion criteria were: (1) history of any psychiatric disorders or dementia; (2) severe or acute unstable medical illness; (3) any other central nervous system diseases; (4) no psychotropic medications use.
Clinical Measurements
Clinical measurements of main interest to the current study included the Hamilton Depression Rating Scale 24-item (HAMD-24) and the Connor-Davidson Resilience Scale (CD-RISC). Additional measurements included the Mini-Mental State Examination (MMSE), and others reported elsewhere (Eyre, Acevedo, et al. 2016; Eyre, Yang, et al. 2016; Yang et al. 2016).
MRI Acquisition
MRI data were acquired on a 3.0 Tesla TIM Trio scanner (Siemens, Germany) using a 32-channel head coil, and head motion was minimized with the adjacent placement of firm cushions. We instructed participants to close eyes and stay awake during image acquisition. Arterial spin-labelled (ASL) images were acquired using a pCASL pulse sequence, post label delay 1200ms, 80 volumes with repetition time 5 seconds, echo time 27 ms, flip angel 90°, field of view 256 × 256 mm2, acquisition matrix 128 × 128, voxel size 2 × 2 × 5, 20 slices (20% dist. factor). Resting-state functional images were acquired with a multi-band gradient-echo echo-planar imaging (EPI) sequence sensitive to BOLD contrast effects. We acquired 275 contiguous EPI resting-state volumes, and the parameters for functional imaging were repetition time 1.24 seconds, echo time 38.2 ms, flip angle 65°, field of view 21.2 × 21.2 cm2, acquisition matrix 118 × 118, 1.8 mm3 iso-voxel size (no gap), 78 slices, and 6 bands. We also acquired anatomic images with 3-dimensional MPRAGE sequence (acquisition matrix 256 × 256 with 1 mm thick contiguous slices) for co-registration with the functional data.
MRI Preprocessing
ASL fMRI images were pre-processed in FSL (FMRIB Software Library, FSL, www.fmrib.ox.ac.uk/fsl) and using the ASLtoolbox (Wang et al. 2008). First, images were corrected for motion in FSL. Then, cerebral blood flow (CBF) was quantified voxelwise using ASLtoolbox using simple subtraction. Images were then registered to each volunteer’s T1-weighted MRI scan, and normalized to MNI space using FSL. Global CBF was quantified using a gray-matter mask, and averaging CBF values across all voxels within that mask.
BOLD fMRI images were pre-processed in FSL for motion correction, high-pass filter (0.01 Hz), image normalization and 5 mm3 Gaussian spatial smoothing. MELODIC (Multivariate Exploratory Linear Decomposition into Independent Components, a tool of FSL) was used to remove significant head motion, scanner, and physiological artifacts using ICA. The processed functional data from all participants were temporally concatenated to form a 4-dimensional data set, which was decomposed into group-level independent components (ICs) using ICA. The MELODIC automated dimensionality estimate was used to determine the number and order of the ICs (Beckmann and Smith 2004). Each component includes brain structures that share the same temporal pattern of signal after mixture modeling was applied. The dual regression approach was subsequently used to back-reconstruct individual-specific connectivity maps associated with each group-level component, which been shown to be an effective and reliable approach to analyses of resting state fMRI data. This approach yielded 36 ICs; 26 of these overlapped grey matter and were considered biologically plausible. Of these 26, we isolated 10 ICs with maps overlapping prefrontal cortex for further analysis (Supplementary Figure 1).
Amygdala Subnuclei
Amygdala nuclei groups were defined using the Jülich atlas (Amunts et al. 2005) for each hemisphere with a threshold of 40%. The atlas was masked such that only voxels with reasonable ASL-CBF data were present for all volunteers (thresholded as per standard procedure in ASLtoolbox (Wang et al. 2008)). Functional metrics were averaged across all voxels within each region, and subjected to further analysis as described below.
Statistical Analyses
In analyses of fMRI data, general linear models were executed in R software, and p-values were corrected for multiple comparisons using the False Discovery Rate (FDR) to corrected p < 0.05. Student’s t tests, chi-squared tests, and linear regression models also examined differences in demographic and clinical variables between depressed and nondepressed volunteers, also executed in R.
Our primary aim was to identify the independent contributions of resilience and depression symptoms on brain function (if any), measured in a dimensional manner (i.e., not binarized or according to depression diagnosis/status). Thus, linear models examined relationships between functional metrics for each nucleus group, with resilience (CD-RISC) and depression (HAMD) scores as factors of interest, and with age and hemisphere as nuisance factors. Global CBF served as an additional nuisance factor in ASL-CBF analyses. In ASL-CBF analyses, we performed an additional test post-hoc where all amygdala nuclei were considered as a repeated within the same model with, the identical covariates as described above.
In cases were significant effects of resilience were identified, we executed follow-up analyses to determine whether this effect was related to binarized depression status (HAMD score > 16 for depressed status). This statistical model targeted a resilience-by-depression-status interaction, with other nuisance regressors including depression scores (HAMD), hemisphere, age, and global CBF (the latter for ASL-CBF only). Because depression status and depression scores are highly correlated (and this multicolinearity could make the beta estimates of these statistical models unstable), we confirmed that results were consistent when removing depression scores as an additional factor in each case.
RESULTS
Relationships between Resilience, Depressive Symptoms, and Depression Status
The main goal of the present study was to examine the independent contributions of resilience and depression symptoms to brain function. As such, we first examined the relationships amongst resilience scores (CD-RISC), depression symptoms (HAMD), and depression status (diagnosis). Depressed volunteers exhibited lower mean resilience scores (CD-RISC) and higher mean depression scores (HAMD) than age-matched controls (Table 1). Resilience scores and depression scores were also negatively correlated, such that volunteers with high resilience tended to have lower depression scores (Pearson’s r = −0.60, p < 0.0001); depression scores explained 35% of the variance in resilience scores. Notably, age was not correlated with depression or resilience scores (p > 0.50 for both; note also the limited age range of the current sample).
Amygdalar Cerebral Blood Flow (CBF, ASL-fMRI)
In our combined resilience-depression dimensional models, resilience scores were negatively correlated with ASL-CBF in the basolateral amygdala (pFDR < 0.05, Table 2); similar trends were also present for centromedial and superficial groups. Indeed, when combining all nuclei in a single repeated-measures statistical model, resilience scores were negatively correlated with ASL-CBF (uncorrected p = 0.04). No significant relationships between amygdalar ASL-CBF and depression scores (HAMD) were detected. In post-hoc tests of depression status, modest interactions were present between resilience and depression status in basolateral amygdala (beta = −0.64, t = −2.32, p = 0.02) and when combining all nuclei (beta = −0.72, t = −2.92, p = 0.004), suggesting that the relationship between resilience and amygdalar CBF was stronger in depressed patients than in nondepressed volunteers (Figure 2). Indeed, when applying the same model separately for depressed and nondepressed groups, resilience was significantly correlated with CBF in basolateral nuclei in depressed patients (beta = −0.79, t = −3.61, p = 0.002), but not in nondepressed volunteers (beta = −0.05, t = −0.24, p = 0.81).
Table 2.
Relationships of Cerebral Blood Flow (ASL fMRI) with Resilience and Depression Measures
| Amygdala Nucleus | Resilience (RISC) | Depression (HAMD) | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| beta | t | p | beta | t | p | |
| Centromedial | −0.23 | −1.299 | 0.198 | −0.358 | −0.932 | 0.354 |
| Basolateral | −0.325 | −2.249 | 0.027* | −0.351 | −1.12 | 0.266 |
| Superficial | −0.309 | −1.779 | 0.079 | −0.368 | −0.978 | 0.331 |
| All (post hoc) | −0.265 | −2.113 | 0.036 | −0.307 | −1.144 | 0.254 |
Figure 2. Resilience Scores Correlate with Amygdala Function Measured with ASL fMRI.
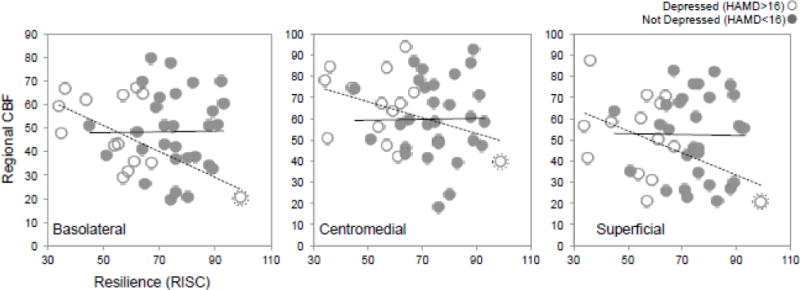
Mean cerebral blood flow (CBF, y axis) is plotted for the basolateral (left), centromedial (middle), and superficial (right) nuclei for each volunteer with respect to resilience scores (x axis), averaged across hemisphere. Depressed and nondepressed groups are shown in open and closed circles respectively. Regression fit lines are also displayed for depressed and nondepressed groups in dotted and solid lines respectively. Note that a potential outlier was identified, and is marked by an enclosing dotted circle.
We identified a single potential outlier, who met the criterion for depressed status (HAMD = 16) and also scored high on the resilience scale (CD-RISC = 99). When removing this potential outlier, a similar pattern of the results described above was detected, in some cases approaching (but not meeting) our criterion for statistical significance in the basolateral amygdala (resilience beta = −0.18, t = −1.12, p = 0.27; resilience-depression interaction beta = −0.61, t = −1.63, p = 0.11). When combining nuclei, the resilience-depression interaction (beta = −0.48, t = −1.48, p = 0.14) still suggested a stronger effect of resilience in patients; however, correlation between resilience and CBF was not present (beta = −0.08, t = −0.60, p = 0.55). The fact that a similar pattern of results was present in this post hoc analysis suggests that this outlier may not be driving all observed effects, and perhaps that the characteristics of our sample (e.g., small sample size, few depressed volunteers with high resilience) negatively impacted statistical power.
Amygdalar Functional Connectivity with Prefrontal Networks (BOLD-fMRI)
In the combined resilience-depression dimensional models, connectivity between the superficial group and the ventral default mode network was negatively correlated with resilience scores (pFDR < 0.05, Table 3 and Figure 3A); volunteers with lower resilience exhibited higher connectivity between superficial amygdala nuclei and ventral DMN. In post-hoc tests, no interaction with depression status was present (pFDR > 0.05; beta = −0.006, t = −1.34, p = 0.18), suggesting that this negative relationship existed for both depressed and nondepressed volunteers.
Table 3.
Functional Connectivity (Resting State BOLD fMRI)
| Network | Amygdala Nucleus | Resilience (RISC) | Depression (HAMD) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| beta | t | p | p-fdr | beta | t | p | p-fdr | ||||||
| Bilateral Frontoparietal | Centromedial | 0.032 | 0.689 | 0.493 | 0.739 | 0.252 | 2.758 | 0.007 | 0.110 | ||||
| Basolateral | −0.037 | −1.067 | 0.289 | 0.612 | 0.056 | 0.830 | 0.408 | 0.693 | |||||
| Superficial | −0.055 | −1.127 | 0.263 | 0.603 | 0.075 | 0.778 | 0.439 | 0.703 | |||||
| Frontal | Centromedial | −0.134 | −2.437 | 0.017 | 0.196 | −0.047 | −0.439 | 0.662 | 0.851 | ||||
| Basolateral | −0.114 | −3.270 | 0.002 | 0.059 | −0.124 | −1.816 | 0.073 | 0.346 | |||||
| Superficial | −0.130 | −2.576 | 0.012 | 0.151 | −0.106 | −1.069 | 0.288 | 0.612 | |||||
| Bilateral Dorsolateral Frontal | Centromedial | −0.082 | −1.517 | 0.133 | 0.503 | −0.382 | −3.572 | 0.001 | 0.033 | ||||
| Basolateral | 0.001 | 0.025 | 0.980 | 0.989 | −0.110 | −1.565 | 0.121 | 0.503 | |||||
| Superficial | −0.053 | −0.987 | 0.326 | 0.612 | −0.199 | −1.876 | 0.064 | 0.346 | |||||
| Somatomotor and Insula | Centromedial | −0.060 | −1.167 | 0.246 | 0.580 | 0.188 | 1.931 | 0.057 | 0.198 | ||||
| Basolateral | 0.008 | 0.224 | 0.823 | 0.926 | 0.194 | 2.737 | 0.007 | 0.110 | |||||
| Superficial | −0.086 | −1.795 | 0.076 | 0.346 | 0.118 | 1.259 | 0.211 | 0.596 | |||||
| Left Fronto-temporo-parietal | Centromedial | −0.034 | −0.748 | 0.457 | 0.722 | −0.022 | −0.246 | 0.806 | 0.917 | ||||
| Basolateral | −0.006 | −0.183 | 0.855 | 0.928 | −0.039 | −0.654 | 0.515 | 0.739 | |||||
| Superficial | −0.014 | −0.320 | 0.750 | 0.914 | 0.062 | 0.694 | 0.490 | 0.739 | |||||
| Bilateral Superior Lateral Frontal | Centromedial | −0.062 | −1.159 | 0.249 | 0.596 | −0.191 | −1.810 | 0.074 | 0.346 | ||||
| Basolateral | −0.031 | −0.947 | 0.346 | 0.612 | −0.062 | −0.968 | 0.336 | 0.612 | |||||
| Superficial | −0.008 | −0.159 | 0.874 | 0.928 | −0.101 | −1.025 | 0.308 | 0.612 | |||||
| Right Frontal | Centromedial | −0.006 | −0.123 | 0.902 | 0.929 | 0.111 | 1.090 | 0.279 | 0.612 | ||||
| Basolateral | 0.021 | 0.556 | 0.580 | 0.798 | −0.057 | −0.778 | 0.439 | 0.703 | |||||
| Superficial | 0.013 | 0.254 | 0.800 | 0.917 | 0.044 | 0.442 | 0.660 | 0.851 | |||||
| Ventral Default Mode | Centromedial | −0.020 | −0.572 | 0.569 | 0.792 | 0.109 | 1.541 | 0.127 | 0.503 | ||||
| Basolateral | −0.055 | −1.861 | 0.066 | 0.346 | 0.018 | 0.307 | 0.759 | 0.914 | |||||
| Superficial | −0.144 | −3.639 | 0.0005 | 0.033 | 0.016 | 0.211 | 0.834 | 0.928 | |||||
| Posterior Default Mode | Centromedial | −0.011 | −0.245 | 0.807 | 0.917 | −0.085 | −0.942 | 0.349 | 0.612 | ||||
| Basolateral | 0.018 | 0.648 | 0.519 | 0.739 | 0.037 | 0.672 | 0.503 | 0.739 | |||||
| Superficial | −0.013 | −0.289 | 0.774 | 0.914 | −0.036 | −0.394 | 0.694 | 0.873 | |||||
| Lateral Frontal Pole | Centromedial | −0.023 | −0.468 | 0.641 | 0.851 | −0.095 | −0.968 | 0.335 | 0.612 | ||||
| Basolateral | 0.005 | 0.142 | 0.887 | 0.928 | −0.019 | −0.296 | 0.768 | 0.914 | |||||
| Superficial | −0.038 | −0.808 | 0.421 | 0.694 | −0.138 | −1.480 | 0.142 | 0.503 | |||||
| Right Frontal | Centromedial | 0.058 | 1.236 | 0.219 | 0.596 | 0.108 | 1.164 | 0.247 | 0.596 | ||||
| Basolateral | −0.004 | −0.109 | 0.914 | 0.930 | 0.063 | 0.950 | 0.345 | 0.612 | |||||
| Superficial | 0.042 | 0.810 | 0.420 | 0.694 | −0.012 | −0.120 | 0.905 | 0.929 | |||||
| Salience | Centromedial | 0.098 | 2.083 | 0.040 | 0.320 | 0.185 | 1.989 | 0.050 | 0.342 | ||||
| Basolateral | 0.038 | 0.966 | 0.337 | 0.612 | 0.184 | 2.385 | 0.019 | 0.198 | |||||
| Superficial | −0.009 | −0.145 | 0.885 | 0.928 | 0.183 | 1.489 | 0.140 | 0.503 | |||||
| Anterior Default Mode | Centromedial | −0.045 | −1.026 | 0.308 | 0.612 | −0.026 | −0.301 | 0.764 | 0.914 | ||||
| Basolateral | −0.028 | −1.140 | 0.257 | 0.602 | −0.048 | −0.983 | 0.328 | 0.612 | |||||
| Superficial | −0.068 | −1.568 | 0.120 | 0.503 | 0.054 | 0.639 | 0.524 | 0.739 | |||||
Figure 3. Resilience and Depression Measures Correlate with Amygdala Connectivity Measured with BOLD fMRI.
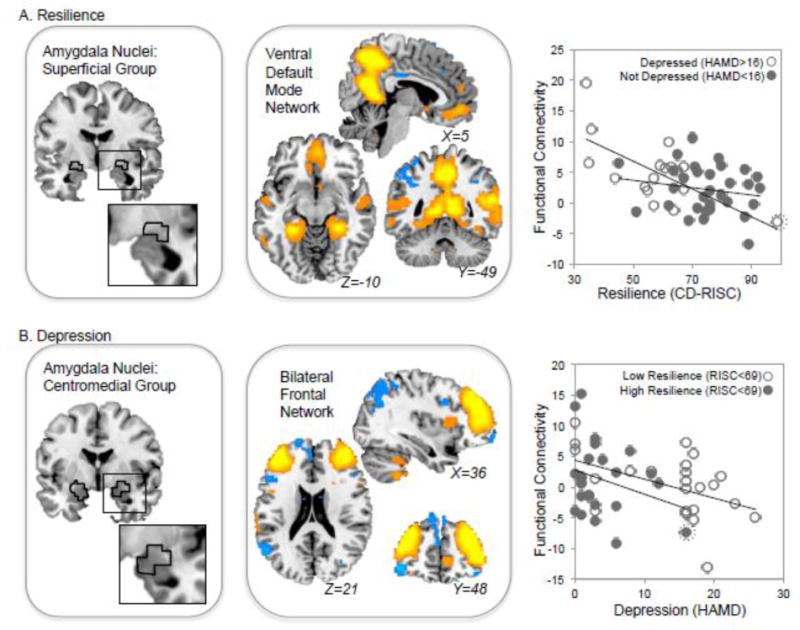
A A significant correlation was identified between resilience and connectivity between the Superficial Group of amygdala nuclei (left) and the Ventral Default Mode Network (middle). The location of the Superficial Group as identified in the Juelich atlas is outlined in black. Regions with resting-state BOLD fMRI timecourses most correlated with the ventral DMN are shown in orange and blue, which indicate opposing functional relationships (i.e., activity in orange voxels is negatively correlated with activity in blue voxels in this network). At right, functional connectivity scores (superficial amygdala nuclei vs. ventral DMN) are plotted against resilience scores (RISC) for each depressed (open circles) and nondepressed (gray circles) volunteer. B. Depression scores (HAMD) were negatively correlated with connectivity between the centromedial group of amygdala nuclei (left) and a Bilateral Frontal Network (middle). At right, this functional connectivity between CM amygdala and this Bilateral Frontal Network is plotted for volunteers with low (open circles) and high (gray circles) resilience scores. Note that a potential outlier is indicated by an enclosing circle (dotted line); this volunteer met the criterion for depressed status and had a high resilience score.
Resilience-depression models also detected a negative relationship between depression scores (HAMD) and functional connectivity between a bilateral frontal resting-state network and the centromedial group of amygdala nuclei (pFDR < 0.05, Table 3 and Figure 3B). Here, volunteers with higher depression scores tended to have low connectivity between this frontal network and centromedial amygdala nuclei.
We performed additional tests post hoc after removing the same outlier discussed above. When removing this potential outlier, again the same effects remained, yet were diminished. Resilience remained negatively correlated with connectivity between the superficial nuclei and ventral DMN (beta = −0.13, t = −2.87, p = 0.005), and there was again no interaction between resilience and depression status (beta = 0.008, t = 0.12, p = 0.9). Depression scores remained negatively correlated with connectivity between the centromedial nuclei and bilateral frontal RSN (beta = −0.34, t = −2.92, p = 0.004).
DISCUSSION
Our results suggest that different aspects of amygdala function are associated with psychological resilience and depression in older adults. Specifically, high resilience was associated with lower amygdala function (CBF) at rest. This effect appeared to be strongest in the basolateral amygdala, and in volunteers with late-life depression. High levels of resilience were also associated with lower connectivity between the amygdala and ventral self-referential networks (i.e., DMN (Sheline et al. 2009)). By contrast, low depression scores were associated with greater connectivity between dorsal frontal networks and the centromedial amygdala, suggesting greater top-down control of negative affect. Taken together, these results suggest that psychological resilience may be more closely tied to ventral limbic networks associated with “bottom-up” generation of emotional state, while depression may be more closely related to the dysregulation of emotions by fronto-limbic circuits.
The role of the amygdala in resilience and depression
The amygdala has been repeatedly linked to negative affect, fear, and emotion in human neuroimaging and in animal models, both in the context of healthy function and in affective disorders (Murray 2007; Siegle et al. 2007; Pessoa and Adolphs 2010). Amygdala hyperactivity is thought to be reflective of overall hyperactivity within ventral limbic circuits in depression and anxiety, perhaps responsible for generating negative affect and/or mood (Mayberg 1997; Drevets 2000; Koenigs and Grafman 2009). Correspondingly, amygdala volume tends to be decreased in patients with depression (Hamilton et al. 2008), including older adults (Burke et al. 2011), and may be affected in other mood and anxiety disorders as well (De Bellis et al. 2000; Woon and Hedges 2009).
One might suspect that resilient individuals would exhibit the opposite pattern, specifically lower amygdala function at rest. Indeed, we report a negative correlation between resilience scores and basal CBF in amygdala nuclei, particularly the basolateral group. Notably, however, a significant interaction between resilience scores and depression status suggests that this effect was driven by depressed patients; depressed older adults with high resilience tended to have lower amygdala basal activity (CBF). These results suggest a complex relationship between amygdala activity, resilience, and late-life depression, where the impact of resilience on amygdala function and structure may depend on disease state. This may explain why previous studies have not shown a clear relationship between resilience and amygdala structure and function (van der Werff et al. 2013), and may reflect the influence of brain aging on these relationships in the context of late-life depression (Steffens 2012).
Amygdala networks, resilience, and depression
The amygdala is a complex structure, including several subnuclei, each of which have a unique set of intrinsic and extrinsic connections.(Amunts et al. 2005) The basolateral group receives input from sensory cortices and other areas, while the centromedial and superficial groups tend to have greater extrinsic connections with frontal cortex and other regions (Amunts et al. 2005; Solano-Castiella et al. 2010). Thus, although on the whole the amygdala can be seen to play a role in the processing of affect, emotion (positive emotion), fear, and related phenomena (Pare and Duvarci 2012; Veinante et al. 2013), its network architecture suggests that its subregions may play differing roles in those and other phenomena.
The circuit-level neurobiology of depression is often described as an imbalance between overly active ventral circuits and under-active dorsal circuits, characterized by decreased top-down regulation of negative emotion/affect leading to depressed mood (Mayberg et al. 1999; Gotlib and Joormann 2010). Our data support this overall pattern, with high depression scores linked to decreased connectivity between dorsal frontal networks and the centromedial amygdala group, while controlling for resilience scores. This is consistent previous MRI studies which have identified decreased function and decreased tissue volume in dorsal frontal brain regions including the dorsolateral and dorsomedial prefrontal corticies (Galynker et al. 1998; Siegle et al. 2007; Amico et al. 2011; Leaver et al. 2015), which is influenced by antidepressant treatment. Network connectivity of the dorsolateral prefrontal cortex has been repeatedly linked to cognitive and self control (Berman et al. 2013). Our results support the model of late-life depression as a “failure” of top-down regulation of ventral limbic circuits by the dorsal prefrontal cortex and associated regions.
Resilience in aging adults, on the other hand, was more closely associated with ventral limbic networks in our study. Specifically, low-resilience individuals exhibited increased connectivity between the amygdala and a ventral default-mode network. This suggests that, when statistically controlling for the presence of depressed symptoms, low resilience is linked with limbic hyperactivity. This complements and perhaps also explains previously reported links between hyperactivity in ventral limbic regions and networks in depression and other affective/mood disorders, where resilience is often not measured or studied as a separate construct (Mayberg 1997; Mayberg et al. 1999; Drevets 2000; Koenigs and Grafman 2009; Gotlib and Joormann 2010; Leaver et al. 2015). This also complements the link we describe above between increased amygdalar CBF in volunteers with low resilience. Taken together, these results also support the idea that lower basal levels of amygdala activity may associate with a greater available range of responsivity, which in turn may increase one’s ability to respond to stressful events (Yamamoto et al. 2017).
Notably, the reported results overlap brain structures known to be influenced by brain aging, cognitive decline, and depression, particularly the medial temporal lobe. Late-life depression may be associated with its own unique set of neuropathophysiological processes (Ajilore et al. 2014; Tadayonnejad and Ajilore 2014; Eyre, Yang, et al. 2016; Yang et al. 2016). However, whether our findings are specific to resilience and depression in later life or can be generalized to younger adults remains to be determined.
Limitations & Conclusions
Our pilot study contributes to a growing body of research demonstrating network disturbances in individuals with late-life depression (Ajilore et al. 2014; Eyre, Yang, et al. 2016). Specifically, we present data suggesting that the neurofunctional architecture underlying emotional resilience is similar to, but not completely overlapping with, the brain-basis of depression in older adults. Taken together, our results could indicate that resilience is more associated with function in ventral “bottom-up” limbic networks, while depression is more closely linked with “top-down” emotion-regulation networks. However, there are several limitations that should be considered. We took a dimensional analysis approach (Insel et al. 2010), constructing a statistical model that measured effects associated with resilience scores while controlling for depression scores, and vice versa. However, recruitment for the current study was not dimensional with respect to resilience, and therefore the design was somewhat imbalanced: depressed patients had on average lower resilience scores than nondepressed volunteers. Although the overlap in resilience scores between these groups was substantial (e.g., see Fig 2), future studies should attempt to recruit a larger sample with a full range of scores (e.g., depressed volunteers with high and low resilience) to measure the brain correlates of resilience with greater statistical power. Future studies could also use MRI sequences with improved temporal and spatial resolution measure amygdala function with more precision. All volunteers for this study also endorsed having subjective memory complaints, which is common in older adults. And finally, our measure of psychological resilience was a widely-used self-report questionnaire; longitudinal studies that measure biomarkers associated with resilience to significant naturalistic stressors (e.g., combat, childbirth, losing a job or loved one) will be better able to understand why some people develop mental disorders and others do not. In the increasingly stressful world, further research into the neurobiology and cultivation of resilience is urgently needed.
Supplementary Material
Figure 1. Amygdala subnuclei.
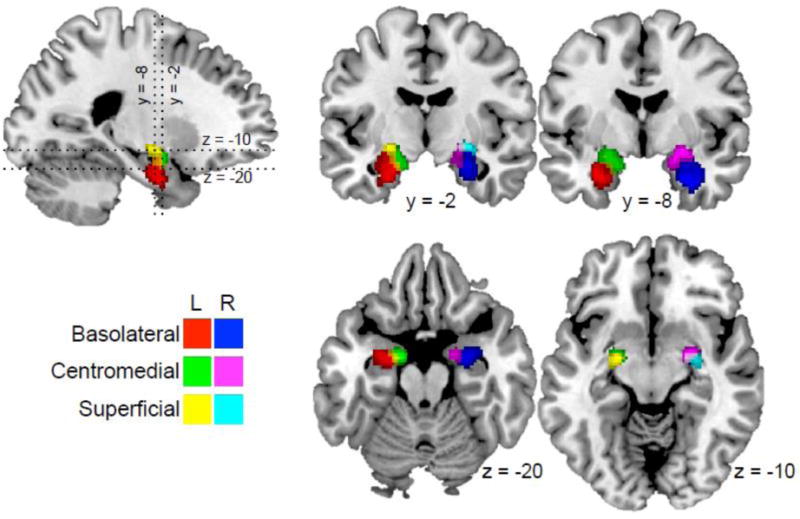
The Jülich histological atlas was used to locate groups of amygdala subnuclei. The basolateral, centromedial, and superficial groups are displayed for each hemisphere, with color key given at lower left.
Highlights.
Psychological resilience and depression are correlated, yet non-overlapping domains
High resilience associated with lower amygdala function
High resilience was linked with decreased ventral amygdala-frontal connectivity
Depressed patients showed decreased dorsal amygdala-frontal connectivity
Acknowledgments
This work was supported by the NIH grants AT008383, AT009198, MH097892, and Alzheimer’s Research and Prevention Foundation to Dr. Lavretsky.
Role of the Funding Source: The sponsors had no role in the study design, data collection, analysis, or interpretation of the data, the writing of this manuscript, or in the decision to submit this manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors have no financial disclosures or conflicts of interest to report.
Contributors: Authors H.L., K.L.N., and P.S. designed the study. Authors H.Y. and N.S.C. collected and managed data collection. Authors A.M.L. and H.Y. conducted MRI preprocessing and analysis. Authors A.M.L., H.Y., and P.S. undertook the statistical analyses. Author A.M.L. wrote the first draft of the manuscript. All authors have contributed to and have approved the final manuscript.
FINANCIAL DISCLOSURES
Authors have no financial disclosures.
References
- Ajilore O, Lamar M, Leow A, Zhang A, Yang S, Kumar A. Graph Theory Analysis of Cortical-Subcortical Networks in Late-Life Depression. Am J Geriatr Psychiatry. 2014;22:195–206. doi: 10.1016/j.jagp.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: the major lobes and a parcellation of the temporal region. Neurobiol Aging. 2005;26:1245-60-82. doi: 10.1016/j.neurobiolaging.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Amico F, Meisenzahl E, Koutsouleris N, Reiser M, Möller H-J, Frodl T. Structural MRI correlates for vulnerability and resilience to major depressive disorder. J Psychiatry Neurosci. 2011;36:15–22. doi: 10.1503/jpn.090186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl) 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Berman MG, Yourganov G, Askren MK, Ayduk O, Casey BJ, Gotlib IH, Kross E, McIntosh AR, Strother S, Wilson NL, Zayas V, Mischel W, Shoda Y, Jonides J. Dimensionality of brain networks linked to life-long individual differences in self-control. Nat Commun. 2013;4:1373. doi: 10.1038/ncomms2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke J, McQuoid DR, Payne ME, Steffens DC, Krishnan RR, Taylor WD. Amygdala Volume in Late-Life Depression. Am J Geriatr Psychiatry. 2011;19:771–776. doi: 10.1097/JGP.0b013e318211069a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles ST, Mather M, Carstensen LL. Aging and emotional memory: The forgettable nature of negative images for older adults. J Exp Psychol Gen. 2003;132:310–324. doi: 10.1037/0096-3445.132.2.310. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Bauer J, Kugel H, Arolt V, Heindel W, Kersting A, Baune BT, Suslow T. Amygdala reactivity to masked negative faces is associated with automatic judgmental bias in major depression: a 3 T fMRI study. J Psychiatry Neurosci. 2007;32:423–429. [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Bauer J, Kugel H, Arolt V, Heindel W, Suslow T. Amygdala reactivity predicts automatic negative evaluations for facial emotions. Psychiatry Res Neuroimaging. 2007;154:13–20. doi: 10.1016/j.pscychresns.2006.05.005. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Casey B, Dahl RE, Birmaher B, Williamson DE, Thomas KM, Axelson DA, Frustaci K, Boring AM, Hall J, Ryan ND. A pilot study of amygdala volumes in pediatric generalized anxiety disorder. Biol Psychiatry. 2000;48:51–57. doi: 10.1016/s0006-3223(00)00835-0. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res. 2000;126:413–431. doi: 10.1016/S0079-6123(00)26027-5. [DOI] [PubMed] [Google Scholar]
- Duara R, Loewenstein DA, Potter E, Appel J, Greig MT, Urs R, Shen Q, Raj A, Small B, Barker W, Schofield E, Wu Y, Potter H. Medial temporal lobe atrophy on MRI scans and the diagnosis of Alzheimer disease. Neurology. 2008;71:1986–1992. doi: 10.1212/01.wnl.0000336925.79704.9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre HA, Acevedo B, Yang H, Siddarth P, Van Dyk K, Ercoli L, Leaver AM, St Cyr N, Narr K, Baune BT, Khalsa DS, Lavretsky H. Changes in Neural Connectivity and Memory Following a Yoga Intervention for Older Adults: A Pilot Study. J Alzheimer’s Dis. 2016;52:673–684. doi: 10.3233/JAD-150653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre HA, Yang H, Leaver AM, Van Dyk K, Siddarth P, St Cyr N, Narr K, Ercoli L, Baune BT, Lavretsky H. Altered resting-state functional connectivity in late-life depression: A cross-sectional study. J Affect Disord. 2016;189:126–133. doi: 10.1016/j.jad.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galynker II, Cai J, Ongseng F, Finestone H, Dutta E, Serseni D. Hypofrontality and negative symptoms in major depressive disorder. J Nucl Med. 1998;39:608–612. [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, Hare TA, Bookheimer SY, Tottenham N. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci U S A. 2013;110:15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage. 2001a;14:685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001b;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Annu Rev Clin Psychol. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Siemer M, Gotlib IH. Amygdala volume in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Mol Psychiatry. 2008;13:993–1000. doi: 10.1038/mp.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Ismail Z, Elbayoumi H, Fischer CE, Hogan DB, Millikin CP, Schweizer T, Mortby ME, Smith EE, Patten SB, Fiest KM. Prevalence of Depression in Patients With Mild Cognitive Impairment. JAMA Psychiatry. 2017;74:58. doi: 10.1001/jamapsychiatry.2016.3162. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Grafman J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav Brain Res. 2009;201:239–243. doi: 10.1016/j.bbr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavretsky H. Resilience and aging : research and practice. Baltimore: Johns Hopkins University Press; 2014. [Google Scholar]
- Lavretsky H, Irwin MR. Resilience and aging. Aging health. 2007;3:309–323. [Google Scholar]
- Leaver AM, Espinoza R, Joshi SH, Vasavada M, Njau S, Woods RPR, Narr KLK. Desynchronization and Plasticity of Striato-frontal Connectivity in Major Depressive Disorder. Cereb cortex. 2015 doi: 10.1093/cercor/bhv207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatr. Clin Neurosci. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT. Reciprocal Limbic-Cortical Function and Negative Mood: Converging PET Findings in Depression and Normal Sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- Murray EA. The amygdala, reward and emotion. Trends Cogn Sci. 2007;11:489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Pare D, Duvarci S. Amygdala microcircuits mediating fear expression and extinction. Curr Opin Neurobiol. 2012;22:717–723. doi: 10.1016/j.conb.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Adolphs R. Emotion processing and the amygdala: from a “low road” to “many roads” of evaluating biological significance. Nat Rev Neurosci. 2010;11:773–783. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed AE, Carstensen LL. The theory behind the age-related positivity effect. Front Psychol. 2012;3:339. doi: 10.3389/fpsyg.2012.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Murrough JW, Han M-H, Charney DS, Nestler EJ. Neurobiology of resilience. Nat Neurosci. 2012;15:1475–1484. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Mintun MA, Wang S, Coalson RS, Raichle ME. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A. 2009;106:1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci. 2010;107:11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can’t shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Solano-Castiella E, Anwander A, Lohmann G, Weiss M, Docherty C, Geyer S, Reimer E, Friederici AD, Turner R. Diffusion tensor imaging segments the human amygdala in vivo. Neuroimage. 2010;49:2958–2965. doi: 10.1016/j.neuroimage.2009.11.027. [DOI] [PubMed] [Google Scholar]
- Steffens DC. Depressive symptoms and mild cognitive impairment in the elderly: an ominous combination. Biol Psychiatry. 2012;71:762–764. doi: 10.1016/j.biopsych.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadayonnejad R, Ajilore O. Brain Network Dysfunction in Late-Life Depression. J Geriatr Psychiatry Neurol. 2014;27:5–12. doi: 10.1177/0891988713516539. [DOI] [PubMed] [Google Scholar]
- van der Werff SJA, van den Berg SM, Pannekoek JN, Elzinga BM, van der Wee NJA. Neuroimaging resilience to stress: a review. Front Behav Neurosci. 2013;7:39. doi: 10.3389/fnbeh.2013.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veinante P, Yalcin I, Barrot M. The amygdala between sensation and affect: a role in pain. J Mol Psychiatry. 2013;1:9. doi: 10.1186/2049-9256-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Aguirre GK, Rao H, Wang J, Fernández-Seara MA, Childress AR, Detre JA. Empirical optimization of ASL data analysis using an ASL data processing toolbox. ASLtbx Magn Reson Imaging. 2008;26:261–269. doi: 10.1016/j.mri.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woon FL, Hedges DW. Amygdala Volume in Adults with Posttraumatic Stress Disorder: A Meta-Analysis. J Neuropsychiatry Clin Neurosci. 2009;21:5–12. doi: 10.1176/jnp.2009.21.1.5. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Toki S, Siegle GJ, Takamura M, Takaishi Y, Yoshimura S, Okada G, Matsumoto T, Nakao T, Muranaka H, Kaseda Y, Murakami T, Okamoto Y, Yamawaki S. Increased amygdala reactivity following early life stress: a potential resilience enhancer role. BMC Psychiatry. 2017;17:27. doi: 10.1186/s12888-017-1201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Leaver AM, Siddarth P, Paholpak P, Ercoli L, St Cyr NM, Eyre HA, Narr KL, Khalsa DS, Lavretsky H. Neurochemical and Neuroanatomical Plasticity Following Memory Training and Yoga Interventions in Older Adults with Mild Cognitive Impairment. Front Aging Neurosci. 2016;8:277. doi: 10.3389/fnagi.2016.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


