Abstract
Objectives
Quantitative T1ρ mapping is a magnetic resonance imaging technique sensitive to pH and other cellular and microstructural factors and is a potentially valuable tool for identifying brain alterations in bipolar disorder. Recently, this technique identified differences in the cerebellum and cerebral white matter of euthymic patients versus healthy controls consistent with reduced pH in these regions, suggesting an underlying metabolic abnormality. The current study builds upon this prior work to investigate brain T1ρ differences across euthymic, depressed, and manic mood states of bipolar disorder.
Methods
Forty participants with bipolar I disorder and 29 healthy control participants balanced for age and gender were enrolled. Participants with bipolar disorder were imaged in one or more mood states, yielding 27, 12, and 13 imaging sessions in euthymic, depressed, and manic mood states, respectively. Three-dimensional, whole-brain anatomical images and T1ρ maps were acquired for all participants, enabling voxel-wise evaluation of T1ρ differences between bipolar mood state and healthy control groups.
Results
All three mood state groups had increased T1ρ relaxation times in the cerebellum compared to the healthy control group. Additionally, the depressed and manic groups had reduced T1ρ relaxation times in and around the basal ganglia compared to the control and euthymic groups.
Conclusions
This study implicates the cerebellum and basal ganglia in the pathophysiology of bipolar disorder and its mood states, the roles of which are relatively unexplored. These findings motivate further investigation of the underlying cause of the abnormalities, and the potential role of altered metabolic activity in these regions.
Keywords: bipolar disorder, T1rho, MRI, cerebellum, basal ganglia
Introduction
Bipolar I disorder is a debilitating psychiatric illness characterized by severe changes in mood from euthymic to depressed and manic states. The pathophysiology underlying these mood-state transitions remains largely unknown. Most in vivo studies of bipolar disorder using advanced imaging technologies have implicated the frontal and limbic regions of the brain, which are central to emotional processing.1,2 These findings are largely based on anatomical, diffusion, functional, and spectroscopic magnetic resonance imaging (MRI) methods, which point to white matter and functional dysconnectivity between brain regions,3-8 structural deficits,9,10 and altered metabolism.11,12
To further study brain variations in bipolar disorder, we recently employed an MRI technique that is new to psychiatric brain imaging called quantitative mapping of T1 relaxation in the rotating frame (T1ρ).13 Compared to the T1 and T2 relaxation times used in conventional MRI, T1ρ relaxation times are uniquely sensitive to factors affecting the chemical exchange of labile protons between free water and the amide and hydroxyl groups of macromolecules, most notably pH and concentration of exchange sites.14-19 We were initially motivated to study T1ρ in bipolar disorder because pH has been found to be reduced in bipolar disorder in select regions of the brain using MR spectroscopy.20,21 Unlike MR spectroscopy, which has very limited spatial resolution and spatial coverage (typically a single ∼8000 mm3 region of interest), T1ρ relaxation times can be quantified (i.e., mapped) over the entire brain with relatively high spatial resolution (<20 mm3). Therefore, T1ρ mapping provides a relatively efficient pH-sensitive method for identifying and studying regional brain alterations in bipolar disorder and its mood states. The utility of T1ρ mapping was recently demonstrated in our study of participants with bipolar I disorder in the euthymic state.13 We found that T1ρ relaxation times were significantly increased in the cerebellum and cerebral white matter in participants with bipolar disorder compared to a group of control participants without a history of psychiatric illness. These findings may be due to reduced pH (e.g., due to increased anaerobic metabolism) and/or reduced macromolecular concentration (e.g., due to brain atrophy). The cerebellar finding is a potentially important observation, and highlights the advantage of technique, as the cerebellum has received little attention in the illness but may in fact have a significant role in mood regulation.22,23
The purpose of the current study was to investigate whether T1ρ differences in bipolar disorder varied across mood states: euthymia, depression, and mania. Expanding upon our prior study, we generated spatial maps of T1ρ relaxation times throughout the brain in groups of participants with bipolar I disorder in the three mood states. We compared the mood-state groups with healthy controls and each other. Given the sensitivity of T1ρ to temporal changes in pH and macromolecular concentrations that may accompany mood state transitions, we hypothesized that T1ρ mapping would reveal mood-state-dependent brain differences.
Methods
Participants
We recruited and imaged 40 participants with bipolar I disorder, 11 of whom had repeated scans in different mood states, and 29 healthy control participants balanced for age and gender. Our previous study reported imaging data from 15 of the participants with bipolar disorder in the euthymic state and 25 of the healthy control participants.13 These data were pooled with new imaging data from the participants with bipolar disorder as well as new healthy control participants to yield a total of 27 scans in the euthymic state, 12 scans in the depressed state, 13 scans in the manic state, and 29 healthy control scans. Twenty-nine of the participants with bipolar disorder were imaged in one mood state (18 in euthymic, four in depressed, and seven in manic mood states), 10 were imaged in two mood states (five in euthymic and depressed, three in euthymic and manic, and two in depressed and manic mood states), and one was imaged in all three mood states. In total, there were 52 imaging sessions across all mood states and 81 sessions for the entire study including controls. All imaging sessions were conducted between November 2012 and April 2015 using the same hardware, software, and protocol. Clinical diagnoses of participants with bipolar I disorder were confirmed by psychiatric evaluation using DSM-IV-TR criteria. Mood state was assessed using the Montgomery-Asberg Depression Rating Scale (MADRS)24 and the Young Mania Rating Scale (YMRS),25 with depression defined as MADRS ≥ 20, mania defined as YMRS ≥ 20, and euthymia defined as MADRS ≤ 10 and YMRS ≤ 12. Participant psychiatric history and current medications were also recorded. Demographics and medication use of the study population are summarized in Table 1. Written informed consent was obtained from all participants in accordance with a protocol approved by the Institutional Review Board at the University of Iowa.
Table 1.
Participant demographics and medication use.
| Control | Euthymic | Depressed | Manic | |
|---|---|---|---|---|
| N (Male/Female) | 29 (16/13) | 27 (14/13) | 12 (6/6) | 13 (7/6) |
| Age (yr)a | 39 ± 14 | 37 ± 14 | 45 ± 14 | 40 ± 14 |
| MADRSa | - | 4.4 ± 2.6 | 30.1 ± 6.4 | 6.3 ± 3.3 |
| YMRSa | - | 1.6 ± 2.1 | 3.2 ± 3.1 | 27.6 ± 4.2 |
| Lithium (N) | - | 11 (41%) | 6 (50%) | 7 (57%) |
| Anticonvulsants (N) | - | 9 (33%) | 7 (58%) | 4 (36%) |
| Antidepressants (N) | - | 11 (41%) | 6 (50%) | 3 (29%) |
| Antipsychotics (N) | - | 11 (41%) | 6 (50%) | 7 (57%) |
| Sedative Hypnotics (N) | - | 9 (33%) | 9 (75%) | 4 (36%) |
Values shown as mean ± standard deviation
MADRS = Montgomery-Asberg Depression Rating Scale (euthymia ≤ 10, depression ≥ 20)
YMRS = Young Mania Rating Scale (euthymia ≤ 12, mania ≥ 20)
Imaging Protocol
Imaging was performed using a 3T MRI system (Magnetom TIM Trio; Siemens Healthcare; Erlangen, Germany) with a vendor-provided 12-channel head receiver coil. The imaging protocol has previously been described in detail13 and is briefly summarized here. First, high-resolution (1.0 mm isotropic) anatomical T1- and T2-weighted brain images were acquired using coronal 3D MP-RAGE and sagittal 3D SPACE sequences respectively. Second, whole-brain T1ρ-weighted images were acquired using a coronal 3D GRE sequence with 1.7×1.7×5.0 mm3 spatial resolution at two different spin-lock times (10 and 55 ms), enabling calculation of T1ρ relaxation times by fitting the voxel intensities of the T1ρ-weighted images versus spin-lock time using a mono-exponential signal decay model with time constant T1ρ.26 The spin-lock pulse frequency was set to 330 Hz.
Image Analysis
T1ρ maps were processed using AFNI27 and MATLAB (Mathworks; Natick, Massachusetts). For each participant, the two spin-lock images were fit to a mono-exponential decay signal model to quantify T1ρ at each voxel. The T1- and T2-weighted anatomical images were used to calculate a nonlinear transformation to a common atlas space28 using BRAINS AutoWorkup29 and ANTs.30 This participant-specific transformation was then applied to the participant's T1ρ map to align it voxel-wise to the atlas coordinate system. A common mask was applied to the aligned T1ρ maps to include only brain tissue voxels that, when averaged across all 81 datasets, had a mean T1ρ relaxation time in the expected range for brain tissue (i.e. 50-120 ms)31 to minimize the influence of cerebral spinal fluid (CSF) partial volume artifacts. The common atlas includes a set of brain tissue labels, including a cerebellar parcellation with 64 labels,32 which were used to delineate anatomical regions.
Statistical Analysis
We assessed how brain T1ρ relaxation times in each separate mood state group (euthymic, depressed, and manic) differed versus the healthy control group. These analyses were done using separate statistical voxel-wise comparisons of the common-atlas-aligned whole-brain T1ρ maps using linear mixed effects modeling in R33 with participant age and gender included as fixed effects. Whole-brain t-score maps were generated, thresholded at an estimated p-value of 0.05, and corrected for multiple comparisons by thresholding significant voxels that did not belong to a cluster at least 440 mm3 in size using AFNI.27 This cluster size threshold was estimated using AFNI's 3dClustSim function and a Type I error rate (α) of 0.05. For the euthymic versus control group comparison, we also investigated whether the 12 euthymic data sets that were not reported in our prior study revealed findings consistent with the original cohort.13
Using the same voxel-wise analysis procedure, we also assessed how brain T1ρ relaxation times in the depressed and manic mood state groups each differed versus the euthymic group. For these analyses, the linear mixed effects model accounted for the fact that some participants were imaged in more than one mood state by including a random intercept term for each unique participant. Effectively, this gives statistical consideration to the repeated observations from participants imaged in multiple mood states while ensuring investigation of the relationship between T1ρ relaxation times and mood states accounts for baseline differences between individuals.
We performed a similar voxel-wise analysis restricted to the cerebellum to investigate potential effects of lithium use on T1ρ relaxation times. We previously reported that euthymic participants with bipolar disorder using lithium had normalized T1ρ relaxation times in the cerebellum compared to participants with bipolar disorder who were not using lithium.13,34 Here, we compared participants with bipolar disorder who were not actively using lithium at the time of their MRI study to those participants who were using lithium, without regard to mood state. For replication, the analysis was restricted to imaging sessions not included in the original cohort.13 For multiple comparisons correction, the cluster size threshold was reduced to 307 mm3 on account of the smaller size of the cerebellum compared to the whole brain.
Lastly, we calculated cerebral and cerebellar gray matter volumes to investigate whether the participants with bipolar disorder had significant gray matter changes that could account for some T1ρ findings (e.g., due to extensive cellular loss and/or increased CSF). Gray matter volumes were calculated as percentage of intracranial volume using participant-specific segmentations calculated as part of the BRAINS AutoWorkup pipeline.29 The percentages were calculated for all imaging sessions and were statistically compared between participants with bipolar disorder (n=52) versus healthy controls (n=29) using two-tailed t-tests with p<0.05 considered significant.
Results
Mood States vs. Controls
We first compared whole-brain T1ρ maps voxel-wise for the euthymic, depressed, and manic groups versus the healthy control group. Statistical maps highlighting the major regions of difference are shown in Figure 1. These regions are also summarized in Table 2 with cerebellar sub-regions summarized in Table 3. Average group T1ρ relaxation times in atlas-defined brain regions implicated in the voxel-wise analyses are tabulated in Supplemental Table S1.
Figure 1.
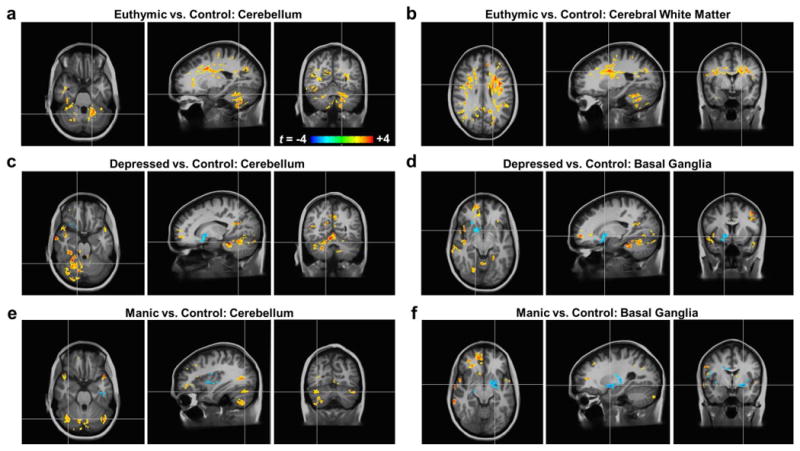
Participants with bipolar disorder have altered mood-state-dependent T1ρ relaxation times in the cerebellum, basal ganglia, and cerebral white matter compared to healthy controls. All brain images show t-score maps of the statistically-significant (p<0.05) voxel-wise differences in T1ρ relaxation times between the (a,b) euthymic, (c,d) depressed, and (e,f) manic groups versus the healthy control group. The statistical maps are overlaid on the average of all N=81 T1-weighted anatomical images in the common atlas space. In the euthymic state, only increased T1ρ relaxation times were found, primarily in the cerebellum (a) and cerebral white matter (b). In the depressed state, increased T1ρ relaxation times were again seen in the cerebellum (c). Additionally, reduced T1ρ relaxation times were found in portions of the right basal ganglia (d). In the manic state, cerebellar T1ρ relaxation times were also increased (e). Similar to the depressed state, regions near the left basal ganglia had reduced T1ρ relaxation times (f).
Table 2.
Regions containing T1ρ differences between participants with bipolar disorder and healthy control participants.
| Group | Euthymic | Depressed | Manic | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Hemisphere | Right | Left | Right | Left | Right | Left |
| Cerebellum | ||||||
| Gray Matter | + | + | + | + | + | + |
| White Matter | + | + | + | + | + | |
|
| ||||||
| Basal Ganglia and Surrounding Tissues | ||||||
| Caudate Nucleus | + | + | ||||
| Putamen | - | |||||
| Nucleus Accumbens | - | |||||
| Globus Pallidus | - | - | ||||
| Amygdala | - | |||||
| Insula | - | - | ||||
| Claustrum | - | - | ||||
| Cerebral White Matter | - | - | ||||
|
| ||||||
| Cerebral White Matter | ||||||
| Excluding Basal Ganglia | + | + | + | + | ||
|
| ||||||
| Cerebral Gray Matter | ||||||
| Anterior Cingulate | + | + | + | |||
| Other Regions | + | + | + | + | +/- | |
+/- = increased/reduced T1ρ relaxation times in the mood state group versus the control group
Table 3.
Cerebellar gray matter regions containing T1ρ differences between participants with bipolar disorder and healthy control participants.
| Group | Euthymic | Depressed | Manic | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||
| Hemisphere | Right | Left | Right | Left | Right | Left | ||||||||||||
|
| ||||||||||||||||||
| Cerebellum (L/V/M) | L | M | V | V | M | L | L | M | V | V | M | L | L | M | V | V | M | L |
| Lobule IV | + | + | ||||||||||||||||
| Lobule V | + | + | + | + | ||||||||||||||
| Lobule VI | + | + | + | |||||||||||||||
| Lobule VIIA Crus I | + | + | + | + | + | |||||||||||||
| Lobule VIIA Crus II | + | + | + | + | + | |||||||||||||
| Lobule VIIB | + | |||||||||||||||||
| Lobule VIIIA | + | + | ||||||||||||||||
| Lobule VIIIB | + | + | + | + | + | |||||||||||||
| Lobule IX | + | + | + | |||||||||||||||
V/M/L = vermal/medial/lateral cerebellar locations; +/- = increased/reduced T1ρ relaxation times in the mood state vs. control group
We found that the euthymic group had clusters of increased T1ρ relaxation times throughout the brain with particularly pronounced clusters in the cerebellum (Figure 1a) and cerebral white matter (Figure 1b). The cerebellum findings included portions of right and left lobules V, VI, VIII, and IX, right lobule VII, and the white matter. Other clusters included portions of the right caudate nucleus and localized regions of the cerebral gray matter including the right anterior cingulate cortex. When separately analyzing the new data sets in the euthymic group from those previously reported,13 clusters of increased T1ρ relaxation times were found in the cerebellum, consistent with the prior findings, but cerebral white matter clusters were less prevalent (see Supplemental Figure S1).
We discovered that the depressed group likewise had increased T1ρ relaxation times in the cerebellum (Figure 1c) but also had reduced times in portions of the right basal ganglia (Figure 1d). The cerebellar clusters included portions of right and left lobules IV and VII, left lobule IX, right lobules V and VI, and the white matter. The right basal ganglia cluster included portions of the nucleus accumbens and globus pallidus. Although the left basal ganglia did not reveal statistically significant differences, it also trended toward reduced T1ρ relaxation times in the depressed group (p=0.055). Additional regions exhibiting increased T1ρ relaxation times included the cerebral white matter (although not to the same extent as the euthymic group) and localized regions of the cerebral gray matter including the right anterior cingulate cortex.
In the manic group, we also found increased T1ρ relaxation times in the cerebellum (Figure 1e) and reduced times near the basal ganglia (Figure 1f). The cerebellar differences were seen in portions of right and left lobule VII, right lobule VIII, and the right white matter. The findings of reduced T1ρ relaxation times in the vicinity of the basal ganglia included portions of the bilateral claustra, insulae, and cerebral white matter and the left putamen, globus pallidus, and amygdala. Localized clusters of increased T1ρ relaxation times were seen in the right caudate nucleus and regions of the cerebral gray matter including the right anterior cingulate cortex.
Depression and Mania vs. Euthymia
To determine if there were any significant mood-state-dependent differences, we compared whole-brain T1ρ maps voxel-wise between (i) the depressed and euthymic groups and (ii) the manic and euthymic groups. The resultant statistical brain maps are shown in Figure 2. Compared to the euthymic participants, the participants in a depressed state had reduced T1ρ relaxation times in the vicinity of the left basal ganglia (putamen and insula) as well as the cerebral white matter and corpus callosum. The depressed group also had increased T1ρ relaxation times in the cerebellum (bilateral vermal lobules IV and V and right medial lobules V and VI), right hippocampus, and left cuneus. The manic versus euthymic group comparison did not reveal any significant clusters; however, it is noteworthy that the largest sub-threshold cluster (317 mm3) consisted of reduced T1ρ relaxation times in portions of the left basal ganglia (putamen and globus pallidus).
Figure 2.
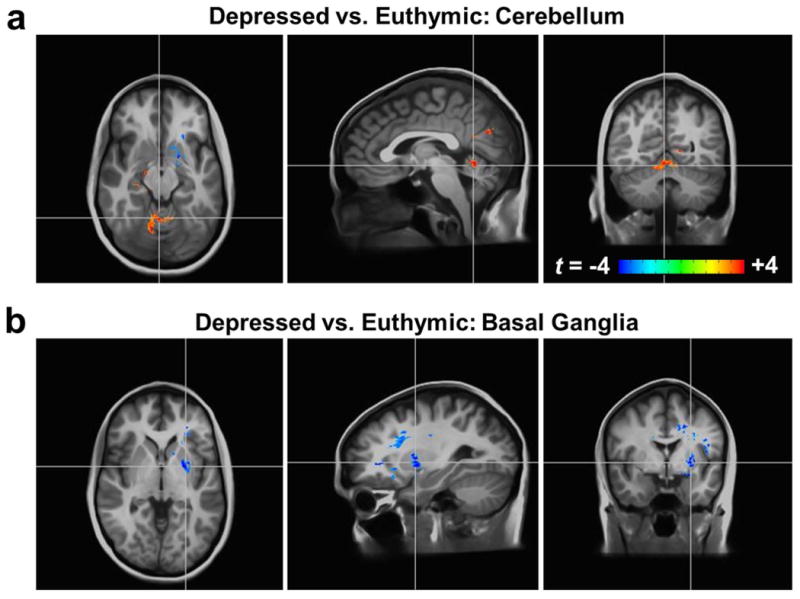
T1ρ relaxation times differ between participants with bipolar disorder in a depressed mood state compared to those in a euthymic state. All brain images show t-score maps overlaid on the average anatomically-aligned T1-weighted images as in Figure 1. The depressed group had (a) increased T1ρ relaxation times in the cerebellum and (b) reduced T1ρ relaxation times in the basal ganglia (p<0.05).
Lithium Effects
We examined the potential effects of lithium in the cerebellum by comparing the T1ρ maps voxel-wise between participants with bipolar disorder not using lithium (N=19) and those using lithium (N=18). No significant clusters were revealed, suggesting that lithium use did not have a normalizing effect on T1ρ when evaluated across mood states.
Gray Matter Volumes
To investigate the possibility that significant gray matter loss contributed to the T1ρ findings, we compared cerebral and cerebellar gray matter volumes between the participants with bipolar disorder and healthy controls. We found that, although there was a trend for reduced gray matter volumes in the participants with bipolar disorder (cerebral gray matter: 32.1±2.6% vs. 33.1±2.1%; cerebellar gray matter: 4.76±0.54% vs. 4.98±4.79%), the differences were not statistically significant (p=0.08 and 0.07, respectively). These findings do not support a significant reduction in gray matter in the participants with bipolar disorder, which suggests that the T1ρ findings in the gray matter are not driven by extensive cellular loss or an associated increase in CSF.
Discussion
This study supports our prior finding of cerebellar alterations in bipolar disorder13 and provides new insight into how mood states may involve the cerebellum and other brain regions such as the basal ganglia. We found abnormalities in the cerebellum for all mood states of bipolar disorder that are consistent with our initial study of participants in the euthymic state only. We also found differences between mood states, particularly in basal ganglia and surrounding tissues in depression and mania, suggesting the involvement of these regions in the manifestation of altered mood states.
Most compellingly, our findings point to cerebellar dysfunction in bipolar disorder, both in emotion and motor processing regions. Cerebellar differences were observed in all three mood states, suggesting that cerebellar alterations may be a trait of the illness. However, the T1ρ signal in the cerebellum also differed between mood states (Table 3), particularly when directly comparing the euthymic and depressed groups (Figure 2a). While cerebellar lobules I-V, VIII, and (to a lesser degree) VI are thought to be involved in motor function, the posterior vermis and lobules VI and VII are most likely involved in emotional regulation.35 Consistent with altered emotional regulation, we observed T1ρ differences in lobule VII of participants with mania and lobules VI and VII of participants in the euthymic and depressed states. In addition, the euthymic and depressed groups had altered T1ρ signal in aspects of lobules IV and/or V, which suggests differences in motor control sites as well and supports previous observations of altered cerebellar activity during motor tasks in bipolar disorder.36-41
One intriguing possibility suggested by our findings is that altered metabolism may interfere with cerebellar function in bipolar disorder. As previously discussed,13 our finding of increased T1ρ relaxation times in the cerebellum is consistent with reduced pH, which may be a consequence of an underlying metabolic variation. To further study potential mood-state-dependent metabolic differences in the cerebellum, it would be interesting to apply MR spectroscopy, which to our knowledge has not been applied to the cerebellum in people with bipolar disorder. However, two MR spectroscopy studies of children at risk for bipolar disorder found evidence of reduced metabolite concentrations (N-acetyl aspartate, creatine, myo-inositol, and choline) in the cerebellar vermis.42,43 A few other studies have found evidence of cerebellar metabolic dysfunction in bipolar disorder including reduced FDG-PET signal in the cerebellar white matter,44 GABAergic dysfunction,45 and altered metabolic associativity between the cerebellum and the prefrontal cortex.46 Although this limited evidence lends support to a cerebellar metabolic dysfunction hypothesis in bipolar disorder, it also emphasizes that much more information is needed to fully understand the pathophysiology of the disorder.
Whereas the observed cerebellar abnormalities may be trait- or state-dependent, our findings in the basal ganglia and surrounding tissues suggest state-dependent changes. We observed differences consistent with higher pH and/or higher macromolecular concentration in the basal ganglia in depressed and manic mood states compared to the euthymic mood state and healthy controls. Several previous observations have linked the basal ganglia to bipolar disorder, including: (i) structural differences such as altered volume and shape;2,47-49 (ii) metabolic alterations including reduced pH in euthymia versus healthy controls21 and increased choline levels in all mood states;47 and (iii) mood-state-dependent functional differences such as response to emotional and reward processing tasks2 and resting-state connectivity to other brain regions involved in emotional regulation.50,51 Furthermore, anatomical studies have shown the existence of connections between the basal ganglia and cerebellum, which suggests an integrated functional network that may, among other functions, influence mood.52,53 Our findings support the view that altered function and/or structure in the basal ganglia and cerebellum combine to play an important role in the etiology or manifestations of different mood states in bipolar disorder.
In addition to cerebellum and basal ganglia differences in bipolar disorder, we also detected increased T1ρ signal in regions of the cortical gray matter in all three mood-state groups compared to the healthy control group, most notably in the right anterior cingulate cortex. The anterior cingulate is thought to have a significant role in bipolar disorder,1,54 and variety of evidence has pointed to metabolic differences in and around this region including reduced pH,11,12,55,56 which is consistent with our findings. However, T1ρ measurements in the cortical gray matter are potentially sensitive to partial volume averaging of CSF, which has a much greater T1ρ relaxation time than brain tissue. Thus, the gray matter findings might be due to a reduction of gray matter volume, and thus increased CSF volume, in the participants with bipolar disorder. This would be consistent with evidence of cortical thinning in bipolar disorder.57 However, our results did not support a marked decrease in gray matter volume in the participants with bipolar disorder compared to the healthy control participants, and the T1ρ data was analyzed in such a way to minimize such partial volume artifacts. Thus, our findings may be driven by abnormal pH and therefore corroborate evidence for reduced pH in the anterior cingulate as a trait of bipolar disorder.
The potential effects of lithium and other medications on the T1ρ differences observed in bipolar disorder remains an important question. Conceivably, quantitative T1ρ mapping may help reveal important therapeutic effects of medications or potentially help identify individuals who may respond to a given therapy. We previously reported that lithium normalized T1ρ relaxation times in the cerebellum of participants with bipolar disorder in the euthymic mood state.13,34 In the present study, we considered the effect of lithium across all new participants with bipolar disorder, regardless of mood state, given the evidence that the cerebellar alterations appear to be a trait of the illness. However, in this new cohort, the lithium effect was not apparent. Additional studies with larger sample sizes and/or prospective study designs will be required to more clearly discern effects of individual or mixed medications, duration of use, dose, compliance, and mood state.
The primary limitation of this study is its relatively small sample sizes. Additional samples are needed to verify the findings by reducing the Type I error rate. The mostly cross-sectional design of this study also presents a limitation. Although the number of participants was adequate to identify major differences between the groups, small regions of difference and potential sub-groups within the population could not be reliably identified. Ideally, a longitudinal study design with more repeated measures would be better able to detect how T1ρ relaxation times change in the brain with mood, and a larger sample could facilitate sub-group comparisons. Additionally, whereas this study limited recruitment to discrete mood state groups, a future study could recruit participants across the spectrum of mood symptoms, which would enable study of how T1ρ relaxation times correlate with symptom severity.
The applied quantitative T1ρ mapping technique also has limitations that may be addressed in future studies. First, in this study, spatial resolution was limited to achieve adequate signal-to-noise ratio (SNR) in a short scan time. Application of more advanced 3D T1ρ mapping sequences can potentially enable higher spatial resolution by providing greater SNR per unit scan time. Longer exam times may also be used to improve spatial resolution and/or SNR, but this will increase the likelihood of problematic participant motion, particularly in people in a manic mood state. Second, there are confounding factors not related to chemical exchange that can influence T1ρ relaxation times. CSF partial volume averaging can cause increased T1ρ signal and may be increased in regions of brain tissue loss. Although there have been some reports of reduced cerebellar volume in people with bipolar disorder,58-60 results have been mixed.61,62 The analyses of cerebellar gray matter volume in this study and our prior report13 further suggest that any volume change is small in our cohort and unlikely to be the dominant driver of our findings. However, we cannot completely eliminate CSF contamination as a potential confound in our study. In future studies, an inversion recovery technique can be used to remove confounding signal from CSF, although this will require a longer scan time.31 Blood volume is another potential confound, although our findings of increased T1ρ signal in the cerebellum is inconsistent with prior reports of decreased blood volume in bipolar disorder.63,64 Additionally, neuro-inflammation, which has been identified in bipolar disorder and may be mood-state dependent,65-67 has been conjectured to potentially influence T1ρ relaxation times,13,68 but there is not yet evidence of this.13,69 It is of note that there are a number of variants of T1ρ mapping such as dispersion imaging and adiabatic T1ρ and RAFF mapping,16,70,71 as well as complementary techniques such as T2 mapping, that may provide increased sensitivity and/or specificity to particular disease processes in the future. However, further study is needed to identify the specific brain pathologies to which T1ρ and its variants are sensitive and how these relate to observed T1ρ abnormalities in psychiatric disorders.
In conclusion, this study adds to the growing evidence for the cerebellum and basal ganglia as areas of interest in the pathophysiology of bipolar disorder and its mood states and motivates further investigation of the underlying causes of the observed abnormalities. Targeted study of these potentially under-appreciated brain regions in bipolar disorder may lead to new insights into the disease mechanisms and identification of new biomarkers and therapeutic targets.
Supplementary Material
Acknowledgments
We thank Autumn Craig, Robin Follmer, Marla Kleingartner, Janie Myers, Ashley Schumacher, and Lois Warren for their assistance with participant recruitment and data collection. This work was supported in part by a gift from Roger Koch through the UI Foundation, NARSAD Young and Independent Investigator Awards from the Brain & Behavior Research Foundation (C.P.J. and V.A.M, respectively), NIMH (R01MH111578), and the University of Iowa Institute for Clinical and Translational Science (U54TR001013). Additionally, J.A.W. was supported by the Carver Foundation, Department of Veterans Affairs (Merit Award), NIMH (R01MH085724), NIDA (R01DA037216), NHLBI (R01HL113863), and a NARSAD Independent Investigator Award and J.G.F. by the NIMH (K23MH083695) and NHLBI (P01HL014388).
References
- 1.Strakowski SM, Adler CM, Almeida J, Altshuler LL, Blumberg HP, Chang KD, DelBello MP, Frangou S, McIntosh A, Phillips ML, Sussman JE, Townsend JD. The functional neuroanatomy of bipolar disorder: a consensus model. Bipolar Disord. 2012;14:313–325. doi: 10.1111/j.1399-5618.2012.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phillips ML, Swartz HA. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am J Psychiatry. 2014;171:829–843. doi: 10.1176/appi.ajp.2014.13081008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brambilla P, Bellani M, Yeh PH, Soares JC, Tansella M. White matter connectivity in bipolar disorder. Int Rev Psychiatry. 2009;21:380–386. doi: 10.1080/09540260902962172. [DOI] [PubMed] [Google Scholar]
- 4.Pan L, Keener MT, Hassel S, Phillips ML. Functional neuroimaging studies of bipolar disorder: examining the wide clinical spectrum in the search for disease endophenotypes. Int Rev Psychiatry. 2009;21:368–379. doi: 10.1080/09540260902962164. [DOI] [PubMed] [Google Scholar]
- 5.Heng S, Song AW, Sim K. White matter abnormalities in bipolar disorder: insights from diffusion tensor imaging studies. J Neural Transm. 2010;117:639–654. doi: 10.1007/s00702-010-0368-9. [DOI] [PubMed] [Google Scholar]
- 6.Vederine FE, Wessa M, Leboyer M, Houenou J. A meta-analysis of whole-brain diffusion tensor imaging studies in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1820–1826. doi: 10.1016/j.pnpbp.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Vargas C, Lopez-Jaramillo C, Vieta E. A systematic literature review of resting state network--functional MRI in bipolar disorder. J Affect Disord. 2013;150:727–735. doi: 10.1016/j.jad.2013.05.083. [DOI] [PubMed] [Google Scholar]
- 8.Marlinge E, Bellivier F, Houenou J. White matter alterations in bipolar disorder: potential for drug discovery and development. Bipolar Disord. 2014;16:97–112. doi: 10.1111/bdi.12135. [DOI] [PubMed] [Google Scholar]
- 9.Emsell L, McDonald C. The structural neuroimaging of bipolar disorder. Int Rev Psychiatry. 2009;21:297–313. doi: 10.1080/09540260902962081. [DOI] [PubMed] [Google Scholar]
- 10.Schneider MR, DelBello MP, McNamara RK, Strakowski SM, Adler CM. Neuroprogression in bipolar disorder. Bipolar Disord. 2012;14:356–374. doi: 10.1111/j.1399-5618.2012.01024.x. [DOI] [PubMed] [Google Scholar]
- 11.Kato T, Kato N. Mitochondrial dysfunction in bipolar disorder. Bipolar Disord. 2000;2:180–190. doi: 10.1034/j.1399-5618.2000.020305.x. [DOI] [PubMed] [Google Scholar]
- 12.Stork C, Renshaw PF. Mitochondrial dysfunction in bipolar disorder: evidence from magnetic resonance spectroscopy research. Mol Psychiatry. 2005;10:900–919. doi: 10.1038/sj.mp.4001711. [DOI] [PubMed] [Google Scholar]
- 13.Johnson CP, Follmer RL, Oguz I, Warren LA, Christensen GE, Fiedorowicz JG, Magnotta VA, Wemmie JA. Brain abnormalities in bipolar disorder detected by quantitative T1rho mapping. Mol Psychiatry. 2015;20:201–206. doi: 10.1038/mp.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kettunen MI, Grohn OH, Silvennoinen MJ, Penttonen M, Kauppinen RA. Effects of intracellular pH, blood, and tissue oxygen tension on T1rho relaxation in rat brain. Magn Reson Med. 2002;48:470–477. doi: 10.1002/mrm.10233. [DOI] [PubMed] [Google Scholar]
- 15.Michaeli S, Burns TC, Kudishevich E, Harel N, Hanson T, Sorce DJ, Garwood M, Low WC. Detection of neuronal loss using T1rho MRI assessment of 1H2O spin dynamics in the aphakia mouse. J Neurosci Methods. 2009;177:160–167. doi: 10.1016/j.jneumeth.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin T, Autio J, Obata T, Kim SG. Spin-locking versus chemical exchange saturation transfer MRI for investigating chemical exchange process between water and labile metabolite protons. Magn Reson Med. 2011;65:1448–1460. doi: 10.1002/mrm.22721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magnotta VA, Heo HY, Dlouhy BJ, Dahdaleh NS, Follmer RL, Thedens DR, Welsh MJ, Wemmie JA. Detecting activity-evoked pH changes in human brain. Proc Natl Acad Sci USA. 2012;109:8270–8273. doi: 10.1073/pnas.1205902109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin T, Mehrens H, Hendrich KS, Kim SG. Mapping brain glucose uptake with chemical exchange-sensitive spin-lock magnetic resonance imaging. J Cereb Blood Flow Metab. 2014;34:1402–1410. doi: 10.1038/jcbfm.2014.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zu Z, Spear J, Li H, Xu J, Gore JC. Measurement of regional cerebral glucose uptake by magnetic resonance spin-lock imaging. Magn Reson Imaging. 2014;32:1078–1084. doi: 10.1016/j.mri.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato T, Murashita J, Kamiya A, Shioiri T, Kato N, Inubushi T. Decreased brain intracellular pH measured by 31P-MRS in bipolar disorder: a confirmation in drug-free patients and correlation with white matter hyperintensity. Eur Arch Psychiatry Clin Neurosci. 1998;248:301–306. doi: 10.1007/s004060050054. [DOI] [PubMed] [Google Scholar]
- 21.Hamakawa H, Murashita J, Yamada N, Inubushi T, Kato N, Kato T. Reduced intracellular pH in the basal ganglia and whole brain measured by 31P-MRS in bipolar disorder. Psychiatry Clin Neurosci. 2004;58:82–88. doi: 10.1111/j.1440-1819.2004.01197.x. [DOI] [PubMed] [Google Scholar]
- 22.Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biol Psychiatry. 2008;64:81–88. doi: 10.1016/j.biopsych.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoppenbrouwers SS, Schutter DJ, Fitzgerald PB, Chen R, Daskalakis ZJ. The role of the cerebellum in the pathophysiology and treatment of neuropsychiatric disorders: a review. Brain Res Rev. 2008;59:185–200. doi: 10.1016/j.brainresrev.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 25.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 26.Johnson CP, Thedens DR, Magnotta VA. Precision-guided sampling schedules for efficient T1rho mapping. J Magn Reson Imaging. 2015;41:242–250. doi: 10.1002/jmri.24518. [DOI] [PubMed] [Google Scholar]
- 27.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 28.Halle M, Talos IF, Jakab M, Makris N, Meier D, Wald L, Fischl B, Kikinis R. Multi-modality MRI-based atlas of the brain. SPL; Boston, MA: 2013. URL http://www.spl.harvard.edu/publications/item/view/2037. [Google Scholar]
- 29.Pierson R, Johnson H, Harris G, Keefe H, Paulsen JS, Andreasen NC, Magnotta VA. Fully automated analysis using BRAINS: AutoWorkup. Neuroimage. 2011;54:328–336. doi: 10.1016/j.neuroimage.2010.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54:2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watts R, Andrews T, Hipko S, Gonyea JV, Filippi CG. In vivo whole-brain T1-rho mapping across adulthood: normative values and age dependence. J Magn Reson Imaging. 2014;40:376–382. doi: 10.1002/jmri.24358. [DOI] [PubMed] [Google Scholar]
- 32.Makris N, Schlerf JE, Hodge SM, Haselgrove C, Albaugh MD, Seidman LJ, Rauch SL, Harris G, Biederman J, Caviness VS, Jr, Kennedy DN, Schmahmann JD. MRI-based surface-assisted parcellation of human cerebellar cortex: an anatomically specified method with estimate of reliability. Neuroimage. 2005;25:1146–1160. doi: 10.1016/j.neuroimage.2004.12.056. [DOI] [PubMed] [Google Scholar]
- 33.Pinheiro JBD, DebRoy S, Sarkar D, R Core Team nlme: linear and nonlinear mixed effects models. R package version 3. 2015:1–120. URL: http://CRAN.R-project.org/package=nlme.
- 34.Johnson CP, Follmer RL, Oguz I, Warren LA, Christensen GE, Fiedorowicz JG, Magnotta VA, Wemmie JA. Quantitative T1rho mapping links the cerebellum and lithium use in bipolar disorder. Mol Psychiatry. 2015;20:149. doi: 10.1038/mp.2015.10. [DOI] [PubMed] [Google Scholar]
- 35.Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46:831–844. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bolbecker AR, Mehta C, Johannesen JK, Edwards CR, O’Donnell BF, Shekhar A, Nurnberger JI, Steinmetz JE, Hetrick WP. Eyeblink conditioning anomalies in bipolar disorder suggest cerebellar dysfunction. Bipolar Disord. 2009;11:19–32. doi: 10.1111/j.1399-5618.2008.00642.x. [DOI] [PubMed] [Google Scholar]
- 37.Bolbecker AR, Hong SL, Kent JS, Forsyth JK, Klaunig MJ, Lazar EK, O’Donnell BF, Hetrick WP. Paced finger-tapping abnormalities in bipolar disorder indicate timing dysfunction. Bipolar Disord. 2011;13:99–110. doi: 10.1111/j.1399-5618.2011.00895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bolbecker AR, Hong SL, Kent JS, Klaunig MJ, O’Donnell BF, Hetrick WP. Postural control in bipolar disorder: increased sway area and decreased dynamical complexity. PLoS One. 2011;6:e19824. doi: 10.1371/journal.pone.0019824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin LF, Olincy A, Ross RG, Du YP, Singel D, Shatti S, Tregellas JR. Cerebellar hyperactivity during smooth pursuit eye movements in bipolar disorder. J Psychiatr Res. 2011;45:670–677. doi: 10.1016/j.jpsychires.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 40.Bolbecker AR, Westfall DR, Howell JM, Lackner RJ, Carroll CA, O’Donnell BF, Hetrick WP. Increased timing variability in schizophrenia and bipolar disorder. PLoS One. 2014;9:e97964. doi: 10.1371/journal.pone.0097964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chrobak AA, Siuda-Krzywicka K, Siwek GP, Arciszewska A, Siwek M, Starowicz-Filip A, Dudek D. Implicit motor learning in bipolar disorder. J Affect Disord. 2015;174:250–256. doi: 10.1016/j.jad.2014.11.043. [DOI] [PubMed] [Google Scholar]
- 42.Cecil KM, DelBello MP, Sellars MC, Strakowski SM. Proton magnetic resonance spectroscopy of the frontal lobe and cerebellar vermis in children with a mood disorder and a familial risk for bipolar disorders. J Child Adolesc Psychopharmacol. 2003;13:545–555. doi: 10.1089/104454603322724931. [DOI] [PubMed] [Google Scholar]
- 43.Singh MK, Spielman D, Libby A, Adams E, Acquaye T, Howe M, Kelley R, Reiss A, Chang KD. Neurochemical deficits in the cerebellar vermis in child offspring of parents with bipolar disorder. Bipolar Disord. 2011;13:189–197. doi: 10.1111/j.1399-5618.2011.00902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Altamura AC, Bertoldo A, Marotta G, Paoli RA, Caletti E, Dragogna F, Buoli M, Baglivo V, Mauri MC, Brambilla P. White matter metabolism differentiates schizophrenia and bipolar disorder: a preliminary PET study. Psychiatry Res. 2013;214:410–414. doi: 10.1016/j.pscychresns.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 45.Fatemi SH, Folsom TD, Rooney RJ, Thuras PD. Expression of GABAA alpha2-, beta1- and epsilon-receptors are altered significantly in the lateral cerebellum of subjects with schizophrenia, major depression and bipolar disorder. Transl Psychiatry. 2013;3:e303. doi: 10.1038/tp.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benson BE, Willis MW, Ketter TA, Speer A, Kimbrell TA, George MS, Herscovitch P, Post RM. Interregional cerebral metabolic associativity during a continuous performance task (Part II) : differential alterations in bipolar and unipolar disorders. Psychiatry Res. 2008;164:30–47. doi: 10.1016/j.pscychresns.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 47.Strakowski SM, Delbello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005;10:105–116. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- 48.Hajek T, Carrey N, Alda M. Neuroanatomical abnormalities as risk factors for bipolar disorder. Bipolar Disord. 2005;7:393–403. doi: 10.1111/j.1399-5618.2005.00238.x. [DOI] [PubMed] [Google Scholar]
- 49.Hwang J, Lyoo IK, Dager SR, Friedman SD, Oh JS, Lee JY, Kim SJ, Dunner DL, Renshaw PF. Basal ganglia shape alterations in bipolar disorder. Am J Psychiatry. 2006;163:276–285. doi: 10.1176/appi.ajp.163.2.276. [DOI] [PubMed] [Google Scholar]
- 50.Liu CH, Li F, Li SF, Wang YJ, Tie CL, Wu HY, Zhou Z, Zhang D, Dong J, Yang Z, Wang CY. Abnormal baseline brain activity in bipolar depression: a resting state functional magnetic resonance imaging study. Psychiatry Res. 2012;203:175–179. doi: 10.1016/j.pscychresns.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 51.Altinay MI, Hulvershorn LA, Karne H, Beall EB, Anand A. Differential resting-state functional connectivity of striatal subregions in bipolar depression and hypomania. Brain Connect. 2016;6:255–265. doi: 10.1089/brain.2015.0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoshi E, Tremblay L, Feger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nat Neurosci. 2005;8:1491–1493. doi: 10.1038/nn1544. [DOI] [PubMed] [Google Scholar]
- 53.Bostan AC, Dum RP, Strick PL. The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci U S A. 2010;107:8452–8456. doi: 10.1073/pnas.1000496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fountoulakis KN, Giannakopoulos P, Kovari E, Bouras C. Assessing the role of cingulate cortex in bipolar disorder: neuropathological, structural and functional imaging data. Brain Res Rev. 2008;59:9–21. doi: 10.1016/j.brainresrev.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 55.Weber WA, Dudley J, Lee JH, Strakowski SM, Adler CM, DelBello MP. A pilot study of alterations in high energy phosphoryl compounds and intracellular pH in unmedicated adolescents with bipolar disorder. J Affect Disord. 2013;150:1109–1113. doi: 10.1016/j.jad.2013.04.047. [DOI] [PubMed] [Google Scholar]
- 56.Chu WJ, Delbello MP, Jarvis KB, Norris MM, Kim MJ, Weber W, Lee JH, Strakowski SM, Adler CM. Magnetic resonance spectroscopy imaging of lactate in patients with bipolar disorder. Psychiatry Res. 2013;213:230–234. doi: 10.1016/j.pscychresns.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 57.Hanford LC, Nazarov A, Hall GB, Sassi RB. Cortical thickness in bipolar disorder: a systematic review. Bipolar Disord. 2016;18:4–18. doi: 10.1111/bdi.12362. [DOI] [PubMed] [Google Scholar]
- 58.Moorhead TW, McKirdy J, Sussmann JE, Hall J, Lawrie SM, Johnstone EC, McIntosh AM. Progressive gray matter loss in patients with bipolar disorder. Biol Psychiatry. 2007;62:894–900. doi: 10.1016/j.biopsych.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 59.Baldacara L, Nery-Fernandes F, Rocha M, Quarantini LC, Rocha GG, Guimaraes JL, Araujo C, Oliveira I, Miranda-Scippa A, Jackowski A. Is cerebellar volume related to bipolar disorder? J Affect Disord. 2011;135:305–309. doi: 10.1016/j.jad.2011.06.059. [DOI] [PubMed] [Google Scholar]
- 60.Eker C, Simsek F, Yilmazer EE, Kitis O, Cinar C, Eker OD, Coburn K, Gonul AS. Brain regions associated with risk and resistance for bipolar I disorder: a voxel-based MRI study of patients with bipolar disorder and their healthy siblings. Bipolar Disord. 2014;16:249–261. doi: 10.1111/bdi.12181. [DOI] [PubMed] [Google Scholar]
- 61.Adler CM, DelBello MP, Jarvis K, Levine A, Adams J, Strakowski SM. Voxel-based study of structural changes in first-episode patients with bipolar disorder. Biol Psychiatry. 2007;61:776–781. doi: 10.1016/j.biopsych.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 62.Laidi C, d’Albis MA, Wessa M, Linke J, Phillips ML, Delavest M, Bellivier F, Versace A, Almeida J, Sarrazin S, Poupon C, Le Dudal K, Daban C, Hamdani N, Leboyer M, Houenou J. Cerebellar volume in schizophrenia and bipolar I disorder with and without psychotic features. Acta Psychiatr Scand. 2015;131:223–233. doi: 10.1111/acps.12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Loeber RT, Sherwood AR, Renshaw PF, Cohen BM, Yurgelun-Todd DA. Differences in cerebellar blood volume in schizophrenia and bipolar disorder. Schizophr Res. 1999;37:81–89. doi: 10.1016/s0920-9964(98)00137-6. [DOI] [PubMed] [Google Scholar]
- 64.Loeber RT, Gruber SA, Cohen BM, Renshaw PF, Sherwood AR, Yurgelun-Todd DA. Cerebellar blood volume in bipolar patients correlates with medication. Biol Psychiatry. 2002;51:370–376. doi: 10.1016/s0006-3223(01)01281-1. [DOI] [PubMed] [Google Scholar]
- 65.Berk M, Kapczinski F, Andreazza AC, Dean OM, Giorlando F, Maes M, Yucel M, Gama CS, Dodd S, Dean B, Magalhaes PV, Amminger P, McGorry P, Malhi GS. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev. 2011;35:804–817. doi: 10.1016/j.neubiorev.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 66.Konradi C, Sillivan SE, Clay HB. Mitochondria, oligodendrocytes and inflammation in bipolar disorder: evidence from transcriptome studies points to intriguing parallels with multiple sclerosis. Neurobiol Dis. 2012;45:37–47. doi: 10.1016/j.nbd.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sayana P, Colpo GD, Simoes LR, Giridharan VV, Teixeira AL, Quevedo J, Barichello T. A systematic review of evidence for the role of inflammatory biomarkers in bipolar patients. J Psychiatr Res. 2017;92:160–182. doi: 10.1016/j.jpsychires.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 68.Mangia S, Carpenter AF, Tyan AE, Eberly LE, Garwood M, Michaeli S. Magnetization transfer and adiabatic T1rho MRI reveal abnormalities in normal-appearing white matter of subjects with multiple sclerosis. Mult Scler. 2014;20:1066–1073. doi: 10.1177/1352458513515084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fiedorowicz JG, Prossin AR, Johnson CP, Christensen GE, Magnotta VA, Wemmie JA. Peripheral inflammation during abnormal mood states in bipolar I disorder. J Affect Disord. 2015;187:172–178. doi: 10.1016/j.jad.2015.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mangia S, Liimatainen T, Garwood M, Michaeli S. Rotating frame relaxation during adiabatic pulses vs. conventional spin lock: simulations and experimental results at 4 T. Magn Reson Imaging. 2009;27:1074–1087. doi: 10.1016/j.mri.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liimatainen T, Mangia S, Ling W, Ellermann J, Sorce DJ, Garwood M, Michaeli S. Relaxation dispersion in MRI induced by fictitious magnetic fields. J Magn Reson. 2011;209:269–276. doi: 10.1016/j.jmr.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


