Abstract
Primary cells cultured in vitro gradually lose features characteristic of the in vivo phenotype. Culture techniques that help maintain cell-specific phenotype are advantageous for development of tissue engineered and bioartificial organs. Here we evaluated the phenotype of primary human renal tubule epithelial cells subjected to fluid shear stress by culturing the cells on an orbital shaker. Transepithelial electrical resistance, cell density, and gene and protein expression of proximal tubule specific functional markers were measured in cells subjected to orbital shear stress. Cells cultured on an orbital shaker had increased transepithelial electrical resistance, higher cell density, and enhanced tubular epithelial specific gene and protein expression. This is likely due at least in part to the mechanical stress applied to the apical surface of the cells, although other factors including increased nutrient and oxygen delivery and improved mixing could also play a role. These results suggest that orbital shaker culture may be a simple approach to augmenting the differentiated phenotype of cultured renal epithelial cells.
Keywords: shear stress, bioartificial kidney, epithelial cell, transepithelial electrical resistance, cell differentiation
Introduction
End stage renal disease (ESRD) affects more than 650,000 individuals in the United States, and its prevalence continues to rise.1 ESRD is a unique case of end stage organ failure for which a treatment option (hemodialysis) is available to substantially extend life. Even so, individuals on maintenance hemodialysis face significantly higher mortality risks as well as major burdens to quality of life associated with treatment. Kidney transplantation is the most effective option for treatment of ESRD, but a lack of a sufficient number of donor organs limits its application to only a fortunate few patients with kidney failure.
Bioartificial and tissue engineered organs have the potential to overcome the shortage of donor organs for kidney disease and a number of other end stage organ failures. One of the promising strategies for development of tissue engineered or bioartificial organs involves removing primary cells or stem cells from a patient or donor and propagating those cells in vitro for use in a natural or synthetic construct.2,3 In order to successfully implement this strategy and provide sufficient tissue replacement or augmentation, propagation of large populations of well-differentiated and functional cells are needed.
Primary cells cultured under standard in vitro culture conditions inevitably lose characteristics of their in vivo phenotype. This is a result of a range of insults generally termed cell culture stress.4 These can include, but are not limited to, altered growth substrate (plastic dish), oxidative stress, altered biochemical microenvironment, and loss of paracrine signaling.5,6 Significant effort has focused on exogenous application of soluble factors such as hormones and growth factors to promote propagation or induce differentiation of primary cells or stem cells. Additional biophysical properties of the cell microenvironment, including application of apical shear stress, also affect cell phenotype, and may provide an additional route to modulate differentiation of primary cultures of kidney cells for tissue engineered and bioartificial organs.
Biophysical forces have been used to improve cell functionality for several tissue engineering applications. In bone, mechanical loading results in fluid motion though the porous bone structure resulting in fluid shear stress that is sensed by osteoblasts.7 Perfusion bioreactors that partially recapitulate this shear stress have been shown to increase mineralized matrix deposition and enhance osteoblastic differentiation in bone cells.8,9 The improvement in cell function has been attributed to the application of shear stress combined with improved nutrient transport. Similarly, shear stress is an important consideration for vascular tissue engineering given the important role of shear stress in regulating endothelial cell phenotype.10,11
Renal tubular epithelial cells are subjected to consistent flow of glomerular filtrate resulting in application of shear stress at the apical cell surface. We have estimated shear stress in the proximal tubule to be in the range of 0.5–5 dyn/cm2 based on previous studies of tubular flow rates and geometries in rodents.12 As such, we have targeted shear stresses of 1–2 dyn/cm2 in our bioartificial constructs in an attempt to recapitulate normal in vivo physiological conditions. Application of physiological levels of apical shear stress in vitro using laminar microfluidic flow systems alters tight junction organization,13 induces actin cytoskeletal remodeling,12–14 increases apical protein uptake,15,16 and induces transporter trafficking to the apical membrane17,18 in renal tubular epithelial cells. Cell culture on a rocker table has also been shown to alter renal tubular epithelial cell phenotype. Atul et al. cultured renal tubular epithelial cells on a rocker table and showed that cells exhibit a more differentiated phenotype with increased dome formation (a marker for active sodium and water transport), increased glucose uptake, and increased pH sensitive ammonia production.19 This was attributed to increased oxygenation of the cells. However, the authors note that additional causes, including biophysical factors, may have played a role in altering phenotype under these conditions. Renal collecting duct epithelial cells cultured under orbital shear stress (OSS) stimulated cilia-mediated mechanosensation, altered sodium currents, and induced actin remodeling similar to that observed in cells cultured in laminar flow systems.19,20 These observed changes in renal tubular epithelial cells suggest that application of apical shear stress alters their differentiated phenotype and may improve the functional capacity of the cells for use in bioartificial or tissue engineered renal replacement devices.
While microfluidic laminar flow systems provide a high degree of flow control and have been useful tools for elucidating the biological significance of fluid shear stress in regulating cell function, scaling these systems to large cell populations presents significant challenges. While orbital shaker culture does not provide the uniform shear stress of a laminar flow system20, this approach has been commonly used to apply shear stress to endothelial cells21–23 and is easily scalable to the large cell populations that would be required to provide adequate replacement of tissue function in tissue engineered/bioartificial constructs.
The goal of this work was to evaluate how application of OSS affects barrier function and differentiation of primary renal tubular epithelial cells. We show that OSS increases transepithelial electrical resistance of the cell monolayer, leads to increased cell density, alters gene expression for tubular epithelial specific markers, and increases protein expression of the apical brush border enzyme gamma-glutamyl transpeptidase (γ-GT). These results suggest that improved cell culture platforms, including culturing cells on an orbital shaker, may be useful for propagating large populations of more differentiated cells for use in tissue engineered and bioartifical organs.
Methods
Cell Culture
Primary human renal tubular epithelial cells were obtained from Innovative Biotherapies, Inc. (Ann Arbor, MI). Cells were maintained in a 1:1 ratio of glucose-free DMEM (Invitrogen, Carlsbad, CA) and F12 (Invitrogen) media for a final glucose concentration of 5 mM. Media was supplemented with 1 mL/L insulin, transferrin, ethanolamine, and selenium (ITES) (Lonza, Basel, Switzerland), 0.7 μg/L triiodothyronine (T3) (Sigma-Aldrich, St. Louis, MO), 50 μg/L epidermal growth factor (EGF) (Peprotech, Rock Hill, NJ) and 10 mL/L penicillin/streptomycin (Invitrogen).24,25
For cell culture experiments, cells were used no later than the second passage following isolation. At final plating, retinoic acid was added to the media at 30 μg/L and EGF was no longer added to the media. Cells were plated on 12 mm Transwell inserts (Corning 3401, Corning, NY) at 5×105 cells/cm2. Cells were cultured under static conditions overnight to facilitate cell attachment. Cells were then moved to an orbital shaker. The frequency needed to achieve a shear stress (τmax) of 2 dyn/cm2 was calculated according to equation 1.21
| (1) |
where a is the radius of rotation of the orbital shaker (0.95 cm), ρ is the density of the cell culture media (1 g/ml), η is the viscosity of the media (9.6×10−4 Pa-sec), and f is the frequency of the orbital shaker. Based on these parameters, a frequency of rotation of 72 rpm is needed to achieve a shear stress of 2 dyn/cm2.
Cell Attachment
To determine if OSS resulted in removal of cells from the culture membranes, cell attachment was evaluated after 3 hours of OSS exposure by fixing the cells in 4% paraformaldehyde for 20 minutes on ice and mounting the membranes in mounting media with DAPI (Vectashield) (Vector Laboratories, Burlingame, CA). Cells grown on membranes with and without OSS exposure were counted, and counts were normalized to area to calculate the cell density.
Cell Viability
To evaluate if OSS induced any significant cell death, viability was evaluated by fluorescence imaging using the LIVE/DEAD cell viability assay kit (L3224, Fisher Scientific, Waltham, MA). Live cells were stained with calcein and dead cells with ethidium homodimer-1. The ratio of live cell to dead cell area measured using ImageJ was used to quantify cell viability in static and OSS exposed cells following 6 hours of exposure and after chronic exposure (≥ 8 days).
N-Acetyl-β-glucosaminidase (NAG) Activity
To determine if OSS exposure resulted in any significant cell damage, NAG activity, a common biomarker of proximal tubule damage,26 was measured using a colorimetric NAG activity assay (ab204705, abcam, Cambridge, UK). Cellular NAG activity was evaluated in cell lysates and normalized to total protein measured by Bradford assay (Bio-Rad Laboratories, Hercules, Ca). Acute effects on cellular NAG activity were evaluated after 6 hours of OSS exposure and chronic effects were evaluated after cells were fully differentiated and confluent (≥ 8 days). To evaluate media NAG activity, culture media from the apical compartment was removed and NAG activity was measured and normalized to apical media volume. Media NAG activity was measured following acute OSS exposure, prior to cell confluence (2 days) and after cells were fully confluent and differentiated.
Immunostaining
To evaluate differentiated phenotype, cells were stained by immunofluorescence for zonula occludens-1 (ZO-1) to visualize cell-cell junctions and acetylated α-tubulin to evaluate primary cilia formation. Cells were fixed in 4% paraformaldehyde on ice for 20 minutes. Cells were washed 3x with PBS, permeabilized with 0.1% Triton-X 100 for 10 minutes, and blocked for 30 minutes in 5% goat serum. Cells were incubated in rabbit anti-ZO-1 (1:200) (Invitrogen 40-2200) and mouse anti-acetylated α-tubulin (1:200) (Invitrogen 32-2700) antibodies in blocking buffer for 1 hour at room temperature. Samples were washed 3x with PBS and incubated with Alexa Fluor 488 goat anti-rabbit and Alexa Fluor 555 goat anti-mouse IgG for 30 minutes at room temperature. Transwell membranes were cut from the plastic frame with a scalpel and mounted with DAPI. Cells were imaged on an epifluorescence microscope at 63x magnification. To evaluate cell density, DAPI images were acquired at 40x magnification from four different locations at the periphery of the insert, where OSS is maximal. Cell nuclei were counted manually in ImageJ. Counts were normalized to the area of each image.
Transepithelial Electrical Resistance
Transepithelial electrical resistance (TEER) was measured daily using an EVOM2 meter (World Precision Instruments, Sarasota, FL). TEER of each membrane was measured prior to cell seeding and this value was subtracted from the subsequent measurements to obtain the resistance of the cell layer. All measurements were normalized to the area of the cell culture insert to give final units of Ω-cm2.
q-RT PCR
Gene expression of proximal tubule specific genes γ-GT, NHE3 and 1α hydroxylase were measured by quantitative real-time polymerase chain reaction (q-RT PCR). Total RNA was isolated using the Micro RNeasy kit (Qiagen, Hilden, Germany). RNA quality was determined by measuring absorbance at 260 nm and 280 nm on a Nanodrop Spectrometer. Complementary DNA was synthesized from RNA by reverse transcription. Forward and reverse primers for γ-GT (Hs00980756_m1), NHE3 (Hs00903842_m1) and 1α hydroxylase (Hs01096154_m1) were obtained from Invitrogen. Expression levels were normalized to GAPDH.
Western Blotting
Cells were collected in lysis buffer, sonicated, and centrifuged for 10 min. at 10,000g. Supernatants were collected and protein was quantified using either Bradford (Bio-Rad) or BCA (Fisher Scientific) assays. Equal amounts of protein were separated on SDS-PAGE gels and transferred to PVDF membranes. Samples were blocked in 5% milk and probed with mouse anti-γGT antibody (Fisher Scientific, MA5-11815) overnight at 4 °C. Membranes were washed 3x in Tris-buffered saline with 0.3% Tween 20 (TBST) and incubated in goat anti-mouse HRP secondary antibody (Fisher Scientific, 32430) for one hour at room temperature. Membranes were washed 3x in TBST and developed with WestFemto Supersignal chemiluminenscence substrate (Fisher Scientific).
Statistical Analysis
Experimental results are given as the mean ± standard deviation (SD) of a minimum of four experimental replicates. Replicate number (n) for each experiment is given in the corresponding figure legend. Statistical significance was evaluated using a Student’s t-test and significant (denoted with *) was based on p≤0.05.
Results
Human primary renal tubule epithelial cells were stained for ZO-1 and actylated α-tubulin to evaluate cell-cell junction and primary cilia formation. Images (Figure 1a) show that cells grown under both static and OSS conditions stained positive for ZO-1 that was highly localized to the cell-cell junctions. Cells also exhibited primary cilia formation with no obvious differences in cellular localization of either ZO-1 or acetylated α-tubulin between static and OSS culture. Increased cell density can be seen in OSS compared to static culture based on the DAPI nuclear counterstain in Figure 1. Quantitative evaluation of cell density is shown in Figure 1b. Cells cultured on an orbital shaker had significantly higher density (p<0.05) than cells grown under static conditions. Cell density increased by approximately 20% when cells were exposed to OSS compared to cells grown under static conditions.
Figure 1.
(a) Immunostaining for zonula occludens 1 (ZO-1) (top), acetylated α-tubulin (α-tub) (middle) and DAPI nuclear staining (bottom) in static and OSS culture. Cell-cell junctions (ZO-1) and cilia (α-tub) were observed under both static and OSS conditions (Scale bar=50 μm). (b) Cell density measured in cells grown under static conditions and on an orbital shaker (n=4). Cells grown on an orbital shaker had a cell density approximately 20% higher than cells grown under static conditions.
Cell attachment was measured following 3 hours of OSS exposure to determine if application of OSS resulted in any significant removal of cells from the substrate. Cell viability was also measured following acute (6 hr) and chronic exposure to OSS to determine if application of shear stress resulted in any significant cell death. No significant differences in cell density were observed following OSS exposure and there was no significant decrease in cell viability in cells exposed to either acute or chronic OSS (Supplementary Figure 1).
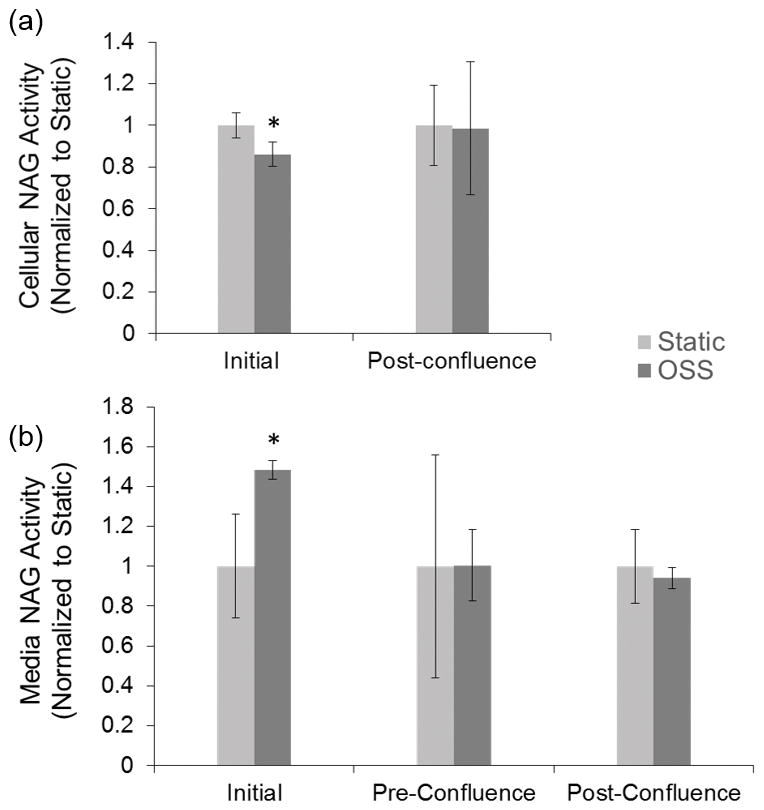
Cellular and media NAG activity were measured at various time points to determine if application of OSS resulted in any short term or long term cellular damage. Initial exposure to OSS resulted in a small but statistically significant decrease in cellular NAG and a corresponding increase in media NAG activity (Figure 2). This was present only after initial exposure to OSS. No difference in cellular NAG activity was observed after cells were fully differentiated (Figure 2a). Further, no change in media NAG activity was observed following chronic exposure to OSS prior to cell confluence (2 days) or after cells were fully differentiated (≥ 8 days) (Figure 2b).
Figure 2.
(a) Cellular and (b) media NAG activity for cells grown under static and OSS conditions. Cellular NAG was measured following initial exposure to OSS (6 hours) and after cells reached full confluence (≥ 8 days). Media was sampled after initial exposure, prior to cell confluence (2 days) and after cell were fully differentiated. There was a small but statistically significantly decrease in cellular NAG activity and an increase in media NAG activity following initial exposure. No differences in cellular or media NAG were measured at later time points.
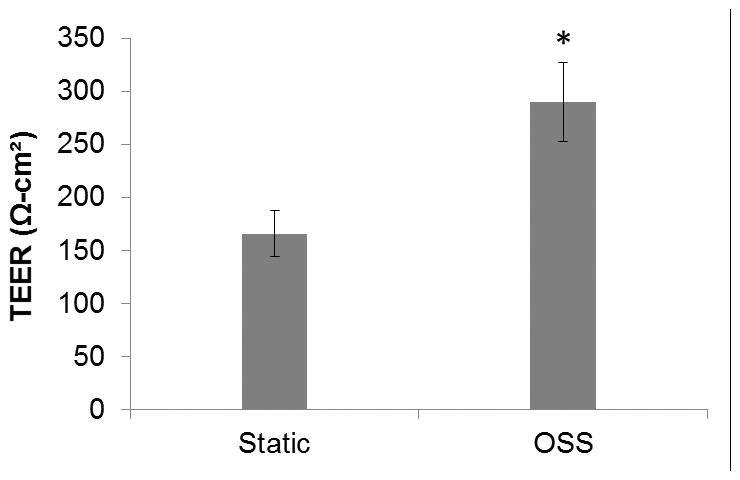
One of the important functions of the renal tubular epithelium is to facilitate removal of toxins by providing a barrier to back-leak of toxins from the glomerular filtrate into the circulation. In vitro, TEER is often used as a surrogate for barrier function as it provides a convenient measure of the relative “tightness” of the epithelial monolayer. To determine if OSS affects barrier function, TEER was measured daily under static and orbital shear stress conditions. The steady state value was taken as the mean TEER measured at day 7–10. Seven days was sufficient for cells to form a monolayer and TEER values were stable after day 7. Steady state TEER was significantly higher (p<0.05) in cells cultured on an orbital shaker (290±37 Ω-cm2) compared to static controls (166±22 Ω-cm2) (Figure 3).
Figure 3.

Steady state transepithelial electrical resistance (TEER) measured in cells grown under static conditions and grown on an orbital shaker. Orbital shaker culture resulted in an increase in average TEER (measured from day 7–10) from 166±22 Ω-cm2 (n=16) in static culture to 290±37 Ω-cm2 (n=16) when cultured on an orbital shaker.
Gene expression of tubule cell specific markers γGT, NHE3, and 1α hydroxylase were analyzed by quantitative real time polymerase chain reaction (q-RT PCR). Gene expression for γ-GT increased more than four-fold under OSS. NHE3 gene expression increased approximately two-fold, while 1α hydroxylase decreased slightly when cells were exposed to orbital shear stress (Figure 4). At the protein level, expression of γ-GT increased approximately three fold when cultured on an orbital shaker as measured by western blotting with GAPDH as a loading control (Figure 5). All changes in gene and protein expression were statistically significant (p<0.05).
Figure 4.
RNA expression of genes encoding γ-GT, NHE3, and 1α hydroxylate for cells grown under static conditions and on an orbital shaker (n=4). Orbital shaker culture resulted in an increase in γ-GT and NHE3 gene expression by approximately 4 and 2 fold respectively. 1α hydroxylate showed a small but statistically significant decrease in expression.
Figure 5.
Protein expression of γ-GT in cells grown under static condition and on an orbital shaker (n=4). Orbital shaker culture resulted in approximately 3 fold increase in protein expression.
Discussion
The strategy of isolating primary tissue cells and propagating a large cell population in vitro is hampered by the difficulty in maintaining in vivo phenotype under standard cell culture conditions. Fluid flow and shear stress are important factors in regulating the phenotype of a number of cells types including tubular epithelial cells. Microfluidic bioreactors are important tools for studying the physiological effects of fluid shear stress and are becoming increasingly important as microphysiological models (organs-on-a-chip) for drug and toxicity screening.14,27,28 However, their inherent small scale is not well-suited for propagation of large cell populations for tissue engineering applications. Culture on an orbital shaker, while not providing the same precise control of flow afforded by laminar flow devices, it is easily scalable to large populations of cells. The analytical approximation of shear stress on the orbital shaker (equation 1), while often cited in the literature, does not capture the complexity of the flow field actually experienced by the cells in culture. More sophisticated computational modeling of fluid dynamics under orbital motion show that at high orbital frequencies, which would be needed to approximate shear stress on endothelial cells, the shear stress is highly non-uniform and cyclical.20 However, at lower frequencies similar to those used here, the shear stress is relatively uniform and steady, although not to the level that would be expected in laminar flow systems. The computational model further suggests that the analytical solution overestimates the shear stress by approximate two fold at low frequencies. Based on our estimate on shear stress in the kidney proximal tubule, we set a target shear stress of 2 dyn/cm2, but based on the computational model, the actual shear stress may be closer to 1 dyn/cm2. This is still within the expected range for shear stress in the proximal tubule.
An additional advantage of orbital shaker culture of renal tubular epithelial cells is straightforward implementation of culture on porous membranes. In static culture on plastic dishes, tubular epithelial cells exhibit a flattened morphology that bears little resemblance to the columnar epithelial morphology of tubule cells in vivo.25,29,30 Growing cells on a porous substrate results in taller, more densely packed cells, although not to the degree observed in vivo. Given that growth on a porous substrate is critical to developing a more differentiated phenotype, all experiment in this study were performed on commercially available Transwell culture membranes. We wanted to determine the additional impact of orbital shaker culture on cell differentiation above what has already been shown by culturing cells on porous substrates.
Analysis of cell attachment, viability, and NAG activity demonstrated the OSS did not results in any significant removal of cells from the membrane nor did OSS induce any short term or long term cell death. NAG activity indicated that initiation of OSS resulted in a small but statistically significant decrease in cellular NAG activity that corresponded to a small increase in media NAG activity. This is likely due to shedding of NAG from the cell membrane into the media upon initiation of OSS. This seems to be an acute response to the initial application of shear stress as later time points showed no difference in cellular or media NAG activity between OSS and static controls. This suggests that slowly ramping the orbital frequency to the desired level of shear stress rather than sudden application may avoid tubular cell damage induced by the initial application of OSS. Despite the initial alterations in NAG activity, cells were able to recover from the initial application of OSS without any lasting tubular damage.
While the mechanisms by which shear stress alters cell density, TEER, and gene and protein expression have not been fully elucidated, previous studies may give some insight into potential targets for further investigation. Cell size and volume are known to be regulated by fluid flow in collecting duct epithelial cells. Boehlke et al.31 showed that cilia-mediated mechanotransduction in response to flow decreases cell size through activation of mTOR. This is consistent with our observed increase in cell density in cells grown under OSS, with a measured increase in cell density even after cells formed confluent monolayers.
Duan et al.13 showed that fluid flow alters the organization of cell-cell junctional complexes in mouse tubule epithelial cells. They showed reinforcement and increased ZO-1 and E-cadherin localization to the apical cell-cell junctions based on quantitative analysis of confocal fluorescence images. There studies were performed on non-porous substrates, precluding any assessment of barrier function. However, our observation of increased TEER in epithelial cells exposed to orbital shear stress is consistent with reinforcement of cell-cell junctions in response to shear stress.
Gene expression for several functional mediators of renal proximal tubular epithelial cell function (γ-GT, NHE3, and 1α hydroxylase) were analyzed under static and OSS conditions. γ-GT is a proximal tubule specific brush border enzyme involved in glutathione metabolism. NHE3 is a sodium/hydrogen exchanger involved in sodium transport and is important for sodium driven water transport in the proximal tubule, and 1α hydroxylase is an enzyme critical for vitamin D metabolism in the proximal tubule. γ-GT and NHE3 both showed increased RNA expression suggesting that these specific markers are positively regulated by exposure to shear stress. 1α hydroxylase showed a slight but statistically significant decrease in RNA expression. Changes in γ-GT expression were also manifested at the protein level. While the markers that we evaluated here have not been characterized previously, gene expression of other markers has been shown to be regulated by shear stress, suggesting the shear stress is an important regulator of a wide variety of genes in renal epithelial cells.32
In summary, we have shown that orbital shaker culture results in an increase in cell density and transepithelial electrical resistance. We also showed that OSS alters gene and protein expression of proximal tubule specific markers. We further demonstrate that application of OSS did not lead to any significant effects on cell attachment or viability and did not induce any significant long term cell toxicity. This simple and scalable method of applying mechanical force may be useful either alone or in combination with other interventions that preserve in vivo like phenotype to allow propagation of more differentiated cells for tissue engineered and bioartificial organs.
Supplementary Material
Acknowledgments
Sources of Funding: National Institutes of Health (DK 092357 to N.F. and EB 021214 to S.R. and W.H.F.)
This work was funded by the National Institutes of Health (DK 092357 and EB 021214).
Footnotes
Conflicts of Interest: S. Roy and W. H. Fissell have ownership in Silicon Kidney. The other authors have no conflicts of interest to report.
References
- 1.United States Renal Data System. 2015 USRDS annual data report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2015. [Google Scholar]
- 2.Vacanti JP, Langer R. Tissue engineering: The design and fabrication of living replacement device for surgical reconstruction and transplantation. The Lancet. 1999;354(Suppl 1):SI32–34. doi: 10.1016/s0140-6736(99)90247-7. [DOI] [PubMed] [Google Scholar]
- 3.Kim S, Fissell WH, Humes HD, Roy S. Current strategies and challenges in engineering a bioartificial kidney. Front Biosci (Elite Ed) 2015;7:215–228. doi: 10.2741/e729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192:547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sherr CJ, DePinho RA. Cellular senscence: Mitotic clock or culture shock? Cell. 2000;102:407–410. doi: 10.1016/s0092-8674(00)00046-5. [DOI] [PubMed] [Google Scholar]
- 6.Halliwell B. Oxidative stress in cell culture: an under-appreciated problem? FEBS Letters. 2003;540:3–6. doi: 10.1016/s0014-5793(03)00235-7. [DOI] [PubMed] [Google Scholar]
- 7.Yeatts AB, Fisher JP. Bone tissue engineering bioreactors: Dynamic culture and the influence of shear stress. Bone. 2011;48:171–181. doi: 10.1016/j.bone.2010.09.138. [DOI] [PubMed] [Google Scholar]
- 8.Bancroft GN, Sikavitsas VI, Dolder Jvd, et al. Fluid flow increases mineralized matrix deposition in 3D perfusion culture of bone marrow stromal osteoblasts in a dose-dependent manner. Proc Natl Acad Sci USA. 2002;99:12600–12605. doi: 10.1073/pnas.202296599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Datta N, Pham QP, Sharma U, Sikavitsas VI, Jansen JA, Mikos AG. In vitro generated extracellular matrix and fluid shear stress synergistically enhance 3D osteoblasic differentation. Proc Natl Acad Sci USA. 2006;103:2488–2493. doi: 10.1073/pnas.0505661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziegler T, Nerem RM. Tissue engineering a blood vessel: Regulation of vascular biology by mechanical stresses. J Cell Biochem. 1994;56:204–209. doi: 10.1002/jcb.240560215. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Riha GM, Yan S, et al. Shear stress induces endothelial differentation from a murine embryonic mesenchymal progenitor cell line. Arterioscler Thromb Vasc Biol. 2005;25:1817–1823. doi: 10.1161/01.ATV.0000175840.90510.a8. [DOI] [PubMed] [Google Scholar]
- 12.Ferrell N, Desai RR, Fleischman AJ, Roy S, Humes HD. A microfluidic bioreactor with integrated transepithelial resistance (TEER) measurement electrodes for evaluation of renal epithelial cells. Biotechnol Bioeng. 2010;107:707–716. doi: 10.1002/bit.22835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duan Y, Gotoh N, Yan Q, et al. Shear-induced reorganization of renal proximal tubule cell actin cytoskeleton and apical juntional complexed. Proc Natl Acad Sci USA. 2008;105:11418–11423. doi: 10.1073/pnas.0804954105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jang K-J, Suh K-Y. A multi-layer microfluidic device for efficient culture and analysis of renal tubular cells. Lab Chip. 2010;10:36–42. doi: 10.1039/b907515a. [DOI] [PubMed] [Google Scholar]
- 15.Ferrell N, Ricci KB, Groszek J, Marmerstein JT, Fissell WH. Albumin handling by renal tubular epithelial cells in a microfluidic bioreactor. Biotechnol Bioeng. 2012;109:797–803. doi: 10.1002/bit.24339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raghavan V, Rbaibi Y, Pastor-Soler NM, Carattino MD, Weisz OA. Shear stress-dependent regulation of apical endocytosis in renal proximal tubule cells mediated by primary cilia. Proc Natl Acad Sci USA. 2014;111:8506–8511. doi: 10.1073/pnas.1402195111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duan Y, Weinstein AM, Weinbaum S, Wang T. Shear stress-induced changes of membrane transporter localization and expression in mouse proximal tubule cells. Proc Natl Acad Sci USA. 2010;107:21860–21865. doi: 10.1073/pnas.1015751107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jang K-J, Cho HS, Kang DH, Bae WG, Kwon T-H, Suh K-Y. Fluid-shear-stress-induced translocation of aquaporin-2 and reorganization of actin cytoskeleton in renal tubular epithelial cells. Integr Biol. 2011;3:134–141. doi: 10.1039/c0ib00018c. [DOI] [PubMed] [Google Scholar]
- 19.Sahai A, Cole LA, Clarke DL, Tannen RL. Rocking promotes differentated properties in LLC-PK1 cells by improved oxygenation. Am J Phyiol Cell Phyiol. 1989;25:C1064–C1069. doi: 10.1152/ajpcell.1989.256.5.C1064. [DOI] [PubMed] [Google Scholar]
- 20.Thomas JMD, Chakroborty A, Sharp MK, Berson RE. Spatial and temporal resolution of shear in an orbiting petri dish. Biotechnol Prog. 2011;27:460–465. doi: 10.1002/btpr.507. [DOI] [PubMed] [Google Scholar]
- 21.Ley K, Lundgren E, Berger E, Arfors K-E. Shear-dependent inhibition of granulocyte adhesion to cultured endothelium by dextran sulfate. Blood. 1989;73:1324–1330. [PubMed] [Google Scholar]
- 22.Tsao PS, Lewis NP, Alpert S, Cooke JP. Exposure to shear stress alters endothelial adhesiveness: Role of nitric oxide. Circulation. 1995;92:3513–3519. doi: 10.1161/01.cir.92.12.3513. [DOI] [PubMed] [Google Scholar]
- 23.Kraiss LW, Weyrich AS, Alto NM, et al. Fluid flow activates a regulator of translation, p70/p85 S6 kinase, in human endothelial cells. Am J Phyiol Heart Circ Physiol. 2000;278:H1537–H1544. doi: 10.1152/ajpheart.2000.278.5.H1537. [DOI] [PubMed] [Google Scholar]
- 24.Detrisac CJ, Sens MA, Garvin AJ, Spicer SS, Sens DA. Tissue culture of human kidney epithelial cells of proximal tubule origin. Kidney Int. 1984;25:383–390. doi: 10.1038/ki.1984.28. [DOI] [PubMed] [Google Scholar]
- 25.Orosz DE, Woost PG, Kolb RJ, et al. Growth, immortalization, and differentation potential of normal adult human proximal tubule cells. In Vitro Cell Dev Biol. 2004;40:22–34. doi: 10.1290/1543-706X(2004)40<22:GIADPO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 26.Vaidya VS, Ferguson MA, Bonventre JV. Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol. 2008;48:463–493. doi: 10.1146/annurev.pharmtox.48.113006.094615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huh D, Torisawa Y-s, Hamilton GA, Kim HJ, Ingber DE. Microengineered physiological biomimicry: Organs-on-Chips. Lab Chip. 2012;12:2156–2164. doi: 10.1039/c2lc40089h. [DOI] [PubMed] [Google Scholar]
- 28.Jang K-J, Mehr AP, Hamilton GA, et al. Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr Biol. 2013;5:1119–1129. doi: 10.1039/c3ib40049b. [DOI] [PubMed] [Google Scholar]
- 29.Steele RE, Preston AS, Johnson JP, Handler JS. Porous-bottom dishes for culture of polarized cells. Am J Phyiol Cell Phyiol. 1986;251:C136–C139. doi: 10.1152/ajpcell.1986.251.1.C136. [DOI] [PubMed] [Google Scholar]
- 30.Parry G, Cullen B, Kaetzel CS, Kramer R, Moss L. Regulation of differentation and polarized secretion in mammary epithelial cells maintained in culture: Extracellular matrix and membrane polarity influences. J Cell Biol. 1987;105:2043–2051. doi: 10.1083/jcb.105.5.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boehlke C, Kotsis F, Patel V, et al. Primary cilia regulate mTORC1 activity and cell size through Lkb1. Nat Cell Biol. 2010;12:1115–1122. doi: 10.1038/ncb2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flores D, Battini L, Gusella GL, Rohatgi R. Fluid shear stress induces renal epithelial gene expression through polycystin-2-dependent trafficking of extracellular regulated kinase. Nephron Physiol. 2011;117:27–36. doi: 10.1159/000321640. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




