Abstract
Objective
Mural angiogenesis and macrophage accumulation are two pathological hallmarks of abdominal aortic aneurysm (AAA) disease. The heterodimeric transcription factor, hypoxia inducible factor (HIF)-1, is an essential regulator of angiogenesis and macrophage fucntion. In this study we investigated HIF-1 expression and activity in clinical and experimental AAA disease.
Methods
Human aortic samples were obtained from 24 AAA patients and six organ donors during open abdominal surgery. Experimental AAAs were created in 10 weeks old male C57BL/6J mice by transient intra-aortic infusion of porcine pancreatic elastase. Expression of HIF-1α and its target gene mRNA levels were assessed in aneurysmal and control aortae. The HIF-1α inhibitors 2-methoxyestradiol or digoxin, or the prolyl-hydroxylase domain-containing protein inhibitors cobalt chloride and JNJ-42041935, or vehicle-alone as control, were administered daily to mice at varying timing points beginning before or after porcine pancreatic elastase infusion. Influences on experimental AAA formation and progression were assessed via serial transabdominal ultrasonographic assessment of aortic diameter and histopathologic analysis at sacrifice.
Results
mRNA levels for HIF-1α, vascular endothelial growth factor-A, glucose transporter-1 and matrix metalloproteinase 2 were significantly increased in both human and experimental aneurysm tissue. Tissue immunostaining detected more HIF-1α protein in both human and experimental aneurysmal, as compared to respective control aortae. Treatment with either HIF-1α inhibitor, beginning either before or after porcine pancreatic elastase infusion, prevented enlargement of experimental aneurysms. Both HIF-1α inhibition regimens attenuated medial elastin degradation, smooth muscle cell depletion, mural angiogenesis, and the accumulation of macrophages, T cells and B cells. While mRNA levels for prolyl-hydroxylase domain-containing protein (PHD) 1 and PHD 2 were elevated in experimental aneurysmal aortae, pharmacological inhibition of PHDs had limited effect on experimental aneurysm progression.
Conclusions
Expression of HIF-1α and its target genes are increased in human and experimental AAAs. Treatment with HIF-1α inhibitors limits experimental AAA progression, with histologic evidence of attenuated mural leukocyte infiltration and angiogenesis. These findings underscore the potential significance of HIF-1α in aneurysm pathogenesis and as a target for pharmacologic suppression of AAA disease.
INTRODUCTION
Abdominal aortic aneurysm (AAA) disease is a potentially fatal, chronic degenerative condition of the distal aorta. Despite significant advances in understanding the underlying pathophysiology and epidemiologic associations of AAAs, to date no pharmacological strategies have proven effective in limiting progression of early disease or reducing the risk for sudden death due to rupture of advanced aneurysms1.
Hypoxia inducible factor (HIF)-1 is a heterodimeric transcription factor, comprised of an oxygen-regulated α subunit and a constitutively expressed β subunit, that regulates angiogenic, proliferative, and cellular metabolic responses to hypoxia. Cellular HIF-1 is rigorously regulated at the post-transcriptional level by oxygen dependent and independent pathways2. Hypoxic conditions stabilize levels of HIF-1α, and transcription is increased in response to inflammatory mediators, growth hormones as well as reactive oxygen species (ROS)3, 4
A growing body of evidence links HIF-1 expression and activity with AAA disease. For example, low oxygen tension and increased ROS generation are present within luminal thrombus in human aneurysmal aortae5–8. Increased levels of HIF-1α as well as key HIF-1 target genes such as vascular endothelial growth factor (VEGF)-A, are consistently reported in aneurysmal, as compared to non-aneurysmal, aortic segments8–14, 9.
Genetically, the HIF-1α 1772T-1790G haplotype, encoding an isoform of HIF-1α with enhanced transcriptional activity, interacting with either the VEGF-A 634C allele or in the presence of cigarette smoking, is associated with increased AAA disease risk15. Cigarette smoking, the most significant environmental risk factor for AAA disease, induces HIF-1α transcription16. Estrogen suppresses HIF-1α transcription, which may, in part, explain the reduced risk for AAA disease present in women16, 17 Diabetes, a condition known to suppress HIF-1 activity, is associated with reduced AAA disease risk in humans and, when induced experimentally in mice, hyperglycemia also suppresses experimental AAAs18–20
Although HIF-1 critically regulates angiogenesis, a characteristic feature of AAA pathobiology, the exact mechanism(s) by which HIF-1 activity promotes aneurysmal aortic degeneration remain poorly understood. Existing insights have been gleaned from exogenous angiotension (Ang) II infusion-dependent murine models, typically created in hyperlipidemic mice. For example, systemic inhibition of HIF-1α by small interfering (si) RNA or chemical inhibitors suppressed AAAs in apoliporoetin E-deficient mice following Ang II infusion21, 22 In contrast, smooth muscle cell (SMC)-specific HIF-1α deficientcy accereated the oneset of aneurysm onsets (abdominal or thoracic anueyrm, not either alone), elastin loss and aortic structural disorganization in lysyl oxidase inhibitor aminopriopionitrile-fed, and Ang II-infused wild type (WT) C57BL/6J mice23. Similarly, myeloid cell-specific HIF-1α and ApoE-deficient mice demonstrated increased aneurysm severity, elastin degradation and aortic macropahge infiltration, in association with reduced aortic mRNA lelevs of tissue inhibitor of metalloproteinases (TIMPs)24. Although imitating certain clinical and pathological features of human AAA disease, this system is dependent on other mechanisms, including the development of focal aortic dissection as a preceding pathological event prior to aneurysm formation, that differ significantly from those present in the human condition25, 26
In the current study, experimental aneurysms were created via segmental, transient, intra-aortic infusion of porcine pancreatic elastase (PPE) in WT mice, a modeling system with greater histological fidelity to the human condition than that achieved by supplemental angiotensin II infusion in hyperlipidemic mice. Expression of message for HIF-1α and its target genes was assessed in human as well as experimental AAAs. Cardiac glycoside digoxin and microtubule-disrupting agent 2-methoxyestradiol (2ME) are two well-charactiezed inhibitors of HIF-1α. Digoxin selectively inhibibits HIF-1α protein expression with limited influence on overall protein synthesis, without affecting the topomerase, mRNA transcription, mechanistic target of rapamycin (mTOR), PHD-VHL-proteasome and Na+/K+ ATPase activity27, 28. 2ME inhibits HIF-1α protein at the posttrancriptional level by destroying the microtubule cytoskeleton, and has no effect on its mRNA transcription or protein degradation. These inhibitors were provided to mice before or after aneurysm creation, to gain additional insight into the role that HIF-1 plays in AAA pathogenesis.
MATERIALS AND METHODS
Experimental aneurysm creation
AAAs were created in 10 weeks old male C57BL/6J mice (the Jackson Laboratory, Bar Harbor, Maine) via intra-aortic infusion of PPE as detailed in our previous studies29, 30. Briefly, under inhaled 2% isoflurane anesthesia, the infrarenal aorta was isolated through a median laparotomy, and temporarily controlled with 6-0 silk suture, and infused for 5 min at a constant pressure via BTPE-010 tube (30 μl of 1.5 U type I PPE/ml in saline, cat# E-1250-100 MG, Sigma-Aldrich, St. Louis, MO). All experimental procedures were conducted in compliance with the Stanford Laboratory Animal Care Guidelines and approved by the Administrative Panel on Laboratory Animal Care at Stanford University (Protocol ID: 11131).
AAA formation and progression were monitored by serial ultrasound measurements of infrarenal aortic diameter at 40 MHz (Vevo 770; Visualsonics, Toronto) at indicated days as previously described30. An AAA was defined as a > 50% increase in infrarenal aortic diameter compared to the baseline level.
Treatment with inhibitors to HIF-1α or PHDs
2ME and digoxin were purchased from Selleck Chemicals, Houston, and MP Biomedicals LLC, Solon, OH, respectively. Both compounds were freshly prepared in 0.5% carboxymethylcellulose/phosphate-buffered saline (PBS), and administered to mice at a dose of 50 mg/kg for 2ME, or 2 mg/kg for digoxin, per day via oral gavage. Dosages were selected based on those proven effective in prior published studies in other disease mouse models31, 32. To evaluate the effect of HIF-1α inhibitor treatment on AAA initiation and progression, treatment was initiated one day prior to, and terminated 14 days following, PPE infusion. To evaluate the effect on progression of existing AAAs, treatment was delayed until 4 days following PPE infusion and administered for a total of 10 days. Control mice for both groups were treated with equal volumes of vehicle over similar timeframes. In additional experiments, mice were treated with one of two mechanistically distinct PHD inhibitors, either cobalt chloride 30 mg/kg/d/ip (Alfa Aesar, Tewksbury, MA) or 1-(5-chloro-6-(trifluoromethoxy)-1H-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid (JNJ-42041935), 100 μmoles/kg/d via oral gavage (Acme Bioscience, Inc., Palo Alto, CA), or vehicle alone, beginning one day prior to PPE infusion and continuing for 14 days33, 34
Human aortic specimen retrieval and preparation
Aneurysm specimens were obtained from 24 patients who underwent open AAA repair surgery at Central South University Xiangya Hospital, Changsha, Hunan, China. Control aortic specimens were obtained at the time of organ harvest from 6 liver transplantation donors at Central South University Xiangya Hospital and Fudan University Zhongshan Hospital, China. The age of normal aortic donors ranged from 37 to 60 years, with an average of 47 years. Table I shows geographic and clinical characteristics of the AAA patients. The respective Human Subject Research Committees at both Xiangya and Zhongshan hospitals approved the collection and use of human samples for this research project, and informed consent was obtained from all participants or their agents. Following harvest, aneurysmal sac specimens were snap frozen in liquid nitrogen and stored at −80°C for further analysis.
Table I.
Main characteristics of 24 aneurysm patients
| Variable | Result |
|---|---|
| Gender (male, %) | 15 (62.5%) |
| Age (mean ± SD, years) | 63.8 ± 10.7 |
| Smoking (cases, %) | 10 (41.7%) |
| Aortic diameter (mean ± SD, mm) | 57.6 ± 12.4 |
| Luminal thrombus (cases, %) | 17 (70.8%) |
| Hypertension (cases, %) | 13 (54.2%) |
| Systolic pressure (mean ± SD, mm Hg) | 138.3 ± 18.3 |
| Diastolic pressure (mean ± SD, mm Hg) | 85.3 ± 13.9 |
| Hyperlipidemia (cases, %) | 5 (20.8%) |
| COPD (cases, %) | 0 (0.0%) |
| Diabetes (cases, %) | 4 (16.7%) |
| Antihypertension medications (cases, %) | 5 (20.8%) |
| Lipid-lowering medication (cases, %) | 1 (4.2%) |
| Diabetic medications (cases, %) | 0 (0%) |
Analysis of gene expression
Total RNA was isolated from mouse hearts and aortae 14 days following PPE infusion, as well as aneurysmal and control human aortae using TRIZOL reagent (Thermo Fisher Scientific Inc, Fremont, CA). Two micrograms of total RNA was reverse transcribed into cDNA with M-MLV reverse transcriptase using oligo-dT as a primer. PCR amplification of cDNA was performed using SYBR-green dye and Taq polymerase on Stratagene’s MX3000 real-time PCR machine. PCR primer sequences or commercial sources for mouse and genes were listed in Supplemental Table I. Message RNA levels in aneurysmal aortae (human or experimental) were expressed as fold changes relative to their respective controls.
Histological assessment
Mice in all groups were euthanized 14 days following PPE infusion. Aortae were harvested, embedded in OCT medium, sectioned (6 μm thickness), and fixed with cold acetone for most histological analyses. HIF-1α staining were performed on formalin-fixed, paraffin-embedded aortic sections (5 μm thick) prepared from mice 14 days after PBS or PPE infusion, or AAA patients or non-aneurysmal organ donors. Medial elastin retention was assessed using Elastica Van Gieson (EVG) staining. Standard three-step biotin-streptavidin-peroxidase tissue immunostaining was performed to evaluate medial SMC integrity, mural leukocyte infiltration, angiogenesis and HIF-1α as previously described35, 36. Primary antibodies for immunostaining were rabbit anti-SMC α-actin polyclonal antibody (Catalogue number 9010-P, Thermo Fisher Scientific Inc., Fremont, CA), rat anti-mouse CD68 mAb (clone FA-11, AbD Serotec, Raleigh, NC), hamster anti-mouse CD3 mAb (clone 145-2C11), rat anti-mouse B220/CD45R mAb (clone RA3-6B2), rat anti-mouse CD31 (clone 390) (Biolegend, San Diego, CA), and mouse anti-human HIF-1α (with cross-reaction to mouse HIF-1α, clone H1alpha67, Novus, USA). Other reagents were biotinylated anti-rabbit, rat, mouse or hamster secondary antibody and streptavidin-peroxidase conjugate from Jackson ImmunoResearch Laboratories Inc, West Grove, PA, and AEC or DAB peroxidase substrate kit (Vector Laboratories Inc., Burlingame, CA). Destruction of medial elastin and SMCs was graded as I (mild) to IV (severe) as previously described35. The degrees of mural leukocyte infiltration and angiogenesis were calculated as the number of subset mAb-positive leukocytes or CD31-positive capillaries per aortic cross-section (ACS).
Flow cytometric analysis of cytokine-producing CD4 T cells
Lymphocyte suspensions were prepared from spleens harvested from experimental mouse cohorts at sacrifice. Cell culture, intracellular cytokine staining and flow cytometric detection of IFN-γ- or IL-17-producing CD4+ T cells were performed using differentially fluochrome-conjugated anti-CD4 (clone GK1.5), IFN- γ (clone XMG1.2) and IL-17 (clone TC11-18H1.1) mAbs (All from Biolegend Inc) as described in our previous studies37.
Determination of significance
Statistical analyses were performed using Prism (Version 6.0g, GrapPad Software Inc., San Diego, CA). Continuous variables were summarized as the mean and standard deviation (SD). Depending on the nature of the data, nonparametric Mann-Whitney test, one sample T-test, or two-way ANOVA followed by the Newman-Keuls post-hoc correction were used to determine significance between groups. Difference in the incidence of experimental AAA following AAA induction between groups was determined by Kaplan-Meier analysis. In all analyses, P < .05 was considered significant.
RESULTS
Expression of HIF-1α, and HIF-1 targeted genes, are increased in human and experimental aneurysm specimens
While mRNA expression for Glut-1 in particular varied among human AAA specimens, significant increases were noted for HIF-1α and its four targeted genes in aneurysmal, as compared to control, aortae (Fig. 1A). All females (9/9), but none of males (0/15), demonstrated NOX2 mRNA expression. HIF-1α or its target gene mRNA levels did not correlate with aneurysmal diameter, and were not influenced by known aneurysmal risks such as cigarette smoking status. Additionally, a large variation (>30-fold) in HIF-1α mRNA expression was noted within the human aneurysm cohort; available demographic information and our experimental methods did not allow for further analysis of this apparent heterogeneity. In mice, as shown in Fig. 1B, HIF-1α mRNA expression was also significantly increased in aneurysmal segments, as were expression of the three HIF-1 targeted genes VEGF-A, Glut-1 and MMP2. Further, immunohistochemistry was perfomed to localize HIF-1α expression in aneurysmal lesions (Fig. 2). In non-aneurysmal aortic sections prepared from PBS-infused mice or organ donor patients, rare or very few cells were stained positively with HIF-1α mAb. In contrast, nuclear HIF-1α expression was observed in the adventitia of experimental and human aortic aneurysms. No staining was seen for negative control antibody in murine and human sepcimens, regardless of aneurysmal status (present or absent). Thus increased expression and activity of HIF-1α was present in both human and experimental AAAs.
Fig. 1. HIF-1α and its target gene mRNA levels are elevated in human and experimental aneurysmal aortae.
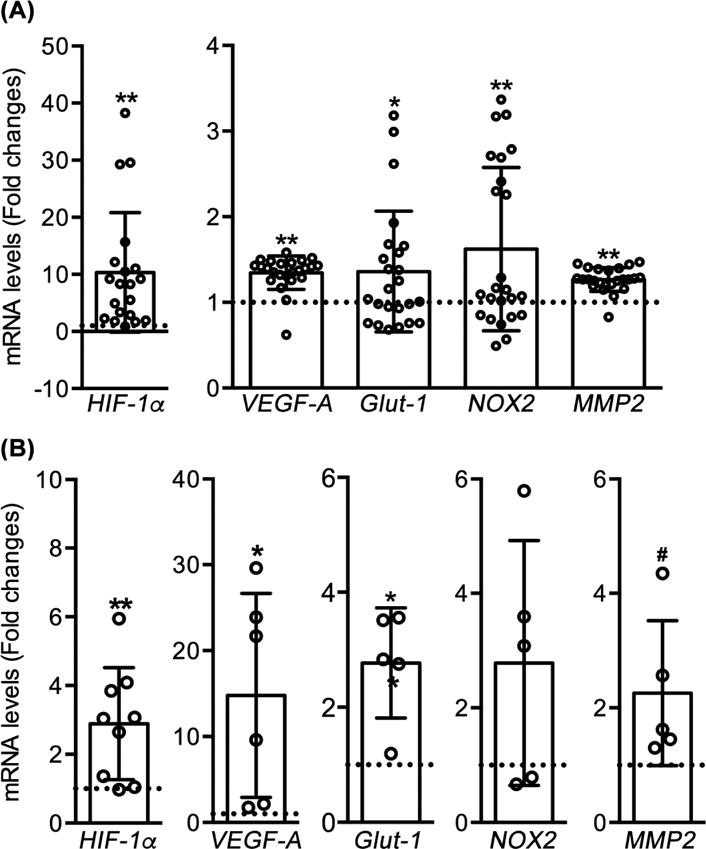
(A): Aneurysmal aortic segments were collected from 24 patients receiving open AAA repair and 6 organ donors for organ transplantation (control). Total RNA was extracted for evaluating expression of HIF-1α and its 4 target genes via quantitative real-time RT-PCR. (B): Ten to twelve weeks old male mice received transient intra-aortic infusion of PPE for aneurysm creation, or PBS as non-aneurysmal control (n=5-8 mice per group). Aortae were collected 2 weeks after the infusion, identical gene expression analyses were performed as in (A). mRNA levels in aneurysmal aortae are presented as the fold changes relative to organ donor human (A) or PBS-infused non-aneurysmal mouse (B) aortae. Data are presented as mean ± SD. One sample T test, .05 <#P< .1, *P< .05 and **P<.01 compared to organ donor aortae (A) or PBS-infused mouse aortae (B), where the value was set at 1 (dotted line).
Fig. 2. Immunostaining of HIF-1α in aneurysmal and non-aneurysmal aortae.

Formalin-fixed, paraffin-embedded sections were prepared from the aortae of mice 14 days following PPE (aneurysmal) or PBS (non-aneurysmal) infusion, as well as AAA patients and non-aneurysmal normal organ donors. Following antigen retrieval, aortic sections were stained with anti-HIF-1α mAb using a standard biotin-streptavidin-peroxidase technique. (A, D): Many nuclear HIF-1α-expressing cells (brown) were observed in aortic adventitia of AAA mice (A) and patients (D). (B, E): Very few cells in the aortic sections from the non-aneurysmal aortae of mice (B) or organ donors (E) stained positively with anti-HIF-1α mAb. (C, F): No cells stained positive for negative control antibody in the aortic sections from aneurysmal mice (C) or patients (F). Additionally, no staining was noted in the aortic section from non-aneurysmal mice or organ donor patients (not shown). These staining patterns were representative from at least 3 individuals in each group.
Treatment with HIF-1α inhibitors suppresses the initiation and progression of experimental AAAs
Increased mRNA levels of HIF-1α and its target genes in aneurysm tissue suggest that HIF-1 plays a role in the formation and progression of AAA disease. To test this hypothesis, two well-characterized HIF-1α inhibitors, 2ME or digoxin, were added to this model. As shown in Fig. 3, in heart samples collected 14 days after PPE infusion, treatment with either inhibitor attenuated expression of VEGF-A, Glut-1 and MMP2 by more than 80%, without affecting HIF-1α mRNA levels. These results indicate that 2ME or digoxin systemically inhibited HIF-1 activity. Neither 2ME nor digoxin treatment affected body weight gain, and digoxin also did not influence heart rate (data not shown) during the followup interval.
Fig. 3. Systemic HIF-1α inhibitor treatment reduces the mRNA levels of HIF-1 target genes.

Mice were given a HIF-1α inhibitor (50 mg/kg/day/gavage for 2ME, or 2 mg/kg/day/gavage for digoxin) or an equal volume of vehicle beginning one day prior to PPE infusion, for a total of 14 days. Total RNA was extracted from the heart 2 weeks following PPE infusion for evaluating HIF-1α and its target gene expression. Message RNA levels in HIF-1α inhibitor-treated mice were presented as the percentage of the value present in the vehicle treatment group (as 100%). n=5-8 in each group. One sample T test, **P<0.05 compared to vehicle treatment where the value is set at 100% (dotted line).
Following PPE infusion, control (vehicle-treated) mice sustained substantial and time-dependent aortic diameter enlargement from day 0 onward (Figs. 4A and 4B), although the degree of enlargement varied between mice and time intervals. Enlargement between days 0–3, 3–7 and 7–14 averaged 0.47, 0.12 and 0.05 mm, respectively. Following either 2ME or digoxin treatment, significantly less aortic enlargement was present compared to mice who received vehicle alone. Additionally, only 60% and 63% of mice treated with 2ME and digoxin, respectively, developed AAA within 14 days as compared to 100% of vehicle-treated mice (Fig. 4C). These results underscore the significant role of HIF-1α in experimental AAA initiation and progression.
Fig. 4. HIF-1α inhibitor treatment suppresses AAA formation.

Male C57BL/6J mice at 10-12 weeks of age were given 2ME (50 mg/kg/day) or digoxin (2 mg/kg/day) by oral gavage beginning one day prior to, and continuing for 13 days following, PPE infusion. There were 5-8 mice in each group. (A): Representative aortic ultrasound images at indicated days in PPE-infused mice treated with either 2ME, digoxin, or vehicle. (B): Mean and SD of aortic diameters at the baseline level (day 0) and indicated days following PPE infusion. Two-way ANOVA followed by Newman-Keuls post-test, *P<.05 and **P <.01 compared to vehicle treatment. (C): Aneurysm incidence. An AAA was defined as a 50% or greater increase in aortic diameter over the baseline level. Kaplan-Meier analysis, *P<.05 and **P<.01 compared to vehicle treatment. (D, E): EVG and SMC α-actin staining were performed and scored (1 (mild) to 4 (severe)) to evaluate medial elastin degradation and SMC depletion. Representative aortic elastin and SMC staining images (D). Quantification (mean and SD) of elastin destruction and SMC depletion in individual groups (E). Nonparametric Mann-Whitney test, *P <.05 and **P <.01 compared to vehicle treatment group.
Histologic correlates of HIF-1α inhibitor treatment in experimental AAAs
Medial elastic lamellae and SMCs
Medial elastin degradation and SMC depletion are key histological features of clinical and experimental AAAs. In these experiments, medial elastic lamellae and SMCs were both markedly preserved in AAA mice treated with either HIF-1α inhibitor (Figs. 4D & 4E). Thus the structural correlates of reduced aortic diameter enlargement in HIF-1α inhibitor-treated mice include preservation of medial elastin and SMC cellularity.
Mural leukocyte infiltration
Mural leukocyte accumulation represents another pathologic hallmark of AAA disease. In experimental AAA harvested from vehicle-treated mice, massive accumulation of CD68-positive macrophages was present predominantly in the adventitia (Figs. 5A & 5B). Large numbers of T and B lymphocytes, identified as CD3 and B220 mAb staining, respectively, were also found in both the media and adventitia (Figs. 5A, 5C & 5D). Treatment with 2ME or digoxin was associated with significant reduction of all 3 subsets of aortic leukocytes (Figs. 5A–5D). These results indicate that HIF-1α inhibition may limit experimental aneurysm progression in part by diminishing aortic accumulation of pro-inflammatory leukocytes.
Fig. 5. HIF-1α inhibitor treatment attenuates mural inflammatory cell accumulation and angiogenesis.
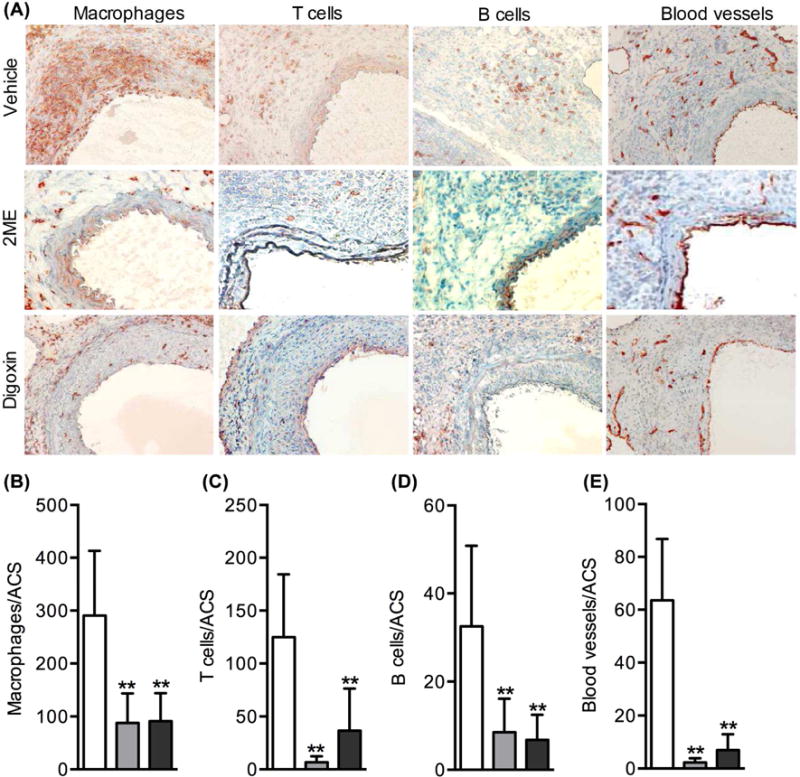
Aortic sections prepared from differentially treated mice were stained with mAbs against CD68 (macrophages), CD3 (T cells), B220 (B cells) and CD31 (neovessels), respectively. (A): Representative aortic immunostaining images for CD68+ macrophages, CD3+ T cells, B220+ B cells and CD31+ neovessels. (B–E): Quantification of aortic macrophages (B), T cells (C), B cells (D) and neoangiogenesis (E) in individual treatment groups. ACS: aortic cross section. Data: mean and SD. n=5-8 mice in each group. Nonparametric Mann-Whitney test, **P<.01 compared to vehicle treatment.
Mural angiogenesis
HIF-1α acts as a critical mediator for angiogenesis in many pathological settings. Thus, we evaluated the influence of HIF-1α inhibitor treatment on mural angiogenesis. As demonstrated by CD31 mAb staining (Figs. 5A & 5E), neovessels in the entire aortic wall, particularly the adventitia, stained positive for CD31 mAb (66.6 ± 23.2 vessels/ACS) in experimental AAAs. Treatment with 2ME or digoxin resulted in a 90% reduction in mural neovessel density (2.3 ± 1.6 vessels/ACS and 6.9 ± 6.0 vessels/ACS for 2ME and digoxin treatments, respectively) (Figs. 5A & 5E). Thus, AAA suppression during HIF-1α inhibitor treatment may arise in large part due to impaired mural angiogenesis during aneurysm formation and progression.
HIF-1α inhibitor treatment status does not influence cytokine-podcuing CD4+ T cell differentiation
To determine whether HIF-1α inhibitor treatment influences systemic differentiation of proinflammatory cytokine-producing CD4+ T cells, intracellular cytokine staining was performed on splenic lymphocytes harvested from mice with experimental AAAs. As shown in Fig. 6, the relative number of IFN-γ-producing CD4+ T cells was comparable between vehicle and HIF-1α inhibitor treatment groups. Similarly, neither treatment with 2ME or digoxin obviously affected the frequency of IL-17-producing splenic CD4+ T cells in splenic lymphocytes.
Fig. 6. HIF-1α inhibitor treatment does not affect the frequency of cytokine-producing CD4+ T cells.

At sacrifice, single lymphocyte suspensions were prepared from the spleens of differentially treated mice. Staining for surface CD4 and intracellular cytokines was conducted following the Biolegend intracellular cytokine stain protocol and FACS-analyzed. Data expressed as mean and SD (in parenthesis) of percentage of cytokine-producing cells in CD4+ T cells. n=4 mice in each group.
HIF-1α inhibitor treatment limits progression of established AAAs
To examine the translational potential of HIF-1α inhibition in existing AAAs, treatment with 2ME or digoxin or vehicle alone was initiated 4 days following PPE infusion and continued until sacrifice. As shown in Fig. 7, aortic diameter enlarged steadily from post-PPE days 3 to 10 in mice following treatment with vehicle alone. Delayed treatment with 2ME or digoxin was effective in reducing the rate of futher enlargement in existing AAAs. While treatment with both inhibitors limited aneurysm enlargement, significance was reached at 3 and 10 days following digoxin treatment and 3 days following 2ME treatment. Lack of significance in the 2ME treatment group following 2ME treatment was seen 10 days following 2ME treatment was likely due to large variations within the responses of individual mice. On histologic analysis, both 2ME and digoxin treatment were associated with elastin preservation and diminished mural angiogenesis and mural leukocyte infiltration. Thus both 2ME and digoxin were noted to limit further progression and transmural inflammation in existing experimental AAAs.
Fig. 7. HIF-1α inhibitor treatment limits progression of existing aneurysms.

Mice were treated with 2 ME (50 mg/kg/day n=7) or digoxin (2 mg/kg/day n=10 mice) or vehicle (n=6) from day 4 following PPE infusion until sacrifice, for a total of 10 days. (A): Representative aortic ultrasound images prior to (day 0), and 3 and 10 days after initiating treatment. (B): Mean and SD of aortic enlargement compared to diameter immediately prior to initiating treatment. Nonparametric Mann-Whitney test, **P<.01. (C–G): Quantification of medial elastin degradation (EVG stain) (C) and SMC depletion (SMC α-actin) (C), mural leukocytes (CD68 for macrophages (D), CD3 for T cells (E) and B220 for B cells (F) and mural neoangiogenesis (CD31) (G). ACS: aortic cross section. Data: mean and SD. Nonparametric Mann-Whitney test, *P<0.05 and **P<.01 compared to vehicle treatment group.
PHD inhibitor treatment has a limited effect on experimental AAAs
Finally, we examined the expression levels and role of PHDs, critical for oxygen-dependent HIF-1α degradation, in experimental AAAs. In real-time quantitative RT-PCR assays, the mRNA levels of PHDs, particularly PHD1 and PHD2, were significantly down-regulated in aneurysmal, as compared to, non-aneurysmal, mouse aortae (Fig. 8A). To test whether PHD inhibitor treatment further accelerated aneurysm progression, mice were treated with either cobalt chloride, JNJ-42041935, or vehicle alone (Fig. 8B). No measureable effect of the cobalt chloride on AAA diameter was noted. In JNJ-42041935-treated mice, average aortic diameters were smaller than those treated with vehicle alone, reaching significance at day 7 only.
Fig. 8. Limited influence of PHD inhibitor treatment on experimental AAAs.

(A): Aortae were collected from mice 14 days follow PBS (non-aneurysm) and PPE (aneurysm) infusion, and mRNA levels for PHD1, PHD2 and PHD3 were analyzed via real-time qRT-PCR assay. mRNA levels in aneurysmal aortae were presented as fold changes relative to PBS-infused mouse aortae, where the value was set at 1 (dotted line). Data are mean and SD from 4 mice in each group. One sample T-test, **P<0.01 compared to 1 (PBS-infused mouse aortae). (B): Mice were treated daily with cobalt chloride (30 mg/kg/i.p.) or JNJ-42041935 (100 μmoles/kg/oral gavage) or vehicle beginning one day following PPE infusion. Data are mean ± SD of aortic diameter from 5 mice in each group. Two-way ANOVA followed by Newman-Keuls post-test, **P<.01 compared to vehicle treatment.
DISCUSSION
This study found increased HIF-1α transcription, HIF-1α protein expression and transcriptional activation of HIF-1α target genes in both human and experimental aneurysmal aortae. Message levels for PHD1 and PHD2, critical for proteasomal degradation of HIF-1α, were also reduced in experimental aneurysmal lesions. Inhibition of HIF-1α by 2ME or digoxin suppressed experimental AAA formation, and the progression of existing AAAs, in conjunction with the attenuation of mural angiogenesis and leukocyte accumulation. This study adds further insight into significance of HIF-1 in AAA pathogenesis, as well as the potential translational value of selective anti-HIF-1 strategies for medical management of aneurysm disease.
Concomitantly elevated expression levels of HIF-1α mRNA, HIF-1α protein and its target gene mRNAs in aneurysmal aortic segments indicate enhanced mRNA transcription, protein expression and activity of HIF-1 in AAA disease. These findings are consistent with previous studies in which mRNA and protein levels of HIF-1α, as well as HIF-1 activity, were elevated in alternative murine AAA modeling systems21, 22. Identifying the precise mechanisms that induce HIF-1α transcription and protein expression in AAAs is beyond the scope of this study, however, reactive oxygen species, Ang II and inflammatory cytokines such as IL-1β, all abundantly present in the aneurysmal aorta and critical for disease progression, are known to upregulate HIF-1α transcription3, 4, 30, 37. Additionally, reduced mRNA levels of PHDs, as demonstrated in the present study, may in part impair oxygen-dependent HIF-1α degradation and consequently stabilize cellular HIF-1α protein.
Both 2ME and digoxin, by interfering with HIF-1α protein synthesis, suppress angiogenesis in oncologic as well as inflammatory diseases27, 28. In the present study, oral administration of either agent reduced the formation, progression and incidence of experimental AAAs while preserving aortic medial elastin and SMC cellularity. More pronounced inhibition was noted for digoxin treatment. Both inhibitors reduced myocardial VEGF-A, Glut-1 and MMP2 expression, suggesting systemic inhibition of HIF-1 activity. These findings complement prior results obtained in alternative murine AAA modeling systems. In the exogenous Ang II infusion in hyperlipidemic mice AAA model, both siRNA technology and oral 2ME or digoxin treatment mitigated aortic HIF-1α protein expression, aneurysm incidence and progression21, 22.
Not all investigations into HIF-1 activity in AAA disease have consistently demonstrated similar findings. Cobalt chloride, a PHD inhibitor, however, abrogated rather than accelerated experimental aneurysms created via abluminal application of calcium chloride38. In those experiments, aortic VEGF-A mRNA levels were slightly but significantly elevated in cobalt-treated mice, suggesting increased HIF-1 activity. In the current experiments, AAAs were only mildly attenuated early following treatment with the PHD inhibitor JNJ-42041935, and no attenuation was noted at all following cobalt chloride administration, at a doses previously reportedly as effective in inhibiting murine PHD activity with both agents33, 34. Given the fact that mural angiogenesis, inflammatory cell accumulation, and medial elastin and SMC depletion are all far more pronounced in the PPE as compared to the calcium chloride model, the observed differences may be model-specific and thus less relevant to the human condition. Specifically, given that intra-aortic PPE infusion substantially reduced mRNA expression of PHD1 and PHD2, further treatment with PHD inhibitors may not augment HIF-1α sufficiently to futher promote angiogenesis and its related pro-aneurysmal effects. Alternatively, the high doses of cobalt treatment previously used (70 vs. 30 mg/kg/d in the current experiments) may have suppressed AAA formation independent of HIF-1-mediated mechanisms.
Additionally, in SMC-specific HIF-1α-deficient mice, abdominal and thoracic aortic aneurysm formation following administration of Ang II and β-aminopropionylnitrile was augmented, rather than suppressed23, and AAA progression (but not incidence) was also accelerated in myeloid cell-HIF-1α deficient mice on a high fat diet following Ang II administration24. Substantial differences between these two modeling systems, despite the common element of exogenous Ang II administration, make it difficult to define the relative significance of SMC vs. myeloid cell-specific HIF-1α deficiency in these experiments. Additionally, putting aside the potential impact of genetic compensation in the setting of cell-specific HIF-1α deficiency, the apparent discrepancy between these and previously cited experiments underscores the importance of further investigations into this potentially significant, and pharmacologically modifiable, mechanism of AAA formation in pathologically relevant modeling systems (such as intra-aortic PPE infusion) and, if possible, clinical applications.
HIF-1 plays a critical role in regulating angiogenesis and activity of myeloid cells, two histological hallmarks of experimental and human AAAs2, 39. The current findings of reduced angiogenesis and macrophage infiltration associated with either HIF-1α inhibitor treatment, as well as our prior published experience with metformin and rapamycin in similarly structured experiments, underscore the significance of these pathological mechanisms in AAA disease35, 36, 40, 41. In addition to producing matrix metalloproteinases (MMPs), neovessels facilitate ingress of inflammatory monocytes, the circulating precursor to tissue macrophages, into the aortic wall. Macrophages themselves secrete large amounts of pro-angiogenic factors, including VEGF-A, MMPs, CCL2, CCL5 and CXCL839, 42 Thus it seems likely that HIF-1-mediated activities contribute to AAA pathogenesis both through the promotion of mural angiogenesis and augmentation of the mural inflammatory macrophage burden in the aneurysmal aorta.
T cells, by producing pro-inflammatory cytokines such as IFN-γ and IL-17, play important roles in experimental AAA pathogenesis43–45. HIF-1α activity is known to influence differentiation of IL-17-producing T-cells46, 47. In these experiments, however, no influence of either inhibitor treatment was noted on IFN-γ- or IL-17 production in splenic CD4+ T cells, although digoxin have previously been shown to down-regulate aortic IFN-γ and IL-17 expression in this model44. Thus, it remains uncertain regarding the influence of HIF-1α inhibitor treatment on IFN-γ and IL-17 in aneurysms. Certainly systemic HIF-1 inhibition may influence IFN-γ and IL-17 production by aortic resident innate immune cells such as lymphocytes and gamma-delta T cells.
A significant demand exists for effective, non-toxic, and non-surgical methods of suppressing early AAA disease. If an acceptable agent could be identified, many “worried well” patients could potentially forestall or avoid surgical management of AAAs altogether. Additionally, patients who have already undergone endovascular aortic aneurysm repair would benefit from adjunctive medical therapy to reduce the rate of secondary interventions necessitated by continued aneurysmal degeneration of the surgical neck and adjacent, non-aneurysmal aorta following the endovascular repair. Digoxin, a glycoside traditionally used for heart failure and arrhythmia management, is enjoying a renaissance of interest across a wide spectrum of diseases. Over 140 trials are ongoing or completed using digoxin to treat conditions varying from cardiovascular disease to rheumatoid arthritis, cancer, type II diabetes and Alzheimer’s Disease (https://clinicaltrials.gov/ct2/results?term=digoxin&pg). 2ME is an orally active, well-tolerated, naturally occurring estradiol derivative with 9 ongoing or completed trials in anti-oncologic applications (https://clinicaltrials.gov/ct2/results?term=2-methoxyestradiol). The therapeutic index of digoxin, while far from ideal, is well-known, and millions of individuals have taken the drug safely for centuries. The safety of 2ME is being established currently. Either of these drugs, if proven effective in suppressing clinical AAA disease in appropriately-designed clinical trials, could dramatically improve the quality of life for millions of patients world-wide.
Several limitations are present in this study. First, HIF-1α regulates the transcription of numerous genes critical for energy metabolism, angiogenesis, cell proliferation, apoptosis, migration, and extracellular matrix and barrier function. A small subset of HIF-1α-targeted genes, including VEGF-A, NOX2, MMP2 and Glut-1, known to be involved in AAA pathogenesis, were selectively evaluated in the present study. The possibility remains that other HIF-1 targeted genes are differentially regulated in AAA disease. Second, unlike genetic interference strategies such as siRNA, neither digoxin nor 2ME are sufficiently specific to confirm a regulatory role for HIF-1α activity and targeted gene expression in AAA pathogenesis. Prior work in complementary AAA models has shown, however, that treatment with both inhibitors is remarkably effective in suppressing HIF-1α protein levels in experimental AAAs21. Hypoxia increases HIF-1α protein levels by inhibiting PHD and FISH-mediated HIF-1α proteasomal degradation2. The current study did not assess aortic mural oxygen tension or the degree to which hypoxia is present in experimental aneurysms. However, reduced mRNA levels of both PHD1 and PHD2, substantitally regulated by oxygen levels, were noted in experimental aneurysmal aorta. Thus, it is possible that increased HIF-1 activity in experimental AAA may also result in part from hypoxia-mediated stabilization of HIF-1α protein. Because inhibition of HIF-1α with digoxin or 2ME substantially diminished the density of mural macropahges and neovessels, principal sources for MMPs and inflammatory mediators, relevant molecular mechanisms by which two inhibitors mediate AAA suppression, including effects on proteolytic mechanisms and proanti-inflammatory mediator expression, were not comprehensively examined. Additionally, attempts at localizing HIF-1α to specific aortic mural cell types were not successful in either frozen or paraffin-embedded specimens, due to technical difficulties with two-color immunostaining. In prior studies, both macropahges and SMCs have stained positively with HIF-1α antibody, suggesting these cells as potential cellular sources for increased HIF-1α expression in AAA disease10.
Hypertension is a known risk factor for AAA disease. While digoxin may affect blood pressure levels and potentially relate to AAA suppression, we and others have previously reported that blood pressure did not contribute to Ang II-induced AAAs in hyperlipidemic mice48 or markedly increased in mice following aneurysm creation via PPE infusion30. Moreover, digoxin treatment was previously shown to suppress Ang II-induced AAAs in hyperlipidemic mice without influencing blood pressure. Additionally, as summarized in the Supplemental Table II, although digoxin treatment has been reported to lower blood pressure in hypertensive rodents or patients wth heart failure, no effect has been recognized in normotensive mammals in the absence of heart failure.
It is also true that HIF-1α-mediated angiogenic activity declines with increasing age in related conditions such as ischemia-induced vascular remodeling49. This is contrast with epidemiological and clinical evidence that advanced age is the single most important demongraphic risk factor for AAA disease50. Thus it remains likely that additional risk factors and mechanisms exist to promote AAA pathogenesis beyond HIF-1 activity, and that additional studies will need to be undertaken to investigate whether age promotes aneurysm disease in a HIF-1-independent manner, or whether HIF-1 mediated influences are more or less significant in the setting of advanced age.
Conclusions
The expression of HIF-1α and its target genes were upregulated in clinical and experimental AAAs. Treatment with chemical inhibitors to HIF-1α suppressed the formation and progression of experimental AAAs. If proven reproducible and relevant in more robust clinical studies, strategies to systemically inhibit HIF-1α expression or activity may hold translational value in the management of AAA disease.
Supplementary Material
Clinical relevance.
Abdominal aortic aneurysm (AAA) is an age-related degenerative disease. Currently, no effective pharmacological treatment is available for limiting or stopping the enlargement of small AAAs. Hypoxia inducible factor (HIF)-1 regulates angiogenesis and myeloid cell activity, both critical for AAA pathogenesis. This study found increased mRNA levels for HIF-1α and its target genes in clinical and experimental aneurysmal aortae. Pharmacological inhibition of HIF-1 with digoxin and 2-methoxyestradiol substantially suppressed the formation and progression of, and further expansion of, existing, experimental AAAs. These results suggest that HIF-1 may be potential therapeutic target for pharmacological management of clinical aneurysm disease.
Acknowledgments
This work was supported in part by grants from the National Heart, Lung and Blood Institute (1R21HL109750-03 and 1R21HL112122-03), a Stanford University Cardiovascular Institute Seed grant, Hunan Province Department of Science and Technology (2013FJ2014 & 2015SK2017) and Xiang Hospital Central South University. Wei Wang was a visiting scholar supported by a fellowship from the China Scholarship Council.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presenatation
This study was in part presented as a poster at the 2013 Society for Vascular Surgery Vascular Annual Meeting, San Francisco, May 30–June 1, 2013.
Author contribution
B.H.X. and R.L.D. designed the study. W.W., B.H.X., H.J.X., Y.B.G., Y.W, and L.X.W performed experiments and collected data. W.W., B.H.X., H.J.X., Y.B.G., Y.W., L.X.W, and S.A.M. analyzed data. B.H.X., W.W., and R.L.D. wrote the manuscript. W.W., L.X.W, W.G.F., J.H.H and S.A.M. contributed to key reagents and human specimens. All authors approved the content and conclusions of the final manuscript.
References
- 1.Golledge J, Norman PE, Murphy MP, Dalman RL. Challenges and opportunities in limiting abdominal aortic aneurysm growth. J Vasc Surg. 2017;65(1):225–33. doi: 10.1016/j.jvs.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Greer SN, Metcalf JL, Wang Y, Ohh M. The updated biology of hypoxia-inducible factor. EMBO J. 2012;31(11):2448–60. doi: 10.1038/emboj.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Movafagh S, Crook S, Vo K. Regulation of hypoxia-inducible factor-1a by reactive oxygen species: new developments in an old debate. J Cell Biochem. 2015;116(5):696–703. doi: 10.1002/jcb.25074. [DOI] [PubMed] [Google Scholar]
- 4.Kuschel A, Simon P, Tug S. Functional regulation of HIF-1alpha under normoxia–is there more than post-translational regulation? J Cell Physiol. 2012;227(2):514–24. doi: 10.1002/jcp.22798. [DOI] [PubMed] [Google Scholar]
- 5.Choke E, Cockerill GW, Dawson J, Chung YL, Griffiths J, Wilson RW, et al. Hypoxia at the site of abdominal aortic aneurysm rupture is not associated with increased lactate. Ann N Y Acad Sci. 2006;1085:306–10. doi: 10.1196/annals.1383.005. [DOI] [PubMed] [Google Scholar]
- 6.Koole D, Zandvoort HJ, Schoneveld A, Vink A, Vos JA, van den Hoogen LL, et al. Intraluminal abdominal aortic aneurysm thrombus is associated with disruption of wall integrity. J Vasc Surg. 2013;57(1):77–83. doi: 10.1016/j.jvs.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Erdozain OJ, Pegrum S, Winrow VR, Horrocks M, Stevens CR. Hypoxia in abdominal aortic aneurysm supports a role for HIF-1alpha and Ets-1 as drivers of matrix metalloproteinase upregulation in human aortic smooth muscle cells. J Vasc Res. 2011;48(2):163–70. doi: 10.1159/000318806. [DOI] [PubMed] [Google Scholar]
- 8.Vorp DA, Lee PC, Wang DH, Makaroun MS, Nemoto EM, Ogawa S, et al. Association of intraluminal thrombus in abdominal aortic aneurysm with local hypoxia and wall weakening. J Vasc Surg. 2001;34(2):291–9. doi: 10.1067/mva.2001.114813. [DOI] [PubMed] [Google Scholar]
- 9.Van Vickle-Chavez SJ, Tung WS, Absi TS, Ennis TL, Mao D, Cobb JP, et al. Temporal changes in mouse aortic wall gene expression during the development of elastase-induced abdominal aortic aneurysms. J Vasc Surg. 2006;43(5):1010–20. doi: 10.1016/j.jvs.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Hu XH, Yang J, Liu CW, Zhang ZS, Zhang Q. The expression and significance of hypoxia-inducible factor-1 alpha and related genes in abdominal aorta aneurysm. Zhonghua Wai Ke Za Zhi. 2004;42(24):1509–12. [PubMed] [Google Scholar]
- 11.Nishibe T, Dardik A, Kondo Y, Kudo F, Muto A, Nishi M, et al. Expression and localization of vascular endothelial growth factor in normal abdominal aorta and abdominal aortic aneurysm. Int Angiol. 2010;29(3):260–5. [PubMed] [Google Scholar]
- 12.Wolanska M, Bankowska-Guszczyn E, Sobolewski K, Kowalewski R. Expression of VEGFs and its receptors in abdominal aortic aneurysm. Int Angiol. 2015;34(6):520–8. [PubMed] [Google Scholar]
- 13.Lim CS, Kiriakidis S, Sandison A, Paleolog EM, Davies AH. Hypoxia-inducible factor pathway and diseases of the vascular wall. J Vasc Surg. 2013;58(1):219–30. doi: 10.1016/j.jvs.2013.02.240. [DOI] [PubMed] [Google Scholar]
- 14.Mayranpaa MI, Trosien JA, Fontaine V, Folkesson M, Kazi M, Eriksson P, et al. Mast cells associate with neovessels in the media and adventitia of abdominal aortic aneurysms. J Vasc Surg. 2009;50(2):388–95. doi: 10.1016/j.jvs.2009.03.055. discussion 95–6. [DOI] [PubMed] [Google Scholar]
- 15.Strauss E, Waliszewski K, Oszkinis G, Staniszewski R. Polymorphisms of genes involved in the hypoxia signaling pathway and the development of abdominal aortic aneurysms or large-artery atherosclerosis. J Vasc Surg. 2015;61(5):1105–13 e3. doi: 10.1016/j.jvs.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Daijo H, Hoshino Y, Kai S, Suzuki K, Nishi K, Matsuo Y, et al. Cigarette smoke reversibly activates hypoxia-inducible factor 1 in a reactive oxygen species-dependent manner. Sci Rep. 2016;6:34424. doi: 10.1038/srep34424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukundan H, Kanagy NL, Resta TC. 17-beta estradiol attenuates hypoxic induction of HIF-1alpha and erythropoietin in Hep3B cells. J Cardiovasc Pharmacol. 2004;44(1):93–100. doi: 10.1097/00005344-200407000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Miyama N, Dua MM, Yeung JJ, Schultz GM, Asagami T, Sho E, et al. Hyperglycemia limits experimental aortic aneurysm progression. J Vasc Surg. 2010;52(4):975–83. doi: 10.1016/j.jvs.2010.05.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bento CF, Pereira P. Regulation of hypoxia-inducible factor 1 and the loss of the cellular response to hypoxia in diabetes. Diabetologia. 2011;54(8):1946–56. doi: 10.1007/s00125-011-2191-8. [DOI] [PubMed] [Google Scholar]
- 20.Takagi H. Association of diabetes mellitus with presence, expansion, and rupture of abdominal aortic aneurysm: “Curiouser and curiouser!” cried ALICE. Semin Vasc Surg. 2016;29(1–2):18–26. doi: 10.1053/j.semvascsurg.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Tsai SH, Huang PH, Hsu YJ, Peng YJ, Lee CH, Wang JC, et al. Inhibition of hypoxia inducible factor-1alpha attenuates abdominal aortic aneurysm progression through the down-regulation of matrix metalloproteinases. Sci Rep. 2016;6:28612. doi: 10.1038/srep28612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang L, Shen L, Li G, Yuan H, Jin X, Wu X. Silencing of hypoxia inducible factor-1alpha gene attenuated angiotensin -induced abdominal aortic aneurysm in apolipoprotein E-deficient mice. Atherosclerosis. 2016;252:40–9. doi: 10.1016/j.atherosclerosis.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 23.Imanishi M, Chiba Y, Tomita N, Matsunaga S, Nakagawa T, Ueno M, et al. Hypoxia-Inducible Factor-1alpha in Smooth Muscle Cells Protects Against Aortic Aneurysms-Brief Report. Arterioscler Thromb Vasc Biol. 2016;36(11):2158–62. doi: 10.1161/ATVBAHA.116.307784. [DOI] [PubMed] [Google Scholar]
- 24.Takahara Y, Tokunou T, Kojima H, Hirooka Y, Ichiki T. Deletion of Hypoxia-Inducible Factor-1alpha in Myeloid Lineage Exaggerates Angiotensin II-Induced Formation of Abdominal Aortic Aneurysm. Clin Sci (Lond) 2017 doi: 10.1042/CS20160865. [DOI] [PubMed] [Google Scholar]
- 25.Senemaud J, Caligiuri G, Etienne H, Delbosc S, Michel JB, Coscas R. Translational Relevance and Recent Advances of Animal Models of Abdominal Aortic Aneurysm. Arterioscler Thromb Vasc Biol. 2017;37(3):401–10. doi: 10.1161/ATVBAHA.116.308534. [DOI] [PubMed] [Google Scholar]
- 26.Lysgaard Poulsen J, Stubbe J, Lindholt JS. Animal Models Used to Explore Abdominal Aortic Aneurysms: A Systematic Review. Eur J Vasc Endovasc Surg. 2016;52(4):487–99. doi: 10.1016/j.ejvs.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Abud EM, Maylor J, Undem C, Punjabi A, Zaiman AL, Myers AC, et al. Digoxin inhibits development of hypoxic pulmonary hypertension in mice. Proc Natl Acad Sci U S A. 2012;109(4):1239–44. doi: 10.1073/pnas.1120385109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mabjeesh NJ, Escuin D, LaVallee TM, Pribluda VS, Swartz GM, Johnson MS, et al. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell. 2003;3(4):363–75. doi: 10.1016/s1535-6108(03)00077-1. [DOI] [PubMed] [Google Scholar]
- 29.Iida Y, Xu B, Xuan H, Glover KJ, Tanaka H, Hu X, et al. Peptide inhibitor of CXCL4-CCL5 heterodimer formation, MKEY, inhibits experimental aortic aneurysm initiation and progression. Arterioscler Thromb Vasc Biol. 2013;33(4):718–26. doi: 10.1161/ATVBAHA.112.300329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iida Y, Xu B, Schultz GM, Chow V, White JJ, Sulaimon S, et al. Efficacy and mechanism of angiotensin II receptor blocker treatment in experimental abdominal aortic aneurysms. PLoS One. 2012;7(12):e49642. doi: 10.1371/journal.pone.0049642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becker CM, Rohwer N, Funakoshi T, Cramer T, Bernhardt W, Birsner A, et al. 2-methoxyestradiol inhibits hypoxia-inducible factor-1 {alpha} and suppresses growth of lesions in a mouse model of endometriosis. Am J Pathol. 2008;172(2):534–44. doi: 10.2353/ajpath.2008.061244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H, Qian DZ, Tan YS, Lee K, Gao P, Ren YR, et al. Digoxin and other cardiac glycosides inhibit HIF-1alpha synthesis and block tumor growth. Proc Natl Acad Sci U S A. 2008;105(50):19579–86. doi: 10.1073/pnas.0809763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xi L, Taher M, Yin C, Salloum F, Kukreja RC. Cobalt chloride induces delayed cardiac preconditioning in mice through selective activation of HIF-1alpha and AP-1 and iNOS signaling. Am J Physiol Heart Circ Physiol. 2004;287(6):H2369–75. doi: 10.1152/ajpheart.00422.2004. [DOI] [PubMed] [Google Scholar]
- 34.Barrett TD, Palomino HL, Brondstetter TI, Kanelakis KC, Wu X, Haug PV, et al. Pharmacological characterization of 1-(5-chloro-6-(trifluoromethoxy)-1H-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid (JNJ-42041935), a potent and selective hypoxia-inducible factor prolyl hydroxylase inhibitor. Mol Pharmacol. 2011;79(6):910–20. doi: 10.1124/mol.110.070508. [DOI] [PubMed] [Google Scholar]
- 35.Fujimura N, Xiong J, Kettler EB, Xuan H, Glover KJ, Mell MW, et al. Metformin treatment status and abdominal aortic aneurysm disease progression. J Vasc Surg. 2016;64(1):46–54 e8. doi: 10.1016/j.jvs.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rouer M, Xu BH, Xuan HJ, Tanaka H, Fujimura N, Glover KJ, et al. Rapamycin limits the growth of established experimental abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2014;47(5):493–500. doi: 10.1016/j.ejvs.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 37.Xuan H, Xu B, Wang W, Tanaka H, Fujimura N, Miyata M, et al. Inhibition or deletion of angiotensin II type 1 receptor suppresses elastase-induced experimental abdominal aortic aneurysms. J Vasc Surg. 2017 doi: 10.1016/j.jvs.2016.12.110. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watanabe A, Ichiki T, Sankoda C, Takahara Y, Ikeda J, Inoue E, et al. Suppression of abdominal aortic aneurysm formation by inhibition of prolyl hydroxylase domain protein through attenuation of inflammation and extracellular matrix disruption. Clin Sci (Lond) 2014;126(9):671–8. doi: 10.1042/CS20130435. [DOI] [PubMed] [Google Scholar]
- 39.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112(5):645–57. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blandino G, Valerio M, Cioce M, Mori F, Casadei L, Pulito C, et al. Metformin elicits anticancer effects through the sequential modulation of DICER and c-MYC. Nat Commun. 2012;3:865. doi: 10.1038/ncomms1859. [DOI] [PubMed] [Google Scholar]
- 41.Land SC, Tee AR. Hypoxia-inducible factor lalpha is regulated by the mammalian target of rapamycin (mTOR) via an mTOR signaling motif. J Biol Chem. 2007;282(28):20534–43. doi: 10.1074/jbc.M611782200. [DOI] [PubMed] [Google Scholar]
- 42.Corliss BA, Azimi MS, Munson JM, Peirce SM, Murfee WL. Macrophages: An Inflammatory Link Between Angiogenesis and Lymphangiogenesis. Microcirculation. 2016;23(2):95–121. doi: 10.1111/micc.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma AK, Lu G, Jester A, Johnston WF, Zhao Y, Hajzus VA, et al. Experimental abdominal aortic aneurysm formation is mediated by IL-17 and attenuated by mesenchymal stem cell treatment. Circulation. 2012;126(11 Suppl 1):S38–45. doi: 10.1161/CIRCULATIONAHA.111.083451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei Z, Wang Y, Zhang K, Liao Y, Ye P, Wu J, et al. Inhibiting the Th17/IL-17A-related inflammatory responses with digoxin confers protection against experimental abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 2014;34(11):2429–38. doi: 10.1161/ATVBAHA.114.304435. [DOI] [PubMed] [Google Scholar]
- 45.Xiong W, Zhao Y, Prall A, Greiner TC, Baxter BT. Key roles of CD4+ T cells and IFN-gamma in the development of abdominal aortic aneurysms in a murine model. J Immunol. 2004;172(4):2607–12. doi: 10.4049/jimmunol.172.4.2607. [DOI] [PubMed] [Google Scholar]
- 46.Chou TF, Chuang YT, Hsieh WC, Chang PY, Liu HY, Mo ST, et al. Tumour suppressor death-associated protein kinase targets cytoplasmic HIF-1alpha for Th17 suppression. Nat Commun. 2016;7:11904. doi: 10.1038/ncomms11904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146(5):772–84. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cassis LA, Gupte M, Thayer S, Zhang X, Charnigo R, Howatt DA, et al. ANG II infusion promotes abdominal aortic aneurysms independent of increased blood pressure in hypercholesterolemic mice. Am J Physiol Heart Circ Physiol. 2009;296(5):H1660–5. doi: 10.1152/ajpheart.00028.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Semenza GL. Hypoxia-inducible factor 1 and cardiovascular disease. Annu Rev Physiol. 2014;76:39–56. doi: 10.1146/annurev-physiol-021113-170322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kent KC, Zwolak RM, Egorova NN, Riles TS, Manganaro A, Moskowitz AJ, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg. 2010;52(3):539–48. doi: 10.1016/j.jvs.2010.05.090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


