Abstract
Recent studies show improved outcomes in ambulated lung failure patients. Ambulation still remains a challenge in these patients. This necessitates development of more compact and less cumbersome respiratory support specifically designed to be wearable. The Paracorporeal Ambulatory Assist Lung (PAAL) is being designed for providing ambulatory support in lung failure patients during bridge to transplant or recovery. We previously published in-vitro and acute in-vivo results of the PAAL. This study further evaluates the PAAL for five days.
Five-day in-vivo studies with the PAAL were conducted in 50-60 kg sheep after heparinization (ACT range: 190-250s) and cannulation with a 27 Fr. Avalon Elite dual lumen cannula. The animals were able to move freely in a stanchion while device flow, resistance, and hemodynamics were recorded hourly. Oxygenation and hemolysis were measured daily. Platelet activation, blood chemistry and comprehensive blood counts are reported for pre-operatively, on POD 0, and POD 5.
Three animals survived for five days. No study termination resulted from device failure. One animal was terminated on POD 0 and one animal was terminated at POD 3. The device was operated between 1.93 – 2.15 L/min. Blood left the device 100% oxygenated. Plasma free hemoglobin ranged 10.8 mg/dL – 14.5 mg/dL. CD62-P expression was under 10%. Minimal thrombus was seen in devices at explant.
Chronic use of the PAAL in awake sheep is promising based on our study. There were no device related complications over the study course. This work represents the next step in our pathway to eventual clinical translation.
Keywords: Artificial Lung, ECMO, Ambulation, Wearable Respiratory Support, In-Vivo Studies
Introduction
Mechanical ventilation (MV) and extracorporeal membrane oxygenation (ECMO) are widely used in treating patients with acute respiratory distress syndrome (ARDS) and end stage chronic lung disease. These treatments are effective in providing respiratory support, but do have some limitations associated with them. Barotrauma and volutrauma due to MV results in poor post-transplant outcomes.1,2 Mechanical ventilation in patients with ARDS can also induce a cytokine response, which can be reduced through protective ventilation strategies3. Ranieri et. al, demonstrate this through their randomized controlled trial. Patients significantly lower inflammatory mediators 36 hours after treatment in the lung protective strategy group. Conventional ECMO is cumbersome and requires confining the patient to an ICU bed. 4,5,6 These patients experience progressive muscle deconditioning which further increases morbidity and mortality.7,8
Significant efforts have been made to improve conventional ECMO. Several centers have implemented ambulation on ECMO using Quadrox oxygenators or the Maquet Cardiohelp, often aided by the use of a cannula such as the Avalon Elite® in an effort to promote patient mobility and reduce muscle deconditioning. 9,10,11 Clinical advances have made ambulation possible in the clinical setting. 12,13,14,15 Biscotti et. al.11 describe the “Sport Model” method of placing VA ECMO through subclavian access. This less invasive procedure provides advantages of central cannulation without need of a thoracotomy and reduces risks of peripheral cannulation. By cannulating through the upper body the patient’s legs are free. This mode of cannulation is thus less invasive and promotes ambulation. Reeb et. al.12 review contemporary modes of cannulation including strategies using the “Sport Model”, and VV ECMO strategies using the DLC and a PA-LA strategy using the Novalung. Rajgopal et. al.13 review the increasing adoption of contemporary ECMO at large centers and conclude that awake and ambulatory ECMO display the best outcomes as deleterious effects of invasive MV, neuropathy and deconditioning can be avoided. Pruijsten et. al.14 describe a custom made “ECMO helmet” constructed using a bicycle helmet that allowed patients on VV ECMO using an Avalon DLC be ambulatory without tubing being held by care givers. More compact and less cumbersome respiratory support devices and modalities would further simplify ambulation. The OxyRVAD approach16 and the newly FDA approved TandemLung17 connect ECMO circuit components in a relatively compact manner. More novel artificial lungs improve compactness and potentially reduce complexity by integrating components further. The Ambulatory Pump Lung (APL)18 and Thoracic Artificial Lung (TAL)19 have shown promise in chronic sheep studies. Breethe Inc. is in the process of commercializing the APL device developed at the University of Maryland20. Novalung GmbH is developing an artificial lung coated with human cells which the goal of improving biocompatibility and increasing durability (Novalung press release 02/12). One could envision a day when even this technology may progress outpatient management.
We are developing the Paracorporeal Ambulatory Assist Lung (PAAL) as another approach to a wearable respiratory assist system.21 The PAAL is a compact, integrated blood pump-oxygenator device that is intended to simplify ambulation of lung failure patients. We previously designed the HFM bundle used in the PAAL22. We increased efficiency to minimize blood contacting surface area. This approach led to a higher gas exchange efficiency than the APL (276 ml/min/m2 vs. 225 ml/min/m2)23. We integrated this HFM bundle with a centrifugal pump capable of pumping against high resistances (such as dual lumen cannula) in our next study. The device was also evaluated for 6 hours in-vivo without complication20. The pump features simple pivot-bearing technology that is driven by permanent magnets, which potentially simplifies design. The integrated pump in the PAAL allows for less invasive cannulation using dual lumen cannula. This approach differs from the TAL approach which uses the heart itself as a pump and requires a thoracotomy for placement.
In this study we tested the PAAL for 5 days in chronic sheep studies. Our goal was to demonstrate low blood hemolysis, low platelet activation below, no increase in HFM bundle resistance and no decrease in oxygenation performance for the PAAL in chronic, awake sheep. This work represents the next step in our pathway to eventual clinical translational of this technology.
Methods
The PAAL integrates a simple pivot bearing centrifugal pump with an efficient HFM bundle into a single compact unit. The overall dimensions of the PAAL are 5.0-inch × 4.8-inch × 4.6-inch and the prototype weighs 4lb. Device weight will be approximately 1 lb once injection molding is used at the product development stage when the design is frozen. Details of the device specifications and bundle manufacturing are described elsewhere20,21. Briefly, the HFM bundle dimensions are 1.75” diameter, 3.12” length and is comprised of 200 stacked sheets. The rated flow (65% inlet saturation and 95% outlet saturation) is ~4L/min and the pressure drop is ~80 mmHg at this flowrate. The gas flow pressure drop is ~1mmHg. The pump can produce over 250 mmHg at 4 L/min operating at 2100 RPM requiring under 14W. Prior to these chronic studies, regions of flow stagnation and high shear in the blood flow path in our previously reported device were minimized using computational fluid dynamics (CFD). The impeller in the CFD designed device had slotted vanes and the flow channel connecting the impeller and fiber bundle was modified. The design improvements through CFD reduced in-vitro hemolysis by 70%. Figure 1 shows the PAAL prototype tested in this study.
Figure 1.
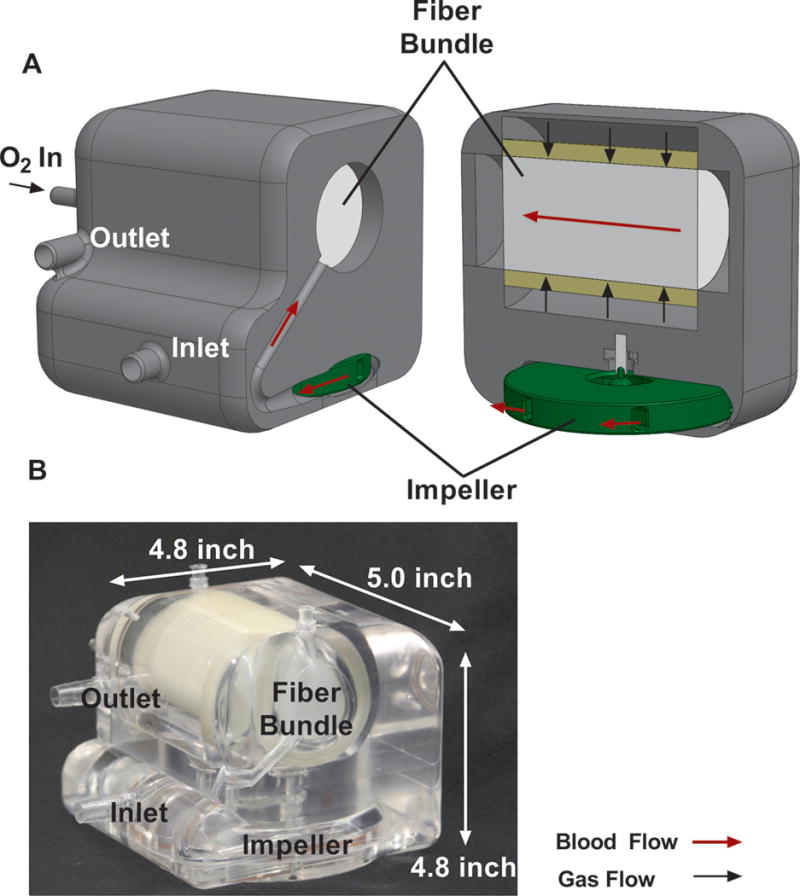
Cross section drawing of the PAAL device showing flow paths (A); and machined prototype (B)
The PAAL was tested for 5 days in adult Dorsett and Suffolk sheep (n=5) at the McGowan Institute’s Center for Preclinical Studies (CPCS). All animals received humane care in accordance with the Guide for Care and Use of Laboratory Animals (NIH publication 86-23, revised 1996). The surgical protocol and animal care were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. Device set up and cannulation are described in detail elsewhere.20 A venous line was placed in the left external jugular via 1 cm cut-down. A 150 U/kg heparin bolus was administered and allowed to circulate in the animal for 10 minutes. The pre-insertion ACT target was 300s. A heparin drip was used to maintain this anticoagulation level. The right external jugular was cannulated with a 27 Fr. Avalon Elite® dual lumen cannula (Maquet Cardiovascular, LLC, Wayne, NJ) via a 4 cm cut-down and checked under fluoroscopy. A saline bolus of no more than 1000 cc was administered if CVP was below 5 mmHg. The cannula was sutured to the skin, and a neck wrap held the cannula in place. The cannula was secured outside of the neck in Animals 2-5 due to prevent kinking seen in Animal 1.
Extracorporeal circulation was initiated at 2.5-3 L/min blood flow and 5-6 L/min pure oxygen sweep gas. The sweep gas was diluted with up to 5% CO2 for maintaining the pCO2 entering the device within a normal range 45 mmHg ± 5 as previously reported21. This is routinely performed for maintaining normocapnia.20,21 Banamine (1 mg/kg) was administered IV while weaning isoflurane and reducing the respiratory rate. The animal was then transferred to a stanchion system shown in Figure 2. The stanchion allowed the animal to sit, stand and move its head freely. This required decreasing pump speeds and lowering flowrates for preventing suction on the cannula. A precautionary nasal cannula was placed following extubation. The PAAL was secured to the animal using a custom designed holster. A seamstress custom manufactured the holster from a polyester lunch-bag (10in × 7.75 in × 3.5in) shown in Figure 2. The walls of the bag were cut to the shape of the device. Polyester back-pack straps were sewn to the side of the animal. The holster was fastened to the side of the animal using the back pack straps.
Figure 2.
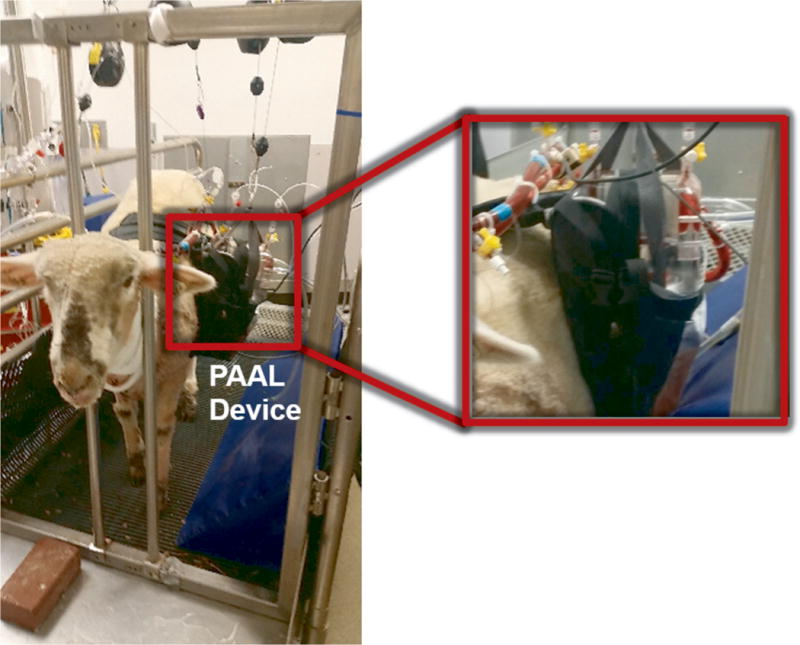
Photograph of the animal wearing the PAAL and standing in the stanchion system.
The post-operative analgesic protocol was butrophanol tartate (0.3 mg/kg intravenous) alternated with banamine (1 mg/kg intravenous) every 6 hours. Reglan (10mg intravenous) was administered every 8 hours postoperatively as a gastric motility stimulant for post anesthetic care. The cannula site dressing and neck wrap were changed at least once a day.
Activated clotting time (ACT) was monitored every hour for the first 12 hours and then at least once in 4 hours when stable. Target ACT range over the study course was 190 s - 250 s (1.5 to 2 times baseline). Arterial blood gases were monitored every 12 hours. ACT was measured using a Medtronic ACT-II machine (Medtronic, Minneapolis, MN). The cartridges of this machine use kaolin as the activating agent. Samples for measuring blood gases were drawn from the device inlet and outlet were drawn two times daily. Plasma free hemoglobin (PfHb) was measured daily using previously described methods23. Blood samples for a comprehensive blood count and blood chemistry test panel (IDEXX Laboratories, Inc., Westbrook, ME) were drawn pre-operatively and on post-operative day (POD) 0,3,5. The pre-operative (baseline) blood sample was drawn 1-3 days before the surgery and the POD 0 sample was drawn after device placement on the day of surgery. Blood count included white blood cell (WBC) counts and hematocrit (Hct). Platelets were reviewed on a blood film microscopically. Blood chemistry included measurements of aspartate transferase and alanine transferase, creatinine kinase, blood urea nitrogen and creatinine as measures of liver function, tissue injury and kidney function respectively. Platelet activation was quantified through a flow cytometric based analysis as previous described in detail24. A MCA2418-Alexa Fluor 488 (an antihuman CD62P antibody, AbD Serotec, NC, USA) was utilized as the platelet activation marker and the isotype control using MCA928-A488 was used for the evaluation of the MCA2418. In addition, platelet activating factor (PAF; Calbiochem, CA, USA)-stimulated samples were also prepared to evaluate platelet functionality. Platelet activation is reported for blood samples drawn pre-operatively, on POD 0, and POD 5.
Animal 3 required emergency device change on POD 2 as air leaked into the circuit from a broken stop-cock. We thus extended the study to POD 7 ensuring the fresh device was tested for a period of 5 days. Data collection for animal 3 started on POD 2. Blood samples were not drawn from Animal 5 between POD 1 and 4 due to low Hct. Device parameters (blood flow, bundle resistance, rotation speed, torque) and hemodynamics (heart rate, arterial pressure, central venous pressure) were recorded every hour. Power consumption was calculated as the product of torque and rotation speed.
Statistical Analyses
Data for the animals that survived the duration of the study are presented as mean ± standard deviation and are averaged for each POD. Hemodynamics, device parameters, platelet status, hematology and blood chemistry were statistically compared with a repeated measures ANOVA. The repeated measures ANOVA was followed up with a Bonferroni pairwise comparison. POD 0 and POD 5 values for oxygenation and hemolysis were statistically compared using a paired t-test, since blood samples for Animal 5 were not drawn on POD 1-4 due to a low hematocrit. All analyses were conducted using IBM SPSS Statistics 24 (IBM Corp., North Castle, NY).
Results
No device related complications were observed in any animal. All sheep were awake and standing post-implant. Animal 1 was terminated early as excessive suction events resulted in flows as low as 0.3 L/min. Necropsy showed that the cannula was kinked in this animal, which likely led to the suction. Animal 2 was terminated as a gastric volvulus prevented the animal from eating. Overnight suction events in Animal 3 led to an isolated spike in plasma-free hemoglobin, PfHb (70mg/dL). Suction was caused by the negative pressure the pump generated, which caused intermittent suctioning of the blood vessel onto the cannula inlet port. Suction was resolved by administering a saline bolus and reducing pump speed. Animal 5 required a device change in the OR as air leaked into the circuit from a broken stop-cock. Animal 5 also had post-operative bleeding around the cannula and venous line sites. Bleeding was controlled by dropping the activated clotting time (ACT) below our target range and transfusing one unit of blood on POD 3.
Table 1 shows recorded device parameters and animal hemodynamics. The PAAL was operated at a blood flow between 1.93 L/min ± 0.21 and 2.15 L/min ± 0.24 L/min. The power ranged 2.16W ± 0.49 to 2.55 ± 0.87 W in this study and the device was operated at ~1500 RPM. Hollow fiber membrane (HFM) bundle resistance did not significantly increase over the study duration. Gross examination of the HFM bundle post-study showed no thrombus in devices used in Animals 1-4, and a few small point deposits in the device used in Animal 5 (Figure 3). Hemodynamics remained stable in all animals and were not significantly altered over the study course. Oxygenation ranged from 98 ml/min ± 14 to 143 ml/min ± 20 (Figure 4A).Oxygenation at the end of the study did not significantly change from POD 0 over the study course (p=0.078). Blood left the device fully saturated at inlet saturations ranging 49.8-58.6% (Figure 4B). In-vivo hemolysis was under 15 mg/dL (Figure 5). Hemolysis at the end of the study did not change significantly from POD 0 (p=0.663). Animal 3 however saw an acute rise in plasma-free hemoglobin to 70 mg/dL on POD 3 which was resolved by POD 4.
Table 1.
Daily measurements of hemodynamics and device parameters
| Parameter | POD 0 | POD 1 | POD 2 | POD 3 | POD 4 | POD 5 |
|---|---|---|---|---|---|---|
| MAP (mmHg) | 100 ± 7.3 | 100 ± 12 | 101 ± 110 | 108 ± 160 | 110 ± 130 | 106 ± 9.60 |
| CVP (mmHg) | 4.44 ± 1.1 | 2.99 ± 0.43 | 3.57 ± 0.250 | 4.14 ± 0.870 | 3.53 ± 0.230 | 3.78 ± 0.390 |
| HR (BPM) | 97.1 ± 19 | 95.4 ± 21 | 91.5 ± 240 | 89.7 ± 210 | 106 ± 240 | 111 ± 150 |
| Flow (L/min) | 2.15 ± 0.24 | 2.04 ± 0.17 | 1.97 ± 0.19 | 1.93 ± 0.21 | 1.94 ± 0.210 | 2.01 ± 0.210 |
| HFM Bundle Resistance (mmHg/L/min) | 12.1 ± 1.3 | 13.4 ± 4.2 | 13.4 ± 30 | 12.9 ± 1.90 | 13.8 ± 2.2 | 13.5 ± 1.80 |
| Power (W) | 2.42 ± 0.53 | 2.55 ± 0.87 | 2.31 ± 0.47 | 2.16 ± 0.49 | 2.3 ± 0.45 | 2.32 ± 0.28 |
| Speed (RPM) | 1510 ± 75 | 1520 ± 170 | 1490 ± 790 | 1430 ± 960 | 1450 ± 1000 | 1460 ± 760 |
POD = Post operative day
MAP = Mean arterial pressure; CVP = Central venous pressure; HR = Heart rate; HFM = Hollow fiber membrane
Figure 3.
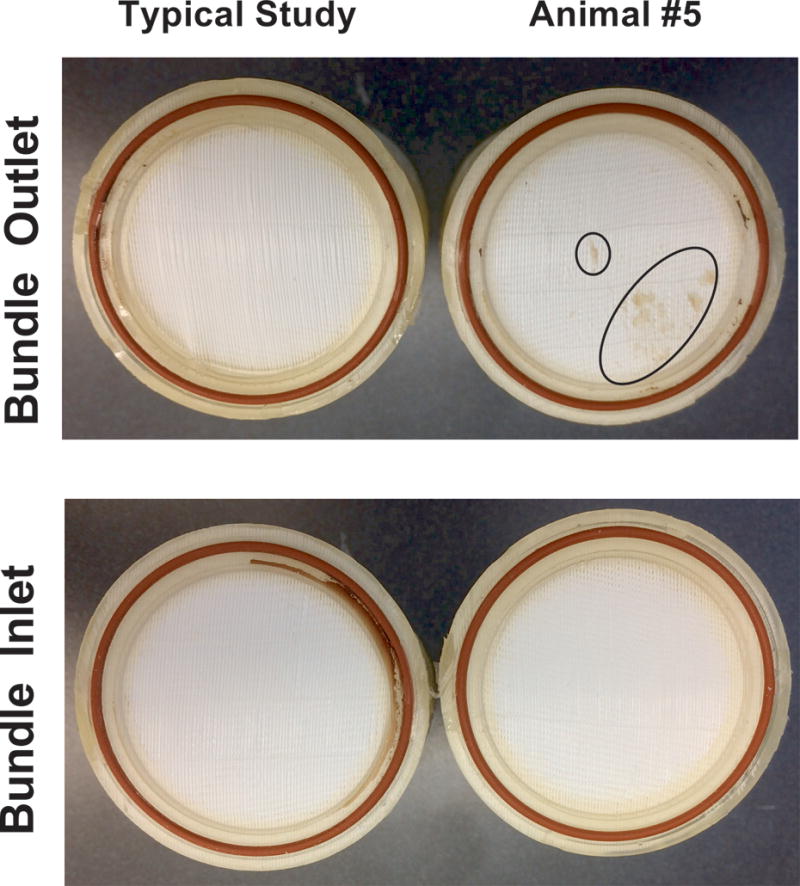
Photographs comparing a typical fiber bundle and the fiber bundle from Animal 5
Figure 4.
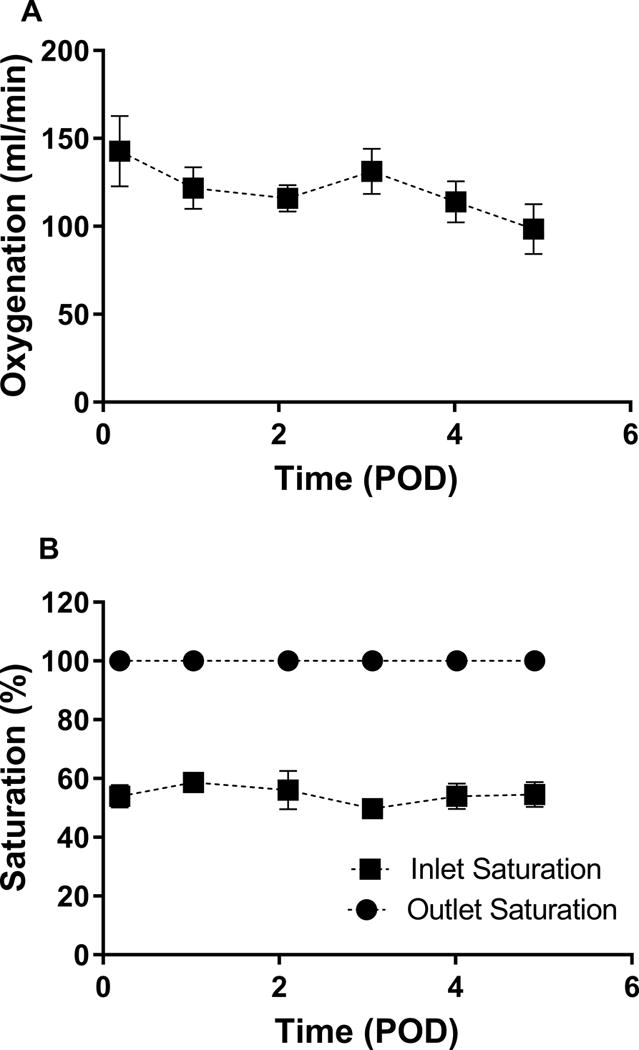
In-vivo oxygenation (A) and blood saturation (B) in the PAAL.
Figure 5.
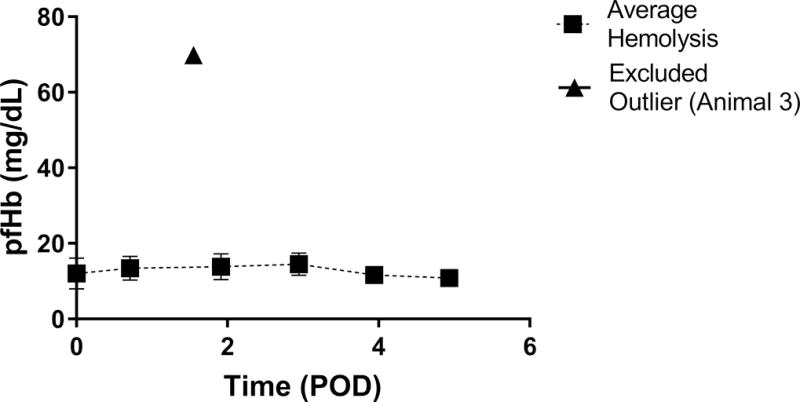
In-vivo hemolysis in the PAAL device. The outlier (triangle) is shown but excluded from the daily average hemolysis.
CD62-P positive platelets remained below 10% during the study (Table 2). Additionally CD62-P positive platelets stimulated with platelet activating factor (PAF) showed increased activation (>60%). Platelets reviewed on a blood film microscopically by a laboratory technician at IDEXX Labs (Westbrook, ME) were adequate in number (>300 K/μL). Blood chemistry parameters measuring kidney function, liver function, and cell and tissue injury were within normal ranges and did not statistically change from baseline over the study course. No damage to the heart, lungs, kidney or liver was seen upon gross examination in a necropsy. Bleeding in Animal 5 led to a hematoma formed around the venous line in the neck.
Table 2.
Hematology and end organ function parameters over the study course
| Parameter | Pre-Op | POD 0 | POD 3b | POD 5 |
|---|---|---|---|---|
| Platelet Status and Hematology | ||||
| CD62-P (%) | 14 ± 19 | 3.7 ± 1 | 5 ± 0 | 8.8 ± 8 |
| PAF-Activated (%) | 73 ± 10 | 69 ± 10 | 59.7 ± 20 | 68 ± 20 |
| Platelet Count | Adequate | Adequate | Adequate | Adequate |
| WBC (k/μL) | 8.6 ± 1 | 2.4 ± 1 | 9.25 ± 4 | 8.6 ± 2 |
| Hct (%) | 30 ± 8 | 21.8 ± 4 | 23.6 ± 3 | 21 ± 4 |
| End Organ Status | ||||
| AST (U/L) | 92 ± 20 | 59 ± 10 | 63 ± 4 | 65 ± 30 |
| ALT (U/L) | 16 ± 4 | 10 ± 3a | 8.5 ± 0.7 | 10 ± 4 |
| CK (U/L) | 110 ± 5 | 120 ± 90 | 129 ± 70 | 100 ± 40 |
| BUN (mg/dL) | 13 ± 3 | 14 ± 3 | 13 ± 1 | 14 ± 2 |
| Creatinine (mg/dL) | 1.1 ± 0.2 | 0.83 ± 0.2 | 0.75 ± 0.07 | 0.87 ± 0.2 |
POD = Post operative day; Pre-Op = Pre-operative
PAF = Platelet activating factor; WBC = White blood cell; Hct = Hematocrit; AST = Aspartate transferase; ALT = Alanine transferase; CK = Creatinine kinase; BUN = Blood urea nitrogen
Significantly different from Pre-Op (p < 0.05)
Animal 5 bloodwork not collected due to low Hct
Discussion
Morbidity and mortality is exacerbated in patients treated with MV and ECMO from confinement to an ICU bed.1,2,6,7 Studies show that ambulating patients on ECMO improves their physical condition and post-transplant outcomes.9,13,14,15 The PAAL design minimizes device size and simplifies ambulation by integrating components into a single compact and wearable artificial lung device. We tested the PAAL in awake sheep for five days in this study. Our study found that the device functioned consistently over five days with no change in oxygenation capacity and resistance to flow through the device. Minimal to no thrombus was seen, and hemolysis remained low. Platelet activation was low (below 10%) and did not increase over the study duration. Pre-operative blood for the platelet activation assay was drawn through venipuncture, which likely led to variability and higher CD62-P expression. These preliminary data indicate that the PAAL can be implanted for five days without change in oxygenation capacity or significant blood damage and blood clotting problems.
All sheep survived the surgery, and three sheep survived the study duration. Suturing the cannula to the neck and using a neck wrap for support kept the cannula free of bends in studies that followed and solved this issue. The neck wrap on our animals was changed daily as the movement of the animal loosened the wrap overnight. Future studies will not include stop-cocks on negative pressure lines of the circuit. The post-operative bleeding in Animal 5 required ACT levels to remain 165-180s (150s at baseline). We noted only few point deposits (Figure 3) at the end of the study and platelet activation remained low (<20%) in Animal 5. This was an encouraging result because we set the ACT between 1.5 and 2 times baseline in other studies. No device related complications were noted in our study and device performance was uncompromised despite these events.
Flowrate in the OR is likely higher than in the ICU because of animal positioning. The animal is placed in a stanchion (Figure 2) when in the ICU. Pump speed and flow for each animal was optimized based on the animal’s behavior in the stanchion. Flowrates in our study are within the range of prior studies in sheep using a 27 Fr. Avalon cannula25. We did not directly measure the amount of blood recirculation between the inlet and outlet lumens of the Avalon cannula. The Avalon cannula design minimizes the recirculation seen in traditional VV-ECMO. Previous investigators have demonstrated that the Avalon cannula has low recirculation ~2% at 2 L/min as long as the cannula tip remains in the IVC13. We placed our cannula under fluoroscopy as mentioned in the methods section. We also checked cannula positioning in the necropsy. Saturation difference across an oxygenator decreases as recirculation rate increases13. We show the inlet and outlet device saturations in Figure 3B are different by almost 50% and the inlet saturation remains within 40% and 60%. This indicates we did not have clinically meaningful recirculation. Recirculation likely does not account for the decrease in flowrate over the study course.
Our study is the first examining a wearable, fully integrated pump-lung in chronic sheep with single site cannulation. Other chronic studies of artificial lungs include the thoracic artificial lung (TAL) and ambulatory pump lung (APL) devices18,19. The TAL is a pump-less artificial lung connected to the patient in a pulmonary artery (PA) to left atrium configuration. The APL integrates a magnetically levitated centrifugal pump with an artificial lung and is connected to the patient in a right atrium (RA) to PA configuration. Neither the PAAL, TAL nor APL have apparent negative effects on hemodynamics in healthy sheep. Creatinine kinase levels in this study were lower than the APL and TAL studies since the PAAL was implanted less invasively through peripheral cannulation and did not require a thoracotomy. Liver function was not impaired during our study based on aspartate transferase and alanine transferase levels which were equal to or lower than TAL and APL studies. The in-vivo performance of the PAAL was comparable to the existing devices.
In-vitro and in-vivo performance of a device are important while assessing gas exchange efficiency. In-vitro performance indicates oxygenation under ideal conditions. This level of oxygenation can deteriorate with clot formation under in-vivo conditions. Rated flow is an important parameter in considering the in-vitro performance of a device and is defined as an inlet saturation of 65% and an outlet saturation of 95%. The rated flow of the TAL is not reported, however is likely over 7 L/min based on in-vitro gas exchange data26. The rated flow of the APL is ~3.5 L/min. The rated flow of the PAAL is ~3.75 L/min. There was minimal to no clotting in the APL over 30 days or in the PAAL over 5 days. The PAAL provides over 180 ml/min oxygenation at 3.5 L/min in-vitro as previously reported23 and has 38% of the surface area of the TAL and 80% of the surface area of the APL. The PAAL has a higher efficiency that the APL (275 ml/min/m2 vs 195 ml/min/m2). A direct comparison to the TAL is not made because of the difference in the principles of operation. Smaller fiber surface area should reduce adverse blood – material interactions. The PAAL is also physically smaller than the current generation of clinically approved devices, the Cardiohelp and the TandemLung, because of the integration of the pump and oxygenator into a single compact unit. In addition, the PAAL has 50% the surface area of the Cardiohelp and 36% of the surface area of the TandemLung.
The hematocrit in our study was 30% ± 8 pre-operatively which decreased to 21.8 ± 4 on POD 0 and was 21% ± 4 on POD 5. Other groups have also reported post-operative decreases in hematocrit. Wu et. al. report a drop in hematocrit from 32% to 27%18. Hematocrit recovered after a period of two weeks. The drop is attributed to blood loss and fluid infusion during surgery. Zhou et. al28. report a drop in hemoglobin from 12.7 g/dL to as low as 9.2 g/dL over the 30 days. The hemoglobin did not recover to baseline values in their study. Hemodilution in our studies could arise from volume infusions during surgery as animals received between 500cc and 1000cc of saline during surgery. In addition, our device and loop priming volume is ~200cc. Blood volume of our sheep was ~3300ml (~60ml/kg). For an initial Hct of 30% the estimated dilution would decrease Hct to 22% to 25%, given a saline infusion of 700cc-1200cc. Stress (from being restrained) and anesthesia can also acutely decrease hematocrit in sheep due to sequestration of red blood cells in the spleen27. A combination of hemodilution from saline infusion and sequestration of red blood cells likely cause the decrease in hematocrit in our study.
Healthy sheep were used for evaluating the PAAL. Healthy animals are commonly used when characterizing the performance of cardiopulmonary devices18,19,24. Furthermore, the FDA also recommends the use of healthy animals for pre-clinical studies28. ECMO has been established to have a role in treating patients with severe lung failure. Our study simply makes a compact and portable system that provides respiratory support in manner similar to more cumbersome systems.
The device presented here would undergo product development before clinical use. “Wall” medical oxygen flowed through the PAAL in this study. We envision that product development prior to human studies will include the design of a portable oxygen cylinder that the patient can roll on a trolley. The PAAL will also be coupled with a motor-controller unit, and a battery pack. We envision the batteries and controllers would be designed in a manner similar to the Heartmate and HeartWare VADs (total ~3.3 to 4.5 lb). The controller and batteries can typically be work on a waist pack or body harness. Patients have been ambulated outside the ICU using the DLC cannula. With a stable cannulation, patients would be able to be ambulatory and leave the hospital. The analogy here is with the progress we have made with VADs in which we routinely send patients home at present. Originally, VADs required confining patients to the ICU and the hospital ward. Evolution in technology has now enabled VAD patients to be cared for at home or in assisted living facilities.
In conclusion, we have developed a compact integrated wearable artificial lung that was implanted in sheep for five days with no device related complications. We improved our cannulation procedure and tested our post-operative management protocol. Chronic use of the PAAL in awake sheep is promising based on our study.
Acknowledgments
This study was supported by NIH grant number RO1 HL117637, the Commonwealth of PA and the McGowan Institute for Regenerative Medicine. The authors thank Robin Geisler for manufacturing the holster that secured the device to the animal.
This study was supported by NIH grant number RO1 HL117637, the Commonwealth of PA and the McGowan Institute for Regenerative Medicine. William J. Federspiel chairs the Scientific Advisory Board and is a Founder of ALung Technologies, in which he has an equity interest. The PAAL technology has not been licensed or optioned to ALung Technologies. Other authors do not have any financial disclosures related to the work presented in this manuscript.
Footnotes
Disclosure Statement
William J. Federspiel chairs the Scientific Advisory Board and is a Founder of ALung Technologies, in which he has an equity interest. The PAAL technology has not been licensed or optioned to ALung Technologies. Other authors do not have any financial disclosures related to the work presented in this manuscript.
References
- 1.Villar J, Blanco J, Añón JM, et al. The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med. 2011;37:1932–1941. doi: 10.1007/s00134-011-2380-4. [DOI] [PubMed] [Google Scholar]
- 2.Fan E, Villar J, Slutsky AS. Novel approaches to minimize ventilator-induced lung injury. BMC Medicine. 11(85):2013. doi: 10.1186/1741-7015-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ranieri VM, Suter PM, Tortorella C, et al. Effect of Mechanical Ventilation on Inflammatory Mediators in Patients With Acute Respiratory Distress Syndrome: A Randomized Controlled Trial. JAMA. 1999;282:54–61. doi: 10.1001/jama.282.1.54. [DOI] [PubMed] [Google Scholar]
- 4.MacLaren G, Combes A, Bartlett RH. Contemporary extracorporeal membrane oxygenation for adult respiratory failure: life support in the new era. Intensive Care Med. 2011;38:210–220. doi: 10.1007/s00134-011-2439-2. [DOI] [PubMed] [Google Scholar]
- 5.Garcia JP, Iacono A, Kon ZN, Griffith BP. Ambulatory extracorporeal membrane oxygenation: a new approach for bridge-to-lung transplantation. J Thorac Cardiovasc Surg. 2010;139:137–139. doi: 10.1016/j.jtcvs.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 6.Strueber M. Artificial Lungs: Are We There yet? Thoracic Surgery Clinics. 2015;25:107–113. doi: 10.1016/j.thorsurg.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Biscotti M, Sonett J, Bacchetta M. ECMO as Bridge to Lung Transplant. Thoracic Surgery Clinics. 2015;25:17–25. doi: 10.1016/j.thorsurg.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Maury G, Langer D, Verleden G, et al. Skeletal Muscle Force and Functional Exercise Tolerance Before and After Lung Transplantation: A Cohort Study. American Journal of Transplantation. 2008;8:1275–1281. doi: 10.1111/j.1600-6143.2008.02209.x. [DOI] [PubMed] [Google Scholar]
- 9.Haneya A, Philipp A, Foltan M, et al. First experience with the new portable extracorporeal membrane oxygenation system Cardiohelp for severe respiratory failure in adults. Perfusion. 2012;27:150–155. doi: 10.1177/0267659111432330. [DOI] [PubMed] [Google Scholar]
- 10.Palanzo D, Qiu F, Baer L, Clark JB, Myers JL, Ündar A. Evolution of the Extracorporeal Life Support Circuitry. Artificial Organs. 2010;34:869–873. doi: 10.1111/j.1525-1594.2010.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D, Zhou X, Liu X, Sidor B, Lynch J, Zwischenberger JB. Wang-Zwische Double Lumen Cannula—Toward a Percutaneous and Ambulatory Paracorporeal Artificial Lung. ASAIO Journal. 2008;54:606–611. doi: 10.1097/MAT.0b013e31818c69ab. [DOI] [PubMed] [Google Scholar]
- 12.Biscotti M, Bacchetta M. The “Sport Model”: Extracorporeal Membrane Oxygenation Using the Subclavian Artery. The Annals of Thoracic Surgery. 2014;98:1487–1489. doi: 10.1016/j.athoracsur.2014.02.069. [DOI] [PubMed] [Google Scholar]
- 13.Reeb J, Olland A, Renaud S, et al. Vascular access for extracorporeal life support: tips and tricks. J Thorac Dis. 2016;8:S353–S363. doi: 10.21037/jtd.2016.04.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajagopal K, Hoeper MM. State of the Art: Bridging to lung transplantation using artificial organ support technologies. The Journal of Heart and Lung Transplantation. 2016;35:1385–1398. doi: 10.1016/j.healun.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Pruijsten R, van Thiel R, Hool S, Saeijs M, Verbiest M, Miranda DR. Mobilization of patients on venovenous extracorporeal membrane oxygenation support using an ECMO helmet. Intensive Care Med. 2014;40:1595–1597. doi: 10.1007/s00134-014-3410-9. [DOI] [PubMed] [Google Scholar]
- 16.Wang D, Zhou X, Lick SD, Liu X, Qian K, Zwischenberger JB. An Ambulatory Pulmonary and Right Heart Assist Device (OxyRVAD) in an Ovine Survival Model. The Journal of Heart and Lung Transplantation. 2007;26:974–979. doi: 10.1016/j.healun.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 17.Heart Transplant Recipient Receives the World’s First 31 Fr ProtekDuoTM Cannula in a Life-Saving TandemLung® Case | Business Wire. 2016 Available at: http://www.businesswire.com. Accessed February 2, 2017.
- 18.Wu ZJ, Zhang T, Bianchi G, et al. 30-Day In-vivo Performance of a Wearable Artificial Pump-Lung for Ambulatory Respiratory Support. Ann Thorac Surg. 2012;93:274–281. doi: 10.1016/j.athoracsur.2011.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato H, Griffith GW, Hall CM, et al. Seven-day artificial lung testing in an in-parallel configuration. The Annals of thoracic surgery. 2007;84:988–994. doi: 10.1016/j.athoracsur.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Babcock S. Breethe, Inc. is making an artificial lung for patients to take home from the hospital. Technically Baltimore. 2015 https://technical.ly/baltimore/2015/04/16/breethe-inc-bartley-griffith-artificial-lung/. Accessed July 6, 2017.
- 21.Madhani SP, Frankowski B, Burgreen GW, Antaki JF, Kormos R, D’Cunha J, Federspiel WJ. In Vitro and In Vivo Evaluation of a Novel Integrated Wearable Artificial Lung. JHLT. 2017 doi: 10.1016/j.healun.2017.02.025. Published Ahead of Print, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madhani SP, Frankowski BJ, Federspiel WJ. Fiber bundle design for an integrated wearable artificial lung [e-pub ahead of print] ASAIO J; . Accessed March 17, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maquet HLS Set Advanced Flyer. https://www.maquet.com/int/products/hls-set-advanced/. Accessed January 19, 2017.
- 24.Johnson CA, Wearden PD, Kocyildirim E, et al. Platelet Activation in Ovines Undergoing Sham Surgery or Implant of the Second Generation PediaFlow™ Pediatric Ventricular Assist Device. Artif Organs. 2011;35:602–613. doi: 10.1111/j.1525-1594.2010.01124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou X, Wang D, Sumpter R, Pattison G, Ballard-Croft C, Zwischenberger JB. Long-term support with an ambulatory percutaneous paracorporeal artificial lung. The Journal of Heart and Lung Transplantation. 2012;31:648–654. doi: 10.1016/j.healun.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schewe RE, Khanafer KM, Arab A, Mitchell JA, Skoog DJ, Cook KE. Design and In Vitro Assessment of an Improved, Low-Resistance, Compliant Thoracic Artificial Lung. ASAIO J. 2012;58:583–589. doi: 10.1097/MAT.0b013e31826dcd23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turner AW, Hodgetts VE. The dynamic red cell storage function of the spleen in sheep. I. Relationship to fluctuations of jugular haematocrit. Aust J Exp Biol Med Sci. 1959;37:399–420. doi: 10.1038/icb.1959.42. [DOI] [PubMed] [Google Scholar]
- 28.FDA Center for Devices and Radiological Health. General Considerations for Animal Studies for Medical Devices – Draft Guidance for Industry and Food and Drug Administration Staff. 2010 Jul 29; Issued. [Google Scholar]


