Abstract
Heavy episodic alcohol consumption (also termed binge drinking) contributes to a wide range of health and cognitive deficits, but the associated brain-based indices are poorly understood. The current study used electroencephalography (EEG) to examine spontaneous neural oscillations in young adults as a function of quantity, frequency, and the pattern of their alcohol consumption. Sixty-one young adults (23.4 ± 3.4 years of age) were assigned to binge drinking (BD) and light drinking (LD) groups that were equated on gender, race/ethnic identity, age, educational background, and family history of alcoholism. EEG activity was recorded during eyes-open and eyes-closed resting conditions. Each participant’s alpha peak frequency (APF) was used to calculate absolute power in individualized theta and alpha frequency bands, with a canonical frequency range used for beta. APF was slower by 0.7 Hz in BD, especially in individuals engaging in high-intensity drinking but there were no changes in alpha power. BD also exhibited higher frontal theta and beta power than LD. Alpha slowing and increased theta power in BD remained after accounting for depression, anxiety, and personality characteristics, while elevated beta power covaried with sensation seeking. Furthermore, APF slowing and theta power correlated with various measures of alcohol consumption including binge episodes and blackouts, but not with measures of working and episodic memory, cognitive flexibility, processing speed, or personality variables, suggesting that these physiological changes may be modulated by high-intensity alcohol intake. These results are consistent with studies of alcohol use disorder (AUD) and support the hypothesis that binge drinking is a transitional stage towards alcohol dependence. The observed thalamocortical dysrhythmia may be indicative of an excitatory-inhibitory imbalance in BD and may potentially serve as an index of the progressive development of AUD with a goal of informing possible interventions to minimize alcohol’s deleterious effects on the brain.
Keywords: Theta, Alpha, Beta, Alpha peak frequency, resting-state EEG, alcohol
Introduction
Binge drinking
Excessive alcohol consumption imposes a tremendous burden on society in terms of economic cost and increased morbidity and mortality rates (Center for Disease Control and Prevention [CDC], 2015). Heavy episodic drinking, also termed binge drinking, accounts for 77% of these costs and is prevalent among young adults and university students (Farke & Anderson, 2007; Grucza, Norberg, & Bierut, 2009; Wechsler et al., 2002). Binge drinking is usually defined as consuming 5 or more standardized alcoholic drinks for men (4 drinks for women) in about 2 hours, which is likely to result in blood alcohol concentration reaching or exceeding 0.08% (National Institute on Alcohol Abuse and Alcoholism [NIAAA], 2016). Research evidence indicates that binge drinking is associated with deficits in affective and memory processing, attention, and inhibitory control (Hartley, Elsabagh, & File, 2004; Mullan, Wong, Allom, & Pack, 2011; Parada et al., 2011; Townshend & Duka, 2005). Such negative implications are critical, considering that the brain continues to mature well into the twenties (Sowell et al., 2003; Westlye et al., 2010) and emerging adults may be particularly vulnerable to deleterious effects of excessive alcohol use (Barron et al., 2005). It has also been proposed that binge drinking reflects a transitional phase toward dependence, as prolonged alcohol consumption induces behavioral and neurological adaptations to alcohol effects (Gilpin & Koob, 2008; Koob, 2006). Thus, further research is needed to examine brain-based indices of the deficits that persist beyond a binge episode and to evaluate their association with drinking levels and patterns.
Resting state EEG in alcohol research
Electro- and magnetoencephalography (EEG and MEG) are used to measure spontaneous neural oscillations during wakeful rest, also termed resting-state, rsEEG/MEG (Rosen et al., 2014; Schomer & Lopes da Silva, 2011). Because it does not require any task engagement, this method has been applied across ages and in diverse populations to examine spontaneous oscillations in various brain-based disorders and conditions (Porjesz et al., 2005; Schomer & Lopes da Silva, 2011) including chronic alcoholism (Begleiter & Porjesz, 2006; Porjesz et al., 2005; Rangaswamy & Porjesz, 2014). The rsEEG signal is analyzed in frequency domain and expressed as a power spectrum (PS) profile across a range of frequencies that traditionally include alpha (8-12 Hz), beta (15-20 Hz), and theta (4-7 Hz) (Nunez & Srinivasan, 2006).
Alpha oscillations feature prominently over the posterior scalp regions especially during an eyes-closed resting condition. They are modulated by altering visual input or attention, such as opening the eyes, and are thought to be sensitive to corticothalamic interactions and levels of arousal (Barry, Clarke, Johnstone, Magee, & Rushby, 2007; Nunez, Wingeier, & Silberstein, 2001; Pfurtscheller & Andrew, 1999). Alpha is quantified by measuring spectral power within its frequency range and by determining its peak frequency (APF) for each participant (Klimesch, 1999). Acute alcohol intoxication induces a decrease in alpha oscillatory frequency and an increase in alpha power (Ehlers, Wall, & Schuckit, 1989; Nikulin, Nikulina, Yamashita, Rossi, & Kahkonen, 2005). Source modeling of the MEG signal has confirmed that alpha power is generated primarily in the medial parieto-occipital area and is increased during alcohol challenge (Rosen et al., 2014). Studies of rsEEG reveal lower alpha power in individuals with alcohol use disorder (AUD) (Begleiter & Platz, 1972; Saletu-Zyhlarz et al., 2004) who also exhibit a low-voltage alpha (LVA) variant that is comorbid with high anxiety (Enoch & Goldman, 1999). Similar lower alpha power was observed by Finn and Justus (1999) in offspring of people with AUD, while another group reported a contradictory higher voltage alpha (Ehlers & Schuckit, 1991).
Spontaneous theta and beta oscillations reflect anteriorly localized rhythms (Rosen et al., 2014), and are altered as a function of acute and chronic alcohol exposure (Rangaswamy et al., 2003; Rangaswamy et al., 2002; Stenberg, Sano, Rosen, & Ingvar, 1994). Increased power in theta and beta bands has previously been observed in acutely intoxicated, healthy participants (Rosen et al., 2014; Stenberg et al., 1994). Elevated beta power has also been found among both people with AUD and their offspring (Bauer & Hesselbrock, 1993; Finn & Justus, 1999; Pollock, Earleywine, & Gabrielli, 1995; Rangaswamy et al., 2002; Rangaswamy et al., 2004; Winterer et al., 1998), whereas higher theta power has only been reported in individuals with AUD (Pollock, Schneider, Zemansky, Gleason, & Pawluczyk, 1992; Propping, Kruger, & Mark, 1981; Rangaswamy et al., 2003).
There is a dearth of studies investigating rsEEG as a function of heavy episodic drinking patterns. Courtney & Polich (2010) recorded EEG during eyes-open (EO) resting condition and reported greater power in fast beta (20-35 Hz) and delta (0-4 Hz) bands in high-binge drinkers than in low- and non-binge drinkers, but no group differences were found within the alpha or theta range. Correas and colleagues (2015) recorded MEG signal during eyes-closed (EC) resting state and observed higher power in theta (4-6 Hz) and narrow alpha (9-11 Hz) range in individuals with a history of binge drinking. Inconsistencies between these studies may have originated from methodological differences including focusing on EO vs EC conditions, the criteria used to define binge drinking and frequency band definitions. This especially pertains to the adjoining frequency bands such as theta, usually defined as spanning 4-7 Hz, and alpha, canonically defined as encompassing 8-12 Hz. The APF is rarely quantified in rsEEG studies and yet slowing of alpha frequency may be reflected in lower alpha and increased theta power, conflating those measures. In an effort to mitigate this problem in a principled way, the present study defined alpha and theta frequency ranges based on individual APF (Angelakis, Lubar, Stathopoulou, & Kounios, 2004; Haegens, Cousijn, Wallis, Harrison, & Nobre, 2014; Klimesch, 1999).
Aims and Hypotheses
The primary objective of the present study was to examine spontaneous oscillations as a function of binge drinking patterns and alcohol consumption levels by quantifying the APF and power of alpha, theta, and beta oscillations within their EEG PS profiles. Continuous EEG signal was recorded during eyes-open and closed (EOC) wakeful rest (Rosen et al., 2014). Alpha and theta spectral bands were determined by taking into account individual APF (Angelakis et al., 2004; Klimesch, 1999), while beta was measured within a fixed frequency band. It was hypothesized that binge drinkers (BD) would exhibit slower APF and higher theta, beta, and alpha mean absolute power than light drinkers (LD), with the expectation that these effects would be particularly evident in individuals engaging in high-intensity drinking.
Methods
Participants
Sixty-one healthy, right-handed young adults were recruited for the current study (M ± SD = 23.4 ± 3.4 years of age, 30 females). None of the participants reported tobacco or illicit drug use at least one month prior to the experiment, a history of neuropsychiatric conditions or brain injury, or medication use at the time of the study. Based on the reported patterns of heavy episodic drinking, the participants were classified into a binge drinking (BD, n = 30), and a light drinking (LD, n = 31) group. A binge episode was defined as consuming 6+ drinks for males and 5+ drinks for females within two hours (Lange & Voas, 2001; NIAAA, 2016). In the previous six months, BDs reported five or more binge episodes (14.8 ± 10.3), whereas LDs had no more than one binge episode in that time period (0.1 ± 0.3). Participants who reported at least one first degree blood relative, excluding their mother, or three or more second degree relatives with AUD were considered to be family history (FH) positive. As shown in Table 1, the groups were matched for gender, race/ethnic identity, age, educational background, and FH of alcoholism, but differed on measures of alcohol consumption as well as some personality traits. The participants gave written informed consent and received monetary compensation for their participation in the study.
Table 1.
Group characteristics
| BD (n = 30)
|
LD (n = 31)
|
U/X2
|
p
|
|
|---|---|---|---|---|
| Age | 23.41 ± 3.45 | 23.32 ± 3.40 | 460 | ns |
| % Male | 50% | 48% | 0.018+ | ns |
| % White (Non-Hispanic) | 66% | 71% | 0.345+ | ns |
| % FH+ for alcoholism | 56% | 44% | 0.205+ | ns |
| Education years | 15.80 ± 2.08 | 15.90 ± 1.99 | 416 | ns |
| Undergraduate GPA | 3.1 ± 0.5 | 3.4 ± 0.4 | 277 | < .05 |
| No. of drinking days/wk | 3.1 ± 1.2 | 1.5 ± 0.7 | 131 | < .001 |
| No. of drinks/occasion | 5.5 ± 1.5 | 1.8 ± 0.9 | 18 | < .001 |
| No. of binge episodes (past 6 mo.) | 14.8 ± 10.3 | 0.1 ± 0.3 | 0 | < .001 |
| No. of alcohol-induced blackouts (past 6 months) | 4.6 ± 3.6 | 0 ± 0.2 | 2 | < .001 |
| No. of drunk occasions/month | 5.6 ± 4.7 | 2.0 ± 1.6 | 149 | < .001 |
| Max no. of drinks in 24 hrs (past 30 days) | 12.6 ± 5.8 | 4.9 ± 2.0 | 25 | < .001 |
| Age of drinking onset | 15.9 ± 1.5 | 18.6 ± 2.0 | 142 | < .001 |
| Severity of alcoholism (SMAST) | 3.2 ± 3.3 | 0.6 ± 1.0 | 190 | < .001 |
| Drinks needed for effects (SRE) | 5.6 ± 1.1 | 4.4 ± 1.3 | 257 | < .01 |
| Drinking motives (DMQ-R) | ||||
| Enhancement | 6.8 ± 1.3 | 5.3 ± 1.6 | 209 | < .01 |
| Social | 7.7 ± 1.3 | 6.4 ± 1.6 | 231 | < .01 |
| Conformity | 4.3 ± 1.5 | 4.0 ± 1.2 | 398 | ns |
| Coping | 5.1 ± 1.8 | 4.0 ± 1.0 | 225 | < .01 |
| Drinking consequences (B-YAACQ) | 11.0 ± 5.3 | 2.0 ± 1.9 | 40 | < .001 |
| Anxiety (GAD-7) | 4.5 ± 5.3 | 2.3 ± 3.2 | 306 | < .05 |
| Depression (PHQ-9) | 4.7 ± 5.0 | 2.0 ± 1.9 | 306 | < .05 |
| Impulsivity (ABIS) | 2.07 ± 0.5 | 1.8 ± 0.3 | 334 | ns |
| Attention | 9.86 ± 2.4 | 9.4 ± 2.2 | 364 | ns |
| Motor | 8.55 ± 2.5 | 7.3 ± 1.9 | 321 | ns |
| Non-Planning | 8.48 ± 2.9 | 6.9 ± 1.7 | 309 | ns |
| Sensation seeking (BSSS) | 3.8 ± 0.7 | 3.4 ± 0.6 | 302 | < .05 |
| Personality (EPQ) | ||||
| Neuroticism | 3.8 ± 3.5 | 3.2 ± 3.1 | 385 | ns |
| Psychoticism | 2.6 ± 2.1 | 2.3 ± 1.6 | 420 | ns |
| Extraversion | 9.5 ± 2.1 | 8.1 ± 3.5 | 352 | ns |
| Working memory | 0.76 ± 0.10 | 0.74 ± 0.11 | 393 | ns |
| Episodic memory | 0.76 ± 0.19 | 0.80 ± 0.14 | 370 | ns |
| Cognitive flexibility | 0.87 ± 0.14 | 0.92 ± 0.05 | 342 | ns |
| Processing speed | 0.58 ± 0.07 | 0.59 ± 0.08 | 377 | ns |
M ± SD were calculated for all measurements. All questionnaire variables were analyzed with Mann Whitney U Test. Gender, race/ethnicity, and family history (FH) of alcoholism were analyzed with χ2 marked with+.
Experimental Protocol
Participants completed a battery of questionnaires (Table 1) including the frequency and quantity of alcohol consumption (modified from Cahalan, Cisin, & Crossley, 1969), daily drinks consumed during the previous month (Timeline Follow-Back, TLFB; Sobell & Sobell, 1992), the level of response to alcohol (Self-rating of the Effects of Alcohol, SRE; Schuckit, Tipp, Smith, Wiesbeck, & Kalmijn, 1997), the severity of alcoholism-related symptoms (Self-Administered Short Michigan Alcoholism Screening Test, SMAST; Selzer, Vinokur, & van Rooijen, 1975), motivation for engaging in alcohol use (Drinking Motive Questionnaire Revised Short Form, DMQ-R SF; Kuntsche & Kuntsche, 2009), and alcohol-related consequences (Brief Young Adult Alcohol Consequencs Questionnaire, B-YAACQ; Read, Kahler, Strong, & Colder, 2006). Additional sets of questionnaires were administered to screen for depression (Patient Health Questionnaire, PHQ-9; Kroenke & Spitzer, 2002) and anxiety (General Anxiety Disorder, GAD-7; Spitzer, Kroenke, Williams, & Lowe, 2006), as well as to assess personality traits (Eysenck Personality Questionnaire, EPQ; Eysenck & Eysenck, 1975), impulsivity traits (Impulsiveness Scale, ABIS; Coutlee, Politzer, Hoyle, & Huettel, 2014), and sensation seeking (Brief Sensation Seeking Scale, BSSS; Hoyle, Stephenson, Palmgreen, Lorch, & Donohew, 2002). As part of the NIH-Toolbox, the List Sorting Working Memory (LSWM) Test, Picture Sequence Memory (PSM) Test, Dimensional Change Card Sort (DCCS) Test, and Pattern Comparison Processing Speed (PCPS) Test were administered to evaluate working memory, episodic memory, cognitive flexibility, and processing speed respectively (Gershon et al., 2013).
Participants were instructed to abstain from alcohol consumption for at least 48 hours preceding the study. Upon arrival, a Discover 12-panel urine drug test (America Screening Corporation, Shreveport, LA) was administered to screen for psychoactive substances. Participants were fitted with an electrocap and took part in an EEG recording during an EOC wakeful resting paradigm. During the EO condition, participants were asked to focus on a fixation cross for 2 minutes. The EC condition commenced as participants kept their eyes closed for two minutes. Two other tasks were also administered, and their results will be reported elsewhere.
Data acquisition and processing
EEG signal was recorded with a 64-channel actiCap BrainVision (Brain Products GmbH, Germany) with a sampling rate of 500 Hz. Electrode impedance level was maintained below 5 kΩ. The nose served as reference and the forehead as ground. Electro-oculogram (EOG) was recorded in a bipolar fashion from two electrodes positioned above and below the left eye.
EEG data analysis was performed with MATLAB (Mathworks, Natick, MA) routines that incorporated publicly available algorithms such as FieldTrip toolbox (Oostenveld, Fries, Maris, & Schoffelen, 2011) and EEGLAB (Delorme & Makeig, 2004). The relevant parts of the analysis script and raw data samples will be provided upon request. Continuous EEG data were bandpass filtered at 0.3-200 Hz and segmented into 4-sec epochs. Epochs containing visible movement artifacts were removed based on visual inspection. Eye blinks, eye movements, and heart beats were further eliminated with Independent Component Analysis (ICA) (Delorme & Makeig, 2004). The complex power spectrum (PS) of the absolute power of each epoch was calculated with a multitapered Fast Fourier Transform method using Hanning tapers (Oostenveld et al., 2011). The PS profiles were computed with 0.25 Hz resolution for the 1-30 Hz frequency range and averaged across all epochs for each EOC condition for each participant. The PS were averaged across electrode clusters to produce spectral profiles for the frontal (AFz, AF3, AF4, Fz, F1, F2, F3, F4, F5, F6), central (Cz, C1, C2, C3, C4, CPz, CP1, CP2, CP3, CP4), and posterior (POz, PO3, PO4, O1, O2, Oz) regions. Group averages for the EC and EO conditions for the three electrode clusters are shown in Fig. 1.
Fig. 1.
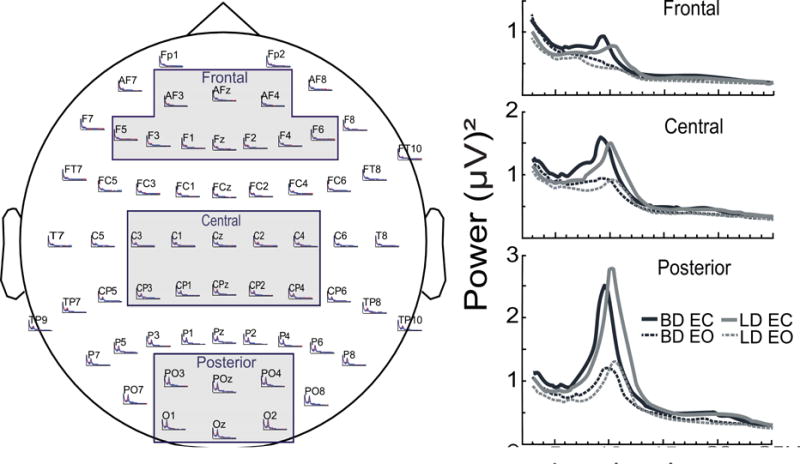
Power Spectra (PS) profiles were averaged across electrode clusters corresponding to frontal, central, and posterior scalp regions. Superimposed are mean absolute PS for the eyes-open (EO) and eyes-closed (EC) conditions for the binge drinking (BD) and light drinking (LD) groups.
Alpha peak frequency (APF) was defined as the frequency at which alpha oscillation exhibits the greatest power. It was determined within the 8-12 Hz frequency range for each participant and each condition across all electrodes with an automatic detection algorithm and was used to define alpha band for each participant. Alpha power was computed within a frequency interval ranging from 2 Hz below to 2 Hz above the individual APF. Theta was defined as a 3 Hz wide band starting at 4 Hz below the lowest alpha boundary. These custom-tailored frequency ranges made it possible to proportionally account for the inter- and intrasubject variability in PS distribution and to mitigate the confounding influences of peak frequency differences on power measures within the adjacent theta and alpha bands. Power in beta frequency band was determined within 15-20 Hz frequency range.
Data analysis
Mixed model ANOVAs were carried out with the between-subject factor of Group (BD and LD) and with EOC as the within-subject factor on APF and power within alpha, theta, and beta bands (SPSS, 2001). Gender and FH were included in the initial analysis but no main effects or interactions regarding these factors were found so they were excluded from further analyses. ANCOVAs were performed to verify whether the observed group differences remained after controlling for non-alcohol related variables including anxiety, depression, and sensation seeking. To further probe the relation between EEG measures and the level of alcohol consumption, subsequent mixed design ANOVAs were performed with participant grouping based on weekly drinking levels as the between-subjects factor and EOC condition as the within-subject factor. Pearson’s chi-square tests were conducted to determine group differences on categorical variables including gender, race, and FH, whereas Mann-Whitney U tests were used for all other variables since they mostly violated the normality assumption (Table 1). Nonparametric Spearman’s rho (denoted as rs) coefficient was utilized to compute correlations between EEG measures and other variables.
Results
Drinking-related questionnaires and personality measures
BD reported higher levels of alcohol consumption and more drinking consequences including blackouts and alcoholism-related symptoms, earlier age of drinking onset, more drinking motives, and higher scores on anxiety, depression, and sensation seeking in comparison to the LD group (Table 1).
Alpha
As expected, the greatest power was found in the alpha band (Fig 1). It was most prominent over the posterior sites where it was possible to measure APF reliably across both EOC conditions, so statistical comparisons focused on that region. Alpha power was greater during EC relative to EO condition, F(1, 59) = 142.6, p < .001 (Fig. 2a) but there was no main effect of Group or Group × EOC interaction. Alpha power did not correlate with any of the alcohol-related variables, but it negatively correlated with enhancement (rs = −0.32, p < .05) and extraversion (rs = −0.33, p < .05).
Fig. 2.
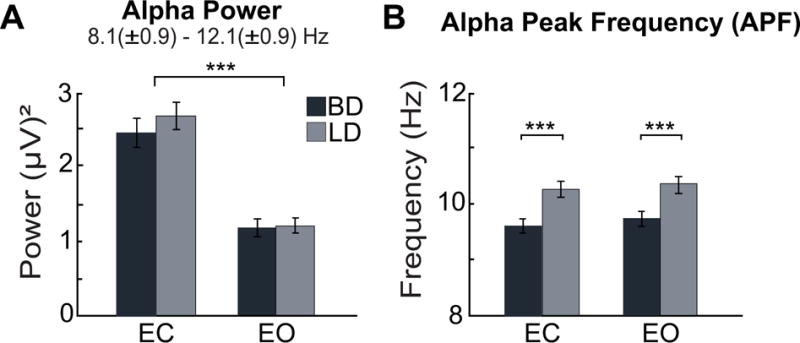
Alpha power and alpha peak frequency (APF) during eyes-closed (EC) and eyes-open (EO) conditions over the posterior scalp in binge (BD) and light drinkers (LD). (A) Alpha power was greater during EC compared to EO but no group differences were observed. (B) Slower APF was found in BDs across both EOC conditions. ***p < 0.001.
In contrast, APF was slower by 0.7 Hz in BD compared to LD, F(1,59) = 17.3, p < .001 (Fig. 2b), and this difference persisted after controlling for anxiety, depression, and sensation seeking (all ps < .01). No significant main effect of EOC nor interaction between the factors of Group and EOC were found. APF correlated negatively with many drinking variables including the number of drinking days per week (rs = −0.44, p < .001), drinks per occasion (rs = −0.43, p < .001), number of binge episodes (rs = −0.47, p < .001), and alcohol-induced blackouts (rs = −0.43, p < .001) in the previous six months, in addition to the number of heavy drinking days (rs = −0.38, p < .01), and the maximum number of drinks in 24 hours (rs = −0.46, p < .001) in the past 30 days. APF also correlated negatively with self-rated drinking consequences (rs = −0.40, p < .01). It correlated positively with the age of drinking onset (rs = 0.28, p < .05) but no association was found between APF and anxiety or depression.
Theta
A main effect of Group on theta power displayed a fronto-posterior gradient with the BD showing greater theta power than LD over the frontal area, F(1, 59) = 10.3, p < .01 (Fig. 3). Group effect on frontal theta power persisted after controlling for anxiety, depression, and sensation seeking (all ps < .01). Theta power was greater during EC for all participants, F(1, 59) = 6.2, p < .05. There was no interaction between the factors of Group and EOC. Nonparametric Spearman’s rho revealed positive correlations between frontal theta power and the number of inebriated occasions per month (rs = 0.32, p < .05), number of binge episodes in the previous six months (rs = 0.32, p < .05) and self-rated drinking consequences scores (rs = 0.31, p < .05). Theta power did not correlate with anxiety or depression.
Fig. 3.
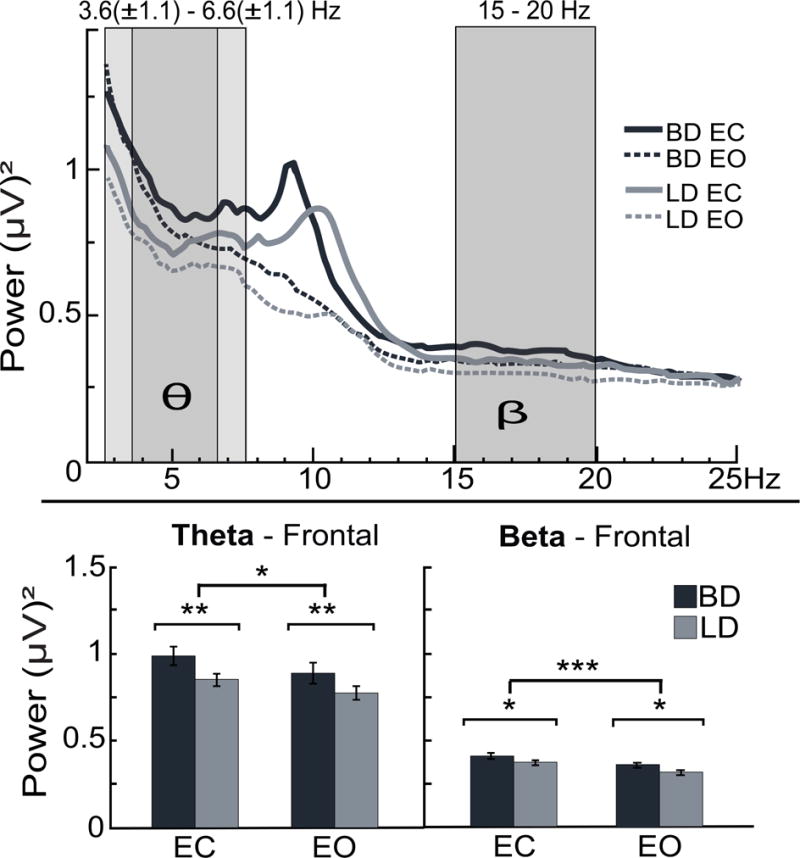
Grand average frontal power spectra are superimposed for binge drinking (BD) and light drinking (LD) groups across eyes-closed (EC) and eyes-open (EO) conditions. Averaged theta band was determined within 3.6 [± 1.1] – 6.6 [± 1.1] Hz, as indicated with darker (average) and lighter [± SD] shaded boxes. Frontal beta was measured within a fixed frequency band of 15-20 Hz. BDs exhibit higher overall theta (θ) and beta (β) power than LDs. Closing the eyes resulted in an enhancement of theta and beta power. *p < 0.05; **p < 0.01; ***p < 0.001.
Beta
Higher beta power was observed in BD compared to LD in frontal regions, F(1, 59) = 4.4, p < .05 (Fig. 3). The main effect of group remained significant when anxiety and depression were included in the analysis as covariates (ps < .05), but not after controlling for sensation seeking. Closing the eyes enhanced frontal beta power in all participants, F(1, 59) = 18.2, p < .001 but there was no interaction between the factors of Group and EOC. Beta power was positively correlated with drinking consequences scores (rs = 0.27, p < .05) and a dimension of the Brief Sensation Seeking Scale, disinhibition (rs = 0.31, p < .05). It did not correlate, however, with any of the variables related to drinking levels.
EEG Measures and Drinking-related Variables
In an effort to reduce the dimensionality of drinking history variables, we have carried out a principal component analysis (PCA). The PCA (SPSS, 2001) encompassed alcohol-related variables including the number of drinking days per week, number of drinks per occasion, number of drunk occasions and maximum number of drinks consumed in 24 hours within the last 30 days, number of binge episodes and alcohol-induced blackouts within the past 6 months, severity of alcoholism (SMAST), drinks needed for effects (SRE), and drinking consequences (B-YAACQ) (Table 2). The first factor had an eigen value of 5.07 and explained 56% of the variance, while the second factor had an eigen value of 1.06 and explained 12%. The eigen values for the other factors did not exceed one (Kaiser, 1960). The Anderson-Rubin method (Anderson & Rubin, 1956) was used to estimate score coefficients for factor 1 representing a combination of variables related to drinking patterns and to examine their associations with EEG features. Posterior alpha peak frequency was negatively correlated with drinking history during EO and EC (rs = −0.30, p < .05 and rs = −0.35, p < .01). Similarly, frontal theta power was positively correlated with drinking history during both resting conditions (rs = 0.38, p < .01 and rs = 0.32, p < .05). Beta power did not significantly correlate with drinking history.
Table 2.
Factor loadings on principal components
| Component 1 (Eigenvalue = 5.07) |
Component 2 (Eigenvalue = 1.06) |
|
|---|---|---|
| No. of drinking days/wk | .708 | .043 |
| No. of drinks/occasion | .891 | .040 |
| Max no. of drinks in 24 hrs | .822 | −.106 |
| No. of binge episodes | .764 | −.378 |
| No. of alcohol-induced blackouts | .773 | −.425 |
| Severity of alcoholism (SMAST) | .582 | .483 |
| Drinks needed for effects (SRE) | .623 | −.378 |
| Drinking consequences (B-YAACQ) | .879 | .023 |
High-intensity regular drinking levels modulate APF
Engaging in binge drinking is associated with alterations of resting EEG indices across the three frequency bands. However, binge episodes are relatively infrequent and may only partly reflect regular drinking patterns. To further investigate the extent to which regular alcohol intake modulates EEG measures, participants were reassigned into three groups based on their weekly levels of alcohol consumption, similar to other studies using this approach (de Bruin, Stam, Bijl, Verbaten, & Kenemans, 2006; Oddy & Barry, 2009). Males who reported consuming 22 or more drinks/week (M ± SD = 28.3 ± 5.62), or females who reported 12 or more drinks/week (16.8 ± 4.43), were assigned into the high alcohol intake group (HiAlc, n = 21). Males who reported 8-20 drinks/week (13.5 ± 5.50), or females who reported 4-10 (5.7 ± 1.70), were assigned into the moderate alcohol intake group (ModAlc, n = 18). Males who reported less than 6 drinks/week (2.7 ± 1.44), or females who reported less than 2 (1.1 ± 0.22), were assigned into the moderate alcohol intake group (LowAlc, n = 19). There was a main effect of group on the mean APF, F(2, 58) = 7.3, p < .01. Tukey HSD post-hoc comparisons revealed that APF was slower among HiAlc (9.6 ± 0.76 Hz) than in ModAlc (10.3 ± 0.67 Hz) and LowAlc (10.4 ± 0.99 Hz) (p < .01). However, no differences in APF were found between ModAlc and LowAlc (Fig. 4). There were no group differences in spectral power for any of the frequency bands.
Fig. 4.
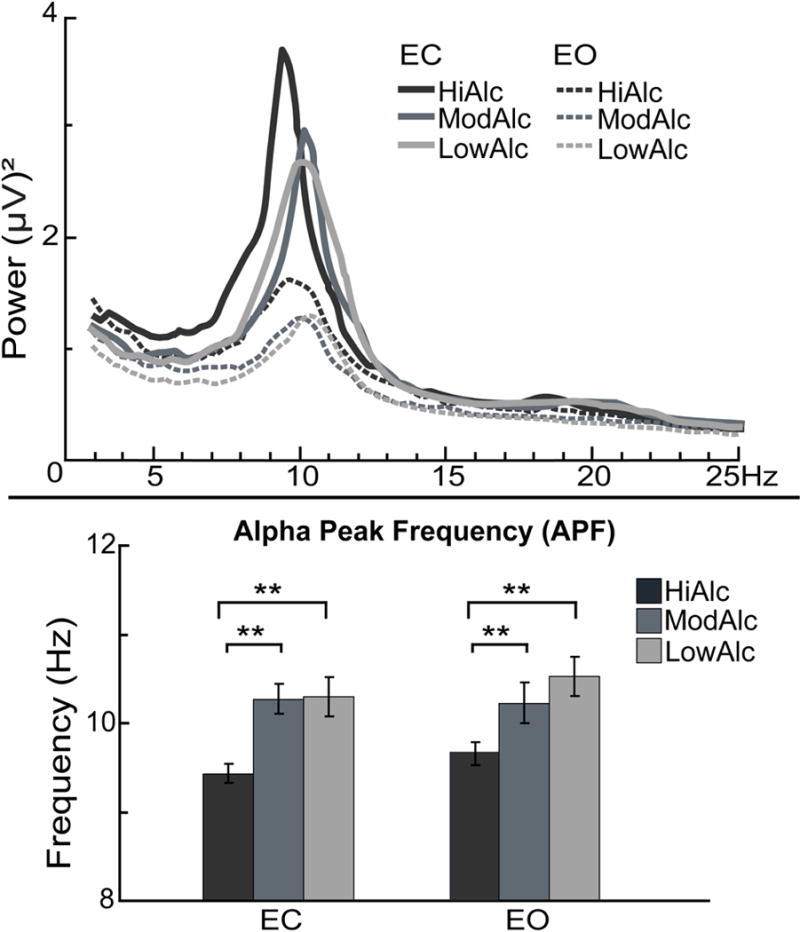
Grand average posterior power spectra are superimposed for high (HiAlc), moderate (ModAlc), and low alcohol intake (LowAlc) groups, in addition to eyes-closed (EC) and eyes-open (EO) conditions. The effect of group was reflected in a slower APF in HiAlc relative to ModAlc and LowAlc, during both EO and EC. *p < 0.05; **p < 0.01; ***p < 0.001.
Discussion
In the present study, spectral characteristics of EEG signal were analyzed during wakeful rest as a function of binge drinking patterns and alcohol consumption levels. Peak frequency of alpha oscillations was slower in binge drinking young adults, even when personality characteristics were accounted for. Alpha slowing was correlated with various measure of increased alcohol consumption but it was not associated with cognitive functions. In fact, the APF was slower among those participants who reported the heaviest levels of weekly drinking. In contrast, alpha power did not differ as a function of binge episodes or drinking levels. Compared to LD, BD participants exhibited greater power in frontal theta and beta frequency bands, with theta power increase correlating with high intensity drinking. As expected, EEG spectral power was elevated in all bands during the EC resting condition, but APF was not sensitive to changes in visual input.
Alpha peak frequency slowing
Although the assessment of individual APF is rarely performed in alcohol research, alpha slowing has been observed during acute intoxication (Ehlers et al., 1989; Lukas, Mendelson, Benedikt, & Jones, 1986). While the participants in the current study were not inebriated, APF was slower by 0.7 Hz in those engaging in heavy episodic drinking and this effect remained after controlling for anxiety, depression, and sensation seeking. Even though the current study could not determine whether the slower APF is a pre-existing characteristic of BDs, its association with drinking habits and insensitivity to personality variables suggests that it may reflect enduring neural adaptation to habitual, hazardous levels of alcohol use. This conjecture is supported by the observation that the most prominent alpha slowing was observed in those who reported hazardous levels of weekly drinking. In the current study, the number of binge episodes reported for the past 6-month period was highly correlated with the weekly alcohol intake (rs = 0.79, p < .001), indicating that the individuals who imbibe at high levels on a regular basis also frequently exceed the criterion for binge drinking. This is consistent with other evidence on heavy drinking patterns and the associated consequences (Courtney & Polich, 2009; Hingson, Zha, & White, 2017; Megan E Patrick, Terry-McElrath, Kloska, & Schulenberg, 2016). Even though future efforts are needed to better characterize the multidimensional phenotype of alcohol consumption, the current results demonstrate that APF reduction is associated with heavy drinking levels. In contrast, individuals drinking at light or moderate levels did not show alpha slowing even if they occasionally engaged in binge drinking. Moreover, APF was slower in participants who began drinking at a younger age, supporting the notion that perpetual alcohol consumption over protracted time period can lead to persistent changes in alpha oscillations. Taken together, these results provide neurophysiological evidence that high-intensity alcohol intake modulates spontaneous oscillations, with more extreme and longer-term consumption resulting in more prominent alpha slowing, consistent with the notion of a greater degree of associated harm (Haber, Harris-Olenak, Burroughs, & Jacob, 2016; M. E. Patrick, 2016; Megan E Patrick et al., 2016). Furthermore, binge drinking in adolescence and emerging young adulthood is associated with neurocognitive deficits confirming increased vulnerability to neurotoxic effects of alcohol during this maturational phase (Jacobus & Tapert, 2013).
Individual APF variability has been shown to relate to cognitive capacity (Angelakis et al., 2004; Grandy et al., 2013), with slower APF at rest predicting poor performance during digit span tasks (Angelakis et al., 2004; Clark et al., 2004), general intelligence tests (Grandy et al., 2013), and memory tasks (Klimesch, Doppelmayr, Pachinger, & Ripper, 1997). In the current experiment, however, NIH-Toolbox cognitive scores did not correlate with APF. Persistent slowing of APF has previously been identified in several neurological and neuropsychiatric disorders including Alzheimer’s disease (Jeong, 2004; Moretti et al., 2004), Parkinson’s disease (Moazami-Goudarzi, Sarnthein, Michels, Moukhtieva, & Jeanmonod, 2008), mild traumatic brain injury (Angelakis et al., 2004), schizophrenia (Boutros et al., 2008), neurogenic (Sarnthein, Stern, Aufenberg, Rousson, & Jeanmonod, 2006) and neuropathic pain (Boord et al., 2008). Based on intracranial EEG recordings in humans and evidence from animal studies, it has been suggested that alpha slowing reflects dysrhythmia of the thalamocortical (TC) system (Llinás, Urbano, Leznik, Ramírez, & Van Marle, 2005; Sarnthein & Jeanmonod, 2007, 2008). Given that it has been observed in a number of brain-based disorders and conditions, alpha slowing may serve as an indicator of a basic-level brain dysfunction resulting from deficits in the TC networks that support sensory and cognitive functions (Hughes & Crunelli, 2005).
Converging evidence suggests that alpha emerges from an interaction between the generators in the thalamus and the neocortex (Bollimunta, Chen, Schroeder, & Ding, 2008; Hughes & Crunelli, 2005; Lopes da Silva, 2011). In vitro work with slices of the lateral geniculate nucleus shows that alpha can be intrinsically generated by high-threshold bursting neurons which are synchronized by gap junction in addition to electrical synapses (Hughes & Crunelli, 2005; Lorincz, Crunelli, & Hughes, 2008). Rhythms in alpha range can be induced by agonists acting on metabotropic glutamate and muscarinic acetylcholine receptors (Hughes et al., 2004; Lorincz et al., 2008). Furthermore, activity of the TC neurons is strongly modulated by the inhibitory input by GABAergic interneurons (Lorincz, Kekesi, Juhasz, Crunelli, & Hughes, 2009). Hyperpolarization of the TC neuronal populations could shift alpha oscillations toward the theta range (Bollimunta, Mo, Schroeder, & Ding, 2011; Hughes & Crunelli, 2005). This oscillatory deceleration is frequently accompanied by increased theta power (Cozac et al., 2016) or reduced alpha-band activity (Boutros et al., 2008; Jeong, 2004; Sarnthein & Jeanmonod, 2007, 2008).
It has been established that ethanol administration potentiates GABAergic signaling (Nevo & Hamon, 1995; Paul, 2006; Santhakumar, Wallner, & Otis, 2007) which can directly affect alpha oscillations by amplifying inhibitory influence on the co-oscillatory connections between the cortex and the thalamus (Steriade, McCormick, & Sejnowski, 1993). In addition to GABA, other neurotransmitter systems are also affected by alcohol which suppresses glutamatergic excitation and stimulates dopaminergic release (Nevo & Hamon, 1995), thereby altering oscillatory functions through multiple neurotransmitter systems (Vengeliene, Bilbao, Molander, & Spanagel, 2008). In contrast, long-term exposure is accompanied with compensatory neuroadaptations counteracting the primary effects of alcohol (Koob & Le Moal, 2008). They are expressed through down-regulation of GABAA (Devaud, Fritschy, Sieghart, & Morrow, 1997; Kang, Spigelman, & Olsen, 1998; Mihic et al., 1997) and nicotinic acetylcholine receptors (Hillmer et al., 2014), and increased glutamatergic function (Nevo & Hamon, 1995). Therefore, the slowing of APF in the BD group may reflect compensatory adaptations in GABAergic, glutamatergic, dopaminergic, and cholinergic systems as a result of prolonged alcohol exposure. The disruption of GABA-mediated inhibition (Llinás et al., 2005) and glutamatergic function (Zhang, Llinás, & Lisman, 2009) has been proposed to result in a dysrhythmic TC state, suggesting that these effects may underlie the observed association of high intensity drinking and slower APF. Alcohol abuse is also associated with damage of the thalamus (Pitel, Segobin, Ritz, Eustache, & Beaunieux, 2015), which can further contribute to dysregulated TC co-oscillations. A significant reversal of thalamic shrinkage was observed after about 8 months of abstinence, suggesting that the structural changes resulted from excessive alcohol consumption rather than just genetic influence (Cardenas, Studholme, Gazdzinski, Durazzo, & Meyerhoff, 2007). Thus, the observed slowing of cortico-thalamic interactions may be a consequence of structural and functional neural adaptations to regular high intensity drinking in young adults.
Alpha power
In contrast, no group differences in alpha power were observed in the current study which is in agreement with EEG results during EO condition (Courtney & Polich, 2010). However, lower alpha power in binge drinkers was reported in a MEG study using an EC resting paradigm (Correas et al., 2015). Methodological disparities may account for inconsistencies with respect to recording during EO vs EC conditions and the way in which alpha frequency band is calculated. When a fixed range is used, as is the case in most studies, alpha slowing may be conflated with decrease in alpha power found in individuals with AUD (Begleiter & Platz, 1972; Saletu-Zyhlarz et al., 2004).
Power increase in theta and beta bands
BD participants exhibited greater frontal theta power that correlated with the frequency of high-intensity drinking in the previous six months. Theta increase has previously been observed in alcoholic (Pollock et al., 1992; Rangaswamy et al., 2003), inebriated (Rosen et al., 2014; Stenberg et al., 1994), and binge drinking cohorts (Correas et al., 2015; de Bruin et al., 2004). Theta power of spontaneous oscillations is also increased in early onset Alzheimer’s disease (Schreiter-Gasser, Gasser, & Ziegler, 1994), individuals with mild traumatic brain injury (Huang et al., 2009) as well as attention deficit hyperactivity disorder (Clarke, Barry, McCarthy, Selikowitz, Brown, et al., 2003; Clarke, Barry, McCarthy, Selikowitz, Clarke, et al., 2003), although some of these effects could potentially be due to alpha peak slowing (Garces et al., 2013; Lansbergen, Arns, van Dongen-Boomsma, Spronk, & Buitelaar, 2011). Obtained in diverse conditions, these findings point to a nonspecific cortico-thalamic dysfunction which may be a result of a neurochemical imbalance in different neurotransmitter systems (Rangaswamy et al., 2003).
In the present study, higher beta power was observed in BD in frontal region, but this increase did not correlate with drinking levels. The group difference remained after controlling for anxiety and depression but not after the sensation seeking trait was used as a covariate. Elevated frontal beta power in BD is consistent with higher beta power exhibited by individuals with AUD (Pollock et al., 1992; Propping et al., 1981; Rangaswamy et al., 2002; Winterer et al., 1998), intoxicated individuals (Rosen et al., 2014; Stenberg et al., 1994), and binge drinkers (Courtney & Polich, 2010). Moreover, beta power is also increased in the relatives of people with AUD (Rangaswamy et al., 2004) and in individuals with antisocial personality and with a family history of alcoholism (Bauer & Hesselbrock, 1993). In contrast, it is reduced in patients with Alzheimer’s disease (Babiloni et al., 2006) and those diagnosed with attention deficit hyperactivity disorder (Lansbergen et al., 2011). This dissociation indicates that increased beta may not be a general indicator of neural dysfunction across a variety of clinical diagnoses but that it may hold specificity for a predisposition towards AUD development (Courtney & Polich, 2010; Rangaswamy et al., 2002; Rangaswamy et al., 2004). Beta oscillations are especially prominent over the sensorimotor cortex and are enhanced by benzodiazepines known to increase GABAA receptor efficiency (Hall, Barnes, Furlong, Seri, & Hillebrand, 2010). Furthermore, GABAA receptor genetic markers have been associated with beta oscillations (Porjesz et al., 2002) and have been proposed as endophenotypes for alcohol use disorder (Rangaswamy & Porjesz, 2008). Thus, a rise in beta spectral power may be induced by GABAA receptor deficiency resulting from an interaction between chronic alcohol use and genetic predisposition and may indicate disrupted excitatory-inhibitory homeostasis in binge drinkers (Porjesz et al., 2002).
Conclusions
In conclusion, binge drinking among young adults is associated with dysregulation of spontaneous EEG signal reflected in the slowing of alpha peak and increased power in theta and beta bands. The present study cannot speak directly to the question of whether these effects are due to premorbid traits or are primarily a result of heavy drinking and longitudinal studies are needed to resolve this important issue. However, the observed correlations between the various measures of alcohol consumption and the slowing of alpha peak and theta power suggest that the levels of habitual alcohol intake modulate these effects. Therefore, prolonged exposure to high doses of alcohol may induce enduring physiological changes in young adult binge drinkers which is consistent with the proposal that binge drinking is a transitional phase towards alcohol dependence, linking impulsive alcohol use to a more compulsive form of consumption (Gilpin & Koob, 2008; Koob, 2006). In contrast to alpha frequency and theta power, group differences in beta power were sensitive to sensation seeking. Together with the evidence of increased beta power in individuals with AUD and their relatives and the reported genetic linkage between beta oscillations and GABA receptor markers, this suggests that beta power may be associated with vulnerability to alcoholism. Therefore, the observed disruptions of the excitatory-inhibitory equilibrium involving a dysrhythmic TC network may serve as possible indices for the progressive development of AUD. In combination with genetic information they may inform possible interventions with a goal of attenuating excessive drinking and minimizing deleterious effects on the brain.
Supplementary Material
Highlights.
Binge drinking is associated with alpha slowing and increased theta and beta power.
Alpha slowing in young adults is especially prominent at hazardous drinking levels
Thalamocortical dysregulation may reflect a transition towards alcohol use disorder
Acknowledgments
This work was supported by start-up funds provided by the College of Sciences at San Diego State University; the National Institute on Alcohol Abuse and Alcoholism [R01-AA016624]; and the National Institutes of General Medical Sciences [5T34GM008303-28]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank Lauren Beaton, Audrey Andrews, Joe Happer, and Martina Knezevic for assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
None of the authors have potential conflicts of interest to be disclosed.
References
- Anderson TW, Rubin H. Statistical inference in factor analysis. Paper presented at the Proceedings of the third Berkeley symposium on mathematical statistics and probability 1956 [Google Scholar]
- Angelakis E, Lubar JF, Stathopoulou S, Kounios J. Peak alpha frequency: an electroencephalographic measure of cognitive preparedness. Clin Neurophysiol. 2004;115:887–897. doi: 10.1016/j.clinph.2003.11.034. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Binetti G, Cassarino A, Dal Forno G, Del Percio C, Ferreri F, et al. Sources of cortical rhythms in adults during physiological aging: a multicentric EEG study. Hum Brain Mapp. 2006;27:162–172. doi: 10.1002/hbm.20175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron S, White A, Swartzwelder HS, Bell RL, Rodd ZA, Slawecki CJ, et al. Adolescent vulnerabilities to chronic alcohol or nicotine exposure: Findings from rodent models. Alcoholism, clinical and experimental research. 2005;29:1720–1725. doi: 10.1097/01.alc.0000179220.79356.e5. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, Johnstone SJ, Magee CA, Rushby JA. EEG differences between eyes-closed and eyes-open resting conditions. Clin Neurophysiol. 2007;118:2765–2773. doi: 10.1016/j.clinph.2007.07.028. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. EEG, autonomic and subjective correlates of the risk for alcoholism. J Stud Alcohol. 1993;54:577–589. doi: 10.15288/jsa.1993.54.577. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Platz A. The effects of alcohol on the central nervous system in humans. In: Kissin B, Begleiter H, editors. The biology of alcoholism. Vol. 2. New York, US: Plenum Publishing Corporation; 1972. pp. 293–343. [Google Scholar]
- Begleiter H, Porjesz B. Genetics of human brain oscillations. Int J Psychophysiol. 2006;60:162–171. doi: 10.1016/j.ijpsycho.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Bollimunta A, Chen Y, Schroeder CE, Ding M. Neuronal mechanisms of cortical alpha oscillations in awake-behaving macaques. J Neurosci. 2008;28:9976–9988. doi: 10.1523/JNEUROSCI.2699-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollimunta A, Mo J, Schroeder CE, Ding M. Neuronal mechanisms and attentional modulation of corticothalamic alpha oscillations. J Neurosci. 2011;31:4935–4943. doi: 10.1523/JNEUROSCI.5580-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boord P, Siddall PJ, Tran Y, Herbert D, Middleton J, Craig A. Electroencephalographic slowing and reduced reactivity in neuropathic pain following spinal cord injury. Spinal Cord. 2008;46(2):118–123. doi: 10.1038/sj.sc.3102077. [DOI] [PubMed] [Google Scholar]
- Boutros NN, Arfken C, Galderisi S, Warrick J, Pratt G, Iacono W. The status of spectral EEG abnormality as a diagnostic test for schizophrenia. Schizophr Res. 2008;99(1–3):225–237. doi: 10.1016/j.schres.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan D, Cisin IH, Crossley HM. American drinking practices: A national study of drinking behavior and attitudes. Monographs of the Rutgers Center of Alcohol Studies. 1969;6:260. [Google Scholar]
- Cardenas VA, Studholme C, Gazdzinski S, Durazzo TC, Meyerhoff DJ. Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage. 2007;34(3):879–887. doi: 10.1016/j.neuroimage.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Fact sheets - Binge drinking. 2015 Retrieved May 10, 2017, from https://www.cdc.gov/alcohol/fact-sheets/binge-drinking.htm.
- Clark CR, Veltmeyer MD, Hamilton RJ, Simms E, Paul R, Hermens D, et al. Spontaneous alpha peak frequency predicts working memory performance across the age span. Int J Psychophysiol. 2004;53:1–9. doi: 10.1016/j.ijpsycho.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, McCarthy R, Selikowitz M, Brown CR, Croft RJ. Effects of stimulant medications on the EEG of children with Attention-Deficit/Hyperactivity Disorder Predominantly Inattentive type. Int J Psychophysiol. 2003;47:129–137. doi: 10.1016/s0167-8760(02)00119-8. [DOI] [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, McCarthy R, Selikowitz M, Clarke DC, Croft RJ. Effects of stimulant medications on children with attention-deficit/hyperactivity disorder and excessive beta activity in their EEG. Clin Neurophysiol. 2003;114:1729–1737. doi: 10.1016/s1388-2457(03)00112-3. [DOI] [PubMed] [Google Scholar]
- Correas A, Rodriguez Holguin S, Cuesta P, Lopez-Caneda E, Garcia-Moreno LM, Cadaveira F, et al. Exploratory analysis of power spectrum and functional connectivity during resting state in young binge drinkers: A MEG study. Int J Neural Syst. 2015;25:1550008. doi: 10.1142/S0129065715500082. [DOI] [PubMed] [Google Scholar]
- Courtney KE, Polich J. Binge drinking in young adults: Data, definitions, and determinants. Psychol Bull. 2009;135:142–156. doi: 10.1037/a0014414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Polich J. Binge drinking effects on EEG in young adult humans. Int J Environ Res Public Health. 2010;7:2325–2336. doi: 10.3390/ijerph7052325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutlee CG, Politzer CS, Hoyle RH, Huettel SA. An abbreviated impulsiveness scale constructed through confirmatory factor analysis of the Barratt Impulsiveness Scale version 11. Archives of Scientific Psychology. 2014;2:1–12. doi: 10.1037/arc0000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozac VV, Gschwandtner U, Hatz F, Hardmeier M, Ruegg S, Fuhr P. Quantitative EEG and cognitive decline in Parkinson’s disease. Parkinsons Dis. 2016;2016:9060649. doi: 10.1155/2016/9060649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin EA, Bijl S, Stam CJ, Bocker KB, Kenemans JL, Verbaten MN. Abnormal EEG synchronisation in heavily drinking students. Clin Neurophysiol. 2004;115(9):2048–2055. doi: 10.1016/j.clinph.2004.04.010. [DOI] [PubMed] [Google Scholar]
- de Bruin EA, Stam CJ, Bijl S, Verbaten MN, Kenemans JL. Moderate-to-heavy alcohol intake is associated with differences in synchronization of brain activity during rest and mental rehearsal. Int J Psychophysiol. 2006;60(3):304–314. doi: 10.1016/j.ijpsycho.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Fritschy JM, Sieghart W, Morrow AL. Bidirectional alterations of GABA(A) receptor subunit peptide levels in rat cortex during chronic ethanol consumption and withdrawal. J Neurochem. 1997;69:126–130. doi: 10.1046/j.1471-4159.1997.69010126.x. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Schuckit MA. Evaluation of EEG alpha activity in sons of alcoholics. Neuropsychopharmacology. 1991;4(3):199–205. [PubMed] [Google Scholar]
- Ehlers CL, Wall TL, Schuckit MA. EEG spectral characteristics following ethanol administration in young men. Electroencephalogr Clin Neurophysiol. 1989;73(3):179–187. doi: 10.1016/0013-4694(89)90118-1. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Goldman D. Genetics of alcoholism and substance abuse. Psychiatr Clin North Am. 1999;22:289–299. viii. doi: 10.1016/s0193-953x(05)70077-0. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ, Eysenck SBG. Manual of the Eysenck Personality Questionnaire (junior and adult) Hodder and Stoughton; 1975. [Google Scholar]
- Farke W, Anderson P. Binge drinking in Europe. Adicciones. 2007;19:333–339. [PubMed] [Google Scholar]
- Finn PR, Justus A. Reduced EEG alpha power in the male and female offspring of alcoholics. Alcohol Clin Exp Res. 1999;23:256–262. [PubMed] [Google Scholar]
- Garces P, Vicente R, Wibral M, Pineda-Pardo JA, Lopez ME, Aurtenetxe S, et al. Brain-wide slowing of spontaneous alpha rhythms in mild cognitive impairment. Front Aging Neurosci. 2013;5:100. doi: 10.3389/fnagi.2013.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon RC, Wagster MV, Hendrie HC, Fox NA, Cook KF, Nowinski CJ. NIH toolbox for assessment of neurological and behavioral function. Neurology. 2013;80(11 Suppl 3):S2–6. doi: 10.1212/WNL.0b013e3182872e5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Koob GF. Neurobiology of alcohol dependence: Focus on motivational mechanisms. Alcohol Research & Health. 2008;31:185–195. [PMC free article] [PubMed] [Google Scholar]
- Grandy TH, Werkle-Bergner M, Chicherio C, Lovden M, Schmiedek F, Lindenberger U. Individual alpha peak frequency is related to latent factors of general cognitive abilities. Neuroimage. 2013;79:10–18. doi: 10.1016/j.neuroimage.2013.04.059. [DOI] [PubMed] [Google Scholar]
- Grucza RA, Norberg KE, Bierut LJ. Binge drinking among youths and young adults in the United States: 1979-2006. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48:692. doi: 10.1097/CHI.0b013e3181a2b32f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber JR, Harris-Olenak B, Burroughs T, Jacob T. Residual Effects: Young Adult Diagnostic Drinking Predicts Late-Life Health Outcomes. J Stud Alcohol Drugs. 2016;77(6):859–867. doi: 10.15288/jsad.2016.77.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens S, Cousijn H, Wallis G, Harrison PJ, Nobre AC. Inter- and intraindividual variability in alpha peak frequency. Neuroimage. 2014;92:46–55. doi: 10.1016/j.neuroimage.2014.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SD, Barnes GR, Furlong PL, Seri S, Hillebrand A. Neuronal network pharmacodynamics of GABAergic modulation in the human cortex determined using pharmaco-magnetoencephalography. Hum Brain Mapp. 2010;31:581–594. doi: 10.1002/hbm.20889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley DE, Elsabagh S, File SE. Binge drinking and sex: effects on mood and cognitive function in healthy young volunteers. Pharmacol Biochem Behav. 2004;78:611–619. doi: 10.1016/j.pbb.2004.04.027. [DOI] [PubMed] [Google Scholar]
- Hillmer AT, Tudorascu DL, Wooten DW, Lao PJ, Barnhart TE, Ahlers EO, et al. Changes in the alpha4beta2* nicotinic acetylcholine system during chronic controlled alcohol exposure in nonhuman primates. Drug Alcohol Depend. 2014;138:216–219. doi: 10.1016/j.drugalcdep.2014.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson RW, Zha W, White AM. Drinking beyond the binge threshold: predictors, consequences, and changes in the US. American journal of preventive medicine. 2017;52(6):717–727. doi: 10.1016/j.amepre.2017.02.014. [DOI] [PubMed] [Google Scholar]
- Hoyle RH, Stephenson MT, Palmgreen P, Lorch EP, Donohew RL. Reliability and validity of a brief measure of sensation seeking. Personality and Individual Differences. 2002;32:401–414. [Google Scholar]
- Huang MX, Theilmann RJ, Robb A, Angeles A, Nichols S, Drake A, et al. Integrated imaging approach with MEG and DTI to detect mild traumatic brain injury in military and civilian patients. J Neurotrauma. 2009;26(8):1213–1226. doi: 10.1089/neu.2008.0672. [DOI] [PubMed] [Google Scholar]
- Hughes SW, Crunelli V. Thalamic mechanisms of EEG alpha rhythms and their pathological implications. Neuroscientist. 2005;11:357–372. doi: 10.1177/1073858405277450. [DOI] [PubMed] [Google Scholar]
- Hughes SW, Lorincz M, Cope DW, Blethyn KL, Kekesi KA, Parri HR, et al. Synchronized oscillations at alpha and theta frequencies in the lateral geniculate nucleus. Neuron. 2004;42:253–268. doi: 10.1016/s0896-6273(04)00191-6. [DOI] [PubMed] [Google Scholar]
- Jacobus J, Tapert SF. Neurotoxic effects of alcohol in adolescence. Annu Rev Clin Psychol. 2013;9:703–721. doi: 10.1146/annurev-clinpsy-050212-185610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J. EEG dynamics in patients with Alzheimer’s disease. Clin Neurophysiol. 2004;115:1490–1505. doi: 10.1016/j.clinph.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Kaiser HF. The application of electronic computers to factor analysis. Educational and psychological measurement. 1960;20(1):141–151. [Google Scholar]
- Kang MH, Spigelman I, Olsen RW. Alteration in the sensitivity of GABA(A) receptors to allosteric modulatory drugs in rat hippocampus after chronic intermittent ethanol treatment. Alcohol Clin Exp Res. 1998;22:2165–2173. [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Brain Res Rev. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Pachinger T, Ripper B. Brain oscillations and human memory: EEG correlates in the upper alpha and theta band. Neurosci Lett. 1997;238:9–12. doi: 10.1016/s0304-3940(97)00771-4. [DOI] [PubMed] [Google Scholar]
- Koob GF. The neurobiology of addiction: a neuroadaptational view relevant for diagnosis. Addiction. 2006;101(Suppl 1):23–30. doi: 10.1111/j.1360-0443.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3113–3123. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL. The PHQ-9 : A new depression measure. Psychiatric Annals. 2002;32:509–515. [Google Scholar]
- Kuntsche E, Kuntsche S. Development and validation of the Drinking Motive Questionnaire Revised Short Form (DMQ-R SF) J Clin Child Adolesc Psychol. 2009;38:899–908. doi: 10.1080/15374410903258967. [DOI] [PubMed] [Google Scholar]
- Lange JE, Voas RB. Defining binge drinking quantities through resulting blood alcohol concentrations. Psychol Addict Behav. 2001;15(4):310–316. doi: 10.1037//0893-164x.15.4.310. [DOI] [PubMed] [Google Scholar]
- Lansbergen MM, Arns M, van Dongen-Boomsma M, Spronk D, Buitelaar JK. The increase in theta/beta ratio on resting-state EEG in boys with attention-deficit/hyperactivity disorder is mediated by slow alpha peak frequency. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:47–52. doi: 10.1016/j.pnpbp.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Llinás R, Urbano FJ, Leznik E, Ramírez RR, Van Marle HJF. Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect. Trends in Neurosciences. 2005;28:325–333. doi: 10.1016/j.tins.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Lopes da Silva FH. Neurocognitive processes and the EEG/MEG. In: Schomer D, Lopes da Silva FH, editors. Niedermeyer’s Electroencephalography: Basic principles, clinical applications, and related fields. Philadelphia: Lippincott Williams & Wilkins; 2011. pp. 1083–1112. [Google Scholar]
- Lorincz ML, Crunelli V, Hughes SW. Cellular dynamics of cholinergically induced alpha (8-13 Hz) rhythms in sensory thalamic nuclei in vitro. J Neurosci. 2008;28:660–671. doi: 10.1523/JNEUROSCI.4468-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorincz ML, Kekesi KA, Juhasz G, Crunelli V, Hughes SW. Temporal framing of thalamic relay-mode firing by phasic inhibition during the alpha rhythm. Neuron. 2009;63:683–696. doi: 10.1016/j.neuron.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas SE, Mendelson JH, Benedikt RA, Jones B. EEG alpha activity increases during transient episodes of ethanol-induced euphoria. Pharmacol Biochem Behav. 1986;25:889–895. doi: 10.1016/0091-3057(86)90403-x. [DOI] [PubMed] [Google Scholar]
- Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, et al. Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature. 1997;389(6649):385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- Moazami-Goudarzi M, Sarnthein J, Michels L, Moukhtieva R, Jeanmonod D. Enhanced frontal low and high frequency power and synchronization in the resting EEG of parkinsonian patients. Neuroimage. 2008;41(3):985–997. doi: 10.1016/j.neuroimage.2008.03.032. [DOI] [PubMed] [Google Scholar]
- Moretti DV, Babiloni C, Binetti G, Cassetta E, Dal Forno G, Ferreric F, et al. Individual analysis of EEG frequency and band power in mild Alzheimer’s disease. Clin Neurophysiol. 2004;115:299–308. doi: 10.1016/s1388-2457(03)00345-6. [DOI] [PubMed] [Google Scholar]
- Mullan B, Wong C, Allom V, Pack SL. The role of executive function in bridging the intention-behaviour gap for binge-drinking in university students. Addict Behav. 2011;36:1023–1026. doi: 10.1016/j.addbeh.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Nevo I, Hamon M. Neurotransmitter and neuromodulatory mechanisms involved in alcohol abuse and alcoholism. Neurochem Int. 1995;26:305–342. doi: 10.1016/0197-0186(94)00139-l. [DOI] [PubMed] [Google Scholar]
- NIAAA. Drinking levels defined. 2016 Retrieved September 31, 2016, from http://www.niaaa.nih.gov/alcohol-health/special-populations-co-occurring-disorders/college-drinking.
- Nikulin VV, Nikulina AV, Yamashita H, Rossi EM, Kahkonen S. Effects of alcohol on spontaneous neuronal oscillations: a combined magnetoencephalography and electroencephalography study. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:687–693. doi: 10.1016/j.pnpbp.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R. Electric fields of the brain: The neurophysics of EEG. New York: Oxford University Press; 2006. [Google Scholar]
- Nunez PL, Wingeier BM, Silberstein RB. Spatial-temporal structures of human alpha rhythms: theory, microcurrent sources, multiscale measurements, and global binding of local networks. Hum Brain Mapp. 2001;13:125–164. doi: 10.1002/hbm.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddy BW, Barry RJ. The relationship of N2 and P3 to inhibitory processing of social drinkers in a Go/NoGo task. Int J Psychophysiol. 2009;72(3):323–330. doi: 10.1016/j.ijpsycho.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;2011:156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada M, Corral M, Caamano-Isorna F, Mota N, Crego A, Holguin SR, et al. Binge drinking and declarative memory in university students. Alcohol Clin Exp Res. 2011;35:1475–1484. doi: 10.1111/j.1530-0277.2011.01484.x. [DOI] [PubMed] [Google Scholar]
- Patrick ME. A Call for Research on High-Intensity Alcohol Use. Alcohol Clin Exp Res. 2016;40(2):256–259. doi: 10.1111/acer.12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick ME, Terry-McElrath YM, Kloska DD, Schulenberg JE. High-Intensity Drinking Among Young Adults in the United States: Prevalence, Frequency, and Developmental Change. Alcohol Clin Exp Res. 2016;40(9):1905–1912. doi: 10.1111/acer.13164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul SM. Alcohol-sensitive GABA receptors and alcohol antagonists. Proc Natl Acad Sci U S A. 2006;103(22):8307–8308. doi: 10.1073/pnas.0602862103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Andrew C. Event-Related changes of band power and coherence: methodology and interpretation. J Clin Neurophysiol. 1999;16:512–519. doi: 10.1097/00004691-199911000-00003. [DOI] [PubMed] [Google Scholar]
- Pitel AL, Segobin SH, Ritz L, Eustache F, Beaunieux H. Thalamic abnormalities are a cardinal feature of alcohol-related brain dysfunction. Neurosci Biobehav Rev. 2015;54:38–45. doi: 10.1016/j.neubiorev.2014.07.023. [DOI] [PubMed] [Google Scholar]
- Pollock VE, Earleywine M, Gabrielli WF. Personality and EEG beta in older adults with alcoholic relatives. Alcohol Clin Exp Res. 1995;19:37–43. doi: 10.1111/j.1530-0277.1995.tb01470.x. [DOI] [PubMed] [Google Scholar]
- Pollock VE, Schneider LS, Zemansky MF, Gleason RP, Pawluczyk S. Topographic quantitative EEG amplitude in recovered alcoholics. Psychiatry Res. 1992;45:25–32. doi: 10.1016/0925-4927(92)90011-r. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Almasy L, Edenberg HJ, Wang K, Chorlian DB, Foroud T, et al. Linkage disequilibrium between the beta frequency of the human EEG and a GABAA receptor gene locus. Proc Natl Acad Sci U S A. 2002;99:3729–3733. doi: 10.1073/pnas.052716399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M, Kamarajan C, Jones KA, Padmanabhapillai A, Begleiter H. The utility of neurophysiological markers in the study of alcoholism. Clin Neurophysiol. 2005;116:993–1018. doi: 10.1016/j.clinph.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Propping P, Kruger J, Mark N. Genetic disposition to alcoholism. An EEG study in alcoholics and their relatives. Hum Genet. 1981;59:51–59. doi: 10.1007/BF00278854. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B. Uncovering genes for cognitive (dys)function and predisposition for alcoholism spectrum disorders: a review of human brain oscillations as effective endophenotypes. Brain Res. 2008;1235:153–171. doi: 10.1016/j.brainres.2008.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B. Understanding alcohol use disorders with neuroelectrophysiology. Handb Clin Neurol. 2014;125:383–414. doi: 10.1016/B978-0-444-62619-6.00023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B, Chorlian DB, Choi K, Jones KA, Wang K, et al. Theta power in the EEG of alcoholics. Alcohol Clin Exp Res. 2003;27:607–615. doi: 10.1097/01.ALC.0000060523.95470.8F. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B, Chorlian DB, Wang K, Jones KA, Bauer LO, et al. Beta power in the EEG of alcoholics. Biol Psychiatry. 2002;52:831–842. doi: 10.1016/s0006-3223(02)01362-8. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B, Chorlian DB, Wang K, Jones KA, Kuperman S, et al. Resting EEG in offspring of male alcoholics: beta frequencies. Int J Psychophysiol. 2004;51:239–251. doi: 10.1016/j.ijpsycho.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Read JP, Kahler CW, Strong DR, Colder CR. Development and preliminary validation of the young adult alcohol consequences questionnaire. Journal of Studies on Alcohol. 2006;67:169–177. doi: 10.15288/jsa.2006.67.169. [DOI] [PubMed] [Google Scholar]
- Rosen BQ, O’Hara R, Kovacevic S, Schulman A, Padovan N, Marinkovic K. Oscillatory spatial profile of alcohol’s effects on the resting state: Anatomically-constrained MEG. Alcohol. 2014;48:89–97. doi: 10.1016/j.alcohol.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saletu-Zyhlarz GM, Arnold O, Anderer P, Oberndorfer S, Walter H, Lesch OM, et al. Differences in brain function between relapsing and abstaining alcohol-dependent patients, evaluated by EEG mapping. Alcohol Alcohol. 2004;39:233–240. doi: 10.1093/alcalc/agh041. [DOI] [PubMed] [Google Scholar]
- Santhakumar V, Wallner M, Otis TS. Ethanol acts directly on extrasynaptic subtypes of GABAA receptors to increase tonic inhibition. Alcohol. 2007;41:211–221. doi: 10.1016/j.alcohol.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnthein J, Jeanmonod D. High thalamocortical theta coherence in patients with Parkinson’s disease. J Neurosci. 2007;27(1):124–131. doi: 10.1523/JNEUROSCI.2411-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnthein J, Jeanmonod D. High thalamocortical theta coherence in patients with neurogenic pain. Neuroimage. 2008;39(4):1910–1917. doi: 10.1016/j.neuroimage.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Sarnthein J, Stern J, Aufenberg C, Rousson V, Jeanmonod D. Increased EEG power and slowed dominant frequency in patients with neurogenic pain. Brain. 2006;129(Pt 1):55–64. doi: 10.1093/brain/awh631. [DOI] [PubMed] [Google Scholar]
- Schomer D, Lopes da Silva F. Basic Principles, Clinical Applications, and Related Fields. Niedermeyer’s electroencephalography. 2011:1296. [Google Scholar]
- Schreiter-Gasser U, Gasser T, Ziegler P. Quantitative EEG analysis in early onset Alzheimer’s disease: correlations with severity, clinical characteristics, visual EEG and CCT. Electroencephalogr Clin Neurophysiol. 1994;90(4):267–272. doi: 10.1016/0013-4694(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Tipp JE, Smith TL, Wiesbeck GA, Kalmijn J. The relationship between Self-Rating of the Effects of alcohol and alcohol challenge results in ninety-eight young men. J Stud Alcohol. 1997;58:397–404. doi: 10.15288/jsa.1997.58.397. [DOI] [PubMed] [Google Scholar]
- Selzer ML, Vinokur A, van Rooijen L. A self-administered Short Michigan Alcoholism Screening Test (SMAST) J Stud Alcohol. 1975;36(1):117–126. doi: 10.15288/jsa.1975.36.117. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-Back. In: Litten RZ, Allen JP, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6(3):309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- SPSS. SPSS for Windows. Chicago, IL: SPSS Inc; 2001. [Google Scholar]
- Stenberg G, Sano M, Rosen I, Ingvar DH. EEG topography of acute ethanol effects in resting and activated normals. J Stud Alcohol. 1994;55:645–656. doi: 10.15288/jsa.1994.55.645. [DOI] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Townshend JM, Duka T. Binge drinking, cognitive performance and mood in a population of young social drinkers. Alcohol Clin Exp Res. 2005;29:317–325. doi: 10.1097/01.alc.0000156453.05028.f5. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. Br J Pharmacol. 2008;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler H, Lee JE, Kuo M, Seibring M, Nelson TF, Lee H. Trends in college binge drinking during a period of increased prevention efforts. Findings from 4 Harvard School of Public Health College Alcohol Study surveys: 1993-2001. J Am Coll Health. 2002;50:203–217. doi: 10.1080/07448480209595713. [DOI] [PubMed] [Google Scholar]
- Westlye LT, Walhovd KB, Dale AM, Bjornerud A, Due-Tonnessen P, Engvig A, et al. Differentiating maturational and aging-related changes of the cerebral cortex by use of thickness and signal intensity. Neuroimage. 2010;52(1):172–185. doi: 10.1016/j.neuroimage.2010.03.056. [DOI] [PubMed] [Google Scholar]
- Winterer G, Kloppel B, Heinz A, Ziller M, Dufeu P, Schmidt LG, et al. Quantitative EEG (QEEG) predicts relapse in patients with chronic alcoholism and points to a frontally pronounced cerebral disturbance. Psychiatry Res. 1998;78:101–113. doi: 10.1016/s0165-1781(97)00148-0. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Llinás RR, Lisman JE. Inhibition of NMDARs in the nucleus reticularis of the thalamus produces delta frequency bursting. Frontiers in Neural Circuits. 2009;3:1–9. doi: 10.3389/neuro.04.020.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


