Abstract
The circadian system orchestrates metabolism in daily 24-hour cycles. Such rhythms organize metabolism by temporally separating opposing metabolic processes and by anticipating recurring feeding-fasting cycles to increase metabolic efficiency. Although animal studies demonstrate that the circadian system plays a pervasive role in regulating metabolism, it is unclear how, and to what degree, circadian research in rodents translates into humans. Here, we review evidence that the circadian system regulates glucose, lipid, and energy metabolism in humans. Using a range of experimental protocols, studies in humans report circadian rhythms in glucose, insulin, glucose tolerance, lipid levels, energy expenditure, and appetite. Several of these rhythms peak in the biological morning or early afternoon, implicating earlier in the daytime as optimal for food intake. Importantly, disruptions in these rhythms impair metabolism and influence the pathogenesis of metabolic diseases. We therefore also review evidence that circadian misalignment induced by mistimed light exposure, sleep, or food intake adversely affects metabolic health in humans. These interconnections among the circadian system, metabolism, and behavior underscore the importance of chronobiology for preventing and treating diabetes, obesity, and hyperlipidemia.
Keywords: circadian, diurnal rhythm, glycemic control, lipids, energy metabolism, circadian misalignment, meal timing
INTRODUCTION
The circadian system organizes metabolism, physiology, and behavior in a daily cycle of circadian rhythms. Circadian derives from the Latin roots circa- meaning around and diēm meaning day, and like all daily or diurnal rhythms, circadian rhythms are periodic patterns that repeat themselves approximately every 24 hours. However, unlike diurnal rhythms, circadian rhythms are generated endogenously within the organism and perpetuate themselves even in the absence of external time cues (Figure 1). Such circadian rhythms have evolved over hundreds of millions of years to orchestrate metabolism by temporally separating opposing metabolic processes (such as anabolism and catabolism) and by anticipating recurring feeding-fasting cycles to optimize metabolic efficiency [1–3].
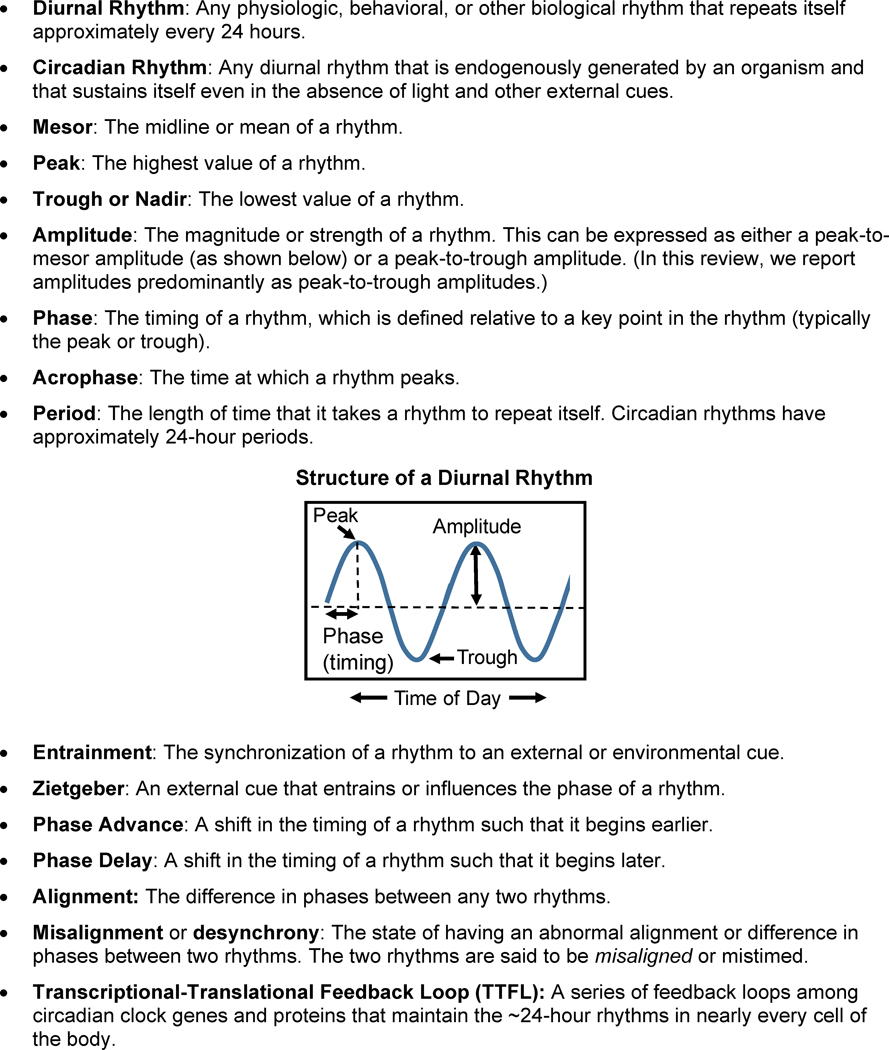
Figure 1.
Diurnal Rhythms Glossary.
The circadian system comprises a central pacemaker in the brain and a series of clocks in peripheral tissues throughout the body, including liver, muscle, and adipose tissue. This system of clocks collectively modulates a wide array of metabolic targets, such as glucocorticoids [4], the master energy sensor AMPK [5], rate-limiting steps in fatty acid and cholesterol synthesis [6, 7], and hepatic CREB to modulate gluconeogenesis [8]. The aggregate effect is that an array of metabolic processes—including insulin sensitivity, insulin secretion, cholesterol synthesis, fat oxidation, and energy expenditure—all follow a rhythm across the 24-hour day [2, 3, 9].
In addition to evidence of circadian rhythms in metabolism, data increasingly suggest that disruption of the circadian system increases the risk of metabolic diseases [9–12]. In rodent studies, clock gene mutants often display obese or diabetic phenotypes and possess defects in core metabolic pathways such as insulin secretion and gluconeogenesis [3, 13–17]. Moreover, misalignment of circadian rhythms in rodents often makes them hyperphagic, insulin resistant, and hyperlipidemic [9–12]. In human trials, circadian misalignment similarly elevates glucose, insulin, and triglyceride levels [18–20] and lowers energy expenditure [21]. Therefore, understanding these rhythms is important for timing when to eat, sleep, be exposed to bright light, be physically active, and even when to take medications to reduce the risk of metabolic diseases [22–24].
While there is ample mechanistic data in animal models demonstrating the wide-sweeping role of the circadian system in metabolism, there are comparatively fewer trials in humans. Given that rodents differ in several key ways from humans—such as being nocturnal, polyphasic (sleeping more than once per day), and having high metabolic rates per body weight—it is unclear how, and to what degree, circadian and diurnal research in rodents translates into humans. In this review, we synthesize evidence for circadian regulation of metabolism in humans. In Section 1, we provide an overview of the architecture of the circadian system and protocols for measuring circadian rhythms in humans. In Section 2, we summarize the evidence for circadian and diurnal rhythms in glucose, lipid, and energy metabolism in humans. In Section 3, we conclude by discussing how circadian alignment or misalignment with three external factors—light, sleep, and food intake—affects metabolism and the risk of metabolic diseases.
1. CIRCADIAN BIOLOGY
1.1. Architecture of the Circadian System
The circadian system consists of two parts: (1) a central clock located in the suprachiasmatic nucleus (SCN) of the hypothalamus and (2) a series of peripheral clocks located in virtually all other tissues of the body, including the liver, pancreas, gastrointestinal tract, skeletal muscle, and adipose tissue (Figure 2). The central clock is thought to regulate metabolism through diffusible factors (primarily cortisol and melatonin) and synaptic projections (including via the autonomic nervous system) [25, 26]. Peripheral tissues integrate these signals from the clock with environmental and behavioral factors (including light, sleep, physical activity, and feeding) and their own autonomous rhythms to regulate metabolism in a rhythmic manner [27]. The autonomous intracellular rhythms are maintained on a molecular level by clock genes and proteins that form a transcriptional-translational feedback loop (TTFL). The TTFL operates in a ~24-hour cycle, activating a rhythmic cascade of transcriptional and posttranscriptional events involving thousands of target genes [28]. In total, about 10% of genetic transcripts exhibit circadian periodicity, and moreover, an even larger number of proteins undergo oscillations arising from circadian rhythms at the post-transcriptional and post-translational levels [28].
Figure 2. The Architecture of the Circadian System.
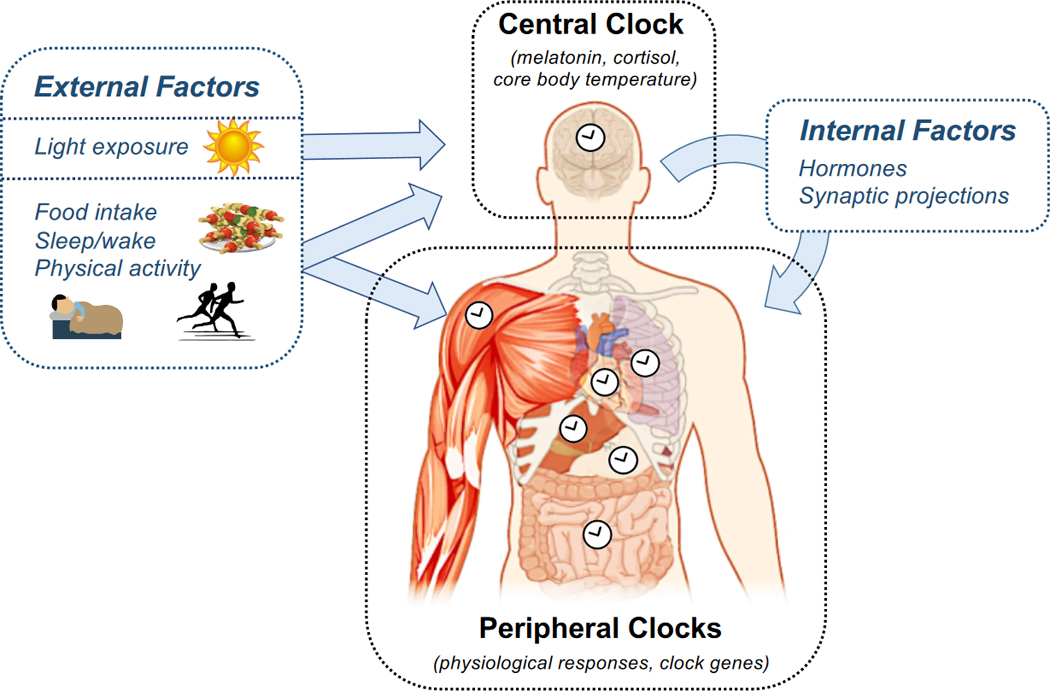
The circadian system comprises a central clock, which is located in the SCN of the hypothalamus, and a series of peripheral clocks located in tissues throughout the body. The central clock is entrained primarily by light, and its rhythm is measured through frequent sampling of melatonin, cortisol, or core body temperature. The central clock affects the phases and amplitudes of peripheral clocks through hormones and synaptic projections. The peripheral clocks are entrained by a combination of these signals from the central clock and external factors, most notably the timing of food intake. Peripheral clock rhythms are measured in humans either by directly measuring the rhythm in a physiologic variable or by measuring the expression of clock genes. Overall, daily rhythms in metabolism are produced by the central and peripheral clocks working in concert.
The timing (phase) of each circadian rhythm is determined by external and internal factors in a process known as entrainment. The central clock’s rhythm is primarily entrained by bright light, whereas the rhythms in peripheral tissues arise from integrating inputs from the central clock, external factors (including light, physical activity, feeding, and sleep), and metabolites [25, 27]. Recently, the timing of food intake has emerged as one of the key zeitgebers or external factors that sets the phase of peripheral clocks [29, 30]. Because different stimuli set the phases of the central and peripheral clocks, the two clock systems become misaligned whenever their respective zeitgebers are out of sync. This misalignment disrupts metabolism since the two clock systems jointly coordinate interdependent metabolic pathways. As we will discuss in Section 3, several controlled trials now suggest that circadian misalignment elevates the risk of developing metabolic diseases.
1.2. Measuring Circadian Rhythms
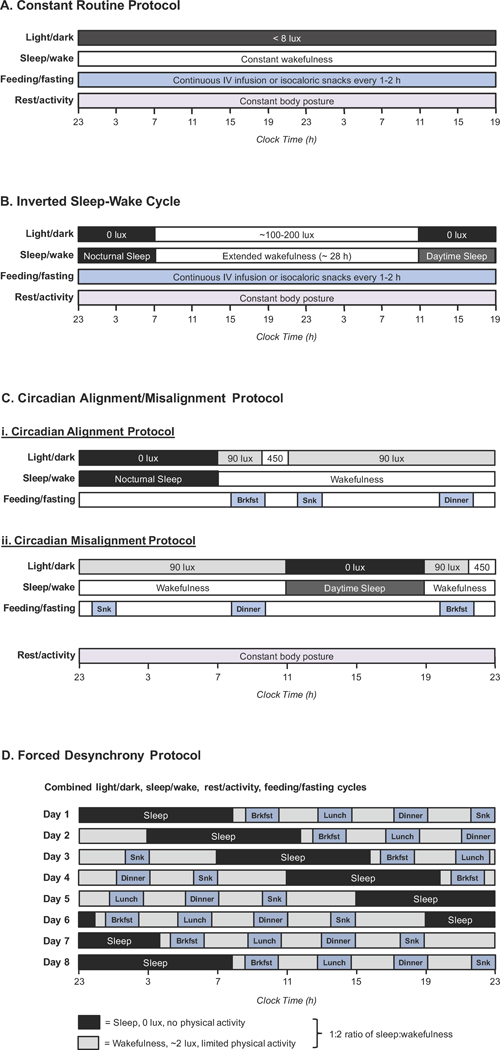
Measuring the circadian component of a rhythm is challenging and requires matching or eliminating all time-dependent external factors in order to isolate the circadian (endogenous) rhythm [31]. To date, four protocols have been developed for measuring circadian rhythms in humans (see Figure 3 for detailed descriptions) [31, 32]. Each of these protocols has unique advantages and disadvantages. The Constant Routine (CR) Protocol involves an extended period of wakefulness (no sleep) longer than 24 hours, during which all external factors (including light, temperature, and feeding) are kept constant. Because all external factors are held constant, any rhythms observed during a CR protocol are assumed to be pure circadian rhythms, generated only by the endogenous circadian system. The other three protocols permit external factors to be present and allow participants to sleep but involve changing the timing of sleep to cycle through different parts of the 24-hour day. Mathematical techniques are then used to extract the circadian component of the rhythm. As a result, these protocols have the advantage of providing information on both the circadian and external (behavioral) components of the rhythm, as well as on the effects of circadian misalignment. The Forced Desynchrony (FD) Protocol entails participants typically living on 20- or 28-hour-long days for 1–2 weeks and allows reconstruction of the full circadian and behavioral rhythms. By contrast, the Circadian Alignment/Misalignment (CA/M) Protocol and Inverted Sleep-Wake Cycle Protocol involve sleeping both during the daytime and at nighttime and therefore enable estimates of the relative contributions of the circadian system versus external factors. In Section 2, we focus on trials using these protocols. Given the scarcity of such trials, we also draw on studies measuring diurnal rhythms to provide historical context and to indicate areas for future inquiry.
Figure 3. Four Protocols for Investigating Circadian Rhythms in Humans.
(A) The Constant Routine Protocol involves a greater than 24-hour period of constant wakefulness wherein all external factors (including light, posture, feeding, and temperature) are kept constant. This protocol allows reconstruction of the entire circadian rhythm but does not enable investigation of circadian misalignment. (B) The Inverted Sleep-Wake Cycle involves periods of nocturnal and daytime sleep, separated by a prolonged period of wakefulness. Feeding and posture are typically kept constant throughout the protocol, but light levels are set to match changes in sleep/wakefulness. This protocol provides insight into both circadian and behavioral cycles. (C) The Circadian Alignment/Misalignment Protocol includes two subprotocols: the alignment protocol with daytime behavioral cycles occurring as they would normally, and a misaligned protocol, with those identical behavioral cycles occurring during the biological night. Light levels are varied, and participants eat normal meals and snacks. While this protocol does not allow reconstruction of the underlying circadian rhythm, it does reveal how much a diurnal rhythm is influenced by the circadian phase (i.e., the time of day), circadian misalignment, and behavioral factors. (D) The Forced Desynchrony Protocol involves following 20- or 28-day hour days for typically 1–2 weeks to cycle through different alignments between circadian rhythms and behavioral rhythms. Light levels during wakefulness are kept very low, and participants consume normal meals and snacks. Mathematical procedures are then used to extract the underlying circadian versus behavioral components of the diurnal rhythm. This protocol also reveals the impact of circadian misalignment on biologic endpoints.
2. CIRCADIAN AND DIURNAL RHYTHMS IN METABOLISM
2.1. Glucose Metabolism
2.1.1 Diurnal Studies
The first evidence for circadian regulation of glucose metabolism emerged in the late 1960’s and 1970’s when several studies reported diurnal variations in glucose tolerance [33–47]. Since then, more than a dozen human studies have reported the existence of a diurnal rhythm in oral glucose tolerance, typically peaking in the morning, with impairments in glucose tolerance in the afternoon and evening [33–49]. Importantly, these time-of-day effects are independent of the fasting duration [40, 48]. Studies using intravenous glucose or insulin tolerance tests [46, 50–54] and mixed meals [55–62] have reported similar findings. (However, fasting glucose is usually lower in the afternoon and evening than in the morning [51, 63].) The size of the diurnal variation in glucose tolerance is strikingly large: adults with normal glucose tolerance in the morning are metabolically equivalent to being prediabetic in the evening [33, 36, 46]. More recently, we reported that oral glucose tolerance in prediabetic adults was 40 mg/dl higher at 19:00 h than at 7:00 h, making prediabetic adults metabolically equivalent to early-stage diabetics at dinnertime [63].
These diurnal variations in glucose tolerance can be partially traced to diurnal rhythms in β-cell responsiveness, insulin secretion, and insulin clearance. Although data on the existence of a diurnal rhythm in fasting insulin is mixed [51, 64, 65], the insulin secretory response varies across the day. β-cell responsiveness—as measured by glucose tolerance, mixed meal, or intravenous tolbutamide testing—is higher in the morning than at other times of day [37, 46, 50, 52, 55, 57, 64, 66]. Yet, the insulin secretion rate and the total insulin secreted in response to a meal appears to peak later in the day [55–57, 62, 67]. One trial using 68-hour euglycemic and hyperglycemic clamps found that the insulin secretion rate peaked in the mid-afternoon (12:00–18:00 h) and was lowest at night while participants were sleeping [67]. Similarly, trials employing intravenous glucose tolerance tests and mixed meal tests using C-peptide deconvolution analysis report that total insulin secretion (AUC of the insulin secretion rate) is 16–51% higher in the afternoon or early evening than in the morning, due to a prolonged secretory period, even when no diurnal rhythm in the peak value of the secretion rate is apparent [55–57, 62]. Insulin clearance also exhibits diurnal variation: hepatic insulin extraction is lower in the morning than the evening [55].
Rhythms in peripheral insulin sensitivity also appear to contribute to the diurnal variation in glycemic control. In one trial employing a frequently-sampled intravenous glucose tolerance test in normal-weight participants, insulin sensitivity was impaired by 34% in the evening relative to the morning [50]. Impairments in insulin sensitivity later in the day have also been confirmed using insulin tolerance tests [46, 68, 69], mixed meal tolerance tests using the triple tracer technique [55], constant glucose infusion procedures using isotope tracers [70], and a 24-hour glucose-controlled insulin infusion procedure reminiscent of a clamp [71]. The diurnal rhythm in peripheral insulin sensitivity is likely due to both core intracellular pathways mediating glucose uptake and circulating factors. About 15% of transcripts in skeletal muscle exhibit a rhythmic pattern [72], including genes involved in glucose and lipid metabolism [61, 72, 73]. Muscle and liver glycogen content exhibit ~17% peak-to-trough variations, peaking in the evening [74]. Subcutaneous, but not visceral, adipose tissue also displays a large-amplitude circadian rhythm in insulin sensitivity: adipose tissue insulin sensitivity is 54% higher at noon than at midnight [75]. Circulating factors also likely contribute. Free fatty acids (FFAs) exhibit diurnal rhythms that mirror the diurnal periodicity in glucose homeostasis [39, 46, 59, 61, 68]. Growth hormone, which induces insulin resistance [76], strongly correlates with glucose levels during nocturnal sleep (22:00 - 2:00 h) in studies where glucose is infused at a constant rate [77]. Cortisol, which is regulated by the central clock, is also likely responsible for the circadian variation in plasma glucose and insulin. Infusing hydrocortisone to raise cortisol levels acutely inhibits insulin secretion and induces peripheral insulin resistance about 4–6 hours later, which lasts up to 12–16 hours [78, 79].
Other pathways involved in mediating glucose uptake may also be under circadian control. Glucose effectiveness—a measure of insulin-independent glucose—uptake is higher in the morning than the evening [50]. However, the data on hepatic insulin sensitivity is conflicting: one trial using a clamp procedure found no rhythm [70], whereas another trial using a triple-tracer mixed meal approach suggests that both endogenous glucose production and hepatic insulin sensitivity are lower in the morning [55]. Since there is no diurnal rhythm in the glucose absorption rate following the ingestion of mixed meals [55], this discrepancy between the two studies may be explained by diurnal rhythms in incretin secretion, which would be apparent during a meal tolerance test but not a clamp procedure.
Intriguingly, diurnal rhythms in glucose tolerance, insulin levels, and peripheral insulin sensitivity are attenuated, phase delayed, or absent in obese individuals [35, 39, 49, 50] and may be altered in aged individuals [39, 54]. Moreover, they are absent or inverted (phase delayed by several hours) in adults with type 2 diabetes [34, 35, 47, 62, 80]. One trial using a clamp procedure reported that adults with type 2 diabetes lack a diurnal rhythm in peripheral insulin sensitivity [80]; they also lack a diurnal rhythm in muscle glycogen storage [74]. Yet, diabetic adults exhibit clear rhythms in hepatic glycogen storage [74] and hepatic insulin sensitivity as measured by a clamp procedure combined with tracers [80, 81]. The net result is that whole-body insulin sensitivity in diabetic adults is highest at ~07:00 h and lowest in the morning. This rhythm in hepatic insulin sensitivity may explain the “dawn phenomenon” of fasting hyperglycemia in the morning in diabetic adults. Why and how these diurnal rhythms are altered in humans with obesity and diabetes is an important area for further research.
2.1.2 Circadian Studies
In the past couple decades, important efforts have clarified the influence of the circadian system on glucose metabolism, both with and without the influence of sleep—a factor known to affect glucose homeostasis.
Using a 24-hour constant glucose infusion in 8 healthy adults, Shapiro et al. reported that peak glucose levels occurred in the early morning (average: 2:28 h; range: 0:45–3:50 h) and were 32% above the daytime nadir [82]. While the insulin secretion rate appeared to have no rhythm when averaged over all participants, in half of the adults, the insulin secretion rate peaked around the same time as the glucose rhythm. (By contrast, most of the remaining participants had no insulin secretion rhythm.) However, a more recent trial using a CR protocol with isocaloric snacks every 2 hours in 6 healthy men reported endogenous circadian rhythms in glucose and insulin, with peak-to-trough amplitudes of 10% and 22%, respectively, and with peak levels occurring approximately at or shortly after the time of habitual awakening [83]. In healthy adults, sleeping from 23:00-7:00 h does not substantially change the mean phase of the glucose rhythm (3:39 h), although it may widen the phase range to 0:15–5:45 h and increase the peak-to-trough variation to ~28% [84]. However, in the same trial which included sleep, the acrophases in insulin levels were distributed over a wide time range (03:00–17:15 h), resulting in no significant group-level peak. The nadir of insulin levels occurred between 21:00-01:00 h in 6 out of 9 subjects, with insulin values declining by 17% below the 24-hour average. Another trial employed a 24-hour glucose infusion procedure in diabetic adults and (obese) BMI-matched control participants and found that glucose levels were highest in the morning and lowest at ~19:00 and ~20:30 h in healthy obese and diabetic adults, respectively [85]. The glucose amplitude was about twice as large in diabetic adults, relative to the BMI-matched control subjects (24% elevation above the nadir vs. 13%). While the nighttime rise in glucose levels correlated quantitatively and temporally with the rise in cortisol levels, the nocturnal rise in insulin secretion rates only paralleled that of glucose only in the healthy subjects but not diabetic adults; in fact, no temporal rhythms in the insulin secretion rate were apparent in diabetic adults. These differences in glucose and insulin rhythms may partially be due to differences in the timing and amplitude of the cortisol rhythm in diabetic versus healthy adults.
Trials have also been performed using FD, CA/M, and inverted sleep-wake cycle protocols. Owens et al. used an FD protocol in 9 healthy young women and found that the 3-hour glucose incremental AUC was lowest at 08:00 h, 58–66% higher at 14:00 and 02:00 h, and highest (99% higher) at 20:00 h; in contrast, fasting glucose exhibited an opposing rhythm, being lowest at 20:00 h [86]. Scheer et al. also used an FD protocol in 10 healthy adults and found that glucose displayed a circadian rhythm, with a small peak-to-trough amplitude of 4% and an acrophase between ~22:30-06:30 h, while insulin lacked a circadian rhythm [18]. More recently, Morris et al. used a CA/M protocol in 14 healthy, nonobese adults and found that although fasting glucose levels were not affected by the circadian phase, glucose tolerance was 17% lower and the 2-hour glucose AUC was 12% higher in the biological evening, compared to the morning [64]. Also, the early-phase insulin AUC was 27% higher and fasting insulin levels were lower in the evening relative to the morning. Finally, Van Cauter et al. combined an inverted sleep/wake cycle protocol with constant glucose infusion in 8 healthy men and found the acrophase for glucose occurred at 02:35±0:33 h, and peak levels were 31% and 17% above afternoon levels for nocturnal sleep versus nocturnal wakefulness, respectively [77]. Glucose levels during daytime sleep were also 16% higher than during wakefulness, demonstrating that sleep does indeed affect the diurnal rhythm in glucose. In this same trial, the acrophase for the insulin secretion rate paralleled that for glucose, but with even larger excursions of 49–60%. Insulin levels largely mirrored the insulin secretion rate, increasing by 41% during nocturnal sleep and by 39% during daytime sleep, except not during nocturnal wakefulness. Correspondingly. insulin clearance was higher during sleep and at night; in particular, insulin clearance was 30–40% higher during nocturnal sleep (23:00 – 3:00 h) than morning wakefulness (8:00 – 11:00 h). The same authors used an analogous protocol comparing 9 obese and 9 lean men and found that the rhythm in glucose tolerance was reversed in obese subjects, indicating an improvement in glucose tolerance as the day progressed [87]. In addition, glucose and insulin rhythms were blunted and phase advanced by ~1.5–2 hours in obese subjects, with glucose levels remaining elevated in the morning after awakening.
Taken together, these findings suggest that glucose tolerance exhibits circadian variation, with poorer glycemic control in the evening and at night in healthy adults. Diurnal rhythms in β-cell responsiveness, peripheral insulin sensitivity (influenced by both internal and circulating factors), insulin clearance, and glucose effectiveness drive these diurnal rhythms in glucose metabolism, whereas hepatic insulin sensitivity may play a lesser role. However, these rhythms are attenuated or phase-delayed in obese and diabetic adults, suggesting that altered circadian rhythms may be a cause or consequence of many metabolic diseases.
2.2. Lipid Metabolism
Although it is well-known that the circadian system regulates several rate-limiting steps in lipid metabolism pathways at both the gene and protein level in rodents [88, 89], very little is known in humans.
2.2.1 Diurnal Studies
A few studies have examined diurnal rhythms in cholesterol and its subcomponents in humans, with conflicting results (e.g. [59, 90–98]). In response to a single meal, one trial reported that postprandial triglycerides were higher, while total cholesterol, LDL-C, HDL-C, and apolipoprotein levels were lower, when a single meal was eaten at night (01:00 h) compared to during the daytime (13:00 h) [90]. In examining 24-hour rhythms, two studies in European Caucasian adults reported total and LDL cholesterol rhythms with modest amplitudes of ~6–7 mg/dl and ~10% [91, 92], while trials in Spanish and Indian adults reported larger amplitudes of ~20–30% in total and LDL cholesterol [93, 94]. The trials did not agree on the acrophase and reported phases ranging from early morning (~8:00–9:00 h) [92] to late afternoon (14:45–18:36 h) [93, 94]. For HDL-C, the evidence for a diurnal rhythm is mixed: the trials in Indian and Spanish adults found evidence of a very small-amplitude rhythm (2–4%) in HDL-C [93, 94], whereas two trials in Caucasians reported that no rhythm exists [91, 92]. Other studies examining healthy men and women reported no significant diurnal variations in total cholesterol or HDL-C levels [95–97].
While most studies agree that triglycerides exhibit a diurnal rhythm [91–93] (with one exception [59]), varying by as much as 33–63% over the day, they do not agree on the phase. For instance, two trials reported an acrophase in the afternoon (~15:00 h) or evening (~17:45–20:00 h) [72, 91], while another reported peak values between noon and early afternoon in males, but not in females [92]. Similar findings were reported in a study based on capillary measurement of triglycerides in healthy men, showing that the largest rise occurred after dinner [98]. Biological sex may explain some of the differences in the triglyceride rhythm. In one study, men clearly showed a two-fold higher maximal increase in serum triglyceride levels following a meal than women and had a higher diurnal variation (36% in men vs. 24% in women) [95]. These sex-related differences may be due to the influence of estrogens on lipid metabolism [99]. In addition, age, metabolic phenotype, and methodological differences—such as measuring serum versus capillary triglycerides [100, 101]—may account for some of the discrepancies.
Similar to the data on plasma cholesterol levels, data on the synthesis of cholesterol lacks consensus. In addition to indirect evidence for a rhythm from cholesterol precursors [102], a study using a simulated jet-lag protocol reported that the cholesterol synthesis rate peaked at ~22:00 h and exhibited a large ~109% amplitude [103]. Yet, a small study in men reported that the free cholesterol and cholesteryl ester fractional synthetic rates instead peaked at ~06:00 h [104]. The discrepancy among trials likely arises both from differences in meal composition and from the fact that the timing of food intake, rather than the circadian system, is the primary determinant of the diurnal patterns in cholesterol synthesis [103, 105]. This suggests that behavioral and other external factors primarily dictate the rhythms in cholesterol synthesis.
Research has also investigated diurnal rhythms in other lipid species. Several trials have reported sizeable diurnal variations in FFAs, including postprandial values, with most studies (but not all [64]) reporting higher AUC values in the afternoon and evening [39, 46, 51, 54, 59, 61, 68, 72, 92]. Apolipoproteins A-1 and B exhibit 24-hour and 12-hour rhythms, respectively, but the amplitudes of their peak-to-trough rhythms are small (~5–6%) [93]. Many other lipid species exhibit diurnal rhythms. A diurnal metabolomics study reported that phospholipids, lysophosphatidylcholines, and lysophosphatidylethanolamines tend to peak in late afternoon and evening [106].
These lipid rhythms may be driven by circadian oscillations in lipid absorption, transport, and partitioning. Novel data from animal studies suggest that the circadian system regulates intestinal lipid absorption [107, 108], yet evidence in humans is still lacking. However, there is evidence for diurnal variations in lipid transport and partitioning. Diurnal rhythms have been observed in acylcarnitines, which participate in fatty acid oxidation by transporting fatty acids from the cytoplasm into the mitochondria: the acrophase of long-chain, unsaturated acylcarnitines occurred earlier in the morning than their long-chain saturated counterparts, whereas short-chain acylcarnitines did not exhibit a homogeneous pattern across the day [106]. Such data suggest that there may be a circadian rhythm in mitochondrial fatty acid transport. In corroboration, mitochondrial oxidative capacity also displays a day-night rhythm: healthy, normal-weight young men, who underwent five skeletal muscle biopsies over a 24-hour period, exhibited a diurnal variation in skeletal muscle mitochondrial lipid metabolism that peaked at ~23:00 h [72]. Another trial that performed muscle biopsies in 13 overweight, healthy women found that genes that regulate fatty acid oxidation are downregulated by 38–82% in the evening relative to the morning, while genes involved in de novo lipogenesis are upregulated by 51–87% [61]. Such a change in gene expression, without a corresponding change in adipose tissue lipolysis, favors a shift in skeletal muscle fatty acid partitioning from oxidation in the morning to lipogenesis in the evening.
2.2.2 Circadian Studies
Circadian studies reveal that a greater fraction of lipids undergo circadian regulation than any other classes of plasma metabolites. In a remarkable metabolomic study in 10 men that used a CR protocol, Dallmann et al. demonstrated that 15% of plasma metabolites exhibit circadian rhythms and that 80% of these rhythmic compounds are lipid metabolites [109]. The majority of lipid species reached their peak levels between mid-morning and noon. Another CR study used a lipidomics-based approach and examined 263 lipid species (glycerolipids, glycerophosphosholipids, sphingolipids, free cholesterol, cholesterol esters, and LDL cholesterol) in plasma obtained from 20 healthy young males [110]. Aside from clear inter-individual differences in the timing and amplitude of rhythms, group-level analysis revealed circadian oscillations in 13% of lipid species, spanning lipids involved in energy storage, transport, and signaling. Most of the lipids exhibiting circadian variation were triglycerides and diglycerides, and the plurality of acrophases was in the morning. By comparison, phosphatidylcholines peaked in the evening, while LDL cholesterol exhibited no circadian rhythm (although values tended to be higher during the daytime). However, the phase of lipid rhythms varied among individuals by up to 12 hours, and intersubject agreement on which lipids were rhythmic was only 18%. Moreover, individuals were clustered according to the strength of rhythmicity for a subset of triglycerides and phosphatidylcholines, suggesting that there may be distinct circadian phenotypes. Such wide variability is supported by third CR study in only 6 young males, which reported high variability in the timing of glycerophospholipid and sphingolipid rhythms [111]. Finally, one study measured the circadian component of lipid rhythms in 14 healthy adults using a CA/M protocol and found that fasting and postprandial FFA levels were not affected by circadian phase [64].
In conclusion, lipids are more extensively regulated by the circadian system than any other plasma metabolites [109], with a plurality of acrophases peaking in the morning or around noon. These rhythms are driven at least in part by differences in lipid synthesis, transport, and partitioning. However, there is wide inter-individual variability in which lipids are rhythmic, as well as in the timing and strength of rhythms, suggesting that there may be distinct circadian metabolic phenotypes. More controlled trials are needed to determine the nature and extent of inter-individual variation in lipid rhythms.
2.3. Energy Metabolism
Animal studies have demonstrated diurnal rhythms in energy metabolism both at the molecular and whole-body levels [1, 89, 112]. In humans, most evidence so far is at the whole-body level. Two trials have used a CR protocol to measure circadian rhythms in energy expenditure and substrate oxidation. One trial used indirect calorimetry in 7 healthy young men and reported a 17% peak-to-trough amplitude in energy expenditure, with peak values occurring between 9:00 and 12:00 h and trough values between 24:00 and 06:00 h [113]. The second trial, in 10 healthy young men, measured oxygen consumption and carbon dioxide production and found a smaller 6% peak-to-trough amplitude in both variables [114]. Both trials reported no significant circadian variation in the respiratory quotient (RQ), which indicates relative substrate oxidation rates [113, 114]. Another trial in 15 obese adults, which included participants fasting throughout the 24-hour cycle, similarly reported a circadian rhythm in energy expenditure with acrophases occurring in the afternoon (13:15–17:23 h) [115]. Research by the same authors, but not under CR or fasting conditions, also reported diurnal rhythms in substrate oxidation [116, 117]. There is some mechanistic evidence supporting a diurnal rhythm in energy metabolism: an uncontrolled study, which employed a protocol that mimics a typical daily lifestyle, reported a 20% diurnal variation in skeletal muscle oxidative capacity, with state 3 mitochondrial respiration (i.e., ADP-stimulated respiration) being highest at 23:00 h [72]. However, mitochondrial markers, content, and biogenesis do not vary across the day [72].
The observed circadian rhythm in energy expenditure is likely due to the postprandial component of energy expenditure. More trials [59, 118, 119] than not [113, 120] report a diurnal variation in postprandial energy expenditure: the thermic effect of food (TEF) is up to 44% higher in the morning relative to the late afternoon and evening; however, there is no difference between morning versus early afternoon values [121]. However, only two trials have attempted to determine whether this diurnal rhythm in TEF is due to the circadian system [113, 119]. One trial, which used a CA/M protocol, found that the diurnal variation in TEF following a meal was entirely driven by the endogenous circadian system, with no contributions from the behavioral cycle; TEF was 50% higher in the biological morning compared to the biological evening [119]. The other trial, which used a CR protocol and isocaloric snacks every two hours, reported no circadian variation in TEF [113]. However, this second study was likely underpowered because the small size of frequent isocaloric snacks makes it more difficult to discern a circadian variation in postprandial energy expenditure and hence in TEF; on this basis, the first study’s conclusion that there is a circadian rhythm in TEF is more likely correct.
In addition to postprandial energy expenditure, postprandial substrate oxidation may also be under circadian control. One trial employing a CA/M protocol in 13 healthy adults, reported a 2% variation in the postprandial RQ between the biological morning and evening, which translated into a 10% variation in postprandial carbohydrate oxidation but no variation in fat oxidation [64]. By contrast, both resting energy expenditure and substrate oxidation in the fasting state are not influenced by the time of day [59, 64, 119–122], with only one trial reporting an exception [72].
Lastly, the circadian system may also play a role in modulating appetite. Trials employing FD and CR protocols have found that self-reported hunger peaks in the evening [86, 123–125], with one trial reporting an acrophase at ~20:00 h and a peak-to-trough amplitude of 17% [123]. However, a trial employing an FD protocol found that this did not translate into a circadian rhythm in food intake [126]. By contrast, data on energy balance hormones suggest that they are regulated by food intake, rather than the circadian system. Initially, a trial using a CR protocol reported a circadian rhythm in leptin in 4 out of 6 individuals, with a 21% peak-to-trough amplitude in those 4 individuals [83]. The same relative amplitude, with a zenith at 24:00 h, was obtained in a diurnal study [127]; however, the same diurnal investigation tested a 6.5-hour meal shift and found that leptin levels are not acutely entrained to the circadian clock but rather to meal timing [127]. Indeed, a more recent trial using an FD protocol found no significant circadian rhythm in leptin [18]. Similarly, levels of the acute hunger hormone ghrelin are not influenced by the time of day but are affected by sleep, habitual meal times, and postprandial glucose levels [58, 128].
In aggregate, these trials suggest that the circadian system regulates 24-hour and postprandial energy expenditure and also subjective appetite, but it does not affect resting energy expenditure, food intake, ghrelin, or leptin. Data on circadian regulation of substrate oxidation are still conflicting, and further research is needed.
3. CIRCADIAN MISALIGNMENT AND TRANSLATIONAL IMPLICATIONS
Diurnal rhythms in metabolism are driven not only by the circadian system, but also by environmental and behavioral factors, including light, sleep, food intake, and physical activity. Increasing evidence suggests that when these external rhythms are out-of-sync with endogenous circadian rhythms—such as through exposure to bright light at night, sleeping during the daytime, or eating at night (Figure 4) —several facets of metabolism are impaired. Here, we review evidence that circadian misalignment induced by the mistiming of three external factors—light exposure, sleep, and food intake—affects metabolic health (summarized in Table 1). For sleep and food intake, we include trials where only the timing (and not the duration) of the external factor is experimentally changed to avoid any confounding effects from sleep loss or daily intermittent fasting (e.g., time-restricted feeding). Thus, we limit our review to trials where the daily fasting duration and sleep duration are unchanged.
Figure 4. Circadian Alignment vs. Misalignment.
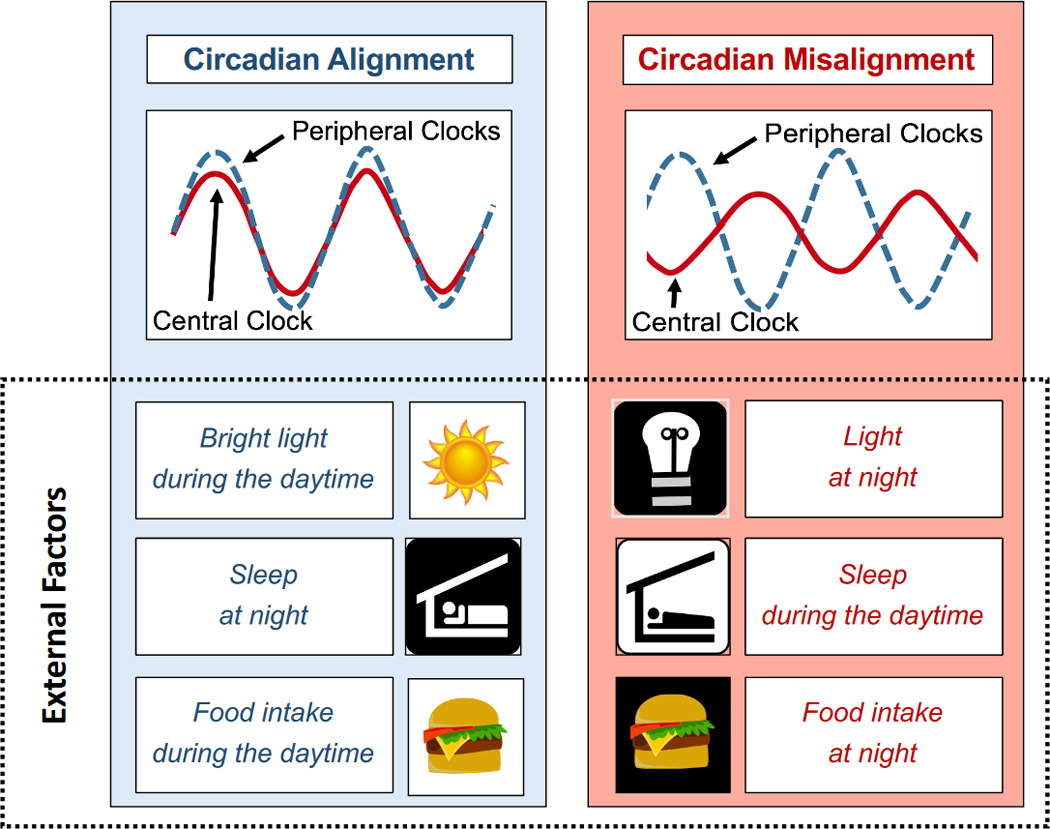
Shown above is a schematic representation of circadian alignment between central and peripheral clocks (left panel) versus misalignment (right panel). Bright light exposure during the daytime, food intake during the daytime, and sleeping during the biological night promote circadian alignment between the central and peripheral clocks. Conversely, light exposure or food intake in the evening/at night, or sleeping during the daytime, misaligns the two clock systems and leads to metabolic dysfunction.
Table 1.
Known Effects of the Circadian System on Metabolism in Humans.1
| Diurnal Rhythm? | Circadian Rhythm? | Affected by Misalignment? | |
|---|---|---|---|
| GLUCOSE METABOLISM | |||
|
| |||
| Glucose tolerance | Yes | Yes | Yes |
| Fasting glucose | Yes | Mixed | No |
| Postprandial glucose2 | Yes | Yes | Yes |
| Fasting insulin | Mixed | No | No |
| Postprandial insulin2 | Yes | Mixed | Yes |
| Beta-cell responsiveness | Yes | ? | ? |
| Insulin secretion | Yes | Yes | Yes |
| Insulin clearance | Yes | Yes | ? |
| Peripheral insulin sensitivity | Yes | Yes | Yes |
| Adipoctye insulin sensitivity | Yes | Yes | ? |
| Hepatic insulin sensitivity | Mixed | ? | ? |
|
| |||
| LIPID METABOLISM | |||
|
| |||
| Cholesterol synthesis rate | Yes | ? | ? |
| Total cholesterol | Yes | ? | ? |
| LDL cholesterol | Yes | ? | ? |
| HDL cholesterol | Mixed | ? | ? |
| Triglycerides | Yes | Yes | No |
| FFAs | Yes | No | Mixed |
| Plasma phospholipids | Yes | At least some | ? |
| Plasma acylcarnitines | Some | ? | ? |
| Diglycerides | Yes | Yes | ? |
| Mitochondrial lipid oxidation | Yes | ? | ? |
|
| |||
| ENERGY METABOLISM | |||
|
| |||
| Energy expenditure | Yes | Yes | Yes |
| Resting energy expenditure | No | No | No |
| TEF | Yes | Yes | No |
| Fasting RQ | No | No | Yes |
| Postprandial RQ | Mixed | Mixed | Yes |
| Subjective hunger | Yes | Yes | Yes |
| Food intake | Yes | No | ? |
| Leptin | Yes | Mixed | Yes |
| Ghrelin | Yes | No | No |
“?” means that the influence of the circadian system is unknown, whereas “Mixed” denotes that the data are conflicting.
Data on the presence of circadian rhythms in glucose and insulin—as determined from CR protocols—are included under postprandial glucose and insulin.
3.1. Light
Light is the primary zeitgeber of the central clock, and the timing, intensity, and duration of exposure to bright light are all linked to metabolic health.
3.1.1 (In)sufficient Daytime Exposure
Bright light exposure during the daytime increases melatonin secretion at night [129–134], so insufficient bright light during the daytime may attenuate the central clock’s rhythm and consequently impair metabolism. For instance, a randomized controlled trial (RCT) found that acutely lowering light exposure from 5,000 lux to 80 lux during the daytime suppressed gastric activity [135]. Conversely, several trials report that morning bright light therapy improves carbohydrate metabolism and fat loss. (All metabolic assessments were performed in the morning unless otherwise mentioned.) Daytime bright light therapy for a few weeks reduced insulin needs in two case studies of insulin-dependent diabetics [136, 137], suggesting that it improves glycemic control. Morning exposure to bright light therapy (1,300–5,000 lux) for 3–20 weeks also can reduce insulin resistance [138], body weight and/or fat mass [138–142], appetite [140], and can increase exercise-induced gains in lean mass [138] in overweight adults. Interestingly, morning exposure to specific wavelengths of red, green, or blue light (60 lux) for one week may also help mitigate sleep deprivation-induced derangements in leptin and ghrelin [143]. However, not all studies report that morning bright light exposure is beneficial: one RCT found that a single session of 4000-lux light exposure for 5 hours in the morning worsened glycemia in diabetic men but not in healthy men [144]. The abnormalities in the circadian rhythms of diabetic individuals (discussed in Section 2.1.1) or an acute increase in sympathetic nervous system activity could explain these divergent effects.
3.1.2 Misalignment
In addition to insufficient exposure to bright light during the daytime, light in the evening or at night is also associated with increased risk of metabolic disturbances. RCTs report that acute exposure to bright light (>500–600 lux) in the evening increases insulin resistance and elevates postprandial insulin, glucose, and GLP-1 levels, relative to dim light (<2–5 lux) [145, 146]. Another RCT reported that acute exposure to 2,000-lux light in the evening impaired carbohydrate digestion, as indicated by the amount of hydrogen exhaled in breath [147], and acute exposure to blue-enriched light (370 lux) in the evening has been found to increase peak postprandial glucose levels [148]. Epidemiologic evidence also suggests that evening or nighttime bright light exposure increases the risk of metabolic diseases. In a cross-sectional analysis of more than 100,000 women, brighter room light while sleeping was strongly associated with a higher BMI, waist circumference, and waist-to-hip ratio [149]. In addition, a prospective cohort study found that elderly adults exposed to light at night (≥3 lux) had a 10% gain in BMI over 10 years [150], had higher triglycerides and LDL cholesterol [151], and had lower HDL cholesterol [151], in comparison to those who slept in dim light (<3 lux). Moreover, increased exposure to light in the evening (18–38 lux) was associated with a 51% increase in the prevalence of diabetes [152], while each hour phase delay in exposure to light above 500 lux was associated with a 1.3 kg/m2 increase in BMI [153]. One study reported that the timing of light exposure explained 35% of the variance in BMI levels [153].
3.2. Sleep Timing
Phase shifts in the timing of sleep—even when sleep duration is kept constant—also induce circadian misalignment, leading to metabolic dysfunction.
3.2.1 Glucose and Lipid Metabolism
Initial studies on simulated night-shift work using a 9-hour phase advance in sleep reported that mistimed sleep worsened glucose and lipid levels [19, 20]. One trial reported that daytime sleep increased the glucose and insulin AUCs by 12% and 39%, respectively, and elevated triglyceride and FFA levels in the late postprandial period, with no effects on GLP-1 [19]. However, a follow-up trial using a similar protocol found that the effects on glucose, insulin, triglycerides, and FFAs were transient and vanished entirely (or nearly entirely) after two days of re-adaptation to habitual sleep time [20]. Recent studies employing more stringent protocols have reported clear effects of circadian misalignment on glucose and lipid metabolism. Scheer et al. used an FD protocol and found that circadian misalignment induced by a 12-hour phase delay in sleep (as well as in the postural cycle, physical activity, meals, etc.) worsened mean daily glucose levels by 6% and insulin levels by 22%, suggesting impairments in insulin sensitivity without adequate β-cell compensation [18]. Three-hour postprandial glucose values were elevated by 11–21% across the three meals (with higher values at breakfast than lunch and dinner), and three out of eight study participants (38%) had prediabetic or diabetic values of glucose tolerance under the misaligned condition but not the aligned condition. However, misalignment did not affect fasting glucose levels. A later trial by Morris et al. with a 12-hour phase delay in sleep, achieved through a CA/M protocol, reported qualitatively similar findings [64]. Circadian misalignment increased 24-hour glucose and insulin AUCs by 2% and 9%, respectively. Misalignment also increased the 2-hour glucose AUC during a meal tolerance test by 6% and the late-phase postprandial insulin AUC by 14%, but fasting glucose, fasting insulin, and the early-phase insulin AUC were unaffected. Mechanistically, reduced insulin sensitivity contributed more than impaired β-cell function to the derangements in glucose tolerance. Misalignment also lowered both the fasting and postprandial RQ by 3% and 2% respectively, favoring an increase in fat oxidation at the expense of carbohydrate oxidation while fasting, and it elevated fasting FFA levels by 15%; however, it did not affect mean 24-hour FFA or triglyceride levels or 2-hour postprandial FFA levels. Circadian misalignment also exacerbates the adverse effects of sleep loss on insulin sensitivity: in a CA/M protocol, circadian misaligned worsened insulin sensitivity relative to the aligned group (−47% vs. −34%) but did not affect insulin secretion, body weight, or food intake [154].
3.2.2 Energy Metabolism
Daytime sleep also reduces energy expenditure and alters substrate oxidation. McHill et al. used a CA/M protocol with a 9-hour phase delay in sleep and reported that participants burned 12–16% fewer calories while sleeping during the daytime than when they slept at night [21]. This, in turn, translated into a 3% decrease in 24-hour energy expenditure, thereby providing a mechanism that could at least partially explain weight gain in night-shift workers. Sleeping during the daytime also increased fat oxidation by 18% and decreased carbohydrate and protein oxidation by 20% and 10%, respectively, at least transiently on the second day of misalignment. In addition, misalignment reduced subjective hunger, despite the fact that peptide YY and leptin were also lower by 11–13% and 35–41%, respectively. This latter effect was confirmed by another trial that used an FD protocol and observed a 17% suppression in mean daily leptin levels in the misaligned condition [18]. However, neither ghrelin [21], resting metabolic rate [119], nor TEF [21, 119] are affected by circadian misalignment.
3.3. Food Intake
Timing of Meals
Several trials have reported that phase-delaying the timing of food intake has adverse metabolic consequences—even when food intake is restricted to the daytime. Shifting the timing of lunch from 13:00 h to 16:30 h increased the glucose incremental AUC by 46%, decreased fasting (but not postprandial energy expenditure) by 4%, and decreased carbohydrate oxidation in the fasting state [155]. Surprisingly, a late lunch also blunted the diurnal cortisol rhythm. Another trial reported that an acute shift in the timing of dinner from 19:00 h to 22:30 h increased the 5-hour glucose AUCs following both dinner and breakfast the following morning by 7–8% and elevated 24-hour glucose levels by 4 mg/dl, although it did not affect 24-hour energy expenditure [156]. Phase-delaying meal timing by a few hours also dramatically increases the amplitude and shifts the phase of the cholesterol synthesis rhythm [105] and shifted the phase but not the mean 24-hour value of the leptin rhythm [127]. More recently, an elegant study investigated the effects of a 5-hour delay in meal timing, maintained for six days, on circadian rhythms in glucose and insulin as measured during a CR protocol [125]. Whereas the late eating phase-delayed the glucose rhythm by 5.7 hours, the rhythms in insulin, triglycerides, cortisol, melatonin, and subjective hunger were unaffected. Surprisingly, however, late eating reduced mean 24-hour glucose levels by 5% during a CR protocol without changing the amplitude of the glucose rhythm; why this occurred is unknown.
In addition, four trials have investigated the effects of consuming a single meal at different times of day for 3–18 days on energy metabolism. Eating a single meal in the evening (18:00 h) altered diurnal rhythms in substrate oxidation, increasing fat oxidation at the expense of carbohydrate oxidation, in comparison to consuming that same meal in the morning (10:00 h) [116, 117, 157]; however, no differences in weight loss after 18 days [116, 157] or in the circadian pattern in energy expenditure after 3 days were found [117].
Caloric Distribution Across Meals
Altering the distribution of calories across meals—even when the timing of meals is not changed—also affects metabolic risk factors. In an RCT, overweight women who ate 70% of their calories before noon lost −0.6 kg more weight over a 6-week period than their late-eating counterparts who ate 70% of their daily calories after mid-afternoon (16:30 h and later) [158]. A single-day trial reported that 20-hour glucose AUC was lower and insulin sensitivity was modestly higher when 60% of calories were eaten at breakfast (09:30 h) rather than at dinner (20:30 h), whereas triglyceride and FFA levels were unaffected [159]. Longer-term studies have reported larger effects. In a 20-week weight loss trial with 420 participants, those who ate lunch before 15:00 h lost 2% more of their baseline body weight than those who ate lunch after 15:00 h, despite no differences in self-reported food intake, breakfast or dinner times, energy expenditure, or sleep [160]. Two 3-month RCTs in obese women [161] or women with polycystic ovary syndrome [162] reported better glucose tolerance when participants ate 50–54% calories at breakfast, rather than at dinnertime, in isocaloric comparisons. In both studies, the large breakfast groups had lower fasting glucose by 7–8%; lower fasting insulin by 22–53%; lower glucose AUC by 7–20%; lower insulin AUC by 28–42%; and correspondingly higher insulin sensitivity indices. In one of the trials [161], the overweight women lost 2.5-fold more body weight and lowered their triglycerides dramatically (−34% vs. +15% in the large dinner group), yet had lower levels of ghrelin and lower subjective hunger [161]. A similar 3-month RCT in diabetes patients found that consuming a large breakfast enriched in protein and fat reduced hemoglobin A1c levels by an additional 0.32% and reduced antihyperglycemic medication dosages and hunger scores, relative to following an isocaloric diet with a large dinner [163]. However, another trial in diabetic adults reported that when participants ate a majority of calories at dinnertime, their daytime insulin secretion was lower, while their glucose levels and nighttime insulin secretion were unchanged [164]. The reduction in insulin secretion when a majority of calories are eaten at dinnertime may be explained by the lower hepatic glucose production in the evening in diabetic adults [80].
DISCUSSION
Although circadian regulation of metabolism is less well-characterized in humans than in rodents, there is clear evidence of circadian rhythms in multiple aspects of metabolism, including glucose, insulin, glucose tolerance, lipid levels, energy expenditure, and appetite. The rhythms in glucose metabolism appear to be driven by diurnal variations in multiple metabolic pathways, including peripheral insulin sensitivity, β-cell responsiveness, insulin clearance, and glucose effectiveness. Similarly, lipid rhythms are influenced by diurnal variations in lipid synthesis, transport, and partitioning. Energy expenditure and appetite also exhibit circadian rhythms, although the data suggest that these rhythms are not driven by ghrelin or leptin, and evidence for an underlying mechanism is lacking. In aggregate, such data indicate that the circadian system plays a pervasive role in regulating metabolism in humans, just like in rodents.
Despite this, many studies do not agree on the amplitudes and/or acrophases of those rhythms. Very likely, many of the discrepancies can be explained by differences in the experimental designs and the control of external or behavioral factors. For instance, in examining rhythms in glucose metabolism, whether the glucose load was administered orally or intravenously may influence both the magnitude and presence of rhythms. As a second example, whether participants sleep and the duration of the extended wakefulness period differs between the CR and FD protocols, which may differentially affect the homeostatic sleep drive and lead to different metabolic outcomes. Currently, however, there is no consistent way to reconcile these methodological differences or distinguish between rhythms driven by the circadian system versus other transient rhythms that may persist for days even after external stimuli are removed. The observed discrepancies may also be explained by small sample sizes since a majority of circadian studies involve 10 or fewer human subjects. Another alternative explanation is that there may be different circadian metabolic phenotypes. Data already suggest that there are sex-based differences and individuals with type 2 diabetes lack or have inverted rhythms in glucose tolerance [34, 35, 62]; moreover, the clustering of acrophases in lipid species across the day suggests more than one circadian phenotype [110, 111]. Regardless, these discrepancies need to be reconciled in future large clinical trials. Lipid metabolism especially merits further investigation, since lipids are more extensively regulated by the circadian system than any other group of plasma metabolites, yet data from both diurnal and circadian trials are particularly varied.
Nonetheless, the evidence seems to be converging that many anabolic rhythms peak in the biological morning or early afternoon in humans. For instance, glucose tolerance is lower and skeletal muscle fatty acid oxidation and the thermic effect of food are higher in the morning than in the evening or at night, which implicates earlier in the daytime as optimal for food intake and nighttime as optimal for sleep and fasting. Indeed, eating in alignment with those rhythms by shifting food intake to earlier during the daytime seems to improve glycemic control and facilitate weight loss in adults, but further well-controlled studies are needed to confirm these preliminary results [155, 156, 158, 160–162]. Interestingly, these benefits may be driven mostly by the circadian variation in postprandial metabolic pathways—particularly those involving peripheral tissues like skeletal muscle and adipose tissue—which seem to be under greater circadian regulation than pathways that are active in the fasting state. In the future, it will be important to clarify which organs and tissues are the most important contributors to these whole-body metabolic rhythms, as well as to determine the underlying molecular mechanisms, such as the relative contributions of circulating factors versus intracellular mediators.
Importantly, disruptions in circadian rhythms impair metabolism and influence the pathogenesis of metabolic disease. Evidence in humans now clearly demonstrates that circadian misalignment induced by mistimed light exposure, sleep, or food intake all worsen glycemic control and adversely affect factors involved in energy balance and weight loss, increasing the risk of diabetes and obesity. Moreover, the effect sizes are large: for instance, an acute bout of circadian misalignment can increase postprandial glucose levels by 11–21% [18], indicating that maintaining circadian alignment is very important for metabolic health. However, future research is needed to determine whether interventions that improve circadian alignment or that influence the circadian system can indeed prevent or reverse metabolic diseases. Overall, these interrelationships among the circadian system, metabolism, and behavior underscore the importance of research into circadian regulation of metabolism. Further research is therefore needed to better understand how the circadian system interacts with external factors and with aging and disease processes in order to prevent and treat diabetes, obesity, and hyperlipidemia.
Acknowledgments
Role of the Funding Source: EP was supported by grant AR7/2016 from Sapienza University, Rome, Italy. CMP was supported by KL2TR001419 from the National Center for Advancing Translational Sciences of the National Institutes of Health. This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding sources had no involvement in any aspect of the research.
Abbreviation List
- CREB
cAMP response element binding protein
- AMPK
adenosine monophosphate-activated protein kinase
- SCN
suprachiasmatic nucleus
- TTFL
transcriptional-translational feedback loop
- CR
constant routine
- FD
forced desynchrony
- CA/M
circadian alignment/misalignment
- h
hour
- AUC
area under the curve
- IRS-1
Insulin receptor substrate-1
- FFAs
free fatty acids
- RQ
respiratory quotient
- TEF
thermic effect of food
- RCTs
randomized controlled trials
- BMI
body mass index
Footnotes
Conflicts of Interest: None.
Author Contributions: EP and CMP conceived of and designed the review article. All authors performed the literature review; analyzed and interpreted the data; wrote multiple subsections of the manuscript; and revised the manuscript for intellectual content. All authors approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Panda S. Circadian physiology of metabolism. Science. 2016;354(6315):1008–1015. doi: 10.1126/science.aah4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerhart-Hines Z, Lazar MA. Circadian metabolism in the light of evolution. Endocr Rev. 2015;36(3):289–304. doi: 10.1210/er.2015-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcheva B, et al. Circadian clocks and metabolism. Handb Exp Pharmacol. 2013;(217):127–55. doi: 10.1007/978-3-642-25950-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balsalobre A, et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289(5488):2344–7. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 5.Jordan SD, Lamia KA. AMPK at the crossroads of circadian clocks and metabolism. Mol Cell Endocrinol. 2013;366(2):163–9. doi: 10.1016/j.mce.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sitaula S, et al. Rev-erb regulation of cholesterologenesis. Biochem Pharmacol. 2017;131:68–77. doi: 10.1016/j.bcp.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng D, et al. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science. 2011;331(6022):1315–9. doi: 10.1126/science.1198125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang EE, et al. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat Med. 2010;16(10):1152–6. doi: 10.1038/nm.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li MD, Li CM, Wang Z. The role of circadian clocks in metabolic disease. Yale J Biol Med. 2012;85(3):387–401. [PMC free article] [PubMed] [Google Scholar]
- 10.Coomans CP, et al. Plasticity of circadian clocks and consequences for metabolism. Diabetes Obes Metab. 2015;17(Suppl 1):65–75. doi: 10.1111/dom.12513. [DOI] [PubMed] [Google Scholar]
- 11.Baron KG, Reid KJ. Circadian misalignment and health. Int Rev Psychiatry. 2014;26(2):139–54. doi: 10.3109/09540261.2014.911149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris CJ, Yang JN, Scheer FA. The impact of the circadian timing system on cardiovascular and metabolic function. Prog Brain Res. 2012;199:337–58. doi: 10.1016/B978-0-444-59427-3.00019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turek FW, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308(5724):1043–5. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marcheva B, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466(7306):627–31. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudic RD, et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2(11):e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci U S A. 2008;105(39):15172–7. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paschos GK, et al. Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat Med. 2012;18(12):1768–77. doi: 10.1038/nm.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scheer FA, et al. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106(11):4453–8. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hampton HS, et al. Postprandial hormone and metabolic responses in simulated shift work. J Endocrinol. 1996;151(2):259–67. doi: 10.1677/joe.0.1510259. [DOI] [PubMed] [Google Scholar]
- 20.Ribeiro D, et al. Altered postprandial hormone and metabolic responses in a simulated shift work environment. Journal of Endocrinology. 1998;158(3):305–310. doi: 10.1677/joe.0.1580305. [DOI] [PubMed] [Google Scholar]
- 21.McHill AW, et al. Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proc Natl Acad Sci U S A. 2014;111(48):17302–7. doi: 10.1073/pnas.1412021111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Giorgi A, et al. Circadian rhythms and medical diseases: does it matter when drugs are taken? Eur J Intern Med. 2013;24(8):698–706. doi: 10.1016/j.ejim.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Dallmann R, Okyar A, Levi F. Dosing-Time Makes the Poison: Circadian Regulation and Pharmacotherapy. Trends Mol Med. 2016;22(5):430–45. doi: 10.1016/j.molmed.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Zhang R, et al. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A. 2014;111(45):16219–24. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–62. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buijs RM, Kalsbeek A. Hypothalamic integration of central and peripheral clocks. Nat Rev Neurosci. 2001;2(7):521–6. doi: 10.1038/35081582. [DOI] [PubMed] [Google Scholar]
- 27.Barclay JL, Tsang AH, Oster H. Interaction of central and peripheral clocks in physiological regulation. Prog Brain Res. 2012;199:163–81. doi: 10.1016/B978-0-444-59427-3.00030-7. [DOI] [PubMed] [Google Scholar]
- 28.Partch CL, Green CB, Takahashi JS. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014;24(2):90–9. doi: 10.1016/j.tcb.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krauchi K, et al. Alteration of internal circadian phase relationships after morning versus evening carbohydrate-rich meals in humans. J Biol Rhythms. 2002;17(4):364–76. doi: 10.1177/074873040201700409. [DOI] [PubMed] [Google Scholar]
- 30.Johnston JD, et al. Circadian Rhythms, Metabolism, and Chrononutrition in Rodents and Humans. Adv Nutr. 2016;7(2):399–406. doi: 10.3945/an.115.010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rietveld WJ, Minors DS, Waterhouse JM. Circadian rhythms and masking: an overview. Chronobiol Int. 1993;10(4):306–12. doi: 10.1080/07420529309059713. [DOI] [PubMed] [Google Scholar]
- 32.Redfern PH, Waterhouse JM, Minors DS. Circadian rhythms: principles and measurement. Pharmacol Ther. 1991;49(3):311–27. doi: 10.1016/0163-7258(91)90061-p. [DOI] [PubMed] [Google Scholar]
- 33.Jarrett RJ, et al. Diurnal variation in oral glucose tolerance: blood sugar and plasma insulin levels morning, afternoon, and evening. Br Med J. 1972;1(5794):199–201. doi: 10.1136/bmj.1.5794.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jarrett RJ, Keen H. Diurnal variation of oral glucose tolerance: a possible pointer to the evolution of diabetes mellitus. Br Med J. 1969;2(5653):341–4. doi: 10.1136/bmj.2.5653.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jarrett RJ, Keen H. Further observations on the diurnal variation in oral glucose tolerance. Br Med J. 1970;4(5731):334–7. doi: 10.1136/bmj.4.5731.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grabner W, et al. Diurnal variation of glucose tolerance and insulin secretion in man (author’s transl) Klin Wochenschr. 1975;53(16):773–8. doi: 10.1007/BF01614859. [DOI] [PubMed] [Google Scholar]
- 37.Aparicio NJ, et al. Circadian variation of the blood glucose, plasma insulin and human growth hormone levels in response to an oral glucose load in normal subjects. Diabetes. 1974;23(2):132–7. doi: 10.2337/diab.23.2.132. [DOI] [PubMed] [Google Scholar]
- 38.Jarrett RJ. Circadian variation in blood glucose levels, in glucose tolerance and in plasma immunoreactive insulin levels. Acta Diabetol Lat. 1972;9(2):263–75. doi: 10.1007/BF01564551. [DOI] [PubMed] [Google Scholar]
- 39.Zimmet PZ, et al. Diurnal variation in glucose tolerance: associated changes in plasma insulin, growth hormone, and non-esterified fatty acids. Br Med J. 1974;1(5906):485–8. doi: 10.1136/bmj.1.5906.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mayer KH, et al. Epidemiologic findings on the relationship of time of day and time since last meal to glucose tolerance. Diabetes. 1976;25(10):936–43. doi: 10.2337/diab.25.10.936. [DOI] [PubMed] [Google Scholar]
- 41.Jarrett RJ, Viberti GC, Sayegh HA. Does “afternoon diabetes” predict diabetes? Br Med J. 1978;1(6112):548–9. doi: 10.1136/bmj.1.6112.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts HJ. Afternoon Glucose Tolerance Testing: A Key to the Pathogenesis, Early Diagnosis and Prognosis of Diabetogenic Hyperinsulinism. J Am Geriatr Soc. 1964;12:423–72. doi: 10.1111/j.1532-5415.1964.tb05730.x. [DOI] [PubMed] [Google Scholar]
- 43.Bowen AJ, Reeves RL. Diurnal variation in glucose tolerance. Arch Intern Med. 1967;119(3):261–4. [PubMed] [Google Scholar]
- 44.Oakley NW, Monier D, Wynn V. Diurnal variation on oral glucose tolerance: insulin and growth hormone changes with special reference to women taking oral contraceptives. Diabetologia. 1973;9(3):235–8. doi: 10.1007/BF01219788. [DOI] [PubMed] [Google Scholar]
- 45.Wojtczak-Jaroszowa J. Physiological and clinical aspects of circadian variations in glucose tolerance. Chronobiologia. 1977;4(4):363–84. [PubMed] [Google Scholar]
- 46.Carroll KF, Nestel PJ. Diurnal variation in glucose tolerance and in insulin secretion in man. Diabetes. 1973;22(5):333–48. doi: 10.2337/diab.22.5.333. [DOI] [PubMed] [Google Scholar]
- 47.Walsh CH, Wright AD. Diurnal patterns of oral glucose tolerance in diabetics. Postgrad Med J. 1975;51(593):169–72. [Google Scholar]
- 48.Hulman A, et al. Effect of time of day and fasting duration on measures of glycaemia: analysis from the Whitehall II Study. Diabetologia. 2013;56(2):294–7. doi: 10.1007/s00125-012-2770-3. [DOI] [PubMed] [Google Scholar]
- 49.Pinkhasov BB, et al. Circadian Rhythms of Carbohydrate Metabolism in Women with Different Types of Obesity. Bull Exp Biol Med. 2016;161(3):323–6. doi: 10.1007/s10517-016-3406-2. [DOI] [PubMed] [Google Scholar]
- 50.Lee A, et al. Diurnal variation in glucose tolerance. Cyclic suppression of insulin action and insulin secretion in normal-weight, but not obese, subjects. Diabetes. 1992;41(6):750–9. doi: 10.2337/diab.41.6.750. [DOI] [PubMed] [Google Scholar]
- 51.Whichelow MJ, et al. Diurnal variation in response to intravenous glucose. Br Med J. 1974;1(5906):488–91. doi: 10.1136/bmj.1.5906.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Melani F, et al. Diurnal variation in blood sugar and serum insulin in response to glucose and/or glucagon in healthy subjects. Horm Metab Res. 1976;8(2):85–8. doi: 10.1055/s-0028-1095597. [DOI] [PubMed] [Google Scholar]
- 53.Verrillo A, de Teresa A, Rucco E. Circadian variation in glucose tolerance and associated changes in plasma insulin and somatostatin levels in normal volunteers. Boll Soc Ital Biol Sper. 1984;60(12):2261–6. [PubMed] [Google Scholar]
- 54.Pisu E, et al. Diurnal variations in insulin secretion and insulin sensitivity in aged subjects. Acta Diabetol Lat. 1980;17(2):153–60. doi: 10.1007/BF02580997. [DOI] [PubMed] [Google Scholar]
- 55.Saad A, et al. Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes. 2012;61(11):2691–700. doi: 10.2337/db11-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Biston P, et al. Diurnal variations in cardiovascular function and glucose regulation in normotensive humans. Hypertension. 1996;28(5):863–71. doi: 10.1161/01.hyp.28.5.863. [DOI] [PubMed] [Google Scholar]
- 57.Van Cauter E, et al. Circadian modulation of glucose and insulin responses to meals: relationship to cortisol rhythm. Am J Physiol. 1992;262(4 Pt 1):E467–75. doi: 10.1152/ajpendo.1992.262.4.E467. [DOI] [PubMed] [Google Scholar]
- 58.Spiegel K, et al. Twenty-four-hour profiles of acylated and total ghrelin: relationship with glucose levels and impact of time of day and sleep. J Clin Endocrinol Metab. 2011;96(2):486–93. doi: 10.1210/jc.2010-1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bo S, et al. Is the timing of caloric intake associated with variation in diet-induced thermogenesis and in the metabolic pattern? A randomized cross-over study. Int J Obes (Lond) 2015;39(12):1689–95. doi: 10.1038/ijo.2015.138. [DOI] [PubMed] [Google Scholar]
- 60.Gibbs M, et al. Diurnal postprandial responses to low and high glycaemic index mixed meals. Clin Nutr. 2014;33(5):889–94. doi: 10.1016/j.clnu.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 61.Yoshino J, et al. Diurnal variation in insulin sensitivity of glucose metabolism is associated with diurnal variations in whole-body and cellular fatty acid metabolism in metabolically normal women. J Clin Endocrinol Metab. 2014;99(9):E1666–70. doi: 10.1210/jc.2014-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Calles-Escandon J, Jaspan J, Robbins DC. Postprandial oscillatory patterns of blood glucose and insulin in NIDDM. Abnormal diurnal insulin secretion patterns and glucose homeostasis independent of obesity. Diabetes Care. 1989;12(10):709–14. doi: 10.2337/diacare.12.10.709. [DOI] [PubMed] [Google Scholar]
- 63.Sonnier T, et al. Glycemic control is impaired in the evening in prediabetes through multiple diurnal rhythms. J Diabetes Complications. 2014;28(6):836–43. doi: 10.1016/j.jdiacomp.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 64.Morris CJ, et al. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc Natl Acad Sci U S A. 2015;112(17):E2225–34. doi: 10.1073/pnas.1418955112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Capani F, et al. Effects of a “single-meal” low calorie diet on the circadian variation of serum cortisol, insulin and somatotropin and urinary excretion of catecholamines. Boll Soc Ital Biol Sper. 1981;57(3):324–6. [PubMed] [Google Scholar]
- 66.Baker IA, Jarrett RJ. Diurnal variation in the blood-sugar and plasma-insulin response to tolbutamide. Lancet. 1972;2(7784):945–7. doi: 10.1016/s0140-6736(72)92471-3. [DOI] [PubMed] [Google Scholar]
- 67.Boden G, et al. Evidence for a circadian rhythm of insulin secretion. Am J Physiol. 1996;271(2 Pt 1):E246–52. doi: 10.1152/ajpendo.1996.271.2.E246. [DOI] [PubMed] [Google Scholar]
- 68.Morgan LM, et al. Diurnal variations in peripheral insulin resistance and plasma non-esterified fatty acid concentrations: a possible link? Ann Clin Biochem. 1999;36(Pt 4):447–50. doi: 10.1177/000456329903600407. [DOI] [PubMed] [Google Scholar]
- 69.Gibson T, Jarrett RJ. Diurnal variation in insulin sensitivity. Lancet. 1972;2(7784):947–8. doi: 10.1016/s0140-6736(72)92472-5. [DOI] [PubMed] [Google Scholar]
- 70.Verrillo A, et al. Differential roles of splanchnic and peripheral tissues in determining diurnal fluctuation of glucose tolerance. Am J Physiol. 1989;257(4 Pt 1):E459–65. doi: 10.1152/ajpendo.1989.257.4.E459. [DOI] [PubMed] [Google Scholar]
- 71.Schulz B, et al. Diurnal rhythm of insulin sensitivity in subjects with normal and impaired glucose tolerance. Exp Clin Endocrinol. 1983;81(3):263–72. doi: 10.1055/s-0029-1210235. [DOI] [PubMed] [Google Scholar]
- 72.van Moorsel D, et al. Demonstration of a day-night rhythm in human skeletal muscle oxidative capacity. Mol Metab. 2016;5(8):635–45. doi: 10.1016/j.molmet.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hansen J, et al. Synchronized human skeletal myotubes of lean, obese and type 2 diabetic patients maintain circadian oscillation of clock genes. Sci Rep. 2016;6:35047. doi: 10.1038/srep35047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Macauley M, et al. Diurnal variation in skeletal muscle and liver glycogen in humans with normal health and Type 2 diabetes. Clin Sci (Lond) 2015;128(10):707–13. doi: 10.1042/CS20140681. [DOI] [PubMed] [Google Scholar]
- 75.Carrasco-Benso MP, et al. Human adipose tissue expresses intrinsic circadian rhythm in insulin sensitivity. FASEB J. 2016;30(9):3117–23. doi: 10.1096/fj.201600269RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moller N, et al. Effects of growth hormone on glucose metabolism. Horm Res. 1991;36(Suppl 1):32–5. [PubMed] [Google Scholar]
- 77.Van Cauter E, et al. Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. J Clin Invest. 1991;88(3):934–42. doi: 10.1172/JCI115396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Plat L, et al. Metabolic effects of short-term elevations of plasma cortisol are more pronounced in the evening than in the morning. J Clin Endocrinol Metab. 1999;84(9):3082–92. doi: 10.1210/jcem.84.9.5978. [DOI] [PubMed] [Google Scholar]
- 79.Plat L, et al. Effects of morning cortisol elevation on insulin secretion and glucose regulation in humans. Am J Physiol. 1996;270(1 Pt 1):E36–42. doi: 10.1152/ajpendo.1996.270.1.E36. [DOI] [PubMed] [Google Scholar]
- 80.Boden G, Chen X, Urbain JL. Evidence for a circadian rhythm of insulin sensitivity in patients with NIDDM caused by cyclic changes in hepatic glucose production. Diabetes. 1996;45(8):1044–50. doi: 10.2337/diab.45.8.1044. [DOI] [PubMed] [Google Scholar]
- 81.Radziuk J, Pye S. Diurnal rhythm in endogenous glucose production is a major contributor to fasting hyperglycaemia in type 2 diabetes. Suprachiasmatic deficit or limit cycle behaviour? Diabetologia. 2006;49(7):1619–28. doi: 10.1007/s00125-006-0273-9. [DOI] [PubMed] [Google Scholar]
- 82.Shapiro ET, et al. Oscillations in insulin secretion during constant glucose infusion in normal man: relationship to changes in plasma glucose. J Clin Endocrinol Metab. 1988;67(2):307–14. doi: 10.1210/jcem-67-2-307. [DOI] [PubMed] [Google Scholar]
- 83.Shea SA, et al. Independent circadian and sleep/wake regulation of adipokines and glucose in humans. J Clin Endocrinol Metab. 2005;90(5):2537–44. doi: 10.1210/jc.2004-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Van Cauter E, et al. Nocturnal decrease in glucose tolerance during constant glucose infusion. J Clin Endocrinol Metab. 1989;69(3):604–11. doi: 10.1210/jcem-69-3-604. [DOI] [PubMed] [Google Scholar]
- 85.Shapiro ET, et al. Nocturnal elevation of glucose levels during fasting in noninsulin-dependent diabetes. J Clin Endocrinol Metab. 1991;72(2):444–54. doi: 10.1210/jcem-72-2-444. [DOI] [PubMed] [Google Scholar]
- 86.Owens DS, et al. A preliminary investigation into individual differences in the circadian variation of meal tolerance: effects on mood and hunger. Chronobiol Int. 1996;13(6):435–47. doi: 10.3109/07420529609020914. [DOI] [PubMed] [Google Scholar]
- 87.Van Cauter EV, et al. Abnormal temporal patterns of glucose tolerance in obesity: relationship to sleep-related growth hormone secretion and circadian cortisol rhythmicity. J Clin Endocrinol Metab. 1994;79(6):1797–805. doi: 10.1210/jcem.79.6.7989487. [DOI] [PubMed] [Google Scholar]
- 88.Reinke H, Asher G. Circadian Clock Control of Liver Metabolic Functions. Gastroenterology. 2016;150(3):574–80. doi: 10.1053/j.gastro.2015.11.043. [DOI] [PubMed] [Google Scholar]
- 89.McGinnis GR, Young ME. Circadian regulation of metabolic homeostasis: causes and consequences. Nat Sci Sleep. 2016;8:163–80. doi: 10.2147/NSS.S78946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Romon M, et al. Circadian variation of postprandial lipemia. Am J Clin Nutr. 1997;65(4):934–40. doi: 10.1093/ajcn/65.4.934. [DOI] [PubMed] [Google Scholar]
- 91.Sennels HP, Jorgensen HL, Fahrenkrug J. Diurnal changes of biochemical metabolic markers in healthy young males - the Bispebjerg study of diurnal variations. Scand J Clin Lab Invest. 2015;75(8):686–92. doi: 10.3109/00365513.2015.1080385. [DOI] [PubMed] [Google Scholar]
- 92.van Kerkhof LW, et al. Diurnal Variation of Hormonal and Lipid Biomarkers in a Molecular Epidemiology-Like Setting. PLoS One. 2015;10(8):e0135652. doi: 10.1371/journal.pone.0135652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rivera-Coll A, Fuentes-Arderiu X, Diez-Noguera A. Circadian rhythmic variations in serum concentrations of clinically important lipids. Clin Chem. 1994;40(8):1549–53. [PubMed] [Google Scholar]
- 94.Singh R, et al. Circadian Time Structure of Circulating Plasma Lipid Components in Healthy Indians of Different Age Groups. Indian J Clin Biochem. 2016;31(2):215–23. doi: 10.1007/s12291-015-0519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Demacker PN, et al. Intra-individual variation of serum cholesterol, triglycerides and high density lipoprotein cholesterol in normal humans. Atherosclerosis. 1982;45(3):259–66. doi: 10.1016/0021-9150(82)90227-1. [DOI] [PubMed] [Google Scholar]
- 96.Statland BE, Winkel P, Bokelund H. Factors contributing to intra-individual variation of serum constituents. 2. Effects of exercise and diet on variation of serum constituents in healthy subjects. Clin Chem. 1973;19(12):1380–3. [PubMed] [Google Scholar]
- 97.Henderson LO, et al. Minimal within-day variation of high density lipoprotein cholesterol and apolipoprotein A-I levels in normal subjects. J Lipid Res. 1980;21(7):953–5. [PubMed] [Google Scholar]
- 98.van Oostrom AJ, et al. Diurnal triglyceride profiles in 30 young healthy men as a function of diet, fasting triglyceride levels, body composition and insulin sensitivity. Ned Tijdschr Geneeskd. 1999;143(37):1868–72. [PubMed] [Google Scholar]
- 99.Halkes CJ, et al. Gender differences in diurnal triglyceridemia in lean and overweight subjects. Int J Obes Relat Metab Disord. 2001;25(12):1767–74. doi: 10.1038/sj.ijo.0801831. [DOI] [PubMed] [Google Scholar]
- 100.Klop B, et al. Daytime triglyceride variability in men and women with different levels of triglyceridemia. Clin Chim Acta. 2011;412(23–24):2183–9. doi: 10.1016/j.cca.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 101.Halkes CJ, et al. Diurnal triglyceridaemia and insulin resistance in mildly obese subjects with normal fasting plasma lipids. J Intern Med. 2004;255(1):74–81. doi: 10.1046/j.0954-6820.2003.01252.x. [DOI] [PubMed] [Google Scholar]
- 102.Miettinen TA. Diurnal variation of cholesterol precursors squalene and methyl sterols in human plasma lipoproteins. J Lipid Res. 1982;23(3):466–73. [PubMed] [Google Scholar]
- 103.Cella LK, Van Cauter E, Schoeller DA. Diurnal rhythmicity of human cholesterol synthesis: normal pattern and adaptation to simulated “jet lag”. Am J Physiol. 1995;269(3 Pt 1):E489–98. doi: 10.1152/ajpendo.1995.269.3.E489. [DOI] [PubMed] [Google Scholar]
- 104.Jones PJ, Schoeller DA. Evidence for diurnal periodicity in human cholesterol synthesis. J Lipid Res. 1990;31(4):667–73. [PubMed] [Google Scholar]
- 105.Cella LK, Van Cauter E, Schoeller DA. Effect of meal timing on diurnal rhythm of human cholesterol synthesis. Am J Physiol. 1995;269(5 Pt 1):E878–83. doi: 10.1152/ajpendo.1995.269.5.E878. [DOI] [PubMed] [Google Scholar]
- 106.Ang JE, et al. Identification of human plasma metabolites exhibiting time-of-day variation using an untargeted liquid chromatography-mass spectrometry metabolomic approach. Chronobiol Int. 2012;29(7):868–81. doi: 10.3109/07420528.2012.699122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hussain MM, Pan X. Circadian regulators of intestinal lipid absorption. J Lipid Res. 2015;56(4):761–70. doi: 10.1194/jlr.R051573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hussain MM. Regulation of intestinal lipid absorption by clock genes. Annu Rev Nutr. 2014;34:357–75. doi: 10.1146/annurev-nutr-071813-105322. [DOI] [PubMed] [Google Scholar]
- 109.Dallmann R, et al. The human circadian metabolome. Proc Natl Acad Sci U S A. 2012;109(7):2625–9. doi: 10.1073/pnas.1114410109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chua EC, et al. Extensive diversity in circadian regulation of plasma lipids and evidence for different circadian metabolic phenotypes in humans. Proc Natl Acad Sci U S A. 2013;110(35):14468–73. doi: 10.1073/pnas.1222647110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kasukawa T, et al. Human blood metabolite timetable indicates internal body time. Proc Natl Acad Sci U S A. 2012;109(37):15036–41. doi: 10.1073/pnas.1207768109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kumar Jha P, Challet E, Kalsbeek A. Circadian rhythms in glucose and lipid metabolism in nocturnal and diurnal mammals. Mol Cell Endocrinol. 2015;418(Pt 1):74–88. doi: 10.1016/j.mce.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 113.Krauchi K, Wirz-Justice A. Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. Am J Physiol. 1994;267(3 Pt 2):R819–29. doi: 10.1152/ajpregu.1994.267.3.R819. [DOI] [PubMed] [Google Scholar]
- 114.Spengler CM, Czeisler CA, Shea SA. An endogenous circadian rhythm of respiratory control in humans. J Physiol. 2000;526(Pt 3):683–94. doi: 10.1111/j.1469-7793.2000.00683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Consoli A, et al. Effect of scheduling of meal times on the circadian rhythm of energy expenditure. Boll Soc Ital Biol Sper. 1981;57(23):2322–4. [PubMed] [Google Scholar]
- 116.Sensi S, Capani F. Chronobiological aspects of weight loss in obesity: effects of different meal timing regimens. Chronobiol Int. 1987;4(2):251–61. doi: 10.3109/07420528709078532. [DOI] [PubMed] [Google Scholar]
- 117.Capani F, et al. Variations in carbohydrate, lipid and protein oxidation evaluated by indirect calorimetry in obese subjects on a “single-meal” low-calorie diet. Boll Soc Ital Biol Sper. 1981;57(3):320–2. [PubMed] [Google Scholar]
- 118.Romon M, et al. Circadian variation of diet-induced thermogenesis. Am J Clin Nutr. 1993;57(4):476–80. doi: 10.1093/ajcn/57.4.476. [DOI] [PubMed] [Google Scholar]
- 119.Morris CJ, et al. The Human Circadian System Has a Dominating Role in Causing the Morning/Evening Difference in Diet-Induced Thermogenesis. Obesity (Silver Spring) 2015;23(10):2053–8. doi: 10.1002/oby.21189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Weststrate JA, et al. Diurnal variation in postabsorptive resting metabolic rate and diet-induced thermogenesis. Am J Clin Nutr. 1989;50(5):908–14. doi: 10.1093/ajcn/50.5.908. [DOI] [PubMed] [Google Scholar]
- 121.Zwiauer KF, Mueller T, Widhalm K. Effect of daytime on resting energy expenditure and thermic effect of food in obese adolescents. J Am Coll Nutr. 1992;11(3):267–71. doi: 10.1080/07315724.1992.10718227. [DOI] [PubMed] [Google Scholar]
- 122.Miles CW, et al. Effect of circadian variation in energy expenditure, within-subject variation and weight reduction on thermic effect of food. Eur J Clin Nutr. 1993;47(4):274–84. [PubMed] [Google Scholar]
- 123.Scheer FA, Morris CJ, Shea SA. The internal circadian clock increases hunger and appetite in the evening independent of food intake and other behaviors. Obesity (Silver Spring) 2013;21(3):421–3. doi: 10.1002/oby.20351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sargent C, et al. Daily Rhythms of Hunger and Satiety in Healthy Men during One Week of Sleep Restriction and Circadian Misalignment. Int J Environ Res Public Health. 2016;13(2):170. doi: 10.3390/ijerph13020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wehrens SMT, et al. Meal Timing Regulates the Human Circadian System. Curr Biol. 2017;27(12):1768–1775 e3. doi: 10.1016/j.cub.2017.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Waterhouse J, et al. Lack of evidence for a marked endogenous component determining food intake in humans during forced desynchrony. Chronobiol Int. 2004;21(3):445–68. doi: 10.1081/cbi-120038628. [DOI] [PubMed] [Google Scholar]
- 127.Schoeller DA, et al. Entrainment of the diurnal rhythm of plasma leptin to meal timing. J Clin Invest. 1997;100(7):1882–7. doi: 10.1172/JCI119717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Natalucci G, et al. Spontaneous 24-h ghrelin secretion pattern in fasting subjects: maintenance of a meal-related pattern. Eur J Endocrinol. 2005;152(6):845–50. doi: 10.1530/eje.1.01919. [DOI] [PubMed] [Google Scholar]
- 129.Park SJ, Tokura H. Bright light exposure during the daytime affects circadian rhythms of urinary melatonin and salivary immunoglobulin A. Chronobiol Int. 1999;16(3):359–71. doi: 10.3109/07420529909116864. [DOI] [PubMed] [Google Scholar]
- 130.Hashimoto S, et al. Midday exposure to bright light changes the circadian organization of plasma melatonin rhythm in humans. Neurosci Lett. 1997;221(2–3):89–92. doi: 10.1016/s0304-3940(96)13291-2. [DOI] [PubMed] [Google Scholar]
- 131.Takasu NN, et al. Repeated exposures to daytime bright light increase nocturnal melatonin rise and maintain circadian phase in young subjects under fixed sleep schedule. Am J Physiol Regul Integr Comp Physiol. 2006;291(6):R1799–807. doi: 10.1152/ajpregu.00211.2006. [DOI] [PubMed] [Google Scholar]
- 132.Lieverse R, et al. Bright light treatment in elderly patients with nonseasonal major depressive disorder: a randomized placebo-controlled trial. Arch Gen Psychiatry. 2011;68(1):61–70. doi: 10.1001/archgenpsychiatry.2010.183. [DOI] [PubMed] [Google Scholar]
- 133.Fukushige H, et al. Effects of tryptophan-rich breakfast and light exposure during the daytime on melatonin secretion at night. Journal of physiological anthropology. 2014;33(1):33. doi: 10.1186/1880-6805-33-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mishima K, et al. Diminished melatonin secretion in the elderly caused by insufficient environmental illumination. J Clin Endocrinol Metab. 2001;86(1):129–34. doi: 10.1210/jcem.86.1.7097. [DOI] [PubMed] [Google Scholar]
- 135.Sone Y, et al. Effects of dim or bright-light exposure during the daytime on human gastrointestinal activity. Chronobiology international. 2003;20(1):123–133. doi: 10.1081/cbi-120017688. [DOI] [PubMed] [Google Scholar]
- 136.Nieuwenhuis R, Spooren P, Tilanus J. Less need for insulin, a surprising effect of phototherapy in insulin-dependent diabetes mellitus. Tijdschrift voor psychiatrie. 2008;51(9):693–697. [PubMed] [Google Scholar]
- 137.Allen N, et al. Insulin sensitivity after phototherapy for seasonal affective disorder. The Lancet. 1992;339(8800):1065–1066. [PubMed] [Google Scholar]
- 138.Sene-Fiorese M, et al. The potential of phototherapy to reduce body fat, insulin resistance and “metabolic inflexibility” related to obesity in women undergoing weight loss treatment. Lasers in surgery and medicine. 2015;47(8):634–642. doi: 10.1002/lsm.22395. [DOI] [PubMed] [Google Scholar]
- 139.Bylesjö EI, Boman K, Wetterberg L. Obesity treated with phototherapy: four case studies. International Journal of Eating Disorders. 1996;20(4):443–446. doi: 10.1002/(SICI)1098-108X(199612)20:4<443::AID-EAT14>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 140.Danilenko KV, Mustafina SV, Pechenkina EA. Bright light for weight loss: results of a controlled crossover trial. Obesity facts. 2013;6(1):28–38. doi: 10.1159/000348549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang P, Tokura H. Thermoregulatory responses in humans during exercise after exposure to two different light intensities. European journal of applied physiology and occupational physiology. 1999;79(3):285–289. doi: 10.1007/s004210050508. [DOI] [PubMed] [Google Scholar]
- 142.Dunai A, et al. Moderate exercise and bright light treatment in overweight and obese individuals. Obesity. 2007;15(7):1749–1757. doi: 10.1038/oby.2007.208. [DOI] [PubMed] [Google Scholar]
- 143.Figueiro MG, Plitnick B, Rea MS. Light modulates leptin and ghrelin in sleep-restricted adults. International journal of endocrinology. 2012;2012 doi: 10.1155/2012/530726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Versteeg RI, et al. Acute effects of morning light on plasma glucose and triglycerides in healthy men and men with type 2 diabetes. Journal of Biological Rhythms. 2017;32(2):130–142. doi: 10.1177/0748730417693480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Albreiki MS, Middleton B, Hampton SM. A single night light exposure acutely alters hormonal and metabolic responses in healthy participants. Endocrine Connections. 2017;6(2):100–110. doi: 10.1530/EC-16-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gil-Lozano M, et al. Short-term sleep deprivation with nocturnal light exposure alters time-dependent glucagon-like peptide-1 and insulin secretion in male volunteers. American Journal of Physiology-Endocrinology and Metabolism. 2016;310(1):E41–E50. doi: 10.1152/ajpendo.00298.2015. [DOI] [PubMed] [Google Scholar]
- 147.Hirota N, Sone Y, Tokura H. Effect of evening exposure to dim or bright light on the digestion of carbohydrate in the supper meal. Chronobiology international. 2003;20(5):853–862. doi: 10.1081/cbi-120024216. [DOI] [PubMed] [Google Scholar]
- 148.Cheung IN, et al. Morning and Evening Blue-Enriched Light Exposure Alters Metabolic Function in Normal Weight Adults. PloS one. 2016;11(5):e0155601. doi: 10.1371/journal.pone.0155601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.McFadden E, et al. The relationship between obesity and exposure to light at night: cross-sectional analyses of over 100,000 women in the breakthrough generations study. American journal of epidemiology. 2014;180(3):245–250. doi: 10.1093/aje/kwu117. [DOI] [PubMed] [Google Scholar]
- 150.Obayashi K, Saeki K, Kurumatani N. Ambient Light Exposure and Changes in Obesity Parameters: A Longitudinal Study of the HEIJO-KYO Cohort. The Journal of Clinical Endocrinology & Metabolism. 2016;101(9):3539–3547. doi: 10.1210/jc.2015-4123. [DOI] [PubMed] [Google Scholar]
- 151.Obayashi K, et al. Exposure to light at night, nocturnal urinary melatonin excretion, and obesity/dyslipidemia in the elderly: a cross-sectional analysis of the HEIJO-KYO study. The Journal of Clinical Endocrinology & Metabolism. 2012;98(1):337–344. doi: 10.1210/jc.2012-2874. [DOI] [PubMed] [Google Scholar]
- 152.Obayashi K, et al. Independent associations of exposure to evening light and nocturnal urinary melatonin excretion with diabetes in the elderly. Chronobiology international. 2014;31(3):394–400. doi: 10.3109/07420528.2013.864299. [DOI] [PubMed] [Google Scholar]
- 153.Reid KJ, et al. Timing and intensity of light correlate with body weight in adults. PloS one. 2014;9(4):e92251. doi: 10.1371/journal.pone.0092251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Leproult R, Holmback U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014;63(6):1860–9. doi: 10.2337/db13-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bandin C, et al. Meal timing affects glucose tolerance, substrate oxidation and circadian-related variables: A randomized, crossover trial. Int J Obes (Lond) 2015;39(5):828–33. doi: 10.1038/ijo.2014.182. [DOI] [PubMed] [Google Scholar]
- 156.Sato M, et al. Acute effect of late evening meal on diurnal variation of blood glucose and energy metabolism. Obes Res Clin Pract. 2011;5(3):e169–266. doi: 10.1016/j.orcp.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 157.Del Ponte A, et al. Modifications in the oscillation of substrates in obese subjects subjected to variations in the pattern of meal-timing. Bollettino Della Societa Italiana di Biologia Sperimentale. 1984;60(11):2099–2104. [PubMed] [Google Scholar]
- 158.Keim NL, et al. Weight loss is greater with consumption of large morning meals and fat-free mass is preserved with large evening meals in women on a controlled weight reduction regimen. J Nutr. 1997;127(1):75–82. doi: 10.1093/jn/127.1.75. [DOI] [PubMed] [Google Scholar]
- 159.Morgan LM, et al. Effect of meal timing and glycaemic index on glucose control and insulin secretion in healthy volunteers. Br J Nutr. 2012;108(7):1286–91. doi: 10.1017/S0007114511006507. [DOI] [PubMed] [Google Scholar]
- 160.Garaulet M, et al. Timing of food intake predicts weight loss effectiveness. Int J Obes (Lond) 2013;37(4):604–11. doi: 10.1038/ijo.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jakubowicz D, et al. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity (Silver Spring) 2013;21(12):2504–12. doi: 10.1002/oby.20460. [DOI] [PubMed] [Google Scholar]
- 162.Jakubowicz D, et al. Effects of caloric intake timing on insulin resistance and hyperandrogenism in lean women with polycystic ovary syndrome. Clin Sci (Lond) 2013;125(9):423–32. doi: 10.1042/CS20130071. [DOI] [PubMed] [Google Scholar]
- 163.Rabinovitz HR, et al. Big breakfast rich in protein and fat improves glycemic control in type 2 diabetics. Obesity (Silver Spring) 2014;22(5):E46–54. doi: 10.1002/oby.20654. [DOI] [PubMed] [Google Scholar]
- 164.Beebe CA, et al. Effect of temporal distribution of calories on diurnal patterns of glucose levels and insulin secretion in NIDDM. Diabetes Care. 1990;13(7):748–55. doi: 10.2337/diacare.13.7.748. [DOI] [PubMed] [Google Scholar]