Abstract
Maternal presence has marked effects on adolescent neurocognition during risk taking, influencing teenagers to make safer decisions. However, it is currently unknown whether maternal buffering changes over the course of adolescence itself, and whether its effects are robust to individual differences family relationship quality. In the current longitudinal study, 23 adolescents completed a risk-taking task under maternal presence during an fMRI scan before and after the transition to high school. Behavioral results reveal that adolescent risk taking increased in the presence of mother across a one year period. At the neural level, we found adolescents reporting higher family conflict showed longitudinal increases in functional coupling between the anterior insula (AI) and ventral striatum (VS) when making safe decisions in the presence of their mother, which was associated with increased real-world risk taking. These findings show that individual differences in family relationship quality undermine effective development of AI-VS connectivity resulting in increased risk taking.
Keywords: ventral striatum, anterior insula, adolescence, maternal buffering, longitudinal, functional connectivity
Adolescence is a formative time of transition between childhood and adulthood characterized by several key changes in biological functioning, such as neural and endocrinological activity (Crone & Dahl, 2012; Peper & Dahl, 2013). Additionally, adolescence also marks a time in which the social ecology is undergoing changes, including increased autonomy from the family as well as heightened parent-adolescent conflicts (e.g., Graber & Brooks-Gunn, 1996; Larson & Richards, 1989; Sumter, Bokhorst, Steinberg, & Westenberg, 2009). Together these changing biological and social contexts are thought to engender notable shifts in behavioral tendencies which contribute to both healthy and unhealthy development. Among other changes in behavior, adolescents begin to engage in greater rates of risky behaviors that subsequently put their well-being at risk (e.g., Mahalik et al., 2013; Steinberg et al., 2008).
Although adolescents are more inclined to take potentially harmful risks, a growing body of research demonstrates that context plays a crucial role in shaping such behavioral tendencies such that adolescents’ risky tendencies are augmented (Chein, Albert, O’Brien, Uckert, & Steinberg, 2011; Gardner & Steinberg, 2005) or attenuated (Silva, Chein, & Steinberg, 2016; Telzer, Ichien, & Qu, 2015), depending on the social and motivational context. Parents, in particular, play a key role in scaffolding their offspring’s self-regulation across juvenile development (Hostinar, Sullivan, & Gunnar, 2014). During childhood, mothers help scaffold the implicit regulation of basic emotional impulses at the neural level (Gee et al., 2014). During adolescence, however, mothers offer aid in other developmentally relevant forms of self-regulation. For instance, maternal presence is associated with decreases in their adolescent’s risk taking via greater activation in brain regions implicated in reward and salience when making safe decisions and less activation while making risky decisions (Telzer et al., 2015), an effect that is specific to mothers and not an unknown adult (Guassi Moreira & Telzer, in press).
Though the potential for maternal presence to reduce adolescent risk taking is promising, much is unknown about the nuances of this phenomenon. One such lacuna is how the quality of family relationships may modulate the strength and direction of maternal buffering. While parent-child relationships are often regarded as having protective characteristics that promote healthy development across childhood and adolescence (Gee et al., 2014; Kerr & Stattin, 2000), parents can also negatively influence their offspring’s development, particularly in more maladaptive family contexts (e.g., Rubin, Burgess, & Hastings, 2002). Accordingly, one feature of adolescent-parent relationships that may undermine the efficacy of maternal buffering on risk taking is the quality of the relationship itself. Western youth—on whom the most research has been conducted—perceive adolescence as a time of blunted family engagement and obligation during the transition to high school and tend to identify less with their families during the course of high school (Qu, Pomerantz, Wang, Cheung, & Cimpian, 2016; Tsai et al., 2013). Importantly, individuals report greater levels of family conflict with their parents during adolescence (Steinberg & Morris, 2001; Tsai, Telzer, & Fuligni, 2013). While increases in family conflict do not necessarily doom adolescents and is relatively normative during this age (Steinberg & Morris, 2001), high levels of family conflict do serve as a potent vulnerability to various forms of risk taking, such as substance use (Gerard, Krishnakumar, & Buehler, 2006; Nash, McQueen, & Bray, 2005; Skeer, Avenue, Mccormick, Normand, & Gilman, 2009).
Such changes in family dynamics across adolescence imply that the effect that parents have on reducing risky behaviors is not static, suggesting that the buffering capacity of maternal presence may wane over time, particularly in high conflict families. Although prior research has found mothers to exhibit a general protective effect in scaffolding their offspring’s decision making (Telzer et al., 2015), it remains unknown whether levels of family conflict moderate this effect. It is plausible that various changes occurring to the parent-adolescent dynamic across adolescence may undermine the effectiveness of maternal presence in buffering risk taking. Higher levels of family conflict likely attenuate the buffering effects of maternal presence on adolescent risk taking, perhaps because adolescents in high conflict families are less motivated to behave safely in the presence of their parents, and the presence of a highly conflictual family member may exploit an existing cognitive vulnerability (e.g., McCormick, Qu, & Telzer, 2016).
Because adolescence is a time of remarkable neural plasticity and a sensitive period for various types of neurodevelopment (Blakemore & Mills, 2014; Hauser, Iannaccone, Walitza, Brandeis, & Brem, 2015; Spear, 2011), changes in close social relationships may affect adolescent risk taking via changes in their neurobiology. In particular, the ventral striatum (VS) is a key brain region involved in approach related behaviors and reward sensitivity. Hyperactivity in the VS, especially in socioemotionally salient contexts, is theorized to underlie adolescent risk taking (Steinberg, 2010) and shows increased activation as individuals age towards mid-adolescence (Crone & Elzinga, 2015). In addition to the VS, prefrontal cortical regions responsible for regulating reward and motivation-related impulses mature steadily throughout adolescence, and are not considered to be fully capable of handling the supposed surge in striatal hyperactivity that accompanies adolescence (Shulman et al., 2016; Steinberg, 2010).
The relationship between subcortical reward regions (e.g., VS) and prefrontal control regions does not describe the totality of the neurobiological underpinnings of adolescent risk taking, as other brain regions serve key roles. Specifically, the anterior insula (AI) has received recent attention as an important player in the development of risky behaviors in adolescence (Smith et al., 2014). The AI is involved in a host of neural functions, ranging from mapping visceral somatosensory inputs (Mufson & Mesulam, 1982), to helping generate bottom-up affective impulses (Ochsner, Silvers, & Buhle, 2012). Many prior studies have observed exaggerated anterior insula activation while adolescents make decisions under uncertainty (e.g., Van Leijenhorst et al., 2010), and recently the AI has been identified as a cortical hub for the integration of affective and cognitive neural signals (Smith, Steinberg, & Chein, 2014; van Duijvenvoorde et al., 2014). The role of the insula as a neural integrator is especially relevant when considering that several models of adolescent neurodevelopment posit that asynchronous development of cognitive and affective neural systems underlie heighted risk taking (Casey, 2015; Shulman et al., 2016). This is a critical, yet overlooked, facet of adolescent neurodevelopment. Adolescents’ predispositions towards risk may not only be the result of a developmental imbalance in potency between reward and cognitive control systems (Casey, 2015; Steinberg, 2010), but also due to poor interfacing between, and integration of, cognitive— affective signals.
These neural changes are central to our understanding of adolescent neurobiology, yet they have not been fully captured in investigations concerned with understanding the buffering effects of maternal influence on adolescent risk taking. Longitudinal neuroimaging studies are essential in order to capture the dynamic changing brain across adolescence, yet such methods are still rare. In the current study, we sought to examine how maternal social buffering on adolescent risk-taking changes across the middle school to high school transition. To this end, we used functional magnetic resonance imaging (fMRI) in a sample of adolescents to understand how functional connectivity with the AI is altered by family conflict and self-reported, real-world risk taking. Adolescents completed measures of family conflict and then underwent an fMRI scan in the 8th grade and again in the 9th grade during which they completed a risk-taking task in the presence of their mother. Because family conflict adversely affects neural processes involved in cognitive control (McCormick et al., 2016) and affective processing (Qu, Galvan, Fuligni, Lieberman, & Telzer, 2015), and the AI serves as a hub between affective (i.e., VS) and cognitive control regions (Smith et al., 2014), we tested whether family conflict affects connectivity between these regions. Lastly, we examined how changes in connectivity between the AI and VS is related to self-reported risk taking.
Methods
Participants
28 adolescents and their mothers from the Midwestern United States were invited to participate. Participants completed a brain scan during their 8th grade year (Wave 1; W1) and again during 9th grade (Wave 2; W2). 5 participants did not participate at both time points for the following reasons: 2 participants moved excessively during the scan at W1, 1 participant’s behavioral data were not recorded properly for one of the waves, 1 participant moved away, and 1 received dental braces between waves of data collection. This yielded a final sample of 23 adolescents (9 female; Mage Wave 1=14.4 years, Mage Wave 2=15.2 years). Participants were ethnically diverse (White=14, African American=6, Asian n=1, and mixed race=2). Written assent and consent was obtained from adolescents and their mothers, respectively, in accordance with the guidelines of the Institutional Review Board. Statistical maps of brain activation will be made available on NeuroVault via the Open Science Framework (osf.io/####).
Self-Report Measures
Family Conflict
Frequency of adolescent-parent conflict at Wave 1 was assessed using the 10-item family conflict scale (Ruiz, Gonzales, & Formoso, 1998). Participants were asked to indicate how often they fight, disagree, or argue with their parents along a five point scale (1=“Almost Never” to 5=“Almost Always”; e.g., “You and your parents ignored each other”). This scale has been utilized to measure family conflict in developmental samples, and its link to risk taking behavior (e.g., Telzer, Gonzales, & Fuligni, 2014), and neural development (McCormick, Qu, & Telzer, 2016). The measure has excellent reliability in our sample (α = 0.94).
Risk Taking
In order to measure subsequent risk-taking behavior at Wave 2, adolescents completed a revised, 12-item version of the Adolescent Risk Taking Scale (Alexander et al., 1990; Telzer, Fuligni, Lieberman, & Galván, 2013). This measure was initially developed and validated on a cohort of youth transitioning from eighth to ninth grade, consistent with the age group of our own sample. Along a four point scale (1=“Never” to 4=“Many Times”), participants rated the extent to which they engaged in a range of risky behaviors such as cheating on a relationship partner, sneaking out of the house at night, or riding in a car without a seatbelt. The measure has excellent reliability in our sample (α = 0.93).
In-Scan Risk Taking Task
In order to assess risk-taking behavior at the neural level, adolescents completed the Stoplight Task (Gardner & Steinberg, 2005), an ecologically valid driving simulation widely used in neuroimaging studies (Chein et al., 2011; Telzer et al., 2015). During the simulation, adolescents were told they were driving a car and would encounter 26 traffic intersections with yellow lights. At each intersection, participants had to choose whether to brake or accelerate through the light by selecting one of two buttons. Instructed to finish the task as quickly as possible, adolescents were told that accelerating (i.e., a ‘go’ decision) through the intersection would result in no delay whereas choosing to brake (i.e., a ‘stop’ decision) would lead to a 3 second delay. However, accelerating through the intersection was associated with the risk of crashing into another car which resulted in a longer (6s) delay than stopping. Eight intersections had approaching cars and resulted in a crash if the participant chose to accelerate instead of stop. This probability of crashing was unknown to the participants—they simply knew they ran some risk of crashing. Prior to the scan, participants were instructed on how to properly complete the task by watching a video and completing two practice runs.
In order to assess the extent to which maternal influences changes in adolescents’ risk taking across the middle school to high school transition, we administered an experimental protocol similar to prior studies in which participants were told that their mothers would be observing them play the stoplight game while in the scanner (e.g., Telzer et al., 2015). Just prior to the start of the task, the participant’s mother announced to her child that she was observing them during the task via the scanner intercom. In order to ensure the announcement was consistent across participants and did not bias behavior, mothers were instructed to recite the following script. “Hi [adolescent name], I just wanted to let you know that I’m here and I’ll be watching you play this round”). The mother stayed in the scan room and observed her child’s behavior for the duration of the task.
fMRI Data Acquisition
A 3T Siemens Trio MRI scanner was used to collect all neuroimaging data at both time points. Scans during the task consisted of T2*-weighted echoplanar images (EPI; 3mm slice thickness; 38 slices; 2s TR; 25ms TE; 92×92 matrix; 230mm FOV; 2.5×2.5×3mm3 voxel size). Structural scans comprised of a T2*weighted, matched-bandwidth (MBW), high resolution, anatomical scan (4s TR; 64ms TE; 230 FOV; 192×192 matrix; 3mm slice thickness; 38 slices) and a T1* magnetization-prepared rapid-acquisition gradient echo (MPRAGE; 1.9s TR; 2.3ms TE; 230 FOV; 256×256 matrix; sagittal plane; slice thickness of 1mm; 192 slices). Both MBW and EPI scans were set to oblique axial in order to maximize brain coverage.
fMRI Data Processing and Analysis
Statistical Parametric Mapping (SPM8; Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK) was used to process and analyze neuroimaging data. Images were spatially realigned to correct head motion (none of the 23 participants exceeded 2.5mm of slice-to-slice motion in any direction). Realigned functional data were coregistered to the high resolution MPRAGE image and segmented into cerebrospinal fluid, gray matter, and white matter. The normalization transformation matrix from the segmentation step was applied to the functional and T2 structural images, transforming them into standard stereotactic space as defined by the Montreal Neurological Institute and International Consortium for Brain Mapping. An 8mm Gaussian kernel, full-width-at-half maximum, was used to smooth normalized data in order to increase the signal-to-noise ratio.
Statistical analyses were performed using Statistical Parametric Mapping (SPM8, Wellcome Department of Cognitive Neurology, London, UK). Each trial was convolved with the canonical hemodynamic response function. High-pass temporal filtering with a cutoff of 128s was applied to remove low-frequency drift in the time-series. A restricted maximum likelihood algorithm, with an autoregressive model order of 1, was used to estimate serial autocorrelations. A GLM for each participant’s fixed-effects analysis was created with four regressors of interest for both waves. Two were decision regressors (Go and Stop) and two were outcome regressors (Crash, Pass). The wait time after stop decisions in addition to a final ‘Game Over’ period after each scan were modeled in order to remove them from the implicit baseline. The duration of decision trials constituted the time between when the yellow light first appeared and when participants indicated a response. Durations for outcomes were modeled as 1 second. Pass events had no specific onset times whereas the onset of crash events was set to when another car hit the participant’s car. Because crash events happened at most 2 seconds following the yellow light, we modeled the pass events as such, corresponding to the point at which the outcome of a risky decision was clear. Null events, consisting of the jittered intertrial intervals between stoplights, were not explicitly modeled, constituting the implicit baseline.
The parameter estimates resulting from the GLM were used to create linear contrast images comparing the conditions of interest. Random effects, group-level analyses were performed on all individual subject contrasts. Our primary interest was in the decision phase of the task, therefore we focused on the contrasts for Stop and Go decisions (W2-W1). Because our primary research questions focused on functional connectivity, we conducted psychophysiological interaction analyses (PPI; O’Reilly et al., 2012) to investigate functional coupling with the insula. We specified the left and right anterior insula (AI) as our two, separate seed regions, structurally defined using the WFUpickatlas (Tzourio-Mazoyer, et al., 2002; Maldjian, Laurienti, Kraft, & Burdette, 2003). The right AI seed yielded no significant findings; results reported below only reflect connectivity with the left AI. We specifically chose the AI because of its theorized role as a hub that integrates cognitive and affective signals. By selecting the AI as our seed, we would be able to investigate changes in both prefrontal-insular and ventral-insular connectivity. A generalized form of context-dependent PPI (gPPI; O’Reilly et al., 2012) was used to extract the deconvolved time series from the insula ROI for each participant to create the physiological variables, convolve each trial type with the canonical HRF to create the psychological regressor, and multiply the time series from the psychological regressors with the physiological variable to create the PPI interaction terms. The interaction terms identified regions that covaried with the insula in a task-dependent manner. For first level models, one regressor representing the deconvolved BOLD signal was included alongside the psychological and PPI interaction terms for each condition in order to create a gPPI model. At the group level, we conducted random-effects whole-brain and regression analyses to compare functional coupling between conditions of interest. Family conflict and risk taking were each entered in separate whole-brain regression analyses to test whether functional connectivity with the insula changed longitudinally depending on adolescent’ family conflict and risk taking.
In order to correct for multiple comparison, we conducted a Monte Carlo simulation using 3dClustSim while estimating smoothing with 3dFWHMx in the AFNI software package (Ward, 2000). Results indicated a voxel-wise threshold of p<.005 combined with a minimum cluster size of 89 voxels for the whole-brain, corresponding to p<.05, Family Wise Error (FWE) corrected. Given that the ventral striatum is an anatomically small region, we did not expect activation in this area to meet this cutoff. Accordingly, we relaxed the threshold for the ventral striatum alone to 20 voxels, consistent with prior work (Giuliani & Pfeifer, 2015).
Results
Behavioral Results
First, we examined whether adolescents made significantly more risky decisions in the presence of their mothers over time. Given our a priori hypothesis that maternal influence would wane across the middle school to high school transition, we conducted a 1-tailed paired samples t-test. Adolescents made more risky decisions in the presence of their mother at Wave 2 (M=56.52%, SD=19.54%) than Wave 1 (M=48.33%, SD=18.39%; t(22) = 1.75, p = .047, 1-tailed). Self-reported risk taking was not associated with risky decisions during the Stoplight task. Although family conflict was not associated with risky decisions on the Stoplight task at Wave 1 or Wave 2, family conflict was correlated with self-reported risk-taking behaviors at Wave 2 (r = .65, p < .001)1.
fMRI Results
Longitudinal changes in Neural Activation during Safe and Risky Decisions
We first examined whole-brain changes in neural activation across time. We observed longitudinal increases in the AI and bilateral VS when adolescents chose to make stop and go decisions in the presence of their mothers (Table 1). We also observed longitudinal increases in the right dorsolateral prefrontal cortex (dlPFC) and anterior cingulate cortex (ACC) when adolescents chose to stop in the presence of their mothers.
Table 1.
Brain regions which showed longitudinal increases in activation during stop and go decisions.
| Region | BA | x | y | z | t | k | |
|---|---|---|---|---|---|---|---|
| Stop Decision W2 > W1 | |||||||
| AI | L | −30 | 17 | −2 | 6.80 | 9093a | |
| VS | L | −24 | 5 | −8 | 4.40 | a | |
| VS | R | 21 | 5 | −5 | 5.62 | a | |
| Fusiform | 37 | R | 27 | −79 | −11 | 7.26 | a |
| ACC | 32/34 | 0 | 26 | 28 | 4.09 | a | |
| TPJ | 39 | R | 57 | −40 | 28 | 4.09 | a |
| TPJ | 39 | L | −54 | −37 | 28 | 3.84 | a |
| dlPFC | 9 | R | 21 | 44 | 28 | 3.49 | a |
| MTG | 21 | R | 42 | −73 | 22 | 2.99 | a |
| SPL | 7 | L | −21 | −61 | 49 | 6.02 | 541b |
| MCC | 24 | L | −6 | −46 | 58 | 4.07 | b |
| Go Decision W2 > W1 | |||||||
| AI | L | −42 | 2 | −5 | 3.82 | 3750c | |
| VS | R | 15 | 14 | −2 | 3.39 | c | |
| VS | L | −18 | 8 | −2 | 5.65 | c | |
| STS | 22 | L | −60 | −28 | 7 | 5.40 | c |
| Thalamus | L | −6 | −19 | −5 | 2.83 | c | |
| Cerebellum | R | 30 | −40 | −20 | 5.69 | c | |
| MTG | 21 | R | 48 | −73 | 19 | 3.92 | 91 |
Note. R refers to right and L refers to left. x, y, and z refer to MNI coordinates; t refers to the t-score at those coordinates (local maxima); AI refers to anterior insula; VS refers to ventral striatum; MTG refers to middle temporal gyrus; ACC refers to anterior cingulate gyrus; vlPFC refers to ventrolateral prefrontal cortex; dlPFC refers to dorsolateral prefrontal cortex; TPJ refers to temporoparietal junction; IPL refers to superior parietal lobule; STS refers to superior temporal sulcus; MCC refers to mid-cingulate cortex. Regions that share the same superscript are part of the same cluster.
Longitudinal changes in AI Functional Connectivity during Safe and Risky Decisions
Next, we examined longitudinal changes in functional connectivity, using the AI as the seed region. Participants evinced longitudinal decreases in functional coupling between the insula and the posterior cingulate cortex (PCC) from W1 to W2 when making stop decisions under maternal presence (Table 2). We found no longitudinal differences in functional coupling with the insula for go decisions under maternal presence.
Table 2.
Brain regions which showed longitudinal changes in connectivity with the anterior insula.
| Region | BA | x | y | z | t | k |
|---|---|---|---|---|---|---|
| Safe Decision W1 > W2 | ||||||
| Posterior Cingulate Cortex | 29/30 | 0 | −40 | 34 | 3.26 | 357 |
Note. R refers to right and L refers to left. x, y, and z refer to MNI coordinates; t refers to the t-score at those coordinates (local maxima).
Longitudinal changes in AI Functional Connectivity and Associations with Family Conflict
In PPI analyses, we ran regression analyses examining how longitudinal changes in functional coupling with the AI during maternal presence varied as a function of self-reported family conflict. Higher levels of family conflict at W1 were related to longitudinal increases in functional coupling between the AI and ventral striatum when adolescents made stop decisions in the presence of their mother (Figure 1). Family conflict was not associated with AI connectivity during go decisions.
Figure 1.
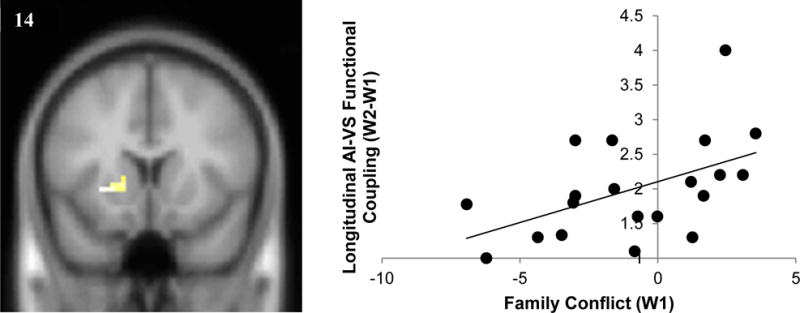
Higher levels of family conflict at W1 were associated with relative increases in VS-AI coupling across time (xyz=−27, 14, −2; k = 27, t = 3.59, p < .005). For descriptive purposes parameter estimates were extracted from the VS which showed functional coupling with the AI and plotted with W1 family conflict. Note. A greater number on the y axis indicates relatively greater coupling between the VS and AI at W2 compared to W1 (W2-W1), highlighting increases in connectivity over time. The x axis represents mean scores on self-reported family conflict. VS cluster reflects a threshold of p<.005, k > 20.
Longitudinal changes in AI Functional Connectivity and Associations with Real World Risk Taking
Next, we ran PPI analyses examining how longitudinal changes in functional connectivity with the AI varied as a function of self-reported risk taking. During stop decisions, adolescents who showed longitudinal increases in functional coupling between the AI and VS showed higher levels of risk taking at W2 (Figure 2). Notably, the AI-VS connectivity clusters in both the family conflict and risk taking regressions were nearly identical between both contrasts. The two regressions shared no other significant clusters of co-activation. For go decisions, self-reported risk-taking was associated with longitudinal decreases in AI connectivity with the bilateral posterior parietal lobe and dorsal prefrontal cortex (see Table 3).
Figure 2.
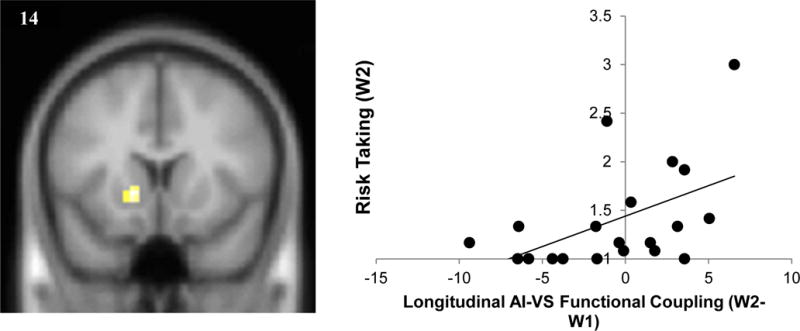
Higher levels of risk taking at W2 were associated with greater VS-AI coupling across time (xyz=−15, 14, −2; k = 26, t = 4.19, p < .005). For descriptive purposes parameter estimates were extracted from the VS cluster which showed functional coupling with the AI and plotted with W2 risk taking. Note. A greater number on the x axis indicates relatively greater coupling between the AI and the VS at W2 compared to W1 (W2-W1), highlighting relative increases in connectivity over time. VS cluster reflects a threshold of p<.005, k > 20.
Table 3.
Brain regions which showed significant associations with self-reported risk-taking and longitudinal connectivity with the anterior insula.
| Region | BA | x | y | z | t | k |
|---|---|---|---|---|---|---|
| Risky Decision W2 > W1 | ||||||
| Posterior Parietal Lobe | 7 | −21 | −46 | 52 | 4.41 | 521 |
| Dorsal Prefrontal Cortex | 9/46 | −33 | 44 | 22 | 3.41 | 163 |
Note. R refers to right and L refers to left. x, y, and z refer to MNI coordinates; t refers to the t-score at those coordinates (local maxima). The majority of the activation in dorsal prefrontal cortex extended into white matter (119 voxels).
Longitudinal changes in AI-VS Connectivity Mediates the Association between Family Conflict and Real World Risk Taking
Given that both family conflict and risk taking were similarly associated with longitudinal increases in AI-VS connectivity, we examined whether changes in AI-VS connectivity mediate the association between family conflict at W1 and risk taking at W2. To this end, we used the MarsBar toolbox in SPM (Brett et al., 2002) to create a functional ROI containing only the overlap in AI-VS connectivity from the clusters that correlated with both family conflict and risk taking. We used this mask to extract parameter estimates of AI-VS connectivity and performed mediation analyses in accordance with the methods specified by Preacher and Hayes (2008). Using the PROCESS macro for SPSS, we bootstrapped with 1,000 samples in order to estimate a bias-corrected confidence interval for our indirect effect. Analyses revealed a significant indirect effect (B = .16, SE = .12, 95% CI: [.02, .50]), such that longitudinal increases in AI-VS connectivity during maternal presence mediated the relationship between family conflict and risk taking (Figure 3).
Figure 3.
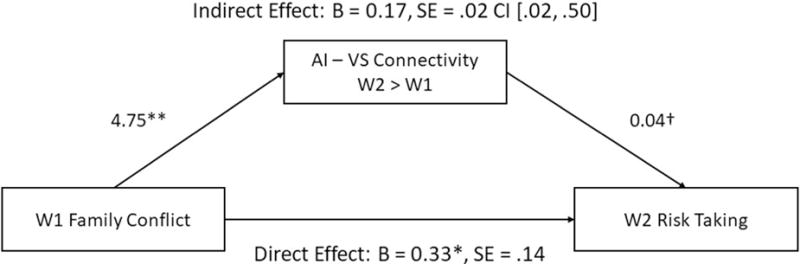
Longitudinal changes in AI-VS connectivity mediated the associated between W1 family conflict and W2 risk taking.
Note. **p<.01, *p<.05, †p<.1; CI denotes 95% confidence interval constructed after 1,000 bootstrapped samples. AI-VS connectivity refers to parameter estimates extracted from W2-W1 contrast.
Discussion
Though burgeoning advances in developmental social and affective neuroscience have highlighted how maternal buffering can promote emotion regulation (Gee et al., 2014) and reduce adolescent risky behavior (Telzer et al., 2015) by modulating neural processes, it is unknown whether this effect is robust to individual differences in family relationship quality, and whether it waxes or wanes across development. Our study shows that individual differences in family relationship quality may undermine the effectiveness of maternal buffering of adolescent risk taking.
In particular, high family conflict was associated with greater risk taking, which was mediated by longitudinal increases in AI-VS connectivity while making safe decisions under maternal presence. That is, individuals who reported greater family conflict showed relatively greater coupling between the AI and VS when making safe decisions in the presence of their mother over time and subsequently engaged in higher rates of dysregulated behavior, as revealed by greater real-world risk taking. We only observed this pattern of activation when individuals chose to make safe decisions, suggesting that these neural signals are only relevant when individuals are trying to self-regulate in the face of a presumably appetitive stimulus. Importantly, recent accounts have argued that the role of the insula may go beyond simply integrating cognitive and affective signals in the brain, possibly serving to recruit contextually relevant brain regions (Smith et al., 2014). Prior work has suggested that AI-VS connectivity during reward processing is critically related to risky decision making in adolescents (van Duijvenvoorde et al., 2014). In addition, this result fits neatly with adult work, which suggests that the insula is capable of integrating cognitive and affective information during decision making (Xue, Lu, Levin, & Bechara, 2010). This raises the possibility that the AI may be poorly integrating cognitive and affective signals and also failing to recruit brain regions that serve important regulatory roles. Curiously, we found null results for AI-PFC connectivity. Although null results are difficult to interpret, this may signify that connectivity between the AI and prefrontal regions is not longitudinally implicated by the quality of parent-adolescent relationships.
Individuals who reported higher levels of family conflict at W1 showed longitudinal increases in coupling between the AI and VS across the course of a year. Since relative decreases in connectivity appeared to facilitate reduced health-compromising risk-taking, and because the AI is involved in integrating affective signals from the VS when making risky decisions, relative increases in connectivity suggest potential dysregulation, as evidenced by greater risk taking. Our results suggest that extant neurobiological models of adolescent risk taking, such as the dual systems model (Shulman et al, 2016; Steinberg, 2010), may require an update insofar that they do not account for the quality of integration of subcortical-cortical signals. Although the relative strength between neural systems likely does matter, the field may be overlooking the notion that the manner in which subcortical-cortical systems interface is also key, with more connectivity between the AI and VS potentially leading to greater risk taking (Smith et al., 2014). Our results are consistent with recent empirical and theoretical work suggesting that integration of affective and cognitive signals via insular connectivity plays an important role in adolescent risk taking (e.g., van Duijvenvoorde et al., 2014; Smith et al., 2014). The AI may be calibrating activity between prefrontal regions and the VS. One potential explanation is that the insula is a relay center that indicates to numerous prefrontal control regions the level of activity in the VS. If the insula is misrepresenting the level of activity in the VS to the PFC, then the latter may not be able to send the proper signals necessary to regulate the former. It is possible that poor integration of prefrontal and striatal signals in the AI predisposes teens towards risky behavior by virtue of regional differences in the neural code—the exact patterns and firing rates of neurons that give rise to behaviors, emotions, cognitions, and psychomotor activity. Strides have been made in using fMRI activity to map out a higher order neural code for advanced mental processes (Guest & Love, 2017), but much remains unknown. Given what is not known, it is still plausible that neural activity encoding emotional salience, motivation, and reward in the striatum needs translating in the AI in order to interface with a separate code used to encode top-down, prefrontal regulation. Our results suggest that the quality of family relationships may render the AI an ineffective ‘translator’ of cognitive and emotional signals.
Our findings are also important for literature surrounding maternal buffering effects. While recent studies indicate that maternal presence represents positive buffering of adolescent risk taking (e.g., Guassi Moreira & Telzer, in press; Telzer et al., 2015), our results suggest there are individual differences in maternal buffering. First, it appears that maternal buffering may wane across the course of adolescence, in an ostensibly similar trajectory as adolescent self-reported levels of family identity (Tsai et al., 2013). This has important ramifications for our understanding of how social influences modulate adolescent neural responses across time. For instance, although the adolescent brain is highly receptive to social influences (e.g., Blakemore & Mills, 2014; Welborn et al., 2015), our results show this is not uniform across development. Additionally, other scholars have recently suggested that risk taking during adolescence may arise as a result of adaptive increases in approach motivation (Spear, 2011; Luciana et al. 2012), exploration, and learning (Goldenberg, Telzer, Lieberman, Fuligni, & Galvan, 2017; McCormick & Telzer, 2017). This raises the curious notion that individual differences in family relationship quality might affect the types of risks taken by adolescents. Not all risks are negative, per se, as some are more beneficial and adaptive than others. For instance, substance use may be a negative risk whereas attempting to establish a new social circle may be a more adaptive risk. One intriguing extension of this work is to determine how differences in relationship quality affect maternal buffering of adaptive and maladaptive risks, something that our task and self-report measures did not allow us to examine.
In addition, we show that individual differences in family relationship quality are associated with the extent to which affective signals are integrated in the insula. Subsequently, disrupted neural integration was related to a greater propensity to engage in risk taking one year later. This is compelling because it highlights how detrimental aspects of family relationship quality can have long-lasting effects on neural functioning. Prior work with children showed that mother-child relationships characterized by greater separation anxiety were less likely to exhibit effective maternal buffering during regulation of basic emotions (Gee et al., 2014). Our results parallel these prior findings, showing that individual differences in relationship quality continue to play an important role in maternal buffering in other developmentally relevant contexts.
A final important implication that these results carry is their relevance for intervention approaches for reducing adolescent risky behavior. Given prior research demonstrating that maternal buffering reduces adolescent risk taking (Telzer et al., 2015), it was suggested that future interventions may benefit from incorporating maternal presence into their protocols (Guassi Moreira & Telzer, in press). However, our results serve as an important qualifier. Adolescents from high conflict homes showed lower maternal buffering effects, as evinced by greater risky decisions in the presence of their mothers over time, which was accompanied by greater AI-VS co-activation. Thus, interventions designed to reduce adolescent risk taking need to consider the quality of the family relationships. Promoting more positive family relationship quality may influence the development of neural integration of the AI and VS, ultimately reducing adolescent risk taking.
Several limitations and future directions should be noted. First, although our sample size is consistent with other longitudinal neuroimaging studies (e.g., van Duijvenvoorde et al., 2014), it was nevertheless relatively small (Poldrack et al., 2017). One area for future improvement is to recruit larger sample sizes. Given the financial and logistical challenges of longitudinal neuroimaging, one feasible future direction is to attempt to replicate these results longitudinally while testing other ancillary hypotheses using behavioral and self-report measures. Future work may consider utilizing both child and adult comparison samples to better pinpoint the exact trajectory of the relative (in)effectiveness of maternal buffering on risky behaviors. In addition, longitudinal studies that include at least three time points can provide stronger and more appropriate tests of mediation as well as developmental trajectories. In this vein, it would be beneficial to collect self-report measures of family conflict a year prior to examining changes in neural processing, as well as collecting real-world Risk-taking measures prior to and a year after the scans. Collecting these measures across at least 3 time points would allow one to examine whether low family conflict alters insula-VS connectivity over time, which then reduces risk taking over time. Another key limitation is that we only probed the effects of maternal influence across time without a baseline condition in which adolescents completed the task alone, making it difficult to determine whether our results are unique to maternal presence or generalize to other contexts. Moreover, we did not include other observers (e.g., peer), so it is difficult to determine whether our effects are unique to mothers or if they generalize to other social agents. Thus, our results should be interpreted with caution, so as to avoid assuming contextual specificity. Finally, our self-reported risk-taking scale was not related to performance on the stoplight task. Nonetheless, neural activation during the task was associated with self-reported risk taking, suggesting that neural indices on the task do predict real-life risk taking. One final limitation lies in that we relaxed our threshold for multiple comparisons for the ventral striatum to 20 voxels. Although consistent with prior work (e.g., Giuliani & Pfeifer, 2015), we caution readers that our findings require careful interpretation and consideration.
In conclusion, our results help delineate the nuances of maternal buffering effects on adolescent neurocognitive development during risk taking. These findings are the first to show that maternal buffering on risky behaviors is not uniformly effective across the course of adolescence, highlighting that the quality of parent-teen relationships may be pivotal in shaping the neural mechanisms that affect maternal buffering. Moreover, our results provide support for nascent theories about the insula’s role in adolescent risk taking (Smith et al., 2014). Therefore, our results may help illuminate future iterations of neurobiological models of adolescent brain development by considering not only the relative strength of neural circuits, but also by how information communicated between such circuits is integrated in the brain. This may help push current theoretical orientations to circuit based accounts with greater explanatory power in describing behavior (Casey, 2015).
Research Highlights.
Adolescent risk taking increased in the presence of mother across a one year period.
Adolescents reporting higher family conflict showed increased anterior insula-ventral striatum functional connectivity.
Longitudinal increases in anterior insula-ventral striatum connectivity were associated with increased real-world risk taking.
Footnotes
We ran additional analyses to determine whether self-reported risk taking changed significantly across both time points. Results from a paired samples t-test indicate that risk taking did not significantly increase across the time points (t(20) = −.042, p > .25, one-tailed), suggesting there may be a unique effect attributable to risk taking in the presence of mothers. This is consistent with prior work showing that self-reported risk taking does not change (increase or decrease) on a group level across one year (Qu et al., 2015), but risk taking does increase to the extent that youth report poorer relationship quality with their family (Qu et al., 2016).
References
- Alexander CS, Kim YJ, Ensminger M, Johnson KE, Smith BJ, Dolan LJ. A measure of risk taking for young adolescents: Reliability and validity assessments. Journal of Youth and Adolescence. 1990;19(6):559–569. doi: 10.1007/BF01537176. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Mills KL. Is adolescence a sensitive period for sociocultural processing? Annual Review of Psychology. 2014;65:187–207. doi: 10.1146/annurev-psych-010213-115202. [DOI] [PubMed] [Google Scholar]
- Casey BJ. Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annual Review of Psychology. 2015;66:295–319. doi: 10.1146/annurev-psych-010814-015156. [DOI] [PubMed] [Google Scholar]
- Chein JM, Albert D, O’Brien L, Uckert K, Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Developmental Science. 2011;14(2):1–10. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Reviews Neuroscience. 2012;13(9):636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Crone EA, Elzinga BM. Changing brains: How longitudinal functional magnetic resonance imaging studies can inform us about cognitive and social-affective growth trajectories. Wiley Interdisciplinary Reviews: Cognitive Science. 2015;6(1):53–63. doi: 10.1002/wcs.1327. [DOI] [PubMed] [Google Scholar]
- Gardner M, Steinberg L. Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: An experimental study. Developmental Psychology. 2005;41(4):625–635. doi: 10.1037/a0026993. [DOI] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam L, Telzer EH, Humphreys KL, Goff B, Shapiro M, Tottenham N. Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychological Science. 2014;25(11):2067–2078. doi: 10.1177/0956797614550878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard JM, Krishnakumar A, Buehler C. Marital conflict, parent-child relations, and youth maladjustment: A longitudinal investigation of spillover effects. Journal of Family Issues. 2006;27(7):951–975. [Google Scholar]
- Giuliani NR, Pfeifer JH. Age-related changes in reappraisal of appetitive cravings during adolescence. NeuroImage. 2015;108:173–181. doi: 10.1016/j.neuroimage.2014.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg D, Telzer EH, Lieberman MD, Fuligni AJ, Galvan A. Greater response variability in adolescents is associated with increased white matter development. Social Cognitive and Affective Neuroscience. 2017;12:436–444. doi: 10.1093/scan/nsw132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graber JA, Brooks-Gunn J. Transitions and turning points: Navigating the passage from childhood through adolescence. Developmental Psychology. 1996;32(4):768–776. doi: 10.1037/0012-1649.32.4.768. [DOI] [Google Scholar]
- Guassi Moreira JF, Telzer EH. Mother still knows best: Maternal influence uniquely modulates adolescent reward sensitivity during risk taking. Developmental Science. :1–11. doi: 10.1111/desc.12484. (in press) [DOI] [PMC free article] [PubMed]
- Guest O, Love BC. What the success of brain imaging implies about the neural code. eLife. 2017;6:1–16. doi: 10.7554/eLife.21397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser TU, Iannaccone R, Walitza S, Brandeis D, Brem S. Cognitive flexibility in adolescence: Neural and behavioral mechanisms of reward prediction error processing in adaptive decision making during development. NeuroImage. 2015;104:347–354. doi: 10.1016/j.neuroimage.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: a review of animal models and human studies across development. Psychological Bulletin. 2014;140(1):256–82. doi: 10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr M, Stattin H. What parents know, how they know it, and several forms of adolescent adjustment: further support for a reinterpretation of monitoring. Developmental Psychology. 2000;36(3):366–380. doi: 10.1037/0012-1649.36.3.366. [DOI] [PubMed] [Google Scholar]
- Larson R, Richards MH. Introduction: The changing life space of early adolescence. Journal of Youth and Adolescence. 1989;18(6):501–509. doi: 10.1007/BF02139070. [DOI] [PubMed] [Google Scholar]
- Mahalik JR, Coley RL, Lombardi CM, Lynch AD, Markowitz AJ, Jaffee SR. Changes in health risk behaviors for males and females from early adolescence through early adulthood. Health Psychology. 2013;32(6):685–694. doi: 10.1037/a0031658. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McCormick EM, Qu Y, Telzer EH. Adolescent neurodevelopment of cognitive control and risk-taking in negative family contexts. NeuroImage. 2016;124:989–996. doi: 10.1016/j.neuroimage.2015.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick EM, Telzer EH. Failure to retreat: Blunted sensitivity to negative feedback supports risky behavior in adolescents. NeuroImage. 2017;147:381–389. doi: 10.1016/j.neuroimage.2016.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam MM. Insula of the old world monkey. II: Afferent cortical input and comments on the claustrum. The Journal of Comparative Neurology. 1982;212(1):23–37. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- Nash SG, McQueen A, Bray JH. Pathways to adolescent alcohol use: Family environment, peer influence, and parental expectations. Journal of Adolescent Health. 2005;37(1):19–28. doi: 10.1016/j.jadohealth.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences. 2012;(1251):E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x.Functional. [DOI] [PMC free article] [PubMed]
- O’Reilly JX, Woolrich MW, Behrens TE, Smith SM, Johansen-Berg H. Tools of the trade: psychophysiological interactions and functional connectivity. Social Cognitive and Affective Neuroscience. 2012;7(5):604–609. doi: 10.1093/scan/nss055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper JS, Dahl RE. Surging hormones: Brain-behavior interactions during puberty. Current Directions in Psychological Science. 2013;22(2):134–139. doi: 10.1177/0963721412473755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Baker CI, Durnez J, Gorgolewski KJ, Matthews PM, Munafò M, Yarkoni T. Scanning the horizon: Towards transparent and reproducible neuroimaging research. Nature Reviews Neuroscience. 2017;18:115–126. doi: 10.1101/059188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Galvan A, Fuligni AJ, Lieberman MD, Telzer EH. Longitudinal changes in prefrontal cortex activation underlie declines in adolescent risk taking. Journal of Neuroscience. 2015;35(32):11308–11314. doi: 10.1523/JNEUROSCI.1553-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Pomerantz EM, Wang M, Cheung C, Cimpian A. Conceptions of adolescence: Implications for differences in engagement in school over early adolescence in the united states andcChina. Journal of Youth and Adolescence. 2016;45(7):1512–1526. doi: 10.1007/s10964-016-0492-4. [DOI] [PubMed] [Google Scholar]
- Rubin KH, Burgess KB, Hastings PD. Stability and social-behavioral consequences of toddlers’ inhibited temperament and parenting behaviors. Child Development. 2002;73(2):483–495. doi: 10.1111/1467-8624.00419. [DOI] [PubMed] [Google Scholar]
- Schlegel A, Barry H., III . Adolescence: An Anthropological Inquiry. Free Press; 1991. [Google Scholar]
- Shulman EP, Smith AR, Silva K, Icenogle G, Duell N, Chein J, Steinberg L. The dual systems model: Review, reappraisal, and reaffirmation. Developmental Cognitive Neuroscience. 2016;17:103–117. doi: 10.1016/j.dcn.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva K, Chein J, Steinberg L. Adolescents in peer groups make more prudent decisions when a slightly older adult is present. Psychological Science. 2016;27(3):322–330. doi: 10.1177/0956797615620379. [DOI] [PubMed] [Google Scholar]
- Skeer M, Avenue H, Mccormick MC, Normand ST, Gilman SE. A prospective study of familial conflict, psychological stress, and the development of substance use disorders in adolescence. Drug and Alcohol Dependence. 2009;104(1–2):65–72. doi: 10.1016/j.drugalcdep.2009.03.017.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AR, Steinberg L, Chein J. The role of the anterior insula in adolescent decision making. Developmental Neuroscience. 2014;36(3–4):196–209. doi: 10.1159/000358918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Rewards, aversions, and affect in adolescence: Emerging convergences across laboratory and human data. Developmental Cognitive Neuroscience. 2011;1:390–403. doi: 10.1016/j.dcn.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A dual systems model of adolescent risk-taking. Developmental Psychobiology. 2010;52(3):216–224. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: Evidence for a dual systems model. Developmental Psychology. 2008;44(6):1764–1778. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Morris AS. Adolescent development. Annual Review of Psychology. 2001;52:83–110. doi: 10.1146/annurev.psych.52.1.83. [DOI] [PubMed] [Google Scholar]
- Sumter SR, Bokhorst CL, Steinberg L, Westenberg PM. The developmental pattern of resistance to peer influence in adolescence: Will the teenager ever be able to resist? Journal of Adolescence. 2009;32(4):1009–1021. doi: 10.1016/j.adolescence.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Telzer EH, Fuligni AJ, Lieberman MD, Galván A. Meaningful family relationships: Neurocognitive buffers of adolescent risk taking. Journal of Cognitive Neuroscience. 2013;25(3):374–387. doi: 10.1162/jocn_a_00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Gonzales N, Fuligni AJ. Family obligation values and family assistance behaviors: Protective and risk factors for Mexican-American adolescents’ substance use. Journal of Youth and Adolescence. 2014;43(2):270–283. doi: 10.1007/s10964-013-9941-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Ichien NT, Qu Y. Mothers know best: Redirecting adolescent reward sensitivity toward safe behavior during risk taking. Social Cognitive and Affective Neuroscience. 2015;10(10):1383–1391. doi: 10.1093/scan/nsv026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai KM, Telzer EH, Fuligni AJ. Continuity and discontinuity in perceptions of family relationships from adolescence to young adulthood. Child Development. 2013;84(2):471–484. doi: 10.1111/j.1467-8624.2012.01858.x. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatom- ical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van Duijvenvoorde ACK, Op de Macks ZA, Overgaauw S, Gunther Moor B, Dahl RE, Crone EA. A cross-sectional and longitudinal analysis of reward-related brain activation: Effects of age, pubertal stage, and reward sensitivity. Brain and Cognition. 2014;89:3–14. doi: 10.1016/j.bandc.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L, Moor BG, Op de Macks ZA, Rombouts SARB, Westenberg PM, Crone EA. Adolescent risky decision-making: Neurocognitive development of reward and control regions. NeuroImage. 2010;51(1):345–355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Welborn BL, Lieberman MD, Goldenberg D, Fuligni AJ, Galván A, Telzer EH. Neural mechanisms of social influence in adolescence. Social Cognitive and Affective Neuroscience. 2015;11(1):100–109. doi: 10.1093/scan/nsv095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G, Lu Z, Levin IP, Bechara A. The impact of prior risk experiences on subsequent risky decision-making: The role of the insula. NeuroImage. 2010;50:709–716. doi: 10.1016/j.neuroimage.2009.12.097. [DOI] [PMC free article] [PubMed] [Google Scholar]


