Abstract
DNA repair pathways are essential for cellular survival as our DNA is constantly under assault from both exogenous and endogenous DNA damaging agents. Five major mammalian DNA repair pathways exist within a cell to maintain genomic integrity. Of these, the DNA mismatch repair (MMR) pathway is highly conserved among species and is well documented in bacteria. In humans, the importance of MMR is underscored by the discovery that a single mutation in any one of four genes within the MMR pathway (MLH1, MSH2, MSH6 and PMS2) results in Lynch syndrome (LS). LS is an autosomal dominant condition that predisposes individuals to a higher incidence of many malignancies including colorectal, endometrial, ovarian, and gastric cancers. In this review, we discuss the role of PMS2 in the MMR pathway, the evolving testing criteria used to identify variants in the PMS2 gene, the LS phenotype as well as the autosomal recessive condition called Constitutional Mismatch Repair Deficiency syndrome, and current methods used to elucidate the clinical impact of PMS2 mutations.
Keywords: PMS2, Lynch syndrome, CMMRD, variants of uncertain significance, pseudogene, DNA mismatch repair
Graphical abstract
1. Introduction and Role of PMS2 in Mismatch Repair
Prior to the ability to perform molecular genetic testing, the transmission of an increased cancer risk throughout generations of some families was recognized by multiple clinicians.1–5 In 1913, Dr. Aldred Scott Warthin was the first to describe a hereditary cancer syndrome.1,2 In studying a large family of German descendants, Warthin observed and documented a high rate of uterine, gastric, and colon cancer incidence starting with a sibship of ten individuals and continuing throughout succeeding generations; he called this family “Family G”.2 In the 1960s, Dr. Henry Lynch began to document cancers from large families with pedigrees that demonstrated an increased incidence of colon, gastric, and endometrial cancer.4,6 Initially termed “Cancer Family Syndrome” by Dr. Lynch, this condition was later renamed Hereditary Nonpolyposis Colorectal Cancer syndrome (HNPCC) to distinguish it from other hereditary cancer syndromes. Currently, the term Lynch syndrome (LS) is used to describe families with germline mutations that disrupt the DNA mismatch repair (MMR) pathway, while HNPCC is now reserved for families effected by a familial colorectal cancer syndrome with features of LS that lack a germline mutation in any of the MMR genes.7,8 Monoallelic mutations in the four main MMR genes: MSH2, MSH6, MLH1, and PMS2, are believed to account for >97% of all LS cases.9 Most recently, a small percentage (~1–3%) of LS families were found to have large deletions in the 3′ terminus of EPCAM (EPithelial Cellular Adhesion Molecule) disrupting transcription of MSH2, which lies downstream of EPCAM.10 For the remainder of this review, we will focus on PMS2. We discuss the current knowledge regarding the role of PMS2 in the MMR pathway, describe the identification of germline PMS2 variants, discuss the clinical phenotype of two distinct conditions caused by pathogenic mutations in this gene, and review current testing methods, which have impacted our understanding of PMS2 mutations.
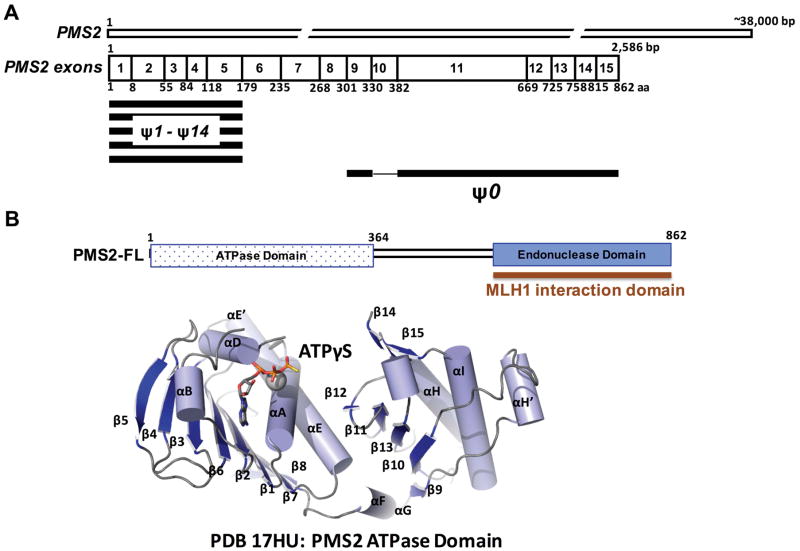
The DNA MMR pathway is one of five major DNA repair pathways in humans and is highly conserved from prokaryotes to eukaryotes.11–15 Mismatches and slippage errors commonly arise during DNA replication and result in non-Watson Crick base pairs or insertion/deletion loops (IDLs).11–15 The MMR pathway increases replication fidelity by 100 – 1000 fold and is essential for the maintenance of genomic integrity.13,14, 16 MMR proceeds as four distinct steps: mismatch recognition (by the MutS complexes), recruitment of downstream proteins (MutL complex) to the site of the mismatch, excision of the DNA strand that contains the mismatch, and resynthesis by a replicative DNA polymerase.11–15 In humans, the MutS homolog (MSH) MSH2-MSH6 complex, also referred to as MutSα, initiates the repair of single base mismatches and short 1 – 2 nt IDLs.11–15 Mismatch recognition and ATP binding induce a conformational change in MutS that is required for the recruitment of the MutLα complex that initiates repair.11–15 MutLα is the predominant MutL heterodimer, which is formed by the MutL homolog 1 (MLH1) and the postmeiotic segregation increased 2 (PMS2) proteins.17,18 PMS2 is located on chromosome 7 and spans ~38,000 base pairs (Figure 1A).19 The full-length PMS2 protein product is translated from 15 exons and is 862 amino acids in length (Figure 1A).19 PMS2 forms a heterodimer with MLH1 via a C-terminal interaction domain (Figure 1B). This interaction is absolutely essential for the stability of PMS2 in vivo.20 In addition, PMS2 functions as a latent endonuclease and is required for 3′ nick-directed MMR.15,21 The crystal structure of the N-terminal 364 amino acids of human PMS2 reveals the ATPase domain of the protein that is able to hydrolyze ATP as well as bind to DNA (Figure 1B).22 While there is no structural information for the C-terminal domain of PMS2, this region harbors an endonuclease domain that is required for protein function during MMR.17,23
Figure 1. Domain map and known structure of PMS2.
(A) PMS2 exons (not to scale) and pseudogenes (ψ). The PMS2 protein spans 15 exons and is 862 aa in length. Portions of pseudogenes ψ1 – 14 overlap with exons 1 – 5 while ψ0 covers exons 9 and 11 – 15. (B) The N-terminal ~364 residues of PMS2 constitute an ATPase domain whose structure has been determined previously,22 whereas the C-terminal end of PMS2 possesses an endonuclease domain that forms a heterodimer with MLH1.
2. PMS2 and Genetic Testing
Of the approximately 3,300 unique MMR gene variants listed in the International Society for Gastrointestinal Hereditary Tumors (InSiGHT) database, only 9% are reported in PMS2, compared to 39% in MLH1, 33% in MSH2 and approximately 19% reported in MSH6 (http://www.insight-database.org/genes). This lower observed frequency of germline variants in PMS2 may be due to the fact that PMS2 has not been studied as rigorously as the other MMR genes. Analysis of PMS2 in clinical settings has been impacted by the presence of PMS2 pseudogenes (Figure 1A) as well as a lack of genetic testing in families who have a low penetrance of cancer.24–26
Pseudogenes
Clinical genetic testing of PMS2 was not offered until 2009, nine years after testing for MLH1 and MSH2 was available.24 Analysis of the PMS2 gene has been complicated by the presence of a large family of pseudogenes that are highly homologous to PMS2 (Figure 1A).19,25 All PMS2-associated pseudogenes are found on chromosome 7.19 It is the presence of these pseudogenes that complicates accurate sequencing of PMS2 to identify variants within this gene. Fourteen pseudogenes (ψ1 – 14) overlap with some or all of exons 1 – 5 of PMS2 and vary in length (Figure 1A) whereas the PMS2CL pseudogene (formerly ψ0) possesses high sequence similarity to exons 9 and 11–15 (Figure 1A). 25,27 While avoiding pseudogenes has presented a challenge in the accurate detection of PMS2 variants, various tactics to circumvent sequencing of these regions during PMS2 analysis have been employed. For instance, a modified long-range PCR method has been used successfully to preferentially amplify PMS2 using a forward primer within exon 10 that does not overlap with the PMS2CL pseudogene.28 The PCR products are sequenced and further compared to the NCBI reference sequence for PMS2 (NT_007819.16) to confirm that the sequence amplified is not from the pseudogene. However, this method alone is not fully reliable for the detection of large-scale deletions.29 To detect large-scale deletions, a combination of the modified long-range PCR method developed by Vaughn et al., and a multiplex ligation-dependent PCR amplification (or MLPA), can be used.28,30 Another approach using long-range RT-PCR followed by sequencing appears to be largely successful at avoiding the detection of variants within the PMS2 pseudogenes.31 A method using direct cDNA sequencing has also been employed to identify splice-site mutations, insertions, and deletions that are often overlooked using traditional PCR-based protocols.32,33
Identification of Families with LS
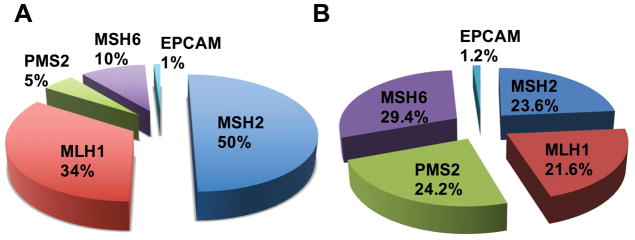
The low frequency of observed PMS2 mutations is in part due to the ascertainment bias inherent in choosing individuals who should undergo testing for mutations in the MMR genes. Families that meet the most stringent of criteria used to identify LS will most often have a germline mutation in MLH1 or MSH2 causing 40% and 34% of LS cases, respectively.34,35 On the other hand, mutations in PMS2 were once thought to account for only 8–15% of LS cases (Figure 2).26,34,35 Traditionally, clinicians recommended molecular genetic testing for LS based on a patient’s personal and/or family history of cancer. Clinical genetic testing for LS first began in families that met Amsterdam I criteria (Box 1).36 The expanded understanding of the clinical presentation of LS lead to the introduction of the Amsterdam II criteria which allowed for the inclusion of multiple LS-associated cancers (Box 1).37 Use of the Amsterdam II testing guidelines facilitated the identification of multiple individuals and families that had LS but did not present with a predominance of early-onset colorectal cancers.38 Based on the discovery that MSI was a hallmark of LS tumors, the National Cancer Institute (NCI) set forth to develop criteria for selecting colon and rectal tumors that warranted MSI testing during a meeting in Bethesda, Maryland, and published in 1997 (Box 2).39,40 Following a cost-effective analysis on the use of MSI and immunohistochemistry (IHC) to screen colorectal cancers for LS, the Evaluation of Genomic Applications in Practice and Prevention (EGAPP) working group published a recommended tumor testing strategy in 2009.41 EGAPP recommended that all newly diagnosed colorectal cancers be screened for LS using preliminary tests that include MSI and/or tumor IHC, allowing for the identification of LS regardless of age at time of cancer diagnosis or family history. The suggested testing algorithms begin with IHC and/or MSI testing of the tumor followed by germline sequencing and deletion/duplication analysis of the LS-associated genes.41 The sensitivity of MSI testing or IHC to screen for the presence of a germline PMS2 mutation is 67% and 75%, respectively.42,43 Therefore, MSI and IHC screening has been found to be helpful in identifying individuals who should further undergo germline testing. The American College of Obstetrics and Gynecology (ACOG) later recommended that all endometrial tumors be screened for LS using MSI/IHC.44
Figure 2. Pie chart displaying the percent mutation in genes causing LS.
(A) LS incidence in individuals ascertained based on meeting Amsterdam or Bethesda criteria (NCBI database).9 (B) LS incidence when ascertained by multigene panel testing, regardless of personal or family history of cancer.46
Box 1. Amsterdam Criteria.
Amsterdam I Criteria
There should be at least three relatives with histologically verified colorectal cancer.
Additional considerations:
One should be a first-degree relative to the other two
At least two successive generations should be affected
At least one relative should be diagnosed with colorectal cancer before age 50
Familial adenomatous polyposis should be excluded
Amsterdam II Criteria
There should be at least three relatives with an LS-associated cancer. This includes CRC, EC, gastric, ovarian, small bowel, ureter or renal pelvis.
Additional considerations:
One should be a first-degree relative to the other two
At least two successive generations should be affected
At least one should be diagnosed before age 50
Familial adenomatous polyposis should be excluded
Tumors should be verified by pathological examination
Box 2. Revised Bethesda Guidelines: Tumors from individuals should be tested for MSI in the following situations.
Colorectal cancer diagnosed in a patient <50 years of age
Presence of synchronous, metachronous colorectal or other LS-associated tumors regardless of age. These include CRC, EC, gastric, ovarian, pancreatic, ureter/renal pelvis, biliary tract
CRC with the MSI-high histology* diagnosed in a patient (<60 years of age)
CRC diagnosed and one or more first-degree relatives with an LS-related tumor, with one of the cancers being diagnosed < 50 years of age.
CRC diagnosed in two or more first- or second-degree relatives with LS-related tumors, regardless of age
*Histological features that are common in tumors with high microsatellite instability include tumor infiltrating lymphocytes, Crohn’s-like lymphocytic reaction, mucinous or signet-ring differentiation, or medullary growth pattern
With each evolving set of guidelines, more families with LS were identified. The diagnosis of LS was further aided by the advent of next-generation sequencing (NGS).45 This technology allows clinicians to simultaneously test multiple genes with massive parallel sequencing in a cost-effective manner. The number of genes in a panel range from two to >100 and the use of gene panels to test for hereditary cancer syndromes became integrated into standard clinical practice starting in 2012.46 PMS2 is included within many NGS panels although the highly homologous PMS2 pseudogenes present a technical challenge even for NGS. To perform accurate mutation detection of PMS2, the genomic testing methods described above are still used including long-range PCR for sequencing and MLPA for large deletion and duplications analyses.46–48 Utilization of these panels has led to the discovery that many families who did not meet any of the previously described guidelines for testing for LS have deleterious mutations in PMS2. Published data from multiple laboratories, clinics, and academic centers now show that PMS2 deleterious mutations are much more common than initially thought.46–50 A comparison of initial MMR mutation frequencies to updated mutation frequencies is available in Figure 2. Recent data suggests that pathogenic mutations in PMS2 are just as frequent as mutations in the other three MMR genes associated with LS (Figure 2A, B) with a population-based carrier frequency of 1 in 714.51
3. The PMS2 Phenotype
Lynch syndrome
Initial studies, which focused primarily on individuals with mutations in MLH1 and MSH2, estimated a 57–80% lifetime risk of colorectal cancer and 32–42% lifetime risk of endometrial cancer for LS.52–55 Individuals with LS had also been shown to be at an increased risk for cancers of the stomach, ovaries, small intestine, biliary tract, brain and urinary tract.52–55 We are now beginning to understand the lower penetrance in LS families with PMS2 mutations. Pathogenic mutations in PMS2 cause an estimated 25 – 32% lifetime risk of LS-associated cancers.26,56 According to a recent report, the age-related risk for colon cancer in PMS2 carriers is 8% for individuals in their 20s, 20% for those in their 30s, 31% for individuals in their 40s, 22% for those in their 50s, and 19% for individuals aged 60 or higher.57 In those diagnosed with cancer, the mean age of diagnosis of the first colorectal cancer is estimated to be 48 years.57 One study, which compared the lifetime risk of colorectal cancer based on gender, reported a higher risk in males (18.75%) compared to females (10.56%).56 In individuals with an identified germline PMS2 mutation, uterine cancer was the second most common cancer diagnosis, with an estimated lifetime risk of 12%–15%.26,56,57 The cancer risks listed above may be overestimated given that many of the cases were based on selecting tumors that were PMS2 deficient by IHC.26,57 Other cancers that have been documented in PMS2 heterozygotes include ovarian, breast, duodenal, small intestine, gastric, urinary tract, and central nervous system cancers.57 Counseling individuals with PMS2 mutations on the risk for cancers beyond colorectal and endometrial is challenging given that most studies performed to elucidate LS cancer risks either do not report data for PMS2 alone or may not have included PMS2 mutations in their analysis. One study looking at 98 families with confirmed pathogenic mutations in PMS2 reported a significant standardized incidence ratio (SIR) for malignancies of the small bowel, renal pelvis, ovaries, and breast.56 An earlier study found that PMS2 carries have a combined 6% lifetime risk of developing cancer of the renal pelvis, stomach, ovary, small bowel, ureter, and brain.26 Additional studies need to be performed to further understand risks beyond colorectal cancer and endometrial cancer in these patients.
Frequently updated guidelines on the medical management recommendations for individuals with a PMS2 mutation can be found at the National Comprehensive Cancer Network (NCCN) website.58 NCCN guidelines originally recommended colonoscopies beginning at age 25 with repeat scope every 1–2 years for any man or woman with LS, regardless of the gene harboring a mutation. The 2.2015 version veered from this initial guideline with respect to timing of initial colonoscopy for those with a PMS2 mutation, suggesting that these individuals begin colonoscopies at age 35. Data indicating a lower overall risk for colorectal cancer and later average age of onset for individuals with PMS2 mutations supports the rationale for delayed colonoscopies.26,56 An expert ad hoc working group later acknowledged that colon cancer is diagnosed prior to age 30 in multiple PMS2 heterozygotes, and concluded that the most appropriate medical management decision would be to initiate colonoscopies at age 25–30 or 2–5 years prior to the earliest diagnosis of colorectal cancer in the family (if it was diagnosed prior to age 30).57
Additional medical management recommendations for carriers of PMS2 mutations are not as clear.58 Clinicians can consider screening for endometrial cancer with endometrial biopsy every one to two years starting at age 30–35 then adding transvaginal ultrasound after menopause.58 While these screening recommendations can be considered, they have not been proven to reduce mortality from endometrial cancer in women with PMS2 mutations.58 Prophylactic hysterectomy and bilateral salpingo-oophorectomy (BSO) should also be discussed as an option. The NCCN guidelines aptly state that the recommendation and timing of prophylactic hysterectomy and BSO should reflect the following: (1) the woman’s desire to have more children, (2) menopause status, (3) comorbidities, (4) presence of gynecologic cancers in the family, and (5) the lower risk of endometrial and ovarian cancer in PMS2 mutations carriers compared to the other MMR genes.58 Screening for any of the other LS-associated cancers can be considered on a case-by-case basis to reflect the types of cancers diagnosed in a family and anticipated efficacy of available screening protocols.58
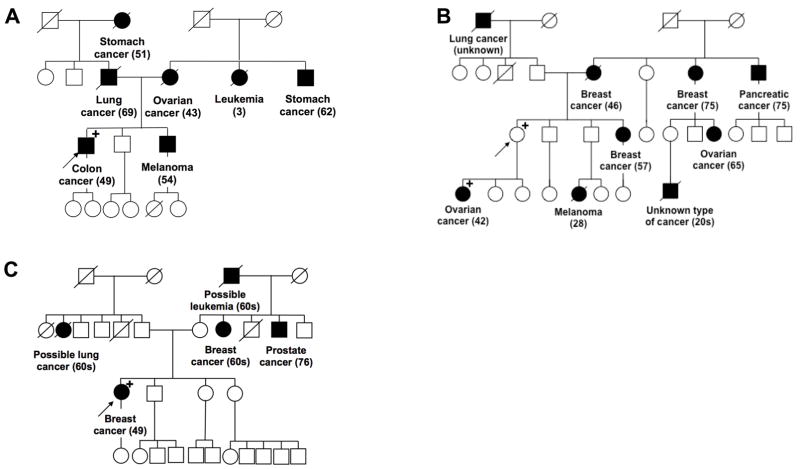
Owing to phenotypic heterogeneity, it should be acknowledged that families with PMS2 mutations might be harder to identify than previously thought. In Figure 3, we provide examples of three pedigrees, which harbor PMS2 variants to demonstrate this phenotypic heterogeneity. The first family (Figure 3A) meets Amsterdam II criteria where the proband (family member who first presents for genetic analysis) also meets Bethesda criteria. In this family, targeted testing for LS identified a PMS2 mutation (Figure 3A). However, many families harboring PMS2 mutations present with a lack of LS-associated cancers and do not meet Amsterdam criteria, leading their clinician to suspect a different hereditary cancer syndrome. The proband in Figure 3B, initially underwent BRCA1 and BRCA2 analysis because she only met testing criteria for Hereditary Breast and Ovarian Cancer syndrome. Due to the suspicious family history, panel testing was later offered, at which point, a pathogenic PMS2 mutation was identified. Figure 3C provides an example of a family in which the proband underwent panel testing following her early-onset breast cancer diagnosis and the only mutation identified was a PMS2 variant of uncertain significance (VUS) where there is a lack of data available regarding the clinical impact the specific mutation. It is encouraging to see that prediction models are now taking into account the lower penetrance of cancer seen in individuals with a PMS2 mutation compared to other LS families. The PREdiction Model for gene Mutations (PREMM1,2,6), developed to predict the likelihood of mutations in MLH1, MSH2 and MSH6, was updated to PREMM5 to allow for identification of families with a mutation in any of the five LS-associated genes.59
Figure 3. Examples of families presenting with PMS2 mutations.
(A) Pedigree of a family with a PMS2 mutation (c.2117delA) that meets Amsterdam II criteria. (B) Pedigree of a family with a PMS2 mutation (c.730C>G) that does not meet Amsterdam II criteria; detected using multi-gene panel next generation sequencing. (C) Family that presents with a PMS2 variant of uncertain significance (c.1420G>T). A circle represents a female and a square represents a male. Black squares/circles represent individuals diagnosed with cancer and a strike-through represents individuals who are deceased. The arrow points to the proband (first person in the family who underwent genetic testing) and the “+” refers to family members who have tested positive for the mutation or VUS.
Constitutional Mismatch Repair Deficiency syndrome
While LS is an autosomal dominant condition, biallelic germline mutations in PMS2 (in addition to MLH1, MSH2, and MSH6) have been observed in an autosomal recessive condition called Constitutional Mismatch Repair Deficiency syndrome (CMMRD), a rare condition that was first described in 1999 within two articles.60,61 It should be noted that over 50% of individuals with molecularly confirmed CMMRD have biallelic mutations in PMS2, a larger percentage compared to the other MMR genes.62 As discussed above, the carrier frequency of PMS2 mutations may be much higher than previously reported.51 In studying CMMRD cases, it was noted that the heterozygous PMS2 family members did not appear to be at an increased risk of cancer, drawing attention to the lower penetrance of cancer in PMS2 heterozygotes.63 The higher incidence of PMS2 mutations in CMMRD patients may also be explained by the severity of disease associated with MLH1 and MSH2 biallelic mutations. These patients may die before age 10 due to their first primary cancer and therefore prior to proper diagnosis of CMMRD. Furthermore, many CMMRD patients are known to be homozygous for a Pakistani founder mutation arising in families with consanguinity.63 Although not yet well studied, it has been suggested that individuals with biallelic mutations in MLH1 or MSH2 may have at least one allele that leads to reduced penetrance whereas inheriting two highly penetrant MLH1 or MSH2 mutations will be embryonic lethal.62
Individuals who are either homozygotes for PMS2 mutations or compound heterozygotes are affected by CMMRD, which primarily increases the risk of childhood-onset hematologic malignancies, brain tumors, and early-onset gastrointestinal tumors.64,65 T-cell lymphoma is the most commonly diagnosed hematological malignancy in CMMRD and typically the first malignancy to present in affected individuals.64 Brain tumors diagnosed in individuals with CMMRD are primarily glioblastomas.66 Colorectal cancers are also common, although they occur at a much younger age compared to LS patients with a mean age of diagnosis at 16.4 years.67 Individuals with CMMRD are also at an increased risk of early-onset duodenal and gastric cancers.68 CMMRD can manifest with non-malignant features that share clinical overlap with Neurofibromatosis Type 1 (NF1) and frequently presents with café au lait macules as well as less common NF1 features such as axillary freckling, Lisch nodules, and plexiform neurofibromas.64,69 Unlike with the tumor-screening algorithm developed for LS patients, MSI is neither sensitive nor specific in diagnosing CMMRD.62,66,67 However, in patients with CMMRD, IHC shows loss of the affected MMR protein in up to 100% of cases.62,66,67
Although the efficacy of surveillance protocols is still not clear, several groups have proposed screening recommendations for patients with CMMRD.66,67,70 A consensus surveillance protocol for individuals with CMMRD recommends the use of brain MRIs starting at the time of diagnosis (as early as birth) and repeated every six months.71 Due to the wide range of tumors that can develop in children with CMMRD, these authors also advocate for the use of annual whole-body MRIs starting at age six or once anesthesia is not needed. Gastrointestinal surveillance should include annual colonoscopy starting between the ages of four and six and annual upper endoscopies with video capsule endoscopy starting at age eight. The frequency of endoscopy should increase to every six months upon identification of polyps and a colectomy should be considered once any polyps display high-grade dysplasia or if the polyp burden cannot be managed with endoscopic excision. Recommendations regarding screening for leukemia and lymphoma include consideration of complete blood count (CBC) and abdominal ultrasounds every six months. Beginning at age 20, genitourinary cancer screening should include a gynecological exam, transvaginal ultrasound, endometrial sampling, and urinalysis with dipstick analysis and urine cytology performed on an annual basis.71
4. Current Methods Used to Classify PMS2 Variants
Germline genetic testing reveals one of three outcomes: a known pathogenic mutation that might explain cancer formation and progression, a benign variant that does not result in a malignant phenotype, or a VUS where little to no data are available regarding the variant.72,73 A majority of mutations found in LS families are pathogenic resulting in an LS-associated cancer due to deficient MMR. Pathogenic variants typically result in premature truncation, deletion, or a deleterious mutation within the particular MMR protein product.73
Approximately 20–30% of missense mutations identified in the MMR genes are VUSs and because they are uncharacterized, pose a challenge to the diagnosis and medical management of LS patients.72,74–77 Some VUSs result in altered, non-functional proteins while others do not result in a change in protein function lending importance to the proper characterization of VUSs as pathogenic or benign in diagnosing LS.78 VUSs include nonsynonymous and synonymous variants that can be single nucleotide changes, insertions or deletions, and splice-site variants. Depending on the location of the VUSs, functional assays can be performed to properly designate these variants and several strategies have been previously proposed to assess the functional impact of VUSs.79 Nonsynonymous variants that alter the amino acid sequence of the resulting PMS2 protein can be scrutinized using in vitro molecular, biochemical, and structural methods.75
The use of recombinant proteins to study in vitro function has become increasingly common.80 For PMS2, while production of the full-length protein requires expression in insect cells, both the N-terminal ATPase domain (residues 1 – 365) and the C-terminal endonuclease domain (residues 506 – 862) in a complex with MLH1 (residues 474 – 756) can be readily expressed in bacteria (E. coli).22,23,81 The ATPase and DNA binding activities of the N-terminal domain of PMS2 have been previously documented.22 Residues in the endonuclease domain of PMS2 are involved with coordinating metal binding (likely zinc) and are necessary for the endonucleolytic activity of PMS2 in vitro.17,23 ATPase, DNA-binding, and endonuclease activities using suitable substrates, as well as complex formation with MLH1 can be measured to compare wild-type (WT) and variant proteins. Structural techniques including small-angle X-ray scattering, and X-ray crystallography can also be further used to assign a functional role for the variant enzymes.82,83 Variant proteins that display aberrant activity compared to the WT protein are more likely to be pathogenic.
While it is useful to study protein function outside of the cell, the recombinant proteins do not recapitulate a cellular environment. Cell-based studies to assess PMS2 protein expression, stability (half-life), and MMR reconstitution from extracts have been used with much success to analyze the impact of a variant on protein function.75,84,85 The latter makes use of plasmid DNA containing a single mismatch that is either repaired or remains unrepaired after treatment with cell extracts containing WT or variant PMS2.86 Furthermore genome-editing tools can be used to directly “knockin” a particular variant to study its function at the endogenous level.87,88 This latter technique is advantageous because it does not involve procedures to first knockdown gene expression prior to transfection with a plasmid harboring the variant. More recently, a cell-free system using in vitro transcription/translation to create the MMR protein variants was used to study the impact of a variant on protein function.89,90 Therefore, a combination of cell-based and in vitro biochemical assays is powerful and will aid in the reclassification of a variant as pathogenic or benign.
Other aspects of MMR function that can be hindered as a result of a VUS include protein localization and mRNA splicing that are essential for maintaining genomic integrity.91,92 Both PMS2 and MLH1 possess nuclear localization signals that are required for import into this organelle where nuclear import appears to be dependent upon heterodimerization.93 A VUS that disrupts heterodimerization will also likely interrupt nuclear localization thereby causing an MMR defect. For the identification of pathogenic variants that impact mRNA splicing, minigene reporter assays can be performed where the splicing patterns of transcripts generated after transient transfection of PCR amplified genomic DNA from the WT and variant constructs are compared.91,94 Sixteen PMS2 variants were previously tested using this assay, where several of the variants displayed aberrant splicing patterns.91 Taken together, these assays are essential to the understanding of the regulation of MMR proteins and are required for the proper designation of a particular VUS as pathogenic or benign.
5. Concluding Remarks
It is of utmost importance when recommending genetic testing of PMS2 to select a laboratory that uses techniques, which provide a comprehensive analysis of this gene (sequencing and deletion/duplication analysis) while also taking into account the hindrance of pseudogenes. Since many LS families with PMS2 mutations remain undiagnosed, panel testing for individuals with a strong family history of cancer is recommended. Panel testing has led to higher detection rates of PMS2 mutations, therefore informing probands as well as family members faced with medical management decisions that are meant to aid in early diagnosis or to prevent tumor formation altogether. These medical interventions would include more frequent colonoscopies or, in some cases, prophylactic surgeries. While PMS2 mutations are associated with a much lower lifetime risk of cancer compared to mutations in the other LS-associated genes, knowledge of a germline PMS2 mutation will also help couples make informed-decisions regarding reproductive risk when both parents are PMS2 heterozygotes.
While universal IHC and/or MSI are widely used as a first step to diagnosing LS, these techniques are not useful in patients not yet diagnosed with cancer. In an effort to provide preventative care, population screening for LS could be considered. Currently, there are several barriers to general population screening: (1) out of pocket expenses for molecular analysis, (2) preventative care costs, (3) interpreting mutations that are low penetrance, and (4) access to genetic experts/testing. The clinical impact of testing for LS is highest when cascade testing is performed throughout a family that allows healthcare providers to implement preventative medical management strategies.95
It is hypothesized that many PMS2 heterozygotes remain undiagnosed because: (1) most PMS2 carriers are not diagnosed with cancer and (2) PMS2 carriers that are affected by a particular cancer do not undergo genetic testing, as they do not meet current LS-testing guidelines.57 Keeping in mind the low penetrance of cancer in some families with a PMS2 germline mutation, it may be reasonable to counsel patients on the phenotypic heterogeneity seen amongst LS families, particularly if the PMS2 mutation was identified through universal tumor screening or panel testing.
Several laboratories are in the process of evaluating the pathogenicity of VUSs in PMS2 and characterizing their impact on MMR function. Reclassification of PMS2 VUSs is hindered by the low penetrance of classic LS-associated tumors and potential inability to use co-segregation studies to calculate likelihood that a variant is pathogenic. Furthermore, some PMS2 variants may be family-specific, and therefore exceedingly rare, leading to the prolonged classification of the variant as a VUS. Since co-segregation studies cannot be solely relied on when analyzing a PMS2 mutation that has low penetrance, we must rely on functional studies to determine the clinical impact of VUSs. Thorough and rigorous analyses of these variants is necessary to aid clinicians in making medical management plans for patients and their families. This would include combining biochemical, molecular, and cell-based data with co-segregation analysis.
Acknowledgments
JB and AP prepared the manuscript. We would like to thank Holly Snyder, MS, CGC and Ms. Jacqueline McKillop for proof reading this manuscript. We would also like to thank Tifany Lewis, MS, CGC for providing a pedigree for Figure 3. AP is supported by a National Institutes of Health (NIH) grant 5R00ES024417-05 awarded by the National Institute of Environmental Health Sciences (NIEHS).
Footnotes
Conflict of Interest: None.
References Cited
- 1.Boland CR, Lynch HT. The history of Lynch syndrome. Fam Cancer. 2013;12(2):145–157. doi: 10.1007/s10689-013-9637-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warthin A. Heredity with reference to carcinoma: As shown by the study of the cases examined in the pathological laboratory of the university of michigan, 1895–1913. Archives of Internal Medicine. 1913;XII(5):546–555. [Google Scholar]
- 3.Savage D. A family history of uterine and gastro-intestinal cancer. Br Med J. 1956;2(4988):341–343. doi: 10.1136/bmj.2.4988.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lynch HT, Shaw MW, Magnuson CW, Larsen AL, Krush AJ. Hereditary factors in cancer. Study of two large midwestern kindreds. Arch Intern Med. 1966;117(2):206–212. [PubMed] [Google Scholar]
- 5.Hauser IJ, Weller CV. A Further Report on the Cancer Family of Warthin. The American Journal of Cancer. 1936;27(3):434–449. [Google Scholar]
- 6.Lynch HT, Snyder CL, Shaw TG, Heinen CD, Hitchins MP. Milestones of Lynch syndrome: 1895–2015. Nat Rev Cancer. 2015;15(3):181–194. doi: 10.1038/nrc3878. [DOI] [PubMed] [Google Scholar]
- 7.Carethers JM, Stoffel EM. Lynch syndrome and Lynch syndrome mimics: The growing complex landscape of hereditary colon cancer. World J Gastroenterol. 2015;21(31):9253–9261. doi: 10.3748/wjg.v21.i31.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jass JR. Hereditary Non-Polyposis Colorectal Cancer: the rise and fall of a confusing term. World J Gastroenterol. 2006;12(31):4943–4950. doi: 10.3748/wjg.v12.i31.4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peltomaki P. Deficient DNA mismatch repair: a common etiologic factor for colon cancer. Hum Mol Genet. 2001;10(7):735–740. doi: 10.1093/hmg/10.7.735. [DOI] [PubMed] [Google Scholar]
- 10.Ligtenberg MJ, Kuiper RP, Chan TL, et al. Heritable somatic methylation and inactivation of MSH2 in families with Lynch syndrome due to deletion of the 3′ exons of TACSTD1. Nat Genet. 2009;41(1):112–117. doi: 10.1038/ng.283. [DOI] [PubMed] [Google Scholar]
- 11.Modrich P. Mechanisms in eukaryotic mismatch repair. J Biol Chem. 2006;281(41):30305–30309. doi: 10.1074/jbc.R600022200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiricny J. The multifaceted mismatch-repair system. Nat Rev Mol Cell Biol. 2006;7(5):335–346. doi: 10.1038/nrm1907. [DOI] [PubMed] [Google Scholar]
- 13.Jiricny J. Postreplicative mismatch repair. Cold Spring Harb Perspect Biol. 2013;5(4):a012633. doi: 10.1101/cshperspect.a012633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kunkel TA, Erie DA. Eukaryotic Mismatch Repair in Relation to DNA Replication. Annu Rev Genet. 2015;49:291–313. doi: 10.1146/annurev-genet-112414-054722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsieh P, Zhang Y. The Devil is in the details for DNA mismatch repair. Proc Natl Acad Sci U S A. 2017;114(14):3552–3554. doi: 10.1073/pnas.1702747114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iyer RR, Pluciennik A, Burdett V, Modrich PL. DNA mismatch repair: functions and mechanisms. Chem Rev. 2006;106(2):302–323. doi: 10.1021/cr0404794. [DOI] [PubMed] [Google Scholar]
- 17.Kadyrov FA, Dzantiev L, Constantin N, Modrich P. Endonucleolytic function of MutLalpha in human mismatch repair. Cell. 2006;126(2):297–308. doi: 10.1016/j.cell.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 18.Guarne A. The functions of MutL in mismatch repair: the power of multitasking. Prog Mol Biol Transl Sci. 2012;110:41–70. doi: 10.1016/B978-0-12-387665-2.00003-1. [DOI] [PubMed] [Google Scholar]
- 19.Nicolaides NC, Carter KC, Shell BK, Papadopoulos N, Vogelstein B, Kinzler KW. Genomic organization of the human PMS2 gene family. Genomics. 1995;30(2):195–206. doi: 10.1006/geno.1995.9885. [DOI] [PubMed] [Google Scholar]
- 20.Chang DK, Ricciardiello L, Goel A, Chang CL, Boland CR. Steady-state regulation of the human DNA mismatch repair system. J Biol Chem. 2000;275(37):29178. [PubMed] [Google Scholar]
- 21.Pluciennik A, Dzantiev L, Iyer RR, Constantin N, Kadyrov FA, Modrich P. PCNA function in the activation and strand direction of MutLalpha endonuclease in mismatch repair. Proc Natl Acad Sci U S A. 2010;107(37):16066–16071. doi: 10.1073/pnas.1010662107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guarne A, Junop MS, Yang W. Structure and function of the N-terminal 40 kDa fragment of human PMS2: a monomeric GHL ATPase. EMBO J. 2001;20(19):5521–5531. doi: 10.1093/emboj/20.19.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kosinski J, Plotz G, Guarne A, Bujnicki JM, Friedhoff P. The PMS2 subunit of human MutLalpha contains a metal ion binding domain of the iron-dependent repressor protein family. J Mol Biol. 2008;382(3):610–627. doi: 10.1016/j.jmb.2008.06.056. [DOI] [PubMed] [Google Scholar]
- 24.Wolf AI, Buchanan AH, Farkas LM. Historical review of Lynch syndrome. Journal of Coloproctology (Rio de Janeiro) 2013;33:95–110. [Google Scholar]
- 25.Nakagawa H, Lockman JC, Frankel WL, et al. Mismatch repair gene PMS2: disease-causing germline mutations are frequent in patients whose tumors stain negative for PMS2 protein, but paralogous genes obscure mutation detection and interpretation. Cancer Res. 2004;64(14):4721–4727. doi: 10.1158/0008-5472.CAN-03-2879. [DOI] [PubMed] [Google Scholar]
- 26.Senter L, Clendenning M, Sotamaa K, et al. The clinical phenotype of Lynch syndrome due to germ-line PMS2 mutations. Gastroenterology. 2008;135(2):419–428. doi: 10.1053/j.gastro.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Vos M, Hayward BE, Picton S, Sheridan E, Bonthron DT. Novel PMS2 pseudogenes can conceal recessive mutations causing a distinctive childhood cancer syndrome. Am J Hum Genet. 2004;74(5):954–964. doi: 10.1086/420796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaughn CP, Robles J, Swensen JJ, et al. Clinical analysis of PMS2: mutation detection and avoidance of pseudogenes. Hum Mutat. 2010;31(5):588–593. doi: 10.1002/humu.21230. [DOI] [PubMed] [Google Scholar]
- 29.Clendenning M, Hampel H, LaJeunesse J, et al. Long-range PCR facilitates the identification of PMS2-specific mutations. Hum Mutat. 2006;27(5):490–495. doi: 10.1002/humu.20318. [DOI] [PubMed] [Google Scholar]
- 30.Clendenning M, Walsh MD, Gelpi JB, et al. Detection of large scale 3′ deletions in the PMS2 gene amongst Colon-CFR participants: have we been missing anything? Fam Cancer. 2013;12(3):563–566. doi: 10.1007/s10689-012-9597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Etzler J, Peyrl A, Zatkova A, et al. RNA-based mutation analysis identifies an unusual MSH6 splicing defect and circumvents PMS2 pseudogene interference. Hum Mutat. 2008;29(2):299–305. doi: 10.1002/humu.20657. [DOI] [PubMed] [Google Scholar]
- 32.Wimmer K, Wernstedt A. PMS2 gene mutational analysis: direct cDNA sequencing to circumvent pseudogene interference. Methods Mol Biol. 2014;1167:289–302. doi: 10.1007/978-1-4939-0835-6_20. [DOI] [PubMed] [Google Scholar]
- 33.Hansen MF, Neckmann U, Lavik LA, et al. A massive parallel sequencing workflow for diagnostic genetic testing of mismatch repair genes. Mol Genet Genomic Med. 2014;2(2):186–200. doi: 10.1002/mgg3.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peltomaki P. Update on Lynch syndrome genomics. Fam Cancer. 2016;15(3):385–393. doi: 10.1007/s10689-016-9882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plazzer JP, Sijmons RH, Woods MO, et al. The InSiGHT database: utilizing 100 years of insights into Lynch syndrome. Fam Cancer. 2013;12(2):175–180. doi: 10.1007/s10689-013-9616-0. [DOI] [PubMed] [Google Scholar]
- 36.Vasen HF, Mecklin JP, Khan PM, Lynch HT. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC) Dis Colon Rectum. 1991;34(5):424–425. doi: 10.1007/BF02053699. [DOI] [PubMed] [Google Scholar]
- 37.Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999;116(6):1453–1456. doi: 10.1016/s0016-5085(99)70510-x. [DOI] [PubMed] [Google Scholar]
- 38.Peltomaki P, Lothe RA, Aaltonen LA, et al. Microsatellite instability is associated with tumors that characterize the hereditary non-polyposis colorectal carcinoma syndrome. Cancer Res. 1993;53(24):5853–5855. [PubMed] [Google Scholar]
- 39.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96(4):261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodriguez-Bigas MA, Boland CR, Hamilton SR, et al. A National Cancer Institute Workshop on Hereditary Nonpolyposis Colorectal Cancer Syndrome: meeting highlights and Bethesda guidelines. J Natl Cancer Inst. 1997;89(23):1758–1762. doi: 10.1093/jnci/89.23.1758. [DOI] [PubMed] [Google Scholar]
- 41.Evaluation of Genomic Applications in P, Prevention Working G. Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genet Med. 2009;11(1):35–41. doi: 10.1097/GIM.0b013e31818fa2ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hendriks YM, Jagmohan-Changur S, van der Klift HM, et al. Heterozygous mutations in PMS2 cause hereditary nonpolyposis colorectal carcinoma (Lynch syndrome) Gastroenterology. 2006;130(2):312–322. doi: 10.1053/j.gastro.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 43.Palomaki GE, McClain MR, Melillo S, Hampel HL, Thibodeau SN. EGAPP supplementary evidence review: DNA testing strategies aimed at reducing morbidity and mortality from Lynch syndrome. Genet Med. 2009;11(1):42–65. doi: 10.1097/GIM.0b013e31818fa2db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Committee on Practice B-G, Society of Gynecologic O. ACOG Practice Bulletin No. 147: Lynch syndrome. Obstet Gynecol. 2014;124(5):1042–1054. doi: 10.1097/01.AOG.0000456325.50739.72. [DOI] [PubMed] [Google Scholar]
- 45.Shendure J, Ji H. Next-generation DNA sequencing. Nat Biotechnol. 2008;26(10):1135–1145. doi: 10.1038/nbt1486. [DOI] [PubMed] [Google Scholar]
- 46.Espenschied CR, LaDuca H, Li S, McFarland R, Gau CL, Hampel H. Multigene Panel Testing Provides a New Perspective on Lynch Syndrome. J Clin Oncol. 2017;35(22):2568–2575. doi: 10.1200/JCO.2016.71.9260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cragun D, Radford C, Dolinsky JS, Caldwell M, Chao E, Pal T. Panel-based testing for inherited colorectal cancer: a descriptive study of clinical testing performed by a US laboratory. Clin Genet. 2014;86(6):510–520. doi: 10.1111/cge.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yurgelun MB, Allen B, Kaldate RR, et al. Identification of a Variety of Mutations in Cancer Predisposition Genes in Patients With Suspected Lynch Syndrome. Gastroenterology. 2015;149(3):604–613. e620. doi: 10.1053/j.gastro.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rohlin A, Rambech E, Kvist A, et al. Expanding the genotype-phenotype spectrum in hereditary colorectal cancer by gene panel testing. Fam Cancer. 2017;16(2):195–203. doi: 10.1007/s10689-016-9934-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ring KL, Bruegl AS, Allen BA, et al. Germline multi-gene hereditary cancer panel testing in an unselected endometrial cancer cohort. Mod Pathol. 2016;29(11):1381–1389. doi: 10.1038/modpathol.2016.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Win AK, Jenkins MA, Dowty JG, et al. Prevalence and Penetrance of Major Genes and Polygenes for Colorectal Cancer. Cancer Epidemiol Biomarkers Prev. 2017;26(3):404–412. doi: 10.1158/1055-9965.EPI-16-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dunlop MG, Farrington SM, Carothers AD, et al. Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet. 1997;6(1):105–110. doi: 10.1093/hmg/6.1.105. [DOI] [PubMed] [Google Scholar]
- 53.Hampel H, Stephens JA, Pukkala E, et al. Cancer risk in hereditary nonpolyposis colorectal cancer syndrome: later age of onset. Gastroenterology. 2005;129(2):415–421. doi: 10.1016/j.gastro.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 54.Jenkins MA, Baglietto L, Dowty JG, et al. Cancer risks for mismatch repair gene mutation carriers: a population-based early onset case-family study. Clin Gastroenterol Hepatol. 2006;4(4):489–498. doi: 10.1016/j.cgh.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 55.Quehenberger F, Vasen HF, van Houwelingen HC. Risk of colorectal and endometrial cancer for carriers of mutations of the hMLH1 and hMSH2 gene: correction for ascertainment. J Med Genet. 2005;42(6):491–496. doi: 10.1136/jmg.2004.024299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.ten Broeke SW, Brohet RM, Tops CM, et al. Lynch syndrome caused by germline PMS2 mutations: delineating the cancer risk. J Clin Oncol. 2015;33(4):319–325. doi: 10.1200/JCO.2014.57.8088. [DOI] [PubMed] [Google Scholar]
- 57.Goodenberger ML, Thomas BC, Riegert-Johnson D, et al. PMS2 monoallelic mutation carriers: the known unknown. Genet Med. 2016;18(1):13–19. doi: 10.1038/gim.2015.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Network NCC. [Accessed Nov. 5, 2017];Genetic/Familial High-Risk Assessment: Colorectal (Version 3.2017) https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf.
- 59.Kastrinos F, Uno H, Ukaegbu C, et al. Development and Validation of the PREMM5 Model for Comprehensive Risk Assessment of Lynch Syndrome. J Clin Oncol. 2017;35(19):2165–2172. doi: 10.1200/JCO.2016.69.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ricciardone MD, Ozcelik T, Cevher B, et al. Human MLH1 deficiency predisposes to hematological malignancy and neurofibromatosis type 1. Cancer Res. 1999;59(2):290–293. [PubMed] [Google Scholar]
- 61.Wang Q, Lasset C, Desseigne F, et al. Neurofibromatosis and early onset of cancers in hMLH1-deficient children. Cancer Res. 1999;59(2):294–297. [PubMed] [Google Scholar]
- 62.Wimmer K, Etzler J. Constitutional mismatch repair-deficiency syndrome: have we so far seen only the tip of an iceberg? Hum Genet. 2008;124(2):105–122. doi: 10.1007/s00439-008-0542-4. [DOI] [PubMed] [Google Scholar]
- 63.De Vos M, Hayward BE, Charlton R, et al. PMS2 mutations in childhood cancer. J Natl Cancer Inst. 2006;98(5):358–361. doi: 10.1093/jnci/djj073. [DOI] [PubMed] [Google Scholar]
- 64.Wimmer K, Kratz CP. Constitutional mismatch repair-deficiency syndrome. Haematologica. 2010;95(5):699–701. doi: 10.3324/haematol.2009.021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suerink M, Potjer TP, Versluijs AB, et al. Constitutional mismatch repair deficiency in a healthy child: On the spot diagnosis? Clin Genet. 2017 doi: 10.1111/cge.13053. [DOI] [PubMed] [Google Scholar]
- 66.Bakry D, Aronson M, Durno C, et al. Genetic and clinical determinants of constitutional mismatch repair deficiency syndrome: report from the constitutional mismatch repair deficiency consortium. Eur J Cancer. 2014;50(5):987–996. doi: 10.1016/j.ejca.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 67.Durno CA, Sherman PM, Aronson M, et al. Phenotypic and genotypic characterisation of biallelic mismatch repair deficiency (BMMR-D) syndrome. Eur J Cancer. 2015;51(8):977–983. doi: 10.1016/j.ejca.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 68.Adam R, Spier I, Zhao B, et al. Exome Sequencing Identifies Biallelic MSH3 Germline Mutations as a Recessive Subtype of Colorectal Adenomatous Polyposis. Am J Hum Genet. 2016;99(2):337–351. doi: 10.1016/j.ajhg.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gallinger S, Aronson M, Shayan K, et al. Gastrointestinal cancers and neurofibromatosis type 1 features in children with a germline homozygous MLH1 mutation. Gastroenterology. 2004;126(2):576–585. doi: 10.1053/j.gastro.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 70.Vasen HF, Ghorbanoghli Z, Bourdeaut F, et al. Guidelines for surveillance of individuals with constitutional mismatch repair-deficiency proposed by the European Consortium “Care for CMMR-D” (C4CMMR-D) J Med Genet. 2014;51(5):283–293. doi: 10.1136/jmedgenet-2013-102238. [DOI] [PubMed] [Google Scholar]
- 71.Tabori U, Hansford JR, Achatz MI, et al. Clinical Management and Tumor Surveillance Recommendations of Inherited Mismatch Repair Deficiency in Childhood. Clin Cancer Res. 2017;23(11):e32–e37. doi: 10.1158/1078-0432.CCR-17-0574. [DOI] [PubMed] [Google Scholar]
- 72.Solomon I, Harrington E, Hooker G, et al. Lynch Syndrome Limbo: Patient Understanding of Variants of Uncertain Significance. J Genet Couns. 2017;26(4):866–877. doi: 10.1007/s10897-017-0066-y. [DOI] [PubMed] [Google Scholar]
- 73.Heinen CD. Genotype to phenotype: analyzing the effects of inherited mutations in colorectal cancer families. Mutat Res. 2010;693(1–2):32–45. doi: 10.1016/j.mrfmmm.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rasmussen LJ, Heinen CD, Royer-Pokora B, et al. Pathological assessment of mismatch repair gene variants in Lynch syndrome: past, present, and future. Hum Mutat. 2012;33(12):1617–1625. doi: 10.1002/humu.22168. [DOI] [PubMed] [Google Scholar]
- 75.Heinen CD. Mismatch repair defects and Lynch syndrome: The role of the basic scientist in the battle against cancer. DNA Repair (Amst) 2016;38:127–134. doi: 10.1016/j.dnarep.2015.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peltomaki P, Vasen H. Mutations associated with HNPCC predisposition -- Update of ICG-HNPCC/INSiGHT mutation database. Dis Markers. 2004;20(4–5):269–276. doi: 10.1155/2004/305058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Borras E, Pineda M, Cadinanos J, et al. Refining the role of PMS2 in Lynch syndrome: germline mutational analysis improved by comprehensive assessment of variants. J Med Genet. 2013;50(8):552–563. doi: 10.1136/jmedgenet-2012-101511. [DOI] [PubMed] [Google Scholar]
- 78.Eggington JM, Bowles KR, Moyes K, et al. A comprehensive laboratory-based program for classification of variants of uncertain significance in hereditary cancer genes. Clin Genet. 2014;86(3):229–237. doi: 10.1111/cge.12315. [DOI] [PubMed] [Google Scholar]
- 79.Heinen CD, Juel Rasmussen L. Determining the functional significance of mismatch repair gene missense variants using biochemical and cellular assays. Hered Cancer Clin Pract. 2012;10(1):9. doi: 10.1186/1897-4287-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Structural Genomics C, China Structural Genomics C, Northeast Structural Genomics C et al. Protein production and purification. Nat Methods. 2008;5(2):135–146. doi: 10.1038/nmeth.f.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tomer G, Buermeyer AB, Nguyen MM, Liskay RM. Contribution of human mlh1 and pms2 ATPase activities to DNA mismatch repair. J Biol Chem. 2002;277(24):21801–21809. doi: 10.1074/jbc.M111342200. [DOI] [PubMed] [Google Scholar]
- 82.Trewhella J, Duff AP, Durand D, et al. 2017 publication guidelines for structural modelling of small-angle scattering data from biomolecules in solution: an update. Acta Crystallogr D Struct Biol. 2017;73(Pt 9):710–728. doi: 10.1107/S2059798317011597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rupp B. Biomolecular crystallography : principles, practice, and application to structural biology. New York: Garland Science; 2010. [Google Scholar]
- 84.Constantin N, Dzantiev L, Kadyrov FA, Modrich P. Human mismatch repair: reconstitution of a nick-directed bidirectional reaction. J Biol Chem. 2005;280(48):39752–39761. doi: 10.1074/jbc.M509701200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kosinski J, Hinrichsen I, Bujnicki JM, Friedhoff P, Plotz G. Identification of Lynch syndrome mutations in the MLH1-PMS2 interface that disturb dimerization and mismatch repair. Hum Mutat. 2010;31(8):975–982. doi: 10.1002/humu.21301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang H, Hays JB. Preparation of DNA substrates for in vitro mismatch repair. Mol Biotechnol. 2000;15(2):97–104. doi: 10.1385/MB:15:2:97. [DOI] [PubMed] [Google Scholar]
- 87.Suzuki K, Tsunekawa Y, Hernandez-Benitez R, et al. In vivo genome editing via CRISPR/Cas9 mediated homology-independent targeted integration. Nature. 2016;540(7631):144–149. doi: 10.1038/nature20565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157(6):1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Drost M, Koppejan H, de Wind N. Inactivation of DNA mismatch repair by variants of uncertain significance in the PMS2 gene. Hum Mutat. 2013;34(11):1477–1480. doi: 10.1002/humu.22426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Drost M, Zonneveld J, van Dijk L, et al. A cell-free assay for the functional analysis of variants of the mismatch repair protein MLH1. Hum Mutat. 2010;31(3):247–253. doi: 10.1002/humu.21180. [DOI] [PubMed] [Google Scholar]
- 91.van der Klift HM, Jansen AM, van der Steenstraten N, et al. Splicing analysis for exonic and intronic mismatch repair gene variants associated with Lynch syndrome confirms high concordance between minigene assays and patient RNA analyses. Mol Genet Genomic Med. 2015;3(4):327–345. doi: 10.1002/mgg3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Borras E, Pineda M, Brieger A, et al. Comprehensive functional assessment of MLH1 variants of unknown significance. Hum Mutat. 2012;33(11):1576–1588. doi: 10.1002/humu.22142. [DOI] [PubMed] [Google Scholar]
- 93.Wu X, Platt JL, Cascalho M. Dimerization of MLH1 and PMS2 limits nuclear localization of MutLalpha. Mol Cell Biol. 2003;23(9):3320–3328. doi: 10.1128/MCB.23.9.3320-3328.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gaildrat P, Killian A, Martins A, Tournier I, Frebourg T, Tosi M. Use of splicing reporter minigene assay to evaluate the effect on splicing of unclassified genetic variants. Methods Mol Biol. 2010;653:249–257. doi: 10.1007/978-1-60761-759-4_15. [DOI] [PubMed] [Google Scholar]
- 95.Hampel H. Genetic counseling and cascade genetic testing in Lynch syndrome. Fam Cancer. 2016;15(3):423–427. doi: 10.1007/s10689-016-9893-5. [DOI] [PubMed] [Google Scholar]