Abstract
Rationale and Objectives
To determine the impact of second opinion assessment on cancer staging and patient management in patients with pancreatic ductal adenocarcinoma.
Methods and Materials
This retrospective study was approved by our IRB with a waiver of informed consent. Second opinion reports between January 1, 2009, and December 31, 2013, alongside outside reports for 65 consecutive cases of biopsy-proven pancreatic adenocarcinomas were presented in random order to two experienced abdominal surgeons who independently reviewed them blinded to the origin of the report, images of the examinations, and patient identifier. Each surgeon filled in a questionnaire for each report recommending cancer staging and patient management. Recommended patient management and staging were evaluated against reference standards (actual patient management at 6 months following second opinion assessment, and pathology or other clinical and imaging reference standards at 6 months or longer, respectively) using Cohen’s kappa.
Results
Cancer staging differed in 13% (9/65) of cases for surgeon 1 and 18,4% (12/65) for surgeon 2. Patient management changed in 38,4% (25/65) of cases for surgeon 1 and 20% (13/65) for surgeon 2. When compared with the pathologic staging gold standard, second opinion was correct 85,7% (6/7) of the time for both surgeons. Recommended patient management from second opinion reports showed good agreement with the reference standard (weighted κ = 0.6467 (0.4014–0.892 and weighted κ = 0.6262 (0.3954–0.857) for surgeon 2.
Conclusion
Second opinion review by subspecialized oncology radiologists can impact patient care, specifically in terms of management decision.
Introduction
The increasing complexity of medical imaging has led to organ/disease sub specialization within the subfields of radiology. One of the latest subspecialties to emerge is oncologic imaging (1). The discrepancy between two radiologists reporting on the same images is a phenomenon that has been known since 1949 (2). Different studies have reported clear clinical benefits in introducing second opinion imaging review in both abdominal radiology gynecological imaging and neuro-radiology (3–6). In most clinical practices, it is not possible for every imaging study to be interpreted by a radiologist with subspecialty training. In this setting, general radiologists often fill the gap of subspecialty radiologists. However, it is common practice at many tertiary referral institutions for clinicians and surgeons to request second opinion review of images by subspecialty radiologists, most of the time without writing a new formal report. This is possible since no new imaging is needed, but only new image interpretation (7). This informal radiologic opinion is often reported in the patients’ chart by the clinicians and surgeons, but without the chance for the second opinion interpreting radiologist to review it. Furthermore, there is no reimbursement for this type of subspecialty second opinion to the interpreting radiologist. Another type of second opinion is asked for during or before multidisciplinary disease management team (DMT) meetings. Nowadays, many tertiary referral centers rely on DMTs for discussion of management of different type of cancers. During these meetings, outside radiological exams are reviewed by a subspecialty-trained radiologist without the issue of a formal report, often changing the management of the disease. In case of other specialties such as surgery or clinical specialties a second opinion practice is commonly accepted and reimbursed, whereas the rationale for reimbursing radiological reassessments and for new reports issuing is still under debate (5). Multiple studies have reported a 1–20% discrepancy in image interpretation (6). A disease-based formal study is needed to assess the benefits of such second opinion reads in terms of changes in patient management.
Our study focused on biologically proven pancreatic ductal adenocarcinoma (PDAC), one of the most lethal cancers with an overall survival at 5 years as low as 5% in the USA (8). We focused on a single type of cancer because of the lack of literature regarding second opinion imaging review of this cancer and because pancreatic cancer treatment is heavily dependent on staging which has a pivotal role in deciding the management of patients with PDAC. Additionally, a significant difference in short- and long-term outcomes has been noted when a pancreatectomy is performed at high-volume PDAC treatment centers (9, 10). Many reasons can account for this better long-term outcome, it might be related to better surgeon skills as well as to the fact that subspecialized radiologists might provide better assessment of tumors in high volume cancer centers. Lastly, general radiologists who often stage this type of cancer may not have the adequate expertise for interpreting all the different imaging patterns which pancreatic cancer can present on cross-sectional imaging, particularly when related with vascular involvement by the tumor (abutment, encasement, vessel deformity and venous teardrop deformity) (11–13). Pancreatic cancers are approached by surgeons with a three ways decision model: resectable, borderline resectable, unresectable (14). According to TNM stage, stage I and II are resectable by definition since the tumor is confined within the pancreas. Stage IV is unresectable since it is characterized by distal metastases. Stage III is the most susceptible to radiologic interpretation mistakes, and it is defined as localized tumor with involvement of a major artery: celiac trunk, common hepatic artery and superior mesenteric artery (SMA). It is further divided into two categories - locally advanced unresectable and borderline resectable. A borderline resectable tumor is defined by a contact between the tumor and the arterial circumference of no more than 180°. However, this definition of borderline resectable is not universally accepted and it might change among institutions, depending on the vessel involved, surgical skills and experience and also on patient status(15). When the tumor surrounds the arterial circumference for more than 180°, the vessel is considered encased and the tumor unresectable(16).
The aim of our study was to determine the clinical impact of second opinion assessment of outside magnetic resonance imaging (MRI) and computed tomography (CT) scans performed by oncologic imaging subspecialized radiologists at our institution, in a population with biopsy-proven PDAC. We compared differences in disease local staging and recommendations in patient management due to the different radiology reports.
Materials and Methods
This single-institution, retrospective study was eligible for a waiver of informed consent according to our institutional review board policy. In this study, we will refer to the outside institution radiologist reports as outside reports, and to our institution’s radiologist reports as second opinion reports. Two junior radiologists (initials blinded for review) with four years of experience respectively and who did not participate in the second opinion assessments independently re-reviewed all outside and second opinion reports, unblinded to the origin of each report, images of the examinations, and patient identifier. They organized the data collection and the database, coordinated the questionnaire filling and kept track of clinical data and follow-ups.
Inclusion criteria
A retrospective search of our institutional database was performed between January 1, 2009, and December 31, 2015. Our insitutional Picture Archiving and Communication System (PACS, GE Centricity PACS, GE Healthcare, Chicago, Illinois, USA) was used to search the radiology reports of all examinations submitted for second opinion reports. The results of this query were combined with the results of a query in the pathology department database giving as results all the biopsy-proven PDACs found in the aforementioned time-frame. The inclusion criteria included the following: biopsy-proven diagnosis of PDAC; abdominal MRI or contrast-enhanced CT performed and interpreted at an outside institution specifically for pancreatic cancer local staging; second opinion interpretation documented in an official report issued by an oncologic imaging subspecialized board-certified radiologist; second opinion report assessment performed between January 1, 2009 and December 31, 2015; and histopathology or ≥ 6 months of follow-up imaging after the second opinion report. Exclusion criteria were: patients who were referred to our hospital for a second opinion consultation but were treated at another institution; and patients who started chemotherapy before the second opinion reports. A total of 307 patients had either MRI or CT studies performed at an outside institution and re-interpreted at our institution in the timeframe selected (Figure 1). For this study, we considered only second opinion report assessments performed between January 1, 2009, and December 31, 2013. Thus, a total of 86 patients were included. To ensure that the second opinion reports did not benefit from extra clinical information, we excluded 15 patients with extra examinations (biopsy, surgery, or additional diagnostic imaging tests) obtained in between the outside report and second opinion interpretation. An additional 6 patients were excluded for lack of follow up information. The final study population consisted of 65 patients (Table 1).
Figure 1.
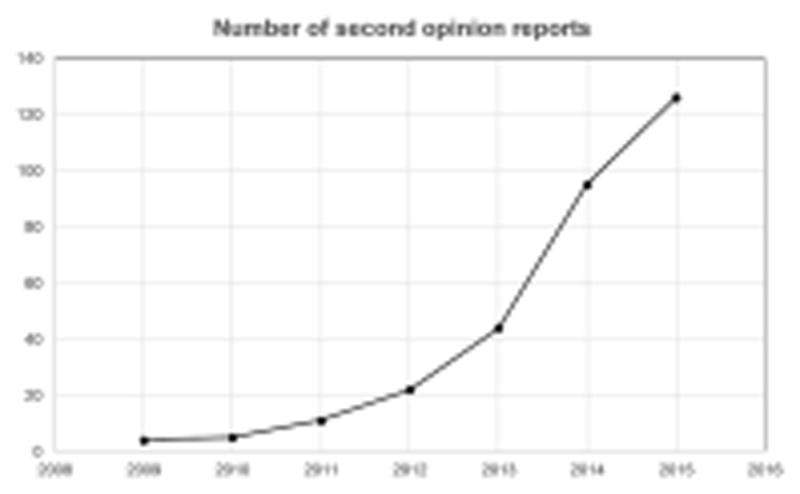
Chart showing the increasing number of MRI and CT scans in patients with biopsy-proven adenocarcinoma, for which an official second opinion report was requested for at our institution, per year, from 2009 to 2015.
Table 1.
Population characteristics and inclusion criteria. CT: computed tomography; MRI: magnetic resonance imaging; n: number
| Population characteristics and patient selection | n= |
|---|---|
| Total number of patients after database search | 307 |
| Number of patients in the timeframe considered (01/2009–12/2013) | 86 |
| Patients excluded because of extra clinical information available (e.g. surgery performed before the study started) | 15 |
| Patients excluded because of lack of follow-up | 6 |
| Final number of patient considered (M:F) | 65 (39:26) |
| Total number of CT exams | 51 |
| Total number of MRI exams | 14 |
Second opinion assessment
Within the study timeframe, a total of 20 different board-certified radiologists with subspecialized expertise in oncologic diagnostic imaging reassessed outside imaging studies, providing second opinion reports that were submitted to surgeons. All studies were digitized into a PACS and reviewed using GE Centricity software (GE Healthcare, Chicago, Illinois, USA). Radiologists had the option of looking at the outside reports during the second opinion assessment as provided for by our institution’s policies.
Retrospective data interpretation
Two highly experienced board-certified oncologic surgeons, both members of our institution’s hepatobiliary cancer DMT, independently re-reviewed both outside and second opinion reports for all cases, blinded to the origin of each report, images of the examinations, and patient identifier. The reports for all patients were unpaired and presented at random to reduce the bias that may occur upon recognizing the report as either an outside or second opinion report. The reports were submitted to the surgeons, who reviewed all the cases within a timeframe of 4 months. Every surgeon was provided with the outside report and the paired second opinion report with several weeks of time interval between the submissions, in order to reduce recognizability of the specific cases. For each report, the surgeons filled in a questionnaire (illustrated in Table 2) provided by two junior radiologists who are described below. On this questionnaire, by referring solely to the report, the surgeons evaluated the clinical stage according to the recent TNM staging system, version 7(17, 18); made a recommendation for patient management; and graded the clarity of the report on a scale from 1 to 5, with “grade 1” being poor, “grade 2” being fair, “grade 3” being average, “grade 4” being good, and “grade 5” being excellent. The grade measured how easily and accurately the surgeons could extract the information needed for proper staging and patient management recommendation.
Table 2.
Questionnaire to fill in provided by the two junior radiologists to two oncologic surgeons. The two surgeons were asked whether they needed further imaging studies in order to propose a certain management on the sole basis of the reports (if yes, they were asked about resectability status, staging according to the TNM staging system, and recommended management); and whether they were satisfied with the report on a scale from one to five.
| Item | Response | ||||
|---|---|---|---|---|---|
| 1. Further imaging recommended to better evaluate an area\finding? (Circle one) | Y | N | |||
|
| |||||
| 2. Resectability status (Circle one) |
|
||||
|
| |||||
| 3. Stage (Circle one) | I IIa IIb III IV | ||||
|
| |||||
| 4. Management (Circle one) |
|
||||
|
| |||||
| 5. Grade of report (Circle one) | Poor | Excellent | |||
| 1 | 2 | 3 | 4 | 5 | |
Two junior radiologists (initials blinded for review) with four years of experience respectively and who did not participate in the second opinion assessments independently re-reviewed all outside and second opinion reports, unblinded to the origin of each report, images of the examinations, and patient identifier. Any additional clinical information regarding patient management or staging from subsequent treatments or follow ups for each patient was noted. Specifically, as patient management was the primary outcome of the study, it was noted whether management proposed by the two surgeons based on each report concurred with the reference standard of actual management after 6 months following second opinion review. Secondarily, to determine staging for each case using the best reference standard available, all patient clinical, pathological, and imaging data after the time of the second opinion assessment, as well as clinical and imaging follow-up at 6 months or longer were reviewed.
Statistical analysis
Confidence intervals (CIs) were used to evaluate the theoretical change in patient management for each surgeon as well as combined. CIs were estimated using the Wilson score interval with continuity correction (19). To assess the level of agreement regarding patient management recommendations made by the two surgeons, Cohen’s kappa statistic (κ) was used for recommendations made based on outside and second opinion reports, respectively. The kappa statistic was interpreted as follows: Poor agreement, κ < 0.20; fair agreement, κ = 0.20 to 0.40; moderate agreement, κ = 0.40 to 0.60; good agreement, κ = 0.60 to 0.80; and very good agreement, κ = 0.80 to 1.00. Weighted kappa with squared weights was used to assess the agreement between patient management recommendations made by the surgeons and actual patient management at 6 months following second opinion assessment, for both outside and second opinion reports, respectively. Additionally, to determine whether there were differences in surgeon ratings of outside and second opinion reports, mean ratings were compared using a paired samples t-test and the significance level was set at p value < 0.01. SPSS for Windows (IBM Corp. Released 2013. IBM, Version 22.0. Armonk, NY: IBM Corp) was used to perform all statistical computations.
Results
Sixty-five patients (26 women and 39 men) were included in the study, with an average age of 65.6 (range, 37 to 82) years. Fifty-one patients had a CT scan from an outside institution, whereas 14 had an MR scan (Table 1).
Review by surgeons – combined results
For 32 of 65 patients (49.2 %; 95% CI: 37.4–61.0), discrepancies in recommended patient management between outside and second opinion reports were reported by at least one of the oncologic surgeons, thus theoretically leading to a change in patient management for each of these cases.
Outside reports were considered insufficient, leading to the recommendation for additional imaging scans for at least one of the two surgeons, in 30 cases (46,1%; 95% CI: 34,5–58,1); second opinion reports led to recommendation for additional imaging scans in 5 cases (7,6%; 95% CI: 3,3–16,7).
Review by surgeons – individual results
Both surgeons asked more additional examinations when basing their interpretations on outside reports, rather than on second reader reports (Table 3).
Table 3.
Results of questionnaire filled in by surgeons, question 1: number of further imaging scans examinations asked by surgeons based on the reports (percentage and 95% confidence interval -CI-)
| Surgeon 1 | Surgeon 2 | |
|---|---|---|
| No further imaging scans asked based on the reports | 27/65 (41,5% CI: 29.6-54-4) | 27/65 (41,5% CI: 29.6-54-4) |
| More imaging asked based on outside reports. | 21/65 (32,3% CI: 22.2–44,3) | 9/65 (13,8% CI:7,4–24,2) |
| More imaging asked based on second reader reports | 3/65 (4,6% CI: 1,5–12,7) | 2/65 (3% CI:0,8–10,5) |
| More imaging asked for both reports | 14/65 (21,5% CI: 13,2–32,9) | 3/65 (4,6% CI:1,5–12,7) |
Surgeon 1 would have theoretically not changed patient management in 44 of 65 cases (67,6%; 95%CI: 55,6–77,9) after reading either of the reports; however, in 21 cases (32,3%; 95%CI: 22,2–44,3), surgeon 1 would have theoretically changed patient management (Table 4). Surgeon 2 would have theoretically not changed patient management in 52 out of 65 cases (80%; 95%CI: 68,7–87,9) after reading either of the reports; however, in 13 cases (20%; 95%CI: 12,1–21,2), surgeon 2 would have theoretically changed patient management (Table 4).
Table 4.
This table shows how surgeons changed the management of the patients after reading second opinion reports, compared with what proposed after reading outside reports: number of cases (percentage and 95% confidence interval -CI-).
| Surgeon 1 | Surgeon 2 | |
|---|---|---|
| Concordance between outside and second reader reports | 44/65 (67,6% CI: 55,6–77.9) | 52/65 (80% CI: 68,7–87,9) |
| Change of therapy: from surgery to chemotherapy | 15/65 (23,1% CI: 14,5–34,6) | 6/65 (9,2% CI: 0,4–18,7) |
| Change of therapy: from chemotherapy to surgery | 0/65 (0% CI: 0–0,58) | 4/65 (6,1% CI: 2,4–14,7) |
| Change of therapy: from neoadjuvant chemotherapy to chemotherapy | 5/65 (7% CI: 3,3–16,6) | 1/65 (1,5% CI:0,2–8,2) |
| Change of therapy: from chemotherapy to neoadjuvant chemotherapy | 1/65 (1,5% CI: 0,2–8,2) | 2/65 (3% CI: 0,8–10,5) |
For each surgeon, patient management proposed based on outside reports had moderate agreement with actual patient management at 6 months following outside reports assessment (Table 5): weighted κ = 0.426 (0,2489–0,6031) for surgeon 1 and weighted κ = 0.4136 (0,2149–0,6129) for surgeon 2, respectively. Meanwhile, there was good agreement between patient management proposed based on second opinion reports and actual management at 6 months following second opinion assessment (Table 5): weighted κ = 0.6467 (0.4014–0.892) for surgeon 1 and weighted κ = 0.6262 (0.3954–0.857) for surgeon 2.
Table 5.
Agreement between three categories of patient management proposed by surgeons based on outside and second opinion reports, compared with actual patient management as found on the clinical report system (surgery summaries, pathology, blood tests, and additional imaging exams). Weighted kappa with squared weights was used to assess agreement.
| PATIENTS’ CLINICAL MANAGEMENT AFTER 6 MONTHS FROM RADIOLOGIC ASSESSMENT | ||||||||
|---|---|---|---|---|---|---|---|---|
| Surgeon 1 | Surgeon 2 | |||||||
|
|
|
|||||||
| Surgery | Neoadjuvant chemotherapy | Chemotherapy | Weighted kappa (95% CI) agreement | Surgery | Neoadjuvant chemotherapy | Chemotherapy | Weighted kappa (95% CI) agreement | |
| MANAGEMENT AS PROPOSED AFTER READING OUTSIDE REPORTS | ||||||||
|
| ||||||||
| Surgery | 6 | 2 | 10 | 3 | 2 | 6 | ||
| Neoadjuvant chemotherapy | 1 | 0 | 8 | 0.426 (0,2489–0,6031) | 3 | 0 | 7 | 0.4136 (0,2149–0,6129) |
| Chemotherapy | 0 | 0 | 38 | 1 | 0 | 43 | ||
|
| ||||||||
| MANAGEMENT AS PROPOSED AFTER READING SECOND OPINION REPORTS | ||||||||
|
| ||||||||
| Surgery | 3 | 0 | 1 | 5 | 0 | 3 | ||
| Neoadjuvant chemotherapy | 3 | 2 | 7 | 0.6467 (0.4014–0.892) | 2 | 2 | 9 | 0.6262 (0.3954–0.857) |
| Chemotherapy | 1 | 0 | 48 | 0 | 0 | 44 | ||
Both surgeons expressed their appreciation for both outside and second opinion reports on a scale from 1 (poor appreciation) to 5 (excellent appreciation). There was a significant difference (p ≤ 0.01) for the rating average of surgeon 1, who graded the outside reports with an average of 2.11 out of 5, and the second opinion reports with an average of 3.14 out of 5 (t= 8.587274; p< 0.00001). There was a significant difference (p ≤ 0.01) for the rating average of surgeon 2, who graded the outside reports with an average of 2.46 out of 5, and the second opinion reports with an average of 3.93 out of 5 (t=16.783127; p< 0.00001).
Discussion
PDAC is the fourth most frequent cause of cancer-related mortality (20). Unlike many other types of cancers, the prognosis of PDAC is still unfavorable with a five-year survival less than 5% (21). Thus, PDAC remains one of the biggest oncologic challenges today (22). Imaging plays an important role in the care of patients with PDAC, contributing to its initial diagnosis, determination of disease extent locally and systemically, treatment selection, and disease follow-up (23–26). However, the added value of radiology subspecialist review of scans of patients with PDAC is unknown. To our knowledge, the added value of sub specialization opinion in PDAC evaluation has been recently demonstrated for pathologists, and not for radiologists (27). For this reason, we analyzed the impact of subspecialized oncologic radiologists’ opinion on patient management in a population of patients with PDAC. Determining the value of subspecialist second opinion of outside imaging examinations is an important issue, particularly when considering the costs of these assessments: unlike many other specialties, radiologists are often asked to review outside scans “informally” and give their opinion without having any reimbursement. It is also important considering the allocation of limited personnel and resources needed to perform these second opinion reviews. And for PDAC in particular, it is important to note that around 20% of diagnosed cancers can be surgically resected, but even in patients with resectable disease, the survival rate at 5 years is less than 30% (20). Other studies have already shown how re-performing scans in a high-volume center can lead to increased short- and long-term survival rates (28). Nevertheless, this approach could lead to increased health costs, resources, and in the case of CT scans, radiation exposure.
CT and MRI have the same accuracy and the similar positive and negative predictive values for PDAC (29). For this reason, we included outside and second opinion reports of both in this study wherein two oncologic surgeons retrospectively recommended patient management blinded to the origin of the report (whether it is an outside or second opinion report) and without any other accompanying clinical or imaging data. Our study found that having second opinion readings of CT and MRI examinations would lead to a significant change in patient management for each surgeon and both surgeons combined. In our study of 65 CT and MRI examinations, recommended patient management was changed in 21/65 (32,3%) cases according to surgeon 1 and 13/65 (20%) cases according to surgeon 2.
Our study found that, importantly, when assessing the agreement of the proposed patient management against the reference standard (actual patient management at 6 months), patient management based on the second opinion report had a significantly higher agreement (weighted κ) than that of the outside report. Additionally, both surgeons had higher appreciation grades for second opinion reports issued by our institution’s subspecialized radiologists. Surgeons also indicated that they would request fewer additional scans after reading the second opinion reports (7.6% vs 46.1% of cases) compared with outside reports.
Overall, our results indicate higher levels of clarity and completeness of the second opinion reports provided by subspecialized radiologists compared with outside reports provided by general radiologists. Second opinion assessments may also lead to higher confidence of surgeons to make decisions regarding patient management and perform surgical operations, as suggested by the growing demand for second opinion assessments at our institution. Ultimately, the difference between the higher quality second opinion assessments and outside assessments could potentially lead to an important reduction in terms of health costs. A further step would be to evaluate the impact of second opinion assessments and survival outcome.
Our study had several limitations. First, this was a retrospective study, potentially leading to several selection biases: only imaging scans for which clinicians requested second opinion review were included; it is possible that differences between second opinion and outside reports would have been less apparent if all outside imaging scans were considered; the time interval between diagnosis and imaging was not defined. Secondly, surgeons were asked to recommend patient management only based on each report, whereas in clinical settings this decision is made based on many other different factors such as clinical data, overall patient status, patient opinion, etc. Furthermore, results could have been affected by institutional bias: surgical decisions at our institution typically involve interpretation of subspecialized radiologists at our institution and the surgeons could have developed familiarity with the type of interpretation given by subspecialized radiologists and recognized which reports were outside reports and which were second opinion. Specifically, despite unpairing and randomizing outside and second opinion reports, the graphic format of them was not changed, making them easier to be recognized by surgeons. Furthermore, our institution reports were only in form of structured reports, as provided by our radiology department policy, whereas outside institution were mostly in an unstructured form, probably making second opinion reports easier to be recognized by surgeons. However, regardless the fact that surgeons recognized or not the origin of the reports, this should have not biased the evaluation whether relevant information was present or not. On the other hand, the structured format versus the freestyle narrative reporting format, despite increasing bias and recognizability, might also be a reason why the appreciation for our institution second opinion reports was higher, in line with existent literature on this topic(30, 31). As concluded by Brook et al. (31) structured reporting of CT performed in pancreatic cancers provides superior evaluation the tumor extension and facilitates surgical planning making surgeons more confident taking decisions about tumor resectability.
An important difference between our study and other second opinion studies in other types of cancers (5, 32, 33) is the advanced stage in which most pancreatic carcinomas are diagnosed. Our study confirmed this trend: most of the patients presented to our attention at stage IV, with distant metastatic disease. There is higher agreement between radiologists if a distant metastasis is present, since the most difficult assessment is local tumor staging (T of the TNM staging) (16, 34). As we discussed in the introduction the differentiation between stage III borderline resectable and locally advanced unresectable is the crucial part of the radiologic assessment of this stage, as shown in Figure 2 and 3 where the differences between outside reports and second opinion reports were exclusively based on the different interpretations of vascular involvement.
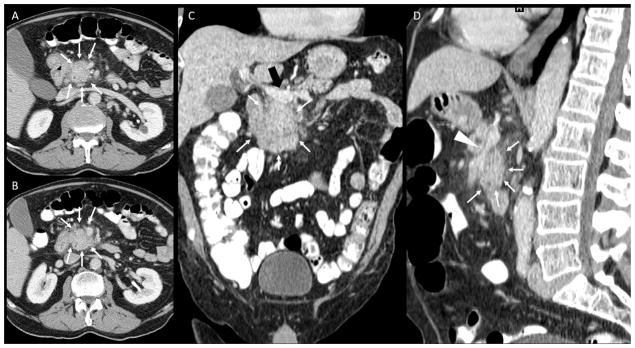
Figure 2.
Contrast CT of the abdomen, portal-venous phase. A, B: axial images; C: coronal reconstruction; D: Sagittal reconstruction. A pancreatic tumor is noted (white arrows in A, B, C, D) with peripancreatic fat infiltration, abuttment of portal vein at the spleno-mesenteric confluence (black arrow in C) and superior mesenteric vein encasement and narrowing (arrowhead in D). No significant artery involvement was described on outside report (T3, stage IIa). Second opinion report described the tumor encasement of the superior mesenteric artery (white arrowhead) changing thus the management of the patient (T4, stage III, unresectable).
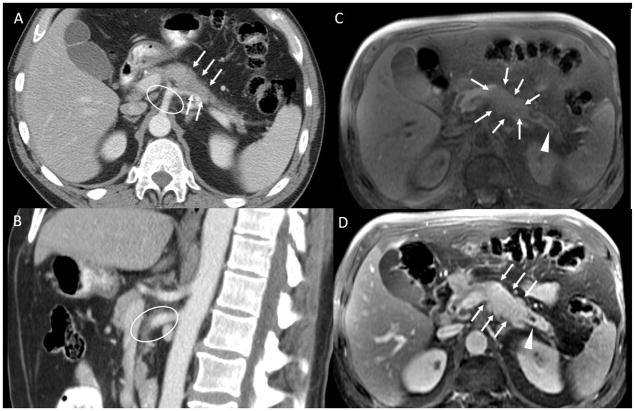
Figure 3.
A: axial CT pancreatic phase shows an isointense pancreatic body tumor, subtly visible in this scan and an encasement of splenic artery B: sagittal CT pancreatic phase at superior mesenteric artery and celiac axis level. C: axial MRI, spoiled Gradient echo fat-sat pre-contrast D: axial MRI, spoiled Gradient echo fat-sat post-contrast pancreatic-phase. The pancreatic head tumor is well depicted in T1 pre-contrast and post contrast images (white arrows in B and C), with a dilatation of the pancreatic duct (white arrowheads). There is mild hyperattenuating tissue surrounding the superior mesenteric artery and the celiac axis (white ellipses in A and C). This was reported as encasement of these arteries by the tumor on the outside report, interpreted by the surgeons as unresectable disease, and as inflammatory tissue on the second opinion report, interpreted by the surgeons as borderline resectable disease. The patient underwent neoadjuvant chemotherapy and was successfully resected.
In conclusion, our results indicate that second opinion review by subspecialized oncology radiologists can impact patient care, specifically in terms of management decision. Our findings support the notion that subspecialty radiological training and subspecialty expertise influence patient care in the setting of multidisciplinary, disease-specific, team-based medicine. Moreover, second opinion consultations should be viewed as a valuable and reimbursable clinical service within the field of radiology.
Footnotes
Institution where work was performed:
Memorial Sloan-Kettering Cancer Center, Department of Radiology, 1275 York Avenue, New York, NY 10065, USA
Conflict of interest disclosure statement:
Grant support was provided by MSK Cancer Center Support Grant/Core Grant P30 CA008748.
Work by Giuseppe Corrias was partially supported by a scholarships awarded by ISSNAF Imaging Science Chapter.
The authors have no conflict of interest to disclose.
The authors were equally involved in acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis, technical, and material support of this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hricak H, Yu KK, Dudley A, Scheidler J, Luft HS. Subspecialty care in radiology: oncologic imaging. Acad Radiol. 1998;5(Suppl 2):S288–90. doi: 10.1016/s1076-6332(98)80334-6. [DOI] [PubMed] [Google Scholar]
- 2.Garland LH. On the Scientific Evaluation of Diagnostic Procedures: Presidential Address Thirty-fourth Annual Meeting of the Radiological Society of North America. Radiology. 1949;52(3):309–28. doi: 10.1148/52.3.309. [DOI] [PubMed] [Google Scholar]
- 3.Bell ME, Patel MD. The degree of abdominal imaging (AI) subspecialization of the reviewing radiologist significantly impacts the number of clinically relevant and incidental discrepancies identified during peer review of emergency after-hours body CT studies. Abdominal imaging. 2014;39(5):1114–8. doi: 10.1007/s00261-014-0139-4. [DOI] [PubMed] [Google Scholar]
- 4.Lindgren EA, Patel MD, Wu Q, Melikian J, Hara AK. The clinical impact of subspecialized radiologist reinterpretation of abdominal imaging studies, with analysis of the types and relative frequency of interpretation discrepancies. Abdominal imaging. 2014;39(5):1119–26. doi: 10.1007/s00261-014-0140-y. [DOI] [PubMed] [Google Scholar]
- 5.Lakhman Y, D’Anastasi M, Micco M, et al. Second-Opinion Interpretations of Gynecologic Oncologic MRI Examinations by Sub-Specialized Radiologists Influence Patient Care. Eur Radiol. 2016;26(7):2089–98. doi: 10.1007/s00330-015-4040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zan E, Yousem DM, Carone M, Lewin JS. Second-opinion consultations in neuroradiology. Radiology. 2010;255(1):135–41. doi: 10.1148/radiol.09090831. [DOI] [PubMed] [Google Scholar]
- 7.Kalbhen C, Yetter E, Olson M, Posniak H, Aranha G. Assessing the resectability of pancreatic carcinoma: the value of reinterpreting abdominal CT performed at other institutions. AJR American journal of roentgenology. 1998;171(6):1571–6. doi: 10.2214/ajr.171.6.9843290. [DOI] [PubMed] [Google Scholar]
- 8.Gillen S, Schuster T, Meyer Zum Buschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7(4):e1000267. doi: 10.1371/journal.pmed.1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshioka R, Yasunaga H, Hasegawa K, et al. Impact of hospital volume on hospital mortality, length of stay and total costs after pancreaticoduodenectomy. Br J Surg. 2014;101(5):523–9. doi: 10.1002/bjs.9420. [DOI] [PubMed] [Google Scholar]
- 10.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349(22):2117–27. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 11.He XY, Yuan YZ. Advances in pancreatic cancer research: moving towards early detection. World J Gastroenterol. 2014;20(32):11241–8. doi: 10.3748/wjg.v20.i32.11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belousova E, Karmazanovsky G, Kriger A, et al. Contrast-enhanced MDCT in patients with pancreatic neuroendocrine tumours: correlation with histological findings and diagnostic performance in differentiation between tumour grades. Clinical radiology. 2017;72(2):150–8. doi: 10.1016/j.crad.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mannelli L, Nougaret S, Vargas HA, Do RK. Advances in diffusion-weighted imaging. Radiol Clin North Am. 2015;53(3):569–81. doi: 10.1016/j.rcl.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vauthey JN, Dixon E. AHPBA/SSO/SSAT Consensus Conference on Resectable and Borderline Resectable Pancreatic Cancer: rationale and overview of the conference. Ann Surg Oncol. 2009;16(7):1725–6. doi: 10.1245/s10434-009-0409-5. [DOI] [PubMed] [Google Scholar]
- 15.Al-Hawary MM, Francis IR, Chari ST, et al. Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the Society of Abdominal Radiology and the American Pancreatic Association. Radiology. 2014;270(1):248–60. doi: 10.1148/radiol.13131184. [DOI] [PubMed] [Google Scholar]
- 16.Zaky AM, Wolfgang CL, Weiss MJ, Javed AA, Fishman EK, Zaheer A. Tumor-vessel relationships in pancreatic ductal adenocarcinoma at multidetector CT: different classification systems and their influence on treatment planning. RadioGraphics. 2016;37(1):93–112. doi: 10.1148/rg.2017160054. [DOI] [PubMed] [Google Scholar]
- 17.Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic Adenocarcinoma, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15(8):1028–61. doi: 10.6004/jnccn.2017.0131. [DOI] [PubMed] [Google Scholar]
- 18.Seufferlein T, Bachet JB, Van Cutsem E, Rougier P, Group EGW. Pancreatic adenocarcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii33–40. doi: 10.1093/annonc/mds224. [DOI] [PubMed] [Google Scholar]
- 19.Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17(8):857–72. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 20.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 21.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA: a cancer journal for clinicians. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 22.Güngör C, Hofmann B, Wolters-Eisfeld G, Bockhorn M. Pancreatic cancer. British journal of pharmacology. 2014;171(4):849–58. doi: 10.1111/bph.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamm EP, Balachandran A, Bhosale PR, et al. Imaging of pancreatic adenocarcinoma: update on staging/resectability. Radiol Clin North Am. 2012;50(3):407–28. doi: 10.1016/j.rcl.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Irie H, Honda H, Kaneko K, Kuroiwa T, Yoshimitsu K, Masuda K. Comparison of helical CT and MR imaging in detecting and staging small pancreatic adenocarcinoma. Abdom Imaging. 1997;22(4):429–33. doi: 10.1007/s002619900226. [DOI] [PubMed] [Google Scholar]
- 25.Tscholakoff D, Hricak H, Thoeni R, Winkler ML, Margulis AR. MR imaging in the diagnosis of pancreatic disease. AJR Am J Roentgenol. 1987;148(4):703–9. doi: 10.2214/ajr.148.4.703. [DOI] [PubMed] [Google Scholar]
- 26.Raman SP, Horton KM, Fishman EK. Multimodality imaging of pancreatic cancer-computed tomography, magnetic resonance imaging, and positron emission tomography. Cancer J. 2012;18(6):511–22. doi: 10.1097/PPO.0b013e318274a461. [DOI] [PubMed] [Google Scholar]
- 27.Liu YJ, Smith-Chakmakova F, Rassaei N, et al. Frozen Section Interpretation of Pancreatic Margins: Subspecialized Gastrointestinal Pathologists Versus General Pathologists. Int J Surg Pathol. 2016;24(2):108–15. doi: 10.1177/1066896915605911. [DOI] [PubMed] [Google Scholar]
- 28.Walters DM, LaPar DJ, de Lange EE, et al. Pancreas-protocol imaging at a high-volume center leads to improved preoperative staging of pancreatic ductal adenocarcinoma. Annals of surgical oncology. 2011;18(10):2764–71. doi: 10.1245/s10434-011-1693-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Megibow AJ, Zhou XH, Rotterdam H, et al. Pancreatic adenocarcinoma: CT versus MR imaging in the evaluation of resectability--report of the Radiology Diagnostic Oncology Group. Radiology. 1995;195(2):327–32. doi: 10.1148/radiology.195.2.7724748. [DOI] [PubMed] [Google Scholar]
- 30.Powell DK, Silberzweig JE. State of structured reporting in radiology, a survey. Acad Radiol. 2015;22(2):226–33. doi: 10.1016/j.acra.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 31.Brook OR, Brook A, Vollmer CM, Kent TS, Sanchez N, Pedrosa I. Structured reporting of multiphasic CT for pancreatic cancer: potential effect on staging and surgical planning. Radiology. 2015;274(2):464–72. doi: 10.1148/radiol.14140206. [DOI] [PubMed] [Google Scholar]
- 32.Ulaner GA, Mannelli L, Dunphy M. Value of second-opinion review of outside institution PET-CT examinations. Nucl Med Commun. 2017;38(4):306–11. doi: 10.1097/MNM.0000000000000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wibmer A, Vargas HA, Donahue TF, et al. Diagnosis of Extracapsular Extension of Prostate Cancer on Prostate MRI: Impact of Second-Opinion Readings by Subspecialized Genitourinary Oncologic Radiologists. AJR Am J Roentgenol. 2015;205(1):W73–8. doi: 10.2214/AJR.14.13600. [DOI] [PubMed] [Google Scholar]
- 34.Cao HST, Balachandran A, Wang H, et al. Radiographic Tumor–Vein Interface as a Predictor of Intraoperative, Pathologic, and Oncologic Outcomes in Resectable and Borderline Resectable Pancreatic Cancer. Journal of Gastrointestinal Surgery. 2014;18(2):269–78. doi: 10.1007/s11605-013-2374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]