Abstract
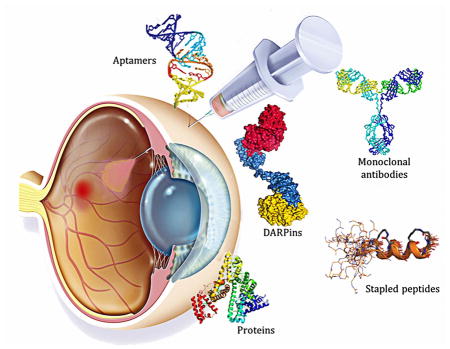
The impact of proteins and peptides on the treatment of various conditions including ocular diseases over the past few decades has been advanced by substantial breakthroughs in structural biochemistry, genetic engineering, formulation and delivery approaches. Formulation and delivery of proteins and peptides, such as monoclonal antibodies, aptamers, recombinant proteins and peptides to ocular tissues poses significant challenges owing to their large size, poor permeation and susceptibility to degradation. A wide range of advanced drug delivery systems including polymeric controlled release systems, cell-based delivery and nanowafers are being exploited to overcome the challenges of frequent administration to ocular tissues. The next generation systems integrated with new delivery technologies are anticipated to generate improved efficacy and safety through the expansion of the therapeutic target space. This review will highlight recent advances in formulation and delivery strategies of protein and peptide based biopharmaceuticals. We will also describe the current state of proteins and peptides based ocular therapy and future therapeutic opportunities.
Keywords: Drug delivery, eye, barriers, targeting, AMD, biopharmaceuticals, controlled release, macromolecules, biologics
Graphical abstract
1. Introduction and current scenario of ophthalmology
In the past few decades, since the approval of a protein based biopharmaceutical in 1982 (Humulin; recombinant human insulin; Eli Lilly, Indianapolis), the approval rate of protein and peptide based biopharmaceuticals has grown significantly[1]. Of the top 10 pharmaceutical products by sales in 2014, a majority was biopharmaceuticals including recombinant therapeutic proteins, peptides, enzymes, monoclonal antibodies and antibody-drug conjugates. From 1982 to 2014, the total number of licensed biopharmaceutical products advanced from 13 to 246 in the United States (US) and European Union (EU; Brussels). The worldwide sales of biopharmaceutical drugs was estimated to be $289 billion in 2014 and are projected to grow to $445 billion by 2019[2]. Among these, the rapidly growing monoclonal antibody (mAb) therapeutics market itself has currently resulted in global sales of over US$50 billion [3]. Likewise with the inception of the anti-vascular endothelial growth factor (anti-VEGF) aptamer in 2004 (Macugen; Pegatanib sodium; OSI Pharmaceuticals, New York) and monoclonal antibody in 2006 (Lucentis; Ranibizumab; Genentech, California), the growth of ophthalmic protein and peptide based biopharmaceutical drug market has accelerated staggeringly. The global sales of biopharmaceutical drugs for ophthalmic indications had exceeded $8 billion in 2016 and is expected to reach $35.7 billion by 2025[4, 5]. A recent survey of ophthalmology market research revealed biologics and drug delivery systems to be the sectors that are anticipated to show strong growth in the next five years[6].
In addition to global sales and market, ophthalmology has garnered quite startling investments in terms of research funding in comparison to other disease areas indicating the urgent need for advanced therapeutic approaches for the treatment of chronic ocular diseases[7].
While over 900 new biopharmaceutical entities are in pipeline, targeting diseases across a wide range of therapeutic areas, the emergence of biosimilars is anticipated to represent the biggest shift in biologic approval landscape[8]. The U.S. patents for blockbuster Lucentis® will be expiring in 2019 and several biosimilar manufacturers are already targeting that molecule[9]. The current ophthalmic drug delivery technologies are tailored to non-targeted small molecules/drugs.
Biopharmaceuticals including proteins and peptides have shown great promise as novel therapeutics in the treatment of ocular diseases. These large molecules offer several advantages compared to small molecule drugs with respect to high potency, activity, low unspecific binding, less toxicity, minimization of drug-drug interaction, biological and chemical diversity [10, 11]. However, these macromolecules also face various challenges such as physical and chemical degradation, short in vivo half-lives, circulation, and distribution. Additionally, macromolecules lack efficient and specific delivery to the target sites. Besides these, clearance by the mononuclear phagocytes (MPS) of the reticuloendothelial system (RES), risk of immunogenic effect, high molecular weight (MW), structural complexity, and failure to permeate cell membranes further reduce their therapeutic efficacy [12]. For these reasons, there is a need to develop novel ophthalmic biopharmaceutical drugs and delivery systems, ideally targeting these macromolecules to biologically relevant ocular tissues.
2. Ocular diseases: current and future biologics based treatments
Millions of people worldwide suffer from a wide variety of ocular diseases. A majority of these pathologies lead to irreversible blindness thereby substantially reducing quality of life. The number of visually impaired people has escalated to 285 million worldwide currently. In the United states alone, one million people were legally blind (visual acuity of 20/200 vision or worse) while 3.2 million suffered from visual impairment and another 8.2 million had vision problems due to uncorrected refractive error in 2015. The number of these conditions are projected to double by 2050[13].
Last few decades have witnessed a considerable growth in the understanding of the pathogenesis and genetics of ocular diseases. Deciphering various compliment pathways, gene associations and pharmacological interventions for retinal diseases have led to substantial development of effective therapies[14]. The major ocular diseases that have significantly impacted vision worldwide include age-related macular degeneration (AMD), cataracts, diabetic retinopathy (DR), dry eye conditions and glaucoma. The treatment market for glaucoma had the largest market share in 2013 with product sales (both branded and generic) exceeding US$ 4.5 billion (£ 2.9 billion) in the United States, Europe and Japan combined. Age related diseases including cataracts, AMD and diabetic retinopathy are expected to become more common with aging populations in developed countries[6]. Table 1 lists FDA approved biopharmaceuticals for ocular indications.
Table 1.
List of FDA approved biopharmaceutical drugs
| Drug | MW (kDa) | Route of administration | Half-life | FDA approval | Indication | Ref. |
|---|---|---|---|---|---|---|
| Adalimumab (Humira®) | 148 | Subcutaneous | ~2 weeks (human) | July 2016 | Uveitis | [15] |
| Aflibercept (Eylea®) | 115 | Intravitreal | 3.63 days (rabbit); 7.1 days (human) | November 2011 | Wet AMD | [16] |
| Ranibizumab (Lucentis®) | 48 | Intravitreal | 2.88 days (rabbit); ~9 days (human) | June 2006, August 2012 | Wet AMD, DME | [17] |
| Pegatanib sodium (Macugen®) | 50 | Intravitreal | ~10 days (human) | December 2004 | Wet AMD | [18] |
| Bevacizumab (Avastin®) | 150 | Intravitreal | 4.32 days (rabbit); 4.9 days (human) | Off-label | Wet AMD | [19] |
2.1. Anti-VEGF agents
The ophthalmology market has grown tremendously over the last 20 years both financially and technologically. The biological milieu of human eye has attracted several proteins, peptides and gene therapy based companies worldwide. Currently, the US Food and Drug Administration (FDA) has approved proteins and peptides based therapy for various ocular indications, involving anti-VEGF agents such as pegatanib (Macugen®), ranibizumab (Lucentis®), aflibercept (Eylea®) that serves as “VEGF trap”, and bevacizumab (Avastin®) which is used off label. Anti-VEGF therapies block the binding of VEGF signaling peptide to its receptors, neutralizing VEGF’s downstream effect of promoting growth of leaky blood vessels from the preexisting ones[20].
Pegatanib, a pegylated anti-VEGF aptamer binds to the major pathological VEGF-A isoform, VEGF165. VEGF165 is primarily responsible for mediating neovascularization in the eye. In contrast, ranibizumab and bevacizumab binds to all isoforms of VEGF-A. Ranibizumab (~48kDa), a monoclonal IgG1 antibody fragment has been reported to exhibit 17-fold higher binding capacity to VEGF receptors (VEGFRs) in comparison to full length bevacizumab (~149kDa). Aflibercept (~97kDa), unlike other VEGF inhibitors, is a recombinant fusion protein that acts as a dummy receptor for VEGF, thus effectively inhibiting the angiogenic response[21]. In addition, it’s ~200 fold higher affinity for VEGF in comparison to ranibizumab may be attributed to strong binding to VEGF-A, VEGF-B and PlGF (placental growth factor) and thereby influencing multiple pathways involved in cell proliferation, migration, extracellular matrix (ECM) degradation as well as pathological angiogenesis[22–24]. These protein and peptide based biopharmaceutical agents have remained relatively effective for the treatment of AMD and related ocular complications for the last few years. However, many patients do not respond to these treatments and some develop decreased responsiveness to the treatment itself. In fact repeated intravitreal injections requires skilled professional execution adding to the treatment cost and serious side effects including ocular pain, infection, or hemorrhage.
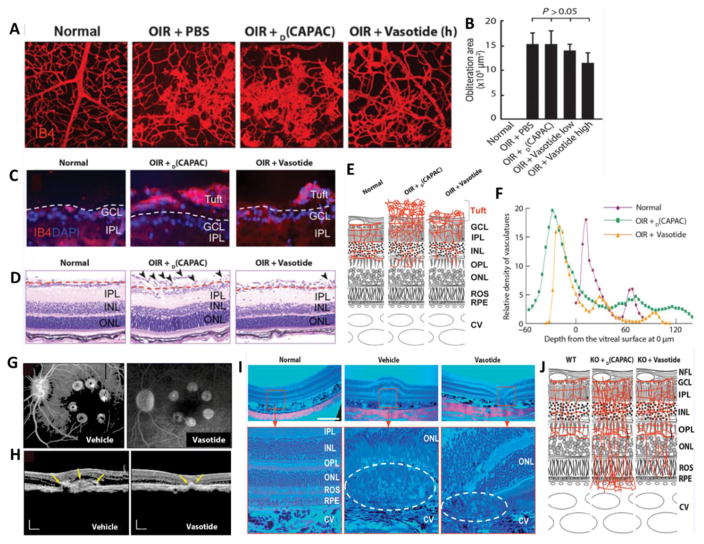
Recently, Vasotide, D(Cys-Leu-Pro-Arg-Cys) (a small cyclic retro-inverted peptidomimetic) developed by Sidman and his co-authors has demonstrated to uniquely block VEGF from binding to two different endothelial receptor molecules i.e. VEGF receptor-1 (VEGFR-1) and neuropilin-1(NRP-1) thus inhibiting retinal angiogenesis. While, VEGFR-1 is known to bind to VEGF ligands: VEGF-A, VEGF-B, and PlGF; NRP-1 modulates several VEGF isoforms including PIGF. Vasotide delivery through eye drops or intraperitoneal injection in three different animal models (a monkey model of human wet AMD, a mouse model of retinal angiomatous proliferation, and a mouse model of retinopathy of prematurity) have demonstrated effectiveness by inhibiting retinal angiogenesis. Such potential of Vasotide peptide in binding two important VEGFRs and at the same time blocking additional mechanisms holds promise for further translation into safer, less-invasive applications in retinal disorders (Fig. 4)[25]. Current anti-VEGF therapies are approved for neovascular (wet) AMD and diabetic macular edema (DME). However, these therapies are often used off-label for other ocular complications including corneal neovascularization and neovascular glaucoma. It has to be taken into consideration that such anti-VEGF therapies are not recommended by FDA to treat diseases such as central serous retinopathy and polypoidal choroidal vasculopathy where VEGF suppression is not the target. In addition, long-term or continuous blocking of VEGF may cause retinal atrophy and/or prevent normal vascular formation, which still remain unanswered.
Fig. 4.
(A) Effect of intraperitoneally injected therapeutic Vasotide peptide on the vasculature and tuft formation in 19 days old (P19) normal and oxygen-induced retinopathy (OIR) mice in comparison to PBS and control D(CAPAC) peptide; (B) Quantitation of tuft areas in wild-type (WT) mice given treatment groups as eye drops; (C) Cryostat sections of vascular tufts extending from the retina into the vitreous with IB4-stained vessels in red and DAPI (4′,6-diamidino-2-phenylindole) counterstained nuclei in blue at P19. Pathological tuft formation is shown above the dashed lines, and reduced vessel formation within the inner retina is shown below the dashed lines; (D) Paraffin sections showing tuft formation above dashed lines and retinal layers below dashed lines; (E) Diagram of the vasculature in different regions of the retina; (F) Quantitation of percent blood vessel area at 4-mm intervals summed through the full retina on a relative scale; (G) 6-min fluorescein angiograms for monkeys treated with eye drops at 29 days after laser-induced photocoagulation; (H) OCT images from monkeys given eye drops at 29 days after the laser-induced lesion. Yellow arrows indicate CNV complex boundaries; (I) H&E-stained monkey retinas at low (upper row) and high (lower row) magnifications showing eosin red–stained vacuolated fibroblast layer outside of the choroid in the upper row. Red boxes indicate macular region; dashed ovals indicate the RPE and ROS zones 29 days after laser-induced lesioning; (J) Diagram showing vascular differences in the retinas of WT mice and vldlr-null (KO)mice treated with treatment groups. NFL, nerve fiber layer; GCL, the ganglion cell layer; IPL, the inner plexiform layer; INL, the inner nuclear layer, OPL, the outer plexiform layer; ONL, outer nuclear layer; ROS, rod (and cone) outer segments; RPE, the retinal pigment epithelium; CV, choroidal vessels. Reprinted from [25].
2.2. Anti-TNF-α agents
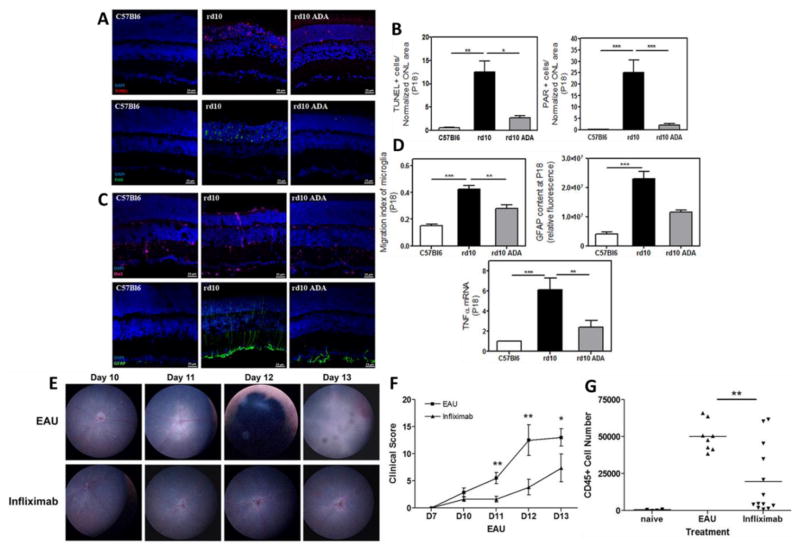
Till date, a number of anti-tumor necrosis factor alpha (TNF-α) agents have been approved by FDA for the treatment of rheumatoid arthritis, ankylosing spondylitis, and psoriasis arthritis. Among these, Adalimumab (Humira®), a recombinant human IgG1 monoclonal antibody received FDA approval recently (July,2016) for the treatment of non-infectious intermediate, posterior, and panuveitis[26]. TNF-α plays an important role in the pathogenesis of inflammatory, edematous, neovascular, and neurodegenerative diseases [27]. In addition, there is increasing evidence of TNF-α involvement in the pathogenesis of experimental retinal neovascularization, proliferative vitreoretinopathy, and macular edema [28–30]. Adalimumab specifically binds to TNF-α and prevents its binding to TNF-α receptors (TNFR) thus blocking inflammatory responses. Fig. 5 depicts effects of anti-TNF-α agents (Adalimumab & infliximab) in treating retinal degeneration and ocular inflammation respectively. There are now enough evidences suggesting the important role of anti-TNF-α therapy in the management of ocular complications specially uveitis[31]. Although, increased risk of serious infections, malignancies and high cost are few drawbacks of such anti-TNF-α therapies [32], further development in delivery strategies for TNF-α blockers in treating diseases of the choroid, retina and macula may hold promise in improving vision and quality of life.
Fig. 5.
(A) Photomicrographs of retinal sections showing significant reduction in photoreceptor cell death in the murine model of human autosomal recessive retinitis pigmentosa, the rd10 mice at postnatal day (P) 18 after Adalimumab (ADA) treatment in comparison to control (C57Bl6) (TUNEL-stained sections revealing dead photoreceptors and PAR content in DAPI-counterstained sections); (B) Bar graph illustrating the effect of ADA on the number of TUNEL-positive nuclei and nuclear poly (ADP) ribose (PAR)-positive cells; (C) Photomicrographs of retinal sections showing reactive gliosis amelioration by ADA in the rd10 mouse retina at P18 (Iba1-labelling to visualize microglial cells and GFAP content in DAPI-counterstained sections); (D) Bar graphs illustrating the effect of ADA on migration index of microglia, the corrected fluorescence of GFAP content and TNFα gene expression; (E) Topical endoscopic fundal imaging (TEFI) images showing intravitreal administration of infliximab suppresses experimental autoimmune uveitis (EAU) in comparison to vehicle control; (F) combined total disease scores demonstrating the difference in clinical disease progression between treatment groups. In EAU control eyes typical disease progression with signs of raised optic disc, vasculitis and severe inflammation; In infliximab treated eyes, only raised optic disc and initial signs of vasculitis are evident; (G) Graph showing total CD45+ infiltrate numbers from individual eyes. Reprinted from [33, 34].
2.3. GLP-1 agonists
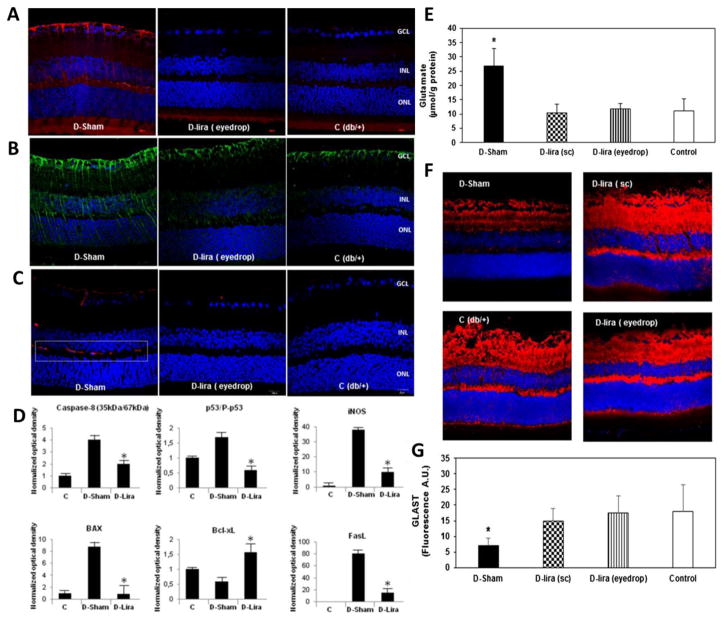
Exenatide (Byetta®/Bydureon®), Liraglutide (Victoza®/Saxenda®), albiglutide (Tanzeum®) and Dulaglutide (Trulicity®) are FDA approved glucagon-like peptide-1 (GLP-1) agonists indicated for the treatment of diabetes mellitus type 2. GLP-1 agonists bind to the glucagon-like peptide 1 receptor (GLP1R) to activate the adenylyl cyclase pathway resulting in increased insulin synthesis and release. GLP1R is highly expressed in pancreatic beta cells and the brain. Retina, being an ontogenetically brain-derived tissue is anticipated to express GLP1R [35]. Recently, Hernandez and co-authors reported abundant expression of GLP1R in human and nonketotic diabetes mice retinas. Retinal degeneration can be treated with systemic administration of liraglutide which was evident from significant reduction in extracellular glutamate levels and increase in prosurvival signaling pathways (Fig. 6). In addition, similar neuroprotective effect was demonstrated after topical administration of native GLP-1 and other GLP-1R agonists without any reduction in blood glucose levels. Such GLP1R expression and activation may open up new approaches for preventing or arresting retinal neurodegeneration with GLP-1 agonists in early stages of diabetic retinopathy [36].
Fig. 6.
Immunofluorescence images from a diabetic mouse (D) after topical administration of GLP-1R agonist, liraglutide (D-lira eye drop) in comparison to vehicle (D-Sham) and a non-diabetic mouse (control, C; db/+). D-lira prevented disruption of the BRB and thus release of VEGF (red) (A), IL-1b (green) (B) and albumin (red) (C), most important players in the pathogenesis of the breakdown of the BRB; (D) Western blotting quantification of proteins from apoptotic (caspase 8, Bax, p53), antiapoptotic (BclxL) and neuroinflammatory (iNOS, FasL) signaling pathways; (E) Retinal concentration of glutamate measured by high-performance liquid chromatography after subcutaneous administration of treatment groups; (F) Comparison of glutamate/aspartate transporter (GLAST) immunofluorescence (red) after topical administration of treatment groups; (G) Quantification of GLAST immunofluorescence in arbitrary units (A.U.). Reprinted from [36].
2.4. Next generation protein and peptide based therapies
Significant developments in protein and peptide based therapies have recently led a number of biologics to enter into clinical trials. For instance, Abicipar pegol (previously AGN-150998 or MP0112, Molecular partners and Allergan) is a genetically engineered mimetic antibody derived from designed ankyrin repeat protein (DARPin®) family. Abicipar is a long-acting mono-DARPin® that binds to all VEGF-A isoforms with high specificity and affinity, thus adding to its good molecular stability, tissue penetration and ease of manufacturing [37]. Abicipar has successfully completed Phase I/IIb clinical trials in wet AMD and DME and is currently recruiting participants for Phase 3 studies (NCT02462928)[38]. Brolucizumab (Alcon Laboratories Inc.), a humanized single-chain variable fragment that binds to all isoforms of VEGF-A with high affinity has completed Phase II clinical trials in wet AMD.
Pegpleranib (Fovista®, Ophthotec) is an anti-platelet-derived growth factor (anti-PDGF) agent that binds to PDGF-BB and prevents PDGF binding to PDGF-β receptors on pericytes, leading to their death via interruption of cell survival signals [39]. Fovista® is currently undergoing Phase 3 clinical trials for the treatment of wet AMD (NCT01940887). Rinucumab (Regeneron), a monoclonal antibody, binds to the PDGF-β receptor, thus prevents the action of PDGF. Rinucumab is in Phase II clinical trial and is being employed in combination with aflibercept in a co-formulated single injection for wet AMD.
Nesvacumab (Regeneron), a monoclonal antibody against angiopoietin-2 (ANG-2) is currently in a Phase II clinical trial and is indicated in combination with aflibercept in a co-formulated single injection for wet AMD. RG7716 (Hoffmann-La Roche), a bispecific antibody that binds both VEGF A and ANG 2 is in Phase II study. It is currently indicated as combination therapy along with Lucentis® for patients with wet AMD (NCT02484690).
Zimura® (Ophthotec), a chemically synthesized anti-C5 aptamer that inhibits complement factor 5 (C5), which is the fifth component participating in cellular inflammatory process. It is in Phase II study for geographic atrophy secondary to dry AMD (NCT02686658), and a combination therapy with Lucentis® has recently completed Phase IIa clinical trials for wet AMD. HI-con1 (Iconic Pharmaceuticals) is a human fusion immunoprotein consisting of two human factor VII as the targeting domains fused to IgG Fc as an effector domain. This chimeric protein binds to tissue factor (TF) with the factor VII component, while the IgG component triggers destruction of the neovascular lesion. It is currently undergoing Phase II study as a monotherapy and/or in combination with Lucentis®. Opt-302 (Opthea) is another fusion protein that binds VEGF-C and VEGF-D, blocking their interaction with VEGFR-2 and VEGFR-3. Opt-302 is also in a Phase I/IIA trial for wet AMD. Apart from these, POT-4 (Alcon, Phase I), Eculizumab (Alexion, Phase II), LFG316 (Novartis, Phase II), FCFD4514S (Genentech, Phase II), Sonepcizumab (Lpath, Phase II), Glatiramer acetate (Teva, Phase II/III), RN6G (Pfizer, Phase II), Daclizumab (Hoffman-La Roche, Phase II) and Infliximab (Janssen, Phase II) are under various stages in clinical trials. Several other protein and peptide based therapeutics are under development [40]. Table 2. lists some of the proteins and peptides currently in clinical trials.
Table 2.
Proteins and peptides currently in clinical trials (Adapted and modified [41])
| Protein/peptide drug | Description | MW (kDa) | Target/activity | Half-life | Company | Current indication | Phase | Ref. |
|---|---|---|---|---|---|---|---|---|
| AGN-150998 (Abiciparpegol) | Recombinant ankyrin repeat protein | 34 | VEGF-A | ~2 weeks | Allergan | Wet AMD | Phase III | NCT02462486; NCT02462928 |
| ALG-1001 (Luminate®) | Integrin peptide | 1 | Integrin receptors | ~3 months | Allegro Ophthalmics | DME, NPDR | Phase II | NCT02348918 |
| Conbercept (Lumitin®) | Recombinant fusion protein | 143 | VEGF-A/B, PGF-1 | ~1 week | Chengdu-Kanghong | Wet AMD, DME | Chinese FDA Phase III/Phase II | NCT01809236 |
| GSK933776 | Anti-amyloid β antibody | NA | Amyloid β fibrils | ~12 days | GlaxoSmithKline | Dry AMD | Phase II | NCT01342926 |
| ISONEP™ (Sphingomab™) | Humanized antibody | ~49 | S1P | More than 4 days | Lpath | Wet AMD | Phase II | NCT01414153 |
| Lampalizumab | Antigen-binding fragment of a humanized monoclonal antibody | 47 | CFD | ~6 days | Roche | Geographic atrophy secondary to AMD | Phase III | NCT02247479 |
| RN6G (PF-4382923) | Anti-amyloid β antibody | NA | Amyloid β fibrils | NA | Pfizer | Dry AMD | Phase II | NCT01003691 |
|
RTH258 ESBA1008 |
Antibody fragment | 26 | VEGF-A | ~5 days | Novartis AG | Wet AMD | Phase III | NCT02507388 |
| VGX-300 (OPT-302) | Recombinant fusion protein | NA | VEGF-C/D | NA | Circadian Opthea | Wet AMD | Phase I | NCT02543229 |
| HI-con1 | Recombinant fusion protein | NA | Tissue factor | NA | Iconic Therapeutics | AMD | Phase II | NCT02358889 |
| Zimura | Aptamer | ~50 | Complement factor C5 | Ophthotec Corporation | AMD, IPCV | Phase II/III |
NCT02686658 NCT02397954 |
NPDR, nonproliferative diabetic retinopathy; S1P, sphingosine 1-phosphate; IPCV, Idiopathic polypoidal choroidal vasculopathy
3. Proteins and peptides: challenges in ocular delivery
Proteins and peptides, a class of biopharmaceuticals poses significant challenges owing to their large size, poor permeation and susceptibility to degradation. The intrinsic properties associated with the complex macromolecular nature of proteins and peptides is often required for achieving high biological activity. However, such structural complexity also renders them as one of the most challenging class of therapeutics to be formulated and delivered. Low stability and short half-lives of peptides and especially protein drugs at physiological pH and temperature or during storage leads to loss of activity, thus putting significant burden on formulation technologies.
3.1. Adverse physicochemical properties of proteins and peptides
3.1.1. Hydrophilicity
Most of the therapeutic proteins and peptides are highly hydrophilic (log P<0) which hinder their permeability across biological membranes. Bioavailability of proteins and peptides depends on their ability to cross these membranes. Poor membrane permeation of macromolecules often embodies added challenge in development of protein and peptide based drug formulations to intracellular target sites. The lipophilic nature of biological membranes restrict these macromolecules from spontaneously entering cells. The absorption of these macromolecules is not governed by simple diffusion or passive absorption. Rather active transport which involves binding to specific receptor, pinocytocis or endocytosis are the major mechanisms responsible for absorption [42, 43]. Permeation of hydrophilic molecules is hindered by the tight junctions present in the cornea and the lipophilic nature of the corneal epithelium [44, 45] whereas hydrophobic molecules permeate corneal epithelium easily. Additionally, the collagen fibers present in the hydrophilic stroma may impede penetration of hydrophobic drugs to some extent. Under certain circumstances, small peptides or even small particles are taken from the extracellular space into cells by an active transport mechanism known as receptor-mediated endocytosis [60]. One of the major disadvantages of proteins and peptides entering into the cell via endocytic pathway is their entrapment into the endosomes and eventually in lysosomes, where majority of the degradation processes undergoes by the action of lysosomal enzymes. This leads to only a small fraction of unaffected proteins/peptides appearing in the cytoplasm. So far, multiple and partially successful attempts have been made to deliver protein and peptide based biopharmaceuticals directly into the cell cytoplasm bypassing the endocytic pathway. Mechanical delivery methods like microinjection and electroporation have been used for decades for cell cytoplasm delivery, but are low-throughput and invasive and require specialized equipment to physically puncture membranes. The delivery of biologics via most favored “oral route” is highly challenging due to GI mucosa and degradative acidic environment. A large fraction of approved and investigational protein and peptide molecules are administered via parenteral routes (IV, IM or SC), intravitreal and sub conjunctival injections. However, non-targeted delivery of protein and peptide based formulations may lead to distribution into normal tissues requiring large quantities of drug administration, which is often not economical and sometimes complicated owing to non-specific toxicity.
3.1.2. Large molecular weight
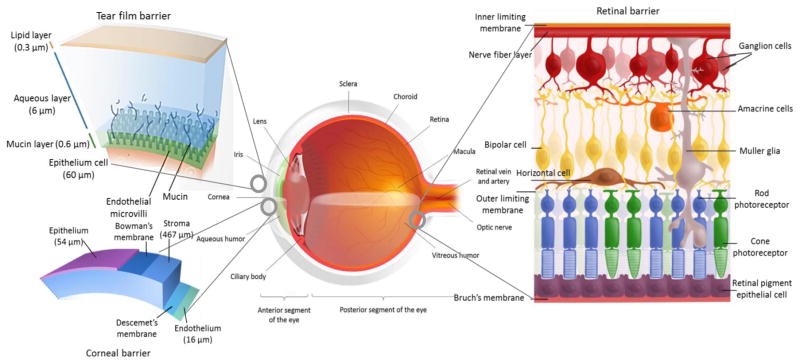
Another major challenge for the delivery of protein and peptide based drugs is their high molecular weight and poor membrane permeability across ocular tissues and barriers. Such challenges have promoted highly invasive intravitreal injection as the primary mode administration for protein and peptide based drugs. The molecular weights of peptides and proteins are generally > 1000 Da with large hydrogen bonding donor/ acceptor groups [46]. Such large size of macromolecules limits diffusion and renders patient compliant topical treatment highly inefficient. The cornea, sclera and retina have tight junctions that significantly limits diffusion of hydrophilic large molecules [47, 48]. The tight junctional space of conjunctival epithelium is generally wider than cornea, but still insufficient for the penetration of these large molecules [5, 49]. The human retina limits the diffusion of molecules greater than 76 kDa due to the inner and outer plexform layers. Macromolecules greater than 150 kDa fail to reach the inner retina [47]. Additionally, choriocapillaries may wash out the molecules that traverse through choroid thus reducing therapeutic concentrations. The ocular anatomy and tissue barriers are shown in Fig. 7.
Fig. 7.
Ocular anatomy and tissue barriers. Reprinted and modified from [50].
3.1.3. Metabolic instability
Proteins and peptides also suffer from a number of physical, chemical and biological instability issues due to their complex secondary, tertiary and quaternary structures. Various physical degradation pathways are involved in the instability of proteins and peptides including denaturation, adsorption, aggregation and precipitation. Moreover, conformational transformation of proteins to inactive forms occur due to pH, temperature, high salt concentration; dissociation of subunit proteins; complexation of enzymes and cofactors; non-covalent complexation with ions, proteolytic degradation under the influence of esterases and proteases; chemical modifications by different compounds (for instance oxidation of SH-groups in sulfhydryl containing enzymes and Fe (II) atoms in heme containing proteins; thiol-disulfide exchange and destruction of labile side-chains of tryptophan and methionine) may also lead to inactivation of various biologically active protein and peptide based drugs in ocular tissues [11].
In the body, the chemical degradation pathways of peptides and proteins include deamidation, oxidation and reduction, proteolysis, disulfide exchange and β-elimination [63]. Any alteration in “active” confirmation may lead to loss of activity and irreversible aggregation of proteins. Vulnerability towards enzymatic degradation under in vivo condition results into shorter half-lives even with parenteral administration. Inside the vitreous humor the half-life of large molecule tends to be in the range of days to weeks [64]. Such short half-lives of proteins require frequent parenteral administrations to maintain therapeutic levels. Frequent parenteral administrations are not patient compliant and/or well tolerated and are often associated with complications including cataract, retinal hemorrhage and detachment [65]. For instance, the average apparent plasma half-life of pegaptanib is 10 days after 3 mg dose whereas ranibizumab remains for 2.88 days in rabbit. Half-life of bevacizumab is 4.32 days with maximum concentration 162 μg/ml in vitreous cavity [66]. In AMD, the vitreous elimination of ranizumab is just 9 days and intrinsic systemic elimination half-life is 2 hours followed by multiple intravitreal injection dose of 0.3–2.0 mg/eye biweekly or monthly [17].
3.2. Challenges in designing protein and peptide based ocular formulations
The formulation of protein and peptide based biotherapeutics poses unique challenges that are not often experienced with small molecules. Overcoming the instability of protein and peptide based agents due to structural properties and environmental factors is one of the key challenges in the development of formulations. Several agents have been incorporated including small sugars (e.g. trehalose) and polysaccharides (e.g. dextrans) to enhance the stability of protein and peptide based biopharmaceuticals [67, 68]. Pluronics and non-ionic surfactants such as polysorbates at low concentrations are widely applied to decrease protein and peptide aggregation [69].
Another major drawback of biopharmaceutical drug formulations is high and variable viscosity. For topical ophthalmic formulations, corneal contact time is longer with increase in viscosity of formulations up to 20 centipoise (cP) [70]. However, a further increase in viscosity leads to reflex tearing and blinking in order to regain the original viscosity of the lacrimal fluid (1.05–5.97 cP). With a rise in clinical application of monoclonal antibodies, the need for high protein doses (concentrated formulations) is often crucial. The FDA does not permit the intravitreal injection of large volumes of drug formulations in patients with ocular diseases. Such requirements render formulation of protein and peptide based biopharmaceuticals very difficult as solutions with high protein content are exceedingly viscous. High viscosity of protein and peptide based biopharmaceuticals also largely affects the syringeability (time required to complete the injection) as well as the force required to deliver the solution with appropriate needles (18 mm in length, 27–30G) [10]. Thus, approaches to achieve lower viscosity formulations with hydrophobic/inorganic salts or lysine and arginine may be useful.
It is also important for protein and peptide based biopharmaceutical formulations to have the same pH as the lacrimal fluid to achieve maximum activity. However, proteins and peptides are often not stable at physiological pH leading to their folding and aggregation. Additionally, the buffer capacity of such formulations is of equal importance for proper preservation. Although, the buffering action of the tears is capable of neutralizing the effect of topically applied biopharmaceutical formulations[71], intraocular hyperosmotic solutions have been reported to elicit transient desiccation of the anterior chamber tissues while hypotonic solutions may cause edema leading to corneal clouding[72]. For this reason, pH of such formulations are compromised and maintained by buffers to achieve maximum activity and maintain stability[73]. The effect of buffers on tonicity should also be taken into account considering the permissible limits of osmolarity for ophthalmic formulations (171–1711 mOsm/kg). Although many of these agents utilized for maintaining the stability and activity of such protein and peptide based biopharmaceutical formulations have been proven to be effective, their use requires careful consideration in terms of local toxicity and potential immunogenicity.
A better understanding of the viscosities of biological solutions, characteristics of nascent proteins and peptides, dynamics and behavior of protein and peptide based topical and injectable formulations is crucial. Towards this goal, utilization of chemical chaperones to inhibit protein misfolding as well as reactivate non-native protein structures[74, 75]; co-administration of recombinant human hyaluronidase with drug to degrade hyaluronic acid (a key structural component of tissues) to facilitate protein and peptide delivery may prove to be useful in addressing the issues poised by formulation challenges[76].
3.2.1. Recombinant human hyaluronidase: penetration enhancer
Hyaluronan (HA), a unique polyanionic and protein-free polysaccharide is highly expressed in the vitreous humor and is primarily responsible for increasing viscosity, expanding volume, and providing structural support to the vitreous body. However, the high viscosity of HA allows it to act as a molecular sieve, thus preventing the penetration of most biopharmaceutical formulations. The property of hyaluronidase (Hyal) to catalyze the degradation of HA have been exploited for decades to increase the penetration of biopharmaceutical drugs across ocular tissue barriers [77, 78].
Human Hyal-1(hHyal-1) is one of the five homologous hyaluronidases encoded in the human genome (Fig. 8A). It is highly expressed in most tissues and cleaves HA substrates of all sizes in a size-independent manner to tetrasaccharides. The amino acid residues present in the N-terminal of hHyal-1 exhibit 31% sequence identity with bee venom hyaluronidase (bvHyal), whose structure has been shown in complex with a HA tetrasaccharide (Figs. 8B and C). In addition, the EGF domains present in Hyal-1 are thought to mediate protein-protein interactions often associated with regulation of growth and development [79]. However, with the development of recombinant human hyaluronidase, some of the significant limitations including immune reactivity with bovine hyaluronidase and lack of catalytic activity at neutral pH with hHyal-1 have been addressed. Such developments have led to approval of HYQVIA (Baxter International Inc.), containing immune globulin infusion 10% (Human) with recombinant human hyaluronidase for adult patients with primary immunodeficiency. PEGylated recombinant human hyaluronidase are currently undergoing Phase III clinical trials in combination with paclitaxel and gemcitabine for treating pancreatic ductal adenocarcinoma (NCT02715804). rHuPH20, another purified form of the recombinant human hyaluronidase has shown promise in elevating dexamethasone levels in ocular tissues (choroid and retina) and the serum [80]. It is currently undergoing Phase I clinical trial for multiple myeloma (NCT02519452) [81]. Although inhibiting a key stromal component such as HA might cause some immunogenic reactions in the body, such potential of recombinant human hyaluronidase in facilitating drug delivery holds promise in the development of protein and peptide based ocular formulations.
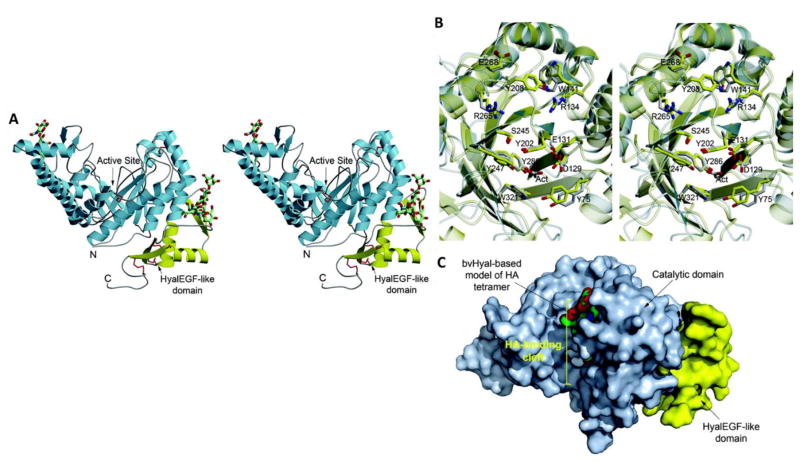
Fig. 8.
(A) Structure of hHyal-1: Stereoscopic representation of a side view. The catalytic and the HyalEGF-like domains are colored light blue and yellow respectively. Disulfide bonds are shown in red. N-linked oligosaccharides are shown as stick models with the atomic color scheme: gray, carbon; red, oxygen; blue, nitrogen; (B) Stereoscopic representation of the active site region of hHyal-1 (gray ribbon) superimposed on that of bvHyal (yellow ribbon). Selected amino acids are colored in the atomic color scheme: red, oxygen; blue, nitrogen; gray (hHyal-1) and yellow (bvHyal), carbon. (C) Molecular surface of the catalytic domain (light blue) and HyalEGF-like (yellow) domains of hHyal-1, illustrating the separation between the HyalEGF-like domain and the active site. A docked tetrasaccharide, inferred from the structure of bvHyal, is shown as a space filling model. Reprinted from [79].
3.2.2. Chemical chaperones: protein aggregation inhibitor
Protein aggregation has remained as one of the primary concerns in the formulation of protein and peptide based biopharmaceuticals for ocular diseases. Previously, several small molecules have been identified for modifying or inhibiting protein aggregation. A novel strategy developed by Sanders et al. utilizes chemical chaperones to inhibit protein misfolding by (kinetic) stabilization and/or inhibit the self-assembly of aggregation-prone sequences of the native protein structures (Fig. 9A)[75].
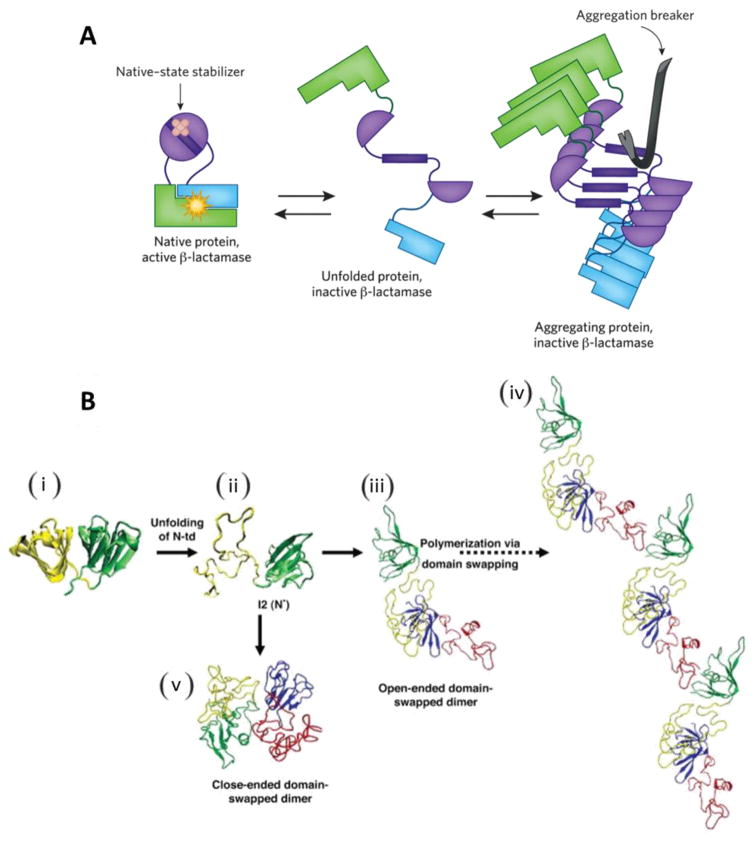
Fig. 9.
(A) Functioning of a chemical chaperone: β-lactamase function is restored when aggregation of the target protein is inhibited. This can occur either through stabilization of the native structure (left) or through inhibition of the process of amyloid self-assembly (right). Reprinted from [86]. (B) Schematic summary of human γD-crystallin (a member of crystallin families) polymerization. (i) Crystal structure of human γD-crystallin. (ii) Simulated monomeric aggregation precursor (I2), often referred as N* in the general mechanism of protein aggregation in literature. (iii) Simulated structure of open-ended domain-swapped dimer. (iv) Simulated structure of close-ended domain-swapped dimer. (v) Model of human γD-crystallin hexamer formed via domain swapping. Reprinted from [84].
In addition, there is growing evidence that several ocular diseases including cataract compromises the folding of the endogenous proteome by sequestering chaperones and chaperonins leading to intra-cytoplasmic aggregation of proteins involved in critical cellular processes [82]. It has been reported that crystallins, constitute 90% of the total proteins in mature lens and undergoes covalent modifications and/or polymerization (Fig. 9B) causing destabilization and aggregation of lens proteins. α-Crystallin, a major chaperone system of mature lens cells recognizes and sequesters misfolded/unfolded conformers, reducing the accessibility of aggregation prone species [83, 84]. Therefore, the application of chaperones provides exciting opportunities for modulating protein aggregation in biopharmaceutical formulations as well as lowering protein aggregate-induced toxicity. Glycerol, 4-Phenylbutyric Acid Sodium Salt (PBA), Tauroursodeoxcholic acid (TUDCA) and trimethylamine-N-oxide (TMAO) have gained wide application as chemical chaperones. Apart from these small molecules, endogenous molecular chaperones (e.g. heat shock proteins, Hsp) and pharmacoperones (e.g. nicotine) are being extensively exploited to promote folding of specific proteins [85].
4. Types of protein and peptide modifications
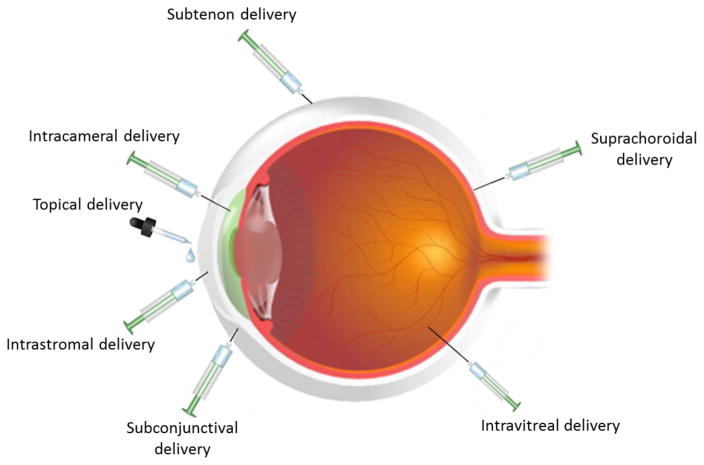
Several intraocular delivery techniques including intrastromal, intracameral, suprachoroidal and intravitreal injections have been explored as possible ways for the delivery of biopharmaceuticals across ocular barriers. Intravitreal injection is currently the most commonly used method for delivering proteins and peptides to the back of the eye. Regardless of the type of injection, most biopharmaceuticals are rapidly cleared from the ocular tissues through posterior transretinal and anterior aqueous humor elimination pathways [87]. Consequently frequent and repeated injections are required which impose a significant treatment burden on the patient, vision care providers, and a cumulative risk of adverse effects from each subsequent injection [88, 89]. Various strategies have been developed and summarized to overcome and address such challenges of proteins and peptides delivery in the next section:
4.1. Chemical modifications
Chemical modification with hydrophilic polymers is a useful strategy to improve the hydrodynamic diameter of the therapeutic constructs which can reduce clearance and promote circulating half-life to an attractive range. PEGylation is one such strategy that involves covalent attachment of a FDA approved polymer, polyethylene glycol (PEG) to a primary amino (-NH2) or sulfhydryl (-SH) groups of proteins or peptides. PEG chains of molecular weight ranging from 5–40 kDa have shown to improve biological activity of therapeutic proteins or peptides and reduce immune responses to a larger extent. Such developments have led to approval of several pegylated drugs in the market [90, 91]. Alternatives to PEG, the negative charge of sialic acid as well as the glycosaminoglycan HA and hydroxyl ethyl starch also holds potential in prolonging half-lives of proteins and peptides and are currently under clinical investigation[92].
4.2. Genetic engineering based modifications
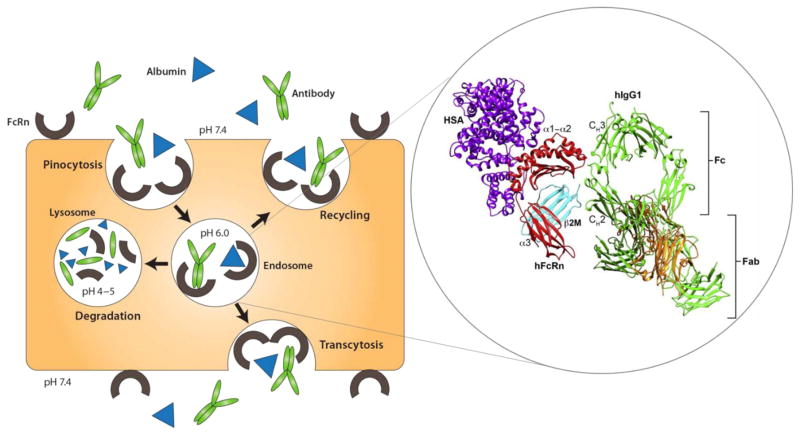
The neonatal Fc receptor (FcRn) is a unique protein encoded by the Fc fragment of IgG receptor and transporter (FCGRT) gene in human. It is similar in structure to the major histocompatibility complex (MHC) class of molecules. FcRn’s exceptional ability to protect IgG and albumin from catabolism (Fig. 10) has guided development of novel genetic fusion based biopharmaceuticals. With higher expression of FcRn in various ocular tissues including corneal epithelium and endothelium, lens epithelium, retinal blood vessel, conjunctiva lymphatic vessel, nonpigmented ciliary epithelium, ciliary blood vessel, iris blood vessel, and optic nerve, approaches to exploit FcRn pathway can be extended to improve circulating time and half-lives of various therapeutic proteins and peptides for ocular delivery [93, 94]. So far only a few approaches to modulate IgG- and albumin-FcRn interactions have been reported. They involve mutations of Fc-domain amino acid residues in the proximity to the FcRn binding site and engineering the IgG– and albumin- FcRn interactions to increase antibody/albumin half-lives [95]. Zalevsky and co-authors demonstrated mutations of two amino acid residues in the human IgG1 VEGF antibody bevacizumab resulted in a ~11-fold improvement in the affinity for human FcRn at pH 6 (Fig. 11) [96]. In addition, bispecific antibodies (bsAbs) co-targeting the PDGF and VEGF pathways to enhance the treatment of AMD have led to tailoring of antibody-like proteins for specific needs [97]. However, the immunogenicity, processing and manufacturability of these bsAbs continue to be a major hurdle for clinical approval. Although, a number of fusion proteins have been recently approved by FDA for various indications and some are undergoing clinical trials, none have been approved for ocular indications.
Fig. 10.
Structure of human FcRn in contact with human IgG1 (hIgG1) and human serum albumin (HAS) and FcRn-mediated recycling of IgG and albumin in vascular endothelial cells; IgG and albumin are internalized into vascular endothelial cells through pinocytosis. The pH of the endosome is 6.0, facilitating association with membrane-bound FcRn. The contents of endosomes can be processed in one of two ways: either recycling back to the apical cell membrane or transcytosis from the apical to the basolateral side. In the case of saturated receptors, excess IgG and albumin are degraded by lysosomes. Top, apical side; bottom, basolateral side. Reprinted and modified from [95].
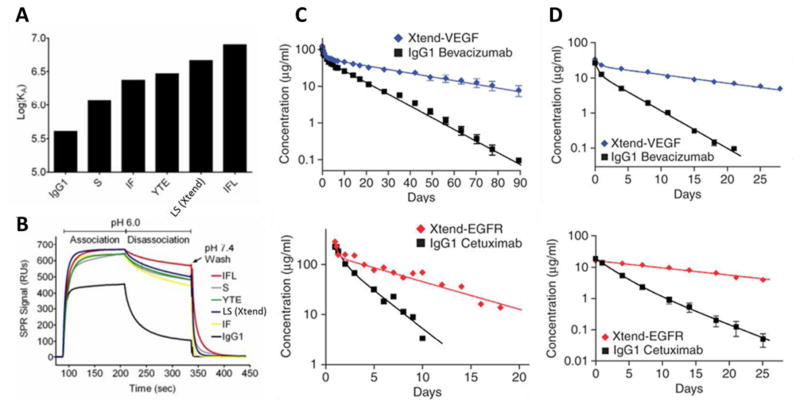
Fig. 11.
(A) The log of the equilibrium association constant KA at pH 6.0 are plotted for various engineered anti-VEGF (bevacizumab) variants demonstrating increased binding to human FcRn in contrast to parent bevacizumab native IgG1 antibody; (B) Binding sensorgrams at pH 6.0 and 7.4 of each variant; Log-linear changes in serum concentrations for anti-VEGF (bevacizumab) and anti-EGFR antibodies in cynomolgus monkeys (C) and hFcRn transgenic mice (D) demonstrating antibodies engineered for higher FcRn affinity (Xtend-VEGF and Xtend-EGFR) promotes half-life extension. Reprinted from [96].
Newer insights on protein and peptide modifications based on medicinal chemistry and structure-activity studies including use of hydroxyl-PEG as an alternative to widely used methoxy-PEG, supramolecular PEGylation of macromolecules for higher binding affinity [98], reversible pegylation to mitigate reduced potency and use of amphiphilic poly(2-oxazoline) polymers which provides better control of the molecular definition of biopharmaceuticals may offer improvements in the pharmacokinetics and potency of protein and peptide based biopharmaceuticals.
5. Routes of protein and peptide delivery to ocular tissues
Challenges to ocular delivery of biopharmaceuticals are noteworthy and considerable opportunities remain to be optimized for delivery approaches, formulation and processing conditions for each peptide and protein based therapeutics.
5.1. Systemic delivery
Oral administration and parenteral injections are typical methods employed to achieve systemic delivery. However, attempts to deliver large hydrophilic protein and peptide based biopharmaceuticals for ocular indications have seen limited success. The miniature size of the eye and presence of ocular barriers prevent ample drug partitioning into the eye. Furthermore, dilution effect of the systemic blood volume, first-pass metabolism by the liver and clearance by kidney require larger drug doses which can result in high costs, systemic side-effects and possible toxicity.
The integrity of ocular barriers seems to play a major role in the penetration of biopharmaceuticals. A study in a clinical set-up showed an increase in visual acuity by 14 letters after treatment with 3 doses of systemic bevacizumab (5mg/kg) in patients with classic choroidal neovascularization (CNV) probably facilitated by the compromised RPE layer [99]. Rohrer and co-authors also reported reduced CNV size and preserved retinal function after intravenous administration of fusion protein CR2-fH (where CR2 is complement receptor 2 and fH is factor H) indicating CR2-fH accesses the site of CNV by way of the impaired BRB. CR2-fH plays a critical role in regulating the inflammatory responses by inhibiting complement activation products in AMD [100, 101]. Although, no serious ocular or systemic side effects were observed in both the cases, high concentration of injected drug or fusion proteins should be taken into consideration. Such shortcomings preclude systemic administration of protein and peptide based biopharmaceuticals for ocular delivery expensive and rare.
5.2. Extraocular delivery
5.2.1. Topical delivery
Topical application of ophthalmic drops has been the method of choice for administering pharmaceutical agents for the treatment of diseases perturbing the ocular surface and/or the anterior segment including dry eye syndrome, conjunctivitis and keratitis. This route has been extensively utilized clinically for the treatment of diseases affecting cornea, conjunctiva, sclera, iris, ciliary body and aqueous humor. However, the limited lacrimal capacity and constant tear drainage from precorneal area leads to wash out of a majority of eye drop within few seconds. Additionally, only a few experimental studies have demonstrated their efficacy for posterior segment diseases. The properties of corneal barriers allow significant passage of moderately lipophilic small molecules, whereas highly hydrophilic large molecular weight biopharmaceuticals undergo restricted permeation generating insufficient concentrations for therapy. Nomoto and co-authors demonstrated the incompetence of topical bevacizumab to reach therapeutic concentrations in the iris, choroid, retina and vitreous of rabbits even after aggressive dosing of 1.25mg/0.05mL six times daily for a week [102]. In another study, topical administration of bevacizumab (10mg/kg, 3 times for 7 days) in mice did not generate any appreciable concentrations into the healthy corneal stroma [103]. In a recent study, Moisseiev and group also failed to generate detectable drug levels in both aqueous and vitreous samples of human eyes after topical administration of bevacizumab (25mg/mL, four drops with 10 minutes interval) [19]. In contrast, Hernandez and coworkers provided the first evidence that somatostatin (SST) eye drops reached the retina not through the cornea but by the trans-scleral route. Such topical administration of SST prevented retinal neurodegeneration in streptozotocin induced diabetes mellitus (STZ-DM) rats and opened up new preventive pharmacological strategy targeted to early stages of DR. [104].
5.2.2. Periocular delivery
5.2.2.1. Subconjunctival delivery
Periocular delivery is frequently achieved through an injection into the subconjunctival area i.e. space underneath the conjunctiva. An injection rooted into the bulbar conjunctiva and superficial to the sclera may provide a way to directly deliver therapeutics into the subconjunctival space. Subconjunctival routes can be used for sustained delivery since a depot can be formed in the space that can expand and accommodate up to 500 μL volume. However, drugs injected into the subconjunctival space are often rapidly cleared via conjunctival blood and lymphatic flow. In addition, pore diameter and intracellular spaces of scleral fiber matrix regulate drug permeation to a large extent. Longer in vivo t1/2 in the iris/ciliary body and retina/ choroid after subconjunctival injection of bevacizumab relative to intravitreal injection may possibly be attributed to binding with negatively charged scleral proteoglycans [102]. In another in vivo study, high bevacizumab concentration was detected in the whole cornea post 24 hours subcutaneous injection which remained almost unchanged in all layers of stroma over the next 14 days [103]. Various drug delivery technologies including microparticles/nanoparticles may be combined with physical techniques such as ultrasound and iontophoresis to achieve therapeutic concentrations of protein and peptide based biopharmaceuticals following periocular administration [105, 106].
5.2.2.2. Sub-tenon delivery
Sub-tenon route is widely utilized for administering anesthetics during ocular surgery. It involves the injection of drug into a fibrous membrane, called tenon’s capsule which along with the sclera binds the sub-tenon space. Although upto 4 mL of drug formulation could be injected through this route, administration complications including pain, chemosis, subconjunctival hemorrhage, retrobulbar and/or orbital hemorrhage, optic nerve damage, retinal ischemia, orbital swelling and rectus muscle dysfunction limit its use for the delivery of protein and peptide based biopharmaceuticals [107, 108]. In patients with clinically significant macular edema, sub-tenon’s injection of bevacizumab (2.5 mg in 0.1 mm volume) resulted in significant short-term visual improvement in eyes [109]. Thus, sub-tenon’s injection may serve as an alternative to intravitreal injection for ocular delivery of biopharmaceuticals.
5.3. Intraocular delivery
Intraocular delivery techniques involve direct delivery of therapeutic agents to the target site thus reducing the distance traversed by the drug to generate higher local drug concentrations, reduced off-target effects and bypassing various ocular barriers to improve ocular drug bioavailability.
5.3.1. Intrastromal delivery
Intrastromal administration entails direct drug delivery into the corneal stroma to overcome the corneal epithelial barrier along with tear fluid drainage. The densely packed collagen fibrils and proteoglycans hinder the diffusion of proteins and peptides inside the corneal stromal structure allowing it to serve as a reservoir for large hydrophilic biopharmaceuticals. Hashemian and co-authors reported intrastromal injection of bevacizumab (2.5 mg/1 mL) using a hypodermic needle led to regression of corneal stromal vascularization in a patient [110]. Recently, in vivo studies by Kim and group have demonstrated corneal vascular regression after intrastromal administration of bevacizumab (4.4 μg) with microneedles (MNs) [111]. These studies further confirm intrastromal delivery as an attractive modality for delivering biopharmaceuticals directly into the cornea.
5.3.2. Intracameral delivery
Intracameral delivery is intended to place the drug solution directly into the anterior segment of the eye. Although, intracameral injection has been extensively explored to improve delivery of biopharmaceuticals to both the anterior as well as posterior segments of the eye, it has not been possible to achieve therapeutic drug concentrations in the posterior segment of the eye following intracameral administration. However, intracameral administration of antibiotic prophylaxis for cataract surgery to prevent endopthalmitis [112, 113] and antifungal agents for deep corneal infections such as fungal keratitis [114] is widely used to deliver drugs to the anterior segment of the eye. Additionally, a combination of intrastromal and intracameral injections has recently shown to be effective in reducing fungal mass not only in the anterior segment but also in the corneal stroma where fungal invasion may lead to corneal perforation [115].
Several in vitro and in vivo studies have demonstrated the effectiveness of intracameral bevacizumab in treating neovascularization with no effects on corneal endothelial cells or thickness [116–118]. Patients with neovascular glaucoma and iris rubeosis have also responded well to the intracameral bevacizumab therapy and did not show any morphological changes of corneal endothelial cells [119–121]. Intracameral injection of bevacizumab loaded polymeric delivery systems may sustain drug release into the anterior segment [122]. However, repeated injections to maintain therapeutic concentrations over prolonged time period and sparse degradation of polymers may obstruct the aqueous flow, thereby elevating intraocular pressure and risk of ocular infections.
5.3.3. Intravitreal delivery
Intravitreal injection is the main modality for delivering biopharmaceuticals to the posterior segment of the eye to date. It is an invasive procedure that involves injection of a drug solution and/or suspension into the vitreous cavity in the center of the eye after penetrating through all layers of the ocular globe. The vitreous cavity can generally accommodate a volume of 20–100μL drug solution/suspension without resentfully altering the visual axis. However, various common complications including edopthalmitis, intraocular inflammation, retinal detachment, intraocular pressure elevation or glaucoma, ocular hemorrhage, floaters and cataract after intravitreal injections may lead to permanent vision loss if untreated.
Currently most of the biopharmaceuticals including pegatanib sodium, ranibizumab, aflibercept and bavcizumab indicated for neovascular or wet AMD are given as intravitreal injections. A comparative pharmacokinetic analysis revealed concentration (Cmax) of bevacizumab in retina/choroid after an intravitreal injection (1.25 mg/0.05 mL) to be ~317-fold higher than a subconjunctival injection at 1 week in rabbits [102]. Intravitreal injection of Avastin® generated significant bevacizumab concentrations in the retina, the retinal pigment epithelium, the choroid and particularly the photoreceptor outer segments in cynomolgus monkeys [123]. Although, biopharmaceutical drugs due to their large molecular weight tend to prevent immediate elimination from the vitreous unlike small molecules, their vitreous half-lives of just few days to weeks may not be sufficient to achieve long-term therapeutic effect. Therefore, novel delivery methods and/or long-term controlled release formulations for protein and peptide based biopharmaceuticals are warranted in order to significantly reduce complications caused by repeated injections.
5.3.4. Suprachoroidal delivery
It is often overlooked that the tissue site of action for most of the biopharmaceuticals is not the vitreous but the choroid and retina. Therefore, delivering drug directly in the target tissues (i.e., choroid and retina) may provide more effective therapy to chorioretinal diseases. Suprachoroidal injections, that involve the placement of a drug in the suprachoridal space (SCS), a conceivable space between the sclera and the choroid holds potential in achieving higher drug levels in target tissues. SCS can expand to accommodate a drug suspension or solution up to 1 mL [124]. Previously, SCS was accessed surgically with a scleral incision and insertion of a long cannula or hypodermic needle through the SCS. Such surgical interventions often lead to SCS collapse resulting from dislocation of the chorioretina and elevated hydrostatic pressure in the eye. Recent advancements in suprachoroidal delivery using MNs, has enabled higher local drug concentrations in the choroid with minimal side effects and least obstruction of the visual acuity. Nonetheless, high blood flow in choriocapillaries render the half-lives of small molecules and biopharmaceuticals in SCS in the order of few minutes to hours. In fact, sustained delivery systems (20 nm – 10 μm) are retained in the SCS for longer periods indicating the suitability of SCS injections [125, 126].
Several studies have demonstrated the effectiveness of suprachoroidal injections for localized delivery of therapeutics to the choroid-retina region [127]. Although, microcannula suprachoroidal injections have shown not to sustain the release of bevacizumab as superiorly as intravitreal injections post one week [128], MNs have demonstrated the potential in delivering bevacizumab (100 μl) to the SCS without any serious adverse effects as noted in Phase I clinical trials [129, 130]. Fig. 12 depicts current and emerging routes for protein and peptide delivery to ocular tissues.
Fig. 12.
Current and emerging routes for protein and peptide delivery to ocular tissues. Reprinted and modified from [50].
Nonetheless, inflammation which is a common side effect of ocular diseases including neovascularization significantly affects the integrity of corneal epithelium, choroid and the RPE layer. Such incompetent barrier function allows protein and peptide based biopharmaceuticals, that have limited access to the intact eye to gain significant access through the compromised barriers of inflamed eyes. Several studies to date have shown the effectiveness of systemic, intravitreal and SCS delivery in compromised tissues and confines compelling implications for other biological approaches in the treatment of ocular diseases. Some characteristics of various routes of administration for ocular drug delivery are provided in Table 4.
Table 4.
Characteristics of various routes of administration for ocular drug delivery
| ROUTES | NOTES |
|---|---|
| TOPICAL | |
| Drug entry pathways | Corneal, conjunctival, and scleral pathways. |
| Delivery barriers | Membrane barriers and elimination pathways on the eye surface, cornea, BRB, and tight junctions. |
| Elimination pathway | Tear wash out; nasolacrimal drainage; choroid, conjunctiva blood flow; lacrimation and blinking. |
| Advantages | High patient compliance; less systemic side effects; relatively easy and safe to administer. |
| Limitations | Small retention time of drug or dosage forms; blurring of vision; irritation; precorneal drug losses; drainage through the nasolacrimal duct; low bioavailability; limited volume of administration (approx. 30 μL); fast clearance from ocular surface; metabolism by tear enzymes; nonproductive uptake into systemic circulation via highly vascularized conjunctiva, choroid, uveal tract and inner retina; aqueous humor outflow gradient. |
| Approaches for improvement in therapeutic efficacy | Bioadhesive formulations may reduce precorneal clearance and increase corneal surface contact time. Positive charge of formulations may enhance the contact time with cornea to interact with negatively charged mucosa. Nanowafers approach may be beneficial for long-term and sustained drug release. |
| SYSTEMIC | |
| Drug entry pathways | Choroid and conjunctiva |
| Delivery barriers | Choroid and BRB (selectively permeable to highly lipophilic molecules). |
| Elimination pathway | Hepatic clearance; conjunctival and choroid capillaries and phagocytic clearance. |
| Advantages | Better patient compliance relative to intraocular injection. |
| Disadvantages | Low bioavailability due to the BRB, hence higher doses required which may produce systemic side effects. |
| Approaches for improvement in therapeutic efficacy | Large molecules and/or hydrophilic drugs are able to penetrate the choroid from the systemic circulation, but are unable to cross the inner BRB into the retina. Therefore, drugs must exit the choroidal circulation and permeate the outer BRB. |
| INTRAVITREAL | |
| Drug entry pathways | Directly to the vitreous chamber |
| Delivery barriers | Diffusion through the vitreous chamber, neural retina, and BRB. |
| Elimination pathway | Movement through aqueous chamber and retina; dynamic clearance mechanisms, such as anterior bulk aqueous flow or posterior vitreoretinal-choroidal flow, and elimination from the site of deposition. |
| Advantages | Local and direct delivery; high therapeutic concentration; no barrier to reach macula. |
| Disadvantages | It is necessary to administer the drug frequently to maintain adequate intraocular concentrations; frequent injections have been associated with adverse events especially retinal detachment, cataract, vitreous hemorrhage and endophthalmitis; linked to degeneration of PRs and cataracts and increase in IOP; only about 50–100 μl is administrable in human via intravitreal; high cost of administration of drugs (anti-VEGF). |
| Approaches for improvement in therapeutic efficacy | Extended drug release formulation for longer duration and/or drug modifications including specific properties such as size, charge, and lipophilicity; also need stimuli-responsive approach for drug release. |
| PERIOCULAR | |
| Drug entry pathways | Trans-scleral pathway to effectively deliver drugs next to the choroid. |
| Delivery barriers | Scleral thickness, choroidal blood circulation and BRBs. |
| Elimination pathway | Conjunctival and choroidal blood and lymphatic flow; losses from the periocular space, BRB, and choroidal circulation; drug binding to tissue proteins. |
| Advantages | Less invasive; high therapeutic drug levels; possible repetitive periocular administration under local anesthesia without direct interference with the vision. High volumes of drug solution can be administered in human and can bypass the BRB without intraocular penetration. |
| Disadvantages | Rapid drug clearance; systemic side effects; tissue hemorrhage; and low retinal bioavailability compared to intravitreal injections; the injected drug still has to traverse the sclera, which is less permeable to larger molecules. The drugs have to pass through several layers including the episclera, sclera, choroid, BM, and RPE-while overcoming choroid circulatory clearance; the delivery is not as effective as intraocular injections in targeting retinal tissue. |
| Approaches for improvement in therapeutic efficacy | Improvements to formulations that either increase residence time or promote diffusion from the middle coat may be effective in overcoming the barriers to periocular delivery; nano-size formulations may provide superior diffusion; charge of formulations determines the interaction or diffusion process. |
| SUPRACHOROIDAL (SC) | |
| Drug entry pathways | Flow across the sclera is quick along the inner surface of the eye and subsequently into the posterior chamber. |
| Delivery barriers | Choroid and basement membrane. |
| Elimination pathway | High blood flow in the chorio-capillaries can wash away therapeutic molecules deposited in the SC space. |
| Advantages | Preferred site for drug delivery to the posterior tissues such as choroid, RPE and macula, due to its non-interference with the optical pathways and improved diffusional access to the choroid; this allows larger volumes of drugs with minimally invasive procedure; SC space can accommodate up to 1 ml of fluid, which rapidly diffuses into the posterior segment; injections of 10–50 μL into the SC space have been demonstrated to be well tolerated with lower risks for ocular complications. |
| Disadvantages | Injection of a drug solution into the SC space can result in rapid drug diffusion to cover the entire SC surface which may potentially induce drug-related toxicities of the surrounding tissues; rapid clearance of macromolecules occurs following suprachoroidal administration; postoperative inflammation and choroidal hemorrhage remain a concern and needs to be overcome while injecting into the SC space. |
| Approaches for improvement in therapeutic efficacy | Diffusion kinetics from the SC space could be optimized using sustained release formulations such as nano and microparticles; drug delivery systems that can provide controlled and continuous drug release are likely to minimize side-effects; such controlled devices might help overcome rapid fluctuation of the dosed drugs from conventional injectable solutions into the SC space and hence reduce toxicity to the surrounding tissues; MNs appear to offer a viable option for delivery of drugs to the back of the eye, especially when delivered through the SC route; these needles help to deposit drug or carrier system into sclera or into the SCS which may facilitate diffusion of drug into deeper ocular tissues, choroid and neural retina. |
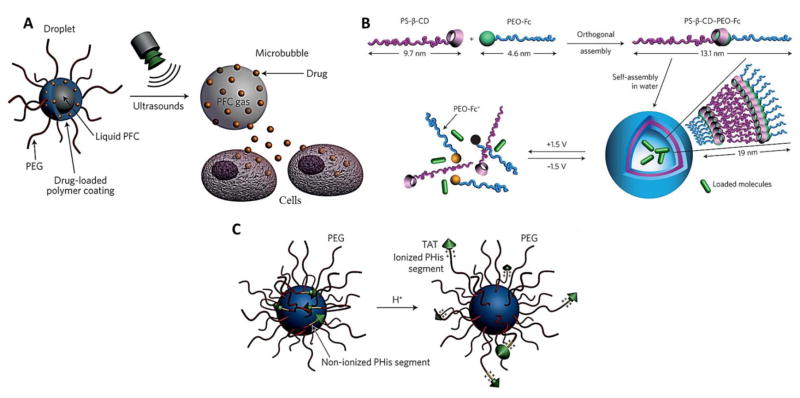
6. Novel formulation approaches for ocular delivery of proteins and peptides
6.1. Biodegradable polymeric micro particles/microspheres
Micro particles or microspheres are generally employed for long-term ocular delivery (1 week or longer) of proteins, peptides and small molecules. The biocompatible polymers constituting the microspheres generate monomers and other nontoxic byproducts upon degradation that are safely cleared out from the eye and eventually from the systemic circulation. Poly(lactic-co-glycolic acid) (PLGA) are the most commonly used polymers with high encapsulation efficiency, sustained release, biocompatibility and ability to degrade into toxicologically acceptable products that are cleared out of ocular tissues [131]. Other potentially constructive materials include polyanhydrides [132] and cyclodextrins [133]. The protein or peptide release rate is closely related to structural properties of microspheres i.e. degradation rate of polymer and/or diffusion of the protein or peptide from the microsphere. In addition, the diffusion rates also depends on the molecular mass of the polymer, protein and peptide, molar ratio of lactic/glycolic acid, entrapment efficiency, surface charge, size and porosity. The shape of the particles also influences their behavior to a great extent [134]. Transcleral delivery of PLGA microspheres provided pegatinib sodium over a period of up to 20 days at the scleral surface [135]. Similarly, intravitreal injection of pegaptanib microspheres sustained release of pegaptanib over several weeks [136]. Gavini and co-workers reported appreciable vancomycin concentrations (0.81 mg/ml) from PLGA micropsheres in the rabbit aqueous humor 180 minutes after topical administration [137]. Such microspheres can be mixed with a fluid carrier in order to achieve better control over the release and pharmacokinetic profiles of the protein and/or peptide based biopharmaceuticals [138]. PLGA microspheres suspended in poly(N-isopropylacrylamide) injectable thermo-responsive hydrogel have shown to sustain the release of ranibizumab (0.153 μg/day) and aflibercept (0.065 μg/day) for 196 days after initial burst release of 22.2 ± 2.2 and 13.1 ± 0.5 μg respectively [139]. The encapsulation process for proteins and peptides is more challenging because these macromolecules often lose their structure and biological activity upon interaction with polymeric materials and biological fluids [140]. For example, formation of covalent dimer by darbepoetin alfa (Aranesp®; Amgen) in a microsphere [141] and acylation of octreotide (Sandostatin® LAR depot, Novartis Pharmaceuticals) in PLGA microspheres [142]. To overcome the challenges of protein/peptide degradation and burst release, various strategies of hydrophobic ion-pairing (HIP) complexation, utilization of biocaompatible block-copolymers and on-demand drug release including pH, thermo, enzyme, light, ultrasound and multi responsive systems have been developed for ocular delivery [143]. Our laboratory has previously demonstrated the potential of such strategies including HIP complexation and block polymers in gel based formulations in sustaining release (~3 months) and minimizing acylation (<7%) of octreotide from microparticles [142, 144]. Fig. 13 shows an example of on-demand micro particle based drug release system. Although several microsphere formulations for ophthalmic indications have reached early stages of clinical trials, but none have yet been approved for commercialization[145]. It is very challenging to achieve long-term release and constant therapeutic levels of biopharmaceuticals for more than a week to months in ocular tissues using polymeric microspheres.
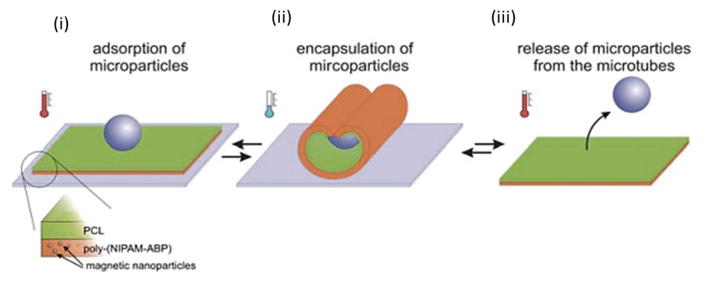
Fig. 13.
Schematic of capture and release of microparticles by self-rolling microtubes. Thin film of poly(N-isopropylacrylamide-co-4-acryloylbenzophenone)(poly(NIPAM-ABP)) and polycaprolactone (PCL) with admixed magnetic nanoparticles (i) is able to form self-rolling tube and to encapsulate microparticles at reduced temperature (ii). The particle can be released at elevated temperature when the microtube is unrolled (iii). Reprinted from [146].
6.2. Biodegradable polymeric nanoparticles/nanospheres
Nanoparticles are generally composed of biodegradable polymers and lipids and include liposomes, dendrimers, micelles and nanowafers that are actively used as carriers for targeted delivery of proteins, peptides and small molecules. Likewise micro particles, drug release from nanoparticles is dependent on the rate of degradation of polymers, molecular mass and other physicochemical factors. Nanoparticles can be administered via various routes including topical, periocular, suprachoroidal and intravitreal. However, intravitreal injection often leads to clouding of the vitreous due to scattering of light by polymeric particles. While micro particles tend to sink to the lower part of the vitreal cavity attributed to their higher molecular mass, nanoparticles are more susceptible to cause clouding in the vitreous. In addition, possible bioactivity loss and low stability of biopharmaceuticals due to interactions with nanoparticle matrix and extensive nanoencapsulation methods may further complicate delivery of proteins and peptides based nano formulations.
While there are several examples of nanoparticle-mediated ocular delivery systems for small-molecules at preclinical and clinical stages, there only few for proteins and peptides which are at early stages of development. A short fragment of antiangiogenic pigment epithelium-derived factor (PEDF), was exhibited to be released from PLGA nanospheres over 40 days in vitro, although 75% of the entrapped PEDF was released in the first 10 days [147]. The surface charge of the nanoparticles also plays a crucial role in ocular penetration. One study demonstrated higher diffusion of anionic human serum albumin based nanoparticles in the vitreous relative to cationic particles [148]. Such negatively charged nanoparticles may be utilized to deliver positively charged proteins and peptides as demonstrated by successful delivery of IgG using anionic gold nanoparticles to the photoreceptor cells and the RPE by subretinal injection [149]. Nanoparticle-mediated expression of natural antiangiogenic factors and pathway regulators offer great therapeutic potential in neovascular disorders. Plasminogen kringle 5 (K5), an 80-amino-acid proteolytic fragment of plasminogen loaded into PLGA nanoparticles exhibited reduced CNV areas and vascular leakage for at least 2 weeks in CNV models suggesting sustained antiangiogenic properties [150, 151]. Another study aimed to down regulate Wnt signaling and ocular neovascularization by increasing very low-density lipoprotein receptor extracellular domain (VLN) expression. A substantial and sustained VLN expression was achieved in cultured cells and retina for ≥4 weeks by encapsulating VLN plasmid in PLGA nanoparticles [152, 153]. Development of core-shell nanoparticles for encapsulating both hydrophobic and hydrophilic cargo [154], PEGylation for prolonging nanoparticle circulation and enhancing tissue penetration, functionalization for stimuli-responsive targeting (Fig. 14) and delivery of nanoparticles in biocompatible gels are some of the future strategies for controlled long term delivery of biotherapeutics. Our laboratory has extensively worked on encapsulating various proteins and peptides including octreotide, insulin, lysozyme, IgG-Fab, IgG, bevacizumab and catalase in a novel patented block copolymer. Our group has demonstrated successful long-term in vitro release of these macromolecules for several weeks to few months (~12 weeks) after suspending such drug-loaded nanoparticles in thermosensitive gels [155–158].
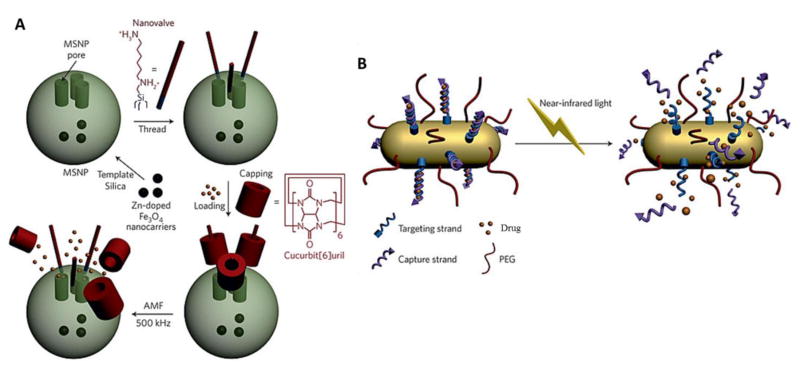
Fig. 14.
(A) Actuation mechanisms based on the heat generated by an alternating magnetic field (AMF) leading to on-demand pulsatile small molecule release from mesoporous silica nanoparticles (MSNPs): Pseudorotaxane-based nanovalves made of cucurbit[6]uril. Reprinted from [160]; (B) Light-triggered small molecule delivery: Drug delivery through the near-infrared-triggered induction of dehybridization of the DNA conjugated at the surface of gold nanorods. Reprinted from [161].
Nanofiber based systems are also being extensively explored due to their potential in generating self-assembling peptide nanofibers and peptide amphiphiles (PA). Recently, a group of researchers have demonstrated significant inhibition of endothelial cell proliferation and migration and aberrant capillary formation by delivering LPPR peptide that binds specifically to the VEGF receptor, NRP-1 as nanofibers. Furthermore, subconjunctival injection of LPPR-PA nanofiber expressively inhibited corneal neovascularization in rat model (81.3%) compared to bevacizumab (51.2%) on day 14 indicating its effectiveness in treating angiogenesis-related disorders [159].
6.2.1. Lipid based nanoparticles
Lipid based nanoacarriers including liposomes, solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) have been utilized as a colloidal system for controlled drug delivery. Liposomes are non-covalent aggregates that present low antigenicity and toxicity. Encapsulation of biopharmaceuticals in liposomes is commonly achieved through dehydration-rehydration method. Although this method yields high association efficiency without utilizing organic solvents and sonication, high developmental cost and particle size instability restricts its wide application. Intravitreal injection of liposomes encapsulated bevacizumab has been reported to be well tolerated through 42 days in rabbits and provided 1.5 times higher drug concentrations in the vitreous for >6 weeks [162]. Annexin A5 associated liposomes were exposed to generate 127 ng/g and 18 ng/g concentrations of bevacizumab in rat and rabbit eyes respectively after 2 hours post topical administration [163]. Furthermore, cationic liposomes offer an additional advantage of greater corneal drug absorption by increasing drug residence time through ionic interactions as shown by Cortesi et al.[164]. Li and group conjugated peptide ATWLPPR to immune-nano-liposome (INL) to deliver PEDF as a targeted therapy for CNV. PEDF-loaded INLs significantly decreased CNV areas in rat models without binding to normal choroidal vessels [165].
SLNs are composed of biocompatible and physiological solid lipids and offer various advantages including avoidance of organic solvents for formulation, improved physical stability, targetibility, controlled release and easy scale-up. However, low drug-loading, burst-effect and rapid elimination by mononuclear phagocytic system (MPS) are some of the drawbacks of SLNs. Chetoni and co-workers demonstrated therapeutic concentrations of an antibiotic, tobramycin was achieved 3 hours in the retina and vitreous following topical and parentral SLN administrations [166, 167]. Cyclosporine-A loaded chitosan based SLNs have shown promising in vitro results with high permeation and biocompatibility in rabbit corneal endothelial cells [168]. To overcome the limited drug-loading and expulsion during phase modifications and higher water content of SLN aqueous dispersions, NLCs have been developed. NLCs are composed of highly disordered solid and liquid lipids and can provide better drug protection and entrapment efficiency in comparison to SLNs [169, 170]. Both SLNs and NLCs have shown potential in delivering small molecules to ocular tissues [171–173]. However, efficiency in delivering protein and peptide based biopharmaceuticals to ocular tissues has not been fully exploited and requires further investigation. A great deal of research has been carried out on stimuli-responsive lipid based nanocarriers for various complications (Fig. 15), and with improved design and technology such stimuli-responsive systems may become feasible for ocular delivery as well.
Fig. 15.
(A) Light-triggered drug delivery: Schematic representation of transcription–translation liposomal system for protein production triggered by irradiating caged DNA with light. Reprinted from [174]; (B) Temperature-based actuation mechanisms for liposomal drug delivery: The temperature-triggered unfolding of a leucine zipper peptide inserted in the membrane of a doxorubicin (Dox)-carrying liposome opens a channel through which the drug is released. Reprinted from [175]; (C) Drug-permeable pores can also be created by the temperature-triggered generation of bubbles from the decomposition of encapsulated ammonium bicarbonate. Reprinted from [176].
Niosomes are self-assembling nanovesicles composed of non-ionic surfactants that behave exactly like liposomes in vivo [177]. Although development of niosomes is still in its infancy, its potential pertinence to many therapeutic agents including small molecules, proteins and peptides can be exploited for various diseases.
6.2.2. Polymeric micelles
Polymeric micelles represent a class of nanocarriers that are composed of amphiphilic polymers which self-assemble in aqueous media to form organized supramolecular structures. Micelles have been actively studied as carriers for ocular delivery of small molecule drugs by our and other laboratories [178]. We have developed a nanomicellar formulation of cyclosporine which generated corneal concentration of 828.25 ng/g, 1 hour post single topical administration and retina/choroid concentration of 53.7 ng/g after multiple administration of 0.1% cyclosporine nanomicellar formulation [179]. In addition, triblock copolymer based positively charged micellar formulation has exhibited to prolong cyclosporine in vitro release and enhance corneal permeation in C57BL/6 mice [180]. Indeed, several micellar formulations are currently undergoing clinical trials including Seciera® (Sun Pharmaceuticals), the formulation developed by our laboratory, which just completed clinical Phase III trial for the treatment of dry eye disease. A similar nanomicellar formulation of voclosporin, VOS® (Merck Animal Health) is under clinical development for the treatment of canine dry eye syndrome.
There are fewer examples of micelles for delivering biopharmaceuticals to ocular tissues which are currently under development [181]. Synthesis and preparation of anti-Flt1 peptide-HA conjugates in the form of micelle, demonstrated to increase stability, residence time and bioavailability of anti-Flt1 peptide over 2 weeks in retinal neovascularization and diabetic retinopathy induced rat model [182, 183]. These anti-Flt1 peptide-HA conjugate micelles were further employed to encapsulate genistein, an inhibitor of tyrosine-specific protein kinases as a combination therapy for ocular neovascularization. The micelles exhibited a sustained release for longer than 24 hours with potent inhibitory effect on vascular hyperpermeability and corneal neovascularization [184].
New ocular delivery technologies such as utilizing micelles as nano-scale microbubble precursor which can be converted into microbubble upon heating or ultrasound irradiation has got tremendous scope for ocular delivery (Fig. 16A). In addition, stimuli-responsive self-assembled intelligent polymeric vesicles capable of elucidating and biomimicking the biological activities of the lipid bilayers can offer controllable small-molecule and biopharmaceutical delivery (Figs. 16B and C).
Fig. 16.
(A) Drug delivery from echogenic perfluorocarbon (PFC)-containing nanoemulsions: The delivery mechanism involves a droplet-to-bubble transition under the action of ultrasound, leading to drug transfer from the bubbles to neighbouring cells. Reprinted from [185]; (B) Voltage-responsive vesicles: Structures of polystyrene-β-cyclodextrin (PS-β-CD) and poly(ethylene oxide)-ferrocene (PEO-Fc), and representation of the voltage-responsive controlled assembly and disassembly of PS-β-CD–PEO-Fc supramolecular vesicles. Reprinted from [186]; (C) pH-sensitive nanocarriers for efficient TAT-peptide exposure: Polyhistidine (PHis)-based micelles responding to acidic microenvironments by an efficient TAT-sequence exposure following ionization of the polyhistidine segments. Reprinted from [187].
6.2.3. Dendrimers
Dendrimers constitute branched, layered architectures composed of synthetic polymers that show promise as nanocarriers in several biomedical applications. The unique branched topologies of dendrimers confer properties completely different from linear polymers. Dendrimers can be divided into different generations (G-1, G-2, G-3) based on the size, number of branches and end groups at the terminal (Fig. 17A). A dendrimer can be composed of any type of polymer which can determine its solubility, stability and biological activity. Some of the commonly used dendrimers are based on polyamidoamines, polyamines, polyamides (polypeptides), poly(aryl ethers), polyesters, carbohydrates and DNA. Among these, polyamidoamine (PAMAM) based dendrimers are most commonly used and commercially available. Unlike linear polymers, the multivalent property of dendrimers provide a means to achieve high concentrations of payloads including small-molecules, biopharmaceuticals and imaging agents. The molecular weight and surface charge of dendrimers also play a crucial role in determining tissue accumulation profiles, drug release rates (from the polymer) and elimination rates. While, high molecular weights of dendrimers (>~40 kDa) prevent rapid clearance, uncharged or negatively charged surface limit nonspecific interactions [188]. In addition, numerous end groups offer a way to precisely control functionality with multiple copies of drugs, chromophores, peptides, proteins and multivalent ligand density. Such surface modifications can not only strengthen ligand-receptor binding and improve the targeting of attached components but can also accelerate dendrimers stimuli-responsive activity [189].
Fig. 17.
(A) Anatomy of a dendrimer: A dendrimer and dendron are represented with solid lines. The colored, broken lines identify the various key regions of the dendrimer. Reprinted from [188]; (B) Generation 4 PAMAM-OH dendrimer. (C) Internally quaternized PAMAM to form QPAMAM-OH dendrimer with inner cationic charges. PAMAM are frequently quaternized by methyl iodide (ICH3) and the terminal surface become very positive allowing the efficient electrostatic binding/loading of negatively charged backbone of siRNA. (D) QPAMAM with different surface modifications, including the addition of acetyl group by direct reaction with acetic anhydride (Ac2O), poly(ethylene glycol) (PEG) and poly-L-lysine (PLL), LHRH peptide. This addition of polymer structures such as PEG and PLL is reported to enhance the surface positive charge and circulation of PAMAM nanoparticles. While, the conjugation to LHRH peptides confers targeting ability in PAMAM based delivery applications. Reprinted from [195].
PAMAM dendrimers as drug delivery vehicles for small-molecules have shown improved biological response, tolerability and lower clearance from ocular surface indicating its utility as an eye drop formulation [190]. A few studies have also confirmed the delivery of therapeutic peptides and proteins using different conjugation techniques with dendrimers (Figs. 17B, C and D) [191–194]. However, targeted delivery of protein and peptide based biopharmaceuticals with dendrimers for ocular complications has not been reported so far. In addition, while there are several in vitro efficacy studies demonstrating the effectiveness of dendrimers as nanocarriers, only a few in vivo efficacy studies exist. Nonetheless, despite several advantages of dendrimers, multistep syntheses, high preparation costs and inadequate quality control assays prohibited significant advancement of dendrimers from the laboratory to the clinic.
6.2.4. Nanowafers
Nanowafers are tiny transparent circular discs that are composed of various polymers including poly(vinyl alcohol) (PVA), polyvinylpyrrolidone (PVP), (hydroxypropyl)methyl cellulose (HPMC), and carboxymethyl cellulose (CMC). These are generally applied with a fingertip on ocular surface and can withstand constant blinking without being displaced unlike topical eye drops. Nanowafers consist of arrays of drug-loaded nanoreservoirs which releases the cargo in a highly controlled manner for a few hours to several days (Fig. 18). The synergistic action between the polymers and the loaded drug leads to slow drug release thus enhancing drug residence time and subsequent absorption into ocular tissues. Importantly, at the end of stipulated drug release time, the nanowafer dissolves and fades away thus rendering ocular surfaces free of polymers. Yuan and group for the first time demonstrated the sustained release and enhanced corneal permeability of doxycycline-loaded PVA nanowafer over 24 hours in mice. The same group reported the efficacy of such PVA fabricated nanowafer loaded with axitinib for treating CNV in a murine ocular burn model [196, 197]. This smart platform integrating nanofabrication as well as drug delivery technologies has been able to establish itself further by demonstrating sustained delivery of dexamethasone and cysteamine for effective treatment of dry eye disease and corneal cystinosis respectively [198–200]. These results provide a strong rationale for their translation and clinical application in biopharmaceuticals delivery.
Fig. 18.
Ocular drug delivery nanowafer: (A) Schematic of nanowafer instilled on the cornea. (B) Diffusion of drug molecules into the corneal tissue. (C) Nanowafer on a fingertip. (D) AFM image of a nanowafer demonstrating an array of 500 nm diameter nanoreservoirs. (E) Fluorescence micrograph of a nanowafer filled with doxycycline (scale bar 5 μm). Reprinted from [196].
6.3. Biodegradable and non-biodegradable polymeric implants
Biodegradable implants can overcome the challenges of particulate systems such as suspension, reconstitution, quantification and manufacturing complexities. In addition, higher drug loading due to larger size and smaller surface-to-volume ratio in comparison to particulate systems, allows prolonged drug release with reduced or minimal burst release. Utilization of additional materials including PEG400 and blends of block co-polymers and PLGA have shown to provide prolonged release of protein drugs with reduced initial burst [172]. Biodegradable implants are capable of sustaining the release from a few days to several months. The devices are introduced directly in the vitreous or onto the sclera by minor surgical techniques [201]. Similar to polymeric particulate systems, biodegradable implants also suffer from poor stability and release rates. Additionally, implantation of such devices requires skilled professional execution leading to their towering price tags. Ozurdex (Allergan®) is one such biodegradable PLGA based implant approved by FDA for macular edema and noninfectious posterior uveitis [202]. The PLGA copolymer matrix releases loaded dexamethasone (0.7 mg) in the vitreal cavity for a period of 6 months [203]. A similar formulation has been evaluated for the delivery of brominidine tartrate in clinical trials (NCT02087085). Surodex (Oculex Pharmaceuticals, Sunnyvale, CA) and Verisome (Icon Biosciences Inc., Sunnyvale, CA) are a few other biodegradable implants that are currently undergoing clinical trials [204]. To date no ophthalmic biodegradable implant for biopharmaceutical drugs has been approved. However, a few biodegradable implants have demonstrated their potential in preclinical studies. An implant loaded with human recombinant tissue plasminogen activator (t-PA) has shown to release t-PA at a rate of 0.5 μg/day for 2 weeks in the vitreous [205].
In contrast, non-biodegradable implants are composed of a core drug reservoir and a semi-permeable membrane allowing continous drug release for up to several months to years. While biodegradable implants are more susceptible to burst and non-linear release kinetics, non-biodegradable implants release drugs following zero-order kinetics. However, non-biodegradable implants requires to be removed or refilled following drug reservoir depletion. Several non-biodegradable implants have been clinically approved including Vitrasert®, Retisert® (Bausch & Lomb, Rochester, NY), Iluvien® (Alimera Sciences, Inc, Alpharetta, GA) for the delivery of small molecule drugs to the vitreous [206–208]. However, large size with the exception Iluvien, surgical procedures and complications such as retinal detachments are a few drawbacks of non-biodegradable implants. Currently, nonbiodegradable implants for biopharmaceutical drugs have not yet reached the market. An osmotic pump, implanted in the subcutaneous space and connected to the sclera using a brain infusion kit has been reported to deliver IgG for 28 days [209]. Ranibizumab port delivery system (PDS) (Genentech, CA) is another non-biodegradable implant that has shown promise in delivering therapeutic concentrations of ranibizumab into the vitreous over an extended period of time and is currently undergoing Phase II clinical trials (NCT02510794). Implants with suitable design, smaller size, minimal surgical procedure, maintenance of biopharmaceutical stability and prolonged release[210] may serve as an attractive system for biopharmaceuticals delivery to ocular tissues.
6.4. In situ gelling formulations
In situ-forming gels are low-viscosity polymeric solutions that undergo phase transition to form a gel following stimulus. Such phase transition can be mediated by changes in temperature, pH, light and ionic composition. Various in-situ gelling systems including chitosan, poloxamer, hydroxypropylmethylcellulose and polycaprolactone have exhibited safe use as ocular depot systems. Topical application of in-situ gelling systems not only aids in increasing precorneal drug residence time but also offers increased stability and bioavailability in ocular tissues. Although, injectable solutions of PLGA in N-methyl pyrollidone and sucrose acetate isobutyrate are known to prolong biopharmaceutical drug delivery, extremely poor permeability of macromolecules across corneal epithelium may present a major obstacle. Till now, several injectable in-situ gelling formulations for ocular delivery of small-molecules have been studied. In fact, such in-situ depot systems are being developed for biopharmaceuticals delivery as well [211]. An example of light activated in-situ gelling system that shows promise is the delivery of bevacizumab in the suprachoroidal space for 60 days in rats [212]. In another study, intravitreal injection of bevacizumab loaded thermosensitive gel exhibited an extended release profile over 18 days in vitro [213] and anti-angiogenic effects in 3-D cultures [214]. Polysaccharides cross-linked hydrogels have shown to sustain bevacizumab release for 3 days with initial burst release for 4 hours [215]. Similarly, Lovett and co-workers demonstrated the ability of silk hydrogels in sustaining bevacizumab release for 3 months in experiments in vitro as well as in vivo in Dutch-belted rabbits [216]. Recently, a reverse thermal gelling system based poly(ethylene glycol)-poly-(serinol hexamethylene urethane) (ESHU) demonstrated sustained release of bevacizumab over 9 weeks in vivo [217]. The unique gelling properties of such depot systems can be optimized to achieve long-term release of biopharmaceuticals for several weeks to few months. In Table 5, we highlight some of the advantages and limitations of various ocular delivery systems for biopharmaceuticals.
Table 5.
A summary of advantages and limitations of various ocular delivery systems for protein and peptide based biopharmaceuticals
| DELIVERY SYSTEMS | ADVANTAGES | LIMITATIONS |
|---|---|---|
| Micro and Nanoparticles |
|
|
| Liposomes |
|
|
| Solid lipid nanocarriers |
|
|
| Dendrimers |
|
|
| Hydrogels |
|
|
| Micelles |
|
|
| Composite formulations (nanocarriers- in-gel) |
|
|
6.5. Delivery using cell penetrating peptides (CPP)
CPPs have shown great potential to quickly translocate attached molecules through the cell membranes into mammalian cells both in vitro and in vivo. Such CPP-conjugated nanocarriers are generally taken up by energy-dependent endocytosis (or macropinocytosis) after electrostatic interactions [218] and can enhance the delivery of small-molecule drugs and biopharmaceuticals to ocular tissues. In this regard, 86-mer trans-activating transcriptional activator (TAT) from HIV-1 has been widely applied to achieve targeted delivery [219]. However, one of the major obstacles that still remains unresolved is the lack of selectivity of TAT. To overcome such a short coming, a novel peptide for ocular delivery (POD, GGG[ARKKAAKA]4) has been recently developed that is capable of delivering protein and peptide based biopharmaceuticals specifically to their target side thus reducing unwanted side effects. Moreover, POD administered topically has shown to reach the dura of the optic nerve within 45 minutes indicating its effectiveness in treating optic nerve diseases. POD also exhibited bacteriostatic activity by reducing bacterial colony number significantly at lower concentrations [220, 221]. Various CPPs of human proteins are being extensively exploited to overcome immunogenicity, poor serum stability and toxicity arising from CPP sequences of non-human origin [222].
Stapled peptides, a class of helical peptides that have recently gained interest focuses on improving membrane penetration of peptides by incorporating modifications such as α-methylation and hydrocarbon based macrocyclic bridging features or mutagenesis to improve hydrophobicity and conformational stabilization of the helix. It has been observed that both noncovalent and covalent constraints to stabilize long peptide sequences have resulted in enhanced binding [223]. However, noncovalently constrained constructs are rapidly proteolyzed and covalently constrained peptides bearing labile cross-links (for example, disulfides and amides) are also vulnerable to proteolysis [224]. Several studies have shown peptide constraints through installation of thioether, lactam or triazole cross-links, or helix promoting non-natural amino acids, have reinforced structure and enhanced peptide-antibody binding affinity [225–227]. Bird and group performed all-hydrocarbon cross-link by olefin metathesis of installed non-natural amino acids bearing olefin tethers to determine the binding of stabilized α-helices of membrane-proximal external region (SAH-MPER) peptide of gp41 with broadly neutralizing anti-HIV-1 antibodies (4E10 and 10E8). The modified SAH-MPER exhibited high-binding affinity towards 4E10 and 10E8 and markedly enhanced protease resistance (Fig. 19) [228]. Such modulations of stapled peptides to enhance protein-protein interactions have been successfully employed in cancer treatment as well. This has led the entry of a stapled peptide, ALRN-5281 (growth-hormone-releasing hormone agonist, Aileron Therapeutics) into clinical trials [229]. Applications of stapled peptides in the context of ocular delivery have not been explored so far. This strategy may prove to be promising for the treatment of ocular complications [230].
Fig. 19.
(A) Chemical optimization of i, i + 3 hydrocarbon stapling: Top, design and synthesis of SAH-MPER(671–683KKK)(q), in which the N-terminal S5 residue was replaced with R3 to lead to efficient i, i + 3 olefin metathesis under standard reaction conditions. (B) Crystal structure of SAH-MPER(671–683KKK)(q) (shown as a blue ribbon and gray transparent van der Waals surface) bound to 4E10 Fab, at 2.9-Å resolution. (C) 2Fo–Fc electron density map (1σ level) of the antibody-bound SAH-MPER(671–683KKK)(q) peptide. (D) Superposition of the native (green; PDB 2FX7)18 and i, i + 3–stapled (gray) MPER(671–683KKK) peptides, highlighting the similarity of antibody-bound structures, aside from the appended C-terminal lysines and the incorporated staple. Z and X represent R3 and S5, respectively, in the staple (red bar above sequence). Reprinted from [231].
6.6. Delivery using living cells
Biomedical applications of cell based drug delivery systems have opened up new perspectives of using our own cells for therapeutic purposes. Such application in improving pharmacokinetic profiles or generating sustained release depots have progressed to clinical evaluations. Encapsulated cell technology (ECT) is one such advancement which utilizes entrapment of genetically modified cells within a semipermeable membrane in order to isolate them from host body. The genetically engineered cells are designed in such a way that allow continuous production of therapeutic proteins. The semipermeable membrane apart from protecting the encapsulated cells, allows passage of nutrients into the cells, while providing sustained release of therapeutic proteins from the implant. NT-501 (Renexus, Neurotech Pharmaceuticals), which aims to protect the retina from degeneration by producing ciliary neurotrophic factor (CNTF), provides sustained delivery of CNTF from the implant encapsulating genetically engineered human retinal pigment epithelial (RPE) cells [232]. A similar ECT, NT-503 has been developed that secretes soluble VEGF receptor protein (Fc-Fusion protein) for a prolonged period of time and has showed significantly higher (20–30 fold higher) VEGF neutralization [233, 234]. ECT seems to be an attractive modality and a better control over the release rate may prove to be exciting in achieving long-term ocular delivery of biopharmaceuticals. Some of the controlled-release systems under investigation for protein and peptide therapeutics indicated for ocular complications are detailed in Table 6.
Table 6.
Controlled-release systems being investigated for protein and peptide therapeutics for ocular implications (Adapted and modified from [41])
| Formulation Approaches | Drug | Description | Release | Status | Ref. |
|---|---|---|---|---|---|
| Particulate systems | |||||
| Biodegradable polymeric microspheres | |||||
| PLGA MPs | Bevacizumab | Fabricated by Double emulsion method; particle size, 2–10 μm | 62% released within 91 days | Preclinical, in vitro | [235] |
| PLA NPs within PLGA MPs | Bevacizumab | Fabricated by supercritical infusion and pressure quench technology | 67% released within 120 days (4 months) | Preclinical, in vivo, rat model | [236] |
| PLGA MPs | Bevacizumab | Fabricated by solid-in-oil-in- hydrophilic oil method; particle size, 2–7 μm | NA | Preclinical, in vivo, rabbit model | [237] |
| Silicon dioxide MPs | Bevacizumab | Synthesized by electrochemical etching and oxidation of silicon wafer in hydrofluoric acid followed by ultrasonic fracture; particles with a pore size of 100 nm | 165 days (5 months) | Preclinical, in vitro | [238] |
| Biodegradable polymeric nanospheres | |||||
| PLGA – albumin NPs | Bevacizumab | Fabricated by w/o/w double emulsion in presence of albumin as a stabilizer; particle size ~ 197 nm | 165 days (5 months) | Preclinical, in vivo, rabbit model | [239], [240] |
| Liposome | Bevacizumab | EPC-Chol and DPC-chol liposomes formed by dehydration and rehydration method followed by freeze drying | NA | Preclinical, in vivo, rabbit model | [162] |
| Liposome–annexin | Bevacizumab | PC-PS-Chol-Toc liposomes fabricated by dehydration and rehydration method and subsequently coated with annexin; particle size, 100 nm | NA | Preclinical, in vivo, rabbit mode | [163] |
| Biodegradable and non-biodegradable polymeric implants | |||||
| ENV705 (intravitreal) Envisia therapeutics | Bevacizumab | Drug dispersed into biodegradable hydrogel-based matrix made by PRINT technology and molded into implants | 2 months | Preclinical animal test | [241] |
| Nano-pores film device (intravitreal) Zordera Inc. | Ranibizumab | Biodegradable DDS based on PCL; drug pellet sandwiched between a nanopore and impermeable layer (total thickness 40 mm) | 3 months | Under investigation | [241] |
| Posterior micropump system (subconjuctival) | Ranibizumab | Nonbiodegradable refillable DDS drug loaded with preprogrammed micropump; drug delivered in controlled nanodroplets | Long- term (refillable ) | Phase I | [242] |
| Port delivery system (PDS) (subconjuctival) Genentech | Ranibizumab | Semipermeable nonbiodegradable membrane with a refillable port; several exit ports to release drug into vitreous humor | Long- term – 1 year (refillable ) | Phase II | [204] |
| Verisome IB20089 (intravitreal) Icon bioscience | Triamcinolo ne/ ranibizumab | Biodegradable with liquid gel or solid core; liquid injectable formulation; coalesces after intravitreal injections to form spherules | 1 year | Phase II | [243] |
| In-situ gelling formulations | |||||
| Hyaluronic acid- dextran | Bevacizumab | Catalyst-free chemical crosslinking between vinylsulfone functionalized HA/thiolated dextranin under physiological conditions; transparent gel formed in vitreous after injection; better sustained release observed in vivo compared with in vitro release | 6 months | Preclinical, in vivo, rabbit model | [244] |
| Alginate-chitosan hydrogel/PLGA microspheres | Bevacizumab / ranibizumab | Antibody-loaded PLGA microspheres encapsulated into alginate hydrogels | 196 days (6.5 weeks) | Preclinical, in vitro | [139] |
| Silk-based hydrogels | Bevacizumab | On the basis of physically crosslinked silk fibroin heavy chain (Mw = 350 kDa); biocompatible crosslinking reaction | Up to 3 months | Preclinical, in vitro | [216] |
| Diels–alder hydrogels | Bevacizumab | On the basis of PEG macro- monomers, chemically crosslinked by Diels–Alder reaction; mechanical stability enhanced | Up to 6 weeks | Preclinical, in vitro | [245] |
| Poly(2-ethyl-2- oxazoline)-b- poly(caprolactone)- b-poly(2-ethyl-2- oxazoline) | Bevacizumab | Reversible sol–gel transition; good in vitro and in vivo biocompatibility | 20 days | Preclinical, in vitro | [213] |
| Poly(n- isopropylacrylamid e) pnipaam and poly(ethylene glycol diacrylate) peg-da | Bevacizumab / ranibizumab | Enhanced mechanical properties; good biocompatibility; thermoresponsive hydrogel; PNIPAAm shows LCST behavior | 3 weeks | Preclinical, in vivo, rat model | [246] |
| PEG-poly- (serinolhexamethyl ene urethane) | Bevacizumab | Sol-to-gel phase transition when kept at in vivo temperatures; good in vitro and in vivo biocompatibility | 17 weeks | Preclinical, in vivo, rabbit model | [217] |
| Delivery using living cells | |||||
| Renexus NT-501 (surgical) Neurotech Inc. | Cell line secreting CNTF | Nonbiodegradable; cell encapsulated in a semipermeable polysulfone capsule; Phase 2 results not encouraging because of adverse effects | 18 months | Phase II/III | [247] |
| NT-503 (surgical) Neurotech Inc. | Cell line secreting VEGFR-Fc | Nonbiodegradable implant; semipermeable hollow fiber membrane encapsulating cells | 12 months | Phase I | [248] |
CNTF, ciliary neurotrophic factor; DDS, drug delivery systems; DPC-chol, 1,2 dipalmitoyl-sn-glycero-3-phosphocholine; EPC-chol, egg phosphatidylcholine-cholesterol; MPs, microparticles; NPs, nanoparticles; PLGA, polylactic-co-glycolic acid; PC-PS-Toc, egg phosphatidylcholine-porcine brain phosphatidylserine-tocopherol; PCL, polycaprolactone; PEG, polyethylene glycol; PLA, polylactic acid.
6.7. Delivery using minimally invasive microneedles and iontophoresis
Microneedles (MNs) as evident from some of the previous examples, have gained wide application due to their potential in circumventing epithelial transport barrier and conjunctival clearance mechanism, and at the same time minimizing retinal damage. Ocular MNs are primarily based on passive [249] and active [125, 250, 251] delivery approaches. Passive MNs typically consist of arrays of solid MNs coated with drug formulations that are intended to dissolve within minutes of insertion, followed shortly by removal of the device. Such simplified nature of passive MNs render them more advantageous for routine clinical use and commercial feasibility. Microneedle pens developed by Song and group have demonstrated to inhibit corneal neovascularization by delivering sunitinib malate in vivo [249]. Nevertheless, the limited drug loading capacity of such devices may constrain their potential for clinical translation. Aimi and coworkers have made an effort to address this limitation by designing titanium-based MNs (Ti MNs) through titanium deep reactive ion etching (Ti DRIE) process. The unique through-thickness fenestrations (i.e. windows) of Ti MNs acts as drug reservoirs and allows increase drug carrying capacity relative to solid MNs [252]. The same group reported a uniform drug deposition and fenestration filling techniques that increased drug carrying capacity of fenestrated Ti MNs five-fold relative to solid MNs of comparable size. Such fenestrated MNs along with micro- or nanoparticle-entrained coatings may hold potential for demonstrating sustained biologics delivery as well and thus reduced treatment frequency. Fig. 20 illustrates scanning and fluorescence micrographs of different types MNs.
Fig. 20.
(A) Hollow microneedle (arrow), 720 μm in length, is shown next to a liquid drop of approximately 50-μL volume from a conventional eye dropper; Scanning electron micrographs of a representative fenestrated TiMN with 200 μm width, 50μm thickness, and 1500μm length: (B) Low magnification image showing the full needle, as well as a portion of its base; (C) Higher magnification image showing the needle tip and fenestrations; (D)Size comparison between a fenestrated TiMN and a conventional 26 gauge hypodermic needle typically used for intravitreal injection; (E) Buckled MN demonstrating graceful, plasticity-based failure mode after mechanical testing; (F) Fluorescence micrographs of PVP/Rhodamine B coated solid and fenestrated TiMNs. Reprinted from [253].
Iontophoresis has been exploited for many years as a non-invasive technique for ocular drug delivery. Although a series of small molecules including ciprofloxacin hydrochloride (ocular infection)[254], gentamicin (pseudomonas keratitis) [255], dexamethasone phosphate [256], methylprednisolone (posterior segment inflammation) [257], carboplatin (retinoblastoma) [258], and methotrexate (inflammatory diseases and intraocular lymphoma) [259] have been delivered successfully, ocular delivery of proteins and peptides using this technique have been rarely explored. A better understanding of electric current application in ocular tissues and advanced designing of devices and probes adapted to the application site may yield efficient intra-ocular penetration of proteins and peptides based therapeutics as well [260]. Zhang and coworkers have recently developed one such flexible ocular iontophoretic device that can be placed under the eyelid and deliver ions through a small area on the eyeball, reducing tissue damage caused by drug during ion penetration [261].
7. Conclusion
Despite the major hurdles in ocular delivery of proteins and peptides, technological breakthroughs in formulation, delivery approaches and manufacturing methods have facilitated the growth and improvement in the biopharmaceutical market. Some of the work with the delivery of biopharmaceutical drugs have shown encouraging results. However, many needs remain unmet for the delivery of relatively smaller biologics, and greater challenges keep arising for developing formulations for larger biopharmaceutical drugs. Current biopharmaceuticals suffer from poor intracellular delivery leading to low ocular bioavailability, reduced stability (including storage, handling and administration), incompetent formulation development strategies and scalability and high manufacturing costs. Developing new biomaterials for effective protection of proteins and peptides and improving intracellular delivery by identifying target-site specific receptors will significantly improve biopharmaceutical delivery.
Another area for future progress is the development of novel formulation and delivery approaches for biopharmaceutical drugs. While extraocular delivery strategies including tropical eye drops and periocular injections have exhibited poor bioavailability and limited targeting, intraocular strategies such as intracameral and intravitreal injections are highly invasive. Efforts should be made to reduce the frequency of intraocular administration (e.g. novel controlled release formulations) and develop new methods or devices (e.g. through non-parenteral routes) that are non-invasive in nature. Expansion of therapeutic target space that are not accessible by the routinely used biologics or small-molecules can pave the way to unexplored therapeutic opportunities. Future research should focus on non-invasive controlled intraocular delivery of highly stable formulations of proteins and peptides.
Supplementary Material
Fig. 1.
Numbers of Phase 3 products by technology type for ophthalmic indications (Till Nov., 2015): MIGS (minimally invasive glaucoma surgery); NCE (New chemical entity)
Fig. 2.
Number of companies classified by technology as well as global areas for ophthalmology market: This analysis does not include multinational companies, as these entities cannot be defined by a single technology and any one country. Note that the classification “Europe” excludes Scotland to avoid double counting.
Fig. 3.
Novel drug R&D venture funding by disease area, 2004–2008 vs 2009–2013
Table 3.
Permeability of proteins, peptides and macromolecules across ocular barriers
| Compound | MWa (Da) | Tissue | Animalb | Permeability | Reference |
|---|---|---|---|---|---|
| Serum albumin | 66000 | cornea | H | 5.48E-07 | [51] |
| Inulin | 5000 | cornea | R | 5.50E-07 | [52] |
| Cyclosporine | 1201 | cornea | R | 1.10E-05 | [53] |
| Deoxycorticosterone | 330 | cornea | R | 4.00E-05 | [54] |
| Progesterone | 314 | cornea | R | 2.00E-05 | [54] |
| Testosterone | 288 | cornea | R | 4.20E-05 | [54] |
| Immunoglobulin | 140000 | stroma | R* | 8.00E-09 | [55] |
| Hemoglobin | 64500 | stroma | O | 5.70E-07 | [56] |
| Serum albumin | 65000 | stroma | R | 1.40E-07 | [57] |
| Dextran | 75000 | endothelium | R | 7.50E-07 | [58] |
| Serum albumin | 65000 | endothelium | R | 8.30E-09 | [55] |
| Poly(vinylpyrrolidone) | 45000 | endothelium | R | 3.80E-07 | [58] |
| Dextran | 16000 | endothelium | R | 2.70E-05 | [58] |
| Inulin | 5000 | endothelium | R | 1.40E-06 | [58] |
| Bevacizumab | 145000 | sclera | H | 5.30E-07 | [59] |
| Dextran-70 | 70000 | sclera | H | 1.90E-06 | [60] |
| Serum albumin | 65000 | sclera | C | 1.30E-07 | [61] |
| Hemoglobin | 64500 | sclera | C | 3.60E-07 | [61] |
| Dextran-40 | 40000 | sclera | H | 4.30E-06 | [60] |
| Dextran-10 | 10000 | sclera | H | 6.20E-06 | [60] |
| Inulin | 5000 | sclera | C | 1.90E-06 | [61] |
| Inulin | 5000 | sclera | H | 9.00E-06 | [60] |
| Inulin | 5000 | sclera | R | 2.50E-06 | [52] |
| Hydrocortisone | 362 | sclera | C | 6.50E-06 | [61] |
| Inulin | 5000 | conjunctiva | R* | 3.80E-06 | [52] |
| FITC-dextran | 77000 | RPE-choroid | C | 2.70E-08 | [62] |
(MW) Molecular weight
Source of tissue (R) rabbit, (C) cow, (O) ox, (H) human
All permeability measurements were obtained from in vitro experiments except those followed by an asterix (*), which were obtained from in vivo.
Acknowledgments
This work was supported by the National Eye Institute (R01-EY09171-14 and R01-EY10659-12).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Walsh G. Biopharmaceutical benchmarks 2014. Nat Biotech. 2014;32:992–1000. doi: 10.1038/nbt.3040. [DOI] [PubMed] [Google Scholar]
- 2.Sengupta S, Kulkarni A. Design principles for clinical efficacy of cancer nanomedicine: a look into the basics. ACS nano. 2013;7:2878–2882. doi: 10.1021/nn4015399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Udpa N, Million RP. Monoclonal antibody biosimilars. Nat Rev Drug Discov. 2016;15:13–14. doi: 10.1038/nrd.2015.12. [DOI] [PubMed] [Google Scholar]
- 4.Syed BA, Evans JB, Bielory L. Wet AMD market. Nat Rev Drug Discov. 2012;11:827–827. doi: 10.1038/nrd3790. [DOI] [PubMed] [Google Scholar]
- 5.Kim YC, Chiang B, Wu X, Prausnitz MR. Ocular delivery of macromolecules. Journal of controlled release : official journal of the Controlled Release Society. 2014;190:172–181. doi: 10.1016/j.jconrel.2014.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.S.I.P.M. Limited. The Ophthalmology Market. 2015:1–46. [Google Scholar]
- 7.Thomas D. Venture Funding of Therapeutic Innovation: A Comprehensive Look at a Decade of Venture Funding of Drug R&D. 2015:1–51. [Google Scholar]
- 8.Walsh G. Biopharmaceutical benchmarks 2010. Nat Biotech. 2010;28:917–924. doi: 10.1038/nbt0910-917. [DOI] [PubMed] [Google Scholar]
- 9.Helzner J. FDA rulings advance biosimilar drugs. Ophthalmology Management. 2016;20:34, 44, 45. [Google Scholar]
- 10.Mitragotri S, Burke PA, Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discov. 2014;13:655–672. doi: 10.1038/nrd4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torchilin V. Intracellular delivery of protein and peptide therapeutics. Drug discovery today. Technologies. 2008;5:e95–e103. doi: 10.1016/j.ddtec.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Truong-Le V, Lovalenti PM, Abdul-Fattah AM. Stabilization challenges and formulation strategies associated with oral biologic drug delivery systems. Advanced drug delivery reviews. 2015;93:95–108. doi: 10.1016/j.addr.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Varma R, Vajaranant TS, Burkemper B, Wu S, Torres M, Hsu C, Choudhury F, McKean-Cowdin R. Visual Impairment and Blindness in Adults in the United States: Demographic and Geographic Variations From 2015 to 2050. JAMA ophthalmology. 2016;134:802–809. doi: 10.1001/jamaophthalmol.2016.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang K, Zhang L, Weinreb RN. Ophthalmic drug discovery: novel targets and mechanisms for retinal diseases and glaucoma. Nat Rev Drug Discov. 2012;11:541–559. doi: 10.1038/nrd3745. [DOI] [PubMed] [Google Scholar]
- 15.Balevic SJ, Rabinovich CE. Profile of adalimumab and its potential in the treatment of uveitis. Drug design, development and therapy. 2016;10:2997–3003. doi: 10.2147/DDDT.S94188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Semeraro F, Morescalchi F, Duse S, Parmeggiani F, Gambicorti E, Costagliola C. Aflibercept in wet AMD: specific role and optimal use. Drug design, development and therapy. 2013;7:711–722. doi: 10.2147/DDDT.S40215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu L, Lu T, Tuomi L, Jumbe N, Lu J, Eppler S, Kuebler P, Damico-Beyer LA, Joshi A. Pharmacokinetics of ranibizumab in patients with neovascular age-related macular degeneration: a population approach. Investigative ophthalmology & visual science. 2013;54:1616–1624. doi: 10.1167/iovs.12-10260. [DOI] [PubMed] [Google Scholar]
- 18.Vinores SA. Pegaptanib in the treatment of wet, age-related macular degeneration. International journal of nanomedicine. 2006;1:263–268. [PMC free article] [PubMed] [Google Scholar]
- 19.Moisseiev E, Waisbourd M, Ben-Artsi E, Levinger E, Barak A, Daniels T, Csaky K, Loewenstein A, Barequet IS. Pharmacokinetics of bevacizumab after topical and intravitreal administration in human eyes. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2014;252:331–337. doi: 10.1007/s00417-013-2495-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrara N, Adamis AP. Ten years of anti-vascular endothelial growth factor therapy. Nat Rev Drug Discov. 2016;15:385–403. doi: 10.1038/nrd.2015.17. [DOI] [PubMed] [Google Scholar]
- 21.Traynor K. Aflibercept approved for macular degeneration. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2012;69:6. doi: 10.2146/news120001. [DOI] [PubMed] [Google Scholar]
- 22.Sharma YRTK, Venkatesh P, Gogia V. Aflibercept – How does it compare with other Anti-VEGF Drugs? Austin Journal of Clinical Ophthalmology. 2014;1:1–8. [Google Scholar]
- 23.Ng EW, Shima DT, Calias P, Cunningham ET, Jr, Guyer DR, Adamis AP. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Discov. 2006;5:123–132. doi: 10.1038/nrd1955. [DOI] [PubMed] [Google Scholar]
- 24.Chang JH, Garg NK, Lunde E, Han KY, Jain S, Azar DT. Corneal neovascularization: an anti-VEGF therapy review. Survey of ophthalmology. 2012;57:415–429. doi: 10.1016/j.survophthal.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sidman RL, Li J, Lawrence M, Hu W, Musso GF, Giordano RJ, Cardo-Vila M, Pasqualini R, Arap W. The peptidomimetic Vasotide targets two retinal VEGF receptors and reduces pathological angiogenesis in murine and nonhuman primate models of retinal disease. Science translational medicine. 2015;7:309ra165. doi: 10.1126/scitranslmed.aac4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neri P, Lettieri M, Fortuna C, Zucchi M, Manoni M, Celani S, Giovannini A. Adalimumab (humira) in ophthalmology: a review of the literature. Middle East African journal of ophthalmology. 2010;17:290–296. doi: 10.4103/0974-9233.71588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodrigues EB, Farah ME, Maia M, Penha FM, Regatieri C, Melo GB, Pinheiro MM, Zanetti CR. Therapeutic monoclonal antibodies in ophthalmology. Progress in retinal and eye research. 2009;28:117–144. doi: 10.1016/j.preteyeres.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 28.Theodossiadis PG, Markomichelakis NN, Sfikakis PP. Tumor necrosis factor antagonists: preliminary evidence for an emerging approach in the treatment of ocular inflammation. Retina. 2007;27:399–413. doi: 10.1097/MAJ.0b013e3180318fbc. [DOI] [PubMed] [Google Scholar]
- 29.Grant MB, Afzal A, Spoerri P, Pan H, Shaw LC, Mames RN. The role of growth factors in the pathogenesis of diabetic retinopathy. Expert opinion on investigational drugs. 2004;13:1275–1293. doi: 10.1517/13543784.13.10.1275. [DOI] [PubMed] [Google Scholar]
- 30.Limb GA, Hollifield RD, Webster L, Charteris DG, Chignell AH. Soluble TNF receptors in vitreoretinal proliferative disease. Investigative ophthalmology & visual science. 2001;42:1586–1591. [PubMed] [Google Scholar]
- 31.Murray PI, Sivaraj RR. Anti-TNF-alpha therapy for uveitis: Behcet and beyond. Eye. 2005;19:831–833. doi: 10.1038/sj.eye.6701792. [DOI] [PubMed] [Google Scholar]
- 32.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 33.Khalili H, Lee RW, Khaw PT, Brocchini S, Dick AD, Copland DA. An anti-TNF-alpha antibody mimetic to treat ocular inflammation. Sci Rep. 2016;6:36905. doi: 10.1038/srep36905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez-Fernandez de la Camara C, Hernandez-Pinto AM, Olivares-Gonzalez L, Cuevas-Martin C, Sanchez-Arago M, Hervas D, Salom D, Cuezva JM, de la Rosa EJ, Millan JM, Rodrigo R. Adalimumab Reduces Photoreceptor Cell Death in A Mouse Model of Retinal Degeneration. Sci Rep. 2015;5:11764. doi: 10.1038/srep11764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Puddu A, Sanguineti R, Montecucco F, Viviani GL. Retinal pigment epithelial cells express a functional receptor for glucagon-like peptide-1 (GLP-1) Mediators of inflammation. 2013;2013:975032. doi: 10.1155/2013/975032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hernandez C, Bogdanov P, Corraliza L, Garcia-Ramirez M, Sola-Adell C, Arranz JA, Arroba AI, Valverde AM, Simo R. Topical Administration of GLP-1 Receptor Agonists Prevents Retinal Neurodegeneration in Experimental Diabetes. Diabetes. 2016;65:172–187. doi: 10.2337/db15-0443. [DOI] [PubMed] [Google Scholar]
- 37.Pluckthun A. Designed ankyrin repeat proteins (DARPins): binding proteins for research, diagnostics, and therapy. Annual review of pharmacology and toxicology. 2015;55:489–511. doi: 10.1146/annurev-pharmtox-010611-134654. [DOI] [PubMed] [Google Scholar]
- 38.Souied EH, Devin F, Mauget-Faysse M, Kolar P, Wolf-Schnurrbusch U, Framme C, Gaucher D, Querques G, Stumpp MT, Wolf S, Group MPS. Treatment of exudative age-related macular degeneration with a designed ankyrin repeat protein that binds vascular endothelial growth factor: a phase I/II study. American journal of ophthalmology. 2014;158:724–732. e722. doi: 10.1016/j.ajo.2014.05.037. [DOI] [PubMed] [Google Scholar]
- 39.Sadiq MA, Hanout M, Sarwar S, Hassan M, Do DV, Nguyen QD, Sepah YJ. Platelet derived growth factor inhibitors: A potential therapeutic approach for ocular neovascularization. Saudi journal of ophthalmology : official journal of the Saudi Ophthalmological Society. 2015;29:287–291. doi: 10.1016/j.sjopt.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ambati J, Atkinson JP, Gelfand BD. Immunology of age-related macular degeneration. Nat Rev Immunol. 2013;13:438–451. doi: 10.1038/nri3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Radhakrishnan K, Sonali N, Moreno M, Nirmal J, Fernandez AA, Venkatraman S, Agrawal R. Protein delivery to the back of the eye: barriers, carriers and stability of anti-VEGF proteins. Drug discovery today. 2017;22:416–423. doi: 10.1016/j.drudis.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 42.Muheem A, Shakeel F, Jahangir MA, Anwar M, Mallick N, Jain GK, Warsi MH, Ahmad FJ. A review on the strategies for oral delivery of proteins and peptides and their clinical perspectives. Saudi pharmaceutical journal : SPJ : the official publication of the Saudi Pharmaceutical Society. 2016;24:413–428. doi: 10.1016/j.jsps.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Renukuntla J, Vadlapudi AD, Patel A, Boddu SH, Mitra AK. Approaches for enhancing oral bioavailability of peptides and proteins. International journal of pharmaceutics. 2013;447:75–93. doi: 10.1016/j.ijpharm.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yi X, Wang Y, Yu FS. Corneal epithelial tight junctions and their response to lipopolysaccharide challenge. Investigative ophthalmology & visual science. 2000;41:4093–4100. [PubMed] [Google Scholar]
- 45.Mitic LL, Van Itallie CM, Anderson JM. Molecular physiology and pathophysiology of tight junctions I. Tight junction structure and function: lessons from mutant animals and proteins. American journal of physiology. Gastrointestinal and liver physiology. 2000;279:G250–254. doi: 10.1152/ajpgi.2000.279.2.G250. [DOI] [PubMed] [Google Scholar]
- 46.Zelikin AN, Ehrhardt C, Healy AM. Materials and methods for delivery of biological drugs. Nature chemistry. 2016;8:997–1007. doi: 10.1038/nchem.2629. [DOI] [PubMed] [Google Scholar]
- 47.Tao Y, Li XX, Jiang YR, Bai XB, Wu BD, Dong JQ. Diffusion of macromolecule through retina after experimental branch retinal vein occlusion and estimate of intraretinal barrier. Current drug metabolism. 2007;8:151–156. doi: 10.2174/138920007779815968. [DOI] [PubMed] [Google Scholar]
- 48.Jackson TL, Antcliff RJ, Hillenkamp J, Marshall J. Human retinal molecular weight exclusion limit and estimate of species variation. Investigative ophthalmology & visual science. 2003;44:2141–2146. doi: 10.1167/iovs.02-1027. [DOI] [PubMed] [Google Scholar]
- 49.Huang AJ, Tseng SC, Kenyon KR. Paracellular permeability of corneal and conjunctival epithelia. Investigative ophthalmology & visual science. 1989;30:684–689. [PubMed] [Google Scholar]
- 50.Delplace V, Payne S, Shoichet M. Delivery strategies for treatment of age-related ocular diseases: From a biological understanding to biomaterial solutions. Journal of controlled release : official journal of the Controlled Release Society. 2015;219:652–668. doi: 10.1016/j.jconrel.2015.09.065. [DOI] [PubMed] [Google Scholar]
- 51.Charalel RA, Engberg K, Noolandi J, Cochran JR, Frank C, Ta CN. Diffusion of protein through the human cornea. Ophthalmic research. 2012;48:50–55. doi: 10.1159/000329794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahmed I, Gokhale RD, Shah MV, Patton TF. Physicochemical determinants of drug diffusion across the conjunctiva, sclera, and cornea. Journal of pharmaceutical sciences. 1987;76:583–586. doi: 10.1002/jps.2600760802. [DOI] [PubMed] [Google Scholar]
- 53.Schoenwald RD. Ocular drug delivery. Pharmacokinetic considerations. Clinical pharmacokinetics. 1990;18:255–269. doi: 10.2165/00003088-199018040-00001. [DOI] [PubMed] [Google Scholar]
- 54.Schoenwald RD, Ward RL. Relationship between steroid permeability across excised rabbit cornea and octanol-water partition coefficients. Journal of pharmaceutical sciences. 1978;67:786–788. doi: 10.1002/jps.2600670614. [DOI] [PubMed] [Google Scholar]
- 55.Allansmith M, de Ramus A, Maurice D. The dynamics of IgG in the cornea. Investigative ophthalmology & visual science. 1979;18:947–955. [PubMed] [Google Scholar]
- 56.Meek KM, Knupp C. Corneal structure and transparency. Progress in retinal and eye research. 2015;49:1–16. doi: 10.1016/j.preteyeres.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maurice DM, Watson PG. The distribution and movement of serum albumin in the cornea. Experimental eye research. 1965;4:355–363. doi: 10.1016/s0014-4835(65)80052-5. [DOI] [PubMed] [Google Scholar]
- 58.Kim JH, Green K, Martinez M, Paton D. Solute permeability of the corneal endothelium and Descemet’s membrane. Experimental eye research. 1971;12:231–238. doi: 10.1016/0014-4835(71)90143-6. [DOI] [PubMed] [Google Scholar]
- 59.Pescina S, Ferrari G, Govoni P, Macaluso C, Padula C, Santi P, Nicoli S. In-vitro permeation of bevacizumab through human sclera: effect of iontophoresis application. The Journal of pharmacy and pharmacology. 2010;62:1189–1194. doi: 10.1111/j.2042-7158.2010.01153.x. [DOI] [PubMed] [Google Scholar]
- 60.Olsen TW, Edelhauser HF, Lim JI, Geroski DH. Human scleral permeability. Effects of age, cryotherapy, transscleral diode laser, and surgical thinning. Investigative ophthalmology & visual science. 1995;36:1893–1903. [PubMed] [Google Scholar]
- 61.Maurice DM, Polgar J. Diffusion across the sclera. Experimental eye research. 1977;25:577–582. doi: 10.1016/0014-4835(77)90136-1. [DOI] [PubMed] [Google Scholar]
- 62.Pitkanen L, Ranta VP, Moilanen H, Urtti A. Permeability of retinal pigment epithelium: effects of permeant molecular weight and lipophilicity. Investigative ophthalmology & visual science. 2005;46:641–646. doi: 10.1167/iovs.04-1051. [DOI] [PubMed] [Google Scholar]
- 63.Bilati U, Allemann E, Doelker E. Strategic approaches for overcoming peptide and protein instability within biodegradable nano- and microparticles. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2005;59:375–388. doi: 10.1016/j.ejpb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 64.Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Advanced drug delivery reviews. 2006;58:1131–1135. doi: 10.1016/j.addr.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 65.Vaishya RD, Khurana V, Patel S, Mitra AK. Controlled ocular drug delivery with nanomicelles. Wiley interdisciplinary reviews. Nanomedicine and nanobiotechnology. 2014;6:422–437. doi: 10.1002/wnan.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Joseph M, Trinh HM, Cholkar K, Pal D, Mitra AK. Recent perspectives on the delivery of biologics to back of the eye. Expert opinion on drug delivery. 2016:1–15. doi: 10.1080/17425247.2016.1227783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Daugherty AL, Mrsny RJ. Formulation and delivery issues for monoclonal antibody therapeutics. Advanced drug delivery reviews. 2006;58:686–706. doi: 10.1016/j.addr.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 68.Sasahara K, McPhie P, Minton AP. Effect of dextran on protein stability and conformation attributed to macromolecular crowding. Journal of molecular biology. 2003;326:1227–1237. doi: 10.1016/s0022-2836(02)01443-2. [DOI] [PubMed] [Google Scholar]
- 69.Kerwin BA. Polysorbates 20 and 80 used in the formulation of protein biotherapeutics: structure and degradation pathways. Journal of pharmaceutical sciences. 2008;97:2924–2935. doi: 10.1002/jps.21190. [DOI] [PubMed] [Google Scholar]
- 70.Kompella UB, Kadam RS, Lee VH. Recent advances in ophthalmic drug delivery. Therapeutic delivery. 2010;1:435–456. doi: 10.4155/TDE.10.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Williams KA, Brereton HM, Farrall A, Standfield SD, Taylor SD, Kirk LA, Coster DJ. Topically applied antibody fragments penetrate into the back of the rabbit eye. Eye. 2005;19:910–913. doi: 10.1038/sj.eye.6701669. [DOI] [PubMed] [Google Scholar]
- 72.Jani R, Lang J, Rodeheaver D, Missel P, Roehrs R, Chowhan M. Modern Pharmaceutics. 4. CRC Press; 2002. Design and Evaluation of Ophthalmic Pharmaceutical Products. [Google Scholar]
- 73.Ali Y, Lehmussaari K. Industrial perspective in ocular drug delivery. Advanced drug delivery reviews. 2006;58:1258–1268. doi: 10.1016/j.addr.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 74.Subrizi A, Toropainen E, Ramsay E, Airaksinen AJ, Kaarniranta K, Urtti A. Oxidative stress protection by exogenous delivery of rhHsp70 chaperone to the retinal pigment epithelium (RPE), a possible therapeutic strategy against RPE degeneration. Pharmaceutical research. 2015;32:211–221. doi: 10.1007/s11095-014-1456-6. [DOI] [PubMed] [Google Scholar]
- 75.Schymkowitz J, Rousseau F. Protein aggregation: A rescue by chaperones. Nat Chem Biol. 2016;12:58–59. doi: 10.1038/nchembio.2006. [DOI] [PubMed] [Google Scholar]
- 76.Mitragotri S, Burke PA, Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discov. 2014;13:655–672. doi: 10.1038/nrd4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Harooni M, Freilich JM, Abelson M, Refojo M. Efficacy of hyaluronidase in reducing increases in intraocular pressure related to the use of viscoelastic substances. Archives of ophthalmology. 1998;116:1218–1221. doi: 10.1001/archopht.116.9.1218. [DOI] [PubMed] [Google Scholar]
- 78.Stern R, Jedrzejas MJ. Hyaluronidases: their genomics, structures, and mechanisms of action. Chemical reviews. 2006;106:818–839. doi: 10.1021/cr050247k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chao KL, Muthukumar L, Herzberg O. Structure of human hyaluronidase-1, a hyaluronan hydrolyzing enzyme involved in tumor growth and angiogenesis. Biochemistry. 2007;46:6911–6920. doi: 10.1021/bi700382g. [DOI] [PubMed] [Google Scholar]
- 80.Kozak I, Kayikcioglu OR, Cheng L, Falkenstein I, Silva GA, Yu DX, Freeman WR. The effect of recombinant human hyaluronidase on dexamethasone penetration into the posterior segment of the eye after sub-Tenon’s injection. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2006;22:362–369. doi: 10.1089/jop.2006.22.362. [DOI] [PubMed] [Google Scholar]
- 81.Frost GI. Recombinant human hyaluronidase (rHuPH20): an enabling platform for subcutaneous drug and fluid administration. Expert opinion on drug delivery. 2007;4:427–440. doi: 10.1517/17425247.4.4.427. [DOI] [PubMed] [Google Scholar]
- 82.Eisele YS, Monteiro C, Fearns C, Encalada SE, Wiseman RL, Powers ET, Kelly JW. Targeting protein aggregation for the treatment of degenerative diseases. Nat Rev Drug Discov. 2015;14:759–780. doi: 10.1038/nrd4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moreau KL, King JA. Protein misfolding and aggregation in cataract disease and prospects for prevention. Trends in molecular medicine. 2012;18:273–282. doi: 10.1016/j.molmed.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Das P, King JA, Zhou R. Aggregation of gamma-crystallins associated with human cataracts via domain swapping at the C-terminal beta-strands. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:10514–10519. doi: 10.1073/pnas.1019152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sauer T, Patel M, Chan CC, Tuo J. Unfolding the Therapeutic Potential of Chemical Chaperones for Age-related Macular Degeneration. Expert Rev Ophthalmol. 2008;3:29–42. doi: 10.1586/17469899.3.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schymkowitz J, Rousseau F. Protein aggregation: A rescue by chaperones. Nat Chem Biol. 2016;12:58–59. doi: 10.1038/nchembio.2006. [DOI] [PubMed] [Google Scholar]
- 87.Edelhauser HF, Rowe-Rendleman CL, Robinson MR, Dawson DG, Chader GJ, Grossniklaus HE, Rittenhouse KD, Wilson CG, Weber DA, Kuppermann BD, Csaky KG, Olsen TW, Kompella UB, Holers VM, Hageman GS, Gilger BC, Campochiaro PA, Whitcup SM, Wong WT. Ophthalmic drug delivery systems for the treatment of retinal diseases: basic research to clinical applications. Investigative ophthalmology & visual science. 2010;51:5403–5420. doi: 10.1167/iovs.10-5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Edelhauser HF, Boatright JH, Nickerson JM, Third APRIWG. Drug delivery to posterior intraocular tissues: third Annual ARVO/Pfizer Ophthalmics Research Institute Conference. Investigative ophthalmology & visual science. 2008;49:4712–4720. doi: 10.1167/iovs.08-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ghate D, Edelhauser HF. Ocular drug delivery. Expert opinion on drug delivery. 2006;3:275–287. doi: 10.1517/17425247.3.2.275. [DOI] [PubMed] [Google Scholar]
- 90.Bailon P, Won CY. PEG-modified biopharmaceuticals. Expert opinion on drug delivery. 2009;6:1–16. doi: 10.1517/17425240802650568. [DOI] [PubMed] [Google Scholar]
- 91.Turecek PL, Bossard MJ, Schoetens F, Ivens IA. PEGylation of Biopharmaceuticals: A Review of Chemistry and Nonclinical Safety Information of Approved Drugs. Journal of pharmaceutical sciences. 2016;105:460–475. doi: 10.1016/j.xphs.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 92.Swami R, Shahiwala A. Impact of physiochemical properties on pharmacokinetics of protein therapeutics. European journal of drug metabolism and pharmacokinetics. 2013;38:231–239. doi: 10.1007/s13318-013-0126-0. [DOI] [PubMed] [Google Scholar]
- 93.Kuo TT, Baker K, Yoshida M, Qiao SW, Aveson VG, Lencer WI, Blumberg RS. Neonatal Fc receptor: from immunity to therapeutics. Journal of clinical immunology. 2010;30:777–789. doi: 10.1007/s10875-010-9468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oganesyan V, Damschroder MM, Cook KE, Li Q, Gao C, Wu H, Dall’Acqua WF. Structural insights into neonatal Fc receptor-based recycling mechanisms. The Journal of biological chemistry. 2014;289:7812–7824. doi: 10.1074/jbc.M113.537563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sockolosky JT, Szoka FC. The neonatal Fc receptor, FcRn, as a target for drug delivery and therapy. Advanced drug delivery reviews. 2015;91:109–124. doi: 10.1016/j.addr.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zalevsky J, Chamberlain AK, Horton HM, Karki S, Leung IW, Sproule TJ, Lazar GA, Roopenian DC, Desjarlais JR. Enhanced antibody half-life improves in vivo activity. Nature biotechnology. 2010;28:157–159. doi: 10.1038/nbt.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mabry R, Gilbertson DG, Frank A, Vu T, Ardourel D, Ostrander C, Stevens B, Julien S, Franke S, Meengs B, Brody J, Presnell S, Hamacher NB, Lantry M, Wolf A, Bukowski T, Rosler R, Yen C, Anderson-Haley M, Brasel K, Pan Q, Franklin H, Thompson P, Dodds M, Underwood S, Peterson S, Sivakumar PV, Snavely M. A dual-targeting PDGFRbeta/VEGF-A molecule assembled from stable antibody fragments demonstrates anti-angiogenic activity in vitro and in vivo. mAbs. 2010;2:20–34. doi: 10.4161/mabs.2.1.10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Webber MJ, Appel EA, Vinciguerra B, Cortinas AB, Thapa LS, Jhunjhunwala S, Isaacs L, Langer R, Anderson DG. Supramolecular PEGylation of biopharmaceuticals. Proceedings of the National Academy of Sciences of the United States of America. 2016 doi: 10.1073/pnas.1616639113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moshfeghi AA, Rosenfeld PJ, Puliafito CA, Michels S, Marcus EN, Lenchus JD, Venkatraman AS. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration: twenty-four-week results of an uncontrolled open-label clinical study. Ophthalmology. 2006;113:2002 e2001–2012. doi: 10.1016/j.ophtha.2006.05.070. [DOI] [PubMed] [Google Scholar]
- 100.Rohrer B, Long Q, Coughlin B, Wilson RB, Huang Y, Qiao F, Tang PH, Kunchithapautham K, Gilkeson GS, Tomlinson S. A targeted inhibitor of the alternative complement pathway reduces angiogenesis in a mouse model of age-related macular degeneration. Investigative ophthalmology & visual science. 2009;50:3056–3064. doi: 10.1167/iovs.08-2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thurman JM, Renner B, Kunchithapautham K, Ferreira VP, Pangburn MK, Ablonczy Z, Tomlinson S, Holers VM, Rohrer B. Oxidative stress renders retinal pigment epithelial cells susceptible to complement-mediated injury. The Journal of biological chemistry. 2009;284:16939–16947. doi: 10.1074/jbc.M808166200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nomoto H, Shiraga F, Kuno N, Kimura E, Fujii S, Shinomiya K, Nugent AK, Hirooka K, Baba T. Pharmacokinetics of bevacizumab after topical, subconjunctival, and intravitreal administration in rabbits. Investigative ophthalmology & visual science. 2009;50:4807–4813. doi: 10.1167/iovs.08-3148. [DOI] [PubMed] [Google Scholar]
- 103.Dastjerdi MH, Sadrai Z, Saban DR, Zhang Q, Dana R. Corneal penetration of topical and subconjunctival bevacizumab. Investigative ophthalmology & visual science. 2011;52:8718–8723. doi: 10.1167/iovs.11-7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hernandez C, Garcia-Ramirez M, Corraliza L, Fernandez-Carneado J, Farrera-Sinfreu J, Ponsati B, Gonzalez-Rodriguez A, Valverde AM, Simo R. Topical administration of somatostatin prevents retinal neurodegeneration in experimental diabetes. Diabetes. 2013;62:2569–2578. doi: 10.2337/db12-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Eljarrat-Binstock E, Orucov F, Aldouby Y, Frucht-Pery J, Domb AJ. Charged nanoparticles delivery to the eye using hydrogel iontophoresis. Journal of controlled release : official journal of the Controlled Release Society. 2008;126:156–161. doi: 10.1016/j.jconrel.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 106.Peeters L, Lentacker I, Vandenbroucke RE, Lucas B, Demeester J, Sanders NN, De Smedt SC. Can ultrasound solve the transport barrier of the neural retina? Pharmaceutical research. 2008;25:2657–2665. doi: 10.1007/s11095-008-9684-2. [DOI] [PubMed] [Google Scholar]
- 107.Kumar CM, Eid H, Dodds C. Sub-Tenon’s anaesthesia: complications and their prevention. Eye. 2011;25:694–703. doi: 10.1038/eye.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mather CM, Kirkpatrick JN. Sub-Tenon’s administration of local anaesthetic: a review of the technique. British journal of anaesthesia. 2003;91:922. author reply 922–923. [PubMed] [Google Scholar]
- 109.Falavarjani KG, Khadamy J, Karimi Moghaddam A, Karimi N, Modarres M. Posterior sub-tenon’s bevacizumab injection in diabetic macular edema; a pilot study. Saudi journal of ophthalmology : official journal of the Saudi Ophthalmological Society. 2015;29:270–273. doi: 10.1016/j.sjopt.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hashemian MN, Zare MA, Rahimi F, Mohammadpour M. Deep intrastromal bevacizumab injection for management of corneal stromal vascularization after deep anterior lamellar keratoplasty, a novel technique. Cornea. 2011;30:215–218. doi: 10.1097/ICO.0b013e3181e291a6. [DOI] [PubMed] [Google Scholar]
- 111.Kim YC, Grossniklaus HE, Edelhauser HF, Prausnitz MR. Intrastromal delivery of bevacizumab using microneedles to treat corneal neovascularization. Investigative ophthalmology & visual science. 2014;55:7376–7386. doi: 10.1167/iovs.14-15257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Braga-Mele R, Chang DF, Henderson BA, Mamalis N, Talley-Rostov A, Vasavada A, Committee ACC. Intracameral antibiotics: Safety, efficacy, and preparation. Journal of cataract and refractive surgery. 2014;40:2134–2142. doi: 10.1016/j.jcrs.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 113.Daien V, Papinaud L, Gillies MC, et al. Effectiveness and safety of an intracameral injection of cefuroxime for the prevention of endophthalmitis after cataract surgery with or without perioperative capsular rupture. JAMA ophthalmology. 2016;134:810–816. doi: 10.1001/jamaophthalmol.2016.1351. [DOI] [PubMed] [Google Scholar]
- 114.Kuriakose T, Kothari M, Paul P, Jacob P, Thomas R. Intracameral amphotericin B injection in the management of deep keratomycosis. Cornea. 2002;21:653–656. doi: 10.1097/00003226-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 115.Hu J, Zhang J, Li Y, Han X, Zheng W, Yang J, Xu G. A Combination of Intrastromal and Intracameral Injections of Amphotericin B in the Treatment of Severe Fungal Keratitis. Journal of ophthalmology. 2016;2016:3436415. doi: 10.1155/2016/3436415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Park HY, Kim SJ, Lee HB, Kim ES, Tchah H. Effect of intracameral bevacizumab injection on corneal endothelium in rabbits. Cornea. 2008;27:1151–1155. doi: 10.1097/ICO.0b013e318180e572. [DOI] [PubMed] [Google Scholar]
- 117.Rusovici R, Sakhalkar M, Chalam KV. Evaluation of cytotoxicity of bevacizumab on VEGF-enriched corneal endothelial cells. Molecular vision. 2011;17:3339–3346. [PMC free article] [PubMed] [Google Scholar]
- 118.Shin JP, Lee JW, Sohn BJ, Kim HK, Kim SY. In vivo corneal endothelial safety of intracameral bevacizumab and effect in neovascular glaucoma combined with Ahmed valve implantation. Journal of glaucoma. 2009;18:589–594. doi: 10.1097/IJG.0b013e3181996ed2. [DOI] [PubMed] [Google Scholar]
- 119.Lim TH, Bae SH, Cho YJ, Lee JH, Kim HK, Sohn YH. Concentration of vascular endothelial growth factor after intracameral bevacizumab injection in eyes with neovascular glaucoma. Korean journal of ophthalmology : KJO. 2009;23:188–192. doi: 10.3341/kjo.2009.23.3.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wolf A, von Jagow B, Ulbig M, Haritoglou C. Intracameral injection of bevacizumab for the treatment of neovascular glaucoma. Ophthalmologica. Journal international d’ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 2011;226:51–56. doi: 10.1159/000327364. [DOI] [PubMed] [Google Scholar]
- 121.Grisanti S, Biester S, Peters S, Tatar O, Ziemssen F, Bartz-Schmidt KU. Intracameral bevacizumab for iris rubeosis. American journal of ophthalmology. 2006;142:158–160. doi: 10.1016/j.ajo.2006.02.045. [DOI] [PubMed] [Google Scholar]
- 122.Han Q, Wang Y, Li X, Peng R, Li A, Qian Z, Yu L. Effects of bevacizumab loaded PEG-PCL-PEG hydrogel intracameral application on intraocular pressure after glaucoma filtration surgery. Journal of materials science. Materials in medicine. 2015;26:225. doi: 10.1007/s10856-015-5556-6. [DOI] [PubMed] [Google Scholar]
- 123.Heiduschka P, Fietz H, Hofmeister S, Schultheiss S, Mack AF, Peters S, Ziemssen F, Niggemann B, Julien S, Bartz-Schmidt KU, Schraermeyer U Tubingen Bevacizumab Study G. Penetration of bevacizumab through the retina after intravitreal injection in the monkey. Investigative ophthalmology & visual science. 2007;48:2814–2823. doi: 10.1167/iovs.06-1171. [DOI] [PubMed] [Google Scholar]
- 124.Seiler GS, Salmon JH, Mantuo R, Feingold S, Dayton PA, Gilger BC. Effect and distribution of contrast medium after injection into the anterior suprachoroidal space in ex vivo eyes. Investigative ophthalmology & visual science. 2011;52:5730–5736. doi: 10.1167/iovs.11-7525. [DOI] [PubMed] [Google Scholar]
- 125.Patel SR, Berezovsky DE, McCarey BE, Zarnitsyn V, Edelhauser HF, Prausnitz MR. Targeted administration into the suprachoroidal space using a microneedle for drug delivery to the posterior segment of the eye. Investigative ophthalmology & visual science. 2012;53:4433–4441. doi: 10.1167/iovs.12-9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.do Rai UJ, Young SA, Thrimawithana TR, Abdelkader H, Alani AW, Pierscionek B, Alany RG. The suprachoroidal pathway: a new drug delivery route to the back of the eye. Drug discovery today. 2015;20:491–495. doi: 10.1016/j.drudis.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 127.Tyagi P, Kadam RS, Kompella UB. Comparison of suprachoroidal drug delivery with subconjunctival and intravitreal routes using noninvasive fluorophotometry. PloS one. 2012;7:e48188. doi: 10.1371/journal.pone.0048188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Olsen TW, Feng X, Wabner K, Csaky K, Pambuccian S, Cameron JD. Pharmacokinetics of pars plana intravitreal injections versus microcannula suprachoroidal injections of bevacizumab in a porcine model. Investigative ophthalmology & visual science. 2011;52:4749–4756. doi: 10.1167/iovs.10-6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Morales-Canton V, Fromow-Guerra J, Salinas Longoria S, Romero Vera R, Widmann M, Patel S, Yerxa B. Suprachoroidal Microinjection of Bevacizumab is Well Tolerated in Human Patients. Investigative ophthalmology & visual science. 2013;54:3299–3299. [Google Scholar]
- 130.Patel SR, Lin AS, Edelhauser HF, Prausnitz MR. Suprachoroidal drug delivery to the back of the eye using hollow microneedles. Pharmaceutical research. 2011;28:166–176. doi: 10.1007/s11095-010-0271-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cohen S, Yoshioka T, Lucarelli M, Hwang LH, Langer R. Controlled delivery systems for proteins based on poly(lactic/glycolic acid) microspheres. Pharmaceutical research. 1991;8:713–720. doi: 10.1023/a:1015841715384. [DOI] [PubMed] [Google Scholar]
- 132.Ron E, Turek T, Mathiowitz E, Chasin M, Hageman M, Langer R. Controlled release of polypeptides from polyanhydrides. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:4176–4180. doi: 10.1073/pnas.90.9.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Davis ME, Brewster ME. Cyclodextrin-based pharmaceutics: past, present and future. Nat Rev Drug Discov. 2004;3:1023–1035. doi: 10.1038/nrd1576. [DOI] [PubMed] [Google Scholar]
- 134.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Carrasquillo KG, Ricker JA, Rigas IK, Miller JW, Gragoudas ES, Adamis AP. Controlled delivery of the anti-VEGF aptamer EYE001 with poly(lactic-co-glycolic)acid microspheres. Investigative ophthalmology & visual science. 2003;44:290–299. doi: 10.1167/iovs.01-1156. [DOI] [PubMed] [Google Scholar]
- 136.Cook GP, Burgess L, Wing J, Dowie T, Calias P, Shima DT, Campbell K, Allison D, Volker S, Schmidt P. Preparation and Characterization of Pegaptanib Sustained Release Microsphere Formulations for Intraocular Application. Investigative ophthalmology & visual science. 2006;47:5123–5123. [Google Scholar]
- 137.Gavini E, Chetoni P, Cossu M, Alvarez MG, Saettone MF, Giunchedi P. PLGA microspheres for the ocular delivery of a peptide drug, vancomycin using emulsification/spray-drying as the preparation method: in vitro/in vivo studies. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2004;57:207–212. doi: 10.1016/j.ejpb.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 138.Kompella UB, Bandi N, Ayalasomayajula SP. Subconjunctival nano- and microparticles sustain retinal delivery of budesonide, a corticosteroid capable of inhibiting VEGF expression. Investigative ophthalmology & visual science. 2003;44:1192–1201. doi: 10.1167/iovs.02-0791. [DOI] [PubMed] [Google Scholar]
- 139.Osswald CR, Kang-Mieler JJ. Controlled and Extended In Vitro Release of Bioactive Anti-Vascular Endothelial Growth Factors from a Microsphere-Hydrogel Drug Delivery System. Current eye research. 2016;41:1216–1222. doi: 10.3109/02713683.2015.1101140. [DOI] [PubMed] [Google Scholar]
- 140.Ye M, Kim S, Park K. Issues in long-term protein delivery using biodegradable microparticles. Journal of controlled release : official journal of the Controlled Release Society. 2010;146:241–260. doi: 10.1016/j.jconrel.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 141.Burke PA, Klumb LA, Herberger JD, Nguyen XC, Harrell RA, Zordich M. Poly(lactide-co-glycolide) microsphere formulations of darbepoetin alfa: spray drying is an alternative to encapsulation by spray-freeze drying. Pharmaceutical research. 2004;21:500–506. doi: 10.1023/B:PHAM.0000019305.79599.a5. [DOI] [PubMed] [Google Scholar]
- 142.Vaishya RD, Mandal A, Gokulgandhi M, Patel S, Mitra AK. Reversible hydrophobic ion-paring complex strategy to minimize acylation of octreotide during long-term delivery from PLGA microparticles. International journal of pharmaceutics. 2015;489:237–245. doi: 10.1016/j.ijpharm.2015.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mahlumba P, Choonara YE, Kumar P, du Toit LC, Pillay V. Stimuli-Responsive Polymeric Systems for Controlled Protein and Peptide Delivery: Future Implications for Ocular Delivery. Molecules. 2016;21 doi: 10.3390/molecules21081002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Vaishya RD, Mandal A, Patel S, Mitra AK. Extended release microparticle-in-gel formulation of octreotide: Effect of polymer type on acylation of peptide during in vitro release. International journal of pharmaceutics. 2015;496:676–688. doi: 10.1016/j.ijpharm.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Eljarrat-Binstock E, Pe’er J, Domb AJ. New techniques for drug delivery to the posterior eye segment. Pharmaceutical research. 2010;27:530–543. doi: 10.1007/s11095-009-0042-9. [DOI] [PubMed] [Google Scholar]
- 146.Zakharchenko S, Puretskiy N, Stoychev G, Stamm M, Ionov L. Temperature controlled encapsulation and release using partially biodegradable thermo-magneto-sensitive self-rolling tubes. Soft Matter. 2010;6:2633–2636. [Google Scholar]
- 147.Li H, Tran VV, Hu Y, Mark Saltzman W, Barnstable CJ, Tombran-Tink J. A PEDF N-terminal peptide protects the retina from ischemic injury when delivered in PLGA nanospheres. Experimental eye research. 2006;83:824–833. doi: 10.1016/j.exer.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 148.Kim H, Robinson SB, Csaky KG. Investigating the movement of intravitreal human serum albumin nanoparticles in the vitreous and retina. Pharmaceutical research. 2009;26:329–337. doi: 10.1007/s11095-008-9745-6. [DOI] [PubMed] [Google Scholar]
- 149.Hayashi A, Naseri A, Pennesi ME, de Juan E., Jr Subretinal delivery of immunoglobulin G with gold nanoparticles in the rabbit eye. Japanese journal of ophthalmology. 2009;53:249–256. doi: 10.1007/s10384-009-0655-x. [DOI] [PubMed] [Google Scholar]
- 150.Jin J, Zhou KK, Park K, Hu Y, Xu X, Zheng Z, Tyagi P, Kompella UB, Ma JX. Anti-inflammatory and antiangiogenic effects of nanoparticle-mediated delivery of a natural angiogenic inhibitor. Investigative ophthalmology & visual science. 2011;52:6230–6237. doi: 10.1167/iovs.10-6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Park K, Chen Y, Hu Y, Mayo AS, Kompella UB, Longeras R, Ma JX. Nanoparticle-mediated expression of an angiogenic inhibitor ameliorates ischemia-induced retinal neovascularization and diabetes-induced retinal vascular leakage. Diabetes. 2009;58:1902–1913. doi: 10.2337/db08-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang Z, Cheng R, Lee K, Tyagi P, Ding L, Kompella UB, Chen J, Xu X, Ma JX. Nanoparticle-mediated expression of a Wnt pathway inhibitor ameliorates ocular neovascularization. Arteriosclerosis, thrombosis, and vascular biology. 2015;35:855–864. doi: 10.1161/ATVBAHA.114.304627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Busik JV, Grant MB. Wnting out ocular neovascularization: using nanoparticle delivery of very-low density lipoprotein receptor extracellular domain as Wnt pathway inhibitor in the retina. Arteriosclerosis, thrombosis, and vascular biology. 2015;35:1046–1047. doi: 10.1161/ATVBAHA.115.305395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mahaling B, Katti DS. Physicochemical properties of core-shell type nanoparticles govern their spatiotemporal biodistribution in the eye. Nanomedicine : nanotechnology, biology, and medicine. 2016;12:2149–2160. doi: 10.1016/j.nano.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 155.Patel SP, Vaishya R, Patel A, Agrahari V, Pal D, Mitra AK. Optimization of novel pentablock copolymer based composite formulation for sustained delivery of peptide/protein in the treatment of ocular diseases. Journal of microencapsulation. 2016;33:103–113. doi: 10.3109/02652048.2015.1134685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Agrahari V, Agrahari V, Hung WT, Christenson LK, Mitra AK. Composite Nanoformulation Therapeutics for Long-Term Ocular Delivery of Macromolecules. Molecular pharmaceutics. 2016;13:2912–2922. doi: 10.1021/acs.molpharmaceut.5b00828. [DOI] [PubMed] [Google Scholar]
- 157.Patel SP, Vaishya R, Pal D, Mitra AK. Novel pentablock copolymer-based nanoparticulate systems for sustained protein delivery. AAPS PharmSciTech. 2015;16:327–343. doi: 10.1208/s12249-014-0196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Patel SP, Vaishya R, Mishra GP, Tamboli V, Pal D, Mitra AK. Tailor-made pentablock copolymer based formulation for sustained ocular delivery of protein therapeutics. Journal of drug delivery. 2014;2014:401747. doi: 10.1155/2014/401747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Senturk B, Cubuk MO, Ozmen MC, Aydin B, Guler MO, Tekinay AB. Inhibition of VEGF mediated corneal neovascularization by anti-angiogenic peptide nanofibers. Biomaterials. 2016;107:124–132. doi: 10.1016/j.biomaterials.2016.08.045. [DOI] [PubMed] [Google Scholar]
- 160.Thomas CR, Ferris DP, Lee JH, Choi E, Cho MH, Kim ES, Stoddart JF, Shin JS, Cheon J, Zink JI. Noninvasive remote-controlled release of drug molecules in vitro using magnetic actuation of mechanized nanoparticles. Journal of the American Chemical Society. 2010;132:10623–10625. doi: 10.1021/ja1022267. [DOI] [PubMed] [Google Scholar]
- 161.Xiao Z, Ji C, Shi J, Pridgen EM, Frieder J, Wu J, Farokhzad OC. DNA self-assembly of targeted near-infrared-responsive gold nanoparticles for cancer thermo-chemotherapy. Angewandte Chemie. 2012;51:11853–11857. doi: 10.1002/anie.201204018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Abrishami M, Zarei-Ghanavati S, Soroush D, Rouhbakhsh M, Jaafari MR, Malaekeh-Nikouei B. Preparation, characterization, and in vivo evaluation of nanoliposomes-encapsulated bevacizumab (avastin) for intravitreal administration. Retina. 2009;29:699–703. doi: 10.1097/IAE.0b013e3181a2f42a. [DOI] [PubMed] [Google Scholar]
- 163.Davis BM, Normando EM, Guo L, Turner LA, Nizari S, O’Shea P, Moss SE, Somavarapu S, Cordeiro MF. Topical delivery of Avastin to the posterior segment of the eye in vivo using annexin A5-associated liposomes. Small. 2014;10:1575–1584. doi: 10.1002/smll.201303433. [DOI] [PubMed] [Google Scholar]
- 164.Cortesi R, Argnani R, Esposito E, Dalpiaz A, Scatturin A, Bortolotti F, Lufino M, Guerrini R, Cavicchioni G, Incorvaia C, Menegatti E, Manservigi R. Cationic liposomes as potential carriers for ocular administration of peptides with anti-herpetic activity. International journal of pharmaceutics. 2006;317:90–100. doi: 10.1016/j.ijpharm.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 165.Li T, Zhang M, Han Y, Zhang H, Xu L, Xiang Y. Targeting therapy of choroidal neovascularization by use of polypeptide- and PEDF-loaded immunoliposomes under ultrasound exposure. Journal of Huazhong University of Science and Technology. Medical sciences = Hua zhong ke ji da xue xue bao. Yi xue Ying De wen ban = Huazhong keji daxue xuebao. Yixue Yingdewen ban. 2010;30:798–803. doi: 10.1007/s11596-010-0661-8. [DOI] [PubMed] [Google Scholar]
- 166.Cavalli R, Gasco MR, Chetoni P, Burgalassi S, Saettone MF. Solid lipid nanoparticles (SLN) as ocular delivery system for tobramycin. International journal of pharmaceutics. 2002;238:241–245. doi: 10.1016/s0378-5173(02)00080-7. [DOI] [PubMed] [Google Scholar]
- 167.Chetoni P, Burgalassi S, Monti D, Tampucci S, Tullio V, Cuffini AM, Muntoni E, Spagnolo R, Zara GP, Cavalli R. Solid lipid nanoparticles as promising tool for intraocular tobramycin delivery: Pharmacokinetic studies on rabbits. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2016;109:214–223. doi: 10.1016/j.ejpb.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 168.Sandri G, Bonferoni MC, Gokce EH, Ferrari F, Rossi S, Patrini M, Caramella C. Chitosan-associated SLN: in vitro and ex vivo characterization of cyclosporine A loaded ophthalmic systems. Journal of microencapsulation. 2010;27:735–746. doi: 10.3109/02652048.2010.517854. [DOI] [PubMed] [Google Scholar]
- 169.Sousa F, Castro P, Fonte P, Kennedy PJ, Neves-Petersen MT, Sarmento B. Nanoparticles for the delivery of therapeutic antibodies: Dogma or promising strategy? Expert opinion on drug delivery. 2016:1–14. doi: 10.1080/17425247.2017.1273345. [DOI] [PubMed] [Google Scholar]
- 170.Seyfoddin A, Shaw J, Al-Kassas R. Solid lipid nanoparticles for ocular drug delivery. Drug delivery. 2010;17:467–489. doi: 10.3109/10717544.2010.483257. [DOI] [PubMed] [Google Scholar]
- 171.Liu R, Liu Z, Zhang C, Zhang B. Nanostructured lipid carriers as novel ophthalmic delivery system for mangiferin: improving in vivo ocular bioavailability. Journal of pharmaceutical sciences. 2012;101:3833–3844. doi: 10.1002/jps.23251. [DOI] [PubMed] [Google Scholar]
- 172.Hippalgaonkar K, Adelli GR, Hippalgaonkar K, Repka MA, Majumdar S. Indomethacin-loaded solid lipid nanoparticles for ocular delivery: development, characterization, and in vitro evaluation. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2013;29:216–228. doi: 10.1089/jop.2012.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang Y, Rajala A, Rajala RV. Lipid Nanoparticles for Ocular Gene Delivery. Journal of functional biomaterials. 2015;6:379–394. doi: 10.3390/jfb6020379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Schroeder A, Goldberg MS, Kastrup C, Wang Y, Jiang S, Joseph BJ, Levins CG, Kannan ST, Langer R, Anderson DG. Remotely activated protein-producing nanoparticles. Nano letters. 2012;12:2685–2689. doi: 10.1021/nl2036047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Al-Ahmady ZS, Al-Jamal WT, Bossche JV, Bui TT, Drake AF, Mason AJ, Kostarelos K. Lipid-peptide vesicle nanoscale hybrids for triggered drug release by mild hyperthermia in vitro and in vivo. ACS nano. 2012;6:9335–9346. doi: 10.1021/nn302148p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chen KJ, Liang HF, Chen HL, Wang Y, Cheng PY, Liu HL, Xia Y, Sung HW. A thermoresponsive bubble-generating liposomal system for triggering localized extracellular drug delivery. ACS nano. 2013;7:438–446. doi: 10.1021/nn304474j. [DOI] [PubMed] [Google Scholar]
- 177.Kazi KM, Mandal AS, Biswas N, Guha A, Chatterjee S, Behera M, Kuotsu K. Niosome: A future of targeted drug delivery systems. J Adv Pharm Technol Res. 2010;1:374–380. doi: 10.4103/0110-5558.76435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mandal A, Cholkar K, Khurana V, Shah A, Agrahari V, Bisht R, Pal D, Mitra AK. Topical Formulation of Self-Assembled Antiviral Prodrug Nanomicelles for Targeted Retinal Delivery. Molecular pharmaceutics. 2017;14:2056–2069. doi: 10.1021/acs.molpharmaceut.7b00128. [DOI] [PubMed] [Google Scholar]
- 179.Cholkar K, Gilger BC, Mitra AK. Topical, Aqueous, Clear Cyclosporine Formulation Design for Anterior and Posterior Ocular Delivery. Translational vision science & technology. 2015;4:1. doi: 10.1167/tvst.4.3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Li J, Li Z, Zhou T, Zhang J, Xia H, Li H, He J, He S, Wang L. Positively charged micelles based on a triblock copolymer demonstrate enhanced corneal penetration. International journal of nanomedicine. 2015;10:6027–6037. doi: 10.2147/IJN.S90347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mandal A, Bisht R, Rupenthal ID, Mitra AK. Polymeric micelles for ocular drug delivery: From structural frameworks to recent preclinical studies. Journal of controlled release : official journal of the Controlled Release Society. 2017;248:96–116. doi: 10.1016/j.jconrel.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Oh EJ, Park K, Choi JS, Joo CK, Hahn SK. Synthesis, characterization, and preliminary assessment of anti-Flt1 peptide-hyaluronate conjugate for the treatment of corneal neovascularization. Biomaterials. 2009;30:6026–6034. doi: 10.1016/j.biomaterials.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 183.Oh EJ, Choi JS, Kim H, Joo CK, Hahn SK. Anti-Flt1 peptide - hyaluronate conjugate for the treatment of retinal neovascularization and diabetic retinopathy. Biomaterials. 2011;32:3115–3123. doi: 10.1016/j.biomaterials.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 184.Kim H, Choi JS, Kim KS, Yang JA, Joo CK, Hahn SK. Flt1 peptide-hyaluronate conjugate micelle-like nanoparticles encapsulating genistein for the treatment of ocular neovascularization. Acta biomaterialia. 2012;8:3932–3940. doi: 10.1016/j.actbio.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 185.Rapoport NY, Kennedy AM, Shea JE, Scaife CL, Nam KH. Controlled and targeted tumor chemotherapy by ultrasound-activated nanoemulsions/microbubbles. Journal of controlled release : official journal of the Controlled Release Society. 2009;138:268–276. doi: 10.1016/j.jconrel.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yan Q, Yuan J, Cai Z, Xin Y, Kang Y, Yin Y. Voltage-responsive vesicles based on orthogonal assembly of two homopolymers. Journal of the American Chemical Society. 2010;132:9268–9270. doi: 10.1021/ja1027502. [DOI] [PubMed] [Google Scholar]
- 187.Lee ES, Gao Z, Kim D, Park K, Kwon IC, Bae YH. Super pH-sensitive multifunctional polymeric micelle for tumor pH(e) specific TAT exposure and multidrug resistance. Journal of controlled release : official journal of the Controlled Release Society. 2008;129:228–236. doi: 10.1016/j.jconrel.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lee CC, MacKay JA, Frechet JM, Szoka FC. Designing dendrimers for biological applications. Nature biotechnology. 2005;23:1517–1526. doi: 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]
- 189.Wang H, Huang Q, Chang H, Xiao J, Cheng Y. Stimuli-responsive dendrimers in drug delivery. Biomaterials Science. 2016;4:375–390. doi: 10.1039/c5bm00532a. [DOI] [PubMed] [Google Scholar]
- 190.Kambhampati SP, Kannan RM. Dendrimer nanoparticles for ocular drug delivery. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2013;29:151–165. doi: 10.1089/jop.2012.0232. [DOI] [PubMed] [Google Scholar]
- 191.McNerny DQ, Kukowska-Latallo JF, Mullen DG, Wallace JM, Desai AM, Shukla R, Huang B, Banaszak Holl MM, Baker JR., Jr RGD dendron bodies; synthetic avidity agents with defined and potentially interchangeable effector sites that can substitute for antibodies. Bioconjugate chemistry. 2009;20:1853–1859. doi: 10.1021/bc900217h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Waite CL, Roth CM. PAMAM-RGD conjugates enhance siRNA delivery through a multicellular spheroid model of malignant glioma. Bioconjugate chemistry. 2009;20:1908–1916. doi: 10.1021/bc900228m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yang H, Kao WJ. Synthesis and characterization of nanoscale dendritic RGD clusters for potential applications in tissue engineering and drug delivery. International journal of nanomedicine. 2007;2:89–99. doi: 10.2147/nano.2007.2.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bayele HK, Ramaswamy C, Wilderspin AF, Srai KS, Toth I, Florence AT. Protein transduction by lipidic peptide dendrimers. Journal of pharmaceutical sciences. 2006;95:1227–1237. doi: 10.1002/jps.20606. [DOI] [PubMed] [Google Scholar]
- 195.Draz MS, Fang BA, Zhang P, Hu Z, Gu S, Weng KC, Gray JW, Chen FF. Nanoparticle-mediated systemic delivery of siRNA for treatment of cancers and viral infections. Theranostics. 2014;4:872–892. doi: 10.7150/thno.9404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yuan X, Marcano DC, Shin CS, Hua X, Isenhart LC, Pflugfelder SC, Acharya G. Ocular drug delivery nanowafer with enhanced therapeutic efficacy. ACS nano. 2015;9:1749–1758. doi: 10.1021/nn506599f. [DOI] [PubMed] [Google Scholar]
- 197.Kim J, Schlesinger EB, Desai TA. Nanostructured materials for ocular delivery: nanodesign for enhanced bioadhesion, transepithelial permeability and sustained delivery. Therapeutic delivery. 2015;6:1365–1376. doi: 10.4155/tde.15.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bian F, Shin CS, Wang C, Pflugfelder SC, Acharya G, De Paiva CS. Dexamethasone Drug Eluting Nanowafers Control Inflammation in Alkali-Burned Corneas Associated With Dry Eye. Investigative ophthalmology & visual science. 2016;57:3222–3230. doi: 10.1167/iovs.16-19074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Coursey TG, Henriksson JT, Marcano DC, Shin CS, Isenhart LC, Ahmed F, De Paiva CS, Pflugfelder SC, Acharya G. Dexamethasone nanowafer as an effective therapy for dry eye disease. Journal of controlled release : official journal of the Controlled Release Society. 2015;213:168–174. doi: 10.1016/j.jconrel.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 200.Marcano DC, Shin CS, Lee B, Isenhart LC, Liu X, Li F, Jester JV, Pflugfelder SC, Simpson J, Acharya G. Synergistic Cysteamine Delivery Nanowafer as an Efficacious Treatment Modality for Corneal Cystinosis. Molecular pharmaceutics. 2016;13:3468–3477. doi: 10.1021/acs.molpharmaceut.6b00488. [DOI] [PubMed] [Google Scholar]
- 201.Yasukawa T, Ogura Y, Sakurai E, Tabata Y, Kimura H. Intraocular sustained drug delivery using implantable polymeric devices. Advanced drug delivery reviews. 2005;57:2033–2046. doi: 10.1016/j.addr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 202.Malcles A, Dot C, Voirin N, Vie AL, Agard E, Bellocq D, Denis P, Kodjikian L. SAFETY OF INTRAVITREAL DEXAMETHASONE IMPLANT (OZURDEX): The SAFODEX study. Incidence and Risk Factors of Ocular Hypertension. Retina. 2016 doi: 10.1097/IAE.0000000000001369. [DOI] [PubMed] [Google Scholar]
- 203.Christoforidis JB, Chang S, Jiang A, Wang J, Cebulla CM. Intravitreal devices for the treatment of vitreous inflammation. Mediators of inflammation. 2012;2012:126463. doi: 10.1155/2012/126463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wang J, Jiang A, Joshi M, Christoforidis J. Drug delivery implants in the treatment of vitreous inflammation. Mediators of inflammation. 2013;2013:780634. doi: 10.1155/2013/780634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhou T, Lewis H, Foster RE, Schwendeman SP. Development of a multiple-drug delivery implant for intraocular management of proliferative vitreoretinopathy. Journal of controlled release : official journal of the Controlled Release Society. 1998;55:281–295. doi: 10.1016/s0168-3659(98)00061-3. [DOI] [PubMed] [Google Scholar]
- 206.Bourges JL, Bloquel C, Thomas A, Froussart F, Bochot A, Azan F, Gurny R, BenEzra D, Behar-Cohen F. Intraocular implants for extended drug delivery: therapeutic applications. Advanced drug delivery reviews. 2006;58:1182–1202. doi: 10.1016/j.addr.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 207.Taban M, Lowder CY, Kaiser PK. Outcome of fluocinolone acetonide implant (Retisert) reimplantation for chronic noninfectious posterior uveitis. Retina. 2008;28:1280–1288. doi: 10.1097/IAE.0b013e31817d8bf2. [DOI] [PubMed] [Google Scholar]
- 208.Jaffe GJ, Martin D, Callanan D, Pearson PA, Levy B, Comstock T Fluocinolone Acetonide Uveitis Study G. Fluocinolone acetonide implant (Retisert) for noninfectious posterior uveitis: thirty-four-week results of a multicenter randomized clinical study. Ophthalmology. 2006;113:1020–1027. doi: 10.1016/j.ophtha.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 209.Ambati J, Gragoudas ES, Miller JW, You TT, Miyamoto K, Delori FC, Adamis AP. Transscleral delivery of bioactive protein to the choroid and retina. Investigative ophthalmology & visual science. 2000;41:1186–1191. [PubMed] [Google Scholar]
- 210.Agarwal P, Rupenthal ID. Injectable implants for the sustained release of protein and peptide drugs. Drug discovery today. 2013;18:337–349. doi: 10.1016/j.drudis.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 211.Agrawal AK, Das M, Jain S. In situ gel systems as ‘smart’ carriers for sustained ocular drug delivery. Expert opinion on drug delivery. 2012;9:383–402. doi: 10.1517/17425247.2012.665367. [DOI] [PubMed] [Google Scholar]
- 212.Tyagi P, Barros M, Stansbury JW, Kompella UB. Light-activated in situ forming gel for sustained suprachoroidal delivery of bevacizumab. Molecular pharmaceutics. 2013;10:2858–2867. doi: 10.1021/mp300716t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wang CH, Hwang YS, Chiang PR, Shen CR, Hong WH, Hsiue GH. Extended release of bevacizumab by thermosensitive biodegradable and biocompatible hydrogel. Biomacromolecules. 2012;13:40–48. doi: 10.1021/bm2009558. [DOI] [PubMed] [Google Scholar]
- 214.Hu CC, Chaw JR, Chen CF, Liu HW. Controlled release bevacizumab in thermoresponsive hydrogel found to inhibit angiogenesis. Bio-medical materials and engineering. 2014;24:1941–1950. doi: 10.3233/BME-141003. [DOI] [PubMed] [Google Scholar]
- 215.Xu X, Weng Y, Xu L, Chen H. Sustained release of Avastin(R) from polysaccharides cross-linked hydrogels for ocular drug delivery. International journal of biological macromolecules. 2013;60:272–276. doi: 10.1016/j.ijbiomac.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 216.Lovett ML, Wang X, Yucel T, York L, Keirstead M, Haggerty L, Kaplan DL. Silk hydrogels for sustained ocular delivery of anti-vascular endothelial growth factor (anti-VEGF) therapeutics. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2015;95:271–278. doi: 10.1016/j.ejpb.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 217.Rauck BM, Friberg TR, Medina Mendez CA, Park D, Shah V, Bilonick RA, Wang Y. Biocompatible reverse thermal gel sustains the release of intravitreal bevacizumab in vivo. Investigative ophthalmology & visual science. 2014;55:469–476. doi: 10.1167/iovs.13-13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kaplan IM, Wadia JS, Dowdy SF. Cationic TAT peptide transduction domain enters cells by macropinocytosis. Journal of controlled release : official journal of the Controlled Release Society. 2005;102:247–253. doi: 10.1016/j.jconrel.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 219.Wang Y, Lin H, Lin S, Qu J, Xiao J, Huang Y, Xiao Y, Fu X, Yang Y, Li X. Cell-penetrating peptide TAT-mediated delivery of acidic FGF to retina and protection against ischemia-reperfusion injury in rats. Journal of cellular and molecular medicine. 2010;14:1998–2005. doi: 10.1111/j.1582-4934.2009.00786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Johnson LN, Cashman SM, Read SP, Kumar-Singh R. Cell penetrating peptide POD mediates delivery of recombinant proteins to retina, cornea and skin. Vision research. 2010;50:686–697. doi: 10.1016/j.visres.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Johnson LN, Cashman SM, Kumar-Singh R. Cell-penetrating peptide for enhanced delivery of nucleic acids and drugs to ocular tissues including retina and cornea. Molecular therapy : the journal of the American Society of Gene Therapy. 2008;16:107–114. doi: 10.1038/sj.mt.6300324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Young Kim H, Young Yum S, Jang G, Ahn D-R. Discovery of a non-cationic cell penetrating peptide derived from membrane-interacting human proteins and its potential as a protein delivery carrier. Scientific Reports. 2015;5:11719. doi: 10.1038/srep11719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ho J, Uger RA, Zwick MB, Luscher MA, Barber BH, MacDonald KS. Conformational constraints imposed on a pan-neutralizing HIV-1 antibody epitope result in increased antigenicity but not neutralizing response. Vaccine. 2005;23:1559–1573. doi: 10.1016/j.vaccine.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 224.Bird GH, Madani N, Perry AF, Princiotto AM, Supko JG, He X, Gavathiotis E, Sodroski JG, Walensky LD. Hydrocarbon double-stapling remedies the proteolytic instability of a lengthy peptide therapeutic. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14093–14098. doi: 10.1073/pnas.1002713107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Cardoso RM, Brunel FM, Ferguson S, Zwick M, Burton DR, Dawson PE, Wilson IA. Structural basis of enhanced binding of extended and helically constrained peptide epitopes of the broadly neutralizing HIV-1 antibody 4E10. Journal of molecular biology. 2007;365:1533–1544. doi: 10.1016/j.jmb.2006.10.088. [DOI] [PubMed] [Google Scholar]
- 226.Leaman DP, Zwick MB. Increased functional stability and homogeneity of viral envelope spikes through directed evolution. PLoS pathogens. 2013;9:e1003184. doi: 10.1371/journal.ppat.1003184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ingale S, Dawson PE. On resin side-chain cyclization of complex peptides using CuAAC. Organic letters. 2011;13:2822–2825. doi: 10.1021/ol200775h. [DOI] [PubMed] [Google Scholar]
- 228.Bird GH, Irimia A, Ofek G, Kwong PD, Wilson IA, Walensky LD. Stapled HIV-1 peptides recapitulate antigenic structures and engage broadly neutralizing antibodies. Nature structural & molecular biology. 2014;21:1058–1067. doi: 10.1038/nsmb.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Chu Q, Moellering RE, Hilinski GJ, Kim Y-W, Grossmann TN, Yeh JTH, Verdine GL. Towards understanding cell penetration by stapled peptides. MedChemComm. 2015;6:111–119. [Google Scholar]
- 230.Brandt CR. Peptide therapeutics for treating ocular surface infections. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2014;30:691–699. doi: 10.1089/jop.2014.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Bird GH, Irimia A, Ofek G, Kwong PD, Wilson IA, Walensky LD. Stapled HIV-1 peptides recapitulate antigenic structures and engage broadly neutralizing antibodies. Nature structural & molecular biology. 2014;21:1058–1067. doi: 10.1038/nsmb.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Emerich DF, Thanos CG. NT-501: an ophthalmic implant of polymer-encapsulated ciliary neurotrophic factor-producing cells. Current opinion in molecular therapeutics. 2008;10:506–515. [PubMed] [Google Scholar]
- 233.Kuno N, Fujii S. Biodegradable intraocular therapies for retinal disorders: progress to date. Drugs & aging. 2010;27:117–134. doi: 10.2165/11530970-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 234.Barar J, Aghanejad A, Fathi M, Omidi Y. Advanced drug delivery and targeting technologies for the ocular diseases. BioImpacts : BI. 2016;6:49–67. doi: 10.15171/bi.2016.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Li F, Hurley B, Liu Y, Leonard B, Griffith M. Controlled release of bevacizumab through nanospheres for extended treatment of age-related macular degeneration. The open ophthalmology journal. 2012;6:54–58. doi: 10.2174/1874364101206010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Yandrapu SK, Upadhyay AK, Petrash JM, Kompella UB. Nanoparticles in porous microparticles prepared by supercritical infusion and pressure quench technology for sustained delivery of bevacizumab. Molecular pharmaceutics. 2013;10:4676–4686. doi: 10.1021/mp400487f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ye Z, Ji YL, Ma X, Wen JG, Wei W, Huang SM. Pharmacokinetics and distributions of bevacizumab by intravitreal injection of bevacizumab-PLGA microspheres in rabbits. International journal of ophthalmology. 2015;8:653–658. doi: 10.3980/j.issn.2222-3959.2015.04.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Freeman WR, Sailor M, Chen M, Cheng L. Nanostructured Porous Silicon Dioxide Microparticles as an Intravitreal Injectable Drug Delivery System for Avastin (Bevacizumab) Lasting Six Months. Investigative ophthalmology & visual science. 2012;53:456–456. [Google Scholar]
- 239.Varshochian R, Jeddi-Tehrani M, Mahmoudi AR, Khoshayand MR, Atyabi F, Sabzevari A, Esfahani MR, Dinarvand R. The protective effect of albumin on bevacizumab activity and stability in PLGA nanoparticles intended for retinal and choroidal neovascularization treatments. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences. 2013;50:341–352. doi: 10.1016/j.ejps.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 240.Varshochian R, Riazi-Esfahani M, Jeddi-Tehrani M, Mahmoudi AR, Aghazadeh S, Mahbod M, Movassat M, Atyabi F, Sabzevari A, Dinarvand R. Albuminated PLGA nanoparticles containing bevacizumab intended for ocular neovascularization treatment. Journal of biomedical materials research. Part A. 2015;103:3148–3156. doi: 10.1002/jbm.a.35446. [DOI] [PubMed] [Google Scholar]
- 241.Lance KD, Bernards DA, Ciaccio NA, Good SD, Mendes TS, Kudisch M, Chan E, Ishikiriyama M, Bhisitkul RB, Desai TA. In vivo and in vitro sustained release of ranibizumab from a nanoporous thin-film device. Drug delivery and translational research. 2016;6:771–780. doi: 10.1007/s13346-016-0298-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Humayun M, Santos A, Altamirano JC, Ribeiro R, Gonzalez R, de la Rosa A, Shih J, Pang C, Jiang F, Calvillo P, Huculak J, Zimmerman J, Caffey S. Implantable MicroPump for Drug Delivery in Patients with Diabetic Macular Edema. Translational vision science & technology. 2014;3:5. doi: 10.1167/tvst.3.6.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Lim JI, Niec M, Wong V. One year results of a phase 1 study of the safety and tolerability of combination therapy using sustained release intravitreal triamcinolone acetonide and ranibizumab for subfoveal neovascular AMD. The British journal of ophthalmology. 2015;99:618–623. doi: 10.1136/bjophthalmol-2014-306002. [DOI] [PubMed] [Google Scholar]
- 244.Yu Y, Lau LC, Lo AC, Chau Y. Injectable Chemically Crosslinked Hydrogel for the Controlled Release of Bevacizumab in Vitreous: A 6-Month In Vivo Study. Translational vision science & technology. 2015;4:5. doi: 10.1167/tvst.4.2.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kirchhof S, Gregoritza M, Messmann V, Hammer N, Goepferich AM, Brandl FP. Diels-Alder hydrogels with enhanced stability: First step toward controlled release of bevacizumab. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2015;96:217–225. doi: 10.1016/j.ejpb.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 246.Alexander A, Ajazuddin, Khan J, Saraf S, Saraf S. Polyethylene glycol (PEG)-Poly(N-isopropylacrylamide) (PNIPAAm) based thermosensitive injectable hydrogels for biomedical applications. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2014;88:575–585. doi: 10.1016/j.ejpb.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 247.Zhang K, Hopkins JJ, Heier JS, Birch DG, Halperin LS, Albini TA, Brown DM, Jaffe GJ, Tao W, Williams GA. Ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for treatment of geographic atrophy in age-related macular degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:6241–6245. doi: 10.1073/pnas.1018987108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Smith J, Ward D, Michaelides M, Moore AT, Simpson S. New and emerging technologies for the treatment of inherited retinal diseases: a horizon scanning review. Eye. 2015;29:1131–1140. doi: 10.1038/eye.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Song HB, Lee KJ, Seo IH, Lee JY, Lee SM, Kim JH, Kim JH, Ryu W. Impact insertion of transfer-molded microneedle for localized and minimally invasive ocular drug delivery. Journal of controlled release : official journal of the Controlled Release Society. 2015;209:272–279. doi: 10.1016/j.jconrel.2015.04.041. [DOI] [PubMed] [Google Scholar]
- 250.Kim YC, Edelhauser HF, Prausnitz MR. Targeted delivery of antiglaucoma drugs to the supraciliary space using microneedles. Investigative ophthalmology & visual science. 2014;55:7387–7397. doi: 10.1167/iovs.14-14651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Thakur RR, Fallows SJ, McMillan HL, Donnelly RF, Jones DS. Microneedle-mediated intrascleral delivery of in situ forming thermoresponsive implants for sustained ocular drug delivery. The Journal of pharmacy and pharmacology. 2014;66:584–595. doi: 10.1111/jphp.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Aimi MF, Rao MP, MacDonald NC, Zuruzi AS, Bothman DP. High-aspect-ratio bulk micromachining of titanium. Nat Mater. 2004;3:103–105. doi: 10.1038/nmat1058. [DOI] [PubMed] [Google Scholar]
- 253.Khandan O, Famili A, Kahook MY, Rao MP. Titanium-based, fenestrated, in-plane microneedles for passive ocular drug delivery. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:6572–6575. doi: 10.1109/EMBC.2012.6347500. [DOI] [PubMed] [Google Scholar]
- 254.Vaka SR, Sammeta SM, Day LB, Murthy SN. Transcorneal iontophoresis for delivery of ciprofloxacin hydrochloride. Current eye research. 2008;33:661–667. doi: 10.1080/02713680802270945. [DOI] [PubMed] [Google Scholar]
- 255.Frucht-Pery J, Raiskup F, Mechoulam H, Shapiro M, Eljarrat-Binstock E, Domb A. Iontophoretic treatment of experimental pseudomonas keratitis in rabbit eyes using gentamicin-loaded hydrogels. Cornea. 2006;25:1182–1186. doi: 10.1097/01.ico.0000243959.14651.18. [DOI] [PubMed] [Google Scholar]
- 256.Raiskup-Wolf F, Eljarrat-Binstock E, Rehak M, Domb A, Frucht-Pery J. Transcorneal and transscleral iontophoresis of the dexamethasone phosphate into the rabbit eye. Cesk Slov Oftalmol. 2007;63:360–368. [PubMed] [Google Scholar]
- 257.Eljarrat-Binstock E, Orucov F, Frucht-Pery J, Pe’er J, Domb AJ. Methylprednisolone delivery to the back of the eye using hydrogel iontophoresis. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2008;24:344–350. doi: 10.1089/jop.2007.0097. [DOI] [PubMed] [Google Scholar]
- 258.Eljarrat-Binstock E, Domb AJ, Orucov F, Dagan A, Frucht-Pery J, Pe’er J. In vitro and in vivo evaluation of carboplatin delivery to the eye using hydrogel-iontophoresis. Current eye research. 2008;33:269–275. doi: 10.1080/02713680701871140. [DOI] [PubMed] [Google Scholar]
- 259.Eljarrat-Binstock E, Domb AJ, Orucov F, Frucht-Pery J, Pe’er J. Methotrexate delivery to the eye using transscleral hydrogel iontophoresis. Current eye research. 2007;32:639–646. doi: 10.1080/02713680701528674. [DOI] [PubMed] [Google Scholar]
- 260.Eljarrat-Binstock E, Domb AJ. Iontophoresis: a non-invasive ocular drug delivery. Journal of controlled release : official journal of the Controlled Release Society. 2006;110:479–489. doi: 10.1016/j.jconrel.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 261.Zhang Y, Chen Y, Yu X, Qi Y, Chen Y, Liu Y, Hu Y, Li Z. A flexible device for ocular iontophoretic drug delivery. Biomicrofluidics. 2016;10:011911. doi: 10.1063/1.4942516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.