Abstract
The suprachoroidal space (SCS) is a potential space between the sclera and choroid that traverses the circumference of the posterior segment of the eye. The SCS is an attractive site for drug delivery because it targets the choroid, retinal pigment epithelium and retina with high bioavailability, while maintaining low levels elsewhere in the eye. Indeed, phase III clinical trials are investigating the safety and efficacy of SCS drug delivery. Here, we review the anatomy and physiology of the SCS; methods to access the SCS; kinetics of SCS drug delivery; strategies to target within the SCS; current and potential clinical indications; and the safety and efficacy of this approach in preclinical animal studies and clinical trials.
Keywords: microneedle, suprachoroidal space, ocular drug delivery, ophthalmic targeting, posterior segment of the eye, uveitis, macular edema
Graphical Abstract
1 The need for novel routes of administration to the posterior segment
Ocular pathology and disease can result in visual impairment and blindness, and consequently, significant loss in quality of life[1–4]. In particular, posterior segment diseases, such as age-related macular degeneration (AMD), diabetic retinopathy (DR), noninfectious uveitis, or central serous chorioretinopathy, can result in permanent vision loss once retinal architecture and physiology are disrupted[5]. The effectiveness of existing and new drugs is limited by the delivery of these drugs to the site of disease in a spatially and temporally controlled manner due to the eye’s small size and unique barriers[6–8].
Traditional routes of delivery, namely topical eye drops and intravitreal injections, are the current gold standards used for treating ophthalmic disease. Topical eye drops result in low bioavailability (1–7%) within the anterior chamber of human eyes, and negligible penetration past the anterior chamber[9, 10]. Thus, topical eye drops have limited applications in the management of posterior segment diseases in humans. Intravitreal injections of corticosteroids and monoclonal antibodies against vascular endothelial growth factor (VEGF) have been used to treat retinal diseases with great effect and have revolutionized the treatment of posterior segment diseases[6, 7, 11–14]. Such a procedure can be performed in the outpatient clinic setting by a trained ophthalmologist under topical or local anesthesia. The vitreous humor acts as a natural depot, slowly releasing drug over ~1 month, depending on formulation[15, 16]. However, the drug diffuses isotropically through the vitreous and can thus diffuse towards non-target regions of the eye (e.g., lens and ciliary body), resulting in side effects[17–21]. An intact blood-retinal barrier can also impede the transport of drugs from the vitreous to the choroid and retinal pigment epithelium [8, 22]. In summary, traditional ophthalmic routes of administration are not able to target specific tissues within the eye, resulting in low bioavailability at the diseased tissue(s) and/or possible side effects due to drug affecting non-target tissues.
1.1 The suprachoroidal space as a novel route of administration
The suprachoroidal space (SCS) is a potential space found between the sclera and choroid (Figure 1). Under typical physiological conditions, the SCS is mostly collapsed due to the intraocular pressure (IOP) and fibers that attach the sclera to the choroid[23, 24]. The SCS has a nominal thickness of 35 μm, so that the choroid can slide against the sclera during accommodation[24, 25]. The SCS plays a role in maintaining IOP via the uveoscleral outflow, which is an alternative drainage route for aqueous humor[26]. Suprachoroidal hemorrhage is an urgent ophthalmic indication, where the choroid bleeds into the SCS. Patients with suprachoroidal hemorrhage report significant pain and can have vision loss. Surgery may be necessary to drain the hematoma and re-appose the sclera and choroid. Therefore, any procedures that access the SCS should avoid causing hemorrhage.
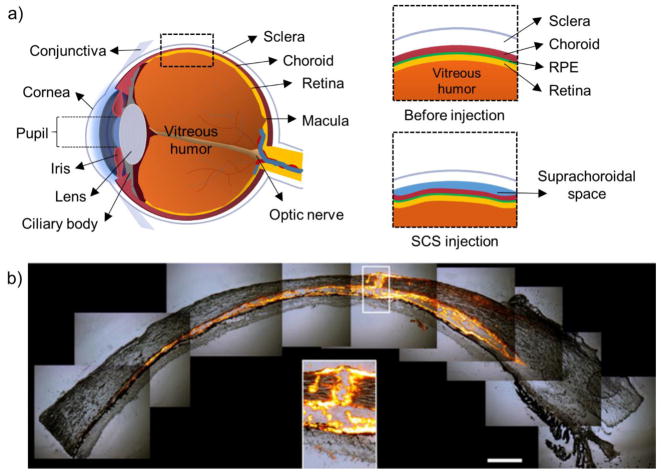
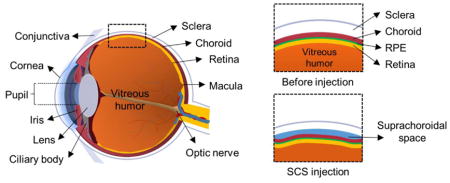
Figure 1.
Diagram of eye before and after suprachoroidal space (SCS) injection. (a) Diagram of eye with relevant anatomy labeled. Insets show before and after SCS injection. (b) Histology of ex vivo porcine eye immediately after microneedle injection of fluorescent particles into SCS.
Due to its proximity to the choroid, the SCS is an attractive site of drug delivery for posterior segment diseases because high bioavailability within the choroid and potentially retina can be achieved[27, 28]. Thus, a lower drug dose (aka, dose sparing) can be used to achieve similar efficacy compared with traditional routes of administration[29]. Furthermore, drug is compartmentalized in the SCS away from non-diseased tissues, which is expected to result in a more favorable side-effect profile[27, 28]. Because of these reasons, drug delivery via the SCS has become an attractive alternative and/or adjunct that is under intense investigation. Most of the research in this field has occurred recently. In fact, of the 39 peer-reviewed research articles indexed on PubMed with the term ‘suprachoroidal drug delivery’, 6 were published in 2017 and 23 were published in 2013 – 2017.
2 Accessing the suprachoroidal space
A method or procedure to access the SCS in a safe, reliable, and efficient manner has been a major hurdle preventing widespread use of this route of administration until recently (Figure 2). In the preclinical setting, access has generally been via sclerotomy (i.e., cutting across the sclera); ab interno surgical approaches have also been used for surgical implantation of glaucoma filters. However, the only method that is currently FDA-cleared is a sclerotomy with micro-cannulation into the SCS (iScience catheter, Ellex Medical, Adelaide, Australia), although this method is not standard of care[30]. As with all surgical approaches, there are risks associated and the procedure must be performed in the operating room. Because an intravitreal injection is easier to perform and carries a relatively low risk, it has been the preferred method of posterior segment delivery. Ongoing clinical trials testing the safety and efficacy of microneedle injections into the SCS have demonstrated that this procedure is reliable and efficient, and can be performed in the outpatient ophthalmologist office under local anesthesia.
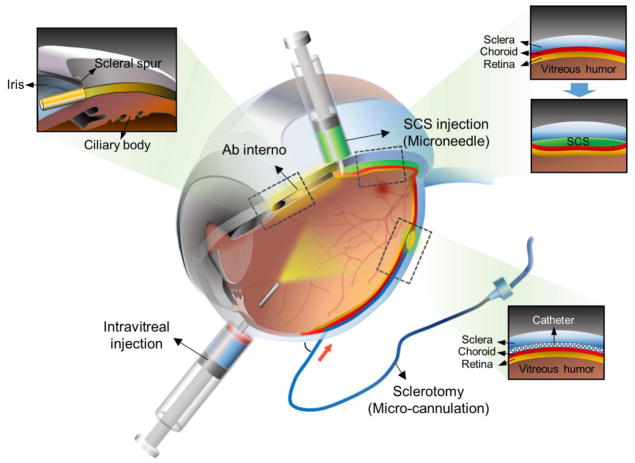
Figure 2.
Diagram of eye highlighting routes of administration to the posterior segment of the eye. Intravitreal injection enables delivery to the posterior segment and is the current gold standard. The SCS can be accessed by ab interno surgical technique, by sclerotomy with subsequent micro-cannulation, or by microneedle injection to the SCS.
2.1 Surgical incision
Drug delivery to the SCS has been achieved by dissecting through the sclera under general or monitored anesthesia[31–37]. Access to the SCS can be achieved via a full-thickness scleral incision, generally with a scalpel, performed in the operating room[28, 31–34, 38–43]. Identification of the scleral-choroidal junction (where the SCS is found) requires slow and delicate dissection so as not to inadvertently cut through the choroid and retina. The retinal pigment epithelium and pigmented choroid serve as a readily identifiable plane to dissect to. Once the sclerotomy is completed, a blunt-tipped catheter can be used to tunnel through the SCS towards the posterior pole[28, 32, 33, 37, 40, 41]. A catheter with a flashing diode has been used to visualize the advancement of the catheter under a microsurgical scope[28, 33]. After infusion, sutures may or may not be used to seal the sclera. The benefits of accessing the SCS via incision and catheterization is that the site of drug delivery can be visualized and chosen by the ophthalmologist. For example, this might be beneficial when targeting chemotherapies to the SCS underlying ocular tumors[44].
Monolithic implants have been placed into the SCS after surgical incision through the sclera. These have been used for a variety of indications, including drug delivery[34, 43, 45–47], glaucoma[48], retinal degeneration[49, 50], and retinal detachments[51–55].
2.2 Ab interno
The Cypass (Transcend[56]) and iStent (Glaukos[57, 58]) micro-stent medical devices are used for lowering IOP in glaucoma refractory to medical therapy. They are surgically implanted within the SCS during cataract surgery using an ab interno approach. During cataract surgery, the micro-stent is guided into the anterior chamber through the keratotomy incision (i.e., corneal incision) and pierced through the scleral spur opposite the keratotomy. Once in position, the device serves as a conduit to enable fluid egress from the anterior chamber to the SCS, thereby enabling IOP reduction.
2.3 Injections
Some groups chose to use a hypodermic needle to directly inject into the SCS[36, 53, 59–63]. This can be a challenging procedure, since visualization of the scleral-choroidal plane is not possible with this method, and instead tactile cues must be used to indicate when the sclera has been penetrated. When used to evacuate suprachoroidal hemorrhage, there is more leeway in targeting an ideal depth because the SCS is expanded with blood[60]. However, when targeting the typically collapsed SCS, this technique is highly dependent on user experience, as a 30 gauge hypodermic needle (~300 μm outer diameter, bevel length > 1 mm) is directed through the ~500 μm thick sclera[64]. Furthermore, demonstrating safety of this method in the clinical setting would be complicated given the sharp learning curve and variability among users. Another issue is targeted drug delivery within the SCS, since the injected material should be targeted towards diseased regions; strategies to guide drugs to specific regions of the SCS are under investigation.
2.3.1 Microneedles
Microneedles have been developed and used to deposit drug into the SCS in a simple and reliable manner[65, 66] (Figure 3). A microneedle is a hollow-bore needle with a length matched to the thickness of the sclera and conjunctiva. The length is chosen so the microneedle is physically unable to penetrate deeper than the SCS (i.e., through the choroid and retina) and perform an inadvertent intravitreal injection. Fluid injected into this space spreads circumferentially within the SCS, bathing the choroid with drug. Furthermore, the same procedure used for intravitreal injection can be used with a microneedle for SCS injection, and can thus be performed in the outpatient clinic setting by ophthalmologists with patients under local anesthesia. To accurately control the insertion depth, the microneedle is positioned perpendicular to the scleral surface and the hard stop at the hub at the microneedles’ base contacts the sclera/conjunctiva. Reflux out the injection site is possible, so the microneedle is often kept in position for ~1 min to minimize reflux. Injection success was found to be dependent on needle length, needle gauge, infusion pressure, and particle size, and did not depend on intraocular pressure[65]. Parameters that affect the force of microneedle penetration through the sclera and into the SCS have been studied, and can be easily administered by hand[67].
Figure 3.
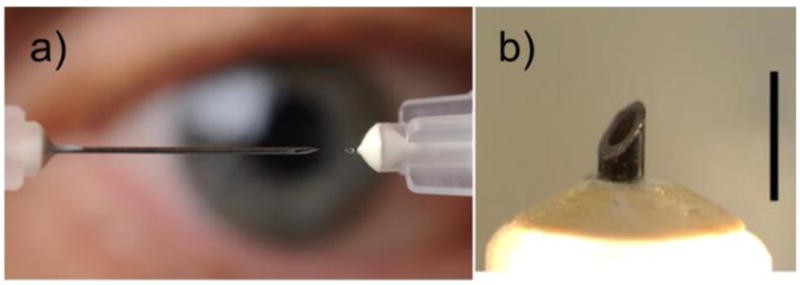
Photographs of microneedle. (a) Photograph of 1½ inch, 30 gauge hypodermic needle (left) and microneedle (right). Reproduced with permission, Gary Meek, Georgia Tech. (b) Magnified photograph of microneedle. Scale bar is 1 mm.
3 Pharmacokinetics within the suprachoroidal space
3.1 Distribution within ocular layers
Immediately after injection into the SCS, higher levels of small molecules and macromolecules were found in the choroidal, retinal pigment epithelium (RPE), and retinal tissues, compared with intravitreal injections[27, 28, 33, 36, 37, 59, 61, 62, 65, 66, 68, 69]. For example, fluorescein was selectively localized to the chorioretina 25–200 times more with than an intravitreal injection[27, 66]. Immediately post-injection of 3mg of bevacizumab, 0.2 μg/mg-tissue and 1.6 μg/mg-tissue of bevacizumab were detected in the choroid after intravitreal and suprachoroidal delivery, respectively[28]. Suprachoroidal injection of bevacizumab resulted in significantly lower drug levels in the aqueous humor and vitreous humor [28]. The concentration of ketorolac detected in the chorioretina was 208 μg/g after intravitreal injection at 30 min and 57 μg/g after suprachoroidal injection at 1 h[37]. Stem cells injected into the SCS using a novel injector were localized to the extravascular choroid [35, 63, 70]. Furthermore, anterior segment tissues, including lens, aqueous humor, and cornea, were largely spared from drug [27, 28, 36, 61, 66]. However, drug was cleared significantly faster with SCS delivery than intravitreal or subconjunctival injections, with small molecules being cleared within a few hours and macromolecules within one day[28, 36, 38]. Particles as small as 20 nm, however were not cleared from the SCS [66, 71]. For this reason, long-acting formulations are needed to enable extended drug delivery in the SCS after a single injection [28, 66, 71–73]. In contrast, the vitreous humor can serve as a depot that slowly releases drug – including small molecules and macromolecules – to the retina and adjacent choroid[15, 16, 28, 74].
3.2 Circumferential distribution
Though fluid delivered into the SCS distributes circumferentially around the eye in the SCS, it does not typically cover the entire space[66, 68, 75]. Similarly, most ocular diseases do not affect the eye uniformly (e.g., AMD affects the macula while sparing the peripheral retina[76–78], while retinitis pigmentosa affects the peripheral retina while sparing the macula[79]). Therefore, targeting to specific regions of the SCS is of interest. Targeting within the SCS can be achieved via the catheterization approach or via formulations and other methods that control spread within the SCS during and after injection.
When particles were injected into the SCS using a microneedle, their flow within the space was limited by the scleral spur and optic nerve[25, 65, 66]. Additional anatomical barriers (the long posterior ciliary arteries in rabbits ex vivo and in vivo and the short posterior ciliary arteries in human cadaver eyes) have also been seen to prevent circumferential particle spread within the SCS[80]. Particles injected in close proximity to these vessels did not spread isotropically like particles injected far from these anatomical features.
Since the SCS is distensible, it is important to determine how injected fluid distributes within the SCS: by increasing circumferential area, by increasing the cross-sectional thickness, or a combination of the two[71, 81]. Multiple studies have shown that increasing injection volume resulted in increasing coverage area[65, 66, 68, 71, 75, 81]. However, it was difficult to cover 100%, likely due to anatomical barriers[80]. Maximal SCS thickness was found to depend on injection volumes, with thickness ranging from 1.7 to 2.8 mm[31]. While the thickness generally increased with injection volume, there did not appear to be a linear trend, especially with IOP at physiological levels[31]. Other studies found that increasing injection volumes resulted in increasing SCS cross-sectional area under optical coherence tomography[68] and, separately, that the median SCS thickness was constant (~160 μm)with injection volumes ranging from 25 μL to 150 μL[71]. The SCS thickness could, however, be further increased by up to 2.8 mm at the site of injection by injecting highly viscous formulations into the SCS[71]. These two findings suggest that SCS thickness is determined by a force balance between the viscous forces of the injected formulation that limit spreading over larger areas in the SCS and the biomechanical elastic forces of the tissue and the SCS fibrils that run between the sclera and choroid that inhibit expansion of SCS thickness [71].
Ultrasound contrast agent injected into the SCS showed a distribution that was near the scleral spur (i.e., the most anterior portion of the SCS) with the cornea facing up, probably due to buoyancy of the low-density particles[31]. High-density particles injected in the SCS could similarly use gravity to control particle distribution[82]. The dense particles sank via decreased buoyancy towards the back of the eye if the eye was oriented upright with respect to gravity, thereby targeting the most-posterior structures like macula.
Other studies have shown that formulations – notably including polymers that impart high viscosity – can influence distribution of particles in the SCS not only during injection but subsequently too[75]. For example, carboxymethyl cellulose localized the injected particles near the site of injection due in part to crosslinking of the polymers to create a gel [29, 75]. In contrast, hyaluronic acid increased viscosity such that spreading during injection was inhibited, but over the course of hours to days after injection, the presence of hyaluronic acid enabled injected particles to spread to cover up to 100% of the SCS. Polymeric formulations (such as hyaluronic acid) are hypothesized to spread well after injections since they exert an osmotic pressure that seeks to imbibe fluid, resulting in expansion of the formulation, and may also coat particles and/or tissue to prevent non-specific binding. By tracking the distribution of particles and fluid formulations simultaneously, the injected fluid traveled further into the SCS than the injected particles when using low-viscosity formulations, suggesting some degree of particle entrapment with the SCS; with high-viscosity formulations, the discrepancy between the travel distance of the particle and polymeric formulation was decreased [81]. These findings suggest that these viscous formulations increased transit time in the SCS, and reduced particle-tissue interactions[81].
3.3 Clearance
3.3.1 Clearance kinetics from the SCS
When comparing the pharmacokinetics and distribution of molecules in the SCS against intravitreal injections, higher levels of injected molecules have been found in the chorioretina with significantly faster clearance after SCS injection. SCS collapse, which can be used as a proxy for clearance of injected liquid (i.e., water) from the SCS was found to reach baseline levels within 40 – 60 min[68, 83].
Considering clearance of molecules, higher levels of bevacizumab were found in the chorioretina 12 h after SCS injection compared with intravitreal injection[28]. However, at 7 days, bevacizumab was undetectable in the SCS group, but remained relatively high in the intravitreal group. Another study reported a clearance half-life of 3.6 – 7.9 h for various macromolecules injected into the SCS[65, 66]. Sodium fluorescein concentration in the chorioretina was found to be 25-fold higher after SCS injection compared with intravitreal injection; however, 2 h after injection, fluorescein levels in the chorioretina were higher in the intravitreal injection group than in the SCS group[62]. Similarly, the elimination half-life of another small molecules, ketorolac, was longer after intravitreal injection than SCS delivery (3.1 h vs. 1.2 h)[36] and, surprisingly, corresponded to a lower Cmax in the chorioretina for the SCS group than the intravitreal group.
Increasing molecular weight up to 500 kDa had only a minor effect on SCS clearance rate[28, 66, 83]. Molecules up to 500 kDa were cleared from the eye within 2 days, but very large macromolecules (2 MDa) had significantly slower clearance than the 500 kDa macromolecules, taking up to 20 days to fully clear[83].
Polystyrene microparticles with diameters as small as 20 nm delivered into the SCS could be found at least 4 months post-injection[65, 66, 75]. Because macromolecules are not rigid, they may be able to adopt a conformation that can exit the eye (e.g., via blood capillaries). On the other hand, polystyrene microspheres are rigid and cannot change conformation and thereby cannot fit into pathways that exit the eye.
3.3.2 Clearance route from the SCS
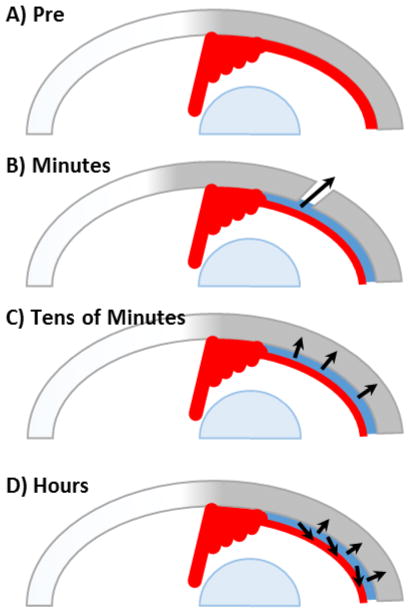
The route of clearance from the SCS is also a topic of interest. After intravitreal injection, xenon and radioactive water were cleared from the eye via choroidal blood flow[84, 85], which has been proposed as the clearance pathway after SCS injection too[28, 61]. Consistent with this hypothesis, fluorescein was cleared from the SCS significantly faster with choroidal perfusion in the living eye than without in a postmortem porcine model[38]. In the context of studies elucidating the uveoscleral outflow pathway, microspheres injected into the anterior chamber were found trapped in the SCS especially where blood vessels penetrated through the sclera (aka., perivascular drainage routes)[86, 87], suggesting this as another pathway for SCS clearance. By systematically studying the clearance routes of fluorescein from the SCS, another study identified three regimes of clearance: (i) initial leakage of fluorescein from the injection site and perivascular leakage sites, which occurred on a timescale of minutes; (ii) pressure-driven trans-scleral movement of fluorescein on a timescale of tens of minutes, (iii) diffusion into the choroid and subsequent intravascular clearance on a timescale of hours[83] (Figure 4). These results were corroborated with a two-dimensional mathematical model of the SCS and surrounding tissues[83].
Figure 4.
Diagram of proposed clearance route kinetic regimes. (A) Diagram of eye pre-injection. (B) Pressure-mediated leakage through injection site and perivascular drainage routes. (C) Pressure-mediated trans-scleral movement. (D) Concentration-mediated diffusion and clearance by the choroidal vasculature.
4 Pharmacodynamics
4.1 Distribution within cell layers
SCS delivery can result in high bioavailability in the sclera, choroid, and RPE[28, 32, 36, 59, 61, 66, 68–70, 88, 89]. For example, a small molecule HIF-1 inhibitor injected into the SCS of rats distributed throughout the choroid and retina, and prevented choroidal neovascularization in an animal model with selective destruction of Bruch’s membrane[69]. In another study, gene transfection was noted in the choroid and RPE after SCS delivery of genetically-engineered adeno-associated virus[32] and nonviral plasmids[88]. And stem cells injected into the SCS were distributed to up to 80% of the SCS, present for at least 10 weeks within the SCS, and were well tolerated without immunosuppression [70]. There is less information about bioavailability within the retina, though steroids injected into the SCS have been shown to resolve macular edema[46, 47, 73, 90]. The cornea, anterior chamber, and lens receive negligible concentrations of drug. Furthermore, dose sparing of drugs that have their mechanism of action at the ciliary body has been reported when using formulations that target the anterior SCS adjacent to the ciliary body[29, 72]. This pattern of distribution is very different than intravitreal injections[28, 36, 66] or topical eye drops[9].
4.2 Drug targets
4.2.1 Choroid
The choroid is a prime drug target for SCS delivery, since the choroid lines the SCS inner surface. Possible diseases of interest that affect the choroid include noninfectious uveitis, dry forms of AMD, choroideremia, uveal melanoma, and central serous chorioretinopathy. The therapeutic effects of corticosteroids[33, 45, 59, 73, 90], small molecules [29, 36, 46, 47, 69, 72], monoclonal antibodies[33, 62], and cells[35, 63] injected into the SCS have been tested in preclinical and clinical trials. Indeed, phase I/II clinical trial results have been promising[73]. Nine participants with noninfectious uveitis were treated with a microneedle injection of triamcinolone into the SCS, and good efficacy and safety were reported. Thirty-eight adverse events were reported, most commonly pain at time of injection. It is worth noting that injection of a corticosteroid like triamcinolone acetonide is expected to reduce inflammatory side effects and thereby affect the safety profile. Phase III clinical trials are underway to test the efficacy of a microneedle injection of triamcinolone acetonide into the SCS to treat noninfectious uveitis (NCT 03097315) and macular edema associated with retinal vein occlusion (NCT 02980874).
4.2.2 Ciliary body
Disease progression of primary open-angle glaucoma can be slowed with IOP-lowering medications and surgeries[9]. The SCS is bordered anteriorly by the ciliary body, which produces aqueous humor and inflates the eye. Furthermore, the SCS plays a role in uveoscleral outflow, one of the routes of aqueous humor drainage from the eye. Two preclinical studies have demonstrated that SCS delivery of ocular hypotensive agents results in IOP reduction[29, 72], as a potential treatment for glaucoma. These studies also showed dose-sparing with SCS injection compared with eye drops. Micro-stents penetrated through the scleral spur to generate a fluid conduit from the anterior chamber into the SCS have been shown to reduce IOP in glaucoma patients[48, 57, 91].
4.2.3 Retinal pigment epithelium
The RPE is a monolayer of epithelium with tight junctions that maintains the outer blood retinal barrier. Bruch’s membrane serves as the basement membrane for the RPE and the underlying choropcapillaris. Preclinical pharmacokinetic studies have demonstrated that high bioavailability is possible at the RPE, compared with other routes of administration[28, 32, 88]. Diseases of interest that are associated with RPE pathology include wet forms of AMD, and central serous chorioretinopathy. Though the pathogenesis of choroidal neovascularization is unknown, dysfunction of the RPE, Bruch’s membrane, and choriocapillaris have been implicated. No studies have yet used SCS delivery for therapeutic effect on the RPE. Studies have shown that choroidal neovascularization is more effectively treated with intravitreal injections of a receptor tyrosine kinase inhibitor than SCS injections, although the authors noted incomplete delivery with the SCS injection[89].
4.2.4 Retina
The retina is not typically the primary site of pathology in acquired posterior segment diseases. Instead, the breakdown of the retinal-RPE-choroidal unit appears to be associated with visual loss[5]. On the other hand, there are genetic diseases, such as retinitis pigmentosa, whereby toxic accumulation of visual cycle proteins results in vision loss. Pharmacokinetic studies have not demonstrated clear advantages of SCS delivery compared with intravitreal or subretinal injections for retinal treatments[28, 32, 88].
4.2.5 Sclera
Few posterior segment diseases affect the sclera. These include scleritis and refractive errors, such as myopia and hyperopia. There have been no published studies targeting these indications via the SCS. Because SCS delivery can achieve high bioavailability within the sclera, if these disease entities are of interest, then approach through the SCS is promising. Subconjunctival delivery can also dose the sclera, although it is hypothesized not to be as effective as SCS injections since IOP induces a natural flow of fluid from the SCS and across/out of the sclera[23, 92]. Scleral permeability was decreased with physiologic and supraphysiologic IOPs compared with IOP of 0 mm Hg[92]. More experiments are needed to explore this further.
4.3 Controlled release
4.3.1 Polymeric controlled release
Because molecules injected into the SCS are cleared quickly, strategies to have controlled release are often desirable. One common strategy is to encapsulate the drug within polymeric microparticles or implants. Monolithic implants have been surgically placed[46, 47] or injected in situ[34, 39, 43, 62] within the SCS. They can result in controlled release over months to the SCS and adjacent tissues surrounding the implant.
Particle suspensions injected into the SCS can be spread circumferential around the eye, where the formulation viscosity plays a role in particle spread. In addition, particles ranging from 20 nm to 2,000 nm have been shown to distribute and behave similarly, independent of particle size[81]. Polymeric microparticles can be found in the eye at least 4 months post-injection, suggesting that they are resistant to clearance by the routes molecules clear by[66]. This means that biodegradable polymer microparticles may be most suitable if the objective is for particles to eventually be cleared from the SCS.
Brimonidine-loaded, biodegradable polymeric microparticles injected into the SCS had a therapeutic effect for up to 1 month due to slow drug release from the microparticles[72]. This system achieved a dose reduction to ~13% of the topical dose, probably due to a combination of slow release and localization of the drug near its site of action in the ciliary body[29]. Dose sparing by bolus injection of just 0.02% of the topical brimonidine dose was also seen.
Various biopolymers that can be used to control drug release have been tested within the SCS with good biocompatibility[34, 39, 43, 46, 62, 66]. A significant foreign body reaction to the polylactic acid microparticles was reported, but it was unclear if this was a sterility or biocompatibility issue[72].
4.3.2 Solubility controlled release
Triamcinolone acetonide has been injected into the SCS clinically and in preclinical models, and shown to have therapeutic benefits that can last for months due to slow dissolution of highly water-insoluble drug microparticles[33, 40, 59, 90]. The controlled release behavior may have been aided by drug properties, such as drug binding, that delayed clearance of free molecules[61].
5 Safety and efficacy
Preclinical animal studies suggest that SCS drug delivery has a similar or better safety profile compared with intravitreal injections[33, 34, 59, 68, 82]. Results from completed Phase I/II and II clinical trials (e.g., NCT01789320) have also reported promising safety profiles[73]. While IOP immediately after injection increased with increasing injection volume, IOP returned to baseline within 1 h post-injection[68, 82]. These IOP changes are similar to those observed with intravitreal injections[15, 93–96], and are not expected to cause long-term ocular damage.
Some studies have examined the eye histologically. For example, SCS delivery of hyaluronic acid resulted in retinal atrophy in one study[34], however, no abnormalities were noted. following SCS delivery of bevacizumab and hyaluronic acid in another study[28]. Observation of the eye in vivo using indirect ophthalmoscopy after SCS injection of triamcinolone acetonide found evidence of choroidal dilation and hyperemia that resolved by 24 h[68]. The study was unable to identify parameters that affected this finding in a systematic way. No changes in electroretinograms before and after injection were reported, indicating that SCS injection did not adversely affect retinal health[59, 68].
5.1 Human clinical trials
5.1.1 Catheter-based techniques
Incision and catheterization to access the SCS has been performed in human patients. Although a device for this purpose has 510(k) approval, it does not have an indication for use nor have clinical trials demonstrated its efficacy compared with currently available methods. A retrospective analysis was performed on the safety and feasibility of the microcatheter approach to the SCS in a total of 21 subjects[40]. A combination of triamcinolone acetonide and bevacizumab was injected, which improved the best corrected visual acuity and decreased foveal thickness at 1 month[40]. Prospective randomized control trials are needed to more fully determine the safety and efficacy of this approach.
5.1.2 Microneedle injections to access the suprachoroidal space
The safety and efficacy of a microneedle injection containing triamcinolone acetonide is being evaluated in the treatment of macular edema following noninfectious uveitis and retinal vein occlusion. An open-label single-arm phase I/II clinical trial (NCT01789320) demonstrated the safety and efficacy of Triescence (triamcinolone acetonide) in patients who have noninfectious posterior uveitis and significant vitreous haze or macular edema[73]. Nine subjects were enrolled to receive a single unilateral microneedle injection of Triensence and were followed for 26 weeks[73]. All subjects had improvements in visual acuity and reduced retinal thickness, and no change in IOP[73]. There were 38 ocular adverse events reported, the most common being eye pain[73].
A phase II clinical trial (NCT02244032) evaluated the safety and efficacy of a microneedle injection of a proprietary triamcinolone formulation for the treatment of macular edema secondary to noninfectious uveitis. Seventeen subjects received a 4.0 mg dose, and had a reduction in retinal thickness and improvement in best corrected visual acuity. A total of 12 adverse events were reported, the most common of which was eye pain (18%). There was no increase in IOP, which is commonly seen with steroid use intravitreally.
A phase III clinical trial (NCT 03097315) is evaluating the safety and efficacy of a microneedle injection of a proprietary triamcinolone formulation in the treatment noninfectious uveitis. In this ongoing trial, subjects are randomized to receive injections containing either sham or triamcinolone. This trial has the two injections at 12 weeks apart, and the subjects are being monitored up to 24 weeks. Another phase III trial (NCT 02595398) is testing suprachoroidal injection of triamcinolone in the treatment of macular edema associated with noninfectious uveitis. Yet another phase III clinical trial (NCT 02980874) is comparing intravitreal aflibercept with and without simultaneous suprachoroidal triamcinolone injection for the management of macular edema due to retinal vein occlusion.
6 Conclusion
The SCS is a potential space found between the sclera and the choroid, and has become increasingly studied as a route of administration to treat posterior segment diseases of the eye. Due to its close proximity to the sclera, choroid, and RPE, high bioavailability is achievable at these tissues compared with traditional ophthalmic drug delivery techniques. While access to the SCS by sclerotomy and catheterization has been FDA-cleared, it is a surgical intervention that is not in clinical use. Instead, microneedle injection into the SCS is receiving significant attention and is undergoing Phase III clinical trials. Noninfectious uveitis has been the most studied indication both preclinically and in clinical trials. Exploration of additional indications is warranted. Optimizing drug delivery strategies and formulations are the subject of ongoing research. In conclusion, the SCS offers a novel route of administration to the posterior segment of the eye that offers great promise for improved drug targeting to sites of action in sclera, choroid and RPE.
Table 1.
Summary of suprachoroidal space access methods
| TECHNIQUE | INJECTION SETTING | TARGETING* |
|---|---|---|
| SCLEROTOMY | Operating room | + |
| CANNULA | Operating room | +++ |
| AB INTERNO | Operating room | +++ |
| HYPODERMIC NEEDLE | Operating room | + |
| MICRONEEDLE | Clinic | +(+)** |
+ indicates poor targeting with little to no control over distribution. ++ indicates acceptable drug targeting with some control over distribution. +++ indicates excellent targeting with near total control over drug deposition.
Targeted can be improved through formulation and other methods.
Acknowledgments
We thank Donna Bondy for administrative support. This work was supported by grants and fellowships from the National Eye Institute (R01EY022097, R01EY025286, T32EY007092 and F30EY025154). BC and MRP are inventors of patents that have been or may be licensed to companies for suprachoroidal drug delivery and MRP is a co-founder and shareholder of Clearside Biomedical, which is developing products for suprachoroidal drug delivery. These potential conflicts of interest have been disclosed and are being managed by Georgia Tech and/or Emory University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Klein R, Klein BE. The prevalence of age-related eye diseases and visual impairment in aging: current estimates. Invest Ophthalmol Vis Sci. 2013;54:ORSF5–ORSF13. doi: 10.1167/iovs.13-12789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kempen GI, Ballemans J, Ranchor AV, van Rens GH, Zijlstra GA. The impact of low vision on activities of daily living, symptoms of depression, feelings of anxiety and social support in community-living older adults seeking vision rehabilitation services. Qual Life Res. 2012;21:1405–1411. doi: 10.1007/s11136-011-0061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rein DB, Zhang P, Wirth KE, Lee PP, Hoerger TJ, McCall N, Klein R, Tielsch JM, Vijan S, Saaddine J. The economic burden of major adult visual disorders in the United States. Arch Ophthalmol-Chic. 2006;124:1754–1760. doi: 10.1001/archopht.124.12.1754. [DOI] [PubMed] [Google Scholar]
- 4.Chia E-M, Wang JJ, Rochtchina E, Smith W, Cumming RR, Mitchell P. Impact of Bilateral Visual Impairment on Health-Related Quality of Life: the Blue Mountains Eye Study. Investigative Opthalmology & Visual Science. 2004;45:71. doi: 10.1167/iovs.03-0661. [DOI] [PubMed] [Google Scholar]
- 5.Bhutto I, Lutty G. Understanding age-related macular degeneration (AMD): relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol Aspects Med. 2012;33:295–317. doi: 10.1016/j.mam.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rowe-Rendleman CL, Durazo SA, Kompella UB, Rittenhouse KD, Di Polo A, Weiner AL, Grossniklaus HE, Naash MI, Lewin AS, Horsager A, Edelhauser HF. Drug and gene delivery to the back of the eye: from bench to bedside. Invest Ophthalmol Vis Sci. 2014;55:2714–2730. doi: 10.1167/iovs.13-13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edelhauser HF, Rowe-Rendleman CL, Robinson MR, Dawson DG, Chader GJ, Grossniklaus HE, Rittenhouse KD, Wilson CG, Weber DA, Kuppermann BD, Csaky KG, Olsen TW, Kompella UB, Holers VM, Hageman GS, Gilger BC, Campochiaro PA, Whitcup SM, Wong WT. Ophthalmic drug delivery systems for the treatment of retinal diseases: basic research to clinical applications. Invest Ophthalmol Vis Sci. 2010;51:5403–5420. doi: 10.1167/iovs.10-5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim YC, Chiang B, Wu X, Prausnitz MR. Ocular delivery of macromolecules. J Control Release. 2014;190:172–181. doi: 10.1016/j.jconrel.2014.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghate D, Edelhauser HF. Barriers to glaucoma drug delivery. J Glaucoma. 2008;17:147–156. doi: 10.1097/IJG.0b013e31814b990d. [DOI] [PubMed] [Google Scholar]
- 10.Ethier CR, Johnson M, Ruberti J. Ocular biomechanics and biotransport. Annu Rev Biomed Eng. 2004;6:249–273. doi: 10.1146/annurev.bioeng.6.040803.140055. [DOI] [PubMed] [Google Scholar]
- 11.Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, Kirchhof B, Ho A, Ogura Y, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Soo Y, Anderesi M, Groetzbach G, Sommerauer B, Sandbrink R, Simader C, Schmidt-Erfurth U View VS Groups. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Group CR, Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicholson BP, Schachat AP. A review of clinical trials of anti-VEGF agents for diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2010;248:915–930. doi: 10.1007/s00417-010-1315-z. [DOI] [PubMed] [Google Scholar]
- 14.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, Group MS. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 15.Bakri SJ, Snyder MR, Reid JM, Pulido JS, Ezzat MK, Singh RJ. Pharmacokinetics of intravitreal ranibizumab (Lucentis) Ophthalmology. 2007;114:2179–2182. doi: 10.1016/j.ophtha.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Nomoto H, Shiraga F, Kuno N, Kimura E, Fujii S, Shinomiya K, Nugent AK, Hirooka K, Baba T. Pharmacokinetics of bevacizumab after topical, subconjunctival, and intravitreal administration in rabbits. Invest Ophthalmol Vis Sci. 2009;50:4807–4813. doi: 10.1167/iovs.08-3148. [DOI] [PubMed] [Google Scholar]
- 17.Li SK, Hao J, Liu H, Lee JH. MRI study of subconjunctival and intravitreal injections. J Pharm Sci. 2012;101:2353–2363. doi: 10.1002/jps.23127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del Amo EM, Urtti A. Current and future ophthalmic drug delivery systems. A shift to the posterior segment. Drug Discov Today. 2008;13:135–143. doi: 10.1016/j.drudis.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv Drug Deliv Rev. 2006;58:1131–1135. doi: 10.1016/j.addr.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 20.Kok H, Lau C, Maycock N, McCluskey P, Lightman S. Outcome of intravitreal triamcinolone in uveitis. Ophthalmology. 2005;112:1916–1921. doi: 10.1016/j.ophtha.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 21.Williamson J, Paterson RW, McGavin DD, Jasani MK, Boyle JA, Doig WM. Posterior subcapsular cataracts and glaucoma associated with long-term oral corticosteroid therapy. In patients with rheumatoid arthritis and related conditions. Br J Ophthalmol. 1969;53:361–372. doi: 10.1136/bjo.53.6.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heiduschka P, Feitz H, Hofmeister S, Schultheiss S, Mack AF, Peters S, Zeimssen F, Niggeman B, Julien S, Bartz-Schmidt KU. Penetration of bevacizumab through the retina after intravitreal injection in the monkey. Invest Ophthalmol Vis Sci. 2007;48:2814–2823. doi: 10.1167/iovs.06-1171. [DOI] [PubMed] [Google Scholar]
- 23.Emi K, Pederson JE, Toris CB. Hydrostatic pressure of the suprachoroidal space. Invest Ophthalmol Vis Sci. 1989;30:233–238. [PubMed] [Google Scholar]
- 24.Tasman W, Jaeger E. Duane’s Ophthalmology. 15. Lippincott Williams & Wilkins; Philadelphia, Pa: 2009. [Google Scholar]
- 25.Krohn J, Bertelsen T. Corrosion casts of the suprachoroidal space and uveoscleral drainage routes in the human eye. Acta Ophthalmol Scand. 1997;75:32–35. doi: 10.1111/j.1600-0420.1997.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 26.Alm A, Nilsson SF. Uveoscleral outflow--a review. Exp Eye Res. 2009;88:760–768. doi: 10.1016/j.exer.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 27.Tyagi P, Kadam RS, Kompella UB. Comparison of suprachoroidal drug delivery with subconjunctival and intravitreal routes using noninvasive fluorophotometry. PLoS One. 2012;7:e48188. doi: 10.1371/journal.pone.0048188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olsen TW, Feng X, Wabner K, Csaky K, Pambuccian S, Cameron JD. Pharmacokinetics of pars plana intravitreal injections versus microcannula suprachoroidal injections of bevacizumab in a porcine model. Invest Ophthalmol Vis Sci. 2011;52:4749–4756. doi: 10.1167/iovs.10-6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim YC, Edelhauser HF, Prausnitz MR. Targeted delivery of antiglaucoma drugs to the supraciliary space using microneedles. Invest Ophthalmol Vis Sci. 2014;55:7387–7397. doi: 10.1167/iovs.14-14651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.U.S.F.a.D.A. (FDA) iScience surgical ophthalmic microcannula. 2004 510(k) Summary. K041108. [Google Scholar]
- 31.Seiler GS, Salmon JH, Mantuo R, Feingold S, Dayton PA, Gilger BC. Effect and distribution of contrast medium after injection into the anterior suprachoroidal space in ex vivo eyes. Invest Ophthalmol Vis Sci. 2011;52:5730–5736. doi: 10.1167/iovs.11-7525. [DOI] [PubMed] [Google Scholar]
- 32.Peden MC, Min J, Meyers C, Lukowski Z, Li Q, Boye SL, Levine M, Hauswirth WW, Ratnakaram R, Dawson W, Smith WC, Sherwood MB. Ab-externo AAV-mediated gene delivery to the suprachoroidal space using a 250 micron flexible microcatheter. PLoS One. 2011;6:e17140. doi: 10.1371/journal.pone.0017140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olsen TW, Feng X, Wabner K, Conston SR, Sierra DH, Folden DV, Smith ME, Cameron JD. Cannulation of the suprachoroidal space: a novel drug delivery methodology to the posterior segment. Am J Ophthalmol. 2006;142:777–787. doi: 10.1016/j.ajo.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 34.Einmahl S, Savoldelli M, D’Hermies F, Tabatabay C, Gurny R, Behar-Cohen F. Evaluation of a novel biomaterial in the suprachoroidal space of the rabbit eye. Invest Ophthalmol Vis Sci. 2002;43:1533–1539. [PubMed] [Google Scholar]
- 35.Tzameret A, Sher I, Belkin M, Treves AJ, Meir A, Nagler A, Levkovitch-Verbin H, Barshack I, Rosner M, Rotenstreich Y. Transplantation of human bone marrow mesenchymal stem cells as a thin subretinal layer ameliorates retinal degeneration in a rat model of retinal dystrophy. Exp Eye Res. 2014;118:135–144. doi: 10.1016/j.exer.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 36.Wang M, Liu W, Lu Q, Zeng H, Liu S, Yue Y, Cheng H, Liu Y, Xue M. Pharmacokinetic comparison of ketorolac after intracameral, intravitreal, and suprachoroidal administration in rabbits. Retina. 2012;32:2158–2164. doi: 10.1097/IAE.0b013e3182576d1d. [DOI] [PubMed] [Google Scholar]
- 37.Kim SH, Galban CJ, Lutz RJ, Dedrick RL, Csaky KG, Lizak MJ, Wang NS, Tansey G, Robinson MR. Assessment of subconjunctival and intrascleral drug delivery to the posterior segment using dynamic contrast-enhanced magnetic resonance imaging. Invest Ophthalmol Vis Sci. 2007;48:808–814. doi: 10.1167/iovs.06-0670. [DOI] [PubMed] [Google Scholar]
- 38.Abarca EM, Salmon JH, Gilger BC. Effect of choroidal perfusion on ocular tissue distribution after intravitreal or suprachoroidal injection in an arterially perfused ex vivo pig eye model. J Ocul Pharmacol Ther. 2013;29:715–722. doi: 10.1089/jop.2013.0063. [DOI] [PubMed] [Google Scholar]
- 39.Wang XH, Li S, Liang L, Xu XD, Zhang XZ, Jiang FG. Evaluation of RGD peptide hydrogel in the posterior segment of the rabbit eye. J Biomater Sci Polym Ed. 2013;24:1185–1197. doi: 10.1080/09205063.2012.745714. [DOI] [PubMed] [Google Scholar]
- 40.Tetz M, Rizzo S, Augustin AJ. Safety of submacular suprachoroidal drug administration via a microcatheter: retrospective analysis of European treatment results. Ophthalmologica. 2012;227:183–189. doi: 10.1159/000336045. [DOI] [PubMed] [Google Scholar]
- 41.Rizzo S, Ebert FG, Bartolo ED, Barca F, Cresti F, Augustin C, Augustin A. Suprachoroidal drug infusion for the treatment of severe subfoveal hard exudates. Retina. 2012;32:776–784. doi: 10.1097/IAE.0b013e3182278b0e. [DOI] [PubMed] [Google Scholar]
- 42.Hou J, Tao Y, Jiang YR, Wang K. In vivo and in vitro study of suprachoroidal fibrin glue. Jpn J Ophthalmol. 2009;53:640–647. doi: 10.1007/s10384-009-0725-0. [DOI] [PubMed] [Google Scholar]
- 43.Einmahl S, Ponsart S, Bejjani RA, D’Hermies F, Savoldelli M, Heller J, Tabatabay C, Gurny R, Behar-Cohen F. Ocular biocompatibility of a poly(ortho ester) characterized by autocatalyzed degradation. J Biomed Mater Res A. 2003;67:44–53. doi: 10.1002/jbm.a.10597. [DOI] [PubMed] [Google Scholar]
- 44.You S, Luo J, Grossniklaus HE, Gou ML, Meng K, Zhang Q. Nanomedicine in the application of uveal melanoma. Int J Ophthalmol. 2016;9:1215–1225. doi: 10.18240/ijo.2016.08.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barbosa Saliba J, Vieira L, Fernandes-Cunha GM, Rodrigues Da Silva G, Ligorio Fialho S, Silva-Cunha A, Bousquet E, Naud MC, Ayres E, Orefice RL, Tekaya M, Kowalczuk L, Zhao M, Behar-Cohen F. Anti-Inflammatory Effect of Dexamethasone Controlled Released From Anterior Suprachoroidal Polyurethane Implants on Endotoxin-Induced Uveitis in Rats. Invest Ophthalmol Vis Sci. 2016;57:1671–1679. doi: 10.1167/iovs.15-18127. [DOI] [PubMed] [Google Scholar]
- 46.Gilger BC, Wilkie DA, Clode AB, McMullen RJ, Jr, Utter ME, Komaromy AM, Brooks DE, Salmon JH. Long-term outcome after implantation of a suprachoroidal cyclosporine drug delivery device in horses with recurrent uveitis. Vet Ophthalmol. 2010;13:294–300. doi: 10.1111/j.1463-5224.2010.00807.x. [DOI] [PubMed] [Google Scholar]
- 47.Gilger BC, Salmon JH, Wilkie DA, Cruysberg LP, Kim J, Hayat M, Kim H, Kim S, Yuan P, Lee SS, Harrington SM, Murray PR, Edelhauser HF, Csaky KG, Robinson MR. A novel bioerodible deep scleral lamellar cyclosporine implant for uveitis. Invest Ophthalmol Vis Sci. 2006;47:2596–2605. doi: 10.1167/iovs.05-1540. [DOI] [PubMed] [Google Scholar]
- 48.Hovakimyan M, Siewert S, Schmidt W, Sternberg K, Reske T, Stachs O, Guthoff R, Wree A, Witt M, Schmitz KP, Allemann R. Development of an Experimental Drug Eluting Suprachoroidal Microstent as Glaucoma Drainage Device. Transl Vis Sci Technol. 2015;4:14. doi: 10.1167/tvst.4.3.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ayton LN, Blamey PJ, Guymer RH, Luu CD, Nayagam DA, Sinclair NC, Shivdasani MN, Yeoh J, McCombe MF, Briggs RJ, Opie NL, Villalobos J, Dimitrov PN, Varsamidis M, Petoe MA, McCarthy CD, Walker JG, Barnes N, Burkitt AN, Williams CE, Shepherd RK, Allen PJ C. Bionic Vision Australia Research. First-in-human trial of a novel suprachoroidal retinal prosthesis. PLoS One. 2014;9:e115239. doi: 10.1371/journal.pone.0115239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fujikado T, Kamei M, Sakaguchi H, Kanda H, Morimoto T, Ikuno Y, Nishida K, Kishima H, Maruo T, Konoma K, Ozawa M, Nishida K. Testing of semichronically implanted retinal prosthesis by suprachoroidal-transretinal stimulation in patients with retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2011;52:4726–4733. doi: 10.1167/iovs.10-6836. [DOI] [PubMed] [Google Scholar]
- 51.El Rayes EN, Oshima Y. Suprachoroidal buckling for retinal detachment. Retina. 2013;33:1073–1075. doi: 10.1097/IAE.0b013e318287daa5. [DOI] [PubMed] [Google Scholar]
- 52.Mittl R, Tiwari R. Suprachoroidal injection of sodium hyaluronate as an internal buckling procedure. Clin Ophthalmol. 1987;19:255–260. doi: 10.1159/000265503. [DOI] [PubMed] [Google Scholar]
- 53.Poole TA, Sudarsky RD. Suprachoroidal implantation for the treatment of retinal detachment. Ophthalmology. 1986;93:1408–1412. doi: 10.1016/s0161-6420(86)33553-x. [DOI] [PubMed] [Google Scholar]
- 54.El Rayes EN, Mikhail M, El Cheweiky H, Elsawah K, Maia A. Suprachoroidal Buckling for the Management of Rhegmatogenous Retinal Detachments Secondary to Peripheral Retinal Breaks. Retina. 2016 doi: 10.1097/IAE.0000000000001214. [DOI] [PubMed] [Google Scholar]
- 55.El Rayes EN, Elborgy E. Suprachoroidal buckling: technique and indications. J Ophthalmic Vis Res. 2013;8:393–399. [PMC free article] [PubMed] [Google Scholar]
- 56.Hoh H, Grisanti S, Grisanti S, Rau M, Ianchulev S. Two-year clinical experience with the CyPass micro-stent: safety and surgical outcomes of a novel supraciliary micro-stent. Klin Monbl Augenheilkd. 2014;231:377–381. doi: 10.1055/s-0034-1368214. [DOI] [PubMed] [Google Scholar]
- 57.Wellik SR, Dale EA. A review of the iStent((R)) trabecular micro-bypass stent: safety and efficacy. Clin Ophthalmol. 2015;9:677–684. doi: 10.2147/OPTH.S57217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fea AM, Belda JI, Rekas M, Junemann A, Chang L, Pablo L, Voskanyan L, Katz LJ. Prospective unmasked randomized evaluation of the iStent inject ((R)) versus two ocular hypotensive agents in patients with primary open-angle glaucoma. Clin Ophthalmol. 2014;8:875–882. doi: 10.2147/OPTH.S59932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen M, Li X, Liu J, Han Y, Cheng L. Safety and pharmacodynamics of suprachoroidal injection of triamcinolone acetonide as a controlled ocular drug release model. J Control Release. 2015;203:109–117. doi: 10.1016/j.jconrel.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 60.Mandelcorn ED, Kitchens JW, Fijalkowski N, Moshfeghi DM. Active aspiration of suprachoroidal hemorrhage using a guarded needle. Ophthalmic Surg Lasers Imaging Retina. 2014;45:150–152. doi: 10.3928/23258160-20140306-09. [DOI] [PubMed] [Google Scholar]
- 61.Kadam RS, Williams J, Tyagi P, Edelhauser HF, Kompella UB. Suprachoroidal delivery in a rabbit ex vivo eye model: influence of drug properties, regional differences in delivery, and comparison with intravitreal and intracameral routes. Mol Vis. 2013;19:1198–1210. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62.Tyagi P, Barros M, Stansbury JW, Kompella UB. Light-activated, in situ forming gel for sustained suprachoroidal delivery of bevacizumab. Mol Pharm. 2013;10:2858–2867. doi: 10.1021/mp300716t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tzameret A, Sher I, Belkin M, Treves AJ, Meir A, Nagler A, Levkovitch-Verbin H, Rotenstreich Y, Solomon AS. Epiretinal transplantation of human bone marrow mesenchymal stem cells rescues retinal and vision function in a rat model of retinal degeneration. Stem Cell Res. 2015;15:387–394. doi: 10.1016/j.scr.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 64.Olsen TW, Aaberg SY, Geroski DH, Edelhauser HF. Human sclera: thickness and surface area. Am J Ophthalmol. 1998;125:237–241. doi: 10.1016/s0002-9394(99)80096-8. [DOI] [PubMed] [Google Scholar]
- 65.Patel SR, Lin AS, Edelhauser HF, Prausnitz MR. Suprachoroidal drug delivery to the back of the eye using hollow microneedles. Pharm Res. 2011;28:166–176. doi: 10.1007/s11095-010-0271-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patel SR, Berezovsky DE, McCarey BE, Zarnitsyn V, Edelhauser HF, Prausnitz MR. Targeted administration into the suprachoroidal space using a microneedle for drug delivery to the posterior segment of the eye. Invest Ophthalmol Vis Sci. 2012;53:4433–4441. doi: 10.1167/iovs.12-9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park SH, Lee KJ, Lee J, Yoon JH, Jo DH, Kim JH, Kang K, Ryu W. Microneedle-based minimally-invasive measurement of puncture resistance and fracture toughness of sclera. Acta Biomater. 2016 doi: 10.1016/j.actbio.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 68.Gu B, Liu J, Li X, Ma Q, Shen M, Cheng L. Real-Time Monitoring of Suprachoroidal Space (SCS) Following SCS Injection Using Ultra-High Resolution Optical Coherence Tomography in Guinea Pig Eyes. Invest Ophthalmol Vis Sci. 2015;56:3623–3634. doi: 10.1167/iovs.15-16597. [DOI] [PubMed] [Google Scholar]
- 69.Zeng M, Shen J, Liu Y, Lu LY, Ding K, Fortmann SD, Khan M, Wang J, Hackett SF, Semenza GL, Campochiaro PA. The HIF-1 antagonist acriflavine: visualization in retina and suppression of ocular neovascularization. J Mol Med (Berl) 2017;95:417–429. doi: 10.1007/s00109-016-1498-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tzameret A, Kalish SE, Sher I, Twito L, Meir A, Levy I, Margel S, Moroz I, Rosner M, Treves AJ, Nagler A, Belkin M, Rotenstreich Y. Long-Term Safety of Transplanting Human Bone Marrow Stromal Cells into the Extravascular Spaces of the Choroid of Rabbits. Stem Cells Int. 2017;2017:4061975. doi: 10.1155/2017/4061975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chiang B, Venugopal N, Grossniklaus HE, Jung JH, Edelhauser HF, Prausnitz MR. Thickness and Closure Kinetics of the Suprachoroidal Space Following Microneedle Injection of Liquid Formulations. Invest Ophthalmol Vis Sci. 2017;58:555–564. doi: 10.1167/iovs.16-20377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chiang B, Kim YC, Doty AC, Grossniklaus HE, Schwendeman SP, Prausnitz MR. Sustained reduction of intraocular pressure by supraciliary delivery of brimonidine-loaded poly(lactic acid) microspheres for the treatment of glaucoma. J Control Release. 2016;228:48–57. doi: 10.1016/j.jconrel.2016.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goldstein DA, Do D, Noronha G, Kissner JM, Srivastava SK, Nguyen QD. Suprachoroidal Corticosteroid Administration: A Novel Route for Local Treatment of Noninfectious Uveitis. Transl Vis Sci Technol. 2016;5:14. doi: 10.1167/tvst.5.6.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113:363–372 e365. doi: 10.1016/j.ophtha.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 75.Kim YC, Oh KH, Edelhauser HF, Prausnitz MR. Formulation to target delivery to the ciliary body and choroid via the suprachoroidal space of the eye using microneedles. Eur J Pharm Biopharm. 2015;95:398–406. doi: 10.1016/j.ejpb.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cheung LK, Eaton A. Age-related macular degeneration. Pharmacotherapy. 2013;33:838–855. doi: 10.1002/phar.1264. [DOI] [PubMed] [Google Scholar]
- 77.Zarbin MA. Pathogenesis of Age-Related Macular Degeneration. Medical Retina. 2012;1:125–133. [Google Scholar]
- 78.Ambati J, Fowler BJ. Mechanisms of age-related macular degeneration. Neuron. 2012;75:26–39. doi: 10.1016/j.neuron.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. The Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 80.Chiang B, Kim YC, Edelhauser HF, Prausnitz MR. Circumferential flow of particles in the suprachoroidal space is impeded by the posterior ciliary arteries. Exp Eye Res. 2016;145:424–431. doi: 10.1016/j.exer.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chiang B, Venugopal N, Edelhauser HF, Prausnitz MR. Distribution of particles, small molecules and polymeric formulation excipients in the suprachoroidal space after microneedle injection. Exp Eye Res. 2016;153:101–109. doi: 10.1016/j.exer.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim YC, Edelhauser HF, Prausnitz MR. Particle-stabilized emulsion droplets for gravity-mediated targeting in the posterior segment of the eye. Adv Healthc Mater. 2014;3:1272–1282. doi: 10.1002/adhm.201300696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chiang B, Wang K, Ethier CR, Prausnitz MR. Clearance Kinetics and Clearance Routes of Molecules From the Suprachoroidal Space After Microneedle Injection. Invest Ophthalmol Vis Sci. 2017;58:545–554. doi: 10.1167/iovs.16-20679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moseley H, Foulds WS, Allan D, Kyle PM. Routes of clearance of radioactive water from the rabbit vitreous. Br J Ophthalmol. 1984;68:145–151. doi: 10.1136/bjo.68.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moseley H, Foulds WS. The movement of xenon-133 from the vitreous to the choroid. Exp Eye Res. 1982;34:169–179. doi: 10.1016/0014-4835(82)90051-3. [DOI] [PubMed] [Google Scholar]
- 86.Inomata H, Bill A. Exit sites of uveoscleral flow of aqueous humor in cynomolgus monkey eyes. Exp Eye Res. 1977;25:113–118. doi: 10.1016/0014-4835(77)90123-3. [DOI] [PubMed] [Google Scholar]
- 87.Bill A. Quantitative determination of uveal blood flow in rabbits. Acta Physiol Scand. 1962;55:101–110. doi: 10.1111/j.1748-1716.1962.tb02423.x. [DOI] [PubMed] [Google Scholar]
- 88.Touchard E, Berdugo M, Bigey P, El Sanharawi M, Savoldelli M, Naud MC, Jeanny JC, Behar-Cohen F. Suprachoroidal electrotransfer: a nonviral gene delivery method to transfect the choroid and the retina without detaching the retina. Mol Ther. 2012;20:1559–1570. doi: 10.1038/mt.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tran J, Craven C, Wabner K, Schmit J, Matter B, Kompella U, Grossniklaus HE, Olsen TW. A Pharmacodynamic Analysis of Choroidal Neovascularization in a Porcine Model Using Three Targeted Drugs. Invest Ophthalmol Vis Sci. 2017;58:3732–3740. doi: 10.1167/iovs.16-21230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gilger BC, Abarca EM, Salmon JH, Patel S. Treatment of acute posterior uveitis in a porcine model by injection of triamcinolone acetonide into the suprachoroidal space using microneedles. Invest Ophthalmol Vis Sci. 2013;54:2483–2492. doi: 10.1167/iovs.13-11747. [DOI] [PubMed] [Google Scholar]
- 91.Garcia-Feijoo J, Rau M, Grisanti S, Grisanti S, Hoh H, Erb C, Guguchkova P, Ahmed I, Grabner G, Reitsamer H, Shaarawy T, Ianchulev T. Supraciliary Micro-stent Implantation for Open-Angle Glaucoma Failing Topical Therapy: 1-Year Results of a Multicenter Study. Am J Ophthalmol. 2015;159:1075–1081 e1071. doi: 10.1016/j.ajo.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 92.Rudnick DE, Noonan JS, Geroski DH, Prausnitz MR, Edelhauser HF. The effect of intraocular pressure on human and rabbit scleral permeability. Invest Ophthalmol Vis Sci. 1999;40:3054–3058. [PubMed] [Google Scholar]
- 93.Rodrigues EB, Grumann A, Jr, Penha FM, Shiroma H, Rossi E, Meyer CH, Stefano V, Maia M, Magalhaes O, Jr, Farah ME. Effect of needle type and injection technique on pain level and vitreal reflux in intravitreal injection. J Ocul Pharmacol Ther. 2011;27:197–203. doi: 10.1089/jop.2010.0082. [DOI] [PubMed] [Google Scholar]
- 94.Doshi RR, Bakri SJ, Fung AE. Intravitreal injection technique. Semin Ophthalmol. 2011;26:104–113. doi: 10.3109/08820538.2010.541318. [DOI] [PubMed] [Google Scholar]
- 95.Jonas JB, Spandau UH, Schlichtenbrede F. Short-term complications of intravitreal injections of triamcinolone and bevacizumab. Eye (Lond) 2008;22:590–591. doi: 10.1038/eye.2008.10. [DOI] [PubMed] [Google Scholar]
- 96.Benz MS, Albini TA, Holz ER, Lakhanpal RR, Westfall AC, Iyer MN, Carvounis PE. Short-term course of intraocular pressure after intravitreal injection of triamcinolone acetonide. Ophthalmology. 2006;113:1174–1178. doi: 10.1016/j.ophtha.2005.10.061. [DOI] [PubMed] [Google Scholar]