Abstract
Chronic stress or inflammation increases tryptophan metabolism along the kynurenine pathway (KP), and the generation of neuroactive kynurenine metabolites contributes to subsequent depressive-like behaviors. Microglia regulate KP balance by preferentially producing oxidative metabolites, including quinolinic acid. Research has focused on the interplay between cytokines and HPA axis-derived corticosteroids in regulating microglial activity and effects of KP metabolites directly on neurons; however, the potential role that KP metabolites have directly on microglial activity is unknown. Here, murine microglia were stimulated with lipopolysaccharide(LPS). After 6 hours, mRNA expression of interleukin(IL)-1β, IL-6, tumor necrosis factor(TNF)-α and inducible nitric oxide synthase(iNOS) was dose-dependently increased along with the rate-limiting enzymes for oxidative KP metabolism, indoleamine-2,3-dioxygenase(IDO)-1 and kynurenine 3-monooxygenase(KMO). By 24 hours post-LPS, kynurenine and quinolinic acid in the media was elevated. Inhibiting KMO with Ro 61-8048 during LPS challenge attenuated extracellular nitrite accumulation and expression of KMO and TNF-α in response to LPS. Similarly, primary microglia isolated from KMO-/- mice exhibited a significantly reduced pro-inflammatory response to LPS compared to WT controls. To determine whether the substrate (kynurenine) or end product (quinolinic acid) of KMO-dependent metabolism modulates the LPS response, microglia were treated with increasing concentrations of L-kynurenine or quinolinic acid in combination with LPS or saline. Interestingly, quinolinic acid did not impact the microglial LPS response. However, L-kynurenine had dose-dependent inhibitory effect on the LPS response. These data are the first to show an anti-inflammatory effect of KMO inhibition on microglia during immune challenge and suggest that KP metabolic balance may play a direct role in regulating microglia activity.
Keywords: microglia, kynurenine, quinolinic acid, inflammation, cytokines, kynurenine metabolism, microglia
Introduction
The kynurenine pathway metabolizes tryptophan to several bioactive metabolites. In the healthy, non-challenged brain, kynurenine is predominantly metabolized to kynurenic acid by astrocytes (Figure 1) (Guillemin et al., 2001). However, disruption of neuroendocrine or neuroinflammatory balance, which involves redistribution and activation of microglia within the brain, can shift the metabolic balance toward oxidative metabolism of excitatory and potentially cytotoxic metabolites (Connor et al., 2008). For example, mice exposed to low-level stress exhibit increased expression of IDO1 and TDO (Dugan et al., 2015), and acute stress increases plasma and brain kynurenine (Dostal et al., 2017). Mice lacking the corticosterone-upregulated enzyme tryptophan 2,3-dioxygenase (TDO) fail to develop stress induced depressive-like behaviors (Gibney et al., 2014).
Fig 1. LPS dose dependently activates BV2 microglia.
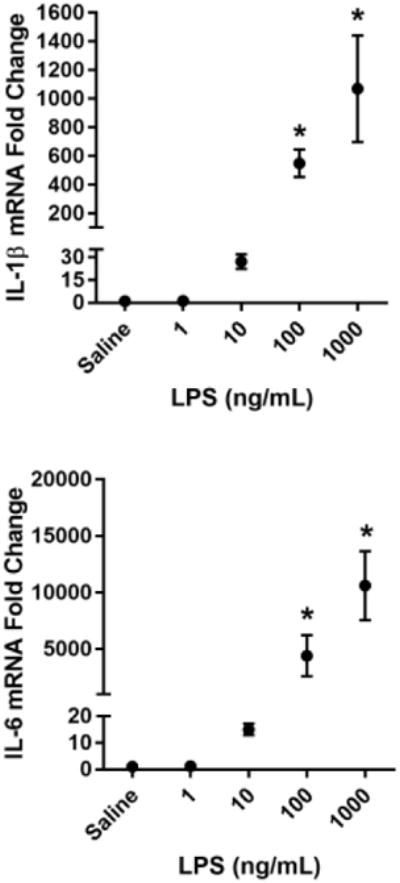
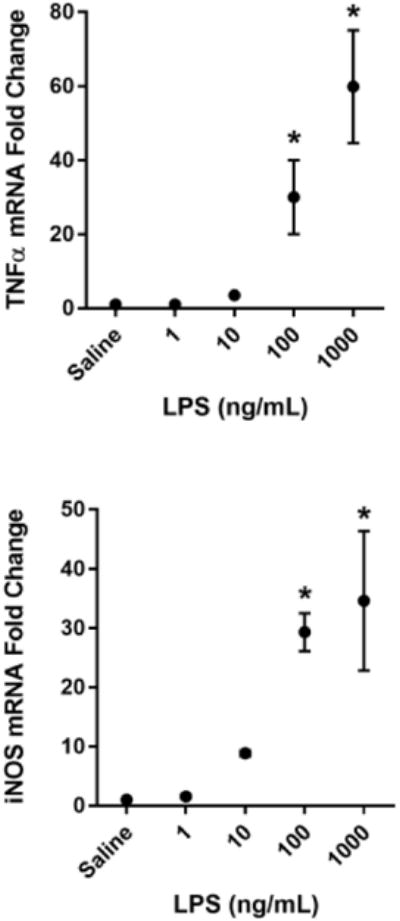
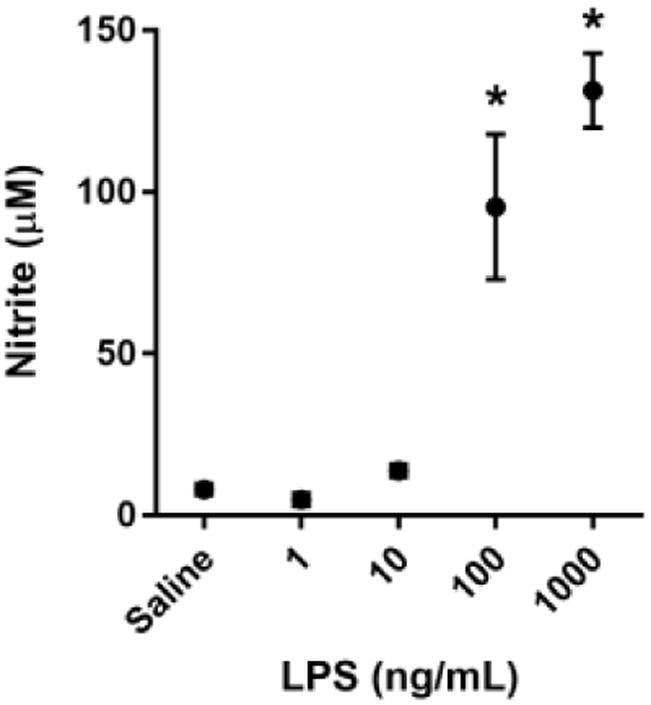
BV2 microglia were treated with saline or LPS (1, 10, 100, or 1000 ng/mL) for 6 h. RNA was isolated and reverse transcribed to cDNA which was analyzed using qPCR to determine expression of: A, Il1b (interleukin-1β); B, Il6 (interlekin-6); C, Tnf (tumor necrosis factor-α); D, Nos2 (inducible nitric oxide synthase). In a separate experiment, BV2 microglia were treated as described above, and 24 h post-LPS, conditioned media was collected and processed using the Griess assay to determine nitrite concentration (E). Bars represent group mean ± SEM, n=3-6, * indicates p<0.05 versus saline-treated controls. Data were analyzed using one-way ANOVA followed by Dunnett's test.
Mice exposed to peripheral lipopolysaccharide (LPS), an immune activator, have elevated levels of kynurenine, 3-hydroxykynurenine (induces free radicals), and quinolinic acid (NMDAR agonist) in the brain (Walker et al., 2013). Recent data suggest that the disruption of kynurenine pathway metabolic balance caused by peripheral inflammation is heterogeneous between distinct brain regions, with the strongest shift towards the oxidative metabolism of kynurenine occurring in the hippocampus (Parrott et al., 2016a; Parrott et al., 2016b). Moreover, altered KP balance has been implicated in neuropathology (Parrott et al., 2015) and psychological stress (Dugan et al., 2015). Increased concentrations of 3-hydroxykynurenine and quinolinic acid have been observed in models of Huntington's disease (Guidetti et al., 2004) and depression (Raison et al., 2010), schizophrenia and autism have been associated with increased kynurenic acid (α7-AChR and NMDAR antagonist) (Schwarcz et al., 2001), and increased 3-hydroxykynurenine and reduced kynurenine and kynurenic acid concentrations have been observed in Parkinson's disease (Ogawa et al., 1992). Together, these studies suggest that disruption of KP metabolic homeostasis may be a pathogenic factor in many neurological conditions. To date, nearly all research examining the role of kynurenine metabolites as mediators of neuropathology have focused on their direct or indirect effects on neurotransmitter systems (Obrenovitch and Urenjak, 2000; Perkins and Stone, 1982; Stone and Perkins, 1981), but the potential effects of shifting KP metabolic balance on the activity of microglia has not been investigated.
Inhibition of kynurenine mono-oxygenase (KMO), the enzyme that oxidatively metabolizes kynurenine to 3-hydroxykynurenine, has a therapeutic effect in several preclinical models of neuropathology including ischemia (Carpenedo et al., 2002), epilepsy (Carpenedo et al., 1994), neuropathic pain (Rojewska et al., 2016), and depression (Parrott and O'Connor, 2016). Additionally, KMO inhibition reduced neuroinflammation normally present in mouse models of trypanosomiasis (Rodgers et al. 2009) and chronic nerve pain (Rojewska et al., 2016). Also, mice with targeted deletion of the KMO gene are protected from behavioral and cognitive disruptions caused by peripheral immune challenge (Heisler and O'Connor, 2015; Parrott and O'Connor, 2016). These data suggest that KMO inhibition can attenuate the pro-inflammatory response in the CNS, which is regulated by microglia. However, the precise mechanisms underlying the anti-inflammatory effect of KMO inhibition were not identified in the aforementioned studies. As KMO is the rate limiting enzyme for the oxidative metabolism of kynurenine to 3-hydroxykynurenine, and subsequently to quinolinic acid, its inhibition reduces the production of these neuroactive metabolites. However, KMO inhibition also results in an accumulation of kynurenine and increased kynurenic acid levels (Zwilling et al., 2011), which could play a role in the neuromodulatory effects of manipulating KP balance, but direct effects on microglia are unknown.
In this study, we tested the hypothesis that KMO inhibition modulates microglial activation. We report that LPS induces a robust increase in pro-inflammatory cytokine expression, production of nitric oxide and KMO-dependent KP metabolism in BV2 murine microglia. The KMO inhibitor Ro 61-8048 selectively reduced expression of the pro-inflammatory factors tumor necrosis factor-α and accumulation of extracellular nitrite, while IDO inhibition had no effect. Similarly, primary microglia with a targeted deletion of the KMO gene exhibited a blunted pro-inflammatory response to LPS. As KMO inhibition results in both reduced quinolinic acid production and increased kynurenine accumulation (Giorgini et al., 2013), the upregulation of pro-inflammatory cytokines and extracellular nitrite levels were measured in the presence of increasing concentrations of quinolinic acid or kynurenine. Interestingly, only kynurenine modulated the BV2 response to LPS. Taken together, these data are the first direct evidence that KMO inhibition dampens the microglial response to LPS, likely by increasing levels of kynurenine. Moreover, the data support the possibility that endogenous KP metabolism plays an important role in regulating microglia activity and provides novel therapeutic opportunities for treating neuroinflammatory conditions.
Methods and Materials
BV2 cell culture
BV2 microglia, a mouse microglial cell line, were obtained from the lab of Jonathan P. Godbout at the Ohio State University (Fenn et al., 2012). Cells were maintained at 37°C in 5% CO2 in Dulbecco's modified eagle's medium (DMEM)/F12+GlutaMAX™ 1X (ThermoFisher Scientific, Waltham, MA) containing 10% fetal bovine serum (FBS, Atlanta Biologicals, Norcross, GA), 50 μg/mL of penicillin and streptomycin (Corning Mediatech, Manassas, VA), and 2.5 μg/mL amphotericin B (ThermoFisher Scientific). Media was changed every three days during expansion of cultures. Cells were passed at approximately 90% confluence up to 10 times per aliquot of stock cells. 0.25% trypsin (ThermoFisher Scientific) was used to detach cells from the culture dish during passage. In all experiments, unless otherwise noted, BV2 microglia were plated at 5×105 cells per well of a 24-well plate, allowed to adhere for 1 hour, gently rinsed with 1X Dulbecco's phosphate buffered saline (PBS, ThermoFisher Scientific), then treated in 1% FBS-DMEM media. Cells were collected after 6 hours of the indicated treatments for isolation of mRNA transcripts, and after 24 hours for collection of conditioned media. Treatments: LPS (saline vehicle, 1, 10, 100, 1000 ng/mL; E. coli serotype 0127:B8, Sigma-Aldrich, St. Louis, MO).; KMO inhibitor (PBS vehicle, 10 μM Ro 61-8048; Tocris Bioscience, Bristol, United Kingdom); IDO inhibitor (PBS vehicle, 10 μM 1-methyltryptophan;Sigma-Aldrich, St. Louis, MO); quinolinic acid (PBS vehicle, 0.006, 0.06, 0., 6.0, 60.0 μM; Sigma-Aldrich); kynurenine (PBS vehicle, 0.048, 0.48, 4.8, 48, 480 μM; Sigma-Aldrich). For Ro 61-8048, 1-methyltryptophan, and kynurenine treatments, concentrated stock solutions were prepared in 0.1 M NaOH (Sigma-Aldrich), neutralized with an equal volume of 0.1 M HCl (Sigma-Aldrich), and buffered with twice the volume of 2X PBS.
Primary glial cell culture
Primary mixed glial cultures were prepared as previously described (Fenn et al., 2012). To isolate primary glial cells, 0-4 day-old C57BL/6J mouse pups from wild type (WT) or KMO knockout (KMO-/-) litters were swiftly decapitated, and brains were removed in a sterile environment. Brains were finely diced then incubated in 0.25% trypsin for 10 minutes. Brain mixture was triturated using a 10 mL serological pipet until all large chunks were broken down. The cell mixture was then pipetted using a glass Pasteur pipet into a 70 μm cell strainer. The strained cell mixture was then centrifuged at 600 g for 6 minutes at 4°C, and the pellet was rescued in 5 mL of 20% FBS-DMEM media per 4 neonatal brains. Cells were then plated on T-175 flasks coated with 0.01% poly-L-lysine (Sigma-Aldrich). Mixed glial cell cultures were maintained at 37°C in 5% CO2 in DMEM-GlutaMAX containing 20% FBS, 50 μg/mL penicillin and streptomycin, and 2.5 μg/mL amphotericin B. Media was changed every three days during expansion of cultures. At confluence, cultures were shaken (orbital) at 120 RPM at 37°C in 5% CO2 for 3.5 hours to isolate microglia from atop the astrocyte monolayer. Supernatants from the shake-off were centrifuged at 600 g for 6 minutes at 4°C, and the cell pellet was rescued in fresh 20% FBS-DMEM media and seeded at 1×105 cells per well of a poly-L-lysine-coated 24-well plate. Cells were allowed to adhere for 48 hours before experimental treatments.
Animals
All animal care and use was conducted in accord with the Guide for the Care and Use of Laboratory Animals, eighth edition (NRC), and protocols were approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio. C57BL/6J mice were obtained from Jackson Laboratory (Bar Harbor, ME; stock# 000664). KMO-/- mice were developed as previously described (Parrott and O'Connor, 2016). Prior to use, WT or KMO-/- breeding pairs were housed in standard shoebox cages, allowed ad libitum food and water access, and general health was monitored daily by veterinary or research staff. Neonates aged 0-4 days old were used for primary microglial cultures.
RNA isolation and qPCR
Microglial cultures were collected in lysis buffer from the RNeasy® RNA Mini Kit (Qiagen, Hilden, Germany) plus 1% β-mercaptoethanol. All cell samples were stored at -80°C until RNA isolation. BV2 and primary microglial cells were homogenized by passing several times through a 27G needle. RNA from microglial cells was isolated using the RNeasy® RNA Mini Kit (Qiagen, Hilden, Germany), according to manufacturer instructions. BV2 RNA was eluted in 30 μL RNase-free water, while primary microglial RNA was eluted in 10 μL RNase-free water. RNA concentration was determined using NanoVue Plus™ spectrophotometer (Biochrom, Cambourne, UK). RNA was then reverse transcribed to cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) following manufacturer instructions. The cDNA reaction was completed using a C1000 thermocycler (Bio-Rad Laboratories, Inc., Hercules, CA). cDNA for the indicated gene targets was amplified using real time qPCR over 40 cycles in a CFX384 Real-Time PCR Detection System (Bio-Rad Laboratories, Inc.). Validated Taqman Gene Expression Assays (ThermoFisher Scientific) were used: Gapdh (assay ID: Mm99999915_g1), Il1b (Mm01336189_m1), Il6 (Mm00446190_m1), Tnf (Mm00443258_m1), Il10 (Mm00439614_m1) Nos2 (Mm00440502_m1), Ido1 (Mm00492586_m1), Kmo (Mm00505511 m1). Gapdh expression was higher than all target genes assayed and was unaffected by experimental treatment; therefore, it was utilized as the sole control housekeeping gene. Data are expressed as relative fold change using the 2ΔΔCt calculation method (User Bulletin #2 ABI PRISM 7700 Sequence Detection System SUBJECT: Relative Quantitation of Gene Expression, 2001).
Griess assay
Before processing using Griess assay, conditioned media samples were store at -80°C. Griess assay was used to measure the accumulation of nitrite in the conditioned media of BV2 and primary microglia. In this assay, standards and samples were incubated with sulfanilic acid (10 mg/mL in 5% phosphoric acid, Sigma-Aldrich) and N-(1-napthyl)-ethylenediamine dihydrochloride (1 mg/mL in deionized water, Sigma-Aldrich) for 15 minutes to produce a color change. Seven concentrations of sodium nitrite as well as a blank sample were diluted in fresh 1% FBS-DMEM media and used as standards. The color change was read by the iMark™ microplate reader (Bio-Rad Laboratories, Inc.) at 500 nm, and sample concentrations were determined by linear regression to the standard curve.
Liquid chromatography mass spectrometry
Liquid chromatography mass spectrometry (LCMS) was used to measure concentrations of kynurenine and quinolinic acid in conditioned media samples as previously described (Parrott et al., 2016). Thawed conditioned media samples were diluted with 5× 0.2% acetic acid and 1 mM standards then put into Amicon Ultra filters (Millipore, Billerica, MA) and centrifuged at 13500 g for 1h at 4 °C. After sample preparation, samples were analyzed by the Mass Spectrometry Core Facility at the University of Texas Health Science Center at San Antonio. This setup consisted of a Q Exactive mass spectrometer (ThermoFisher Scientific) with on-line separation by a Dionex UltiMate 3000 HPLC system (ThermoFisher Scientific), and the data collected were analyzed using Xcalibur 2.2 software (ThermoFisher Scientific).
Statistics
All data were analyzed using Sigma Plot 12 statistical software package (San Jose, CA) and alpha was set to 0.05. For figure 1, data were analyzed using one-way ANOVA followed by Dunnett's post hoc test to compare means of treatment groups to means of control group when a significant main effect was identified. For figure 2, data were analyzed using t-test. For figures 3-6, data were analyzed using two-way ANOVA followed by Holm-Sidak post hoc analysis for between group analysis when a significant interaction was identified. Chauvenet's test was used to identify spurious data as previously described (Taylor, 1997).
Fig 2. LPS increases expression of rate-limiting kynurenine pathway enzymes for oxidative metabolism and generation of quinolinic acid.
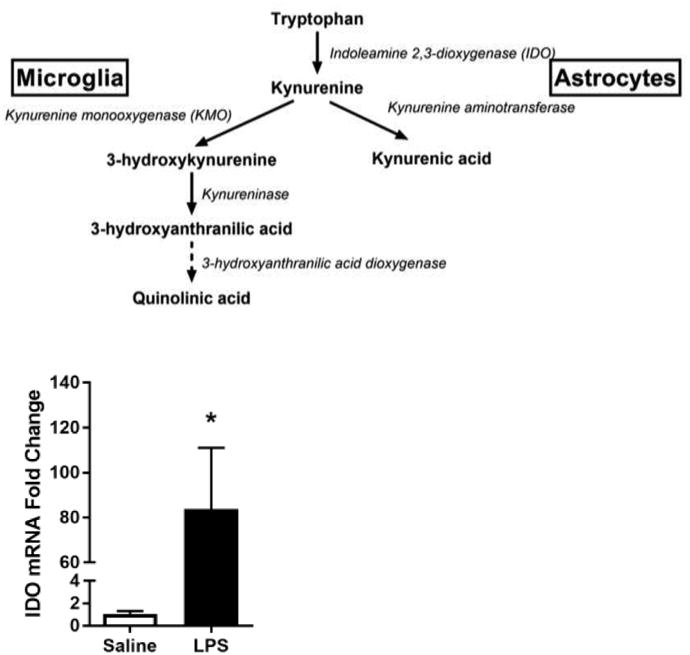
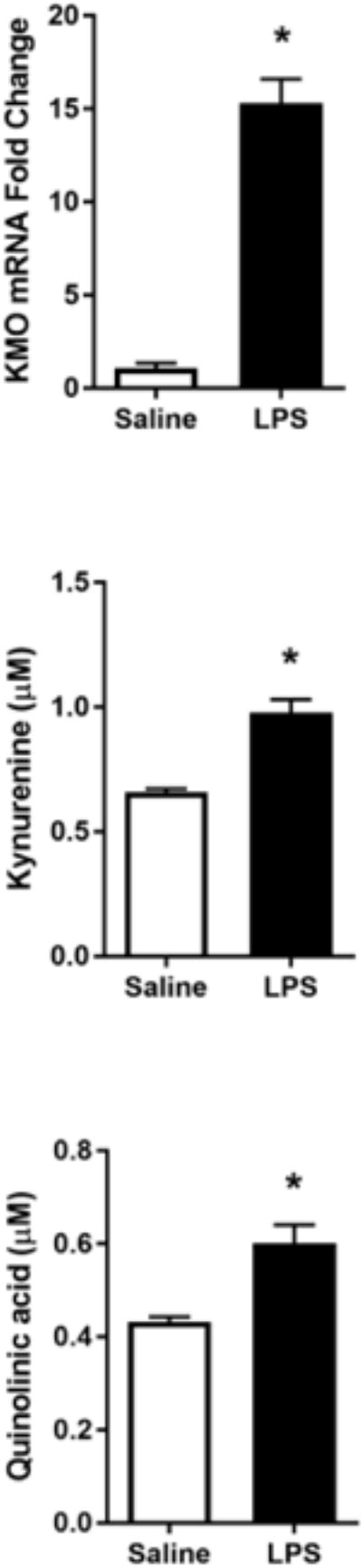
A, Kynurenine pathway diagram. BV2 microglia were treated with saline or LPS (100 ng/mL) for 6 h. RNA was isolated and reverse transcribed to cDNA which was analyzed using qPCR to determine expression of: B, Ido (indoleamine-2,3-dioxygenase-1); C, Kmo (kynurenine monooxygenase). After 24 h LPS exposure, conditioned media was collected and processed using LC-MS to determine concentration of kynurenine (D) and quinolinic acid (E). Bars represent group mean ± SEM, n=3-6, * indicates p<0.05 versus saline-treated controls. Data were analyzed using t-test.
Fig 3.
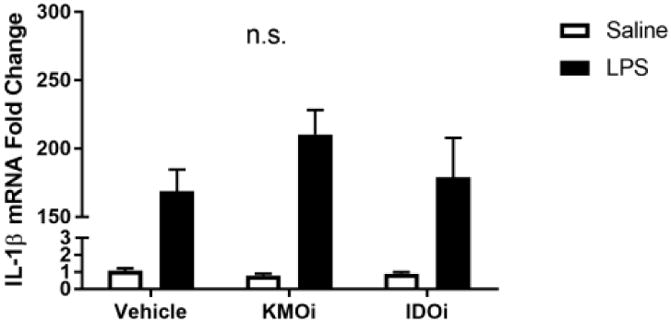
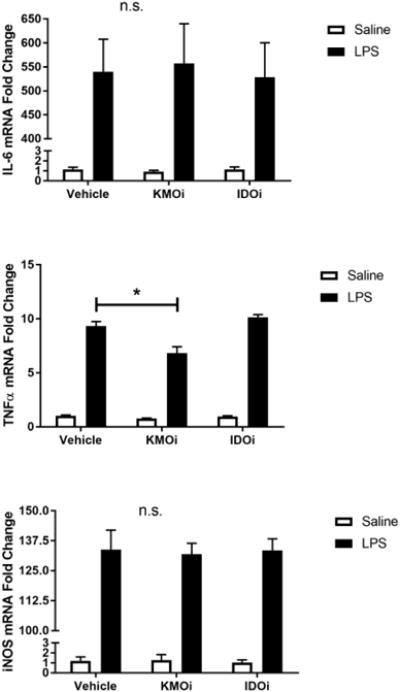
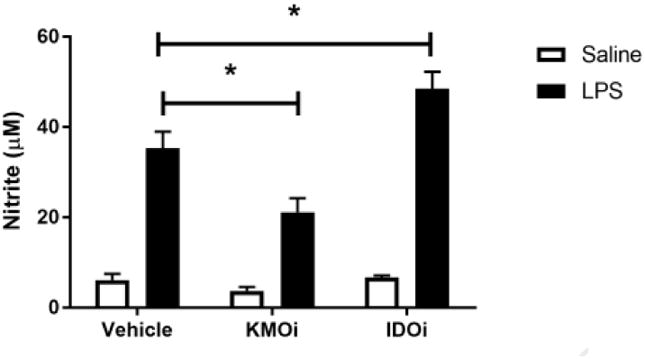
Inhibition of KMO, but not IDO, attenuates TNF-α upregulation and nitrite accumulation. BV2 microglia were treated with saline or LPS (100 ng/mL), then immediately followed by treatment with KMO inhibitor (KMOi, Ro 60-8048, 10 μM) or IDO inhibitor (IDOi, 1-methyl-tryptophan, 10 μM). After 6 h, RNA was isolated and reverse transcribed to cDNA which was analyzed using qPCR to determine expression of: A, Il1b (interleukin-1β); B, Il6 (interlekin-6); C, Tnf (tumor necrosis factor-α); D, Il10 (interleukin-10); E, Nos2 (inducible nitric oxide synthase). After 24 h LPS exposure, conditioned media was collected and processed using Griess assay to determine concentration of nitrite (F). Bars represent group mean ± SEM, n=3-8, * indicates p<0.05 versus indicated group. Data were analyzed using two-way ANOVA followed by Holm Sidak post hoc analysis. Not significant (n.s.)
Fig 6. Kynurenine administration reduces microglial activation state.
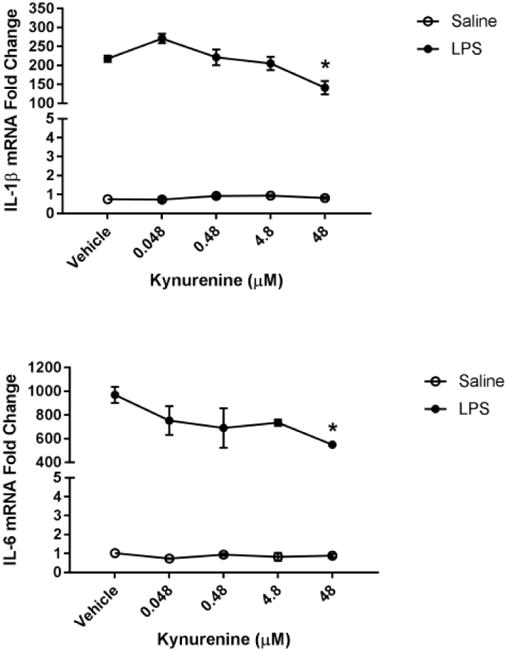
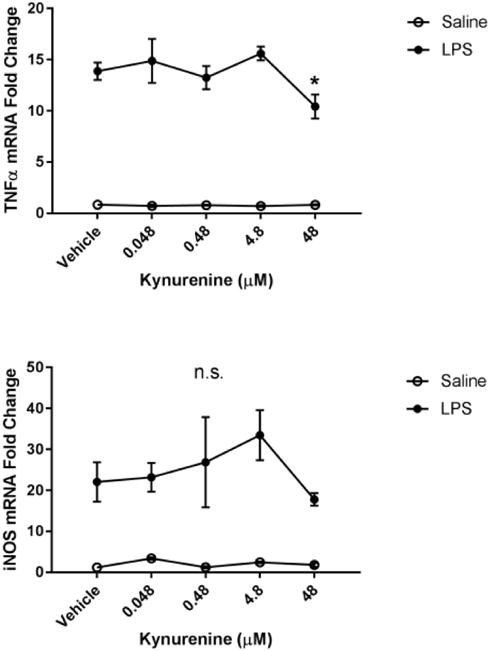
BV2 microglia were treated with saline or LPS (100 ng/mL), then immediately followed by treatment with kynurenine (vehicle, 0.048, 0.48, 4.8, 48, 480 μM). After 4 h, cells were collected for qPCR analysis to determine expression of: A, Il1b (interleukin-1-b); B, Il6 (interlekin-6); C, Tnf (tumor necrosis factor-a); D, Il10 (interleukin-10); E, Nos2 (inducible nitric oxide synthase). Data were analyzed using two-way ANOVA followed by Holm Sidak post hoc analysis.
Results
LPS dose dependently activates BV2 microglia
To identify a sub-maximal dose of lipopolysaccharide (LPS) that significantly increased steady-state mRNA transcript expression of pro-inflammatory cytokines, BV2 cells were incubated with increasing concentrations of LPS for 6h. A dose of 100 ng/mL LPS was required to reliably and significantly increase BV2 microglia mRNA expression of the pro-inflammatory factors interleukin-1β (Fig. 1A- IL-1β p<0.01), interleukin-6 (Fig 1B- IL-6; p<0.05), tumor necrosis factor-α (Fig 1C- TNFα; p<0.05), and inducible nitric oxide synthase (Fig. 1D- iNOS; p<0.001) compared to saline-treated controls. Similarly, 100 ng/mL of LPS was sufficient to induce a significant accumulation of extracellular nitrite (Fig. 1E- p<0.001), a breakdown product of nitric oxide and indicator of oxidative stress, by 24h post-LPS. Using these data, 100 ng/mL of LPS was selected as a robust, but sub-maximal dose of LPS to induce microglial upregulation for all subsequent experiments.
LPS increases expression of rate limiting kynurenine pathway enzymes for oxidative metabolism and generation of quinolinic acid
Microglia predominantly metabolize kynurenine along the KMO-dependent oxidative pathway (Figure 2A), and immune challenge with LPS increases IDO and KMO-dependent metabolism in mouse brain tissue (Parrott and O'Connor, 2016; Parrott et al., 2016; Walker et al., 2013).To determine whether the BV2 microglial response to LPS recapitulates the increased KP metabolic response reported in vivo, BV2 mRNA expression was measured 6h following LPS challenge (Figure 2). LPS treatment induced BV2 microglial mRNA expression of IDO (p<0.05) and KMO (p<0.001). Further, LPS treatment resulted in extracellular accumulation of kynurenine (p<0.05) and quinolinic acid (p<0.05) following 24h LPS. These data support the notion that inflammation promotes induction of the KP in microglia.
Inhibition of KMO, but not IDO, attenuates TNF-α upregulation and nitrite accumulation
Because microglia are among the predominant KMO-expressing cells in the CNS and the anti-inflammatory effect of KMO inhibition in whole animals, we sought to probe the effects of KMO inhibition on microglia. BV2 microglia were co-treated with LPS or saline vehicle and the KMO inhibitor Ro 61-8048 or PBS vehicle (Figure 3). KMO inhibition did not alter LPS-induced expression of IL-1β, IL-6, or iNOS compared to LPS-vehicle treated groups. However, KMO inhibition significantly reduced BV2 microglial expression of TNFα (p<0.01) and the accumulation of nitrite in the media (p<0.05) compared to LPS-vehicle treated controls. In addition, inhibition of IDO using 1-methyltryptophan did not result in any significant changes in the microglial response to LPS. Of note, increasing the concentration of Ro 61-8048 by one log unit resulted in significant cytotoxicity (data not shown). These data suggest an immunomodulatory role of KMO in microglia and represent the first report that inhibition of the KP attenuates microglial inflammatory response.
Genetic deletion of kynurenine monooxygenase reduces activation in response to LPS in primary microglia
We sought to confirm the anti-inflammatory effect of microglial KMO inhibition in a primary culture system by using a genetic approach. WT or KMO-/- primary microglia were treated with vehicle or LPS (Figure 4). KMO-/- prevented LPS-induction of IL-1β (p<0.05), TNFα (p<0.05), and extracellular accumulation of nitrite (p<0.05). Also, there is a basal reduction in extracellular nitrite accumulation from KMO-/- microglia. These data support that KMO knockout protects microglia from LPS-induced pro-inflammatory phenotype.
Fig 4. Genetic deletion of kynurenine monooxygenase reduces activation in response to LPS in primary microglia.
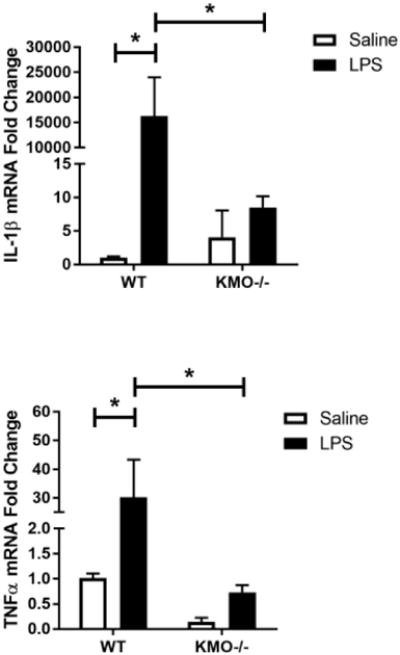
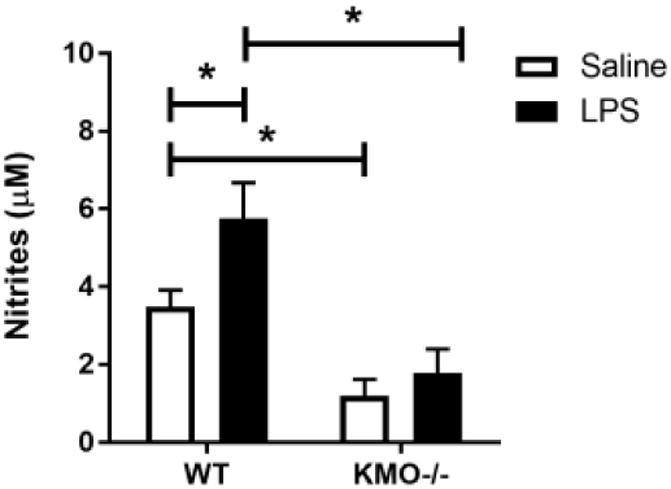
Primary microglia from WT or KMO-/- C57BL/6J pups were treated with LPS (10 ng/mL). After 24 h, cells were analyzed using qPCR to determine relative expression of: A, Il1b (interleukin-1β) and B, Tnf (tumor necrosis factor-α). Conditioned media was collected and processed using Griess assay to determine concentration of nitrite (C). Bars represent group mean ± SEM, n=3-7, * indicates p<0.05 versus indicated group. Data were analyzed using two-way ANOVA followed by Holm Sidak post hoc analysis. Not significant (n.s.)
L-Kynurenine administration reduces microglial activation state
After gathering evidence that KMO inhibition reduces a pro-inflammatory microglial phenotype following LPS, we sought to explore the potential mechanism. Previous reports suggest that NMDA receptor activation potentiates neuroinflammation (Kaindl et al., 2012). Therefore, BV2 microglia were treated with increasing doses of quinolinic acid in the presence or absence of LPS (Figure 5). Although LPS significantly increased expression of IL-1β, IL-6, TNFα, and extracellular nitrite, quinolinic had no significant effect on these parameters following 6h of LPS.
Fig 5. Quinolinic acid treatment does not affect microglial activation.
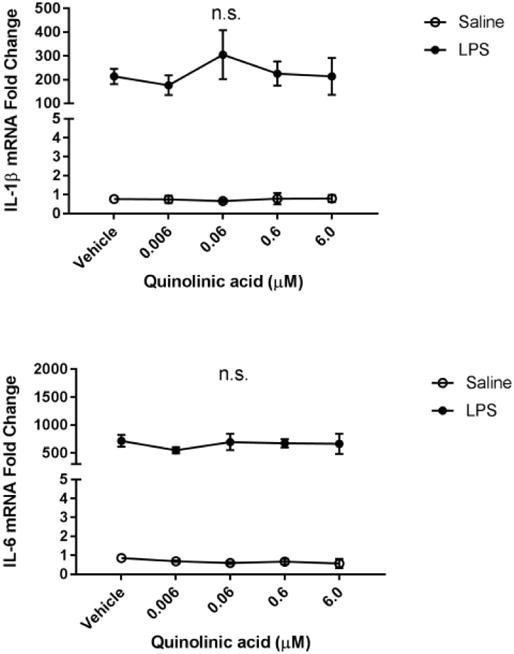
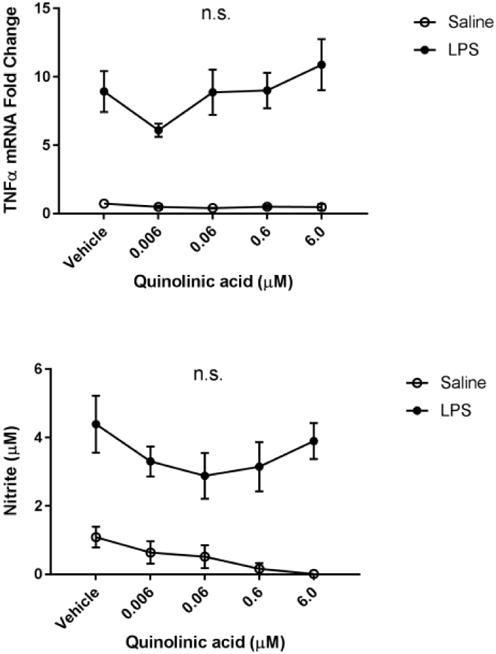
BV2 microglia were treated with saline or LPS (100 ng/mL), then immediately followed by treatment with kynurenine (vehicle, 0.006, 0.06, 0.6, 6.0, 60.0 μM). After 4 h, cells were collected for qPCR analysis to determine expression of: A, Il1b (interleukin-1-b); B, Il6 (interlekin-6); C, Tnf (tumor necrosis factor-a); D, Il10 (interleukin-10); E, Nos2 (inducible nitric oxide synthase). Data were analyzed using two-way ANOVA followed by Holm Sidak post hoc analysis. Not significant n.s.
Next, we investigated the potential immunomodulatory role of kynurenine on microglia. Previous studies report that KMO inhibition or knockout results in an accumulation of peripheral and central kynurenine (Giorgini et al., 2013; Parrott et al., 2016). Therefore, BV2 microglia were co-treated with LPS and increasing doses of kynurenine (Figure 6). Kynurenine (48 μM) reduced LPS-induced microglial expression of IL-1β (p<0.01), IL-6 (p<0.05), and TNFα (p<0.05) compared to LPS-vehicle controls. There was no significant effect of kynurenine on LPS-induced expression of the anti-inflammatory cytokine interleukin-10 and iNOS compared to LPS-vehicle controls. These data suggest that kynurenine negatively regulates the microglial response to LPS.
Discussion
KP metabolic balance is shifted towards oxidative/excitatory metabolites in stress and inflammation and provides a central node that mediates subsequent behavioral consequences. While many studies have focused effects of KP metabolites directly on neurons, the potential role that KP metabolites have directly on microglial activity is unknown. Our data are the first to suggest that kynurenine metabolites directly modulate microglial activity. We found that both pharmacological inhibition and genetic deletion of microglial KMO ameliorates LPS responses (Fig. 3-4). The role of kynurenine metabolites as mediators of neuropathology have focused on their direct or indirect effects on neurotransmitter systems. For example, kynurenic acid and quinolinic acid have opposing effects on NMD A excitability, and 3-hydroxyanthranillic acid is toxic to neuronal cultures (Obrenovitch and Urenjak, 2000; M.N. Perkins and Stone, 1983; Smith et al., 2007). However, the effects of kynurenine metabolites on the inflammatory response of microglia, which has been implicated in many neuropathologies (Nakagawa and Chiba, 2015), are unknown. To address this gap in knowledge, we utilized an in vitro approach targeting enzymes and metabolites of the kynurenine pathway in the context of microglial activation.
Microglia are the innate immune cells resident to the CNS, and as such are the drivers of neuroinflammation and brain cytokine expression (Ransohoff and Perry, 2009). Peripheral LPS in vivo results in acute sickness behaviors that peak around 6 hours post injection, as well as depressive-like behaviors that peak 24-48 hours post injection (Frenois et al., 2007), and microglial expression of pro-inflammatory cytokines is increased (Wohleb et al., 2012). In vivo, LPS does not cross the blood brain barrier, and instead microglia are activated by downstream signaling induced by LPS in the periphery via neural and humoral routes (Bluthé et al., 1994; Quan et al., 1998). However, LPS administered to mice centrally via intracranioventricular injection or peripherally via intraperitoneal injection both develop the same behavioral and cytokine responses (Henry et al., 2008; Lawson et al., 2013). To this end, treating microglia directly with LPS is an appropriate model of neuroinflammation. Furthermore, BV2 cells are a mouse microglial cell line derived from C57BL6 mice (Blasi et al., 1990). BV2 cells treated with LPS in vitro resemble the in vivo microglial response, characterized by increased expression of pro-inflammatory factors (Henn et al., 2009). Any in vitro approach has limitations because the in vivo condition is encompassed by multiple cell types and responses. However, it affords the ability to study this process in isolation without the complexities of the native tissue.
The rate limiting enzymes of KP, IDO or TDO can be upregulated by neuroendocrine and neuroimmune factors; corticosterone upregulates TDO, while several cytokines upregulate IDO. This can occur in the periphery resulting in an increased supply of kynurenine to the brain for further metabolism or it can occur directly in the brain. Downstream of IDO/TDO, KMO metabolizes kynurenine, and is the rate-limiting enzyme for the production of quinolinic acid (Connor et al., 2008). In the CNS, the kynurenine pathway is segregated across cell types; microglia more highly express KMO, while astrocytes highly express kynurenine aminotransferase isoforms which metabolize kynurenine into kynurenic acid (Zmarowski et al., 2009). This cellular segregation suggests that these cell types exert opposing effects of the kynurenine pathway during inflammation. Moreover, preclinical rodent studies have found that IDO is necessary for mice to develop depressive-like behaviors in response to inflammation and KMO is required for this anhedonic response (O'Connor et al., 2009; Parrott et al., 2016). These studies and others provide evidence that the kynurenine pathway can exert in vivo effects, but the direct effects of kynurenine metabolites at the cellular level has received little attention.
As previously mentioned, kynurenine and several of its metabolites are biologically active. Quinolinic acid promotes reactive oxygen and nitrogen species, excitotoxicity via NMDA receptor activation, and neuronal death (M N Perkins and Stone, 1983; Santamaria et al., 2001; Sumit et al., 2015). NMDA receptor activation has been reported to potentiate microglial activation in vitro (Kaindl et al., 2012). One can speculate that by inhibiting KMO action, there is reduced quinolinic acid (Giorgini et al., 2013) and therefore less interaction with the LPS response. Interestingly, we found that BV2 microglia treated with quinolinic acid in the presence or absence of LPS does not alter microglial inflammatory response. Although NMDA receptor activation has been observed to play a role in microglial activity in culture, in vivo microglial expression of NMDA receptors is relatively low and therefore may not play a large role (Domercq et al., 2013). An alternative pathway of the protective effects of KMO inhibition may be the resultant accumulation of the upstream metabolite kynurenine (Giorgini et al., 2013). We found that kynurenine treatment reduced microglial LPS response (Fig. 6A-C). These data suggest that kynurenine, which is increased with LPS treatment (Fig. 2D-E) can feed back onto microglial activity, but mechanisms of this action are unknown. However, kynurenine is an agonist for the aryl hydrocarbon receptor (AhR), an intracellular receptor that has been observed to reduce pro-inflammatory responses in several models (Bessede et al., 2014; Kimura et al., 2009; C. F. A. Vogel et al., 2007; C. F. a Vogel et al., 2007). It is possible that kynurenine action on AhR in microglia can ameliorate LPS response.
We inhibited IDO and KMO as the rate limiting enzymes for the KP as a whole, or the neurotoxic branch that produces quinolinic acid, respectively. We found that inhibition of IDO had no effect on microglial LPS activation, but KMO inhibition reduced expression of TNFα and extracellular nitrites (Fig. 3). The lack of effect by IDO inhibition may be explained by the presence and activity of other dioxygenase enzymes that can metabolize tryptophan to kynurenine and proceed down the KMO branch. Our data point to relative specificity in that TNFα and extracellular nitrites are most affected by KMO inhibition after LPS treatment in BV2 cells (Fig. 3). Differential regulation by subunits of the canonical inflammatory-response transcription factor NF-kB may explain this specificity. However, data from our KMO-/- microglia show changes in all measures shown (Fig. 4). It is possible that the in vivo effects of KMO knockout impact several cell types that then feed back onto microglial regulation of the expression of several cytokines. Indeed, the reduction of several cytokines in microglia after KMO inhibition is in line with previous reports that KMO inhibition can ameliorate inflammation in vivo (Clark et al. 2005; Rojewska et al. 2016). Previous reports have also shown that primary microglia undergo more pronounced changes in gene expression as compared to BV-2 cells (Henn et al., 2009) which could result in a magnified inhibitory effect of gene deletion as opposed to pharmacological inhibition in BV-2 microglia. Targeted deletion of the KMO gene could also result in other compensatory changes in the expression of cellular components that remain to be characterized.
We found that inhibition of KMO, but not IDO, significantly reduced cytokine expression and nitrite production by LPS-activated microglia. These data indicated that manipulation of the kynurenine pathway via KMO can ameliorate elements of the microglial inflammatory responses. KMO inhibition can result in reduced quinolinic acid, as well as accumulations of kynurenine, and these changes suggest that these metabolites may be able to feed back onto microglia activation. We treated BV2 microglia with quinolinic acid and observed no effect on the production of pro-inflammatory factors. This was in contrast to previous studies that found that activation of microglial NMDARs using NMDA resulted in accumulation of nitrite and secretion of several pro-inflammatory cytokines, including TNF-α (Kaindl et al., 2012)a. Our initial hypothesis was that quinolinic acid would potentiate the effects of LPS, and although BV-2 microglia are known to express functional NMDARs (Tsai et al., 2013), our data suggest that quinolinic acid and NMDA act differently on microglia or that the time course of our experiment was not sufficient to observe an NMDA-mediated effect of quinolinic acid. However, upon treatment with kynurenine, LPS-activated BV2 microglia expression of pro-inflammatory cytokines was significantly reduced. The mechanisms by which kynurenine treatment can ameliorate inflammatory responses is unknown. Kynurenine is an endogenous ligand for the AhR, an immunodmodulatory intracellular receptor. We speculate that increased kynurenine can reduce microglial inflammatory responses via AhR, and this mechanism will be examined in a follow-up study. The data presented here are the first to show direct kynurenine metabolite interactions with microglial activity. KMO has been implicated in a number of stress- and immune-associated neuropathology. This study provides support for potential new targets to modulate microglial responses.
Highlights.
KMO inhibition attenutates specific elements of microglia activation
Primary KMO -/- microglia recapitulate pharmacologic inhibition of KMO
Kynurenine negatively modulates microglia responses to LPS
Exogenous quinolinic acid did not potentiate LPS-induced activity
Acknowledgments
This research was supported by National Institute of Mental Health grant R01-MH090127, VA Merit Award I01BX003195-01, NIH National Centers for Advancing Translational Sciences grant UL1 TR001120 and Texas Higher Education Coordinating Board NHARP 003659-0010-2012 (JCO), NIH Institutional training grant T32 NS 082145 (AMG), and the NIH National Research Service Award grant 1F31 MH102070-01A1 (JMP). We would like to thank Susan T. Weintraub, PhD and Xiaoli Gao, PhD at the University of Texas Health Science Center at San Antonio Mass Spectrometry Core Facilities for their assistance with LC/MS method design, sample preparation, sample analysis, and data collection. Mass spectrometry analyses were conducted on instrumentation obtained with funding from the National Institutes of Health (1S10OD016417-01 to STW). The content is the sole the responsibility of the authors and does not necessarily represent the views of the National Institute of Mental Health or the National Institutes of Health.
Footnotes
Conflict of Interest Statement: Dr. O'Connor has had previous consulting agreements with Lundbeck Research USA and Janssen Research and Development, LLC unrelated to the work contained in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bessede A, Gargaro M, Pallotta MT, Matino D, Servillo G, Brunacci C, Bicciato S, Mazza EMC, Macchiarulo A, Vacca C, Iannitti R, Tissi L, Volpi C, Belladonna ML, Orabona C, Bianchi R, Lanz TV, Platten M, Della Fazia MA, Piobbico D, Zelante T, Funakoshi H, Nakamura T, Gilot D, Denison MS, Guillemin GJ, DuHadaway JB, Prendergast GC, Metz R, Geffard M, Boon L, Pirro M, Iorio A, Veyret B, Romani L, Grohmann U, Fallarino F, Puccetti P, Alban B, Marco G, Maria TP, Davide M, Giuseppe S, Cinzia B, Silvio B, Emilia MCM, Antonio M, Carmine V, Rossana I, Luciana T, Claudia V, Maria LB, Ciriana O, Roberta B, Tobias VL, Michael P, Maria ADF, Danilo P, Teresa Z, Hiroshi F, Toshikazu N, David G, Michael SD, Gilles JG, James BD, George CP, Richard M, Michel G, Louis B, Matteo P, Alfonso I, Bernard V, Luigina R, Ursula G, Francesca F, Paolo P. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature. 2014;511:184–190. doi: 10.1038/nature13323. https://doi.org/10.1038/nature13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol. 1990;27:229–37. doi: 10.1016/0165-5728(90)90073-v. [DOI] [PubMed] [Google Scholar]
- Bluthé RM, Walter V, Parnet P, Layé S, Lestage J, Verrier D, Poole S, Stenning BE, Kelley KW, Dantzer R. Lipopolysaccharide induces sickness behaviour in rats by a vagal mediated mechanism. C R Acad Sci III. 1994;317:499–503. [PubMed] [Google Scholar]
- Carpenedo R, Chiarugi A, Russi P, Lombardi G, Carlà V, Pellicciari R, Mattoli L, Moroni F. Inhibitors of kynurenine hydroxylase and kynureninase increase cerebral formation of kynurenate and have sedative and anticonvulsant activities. Neuroscience. 1994;61:237–244. doi: 10.1016/0306-4522(94)90227-5. https://doi.org/10.1016/0306-4522(94)90227-5. [DOI] [PubMed] [Google Scholar]
- Carpenedo R, Meli E, Peruginelli F, Pellegrini-Giampietro DE, Moroni F. Kynurenine 3-mono-oxygenase inhibitors attenuate post-ischemic neuronal death in organotypic hippocampal slice cultures. J Neurochem. 2002;82:1465–1471. doi: 10.1046/j.1471-4159.2002.01090.x. https://doi.org/10.1046/j.1471-4159.2002.01090.x. [DOI] [PubMed] [Google Scholar]
- Clark CJ, Mackay GM, Smythe GA, Bustamante S, Stone TW, Phillips RS. Prolonged Survival of a Murine Model of Cerebral Malaria by Kynurenine Pathway Inhibition. Infect Immun. 2005;73:5249–5251. doi: 10.1128/IAI.73.8.5249-5251.2005. https://doi.org/10.1128/IAI.73.8.5249-5251.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor TJ, Starr N, O'Sullivan JB, Harkin A. Induction of indolamine 2,3-dioxygenase and kynurenine 3-monooxygenase in rat brain following a systemic inflammatory challenge: A role for IFN-γ? Neuroscience Letters. 2008 doi: 10.1016/j.neulet.2008.06.007. https://doi.org/doi:10.1016/j.neulet.2008.06.007. [DOI] [PubMed]
- Domercq M, Vázquez-Villoldo N, Matute C. Neurotransmitter signaling in the pathophysiology of microglia. Front Cell Neurosci. 2013;7:49. doi: 10.3389/fncel.2013.00049. https://doi.org/10.3389/fncel.2013.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostal C, Carson Sulzer M, Kelley K, Freund G, McCusker R. Glial and tissue-specific regulation of Kynurenine 1 Pathway dioxygenases by acute stress of mice. Neurobiol Stress. 2017;7:1–5. doi: 10.1016/j.ynstr.2017.02.002. https://doi.org/10.1016/j.ynstr.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugan AM, Parrott JM, Redus L, Hensler JG, O'Connor JC, O'Connor JC. Low-level stress induces production of neuroprotective factors in wild-type but not BDNF+/- mice: interleukin-10 and kynurenic acid. Int J Neuropsychopharmacol. 2015 doi: 10.1093/ijnp/pyv089. pyv089. https://doi.org/10.1093/ijnp/pyv089. [DOI] [PMC free article] [PubMed]
- Fenn AM, Henry CJ, Huang Y, Dugan A, Godbout JP. Lipopolysaccharide-induced interleukin (IL)-4 receptor-α expression and corresponding sensitivity to the M2 promoting effects of IL-4 are impaired in microglia of aged mice. Brain Behav Immun. 2012;26:766–777. doi: 10.1016/j.bbi.2011.10.003. https://doi.org/10.1016/j.bbi.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenois F, Moreau M, O'Connor J, Lawson M, Micon C, Lestage J, Kelley KW, Dantzer R, Castanon N. Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology. 2007;32:516–31. doi: 10.1016/j.psyneuen.2007.03.005. https://doi.org/10.1016/j.psyneuen.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibney SM, Fagan EM, Waldron AM, O'Byrne J, Connor TJ, Harkin A. Inhibition of stress-induced hepatic tryptophan 2,3-dioxygenase exhibits antidepressant activity in an animal model of depressive behaviour. Int J Neuropsychopharmacol. 2014;17:917–928. doi: 10.1017/S1461145713001673. https://doi.org/10.1017/S1461145713001673. [DOI] [PubMed] [Google Scholar]
- Giorgini F, Huang SY, Sathyasaikumar KV, Notarangelo FM, Thomas MAR, Tararina M, Wu HQ, Schwarcz R, Muchowski PJ. Targeted Deletion of Kynurenine 3-Monooxygenase in Mice: A new tool for studying kynurenine pathway metabolism in periphery and brain. J Biol Chem. 2013;288:36554–36566. doi: 10.1074/jbc.M113.503813. https://doi.org/10.1074/jbc.M113.503813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidetti P, Luthi-Carter RE, Augood SJ, Schwarcz R. Neostriatal and cortical quinolinate levels are increased in early grade Huntington's disease. Neurobiol Dis. 2004;17:455–61. doi: 10.1016/j.nbd.2004.07.006. https://doi.org/10.1016/j.nbd.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Kerr SJ, Smythe GA, Smith DG, Kapoor V, Armati PJ, Croitoru J, Brew BJ. Kynurenine pathway metabolism in human astrocytes: a paradox for neuronal protection. J Neurochem. 2001;78:842–853. doi: 10.1046/j.1471-4159.2001.00498.x. https://doi.org/10.1046/j.1471-4159.2001.00498.x. [DOI] [PubMed] [Google Scholar]
- Heisler JM, O'Connor JC. Indoleamine 2,3-dioxygenase-dependent neurotoxic kynurenine metabolism mediates inflammation-induced deficit in recognition memory. Brain Behav Immun. 2015;50:115–124. doi: 10.1016/j.bbi.2015.06.022. https://doi.org/10.1016/j.bbi.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henn A, Lund S, Hedtjärn M, Schrattenholz A, Pörzgen P, Leist M. The Suitability of BV2 Cells as Alternative Model System for Primary Microglia Cultures or for Animal Experiments Examining Brain Inflammation. ALTEX Altern to Anim Exp. 2009;26:83–94. doi: 10.14573/altex.2009.2.83. [DOI] [PubMed] [Google Scholar]
- Henry CCJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, Sheridan JF, Godbout JPJ. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflammation. 2008;5:15. doi: 10.1186/1742-2094-5-15. https://doi.org/10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaindl AM, Degos V, Peineau S, Gouadon E. Activation of microglial N‐ methyl‐ D‐ aspartate receptors triggers inflammation and neuronal cell death in the developing and mature brain. Ann Neurol. 2012;72:536–49. doi: 10.1002/ana.23626. https://doi.org/10.1002/ana.23626. [DOI] [PubMed] [Google Scholar]
- Kimura A, Naka T, Nakahama T, Chinen I, Masuda K, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor in combination with Stat1 regulates LPS-induced inflammatory responses. J Exp Med. 2009;206:2027–2035. doi: 10.1084/jem.20090560. https://doi.org/10.1084/jem.20090560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson MA, Parrott JM, McCusker RH, Dantzer R, Kelley KW, O'Connor JC. Intracerebroventricular administration of lipopolysaccharide induces indoleamine-2,3-dioxygenase-dependent depression-like behaviors. J Neuroinflammation. 2013;10:87. doi: 10.1186/1742-2094-10-87. https://doi.org/10.1186/1742-2094-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y, Chiba K. Diversity and plasticity of microglial cells in psychiatric and neurological disorders. Pharmacol Ther. 2015;154:21–35. doi: 10.1016/j.pharmthera.2015.06.010. https://doi.org/10.1016/j.pharmthera.2015.06.010. [DOI] [PubMed] [Google Scholar]
- O'Connor JC, Lawson MA, André C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009;14:511–522. doi: 10.1038/sj.mp.4002148. https://doi.org/10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrenovitch TP, Urenjak J. In vivo assessment of kynurenate neuroprotective potency and quinolinate excitotoxicity. Amino Acids. 2000;19:299–309. doi: 10.1007/s007260070061. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Matson WR, Beal MF, Myers RH, Bird ED, Milbury P, Saso S. Kynurenine pathway abnormalities in Parkinson's disease. Neurology. 1992;42:1702–6. doi: 10.1212/wnl.42.9.1702. [DOI] [PubMed] [Google Scholar]
- Parrott J, O'Connor JC. Neurotoxic kynurenine metabolism is increased in the dorsal hippocampus and drives distinct depressive behaviors during inflammation. Transl Psychiatry. 2016;18 doi: 10.1038/tp.2016.200. https://doi.org/10.1038/tp.2016.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott JM, O'Connor JC. Kynurenine 3-monooxygenase: an influential mediator of neuropathology. Front Psychiatry. 2015;6:1–17. doi: 10.3389/fpsyt.2015.00116. https://doi.org/10.3389/fpsyt.2015.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott JM, Redus L, O'Connor JC. Kynurenine metabolic balance is disrupted in the hippocampus following peripheral lipopolysaccharide challenge. J Neuroinflammation. 2016;13 doi: 10.1186/s12974-016-0590-y. https://doi.org/10.1186/s12974-016-0590-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins MN, Stone TW. Quinolinic acid: regional variations in neuronal sensitivity. Brain Res. 1983;259:172–176. doi: 10.1016/0006-8993(83)91084-3. https://doi.org/10.1016/0006-8993(83)91084-3. [DOI] [PubMed] [Google Scholar]
- Perkins MN, Stone TW. Pharmacology and regional variations of quinolinic acid-evoked excitations in the rat central nervous system. J Pharmacol Exp Ther. 1983;226:551–7. [PubMed] [Google Scholar]
- Perkins MN, Stone TW. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 1982;247:184–187. doi: 10.1016/0006-8993(82)91048-4. https://doi.org/10.1016/0006-8993(82)91048-4. [DOI] [PubMed] [Google Scholar]
- Quan N, Whiteside M, Herkenham M. Time course and localization patterns of interleukin-1beta messenger RNA expression in brain and pituitary after peripheral administration of lipopolysaccharide. Neuroscience. 1998;83:281–93. doi: 10.1016/s0306-4522(97)00350-3. [DOI] [PubMed] [Google Scholar]
- Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, Spivey JR, Saito K, Miller AH. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry. 2010;15:393–403. doi: 10.1038/mp.2009.116. https://doi.org/10.1038/mp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH. Microglial Physiology: Unique Stimuli, Specialized Responses. Immunology. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. https://doi.org/10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Rodgers J, Stone TW, Barrett MP, Bradley B, Kennedy PGE. Kynurenine pathway inhibition reduces central nervous system inflammation in a model of human African trypanosomiasis. Brain. 2009a;132:1259–67. doi: 10.1093/brain/awp074. https://doi.org/10.1093/brain/awp074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers J, Stone TW, Barrett MP, Bradley B, Kennedy PGE. Kynurenine pathway inhibition reduces central nervous system inflammation in a model of human African trypanosomiasis. Brain. 2009b;132:1259–1267. doi: 10.1093/brain/awp074. https://doi.org/10.1093/brain/awp074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojewska E, Piotrowska A, Makuch W, Przewlocka B, Mika J. Pharmacological kynurenine 3-monooxygenase enzyme inhibition significantly reduces neuropathic pain in a rat model. Neuropharmacology. 2016;102:80–91. doi: 10.1016/j.neuropharm.2015.10.040. https://doi.org/10.1016/j.neuropharm.2015.10.040. [DOI] [PubMed] [Google Scholar]
- Santamaria A, Galvan-Arzate S, Lisy V, Ali S, Dunhart H, Osorio-Rico L, Rios C, Sut'astny F. Quinolinic acid induces oxidative stress in rat brain synaptosomes. Neuroreport. 2001;12:871–874. doi: 10.1097/00001756-200103260-00049. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, Roberts RC. Increased cortical kynurenate content in schizophrenia. Biol Psychiatry. 2001;50:521–30. doi: 10.1016/s0006-3223(01)01078-2. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Stone TW, Smith RA. Neurotoxicity of tryptophan metabolites. Biochem Soc Trans. 2007;35:1287–1289. doi: 10.1042/BST0351287. [DOI] [PubMed] [Google Scholar]
- Stone TW, Perkins MN. Quinolinic acid: a potent endogenous excitant at amino acid receptors in CNS. Eur J Pharmacol. 1981;72:411–2. doi: 10.1016/0014-2999(81)90587-2. [DOI] [PubMed] [Google Scholar]
- Sumit J, Shamsher S, Navneet K, Puneet K. Protective Effect of Spermidine Against Excitotoxic Neuronal Death Induced by Quinolinic Acid in Rats: Possible Neurotransmitters and Neuroinflammatory Mechanism. Neurotox Res. 2015;28:171–184. doi: 10.1007/s12640-015-9535-y. https://doi.org/10.1007/s12640-015-9535-y. [DOI] [PubMed] [Google Scholar]
- Taylor JR. An introduction to error analysis : the study of uncertainties in physical measurements. University Science Books; 1997. [Google Scholar]
- Tsai KL, Chang HF, Wu SN. The Inhibition of Inwardly Rectifying K + Channels by Memantine in Macrophages and Microglial Cells. Cell Physiol Biochem. 2013;31:938–951. doi: 10.1159/000350112. https://doi.org/10.1159/000350112. [DOI] [PubMed] [Google Scholar]
- User Bulletin #2 ABI PRISM 7700 Sequence Detection System SUBJECT: Relative Quantitation of Gene Expression. 2001 [Google Scholar]
- Vogel CFA, Sciullo E, Li W, Wong P, Lazennec G, Matsumura F. RelB, a new partner of aryl hydrocarbon receptor-mediated transcription. Mol Endocrinol. 2007;21:2941–55. doi: 10.1210/me.2007-0211. https://doi.org/10.1210/me.2007-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CFa, Sciullo E, Matsumura F. Involvement of RelB in aryl hydrocarbon receptor-mediated induction of chemokines. Biochem Biophys Res Commun. 2007;363:722–726. doi: 10.1016/j.bbrc.2007.09.032. https://doi.org/10.1016/j.bbrc.2007.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AK, Budac DP, Bisulco S, Lee AW, Smith RA, Beenders B, Kelley KW, Dantzer R. NMDA Receptor Blockade by Ketamine Abrogates Lipopolysaccharide-Induced Depressive-Like Behavior in C57BL/6J Mice. Neuropsychopharmacology. 2013;38:1609–1616. doi: 10.1038/npp.2013.71. https://doi.org/10.1038/npp.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Fenn AM, Pacenta AM, Powell ND, Sheridan JF, Godbout JP. Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory CNS macrophages, and prolonged social withdrawal in socially defeated mice. Psychoneuroendocrinology. 2012;37:1491–1505. doi: 10.1016/j.psyneuen.2012.02.003. https://doi.org/10.1016/j.psyneuen.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmarowski A, Wu HQ, Brooks JM, Potter MC, Pellicciari R, Schwarcz R, Bruno JP. Astrocyte-derived kynurenic acid modulates basal and evoked cortical acetylcholine release. Eur J Neurosci. 2009;29:529–538. doi: 10.1111/j.1460-9568.2008.06594.x. https://doi.org/10.1111/j.1460-9568.2008.06594.x. [DOI] [PubMed] [Google Scholar]
- Zwilling D, Huang SY, Sathyasaikumar KV, Notarangelo FM, Guidetti P, Wu HQ, Lee J, Truong J, Andrews-Zwilling Y, Hsieh EW, Louie JY, Wu T, Scearce-Levie K, Patrick C, Adame A, Giorgini F, Moussaoui S, Laue G, Rassoulpour A, Flik G, Huang Y, Muchowski JM, Masliah E, Schwarcz R, Muchowski PJ. Kynurenine 3-Monooxygenase Inhibition in Blood Ameliorates Neurodegeneration. Cell. 2011;145:863–874. doi: 10.1016/j.cell.2011.05.020. https://doi.org/10.1016/j.cell.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]


