Abstract
Complement-mediated allograft injury, elicited by donor specific HLA antibodies (DSA), is a defining pathophysiological characteristic of allograft damage. We aimed to study DSA-induced complement activation as a diagnostic marker of antibody mediated rejection (AMR) and a risk stratification tool for graft loss in the context of lung transplantation (LT). We identified 38 DSA positive patients whose serum samples were submitted for C3d deposition testing via the C3d assay. Among these 38 patients, 15 had AMR (DSAPosAMRPos). Results were reported for each patient as the C3d ratio for each DSA, the immunodominant DSA, and the C3d ratio for all DSA present in a sample (C3d ratioSUM). DSAPosAMRPos patients had higher C3d ratioSUM values (58.66 (−1.32–118.6) vs. 1.52 (0.30–2.74), p=0.0016) and increased immunodominant C3d ratios (41.87 (1.72–82.02) vs. 0.69 (0.21–1.19), p=0.001) when compared to DSAPosAMRNeg patients. Specificity and calculated positive predictive value of the immunodominant C3d ratio and BCMsum tests for AMR diagnosis were both 100% (CI=17.4–100) in this cohort. Worst graft survival was associated with both immunodominant C3d ratio≥4 or C3d ratioSUM≥10 or BCMsum >7000, suggesting that the antibody composition and/or strength are the principal determinants of an HLA DSA’s capacity to activate complement.
Keywords: complement, donor specific HLA antibodies, lung transplant, antibody-mediated rejection
Introduction
In response to alloimmunization via organ transplantation, patients develop alloantibodies to HLA expressed by donor tissue and are referred to as donor specific antibodies (DSA). In heart and kidney transplantation, DSA mediate damage to the allograft and lower graft survival via multiple mechanisms including complement-dependent and independent actions (1). Criteria for diagnosis of antibody-mediated rejection (AMR) comprise the presence of circulating DSA and histological patterns in the graft biopsy, including endothelial swelling, leukocytic infiltrate, and the complement split product C4d (2, 3). Using data obtained in heart and renal transplantation as a working hypothesis and a series of histological case reports of AMR in lung transplantation (LT) (4, 5), work over the past decade has attempted to define the features of AMR in the field of LT (2). Recently, a consensus was reached on the diagnostic criteria of AMR in LT: the presence of DSA and evocative lung pathology, with or without the presence of C4d in the graft associated with or without graft failure (clinical AMR or subclinical AMR, respectively) (6). With these criteria in hand, the lung transplant community is now in pursuit of new technologies and algorithms that allow for risk stratification of DSA+ patients to guide management and therapy.
As AMR is the main cause of late-stage graft failure across most solid organ transplants (7), a large number of studies have attempted to identify features of DSA that may be indicative of graft failure. Historically, the presence of strong DSA levels based on MFI values, a semi-quantitative measurement of the quantity of antibody bound to antigen-coupled luminex beads, has been the major approach used to guide clinical management during transplant care (8). A further step to stratify the pathogenic potential of DSA has been to measure their ability to activate complement. The C1q platform has been instrumental in identifying patients with DSA that bind C1q, the major mediator of classical complement activation, which are more likely to result in episodes of rejection and late-stage graft failure in cardiac and renal transplantation (9–11). While the C1q technology has been evaluated as a diagnostic tool in both cardiac and renal transplant, the field of LT underutilizes these platforms for risk stratification.
Recently, a new solid-phase C3d assay was developed to assess the ability of HLA DSA to both bind and activate complement. The principle of the assay is similar to the commonly used single antigen platform, whereby DSA in patient serum binds to single antigen beads. Instead of detecting antibody bound to beads with an anti-human IgG secondary antibody, the DSA-bound beads are mixed with human complement, which results in classical complement activation and C3d deposition on the bead surface. An anti-human C3d antibody is then used to detect bead-bound C3d. Therefore, the C3d assay is a direct measure of HLA DSA activation of human complement. We hypothesize that complement activation by DSA in the C3d assay will be a strong indicator of AMR diagnosis and poor graft outcome.
To test this hypothesis, we used a well-defined cohort of LT recipients (12) and determined whether the C3d assay identified LT patients with AMR. We identified DSA positive patients and tested their sera for the presence of complement activating antibodies using the C3d assay. Moreover, as our patients at Foch Hospital are prospectively monitored for AMR diagnosis, we directly compared levels of DSA-mediated C3d activation between AMR positive and negative LT patients. DSAPosAMRPos LT patients had DSA which induced significantly higher levels of complement activation when compared to DSAPosAMRNeg patients. Furthermore, DSAPos patients with increased C3d deposition had significantly lower graft survival than DSAPos patients without C3d activation.
Materials and Methods
Ethics
This observational study was approved by the research protocol evaluation committee of the Institutional Review Board of the French Learned Society For Respiratory Medicine -Société de Pneumologie de Langue Française.
Study Population
All patients receiving bilateral LT at Foch Hospital between January 2010-December 2013 and 3 more patients with AMR diagnosis and serum available for analysis (2 transplanted between August 2008-January 2010 and one in March 2014) were included in this monocentric retrospective study. All patients were routinely screened post-operatively for DSA at D1, 7, 21, 30, then M2, 3, 4, 5, 6, 9, 12 then every 6 months thereafter, using the One Lambda® single antigen test. Of 209 patients, 108 tested positive for DSA during routine single antigen screening. We used these 108 patients as our cohort for analyses using the Immucor® (Lifecodes, Norcross, GA) LSA luminex-based assays for single antigen and C3d testing.
AMR Diagnosis
Protocol patient biopsies were mostly retrieved trans-bronchially (TBB, routinely at M1, M3, M4, M6, M9, M12 and for cause), or in some cases acquired through thoracotomy or explantation. Biopsies were scored as previously described (2, 12). If biopsies scored positive for histological patterns suggestive of AMR with circulating DSA, biopsies were further characterized by C4d immunohistochemistry. AMR was diagnosed using the following criteria: (i) clinical dysfunction; (ii) DSA positivity; (iii) presence of C4d in lung biopsies; (iv) histological patterns suggestive of AMR in the absence of other causes (i.e. ischemia-reperfusion, infection, aspiration, drug toxicity). If C4d was detected in biopsies, patients were categorized as AMRPosC4dPos (n=10) despite the presence or absence of histological patterns. If C4d was not detected in biopsies, yet there were histological patterns suggestive of AMR in the biopsy, patients were categorized as AMRPosC4dNeg (n=5). Notably, each AMR patient met the diagnostic criteria for certain or probable AMR with DSA positivity.
HLA Typing, HLA Antibody Testing and Criteria for DSA assignment
Among 108 DSA positive patients with clinical monitoring, the presence of DSA in DTT treated sera was determined using LSA Single Antigen Class I and II platforms according to manufacturer’s protocol (Immucor®). Clinically validated sera were used as controls (serum without HLA antibodies (negative serum, NS); pooled sera containing HLA antibodies with≥80% cPRA (PS). Intermediate resolution HLA typing of recipient and donor HLA-A, B, C, DRB, DQA1, DQB1 was performed using molecular methods (One Lambda, Canoga Park, CA). The background corrected MFI (BCM) was calculated as such: Raw MFI(allele) – Background MFI(allele) = BCM. The background MFI for each single antigen bead was provided by the manufacturer. Patients were categorized DSAPos (n=40) if their sera contained DSA with BCM>500. The HLA class I and/or class II specificity, number of DSA specificities, immunodominant DSA specificity (i.e. the DSA with highest BCM), and MFI of the immunodominant DSA were all recorded for comparison between AMR positive and negative patients. The BCMSUM was determined by adding the BCM of each DSA in a given sample (BCMSUM = BCM(DSA#1) + BCM(DSA#2) +…).
C3d Assay
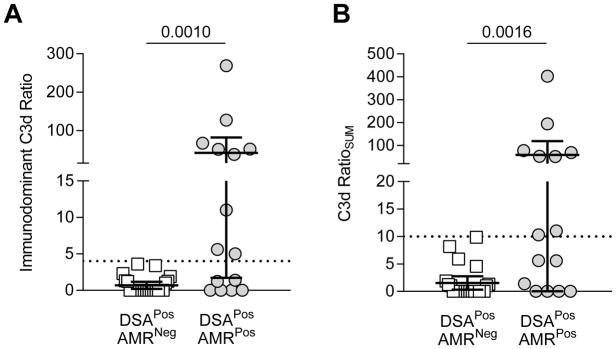
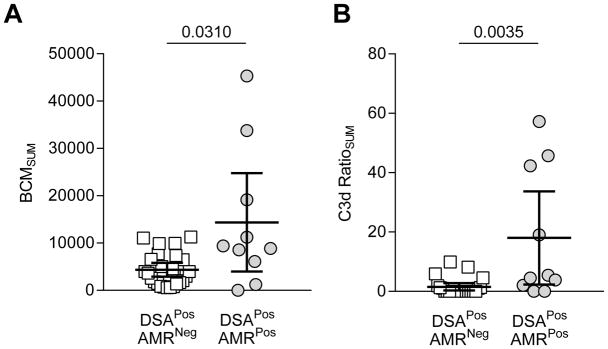
We used the solid phase SAB-based C3d assay (Immucor®) to detect DSA-mediated C3d deposition via Luminex as previously described (13). NS and PS were used as controls for complement activation. Of the 40 DSAPos patients, only 38 had enough sera for subsequent C3d testing. The level of C3d deposition was represented as the C3d ratio for each bead which was calculated as the ratio of MFI with patient serum/MFI with negative control serum (NS) (C3d ratio= C3d MFIpatient/C3d MFINS). The C3d ratioSUM was determined by adding the C3d ratio of each DSA in a given serum sample (C3d ratioSUM = C3d ratio(DSA#1) + C3d ratio(DSA#2) +…). We determined a cutoff of 4 for the immunodominant C3d ratio and 10 for the C3d ratioSUM according to ROC analysis (Supplemental Table 1) for AMR diagnosis. These chosen cutoffs are represented in Figure 4A. No DSAPosAMRNeg patients had an immunodominant C3d ratio>4. Similarly, no DSAPosAMRNeg patients had a C3d ratio>10 (Figure 4B).
Figure 4. Use of Immunodominant C3d MFI and C3d ratioSUM Values to Predict AMR Diagnosis.
(A) The immunodominant C3d value was graphed for each patient; each symbol is the value of a single immunodominant DSA for a given patient. DSAPosAMRPos patients had significantly higher immunodominant C3d ratios than DSAPosAMRNeg patients (mean±SD: 41.9 ±72.5 vs. 0.7±1.1, p= 0.0010). Immunodominant C3d ratio >4 (indicated by a dashed line) was only found in DSAPosAMRPos. (B) The C3d RatioSUM was calculated for each patient in each group; each symbol represents the sum total of C3d activation for the given patient. DSAPosAMRPos patients had significantly higher C3d RatioSUM (mean±SD: 58.7±108.3 vs. 1.5±2.8, p= 0.0016). C3d RatioSUM >10 (indicated by a dashed line) was only found in DSAPosAMRPos. DSAPosAMRNeg patients, white squares, n=23; DSAPosAMRPos patients, gray circles, n=15. Mann-Whitney analysis was performed, p values as depicted in each graph.
Time Points for Analysis
We identified specific time points, based on DSA levels and episodes of rejection, to compare BCM values and C3d ratios between patient groups. For DSAPosAMRPos patients, we used sera samples taken at the time of biopsy-proven rejection. For DSAPosAMRNeg patients, we used the peak post-transplant serum sample with the highest MFI value (according to the routine monitoring) for comparison to DSAPosAMRPos patients. The time of post transplantation sample collection among DSAPosAMRPos and DSAPosAMRNeg patients did not differ significantly (mean ±SD), 146 days ±163.9 vs. 153 days ±245.7 p= 0.52, respectively). Additionally, when available, we tested sera obtained from DSAPosAMRNeg patients prior to the peak DSA and from DSAPosAMRPos patients prior to the rejection episode.
Diagnostic value of C3d ratio
To evaluate the contribution of the C3d ratio to AMR diagnosis, we assessed intrinsic values (sensitivity (Se), specificity (Sp)) and extrinsic values (negative and positive predictive value (NPV and PPV respectively)). If true positive=a, false positive=b, false negative=c, true negative=d, then values were calculated as follow: Se=a/(a+c) and Sp=d/(d+b). PPV and NPV were calculated using Bayes Theorem, values of Se and Sp, and an a priori prevalence of antibody-mediated rejection equal to 10.6%, a value consistent with a non-selected patient cohort based on our previous publication (12).
Statistical Analyses
Baseline demographics and clinical characteristics were compared between DSAPosAMRNeg and DSAPosAMRPos groups. Categorical variables were expressed as a percentage and a number, while quantitative variables were expressed either as mean±SD or as median with 25–75 interquartile range (IQR). Fisher or Chi-square tests were used for categorical variables, whereas Kruskall-Wallis and Mann-Whitney tests were used for comparison of quantitative variables. Kaplan-Meier curves were used to determine graft survival with respect to C3d ratios. Univariate analyses of categorical variables were performed using the log-rank method, with hazard ratios determined as described (14). Correlation testing was performed using the Spearman Test. Confidence intervals for diagnostic values were estimated using STATA statistical software (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP). All other analyses were performed using GraphPad Prism® v6.0 for Mac OS X (GraphPad Software, San Diego, CA). Statistical significance was assigned based on a p≤0.05.
Results
Population description
For these studies, we used our well-defined and historic lung transplant cohort at Foch Hospital (12). Routine DSA monitoring of these transplant recipients identified 108 patients with DSA positive samples that were used to characterize the utility of the C3d assay for AMR risk stratification. These 108 DSA positive historic samples were tested for HLA DSA using the LSA HLA class I and class II test, and we found 40/108 patients were DSAPos (background corrected mean fluorescence intensity (BCM)≥500). Two of these patients had limited sample volumes, and were excluded from further analyses (Figure 1). The final population for C3d testing included 38 DSAPos patients: 15 with AMR (DSAPosAMRPos) and 23 without AMR (DSAPosAMRNeg). Demographics, disease etiology, and induction treatment were not significantly different between the DSAPosAMRNeg and DSAPosAMRPos groups (Table 1). DSAPosAMRPos patients had significantly more donor HLA mismatches and a higher incidence of acute cellular rejection (AR) during the first posttransplant year (Table 1). The specificity (Class I or II) and the number of DSA specificities were not statistically distinct between DSAPosAMRNeg and DSAPosAMRPos groups (Table 2). However, the strength (BCM of immunodominant and BCMSUM) of the DSA from DSAPosAMRPos patients was significantly higher than DSAPosAMRNeg patients (Table 2).
Figure 1.
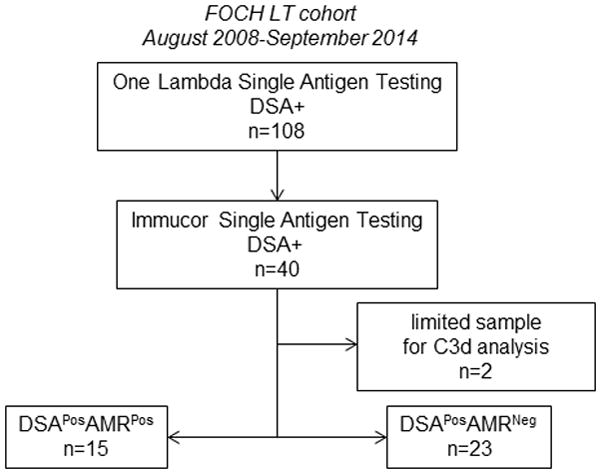
Study population: flow chart
Table 1. Patient Characteristics of DSAPosAMRNeg and DSAPosAMRPos Populations.
Categorical variables were expressed as a percentage and a number, while quantitative variables were expressed either as median with 25–75 interquartile range (IQR) unless specify as § for mean±SD. Fisher or Chi-square tests were used for categorical variables. Mann-Whitney test was used for continuous variables. Abbreviations: AR: acute cellular rejection; BMI: Body Mass Index; CF: Cystic Fibrosis; ECMO: extra corporeal Membrane Oxygenation; EVLP: ex vivo lung preconditioning; GERD: gastro-esophagial reflux; ILD: Interstitial Lung Disease; LAS: Lung Allocation Score; PGD3: Primary Graft Dysfunction grade 3.
| Clinical Variable | DSAPosAMRNeg (n=23) | DSAPosAMRPos (n=15) | p value |
|---|---|---|---|
| Recipient Age (years) | 38.8 (28.9–50) | 33.2 (21.6–56.5) | 0.45 |
| Underlying Disease (Emphysema/ILD/CF/others) | 7/5/9/2 | 3/4/6/2 | 0.88 |
| Gender (F) | 16 | 6 | 0.1 |
| BMI | 19.5 (17.6–22.4) | 20.3 (17.9–30) | 0.38 |
| LAS | 36.40 (32.84–45.40) | 35.70 (33.30–41.10) | 0.95 |
| Donor Age (years) | 49 (40–57) | 48 (37–62) | 0.71 |
| Donor P/F | 372 (307–433) | 380 (323–424) | 0.67 |
| Ischemia Duration (minutes) | 380 (270–442) | 379 (340–474) | 0.42 |
| HELT | 4 (16%) | 2 (13%) | 1 |
| Peri-op ECMO | 9 (40%) | 9 (60%) | 0.32 |
| EVLP | 7 (32%) | 1 (6%) | 0.11 |
| PGD3 at H72 | 8 (32%) | 1 (6%) | 0.06 |
| CMV DR (D−R−/D+R+/D−R+/D+R+) | 4/9/4/6 | 3/5/6/1 | 0.29 |
| HLA Mismatch § | 6.09±1.02 | 6.93±0.96 | 0.03 |
| Induction | 17 (68%) | 10 (66%) | 1 |
| AR at M12 § | 0.78±0.9 | 2±1.5 | 0.01 |
| GERD | 16 (84%) | 6 (75%) | 0.62 |
Table 2. DSA Characteristics of DSAPosAMRNeg and DSAPosAMRPos Populations.
Categorical variables were expressed as a percentage and a number, while quantitative variables were expressed either as median with 25–75 interquartile range (IQR) unless specify as § for mean±SD. Fisher or Chi-square tests were used for categorical variables. Mann-Whitney test was used for continuous variables.
| DSA Characteristics | DSAPosAMRNeg (n=23) | DSAPosAMRPos (n=15) | p value |
|---|---|---|---|
| Class I/II/both | 3/18/2 | 2/10/3 | 0.59 |
| Number of specifcities § | 2.7±1.5 | 3.4±2.1 | 0.36 |
| Immunodominant DQ | 7 (32%) | 10 (67%) | 0.04 |
| Immunodominant BCM | 1894 (965–3643) | 8965 (3336–13589) | <0.01 |
| Sum BCM | 3994 (1824–7713) | 19406 (5624–23978) | <0.01 |
BCM and C3d ratio by DSA bead between DSAPosAMRPos and DSAPosAMRNeg patients
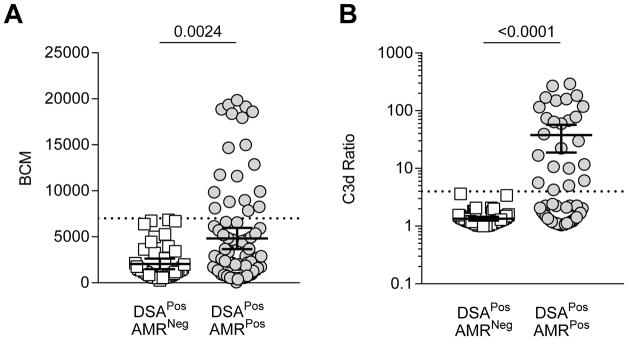
We first determined if there were inherent differences in BCM and C3d ratio values between DSAPosAMRPos and DSAPosAMRNeg patients by comparing these values at the time of rejection or when the DSA levels were maximal (“peak” according to historical DSA testing). Knowing donor specificity allowed us to restrict our analysis to beads containing donor specific antigen for each patient. Analysis of BCM and C3d ratio for each DSA bead between patient populations revealed a significant disparity in the range between patient groups. DSA from DSAPosAMRPos patients showed a significant increase in BCM values and in the capacity to activate C3d compared to patients in the DSAPosAMRNeg group (BCM, mean±SD: 4814±5407 vs. 2060± 1908; C3d ratio, mean±SD: 37.8±68.7 vs. 1.3± 0.4, p<0.0001 respectively) (Figure 2). There was a clear distinction between the two patient groups with respect to BCM and the range of C3d activation: DSAPosAMRNeg patients never had a C3d ratio>4, whereas some DSA from DSAPosAMRPos patients resulted in over 100-fold increase in C3d activation. In summary, DSA present at the time of AMR diagnosis demonstrated increased ability to activate the classical complement pathway.
Figure 2. Comparison of BCM and C3d ratio for each DSA bead for each DSA+ patient between DSAPosAMRPos and DSAPosAMRNeg patients.
(A) Each dot represents BCM value for a single bead. Only DSA beads with BCM>500 are represented. Beads of DSAPosAMRPos patients (n= 85) have significantly higher BCM than DSAPosAMRNeg patients (n=45) (mean±SD respectively 4814±5407 vs. 2060±1908, p=0.0024 Mann-Whitney). Only beads from DSAPosAMRPos patients had a BCM >7000 (dashed line). (B) Each dot represents the C3d ratio value for a single bead. Only DSA beads with a C3d ratio >1 are represented. Beads of DSAPosAMRPos patients (n=81) have significantly higher C3d ratios than beads of DSAPosAMRNeg patients (n=49) (mean±SD respectively 21.84±49.88 vs. 1.5±0.48, p<0.001 Mann-Whitney). Only beads from DSAPosAMRPos patients had a C3d ratio >4 (dashed line). Data graphed as mean ± CI 95.
C3d ratio correlation with BCM between DSAPosAMRPos and DSAPosAMRNeg patients
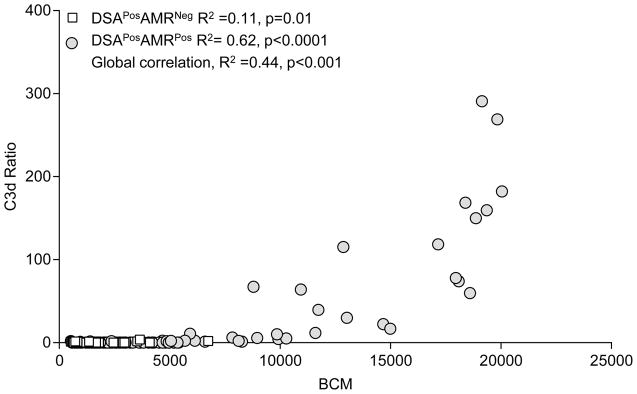
To explore if increased capacity to activate complement was also associated with an increased quantity of DSA, we looked to see if there was a correlation between BCM values and C3d ratios. In the total population of DSAPosAMRPos and DSAPosAMRNeg, we found a moderate correlation between BCM and C3d ratio with (R2=0.44, p<0.0001). Considering correlation only in DSAPosAMRPos patients, we found a much stronger positive correlation (R2=0.63, p<0.0001) between the C3d ratio and BCM. Of note only three beads with BCM>7000 had a C3d ratio<4. There was no correlation between BCM or C3d ratio for DSAPosAMRNeg patients (R2=0.11, p=0.01) (Figure 3).
Figure 3. Correlation Between BCM & C3d Ratio in Lung Transplant Recipients with Respect to AMR Diagnosis.
The BCM and C3d ratio were plotted for each DSA bead with white squares for DSAPosAMRNeg patients and gray circles for DSAPosAMRPos patients. Correlation between BCM and C3d ratio was calculated for each group of patients. R2 and p values are reported.
Immunodominant C3d ratio and C3d ratioSUM for AMR diagnosis
The C3d ratio of the immunodominant DSA was found to be significantly higher in the patients who experienced rejection over those who did not (Figure 4A). Another measure currently being assessed is the sum of the BCM values for each DSA in an individual patient sample, thereby accounting for the total quantity of antibody capable of inducing graft injury. Thus, we calculated the C3d ratioSUM by adding the C3d ratios for each DSA bead in a patient sample. The C3d ratioSUM was significantly higher in DSAPosAMRPos versus DSAPosAMRNeg patients (Figure 4B).
Diagnostic value of C3d for AMR diagnosis
ROC analyses showed a cutpoint of >4 for the Immunodominant C3d ratio provides a sensitivity of 60% (CI=32.3%–83.6%) and specificity of 100% (CI= 85.2%–100%). An identical sensitivity and specificity was achieved using a cutpoint of 7000 MFI for the BCMsum.
Using these thresholds based on ROC analysis (Supplemental Table 1) and our previously published 10.6% prevalence of AMR (12) the calculated NPV was 95.5% (CI 91.8–97.4), 95.5% (CI 91.8–97.4) and 94.8% (CI 91.2–96.8) for immunodominant BCM, immunodominant C3d ratio, and C3d ratioSUM (Table 3). The expected probability of no AMR without the C3d Ratio and BCMsum tests is 89.4% [ie. one minus the prevalence or (1–0.106) × 100%]. With these tests included, the probability of no AMR increased to 95.5%. Although the PPV is strong, its uncertainty is great because the number of AMR cases in our cohort is low and there is an overall low prevalence of AMR in the lung transplant population.
Table 3.
Immunodominant C3d Ratio and C3d RatioSUM diagnostic values.
| BCMSUM | Immunodominant C3d Ratio | C3d RatioSUM | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Threshold | <7000 | >7000 | <4 | >4 | <10 | >10 |
|
|
||||||
| DSAPosAMRNeg (n) | 23 | 0 | 23 | 0 | 23 | 0 |
|
| ||||||
| DSAPosAMRPos (n) | 6 | 9 | 6 | 9 | 7 | 8 |
|
| ||||||
| Sensitivity† | 60 (32.3–83.6) | 60 (32.3–83.6) | 53 (26.6–78.7) | |||
|
| ||||||
| Specificity† | 100 (85.2–100) | 100 (85.2–100) | 100 (85.2–100) | |||
|
| ||||||
| Positive Predictive Value* | 100 (17.4–100) | 100 (17.4–100) | 100 (15.8–100) | |||
|
| ||||||
| Negative Predictive Value* | 95.5 (91.8–97.4) | 95.5 (91.8–97.4) | 94.8 (91.2–96.8) | |||
value based on a (95% Confidence interval)
value based on an AMR prevalence of 10.6% (12) with (95% Confidence interval)
C3d ratio and graft survival
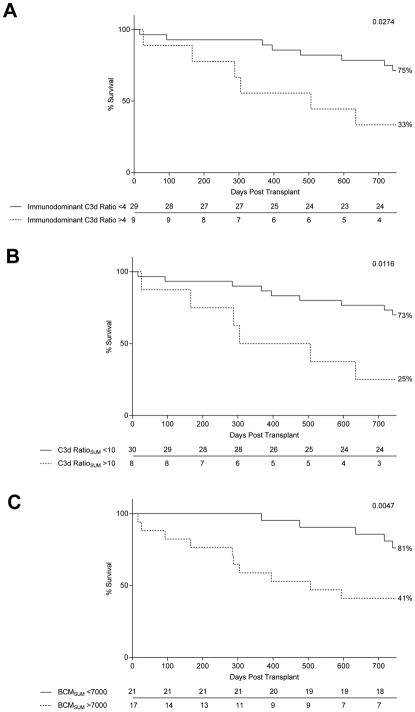
Next, we assessed graft survival with respect to C3d ratio. Two-year graft survival in patients with an immunodominant DSA with a C3d ratio>4 was 35%, compared to 75% in patients with an immunodominant DSA with a C3d ratio<4 (Figure 5A). Patients with C3d ratioSUM>10 had severely impaired two-year graft survival (25% vs. 73% for patients with C3d ratioSUM<10) (Figure 5B). Lastly, using BCM values as a stratification tool, we found that patients with BCM>7000 had 41% two-year graft survival, while patients with BCM<7000 had 81% graft survival two years post LT (Figure 5C).
Figure 5. C3d Ratio is Associated with Poor Graft Survival.
Kaplan-Meier curves were used to determine graft survival with respect to an immunodominant C3d ratio threshold of 4 (A), a C3d ratioSUM threshold of 10 (B), a BCMSUM threshold of 7000 (C).
C3d assay prior to AMR diagnosis
To see if the C3d assay identified pathogenic DSA prior to clinical dysfunction, we analyzed whether C3d deposition occurred in the presence of DSA from samples taken at the time point preceding AMR diagnosis. To do so, we tested the serum sample obtained prior to the rejection sample for each DSAPosAMRPos patient. Ten of 15 patients had available pre-AMR samples that ranged from 13–581 days before rejection, and we compared the C3d ratioSUM to the 23 DSAPosAMRNeg patients at peak. Interestingly, the comparison of BCMSUM between pre-AMR DSAPosAMRPos samples and DSAPosAMRNeg patients revealed a small, yet significant difference (Figure 6A), whereas the difference in the capacity to activate complement was strikingly different (Figure 6B).
Figure 6. Detection of Complement Activating DSA Prior to Episodes of AMR.
Sera from DSAPosAMRNeg patients at the peak DSA and from DSAPosAMRPos patients prior to the rejection episode (ranging 13–581 days before rejection) were assessed for BCMSUM (A) (n=23 and n=10 respectively) and C3d ratioSUM (B) (n=23 and n=10 respectively). Mann-Whitney analysis was performed, p values as indicated.
C3d serum analysis vs. C4d biopsy staining
Positive C4d staining in the allograft biopsy is a hallmark of complement activation in the graft. We decided to compare complement activation across two different biological compartments: serum (C3d) and biopsy (C4d) (Table 4). Only 5 of the 10 AMRPosC4dPos patients tested had immunodominant DSA with a C3d ratio>4. Of note, all 5 AMRPosC4dNeg patients had DSA capable of activating complement. Taking into account both C4d and C3d tests, every AMRPos patient had detectable complement activation.
Table 4. C4d and C3d as Indicators of Complement Activation.
C3d ratios were retrospectively assessed in conjunction with C4d status in AMR positive patients. The number of patients within each category (AMRPosC4dPos, n=10; AMRPosC4dNeg, n=5) were binned in either the upper or lower threshold for each ratio.
| Immunodominant C3d | C3d RatioSUM | |||
|---|---|---|---|---|
| Threshold | <4 | >4 | <10 | >10 |
| AMRPosC4dPos (n=10) | 5 | 5 | 6 | 4 |
| AMRPosC4dNeg (n=5) | 0 | 5 | 1 | 4 |
Discussion
There is an urgent need to develop tools that will allow for risk stratification of DSAPos LT patients most likely to experience AMR episodes. Repeated episodes of rejection lead to clinical dysfunction, short-term allograft survival, and cause mortality of LT patients (4, 12, 15–17). The presence of DSA is a known indicator of poor graft prognosis (18) (19) (20), yet other studies have delineated that not all DSA have similar pathogenicity (1). As DSA-mediated complement activation has been shown to increase the frequency of graft loss, we hypothesized that measuring complement activation by DSA in vitro would be associated with AMR. In this study, we leveraged a well-defined LT cohort with prospective AMR diagnosis to analyze how complement activating DSA using a new platform, the C3d assay, contribute to AMR diagnosis and predict LT at risk of a subsequent occurrence of AMR.
Three other studies reported the use of the C3d assay to assess DSA-mediated complement activation. Sicard et al. demonstrated DSA from renal transplant patients undergoing AMR not only activated complement, but that the level of C3d activation was an independent predictor of AMR-related graft loss (21). We reported DSA from cardiac transplant recipients at the time of biopsy-proven AMR activated complement in the C3d assay, and this was inhibited using a novel complement inhibitor (13). Moreover, Comoli et al. found that C3d+ de novo DSA were significant indicators of poor graft outcome ten years post kidney transplant (22). The data presented in this study align with these previous reports, as DSA from LT patients experiencing AMR were significantly more prone to activate complement in the C3d assay, and that C3d+ DSA were indicative of extremely poor graft outcome. Collectively, these reports of complement activating DSA across solid organ transplant reiterate the importance of understanding the physiological contributions of complement during AMR.
Consistent with previous reports, a higher C3d ratio correlates with high BCM in DSAPosAMRPos patients (23, 24). Since the C3d ratio does not discriminate graft outcome better than BCMsum values the added clinical value of this assay is questionable. However, the C3d assay does in fact supply a mechanism by which increased quantities of DSA can promote graft damage. Specifically, the greater the amount of DSA, the more likely complement activation is to occur, which may result in more complement-mediated pathology in the lungs and subsequent rejection. Indeed, a C3d ratio>4 mainly occurs in the range of BCM values rarely reached by those patients who do not experience rejection (DSAPosAMRNeg). Despite a strong correlation of high BCM and C3d ratio, not every DSA with BCM>7000 is capable of inducing complement as measured by C3d. This phenomenon could be explained by other DSA intrinsic factors, such as affinity, subclass, and Fc glycosylation. Altered Fc glycosylation profiles are known to modulate complement activation, and different IgG subclasses have varying rates of complement activation (1). Whatever the underlying mechanism, the discrepancy between BCM and C3d highlight that even in our small population, single antigen and C3d assays are not exactly equivalent and the C3d assay may be beneficial for identifying unique DSA with high levels in circulation, but varying pathogenicity.
Our results suggest interesting specificity and PPV of the C3d assay for AMR diagnosis. Due to the small size of our population those predictive values (that depend on the frequency of event) might be cautiously interpreted. The other diagnostic values such as NPV and sensitivity are moderately convincing. However, the additional value of the C3d assay compared to current single antigen testing (BCM/MFI) remains unclear. While perhaps not a better diagnostic indicator than BCM, the C3d assay may be useful in stratifying patients for treatment strategies. As current complement inhibitor therapies are quite expensive, it would be useful to know which patients would benefit most from these treatments. For example, use of Eculizumab may not be required for patients with no proof of complement activation(25). Conversely, those patients with DSA that potently induce C3d may greatly benefit from anti-complement treatment. We found that C3d deposition was increased in DSAPos samples drawn prior to the time of diagnosed rejection. Having knowledge of the pathogenic potential of DSA prior to an episode of rejection would allow for early therapeutic intervention with treatments that may dampen the effects of complement activation.
We found that patients with DSA which significantly activated complement had extremely poor graft survival rates (<35% two-year graft survival) compared to DSAPos patients with minimal complement activation (>70% two-year graft survival). Others have also demonstrated that the complement-binding potential of DSA, via C1q interactions, is a clear indicator of patients more likely to experience AMR (26). A patient sample containing DSA with elevated levels of complement activation, assessed by C3d assay, would indicate the increased likelihood of subsequent AMR. Using DSA strength to discriminate two-year graft survival led to similar trends (~80% (weak BCM) vs ~40% (strong BCM)) as when complement deposition was used to assess survival. Use of the immunodominant C3d ratio versus the C3d ratioSUM to examine two-year graft survival highlighted that both values similarly identified those patients which would succumb to graft loss.
There are multiple potential explanations for the discrepancy between the serum-based C3d test and C4d deposition in the graft, beyond those of the basic sensitivity/specificity issues intrinsic to each assay. On one hand, complement activation by circulating DSA partly depends on the amount of circulating DSA. The “sponge effect” is probably greater in the lung than in other organs, as the capillary surface is 100-fold higher in a lung than in a kidney. This could account for several AMR patients who have circulating DSA with low to intermediate BCM values. The strength measured during single antigen testing, or “circulating strength,” does not preclude intragraft DSA concentration (27). On the other hand, intragraft complement activation depends on the number of DSA specificities, respective expression of each HLA molecule on the endothelial surface, and the level of efficacy of the intrinsic complement inhibitory system at the surface of the targeted cells (i.e. CD59, CD55, CD46). Taken together, clinicians may consider the information gained from these two assays as complementary to understanding the ongoing AMR process. Indeed, both assays indicate complement activation, and should be helpful to indicate anti-complement therapy(25).
There are several limitations in this study including nonconsecutive and small numbers of patients, and the retrospective nature of the analysis. But these retrospective analyses also allowed for the assessment of samples from multiple time points from DSAPosAMRNeg patients including the time point with the highest BCM. The diagnostic value of the C3d assay will have to be reassessed in a larger prospective multicenter cohort including consecutive patients.
Testing our samples with the two different platforms highlighted the discrepancy between the two kits. The decrease from 108 DSA positive patients to 40 might be explained by the known differences between the two platforms due to HLA antigen quality and density, specificity and sensitivity of the beads (28). Small differences can also contribute to variations in MFI between two laboratories (29).
In summary, DSA MFI or BCM values are used in conjunction with clinical dysfunction and graft biopsy pathology to help guide treatment during episodes of rejection in LT. Our results suggest that both high BCM and C3d ratios might be helpful for AMR diagnosis and graft loss prediction. The C3d assay may be valuable in identifying patients most likely to benefit from anti-complement therapeutic intervention. Moreover, the C3d assay was useful in identifying DSA activating complement in some serum samples drawn prior to AMR diagnosis, and may be worth exploring in larger cohorts. Continual monitoring of DSA using the C3d assay may be able to identify patients who will have AMR, and allow for early therapeutic intervention to minimize DSA-mediated graft damage.
Supplementary Material
ROC analysis for AMR diagnosis for immunodominant C3d ratio (A), C3d ratioSUM (B) and BCM (C). Highlighted line shows the chosen cutoff.
Acknowledgments
Funding Sources
This work was financially supported by both contributions from the “Association Gregory Lemarchal”, the “Fondation du souffle” and “Fondation Foch,” and funding from the NIH (R01 AI081249 and U01 AI124319 to EFR).
The authors would like to thank the following members of the UCLA Immunogenetics Center: George Banzuela, Gertrudes Aguas, Gemalene Sunga, Fadi Kandarian. Additionally, this work was financially supported by both contributions from the “Association Gregory Lemarchal”, the “Fondation du souffle” and “Fondation Foch,” and funding from the NIH (R01 AI081249 and U01 AI124319 to EFR).
Abbreviations
- AR
acute cellular rejection
- AMR
antibody mediated rejection
- BCM
background corrected MFI
- BMI
body mass Index
- BOS
bronchiolitis obliterans syndrome
- CF
cystic fibrosis
- DSA
donor specific antibody
- ECMO
extra corporeal membrane oxygenation
- EVLP
ex vivo lung preconditioning
- GERD
gastro esophageal reflux
- HELT
high emergency lung transplantation
- HLA
human leukocyte antigen
- HLA-Ab
HLA antibodies
- ILD
interstitial lung disease
- IVIG
intravenous immunoglobulin
- LT
lung transplantation
- MFI
mean fluorescence intensity
- NPV
negative predictive value
- PGD3
primary graft dysfunction grade 3
- PPV
positive predictive value
- RAS
restrictive allograft syndrome
- Se
sensitivity
- Sp
specificity
- SAB
single antigen beads
Footnotes
Author’s contribution
Designed study: AR, KAT, EFR
Performed study: AR, KAT, NH
Collected data: AR, KAT, CSB, ES, LBA, AMH, ML, FP
Analyzed data: AR, KAT, EFR, FP
Wrote the paper: AR, KAT, EFR
Disclosures
Dr. Antoine Roux has conflicts of interest to disclose as described by Transplant International: he served as a consultant for Novartis France (concerning CMV in solid organ transplantation). The other authors have no conflicts of interest to disclose as described by the Transplant International journal. C3d reagents were provided by Immucor® (Lifecodes, Norcross, GA, USA) for this study.
References
- 1.Thomas KA, Valenzuela NM, Reed EF. The perfect storm: HLA antibodies, complement, FcγRs, and endothelium in transplant rejection. Trends Mol Med. 2015 doi: 10.1016/j.molmed.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berry GJ, Burke MM, Andersen C, Bruneval P, Fedrigo M, Fishbein MC, et al. The 2013 International Society for Heart and Lung Transplantation Working Formulation for the standardization of nomenclature in the pathologic diagnosis of antibody-mediated rejection in heart transplantation. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2013;32(12):1147–62. doi: 10.1016/j.healun.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Haas M. An updated Banff schema for diagnosis of antibody-mediated rejection in renal allografts. Curr Opin Organ Transplant. 2014;19(3):315–22. doi: 10.1097/MOT.0000000000000072. [DOI] [PubMed] [Google Scholar]
- 4.DeNicola MM, Weigt SS, Belperio JA, Reed EF, Ross DJ, Wallace WD. Pathologic findings in lung allografts with anti-HLA antibodies. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2013;32(3):326–32. doi: 10.1016/j.healun.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yousem SA, Zeevi A. The histopathology of lung allograft dysfunction associated with the development of donor-specific HLA alloantibodies. Am J Surg Pathol. 2012;36(7):987–92. doi: 10.1097/PAS.0b013e31825197ae. [DOI] [PubMed] [Google Scholar]
- 6.Levine DJ, Glanville AR, Aboyoun C, Belperio J, Benden C, Berry GJ, et al. Antibody-mediated rejection of the lung: A consensus report of the International Society for Heart and Lung Transplantation. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2016;35(4):397–406. doi: 10.1016/j.healun.2016.01.1223. [DOI] [PubMed] [Google Scholar]
- 7.Sellarés J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12(2):388–99. doi: 10.1111/j.1600-6143.2011.03840.x. [DOI] [PubMed] [Google Scholar]
- 8.Hachem R. Antibody-Mediated Lung Transplant Rejection. Current respiratory care reports. 2012;1(3):157–61. doi: 10.1007/s13665-012-0019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen G, Sequeira F, Tyan DB. Novel C1q assay reveals a clinically relevant subset of human leukocyte antigen antibodies independent of immunoglobulin G strength on single antigen beads. Hum Immunol. 2011;72(10):849–58. doi: 10.1016/j.humimm.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Tyan DB. New approaches for detecting complement-fixing antibodies. Curr Opin Organ Transplant. 2012;17(4):409–15. doi: 10.1097/MOT.0b013e328355fb9b. [DOI] [PubMed] [Google Scholar]
- 11.Loupy A, Lefaucheur C, Vernerey D, Prugger C, Duong van Huyen JP, Mooney N, et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med. 2013;369(13):1215–26. doi: 10.1056/NEJMoa1302506. [DOI] [PubMed] [Google Scholar]
- 12.Roux A, Bendib Le Lan I, Holifanjaniaina S, Thomas KA, Hamid AM, Picard C, et al. Antibody-Mediated Rejection in Lung Transplantation: Clinical Outcomes and Donor-Specific Antibody Characteristics. Am J Transplant. 2016;16(4):1216–28. doi: 10.1111/ajt.13589. [DOI] [PubMed] [Google Scholar]
- 13.Thomas KA, Valenzuela NM, Gjertson D, Mulder A, Fishbein MC, Parry GC, et al. An Anti-C1s Monoclonal, TNT003, Inhibits Complement Activation Induced by Antibodies Against HLA. Am J Transplant. 2015 doi: 10.1111/ajt.13273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Machin D, Cheung YB, Parmar MK. Survival Analysis: A Practical Approach. 2. John Wiley & Sons, Ltd; 2006. [Google Scholar]
- 15.Bohmig GA, Wahrmann M, Regele H, Exner M, Robl B, Derfler K, et al. Immunoadsorption in severe C4d-positive acute kidney allograft rejection: a randomized controlled trial. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2007;7(1):117–21. doi: 10.1111/j.1600-6143.2006.01613.x. [DOI] [PubMed] [Google Scholar]
- 16.Lobo LJ, Aris RM, Schmitz J, Neuringer IP. Donor-specific antibodies are associated with antibody-mediated rejection, acute cellular rejection, bronchiolitis obliterans syndrome, and cystic fibrosis after lung transplantation. J Heart Lung Transplant. 2013;32(1):70–7. doi: 10.1016/j.healun.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Witt CA, Gaut JP, Yusen RD, Byers DE, Iuppa JA, Bennett Bain K, et al. Acute antibody-mediated rejection after lung transplantation. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2013;32(10):1034–40. doi: 10.1016/j.healun.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tikkanen JM, Singer LG, Kim SJ, Li Y, Binnie M, Chaparro C, et al. De Novo DQ Donor-Specific Antibodies Are Associated with Chronic Lung Allograft Dysfunction after Lung Transplantation. Am J Respir Crit Care Med. 2016;194(5):596–606. doi: 10.1164/rccm.201509-1857OC. [DOI] [PubMed] [Google Scholar]
- 19.Roux A, Bendib Le Lan I, Holifanjaniaina S, Thomas KA, Picard C, Grenet D, et al. Characteristics of Donor-Specific Antibodies Associated With Antibody-Mediated Rejection in Lung Transplantation. Front Med (Lausanne) 2017;4:155. doi: 10.3389/fmed.2017.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verleden SE, Vanaudenaerde BM, Emonds MP, Van Raemdonck DE, Neyrinck AP, Verleden GM, et al. Donor-specific and -nonspecific HLA antibodies and outcome post lung transplantation. The European respiratory journal. 2017;50(5) doi: 10.1183/13993003.01248-2017. [DOI] [PubMed] [Google Scholar]
- 21.Sicard A, Ducreux S, Rabeyrin M, Couzi L, McGregor B, Badet L, et al. Detection of C3d-binding donor-specific anti-HLA antibodies at diagnosis of humoral rejection predicts renal graft loss. J Am Soc Nephrol. 2015;26(2):457–67. doi: 10.1681/ASN.2013101144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Comoli P, Cioni M, Tagliamacco A, Quartuccio G, Innocente A, Fontana I, et al. Acquisition of C3d-Binding Activity by De Novo Donor-Specific HLA Antibodies Correlates With Graft Loss in Nonsensitized Pediatric Kidney Recipients. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2016;16(7):2106–16. doi: 10.1111/ajt.13700. [DOI] [PubMed] [Google Scholar]
- 23.Wiebe C, Gareau AJ, Pochinco D, Gibson IW, Ho J, Birk PE, et al. Evaluation of C1q Status and Titer of De Novo Donor-Specific Antibodies as Predictors of Allograft Survival. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2017;17(3):703–11. doi: 10.1111/ajt.14015. [DOI] [PubMed] [Google Scholar]
- 24.Yell M, Muth BL, Kaufman DB, Djamali A, Ellis TM. C1q Binding Activity of De Novo Donor-specific HLA Antibodies in Renal Transplant Recipients With and Without Antibody-mediated Rejection. Transplantation. 2015;99(6):1151–5. doi: 10.1097/TP.0000000000000699. [DOI] [PubMed] [Google Scholar]
- 25.Lefaucheur C, Viglietti D, Hidalgo LG, Ratner LE, Bagnasco SM, Batal I, et al. Complement-Activating Anti-HLA Antibodies in Kidney Transplantation: Allograft Gene Expression Profiling and Response to Treatment. Journal of the American Society of Nephrology: JASN. 2017 doi: 10.1681/ASN.2017050589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lefaucheur C, Viglietti D, Bentlejewski C, Duong van Huyen JP, Vernerey D, Aubert O, et al. IgG Donor-Specific Anti-Human HLA Antibody Subclasses and Kidney Allograft Antibody-Mediated Injury. Journal of the American Society of Nephrology: JASN. 2016;27(1):293–304. doi: 10.1681/ASN.2014111120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Visentin J, Chartier A, Massara L, Linares G, Guidicelli G, Blanchard E, et al. Lung intragraft donor-specific antibodies as a risk factor for graft loss. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2016;35(12):1418–26. doi: 10.1016/j.healun.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Minucci PB, Resse M, Sabia C, Esposito A, De Iorio G, Napoli C. Anti-HLA Antibodies Testing on Solid Phase: Comparative Evaluation of Different Kit Vendors Through Luminex Technology. Exp Clin Transplant. 2017;15(6):636–40. doi: 10.6002/ect.2016.0199. [DOI] [PubMed] [Google Scholar]
- 29.Reed EF, Rao P, Zhang Z, Gebel H, Bray RA, Guleria I, et al. Comprehensive assessment and standardization of solid phase multiplex-bead arrays for the detection of antibodies to HLA. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13(7):1859–70. doi: 10.1111/ajt.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ROC analysis for AMR diagnosis for immunodominant C3d ratio (A), C3d ratioSUM (B) and BCM (C). Highlighted line shows the chosen cutoff.