Abstract
Neonatal seizures associated with hypoxic-ischemic encephalopathy (HIE) pose a challenge in their acute clinical management and are often followed by long-term neurological consequences. We used a newly characterized CD-1 mouse model of neonatal ischemic seizures associated with age-dependent (P7 vs. P10) seizure severity and phenobarbital efficacy (i.e.; PB-resistant vs. PB-efficacious respectively) following unilateral carotid ligation. The long-term consequences following untreated neonatal seizures in P7 vs. P10 ligated pups were investigated using neurobehavioral testing, 24h v- quantitative EEG-EMG (qEEG, qEMG), and western blot analyses in adult mice. Significant hyperactivity emerged in a small sub-set of mice in both age-groups associated with a failure to habituate during open-field (OF) testing. 24h continuous qEEGs detected significantly altered sleep architecture due to long-wake cycles in both age-groups. Delta power (0.5-4 Hz) quantification during slow-wave-sleep (SWS) revealed significant SWS compensation in P10 ligates following periods of increased sleep pressure which the P7 ligate group failed to show. Theta/beta ratios deemed as negative correlation markers of attentional control were significantly higher only in the P10 ligates. These results indicate that neonatal age-dependent differences in the characteristics of ischemic neonatal seizures in CD-1 pups differentially modulate long-term outcomes, when evaluated with v-qEEG/EMG as adults.
Keywords: hypoxic-ischemic encephalopathy, ischemia, neonatal seizures, hyperactivity, sleep structure, delta power
Introduction
Neonatal seizures affect 1.5-3/1000 newborns and result in long-term neurological consequences (Volpe, 2001). Hypoxic-ischemic encephalopathy (HIE) is the predominant underlying etiology of neonatal seizures, with adverse long-term outcomes such as epilepsy, cerebral palsy, developmental delay, and intellectual disabilities (Pisani and Spagnoli, 2016; Tekgul et al., 2006; Vasudevan and Levene, 2013).
The roles of etiology and acute seizure severity on long-term outcomes of neonatal seizures have been debated clinically (Glass et al., 2011; Kwon et al., 2011). At 5 years of age, patients with mild HIE, graded by early EEG and clinical assessment, have significantly lower full-scale IQ, verbal IQ, and performance IQ than their peers (Murray et al., 2016). Neonates with a total electrographic seizure burden of more than 40 minutes had a nine-fold increase in odds of neurological consequences (Kharoshankaya et al., 2016). More severe electrographic seizure burdens in HIE are significantly associated with abnormal outcomes. Few animal model studies have been done to determine the factors responsible for the long-term co-morbidities of neonatal seizures (Kang and Kadam, 2015). Even fewer use EEG to determine seizure burdens at both the acute and chronic stages.
Using electro-clinical, molecular, and behavioral assays in C57BL/6J P7 pups, (Rodriguez-Alvarez et al., 2015) showed increased long-term seizure susceptibility but minimal injury and no spatial memory deficits. Similarly, a model of perinatal hypoxia/ischemia in Sprague Dawley rat pups has shown chronically progressive epilepsy associated exclusively with injury (Kadam and Dudek, 2007). We have previously characterized a mouse model of neonatal ischemic seizures in CD-1 pups, reporting a significant age-dependent difference in acute seizure severity and phenobarbital refractoriness to the same ischemic insult (Kang et al., 2015). In contrast to chemoconvulsant models including kainic acid, this new model of neonatal ischemic seizures shows electrographic seizure burdens and brain injury profiles similar to those reported in HIE, highlighting clinical relevance (Kharoshankaya et al., 2016; Kang and Kadam, 2014). In the current study, the long-term co-morbidities of hypoxic/ischemic insult were investigated using standard neurobehavioral tests and chronic 24h v-qEEG/EMGs to investigate: 1) the long-term developmental consequences of untreated neonatal ischemic seizures at two distinct developmental ages (i.e.; P7 vs. P10); 2) the use of acute seizure parameters to predict acute and long-term outcomes; and 3) qEEG biomarkers identifying long-term neurophysiological outcomes by neonatal age of ischemic insult.
Materials and Methods
1. Animals and acute surgical procedures
All animal care and procedures were carried out in accordance with the recommendations in the Guide for Care and Use of Laboratory Animals of the National Institutes of Health and the protocols used in this study were approved by the Committee on the Ethics of Animal Experiments of the Johns Hopkins University. All surgical procedures were performed under isoflurane anesthesia. All litters of CD-1 mice were purchased from Charles River Laboratories Inc. (Wilmington, MA, USA) with newly born litters of pups (n=10; equal number for both sexes) arriving on postnatal day (PND) 3 day. Pups were allowed to acclimate in polycarbonate cages on a 12h light-dark cycle. Food was provided ad libitum (see Table 1 for sample size and sex distribution and Fig. 1A for timelines).
Table 1. Sample size by age and sex.
| Control (♂/♀) | Ligated (♂/♀) | Total (♂/♀) | |
|---|---|---|---|
| P7 | 9 (4/5) | 10 (5/5) | 19 (9/10) |
| P10 | 10 (5/5) | 10 (5/5) | 20 (10/10) |
| Total | 19 (9/10) | 20 (10/10) | 39 (19/20) |
Figure 1.
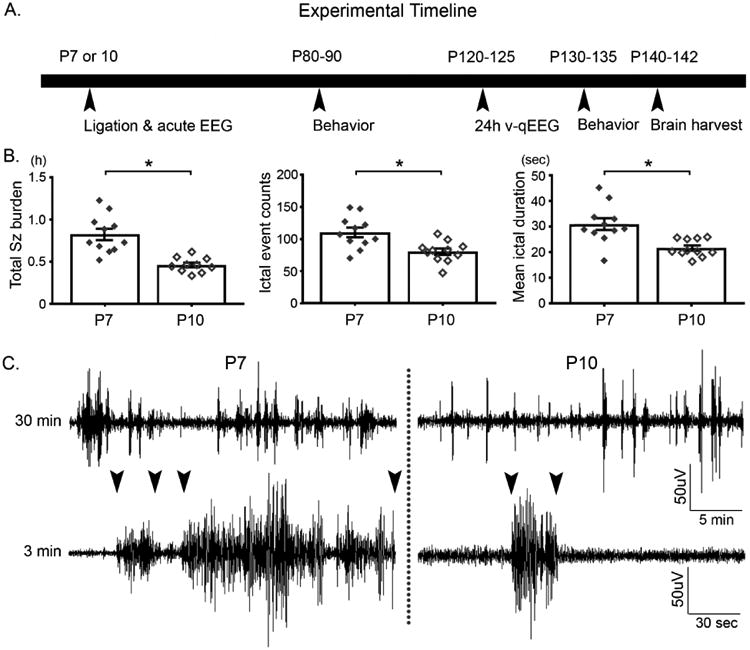
A. Experimental paradigm B. Seizure severity was higher in P7 ligates compared to P10, driven by significantly higher number of ictal events and mean ictal duration (n=11 for each age; students t-test, p<0.05). The error bars shown denote ± 1 S.E.M. C. Representative raw EEG traces for P7 and P10 ligate respectively. Arrowheads indicate representative seizure duration for each group.
1.1 Surgical procedure for ischemic insult and in-vivo synchronous video-EEG recording
Briefly, pups were anesthetized with 4% isoflurane for induction and maintained at 1.5% as previously described (Kang et al., 2015). Neonatal ischemia was induced by permanent double-ligation of the right common carotid artery. Sham-controls were treated identically except for the carotid ligation. Three sub-dermal EEG silver electrodes (1 recording, 1 reference, 1 ground) were implanted on the pups' skull overlying the parietal cortex using bregma as a reference. A third ground electrode was implanted over the rostrum. The electrodes (IVES EEG; Model # SWE-L25 – IVES EEG solutions, MA, USA) were fixed with minimal cyanoacrylate adhesive (KrazyGlue). Pups were allowed to recover from the anesthesia in a 36°C isothermal chamber followed by continuous 3h recordings of video-EEG. EEG and video data were acquired using Sirenia Acquisition software (Pinnacle Technology Inc.). Raw EEG traces with synchronous EMG and video were used to manually score behavioral state transitions within Sirenia (i.e.; sleep score module), blind to the treatment group identity. A seizure was defined as epileptiform discharges lasting ≥6s. At the end of the recording session and following removal of the electrodes and application of topical anesthetic, the pups were returned to the dams. Pups were monitored daily for the next week to ensure post-surgery recovery.
1.2 Experimental design
Equal number of pups of both sexes from each litter (n=10 each) at both P7 and P10 were randomly assigned to one of the two experimental groups: 1) sham: naïve; 2) ligation control. During the neonatal ligation, pups underwent permanent toe-clipping under anesthesia for all future identification for each litter. Experimenters used toe-clip identity only and remained blind to treatment category for both long-term behavioral studies and EEG recordings as adults. Experimenters scoring and analyzing qEEG were similarly blinded.
1.3 Animal housing
At PND 21-23, mice were weaned from the dam and housed 4-5 mice per cage, separated by sex. Housing was in polycarbonate cages on a 12h light-dark cycle and food was provided ad libitum as before. After head mount implantation surgery at P120, animals were single-housed along with piece of bedding from its home cage and a water-gel pack to prevent dehydration during recovery. Mice were monitored daily for a week to ensure satisfactory post-surgery recovery.
2. Behavior tests
All procedures were conducted with minimal handling under strict supervision of the Behavior Core at the Brain Science Institute, Johns Hopkins University. Adult mice underwent testing at ages PND 80±4 or 130±4. Mice were habituated daily for 5 min prior to the initiation of behavior tests. Habituation period and the behavioral tests covered a period of two weeks, with at least 48h breaks in between tests to minimize stress. All experimental groups went through the same tests, which were conducted during daylight hours (8AM to 6PM), in the same sequence: for P80 open-field, Y-maze, EPM, social interaction, and PPI; for P130, open-field, social interaction, and PPI. Each session was run in a sex-matched manner, and no cross-sex interaction or presence in same space occurred. Minimal standardization for the mouse cohorts other than age were employed (i.e.; body weight etc.) for the behavior tests conducted in this study, since heterogenization within experiments has been purported to improve reproducibility among experiments (Richter et al., 2010).
2.1 Open-Field (OF) Test
All testing procedures were carried out in a square PAS- Open field station (16″W × 16″D × 15H; San Diego Instruments, CA) equipped with photo-beam configuration). Data was acquired through PAS software based on the grid of invisible infrared light beams (16 × 16) set on the four sides of the walls of the station. Each station was air/humidity controlled and had ambient light. Mice were placed at the center of the station and remained in the enclosure for 25 minutes while the mice were allowed to explore the field freely. The station was cleaned with 70% ethanol after completion of each session. Total activity levels for the entire duration (25 min) and the 5 min habituation interval were quantitated. Each mouse underwent OF test session twice: once at PND 80 and again at PND 130.
2.12 Identification of hyperactive mice by OF
Identification of hyperactivity was based on beam breaks in OF test, and thus defined as the animals which met Rosner's Extreme Studentized Deviate test for multiple outliers (two-sided test, significance level set at 0.00001, maximum number of outliers allowed set at 3 out of total 39); increasing the maximum number of outliers allowed to 10 did not identify any other animals as an outlier. Further statistical justification was provided by Shapiro-Wilks test, a goodness-of-the-fit test allowing the comparison of sample distributions pre- and post-outlier removal. Inclusion of the three hyperactive animals resulted in a p<0.001 by Shapiro-Wilks test, indicating the non-normal distribution of those 3 mice. In contrast, the same test justified the normal distribution (Shapiro-Wilks test, p=0.602), after the exclusion of 3 hyperactive mice, and thus the identification of hyperactive animals as a cohort.
2.2 Y-maze
Y-maze testing for spatial memory consisted of two sessions. For the first session, mice were placed in a Y-shaped apparatus at one arm and were allowed to explore each arm. The sequence in which it navigated each arm was recorded, to examine if it preferred a new arm over the one it just exited from. Three days later, a different Y-shaped apparatus was used to conduct the second session. Mice were allowed to habituate in the apparatus for 5 min, while one arm was blocked with a transparent barrier. After 20 min, mice were put into the same Y-shaped apparatus to examine whether it would prefer the novel arm that it was not allowed to explore during the habituation session. Percent scores were calculated as follows: for YM1, percent score = [(# of Alternations/Total # of triads) *100]: for YM2, percent score = 100*(time spent in novel arm / total test duration).
2.3 Elevated Plus Maze
Elevated plus maze (EPM) assessed anxiety levels of mouse using an apparatus that has a plus-sign shape with crossing of an open arm and a closed arm at an elevation of 2 feet. Each mouse was placed at the center of the square, facing an open arm, and was allowed to explore the apparatus for 5 min; % score = 100*(time spent in open arm / total test duration). The time spent in the open arms and closed arms were manually scored by an observer blind to experimental groups.
2.4 Pre-pulse Inhibition (PPI)
The pre-pulse inhibition test was conducted using a startle reflex measurement system (Ohara Ika Sangyo, Tokyo, Japan). Each mouse was put in a Plexiglas cylindrical tube with limited space adjustment capability. Each session started with a 5 min habituation period when mice were left undisturbed in 70dB background noise. Testing began with presentations of a 120dB (100ms, broadband burst) pulse repeated 10 times with 20s intervals. This was followed by a series of randomized presentations of pre-pulses (20ms broadband burst) of levels (74-120), (78-120), (82-120), (86-120), (90-120), 80ms prior to the 120 pulse. Each session contained six presentations of each pre-pulse, and the startle response was measured. Five blocks of the six trial types were presented in a pseudorandom order, while each trial type was presented at least once within a block.
2.5 Social Interaction (SI)
Social behavior was tested at PND 80 and 130 using the three chamber paradigm (Billingslea, 2007). Each subject was habituated to the Plexiglas apparatus(30×36×17cm3) for 5 min, two days prior to the day of test. The testing consisted of two parts that were 30 min apart: 1) social recognition, 2) social preference. Each mouse started at the center (“start”) chamber (15×10 cm; Habitest Runway; Coulbourn Instruments) separated from the other (left and right) chambers by drop doors. The left and right chambers each contained a small conical cage (radius=2 inch, height=6 inch) with an alien conspecific adult mouse, and access to the alien mouse was limited to nose pokes through a grid with 1-cm holes. This paradigm and protocol has been previously described (Nadler et al., 2004). The SI index was calculated as novel chamber exploration time / (total exploration time – time spent in the middle starting chamber) during the test phase. As an alternative parameter of rodent sociability, the number of times the mouse showed sniffing behavior while interacting with the alien mouse that was specific to the small conical cage was quantitated and compared (data not shown).
3. Chronic EEG surgery and quantitative EEG (qEEG) analysis
3.1 Head mount implantation surgery
The surgical procedures were done as previously described (Adler et al., 2014; Johnston et al., 2014), under isoflurane anesthesia (4-2.5%) that lasted approximately 30-40 min each. At P120 (±10 days), mice were surgically implanted with EEG (subdural; 0.5mm caudal to bregma and 3.5mm bilateral to midline) and EMG (suprascapular) recording electrodes at brain coordinates referenced to bregma. The electrode remaining implantation coordinates were the same as previously reported (Adler et al., 2014).
3.2 24h EEG/EMG recording
After recovery from head mount surgery (4-6 days), each mouse was placed in a recording chamber with food and water provided at ad libitum. 24h continuous vEEG/EMG was recorded using Sirenia Acquisition software (Pinnacle Technology Inc., Lawrence, KS, USA). Each chamber had a tethered pre-amplifier and commutator attached with a top-view infrared camera. Each mouse had a new piece of nesting material, and a small piece of nesting from its home-cage at start of the recording session. Nesting scores were assigned at end of 24h video.
3.3 Quantitative EEG (qEEG) and sleep analysis
All acquired raw EEG data were scored as 10s epochs of Wake, NREM, and REM sleep by a scorer blinded to groups; similar to previously described protocols (Adler et al., 2014; Johnston et al., 2014). One mouse out of total 39 had an EEG artifact of less than 60min, which was excluded from the delta and gamma analyses: the reason for this artifact is unclear. Synchronous EMG and video were used to confirm wake-inactive states and possible artifacts which were then excluded. Basic analyses included: 1) percentage, duration, and the cycle count of each sleep state; 2) duration of and latency to the first and longest event for sleep cycles. The quality of nesting was visually scored at the end of the recording from score 1 (poorest) to 5 (complete) as previously reported (Johnston et al., 2014).
3.4 Computational and algorithmic analysis of EEG power
Power data extracted from the EEG1 lead were analyzed. Frequency data were binned as delta (0.5–4.0 Hz), theta (5.5–8.0 Hz), alpha (8.0–13.0 Hz), beta (13.0–30.0 Hz) and gamma (35–44 Hz). To compare the total delta power during the 24h period, we used area under the curve (AUC) analysis with delta power vs. time graphs. The delta-power AUC was calculated for the sleep and wake cycles of each mouse with trapezoidal summations using R statistical software (Zeileis and Grothendieck, 2005; Bilodeau et al., 2015; Trapletti and Hornik, 2013). Data were analyzed by separating the sleep and wake delta power and filtered by removing points 2.5 deviations away from the average delta power in a set window of 200, 10s epochs using published protocols (Johnston et al., 2014). The sleep and wake files were then concatenated. The AUC was also analyzed during the transition periods for sleep cycles greater than ten minutes in duration. The first 10 minutes of the sleep cycle were isolated, and the preceding 10 minutes of wake before the cycle were isolated. The AUC was then calculated for the sleep transition and its preceding wake, and the data were normalized by dividing the 10 minutes of sleep AUC by its preceding wake AUC to evaluate delta changes during behavioral state transitions. The NREM power at each of the above listed frequencies was then divided by the corresponding wake power to provide a ratio between behavioral states. The theta-beta ratio (TBR) was calculated by dividing theta power at each epoch by its corresponding beta power: TBR = Theta power/beta power.
3.5 Spectral power analyses (Gamma, Theta and Beta)
The spectral power datasets were represented as a scatterplot of the recorded values per 10 seconds epoch. Each epoch was labeled wake and sleep by a blind scorer. These datasets were then input into an R-stats program to calculate the area under the curve (AUC) for each spectral power. The AUC was calculated the using trapezoidal rule between each epoch similar to previously reported (Johnston et al., 2014). The trapezoidal rule works by approximating the area of the region under the gamma power graph by calculating the area of every epoch as a trapezoid, then does a summation of those trapezoids to get AUC over the 24h recording period. The wake gamma AUC was calculated by summation of all the AUCs during each individual wake cycle within a 24h period. Sleep gamma, which was graded over time spent sleeping within each sleep cycle and therefore dependent on the duration of each sleep cycle, was calculated as: total gamma AUC – wake gamma AUC over the 24 h recording period. Theta and Beta powers were summated over the light and dark cycles to look at diurnal variation. All values were generated by automated code in the R-stats program.
4. Immunoblot
All animals underwent trans-cardiac perfusion (ice-cold PBS) after being anesthetized with chloral hydrate (90 mg/ml; IP). At P140±10, whole brains were harvested and frozen as ipsilateral/ contralateral hemisphere, neocortex and deep matter, and stored at -80°C until further use. After brain tissue homogenization and lysis, protease/phosphatase inhibitor cocktail was added to the lysate. Bradford Assay (Bio-Rad) was performed to determine the protein concentration. For each sample 50ug of protein in 20ul was loaded for gel electrophoresis; 4-20% gradient 1.5mm 15 wells SDS gels (Invitrogen) for 100-120 min with 130V. Samples were transferred onto nitrocellulose membranes for overnight wet-transfer for a minimum of 24 hours at 30V, followed by 1h blocking step and overnight incubation in primary antibodies: rabbit α-KCC2 (1:1000, Millipore; Cat.No. 07-432; Antibody registry AB 11213615); mouse α-actin (1:10000, LI-COR Biosciences, Cat.No. 926-42214). Membranes were then incubated in secondary antibodies for 1 h (1:5000 for both goat α-mouse 680LT and goat α-rabbit 800CW, LI-COR Biosciences), followed by detergent-based washing steps. Protein bands were analyzed using the Odyssey imaging system 2.1 (LI-COR Biosciences) and the optical density of each KCC2 band was normalized to the actin bands run on the same lane.
Statistical analyses
All statistical tests were performed using SPSS21 (IBM, Armonk, NY, USA). Group means of acute total electrographic seizure burden and ictal duration within each group were compared using student's t-test; Shapiro-Wilk test was used to ensure distribution normality Group means for all other experiments were compared using one-way ANOVA with Bonferroni correction for between group comparisons. For open field habituation, within-group activity levels were analyzed with repeated-measures ANOVA, and the F statistic was reported as F (dftime, dferror) and p-values. The assumption of sphericity for data was validated with Mauchly's test. Pairwise t-tests within groups are also reported for some experiments. A two-way ANOVA was conducted to assess paired light-dark and sleep-wake differences across groups, for which the results were reported as F (dftime, dferror) and p-values. P-values less than or equal to the alpha at 0.05 (p≤0.05) were considered statistically significant. All data are reported as mean±1 S.E.M.
Results
Age-dependent seizure severity
Age-dependent seizure severity is one of the hallmarks of the newly characterized model for neonatal ischemia (Kang et al., 2015) used in this study. Total seizure burden over the 3h duration was significantly higher (192%) in P7 ligates compared to P10 (Fig 1B; P<0.001; n=11 for each), driven by significant increases in both the number of ictal events and mean ictal durations (Fig. 1B; P<0.001 and P<0.001 respectively).
Emergence of hyperactivity in ligate mice
Mice underwent OF testing to assess their daytime activity levels and habituation learning abilities. At P80 and P130, the activity levels in the first 5 minutes were not significantly different among groups, indicating that the ligated animals did not have a difference in novelty driven exploration (Fig 2A, P80). At P130, P7 ligates took longer to habituate but P10 ligates habituated similar to age-matched sham controls (Fig 2B; P130, repeated measures ANOVA, p<0.05 in comparison to the 1st 5min interval). All mice in study habituated by the end of testing period (i.e.; 25 min), but shams habituated significantly within the first 10 min of the testing period (i.e.; 1st 5 min interval vs. 2nd 5 min interval; repeated measures ANOVA).
Figure 2.
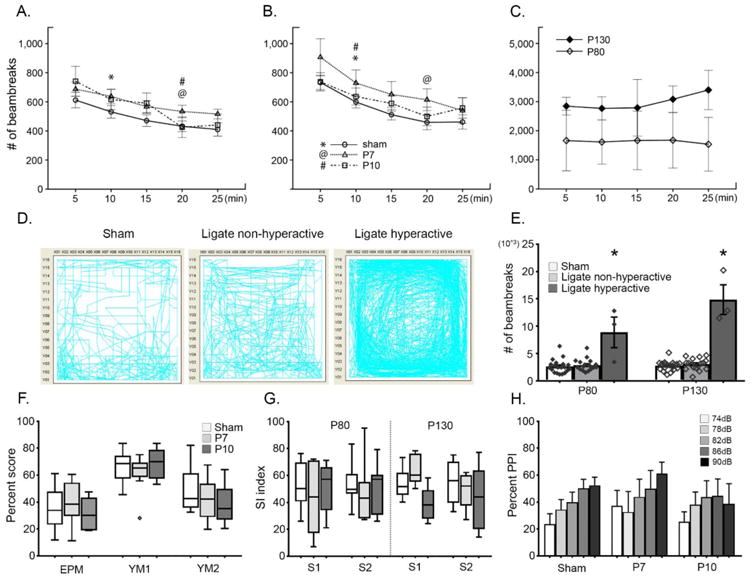
A-B. Open-field (OF) activity levels were assessed at P80 and P130 respectively. Open-field habituation shows relatively intact explorative learning for all three groups tested. Symbols denote where habituation occurred: the interval during which the # of beambreaks compared to that of the 1st interval became statistically significant; repeated measures ANOVA, F(4, 76) = 9.82, F(4, 32) = 5.97, F(4, 32) = 8.18; n=20, 10, 9 for sham, P7, P10 ligates respectively for P80 OF; F(4, 72) = 24.37, F(4, 28) = 6.42, F(4, 28) = 4.62; n=19, 8, 8 for sham, P7, P10 ligates respectively for P130 OF) All groups habituated by the end. C. Significantly impaired habituation of hyperactive cohort (n=3) in OF habituation curve; F(4, 4) = 0.28, F(4, 8) = 0.76, for P80 and P130 respectively. No habituation occurred in this cohort. D. Representative OF maps revealed few animals (n=1 P7, n=2 P10) in ligated cohort that are significantly hyperactive compared to sham and non-hyperactive ligates. E. Total OF activity quantitated by the number of beam-breaks shows extremely high activity of hyperactive cohorts [# of beam-breaks > 6000s]; ANOVA p<0.001 for HA for both P80 and 130, although the assumption of homogenous variance is violated due to the small sample size of ligate hyperactive group). F. Behavioral tests for anxiety and spatial memory show no significant impairment in P7 or P10 ligates (One-way ANOVA; in order of tests, F=0.40, 1.72, 0.85 and p=0.68, 0.20, 0.44; n=20, 9, and 8 for groups respectively); please see methods for percent score calculations; the diamond symbol denotes an outlier. G. Social interaction tests show no significant deficit in ligates compared to sham (S1 for social recognition and S2 for social preference); One-way ANOVA; in order of tests, F=0.50, 0.51, 0.82, 0.05 and p=0.61, 0.61, 0.46, p=0.95; n=16, 5, and 7 for groups respectively. H. Prepulse inhibition failed to detect any clear alteration in acoustic sensitivity for P7 and P10 ligates compared to sham (neither the one-way ANOVA among groups nor the repeated measures ANOVA across decibels).
Remarkable hyperactivity was detected in a subset of ligate mice (Fig. 2C-E; n=3, 1 P7 ligate and 2 P10 ligates, all females, see methods), with failure in habituation (Fig. 2C). As shown in OF activity maps (Fig. 2D), total activity for this hyperactive cohort was significantly higher compared to shams and their non-hyperactive ligate littermates (Fig. 2E; 2644±1243 counts of light beam breaks for sham, 3012±1054 for non-hyperactive ligates, and 8893±4858 for hyperactive group, evaluated at P80; p<0.001). Therefore, OF testing identified a cohort of hyperactive ligates at both ages. The nesting scores at end of 24h video period did not differ significantly amongst groups or for hyperactive ligates (data not shown).
Behavioral tests for cognitive and social abilities
A standard battery of behavioral tests for assessing neuropsychiatric phenotypes was conducted. EPM and Y-maze tests failed to identify a significant difference in anxiety and spatial memory in both ligate groups (Fig. 2F, one-way ANOVA; F=0.40, 1.72, 0.95 for YM1, YM2, EPM respectively; n=20, 9, 8, for sham, P7, P10 ligates respectively). Neither of ligate groups had any significant social deficits (Fig. 2G, one-way ANOVA; F=0.50, 0.51, 0.82, 0.05 from left to right respectively); the number of sniffs made also did not differ among groups (data not shown). Pre-pulse inhibition tests failed to detect significant alteration in the acoustic sensitivity profile in either P7 or P10 ligates (Fig. 2H). The comparison of PPI response at any specific interval tested was not significant among groups (One-way ANOVA). In summary, a standard battery of behavioral tests failed to identify any significant cognitive or social deficits in ligated group of mice at both ages.
Altered hypnograms in ligates
The effects of untreated transient neonatal ischemic seizures on long-term sleep architecture were investigated (Fig. 3A). P10 ligates had significantly altered percent sleep/wake states during the light cycle compared to both sham and P7 ligates (Fig. 3B; paired t-tests n=19, 10, 10 for sham, P7, P10 ligates respectively). As expected, percent wake state was significantly higher in all groups during the dark cycle (i.e.; nocturnal period), and not significantly different among groups (Fig. 3B). Both P7 and P10 ligates however, had significantly longer duration of their longest wake cycle compared to shams (4.63±0.47h for sham, 7.38±1.20h for P7 ligates 8.15±0.87h for P10 ligates Fig. 3C; one-way ANOVA, F = 6.315, p=0.047 for P7 and p=0.011 for P10). The diurnal variation in sleep microarchitecture, where NREM cycles were longer in the light cycle and REM cycles were longer in the dark cycle were detected in all groups. However, the significant diurnal variation within groups between light and dark cycle for NREM and REM durations detected in shams was lost in P7 ligates (Figure 3D&E; two-way ANOVA). P10 ligates retained diurnal variation of NREM but not for REM.
Figure 3.
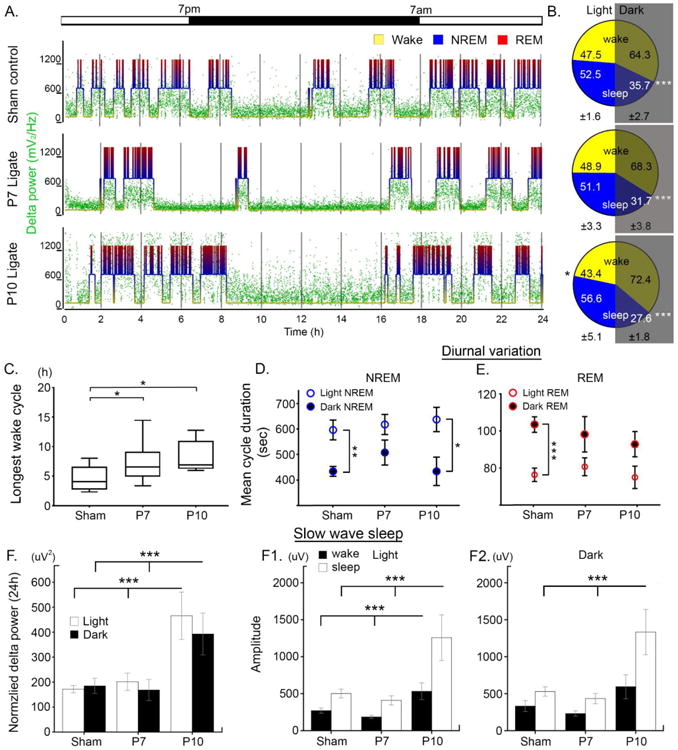
A. Representative 24h hypnograms and associated delta power for each group. B. Mean percentage of wake and sleep by light and dark cycle: ±1 S.E.M are denoted for light and dark cycle at the bottom of each pie chart. P10 ligates had significantly higher % sleep during day (black asterisk, n=19, 10, and 10 for sham, P7, and P10 ligates respectively; paired t-test, p=0.006 for P10). All groups showed significantly higher percent wake vs. sleep (p<0.001; white asterisks). C. Longest wake cycle for each group. Ligates had significantly longer duration of longest wake compared to sham; one-way ANOVA (F=6.32, p=0.005), Bonferroni correction; p=0.046 for P7 and p=0.011 for P10 compared to sham (n=18, 9, 8 for sham, P7, and P10). D-E. Mean NREM and REM duration by light and dark cycle. Significantly longer duration of NREM during day and REM during night were observed in sham; two-way ANOVA, Bonferroni correction: p=0.003 and 0.0001 for sham NREM and REM respectively. These significances were lost in P7 ligates; p= 0.44 and 0.30. P10 ligates retained the significant NREM duration but not REM; p=0.01 and 0.19 respectively. F. Normalized delta power was quantitated for each light and dark cycle, and P10 ligates have significantly higher delta compared to sham and P7 ligates (One-way ANOVA, p=0.014 for light and p=0.021 for dark; n=16, 9, 8 for sham, P7, P10 ligates respectively). F1-2 Delta amplitude for wake and sleep cycles were significantly higher in P10 ligates for both light and dark cycle (One-way ANOVA, Bonferroni correction; F=5.54, 6.9, 1.8, 8.0 and p=0.009, 0.003, 0.185, 0.002 in order of light wake, sleep, dark wake, sleep).
Analyzed by sex, a few additional differences were observed. During the dark cycle, the average duration of wake cycles were significantly longer in female P10 ligates compared to male P10 ligates (student's t-test, p=0.01). Additionally, average duration of the longest wake cycle was significantly longer in female P10 ligates (student's t-test, p=0.04), compared to male P10 ligates (data not shown). Conversely, male P10 ligates had significantly longer NREM and REM cycle durations during the dark cycle (student's t-test, p=0.01 for both) compared to female P10 ligates. Therefore, sleep structure hypnograms in adult mice with history of neonatal ischemic seizures were significantly modulated by neonatal age at ischemia and sex.
Slow wave sleep (SWS) dysfunction
Delta wave (0.5-4Hz) frequency and amplitude represent sleep quality during SWS (NREM). Both P7 and P10 ligates were found to have significantly longer wake cycles compared to shams (Fig. 3C; one-way ANOVA). Long wake cycles are usually compensated for by increased delta power during sleep (Roth, 2009). However, only P10 ligates had significantly higher normalized delta power (i.e.; SWS delta power normalized to wake delta power for each mouse for its own behavioral states) during sleep in both light and dark cycles, unlike P7 ligates which remained similar to shams in spite of long wakes (185±30 uV2/Hz for sham, 169±42 uV2/Hz for P7 ligates, 393±84 uV2/Hz for P10 ligates; Fig. 3F, one-way ANOVA). Delta amplitude for P10 ligates in wake and sleep states was significantly higher during both light and dark cycles (Fig. 3 F1&2, one-way ANOVA). This suggests a potential compensation for the long wakes in P10 ligates through higher delta power and amplitudes, which were absent in P7 ligates. This suggests that the P7 ligate group loses both diurnal variation in NREM and REM states and the ability to compensate for lost sleep.
Gamma power as a function of behavioral state
Gamma oscillation reflects a cognitive-behavioral state at which cortical activity peaks. Total gamma AUC of 24h duration was differentially affected by the age at ligation (Fig 4B, One-way ANOVA: p=0.047; Bonferroni correction P7 vs. P10: p=0.053). Total gamma AUC was significantly different between P7 and P10 ligates but neither group was statistically different from sham controls. When analyzed by behavioral state (i.e.; sleep or wake) the total gamma AUC during wake was higher than sleep in all groups, although not statistically significant (Fig 4C&D, two-way ANOVA); this pattern is also seen in human patients (Cantero et al., 2004; Cantero and Atienza, 2005). Pairwise t-test detected weak statistical significance between wake and sleep gamma in sham and P10 ligates but not in P7 ligates; Fig 4C&D; paired t-test, sham, p=0.0003; P7, p=0.067; P10, p<0.05, n=18, 10, 9 for sham, P7, P10 ligates respectively). However, 2-way ANOVAs showed no statistical differences for wake-sleep state pairs across treatment groups. This may indicate that both total gamma power and its modulation by activity state were not significantly differentially affected by ischemic insult unlike delta power dysfunction which was differential by age of insult..
Figure 4.
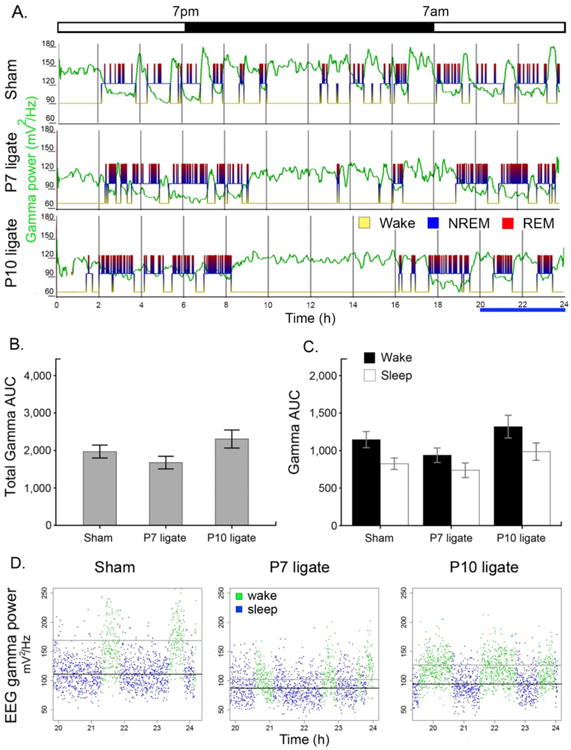
Gamma power analyses was not statistically significant unlike delta power. A. Representative traces of gamma power along with sleep hypnogram for each group. B. Total gamma AUC of ligates were significantly different from each other (p=0.053), but not from sham (One-way ANOVA, Bonferroni correction; F=3.347, p=0.047, n= 18, 10, 10 for sham, P7, P10 ligates respectively). C. Gamma AUC by wake/sleep cycle was not significant by 2-way ANOVA across treatment groups (paired t-test within each group showed p values, p=0.0003, p=0.067, p=0.026 and n=18, 10, 9 for sham, P7, and P10 respectively). D. Scatter plot of EEG gamma power in duration of 4h for each group. (black line: mean for sleep; gray line: mean for wake).
Theta power AUC and TBR ratios
Theta/beta ratio denotes a negative relationship with attentional control. The theta/beta ratio, now commonly investigated in attention deficit syndromes, likely reflects prefrontal mediated attentional control and has been proposed as a useful biomarker of ADD/ADHD/attention disorders (Putman et al., 2014). In this study, P10 ligates had a significantly higher theta power AUC during light and dark cycle (Fig. 5A, one-way ANOVA, F=9.3 and 13.2 for light and dark, p=0.001 vs. Sham and 0.002 vs. P7 ligates, n=18, 10, 10 for sham, P7, P10 ligates respectively). Similar results were observed for beta AUC (Fig 5B, one-way ANOVA, F=4.2 and 7.2 for light and dark n=18, 10, 10 for sham, P7, P10 ligates respectively; see Table 2). Additionally, theta/beta ratios during the dark cycle were significantly higher in P10 ligates than sham controls (one-way ANOVA, F=4.4 n=18, 10, 10 for sham, P7, P10 ligates respectively; p=0.004) but not in P7 ligates. Overall, the power AUCs for theta and beta and TBR ratio were only significant different in the P10 ligate group.
Figure 5.
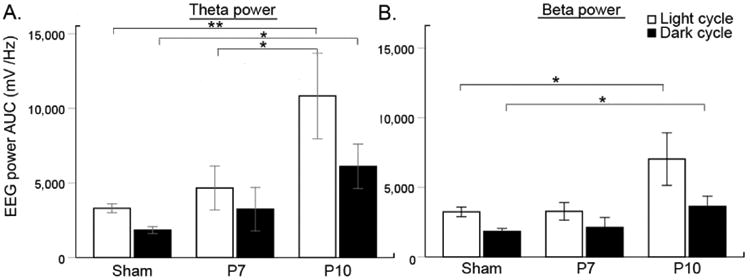
Theta beta power analyses A. Significant increase in theta power was detected during the light and dark cycle in the P10 mice compare to controls (One-way ANOVA; F=6.45, p=0.004 and F=4.99, p=0.012 for theta light and dark, n=18, 10, 11 for sham, P7, and P10 respectively. P10 mice had significantly higher theta light and dark compared to sham and theta light compared to P7 ligate mice during the light cycle (Bonferroni correction for P10: p=0.004 and p=0.01 vs. sham theta light and dark respectively; and p=0.048 vs. P7 theta dark). B. Beta power showed similar trends in P10 mice compared to control mice but not to P7 (One-way ANOVA; F=4.40, p=0.020 and F=3.50, p=0.041 for beta light and dark, same number of n as in theta analysis; Bonferroni correction for P10: p=0.027 and p=0.043 vs. sham beta light and dark respectively).
Table 2. qEEG spectral analyses means ± SEM.
| Sham | P7 ligate | P10 ligate | |
|---|---|---|---|
| Normalized Delta Power (uV2/Hz) – Light cycle | 171.6±14.5 | 201.6±34.5 | ***### 465.7±94.5 |
| Normalized Delta Power (uV2/Hz) – Dark cycle | 185.0±30.4 | 168.6±42.2 | ***### 392.6±83.7 |
| Delta Amplitude Wake (uV) - Light cycle | 271.4±34.6 | 185.5±21.5 | ***### 532.4±113.9 |
| Delta Amplitude Sleep (uV) - Light cycle | 501.8±59.2 | 410.1±61.3 | ***### 1256.8±309.3 |
| Delta Amplitude Wake (uV) - Dark cycle | 332.5±74.5 | 231.7±35.7 | ***### 594.4±162.1 |
| Delta Amplitude Sleep (uV) - Dark cycle | 529.5±63.1 | 434.0±70.0 | ***### 1332.9±304.8 |
| Total Gamma AUC (uV2/Hz) | 1971.0±172.5 | 1675.9±168.9 | # 2683.7±435.1 |
| Wake Gamma AUC (uV2/Hz) | 1146.3±107.7 | 938.3±96.5 | 1319.6±151.5 |
| Sleep Gamma AUC (uV2/Hz) | 824.6±76.6 | 737.6±97.9 | 986.2±116.5 |
| Theta AUC (uV2/Hz) - Light cycle | 3307.0±295.0 | 3279.7±567.6 | ***## 11760.6±3001.1 |
| Theta AUC (uV2/Hz) - Dark cycle | 1838.0±233.5 | 1827.0±388.6 | ***## 6619.4±1552.5 |
| Beta AUC (uV2/Hz) - Light cycle | 3237.7±348.4 | 2754.7±395.0 | **# 7667.9±1965.9 |
| Beta AUC (uV2/Hz) - Dark cycle | 1835.6±212.0 | 1432.5±228.4 | *3945.7±735.1 |
| Theta/Beta Ratio - Light cycle | 1.1±0.1 | 1.3±0.1 | 1.4±0.2 |
| Theta/Beta Ratio - Dark cycle | 1.0±0.1 | 1.4±0.2 | **## 1.5±0.2 |
One-way ANOVA; Bonferroni correction
p<0.01,
p<0.001.
compared to Sham
One-way ANOVA; Bonferroni correction
p<0.05,
p<0.01,
p<0.001.
compared to P7 ligate
EMG power analyses
EMG lead power analyses (10-50 Hz) showed significant motor activity related increases during wake cycles and silent leads during sleep and during wake inactivity. Total EMG power during the 24h cycles (Fig.6 A) not only correlated well with hyperactivity during OF testing (Fig. 2D) but also captured other ligate mice that were not hyperactive during OF. 24h EMG allowed analyses of activity levels by light and dark cycles over a 24h period. The expected nocturnal increase in exploratory activity for mice led to a significant increase in EMG lead power during the dark cycle in sham mice (Fig 6B). Both the P7 and P10 ligate mice showed similar increases; however the significant diurnal difference in activity levels on EMG were lost due to high variability introduced by the hyperactive mice in this cohort. In, summary, EMG power analyses are a powerful tool to quantitate mouse activity levels over 24h periods allowing investigation of activity levels in the dark cycle when they are the most active.
Figure 6.
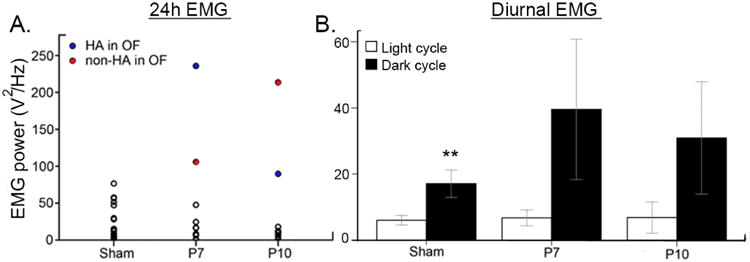
24h EMG lead power analyses. A. Total EMG power during 24h EEG recording identified hyperactive mice in the ligate groups. B. Significant diurnal variation in EMG activity detected in control mice was lost in both ligate groups due to the variability in hyperactivity (paired t-test p=0.003, 0.123, 0.101 and n=19, 10, 10 for sham, P7, and P10 respectively).
KCC2 in adult cortex
The effect of neonatal ischemia on long-term expression levels of chief neuronal chloride extruder KCC2 in the neocortex has not been evaluated to date. We have previously shown that KCC2 levels were significantly reduced in the days following the neonatal ischemic insult in this model. However the KCC2 hypofunction was transient and showed recovery over a four day period (Kang et al., 2015). In this study, KCC2 expression in the superficial grey matter in the ligated mice was investigated in adult (i.e.; 4 month old) mice with history of acute neonatal ischemic-seizures (Fig. 1 B and C) and found to similar between treatment groups as 4-month-old adults (Supplementary Fig. 1; One way ANOVA; p=0.31 for left and p=0.46 for right). This indicates KCC2 hypofunction does not persist into adult-hood in the model and may not underlie the emergence of the chronic and spontaneous epileptiform discharges and seizures (Suppl. Fig. 2).
Discussion
Neonatal ischemic seizures, although transient, can have long-lasting neurobehavioral consequences as adults. The frequency and impact of cognitive and behavioral comorbidities associated with epilepsy have been recently highlighted in the 2012 Institute of Medicine report on epilepsy. Comorbidities have also been acknowledged as a National Institutes of Health (NIH) Benchmark area for research in epilepsy (Curing the Epilepsies:Pathways Forward, 2013). However, very few studies have investigated long-term comorbidities following neonatal seizures where the etiology and immediate treatment protocols may dictate outcomes (Ajayi et al., 1998; Forcelli et al., 2012). 24h continuous EEG/EMG with synchronous video and qEEG spectral analyses in this study detected differential alterations in sleep architecture and qEEG biomarkers in both the P7 and P10 ligated group.
Chronic study replicated the acute age-dependent electrographic seizure burden characteristics previously described for the model
We have previously characterized a mouse model of ischemic neonatal seizures in CD-1 pups that shows age-dependent seizure susceptibility and PB-resistance (Kang et al., 2015). The replicated results for the higher seizure severity observed in P7 compared to P10 suggest that the age-dependent PB-efficacy as well as post-ischemic downregulation of KCC2 expression are expected. The developmental and post-insult regulation of chloride ion gradients and chloride co-transporters function have been proposed to heavily impact age-dependent seizure susceptibility as well as PB efficacy (Kang et al., 2015) (Ben-Ari, 2002) (Dzhala et al., 2005; Morita et al., 2014). As the chief Cl- extruder in the brain, KCC2 is the central regulator of neuronal Cl- homeostasis critical for enabling strong hyperpolarizing synaptic inhibition in neurons. The role of KCC2 hypofunction in neuronal excitability and seizure susceptibility has been significantly strengthened by recent studies (Kahle et al., 2016; Stodberg et al., 2015). Evaluation of KCC2 expression levels in superficial grey matter showed no difference among groups (Supplementary Fig. 1), as post-ischemic downregulation of KCC2 expression is known to be transient (Kang et al., 2015), and we did not expect to observe any difference either across different age groups or between hemispheres. Not many studies have assessed the chronic alterations in the expression levels or localizations of proteins involved in glutamatergic or GABAergic neurotransmission in the context of neonatal seizures. Future proteomic and slice electrophysiology studies using chronic animal models will be able to address the mechanisms underlying epileptogenesis in this model.
Standard behavioral tests fail to detect consistent neurobehavioral differences in adult mice
Behavioral testing in mice is known to be influenced by multiple factors (Chesler et al., 2002). Recently published literature highlights the multitude of factors unrelated to the standardized behavioral test itself that can alter mouse behavior, such as handling and gender of the experimenter (Sorge et al., 2014). Our behavioral tests, however, failed to identify a clear phenotype for ligate vs. sham-control mice with the exception of the OF test for hyperactive ligates that occurred in both groups; the gross locomotor behavior and the exploratory response to a novel environment were not impaired in either P7 or P10 ligates. High within-group variability between the P7 and P10 group responses for the PPI and social interaction test resulted in varied but non-significant results. However, the non-identical slope of PPI response profile among groups (exponential in P7 vs. positive non-linear in sham and P10 ligates) is open to interpretation, and may require further examination in a wider range of acoustic stimuli. Recent studies in neonatal mice following hypoxia have reported a similar failure to detect significant differences with spatial memory (Rodriguez-Alvarez et al., 2015). Additionally, the same hypoxia-only model documented hypoactivity within the OF test, in contrast to the hyperactivity results reported here following ischemic seizures, indicating etiology of the neonatal insult may play a pivotal role in determining long-term outcomes.
Hyperactivity detected in a subset of post-ischemic mice as adults correlated with high EMG power
Adult onset hyperactivity was a feature noted for a subset of both P7 (20%) and P10 ligates (30%). These OF hyperactive mice failed to habituate within the OF session, unlike their non-hyperactive littermates. EMG power analysis synchronously acquired during the 24h video/EEG/EMG not only identified nocturnal hyperactivity in all three OF hyperactive mice, but also detected an additional nocturnally hyperactive mouse which was not hyperactive during the OF. 24h EMG power therefore was a more robust technique for identification of hyperactivity in this study, as it allowed diurnal comparison in activity levels as well as longer duration of quantification of activity compared to OF testing periods. The development of hyperactivity detected in this study replicated the hyperkinesia reported in long-term follow-up studies done in HIE patients (George et al., 2009). A similar hyperactivity has been noted in adult mice following ischemic neonatal seizures in CD-1 mice following ligation at P12 (Kadam et al., 2010) which was progressive. Because hyperactive mice has been detected across P7, P10, and P12 groups, this indicates that the emergence of hyperactivity in the model may be related to the neonatal ischemic insult itself rather than the age-dependent seizure phenotypes
Development of chronic epilepsy following neonatal ischemic seizures
Adult mice from both ligated age groups displayed spontaneous epileptiform discharges (i.e., spikes) on their 24h chronic EEGs. Generalized tonic-clonic seizures were also recorded on the same EEGs, in one mouse from the P7 ligated group implicating development of full blown epilepsy in atleast one ligated mouse (Supplementary Fig. 2). This indicated that development of chronic epilepsy may be an important feature of the CD1 mouse model of neonatal seizures that requires further investigation.
SWS compensation for sleep deprivation due to long wake cycles was significantly different between P7 and P10 ligates
Developmental disabilities are commonly associated with sleep dysfunction (Doran et al., 2006; Richdale and Baker, 2014). A large body of evidence suggests a bidirectional relationship between sleep and epilepsy, where sleep disorders are common comorbidities in individuals with epilepsy (Dewolfe et al., 2013; Rocamora et al., 2008) and seizures are known to often arise from SWS. The depth and quality of SWS occurring during NREM are critical to the health of the developing brain (Greene and Frank, 2010; Roth, 2009). The EEG pattern of NREM sleep is dominated by slow wave activity (SWA). SWA is defined as EEG power in the 0.5–4 Hz frequency during NREM sleep, and is a reliable measure of the amplitude and density of slow waves during NREM sleep cycles. SWA is an established marker of sleep pressure (Greene and Frank, 2010), increasing with the duration of waking and dissipating throughout sleep. Sleep deprivation studies in WT rodents and humans have shown a tendency towards NREM compensation by increase in SWA during sleep cycles following periods of sleep deprivation (Roth, 2009; Brunner et al., 1993). Despite the longer longest wake in dark cycle detected in P7 and P10 ligates (Fig. 3), the NREM cycles in the P7 ligate group failed to show any compensatory increase in SWA contrary to what would be expected. In rats, the rate of decrease in glutamate during sleep after sleep deprivation was twice as fast as the average decrease during spontaneous NREM sleep (Dash et al., 2009). High sleep pressure seems to enhance the NREM-related decline in glutamate levels in naïve brains that are sleep deprived. Since this compensation occurred in the P10 group but failed to occur in the P7 group, we hypothesize that the behavioral state glutamate homeostasis may be significantly impaired in the P7 group. Future studies could investigate this hypothesis using in-vivo glutamate biosensors (Johnston et al., 2014).
In addition, a loss of statistical significance in the mean duration of NREM or REM sleep in P7 ligates in light vs. dark cycle can certainly have a significant biological implication, despite the same amount of total duration of NREM and REM. Sleep architecture employs the separation of sleep elements into distinct cycles (light vs. dark or REM vs. NREM), and this difference (10 vs. 20sec), although seemingly not biologically significant, may imply a compensatory mechanism in a way that the boundaries for light cycle-specific sleep elements are being obscured.
Gamma oscillations and behavioral state
EEG gamma frequency oscillations have been proposed to account for the neural synchronization crucial for perceptual integration both during cortical arousal in active wake cycles and during sleep (Hwang et al., 2013; Maloney et al., 1997). Increased gamma power and synchronization is generally observed during cognitive tasks performed during active wake (Cantero et al., 2004). Gamma power is also associated with paradoxical sleep (REM), highlighting the unique characteristics and role of gamma oscillations in conscious and unconscious processing (Gross and Gotman, 1999). In this study, a similar behavioral state-dependent modulation of gamma power was noted. The increase in gamma power from sleep to wake transitions was immediate. In contrast, the wake to sleep gamma transition was graded over time with a few peaks of phasic activity within the sleep cycles. Overall gamma power (AUC) was higher during wakefulness and lower during sleep (Gross and Gotman, 1999), and thus had the opposite trend compared to delta frequency (AUC) which was prominent during SWA as noted above. This study showed that although there was a behavioral state modulation in gamma power in all groups, these were not significantly different from one another. Other parameters including rate-of-fall or rise of gamma during wake-sleep transitions will be of interest to investigate in future studies, because gamma oscillations during different behavioral states reflect rhythmic firing of inhibitory interneurons, entraining neuronal firing (Buzsaki and Wang, 2012).
Theta/Beta power AUC and ratios
Dysfunction of the prefrontal cortex mediated attention as seen in attention deficit/hyperactivity disorder (ADHD) has been robustly related to an increased ratio between power in the slow theta frequency band and the fast beta band (Barry et al., 2003; Clarke et al., 2002). Theta beta ratios (TBR) derived using single-channel quantitative EEG have recently been in the spotlight in autism research (Chan and Leung, 2006) and have been proposed to underlie impaired connectivity associated with the ADHD symptoms in autistic children. Theta amplitude and therefore AUC power usually goes up during sleep. Therefore, the theta power AUC during the light cycle will be higher than during the dark cycle which is a period of exploration, nesting and grooming activity, making TBR ratios higher in light vs. dark cycles. A higher theta/beta ratio may also reflect more involvement of subcortical (limbic) as opposed to cortical structures in inhibitory cortical control that have been shown to underlie disadvantageous decision making (Schutter and Van Honk, 2005). The higher TBR detected during the dark cycles (Table 2, significantly higher only in P10 ligates) in the ligate groups during the active exploration period where attentional control would be vital was opposite of the same ratio detected as being lower in sham controls during the dark cycle when compared to their light cycle TBRs. This switch in TBRs was consistent in both P7 and P10 ligate groups and an interesting finding as related to a possible biomarker of attentional control during the nocturnal period when it would be most relevant for rodents. In addition, a recent study has reported a change in theta band power associated with neuropsychiatric phenotypes in rodent models of post-injury epilepsy, further supporting the diagnostic potential of spectral power analyses in predicting long-term outcomes of neonatal seizures (Milikovsky et al., 2017).
Correlations
Correlation analyses (data not shown) run for between the following parameters were not statistically significant: 1) Ischemic lesion size (quantified by visual inspection during brain harvest, as percent volume loss in ipsilateral hemisphere), 2) the occurrence of spontaneous seizure and epileptiform discharges as adults, 3) OF activity levels, and 4) the severity of neonatal seizures, 5) qEEG parameters. However, these analyses are limited by: 1) qualitative assessment of ischemic lesions, and 2) single 24h EEG recording session, which can resolved in future studies by recording on multiple days for a better estimate of spontaneous seizure occurrence as adults, and 3) genetic differences in outbred mice which may account for diverse compensatory mechanism.
Conclusions
The findings of this study indicate that the significantly higher acute seizure burdens at P7 compared to P10 have distinct long-term consequences, identified by 24h qEEG/EMG recorded as adults in the same mice. qEEG biomarkers identified a significant lack of SWS compensation following long-wake cycles and poor gamma power delineation between sleep and wake states, in the P7 ligate group, both of which were preserved in the P10 ligate group of mice. In contrast, the P10 ligate group showed significant increases in theta/beta ratios not seen in the P7 group indicating age at which neonatal seizures occur can dictate long-term outcomes. These findings align with the recent findings in a clinical study done in HIE neonates where high electrographic seizure burdens were predictive of abnormal long-term outcomes (Weeke et al., 2016).
Supplementary Material
Supplementary Figure 1. Western blot analyses for KCC2, A. KCC2 expression levels in the superficial gray matter were not different among groups (One-way ANOVA: F=0.673, p=0.57 for left and F=1.126, p=0.35 for right; n=14, 9, and 6 for sham, P7, and P10 respectively).
Supplementary Figure 2. EEG trace shows a spontaneous tonic-clonic seizure occurring in 4-month-old mouse that developed epilepsy following transient ischemic neonatal seizures at P7. Image (inset) shows tonic posture of seizing mouse at time point denoted by black bar on the EEG trace. Epileptiform EEG discharges were detected in 24h EEGs of other mice with history of neonatal seizures (n=8). Control mice did not show any epileptiform activity at 4 months.
Highlights.
Open-field test identified a subset of hyperactive mice that fail to habituate
qEEG reveal significantly disturbed diurnal and sleep-dependent spectral power modulation
qEMG allow reliable quantification of diurnal and activity-dependent motor activity
Acknowledgments
We thank Brandon Carter for his technical support during this study.
Funding: Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R01HD090884 (SDK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- EEG
electroencephalogram
- EMG
electromyogram
- PB
phenobarbital
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Adler DA, Ammanuel S, Lei J, Dada T, Borbiev T, Johnston MV, Kadam SD, Burd I. Circadian cycle-dependent EEG biomarkers of pathogenicity in adult mice following prenatal exposure to in utero inflammation. Neuroscience. 2014 doi: 10.1016/j.neuroscience.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajayi OA, Oyaniyi OT, Chike-Obi UD. Adverse effects of early phenobarbital administration in term newborns with perinatal asphyxia. Trop Med Int Health. 1998;3:592–595. doi: 10.1046/j.1365-3156.1998.00274.x. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, Johnstone SJ. A review of electrophysiology in attention-deficit/hyperactivity disorder: I. Qualitative and quantitative electroencephalography. Clinical Neurophysiology. 2003;114:171–183. doi: 10.1016/s1388-2457(02)00362-0. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Bilodeau M, Micheaux PLd, Mahdi S. The R Package groc for Generalized Regression on Orthogonal Components. Journal of Statistical Software. 2015;1(1) 2015. [Google Scholar]
- Brunner DP, Dijk DJ, Borbely AA. Repeated partial sleep deprivation progressively changes in EEG during sleep and wakefulness. Sleep. 1993;16:100–113. doi: 10.1093/sleep/16.2.100. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Wang XJ. Mechanisms of gamma oscillations. Annu Rev Neurosci. 2012;35:203–225. doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantero JL, Atienza M. The role of neural synchronization in the emergence of cognition across the wake-sleep cycle. Rev Neurosci. 2005;16:69–83. doi: 10.1515/revneuro.2005.16.1.69. [DOI] [PubMed] [Google Scholar]
- Cantero JL, Atienza M, Madsen JR, Stickgold R. Gamma EEG dynamics in neocortex and hippocampus during human wakefulness and sleep. NeuroImage. 2004;22:1271–1280. doi: 10.1016/j.neuroimage.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Chan AS, Leung WWM. Differentiating Autistic Children with Quantitative Encephalography: A 3-Month Longitudinal Study. J Child Neurol. 2006;21:391–399. doi: 10.1177/08830738060210050501. [DOI] [PubMed] [Google Scholar]
- Chesler EJ, Wilson SG, Lariviere WR, Rodriguez-Zas SL, Mogil JS. Influences of laboratory environment on behavior. Nat Neurosci. 2002;5:1101–1102. doi: 10.1038/nn1102-1101. [DOI] [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, McCarthy R, Selikowitz M, Brown CR. EEG evidence for a new conceptualisation of attention deficit hyperactivity disorder. Clinical Neurophysiology. 2002;113:1036–1044. doi: 10.1016/s1388-2457(02)00115-3. [DOI] [PubMed] [Google Scholar]
- Curing the Epilepsies: Pathways Forward. 2013 https://meetings.ninds.nih.gov/assets/2013epilepsies/CuringEpilepsies2013_PROGRAMBOOK.pdf.
- Dash MB, Douglas CL, Vyazovskiy VV, Cirelli C, Tononi G. Long-term homeostasis of extracellular glutamate in the rat cerebral cortex across sleep and waking states. J Neurosci. 2009;29:620–629. doi: 10.1523/JNEUROSCI.5486-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewolfe JL, Malow B, Huguenard J, Stickgold R, Bourgeois B, Holmes GL. Sleep and epilepsy: a summary of the 2011 merritt-putnam symposium. Epilepsy Curr. 2013;13:42–49. doi: 10.5698/1535-7511-13.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran SM, Harvey MT, Horner RH. Sleep and Developmental Disabilities: Assessment, Treatment, and Outcome Measures. Mental Retardation. 2006;44:13–27. doi: 10.1352/0047-6765(2006)44[13:SADDAT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, Delpire E, Jensen FE, Staley KJ. NKCC1 transporter facilitates seizures in the developing brain. Nat Med. 2005;11:1205–1213. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- Forcelli PA, Kozlowski R, Snyder C, Kondratyev A, Gale K. Effects of Neonatal Antiepileptic Drug Exposure on Cognitive, Emotional, and Motor Function in Adult Rats. Journal of Pharmacology and Experimental Therapeutics. 2012;340:558–566. doi: 10.1124/jpet.111.188862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George B, Padmam MS, Nair MK, Indira MS, Syamalan K, Padmamohan J. Hypoxic ischemic encephalopathy developmental outcome at 12 years. Indian Pediatr. 2009;46 Suppl:s67–s70. [PubMed] [Google Scholar]
- Glass HC, Ferriero DM, Miller SP. Correspondence on “clinical seizures in neonatal hypoxic-ischemic encephalopathy have no independent impact on neurodevelopmental outcome: secondary analyses of data from the neonatal research network hypothermia trial”. J Child Neurol. 2011;26:532–534. doi: 10.1177/0883073811399801. [DOI] [PubMed] [Google Scholar]
- Greene RW, Frank MG. Slow Wave Activity During Sleep: Functional and Therapeutic Implications. The Neuroscientist. 2010;16:618–633. doi: 10.1177/1073858410377064. [DOI] [PubMed] [Google Scholar]
- Gross DW, Gotman J. Correlation of high-frequency oscillations with the sleeprÇôwake cycle and cognitive activity in humans. Neuroscience. 1999;94:1005–1018. doi: 10.1016/s0306-4522(99)00343-7. [DOI] [PubMed] [Google Scholar]
- Hwang E, McNally J, Choi JH. Reduction in cortical gamma synchrony during depolarized state of slow wave activity in mice. Frontiers in Systems Neuroscience. 2013;7:107. doi: 10.3389/fnsys.2013.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston MV, Ammanuel S, O'Driscoll C, Wozniak A, Naidu S, Kadam SD. Twenty-four hour quantitative-EEG and in-vivo glutamate biosensor detects activity and circadian rhythm dependent biomarkers of pathogenesis in Mecp2 null mice. Front Syst Neurosci. 2014;8:118. doi: 10.3389/fnsys.2014.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam SD, Dudek FE. Neuropathogical features of a rat model for perinatal hypoxic-ischemic encephalopathy with associated epilepsy. J Comp Neurol. 2007;505:716–737. doi: 10.1002/cne.21533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam SD, Smith-Hicks CL, Smith DR, Worley PF, Comi AM. Functional integration of new neurons into hippocampal networks and poststroke comorbidities following neonatal stroke in mice. Epilepsy & Behavior. 2010;18:344–357. doi: 10.1016/j.yebeh.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle KT, Khanna AR, Duan J, Staley KJ, Delpire E, Poduri A. The KCC2 Cotransporter and Human Epilepsy: Getting Excited About Inhibition. The Neuroscientist. 2016 doi: 10.1177/1073858416645087. [DOI] [PubMed] [Google Scholar]
- Kang S, Kadam S. Pre-Clinical Models of Acquired Neonatal Seizures: Differential Effects of Injury on Function of Chloride Co-Transporters. Austin J Cerebrovasc Dis Stroke. 2014;1 [PMC free article] [PubMed] [Google Scholar]
- Kang SK, Kadam SD. Neonatal Seizures: Impact on Neurodevelopmental Outcomes. Front Pediatr. 2015;3:101. doi: 10.3389/fped.2015.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SK, Markowitz GJ, Kim ST, Johnston MV, Kadam SD. Age- and sex-dependent susceptibility to phenobarbital-resistant neonatal seizures: role of chloride co-transporters. Front Cell Neurosci. 2015;9:173. doi: 10.3389/fncel.2015.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharoshankaya L, Stevenson NJ, Livingstone V, Murray DM, Murphy BP, Ahearne CE, Boylan GB. Seizure burden and neurodevelopmental outcome in neonates with hypoxic-ischemic encephalopathy. Dev Med Child Neurol. 2016 doi: 10.1111/dmcn.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JM, Guillet R, Shankaran S, Laptook AR, McDonald SA, Ehrenkranz RA, Tyson JE, O'Shea TM, Goldberg RN, Donovan EF, Fanaroff AA, Poole WK, Higgins RD, Walsh MC. Clinical seizures in neonatal hypoxic-ischemic encephalopathy have no independent impact on neurodevelopmental outcome: secondary analyses of data from the neonatal research network hypothermia trial. J Child Neurol. 2011;26:322–328. doi: 10.1177/0883073810380915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney KJ, Cape EG, Gotman J, Jones BE. High-frequency electroencephalogram activity in association with sleep-wake states and spontaneous behaviors in the rat. Neuroscience. 1997;76:541–555. doi: 10.1016/s0306-4522(96)00298-9. [DOI] [PubMed] [Google Scholar]
- Milikovsky DZ, Weissberg I, Kamintsky L, Lippmann K, Schefenbauer O, Frigerio F, Rizzi M, Sheintuch L, Zelig D, Ofer J, Vezzani A, Friedman A. Electrocorticographic Dynamics as a Novel Biomarker in Five Models of Epileptogenesis. The Journal of Neuroscience. 2017;37:4450. doi: 10.1523/JNEUROSCI.2446-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita Y, Callicott JH, Testa LR, Mighdoll MI, Dickinson D, Chen Q, Tao R, Lipska BK, Kolachana B, Law AJ, Ye T, Straub RE, Weinberger DR, Kleinman JE, Hyde TM. Characteristics of the cation cotransporter NKCC1 in human brain: alternate transcripts, expression in development, and potential relationships to brain function and schizophrenia. J Neurosci. 2014;34:4929–4940. doi: 10.1523/JNEUROSCI.1423-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray DM, O'Connor CM, Ryan CA, Korotchikova I, Boylan GB. Early EEG Grade and Outcome at 5 Years After Mild Neonatal Hypoxic Ischemic Encephalopathy. Pediatrics. 2016 doi: 10.1542/peds.2016-0659. [DOI] [PubMed] [Google Scholar]
- Nadler JJ, Moy SS, Dold G, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN. Automated apparatus for quantitation of social approach behaviors in mice. Genes, Brain and Behavior. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- Pisani F, Spagnoli C. Neonatal Seizures: A Review of Outcomes and Outcome Predictors. Neuropediatrics. 2016;47:12–19. doi: 10.1055/s-0035-1567873. [DOI] [PubMed] [Google Scholar]
- Putman P, Verkuil B, Arias-Garcia E, Pantazi I, van Schie C. EEG theta/beta ratio as a potential biomarker for attentional control and resilience against deleterious effects of stress on attention. Cognitive, Affective, & Behavioral Neuroscience. 2014;14:782–791. doi: 10.3758/s13415-013-0238-7. [DOI] [PubMed] [Google Scholar]
- Richdale AL, Baker EK. Sleep in Individuals with an Intellectual or Developmental Disability: Recent Research Reports. Current Developmental Disorders Reports. 2014;1:74–85. [Google Scholar]
- Richter SH, Garner JP, Auer C, Kunert J, Wurbel H. Systematic variation improves reproducibility of animal experiments. Nat Meth. 2010;7:167–168. doi: 10.1038/nmeth0310-167. [DOI] [PubMed] [Google Scholar]
- Rocamora R, Sanchez-Alvarez JC, Salas-Puig J. The relationship between sleep and epilepsy. Neurologist. 2008;14:S35–S43. doi: 10.1097/01.nrl.0000340790.15295.59. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Alvarez N, Jimenez-Mateos EM, Dunleavy M, Waddington JL, Boylan GB, Henshall DC. Effects of hypoxia-induced neonatal seizures on acute hippocampal injury and later-life seizure susceptibility and anxiety-related behavior in mice. Neurobiology of Disease. 2015;83:100–114. doi: 10.1016/j.nbd.2015.08.023. [DOI] [PubMed] [Google Scholar]
- Roth T. Slow Wave Sleep: Does it Matter? J Clin Sleep Med. 2009;5:S4–S5. [PMC free article] [PubMed] [Google Scholar]
- Schutter DJLG, Van Honk J. Electrophysiological ratio markers for the balance between reward and punishment. Cognitive Brain Research. 2005;24:685–690. doi: 10.1016/j.cogbrainres.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Sorge RE, et al. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Meth. 2014;11:629–632. doi: 10.1038/nmeth.2935. [DOI] [PubMed] [Google Scholar]
- Stodberg T, et al. Mutations in SLC12A5 in epilepsy of infancy with migrating focal seizures. Nat Commun. 2015;6:8038. doi: 10.1038/ncomms9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekgul H, Gauvreau K, Soul J, Murphy L, Robertson R, Stewart J, Volpe J, Bourgeois B, Du Plessis AJ. The current etiologic profile and neurodevelopmental outcome of seizures in term newborn infants. Pediatrics. 2006;117:1270–1280. doi: 10.1542/peds.2005-1178. [DOI] [PubMed] [Google Scholar]
- Trapletti A, Hornik K. tseries: Time Series Analysis and Computational Finance. R package version 0. 2013:10–32. [Google Scholar]
- Vasudevan C, Levene M. Epidemiology and aetiology of neonatal seizures. Semin Fetal Neonatal Med. 2013;18:185–191. doi: 10.1016/j.siny.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Neurology of the newborn. Major Probl Clin Pediatr. 2001;22:1–648. [PubMed] [Google Scholar]
- Weeke LC, Boylan GB, Pressler RM, Hallberg B, Blennow M, Toet MC, Groenendaal F, De Vries LS. Role of EEG background activity, seizure burden and MRI in predicting neurodevelopmental outcome in full-term infants with hypoxic-ischaemic encephalopathy in the era of therapeutic hypothermia. Eur J Paediatr Neurol. 2016;20:855–864. doi: 10.1016/j.ejpn.2016.06.003. [DOI] [PubMed] [Google Scholar]
- Zeileis A, Grothendieck G. zoo: S3 Infrastructure for Regular and Irregular Time Series. Journal of Statistical Software. 2005;1(6) 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Western blot analyses for KCC2, A. KCC2 expression levels in the superficial gray matter were not different among groups (One-way ANOVA: F=0.673, p=0.57 for left and F=1.126, p=0.35 for right; n=14, 9, and 6 for sham, P7, and P10 respectively).
Supplementary Figure 2. EEG trace shows a spontaneous tonic-clonic seizure occurring in 4-month-old mouse that developed epilepsy following transient ischemic neonatal seizures at P7. Image (inset) shows tonic posture of seizing mouse at time point denoted by black bar on the EEG trace. Epileptiform EEG discharges were detected in 24h EEGs of other mice with history of neonatal seizures (n=8). Control mice did not show any epileptiform activity at 4 months.


