Abstract
Previous findings that skill learning is associated with the formation and preferential stabilization of new dendritic spines in cortex have raised the possibility that this preferential stabilization is a mechanism for lasting skill memory. We investigated this possibility in adult mice using in vivo two-photon imaging to monitor spine dynamics on superficial apical dendrites of layer V pyramidal neurons in motor cortex during manual skill learning. Spine formation increased over the first 3 days of training on a skilled reaching task, followed by increased spine elimination. A greater proportion of spines formed during the first 3 training days were lost if training stopped after 3, compared with 15 days. However, performance gains achieved in 3 training days persisted, indicating that preferential new spine stabilization was non-essential for skill retention. Consistent with a role in ongoing skill refinement, the persistence of spines formed early in training strongly predicted performance improvements. Finally, while we observed no net spine density change on superficial dendrites, the density of spines on deeper apical branches of the same neuronal population was increased regardless of training duration, suggestive of a potential role in the retention of the initial skill memory. Together, these results indicate dendritic subpopulation-dependent variation in spine structural responses to skill learning, which potentially reflect distinct contributions to the refinement and retention of newly acquired motor skills.
Keywords: dendritic subpopulations, in vivo imaging, motor-skill learning, spine dynamics
1. Introduction
While it is generally well accepted that major behavioral changes must reflect altered neural activity patterns, the nature of the synaptic changes supporting these alterations is poorly resolved (Chen et al., 2014; Gibson & Olive, 2017; Kolb & Whishaw, 1998; Makino et al., 2016). Repeatedly imaging synaptic elements over time in vivo, including the dendritic spines which form the majority of excitatory synapses in the brain (Bourne & Harris, 2008; Harms & Dunavesky, 2007; Yuste & Bonhoeffer, 2004; Yuste & Denk 1995) is powerful for revealing interrelationships between synaptic and behavioral change. Using transcranial two-photon imaging in transgenic mice expressing fluorescent proteins in cortical neurons, spine dynamics on superficial dendrites within the first several hundred microns of cortex can be monitored over time as an animal learns. In naive adult mouse cortex, spines are remarkably stable, with as little as 10% of spines turning over within a two-week period (Grutzlender et al., 2002). The extent of structural stability is thought to reflect the stability of synaptic connections in the mature brain. However, mice retain the ability to learn new tasks throughout their lifespan (Tennant & Jones, 2012; Whishaw, 1998), and this new learning presumably reflects modifications in synaptic connections. Motor skill learning, even in aged mice, results in reorganization of movement representations in motor cortex (Tennant & Jones, 2012). Similarly, in adult rats, regions in which movement representations have reorganized in response to motor skill training have been found to have increased quantities of synapses in layer V compared with untrained controls (Kleim et al., 2002).
Previous studies have examined how motor skill training affects synaptic plasticity in the motor cortex over time in vivo (Chen & Zuo, 2014; Fu et al., 2012; Harms et al., 2008; Padmashiri et al., 2013; Reiner & Dunavesky, 2015; Xu et al., 2009; Yang et al., 2009). Training mice on a novel manual (skilled reaching) task promotes immediate formation followed by selective elimination of dendritic spines on the apical branches of layer V pyramidal neurons in the motor cortex contralateral to the trained forelimb (Xu et al., 2009). A similar pattern of increased spine formation followed by increased elimination has been found in mice trained on an accelerating rotorod (Yang et al., 2009; Yang et al., 2014). Continued training on either task is associated with an increase in the proportion of new spines that persist compared with new spines in untrained controls (Xu et al., 2009; Yang et al., 2009). These findings have raised the possibility that the preferential stabilization of the spines that are formed during learning is a mechanism for the long-term retention of motor skills (Xu et al., 2009; Yang et al., 2009), but this possibility had not been directly tested. The main goal of the present study was to test the hypothesis that the long-term retention of motor skills is dependent upon the preferential stabilization of apical dendritic spines that are formed during the process of acquiring those skills.
An additional goal of the present study was to investigate the possibility that patterns of spine change may vary between superficial and deeper dendrites. Numerous examinations of spine turnover on superficial dendrites in various regions of the cortex have yet to reveal net changes in spine density over time (Holtmatt et al., 2006; Trachtenberg et al., 2002; Xu et al., 2008; Yang et al., 2009). This contradicts findings of learning-related changes in spine densities on various dendritic populations of Golgi-stained pyramidal neurons in the motor cortex of rats (Greenough & Withers, 1985; Withers & Greenough, 1989; Wang et al., 2012) and nonhuman primates (Kleim et al., 1997), as well as transmission electron microscopy evidence of increases in synapse quantities in the same species (Adkins et al., 2009; Jones et al., 1997; Kleim et al., 1998; Keim et al., 2002; Kleim et al., 2003). Besides the species differences, a major difference between these two sets of studies has been the dendritic subpopulations examined. Most in vivo analyses of spine density have been restricted to the first several hundred microns of superficial cortex, which contains fanlike dendritic arbors of pyramidal neurons called apical tufts that extend into layer I of cortex. Most histological studies have focused on deeper dendrites, primarily those located within layers II/III and V. We therefore sought to clarify whether spines on different subpopulations of dendrites of the same neuronal population respond differently to manual skill learning.
2. Methods
2.1. Subjects
Eighteen male C57/BL6 Yellow Fluorescent Protein (YFP)-H line transgenic mice (B6/Cg-Tg (thy-1 YFPH) 2Jrs/J) expressing YFP in a subset of layer five pyramidal neurons (Feng et al., 2000) and 5 male wild type C57/BL6 mice were used. All animals were bred at the Animal Resource Center at the University of Texas at Austin (ARC) and were between 4 and 5 months old at the time of cranial window implantation (M ± SE weight, 24.69 ± 0.64g). Mice were placed into one of three groups that underwent: (1) training for 15 consecutive days (Trained 15D, n = 5), (2) training that stopped after 3 days (Trained 3D, n = 5) or (3) no-training control procedures (Control, n=5). The timeline of experimental procedures is summarized in Figure 1.
Figure 1. Experimental approach.
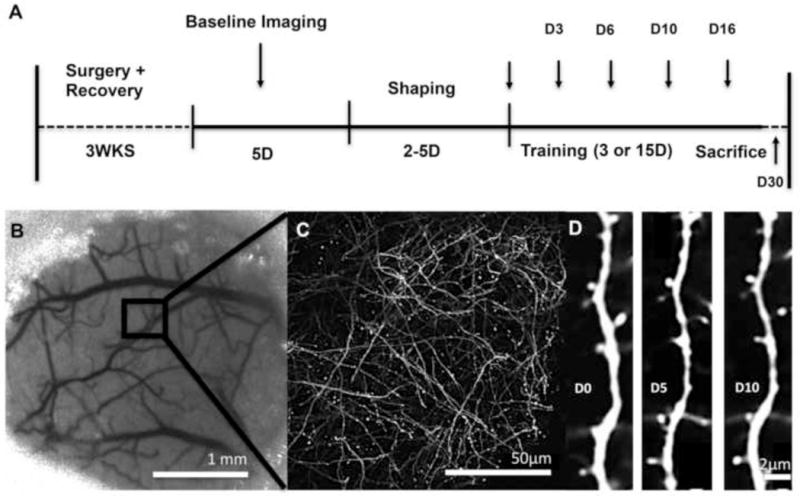
(A) Experimental timeline. (B) Sample region within cranial window. (C) Low magnification image of YFP fluorescence. (D) High magnification time-lapse imaging of an individual dendritic branch within the sample region of an untrained control
Three mice with extremely dense labeling of YFP and two mice that died during early imaging sessions were omitted from the experiment. Of the remaining 15 YFP expressing mice, data from the last imaging session of 3 mice (n = 1 per group) were not available due to issues with window clarity or altered fluorescence. The wild type mice were used to corroborate the behavioral results of the Trained 3D condition. Mice were housed in groups of two to four on a 12:12 hour light/dark cycle and received food and water ad libitum prior to behavioral testing. Each cage had standardized supplementation including wooden toys, bedding and polyvinyl chloride pipes which were replaced weekly. During behavioral procedures, mice were placed on scheduled feeding (2.5-3g food once per day) to avoid satiation during behavioral training. Body weights were not permitted to fall below 90% of free feeding weights (M ± SE body weight: 21.91 ± 0.49g, over the experimental time course). All animal use was in accordance with a protocol approved by the Animal Care and Use Committee of the University of Texas at Austin.
2.2. Cranial Window Implantation
Mice were anesthetized with ketamine (4 mg/kg, i.p.) and xylazine (3 mg/kg, i.p.). Dexamethasone (2 mg/kg, i.p.) and carprofen (2.5 mg/kg, i.p.) were administered to help minimize cortical swelling during surgical procedures. Booster injections of ketamine (50 mg/kg) were given as needed to maintain anesthetic plane. Following midline incision, a 3 mm circular region was thinned using a high-speed dental drill and a 0.5mm drill bit, and skull was removed leaving dura intact. Saline was frequently applied to protect the brain from overheating. Skull was then replaced with a thin No. 1 coverglass (Warner Instruments, #64-0720) and sealed with cyanoacrilade (3M Vetbond Tissue Adhesive, #1469) and dental cement. All windows were made over the forelimb area of the right motor cortex as defined previously from intracortical microstimulation mapping experiments (Tennant et al., 2011). Briefly, windows were placed approximately 2 mm rostral to bregma and 0.5 mm lateral to midline. Following surgery animals were given buprenorphine (3 mg/kg, s.c.) for pain management and allowed to recover in their cage for three weeks with food and water ad libitum prior to behavioral procedures. For the first week, animals were given daily injections of carprofen (2.5 mg/kg, i.p.) to help minimize inflammation following surgery. A three week recovery period was chosen to ensure that spine dynamics were unaffected by any residual inflammation from surgery (Xu et al., 2007).
2.3. Behavioral Training
Mice were trained on a skilled reaching task, the Pasta Matrix Reaching Task, as previously described (Ballermann et al., 2001; Tennant & Jones, 2012). Briefly, mice were trained to reach for and break small pieces of uncooked capellini pasta (3.2 cm in height and 1 mm diameter; DeCecco brand, Fratelli De Cecco di Filippo Fara San Martino S.p.A., Italy), arranged vertically in a matrix outside of the chamber opening (Fig. 2). In order to successfully retrieve a pasta piece, mice needed to reach outside of the chamber opening, grasp the pasta and break it by pulling it. Pasta pieces were not replaced once broken, such that on each successive attempt animals were required to change the reach trajectory in order to obtain the next piece of pasta.
Figure 2. The Pasta Matrix Reaching Task.
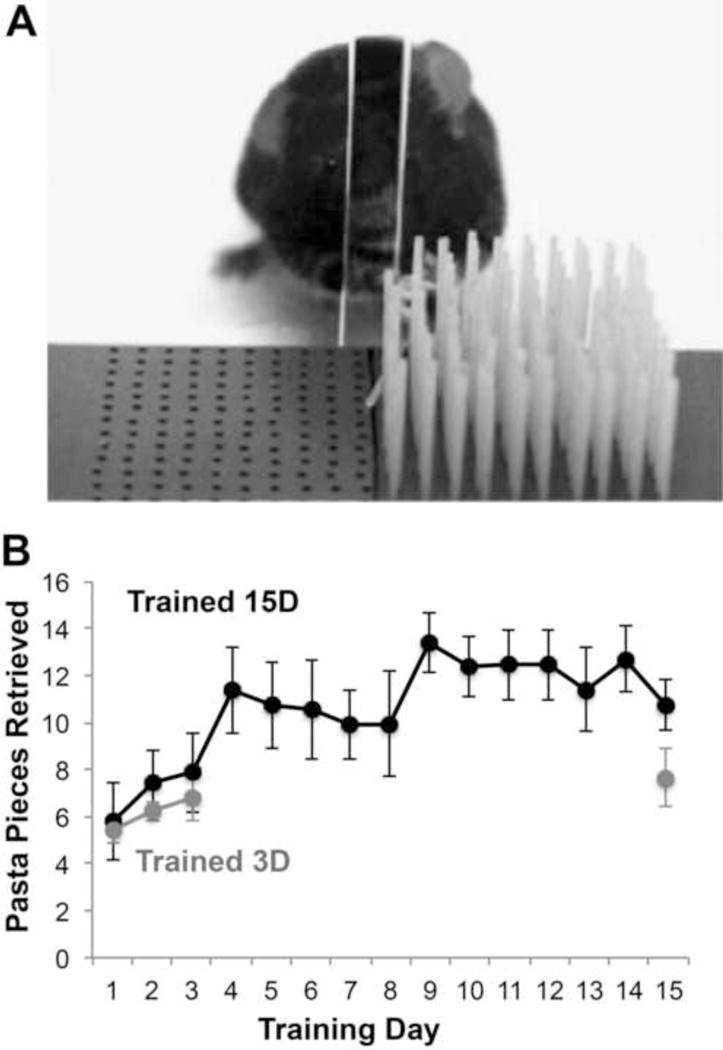
(A) Animals learned to reach for pasta arranged in a 10 × 10 matrix through an opening outside of a plexiglass chamber. (B) Performance on the pasta matrix task in animals trained for either 15 days (Trained 15D) or 3 days (Trained 3D). Data are M ± SE. See also Supplementary Figures 1–3.
Reaching practice was restricted to the animal’s left forelimb, contralateral to cranial window implantation. For shaping, animals were given small pieces of pasta on the chamber floor, too small for pasta handling (Xu et al., 2009) and encouraged to reach towards a matrix of pasta that was placed just outside the chamber opening. To ensure that animals did not reach with their right forelimb, pasta was arranged in matrix positions on the right hand side of the chamber opening only. Given that practice in pasta handling alone can increase spine turnover in motor cortex of young animals (Xu et al., 2009), a strict criterion for the cessation of shaping was used to minimize its contribution to training effects on spine turnover. Training commenced once the animal made a total of five reach attempts or was able to break two pieces of pasta.
Training procedures lasted for either 3 (Trained 3D) or 15 days (Trained 15D). Each day, mice were placed in the chamber for either 15 minutes or until they made 100 reach attempts. Mice in the Trained 3D condition received control procedures for the remaining eleven days, followed by a final test on day 15 to probe for reaching performance. Control procedures consisted of placing animals into reaching chambers without a pasta matrix, but with small pasta pieces (too small for pasta handling) on the chamber floor at the same time and for the same duration that a yoked mouse was being trained on the Pasta Matrix Reaching Task (Tennant & Jones, 2012).
2.4. In Vivo Imaging of Dendrites
Animals were anesthetized with 1.5-2.5% isoflurane and inserted into a custom made stereotaxic apparatus fitted with a headbar to minimize breathing artifact. All images were gathered within the caudal forelimb area (CFA) of the motor cortex defined in a previous intracortical microstimulation study (Tennant & Jones, 2011). The localization of sample sites across imaging sessions was guided by superficial vascular landmarks (Fig. 1B). Images were acquired using a Prairie Ultima standard two-photon microscope with a Ti:Sapphire laser tuned to 920 nm (YFP) at low laser power (~30 mW) to minimize phototoxicity. Laser power was adjusted through a Pockels cell in order to obtain near identical fluorescence at each imaging location and across imaging days. High-resolution three dimensional image stacks (Fig. 1D) were gathered between 50 and 200 μm from the pial surface using a water immersion objective (40×, 0.8 NA, Olympus) and a digital zoom of 4×. Image stacks consisted of 150-200 optical sections spaced 0.7 μm apart covering an area of 240 μm × 240 μm (512 × 512 pixels, 0.13 μm/pixel). Four to five image stacks spaced 250 μm apart in any direction and containing at least ten dendrites with visible spines were obtained for each animal. Animals received two baseline imaging sessions spaced five days apart, with the second imaging session following the second shaping day but prior to the onset of formal behavioral training. Animals were imaged on training days 3, 6, 10 and one day following the final training session (on day 16).
2.5. Spine Dynamics Analyses
All analyses of dendritic spine turnover and spine density were performed blind to experimental condition. For analysis of in vivo spine dynamics a total of 8-10 dendritic segments per image stack, each at least 20 μm in length, were analyzed (~150-250 spines per animal) using ImageJ. (Rasband, 1997–2016). Spines were considered to be the same between imaging sessions based on their relative position to adjacent dendrites and spines. Only dendrites parallel to the imaging plane in both views were used for analyses to minimize possible rotational artifacts, and dendrites containing saturated pixels were excluded. If the distance between a given spine and adjacent landmark was more than 0.7 μm from its relative position in the previous image, it was counted as different, a distance chosen based on both the resolution of the two-photon microscope (~0.7 μm) and previous reports that spines can move up to 0.3 μm in either direction due to changes in spine morphology, slight rotation of the head, or breathing artifact (Grutzlender et al., 2002). Spine turnover was measured by comparing dendritic protrusions in the image being analyzed to those in the previous imaging session. A spine was counted as stable if it appeared in both the previous imaging session and the one being analyzed, as newly formed if it was only present in the image being analyzed, and as eliminated if it was visible in the previous imaging session but not the one being analyzed. Filopodia, defined as long dendritic protrusions with no head, were rarely observed and excluded from analyses of spine turnover (Padmashri et al., 2013). The percentage of spine formation and elimination was calculated as the number of spines gained or lost divided by the total number of stable spines in the analyzed imaging session (Xu et al., 2009). Analyses were performed on raw unprocessed image stacks but for presentation purposes, images are shown as maximum intensity projections consisting of 5-10 optical sections with median and Gaussian filters applied.
2.6. Histological Preparation and Spine Density Analysis
Mice were overdosed with a lethal injection of sodium pentobarbital and transcardially perfused with 0.1M phosphate buffer (PB) saline and 4% paraformaldehyde two weeks following the final imaging time point. The two week delay was to ensure that any training effects on spine density could conceivably be related to skill retention. Coronal sections (50 μm) were cut using a Leica VT1000S vibratome and mounted on slides for subsequent analysis of spine density on YFP+ pyramidal neurons using confocal microscopy. Image stacks were acquired using the confocal mode on the two-photon microscope. The dichroic mirror was replaced with a lens tuned to 488nm (FITC) and image stacks containing apical dendritic branches within layer I (≤200 μm from the surface) and layer II/III (between 200-400 μm from the surface) of motor cortex were gathered using a water immersion objective (40×, 0.8 NA; Olympus). All spine density analyses were performed using Image J software. For layer II/III dendrites, a total of ten apical dendrites measuring at least 50 μm in length within cytoarchitectural motor cortex boundaries were sampled per hemisphere in each animal (Tennant et al., 2011). For layer 1 dendrites (the sample region for in vivo imaging) we took a slightly more liberal approach due to observed mechanical damage (presumably from sectioning) of this sampling region in several animals. Between 8-10 dendrites per hemisphere per animal measuring at least 35 μm in length were analyzed. Data from one animal in the Trained 3D group had to be omitted from spine density analyses due to tissue damage incurred at the time of extraction. Data from an additional 3 animals (n = 2 Trained 15D, n = 1 Trained 3D) had to be omitted from the layer 1 measures due to an inability to localize a sufficient sample of intact dendrites of this length. For all dendritic analyses, spines along the length of measured dendrite were manually counted, and density was calculated as total spines per length of dendrite.
2.7. Statistical Analyses
All statistical analyses were performed using the SPSS software package. Paired and independent samples t-tests were used for within and between group examinations of behavioral performance on the pasta matrix reaching task respectively. Repeated measures analyses of variance (ANOVAs) were used to examine group differences in spine measures over time in trained animals versus controls and between the two training durations, and the Shapiro-Wilks test was used to check for normality. When warranted by significant Group by Day interactions, post hoc group comparisons per time point were performed using Holm-Bonferroni corrected t-tests. The last imaging time point (Day 16) was omitted from the ANOVAs because of animal attrition at this time point, and instead, within-animal comparisons between Day 16 and pre-training time points were analyzed with t-tests. Day 16 was also counted as a comparison in the Bonferroni-Holm’s correction when post-hoc tests per time point were warranted by ANOVA results. Pearson correlations were used to probe for relationships between behavioral and new spine survival. Paired t-tests were used to probe for within-animal differences in layer II/III spine density in trained compared to untrained motor cortices.
3. Results
3.1 Manual Skill Training Increased Dendritic Spine Turnover
In adult mice, spines along the superficial dendrites of YFP-labeled pyramidal neurons have been found to be remarkably stable, with only ~3% of spines forming and disappearing over a one-week period (Grutzendler et al., 2002). We found that over 3 days of training on a skilled reaching task (Fig. 2) spine formation in adult animals more than doubled compared to baseline (Fig. 3A). Prior to training, the M ± SE percentage of new-to-pre-existing spines was 3.0 ± 0.3, which increased to 7.2 ± 0.3 after 3 days of training, whereas in untrained controls there was minimal change in the percentage of new-to-pre-existing across the same time points (3.2 ± 1.3 and 3.0 ± 1.2, respectively; Fig. 3B). The increase in spine formation in trained animals was followed by an equal and opposite increase in spine elimination by day 6 of training. Spine formation and elimination returned to baseline levels for the remainder of the training period, and there was no net change in spine density by either of the final two imaging sessions. Animals that received no-training control procedures showed minimal changes in spine turnover across the same imaging intervals (Fig. 3B). In repeated measures ANOVAs for the Trained 15D group compared with Controls, there was a main effect of time on both spine formation (F(4,24) = 11.85, p < .001) and spine elimination (F(4, 24) = 12.99, p < .001), as well as a significant group by time interaction for both (formation: F(4, 24) = 13.05, elimination: F(4, 24) = 13.05, p’s < .001), indicating that group differences depended on the time point.
Figure 3. Skill training increased spine formation and subsequent elimination in trained motor cortex.
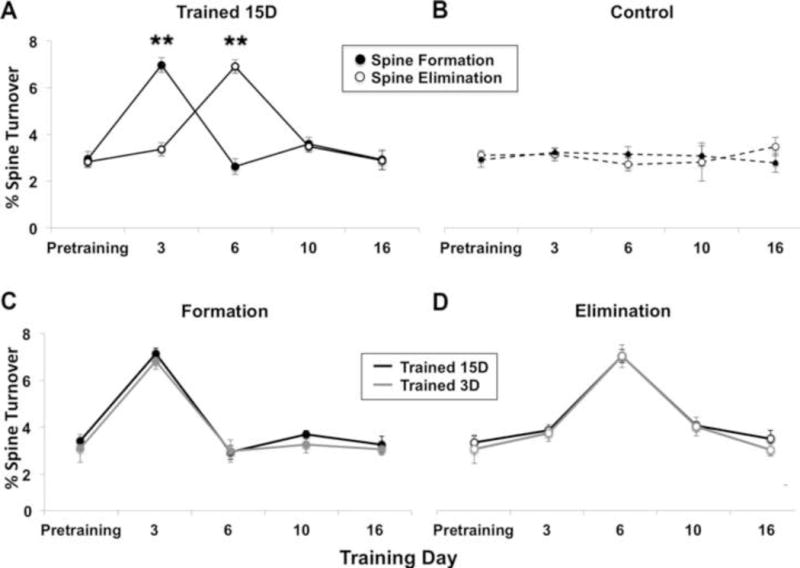
(A) M ± SE rate of spine turnover throughout the period of training on the pasta matrix task. In animals trained for 15 days (Trained 15D), spine formation increased over 3 days of training compared to baseline (**p < .001) and was followed by an equal and opposite increase in spine elimination by day 6 (**p < .001 versus baseline). Turnover rates returned to baseline levels by day 10. (B) There was little variation in the rate of spine turnover during the same time course in untrained controls. (C-D) The pattern and rate of spine turnover was comparable in animals that were trained for 3 days (Trained 3D) and 15 days. The data from the Trained 15D group in (C) and (D) are the same as that shown in (A).
Among the groups receiving either 3 or 15 days of training, behavioral performance in the first three days of training was similar (Fig. 2B) as were patterns of spine turnover (Fig. 3C and 3D). There was a significant main effect of time on both formation (F(4, 24) = 36.39 p < .001) and elimination (F(4,24) = 51.34, p < .001) but no group by time interaction (Fig 3B, Formation: F(4,24) = 0.143; Elimination: F(4,24) > 1.97, p’s > 0.05), indicating that both groups show similar rates of spine turnover across time points.
Similar results have been found in adolescent mice receiving training on a skilled reaching task; however the rates of spine turnover were much higher and increases were observed as quickly as one hour after the first training session (Xu et al., 2009). We imaged a subset of trained animals (n=3) after the first day of training and found no change in spine turnover compared to baseline turnover rates (t(2) = 3.00, p > .05; M ± SE % turnover at baseline: 3.50 ± 0.15 formation, 2.77 ± 0.58 elimination; on training day 1: 3.27 ± 0.15 formation, 3.28 ± 0.14 elimination). Together, these results indicate that novel manual skill learning produces similar, albeit of lower magnitude and slower in time course, alterations in spine turnover on the superficial dendritic branches of layer V pyramidal neurons in the motor cortex of adults as it does in juveniles.
3.2 Preferential Stabilization of New Spines, but Not Newly Learned Skill, Depended on Continued Training
Spines formed during acquisition of a fine motor skill task in juveniles have been found to persist in much higher proportions over a period of continued training than do new spines in untrained controls, consistent with the possibility that the preferential stabilization of newly formed spines is a mechanism that contributes to retention of newly acquired skill (Xu et al., 2009). We tracked the fates of newly formed spines (Fig. 4A) and found that in animals with ongoing training new spines that appeared by day 3 persisted in much greater proportions at later time points than did newly formed spines in controls over the same time span (Fig. 4B). In contrast, spines that were present prior to the onset of training were more likely to disappear over the same time period in trained animals than in controls (Fig. 4C). In both groups, new spines disappeared in greater proportions than pre-existing spines, but the relative proportion of new spines that persisted was doubled in animals with continued training (Fig. 4D). Repeated measures ANOVAs of the fates of new and pre-existing spines after day 3 revealed significant effects of Group (Trained 15D versus Control, F’s(1, 8) = 13.94 and 26.03, respectively, p’s < .001) but no Group by Time interactions. By day 16, the proportions of persisting new and pre-existing spines continued to be significantly greater in the Trained 15D group compared with controls (p’s < .03). These results support that, as with juveniles, fine motor skill learning in adults is associated with the preferential stabilization of spines that are formed early in the process of skill acquisition on the superficial dendrites of layer V pyramidal neurons.
Figure 4. Newly formed spines were preferentially stabilized by continued training.
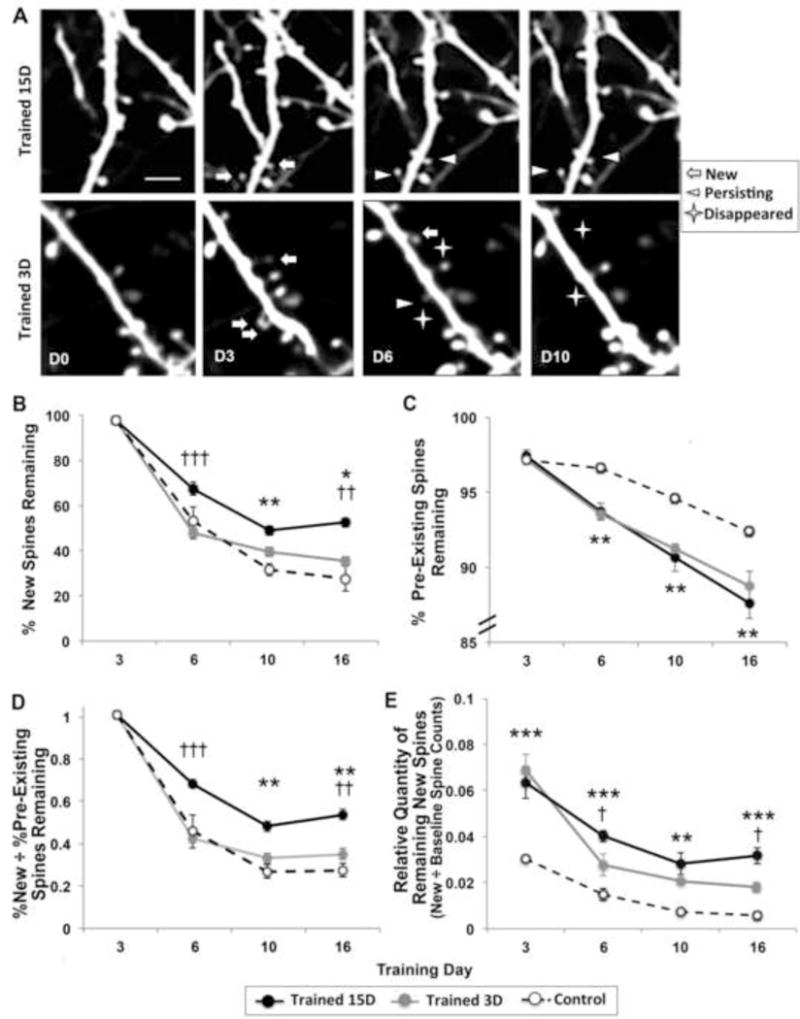
(A) Examples of spine dynamics from animals receiving 15 and 3 days of training. Similar rates of spine formation (“New”) were found across training conditions on day 3. On subsequent days newly formed spines were more likely to remain (“Persisting”) in animals with ongoing training and more likely to disappear in animals that stopped training. Scale bar = 2 μm. (B) The proportion of new spines generated by the third training day that persisted to the final imaging time point was significantly greater in the Trained 15D group compared to both controls and the Trained 3D group. (C). Pre-existing spines were more likely to disappear in Trained 15D versus Control, but the two training duration groups lost pre-existing spines in similar proportions that were not significantly different. Note the difference in scales between (B) and (C). (D) The Trained 15D group showed the greatest new spine persistence in proportion to pre-existing spine persistence compared to both Trained 3D and Control groups. (E) Despite high rates of new spine elimination, the Trained 3D group had a significantly increased relative quantity of persisting new spines compared with controls on days 10 and 16 (p’s < .017), though these quantities were diminished relative to the Trained 15D group. *p < .017, **p < .005, ***p < .0005, Trained 15D vs. Control; †p < .017, ††p < .0005, †††p < .0005, Trained 15D vs. Trained 3D. Data are M ± SE.
We next asked whether continued training was necessary for the preferential stabilization of newly formed spines. In a subset of animals, we stopped training procedures after day 3 (Trained 3D), the peak of spine formation, and followed the fates of newly formed spines over the next two weeks (Fig. 4A). The percentage of new spines that remained after day 3 was greatly reduced in animals that stopped training compared to those with continued training (ANOVA Trained 15D vs. Trained 3D Group effect: F(1, 8) = 70.94, p < .0001). The Trained 3D group showed a similar pattern of increased spine formation followed by increased spine elimination (Fig. 3C and 3D), including increased elimination of pre-existing spines (Fig. 4C), as those trained for 15 days, but in the absence of further training, the newly formed spines were not preferentially stabilized, disappearing at very high rates similar to controls (Fig. 4B). These results support that the preferential stabilization of new spines that are generated early in training is dependent on continued skill practice.
Unlike the stabilization of new spines, the persistence of skills learned in the early training period was not dependent on continued practice. When tested after 12 days of no-training control procedures, briefly trained mice performed as well as they did on day 3 as measured by the number of pasta pieces retrieved (Fig. 2B; t(4) = 2.78, p = .24). Performance improvements similarly endured in a group of wild type mice that underwent the same brief training procedure (Suppl. Fig. 1). While the difference between the two training groups at day 15 in the number of pasta pieces retrieved failed to reach significance (t(8) = 2.30, p = .062), the efficiency with which mice retrieved pasta, as measured by the % retrievals per reach attempt, was significantly improved with continued training (Suppl. Figs. 2–3). Nevertheless, gains in reaching efficiency that were achieved by the third training day also endured in the absence of continued training (Suppl. Fig. 3B). These results indicate that the persistence of skill learned during the early training period did not depend on the preferential stabilization of spines that were formed during that period.
3.3. Training Increased the Population of Persisting New Spines Without Preferential Stabilization
The major increase in spine formation in the first three training days could be expected to increase the total population of persisting new spines relative to controls even in the absence of their preferential stabilization. We estimated new spine quantities as a relative factor of total spines counted at baseline to account for variability in the number of spines sampled between animals (Fig. 4E). Mice with 3 days of training had relative quantities of persisting new spines that were intermediate between those of controls and mice with 15 days of training. In comparing mice with 3 versus 15 days of training, there was a significant effect of Group on the relative quantities of new spines that persisted on days 6 and 10 (F(1, 8) = 5.51, p = .04). By day 16, the relative quantities of new spines continued to be significantly increased in the Trained 15D group compared with the Trained 3D group (p = .027). Both training durations, however, had increased relative quantities of persisting new spines compared with controls (ANOVA Group effects, Trained 15D vs. Control: F(1, 8) = 35.41, p < .001, Trained 3D vs. Control: F(1,8) = 31.90, p <.001). Thus, while the present results indicate that the endurance of newly learned skill cannot be attributed to preferential stabilization of new spines, it remains possible that the persistence, after training stopped, of a modest proportion of the new spines that were formed early in training contributed to the endurance of skills learned in the same time period.
3.4. Persisting New Spines Predicted Performance Gains
To probe whether preferential new spine stabilization was associated behavioral improvement, we analyzed the relationship between the proportion of persisting new spines and performance gains between days 3 and 15, as measured by the difference in pasta pieces broken. Performance gains were positively correlated with the % of new spines formed on day 3 that were still present on days 10 (r = 0.82, t(8) = 4.18, p = .015; Fig. 5A) and 16 (r = 0.87, t(6) = 4.33, p = .004), indicating that the stabilization of the spines that were formed during the first 3 days of training is associated with subsequent improvements in behavioral performance. These performance gains were similarly correlated with relative quantities of new spines remaining on day 10 (r = 0.84, t(8) = 4.44, p = .002; Fig. 5B). These results indicate that both the preferential stabilization and the persisting quantity of new spines that are formed on superficial dendrites of layer V pyramidal neurons early in the process of acquiring a new motor skill are associated with skill refinement.
Figure 5. New spine stabilization predicted performance gains.
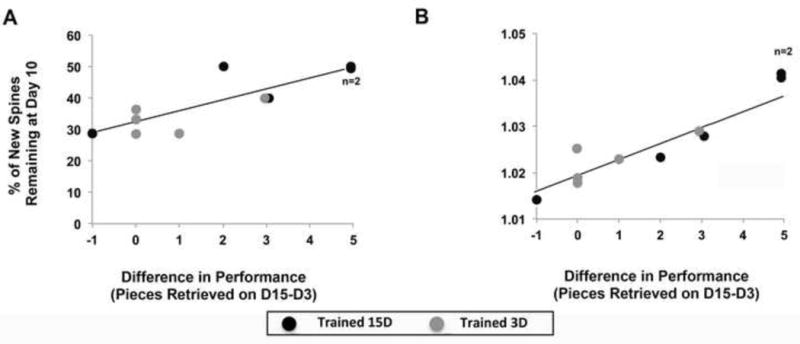
(A) Performance gains, measured by the difference in pasta retrieved on day 15 versus 3, positively correlate with the percentage of new persisting spines and (D) the relative quantities of new persisting spines at day 10 in both training groups.
3.5 Skills Training Induced Subpopulation-Specific Increases in Dendritic Spine Density
While training was associated with a significant increase in spine formation and subsequent elimination on the superficial apical branches of layer V pyramidal neurons in motor cortex in vivo, rates of formation and elimination were nearly equal, and thus no net change in spine density was observed. The absence of net changes in spine quantities on these dendrites has been consistently reported in in vivo imaging studies (Grutzlendler et al., 2002; Trachtenberg et al., 2001; Zuo et al., 2005) but it contradicts reports of training induced alterations in spine density on dendrites that are deeper in the cortex (e.g., Greenough & Withers, 1985; Withers & Greenough, 1989; Wang et al., 2012). We examined the possibility that this contradiction reflects dendritic subpopulation-dependent variation. In the same mice imaged in vivo, we histologically examined spine density on the apical branches of YFP expressing layer V pyramidal neurons within layers I and layer II/III of motor cortex using confocal microscopy (Fig. 6A and B). Consistent with the in vivo results, spine density in layer I was found to be similar in the trained and untrained hemispheres of both groups (Fig. 6C; Trained 15D, t(2) = 4.30, p =.49; Trained 3D, t(2) = 4.30, p = .90). In contrast, spine density in layer II/III was significantly greater in the trained compared to the untrained hemisphere in both training groups (Fig. 6D; Trained 15D, t(4) = 2.78, p < .01; Trained 3D, t(3) = 2.41, p < .01). These results suggest that there are dendritic subpopulation-specific structural responses to training, which can explain differences between in vivo and fixed tissue results. That spine density was similarly increased in the Trained 3D group versus Trained 15D group (t(7) = 1.93, p = .98, Fig. 6D) is also generally consistent with the possibility that the net increases in spine density on this dendritic subpopulation could be a structural mechanism for long-term retention of the skill that was learned within 3 training sessions.
Figure 6. Brief and extended training increased spine densities on apical branches of layer V neurons in layer II/III but not layer I of trained motor cortex.
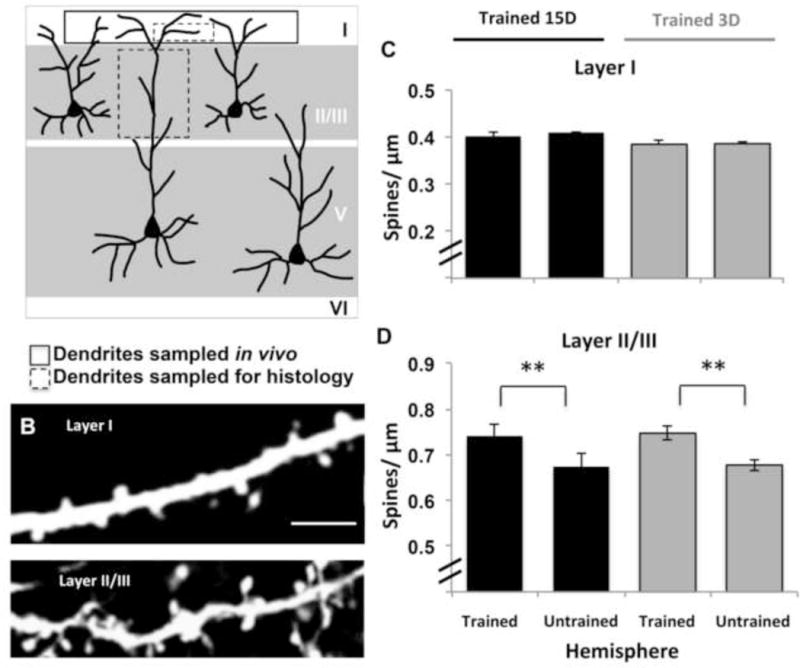
(A) Illustration of apical dendritic subpopulations of layer V pyramidal neurons that were sampled in vivo and histologically. (B) Confocal images of apical dendritic branches sampled from layer I and layer II/III in histological samples. Scale bar = 10 μm. (C) Consistent with in vivo results, spine densities on layer I dendrites were similar between trained and untrained hemispheres in Trained 15D (n = 3, p = .49) and Trained 3D (n = 3, p = .90) groups. (D) Spine density on layer II/III dendrites was increased in trained versus untrained motor cortex in both the Trained 15D (n = 5, **p = 0.002) and Trained 3D (n = 4, **p = .002) group. Data are M ± SE.
4. Discussion
Previous findings have raised the possibility that the preferential stabilization of new dendritic spines that are formed early in the process of learning a new motor skill in superficial motor cortex could be a mechanism contributing to lasting memory for that skill (Xu et al., 2009; Yang et al., 2009). Newly formed spines tend to be transient, disappearing in much greater proportions than do pre-existing spines (Chen et al., 2014). In mice trained in a skilled reaching task (Xu et al., 2009) or on an accelerating rotarod (Yang et al., 2009), the spines that are formed early in training during a period of increased spine turnover persist in greater proportions than do new spines in untrained controls, supporting that new spines are preferentially stabilized during motor skill learning. Whether this new population of stabilized spines was necessary for long-term retention of the newly acquired had not been determined. In the present study, by examining the maintenance of newly formed spines in mice with ongoing training compared to mice that were only briefly trained, we found that continued training is required for the preferential stabilization of spines that are formed in response to the initial training. In proportions and relative quantities, stabilized new spines were correlated with the magnitude of performance improvements between the third and final training sessions. However, the preferential stabilization of new spines could not have been essential for the retention of the new skill because mice in the briefly trained condition maintained the skill that they learned while losing new spines in proportions similar to untrained controls. A summary of these experimental results is shown in Figure 7.
Figure 7. Illustrative summary of experimental results.
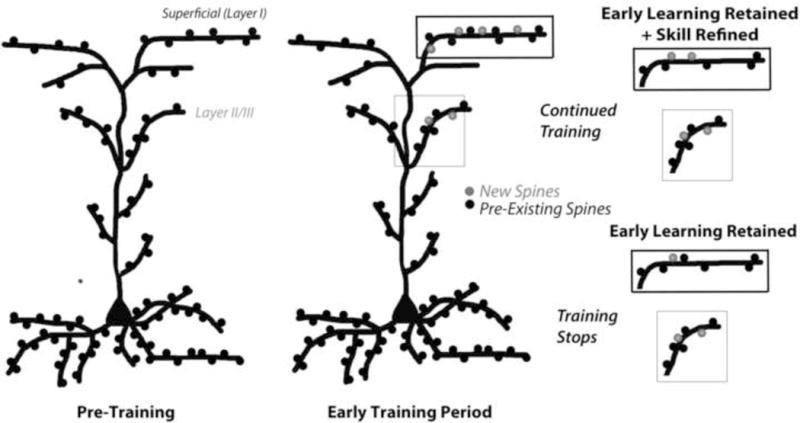
In adult mice, in the early phase of learning a new motor skill there are increases in spine formation on layer I apical branches in trained motor cortex. Upon continued training, the new spines are preferentially stabilized, which is predictive of performance improvements, and this occurs at the expense of pre-existing spines such that the relative spine density remains unchanged. In the absence of continued training, new spines are more likely to disappear, but the skill that was learned earlier does not. The early phase of learning also increases the density of spines on apical dendrites in layer II/III (although when that occurs is unknown) and this increase does not depend on continued training.
Motor skill learning involves the acquisition of complex movement combinations that are refined with practice and retained for long time periods (Luft & Buitrago, 2005). Across training sessions, motor skill learning curves are characterized by an early phase of rapid performance improvements followed by a period of more gradual improvements (Diedrichsen & Kornysheva, 2015; Karni et al., 1998; Kleim et al., 1996; Kleim et al., 2005; Luft & Buitrago, 2005). In the present study, the end of the brief training period was near the end of this early phase of rapid performance improvements (~ the first 4 days), which was also during the period of increased spine formation. This was followed in both training groups by an equal amount of spine elimination. However, in mice with continued training, new spines survived this elimination process in almost twice the proportion as did the new spines of mice that had ceased training. The stabilization of new spines during motor skill learning is associated with increases in their size (Fu et al., 2012), and spine size reflects the strength of the synapse (Hofer et al., 2009; Matsuzaki et al., 2001; Roberts et al., 2010;Yasumatsu et al., 2008). Thus, spine stabilization can reflect synapse maturation. Since the survival and maturation of synapses is neural activity-dependent (Katz & Shatz, 1996; Marrs et al., 2001; Star et al., 2002; Wong & Ghosh, 2002; Yasumatsu et al., 2008), it seems likely that the preferential survival of nascent spines only in mice with continued training reflects that these spines were sufficiently activated to promote the maturation of their synapses as the animals practiced, and continued to subtly improve performance in the reaching task.
Given its dependency on continued training and correlation with performance improvements, the overall pattern of the present results is consistent with a greater involvement of the preferential stabilization of new spines in ongoing skill refinement than in the memory of the skill that was learned as the new spines were formed. This obviously does not rule out that the modest proportion of new spines that persisted in the absence of continued training contributed to memory for skill learned in the early training period. It also does not rule out a contribution of the preferential stabilization of this population of spines in skill memory. In the process of learning motor skills, the more subtle performance improvements that occur after the early learning phase presumably reflect the accumulation of subtle refinements in movement strategy, which depend on retention of those refinements that were established before. It may very well be that the preferential stabilization of new spines is involved in this accumulated memory of skill refinements in the gradual learning phase.
About a third of new spines that appear during the early learning phase form in clusters, and such clusters are more likely to persist with prolonged training compared to individual spines (Fu et al., 2012). Formation of new spine clusters over 4 days depends on task practice, and successive new spines accompany strengthening of the first spine in the cluster (Fu et al., 2012). Thus, it may be the stabilization of the new spine clusters specifically, rather than newly formed spines in general, that is associated with improvements in motor performance. Consistent with this, fmr1 knockout mice (a fragile × syndrome model) have more limited performance improvements with ongoing motor training compared with wildtype mice (Padmashri et al., 2013) as well as reduced spine formation and clustering, but they do not have reductions in the proportions of new spines that are stabilized (Reiner & Dunavesky, 2015). Thus, the greater spine stabilization that was associated with performance improvements in our study was potentially mediated by repeated practice-dependent activation of newly formed spine clusters (Fu et al., 2012; Kasai et al., 2010).
We also found that spine changes varied between the superficial and deeper dendrites of layer V pyramidal neurons. Similar to previous studies (Holtmaat et al., 2006; Trachtenberg et al., 2002; Xu et al., 2009; Yang et al., 2009) we found no net change in spine density on the superficial apical dendrites of layer V pyramidal neurons imaged in vivo. Histological analyses of this superficial population of dendrites confirmed a lack of net change in spine densities between the trained and untrained hemispheres. In contrast, we found that spine density on deeper apical dendrites within layer II/III was increased in the trained compared with untrained motor cortex. These results indicate variation in the structural effects of skill learning even across nearby dendritic subpopulations of the same neuronal population. Such variation is not surprising in the context of abundant fixed-tissue evidence for laminar specific (Adkins et al., 2002; Gonzalez et al., 2005; Uylings et al., 1978; Wang et al., 2012) and neuron population specific (Uylings et al., 1978; Wang et al., 2012; Withers & Greenough, 1989) dendritic structural plasticity in response to various manipulations of behavioral experience in rats. Motor skill training in rats also affects the structure of different pyramidal neuron populations differently (Kleim, et al., 1997; Greenough & Withers, 1985; Withers & Greenough, 1989). Wang and colleagues (2012) have found that skilled reach training in rats induces distinct structural plasticity in different subsets of the same neuronal population (layer V apical versus basilar dendrites). The pattern of structural changes across subpopulations of pyramidal neurons and their dendrites also vary with different motor tasks (Kolb, 2008). Some differences in how spine dynamics on the superficial apical branches of layer II/III (Ma et al., 2016) versus layer V (Yang et al., 2014) pyramidal neurons of mice are affected by rotorod or treadmill training have also been reported. Thus, evidence from histological and in vivo studies converge to support that dendritic structural responses to skill learning vary across different dendritic subpopulations of motor cortex.
That spine densities within layer II/III were similarly increased in both training groups, as assessed two weeks after the final (Day 15) training session is generally consistent with the potential involvement of this structural change in the persistence of skill that was learned during the initial training period. However, because measurements of spine density were assessed histologically, the time course of the spine addition remains unknown, and it is also possible that there were earlier differences in spine densities between the two training groups that were missed. However, Gloor et al. (2015) have found that the basilar and apical dendrites of layer V pyramidal neurons in rats continue to increase in length after skilled reach training stops, peaking one month after the end of training and shrinking thereafter. Therefore, it is possible that the observed increases in spine density would not have been as evident earlier and also that they may in fact return to baseline levels at later time points after the end of training.
Superficial dendrites have not been a popular choice as the focus of histological analyses, possibly due to challenges related to the high density of dendrites in superficial cortex and its tendency to accrue damage during histological tissue processing. For in vivo imaging, the clarity with which superficial dendrites can be visualized with two photon microscopy to enable real time assays of experience-induced synaptic plasticity is compelling of the focus on this population. Still, the exact functional relevance of the observed structural changes remains poorly understood. Here we have shown that while motor skill learning is associated with the formation and preferential stabilization of new spines on superficial dendrites of layer V pyramidal neurons, the latter is not required for the retention of the newly acquired skill. Instead, the degree to which new spines are preferentially stabilized could reflect the degree to which the process of skill mastery continues. In addition, we found strikingly different structural responses to skill learning on dendrites slightly deeper in cortex. In layers II/III, but not in layer I, spine densities on apical dendrites of layer V pyramidal neurons increased, similarly so in mice with brief or more prolonged training, indicating that spine changes even on the same dendritic population can vary greatly with dendritic location, and that net increases in spines on the deeper subpopulation is potentially a structural substrate for the long-term retention of skills acquired early in training.
Motor skill learning involves the generation of complex movement patterns that are learned with repeated practice. Mastery of a motor skill is reflected in increases in efficiency and accuracy of the movements (Diedrichsen & Kornysheva, 2015). This complex learning process is likely the product of harmonized changes in the synaptic connectivity of numerous neuronal and dendritic populations occurring both within and beyond motor cortex. Ours and previous results highlight that while in vivo imaging is an invaluable approach for examining the structural basis of experience-dependent plasticity, spine alterations occurring on the superficial apical branches of cortical pyramidal neurons provide only a small window into training-induced structural plasticity, which undoubtedly requires coordination with synaptic changes across diverse neuronal and dendritic populations.
Supplementary Material
Dendritic spines formed during skill learning need not persist for skill memory to.
Their selective persistence strongly predicts further skill refinement.
Spine changes during skill learning vary with motor cortical depth.
They increase in net quantity on deeper, but not superficial, apical dendrites.
The former is a potential substrate for lasting skill memory.
Acknowledgments
This work is supported by the National Institutes of Health (NINDS NS078791 to T.A.J, A.K.D, Y.Z. and NS056839 to T.A.J.).
Abbreviations
- MC
motor cortex
- CFA
caudal forelimb area
- YFP
yellow fluorescent protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Financial Interest
The authors have no competing financial interests to declare
References
- Adkins DL, Bury SD, Jones TA. Laminar-dependent dendritic spine alterations in motor cortex of adult rats following callosal transection and forced forelimb use. Neurobio Learn Mem. 2002;78:35–52. doi: 10.1006/nlme.2001.4045. [DOI] [PubMed] [Google Scholar]
- Ballermann M, Metz GA, McKenna JE, Klassen F, Whishaw IQ. The pasta matrix reaching task: a simple test for measuring skilled reaching distance, direction, and dexterity in rats. Journal of Neuroscience Methods. 2001;106(1):39–45. doi: 10.1016/s0165-0270(01)00326-0. [DOI] [PubMed] [Google Scholar]
- Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annual Review Neurosci. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Lu J, Zuo Y. Spatiotemporal dynamics of dendritic spines in the living brain. Front Neuroanat. 2014;8 doi: 10.3389/fnana.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J, Kornysheva K. Motor skill learning between selection and execution. Trends in Cognitive Sciences. 2015;19(4):227–233. doi: 10.1016/j.tics.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M, Yu X, Lu J, Zuo Y. Repetitive motor learning induces coordinated formation of clustered dendritic spines in vivo. Nature. 2012;483(7387):92–95. doi: 10.1038/nature10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Olive MF. Structural and functional plasticity of dendritic spines - root or result of behavior? Genes, Brain, and Behavior. 2017;16(1):101–117. doi: 10.1111/gbb.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloor C, Luft AR, Hosp JA. Biphasic plasticity of dendritic fields in layer V motor neurons in response to motor learning. Neurobiol Learn Mem. 2015;125:189–194. doi: 10.1016/j.nlm.2015.08.009. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Larson JR, Withers GS. Effects of unilateral and bilateral training in a reaching task on dendritic branching of neurons in the rat motor-sensory forelimb cortex. Behavioral and Neural Biology. 1985;44(2):301–314. doi: 10.1016/s0163-1047(85)90310-3. [DOI] [PubMed] [Google Scholar]
- Grutzendler J, Kasthuri N, Gan WB. Long-term dendritic spine stability in the adult cortex. Nature. 2002;420:812–816. doi: 10.1038/nature01276. [DOI] [PubMed] [Google Scholar]
- Harms KJ, Dunaevsky A. Dendritic spine plasticity: looking beyond development. Brain Research. 2007;1184:65–71. doi: 10.1016/j.brainres.2006.02.094. [DOI] [PubMed] [Google Scholar]
- Harms KJ, Rioult-Pedotti MS, Carter DR, Dunaevsky A. Transient spine expansion and learning-induced plasticity in layer 1 primary motor cortex. J Neurosci. 2008;28(22):5686–5690. doi: 10.1523/JNEUROSCI.0584-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hübener M. Experience leaves a lasting structural trace in cortical circuits. Nature. 2009;457(7227):313–317. doi: 10.1038/nature07487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat Anthony, Wilbrecht L, Knott GW, Welker E, Svoboda K. Experience-dependent and cell-type-specific spine growth in the neocortex. Nature. 2006;441(7096):979–983. doi: 10.1038/nature04783. [DOI] [PubMed] [Google Scholar]
- Jones TA, Klintsova AY, Kilman VL, Sirevaag AM, Greenough WT. Induction of multiple synapses by experience in the visual cortex of adult rats. Neurobiol Learn Mem. 1997;68:13–20. doi: 10.1006/nlme.1997.3774. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, Turner R, Ungerlieder LG. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. PNAS. 1998;95:861–868. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H, Hayama T, Ishikawa M, Watanabe S, Yagishita S, Noguchi J. Learning rules and persistence of dendritic spines. Eur J Neurosci. 2010;32(2):241–249. doi: 10.1111/j.1460-9568.2010.07344.x. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Lussnig E, Schwarz ER, Comery TA, Greenough WT. Synaptogenesis and Fos expression in the motor cortex of the adult rat after motor skill learning. J Neurosci. 1996;16:4529–4535. doi: 10.1523/JNEUROSCI.16-14-04529.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim JA, Barbay S, Cooper NR, Hogg TM, Reidel CN, Remple MS, Nudo RJ. Motor learning-dependent synaptogenesis is localized to functionally reorganized motor cortex. Neurobiol Learn Mem. 2002;77(1):63–77. doi: 10.1006/nlme.2000.4004. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Jones TA, Schallert T. Motor enrichment and the induction of plasticity before or after brain injury. Neurochemical Research. 2003;28(11):1757–1769. doi: 10.1023/a:1026025408742. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Hogg TM, VandenBerg PM, Cooper NR, Bruneau R, Remple M. Cortical Synaptogenesis and motor map reorganization occur during late, but not early, phase of motor skill learning. J Neurosci. 2005;24:628–633. doi: 10.1523/JNEUROSCI.3440-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Wishaw IQ. Brain plasticity and behavior. Annu Revu Psychol. 1998;49:43–64. doi: 10.1146/annurev.psych.49.1.43. [DOI] [PubMed] [Google Scholar]
- Luft AR, Buitrago MM. Stages of motor skill learning. Mol Neurobiol. 2005;32:205–216. doi: 10.1385/MN:32:3:205. [DOI] [PubMed] [Google Scholar]
- Makino H, Hwang EJ, Hedrick NG, Komiyama T. Circuit Mechanisms of Sensorimotor Learning. Neuron. 2016;92(4):705–721. doi: 10.1016/j.neuron.2016.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs GS, Green SH, Dailey ME. Rapid formation and remodeling of postsynaptic densities in developing dendrites. Nat Neurosci. 2001;4:1006–1013. doi: 10.1038/nn717. [DOI] [PubMed] [Google Scholar]
- Masamizu Y, Tanaka YR, Tanaka YH, Hira R, Ohkubo F, Kitamura K, Matsuzaki M. Two distinct layer-specific dynamics of cortical ensembles during learning of a motor task. Nat Neurosci. 2014;17(7):987–994. doi: 10.1038/nn.3739. [DOI] [PubMed] [Google Scholar]
- Padmashri R, Reiner BC, Suresh A, Spartz E, Dunaevsky A. Altered structural and functional synaptic plasticity with motor skill learning in a mouse model of fragile X syndrome. J Neurosci. 2013;33(50):19715–19723. doi: 10.1523/JNEUROSCI.2514-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband WS, Image J. US National Institutes of Health. Bethesda MD, USA: 1997–2016. [Google Scholar]
- Reiner BC, Dunaevsky A. Deficit in Motor Training-Induced Clustering, but Not Stabilization, of New Dendritic Spines in fmr1 Knock-Out Mice. PLoS ONE. 2015;10(5) doi: 10.1371/journal.pone.0126572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TF, Tschida KA, Klein ME, Mooney R. Rapid spine stabilization and synaptic enhancement at the onset of behavioural learning. Nature. 2010;463(7283):948–952. doi: 10.1038/nature08759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Star EN, Kwiatkowski DJ, Murthy VN. Rapid turnover of actin in dendritic spines and its regulation by activity. Nat Neurosci. 2002;5:239–246. doi: 10.1038/nn811. [DOI] [PubMed] [Google Scholar]
- Tennant KA, Adkins DL, Donlan NA, Asay AL, Thomas N, Kleim JA, Jones TA. The organization of the forelimb representation of the C57BL/6 mouse motor cortex as defined by intracortical microstimulation and cytoarchitecture. Cerebral Cortex. 2011;21(4):865–876. doi: 10.1093/cercor/bhq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant KA, Adkins DL, Scalco MD, Donlan NA, Asay AL, Thomas N, Jones TA. Skill learning induced plasticity of motor cortical representations is time and age-dependent. Neurobiol Learn Mem. 2012;98(3):291–302. doi: 10.1016/j.nlm.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, Svoboda K. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Kuypers K, Diamond MC, Veltman WA. Effects of differential environments on plasticity of dendrites of cortical pyramidal neurons in adult rats. Experim Neurol. 1978;62(3):658–677. doi: 10.1016/0014-4886(78)90276-5. [DOI] [PubMed] [Google Scholar]
- Volkmar FR, Greenough WT. Rearing complexity affects branching of dendrites in the visual cortex of the rat. Science. 1972;176(4042):1445–1447. doi: 10.1126/science.176.4042.1445. [DOI] [PubMed] [Google Scholar]
- Wang L, Conner JM, Rickert J, Tuszynski MH. Structural plasticity within highly specific neuronal populations identifies a unique parcellation of motor learning in the adult brain. PNAS. 2011;108(6):2545–2550. doi: 10.1073/pnas.1014335108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RO, Ghosh A. Activity-dependent regulation of dendritic growth and patterning. Nat Rev Neurosci. 2002;3:803–812. doi: 10.1038/nrn941. [DOI] [PubMed] [Google Scholar]
- Xu Hua-Tai, Feng Pan, Guang Yang, Gan WB. Choice of Cranial Window Type for in Vivo Imaging Affects Dendritic Spine Turnover in the Cortex. Nat Neurosci. 2007;10(5):549–551. doi: 10.1038/nn1883. [DOI] [PubMed] [Google Scholar]
- Xu T, Xinzhu Y, Perlik A, Tobin W, Zweig J, Tennant K, Jones T, Zuo Y. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 2009;465:915–919. doi: 10.1038/nature08389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462:920–924. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Lai CS, Cichon J, Ma L, Li W, Gan WB. Sleep promotes branch-specific formation of dendritic spines after learning. Science. 2014;344:1173–1178. doi: 10.1126/science.1249098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumatsu N, Matsuzaki M, Miyazaki T, Noguchi J, Kasai H. Principles of long-term dynamics of dendritic spines. J Neurosci. 2008;28:13592–13608. doi: 10.1523/JNEUROSCI.0603-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R, Denk W. Dendritic spines as basic functional units of neuronal integration. Nature. 1995;375:682–684. doi: 10.1038/375682a0. [DOI] [PubMed] [Google Scholar]
- Yuste R, Bonhoeffer T. Genesis of dendritic spines: insights from ultrastructural and imaging studies. Nat Rev Neurosci. 2004;5:24–34. doi: 10.1038/nrn1300. [DOI] [PubMed] [Google Scholar]
- Yuste R. Dendritic spines and distributed circuits. Neuron. 2011;71:772–781. doi: 10.1016/j.neuron.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


