Abstract
Human influenza viruses passaged in eggs often acquire mutations in the hemagglutinin (HA) receptor binding site (RBS). To determine if egg-adapted H1N1 vaccines commonly elicit antibodies targeting the egg-adapted RBS of HA, we completed hemagglutinin-inhibition assays with A/California/7/2009 HA and egg-adapted A/California/7/2009-X-179A HA using sera collected from 159 humans vaccinated with seasonal influenza vaccines during the 2015-16 season. We found that ~5% of participants had ≥4-fold higher antibody titers to the egg-adapted viral strain compared to wild type viral strain. We used reverse-genetics to demonstrate that a single egg-adapted HA RBS mutation (Q226R) was responsible for this phenotype.
Keywords: influenza, hemagglutinin, vaccines
1. Introduction
Influenza viruses attach to cells through specific interactions between the viral hemagglutinin (HA) protein and terminally-linked sialic acids on target cells [1]. Most human influenza vaccine antigens are prepared from viruses grown in fertilized chicken eggs. Human influenza viruses grown in eggs often acquire mutations in or near the HA receptor binding site (RBS) that increase binding to α-2,3-linked sialic acids, and some of these mutations lead to large antigenic changes [2]. For example, the 2016-17 H3N2 egg-grown vaccine was antigenically mismatched compared to circulating H3N2 strains due to a T160K HA mutation that arose during egg passage [3]. In this case, the egg-adapted T160K HA mutation was located in a classic antigenic site adjacent to the RBS [3].
Recent studies have identified antibodies with long CDR3 domains that act like sialic acid mimics which make physical contact with HA RBS residues [4, 5]. There is considerable interest in developing vaccines that elicit these types of antibodies since they are able to neutralize a wide range of different influenza virus strains. It is unclear if vaccine strains with egg-adapted RBSs are able to elicit these broadly reactive antibodies, given that most egg-grown vaccine strains possess RBS mutations that facilitate growth in eggs. In a landmark study, Raymond and colleagues isolated monoclonal antibodies from vaccinated humans that bind to the egg-adapted RBS of H1N1 but not to circulating H1N1 viral strains [6]. These antibodies bind to egg-grown H1N1 viral strains that utilize α-2,3-linked sialic acids but not to viral strains that actually circulate in humans that utilize α-2,6-linked sialic acids [6]. It is unknown if these types of antibodies are commonly elicited by egg-adapted H1N1 vaccine strains. To address this, we completed hemagglutination-inhibition (HAI) assays with ‘wild-type’ H1N1 HA (A/California/7/2009) and ‘egg-adapted’ H1N1 HA (A/California/7/2009-X-179A) using sera collected from 159 individuals pre- and post-vaccination with the egg-adapted 2015-2016 seasonal influenza vaccine.
2. Materials and Methods
2.1 Cohort description
Prior to the 2015-2016 influenza season, individuals were enrolled in the University of Michigan Household Influenza Vaccine Effectiveness (HIVE) study, as previously described [7, 8]. Households were defined as having ≥4 members, of which ≥2 were children under age 18. Over the course of the 2015-16 influenza season, nasal and throat swab samples were collected from participants that displayed symptoms of acute respiratory illnesses and these samples were tested for influenza virus by real-time reverse-transcription polymerase chain reaction (RT-PCR). For this study, we analyzed sera from participants that received a seasonal influenza vaccine (97% received Sanofi Pasteur vaccine, 1% received GSK vaccine, 2% vaccine type unknown). Serum samples were collected from participants ages ≥13 years at the time of enrollment and also ≥14 days following vaccination. Informed consent was obtained and the study was approved by the University of Michigan Medical School Institutional Review Board.
2.2 HAI assays
HAI assays were performed using de-identified sera collected pre- and post-vaccination from 159 individuals with the approval of the University of Pennsylvania Institutional Review Board. Sera were pre-treated with receptor-destroying enzyme for 2 hours at 37°C and inactivated for 30 minutes at 55°C. Sera were also absorbed with 10% turkey red blood cell solution for 1 hour at 4°C prior to completing HAI assay. We used influenza virus-like particles (VLPs) for the HAI assays in this study as previously described [9], since it is difficult to grow human H1N1 viruses without adaptive mutations. We used VLPs that expressed the ‘wild-type’ A/California/7/2009 H1N1 HA or the egg-adapted A/California/7/2009-X-179A H1N1 HA. The VLPs for these experiments possessed an N3 neuraminidase (A/duck/Alberta/300/77) that most humans have not been exposed to previously. We constructed our VLPs in this manner to avoid potential complications from neuraminidase-reactive antibodies. We also included 20nM of oseltamavir in our HAI assays to prevent neuraminidase binding of turkey red blood cells [10]. The A/California/7/2009 wild-type HA and the A/California/7/2009-X-179A HA in our VLP constructs differ at 2 residues (H3 numbering used throughout); the X-179A strain possesses a glutamine to arginine mutation at position 226 (Q226R) and from lysine to threonine at position 212 (K212T). For some experiments we completed additional HAI assays using VLPs that expressed A/California/7/2009 HAs that were engineered to possess only the Q226R or K212T mutations.
2.2 Absorption assays
Suspension 293F cells (Thermo Fisher) were transfected with plasmids expressing A/California/7/2009 HA WT or X-179A. We also included 293F with no plasmid transfection as a control. The 293F cells (1×108 cells/mL) were harvested 48 hours post-transfection and incubated with sera for 1 hour at room temperature. Cell/sera mixtures were then centrifuged for 2000 rpm for 5 minutes, and sera were isolated. The absorbed sera were used in direct binding ELISAs using plated coated with either A/California/7/2009 HA WT or X-179A recombinant antigen.
3. Results
We completed two independent HAI assays using sera from 159 individuals collected prior to and ≥14 days after vaccination. Turkey red blood cells were used for these assays. Vaccinated subjects ranged in age from 13 to 76 years old: 22 (14%) were ≤18 years old and 22 (14%) were ≥50 years old. We completed HAI assays with VLPs that possessed the A/California/7/2009 H1N1 wild-type HA or the A/California/7/2009-X-179A HA. The wild-type HA differs from the egg-adapted X-179A HA at residues 212 (K212T) and 226 (Q226R) (Figure 1). Neither of these mutations is predicted to introduce or remove a glycosylation site. Residue 212 is somewhat buried within the trimer interface, whereas residue 226 is located directly in the RBS (Figure 1) and is known to impact receptor binding [11].
Figure 1. Differences between WT and X-179A HAs.
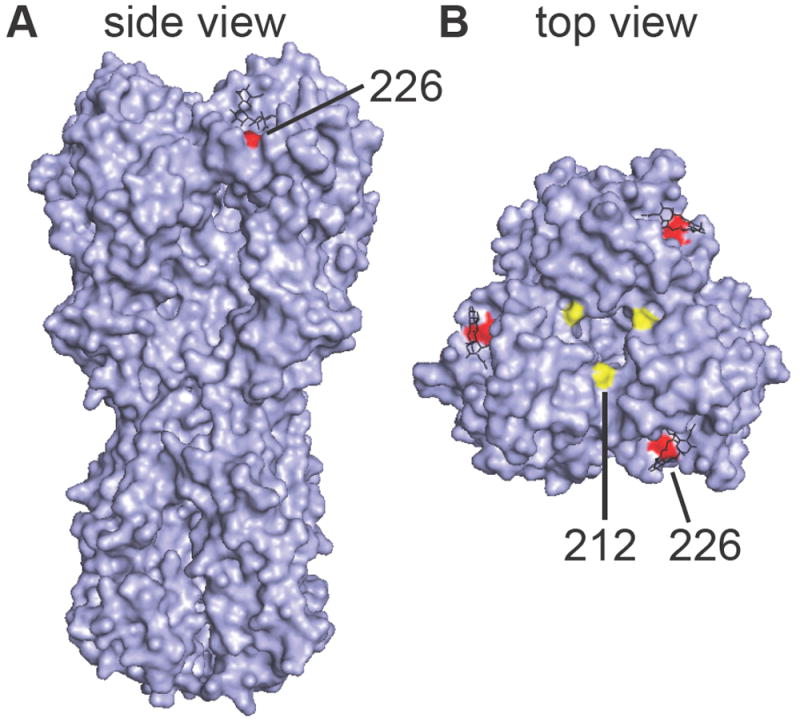
A side view (A) and top view (B) of the H1 trimer are shown (PDB ID code 3UBN). Residue 212 is shown in yellow and residue 226 is shown in red. The glycan receptor is shown in black.
We identified 8 participants (~5% of participants that we tested) that had post-vaccination antibody titers that were ≥4-fold higher to the egg-adapted X-179A HA compared to the wild-type HA following vaccination in 2 independent HAI experiments (Table 1). Most of these individuals possessed antibodies that reacted to the X-179A HA better than the WT HA prior to vaccination, which is likely the case due to past vaccinations with the X-179A vaccine strain that has been utilized in the human population since 2009. Only 22 (13.8%) of participants had a ≥4 fold rise in titer against either HA (Table 1), which is consistent with previous studies using this highly vaccinated cohort [7]. Of those who did respond to the vaccine, 4 (3%) responded only to the WT HA, 3 (2%) responded only to the egg-adapted X-179A HA, and 15 (9%) responded to both strains. Despite the overall low levels of seroconversion in this cohort, some individuals clearly seroconverted against the X-179A HA but not the WT HA following vaccination in this study. For example, participant #81’s HAI titer against X-179A HA rose from a value of 15 pre-vaccination to a value of 100 post-vaccination but HAI titers in this individual against WT HA were nearly the same pre- and post-vaccination (pre-vaccination titer of 10 and post-vaccination titer of 25) (Table 1; average HAI titer from 2 experiments described in text). Similarly, participant #147 was HAI negative against both HAs prior to vaccination and had a much higher HAI titer against X-179A HA (HAI titer of 70) compared to WT HA (HAI titer of 10) following vaccination (Table 1; average HAI titer from 2 experiments described in text). These data indicate that the X-179A egg-adapted vaccine strain elicits and/or reinforces antibody responses that recognize the X-179A egg-adapted H1N1 HA more efficiently than the WT H1N1 HA in some individuals.
Table 1.
HAI titers against the WT and X179A strains pre- and post-vaccination.1
| Experiment 1 | Experiment 2 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-vaccination | Post-vaccination | Pre-vaccination | Post-vaccination | ||||||||||||||
|
|
|||||||||||||||||
| Study ID | Age (years) | WT | X179A | X179A/WT | WT | X179A | X179A/WT | WT | X179A | X179A/WT | WT | X179A | X179A/WT | Infecte d2 | |||
|
|
|||||||||||||||||
| 1 | 49 | 10 | 30 | 3 | 10 | 30 | 3 | 10 | 30 | 3 | 10 | 30 | 3 | ||||
| 2 | 18 | 160 | 160 | 1 | 320 | 480 | 1.5 | 160 | 120 | 0.75 | 320 | 240 | 0.75 | ||||
| 3 | 46 | 5 | 5 | 1 | 5 | 5 | 1 | 5 | 10 | 2 | 5 | 10 | 2 | ||||
| 4 | 35 | 5 | 5 | 1 | 10 | 10 | 1 | 5 | 5 | 1 | 10 | 5 | 0.5 | ||||
| 5 | 40 | 40 | 30 | 0.75 | 40 | 30 | 0.75 | 30 | 20 | 0.67 | 30 | 30 | 1 | ||||
| 6 | 40 | 40 | 30 | 0.75 | 40 | 30 | 0.75 | 40 | 30 | 0.75 | 40 | 30 | 0.75 | ||||
| 7 | 49 | 20 | 30 | 1.5 | 60 | 60 | 1 | 20 | 20 | 1 | 40 | 60 | 1.5 | ||||
| 8 | 17 | 30 | 30 | 1 | 120 | 80 | 0.67 | 30 | 20 | 0.67 | 120 | 80 | 0.67 | ||||
| 9 | 47 | 80 | 60 | 0.75 | 120 | 120 | 1 | 80 | 80 | 1 | 160 | 120 | 0.75 | ||||
| 10 | 21 | 240 | 240 | 1 | 240 | 160 | 0.67 | 240 | 160 | 0.67 | 240 | 160 | 0.67 | ||||
| 11 | 19 | 20 | 20 | 1 | 60 | 40 | 0.67 | 20 | 10 | 0.5 | 40 | 30 | 0.75 | ||||
| 12 | 48 | 5 | 5 | 1 | 5 | 5 | 1 | 5 | 5 | 1 | 5 | 5 | 1 | ||||
| 13 | 48 | 240 | 480 | 2 | 240 | 320 | 1.33 | 240 | 480 | 2 | 160 | 320 | 2 | ||||
| 14 | 18 | 20 | 40 | 2 | 30 | 60 | 2 | 10 | 40 | 4 | 20 | 60 | 3 | ||||
| 15 | 53 | 5 | 5 | 1 | 5 | 5 | 1 | 5 | 5 | 1 | 5 | 5 | 1 | ||||
| 16 | 44 | 5 | 5 | 1 | 10 | 10 | 1 | 5 | 5 | 1 | 5 | 10 | 2 | ||||
| 17 | 41 | 5 | 5 | 1 | 30 | 30 | 1 | 5 | 5 | 1 | 30 | 20 | 0.67 | ||||
| 18 | 46 | 5 | 5 | 1 | 30 | 30 | 1 | 5 | 5 | 1 | 30 | 20 | 0.67 | ||||
| 19 | 48 | 5 | 5 | 1 | 5 | 5 | 1 | 5 | 5 | 1 | 5 | 5 | 1 | ||||
| 20 | 49 | 5 | 5 | 1 | 5 | 5 | 1 | 5 | 5 | 1 | 5 | 5 | 1 | ||||
| 21 | 17 | 480 | 480 | 1 | 320 | 240 | 0.75 | 320 | 480 | 1.5 | 160 | 320 | 2 | ||||
| 22 | 45 | 320 | 240 | 0.75 | 240 | 240 | 1 | 160 | 240 | 1.5 | 240 | 240 | 1 | ||||
| 23 | 59 | 40 | 40 | 1 | 30 | 30 | 1 | 30 | 40 | 1.33 | 20 | 30 | 1.5 | ||||
| 24 | 20 | 240 | 240 | 1 | 160 | 160 | 1 | 160 | 240 | 1.5 | 160 | 160 | 1 | ||||
| 25 | 53 | 10 | 10 | 1 | 30 | 30 | 1 | 10 | 10 | 1 | 30 | 30 | 1 | ||||
| 26 | 56 | 40 | 5 | 0.13 | 60 | 20 | 0.33 | 40 | 5 | 0.13 | 60 | 20 | 0.33 | ||||
|
| |||||||||||||||||
| 27 | 43 | 20 | 60 | 3 | 10 | 60 | 6 | 10 | 60 | 6 | 20 | 80 | 4 | ||||
|
| |||||||||||||||||
| 28 | 40 | 30 | 40 | 1.33 | 20 | 30 | 1.5 | 20 | 40 | 2 | 20 | 30 | 1.5 | ||||
| 29 | 38 | 60 | 40 | 0.67 | 60 | 30 | 0.5 | 40 | 40 | 1 | 40 | 30 | 0.75 | ||||
|
| |||||||||||||||||
| 30 | 42 | 5 | 10 | 2 | 5 | 30 | 6 | 5 | 20 | 4 | 5 | 30 | 6 | ||||
|
| |||||||||||||||||
| 31 | 42 | 40 | 60 | 1.5 | 40 | 60 | 1.5 | 30 | 40 | 1.33 | 40 | 40 | 1 | ||||
| 32 | 38 | 40 | 30 | 0.75 | 40 | 60 | 1.5 | 30 | 30 | 1 | 40 | 40 | 1 | ||||
| 33 | 37 | 20 | 30 | 1.5 | 20 | 30 | 1.5 | 20 | 30 | 1.5 | 10 | 20 | 2 | ||||
| 34 | 38 | 40 | 40 | 1 | 40 | 40 | 1 | 30 | 40 | 1.33 | 40 | 40 | 1 | ||||
| 35 | 39 | 40 | 30 | 0.75 | 60 | 40 | 0.67 | 40 | 30 | 0.75 | 60 | 40 | 0.67 | ||||
| 36 | 16 | 320 | 240 | 0.75 | 640 | 480 | 0.75 | 240 | 160 | 0.67 | 320 | 320 | 1 | ||||
| 37 | 44 | 60 | 60 | 1 | 60 | 60 | 1 | 60 | 60 | 1 | 40 | 40 | 1 | ||||
| 38 | 46 | 30 | 20 | 0.67 | 20 | 10 | 0.5 | 20 | 20 | 1 | 20 | 20 | 1 | ||||
| 39 | 52 | 5 | 5 | 1 | 5 | 5 | 1 | 5 | 5 | 1 | 5 | 5 | 1 | ||||
| 40 | 47 | 20 | 10 | 0.5 | 20 | 20 | 1 | 10 | 20 | 2 | 20 | 20 | 1 | ||||
| 41 | 43 | 5 | 5 | 1 | 30 | 30 | 1 | 5 | 10 | 2 | 30 | 30 | 1 | ||||
| 42 | 41 | 10 | 10 | 1 | 20 | 30 | 1.5 | 10 | 20 | 2 | 20 | 30 | 1.5 | ||||
| 43 | 51 | 160 | 120 | 0.75 | 160 | 120 | 0.75 | 160 | 120 | 0.75 | 120 | 120 | 1 | ||||
| 44 | 40 | 60 | 40 | 0.67 | 40 | 30 | 0.75 | 40 | 40 | 1 | 40 | 40 | 1 | ||||
|
| |||||||||||||||||
| 45 | 43 | 5 | 120 | 24 | 20 | 240 | 12 | 5 | 120 | 24 | 20 | 240 | 12 | ||||
|
| |||||||||||||||||
| 46 | 69 | 5 | 5 | 1 | 5 | 5 | 1 | 5 | 5 | 1 | 5 | 5 | 1 | ||||
| 47 | 46 | 40 | 80 | 2 | 60 | 120 | 2 | 40 | 60 | 1.5 | 80 | 80 | 1 | ||||
| 48 | 46 | 5 | 5 | 1 | 5 | 5 | 1 | 5 | 5 | 1 | 5 | 5 | 1 | ||||
| 49 | 33 | 60 | 60 | 1 | 80 | 60 | 0.75 | 60 | 40 | 0.67 | 80 | 60 | 0.75 | ||||
| 50 | 41 | 20 | 20 | 1 | 20 | 20 | 1 | 20 | 20 | 1 | 20 | 20 | 1 | ||||
| 51 | 46 | 80 | 60 | 0.75 | 80 | 80 | 1 | 80 | 60 | 0.75 | 80 | 60 | 0.75 | ||||
| 52 | 17 | 640 | 480 | 0.75 | 640 | 480 | 0.75 | 480 | 320 | 0.67 | 640 | 480 | 0.75 | ||||
| 53 | 15 | 480 | 320 | 0.67 | 480 | 320 | 0.67 | 320 | 240 | 0.75 | 320 | 320 | 1 | ||||
| 54 | 44 | 10 | 5 | 0.5 | 10 | 5 | 0.5 | 5 | 5 | 1 | 5 | 5 | 1 | ||||
| 55 | 52 | 20 | 20 | 1 | 30 | 30 | 1 | 20 | 20 | 1 | 30 | 20 | 0.67 | ||||
| 56 | 19 | 120 | 120 | 1 | 80 | 60 | 0.75 | 120 | 80 | 0.67 | 80 | 60 | 0.75 | ||||
| 57 | 41 | 120 | 60 | 0.5 | 120 | 80 | 0.67 | 80 | 60 | 0.75 | 80 | 60 | 0.75 | ||||
| 58 | 43 | 20 | 30 | 1.5 | 320 | 480 | 1.5 | 20 | 30 | 1.5 | 320 | 320 | 1 | ||||
| 59 | 41 | 20 | 10 | 0.5 | 20 | 20 | 1 | 20 | 10 | 0.5 | 20 | 20 | 1 | ||||
| 60 | 51 | 5 | 5 | 1 | 30 | 40 | 1.33 | 5 | 5 | 1 | 30 | 30 | 1 | ||||
| 61 | 50 | 10 | 10 | 1 | 20 | 20 | 1 | 10 | 20 | 2 | 10 | 20 | 2 | ||||
| 62 | 44 | 80 | 60 | 0.75 | 80 | 80 | 1 | 80 | 40 | 0.5 | 80 | 60 | 0.75 | ||||
| 63 | 38 | 5 | 5 | 1 | 20 | 30 | 1.5 | 5 | 5 | 1 | 20 | 30 | 1.5 | ||||
| 64 | 17 | 240 | 240 | 1 | 240 | 240 | 1 | 240 | 160 | 0.67 | 240 | 160 | 0.67 | ||||
| 65 | 50 | 30 | 40 | 1.33 | 5 | 5 | 1 | 10 | 40 | 4 | 5 | 5 | 1 | ||||
| 66 | 53 | 20 | 10 | 0.5 | 30 | 30 | 1 | 20 | 5 | 0.25 | 30 | 20 | 0.67 | ||||
| 67 | 46 | 60 | 40 | 0.67 | 120 | 120 | 1 | 40 | 30 | 0.75 | 80 | 60 | 0.75 | ||||
| 68 | 54 | 80 | 60 | 0.75 | 30 | 40 | 1.33 | 60 | 40 | 0.67 | 30 | 20 | 0.67 | ||||
| 69 | 36 | 160 | 120 | 0.75 | 320 | 240 | 0.75 | 120 | 80 | 0.67 | 320 | 160 | 0.5 | ||||
| 70 | 42 | 10 | 20 | 2 | 30 | 30 | 1 | 20 | 20 | 1 | 20 | 20 | 1 | ||||
| 71 | 45 | 120 | 120 | 1 | 160 | 160 | 1 | 120 | 80 | 0.67 | 120 | 80 | 0.67 | ||||
| 72 | 34 | 240 | 120 | 0.5 | 320 | 160 | 0.5 | 240 | 120 | 0.5 | 320 | 160 | 0.5 | ||||
| 73 | 47 | 30 | 20 | 0.67 | 20 | 10 | 0.5 | 20 | 10 | 0.5 | 20 | 10 | 0.5 | ||||
| 74 | 42 | 5 | 5 | 1 | 20 | 10 | 0.5 | 5 | 5 | 1 | 20 | 10 | 0.5 | ||||
| 75 | 20 | 160 | 120 | 0.75 | 240 | 240 | 1 | 160 | 80 | 0.5 | 320 | 160 | 0.5 | ||||
| 76 | 17 | 120 | 120 | 1 | 160 | 120 | 0.75 | 160 | 80 | 0.5 | 160 | 80 | 0.5 | ||||
| 77 | 35 | 320 | 240 | 0.75 | 480 | 320 | 0.67 | 320 | 160 | 0.5 | 320 | 160 | 0.5 | ||||
| 78 | 38 | 240 | 240 | 1 | 160 | 160 | 1 | 240 | 160 | 0.67 | 160 | 120 | 0.75 | ||||
| 79 | 39 | 30 | 30 | 1 | 30 | 40 | 1.33 | 20 | 30 | 1.5 | 30 | 40 | 1.33 | ||||
| 80 | 44 | 160 | 120 | 0.75 | 160 | 120 | 0.75 | 160 | 80 | 0.5 | 160 | 80 | 0.5 | ||||
|
| |||||||||||||||||
| 81 | 41 | 10 | 20 | 2 | 30 | 120 | 4 | 10 | 10 | 1 | 20 | 80 | 4 | ||||
|
| |||||||||||||||||
| 82 | 44 | 30 | 30 | 1 | 40 | 40 | 1 | 30 | 20 | 0.67 | 30 | 30 | 1 | + | |||
| 83 | 50 | 5 | 5 | 1 | 5 | 5 | 1 | 5 | 5 | 1 | 5 | 5 | 1 | ||||
| 84 | 19 | 120 | 80 | 0.67 | 160 | 120 | 0.75 | 120 | 80 | 0.67 | 160 | 120 | 0.75 | ||||
| 85 | 42 | 10 | 10 | 1 | 20 | 10 | 0.5 | 10 | 10 | 1 | 20 | 10 | 0.5 | ||||
| 86 | 40 | 30 | 30 | 1 | 40 | 30 | 0.75 | 30 | 30 | 1 | 40 | 30 | 0.75 | ||||
| 87 | 66 | 30 | 30 | 1 | 30 | 30 | 1 | 30 | 30 | 1 | 20 | 30 | 1.5 | ||||
| 88 | 50 | 5 | 5 | 1 | 5 | 5 | 1 | 5 | 5 | 1 | 5 | 5 | 1 | ||||
| 89 | 36 | 40 | 40 | 1 | 40 | 40 | 1 | 40 | 40 | 1 | 40 | 30 | 0.75 | ||||
| 90 | 43 | 5 | 5 | 1 | 40 | 40 | 1 | 5 | 5 | 1 | 40 | 40 | 1 | + | |||
| 91 | 46 | 5 | 10 | 2 | 30 | 40 | 1.33 | 5 | 5 | 1 | 20 | 30 | 1.5 | ||||
| 92 | 41 | 5 | 5 | 1 | 5 | 5 | 1 | 5 | 5 | 1 | 5 | 5 | 1 | ||||
|
| |||||||||||||||||
| 93 | 47 | 10 | 30 | 3 | 10 | 40 | 4 | 10 | 40 | 4 | 10 | 40 | 4 | ||||
|
| |||||||||||||||||
| 94 | 41 | 80 | 60 | 0.75 | 40 | 30 | 0.75 | 80 | 80 | 1 | 40 | 20 | 0.5 | ||||
| 95 | 39 | 5 | 5 | 1 | 5 | 5 | 1 | 5 | 5 | 1 | 5 | 5 | 1 | ||||
| 96 | 42 | 80 | 80 | 1 | 80 | 60 | 0.75 | 80 | 60 | 0.75 | 80 | 40 | 0.5 | ||||
| 97 | 41 | 60 | 120 | 2 | 40 | 80 | 2 | 40 | 80 | 2 | 40 | 80 | 2 | ||||
| 98 | 54 | 5 | 5 | 1 | 320 | 320 | 1 | 5 | 5 | 1 | 320 | 240 | 0.75 | ||||
| 99 | 46 | 5 | 10 | 2 | 5 | 20 | 4 | 5 | 10 | 2 | 5 | 10 | 2 | ||||
| 100 | 47 | 40 | 40 | 1 | 30 | 40 | 1.33 | 30 | 40 | 1.33 | 30 | 30 | 1 | ||||
| 101 | 49 | 20 | 10 | 0.5 | 10 | 10 | 1 | 20 | 5 | 0.25 | 10 | 5 | 0.5 | ||||
| 102 | 20 | 240 | 240 | 1 | 240 | 240 | 1 | 240 | 160 | 0.67 | 240 | 160 | 0.67 | ||||
| 103 | 20 | 640 | 640 | 1 | 640 | 640 | 1 | 640 | 640 | 1 | 480 | 320 | 0.67 | ||||
| 104 | 17 | 640 | 480 | 0.75 | 480 | 480 | 1 | 480 | 320 | 0.67 | 480 | 320 | 0.67 | ||||
| 105 | 41 | 40 | 60 | 1.5 | 40 | 60 | 1.5 | 30 | 40 | 1.33 | 40 | 40 | 1 | ||||
| 106 | 39 | 5 | 20 | 4 | 10 | 30 | 3 | 5 | 10 | 2 | 10 | 20 | 2 | ||||
| 107 | 43 | 60 | 60 | 1 | 60 | 60 | 1 | 40 | 40 | 1 | 40 | 40 | 1 | ||||
| 108 | 45 | 5 | 5 | 1 | 5 | 5 | 1 | 5 | 5 | 1 | 5 | 5 | 1 | + | |||
| 109 | 49 | 120 | 120 | 1 | 120 | 120 | 1 | 80 | 80 | 1 | 80 | 80 | 1 | ||||
| 110 | 50 | 30 | 120 | 4 | 60 | 120 | 2 | 30 | 120 | 4 | 40 | 80 | 2 | ||||
| 111 | 62 | 5 | 5 | 1 | 5 | 5 | 1 | 5 | 5 | 1 | 5 | 5 | 1 | ||||
| 112 | 78 | 5 | 5 | 1 | 5 | 5 | 1 | 5 | 5 | 1 | 5 | 5 | 1 | ||||
| 113 | 18 | 120 | 80 | 0.67 | 120 | 80 | 0.67 | 80 | 60 | 0.75 | 80 | 60 | 0.75 | ||||
|
| |||||||||||||||||
| 114 | 53 | 5 | 30 | 6 | 10 | 40 | 4 | 5 | 20 | 4 | 10 | 40 | 4 | ||||
|
| |||||||||||||||||
| 115 | 50 | 60 | 60 | 1 | 60 | 80 | 1.33 | 60 | 40 | 0.67 | 60 | 60 | 1 | ||||
| 116 | 22 | 240 | 240 | 1 | 120 | 120 | 1 | 240 | 160 | 0.67 | 160 | 120 | 0.75 | ||||
| 117 | 19 | 80 | 80 | 1 | 80 | 60 | 0.75 | 80 | 60 | 0.75 | 80 | 40 | 0.5 | ||||
| 118 | 47 | 20 | 40 | 2 | 30 | 60 | 2 | 20 | 30 | 1.5 | 30 | 40 | 1.33 | ||||
| 119 | 78 | 5 | 5 | 1 | 5 | 10 | 2 | 5 | 5 | 1 | 5 | 5 | 1 | ||||
| 120 | 75 | 5 | 5 | 1 | 10 | 20 | 2 | 5 | 5 | 1 | 5 | 10 | 2 | ||||
| 121 | 42 | 80 | 60 | 0.75 | 80 | 60 | 0.75 | 80 | 60 | 0.75 | 80 | 60 | 0.75 | ||||
| 122 | 38 | 80 | 80 | 1 | 120 | 120 | 1 | 80 | 60 | 0.75 | 80 | 80 | 1 | ||||
| 123 | 57 | 5 | 5 | 1 | 5 | 10 | 2 | 5 | 5 | 1 | 5 | 5 | 1 | ||||
| 124 | 40 | 40 | 60 | 1.5 | 80 | 120 | 1.5 | 40 | 40 | 1 | 60 | 60 | 1 | ||||
| 125 | 40 | 5 | 10 | 2 | 30 | 30 | 1 | 5 | 10 | 2 | 20 | 20 | 1 | ||||
| 126 | 41 | 160 | 160 | 1 | 160 | 160 | 1 | 160 | 120 | 0.75 | 160 | 120 | 0.75 | ||||
| 127 | 34 | 20 | 30 | 1.5 | 30 | 40 | 1.33 | 20 | 20 | 1 | 20 | 30 | 1.5 | ||||
| 128 | 16 | 40 | 30 | 0.75 | 120 | 120 | 1 | 40 | 30 | 0.75 | 120 | 80 | 0.67 | ||||
| 129 | 38 | 20 | 30 | 1.5 | 30 | 30 | 1 | 20 | 20 | 1 | 30 | 30 | 1 | ||||
| 130 | 40 | 60 | 60 | 1 | 60 | 60 | 1 | 40 | 60 | 1.5 | 40 | 60 | 1.5 | ||||
|
| |||||||||||||||||
| 131 | 44 | 5 | 10 | 2 | 5 | 30 | 6 | 5 | 30 | 6 | 5 | 30 | 6 | + | |||
|
| |||||||||||||||||
| 132 | 47 | 5 | 5 | 1 | 40 | 60 | 1.5 | 5 | 5 | 1 | 40 | 40 | 1 | ||||
| 133 | 43 | 40 | 30 | 0.75 | 40 | 30 | 0.75 | 40 | 30 | 0.75 | 30 | 20 | 0.67 | ||||
| 134 | 28 | 120 | 80 | 0.67 | 120 | 120 | 1 | 80 | 60 | 0.75 | 120 | 80 | 0.67 | ||||
| 135 | 44 | 10 | 10 | 1 | 20 | 20 | 1 | 10 | 5 | 0.5 | 20 | 10 | 0.5 | ||||
| 136 | 45 | 120 | 120 | 1 | 120 | 120 | 1 | 120 | 80 | 0.67 | 160 | 120 | 0.75 | ||||
| 137 | 49 | 40 | 40 | 1 | 30 | 40 | 1.33 | 40 | 30 | 0.75 | 30 | 30 | 1 | + | |||
| 138 | 52 | 30 | 20 | 0.67 | 30 | 30 | 1 | 20 | 20 | 1 | 20 | 20 | 1 | + | |||
| 139 | 46 | 480 | 320 | 0.67 | 480 | 240 | 0.5 | 480 | 240 | 0.5 | 320 | 160 | 0.5 | ||||
| 140 | 40 | 5 | 20 | 4 | 10 | 30 | 3 | 5 | 10 | 2 | 10 | 20 | 2 | ||||
| 141 | 43 | 240 | 240 | 1 | 240 | 240 | 1 | 160 | 160 | 1 | 160 | 160 | 1 | ||||
| 142 | 44 | 40 | 30 | 0.75 | 20 | 10 | 0.5 | 40 | 20 | 0.5 | 20 | 10 | 0.5 | ||||
| 143 | 45 | 10 | 10 | 1 | 30 | 30 | 1 | 10 | 5 | 0.5 | 20 | 10 | 0.5 | ||||
| 144 | 16 | 320 | 240 | 0.75 | 480 | 480 | 1 | 320 | 240 | 0.75 | 480 | 240 | 0.5 | ||||
| 145 | 51 | 20 | 20 | 1 | 160 | 120 | 0.75 | 20 | 10 | 0.5 | 160 | 120 | 0.75 | ||||
| 146 | 54 | 10 | 40 | 4 | 30 | 60 | 2 | 10 | 30 | 3 | 30 | 40 | 1.33 | ||||
|
| |||||||||||||||||
| 147 | 36 | 5 | 5 | 1 | 10 | 80 | 8 | 5 | 5 | 1 | 10 | 60 | 6 | ||||
|
| |||||||||||||||||
| 148 | 38 | 40 | 40 | 1 | 60 | 60 | 1 | 40 | 30 | 0.75 | 60 | 40 | 0.67 | + | |||
| 149 | 38 | 10 | 20 | 2 | 20 | 30 | 1.5 | 10 | 10 | 1 | 20 | 20 | 1 | ||||
| 150 | 41 | 10 | 5 | 0.5 | 40 | 30 | 0.75 | 5 | 5 | 1 | 40 | 20 | 0.5 | ||||
| 151 | 19 | 5 | 5 | 1 | 160 | 160 | 1 | 5 | 5 | 1 | 480 | 120 | 0.25 | ||||
| 152 | 49 | 60 | 40 | 0.67 | 80 | 60 | 0.75 | 60 | 40 | 0.67 | 80 | 40 | 0.5 | ||||
| 153 | 52 | 40 | 30 | 0.75 | 60 | 60 | 1 | 30 | 20 | 0.67 | 40 | 40 | 1 | ||||
| 154 | 52 | 5 | 5 | 1 | 40 | 30 | 0.75 | 5 | 5 | 1 | 40 | 20 | 0.5 | ||||
| 155 | 35 | 40 | 40 | 1 | 40 | 40 | 1 | 40 | 80 | 2 | 40 | 60 | 1.5 | ||||
| 156 | 46 | 20 | 40 | 2 | 120 | 240 | 2 | 10 | 40 | 4 | 80 | 160 | 2 | ||||
| 157 | 43 | 40 | 30 | 0.75 | 80 | 60 | 0.75 | 40 | 20 | 0.5 | 80 | 40 | 0.5 | ||||
| 158 | 44 | 5 | 5 | 1 | 5 | 5 | 1 | 5 | 5 | 1 | 5 | 5 | 1 | ||||
| 159 | 53 | 5 | 5 | 1 | 5 | 5 | 1 | 5 | 5 | 1 | 5 | 5 | 1 | ||||
Grey indicates post-vaccination sera samples that had 4-fold higher HAI titers using X179A virus compared to WT virus in 2 independent experiments. Undetectable titers were assigned a value of 5.
The infected column indicates whether the donor was infected after vaccination during the 2015-2016 influenza season.
To confirm that the difference in WT HA versus X-179A HA reactivity was due to RBS differences, we completed additional HAI experiments using A/California/7/2009 HAs that were engineered to possess only K212T or Q226R HA mutations. We completed experiments with serum samples from the 8 participants that had HAI titers that were ≥4-fold higher to the egg-adapted X-179A HA compared to the wild-type HA. HAI titers were lower using the WT HA and HA with the K212T mutation compared to the X-179A HA and the HA with the Q226R mutation (Figure 2A), indicating that the 8 individuals that we identified in our study possessed antibodies that targeted the egg-adapted RBS of the X-179A H1N1 vaccine strain. As a control, we also tested serum samples from participants that had similar HAI titers using the X-179A HA and WT HA. These control samples had similar HAI titers when assays were performed with WT HA, X-179A HA, or HAs with either the K212T or Q226R mutations (Figure 2B).
Figure 2. Identification of individuals that possess antibodies that target the RBS of X-179A following vaccination.
HAI assays using VLPs with WT HA, X-179A HA, and HA engineered to possess the Q226R or K212T mutations were completed using post-vaccination sera from (A) participants that had ≥4-fold higher HAI titers to the egg-adapted X-179A HA compared to the WT HA or from (B) participants that had similar HAI titers to the egg-adapted X-179A HA and WT HA. Shown is % HAI titer compared to HAI titer using X-179A HA. Mean percentages from three HAI experiments + SD is shown. *, p<0.05
HAI assays can be difficult to interpret when comparing HAs with different receptor binding properties [12]. For this reason, we completed additional direct antibody binding assays. We incubated serum samples with 293 cells expressing WT or X-179A HA and then we completed ELISAs with the absorbed sera using plates coated with WT and X-179A HA. We completed experiments with two sera samples that had large X-179A /WT HAI differences and one control serum sample that did not have HAI differences using X-179A HA and WT HA (Figure 3). X-179A HA absorption removed nearly all antibodies from serum that had high X-179A/WT HAI ratios, whereas WT HA failed to absorb a large fraction of X-179A HA-reactive antibodies from these sera (Figure 3A-B). In contrast, both X-179A HA and WT absorption removed nearly all X-179A HA- and WT HA-reactive antibodies from a control serum sample (Figure 3C). These data suggest that the HAI differences in Table 1 and Figure 2 are due to differences in antibody binding rather than differences in WT and X-179A receptor binding properties.
Figure 3. Serum antibodies from some participants bind strongly to X-179A HA but not WT HA.
Sera were absorbed with 293 cells expressing WT or X-179A HA and then ELISAs were completed using WT HA (black) or X-179A HA (grey). Sera from two donors that had large HAI differences using WT HA and X-179A HA (A-B) and one donor that had similar HAI titers using WT HA and X-179A HA (C) were tested. Area under the curve analyses were completed using ELISA data. Data are expressed as % binding relative to mock absorption conditions. Mean of triplicate samples + SD is shown. *, p<0.05
Seven out of the 159 vaccinated individuals in this study had PCR-confirmed H1N1 infection during the course of the 2015-16 season. All 7 of these individuals possessed ≤60 antibody titers against the WT H1N1 strain after vaccination (Table 1). Importantly, one of the H1N1-infected participants (#131) had a higher HAI titer to the X-179A HA compared to the WT HA following vaccination, albeit titers to both HAs were low in this individual. From these studies, we conclude that some individuals vaccinated with the egg-adapted X-179A vaccine strain produce antibodies that recognize egg-adapted epitopes in the HA RBS and we speculate that this might contribute to reduced vaccine effectiveness.
4. Discussion
Human influenza viruses typically acquire mutations in and around the HA RBS that enhance virus binding to α-2,3-linked sialic acids when grown in fertilized chicken eggs. Sometimes these mutations are located in classical antibody binding sites that lead to substantial vaccine mismatches, as was the case during the 2016-2017 H3N2-dominated influenza season [3]. More commonly, egg-grown vaccine strains possess adaptive mutations ‘buried’ in the RBS, and it has been historically thought that these mutations are not as antigenically important. However, it is becoming clear that some human antibodies with long CDR3 domains physically make contact with residues deep within the RBS [4, 5], and a recent study isolated monoclonal antibodies from a vaccinated human that could bind to HAs with an egg-adapted RBS but not to non-egg adapted HAs that were present in viruses that circulated in humans [6]. Here, we set out to determine how common these types of antibodies are in vaccinated humans and if they are associated with vaccine failure.
We found that ~5% of individuals vaccinated with the 2015-2016 seasonal influenza vaccine possessed antibodies that recognized the X-179A H1N1 HA ≥4-fold more efficiently compared to the WT H1N1 HA. Given that >140 million people receive a seasonal influenza vaccine in the U.S. each year [13], these data indicate that a large number of individuals (>7 million in the U.S.!) possess antibodies that preferentially recognize the egg-adapted HA RBS of H1N1 rather than the HA of H1N1 viruses that circulate in humans. For our study, we focused on individuals that had ≥4-fold differences in HAI titers using the different HAs, and this conservative fold difference cutoff likely underestimates the number of individuals that possess antibodies that bind to the egg-adapted H1N1 strain but not the circulating H1N1 strain.
It is notable that participants in our study come from a population with high annual vaccine uptake rates, and therefore they are likely to have had multiple previous influenza vaccines [14]. Consistent with this, very few participants responded to vaccination with a ≥4-fold rise in titer. All participants who responded to X-179A HA had pre-vaccination titers ≤40. Most participants that possessed X-179A HA-specific antibodies after vaccination also possessed these antibodies prior to vaccination. Since the egg-adapted H1N1 component of seasonal influenza vaccines remained unchanged between 2010-2016, it is possible that these participants developed this phenotype during an earlier vaccination and that these antibody responses were continually boosted by subsequent immunizations.
While most studies of egg-adaptation have focused on H3N2 viruses, our study clearly demonstrates that egg-adaptations in the HA RBS of H1N1 viruses can also affect antigenicity. Antibodies targeting the RBS of HA have the potential to be broadly neutralizing if they are not restricted to binding only egg-adapted HAs [15, 16]. Our current reliance on eggs for the production of most seasonal influenza vaccines disfavors the generation of these types of broadly reactive antibodies. It is important to continue to develop new systems to prepare influenza vaccine antigens that are not dependent on viral growth in eggs, such as baculovirus and mammalian cell-based systems. Future studies should address if antigens produced in these alternative systems are more effective at eliciting antibodies that target the RBS of HAs that are present in human influenza virus strains.
Highlights.
egg-adapted H1N1 vaccines elicit Abs that react to egg-epitopes in some adults
5% of human vaccinees possess Abs that react to H1N1 egg-adapted epitopes
we speculate that these Abs contribute to reduced vaccine effectiveness
Acknowledgments
This work was supported by the National Institute of Allergy and Infectious Diseases (1R01AI113047, SEH; 1R01AI108686, SEH; 1R01AI097150, ASM; CEIRS HHSN272201400005C, SEH and ASM) and Center for Disease Control (U01IP000474, ASM). Scott E. Hensley, Ph.D. holds an Investigators in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund.
Footnotes
Conflict of interest statement
ASM has received grant support from Sanofi Pasteur and consultancy fees from Sanofi, GSK, and Novavax for work unrelated to this report. SEH has received consultancy fee from Lumen, Novavax, and Merck for work unrelated to this report. All other authors report no potential conflicts. All authors will submit the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Skehel JJ, Wiley DC. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annual review of biochemistry. 2000;69:531–69. doi: 10.1146/annurev.biochem.69.1.531. [DOI] [PubMed] [Google Scholar]
- 2.Schultz-Cherry S, Jones JC. Influenza vaccines: the good, the bad, and the eggs. Adv Virus Res. 2010;77:63–84. doi: 10.1016/B978-0-12-385034-8.00003-X. [DOI] [PubMed] [Google Scholar]
- 3.Zost SJ, Parkhouse K, Gumina ME, Kim K, Diaz Perez S, Wilson PC, et al. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:12578–83. doi: 10.1073/pnas.1712377114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whittle JR, Zhang R, Khurana S, King LR, Manischewitz J, Golding H, et al. Broadly neutralizing human antibody that recognizes the receptor-binding pocket of influenza virus hemagglutinin. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:14216–21. doi: 10.1073/pnas.1111497108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt AG, Therkelsen MD, Stewart S, Kepler TB, Liao HX, Moody MA, et al. Viral receptor-binding site antibodies with diverse germline origins. Cell. 2015;161:1026–34. doi: 10.1016/j.cell.2015.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raymond DD, Stewart SM, Lee J, Ferdman J, Bajic G, Do KT, et al. Influenza immunization elicits antibodies specific for an egg-adapted vaccine strain. Nature medicine. 2016;22:1465–9. doi: 10.1038/nm.4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohmit SE, Petrie JG, Malosh RE, Johnson E, Truscon R, Aaron B, et al. Substantial Influenza Vaccine Effectiveness in Households With Children During the 2013-2014 Influenza Season, When 2009 Pandemic Influenza A(H1N1) Virus Predominated. The Journal of infectious diseases. 2016;213:1229–36. doi: 10.1093/infdis/jiv563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrie JG, Parkhouse K, Ohmit SE, Malosh RE, Monto AS, Hensley SE. Antibodies Against the Current Influenza A(H1N1) Vaccine Strain Do Not Protect Some Individuals From Infection With Contemporary Circulating Influenza A(H1N1) Virus Strains. The Journal of infectious diseases. 2016;214:1947–51. doi: 10.1093/infdis/jiw479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giles BM, Bissel SJ, Craigo JK, Dealmeida DR, Wiley CA, Tumpey TM, et al. Elicitation of anti-1918 influenza virus immunity early in life prevents morbidity and lower levels of lung infection by 2009 pandemic H1N1 influenza virus in aged mice. Journal of virology. 2012;86:1500–13. doi: 10.1128/JVI.06034-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chambers BS, Li Y, Hodinka RL, Hensley SE. Recent H3N2 Influenza Virus Clinical Isolates Rapidly Acquire Hemagglutinin or Neuraminidase Mutations When Propagated for Antigenic Analyses. Journal of virology. 2014;88:10986–9. doi: 10.1128/JVI.01077-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicholls JM, Chan RW, Russell RJ, Air GM, Peiris JS. Evolving complexities of influenza virus and its receptors. Trends in microbiology. 2008;16:149–57. doi: 10.1016/j.tim.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Bostick DL, Sullivan CB, Myers JL, Griesemer SB, Stgeorge K, et al. Single Hemagglutinin Mutations That Alter both Antigenicity and Receptor Binding Avidity Influence Influenza Virus Antigenic Clustering. Journal of virology. 2013;87:9904–10. doi: 10.1128/JVI.01023-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.https://www.cdc.gov/flu/fluvaxview/nifs-estimates-nov2017.htm
- 14.Petrie JG, Malosh RE, Cheng CK, Ohmit SE, Martin ET, Johnson E, et al. The Household Influenza Vaccine Effectiveness Study: Lack of Antibody Response and Protection Following Receipt of 2014-2015 Influenza Vaccine. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2017;65:1644–51. doi: 10.1093/cid/cix608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu NC, Wilson IA. A Perspective on the Structural and Functional Constraints for Immune Evasion: Insights from Influenza Virus. J Mol Biol. 2017;429:2694–709. doi: 10.1016/j.jmb.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krammer F, Palese P, Steel J. Advances in universal influenza virus vaccine design and antibody mediated therapies based on conserved regions of the hemagglutinin. Curr Top Microbiol Immunol. 2015;386:301–21. doi: 10.1007/82_2014_408. [DOI] [PubMed] [Google Scholar]


