Abstract
Background Despite much progress in the surgical and endovascular treatment of thoracoabdominal aortic diseases (TAADs), there is no consensus regarding the optimal approach to minimize operative mortality and end-organ dysfunction. We report our experience in the past 16 years treating TAAD by open surgery.
Methods A retrospective review of all TAAD patients who underwent an open repair since January 2000 was performed. The primary endpoints included early morbidity and mortality, and the secondary endpoints were overall death and rate of aortic reintervention.
Results There were 112 patients treated by open surgery for TAAD. Mean age was 66 ± 10 years and 61 (54%) were male. Seventy-seven (69%) patients had aneurysmal degeneration without aortic dissection and the remaining 35 (31%) had a concomitant aortic dissection. There were 12 deaths (10.7%) and they were equally distributed between the aneurysm and dissection groups ( p = 0.8). The mortality for elective surgery was 3.2% (2/61). The rate of permanent paraplegia and stroke were each 2.6% (3/112). The rate of cerebrovascular accident was significantly higher in the dissection group (8.5% vs. 1.2%, p = 0.05). The survival at 1, 5, and 10 years was 80.6, 56.1, and 32.7%, respectively.
Conclusion Our data confirm that open replacement of the thoracoabdominal aorta can be performed in expert centers quite safely. Different aortic pathologies (degenerative aneurysm vs. dissection) do not influence the short- and long-term outcomes. Open surgery should still be considered the standard in the management of TAAD.
Keywords: thoracoabdomnial aortic disease, thoracoabdominal aortic aneurysm
Since the early era of the 1950s, 1 2 much progress has been made in the surgical treatment of thoracoabdominal aortic disease (TAAD). In the 1970s, Crawford introduced many new concepts that are still used today including endoaortic graft inclusion and patch reattachment of visceral vessels. 3 Until the late 1980s, the cross-clamp technique was the mainstay of surgical repair. Although results from that era were impressive, the rate of neurological dysfunction (especially spinal cord injury [SCI]) remained high. 4 Many adjunct techniques were proposed in the 1990s to address this complication, including distal aortic perfusion, intraoperative and postoperative cerebrospinal fluid drainage, systemic cooling, spinal cooling, and monitoring of somatosensory-evoked potentials. 5 6 7 8 9 More recently, alternative strategies such as endovascular 10 11 12 or hybrid 13 14 15 approaches have been proposed.
Despite this progress, the best strategy to reduce surgical-related morbidity remains contentious. Here, we report our experience in the open treatment of TAAD from 2000 to 2016, evaluating overall outcomes and the effect of different aortic pathology on successful repair.
Materials and Methods
Study Design and Definitions
A retrospective analysis of our institutional database was performed to identify all cases of open thoracoabdominal aortic replacement between 2000 and 2016. Although our experience with this operation began in 1983, we restricted this analysis to the modern era. Data regarding preoperative risk factors, extent of replacement, use of the adjuncts, and postoperative course were collected from the patients' medical charts. All patients were followed-up with cross-sectional imaging at 1 month after surgery and clinically every 6 months to a year thereafter. Long-term survival information was based on clinical follow-up, routine radiographic imaging, Social Security Death Index, Epic (Epic Systems Corporation, Verona, WI) death information, and online obituary check (Aortic Institute methodology). 16 Survival follow-up was 98.2% complete (2 patients were lost to follow-up).
The Human Investigation Committee of Yale University approved this study. Informed consent was not required as anonymous clinical data were used.
Early mortality is defined as death within 30 days of the index operation. SCI includes both paraplegia and paraparesis. Global neurological deficit includes both delirium requiring drug treatment, and decreased level of consciousness with inability to wean from mechanical ventilation (without localizing neurology). Focal neurological deficit is defined as localized sensory or motor deficit. Acute kidney injury (AKI) is defined as an increase of creatinine to more than 1.5 times the baseline values. 17
Indications for surgery were based on aortic diameter (generally > 5.5 cm) and clinical presentation (general, pain). Patients requiring emergent/urgent operative intervention included those with aortic rupture, end-organ malperfusion, or severe, unrelenting pain. These patients were operated on regardless of aortic diameter. 18 Elective surgery was indicated when maximum aortic diameter exceeded 5.5 cm, or when rapid enlargement was evident (> 5 mm in 6 months). 18 19 The extent of aortic replacement was based on anatomic involvement. Aneurysms limited to the descending thoracic aorta were not considered in this analysis.
Surgical Technique
Whenever the clinical condition allows, a spinal drain is inserted and a computed tomography angiography (CTA) is performed to detect the anterior spinal artery (artery of Adamkiewicz). Since 2009, we have routinely used neuromonitoring with somatosensory-evoked potentials 20 (26.7%, 30/112).
When technically feasible, left heart bypass (LHB) was used for distal aortic perfusion. This was used in 79.5% (89/112) of cases. When the left inferior pulmonary vein was unavailable for cannulation, we used cardiopulmonary bypass (CPB) with femoro-femoral cannulation. This was used in nine patients (8%). If the proximal aorta was considered “unclampable,” we used deep hypothermic circulatory arrest (DHCA), which was necessary in four patients (3.5%).
The clamp-and-sew technique was reserved for patients with vessels unsuitable for peripheral cannulation, or electively in Crawford type IV replacement when the spinal artery was not involved in the replacement. This technique was used in 12.5% (14/112) of patients.
Our surgical technique is as follows: After a standard thoracoabdominal incision, the diaphragm is divided. We usually utilize a circumferential incision of the diaphragm ∼2 cm from the costal margin. This incision is carried into the hiatus permitting access in the retroperitoneal space, medial visceral rotation, and access to the aorta.
LHB is started specifically and only after clamping the aorta; we have demonstrated that this lowers the risk of embolic stroke from the retroperfusion. 21 The sequential clamping technique is used in a craniocaudal fashion. Intercostal and lumbar arteries in the portion corresponding to the previously identified spinal artery are preserved if possible or directly anastomosed to the graft. In 22 patients, a separate graft was used (19.6%). The visceral arteries are preferably reattached as a patch (or individually if necessary). The patch usually includes the celiac artery, the superior mesenteric artery (SMA), and the right renal arteries. The left renal artery is reattached separately. In patients with a connective tissue disease, we use a branched graft for the visceral vessels. At the end of the procedure, the aneurysmal sac is closed over the prosthesis.
Statistical Analysis
Continuous variables are presented as mean ± standard deviation or as median as appropriate. Categorical variables are presented as number and percentage. Univariate analysis was done with Pearson's chi-square test, Fischer's exact test, Student's t -test, and analysis of variance (ANOVA) analysis, as appropriate. All the preoperative and intraoperative variables with a p -value of < 0.01 were entered in a multivariate analysis with a stepwise logistic regression to identify independent predictors of operative mortality. However, the number of events was too low to reach statistical significance according to overfitting rules. 22 Long-term survival was estimated using the Kaplan–Meier method, and the Gehan–Breslow–Wilcoxon test was used to make intergroup comparisons. All the statistical analysis was performed using IBM SPSS Statistics for Macintosh (Version 22.0, IBM Corp, Armonk, NY).
Patients
From 2000 to 2016, 112 consecutive patients underwent open repair of TAAD by one surgeon (J.A.E.). Mean age was 66 years ( ± 10) and 54% (61/112) were male. In 77 patients, a degenerative aneurysm was present, while the remaining 35 patients had a dissection (acute/subacute or chronic). Patients with dissection were significantly younger ( p < 0.00001). According to the Crawford classification for the extent of repair, we preformed 39 type I, 27 type II, 28 type III, and 18 type IV repairs ( Fig. 1 ). Hypertension was present in 91.1% of patients. Coronary artery disease was present in 33.9% and was significantly more common in aneurysm patients than dissectors ( p = 0.003). As expected, previous ascending aortic replacement was more common in dissection patients ( p = 0.008), and previous abdominal aortic aneurysm (AAA) repair was more common in aneurysm patients ( p = 0.004). Preoperative patient variables are listed in Table 1 .
Fig. 1.
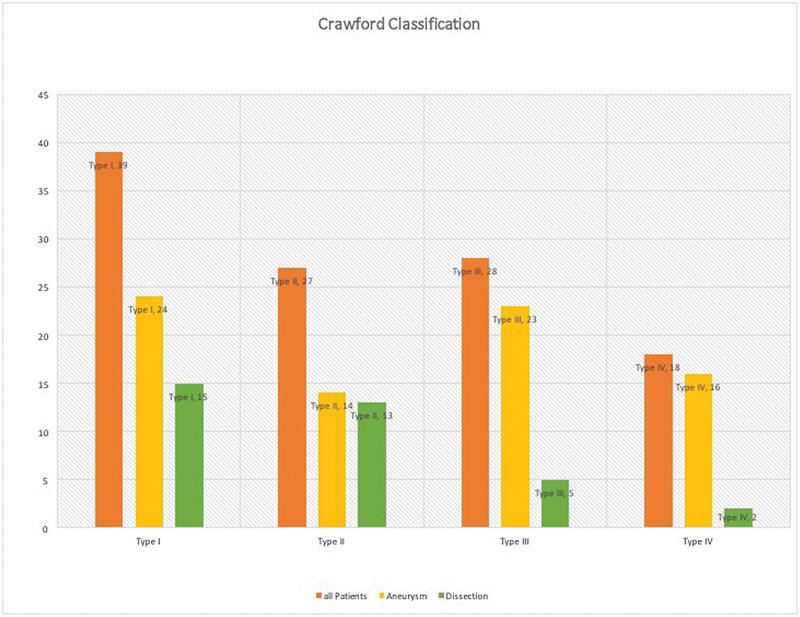
Number of operations by Crawford classification.
Table 1. Pre-operative patient demographics, cardiovascular risk factors and aortic anatomy (aneurysm vs. dissection cases).
| Preoperative variables | All patients | Aneurysm | Dissection | p -Value | |||
|---|---|---|---|---|---|---|---|
| Age (y) | 66 | ±10 | 70 | ±8 | 58 | ±11 | < 0.00001 |
| Male | 61 | 54% | 41 | 53.2% | 20 | 57.1% | 0.7 |
| Aortic aneurysm without dissection | 77 | 68.75% | |||||
| Aortic dissection | 35 | 31.25% | |||||
| Acute/subacute | 10 | 28.5% | |||||
| Chronic | 25 | 71.5% | |||||
| Type I | 10 | 28.6% | |||||
| Type IIIa | 10 | 28.6% | |||||
| Type IIIb | 15 | 42.8% | |||||
| Largest aortic diameter (cm) | 6.19 | ±1.26 | 6.38 | ±1.12 | 5.7 | ±1.45 | 0.1 |
| Hypertension | 102 | 91.1% | 68 | 88.3% | 34 | 97.1% | 0.1 |
| Smoking | 63 | 56.2% | 45 | 58.4% | 18 | 51.4% | 0.4 |
| Dyslipidemia | 46 | 41.1% | 32 | 41.5% | 14 | 40% | 0.8 |
| Obesity | 20 | 17.8% | 14 | 18.1% | 6 | 17.1% | 0.8 |
| Diabetes mellitus (DM) | 17 | 15.1% | 13 | 16.8% | 4 | 11.4% | 0.4 |
| Congestive heart failure | 15 | 13.3% | 10 | 12.9% | 5 | 14.2% | 0.8 |
| Coronary artery disease | 38 | 33.9% | 33 | 42.8% | 5 | 14.2% | 0.003 |
| Chronic obstructive pulmonary disease | 45 | 40.1% | 29 | 37.6% | 16 | 45.7% | 0.4 |
| Peripheral vascular disease | 28 | 25% | 22 | 28.5% | 6 | 17.1% | 0.1 |
| Chronic renal failure | 25 | 20.4% | 16 | 20.7% | 9 | 25.7% | 0.5 |
| Previous cardiac surgery | 44 | 39.2% | 28 | 36.3% | 16 | 45.7% | 0.3 |
| Previous ascending aortic repair | 27 | 24.1% | 13 | 16.8% | 14 | 40% | 0.008 |
| Previous AAA repair | 25 | 22.3% | 23 | 29.8% | 2 | 5.7% | 0.004 |
| Genetically triggered disorder | 11 | 9.8% | 6 | 7.7% | 5 | 14.2% | 0.2 |
| Elective | 61 | 54.4% | 43 | 55.8% | 18 | 51.4% | 0.6 |
| Urgent or emergent | 51 | 45.6% | 34 | 44.2% | 17 | 48.6% | 0.6 |
Abbreviation: AAA, abdominal aortic aneurysm.
Results
Early Outcome
The early mortality was 10.7% (12/112), with no significant difference between aneurysm and dissection patients ( p = 0.8). In elective operations, the early mortality was 3.2% (2/61). There were 5 intraoperative deaths, 2 due to uncontrollable hemorrhage and 3 due to cardiogenic shock. Other causes of early death were anoxic brain injury in 3, multisystem organ failure in 2, respiratory failure in 1, and sudden death in 1. On univariate analysis, previous cardiac surgery ( p = 0.004) and urgent/emergent repair ( p = 0.005) were strongly associated with early mortality. SCI was seen in 11 patients (9.8%) with 3 cases (2.6%) of permanent paraplegia and the remaining 8 transient (7.1%). A focal neurological brain deficit was seen in 4 patients (3.5%) and was significantly more common in patients with dissection ( p = 0.05). Global neurological deficit was evident in 14 patients (12.5%), 5 of which resolved spontaneously within 24 hours, 5 within 72 hours, and the last 4 lasted for more than 72 hours. On univariate analysis, chronic renal failure (CRF) ( p = 0.008) and previous cardiac surgery ( p = 0.008) were associated with postoperative global neurological deficit. Twenty-five (22.3%) patients developed AKI, 9 (8%) of whom required dialysis. All early outcomes are listed in Table 2 .
Table 2. Post-operative morbidity and mortality.
| All patients | Aneurysm | Dissection | p -Value | ||||
|---|---|---|---|---|---|---|---|
| Early mortality (30 d mortality) | 12 | 10.7% | 8 | 10.3% | 4 | 11.4% | 0.8 |
| Early mortality in elective patients | 2 | 3.2% | 1 | 1.2% | 1 | 2.8% | 0.5 |
| Spinal cord injury | 11 | 9.8% | 8 | 10.3% | 3 | 8.5% | 0.7 |
| Permanent paraplegia | 3 | 2.6% | 2 | 2.5% | 1 | 2.8% | 0.9 |
| Resolved within 72 h or with reinstatement of spinal drainage | 8 | 7.1% | 6 | 7.7% | 2 | 5.7% | |
| Global neurological deficit | 14 | 12.5% | 12 | 15.5% | 2 | 5.7% | 0.1 |
| Resolved in 24 h | 5 | 4.4% | 4 | 5.1 | 1 | 2.8 | 0.5 |
| Resolved in 24–72 h | 5 | 4.4% | 5 | 6.4% | 0 | ||
| Focal neurological deficit | 4 | 3.5% | 1 | 1.2% | 3 | 8.5% | 0.05 |
| Resolved in 24 h | 1 | 0.8% | 0 | 1 | 2.8% | ||
| Need for tracheostomy or reintubation | 23 | 20.5% | 14 | 18.1% | 9 | 25.7% | 0.3 |
| Pleural effusion requiring thoracentesis | 15 | 13.3% | 7 | 9.1% | 8 | 22.8% | 0.04 |
| Acute kidney injury | 25 | 22.3% | 18 | 23.3% | 7 | 20% | 0.6 |
| Temporary dialysis | 9 | 8% | 8 | 10.3% | 1 | 2.8% | |
| Reexploration for bleeding | 12 | 10.7% | 7 | 9.1% | 5 | 14.2% | 0.4 |
Late Outcome
The median follow-up duration was 36 months (range, 1–180 months). The Kaplan–Meier survival at 1, 2, 5, and 10 years was 80.6, 73.9, 56.1, and 32.7%, respectively ( Fig. 2 ). On univariate analysis, the need for tracheostomy or reintubation in the early postoperative period was associated with late mortality ( p < 0.0001). Although patients with degenerative aneurysms were older, the hazard ratio (HR) of death for this group was not significantly higher than the cohort of dissection patients (HR, 1.11, 95% confidence interval [CI], 0.6–2.05, p = 0.7) ( Fig. 3 ).
Fig. 2.
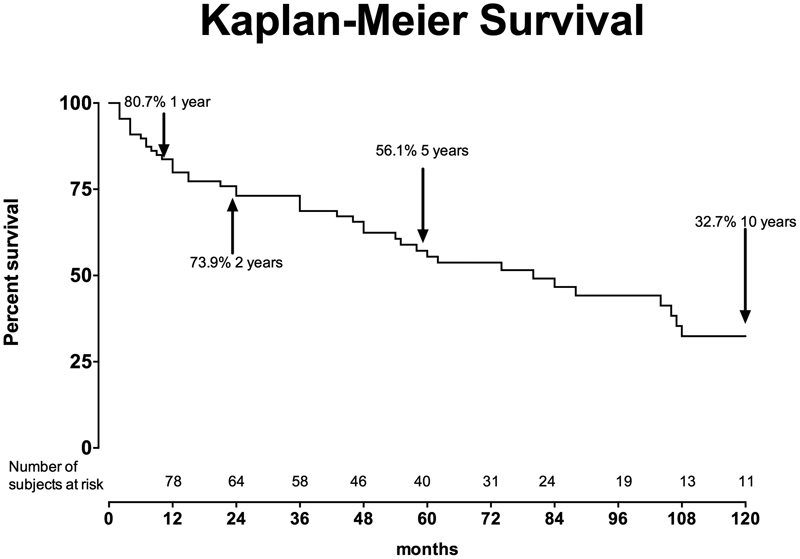
Ten-year survival with the Kaplan–Maier method.
Fig. 3.
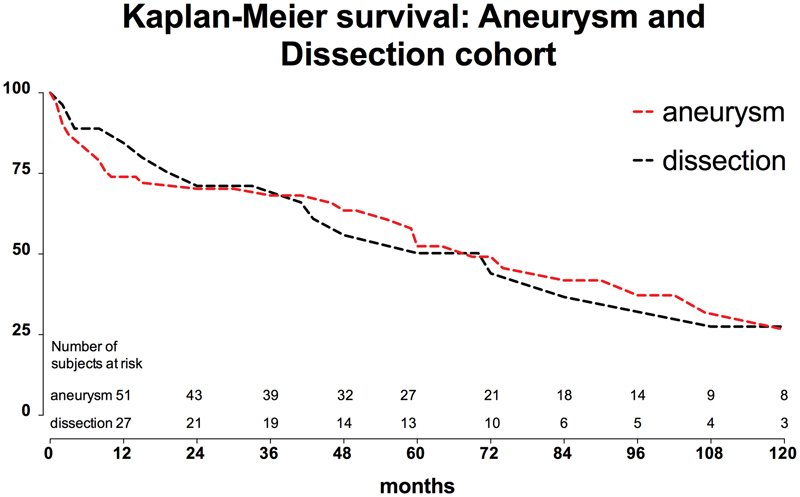
Ten-year survival with the Gehan–Breslow–Wilcoxon test for the two groups of patients (aneurysm vs. dissection).
Aortic reintervention was necessary in 10 patients at a time interval of 1 to 15 years (median, 3 years); the procedures performed are shown in Table 3 . Nine patients underwent an open procedure and one an endovascular procedure.
Table 3. Number and location of late reoperation.
| Aortic segment | N | Aneurysm | Dissection |
|---|---|---|---|
| Root and ascending | 1 | 1 | 0 |
| Proximal contiguous aorta | 2 | 2 | 0 |
| Visceral artery patch | 1 | 1 | 0 |
| Distal contiguous aorta | 2 | 1 | 1 |
| Subrenal aorta | 2 | 2 | 0 |
Discussion
In the past two decades, intra- and perioperative management of patients undergoing thoracoabdominal aortic replacement has evolved substantially, resulting in improved early survival and decreased morbidity. 23 24 Remarkably, in the late 1980s, using the clamp-and-sew technique, Crawford et al reported an early mortality of less than 10%, 4 but the rate of SCI was very high, with an incidence of up to 50% in type II aortas when the clamp time exceeded 45 minutes. 25 The introduction of distal aortic perfusion with LHB and of preoperative use of spinal drainage has brought a meaningful reduction of SCI from 39 to 10% in type II cases. 26 27 In our series, the overall rate of SCI was 9.8%, but most of these cases were transient paraplegia/paraparesis, with a rate of permanent paraplegia of 2.6%, results that are consistent with Coselli et al's remarkably extensive experience. 23
Another important factor with regards to SCI rate is the aggressive reattachment of intercostal arteries in the critical zone between T8 and L1. In recent years, the use of the CTA has superseded selective angiography for the detection of the spinal artery. 28 Knowledge of the precise location of the spinal artery permits selective reattachment, lowering the clamping time. Some authors have also recently proposed a selective reattachment of the intercostal vessel guided by neuromonitoring, 29 aiming to enhance spinal cord protection.
Although modern adjuncts have facilitated an impressive drop in the SCI rate, the rate of early mortality has not followed this trend. In tertiary referral centers, open surgical repair is accomplished with an early mortality of 10% or less. 23 24 30 However, these results are not achieved outside of the tertiary referral centers. A recent review of the Nationwide Inpatient Sample database (a discharge database representing ∼20% of United States hospitals) shows a mortality after elective TAAD repair of 22.3%, with higher rates in hospitals with lower operative volume. 31 Furthermore, a greater than threefold higher mortality has been reported when thoracoabdominal aortic replacement is accomplished in centers with limited experience. 32 We report an overall early mortality of 10.7% for all cases and 3.2% for elective cases, in line with data from most expert centers.
Although most of the patients with TAAD have many risk factors for cerebrovascular disease, the rate of permanent stroke after thoracoabdominal aortic replacement is relatively low. Our incidence of 2.6% falls within the literature range. 23 33 We noticed, however, a difference between the aneurysm (1.2%) and dissection groups (8.5%) ( p = 0.05). This may be related to embolism from retroperfusion of the dissected aorta or to the inherent weakness of the intracranial vessels in dissectors.
While open surgery is an accepted option in the treatment of TAAD, uncertainty remains regarding the role of endovascular procedures and hybrid techniques. A fenestrated or branched graft can be used for the endovascular treatment of TAAD. However, manufacture of bespoke grafts for individual patients can be time-consuming and potentially unsuitable in urgent/emergent situations. Verhoeven et al report an early mortality of 7.8% and a SCI rate of 9% in the elective endovascular repair of TAAD. 10 Haulon et al 11 report an early mortality of 9% and a rate of SCI of 12%. In expert tertiary care centers, open TAAD repair is accomplished, as in our experience, with equal or lower mortality and SCI rates in the elective setting. In our series, the early mortality was 3.2% (2/61), in the elective setting. Although the endovascular option may be noninferior in terms of mortality and paraplegia, the rate of reintervention is high with endovascular repair in the mid-term (24% at 3 years 10 ).
In the setting of chronic thoracoabdominal dissection, experience with fenestrated and branched endovascular grafts remains extremely limited. 34 There may be some important role for thoracic endovascular aortic repair (TEVAR) in aortic dissection. 35 36 37 However, the appropriateness of TEVAR for chronic dissection is questionable, especially as regards the histologic change in a chronic dissected aorta (progressive fibrosis and immobility of the dissection flap). 38 Also, the presence of multiple fenestrations decreases the likelihood of complete false lumen thrombosis in TEVAR for chronic dissection. A meta-analysis 37 of 39 studies involving 197 patients treated with TEVAR for complicated chronic type B dissection reported a 30-day mortality of 3.2% and 2-year survival of 91%. Reintervention rates ranged from 0 to 60% in those studies with a median follow-up of 31 months.
Hybrid procedures represent another alternative in the treatment of the TAAD. They combine an extra-anatomic open surgical debranching of the visceral vessels with staged or immediate endovascular aortic repair. The avoidance of thoracotomy and single lung ventilation and the lack of aortic cross clamping are expected to limit the physiologic derangements compared with the standard surgical approach. Like other endovascular procedures, hybrid procedures are potentially unsuitable in urgent/emergency situations. In a meta-analysis 39 that included 19 studies and 660 patients, the overall early mortality of the hybrid approach was 12.6% (range from 0 to 44%) and the overall rate of SCI was 3.4% (range, 0–15.3%). Moreover, the overall rate of perioperative morbidity is disturbingly high (varying from 35.5 40 to 61% 15 ). Also, visceral graft occlusion is disturbingly more common than anticipated, with disastrous consequences. 39 Hybrid repair has not lived up to its expectations as an option for TAAD repair. 40
It should be noted, however, that in many studies the patients who underwent alternative (nonopen) procedures were considered unfit for open surgery. Thus, the role of open and endovascular procedures should be considered complementary and not strictly competitive.
This study adds to the literature demonstrating that dedicated tertiary centers can deliver open TAAD repair safely, from a mortality, stroke, and paraplegia point of view. This is not unfortunately true across a large spectrum of hospitals of varying experience levels. 31 Regionalization of care for TAAD patients should be considered.
Limitations
This study is observational in nature and involved retrospective review of data collected in a single tertiary care center. These outcomes may not be typical of the general experience at centers of varying experience levels.
Conclusion
Our data confirm that open replacement of the thoracoabdominal aorta can be performed quite safely in experienced centers. Different aortic pathologies (degenerative aneurysm vs. dissection) do not influence the short- and long-term outcomes. We are not aware of papers from the literature which show outcomes of endovascular and hybrid procedures that can justify a shift toward an alternative approach when compared with such results of open surgery.
Ongoing progress in endovascular techniques has altered the approach to TAAD repair, especially in patients who are poor candidates for open surgery. However, open repair should fairly be considered the standard in the treatment of TAAD except for highest risk, very unsuitable candidates. It is best to consider open and endovascular repair as complementary rather than competing approaches to provide the best individualized treatment for all patient categories.
Funding Statement
Funding None.
Footnotes
Conflict of Interest None.
References
- 1.De Bakey M E, Cooley D A. Successful resection of aneurysm of thoracic aorta and replacement by graft. J Am Med Assoc. 1953;152(08):673–676. doi: 10.1001/jama.1953.03690080017005. [DOI] [PubMed] [Google Scholar]
- 2.Etheredge S N, Yee J, Smith J V, Schonberger S, Goldman M J. Successful resection of a large aneurysm of the upper abdominal aorta and replacement with homograft. Surgery. 1955;38(06):1071–1081. [PubMed] [Google Scholar]
- 3.Crawford E S. Thoraco-abdominal and abdominal aortic aneurysms involving renal, superior mesenteric, celiac arteries. Ann Surg. 1974;179(05):763–772. doi: 10.1097/00000658-197405000-00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crawford E S, Crawford J L, Safi H J et al. Thoracoabdominal aortic aneurysms: preoperative and intraoperative factors determining immediate and long-term results of operations in 605 patients. J Vasc Surg. 1986;3(03):389–404. doi: 10.1067/mva.1986.avs0030389. [DOI] [PubMed] [Google Scholar]
- 5.Cambria R P, Davison J K, Zannetti Set al. Clinical experience with epidural cooling for spinal cord protection during thoracic and thoracoabdominal aneurysm repair J Vasc Surg 19972502234–241., discussion 241–243 [DOI] [PubMed] [Google Scholar]
- 6.Coselli J S, LeMaire S A.Left heart bypass reduces paraplegia rates after thoracoabdominal aortic aneurysm repair Ann Thorac Surg 199967061931–1934., discussion 1953–1958 [DOI] [PubMed] [Google Scholar]
- 7.Griepp R B, Ergin M A, Galla J D, Klein J J, Spielvogel D, Griepp E B. Minimizing spinal cord injury during repair of descending thoracic and thoracoabdominal aneurysms: the Mount Sinai approach. Semin Thorac Cardiovasc Surg. 1998;10(01):25–28. doi: 10.1016/s1043-0679(98)70013-9. [DOI] [PubMed] [Google Scholar]
- 8.Kouchoukos N T, Daily B B, Rokkas C K, Murphy S F, Bauer S, Abboud N.Hypothermic bypass and circulatory arrest for operations on the descending thoracic and thoracoabdominal aorta Ann Thorac Surg 1995600167–76., discussion 76–77 [PubMed] [Google Scholar]
- 9.Schepens M AAM, Defauw J JAM, Hamerlijnck R PHM, Vermeulen F EE. Use of left heart bypass in the surgical repair of thoracoabdominal aortic aneurysms. Ann Vasc Surg. 1995;9(04):327–338. doi: 10.1007/BF02139403. [DOI] [PubMed] [Google Scholar]
- 10.Verhoeven E L, Katsargyris A, Bekkema F et al. Editor's choice - ten-year experience with endovascular repair of thoracoabdominal aortic aneurysms: results from 166 consecutive patients. Eur J Vasc Endovasc Surg. 2015;49(05):524–531. doi: 10.1016/j.ejvs.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 11.Haulon S, D'Elia P, O'Brien N et al. Endovascular repair of thoracoabdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2010;39(02):171–178. doi: 10.1016/j.ejvs.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg R, Eagleton M, Mastracci T.Branched endografts for thoracoabdominal aneurysms J Thorac Cardiovasc Surg 2010140(6, Suppl):S171–S178. [DOI] [PubMed] [Google Scholar]
- 13.Pacini D, Di Marco L, Murana G, Pantaleo A, Leone A, Di Bartolomeo R. Hybrid repair of thoracoabdominal aneurysm: a two-stage approach. Ann Thorac Surg. 2013;96(04):1496–1498. doi: 10.1016/j.athoracsur.2013.04.115. [DOI] [PubMed] [Google Scholar]
- 14.Chiesa R, Tshomba Y, Melissano G et al. Hybrid approach to thoracoabdominal aortic aneurysms in patients with prior aortic surgery. J Vasc Surg. 2007;45(06):1128–1135. doi: 10.1016/j.jvs.2006.10.057. [DOI] [PubMed] [Google Scholar]
- 15.Black S A, Wolfe J H, Clark M, Hamady M, Cheshire N J, Jenkins M P.Complex thoracoabdominal aortic aneurysms: endovascular exclusion with visceral revascularization J Vasc Surg 200643061081–1089., discussion 1089 [DOI] [PubMed] [Google Scholar]
- 16.Peterss S, Charilaou P, Ziganshin B A, Elefteriades J A. Assessment of survival in retrospective studies: the Social Security Death Index is not adequate for estimation. J Thorac Cardiovasc Surg. 2017;153(04):899–901. doi: 10.1016/j.jtcvs.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 17.Bellomo R, Kellum J A, Ronco C. Defining and classifying acute renal failure: from advocacy to consensus and validation of the RIFLE criteria. Intensive Care Med. 2007;33(03):409–413. doi: 10.1007/s00134-006-0478-x. [DOI] [PubMed] [Google Scholar]
- 18.Elefteriades J A.Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks Ann Thorac Surg 20027405S1877–S1880., discussion S1892–S1898 [DOI] [PubMed] [Google Scholar]
- 19.Erbel R, Aboyans V, Boileau C et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. Eur Heart J. 2014;35(41):2873–2926. doi: 10.1093/eurheartj/ehu281. [DOI] [PubMed] [Google Scholar]
- 20.Liu L Y, Callahan B, Peterss S et al. Neuromonitoring using motor and somatosensory evoked potentials in aortic surgery. J Card Surg. 2016;31(06):383–389. doi: 10.1111/jocs.12739. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein L J, Davies R R, Rizzo J A et al. Stroke in surgery of the thoracic aorta: incidence, impact, etiology, and prevention. J Thorac Cardiovasc Surg. 2001;122(05):935–945. doi: 10.1067/mtc.2001.117276. [DOI] [PubMed] [Google Scholar]
- 22.Rizzo J A, Chen J, Fang H, Ziganshin B A, Elefteriades J A. Statistical challenges in identifying risk factors for aortic disease. Aorta (Stamford) 2014;2(02):45–55. doi: 10.12945/j.aorta.2014.14-019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coselli J S, LeMaire S A, Preventza O et al. Outcomes of 3309 thoracoabdominal aortic aneurysm repairs. J Thorac Cardiovasc Surg. 2016;151(05):1323–1337. doi: 10.1016/j.jtcvs.2015.12.050. [DOI] [PubMed] [Google Scholar]
- 24.Conrad M F, Crawford R S, Davison J K, Cambria R P.Thoracoabdominal aneurysm repair: a 20-year perspective Ann Thorac Surg 20078302S856–S861., discussion S890–S892 [DOI] [PubMed] [Google Scholar]
- 25.Svensson L G, Crawford E S, Hess K R, Coselli J S, Safi H J.Experience with 1509 patients undergoing thoracoabdominal aortic operations J Vasc Surg 19931702357–368., discussion 368–370 [PubMed] [Google Scholar]
- 26.Safi H J, Miller C C, III, Huynh T Tet al. Distal aortic perfusion and cerebrospinal fluid drainage for thoracoabdominal and descending thoracic aortic repair: ten years of organ protection Ann Surg 200323803372–380., discussion 380–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Epstein N E. Cerebrospinal fluid drains reduce risk of spinal cord injury for thoracic/thoracoabdominal aneurysm surgery: a review. Surg Neurol Int. 2018;9:48. doi: 10.4103/sni.sni_433_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka H, Ogino H, Minatoya K et al. The impact of preoperative identification of the Adamkiewicz artery on descending and thoracoabdominal aortic repair. J Thorac Cardiovasc Surg. 2016;151(01):122–128. doi: 10.1016/j.jtcvs.2015.07.079. [DOI] [PubMed] [Google Scholar]
- 29.Estrera A L, Sandhu H K, Charlton-Ouw K M et al. A quarter century of organ protection in open thoracoabdominal repair. Ann Surg. 2015;262(04):660–668. doi: 10.1097/SLA.0000000000001432. [DOI] [PubMed] [Google Scholar]
- 30.Gaudino M, Lau C, Munjal M, Girardi L N. Open repair of ruptured descending thoracic and thoracoabdominal aortic aneurysms. J Thorac Cardiovasc Surg. 2015;150(04):814–821. doi: 10.1016/j.jtcvs.2015.06.077. [DOI] [PubMed] [Google Scholar]
- 31.Cowan J A, Jr, Dimick J B, Henke P K, Huber T S, Stanley J C, Upchurch G R., Jr Surgical treatment of intact thoracoabdominal aortic aneurysms in the United States: hospital and surgeon volume-related outcomes. J Vasc Surg. 2003;37(06):1169–1174. doi: 10.1016/s0741-5214(03)00085-5. [DOI] [PubMed] [Google Scholar]
- 32.Gopaldas R R, Huh J, Dao T K et al. Superior nationwide outcomes of endovascular versus open repair for isolated descending thoracic aortic aneurysm in 11,669 patients. J Thorac Cardiovasc Surg. 2010;140(05):1001–1010. doi: 10.1016/j.jtcvs.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Kouchoukos N T, Kulik A, Castner C F.Open thoracoabdominal aortic repair for chronic type B dissection J Thorac Cardiovasc Surg 2015149(2, Suppl)S125–S129. [DOI] [PubMed] [Google Scholar]
- 34.Kitagawa A, Greenberg R K, Eagleton M J, Mastracci T M, Roselli E E. Fenestrated and branched endovascular aortic repair for chronic type B aortic dissection with thoracoabdominal aneurysms. J Vasc Surg. 2013;58(03):625–634. doi: 10.1016/j.jvs.2013.01.049. [DOI] [PubMed] [Google Scholar]
- 35.Nienaber C A, Rousseau H, Eggebrecht H et al. Randomized comparison of strategies for type B aortic dissection: the INvestigation of STEnt Grafts in Aortic Dissection (INSTEAD) trial. Circulation. 2009;120(25):2519–2528. doi: 10.1161/CIRCULATIONAHA.109.886408. [DOI] [PubMed] [Google Scholar]
- 36.Nienaber C A, Kische S, Rousseau H et al. Endovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv. 2013;6(04):407–416. doi: 10.1161/CIRCINTERVENTIONS.113.000463. [DOI] [PubMed] [Google Scholar]
- 37.Thrumurthy S G, Karthikesalingam A, Patterson B O et al. A systematic review of mid-term outcomes of thoracic endovascular repair (TEVAR) of chronic type B aortic dissection. Eur J Vasc Endovasc Surg. 2011;42(05):632–647. doi: 10.1016/j.ejvs.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Peterss S, Mansour A M, Ross J A et al. Changing pathology of the thoracic aorta from acute to chronic dissection: literature review and insights. J Am Coll Cardiol. 2016;68(10):1054–1065. doi: 10.1016/j.jacc.2016.05.091. [DOI] [PubMed] [Google Scholar]
- 39.Canaud L, Karthikesalingam A, Jackson D et al. Clinical outcomes of single versus staged hybrid repair for thoracoabdominal aortic aneurysm. J Vasc Surg. 2013;58(05):1192–1200. doi: 10.1016/j.jvs.2013.04.061. [DOI] [PubMed] [Google Scholar]
- 40.Chiesa R, Tshomba Y, Melissano G, Logaldo D. Is hybrid procedure the best treatment option for thoraco-abdominal aortic aneurysm? Eur J Vasc Endovasc Surg. 2009;38(01):26–34. doi: 10.1016/j.ejvs.2009.03.018. [DOI] [PubMed] [Google Scholar]


