Abstract
Abdominal aortic aneurysm (AAA) is defined as a permanent dilatation of the abdominal aorta that exceeds 3 cm. Most AAAs arise in the portion of abdominal aorta distal to the renal arteries and are defined as infrarenal. Most AAAs are totally asymptomatic until catastrophic rupture. The strongest predictor of AAA rupture is the diameter. Surgery is indicated to prevent rupture when the risk of rupture exceeds the risk of surgery. In this review, we aim to analyze this disease comprehensively, starting from an epidemiological perspective, exploring etiology and pathophysiology, and concluding with surgical controversies. We will pursue these goals by addressing eight specific questions regarding AAA: (1) Is the incidence of AAA increasing? (2) Are ultrasound screening programs for AAA effective? (3) What causes AAA: Genes versus environment? (4) Animal models: Are they really relevant? (5) What pathophysiology leads to AAA? (6) Indications for AAA surgery: Are surgeons over-eager to operate? (7) Elective AAA repair: Open or endovascular? (8) Emergency AAA repair: Open or endovascular?
Keywords: abdominal aortic aneurysm, AAA, risk factors, animal models, indication for AAA surgery, rupture AAA, endovascular aortic repair
Key Points
Ultrasound screening programs for AAAs are immensely effective.
Both genes and environmental factors contribute to the development of AAAs.
Three key processes contribute to AAAs development: proteolysis, inflammation, and vascular smooth muscle cell (VSMC) apoptosis.
Surgical indications for AAA depend on an accurate balance between the risk of rupture and the risk of surgery.
Endovascular repair offers a lower early procedural mortality and morbidity, but open surgery achieves greater survival and freedom from reintervention in the long term.
Dilatation of the abdominal aorta is a complex and dynamic process that eventually leads to the formation of an abdominal aortic aneurysm (AAA). The term aneurysm derives from the Greek ανευρυσμα (aneurusma) that means widening. Aneurysm can be defined as a permanent, irreversible, and localized dilatation of a vessel that exceeds 1.5 times the normal diameter of the vessel. For the abdominal aorta, the threshold is a diameter of more than 3 cm. Most AAAs develop in the portion of aorta 1 to 2 cm distal to the renal artery and are termed infrarenal AAA. These occur mainly in men older than 65 years. A key risk factor is cigarette smoking. From a molecular perspective, three processes are involved in the development of AAA: proteolysis, inflammation, and smooth muscle cell (SMC) apoptosis. Although some symptoms can be linked to AAA, most aneurysms are totally asymptomatic until rupture, which leads to death in 65% of patients (patients who die outside the hospital plus perioperative mortality). 1 2 The strongest predictor of AAA rupture is the diameter. Surgery is indicated to prevent rupture and should be performed when the risk of rupture exceeds the risk of surgery. For AAA repair, two options are currently available: standard open surgery and endovascular aortic repair (EVAR). In this review, we aim to analyze this disease comprehensively, starting from an epidemiological perspective, exploring etiology and pathophysiology, aspect, and concluding with surgical controversies. We will pursue these goals by addressing eight specific questions regarding AAA:
Is the incidence of AAA increasing?
Are ultrasound screening programs for AAA effective?
What causes AAA: Genes versus environment?
Animal models: Are they really relevant?
What pathophysiology leads to AAA?
Indications for AAA surgery: Are surgeons over-eager to operate?
Elective AAA repair: Open or endovascular?
Emergency AAA repair: Open or endovascular?
Is the Incidence of AAA Increasing?
During the second half of the 20th century, there has been a steady increase in incidence and mortality from AAA. Important articles documenting this trend are summarized in Table 1 , which shows compelling, extensive, consistent, and worldwide evidence of increased aneurysm mortality over the last half century. This rise in incidence and mortality from AAA has usually been ascribed to the aging of the population, increased detection (from increased imaging), and also, perhaps, to a bona fide increase of this disease in the human population. 3
Table 1. Increasing incidence of AAA and death due to AAA in the prescreening era.
| Population | Year | Key findings regarding AAA |
|---|---|---|
| Rochester, MN 236 | 1984 | Incidence increased sevenfold between 1951 and 1980 |
| United States 237 | 1987 | From 1951 to 1968, age-specific and age-adjusted mortalities increased constantly (average annual increase of 17% for white males, 12% for white females, 14% for nonwhite males, and 15% for nonwhite females) |
| England and Wales 238 | 1989 | Deaths due to AAA increased by 53% between 1974 and 1984 |
| Kansas City, KS 239 | 1991 | Prevalence increased between 1950–1959 and 1970–1984 in Kansas City among both men (1.5-fold increase) and women (2.5-fold increase) |
| Australia 240 | 1991 | From 1980 to 1988, age-standardized AAA mortality rate increased 36% in men and 24% in women |
| Sweden 241 | 1992 | A necropsy study showed that from 1958 to 1986 mean annual age-standardized increase of aortic aneurysmal disease was 4.7% among men and 3.0% among women |
| Sweden 242 | 1992 | From 1960 to 1988, the annual rate of rupture of AAA standardized for age increased by 2.4% yearly |
| Canada 243 | 1995 | From 1969 to 1991, an increasing number of AAA was diagnosed |
| United States 244 | 1999 | From 1979 to 1991, there was a 20% increase of deaths due to and 50% increase in AAA hospitalizations |
| England and Wales 245 | 2005 | From 1979 to 1999, AAA mortality rate and hospital admissions for AAA increased steadily |
Abbreviation: AAA, abdominal aortic aneurysm.
As we shall see immediately below, a crucial advance in diagnosis of AAA profoundly counteracted this rising toll of AAA on the human population.
Are Ultrasound Screening Programs for AAA Effective?
During the early part of the era depicted in Table 1 , the overall mortality for ruptured AAA (rAAA) (combining mortality outside the hospital and mortality from emergency surgery) overall exceeded 80%. 4 5
Subsequently, the advent of a noninvasive diagnostic tool completely altered this bleak picture. It was found that abdominal ultrasonography could detect AAA in 97.3% of affected patients. 6 Subsequently, multiple randomized, controlled studies demonstrated the tremendous effectiveness and epidemiologic impact of ultrasound-based screening programs for AAA (see Table 2 ). The Aneurysm Detection and Management (ADAM) Study (VA Cooperative investigation) also showed vividly the extremely strong impact of smoking on incidence of AAA (5.7-fold increase). 7
Table 2. Randomized controlled studies of ultrasound screening for AAA.
| Population | Year | Number of patients | Key findings regarding AAA |
|---|---|---|---|
| United Kingdom 246 | 1995 | 15,777 | AAA detected in 4% overall and 7.6% of men. Screening lowered incidence of rupture by 55% in men, while of no benefit in women |
| Denmark 247 | 2005 | 12,639 M Age > 65 y |
AAA found in 4% of men. Need for emergency surgery for AAA lowered by 75% and death from AAA lowered by 67% |
| MASS (multicenter aneurysm screening study) 13 | 2004 | 65,800 M Age 64–72 y |
Aneurysm mortality reduced by 42% |
| Australia 248 | 2004 | 41,000 Age 65–83 y |
AAA prevalence 7.2%. Mortality ratio 0.61 (screened group to nonscreened group), but difference NS (unfortunately multiple mortalities occurred in patients randomized to scanning who did not comply to be scanned, skewing results adversely) |
| Cochrane review 249 | 2007 | 127,891 men, 9,342 women | No reduction in all-cause mortality. Significant reduction in AAA-related death in men (OR, 0.60) |
| Aneurysm Detection and Management study (VA study) 7 | 1997 | 73,451 veterans Age 50–79 y |
Smoking raises incidence of aneurysm 5.7-fold Aneurysms found in 1.4% of patients |
Abbreviation: AAA, abdominal aortic aneurysm.
Based on this powerful data, in 2005, the U.S. preventive service task force (USPSTF) recommend one-time screening with ultrasonography for all men aged 65 to 74 years who have ever smoked (recommendation Class B). 8 In the same document, the USPSTF recommended against routine screening for women (recommendation Class D). 8 Three to four years later, England, 9 Scotland, 10 and Sweden 11 followed suit, recommending AAA screening for all men older than 65 years, this time regardless of history of smoking.
Recently, updates of these screening studies with longer follow-up have been published ( Table 3 ). Now, with follow-up up to 15 years, the strong beneficial impact of routine ultrasonographic screening for AAA in preventing AAA-related death in elderly men has been unequivocally confirmed.
Table 3. Follow-up screening studies with longer follow-up.
| Population | Year | Years of follow-up | Key findings regarding AAA |
|---|---|---|---|
| MASS 250 | 2012 | 13 | 42% reduction in AAA-related mortality with screening |
| Denmark 251 | 2010 | 14 | AAA-related mortality decreased by 66%. Screening was cost-effective |
| United Kingdom 252 | 2007 | 15 | AAA-related mortality reduced by 11% (NS)—small trial |
Abbreviations: AAA, abdominal aortic aneurysm; NS, nonsignificant.
The U.S. recommendations for screening were updated in 2014 by the USPSTF. 12 The recommendation for the ultrasonography for men older than 65 years who have ever smoked was confirmed (Class B). The last recommendations for the screening from the European Society of Cardiology are listed in Table 4 . The evidence regarding the balance of benefits and harms of screening for AAA in women smokers aged 65 to 75 years was considered insufficient to recommend screening at this time.
Table 4. Recommendations for AAA screening—European Society of Cardiology guidelines 168 .
| Recommendation | Level | Class |
|---|---|---|
| Ultrasound is recommended in all men older than 65 y | I | A |
| Ultrasound may be considered in women older than 65 y with a history of current/past smoking or positive familiar history | IIb | C |
| Ultrasound is not recommended in women with no history of smoking or familiar history | III | C |
| Ultrasound should be considered in first-degree siblings of patients with AAA | IIa | B |
| In patients with AAA with a diameter between 30 and 39 mm imaging should be considered every 3 y | IIa | B |
| In patients with AAA with a diameter between 40 and 44 mm imaging should be considered every 2 y | IIa | B |
| In patients with AAA diameter between 45 and 50 mm imaging should be considered yearly | IIa | B |
Abbreviation: AAA, abdominal aortic aneurysm.
Traditionally, prevalence of AAA in screened populations has ranged largely from 1.1 to 5.2%. 13 14 15 16 17 18 19 20 21 The most accurate studies useful to detect the AAA prevalence in the postscreening era are listed in Table 5 . What is most interesting, however, is that the mortality from AAA has been decreasing—reflecting the beneficial impact of ultrasonographic screening programs. Drops in mortality in the most recent years have been shown widely, including Australia, 22 New Zealand, 23 and England and Wales. 24
Table 5. Most accurate and recent studies on the prevalence of AAA in the postscreening era.
| Study or authors | Year | Total number of patients (men older than 65 y) | Prevalence of AAA |
|---|---|---|---|
| ADAM study 15 | 2000 | 126,196 | 4.2% (5,283/126,196) |
| MASS trial 13 | 2002 | 27,147 | 4.9% (1,333/27,147) |
| Svensjo et al 18 | 2011 | 22,187 | 2.2% (500/22,187) |
| GASP program 16 | 2012 | 52,690 | 3.82% (2,013/52,690) |
| VIVA trial 20 | 2015 | 18,749 | 3.3% (618/18,749) |
| Benson et al 19 | 2016 | 24,891 | 1.18% (292/24,891) |
| Wanhainen et al 21 | 2016 | 253,896 | 1.5% (3,891/253,896) |
Abbreviations: AAA, abdominal aortic aneurysm; ADAM, Aneurysm Detection and Management; GASP, Gloucestershire Aneurysm Screening Programme; MASS, Multicentre Aneurysm Screening Study; VIVA, viborg vascular.
Thus, in summary, we can say that over the latter half of the 20th century, incidence and mortality from AAA showed a progressive increase. However, in the last decade, medical science has succeeded, via the implementation of increased echocardiographic screening for AAA, in beneficially impacting the mortality toll taken by this disease.
What Causes Abdominal Aortic Aneurysm: Genes versus Environment
Impact of Male Gender
In general, AAA is mainly a disease of elderly males ( Fig. 1 ). 25 Its prevalence in individuals older than 65 years is three to four times higher in men than in women, 26 and the risk of AAA increase by 40% every 5 years after the age of 65 years. 27
Fig. 1.
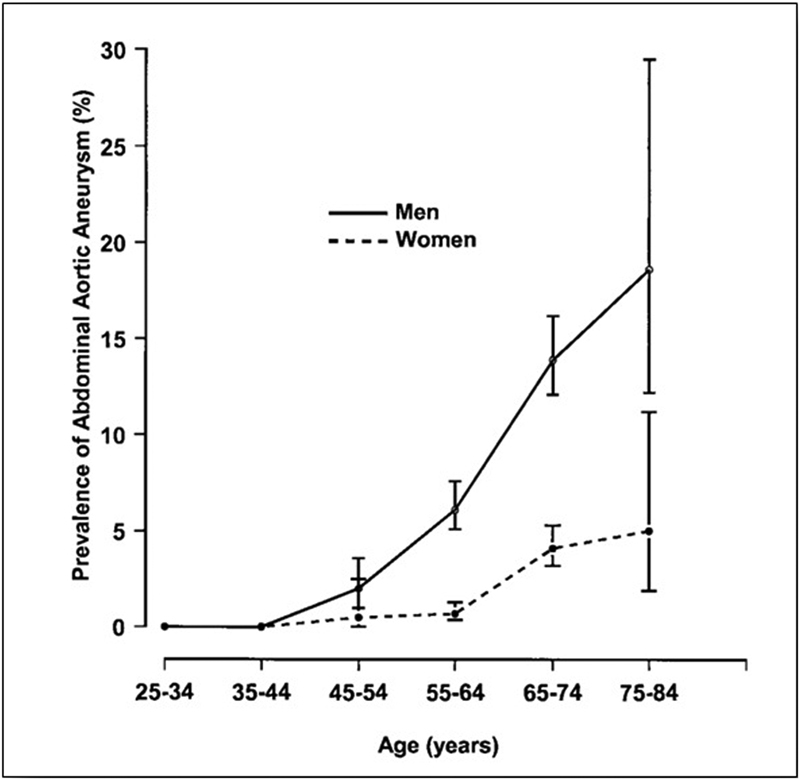
Prevalence of abdominal aortic aneurysm according to age and gender in 6,836 men and women aged 25 to 83 years analyzed in 1994 to 1995 in the city of Tromsø, Norway. Note the sharp rise of the prevalence in men after 60 years of age. (Reproduced with permission from Singh et al. 255 ).
The reason men have much higher risk of AAA than women is unclear, but probably it is the result of hormonal factors and genetic susceptibility. 28 The protective role of the female sex hormone milieu has been shown creatively in an animal model. Normally, after AAA induction, AAAs grow more slowly in female rats than in males. 29 30 However, after transplanting the female aorta into male rats, the rate growth of the AAA equals that in male rats. Estrogens are thought to exert an immunomodulatory effect; particularly, they reduce macrophage matrix metalloproteinase (MMP) production, thus decreasing the collagen degradation and slowing progression of the AAA. 31 Although less common, AAA in women have a worse prognosis, 32 with a fourfold higher risk of rupture, 33 and increased short-term mortality after both EVAR and open repair in both elective and emergent condition. 34 35 36 (The main risk factors for AAA are listed in Table 6 .)
Table 6. Environmental risk factors for AAA.
| Risk factor | OR | 95% CI | |
|---|---|---|---|
| AAA development | Male sex | ||
| Advanced age | |||
| Smoking | |||
| Former smoker vs. never smoker 253 | 2.3 | 1.9–2.8 | |
| 20 cigarettes/d vs. never smoker 37 | 13.72 | 6.12–30.78 | |
| Family history 69 | 2.2 | 1.6–3.2 | |
| Hypertension 40 | 1.25 | 1.21–1.28 | |
| Diabetes (protective role) 7 | 0.68 | 0.60–0.77 | |
| Obesity 40 | 1.20 | 1.17–1.22 | |
| AAA expansion | Smoking | ||
| AAA diameter | |||
| Cardiac transplant 254 | |||
| AAA rupture | Smoking 33 | 2.2 | 1.33–3.06 |
| Female sex | |||
| AAA diameter | |||
| Hypertension 164 | 1.04 | 1.02–1.07 | |
| Family history |
Abbreviations: AAA, abdominal aortic aneurysm; CI, confidence interval; OR, odds ratio.
Role of Cigarette Smoking
The principal modifiable risk factor for AAA is smoking. 37 38 This association was first described in 1958. 39 Since then many reports have showed the extremely strong correlation between smoking and AAA—with odds ratios (ORs) between smokers and nonsmokers ranging from 2.3 to 13.72. 37 Moreover, a linear association between number of cigarette smoked or years of smoking and prevalence of AAA has been shown. 40 In the same article, also an association shown between a decline in the prevalence of AAA and the years of widespread smoking cessation was evident. 40 Intriguingly, smoking seems to be a substantially greater risk factor for AAA than for occlusive atherosclerotic disease. 38 Smoking is also an important factor in the progression of AAA. In a recent meta-analysis using data from 15,475 patients with small (3–5.5 cm) AAAs, current smoking was associated with an increased rate of expansion (compared with nonsmokers) of 3.5 mm/year (95% confidence interval [CI], 0.23–0.48). 33 In the same article, smoking was also associated with an increased risk of rupture (hazard ratio [HR], 2.02; 95% CI, 1.33–3.06) regardless the AAA diameter. 33
This strong association between smoking and AAA has led many to investigate the molecular mechanism that can explain this deleterious effect. In a mouse AAA model treated with benzo(a)pyrene (an important constituent of cigarette smoke), increased gene expression of MMPs was evident, with degeneration of the lamellar unit and loss of SMCs. 41 Exposure to tobacco smoke in an animal model of AAA showed increased progression of AAA even in mice deficient for MMP and elastase. This progression was explained by altered activity of the immune system. 42 Moreover, nicotine (a major component of cigarette smoke) can promote the developing of AAA in an animal model through activation of adenosine monophosphate-activated kinase α2, resulting in the phosphorylation in the VSMC of the activator protein 2α, which causes increase MMP-2 gene expression. 43 Finally, it has been demonstrated in vitro that extract of cigarette smoke can inhibit expression of prolyl-4-hydroxylase in VSMC, thus decreasing collagen synthesis. 44
The impact of cigarette smoking is so powerful that Lederle 45 has shown that the dramatic rise in aneurysm mortality that characterized the second half of the 20th century was curtailed as cigarette smoking fell in the past decades of the century (see Fig. 2A , B ).
Fig. 2.
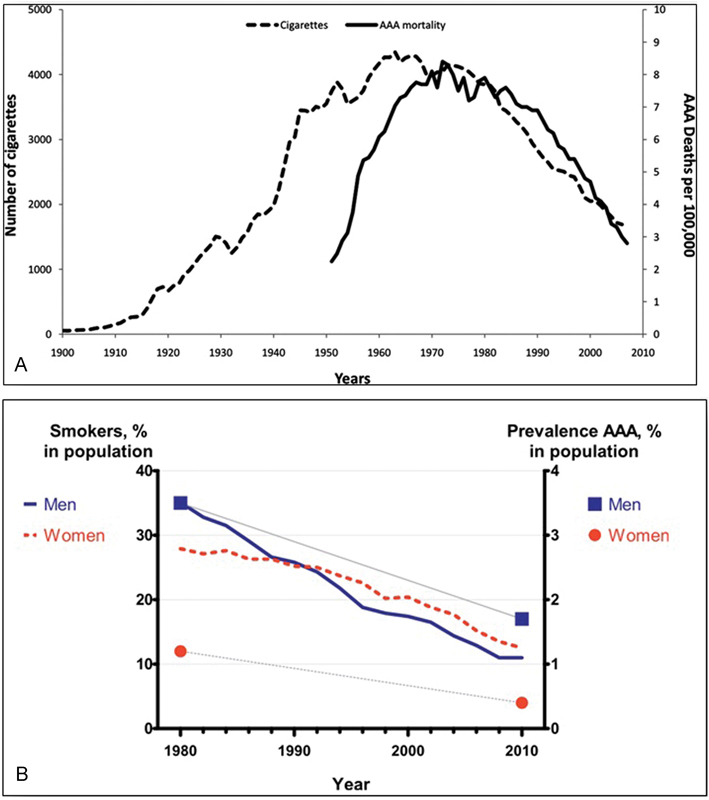
( A ) Linear correlation between number of cigarettes smoked and AAA mortality in the United States. (Reproduced with permission from Lederle. 45 ) ( B ) Historical and contemporary AAA prevalence rates compared with time trends in smoking in the Swedish population. Again, a linear correlation between smoking and AAA prevalence is evident. (Reproduced with permission from Svensjö et al. 256 ) AAA, abdominal aortic aneurysm.
Hypertension
While a strong association between smoking and AAA is evident, the association between hypertension and AAA is weak ( Fig. 3 ). In a retrospective study with a cohort more than 3 million people, hypertension was associated with AAA with an OR of 1.25 (95% CI, 1.21–1.28), 40 and in a prospective study with 7-year follow-up, the OR for AAA in patients with hypertension was slightly but significantly higher (OR, 1.54). 37 Finally, in a population-based study with both historical and current data, the association between hypertension and AAA failed to reach the statistical significance. 46 Where hypertension does matter is in the fact that high blood pressure seems to be a more important risk factor for growth and rupture of AAA. In the analysis of 2,257 patients involved in the United Kingdom small aneurysm trial (UKSAT) 47 and United Kingdom small aneurysm study, 48 after variable adjustment with the Cox's regression, the HR of rupture for patient with hypertension was 1.04 (95% CI, 1.02–1.07). 25 49 The association between hypertension and AAA rupture was confirmed in a recent meta-analysis. 33 In particular, this study, after variable adjustment, showed an increased risk of rupture of 1.11-folds (95% CI, 1.02–1.22) for each 10 mm Hg increase of the mean pressure.
Fig. 3.
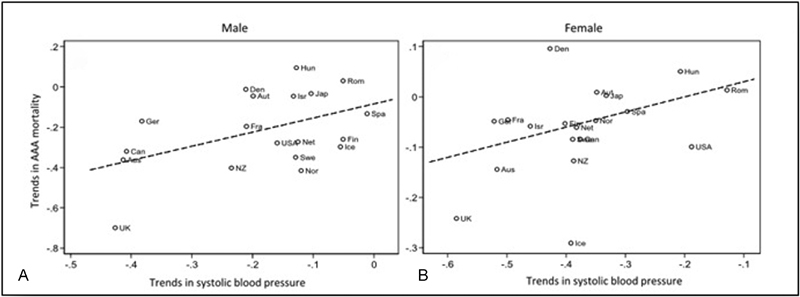
Linear regression revealing the positive association between temporal trends in (A) male and (B) female mean systolic blood pressure and AAA mortality. (Reproduced with permission from Sidloff et al. 54 ) AAA, abdominal aortic aneurysm.
Obesity
Discordant data exist about the association of AAA with obesity: in a large retrospective analysis involving more than 3 million people, body mass index (BMI) > 25 was associated with an increased risk of AAA. 40 In analysis of ultrasonography in 12,203 men aged 65 to 83 years, a correlation between obesity and AAA was shown, 50 with a stronger correlation in obese patients with a high waist circumference. 51 However, in other prospective studies, high BMI was not associated with risk of AAA. 52 53 In a recent population-based cohort study, waist circumference was associated with increased risk of AAA, while high BMI was not. 53 While BMI reflects total adiposity, waist circumference is more reflective of visceral adiposity. Therefore, it may be that visceral adiposity, rather than total adiposity, is important in the development of AAA. 54
Diabetes
Further confirming the difference between classic occlusive cardiovascular diseases (CAD and peripheral artery disease, where diabetes is one of the most important risk factors), in AAA, diabetes appears to have a protective effect. This was first proposed in 1997 after analysis of 73,451 males who underwent ultrasonography. 7 The patients with diabetes had an OR for AAA of 0.68. The authors were initially dubious regarding this result, 55 but became convinced when a similar result was found in a study with a different design, 56 convincing the authors of the protective role of diabetes against developing an AAA. 7 Since then, many other reports have confirmed the protective role of diabetes. 15 18 40 50 57 58 Finally, in 2016, an article from the ALICE group (All Literature Investigation Cardiovascular Evidence) summarized the results of seven different meta-analyses and confirmed the protective role of diabetes for AAA. 59 The protective role of diabetes is evident not only for the development of AAA but also in decreasing the growth rate of the aneurysm. A recent meta-analysis estimated an annual mean effect of diabetes on grow rate of −0.6 mm/year. 33 58 The physiopathological explanation for the protective effect of diabetes remains elusive. Both mechanics and molecular mechanisms have been postulated. In diabetic patients, a thickening of the aortic wall is evident—a factor well known to aortic surgeons. 60 According to Laplace's law, a thicker aortic wall decreases wall stress. Wall stress is considered pivotal for progression of AAA. 61 From a molecular point of view, different mechanisms have been proposed. The advanced glycation end products typical of diabetes cause cross-linking of collagen fibers. 62 In vitro, this cross-linking inhibits the proteolysis 63 and secretion of MMPs that are involved in AAA formation. 64 Moreover, the presence of the end products advanced glycation promotes proliferation of the SMCs in the media. 65 Hyperglycemia also suppresses plasmin, itself an activator of MMPs 66 leading to a further decrease in overall MMP activity.
Atherosclerosis
Although atherosclerotic changes are often seen in AAA, the relationship is not a casual one. Both epidemiological data and molecular studies provide evidence that AAA is a different disease from classical atherosclerotic occlusive disease. Interestingly, almost every factor associated with AAA is also associated with DNA methylation, and analysis could be conducted to elucidate this link. 67
Genetics
After smoking the second most important risk factor for AAA is the family history, 68 69 70 with a positive history raising the OR of AAA development by as much as 1.96 (6) to 2.2. 69 Interestingly, patients with a female relative with AAA are even more strongly affected, manifesting a 2- and 0.5-fold higher risk than patients with a male relative with AAA. 69 The strong association of positive family history and AAA in wide epidemiological studies, together with the growing evidence number of specific gene association (see later) strongly supports genetic influence on the AAA development.
The first report of clustering of AAA in a single family goes back to 1977, when three affected brothers were reported. 71 Following this first report of a single family, in 1984, Tilson and Seashore reported 50 families with AAA in two or more first-order relatives, 72 demonstrating the genetic etiology of AAA. (The senior author J.A.E. of this article was a trainee in the audience when Tilson and Seashore presented their ground-breaking findings at Surgical Grand Rounds at Yale.)
Since those pioneering observations, the genetic influence on AAA has been confirmed from many different perspectives. The higher prevalence of AAA in white men compared with other races 40 suggests a genetic predisposition. 73 Based on interviews of patients with AAA, the percentage of positive family ranges from 6.1 74 to 19.2 75 to 35.7%, 76 with a mean around 15%. 77 The observed prevalence of AAA in first-degree family members after ultrasonography screening ranges between 9 78 and 19 79 and 29%. 80 This high level of concurrence of AAA between first-degree relatives confirms a genetic influence in the development of AAA. From a clinical perspective, it was noted that familial AAA (FAAA) tends to present and to rupture at a younger age compared with sporadic AAA (SAAA). 73 81 Moreover, FAAA manifests a greater incidence of rupture when compared with SAAA. 82 83 The different clinical behavior of FAAA compared with SAAA corroborates the importance of genetic predisposition. Finally, the Swedish twin registry revealed that the twin of a monozygotic twin with AAA suffered a risk of AAA that was 71 times that of the monozygotic twin of a person without AAA. 84
Many studies have attempted to characterize the specific pattern of genetic inheritance. In 1991, Majumder et al performed segregation analysis of patients who underwent emergency repair for rAAA and suggested a recessive model of inheritance. 85 In 2003, Kuivaniemi et al examined 233 families with at least two members with AAA, reporting that ∼75% of their data fitted an autosomal recessive inheritance pattern, while in the remaining 25%, an autosomal dominant pattern better explained their results. They conclude that the lack of consistency in the mode of inheritance may be indicative of multifactorial disease with multiple genetic and environmental risk factors. 86
In the last 25 years, an impressive number of genes have been investigated to evaluate their possible role of the pathogenesis of AAA. While for thoracic aortic aneurysm (TAA), there exists a very specific list of genes that are undoubtedly involved in pathogenesis of TAA, 87 88 no single mutation can be undoubtedly associated with AAA. Results from a recent meta-analysis show that 263 genes have been investigated and an association with AAA was reported with variants in 87 of these. 89 In general, most of these studies have focused mainly on three classes of genes: 90 genes for the structural component of the aortic wall (collagens, elastin), 91 genes for the enzymes responsible for degrading the structural molecules of the aortic wall (MMPs and their inhibitors), 92 93 and genes for proteins involved in the immune response. 94
Genome-wide association studies (GWASs) have also been applied in search of greater understanding of the genetics of AAA. 95
The first association of AAA with a single polymorphism in a GWAS emerged in 2008. 96 The G-allele of a single nucleotide polymorphism (SNP), rs10757278, located on chromosome 9p21.3 was significantly associated with AAA, with an OR of 1.31 (95% CI, 1.22–1.41) and a highly significant p = 1.2× 10 −12 . This mutation can stimulate apoptosis of SMCs via enhancement of the p53 signaling pathway. 97 Later, in 2010, a study of 1,292 individuals with AAA and 30,503 controls from Iceland and the Netherlands showed that the [A] allele of rs7025486 on 9q33 was associated with AAA, with an OR of 1.21 (95% CI, 1.11–1.32). 98 rs7025486 [A] codes for DAB2IP, a member of the RAS-GTPase–activating protein family. 99 DAB2IP has been shown to suppress cell survival and proliferation and to enhance apoptosis. 100 In a similarly designed study with 1,866 patients with AAA and 5,435 controls, another polymorphism, rs1466535, located within intron 1 of low-density lipoprotein receptor (LDLR)–related protein 1 (LRP1), demonstrated significant association ( p = 0.0042) with AAA but not with coronary artery disease, blood pressure, diabetes, or hyperlipidemia—suggesting that this locus could be specific to AAA. 101 The role of LRP1 in the development of AAA may reflect regulation of extracellular matrix (ECM) remodeling and VSMC migration and proliferation. 102 In 2013, a meta-analysis showed that patients with AAA had higher level of circulating interleukin (IL)-6. 103 Pooling data from 4,524 cases with AAA and 15,710 controls demonstrated that rs7529229 (codifying for a variant of IL-6R named ala358) was significantly associated with a lower risk of AAA (OR, 0.84; 95% CI, 0.80–0.89). A subsequent in vitro analysis using lymphoblastoid cells showed that, after stimulation with IL-6, the presence of the IL-6R ala358 was associated with a reduction of STAT3, MYC, and ICAM-1. These results gave evidence that IL-6 is likely a causative pathway in the developing of AAA. 103
Finally, in 2017, a meta-analysis of all six available GWAS datasets for AAA (total 4,972 cases and 99,858 controls, with a validation cohort of 5,232 cases and 7,908 controls) confirmed five of the previous six identified SNPs and found four novels SNP associated with AAA. 104 Among the novel identified loci, one deserves major interest: the rs3827066 on chromosome 20q13.12; this codes for MMP-9, which is known to play an important role in the developing of AAA and TAA. 105 The confirmed SNP are all the previous cited but rs1466535 coding for the LRP1 which demonstrated a borderline association with AAA in the combined analysis ( p = 6.4 × 10 −7 ); the other two SNP are the rs599839 coding for PSRC1-CELSR2-SORT1 106 and rs6511720 coding for LDL-R 107 ( Table 7 ).
Table 7. SNP associated with AAA risk in GWAS.
| SNP in GWAS associated with AAA | OR | 95% CI |
|---|---|---|
|
rs10757278
96
Apoptosis vascular smooth muscle cell through p53 |
1.31 | 1.22–1.41 |
|
rs7025486
98
Apoptosis vascular smooth muscle cell through DAB2IP (member of the RAS-GTPase-activating protein family) |
1.21 | 1.11–1.32 |
|
rs7529229
103
IL-6R ala358 (decreasing the inflammatory response after stimulation with IL-6) |
0.84 | 0.80–0.89 |
|
rs1466535
a
101
LRP1: it could act in the regulation of ECM remodeling and in the vascular smooth muscle cell migration and proliferation |
1.15 | 1.10–1.21 |
|
rs599839
106
CELSR2 , PSRC1 , and SORT1 genes: using RT-PCR RNA of sort-1 was find expressed in AAA tissue |
0.81 | 0.76–0.85 |
|
rs6511720
107
Code for LDR-R: similar to LRP1 |
0.76 | 0.70–0.83 |
|
rs3827066
104
Codes for matrix metalloproteinase 9: involved in the degradation of the ECM |
1.22 | 1.16–1.28 |
Abbreviations: AAA, abdominal aortic aneurysm; CI, confidence interval; ECM, extracellular matrix; GWAS, Genome-wide association study; IL, interleukin; LRP1, low-density lipoprotein receptor-related protein 1; OR, odds ratio; SNP, single nucleotide polymorphism.
In the last meta-analysis, it did not reach the statistical significance for GWAS. 104
In conclusion, the development of AAA is a combination of the genetic predispositions and environmental factors enumerated earlier.
It is worth noting some important parameters along which AAAs differ from TAAs. Specifically, AAAs show older mean age of presentation, absence of specific causative genes (just increased risk with some mutations), and very strong association with the cigarette smoking. Thus, environmental factors appear to be more important for AAA than for TAA, and genetic factors more important for TAA than for AAA.
Animal Models: Are Really Relevant?
A deep understanding of the mechanisms that underlie formation and progression of AAA is of paramount importance if we are to develop therapeutic and preventative strategies. Animal models are vital to these issues.
The first animal model of aneurysm was developed in the 1980s by Gertz et al. 108 They noticed an aneurysm of the common carotid artery in a rabbit 3 weeks after the periadventitial application of calcium chloride (CaCl 2 ). Histologically, the CaCl 2 diffuses into the media of the aortic wall, binding preferentially the internal elastic lamina and the elastic fibers in the lamellar network. The calcium-elastic tissue complex attracts inflammatory cells, predominantly monocytes and macrophages, which disrupt the integrity of lamellar units in the media, causing progressive luminal dilatation. For the first time, this work implicated the immune system in the development of AAA. Many other studies have utilized CaCl 2 induction of aneurysm, 109 110 111 112 113 114 often in mice and rats. It has been shown that by adding phosphate to the CaCl 2 , the extent of aortic medial calcification is increased. 115 It has been shown that periaortic application of CaCl 2 has other important effects beyond immune stimulation, including and increased oxidative stress, 116 induced VSMC apoptosis, and increased production of MMP-2 and MMP-9. 117 Beyond monocytes and macrophages, the periadventitial application of CaCl 2 also provokes migration and degranulation of mast cells. 118 A positive correlation between the number of mast cells in the adventitia and the AAA diameter has been noted 118 both in animal model and in human AAA. In the CaCl 2 model, macrophages also secrete proinflammatory cytokines such as IL-1 and IL-6, causing further increase of the inflammatory infiltrates and MMP activity. 119 Finally, adventitial neovascularization has been demonstrated in CaCl 2 -induced AAA in both mice 116 and rats. 118
In conclusion, the animal model of AAA induced by CaCl 2 shares many pathological characteristics with human AAAs, such as calcification, inflammatory cell infiltrate, oxidative stress, neovascularization, degradation of the ECM, and VSMC apoptosis. However, CaCl 2 -induced animal AAAs do not display intraluminal thrombus, atherosclerosis, and rupture which are important features of human AAA. Moreover, a laparotomy is necessary to induce the AAA.
The chronologically second animal model of AAA is the elastase model introduced by Anidjar et al in 1990. 120 In this model (see Fig. 4 ), porcine pancreatic elastase is infused into the lumen of the abdominal aorta of rats, causing AAA. Other studies showed similar results in mice 121 and rabbits 122 as well as after the periadventitial application of elastase. 123 The infusion of elastase inside the abdominal aorta results in a dense inflammatory infiltrate visible 2 weeks after the infusion, as well as extensive degradation of elastic fibers in the media. 120 The inflammatory infiltrate is composed predominately by macrophages, but neutrophils are present as well. 121 In these models, porcine pancreatic elastase was not detectable in aortic wall extracts within 24 hours of elastase perfusion, implying that pancreatic elastase is not directly responsible for the late degradation of aortic wall elastin associated with aneurysmal dilatation. 121 Similar to the CaCl 2 model, the elastase model induces calcification in the aortic wall. 124 Moreover, differently from CaCl 2 model, elastase-induced AAAs do present intraluminal thrombosis and do manifest rupture. 125
Fig. 4.
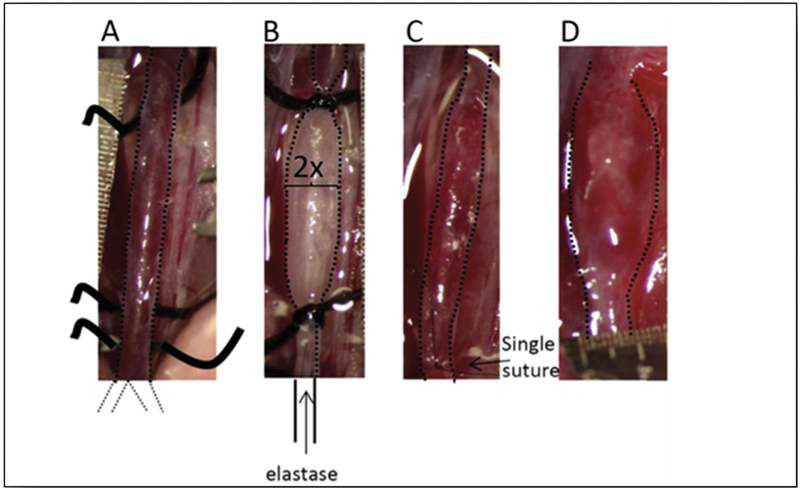
( A ) Isolation of the aorta from the left renal vein to the bifurcation. ( B ) A 5-minute type 1 porcine pancreatic elastase infusion at a pressure of 100 mm Hg for 5 minutes. ( C ) Incision is closed with a single suture and blood flow is re-established. ( D ) Aneurysm is formed 14 days after elastase infusion. (Reproduced with permission from Lysgaard Poulsen et al. 125 )
The third animal model of AAA was developed by Daugherty et al's group in Kentucky. 126 They founded that the intravenous infusion of angiotensin II in the hyperlipidemic apoE –/– mouse induces AAA in 2 to 3 weeks ( Fig. 5 ). The development of AAA after angiotensin II infusion has been noted also in the LDLR –/– mouse. 127 LDLR contributes to the disposal of low-density lipoproteins. Although no atherosclerotic lesions are visible, the presence of hyperlipidemia facilitates the development of AAA. In this model, transmural disruptions of the media are evident. 128 These medial disruptions are accompanied by extensive inflammatory infiltrates (predominantly macrophages and lymphocytes) at sites of disrupted elastic lamellae and damaged SMCs, with reactive fibromuscular hyperplasia. 126 It is not clear whether the macrophage and lymphocytes accumulation acts as a stimulus for elastin degradation or vice versa. 129 As in humans, also in the mouse model of AAA, angiotensin II-induced males are much more prone to AAA development. 130 Rupture is also common. 125 While in the other models, the infrarenal aorta is the site of AAA development, and in the angiotensin II-induced model, the suprarenal aorta is involved in the dilatation. 126 127 131
Fig. 5.
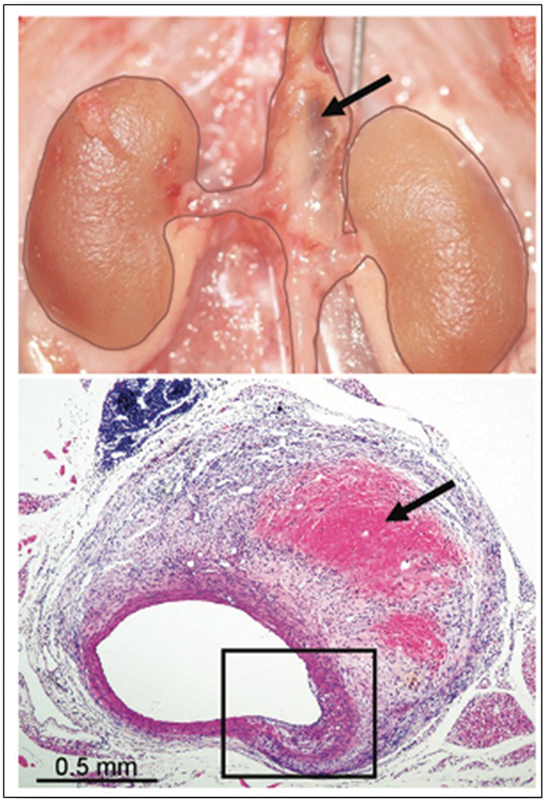
Suprarenal AAA 4 weeks after Ang-II infusion in the apoE –/– mouse; note hemorrhage into the wall in the macroscopic (upper, arrow) and H&E section (lower; arrow). (Reproduced with permission from Gertz et al. 132 ) AAA, abdominal aortic aneurysm; Ang-II, angiotensin-II; H&E, hematoxylin and eosin.
This angiotensin II model has several advantages over the other models: a minor surgery is suffices, laparotomy and arteriotomy are not required, the model is reproducible, and rupture is common. 125 For these reasons, the angiotensin II model is the most common model currently used. 132 However, unlike the other two models, calcification is not seen in the aortic wall.
Another small animal model is the xenograft approach. In this model, transplantation of the infrarenal aorta is performed from one species to another, for example, guinea pig to rat, to induce aneurysms. 133 134 135 Prior to implantation of the aorta, the donor aorta must be decellularized. The decellularization of the donor graft is needed to trigger a slower immunological response and not an acute fatal rejection episode. 125 This model is extremely demanding from a technical point of view. While intraluminal thrombosis is seen, the induced aneurysms do not rupture. Like, the angiotensin II model, calcification is absent in the aortic wall. 125
Interestingly, administration of doxycycline (a broad-based inhibitor of MMPs) before AAA induction in elastase, 121 CaCl 2 , 136 and angiotensin II 127 infusion models attenuates the formation of experimental AAAs. Also, the administration of rapamycin in the elastase model limits the AAA progression in elastase model. 109 To date, the only therapy that has been shown to induce regression of established AAAs animal models is the inhibition of JNK 110 (c-Jun-N-terminal kinase can cause downregulation of gene expression of some crucial ECM biosynthetic enzymes [lysyl hydroxylase, lysyl oxidase, and prolyl 4-hydroxylase] and it can activate the MMPs). 110
Finally, a more physiological porcine model of AAA based on laparoscopic delivery of CaCl 2 to the periadventitial surface of the aorta combined with angiotensin-II infusion has been proposed. 132
The animal models of AAA, including their advantages and shortcomings, are summarized in Table 8 .
Table 8. Characteristics of various of animal models of AAA.
| Animal models | CaCl 2 | Elastase | Angiotensin 2 |
|---|---|---|---|
| Mechanism | Calcification and inflammation | Calcification and inflammation | Inflammation |
| Rupture | No | Yes | Yes |
| Intraluminal t hrombosis | No | Yes | No |
| Need for major surgery | Yes | Yes | No |
Abbreviations: AAA, abdominal aortic aneurysm; CaCl 2 , calcium chloride.
Models of AAA in small size animals do not permit evaluation of novel medical devices where an aortic diameter similar to that of humans is deemed necessary. To address this issue, animal models in large animals have been developed, largely by surgically enlarging the aorta by surgically implanted patches. 111 112 These models are suitable for study of surgical devices (e.g., stent grafts) but are not valuable as pathophysiological replicas.
In conclusion, for small animal models, the biggest limitation in the fidelity by which the available models recapitulate the pathological features of human aortic aneurysms is the deficiency in clarifying the very first phase of the human disease. 128 In all the models, the AAA induction is nonphysiologic and not reflective of human disease. Moreover, the induced aneurysms do not expand indefinitely over time and are characterized by a stabilization of the biological process after a few days or weeks, reflecting cessation of the initial insult and subsequent healing. This is another import difference from human AAA. Therefore, the available animal models of AAA, imaginative and creative, resemble the human aneurysm in many but not in all respects. These models have permitted extensive investigation of pathophysiology and treatment of experimentally induced AAAs, but a more “naturally” occurring experimental model of human AAA (e.g., genetically induced) would be a welcome advance.
What Pathophysiology Leads to AAA?
Three key processes contribute to the AAA development: proteolysis, inflammation, and VSMC apoptosis. 25
Proteolysis
Two classes of proteases are commonly considered responsible for the degradation of the ECM in AAA: MMPs and cathepsins. 113 114 137 Cathepsins are a group of enzymes with both elastolytic and collagenolytic activities. 138 In the animal model, deficiency of cathepsins protects from AAA formation. 139 140 141 Also, it has been demonstrated, in population-based study, that high level of cathepsin-S 142 and cathepsin-L 113 are associated with a higher risk of AAA, with ORs, respectively, of 1.31 and 3.04.
The most studied classes of proteases are the MMPs. MMPs are a family of zinc-dependent enzymes with collagenolytic activity. They are physiologically involved in many processes, such as wound healing, bone and tendon homeostasis, pregnancy and parturition, and mammary involution. 143 144 All members of the MMP family are secreted in a latent form, requiring activation for proteolytic activity. The MMPs are inhibited by tissue inhibitors of MMPs (TIMPs). From a chemical perspective, MMPs share common amino acid sequences. 145 TIMPs are a family of enzymes capable of inhibiting the activity of the MMPs; 146 there are four members of the TIMP family. Several studies evaluating messenger RNA levels have shown that aneurysmal tissues have an imbalance between MMP and TIMP activities. 147 148 149 Also, high plasma levels of MMP-9 have been identified in large aneurysms 150 and plasma levels of MMP-9 decrease in patients after AAA repair. 151 152 However, not all the results are concordant: in a large study, plasma levels of MMP-9 failed to show relevance as serum marker for aortic dilatation. 153
Interestingly, after treatment with a nonselective inhibitor of MMPs (the antibiotic doxycycline) in mice, the development of AAA was suppressed; the same result was noted in MMP-9 deficient mouse but not in an MMP-12 deficient mouse. 121 On the basis of this observation, first small randomized controlled trial (RCT) was designed to evaluate the effect of doxycycline on the progression of human AAA. The initial results were encouraging; 154 unexpectedly a second RCT with 286 patients with small AAAs (<5 cm) showed that the use of doxycycline was associated with an increased expansion of the AAA. 155
Inflammation
Several classes of inflammatory cells have been identified in human AAAs, particularly macrophages. 73 137 In animal models, deficiency of C–C chemokine receptor type 2 (an important receptor for macrophage mediation of response to inflammation) attenuates the progression of AAA, 156 suggesting a role of macrophages more in the progression than in the formation of AAA. Another important macrophage receptor that has shown to be upregulated in human AAA is the CXCR4; 157 in an animal model, blockade of this receptor with the antagonist AMD3100 inhibits the formation and progression of AAA. 157 Lymphocytes are also present both in human 158 159 and experimental AAA. 125 160 Proinflammatory cytokines have also been implicate in the formation and development of AAA, including epidermal growth factor, IL-1B, IL-17, IL-23, transforming growth factor-β, interferon-γ, and tumor necrosis factor-α.
VSMC Apoptosis
Although a decreased number of VSMC in AAA tissue is documented extensively, it is not entirely clear whether cell death is an active pathological event or a consequence of tissue deterioration. 137 A few data exist from animal models regarding the process of apoptosis of VSMC. Wang et al showed that deletion of the receptor serine–threonine protein kinase 3 involved in the process of VSMC apoptosis inhibits the development of AAA in an animal model. 161 Moreover, TNF-α secreted by macrophages can cause VSMC apoptosis 162 and also the release of chymase, a protease secreted by the mast cells can induce SVMC apoptosis. 163 Increased clarification of the underlying pathophysiology of AAA holds promise for new preventive and therapeutic approaches.
Indications for AAA Surgery: Are Surgeons Over-eager to Operate?
The decision about whether an AAA requires repair depends on an accurate balance between the risk of mortality from AAA rupture and the risk of surgery. Considerations regarding patient general life expectancy also enter into the equation. AAA diameter is the strongest predictor of aneurysm rupture, 164 165 and the rupture risk increases exponentially with increase in aneurysm diameter. 166 167
Although diameter is undoubtedly the key factor, it cannot be the unique criterion for the decision. The overall characteristics of every single patient and the specific characteristics of the AAA (e.g., familial vs. sporadic) must be considered as well. International (both European and North American) guidelines recommend surgery when the AAA diameter exceeds 55 mm in men and 50 mm in women (level of evidence I-B) 168 169 170 ( Table 9 ). The older guidelines of the European Society for Vascular Surgery guidelines recommend a threshold of 52 mm for women. 171
Table 9. Indications for surgery (from most recent European Society of Cardiology guidelines) 168 .
| Recommendation | Class | Level |
|---|---|---|
| Surveillance is safe and indicated in patients with AAA < 55 mm | I | A |
| AAA repair is indicated in male patients with AAA > 55 mm | I | B |
| AAA repair is indicated in female patients with AAA > 50 mm | I | C |
| AAA repair is indicated when AAA grow rate exceed 10 mm/y | I | B |
| In patients deemed fit for open repair with AAA anatomically suitable for EVAR both open repair and EVAR are recommend | I | A |
| If AAA is unsuitable for EVAR, open repair is recommended | I | C |
| In patients deemed unfit for open repair, EVAR along with best medical therapy could be considered | IIb | B |
Abbreviations: AAA, abdominal aortic aneurysm; EVAR, endovascular aortic repair.
These thresholds have been established on the basis of many observational studies demonstrating a dramatic increase in the rate of rupture when the maximum aneurysm diameter exceeds 50 mm. Particularly, Reed et al 167 estimated an annual risk of rupture of 1% for diameter < 50 mm, 11% for diameter between 50 and 59 mm, and 26% for a diameter > 60 mm. Similar results have been showed by the analysis of Brown and Powell, 164 who calculated an annual rupture rate of 6.5% when the diameter exceeds 50 mm. Analysis of outcomes in elderly patients unfit for surgical repair revealed an annual rate of rupture of 12% with a diameter between 50 and 59 mm and 14% with a diameter > 60 mm. 172 Finally, similar results were reported analyzing the data of patients unfit for surgery from the ADAM study, with a 1-year incidence of probable rupture of 9.4% for AAA of 5.5 to 5.9 cm, 10.2% for AAA of 6.0 to 6.9 cm (19.1% for the subgroup of 6.5–6.9 cm), and 32.5% for AAA of 7.0 cm or more. 166 Finally, a 2013 meta-analysis of 18 studies analyzing growth rate and risk of rupture of small AAA (< 5 cm) estimated a rate of rupture of 6.4 per 1,000 person/year for male with a AAA of 50 mm of diameter. For women, a similar rate of rupture was incurred at an AAA diameter of only 40 mm (rate of rupture 7.9 per 1,000 person/year). 173
The issue of surgical repair before the threshold of 55 mm has been a matter of debate during the 1990s. In fact, in 1992, elective repair had been recommended for AAA of 40 mm or more for patients without contraindication, 174 although others had advocated the use of surveillance by means of imaging until the diameter reaches 50 175 or 60 mm. 176 To address this issue, two RCTs have been undertaken: the ADAM study 177 and the UKSAT. 178 The design of these RCTs was similar: patients with AAA diameter between 40 and 54 mm considered fit for open surgery where randomly assigned to immediate open repair or to surveillance by means of ultrasonography or computed tomography (CT) scan every 6 months with repair reserved until the diameter exceeds 55 mm or the aneurysm become symptomatic. Both these studies showed no improved in survival in the early repair group, although the operative mortality was significantly lower in the ADAM study (2.0 vs. 5.8%). 177 178 It should be noted that in both the ADAM trial and the UKSAT, 70% of patients assigned to observation ended up with open surgery.
Two other RCTs comparing EVAR and surveillance have been performed: the Comparison of Surveillance versus Aortic Endografting for Small Aneurysm Repair (CAESAR) study and the Positive Impact of Endovascular Options for treating Aneurysms Early (PIVOTAL) study. 179 180 The design of these two studies recapitulated the two prior open surgery trials: patients with small AAAs (40–54 mm in CAESAR and 40–50 in PIVOTAL) considered suitable for endovascular repair were randomly assigned to early EVAR or watchful waiting, with repair reserved until diameter exceeds 55 mm or symptoms appears. As in the former open trials, no benefit in survival was observed after, respectively, 54 and 20 ± 12 months of follow-up in the early treatment group. 179 180
Finally, a Cochrane meta-analysis collecting data from all these four RCTs concluded that early repair of AAA does not yield any survival advantage compared with surveillance. 181
On the basis of these results, the latest guidelines assert that surveillance is indicated and safe in male patients with AAA < 55 mm and slow (<10 mm/year) growth (level of evidence I-A). 168
Analysis of aneurysm diameter at the time of surgical intervention has been performed for many counties around the world (Europe, North America, and Australia). 182 183 184 Mean diameters at surgery ranged from 6.2 to 6.7 cm. In both the United Kingdom and the United States, the rates of surgery for ruptured aneurysm have decreased substantially. 184 This development has been attributed to the increasing number of elective operation each year in both countries 184 and considered an indicator of the dramatic efficiency of the screening.
In the United States, AAA repair is often pursued with a more aggressive posture 183 184 ; 40% of all intact AAA repairs in men in the United States were performed at a diameter between 50 and 55 mm. Proponents point to the drop-in need for operations for rAAA as a validation for such an aggressive approach. Others, Lederle included, bemoan this aggressive deviation from evidence-based guidelines. 185 The authors wish to point out that essentially every experienced aortic surgeon has experienced and treated patients with rupture before the criterion of 5.5 cm for males (or 5.0 cm for females) is reached. Regardless of the strength of the randomized trials, the behavior of aneurysms is not fully predictable. This is seen vividly in thoracic aortic disease, where genetic characteristics have been better clarified, and subgroups with specific mutations (such as ACTA2 and MYLK) dissect at very small diameters, often without aneurysmal dilatation. 87 If a surgeon's judgment and experience (or even his “instinct”) have the surgeon concerned, the authors would not object to an early operation.
One must recognize also that the RCT discussed earlier 177 180 showing no benefit from early surgery (and even an additional supportive meta-analysis) 186 187 are, at this point, somewhat dated. All these trials began recruitment at least a decade ago, and clinical practice has changed considerably since then. 188 189 Surgical safety continues to improve, altering the risk/benefit ratios. Therefore, a change in the threshold of the guidelines has been proposed. 190 This change will require new RCTs. The previous RCTs enrolled for early repair male patients with a diameter between 40 and 55 mm. However, it has been clearly demonstrated that when the diameter reaches 50 mm, the growth accelerates and the risk of rupture rises, compared with a diameter < 45 mm. 164 165 191 A RCT comparing surgery against watchful waiting in patients with AAA of a diameter of between 50 and 55 mm would be valuable. Given the high level of evidence of the current guidelines, only a large RCT so designed could justify an earlier criterion for intervention.
Another important issue that has been addressed in recent years regards the possibility of endovascular repair in patients considered unfit for open surgery. In this group of patients, the possible survival benefit of EVAR versus observation has been evaluated with a RCT: the EVAR II study. 192 This trial showed a high perioperative mortality in the endovascular repair group (9% 13/150) (significantly higher than the perioperative mortality in the EVAR-I trial done in the same centers [1.7%]). 193 There was no difference in survival at 4 years after randomization ( Fig. 6 ). 192 Also, the need for continued surveillance after endovascular repair, and the high rates of reintervention caused substantial increase of the costs. Late follow-up at 8 years after intervention 194 confirmed the absence of reduction of mortality in the repair group, although a lower rate of AAA-related mortality was shown.
Fig. 6.
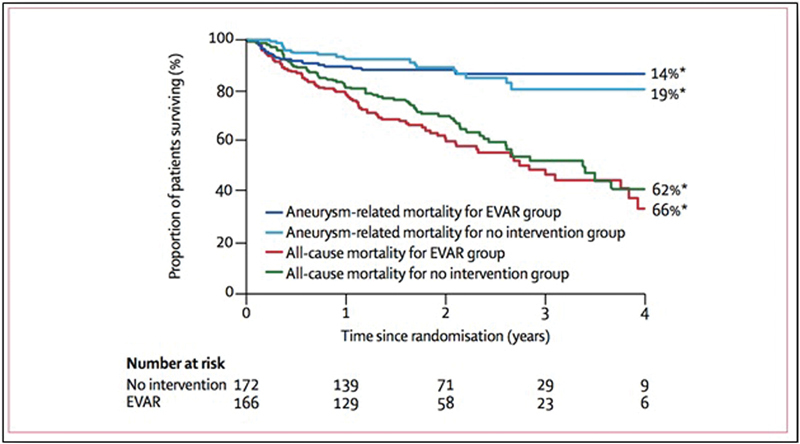
Kaplan–Meier's method to estimate all-cause mortality and AAA-related mortality in patient unfit for open surgery treated with EVAR or with no intervention. EVAR does not offer benefit in survival respect, no intervention in patient deemed unfit for open surgery. (Reproduced with permission from EVAR trial participants. 192 ) AAA, abdominal aortic aneurysm; EVAR, endovascular aortic repair.
Therefore, one might say that “you get what you pay for”—not financially, but in terms of invasiveness. The open procedure is more effective and durable than EVAR, but it requires open surgery. The open procedure also trades a slightly higher early mortality for improved long-term survival.
Elective AAA Repair: Open or Endovascular?
What is the best approach to repair an infrarenal AAA? This has been one of the most debated topics in the field of aortic surgery during the past 15 years. Since 1991 when the feasibility of EVAR was demonstrated by Parodi et al 111 and Volodos et al, 195 this procedure has progressively become more popular. Initially, endovascular repair was reserved for patients deemed unfit for open surgery, while today more than three quarters of all the infrarenal AAA repairs are accomplished endovascularly. 196 (We worry that new trainees may lack sufficient open AAA skills.)
The safety and efficacy of endovascular repair was established in the 1990s with retrospective cohort studies and prospective registries. These include the Registry of Endovascular Treatment of Abdominal Aortic Aneurysm 197 and the European Collaborators on Stent Graft Techniques for Abdominal Aortic Aneurysm Repair Registry. 198 Both these registries began patient recruitment in 1996 and reported 30-day procedural mortality of 2.9 and 3.1%, respectively.
Although the reported 30-day mortality was low, the retrospective nature raised the potential of selection bias; therefore, RCTs were undertaken to evaluate differences between EVAR and open surgery. The three biggest pertinent RCTs are EVAR I, 193 DREAM, 199 and OVER. 200 The design of these RCTs is quite similar: patients with AAA > 5.5 cm deemed fit for open surgery and also suitable for EVAR were randomly assigned to open repair or EVAR. The results of these studies are similar: EVAR is associated with a significantly lower 30-day mortality (EVAR I: 1.6 vs. 4.6%, 193 DREAM: 1.2 vs. 4.6%, 199 and OVER: 0.5 vs. 3.0% 200 ), as well as shorter intensive care unit and in-hospital length of stay. However, the survival advantage is lost after 2 or 3 years of follow-up. 200 201 202 203 204 The results of these RCTs are summarized in a meta-analysis that confirms the immediate survival advantage of EVAR is lost after 2 years of follow-up. 205
Other than the RCTs, reports also from the real world have confirmed the early survival advantage of EVAR over open surgery (relative risk of death with open repair: 3.22). 189 As in the randomized trials, the early survival benefit of the endovascular approach is lost after 3 years of follow-up. 189
Beyond the loss of late survival benefit, other most worrisome aspect of endovascular repair is the continued risk of AAA rupture after the repair. This is related to the mechanism of the endovascular repair itself. 206 To remain in situ, the stent graft needs to exert a radial force against the “neck” of the aneurysm (really, against the proximal and distal stent landing zones). This force can cause dilatation of the proximal neck, permitting device migration and development of endoleak termed type Ia proximal and type Ib distal. However, this is theoretic in nature and the incidence of clinically significant proximal neck dilatation is quite small. Type I endoleak are extremely dangerous because of the sharp rise in the pressure in the aneurismal sac, with subsequent high risk of aneurysm rupture. This difference in the technical efficacy and durability of the two therapies is demonstrated by the much higher rate of aortic reintervention in the endovascular groups (EVAR I: 6.3 vs. 2.1%, 201 DREAM: 20.8 vs. 2.2%, 204 and 2.3 vs. 0.8% in the Medicare population analysis). 207 In meta-analysis, the relative risk of reintervention is 2.53 for the endovascular groups Other than a greater rate of aortic reintervention, the lesser efficacy of the EVAR is demonstrated by the significantly higher rate of AAA relate mortality in long-term follow-up: EVAR I: 0.8 versus 0.2% 201 ; six cases of late rupture in the EVAR group versus zero in the open group in the OVER trial 203 ; and in the Medicare population 5.4 versus 1.4%. 189 In addition, type II “side branch endoleaks” are not innocuous, as a long-term report noted there can be continued sac expansion in this cohort. 208
Very recently, in 2016, the results of very long-term follow-up (up to 15 years) of the EVAR I patients have been published ( Fig. 7 ). 209 Of the 1,252 patients initially randomized, about one-third were still alive. Patients in the open repair group manifested a superior survival over the EVAR group (53 vs. 46%), with a HR for death of 1.25 for EVAR patients. Also, the AAA related mortality was significantly higher in the EVAR group (5 vs. 1%), with a very significant increased risk for the EVAR group (HR, 5.82). The increased aneurysm-related mortality in the EVAR group was mainly attributable to secondary aneurysm sac rupture (43 ruptures in EVAR vs. 1 in open repair), 210 with increased cancer mortality also observed in the EVAR group (adjusted HR, 1.87). 209 Also, in 2016, the results of very long-term follow-up of the DREAM trial have been presented. 211 After 12 to 15 years of follow-up, patients randomized to EVAR showed comparable survival, but at the expense of a threefold higher reintervention rate. 211 These trial results are tabulated in Table 10 .
Fig. 7.
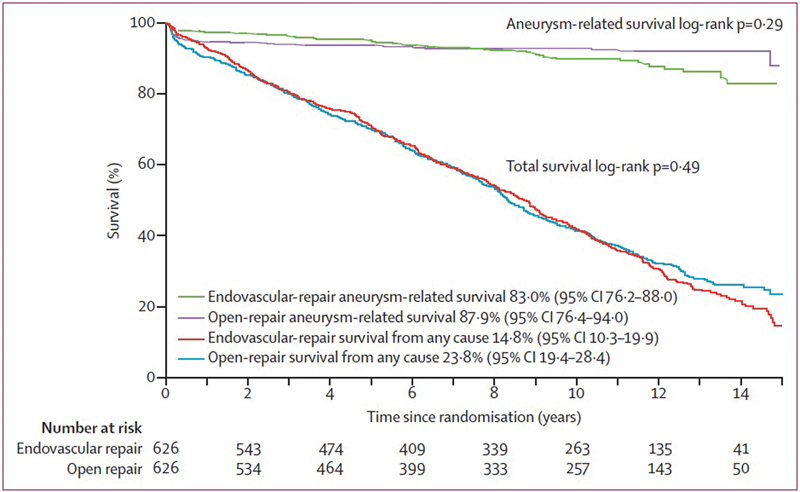
Kaplan–Meier's method to estimate survival from any cause and AAA-related survival in the very long-term follow-up in the EVAR I trial. In the first 2 years, EVAR gives an advantage in survivals, but this advantage is lost after 2 years of follow-up and after 12 years, the open repair offers an advantage in survival. (Reproduced with permission from Patel et al. 209 ) AAA, abdominal aortic aneurysm; EVAR, endovascular aortic repair.
Table 10. Result of trials of open surgery versus EVAR in elective circumstances.
| Name of the trial | 30 d OR mortality | 30 d EVAR mortality | p -Value | Medium-term OR mortality | Medium-term EVAR mortality | p -Value | Long-term OR mortality | Long-term EVAR mortality | p -Value |
|---|---|---|---|---|---|---|---|---|---|
| EVAR I 192 | 4.6% | 1.6% | 0.007 | 19.9% | 20.08% | 0.3 | 23.1% | 22.3% | 0.5 |
| DREAM 199 | 4.6% | 1.2% | 0.1 | 10.3% | 10.4% | 0.8 | 33.7% | 33.5% | 0.97 |
| OVER 200 | 3.0% | 0.5% | 0.004 | 9.8% | 7% | 0.1 | 33.4% | 32.9% | 0.81 |
Abbreviations: AAA, abdominal aortic aneurysm; EVAR, endovascular aortic repair; OR, odds ratio.
Emergency AAA Repair: Open or Endovascular?
Despite the increasing detection of asymptomatic AAA and the subsequent growing number of protective elective repairs, rAAA caused more than 2,400 deaths in the United States in 2015. 212 Open repair is still associated with high mortality, and evidence does not suggest a great improvement in outcome over time. 182 213 The feasibility of EVAR in the treatment of rAAA was demonstrated in 1994. 214 Since then growing experience in its application in elective cases has led to an increased its use also in the emergency setting.
The theoretical advantages of EVAR in the treatment of rAAA are clear: offers to decrease visceral and lower extremity ischemia time by balloon inflation control, which is significantly shorter than cross-clamping, and also avoids bloody periaortic dissection. However, only 40 to 64% of patients with rAAA have aortic anatomy suitable for EVAR. 1
Observational studies have reported improved short-term outcomes for EVAR ( Table 11 ). 215 216 217 218 219 A report from the Nationwide Impatient Sample (a database representative of around 20% of nonfederal U.S. hospitals) for the period 2001 to 2006 (27,750 patients) reported a mortality for the EVAR group of 31.7% compared with 40.7% in the open repair group. 216 Analysis of the Medicare population in the period 2001 to 2008 also reports a significant survival advantage in the EVAR group (mortality 33.8 vs. 44.7%). 219 Retrospective analysis from two centers in Europe who adopted an “EVAR-whenever-possible” approach in a cohort of 361 patients shows a major advantage for EVAR (15.7 vs. 37.4% mortality, with OR for death in the open repair group 3.3). 220
Table 11. Emergent AAA repair: EVAR versus open.
| Author and year of publication | Type of study | Period | No. of rAAA | EVAR | OR | 30-d mortality EVAR | 30-d mortality OR | p -Value | Overall 30-d mortality |
|---|---|---|---|---|---|---|---|---|---|
| Hinchliffe et al (2006) 222 | RCT | 2002–2004 | 32 | 15 | 17 | 53% (8/15) |
53% (9/17) |
NS | 53% (17/32) |
| Desgranges et al (2015) 223 | RCT | 2007–2013 | 107 | 56 | 51 | 18% (10/56) |
23.5% (12/51) |
NS | 20.5% (22/107) |
| Reimerink et al (2013) 224 | RCT | 2004–2011 | 116 | 57 | 59 | 42% (24/57) |
47% (28/59) |
NS | 44.8% (52/116) |
| IMPROVE (2014) 225 | RCT | 2009–2013 | 613 | 316 | 297 | 35.4% (112/316) |
37.4% (111/297) |
NS | 36.3% (223/613) |
| Ruptured Aneurysm Trialists (2015) 227 | M-A of RCT | – | 836 | 429 | 407 | 31.2% (134/429) |
33.9% (138/407) |
NS | 32.5% (272/836) |
| McPhee et al (2009) 216 | Registry | 2001–2006 | 27,750 | 3,179 | 24,571 | 31.7% (1,008/3,179) | 40.7% (10,000/24,571) | <0.001 | 39.6% (11,008/27,750) |
| Giles et al (2009) 217 | Registry | 2001–2005 | 23,335 | 2,499 | 20,836 | 32.3% (807/2,499) |
40.8% (8,501/20,836) |
<0.001 | 39.8% (9,308/23,335) |
| Edwards et al (2014) 219 | PM on registry | 2001–2008 | 2,198 | 1,099 | 1,099 | 33.8% (371/1,099) |
47.7% (524/1,099) |
<0.001 | 40.7% (895/2,198) |
Abbreviations: AAA, abdominal aortic aneurysm; EVAR, endovascular aortic repair; M-A, meta-analysis; OR, odds ratio; rAAA, ruptured AAA; RCT, randomized controlled trial.
It must be recognized that RCTs for rAAA suffer from methodological issues (exclusion of hemodynamically unstable patients, anatomically unsuitable patients, randomization before or after CT scan). Some authors have also criticized the ethics of organizing an RCT for rAAA. 221 Nonetheless, four RCTs have been performed to compare treatment of rAAA by EVAR or open surgery. The results of these trials (and a large registry) are found in Table 11 . These four studies (one from the UK, ECAR in France, AJAX in the Netherlands, and IMPROVE in the UK) showed, surprisingly, no significant difference in surgical mortality between EVAR and open repair for rAAA. 222 223 224 225 Subgroup analysis of IMPROVE, however, suggested that women did better after endovascular repair than open repair, due to a high mortality in the female open repair group (57%). 225
An editorial accompanying this last trial suggested that 90-day survival should have been chosen as the primary outcome instead of 30-day survival. 226 This was done in a meta-analysis of three of the trials. 227 Again, no difference between the two groups was evident at either 30 or 90 days. Also, analyses of 1-year outcomes revealed no difference between EVAR and open surgery ( Fig. 8 ). 223 228
Fig. 8.
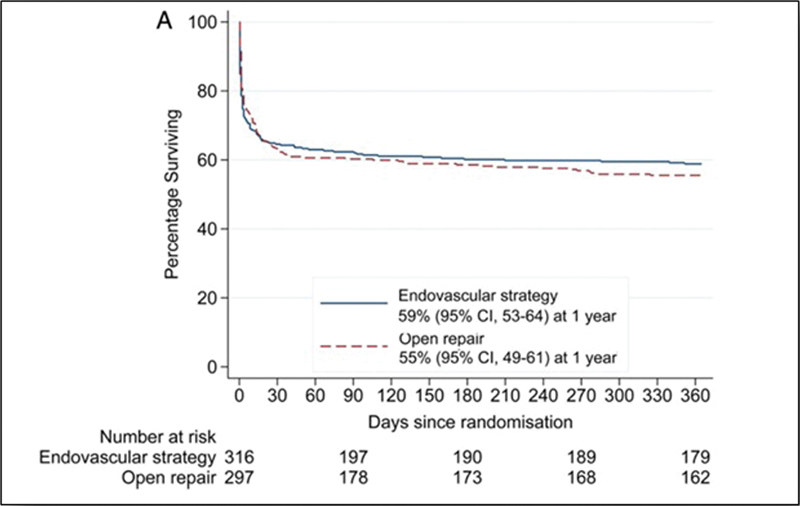
Kaplan–Meir's survival in patients with ruptured AAA treated with EVAR or open surgery. No significant difference is seen at 30, 90 days, and after 1 year of follow-up. (Reproduced with permission from IMPROVE Trial Investigators. 228 ) AAA, abdominal aortic aneurysm; EVAR, endovascular aortic repair.
Therefore, the promise shown for EVAR for rAAA in the observational studies was not confirmed in RCTs and meta-analyses, likely reflecting selection bias in the observational studies. 229 Severely hemodynamically patients are likely to have been triaged to open surgery in observational studies. Also, length of the proximal “neck” is very pertinent. Analysis of the IMPROVE data has shown that a short neck increases mortality both after open repair or EVAR. 207 Obviously, in case of a short neck EVAR is contraindicated. In case of open repair with a neck shorter than 15 mm, the clamp must be often placed above the renal arteries, with inevitable compromise of the visceral circulation, especially poorly tolerated in shocked patients. These considerations help explain the contradiction between the results of the observational trials and the RCTs.
In conclusion, no distinct advantage can be claimed for EVAR or open surgery for these very compromised patients with rAAA. Anatomic considerations and institutional and surgeon experience and preference can fairly be permitted to predominate.
Hypotension Management—New Data on a Perpetual Controversy
Permissive hypotension in the preoperative management of rAAA has for many years been advised to reduce bleeding prior to repair. 230 However, recent data from the IMPROVE trial report a significant higher mortality in patients with systolic blood pressure lower than 70 mm Hg when compared with patients with a systolic blood pressure higher than 70 mm Hg. 225 These data, together with the results of a recent meta-analysis 231 show that excessive hypotension (<70 mm Hg) is a negative prognostic factor in patients with rAAA.
Permissive hypotension is linked to the other cornerstone of the preoperative management of rAAA: fluid restriction. Aggressive fluid resuscitation may exacerbate bleeding for two reasons. 171 232 First, the increased blood pressure exacerbates bleeding, and second, the accompanying hemodilution adversely affects the clot formation, further increasing bleeding and third space fluid accumulation. 233 In a retrospective analysis of 154 patients, the administration of more than 3.5 L of fluid was associated with an OR for death of 3.54. 194 Moreover, it has been demonstrated that for each additional liter of fluid administered per hour before the aortic cross-clamp or the endoprosthesis sealing, the odds of perioperative death increase of 1.57-fold. 234
Therefore, recent data indicate that the benefit of the decreased bleeding from permissive hypotension should be balanced against the risk of end organ ischemia. It is best to limit fluid administration as much as possible (boluses of 250 mL), yet maintaining systolic blood pressure > 70 mm Hg ( Fig. 9 ). 230 235
Fig. 9.
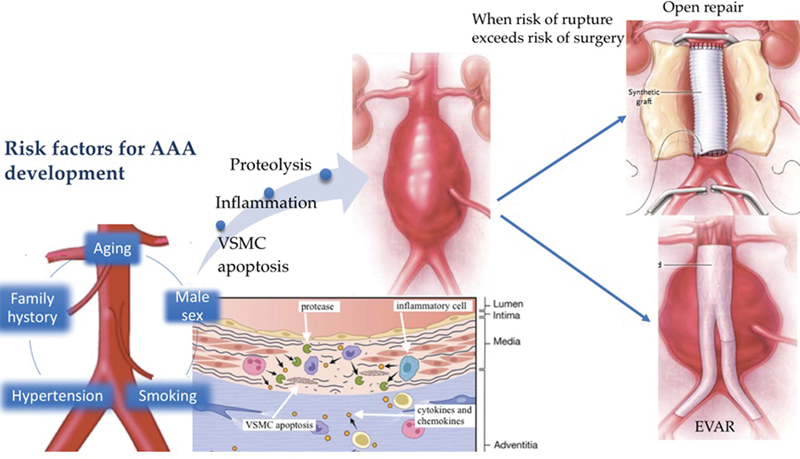
Abdominal aortic aneurysm: from the development to management. Smoking, family history, aging, male sex, and hypertension are the main risk factors for AAA development. VSMC apoptosis, inflammation, and proteolysis are the molecular mechanism that causes AAA. When the risk of rupture exceeds the risk of surgery, there are two options: EVAR and open surgery. (Portions of this figure are modified from Kent 196 and Davis FM, Rateri DL, Daugherty A. Mechanisms of aortic aneurysm formation: translating preclinical studies into clinical therapies. Heart 2014;100:1498–1505.) AAA, abdominal aortic aneurysm; EVAR, endovascular aortic repair.
Summary
Is the incidence of AAA increasing ? There is no doubt that the incidence and mortality of AAA increased progressively during the second half of the 20th century, probably reflecting increased imaging diagnosis as well as a bona fide (cryptogenic) increase in aneurysm disease. The advent of ultrasound screening and effective, safe surgical techniques have decreased the toll of this disease in recent decades.
Are ultrasound screening programs for AAA effective ? Yes, immensely effective. Guidelines and Medicare reimbursement permit an abdominal ultrasound for AAA at the age of 65 years in males. This screening is extremely accurate in identifying AAAs for follow-up and surgical intervention. If there is no AAA at that point, it is extremely unlikely that the patient will die of AAA.
What causes AAA: Genes versus environment ? Both contribute. Genes clearly play a role, but specific genes and mutations, so clearly outlined for TAA, remain elusive for AAA. Cigarette smoking is far and away the main environmental culprit.
Animal models: Are they really relevant ? Excellent animal models, especially the angiotensin-induced AAA of Daugherty et al, which mimic many characteristics of human aneurysm, have proven immensely helpful in clarifying pathophysiology and suggesting novel therapies. However, all models rely on a very “artificial” injury to the aortic wall, an instigation of AAA that is not reflective of human disease. A genetic model or other physiologically based preparation, as for TAA (e.g., FBN1 knockouts for Marfan's disease), is sorely needed.
What pathophysiology leads to AAA ? Inflammation, elastin and collagen degradation by MMPs, and SMC loss are the predominant factors thus far identified.
Indications for AAA surgery: Are surgeons over-eager to operate ? Although an intervention criterion of 5.5 cm for males and 5.0 cm for females are supported by abundant observational and randomized clinical studies, earlier operation, based on surgeon “instinct” and unpredictability of AAA behavior in specific individuals, is understandable and not to be discouraged.
Elective AAA repair: Open or endovascular ? While EVAR offers somewhat lower early procedural mortality, open surgery offers greater survival and freedom from reintervention in the long term. We hope that current trainees will achieve adequate facility in open AAA procedures in the current endovascular era.
Emergency AAA repair: Open or endovascular ? This is a toss-up. Anatomic features (neck length), degree of hemodynamic instability, and institutional and individual surgeon experience can fairly indicate either an open or endovascular approach to these critically ill patients. The fluid restrictive, hypotensive preoperative management that decreases bleeding before open or endovascular treatment must be moderated to maintain systolic blood pressure above 70 mm Hg.
Funding Statement
Funding None.
Footnotes
Conflict of Interest No conflict of interest in relation to this article.
References
- 1.van Beek S C, Conijn A P, Koelemay M J, Balm R. Editor's choice - endovascular aneurysm repair versus open repair for patients with a ruptured abdominal aortic aneurysm: a systematic review and meta-analysis of short-term survival. Eur J Vasc Endovasc Surg. 2014;47(06):593–602. doi: 10.1016/j.ejvs.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Sakalihasan N, Limet R, Defawe O D.Abdominal aortic aneurysm Lancet 2005365(9470):1577–1589. [DOI] [PubMed] [Google Scholar]
- 3.Elefteriades J A, Rizzo J A. NY: Informa Healthcare USA; 2007. Epidemiology: incidence, prevalence, trends; pp. 89–97. [Google Scholar]
- 4.Ingoldby C J, Wujanto R, Mitchell J E. Impact of vascular surgery on community mortality from ruptured aortic aneurysms. Br J Surg. 1986;73(07):551–553. doi: 10.1002/bjs.1800730711. [DOI] [PubMed] [Google Scholar]
- 5.Johansson G, Swedenborg J. Ruptured abdominal aortic aneurysms: a study of incidence and mortality. Br J Surg. 1986;73(02):101–103. doi: 10.1002/bjs.1800730205. [DOI] [PubMed] [Google Scholar]
- 6.Scott R A, Ashton H A, Kay D N. Abdominal aortic aneurysm in 4237 screened patients: prevalence, development and management over 6 years. Br J Surg. 1991;78(09):1122–1125. doi: 10.1002/bjs.1800780929. [DOI] [PubMed] [Google Scholar]
- 7.Lederle F A, Johnson G R, Wilson S E et al. Prevalence and associations of abdominal aortic aneurysm detected through screening. Ann Intern Med. 1997;126(06):441–449. doi: 10.7326/0003-4819-126-6-199703150-00004. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Preventive Services Task Force.Screening for abdominal aortic aneurysm: recommendation statement Ann Intern Med 200514203198–202. [DOI] [PubMed] [Google Scholar]
- 9.NHS. Abdominal aortic aneurysm screening: programme overview
- 10.Goverment S.NHS Abdominal Aortic Aneurysm (AAA) Screening Programme Ann Rep; 2008 [Google Scholar]
- 11.ASSESSMENT SAFHT. Screening for Abdominal Aortic Aneurysm
- 12.LeFevre M L; U.S. Preventive Services Task Force.Screening for abdominal aortic aneurysm: U.S. Preventive Services Task Force recommendation statement Ann Intern Med 201416104281–290. [DOI] [PubMed] [Google Scholar]
- 13.Ashton H A, Buxton M J, Day N Eet al. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial Lancet 2002360(9345):1531–1539. [DOI] [PubMed] [Google Scholar]
- 14.Stather P W, Sidloff D A, Rhema I A, Choke E, Bown M J, Sayers R D. A review of current reporting of abdominal aortic aneurysm mortality and prevalence in the literature. Eur J Vasc Endovasc Surg. 2014;47(03):240–242. doi: 10.1016/j.ejvs.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Lederle F A, Johnson G R, Wilson S E et al. The aneurysm detection and management study screening program: validation cohort and final results. Arch Intern Med. 2000;160(10):1425–1430. doi: 10.1001/archinte.160.10.1425. [DOI] [PubMed] [Google Scholar]
- 16.Darwood R, Earnshaw J J, Turton G et al. Twenty-year review of abdominal aortic aneurysm screening in men in the county of Gloucestershire, United Kingdom. J Vasc Surg. 2012;56(01):8–13. doi: 10.1016/j.jvs.2011.12.069. [DOI] [PubMed] [Google Scholar]
- 17.Darwood R J, Brooks M J. The impact of decreasing abdominal aortic aneurysm prevalence on a local aneurysm screening programme. Eur J Vasc Endovasc Surg. 2012;44(01):45–50. doi: 10.1016/j.ejvs.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Svensjö S, Björck M, Gürtelschmid M, Djavani Gidlund K, Hellberg A, Wanhainen A. Low prevalence of abdominal aortic aneurysm among 65-year-old Swedish men indicates a change in the epidemiology of the disease. Circulation. 2011;124(10):1118–1123. doi: 10.1161/CIRCULATIONAHA.111.030379. [DOI] [PubMed] [Google Scholar]
- 19.Benson R A, Poole R, Murray S, Moxey P, Loftus I M. Screening results from a large United Kingdom abdominal aortic aneurysm screening center in the context of optimizing United Kingdom National Abdominal Aortic Aneurysm Screening Programme protocols. J Vasc Surg. 2016;63(02):301–304. doi: 10.1016/j.jvs.2015.08.091. [DOI] [PubMed] [Google Scholar]
- 20.Grøndal N, Søgaard R, Lindholt J S. Baseline prevalence of abdominal aortic aneurysm, peripheral arterial disease and hypertension in men aged 65-74 years from a population screening study (VIVA trial) Br J Surg. 2015;102(08):902–906. doi: 10.1002/bjs.9825. [DOI] [PubMed] [Google Scholar]
- 21.Wanhainen A, Hultgren R, Linné A et al. Outcome of the Swedish Nationwide abdominal aortic aneurysm screening program. Circulation. 2016;134(16):1141–1148. doi: 10.1161/CIRCULATIONAHA.116.022305. [DOI] [PubMed] [Google Scholar]
- 22.Norman P E, Spilsbury K, Semmens J B. Falling rates of hospitalization and mortality from abdominal aortic aneurysms in Australia. J Vasc Surg. 2011;53(02):274–277. doi: 10.1016/j.jvs.2010.08.087. [DOI] [PubMed] [Google Scholar]
- 23.Sandiford P, Mosquera D, Bramley D. Trends in incidence and mortality from abdominal aortic aneurysm in New Zealand. Br J Surg. 2011;98(05):645–651. doi: 10.1002/bjs.7461. [DOI] [PubMed] [Google Scholar]
- 24.Choke E, Vijaynagar B, Thompson J, Nasim A, Bown M J, Sayers R D. Changing epidemiology of abdominal aortic aneurysms in England and Wales: older and more benign? Circulation. 2012;125(13):1617–1625. doi: 10.1161/CIRCULATIONAHA.111.077503. [DOI] [PubMed] [Google Scholar]
- 25.Nordon I M, Hinchliffe R J, Loftus I M, Thompson M M. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat Rev Cardiol. 2011;8(02):92–102. doi: 10.1038/nrcardio.2010.180. [DOI] [PubMed] [Google Scholar]
- 26.Svensjö S, Björck M, Wanhainen A. Current prevalence of abdominal aortic aneurysm in 70-year-old women. Br J Surg. 2013;100(03):367–372. doi: 10.1002/bjs.8984. [DOI] [PubMed] [Google Scholar]
- 27.Vardulaki K A, Walker N M, Day N E, Duffy S W, Ashton H A, Scott R A. Quantifying the risks of hypertension, age, sex and smoking in patients with abdominal aortic aneurysm. Br J Surg. 2000;87(02):195–200. doi: 10.1046/j.1365-2168.2000.01353.x. [DOI] [PubMed] [Google Scholar]
- 28.Lo R C, Schermerhorn M L. Abdominal aortic aneurysms in women. J Vasc Surg. 2016;63(03):839–844. doi: 10.1016/j.jvs.2015.10.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ailawadi G, Eliason J L, Roelofs K J et al. Gender differences in experimental aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2004;24(11):2116–2122. doi: 10.1161/01.ATV.0000143386.26399.84. [DOI] [PubMed] [Google Scholar]
- 30.Cho B S, Woodrum D T, Roelofs K J, Stanley J C, Henke P K, Upchurch G R., Jr Differential regulation of aortic growth in male and female rodents is associated with AAA development. J Surg Res. 2009;155(02):330–338. doi: 10.1016/j.jss.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu X-F, Zhang J, Paskauskas S, Xin S-J, Duan Z-Q. The role of estrogen in the formation of experimental abdominal aortic aneurysm. Am J Surg. 2009;197(01):49–54. doi: 10.1016/j.amjsurg.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 32.Norman P E, Powell J T. Abdominal aortic aneurysm: the prognosis in women is worse than in men. Circulation. 2007;115(22):2865–2869. doi: 10.1161/CIRCULATIONAHA.106.671859. [DOI] [PubMed] [Google Scholar]
- 33.Sweeting M J, Thompson S G, Brown L C, Powell J T; RESCAN collaborators.Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms Br J Surg 20129905655–665. [DOI] [PubMed] [Google Scholar]
- 34.Fillinger M F, Marra S P, Raghavan M L, Kennedy F E. Prediction of rupture risk in abdominal aortic aneurysm during observation: wall stress versus diameter. J Vasc Surg. 2003;37(04):724–732. doi: 10.1067/mva.2003.213. [DOI] [PubMed] [Google Scholar]
- 35.Dueck A D, Johnston K W, Alter D, Laupacis A, Kucey D S. Predictors of repair and effect of gender on treatment of ruptured abdominal aortic aneurysm. J Vasc Surg. 2004;39(04):784–787. doi: 10.1016/j.jvs.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 36.Egorova N N, Vouyouka A G, McKinsey J Fet al. Effect of gender on long-term survival after abdominal aortic aneurysm repair based on results from the Medicare national database J Vasc Surg 201154011–1.2E7., discussion 11–12 [DOI] [PubMed] [Google Scholar]
- 37.Forsdahl S H, Singh K, Solberg S, Jacobsen B K. Risk factors for abdominal aortic aneurysms: a 7-year prospective study: the Tromsø Study, 1994-2001. Circulation. 2009;119(16):2202–2208. doi: 10.1161/CIRCULATIONAHA.108.817619. [DOI] [PubMed] [Google Scholar]
- 38.Lederle F A, Nelson D B, Joseph A M. Smokers' relative risk for aortic aneurysm compared with other smoking-related diseases: a systematic review. J Vasc Surg. 2003;38(02):329–334. doi: 10.1016/s0741-5214(03)00136-8. [DOI] [PubMed] [Google Scholar]
- 39.Hammond E C, Horn D. Smoking and death rates: report on forty-four months of follow-up of 187,783 men. 2. Death rates by cause. J Am Med Assoc. 1958;166(11):1294–1308. doi: 10.1001/jama.1958.02990110030007. [DOI] [PubMed] [Google Scholar]
- 40.Kent K C, Zwolak R M, Egorova N N et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg. 2010;52(03):539–548. doi: 10.1016/j.jvs.2010.05.090. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Ramos K S. The development of abdominal aortic aneurysms in mice is enhanced by benzo(a)pyrene. Vasc Health Risk Manag. 2008;4(05):1095–1102. doi: 10.2147/vhrm.s3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jin J, Arif B, Garcia-Fernandez F et al. Novel mechanism of aortic aneurysm development in mice associated with smoking and leukocytes. Arterioscler Thromb Vasc Biol. 2012;32(12):2901–2909. doi: 10.1161/ATVBAHA.112.300208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang S, Zhang C, Zhang M et al. Activation of AMP-activated protein kinase α2 by nicotine instigates formation of abdominal aortic aneurysms in mice in vivo. Nat Med. 2012;18(06):902–910. doi: 10.1038/nm.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raveendran M, Senthil D, Utama B et al. Cigarette suppresses the expression of P4Halpha and vascular collagen production. Biochem Biophys Res Commun. 2004;323(02):592–598. doi: 10.1016/j.bbrc.2004.08.129. [DOI] [PubMed] [Google Scholar]
- 45.Lederle F A. The rise and fall of abdominal aortic aneurysm. Circulation. 2011;124(10):1097–1099. doi: 10.1161/CIRCULATIONAHA.111.052365. [DOI] [PubMed] [Google Scholar]
- 46.Wanhainen A, Bergqvist D, Boman K, Nilsson T K, Rutegård J, Björck M. Risk factors associated with abdominal aortic aneurysm: a population-based study with historical and current data. J Vasc Surg. 2005;41(03):390–396. doi: 10.1016/j.jvs.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 47.Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. The UK Small Aneurysm Trial Participants Lancet 1998352(9141):1649–1655. [PubMed] [Google Scholar]
- 48.The U.K. Small Aneurysm Trial: design, methods and progress. The UK Small Aneurysm Trial participants. Eur J Vasc Endovasc Surg. 1995;9(01):42–48. doi: 10.1016/s1078-5884(05)80223-0. [DOI] [PubMed] [Google Scholar]
- 49.Brown L C, Powell J T.Risk factors for aneurysm rupture in patients kept under ultrasound surveillance. UK Small Aneurysm Trial Participants Ann Surg 199923003289–296., discussion 296–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Le M T, Jamrozik K, Davis T M, Norman P E. Negative association between infra-renal aortic diameter and glycaemia: the Health in Men Study. Eur J Vasc Endovasc Surg. 2007;33(05):599–604. doi: 10.1016/j.ejvs.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 51.Golledge J, Clancy P, Jamrozik K, Norman P E. Obesity, adipokines, and abdominal aortic aneurysm: health in Men study. Circulation. 2007;116(20):2275–2279. doi: 10.1161/CIRCULATIONAHA.107.717926. [DOI] [PubMed] [Google Scholar]
- 52.Iribarren C, Darbinian J A, Go A S, Fireman B H, Lee C D, Grey D P. Traditional and novel risk factors for clinically diagnosed abdominal aortic aneurysm: the Kaiser multiphasic health checkup cohort study. Ann Epidemiol. 2007;17(09):669–678. doi: 10.1016/j.annepidem.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 53.Stackelberg O, Björck M, Sadr-Azodi O, Larsson S C, Orsini N, Wolk A. Obesity and abdominal aortic aneurysm. Br J Surg. 2013;100(03):360–366. doi: 10.1002/bjs.8983. [DOI] [PubMed] [Google Scholar]
- 54.Sidloff D, Stather P, Dattani N et al. Aneurysm global epidemiology study: public health measures can further reduce abdominal aortic aneurysm mortality. Circulation. 2014;129(07):747–753. doi: 10.1161/CIRCULATIONAHA.113.005457. [DOI] [PubMed] [Google Scholar]
- 55.Lederle F A. The strange relationship between diabetes and abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2012;43(03):254–256. doi: 10.1016/j.ejvs.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 56.LaMorte W W, Scott T E, Menzoian J O. Racial differences in the incidence of femoral bypass and abdominal aortic aneurysmectomy in Massachusetts: relationship to cardiovascular risk factors. J Vasc Surg. 1995;21(03):422–431. doi: 10.1016/s0741-5214(95)70284-9. [DOI] [PubMed] [Google Scholar]
- 57.Shah A D, Langenberg C, Rapsomaniki E et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1·9 million people. Lancet Diabetes Endocrinol. 2015;3(02):105–113. doi: 10.1016/S2213-8587(14)70219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiong J, Wu Z, Chen C, Wei Y, Guo W. Association between diabetes and prevalence and growth rate of abdominal aortic aneurysms: A meta-analysis. Int J Cardiol. 2016;221:484–495. doi: 10.1016/j.ijcard.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 59.Takagi H; Takuya Umemoto for the ALICE (All-Literature Investigation of Cardiovascular Evidence) Group.Association of diabetes mellitus with presence, expansion, and rupture of abdominal aortic aneurysm: “Curiouser and curiouser!” cried ALICE Semin Vasc Surg 201629(1-2):18–26. [DOI] [PubMed] [Google Scholar]
- 60.Astrand H, Rydén-Ahlgren A, Sundkvist G, Sandgren T, Länne T. Reduced aortic wall stress in diabetes mellitus. Eur J Vasc Endovasc Surg. 2007;33(05):592–598. doi: 10.1016/j.ejvs.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 61.Vorp D A. Biomechanics of abdominal aortic aneurysm. J Biomech. 2007;40(09):1887–1902. doi: 10.1016/j.jbiomech.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shantikumar S, Ajjan R, Porter K E, Scott D J. Diabetes and the abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2010;39(02):200–207. doi: 10.1016/j.ejvs.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 63.Norman P E, Davis T ME, Le M TQ, Golledge J. Matrix biology of abdominal aortic aneurysms in diabetes: mechanisms underlying the negative association. Connect Tissue Res. 2007;48(03):125–131. doi: 10.1080/03008200701331524. [DOI] [PubMed] [Google Scholar]
- 64.Golledge J, Karan M, Moran C S et al. Reduced expansion rate of abdominal aortic aneurysms in patients with diabetes may be related to aberrant monocyte-matrix interactions. Eur Heart J. 2008;29(05):665–672. doi: 10.1093/eurheartj/ehm557. [DOI] [PubMed] [Google Scholar]
- 65.Sakata N, Meng J, Takebayashi S. Effects of advanced glycation end products on the proliferation and fibronectin production of smooth muscle cells. J Atheroscler Thromb. 2000;7(03):169–176. doi: 10.5551/jat1994.7.169. [DOI] [PubMed] [Google Scholar]
- 66.Dua M M, Miyama N, Azuma J et al. Hyperglycemia modulates plasminogen activator inhibitor-1 expression and aortic diameter in experimental aortic aneurysm disease. Surgery. 2010;148(02):429–435. doi: 10.1016/j.surg.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Toghill B J, Saratzis A, Harrison S C, Verissimo A R, Mallon E B, Bown M J. The potential role of DNA methylation in the pathogenesis of abdominal aortic aneurysm. Atherosclerosis. 2015;241(01):121–129. doi: 10.1016/j.atherosclerosis.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 68.Larsson E, Granath F, Swedenborg J, Hultgren R.A population-based case-control study of the familial risk of abdominal aortic aneurysm J Vasc Surg 2009490147–50., discussion 51 [DOI] [PubMed] [Google Scholar]
- 69.Joergensen T MM, Houlind K, Green A, Lindholt J S. Abdominal aortic diameter is increased in males with a family history of abdominal aortic aneurysms: results from the Danish VIVA-trial. Eur J Vasc Endovasc Surg. 2014;48(06):669–675. doi: 10.1016/j.ejvs.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 70.Linné A, Lindström D, Hultgren R. High prevalence of abdominal aortic aneurysms in brothers and sisters of patients despite a low prevalence in the population. J Vasc Surg. 2012;56(02):305–310. doi: 10.1016/j.jvs.2012.01.061. [DOI] [PubMed] [Google Scholar]
- 71.Clifton M A. Familial abdominal aortic aneurysms. Br J Surg. 1977;64(11):765–766. doi: 10.1002/bjs.1800641102. [DOI] [PubMed] [Google Scholar]
- 72.Tilson M D, Seashore M R. Fifty families with abdominal aortic aneurysms in two or more first-order relatives. Am J Surg. 1984;147(04):551–553. doi: 10.1016/0002-9610(84)90020-5. [DOI] [PubMed] [Google Scholar]
- 73.Kuivaniemi H, Platsoucas C D, Tilson M D., III Aortic aneurysms: an immune disease with a strong genetic component. Circulation. 2008;117(02):242–252. doi: 10.1161/CIRCULATIONAHA.107.690982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johnston K W, Scobie T K. Multicenter prospective study of nonruptured abdominal aortic aneurysms. I. Population and operative management. J Vasc Surg. 1988;7(01):69–81. [PubMed] [Google Scholar]
- 75.Johansen K, Koepsell T. Familial tendency for abdominal aortic aneurysms. JAMA. 1986;256(14):1934–1936. [PubMed] [Google Scholar]
- 76.Powell J T, Greenhalgh R M. Multifactorial inheritance of abdominal aortic aneurysm. Eur J Vasc Surg. 1987;1(01):29–31. doi: 10.1016/s0950-821x(87)80020-8. [DOI] [PubMed] [Google Scholar]
- 77.Kuivaniemi H, Kyo Y, Lenk G, Tromp G. Genome-wide approach to finding abdominal aortic aneurysm susceptibility genes in humans. Ann N Y Acad Sci. 2006;1085:270–281. doi: 10.1196/annals.1383.022. [DOI] [PubMed] [Google Scholar]
- 78.Jaakkola P, Kuivaniemi H, Partanen K, Tromp G, Liljeström B, Ryynänen M. Familial abdominal aortic aneurysms: screening of 71 families. Eur J Surg. 1996;162(08):611–617. [PubMed] [Google Scholar]
- 79.Baird P A, Sadovnick A D, Yee I M, Cole C W, Cole L.Sibling risks of abdominal aortic aneurysm Lancet 1995346(8975):601–604. [DOI] [PubMed] [Google Scholar]
- 80.Bengtsson H, Norrgård O, Angquist K A, Ekberg O, Oberg L, Bergqvist D. Ultrasonographic screening of the abdominal aorta among siblings of patients with abdominal aortic aneurysms. Br J Surg. 1989;76(06):589–591. doi: 10.1002/bjs.1800760620. [DOI] [PubMed] [Google Scholar]
- 81.Darling R C, III, Brewster D C, Darling R C et al. Are familial abdominal aortic aneurysms different? J Vasc Surg. 1989;10(01):39–43. doi: 10.1067/mva.1989.vs0100039. [DOI] [PubMed] [Google Scholar]
- 82.Limet R. Familial risk of abdominal aortic aneurysm and its consequences for organization of selective detection [in French] J Mal Vasc. 1995;20(04):285–287. [PubMed] [Google Scholar]
- 83.Verloes A, Sakalihasan N, Koulischer L, Limet R. Aneurysms of the abdominal aorta: familial and genetic aspects in three hundred thirteen pedigrees. J Vasc Surg. 1995;21(04):646–655. doi: 10.1016/s0741-5214(95)70196-6. [DOI] [PubMed] [Google Scholar]
- 84.Wahlgren C M, Larsson E, Magnusson P K, Hultgren R, Swedenborg J.Genetic and environmental contributions to abdominal aortic aneurysm development in a twin population J Vasc Surg 201051013–7., discussion 7 [DOI] [PubMed] [Google Scholar]
- 85.Majumder P P, St Jean P L, Ferrell R E, Webster M W, Steed D L. On the inheritance of abdominal aortic aneurysm. Am J Hum Genet. 1991;48(01):164–170. [PMC free article] [PubMed] [Google Scholar]
- 86.Kuivaniemi H, Shibamura H, Arthur C et al. Familial abdominal aortic aneurysms: collection of 233 multiplex families. J Vasc Surg. 2003;37(02):340–345. doi: 10.1067/mva.2003.71. [DOI] [PubMed] [Google Scholar]
- 87.Ziganshin B A, Bailey A E, Coons C et al. Routine genetic testing for thoracic aortic aneurysm and dissection in a clinical setting. Ann Thorac Surg. 2015;100(05):1604–1611. doi: 10.1016/j.athoracsur.2015.04.106. [DOI] [PubMed] [Google Scholar]
- 88.Isselbacher E M, Lino Cardenas C L, Lindsay M E. Hereditary influence in thoracic aortic aneurysm and dissection. Circulation. 2016;133(24):2516–2528. doi: 10.1161/CIRCULATIONAHA.116.009762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bradley D T, Badger S A, McFarland M, Hughes A E. Abdominal aortic aneurysm genetic associations: mostly false? A systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2016;51(01):64–75. doi: 10.1016/j.ejvs.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 90.Golledge J, Muller J, Daugherty A, Norman P. Abdominal aortic aneurysm: pathogenesis and implications for management. Arterioscler Thromb Vasc Biol. 2006;26(12):2605–2613. doi: 10.1161/01.ATV.0000245819.32762.cb. [DOI] [PubMed] [Google Scholar]
- 91.Armstrong P J, Johanning J M, Calton W C, Jr et al. Differential gene expression in human abdominal aorta: aneurysmal versus occlusive disease. J Vasc Surg. 2002;35(02):346–355. doi: 10.1067/mva.2002.121071. [DOI] [PubMed] [Google Scholar]
- 92.Jones G T, Phillips V L, Harris E L, Rossaak J I, van Rij A M. Functional matrix metalloproteinase-9 polymorphism (C-1562T) associated with abdominal aortic aneurysm. J Vasc Surg. 2003;38(06):1363–1367. doi: 10.1016/s0741-5214(03)01027-9. [DOI] [PubMed] [Google Scholar]
- 93.Ogata T, Shibamura H, Tromp G et al. Genetic analysis of polymorphisms in biologically relevant candidate genes in patients with abdominal aortic aneurysms. J Vasc Surg. 2005;41:1036–1042. doi: 10.1016/j.jvs.2005.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rasmussen T E, Hallett J W, Jr, Schulte S, Harmsen W S, O'Fallon W M, Weyand C M. Genetic similarity in inflammatory and degenerative abdominal aortic aneurysms: a study of human leukocyte antigen class II disease risk genes. J Vasc Surg. 2001;34(01):84–89. doi: 10.1067/mva.2001.115603. [DOI] [PubMed] [Google Scholar]
- 95.Tromp G, Kuivaniemi H. Developments in genomics to improve understanding, diagnosis and management of aneurysms and peripheral artery disease. Eur J Vasc Endovasc Surg. 2009;38(06):676–682. doi: 10.1016/j.ejvs.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 96.Helgadottir A, Thorleifsson G, Magnusson K P et al. The same sequence variant on 9p21 associates with myocardial infarction, abdominal aortic aneurysm and intracranial aneurysm. Nat Genet. 2008;40(02):217–224. doi: 10.1038/ng.72. [DOI] [PubMed] [Google Scholar]
- 97.Leeper N J, Raiesdana A, Kojima Y et al. Loss of CDKN2B promotes p53-dependent smooth muscle cell apoptosis and aneurysm formation. Arterioscler Thromb Vasc Biol. 2013;33(01):e1–e10. doi: 10.1161/ATVBAHA.112.300399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gretarsdottir S, Baas A F, Thorleifsson G et al. Genome-wide association study identifies a sequence variant within the DAB2IP gene conferring susceptibility to abdominal aortic aneurysm. Nat Genet. 2010;42(08):692–697. doi: 10.1038/ng.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Iwashita S, Song S Y. RasGAPs: a crucial regulator of extracellular stimuli for homeostasis of cellular functions. Mol Biosyst. 2008;4(03):213–222. doi: 10.1039/b716357f. [DOI] [PubMed] [Google Scholar]
- 100.Xie D, Gore C, Zhou J et al. DAB2IP coordinates both PI3K-Akt and ASK1 pathways for cell survival and apoptosis. Proc Natl Acad Sci U S A. 2009;106(47):19878–19883. doi: 10.1073/pnas.0908458106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bown M J, Jones G T, Harrison S C et al. Abdominal aortic aneurysm is associated with a variant in low-density lipoprotein receptor-related protein 1. Am J Hum Genet. 2011;89(05):619–627. doi: 10.1016/j.ajhg.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rolph R C, Waltham M, Smith A, Kuivaniemi H. Expanding horizons for abdominal aortic aneurysms. Aorta (Stamford) 2015;3(01):9–15. doi: 10.12945/j.aorta.2015.14-041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Harrison S C, Smith A J, Jones G T et al. Interleukin-6 receptor pathways in abdominal aortic aneurysm. Eur Heart J. 2013;34(48):3707–3716. doi: 10.1093/eurheartj/ehs354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jones G T, Tromp G, Kuivaniemi H et al. Meta-analysis of genome-wide association studies for abdominal aortic aneurysm identifies four new disease-specific risk loci. Circ Res. 2017;120(02):341–353. doi: 10.1161/CIRCRESAHA.116.308765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Koullias G J, Ravichandran P, Korkolis D P, Rimm D L, Elefteriades J A.Increased tissue microarray matrix metalloproteinase expression favors proteolysis in thoracic aortic aneurysms and dissections Ann Thorac Surg 200478062106–2110., discussion 2110–2111 [DOI] [PubMed] [Google Scholar]
- 106.Jones G T, Bown M J, Gretarsdottir S et al. A sequence variant associated with sortilin-1 (SORT1) on 1p13.3 is independently associated with abdominal aortic aneurysm. Hum Mol Genet. 2013;22(14):2941–2947. doi: 10.1093/hmg/ddt141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bradley D T, Hughes A E, Badger S A et al. A variant in LDLR is associated with abdominal aortic aneurysm. Circ Cardiovasc Genet. 2013;6(05):498–504. doi: 10.1161/CIRCGENETICS.113.000165. [DOI] [PubMed] [Google Scholar]
- 108.Gertz S D, Kurgan A, Eisenberg D. Aneurysm of the rabbit common carotid artery induced by periarterial application of calcium chloride in vivo. J Clin Invest. 1988;81(03):649–656. doi: 10.1172/JCI113368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rouer M, Xu B H, Xuan H J et al. Rapamycin limits the growth of established experimental abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2014;47(05):493–500. doi: 10.1016/j.ejvs.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 110.Yoshimura K, Aoki H, Ikeda Y et al. Regression of abdominal aortic aneurysm by inhibition of c-Jun N-terminal kinase. Nat Med. 2005;11(12):1330–1338. doi: 10.1038/nm1335. [DOI] [PubMed] [Google Scholar]
- 111.Parodi J C, Palmaz J C, Barone H D. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg. 1991;5(06):491–499. doi: 10.1007/BF02015271. [DOI] [PubMed] [Google Scholar]
- 112.Jordan W D, Jr, Sampson L K, Iyer S et al. Abdominal aortic aneurysm repair via percutaneous endovascular stenting in the swine model. Am Surg. 1998;64(11):1070–1073. [PubMed] [Google Scholar]
- 113.Lv B J, Lindholt J S, Wang J, Cheng X, Shi G P. Plasma levels of cathepsins L, K, and V and risks of abdominal aortic aneurysms: a randomized population-based study. Atherosclerosis. 2013;230(01):100–105. doi: 10.1016/j.atherosclerosis.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kadoglou N P, Liapis C D. Matrix metalloproteinases: contribution to pathogenesis, diagnosis, surveillance and treatment of abdominal aortic aneurysms. Curr Med Res Opin. 2004;20(04):419–432. doi: 10.1185/030079904125003143. [DOI] [PubMed] [Google Scholar]
- 115.Yamanouchi D, Morgan S, Stair C et al. Accelerated aneurysmal dilation associated with apoptosis and inflammation in a newly developed calcium phosphate rodent abdominal aortic aneurysm model. J Vasc Surg. 2012;56(02):455–461. doi: 10.1016/j.jvs.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kaneko H, Anzai T, Morisawa M et al. Resveratrol prevents the development of abdominal aortic aneurysm through attenuation of inflammation, oxidative stress, and neovascularization. Atherosclerosis. 2011;217(02):350–357. doi: 10.1016/j.atherosclerosis.2011.03.042. [DOI] [PubMed] [Google Scholar]
- 117.Kothapalli C R, Gacchina C E, Ramamurthi A. Utility of hyaluronan oligomers and transforming growth factor-beta1 factors for elastic matrix regeneration by aneurysmal rat aortic smooth muscle cells. Tissue Eng Part A. 2009;15(11):3247–3260. doi: 10.1089/ten.tea.2008.0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tsuruda T, Kato J, Hatakeyama K et al. Adventitial mast cells contribute to pathogenesis in the progression of abdominal aortic aneurysm. Circ Res. 2008;102(11):1368–1377. doi: 10.1161/CIRCRESAHA.108.173682. [DOI] [PubMed] [Google Scholar]
- 119.Tazume H, Miyata K, Tian Z et al. Macrophage-derived angiopoietin-like protein 2 accelerates development of abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 2012;32(06):1400–1409. doi: 10.1161/ATVBAHA.112.247866. [DOI] [PubMed] [Google Scholar]
- 120.Anidjar S, Salzmann J L, Gentric D, Lagneau P, Camilleri J P, Michel J B. Elastase-induced experimental aneurysms in rats. Circulation. 1990;82(03):973–981. doi: 10.1161/01.cir.82.3.973. [DOI] [PubMed] [Google Scholar]
- 121.Pyo R, Lee J K, Shipley J M et al. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest. 2000;105(11):1641–1649. doi: 10.1172/JCI8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kallmes D F, Fujiwara N H, Berr S S, Helm G A, Cloft H J. Elastase-induced saccular aneurysms in rabbits: a dose-escalation study. AJNR Am J Neuroradiol. 2002;23(02):295–298. [PMC free article] [PubMed] [Google Scholar]
- 123.White J V, Mazzacco S L. Formation and growth of aortic aneurysms induced by adventitial elastolysis. Ann N Y Acad Sci. 1996;800:97–120. doi: 10.1111/j.1749-6632.1996.tb33302.x. [DOI] [PubMed] [Google Scholar]
- 124.Basalyga D M, Simionescu D T, Xiong W, Baxter B T, Starcher B C, Vyavahare N R. Elastin degradation and calcification in an abdominal aorta injury model: role of matrix metalloproteinases. Circulation. 2004;110(22):3480–3487. doi: 10.1161/01.CIR.0000148367.08413.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lysgaard Poulsen J, Stubbe J, Lindholt J S. Animal models used to explore abdominal aortic aneurysms: a systematic review. Eur J Vasc Endovasc Surg. 2016;52(04):487–499. doi: 10.1016/j.ejvs.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 126.Daugherty A, Manning M W, Cassis L A. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105(11):1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Manning M W, Cassis L A, Daugherty A. Differential effects of doxycycline, a broad-spectrum matrix metalloproteinase inhibitor, on angiotensin II-induced atherosclerosis and abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2003;23(03):483–488. doi: 10.1161/01.ATV.0000058404.92759.32. [DOI] [PubMed] [Google Scholar]
- 128.Bruemmer D, Daugherty A, Lu H, Rateri D L. Relevance of angiotensin II-induced aortic pathologies in mice to human aortic aneurysms. Ann N Y Acad Sci. 2011;1245:7–10. doi: 10.1111/j.1749-6632.2011.06332.x. [DOI] [PubMed] [Google Scholar]
- 129.Daugherty A, Cassis L A. Mouse models of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2004;24(03):429–434. doi: 10.1161/01.ATV.0000118013.72016.ea. [DOI] [PubMed] [Google Scholar]
- 130.Henriques T A, Huang J, D'Souza S S, Daugherty A, Cassis L A. Orchidectomy, but not ovariectomy, regulates angiotensin II-induced vascular diseases in apolipoprotein E-deficient mice. Endocrinology. 2004;145(08):3866–3872. doi: 10.1210/en.2003-1615. [DOI] [PubMed] [Google Scholar]
- 131.Deng G G, Martin-McNulty B, Sukovich D A et al. Urokinase-type plasminogen activator plays a critical role in angiotensin II-induced abdominal aortic aneurysm. Circ Res. 2003;92(05):510–517. doi: 10.1161/01.RES.0000061571.49375.E1. [DOI] [PubMed] [Google Scholar]
- 132.Gertz S D, Mintz Y, Beeri R et al. Lessons from animal models of arterial aneurysm. Aorta (Stamford) 2013;1(05):244–254. doi: 10.12945/j.aorta.2013.13-052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Allaire E, Guettier C, Bruneval P, Plissonnier D, Michel J-B. Cell-free arterial grafts: morphologic characteristics of aortic isografts, allografts, and xenografts in rats. J Vasc Surg. 1994;19(03):446–456. doi: 10.1016/s0741-5214(94)70071-0. [DOI] [PubMed] [Google Scholar]
- 134.Dai J, Louedec L, Philippe M, Michel J-B, Houard X. Effect of blocking platelet activation with AZD6140 on development of abdominal aortic aneurysm in a rat aneurysmal model. J Vasc Surg. 2009;49(03):719–727. doi: 10.1016/j.jvs.2008.09.057. [DOI] [PubMed] [Google Scholar]
- 135.Allaire E, Bruneval P, Mandet C, Becquemin J-P, Michel J-B. The immunogenicity of the extracellular matrix in arterial xenografts. Surgery. 1997;122(01):73–81. doi: 10.1016/s0039-6060(97)90267-1. [DOI] [PubMed] [Google Scholar]
- 136.Prall A K, Longo G M, Mayhan W G et al. Doxycycline in patients with abdominal aortic aneurysms and in mice: comparison of serum levels and effect on aneurysm growth in mice. J Vasc Surg. 2002;35(05):923–929. doi: 10.1067/mva.2002.123757. [DOI] [PubMed] [Google Scholar]
- 137.Davis F M, Rateri D L, Daugherty A. Abdominal aortic aneurysm: novel mechanisms and therapies. Curr Opin Cardiol. 2015;30(06):566–573. doi: 10.1097/HCO.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yasuda Y, Li Z, Greenbaum D, Bogyo M, Weber E, Brömme D. Cathepsin V, a novel and potent elastolytic activity expressed in activated macrophages. J Biol Chem. 2004;279(35):36761–36770. doi: 10.1074/jbc.M403986200. [DOI] [PubMed] [Google Scholar]
- 139.Qin Y, Cao X, Guo J et al. Deficiency of cathepsin S attenuates angiotensin II-induced abdominal aortic aneurysm formation in apolipoprotein E-deficient mice. Cardiovasc Res. 2012;96(03):401–410. doi: 10.1093/cvr/cvs263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sun J, Sukhova G K, Zhang J et al. Cathepsin K deficiency reduces elastase perfusion-induced abdominal aortic aneurysms in mice. Arterioscler Thromb Vasc Biol. 2012;32(01):15–23. doi: 10.1161/ATVBAHA.111.235002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sun J, Sukhova G K, Zhang J et al. Cathepsin L activity is essential to elastase perfusion-induced abdominal aortic aneurysms in mice. Arterioscler Thromb Vasc Biol. 2011;31(11):2500–2508. doi: 10.1161/ATVBAHA.111.230201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lv B J, Lindholt J S, Cheng X, Wang J, Shi G P. Plasma cathepsin S and cystatin C levels and risk of abdominal aortic aneurysm: a randomized population-based study. PLoS One. 2012;7(07):e41813. doi: 10.1371/journal.pone.0041813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92(08):827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 144.Brinckerhoff C E, Matrisian L M. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3(03):207–214. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- 145.Aziz F, Kuivaniemi H. Role of matrix metalloproteinase inhibitors in preventing abdominal aortic aneurysm. Ann Vasc Surg. 2007;21(03):392–401. doi: 10.1016/j.avsg.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gomez D E, Alonso D F, Yoshiji H, Thorgeirsson U P. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol. 1997;74(02):111–122. [PubMed] [Google Scholar]
- 147.Freestone T, Turner R J, Coady A, Higman D J, Greenhalgh R M, Powell J T. Inflammation and matrix metalloproteinases in the enlarging abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 1995;15(08):1145–1151. doi: 10.1161/01.atv.15.8.1145. [DOI] [PubMed] [Google Scholar]
- 148.McMillan W D, Tamarina N A, Cipollone M, Johnson D A, Parker M A, Pearce W H. Size matters: the relationship between MMP-9 expression and aortic diameter. Circulation. 1997;96(07):2228–2232. doi: 10.1161/01.cir.96.7.2228. [DOI] [PubMed] [Google Scholar]
- 149.Elmore J R, Keister B F, Franklin D P, Youkey J R, Carey D J. Expression of matrix metalloproteinases and TIMPs in human abdominal aortic aneurysms. Ann Vasc Surg. 1998;12(03):221–228. doi: 10.1007/s100169900144. [DOI] [PubMed] [Google Scholar]
- 150.McMillan W D, Patterson B K, Keen R R, Shively V P, Cipollone M, Pearce W H. In situ localization and quantification of mRNA for 92-kD type IV collagenase and its inhibitor in aneurysmal, occlusive, and normal aorta. Arterioscler Thromb Vasc Biol. 1995;15(08):1139–1144. doi: 10.1161/01.atv.15.8.1139. [DOI] [PubMed] [Google Scholar]
- 151.Lorelli D R, Jean-Claude J M, Fox C J et al. Response of plasma matrix metalloproteinase-9 to conventional abdominal aortic aneurysm repair or endovascular exclusion: implications for endoleak. J Vasc Surg. 2002;35(05):916–922. doi: 10.1067/mva.2002.123676. [DOI] [PubMed] [Google Scholar]
- 152.Watanabe T, Sato A, Sawai T et al. The elevated level of circulating matrix metalloproteinase-9 in patients with abdominal aortic aneurysms decreased to levels equal to those of healthy controls after an aortic repair. Ann Vasc Surg. 2006;20(03):317–321. doi: 10.1007/s10016-006-9038-7. [DOI] [PubMed] [Google Scholar]
- 153.Eugster T, Huber A, Obeid T, Schwegler I, Gürke L, Stierli P. Aminoterminal propeptide of type III procollagen and matrix metalloproteinases-2 and -9 failed to serve as serum markers for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2005;29(04):378–382. doi: 10.1016/j.ejvs.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 154.Mosorin M, Juvonen J, Biancari F et al. Use of doxycycline to decrease the growth rate of abdominal aortic aneurysms: a randomized, double-blind, placebo-controlled pilot study. J Vasc Surg. 2001;34(04):606–610. doi: 10.1067/mva.2001.117891. [DOI] [PubMed] [Google Scholar]
- 155.Meijer C A, Stijnen T, Wasser M N, Hamming J F, van Bockel J H, Lindeman J H; Pharmaceutical Aneurysm Stabilisation Trial Study Group.Doxycycline for stabilization of abdominal aortic aneurysms: a randomized trial Ann Intern Med 201315912815–823. [DOI] [PubMed] [Google Scholar]
- 156.Daugherty A, Rateri D L, Charo I F, Owens A P, Howatt D A, Cassis L A. Angiotensin II infusion promotes ascending aortic aneurysms: attenuation by CCR2 deficiency in apoE-/- mice. Clin Sci (Lond) 2010;118(11):681–689. doi: 10.1042/CS20090372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Michineau S, Franck G, Wagner-Ballon O, Dai J, Allaire E, Gervais M. Chemokine (C-X-C motif) receptor 4 blockade by AMD3100 inhibits experimental abdominal aortic aneurysm expansion through anti-inflammatory effects. Arterioscler Thromb Vasc Biol. 2014;34(08):1747–1755. doi: 10.1161/ATVBAHA.114.303913. [DOI] [PubMed] [Google Scholar]
- 158.Forester N D, Cruickshank S M, Scott D J, Carding S R. Functional characterization of T cells in abdominal aortic aneurysms. Immunology. 2005;115(02):262–270. doi: 10.1111/j.1365-2567.2005.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bobryshev Y V, Lord R S. Vascular-associated lymphoid tissue (VALT) involvement in aortic aneurysm. Atherosclerosis. 2001;154(01):15–21. doi: 10.1016/s0021-9150(00)00441-x. [DOI] [PubMed] [Google Scholar]
- 160.Xiao J, Angsana J, Wen J et al. Syndecan-1 displays a protective role in aortic aneurysm formation by modulating T cell-mediated responses. Arterioscler Thromb Vasc Biol. 2012;32(02):386–396. doi: 10.1161/ATVBAHA.111.242198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang Q, Liu Z, Ren J, Morgan S, Assa C, Liu B. Receptor-interacting protein kinase 3 contributes to abdominal aortic aneurysms via smooth muscle cell necrosis and inflammation. Circ Res. 2015;116(04):600–611. doi: 10.1161/CIRCRESAHA.116.304899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Boyle J J, Weissberg P L, Bennett M R. Tumor necrosis factor-alpha promotes macrophage-induced vascular smooth muscle cell apoptosis by direct and autocrine mechanisms. Arterioscler Thromb Vasc Biol. 2003;23(09):1553–1558. doi: 10.1161/01.ATV.0000086961.44581.B7. [DOI] [PubMed] [Google Scholar]
- 163.Leskinen M, Wang Y, Leszczynski D, Lindstedt K A, Kovanen P T. Mast cell chymase induces apoptosis of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2001;21(04):516–522. doi: 10.1161/01.atv.21.4.516. [DOI] [PubMed] [Google Scholar]
- 164.Brown L C, Powell J T.Risk factors for aneurysm rupture in patients kept under ultrasound surveillance. UK Small Aneurysm Trial Participants Ann Surg 199923003289–296., discussion 296–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Brady A R, Thompson S G, Fowkes F GR, Greenhalgh R M, Powell J T; UK Small Aneurysm Trial Participants.Abdominal aortic aneurysm expansion: risk factors and time intervals for surveillance Circulation 20041100116–21. [DOI] [PubMed] [Google Scholar]
- 166.Lederle F A, Johnson G R, Wilson S E et al. Rupture rate of large abdominal aortic aneurysms in patients refusing or unfit for elective repair. JAMA. 2002;287(22):2968–2972. doi: 10.1001/jama.287.22.2968. [DOI] [PubMed] [Google Scholar]
- 167.Reed W W, Hallett J W, Jr, Damiano M A, Ballard D J. Learning from the last ultrasound. A population-based study of patients with abdominal aortic aneurysm. Arch Intern Med. 1997;157(18):2064–2068. doi: 10.1001/archinte.157.18.2064. [DOI] [PubMed] [Google Scholar]
- 168.Erbel R, Aboyans V, Boileau C et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. Eur Heart J. 2014;35(41):2873–2926. doi: 10.1093/eurheartj/ehu281. [DOI] [PubMed] [Google Scholar]
- 169.Brewster D C, Cronenwett J L, Hallett J W, Jr, Johnston K W, Krupski W C, Matsumura J S; Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery.Guidelines for the treatment of abdominal aortic aneurysms. Report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery J Vasc Surg 200337051106–1117. [DOI] [PubMed] [Google Scholar]
- 170.Chaikof E L, Brewster D C, Dalman R Let al. The care of patients with an abdominal aortic aneurysm: the Society for Vascular Surgery practice guidelines J Vasc Surg 200950(4, Suppl):S2–S49. [DOI] [PubMed] [Google Scholar]
- 171.Moll F L, Powell J T, Fraedrich G et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg. 2011;41 01:S1–S58. doi: 10.1016/j.ejvs.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 172.Jones A, Cahill D, Gardham R. Outcome in patients with a large abdominal aortic aneurysm considered unfit for surgery. Br J Surg. 1998;85(10):1382–1384. doi: 10.1046/j.1365-2168.1998.00947.x. [DOI] [PubMed] [Google Scholar]
- 173.Bown M J, Sweeting M J, Brown L C, Powell J T, Thompson S G; RESCAN Collaborators.Surveillance intervals for small abdominal aortic aneurysms: a meta-analysis JAMA 201330908806–813. [DOI] [PubMed] [Google Scholar]
- 174.Hollier L H, Taylor L M, Ochsner J. Recommended indications for operative treatment of abdominal aortic aneurysms. Report of a subcommittee of the Joint Council of the Society for Vascular Surgery and the North American Chapter of the International Society for Cardiovascular Surgery. J Vasc Surg. 1992;15(06):1046–1056. [PubMed] [Google Scholar]
- 175.Herrin J, Etchason J A, Kahan J P, Brook R H, Ballard D J. Effect of panel composition on physician ratings of appropriateness of abdominal aortic aneurysm surgery: elucidating differences between multispecialty panel results and specialty society recommendations. Health Policy. 1997;42(01):67–81. doi: 10.1016/s0168-8510(97)00055-9. [DOI] [PubMed] [Google Scholar]
- 176.Scott R A, Wilson N M, Ashton H A, Kay D N.Is surgery necessary for abdominal aortic aneurysm less than 6 cm in diameter? Lancet 1993342(8884):1395–1396. [DOI] [PubMed] [Google Scholar]
- 177.Lederle F A, Wilson S E, Johnson G R et al. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346(19):1437–1444. doi: 10.1056/NEJMoa012573. [DOI] [PubMed] [Google Scholar]
- 178.Powell J T, Brady A R, Brown L Cet al. Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. The UK Small Aneurysm Trial Participants Lancet 1998352(9141):1649–1655. [PubMed] [Google Scholar]
- 179.Cao P, De Rango P, Verzini F, Parlani G, Romano L, Cieri E; CAESAR Trial Group.Comparison of surveillance versus aortic endografting for small aneurysm repair (CAESAR): results from a randomised trial Eur J Vasc Endovasc Surg 2011410113–25. [DOI] [PubMed] [Google Scholar]
- 180.Ouriel K, Clair D G, Kent K C, Zarins C K; Positive Impact of Endovascular Options for treating Aneurysms Early (PIVOTAL) Investigators.Endovascular repair compared with surveillance for patients with small abdominal aortic aneurysms J Vasc Surg 201051051081–1087. [DOI] [PubMed] [Google Scholar]
- 181.Filardo G, Powell J T, Martinez M A, Ballard D J. Surgery for small asymptomatic abdominal aortic aneurysms. Cochrane Database Syst Rev. 2012;(03):CD001835. doi: 10.1002/14651858.CD001835.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mani K, Lees T, Beiles B et al. Treatment of abdominal aortic aneurysm in nine countries 2005-2009: a vascunet report. Eur J Vasc Endovasc Surg. 2011;42(05):598–607. doi: 10.1016/j.ejvs.2011.06.043. [DOI] [PubMed] [Google Scholar]
- 183.Beck A W, Sedrakyan A, Mao J et al. Variations in abdominal aortic aneurysm care: a report from the International Consortium of Vascular Registries. Circulation. 2016;134(24):1948–1958. doi: 10.1161/CIRCULATIONAHA.116.024870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Karthikesalingam A, Vidal-Diez A, Holt P J et al. Thresholds for abdominal aortic aneurysm repair in England and the United States. N Engl J Med. 2016;375(21):2051–2059. doi: 10.1056/NEJMoa1600931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lederle F.Distinguished Lecture Given at the Opening of the 5th International Meeting on Aortic Disease, Liège, Belgium (September 15, 2016) Stamford: AORTA; 2016;4:3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Filardo G, Lederle F A, Ballard D J et al. Immediate open repair vs surveillance in patients with small abdominal aortic aneurysms: survival differences by aneurysm size. Mayo Clin Proc. 2013;88(09):910–919. doi: 10.1016/j.mayocp.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 187.Filardo G, Lederle F A, Ballard D J et al. Effect of age on survival between open repair and surveillance for small abdominal aortic aneurysms. Am J Cardiol. 2014;114(08):1281–1286. doi: 10.1016/j.amjcard.2014.07.055. [DOI] [PubMed] [Google Scholar]
- 188.De Martino R R, Hoel A W, Beck A W et al. Participation in the Vascular Quality Initiative is associated with improved perioperative medication use, which is associated with longer patient survival. J Vasc Surg. 2015;61(04):1010–1019. doi: 10.1016/j.jvs.2014.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Schermerhorn M L, Buck D B, O'Malley A J et al. Long-term outcomes of abdominal aortic aneurysm in the medicare population. N Engl J Med. 2015;373(04):328–338. doi: 10.1056/NEJMoa1405778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Paraskevas K I, Mikhailidis D P, Veith F J. The rationale for lowering the size threshold in elective endovascular repair of abdominal aortic aneurysm. J Endovasc Ther. 2011;18(03):308–313. doi: 10.1583/10-3371.1. [DOI] [PubMed] [Google Scholar]
- 191.Schlösser F J, Tangelder M J, Verhagen H J et al. Growth predictors and prognosis of small abdominal aortic aneurysms. J Vasc Surg. 2008;47(06):1127–1133. doi: 10.1016/j.jvs.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 192.EVAR trial participants.Endovascular aneurysm repair and outcome in patients unfit for open repair of abdominal aortic aneurysm (EVAR trial 2): randomised controlled trial Lancet 2005365(9478):2187–2192. [DOI] [PubMed] [Google Scholar]
- 193.Greenhalgh R M, Brown L C, Kwong G P, Powell J T, Thompson S G; EVAR trial participants.Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomised controlled trial Lancet 2004364(9437):843–848. [DOI] [PubMed] [Google Scholar]
- 194.Hardman D TA, Fisher C M, Patel M I et al. Ruptured abdominal aortic aneurysms: who should be offered surgery? J Vasc Surg. 1996;23(01):123–129. doi: 10.1016/s0741-5214(05)80042-4. [DOI] [PubMed] [Google Scholar]
- 195.Volodos N L, Karpovich I P, Troyan V I et al. Clinical experience of the use of self-fixing synthetic prostheses for remote endoprosthetics of the thoracic and the abdominal aorta and iliac arteries through the femoral artery and as intraoperative endoprosthesis for aorta reconstruction. Vasa Suppl. 1991;33:93–95. [PubMed] [Google Scholar]
- 196.Kent K C. Clinical practice. Abdominal aortic aneurysms. N Engl J Med. 2014;371(22):2101–2108. doi: 10.1056/NEJMcp1401430. [DOI] [PubMed] [Google Scholar]
- 197.Thomas S M, Gaines P A, Beard J D; Vascular Surgical Society of Great Britain and Ireland; British Society of Interventional Radiology.Short-term (30-day) outcome of endovascular treatment of abdominal aortic aneurism: results from the prospective Registry of Endovascular Treatment of Abdominal Aortic Aneurism (RETA) Eur J Vasc Endovasc Surg 2001210157–64. [DOI] [PubMed] [Google Scholar]
- 198.Harris P L, Vallabhaneni S R, Desgranges P, Becquemin J P, van Marrewijk C, Laheij R J. Incidence and risk factors of late rupture, conversion, and death after endovascular repair of infrarenal aortic aneurysms: the EUROSTAR experience. European Collaborators on Stent/graft techniques for aortic aneurysm repair. J Vasc Surg. 2000;32(04):739–749. doi: 10.1067/mva.2000.109990. [DOI] [PubMed] [Google Scholar]
- 199.Prinssen M, Verhoeven E LG, Buth J et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2004;351(16):1607–1618. doi: 10.1056/NEJMoa042002. [DOI] [PubMed] [Google Scholar]
- 200.Lederle F A, Freischlag J A, Kyriakides T C et al. Outcomes following endovascular vs open repair of abdominal aortic aneurysm: a randomized trial. JAMA. 2009;302(14):1535–1542. doi: 10.1001/jama.2009.1426. [DOI] [PubMed] [Google Scholar]
- 201.Greenhalgh R M, Brown L C, Powell J T, Thompson S G, Epstein D, Sculpher M J; United Kingdom EVAR Trial Investigators.Endovascular versus open repair of abdominal aortic aneurysm N Engl J Med 2010362201863–1871. [DOI] [PubMed] [Google Scholar]
- 202.Blankensteijn J D, de Jong S E, Prinssen M et al. Two-year outcomes after conventional or endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2005;352(23):2398–2405. doi: 10.1056/NEJMoa051255. [DOI] [PubMed] [Google Scholar]
- 203.Lederle F A, Freischlag J A, Kyriakides T C et al. Long-term comparison of endovascular and open repair of abdominal aortic aneurysm. N Engl J Med. 2012;367(21):1988–1997. doi: 10.1056/NEJMoa1207481. [DOI] [PubMed] [Google Scholar]
- 204.De Bruin J L, Baas A F, Buth J et al. Long-term outcome of open or endovascular repair of abdominal aortic aneurysm. N Engl J Med. 2010;362(20):1881–1889. doi: 10.1056/NEJMoa0909499. [DOI] [PubMed] [Google Scholar]
- 205.Dangas G, O'Connor D, Firwana B et al. Open versus endovascular stent graft repair of abdominal aortic aneurysms: a meta-analysis of randomized trials. JACC Cardiovasc Interv. 2012;5(10):1071–1080. doi: 10.1016/j.jcin.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 206.Elefteriades J A, Farkas E A. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J Am Coll Cardiol. 2010;55(09):841–857. doi: 10.1016/j.jacc.2009.08.084. [DOI] [PubMed] [Google Scholar]
- 207.IMPROVE Trial Investigators.The effect of aortic morphology on peri-operative mortality of ruptured abdominal aortic aneurysm Eur Heart J 201536211328–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sarac T P, Gibbons C, Vargas L et al. Long-term follow-up of type II endoleak embolization reveals the need for close surveillance. J Vasc Surg. 2012;55(01):33–40. doi: 10.1016/j.jvs.2011.07.092. [DOI] [PubMed] [Google Scholar]
- 209.Patel R, Sweeting M J, Powell J T, Greenhalgh R M; EVAR trial investigators.Endovascular versus open repair of abdominal aortic aneurysm in 15-years' follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial Lancet 2016388(10058):2366–2374. [DOI] [PubMed] [Google Scholar]
- 210.Powell J T. Prophylactic abdominal aortic aneurysm repair? Open repair brings early pain but later gain. Eur J Vasc Endovasc Surg. 2016;52(06):719–720. doi: 10.1016/j.ejvs.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 211.van Schaik T G, de Bruin J, van Sambeek M et al. RS09. Very long-term follow-up (12–15 years) of the Dutch Randomized Endovascular Aneurysm Repair Management (DREAM) Trial. J Vasc Surg. 2016;63:143S. [Google Scholar]
- 212.Prevention Cfdca. Centers for Disease Control and Prevention, National Center for Health Statistics.Compressed Mortality File 1999–2015 on CDC WONDER Online Database, released December 2015. Data are from the Compressed Mortality File 1999–2015 Series 20 No. 2U, 2016, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program2017
- 213.Bown M J, Sutton A J, Bell P R, Sayers R D. A meta-analysis of 50 years of ruptured abdominal aortic aneurysm repair. Br J Surg. 2002;89(06):714–730. doi: 10.1046/j.1365-2168.2002.02122.x. [DOI] [PubMed] [Google Scholar]
- 214.Yusuf S W, Whitaker S C, Chuter T A, Wenham P W, Hopkinson B R.Emergency endovascular repair of leaking aortic aneurysm Lancet 1994344(8937):1645. [DOI] [PubMed] [Google Scholar]
- 215.Lesperance K, Andersen C, Singh N, Starnes B, Martin M J.Expanding use of emergency endovascular repair for ruptured abdominal aortic aneurysms: disparities in outcomes from a nationwide perspective J Vasc Surg 200847061165–1170., discussion 1170–1171 [DOI] [PubMed] [Google Scholar]
- 216.McPhee J, Eslami M H, Arous E J, Messina L M, Schanzer A. Endovascular treatment of ruptured abdominal aortic aneurysms in the United States (2001-2006): a significant survival benefit over open repair is independently associated with increased institutional volume. J Vasc Surg. 2009;49(04):817–826. doi: 10.1016/j.jvs.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 217.Giles K A, Pomposelli F, Hamdan A, Wyers M, Jhaveri A, Schermerhorn M L.Decrease in total aneurysm-related deaths in the era of endovascular aneurysm repair J Vasc Surg 20094903543–550., discussion 550–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Davenport D L, O'Keeffe S D, Minion D J, Sorial E E, Endean E D, Xenos E S. Thirty-day NSQIP database outcomes of open versus endoluminal repair of ruptured abdominal aortic aneurysms. J Vasc Surg. 2010;51(02):305–90. doi: 10.1016/j.jvs.2009.08.086. [DOI] [PubMed] [Google Scholar]
- 219.Edwards S T, Schermerhorn M L, O'Malley A J et al. Comparative effectiveness of endovascular versus open repair of ruptured abdominal aortic aneurysm in the Medicare population. J Vasc Surg. 2014;59(03):575–582. doi: 10.1016/j.jvs.2013.08.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Mayer D, Aeschbacher S, Pfammatter Tet al. Complete replacement of open repair for ruptured abdominal aortic aneurysms by endovascular aneurysm repair: a two-center 14-year experience Ann Surg 201225605688–695., discussion 695–696 [DOI] [PubMed] [Google Scholar]
- 221.Veith F J, Powell J T, Hinchliffe R J. Is a randomized trial necessary to determine whether endovascular repair is the preferred management strategy in patients with ruptured abdominal aortic aneurysms? J Vasc Surg. 2010;52(04):1087–1093. doi: 10.1016/j.jvs.2010.05.142. [DOI] [PubMed] [Google Scholar]
- 222.Hinchliffe R J, Bruijstens L, MacSweeney S T, Braithwaite B D.A randomised trial of endovascular and open surgery for ruptured abdominal aortic aneurysm - results of a pilot study and lessons learned for future studies Eur J Vasc Endovasc Surg 20063205506–513., discussion 514–515 [DOI] [PubMed] [Google Scholar]
- 223.Desgranges P, Kobeiter H, Katsahian S et al. Editor's choice - ECAR (Endovasculaire ou Chirurgie dans les Anévrysmes aorto-iliaques Rompus): a French randomized controlled trial of endovascular versus open surgical repair of ruptured aorto-iliac aneurysms. Eur J Vasc Endovasc Surg. 2015;50(03):303–310. doi: 10.1016/j.ejvs.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 224.Reimerink J J, Hoornweg L L, Vahl A C et al. Endovascular repair versus open repair of ruptured abdominal aortic aneurysms: a multicenter randomized controlled trial. Ann Surg. 2013;258(02):248–256. doi: 10.1097/SLA.0b013e31828d4b76. [DOI] [PubMed] [Google Scholar]
- 225.Powell J T, Sweeting M J, Thompson M M et al. Endovascular or open repair strategy for ruptured abdominal aortic aneurysm: 30 day outcomes from IMPROVE randomised trial. BMJ. 2014;348:f7661. doi: 10.1136/bmj.f7661. [DOI] [PubMed] [Google Scholar]
- 226.Björck M. Surgery for ruptured abdominal aortic aneurysm. BMJ. 2014;348:g95. doi: 10.1136/bmj.g95. [DOI] [PubMed] [Google Scholar]
- 227.Sweeting M J, Balm R, Desgranges P, Ulug P, Powell J T; Ruptured Aneurysm Trialists.Individual-patient meta-analysis of three randomized trials comparing endovascular versus open repair for ruptured abdominal aortic aneurysm Br J Surg 2015102101229–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.IMPROVE Trial Investigators.Endovascular strategy or open repair for ruptured abdominal aortic aneurysm: one-year outcomes from the IMPROVE randomized trial Eur Heart J 201536312061–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sarac T P, Bannazadeh M, Rowan A F et al. Comparative predictors of mortality for endovascular and open repair of ruptured infrarenal abdominal aortic aneurysms. Ann Vasc Surg. 2011;25(04):461–468. doi: 10.1016/j.avsg.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 230.Hope K, Nickols G, Mouton R. Modern anesthetic management of ruptured abdominal aortic aneurysms. J Cardiothorac Vasc Anesth. 2016;30(06):1676–1684. doi: 10.1053/j.jvca.2016.03.147. [DOI] [PubMed] [Google Scholar]
- 231.Luebke T, Brunkwall J. Risk-adjusted meta-analysis of 30-day mortality of endovascular versus open repair for ruptured abdominal aortic aneurysms. Ann Vasc Surg. 2015;29(04):845–863. doi: 10.1016/j.avsg.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 232.Hinchliffe R J, Powell J T. Improving the outcomes from ruptured abdominal aortic aneurysm: interdisciplinary best practice guidelines. Ann R Coll Surg Engl. 2013;95(02):96–97. doi: 10.1308/003588413X13511609956778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Crawford E S. Ruptured abdominal aortic aneurysm. J Vasc Surg. 1991;13(02):348–350. doi: 10.1016/0741-5214(91)90228-m. [DOI] [PubMed] [Google Scholar]
- 234.Dick F, Erdoes G, Opfermann P, Eberle B, Schmidli J, von Allmen R S. Delayed volume resuscitation during initial management of ruptured abdominal aortic aneurysm. J Vasc Surg. 2013;57(04):943–950. doi: 10.1016/j.jvs.2012.09.072. [DOI] [PubMed] [Google Scholar]
- 235.Mayer D, Pfammatter T, Rancic Z et al. 10 years of emergency endovascular aneurysm repair for ruptured abdominal aortoiliac aneurysms: lessons learned. Ann Surg. 2009;249(03):510–515. doi: 10.1097/SLA.0b013e31819a8b65. [DOI] [PubMed] [Google Scholar]
- 236.Melton L J, III, Bickerstaff L K, Hollier L H et al. Changing incidence of abdominal aortic aneurysms: a population-based study. Am J Epidemiol. 1984;120(03):379–386. doi: 10.1093/oxfordjournals.aje.a113902. [DOI] [PubMed] [Google Scholar]
- 237.Lilienfeld D E, Gunderson P D, Sprafka J M, Vargas C. Epidemiology of aortic aneurysms: I. Mortality trends in the United States, 1951 to 1981. Arteriosclerosis. 1987;7(06):637–643. doi: 10.1161/01.atv.7.6.637. [DOI] [PubMed] [Google Scholar]
- 238.Fowkes F G, Macintyre C C, Ruckley C V.Increasing incidence of aortic aneurysms in England and Wales BMJ 1989298(6665):33–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.McFarlane M J. The epidemiologic necropsy for abdominal aortic aneurysm. JAMA. 1991;265(16):2085–2088. [PubMed] [Google Scholar]
- 240.Norman P E, Castleden W M, Hockey R L. Prevalence of abdominal aortic aneurysm in Western Australia. Br J Surg. 1991;78(09):1118–1121. doi: 10.1002/bjs.1800780928. [DOI] [PubMed] [Google Scholar]
- 241.Bengtsson H, Bergqvist D, Sternby N H. Increasing prevalence of abdominal aortic aneurysms. A necropsy study. Eur J Surg. 1992;158(01):19–23. [PubMed] [Google Scholar]
- 242.Drott C, Arfvidsson B, Ortenwall P, Lundholm K. Age-standardized incidence of ruptured aortic aneurysm in a defined Swedish population between 1952 and 1988: mortality rate and operative results. Br J Surg. 1992;79(02):175–179. doi: 10.1002/bjs.1800790228. [DOI] [PubMed] [Google Scholar]
- 243.Millar W J, Cole C W, Hill G B.Trends in mortality and hospital morbidity due to abdominal aortic aneurysms Health Rep 199570119–27., 21–30 [PubMed] [Google Scholar]
- 244.Blanchard J F. Epidemiology of abdominal aortic aneurysms. Epidemiol Rev. 1999;21(02):207–221. doi: 10.1093/oxfordjournals.epirev.a017997. [DOI] [PubMed] [Google Scholar]
- 245.Filipovic M, Goldacre M J, Roberts S E, Yeates D, Duncan M E, Cook-Mozaffari P. Trends in mortality and hospital admission rates for abdominal aortic aneurysm in England and Wales, 1979-1999. Br J Surg. 2005;92(08):968–975. doi: 10.1002/bjs.5118. [DOI] [PubMed] [Google Scholar]
- 246.Scott R A, Wilson N M, Ashton H A, Kay D N. Influence of screening on the incidence of ruptured abdominal aortic aneurysm: 5-year results of a randomized controlled study. Br J Surg. 1995;82(08):1066–1070. doi: 10.1002/bjs.1800820821. [DOI] [PubMed] [Google Scholar]
- 247.Lindholt J S, Juul S, Fasting H, Henneberg E W.Screening for abdominal aortic aneurysms: single centre randomised controlled trial BMJ 2005330(7494):750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Norman P E, Jamrozik K, Lawrence-Brown M Met al. Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm BMJ 2004329(7477):1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Cosford P A, Leng G C. Screening for abdominal aortic aneurysm. Cochrane Database Syst Rev. 2007;(02):CD002945. doi: 10.1002/14651858.CD002945.pub2. [DOI] [PubMed] [Google Scholar]
- 250.Thompson S G, Ashton H A, Gao L, Buxton M J, Scott R AP; Multicentre Aneurysm Screening Study (MASS) Group.Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening Br J Surg 201299121649–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Lindholt J S, Sørensen J, Søgaard R, Henneberg E W. Long-term benefit and cost-effectiveness analysis of screening for abdominal aortic aneurysms from a randomized controlled trial. Br J Surg. 2010;97(06):826–834. doi: 10.1002/bjs.7001. [DOI] [PubMed] [Google Scholar]
- 252.Ashton H A, Gao L, Kim L G, Druce P S, Thompson S G, Scott R A. Fifteen-year follow-up of a randomized clinical trial of ultrasonographic screening for abdominal aortic aneurysms. Br J Surg. 2007;94(06):696–701. doi: 10.1002/bjs.5780. [DOI] [PubMed] [Google Scholar]
- 253.Jamrozik K, Norman P E, Spencer C A et al. Screening for abdominal aortic aneurysm: lessons from a population-based study. Med J Aust. 2000;173(07):345–350. doi: 10.5694/j.1326-5377.2000.tb125684.x. [DOI] [PubMed] [Google Scholar]
- 254.Englesbe M J, Wu A H, Clowes A W, Zierler R E. The prevalence and natural history of aortic aneurysms in heart and abdominal organ transplant patients. J Vasc Surg. 2003;37(01):27–31. doi: 10.1067/mva.2003.57. [DOI] [PubMed] [Google Scholar]
- 255.Singh K, Bønaa K H, Jacobsen B K, Bjørk L, Solberg S. Prevalence of and risk factors for abdominal aortic aneurysms in a population-based study: the Tromsø study. Am J Epidemiol. 2001;154(03):236–244. doi: 10.1093/aje/154.3.236. [DOI] [PubMed] [Google Scholar]
- 256.Svensjö S, Björck M, Wanhainen A. Update on screening for abdominal aortic aneurysm: a topical review. Eur J Vasc Endovasc Surg. 2014;48(06):659–667. doi: 10.1016/j.ejvs.2014.08.029. [DOI] [PubMed] [Google Scholar]


