Abstract
Thrombocytopenia, ascites, myelofibrosis, renal dysfunction, and organomegaly (TAFRO) syndrome is a newly recognized but rare disease, and its treatment has not yet been established. We reported a 50-year-old woman with TAFRO syndrome diagnosed 2 years after the initial symptoms of a fever, fatigue, epigastric pain, edema, ascites, lymphadenopathy, thrombocytopenia and renal insufficiency. The patient showed refractory ascites and required hemodialysis under corticosteroid mono-therapy for suspected immune-mediated disease but was successfully treated with additive rituximab, resulting in improvement in her laboratory data, the withdrawal of hemodialysis and the disappearance of ascites. This case underscores the therapeutic utility of rituximab in patients with corticosteroid-resistant TAFRO syndrome, even long after the onset of the disease.
Keywords: ascites, corticosteroid, hemodialysis, TAFRO syndrome, thrombocytopenia, rituximab
Introduction
Thrombocytopenia, ascites, myelofibrosis, renal dysfunction, and organomegaly (TAFRO) syndrome as a novel clinical entity is considered to be a sub-type of human herpes virus-8 (HHV-8)-negative/idiopathic multicentric Castleman's disease (iMCD) that shares common symptoms and laboratory abnormalities with a cytokine storm (1-3). The etiology of TAFRO syndrome is unknown, but cytokine storm triggered by infection, immune dysfunction or tumorigenesis (4,5) may be involved. With the increased recognition of TAFRO syndrome, there have been an increasing number of reports in which patients were successfully treated with corticosteroids and a variety of immunomodulating drugs, such as tocilizumab, cyclosporine A and rituximab, mostly at an early stage of the disease (2,3). However, there is no established treatment, and information on effective treatments with regard to the long-term clinical course is quite limited. Unfortunately, a delayed diagnosis and a lack of efficient treatment sometimes lead to multiple organ dysfunction and even death in cases of TAFRO syndrome (2,3,6,7). As such, the accumulation of experience concerning the treatment of TAFRO syndrome is necessary.
We herein report a case of TAFRO syndrome diagnosed two years after the initial presentation of symptoms that showed refractory ascites and required hemodialysis under corticosteroid mono-therapy. Our patient was successfully treated with rituximab. There are still few reports of the treatment result of rituximab in cases of TAFRO syndrome. Therefore, our case report can add new information regarding the treatment options for patients with TAFRO syndrome, even long after the onset.
Case Report
A 50-year-old Japanese woman was admitted to our hospital for the evaluation of a fever, fatigue and epigastric pain lasting for 10 days. She had a history of Pityriasis roses Gilbert. On admission she showed a high fever, leg edema, and right pleural effusion and multiple lymphadenopathy. The laboratory data at admission are shown in the Table. She showed severe thrombocytopenia, a high level of serum alkaline phosphatase, hypoalbuminemia, mild renal insufficiency and positive inflammatory signs. Although she was treated with antibiotics, she had no focus of infection and no evidence of a viral infection, including HHV-8. Since the patient did not show any signs or symptoms relevant to specific infectious diseases throughout the clinical course, we did not perform specific tests to exclude rare infectious diseases, such as acid-fast bacterial infections, rickettsial disease, lyme disease and a severe fever with thrombocytopenia syndrome. Various autoantibodies were positive, but no autoimmune disorder was definitively diagnosed. Fluorodeoxyglucose-positron emission tomography/computed tomography showed positive lesions in multiple lymph nodes (SUV max 2-3), some bone marrow (Fig. 1) and the spleen, suggesting lymphoma. However, a right axillary lymph node biopsy revealed no apparent lymphoma cells and few IgG4-positive plasma cells, but the specimen resembled multicentric Castleman's disease of mixed-type-like findings (Fig. 2). A bone marrow biopsy showed increased density of megakaryocytes and mild reticulin fibrosis. Ascites appeared soon after admission.
Table.
Laboratory Data on Admission.
| Urine | ||
| Protein | ± | |
| Occult blood | 2+ | |
| Red blood cell | 5-9 | /high power field |
| Wight blood cell | 1-4 | /high power field |
| NAG | 21.1 | U/L |
| α1-microglobulin | 28.1 | mg/L |
| Complete blood count | ||
| WBC | 8,200 | /μL |
| Hb | 12.7 | g/dL |
| Platelet | 1.9×104 | /μL |
| Blood chemistry | ||
| Total protein | 6.1 | g/dL |
| Albumin | 2.0 | g/dL |
| Urea nitrogen | 13.3 | mg/dL |
| Creatinine | 1.07 | mg/dL |
| Aspartate aminotransferase | 23 | IU/L |
| Alanine aminotransferase | 14 | IU/L |
| Total bilirubin | 0.32 | mg/dL |
| Alkaline phosphatase | 392 | IU/L |
| γ glutamyltransferase | 42 | IU/L |
| Lactate dehydrogenase | 264 | IU/L |
| Na | 134 | mEq/L |
| K | 3.9 | mEq/L |
| Cl | 100 | mEq/L |
| Ferritin | 355.2 | ng/dL |
| estimated GFR | 58.6 | mL/min/1.73 m2 |
| Immunologic test | ||
| IgG | 1,460 | mg/dL |
| IgG4 | 25.2 | mg/dL |
| IgA | 436 | mg/dL |
| IgM | 42 | mg/dL |
| IgE | 216 | mg/dL |
| C3 | 72 | mg/dL |
| C4 | 14 | mg/dL |
| C-reactive protein | 18.2 | mg/dL |
| Procalcitonin | 1.28 | ng/dL (<0.05) |
| Soluble interleukin-2 receptor | 2,013 | U/mL |
| Antinuclear antibody | ×320 | |
| Anti-DNA antibody | 8.0 | IU/mL (<9.0) |
| Anti-SS-A antibody | 240 | U/mL (<6.0) |
| Anti-SS-B antibody | 157.6 | U/mL (<6.0) |
| MPO-ANCA | <1.0 | U/mL (<3.4) |
| PR3-ANCA | <1.0 | U/mL (<3.4) |
| Thyroglobulin antibody | 157 | IU/mL |
| Thyroperoxidase antibody | 16 | IU/mL |
| Platelet associated-IgG | 218 | ng/107 cells |
| Virologic test | ||
| HBs antigen | negative | |
| HCV antibody | negative | |
| HIV-antibody | negative | |
| HHV-8-DNA | negative | |
| EBV capsid antigen-IgG (index) | ×80 | |
| EBV capsid antigen-IgM (index) | ×10 | |
| EBV nuclear antigen | ×20 | |
| CMV IgM | negative | |
| C7-HRP (/50,000 WBC) | 0 |
The values in the parentheses show the normal range.
NAG: N-acetyl-β-D-glucosaminidase, GFR: glomerular filtration rate, MPO-ANCA: myeloperoxidase-anti-neutrophil cytoplasmic antibody, PR3-ANCA: proteinase 3-anti-neutrophil cytoplasmic antibody, HBs: hepatitis B surface antigen, HCV: hepatitis C virus, HIV: human immunodeficiency virus, HHV-8: human herpesvirus-8, EBV: Epstein-Barr virus, CMV: cytomegalovirus
Figure 1.
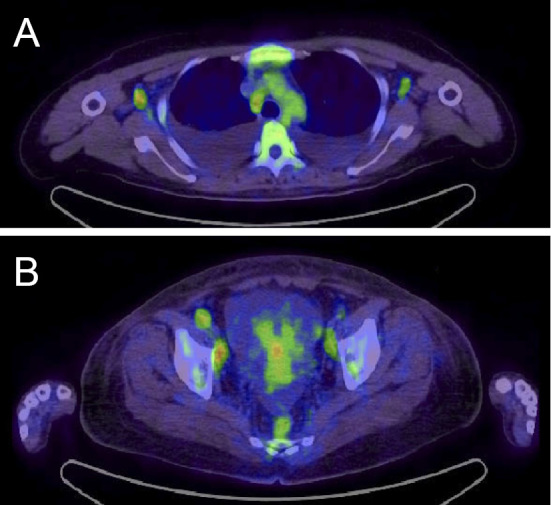
FDG positron emission tomography/computed tomography. Maximum intensity projection images show the intense tracer accumulation in bilateral axillary and mediastinum lymph nodes (A) and pelvic lymph nodes (B). The tracer accumulation is also seen in bone marrows (A, B). The maximum standardized uptake value (SUV) of the mass is 2-3.
Figure 2.
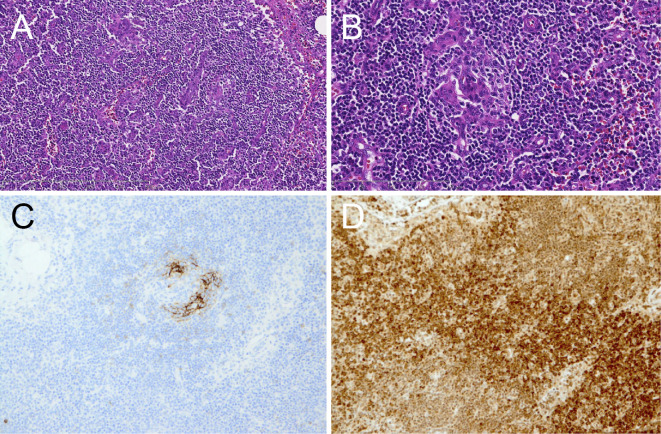
A right axillary lymph node biopsy. Micrograph showing interfollicular vascular proliferation in the pattern of arborizing or anastomosis and interfollicular infiltration of lymphocytes and plasma cells in the mixed nature (A). [Hematoxylin and Eosin (H&E) staining] ×100. Blood vessel hyperplasia with prominent atrophic germinal centers in lymphoid follicles (B). (H&E staining) ×200. CD23 immunostaining for dendritic cells showing disrupted patterns of follicular dendritic cell networks and their loss with blood vessel hyperplasia (C). ×200. Numerous plasma cells stained with anti-IL-6 are found in the interfollicular region (D). ×200.
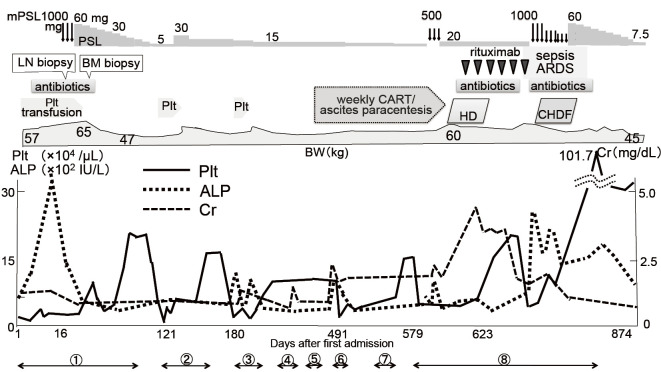
The clinical course of the patient after admission is shown in Fig. 3. She was treated with platelet transfusion and methylprednisolone (mPSL) pulse therapy [1,000 mg/day intravenously (i. v.) 3 consecutive days], and then oral prednisolone (PSL) at 60 mg/day under suspicion of immune-mediated disease, resulting in partial improvement in her symptoms and laboratory data. On the 85th day after admission, the patient was discharged with a platelet count of 23.7×103/μL, alkaline phosphatase 421 U/L, creatinine (Cr) 0.69 mg/dL and C-reactive protein 0.43 mg/dL under PSL at 25 mg/day.
Figure 3.
The clinical course of the patient with PSL and rituximab therapy. After the introduction of rituximab, ascites began to decrease without concentrated ascites reinfusion therapy or ascites paracentesis, and the renal function recovered. The body weight reflects the degree of ascites. ① to ⑧ indicate the length of the hospital stay. mPSL: methylprednisolone pulse therapy, PSL: prednisolone, LN: lymph node, BM: bone marrow, ARDS: adult respiratory distress syndrome, CART: concentrated ascites reinfusion therapy, HD: hemodialysis, CHDF: continuous hemodiafiltration, Plt: platelet, ALP: alkaline phosphatase, Cr: creatinine
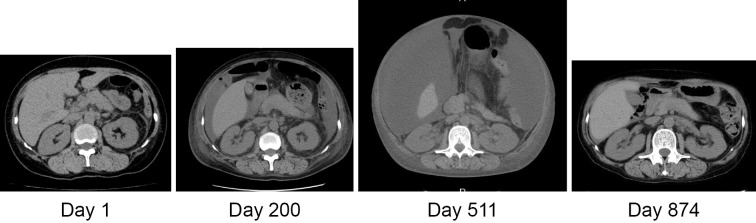
On the 2nd and 3rd admissions (121st and 180th days after the first admission, respectively) for the relapse of the disease with ascites, abdominal pain and thrombocytopenia, the patient was treated with an increased dose of PSL, platelet transfusion, diuretics and the oral thrombopoietin mimetic eltrombopag. Controlling edema, ascites and renal dysfunction became difficult while tapering the dose of PSL, and the patient complained of appetite loss due to abdominal distention. On the 4th admission (307th day) after confirming exudative ascites, she was treated with albumin transfusion, ascites paracentesis and cell-free and concentrated ascites reinfusion therapy (CART), and her urinary volume was increased and the renal function transiently recovered. To control ascites, as shown in Fig. 4, she underwent ascites paracentesis by taking PSL at 15 mg/day, furosemide at 60 mg/day, spironolactone at 25 mg/day, trichlormethiazide at 1 mg/day and tolvaptan at 15 mg/day on the 5th and 6th admissions.
Figure 4.
Clinical course of ascites on abdominal plain CT. The large amount of ascites on the 511th after the first admission disappeared on the 874th day after the first admission.
On the 7th admission (588th day) for appetite loss due to ascites, she developed a fever and chills when concentrated ascitic fluid was returned to the patient during CART. A clinically significant fever is relatively rare during CART (8). However, our patient developed high fever and chillness during reinfusion at every CART; we therefore suspected that the concentrated cytokines infused into the systemic circulation might have induced the fever. Indeed, the interleukin (IL)-6 level in the serum and ascitic fluids was elevated (15.9 pg/mL and 5,480 pg/mL, respectively), and vascular endothelial growth factor (VEGF) in the serum and ascitic fluid was also high (68 pg/dL and 542 pg/mL, respectively). The symptoms, laboratory data, imaging findings and pathology of the patient were re-evaluated and confirmed a definitive diagnosis of TAFRO syndrome (3).
On the 8th admission (609th day) for exacerbation of the disease at 7.5 mg/day PSL, the patient was treated with mPSL pulse therapy (500 mg/day i.v. 3 consecutive days) and then oral 20 mg/day PSL. Ascites and renal failure were resistant to corticosteroid treatment. Hemodialysis due to oliguria and serum creatinine of 4.14 mg/dL was introduced, and extracorporeal ultrafiltration, online-hemodiafiltration, packed red blood cell and albumin transfusions were also needed to prevent hypotension during hemodialysis. We therefore decided to treat the patient with a total of 8 cycles of rituximab (375 mg/m2, weekly) as a second-line drug for TAFRO syndrome.
Although the complication of pneumonia and cytomegalovirus infection prolonged the interval of rituximab administration, after the 4th administration of rituximab the patient was able to discontinue hemodialysis and showed prominent improvement in her laboratory data and edema and a moderate reduction in ascites. After the 6th administration of rituximab, septic shock occurred due to cholecystitis by Escherichia coli, and the patient became complicated with acute respiratory distress syndrome. The patient received antibiotics, mechanical ventilation and continuous hemodiafiltration (CHDF) for cytokine removal, mPSL pulse therapy and oral 60 mg/day PSL. The patient's condition gradually improved, and mechanical ventilation and CHDF were both withdrawn with the stabilization of the renal function and platelet count. Ascites aggravated by sepsis remained when she was discharged with 7.5 mg/day PSL, but it had disappeared on CT at 2 months after the discharge (874th day) (Fig. 4).
TAFRO syndrome with a fever, thrombocytopenia and ascites relapsed in the patient 4 months after the disappearance of ascites (987th day) and she was successfully treated again with high-dose corticosteroids and a total of 3 cycles of rituximab (375 mg/m2, weekly). We then extended the interval of rituximab administration to 3, 6 and 8 weeks, after which the patient was well without thrombocytopenia or ascites. Thus, maintenance treatment with rituximab may be an effective alternative way of controlling the long-term clinical course of TAFRO syndrome.
Discussion
Several clinical and pathological characteristics of TAFRO syndrome, such as severe inflammation with a high level of serum IL-6 and the histopathological findings of lymph nodes, resemble those of iMCD. However, other characteristic TAFRO findings, such as a normal immunoglobulin level, thrombocytopenia, relatively small lymphadenopathy, marked pleural effusion, ascites and edema, renal insufficiency, reticulin myelofibrosis and acute or subacute onset and clinical course are not considered typical findings for iMCD (3). Therefore, TAFRO syndrome may be a disease entity distinct from iMCD.
Although cytokine storm seems to be the main pathophysiology, the etiology of TAFRO syndrome is not known (1-3). At present, there is no standardized therapy. Most patients with TAFRO syndrome are treated with corticosteroids and additional immunomodulating drugs, but some have died of organ failure due to the ineffectiveness of treatment (2,3,6,7).
The consistency in the findings of TAFRO syndrome suggests a single cause. If so, the differing effects of the same drug on TAFRO syndrome might be due to being administered at different phases of the disease or to dysregulation of multiple immunological processes. Iwaki et al. reported that there was little evidence of an association between the serum IL-6 concentration and effectiveness of anti-IL-6 therapy with tocilizumab or siltuximab in TAFRO syndrome/iMCD, suggesting that elevated IL-6 may not be the primary pathological driver of the proinflammatory hypercytokinemia responsible for TAFRO syndrome/iMCD (2). Furthermore, iMCD shows thrombocytosis due to cytokine storm, whereas TAFRO syndrome is associated with thrombocytopenia with hyper-/normoplasia of megakaryocytes, suggesting that not only cytokine storm but also other factors, such as immune dysfunction, may induce the consumption of thrombocytes in TAFRO syndrome (2). Considering that immune dysfunction is possible with TAFRO syndrome, choosing an appropriate treatment including immunomodulating drugs is important.
Most patients with TAFRO syndrome are treated initially with corticosteroids. Additional immunomodulating drugs, such as tocilizumab, cyclosporine A and rituximab, are then introduced (usually at an early phase of the disease) in case of a poor response to corticosteroids (2,3). We did not treat our patient with cyclosporine A because a recovery of the renal function was still possible. Given that immune dysfunction may contribute to TAFRO syndrome and that our patient had various autoantibodies but a relatively low C-reactive protein level under corticosteroid therapy, we chose rituximab over tocilizumab as the second-line drug. Six cases of TAFRO syndrome have reported clinical improvement with rituximab, a monoclonal antibody directed against the CD20 antigen on B-lymphocytes, in addition to corticosteroids (9-14). In our case, rituximab soon began to show effectiveness for symptoms, laboratory abnormalities and organ failure. Furthermore, the relapse of TAFRO syndrome about one year after the initial treatment with rituximab was successfully treated with rituximab again. This result may support the notion that an autoimmune mechanism is largely involved in TAFRO syndrome. Rituximab might have regulated the disease activity due to immune dysfunction in our patient. We are now examining the proper interval of rituximab administration as maintenance therapy in our patient.
Of note: the refractory infiltrative ascites was reversible even long after the onset of disease in our case, but the disappearance of ascites occurred later than the improvement of the abnormal laboratory data. Vasculogenesis has been suggested to contribute to ascites in TAFRO syndrome because thalidomide, which can inhibit vasculogenesis, was found to effectively treat tocilizumab-resistant refractory ascites in TAFRO syndrome (15). The contribution of vasculogenesis to ascites and whether or not rituximab inhibited vasculogenesis in our patient remain unclear at present.
Renal dysfunction in patients with TAFRO syndrome may be due to hemodynamic alterations and/or glomerular factors. A limited number of renal biopsies in patients with TAFRO syndrome presenting with hematuria and proteinuria revealed mild to moderate proliferation of mesangial cells and changes of glomerular capillary walls (16). Our patient did not have markedly abnormal urinalysis findings, so the rapid deterioration of the renal function with oliguria and need for hemodialysis may have been due to the contribution of hemodynamic alterations due to severe ascites. However, our patient was able to discontinue hemodialysis after additional rituximab treatment despite the persistence of ascites, suggesting that intra-renal hemodynamic alterations rather than pre-renal factors are involved in severe renal dysfunction.
Infection is considered to be a factor that triggers cytokine storm in iMCD. Patients with TAFRO syndrome, including our case, often show abdominal pain and elevated alkaline phosphatase (ALP) (liver type dominant), CRP and procalcitonin (17). A liver biopsy has revealed neutrophilic infiltration and microabscesses, mostly in the portal region in some patients (17) and cholangitis in one case with TAFRO syndrome (13). Iwaki et al. suggested that cytokine storm in TAFRO syndrome might be triggered by an undetected infection in the hepatobiliary system (17). Escherichia coli-induced cholecystitis in our patient may be a mere comorbidity; however, a relationship may exist between infection of the hepatobiliary system and TAFRO syndrome in our patient.
In summary, we experienced a patient with TAFRO syndrome with refractory ascites and renal failure requiring hemodialysis that was treated successfully with rituximab, even after two-year-long corticosteroid treatment, suggesting an important role of rituximab in treating patients with TAFRO syndrome. Any therapeutic experiences of TAFRO syndrome are valuable at present; however, clarifying the etiology, such as an undetected infection, is of vital importance to the development of a targeted treatment.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Takai K, Nikkuni K, Shibuya H, Hashidate H. Thrombocytopenia with mild bone marrow fibrosis accompanied by fever, pleural effusion, ascites and hepatosplenomegaly. Rinsho Ketsueki (Jpn J Clin Hematol) 51: 320-325, 2010. (in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 2. Iwaki N, Fajgenbaum DC, Nabel CS, et al. Clinicopathologic analysis of TAFRO syndrome demonstrates a distinct subtype of HHV-8-negative multicentric Castleman disease. Am J Hematol 91: 220-226, 2016. [DOI] [PubMed] [Google Scholar]
- 3. Masaki Y, Kawabata H, Takai K, et al. Proposed diagnostic criteria, disease severity classification and treatment strategy for TAFRO syndrome, 2015 version. Int J Hematol 103: 686, 2016. [DOI] [PubMed] [Google Scholar]
- 4. Suda T, Katano H, Delsol G, et al. HHV-8 infection status of AIDS-related and AIDS-associated multicentric Castleman's disease. Pathol Int 51: 671-679, 2001. [DOI] [PubMed] [Google Scholar]
- 5. Dossier A, Meignin V, Fieshi C, Boutboul D, Oksenhendler E, Galicier L. Human herpesvirus 8-related Castleman disease in the absence of HIV infection. Clin Infect Dis 56: 833-843, 2013. [DOI] [PubMed] [Google Scholar]
- 6. Masaki Y, Nakajima A, Iwao H, et al. Japanese variant of multicentric Castleman's disease associated with serositis and thrombocytopenia-a report of two cases: is TAFRO syndrome (Castleman-Kojima disease) a distinct clinicopathological entity? J Clin Exp Hematop 53: 79-85, 2013. [DOI] [PubMed] [Google Scholar]
- 7. Takai K, Nikkuni K, Shibuya H, Hashidate H. Thrombocytopenia with mild bone marrow fibrosis accompanied by fever, pleural effusion, ascites and hepatosplenomegaly. Jpn J Clin Hematol 51: 320-325, 2010. [PubMed] [Google Scholar]
- 8. Hanafusa N, Isoai A, Ishihara T, et al. Safety and efficacy of cell-free and concentrated ascites reinfusion therapy (CART) in refractory ascites: Post-marketing surveillance results. PLoS One 12: e0177303, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ozawa T, Kosugi S, Kito M, et al. Efficacy of rituximab for TAFRO syndrome, a variant type of multicentric Castleman's disease. Rinsho Ketsueki (Jpn J Clin Hematol) 55: 350-355, 2014. (in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 10. Jain P, Verstovsek S, Loghavi S, et al. Durable remission with rituximab in a patient with an unusual variant of Castleman's disease with myelofibrosis-TAFRO syndrome. Am J Hematol 90: 1091-1092, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tedesco S, Postacchini L, Manfredi L, et al. Successful treatment of a Caucasian case of multifocal Castleman's disease with TAFRO syndrome with a pathophysiology targeted therapy - a case report. Exp Hematol Oncol 4: 3, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hiramatsu S, Ohmura K, Tsuji H, et al. Successful treatment by rituximab in a patient with TAFRO syndrome with cardiomyopathy. Jpn J Clin Immunol 39: 64-71, 2016. [DOI] [PubMed] [Google Scholar]
- 13. Nagai Y, Ando S, Honda N, et al. TAFRO syndrome showing cholangitis on liver biopsy. Rinsho Ketsueki (Jpn J Clin Hematol) 57: 2490-2495, 2016. (in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 14. Nara M, Komatsuda A, Itoh F, et al. Two cases of thrombocytopenia, anasarca, fever, reticulin fibrosis/renal failure, and organomegaly (TAFRO) syndrome with high serum procalcitonin levels, including the first case complicated with adrenal hemorrhaging. Intern Med 56: 1247-1252, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tatekawa S, Umemura K, Fukuyama R, et al. Thalidomide for tocilizumab-resistant ascites with TAFRO syndrome. Clin Case Rep 3: 472-478, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kawashima M, Usui T, Okada H, et al. TAFRO syndrome: 2 cases and review of the literature. Mod Rheumatol 20: 1-5, 2015. [DOI] [PubMed] [Google Scholar]
- 17. Iwaki N, Gion Y, Kondo E, et al. Elevated serum interferon γ-induced protein 10 kDa is associated with TAFRO syndrome. Sci Rep 7: 42316, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]