Abstract
We herein report the case of a 25-year-old Japanese woman with left-main-trunk acute myocardial infarction (LMT-AMI). She had cardiogenic shock, so emergency percutaneous intervention was performed. Intravascular ultrasound of LMT-AMI showed that the three-layered structure of the intima, tunica media, and adventitia was not clearly visible, and the vessel was concentrically thickened; unstable plaque and calcification were not seen. AMI is rarely seen in young women, but Takayasu's arteritis is one major cause. If a young woman complaining of typical chest pain as acute coronary syndrome is encountered, systemic diseases must be considered.
Keywords: acute myocardial infarction, Takayasu's arteritis, young woman, chest pain, cardiogenic shock
Introduction
The prevalence of juvenile-onset acute myocardial infarction (AMI) has been increasing due to the increasing exposure to coronary risk factors, such as smoking, diabetes mellitus and familial hypercholesterolemia. The reported incidence of AMI in patients <45 years of age was 2-6% in the 1980s (1) and 4-10% in the 1990s (2,3). Classic coronary risk factors are not commonly seen in young women, and relatively uncommon causes, such as congenital heart disease, Kawasaki disease, vasculitis syndrome, and hemostatic abnormalities are considered (4-6). Among them, Takayasu's arteritis is an uncommon disease. The incidence of new cases of Takayasu's arteritis is 1-3/million person·years in the United States and Europe (7), but these cases can have a dramatic clinical presentation and are sometimes fatal.
We encountered a patient with new-onset Takayasu's arteritis who presented with a left-main-trunk (LMT) AMI. She was successfully treated with emergent coronary intervention and steroid therapy.
Case Report
A 25-year-old Japanese woman was referred to our hospital with severe chest pain and consciousness disturbance. She had no known history of heart disease or other chronic diseases, including connective tissue diseases, nor any medication or smoking histories.
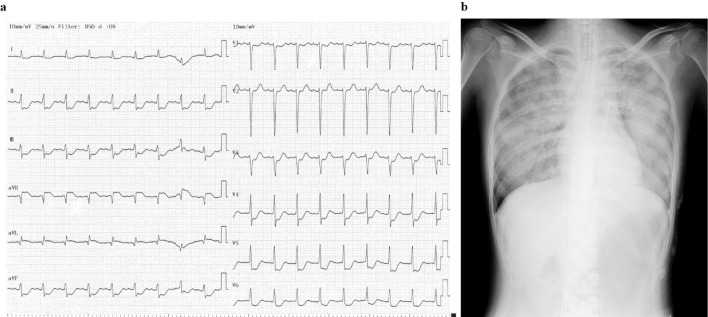
From four days before the hospitalization, she began to feel chest pain on exercise, and it gradually worsened, sometimes accompanied by cold sweat. Several hours prior to her visit to our hospital, she felt severe chest pain with cold sweat and was taken to a nearby clinic. She fainted in the waiting room and was transferred to a nearby general hospital. Electrocardiogram showed marked ST-T changes (Fig. 1a). On echocardiography, the wall motion of the left ventricle was diffusely reduced, but the motion of only the basal inferior wall was preserved. Thus, severe myocardial ischemia due to LMT lesions was suspected, and she was transferred to our hospital.
Figure 1.
a: 12-lead electrocardiogram showed ST elevation in the aVR lead and extensive ST depression in the other leads. b: Chest X-ray on admission showed pulmonary congestion.
On admission, her consciousness was Glasgow Coma Scale E1V1M5, and her blood pressure and percutaneous oxygen saturation could not be measured. The heart rate was 113 beats/min, irregular; the jugular vein was dilated; and a coarse crackle was audible in her chest, but no abnormal heart sounds or murmurs were audible. No edema was detected in her extremities.
As shown in Table 1, elevations in the white blood cell count and levels of aspartate aminotransferase, lactate dehydrogenase, creatine kinase (CK), CK-muscular brain type, and troponin T were present, but other laboratory values were within normal ranges. IgG4 was not measured. A blood gas analysis showed lactic acidosis, and several inflammatory markers were elevated, but the measured antibodies were all within normal ranges. Chest X-ray showed pulmonary congestion (Fig. 1b).
Table 1.
Laboratory Findings.
| WBC | 19,400 | /μL | CRP | 8.18 | mg/dL |
| Hemoglobin | 12.5 | g/dL | Total protein | 8.8 | g/dL |
| Platelets | 56.7 | ×10³/μL | Albumin | 2.9 | g/dL |
| PT-INR | 1.17 | BUN | 15.0 | mg/dL | |
| APTT | 36.8 | sec | Creatinine | 0.56 | mg/dL |
| FDP | 9.9 | μg/mL | Uric acid | 6.5 | mg/dL |
| Fibrinogen | 704 | mg/dL | Total bilirubin | 0.44 | mg/dL |
| D dimer | 4.2 | μg/mL | AST | 75 | IU/L |
| ANA | 80 | times | ALT | 14 | IU/L |
| dsDNA antibody | 8.4 | IU/mL | LDH | 417 | IU/L |
| C3 | 91 | mg/dL | γ-GT | 31 | IU/L |
| C4 | 40 | mg/dL | Creatine kinase | 1,100 | IU/L |
| CH50 | 46.9 | IU/mL | CK-MB | 89 | IU/L |
| Anti cardiolipin IgG | <8.0 | Sodium | 136 | mmol/L | |
| CLβ2GPI antibody | <0.7 | Potassium | 4.9 | mmol/L | |
| MPO-ANCA | <1.3 | Chloride | 100 | mmol/L | |
| PR3-ANCA | <3.5 | Magnesium | 2.8 | mg/dL | |
| pH | 7.016 | Glucose | 341 | mg/dL | |
| PCO2 | 81.2 | Torr | Hemoglobin A1c | 4.6 | % |
| PO2 | 37.7 | Torr | Total cholesterol | 158 | mg/dL |
| HCO3- | 21 | mmol/L | LDL-C | 100 | mg/dL |
| Base excess | -11.4 | mmol/L | HDL-C | 48 | mg/dL |
| Anion gap | 19.8 | mmol/L | Triglycerides | 47 | mg/dL |
| Lactate | 10.3 | mmol/L |
ALT: alanine aminotransferase, ANA: antinuclear antibody, APTT: activated partial thromboplastin time, AST: aspartate aminotransferase, BUN: blood urea nitrogen, CK-MB: Creatine kinase-muscular brain type, CLβGPI: cardiolipin β glycoprotein I, CRP: C-reactive protein, dsDNA: double stranded deoxyribonucleic acid, FDP: fibrin degradation product, γ-GT: γ-glutamyl transferase, HDL-C: high-density lipoprotein cholesterol, LDH: lactate dehydrogenase, LDL-C: low-density lipoprotein cholesterol, MPO-ANCA: myeloperoxidase-anti-neutrophil cytoplasmic antibodies, PR3-ANCA: proteinase-3-anti-neutrophil cytoplasmic antibodies, PT-INR: prothrombin time-international ratio, WBC: white blood cell
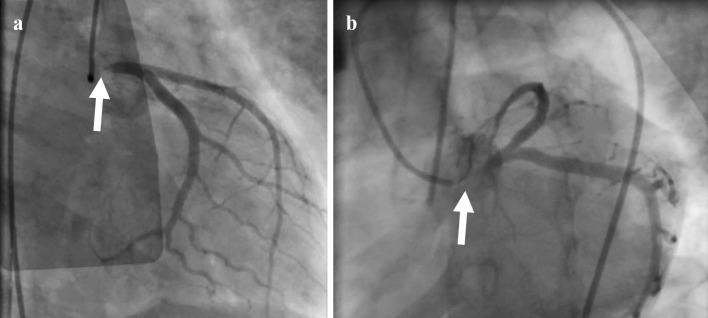
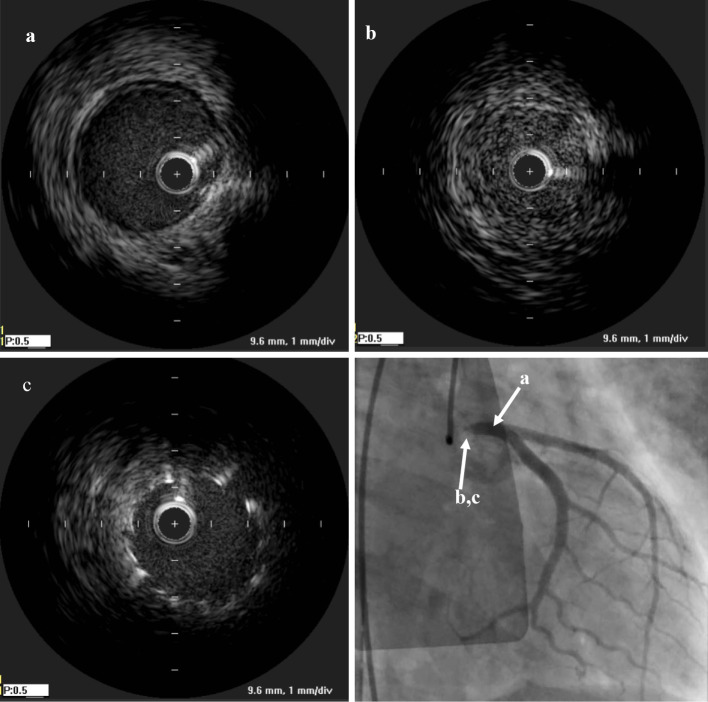
Because of cardiogenic shock with pulseless electrical activity and ventricular tachycardia, cardiopulmonary resuscitation was initiated. After the insertion of venoarterial extracorporeal membrane oxygenation (VA-ECMO), we performed coronary angiography, and it showed 99% stenosis at the LMT (Fig. 2). Intravascular ultrasound (IVUS; OptiCross, Boston Scientific, Freemont, USA) showed that there was no calcification and that the three-layered structure of the intima, tunica media, and adventitia was unclear and concentrically thickened (Fig. 3).
Figure 2.
Coronary angiography showed 99% stenosis in the left main trunk. a: Right anterior oblique view. b: Left caudal view
Figure 3.
Intravascular ultrasound showed (a) intact lesion of LMT, (b) the three-layered structure of the intima, tunica media, and adventitia was unclear and concentrically thickened in LMT. (c) Post-stenting
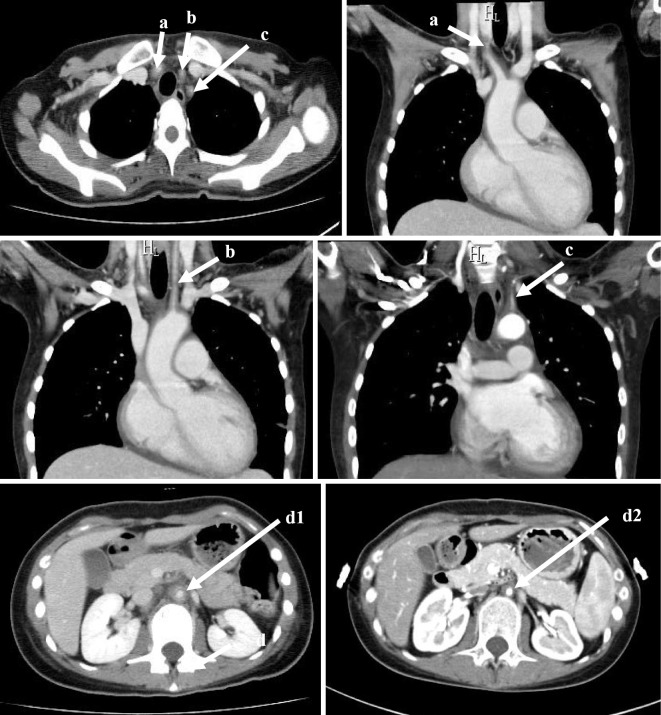
After percutaneous transluminal coronary angioplasty was performed, a bare metal stent (3.5 mm×12 mm) was successfully placed (Fig. 3c), and thrombolysis in myocardial infarction grade 3 flow was obtained. We applied intra-aortic balloon pumping (IABP), and the patient was transferred to the coronary care unit. The maximum value of CK was 9,770 IU/L, and the VA-ECMO and IABP were withdrawn on the 3rd and 8th hospital days, respectively. Regarding the diagnosis of Takayasu's arteritis, based on the American College of Rheumatology 1990 criteria for the classification of Takayasu's arteritis (7), four of the six diagnostic criteria of Takayasu's arteritis (less than 40 years of age, pulse weakness of the brachial artery, vascular murmur at the subclavian artery or aorta, abnormality of arteriography) were met in this case (Fig. 4). In addition, based on the Japanese Circulation Society 2008 criteria for the diagnosis of Takayasu's arteritis, the diagnosis of Takayasu's arteritis was definite: computed tomography showed multiple vascular wall thickening and stenosis in the aorta and its first branch, and C-reactive protein (CRP) was high at the time of the visit. Differential diagnoses, such as atherosclerosis, inflammatory aneurysm, and giant cell arteritis, were completely ruled out.
Figure 4.
Computed tomography showed wall thickening and narrowing in the right brachiocephalic artery (a), left common carotid artery (b), left subclavian artery (c), abdominal aorta under the branch of renal artery (d1), and abdominal aorta at the 4th months from the start of prednisolone (d2). The vascular wall thickening improved.
We started prednisolone at the 43rd hospital day. At this point, 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) revealed weak positivity, and the CRP decreased to 0.68 mg/dL (8.18 mg/dL at the emergency room); the erythrocyte sedimentation rate also decreased to 26 mm (44 mm at the 18th hospital day). We therefore considered that the extent of systemic inflammation was weak, and a rheumatologist advised us to start low-dose prednisolone at 10 mg/day. The patient was released from the hospital on the 67th hospital day.
Discussion
We encountered a patient with Takayasu's arteritis who presented with AMI due to LMT occlusion. She recovered with intensive therapy including emergent percutaneous coronary intervention. This case contains several important clinical messages about juvenile-onset AMI.
AMI in the young is extremely rare. It is reported that the incidence of AMI in those younger than 40 years of age is only about 5% in total, and 90% of these cases are men (8). In the Framingham Study (4), the incidence of AMI in women 45-54 years of age was 0.7/1,000 person・years, and 0.2/1,000 person・years in women 35-44 years of age. The rarity of AMI in young women is due to the anti-arteriosclerotic effect of estrogen. The causes of AMI in women under 45 years of age were arteriosclerotic in 80% of cases, cardio-embolic in 5% of cases (i.e. paradoxical embolism or infective endocarditis), a hypercoagulant state in 5% of cases, congenital coronary artery abnormality in 4% of cases, and others in 6% of cases, including coronary artery spasm, coronary artery dissection, vasculitis, chest trauma, and irradiation of the mediastinum (5).
A Japanese study examined the etiology of AMI in young women (6). Among 2,457 cases of AMI, the number of women under 50 years of age was 24, and 10 of these cases were non-atherosclerotic on angiography; only 3 cases had Takayasu's arteritis. In the same study, there were 251 men under 50 years of age with AMI, and a non-atherosclerotic etiology was noted in only 1 case of embolism. Although it is rare, AMI can occur in young women, and rigorous surveys for systemic causes are essential.
In the present case, the patient fell into a very severe condition and was rescued by intensive treatments. We ruled out other causes of cardiogenic shock, such as cardiac rupture, mitral chordal rupture, and ventricular septal perforation. There were no potential causes of the shock other than AMI at the LMT lesion. The early detection and diagnosis of Takayasu's arteritis before progression to such a severe condition are important. Regarding the initial symptoms of Takayasu's arteritis, a fever (17.1%) is most common, followed by dizziness (12.9%), pulse deficit (11.2%), and general malaise (7.8%), but angina pectoris (2.8%) is uncommon. Ischemic heart disease occurs in 10.7% of Takayasu's arteritis cases during the entire course, but cases diagnosed by the presentation as AMI have been reported as case reports (Table 2) (9-15).
Table 2.
Case Reports of Takayasu’s Arteritis Developed as Acute Myocardial Infarction.
| Reference | Age(yrs), Sex | Lesion | CK (IU/L) |
|---|---|---|---|
| 9 | 23, F | LAD aneurysm | 911 (admission) |
| 10 | 55, M | RCA middle | n.a. |
| 11 | 22, F | LMT | n.a. |
| 12 | 16, F | RCA ostium+LMT | 6,748 (max) |
| 13 | 19, M | RCA proximal aneurysm | 1,524 (admission) |
| 14 | 39, F | RCA ostium | n.a. |
| 15 | 17, M | RCA middle | n.a. |
M: Male, F: Female, LAD: left anterior descending artery, RCA: right coronary artery, LMT: left main trunk, n.a.: not available
Coronary artery lesions are present in 70-80% of Takayasu's arteritis cases, as pathologically, the inflammatory process extends to the intima and proliferates, which contracts the fibrotic media and adventitia from the ascending aorta (16,17). Especially in the active phase of Takayasu's arteritis, increases in a thrombotic tendency due to endothelial dysfunction, platelet aggregation, and coagulation activity (18-20) contribute to the development of AMI. Therefore, percutaneous coronary intervention combined with steroid therapy is effective for preventing restenosis (21). However, in the present case, Takayasu's arteritis was not in the very active phase, as FDG-PET showed only a weak uptake at the LMT.
Only one IVUS finding of LMT of effort angina due to Takayasu's arteritis has been reported (22), and LMT-AMI has never been reported as a cause, to our knowledge. In the present case, unstable plaque and calcification were not seen, but the three-layered structure of the intima, tunica media, and adventitia was not clearly visible, and the vessel was concentrically thickened (Fig. 3). Therefore, the pathophysiological mechanism of LMT-AMI in this case is not plaque rupture but concentric narrowing of the vessel itself.
In conclusion, in young women who complain of chest pain, a specific cause of coronary artery disease may exist, and systemic causes of vascular diseases, including Takayasu's arteritis, should be considered.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Jalowiec DA, Hill JA. Myocardial infarction in the young and in women. Cardiovasc Clin 20: 197-206, 1998. [PubMed] [Google Scholar]
- 2. Fournier JA, Sánchez A, Quero J, Fernández-Cortacero JA, González-Barrero A, et al. . Myocardial infarction in men aged 40 years or less: a prospective clinical angiographic study. Clin Cardiol 19: 631-636, 1996. [DOI] [PubMed] [Google Scholar]
- 3. Doughty M, Mehta R, Bruckman D, et al. . Acute myocardial infarction in the young-the university of Michigan experience. Am Heart J 143: 56-62, 2002. [DOI] [PubMed] [Google Scholar]
- 4. Lerner DJ, Kannel WB. Patterns of coronary heart disease morbidity and mortality in the sexes: A 26-year follow-up of the Framingham population. Am Heart J 111: 383-390, 1986. [DOI] [PubMed] [Google Scholar]
- 5. Lubna C, James DM. Myocardial infarction in young patients. Am J Med 107: 254-261, 1999. [DOI] [PubMed] [Google Scholar]
- 6. Toyofuku M, Goto Y, Matsumoto T, et al. . Acute myocardial infarction in young Japanese women. J Cardiol 28: 313-319, 1996. (in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 7. Arend WP, Michel BA, Bloch DA, et al. . The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum 33: 1129-1134, 1990. [DOI] [PubMed] [Google Scholar]
- 8. Hamsten A, Norberg R, Bjorkholm M, et al. . Antibodies to cardiolipin in young survivors of myocardial infarction: an association with recurrent cardiovascular events. Lancet 327: 113-116, 1986. [DOI] [PubMed] [Google Scholar]
- 9. Araszkiewicz A, Prech M, Hrycaj P, Lesiak M, Grajek S, Cieslinski A. Acute myocardial infarction and rapid development of coronary aneurythms in a young woman - unusual presentation of Takayasu arteritis? Can J Cardiol 23: 61-63, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miyagawa K, Shiraishi J, Nasu M, et al. . Usefulness of ultrasonography in carotid arteries and combined positron emission tomography for diagnosis of Takayasu arteritis with unusual presentation as acute myocardial infarction: a case report. J Cardiol 50: 317-324, 2007. [PubMed] [Google Scholar]
- 11. Hamdan A, Porter A, Georghiou GP, et al. . Unusual presentation of Takayasu arteritis. Int J Cardiol 119: 249-250, 2007. [DOI] [PubMed] [Google Scholar]
- 12. Roghi A, Pedrotti P, Milazzo A, et al. . Acute myocardial infarction and cardiac arrest in atypical Takayasu aortitis in a young girl: unusual diagnostic role of cardiac magnetic resonance imaging in emergency. Circulation 121: e370-e375, 2010. [DOI] [PubMed] [Google Scholar]
- 13. Ouali S, Kacem S, Fradj FB, et al. . Takayasu arteritis with coronary aneurysms. Tex Heart Inst J 38: 183-186, 2011. [PMC free article] [PubMed] [Google Scholar]
- 14. Yoshida T, Oi K, Shinohara N, Mihara A, Yokoyama K. Emergency surgery for coronary ostial occlusion and aortic root aneurysm associated with Takayasu's arteritis; report of a case. Kyobu Geka 66: 1196-1198, 2013. (in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 15. Chen T, Mi J, Zhong MH, et al. . Acute inferior myocardial infarction as the first manifestation of Takayasu arteritis in a young boy. Chin Med J 128: 2414, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matsubara O, Kuwata T, Nemoto T, Kasuga T, Numano F. Coronary artery lesions in Takayasu arteritis - pathological considerations. Heart Vessels 7 (Suppl.): 26-31, 1992. [DOI] [PubMed] [Google Scholar]
- 17. Amano J, Suzuki A. Coronary artery involvement in Takayasu's arteritis. Collective review and guideline for surgical treatment. J Thorac Cardiovasc Surg 102: 554-560, 1991. [PubMed] [Google Scholar]
- 18. Akazawa H, Ikeda U, Yamamoto K, Kuroda T, Shimada K. Hypercoagulable state in patients with Takayasu's arteritis. Thromb Haemost 75: 712-716, 1996. [PubMed] [Google Scholar]
- 19. Watanabe T, Kishi Y, Numano F, Isobe M. Enhanced platelet sensitivity to prostacyclin in patients in an active stage of Takayasu arteritis. Thromb Res 104: 77-83, 2001. [DOI] [PubMed] [Google Scholar]
- 20. Kasuya N, Kishi Y, Isobe M, Yoshida M, Numano F. P-selection expression, but not GPIIb/IIIa activation, is enhanced in the inflammatory stage of Takayasu's arteritis. Circ J 70: 600-604, 2006. [DOI] [PubMed] [Google Scholar]
- 21. Yokota K, Shimpo M, Iwata T, et al. . A case of Takayasu arteritis with repeated coronary artery restenosis after drug-eluting stent implantation successfully treated with a combination of steroids. Intern Med 51: 739-743, 2012. [DOI] [PubMed] [Google Scholar]
- 22. Park H, Otani H, Yamamoto Y, et al. . Percutaneous coronary intervention for left main trunk ostial stenosis in a patient with Takayasu's arteritis. Cardiovasc Interv Ther 26: 70-73, 2011. [DOI] [PubMed] [Google Scholar]