Abstract
Objective
Sorafenib is a standard therapy for advanced hepatocellular carcinoma (HCC), whereas radiotherapy is effective for local control of extrahepatic spread (EHS) or macrovascular invasion (MVI). This study investigated the safety and efficacy of this combined therapy to treat advanced HCC.
Methods
This retrospective study reviewed 62 patients with advanced-stage HCC with EHS or MVI who received sorafenib therapy, excluding the patients with only lung metastases.
Results
Of the 62 patients, 15 were treated using the combined therapy of sorafenib and radiotherapy (group RS), and 47 were treated with sorafenib monotherapy (group S). In group RS, patients were treated using three-dimensional conformal radiotherapy with a total irradiation dose of 30-60 Gy (median, 50 Gy). Irradiation was targeted at the bone, lymph nodes, adrenal gland, and MVI in 6, 5, 1, and 4 patients, respectively. The overall incidence of adverse events was 93.3% in group RS and 91.5% in group S (p=N.S.). Incidences of thrombocytopenia, leukopenia, and skin reaction were significantly higher in group RS (73.3%, 40.0%, and 66.7%, respectively) than in group S (36.2%, 10.6%, and 27.7%, respectively, p=0.02, 0.02, and <0.01, respectively). The incidence of severe adverse events, however, was comparable in the 2 groups: 20% in group RS and 19.2% in group S. The median progression-free survival (PFS) of EHS or MVI, PFS of whole lesions, and overall survival were longer in group RS (13.5, 10.6, and 31.2 months, respectively) than in group S (3.3, 3.5, and 12.1 months, respectively) (p<0.01 for all).
Conclusion
Sorafenib in combination with radiotherapy is a feasible and tolerable treatment option for advanced HCC.
Keywords: sorafenib, hepatocellular carcinoma, chemotherapy, radiation, radiotherapy, metastasis
Introduction
Hepatocellular carcinoma (HCC) is a major cause of mortality and the third-most common cause of cancer-related deaths worldwide (1,2). HCC is often detected at an advanced stage or when the patient presents with advanced liver cirrhosis at the time of the diagnosis; patients are therefore treated with potentially curative therapies, such as resection, liver transplantation, or other locoregional therapies (3). Furthermore, the potential for recurrence is high after radical treatment for early- or intermediate-stage HCC, and recurrence sometimes leads to spread to extrahepatic sites such as lung, bone, adrenal gland, and lymph node or invasion into the portal or hepatic vein. Extrahepatic spread (EHS) or macrovascular invasion (MVI) is recognized as a fatal factor, owing to which therapeutic selection is very limited.
Sorafenib is an orally active targeted anticancer agent that inhibits proliferation and angiogenesis by blocking Raf kinase and the receptors for vascular endothelial growth factor (VEGF) and platelet-derived growth factor (4). Two large-scale, phase III randomized, double blind, placebo-controlled studies-the Sorafenib HCC Assessment Randomized Protocol trial study (5) and the Asia-Pacific Study (6)-demonstrated that sorafenib significantly delays the time to progression and prolongs the overall survival (OS) in patients with advanced HCC. Accordingly, sorafenib is the only recognized systemic chemotherapeutic agent for patients with advanced HCC expanding to extrahepatic sites or invading the portal or hepatic vein (5-7).
Radiotherapy may improve the survival of patients with advanced HCC with EHS (8-10) or MVI (11-13). Since only a few studies (14-16) have focused on this combination therapy, its safety and efficacy remain unclear. Therefore, in this study, we aimed to evaluate the safety and feasibility of the combination of sorafenib therapy and radiotherapy for patients with advanced HCC with EHS or MVI.
Materials and Methods
Patients
We retrospectively reviewed data collected prospectively from 99 consecutive patients who received sorafenib (NexavarⓇ; Bayer HealthCare Pharmaceuticals, Pittsburg, USA) for advanced HCC with Barcelona Clinic Liver Cancer stage C in the Department of Hepato-Biliary-Pancreatic Surgery at the National Hospital Organization Kyushu Medical Center between July 2009 and June 2015.
HCC was diagnosed through a pathological examination or a combination of specific radiologic findings of dynamic multidetector-row computed tomography (MDCT) or magnetic resonance imaging (MRI) according to the criteria of the American Association for the Study of Liver Diseases (7).
Sorafenib was administered to patients with advanced HCC if (i) their Eastern Cooperative Oncology Group performance status score was 0-1; (ii) their liver function was classified as Child-Pugh A or B; and (iii) they had an adequate hepatic function (albumin level >2.5 g/dL, total bilirubin level <3.0 mg/dL, and alanine aminotransferase and aspartate aminotransferase levels <5 times the upper limit of the normal range).
Of the 99 patients, 8 were excluded due to radiologic confirmation, 10 were excluded due to the presence of Child-Pugh B or C, and 19 were excluded for EHS to only the lung, because lung metastasis was not considered an appropriate target for radiotherapy. A total of 62 patients were ultimately enrolled in this retrospective study. The patients were divided into two groups: a group that received combined therapy of sorafenib and radiotherapy (group RS) and a group that received sorafenib monotherapy (group S).
This study was approved by the Ethics Committee of the National Hospital Organization Kyushu Medical Center and performed in compliance with the Declaration of Helsinki. All patients provided their written informed consent.
Group S
The starting dosage of sorafenib was 800 mg/day orally (p.o.). However, considering the possibility of having to discontinue sorafenib treatment at an early stage due to adverse events, the initial dosage for patients with comorbidities was reduced to 400 mg/day. The initial dosage for patients ≥75 years of age or those with a body weight ≤40 kg or a history of treatment for varices or ascites was 200-400 mg/day. The dose was increased to the standard dose level according to each patient's tolerance. Treatment was continued until tumor progression or unacceptable toxicity associated with sorafenib, or withdrawal of consent.
Group RS
In the treatment guidelines for HCC, including the American Association for the Study of Liver Diseases guidelines (7) or the Barcelona Clinical Liver Cancer guidelines (17), radiation is not routinely recommended. At our institution, the indications for radiotherapy in patients with HCC are as follows: (i) localized bone metastases, especially vertebral metastases with high risk of spinal damage, or painful bone metastases; (ii) solitary lymph node metastasis or lymph node metastases localized in a region; (iii) adrenal metastasis refractory to transcatheter arterial chemoembolization (TACE); (iv) macrovascular invasion refractory to TACE or hepatic arterial infusional chemotherapy; and (v) informed consent of the patient. We excluded patients with only lung metastasis as EHS from assessment, owing to the severe adverse effect of radiation pneumonitis resulting from radiotherapy of the lung. Radiotherapy was started within one month of sorafenib initiation in all patients. Only one patient received sequential treatment with radiotherapy followed by sorafenib. All patients received three-dimensional conformal radiotherapy (3D-CRT). Target lesions of radiotherapy were EHS or MVI, but not intrahepatic lesions. The biologically effective dose was converted to conventional fractionation of each dose due to variation in the dose-fractionation schedule. The α/β ratio was 10.
The starting dosage of sorafenib was 800 mg/day. The conditions for reducing the dosage were the same as described above for group S. Considering the possibility of having to discontinue sorafenib and radiotherapy treatment at an early stage due to adverse events or bone marrow suppression, one-level reduction of the initial dosage was permitted. The dose was then increased to the standard dose level according to each patient's tolerance. Treatment was continued until tumor progression or unacceptable toxicity associated with sorafenib, or withdrawal of consent.
The combination of radiotherapy and sorafenib therapy was selected by the attending physicians. After explaining the methods, comorbidity, and expected efficacy of combination therapy of sorafenib and radiotherapy to the patients, they finally selected the combination therapy or sorafenib monotherapy.
Evaluation of treatment response
Images were acquired using dynamic MDCT or dynamic MRI at baseline and every 6-8 weeks after treatment initiation. The best radiologic tumor response was assessed in accordance with the modified Response Evaluation Criteria in Solid Tumors (18) at 6-8 or 14-16 weeks after the initiation of sorafenib treatment or radiotherapy. For measurable target lesions, the best radiologic response was classified as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). We documented the cause of PD (pattern of progression) as follows: ≥20% increase in tumor size compared with the size of a baseline lesion (intrahepatic growth or extrahepatic growth), a new intrahepatic lesion, or a new extrahepatic lesion and/or vascular invasion. For MVI identified as a nonmeasurable lesion, the radiologic response was classified as a CR, PD, or incomplete response (IR)/SD (18).
Safety
Toxicity was monitored through physical examinations and laboratory tests during the treatments. The adverse events were graded based on the Common Terminology Criteria for Adverse Events v3.0.
Follow-up
All patients were followed-up at our outpatient clinic according to a standardized protocol that included tumor marker tests every 1-2 months and MDCT or MRI every 6-8 weeks until the patient's death or last visit. At the end of the study period (on August 1, 2015), the median follow-up time was 14.3 months, and 46 patients had died; no patients were lost to follow-up.
Statistical analyses
Statistical analyses were performed using the JMP software program, version 11.0 (SAS Institute, Cary, USA). Categorical variables were analyzed using the chi-squared test or Fisher's exact test, as appropriate. Continuous variables were analyzed using Student's t-test or the Mann-Whitney U test, as appropriate. The time to progression and OS were analyzed using the Kaplan-Meier method, and groups were compared using the log-rank test. Subgroup analyses, univariate analyses, and multivariate analyses were performed using a Cox proportional hazards model and the backward elimination procedure. A value of p<0.05 indicated a statistically significant difference.
Results
Patient characteristics
There were more women in group RS than in group S; however, the liver function and tumor condition (distribution of metastatic organ, concomitant intrahepatic lesion, and tumor markers) did not differ markedly between the groups (Table 1).
Table 1.
Characteristics of the Patients.
| Variables | Group RS(n=15) (n=15) |
Group S (n=47) |
p value |
|---|---|---|---|
| Age (median, IQR) | 75 (61-78) | 68 (62-76) | 0.30 |
| Sex (Male/Female) | 9 /6 | 40 /7 | 0.048 |
| Etiology (HBV/HCV/ NBNC) | 2/8/5 | 7 /33 /7 | 0.88 |
| Child-Pugh score (5/6) | 11 /4 | 25/22 | 0.16 |
| Metastastic lesions | |||
| Lung | 2 | 12 | 0.3 |
| Bone | 6 | 17 | 0.84 |
| Lymph node | 7 | 15 | 0.3 |
| Adrenal gland | 1 | 4 | 0.78 |
| Maxium diameter of metastastic lesions (median, IQR) (mm) | 30 (18-43) | 24 (20-34.3) | 0.30 |
| Macrovascular invasion | 4 | 18 | 0.54 |
| Portal vein invasion (1/2/3/4) | 0/0/2/0 | 0/9/5/2 | 0.40 |
| Hepatic vein invasion (1/2/3) | 0/0/2 | 0/1/3 | 0.44 |
| Concomitant intrahepatic lesions | 10 | 38 | 0.30 |
| Initial dose of sorafenib [800/400/200 (mg)] | 1/9/5 | 11/28/8 | 0.21 |
| AFP (median, IQR) (ng/mL) | 17.2 (6.4-2,672) | 129 (13-469) | 0.63 |
| AFP-L3 (median, IQR) (%) | 30 (0.38-61.9) | 15 (1.4-64.7) | 0.61 |
| DCP (median, IQR) (mAu/mL) | 228 (24.5-1,255) | 180 (26.5-7,708) | 0.4 |
IQR: interquartile range, HBV: hepatitis B, HCV: hepatitis C, NBNC: non hepatitis B and non hepatitis C, AFP: α-fetoprotein, AFP-L3: Lens culinaris agglutinin-reactive fraction of AFP, DCP: des-γ-carboxy prothrombin
The starting dose of sorafenib was not statistically different between the 2 groups; however, 1 patient received 800 mg sorafenib as the starting dose in group RS, whereas 11 patients received 800 mg sorafenib in group S.
In group RS, radiotherapy was given to target lymph node metastases in six patients, bone metastases in five patients, adrenal metastasis in one patient, and MVI in four patients. Of these, one patient received radiotherapy for three lymph node metastases, and another patient received radiotherapy for lymph node metastasis and bone metastasis. All other patients received radiotherapy for solitary target lesions. The total radiation dose ranged from 30 to 60 Gy (median, 50 Gy). The fraction size of radiotherapy ranged from 2 to 3 Gy (median, 2 Gy). After the conversion, the biologically effective dose ranged from 39 to 78 Gy (median, 60 Gy).
No patients in either group had a history of TACE for EHS lesions.
Tumor response
The tumor response was evaluated by the modified Response Evaluation Criteria in Solid Tumors. First, we evaluated the tumor response of only EHS or MVI targeted by radiotherapy. EHS was evaluated as measurable lesions, and MVI was evaluated as nonmeasurable lesions (Table 2). Among those with measurable lesions in group RS, six patients exhibited PR, and five exhibited SD. No CR or PD was noted in group RS. In group S, wherein 28 patients had measurable lesions, 1 patient exhibited PR, 10 exhibited SD, and 17 exhibited PD. With regard to the local control of metastatic lesions, group RS showed a significantly more effective response than group S (p<0.01).
Table 2.
Tumor Response of Extrahepatic Spread or Macrovascular Invasion.
| Variable | Group RS | Group S | p value | |
|---|---|---|---|---|
| Measurable lesions | (n=11) | (n=28) | <0.01 | |
| CR | 0 | 0 | ||
| PR | 6 | 1 | ||
| SD | 5 | 10 | ||
| PD | 0 | 17 | ||
| Nonmeasurable lesion | (n=4) | (n=19) | ||
| CR | 1 | 0 | <0.01 | |
| IR/SD | 3 | 3 | ||
| PD | 0 | 16 |
CR: complete response, PR: partial response, SD: stable disease, PD: progressive disease, IR: imcomplete response
Among patients with nonmeasurable lesions in group RS, one patient exhibited CR, and three exhibited IR/SD. Furthermore, three patients with IR/SD showed no progression and a slight decrease in MVI. In group S, 19 patients had nonmeasurable lesions of MVI, of which 16 (84.2%) exhibited PD (p<0.01).
The OS and progression-free survival (PFS) in patients treated with combination therapy of radiotherapy and sorafenib
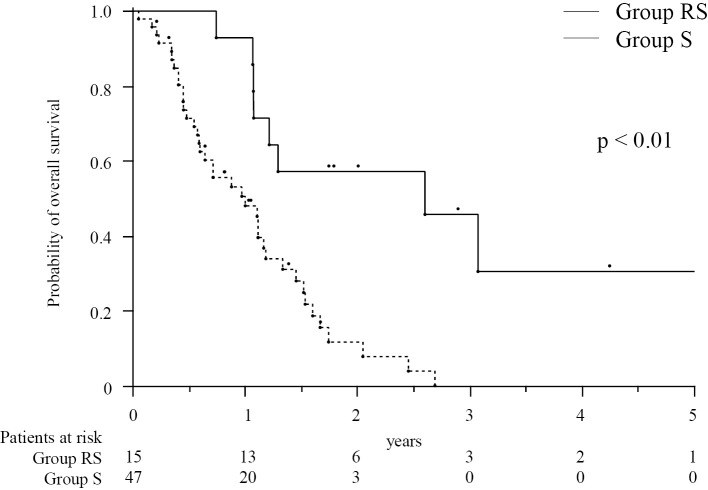
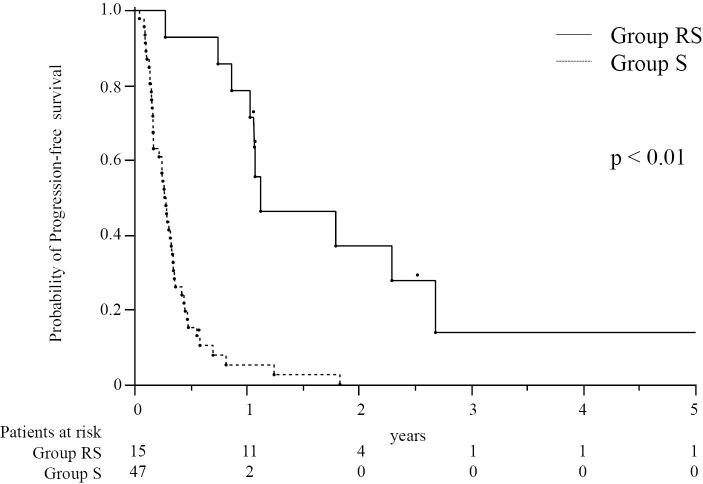
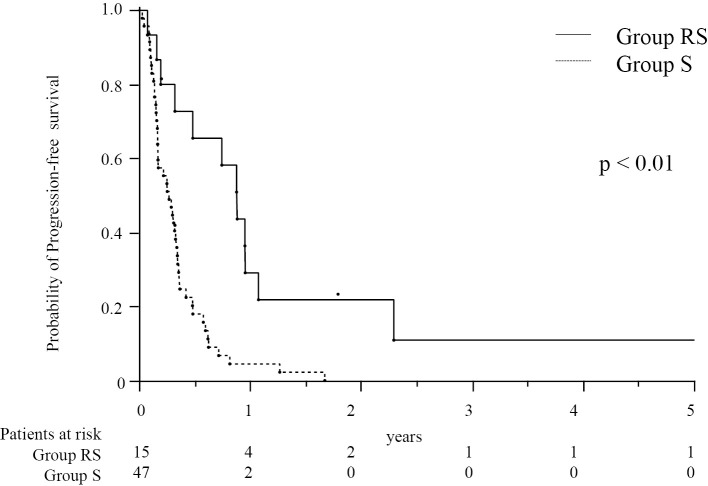
The median OS in group RS (31.2 months) was significantly longer than that in group S (12.1 months, p<0.01, Fig. 1). The median PFS of EHS or MVI was longer in group RS (13.5 months) than in group S (3.3 months, p<0.01, Fig. 2). However, many patients had EHS or MVI along with concomitant intrahepatic lesions. As concomitant intrahepatic lesions influence the survival, we also evaluated the PFS of whole lesions: The median PFS of whole lesions was longer in group RS (10.6 months) than in group S (3.3 months, p<0.01, Fig. 3).
Figure 1.
The cumulative overall survival in patients treated with a combination of radiotherapy and sorafenib therapy (group RS, n=15) and those treated with sorafenib monotherapy (group S, n=47). The numbers below the X-axis indicate the number of patients at risk.
Figure 2.
The cumulative progression-free survival of patients with extrahepatic lesions or macrovascular invasion treated with a combination of radiotherapy and sorafenib therapy (group RS, n=15) and those treated with sorafenib monotherapy (group S, n=47). The numbers below the X-axis indicate the number of patients at risk.
Figure 3.
The cumulative progression-free survival of patients with whole lesions treated with a combination of radiotherapy and sorafenib therapy (group RS, n=15) and those treated with sorafenib monotherapy (group S, n=47). The numbers below the X-axis indicate the number of patients at risk.
Furthermore, patients with only MVI (4 and 20 patients in groups RS and S, respectively) and patients with only EHS (11 and 27 patients in groups RS and S, respectively) were separated into two categories. In patients with MVI, the median OS in group RS (26.2 months) showed a tendency to improve compared to the median OS in group S (11.7 months, p<0.09). The median PFS for the MVI category was longer in group RS (33.5 months) than in group S (3.0 months, p<0.01). The median PFS for patients with whole lesions was longer in group RS (11.0 months) than in group S (2.6 months, p=0.02).
In patients with only EHS without MVI, the median OS for group RS (31.3 months) was longer than that for group S (13.3 months, p<0.01). The median PFS for the EHS category was longer in group RS (17.3 months) than in group S (4.1 months, p<0.01). The median PFS for patients with whole lesions was longer in group RS (10.6 months) than in group S (4.2 months, p<0.01).
Prediction of the survival
A univariate analysis showed a significant correlation between the survival and the following parameters in patients: the α-fetoprotein (AFP) level and the combination of sorafenib treatment and radiotherapy (Table 3). Furthermore, a multivariate analysis identified the AFP level and a combination of sorafenib and radiotherapy as independent predictors of the OS in patients.
Table 3.
Factors Associated with Survival in HCC Patients with Extrahepatic Spread or Macrovascular Invasion.
| Variable | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| HR | (95% CI) | p value | HR | (95% CI) | p value | ||
| Age | ≥70 | 0.84 | (0.46-1.51) | 0.55 | 1.60 | (0.82-3.17) | 0.17 |
| Sex | Male | 1.37 | (0.69-3.03) | 0.38 | 1.08 | (0.49-2.56) | 0.86 |
| Child-Pugh score | 5 | 0.57 | (0.32-1.03) | 0.06 | 0.58 | (0.30-1.13) | 0.11 |
| HBV | yes | 0.81 | (0.33-1.70) | 0.59 | |||
| HCV | yes | 1.27 | (0.69-2.47) | 0.45 | |||
| Extrahepatic spread | yes | 1.22 | (0.64-2.54) | 0.55 | |||
| Lung | yes | ||||||
| Bone | yes | 1.45 | (0.78-2.63) | 0.24 | |||
| Lymph node | yes | 0.9 | (0.47-1.64) | 0.73 | |||
| Adrenal gland | yes | 1.85 | (0.63-4.33) | 0.24 | |||
| Macrovascular invasion | yes | 1.22 | (0.66-2.20) | 0.52 | |||
| Concomitant intrahepatic lesions | no | 1.51 | (0.72-3.70) | 0.29 | |||
| AFP (ng/mL) | ≥400 | 2.35 | (1.08-4.81) | 0.03 | 3.82 | (1.64-8.67) | <0.01 |
| DCP (mAu/mL) | ≥1,000 | 1.5 | (0.78-2.76) | 0.22 | |||
| Combination of sorafenib and radiotherapy | yes | 0.25 | (0.10-0.54) | <0.01 | 0.20 | (0.12-0.61) | <0.01 |
HR: hazard ratio, CI: confidence interval, HBV: hepatitis B, HCV: hepatitis C, AFP: α-fetoprotein, DCP: des-γ-carboxy prothrombin
Safety
As shown in Table 4, the overall incidence of adverse events was 93.3% in group RS and 91.5% in group S (p=N.S.). The most common toxicity was thrombocytopenia (n=11, 73.3%) in group RS, as reported previously (14); however, none of the patients showed grade ≥ 3 thrombocytopenia. The second-most common adverse event was other skin reactions, including pigmentation found in 10 patients (66.7%); however, none of the patients showed severe pigmentation with skin ulcer or burn. On comparing groups RS and S, the incidences of thrombocytopenia, leukopenia, and skin reaction were significantly higher in group RS (73.3%, 40.0%, and 66.7%, respectively) than in group S (36.2%, 10.6%, and 27.7%, respectively, p=0.02, 0.02, and <0.01, respectively); however, all of these events were grade 1 or 2, and they did not interrupt the treatment in either group. Severe adverse events, including anorexia, fatigue, and gastrointestinal bleeding of grade 3, were seen in one patient in group RS. However, the incidence of adverse events higher than grade 3 was not significantly different between the two groups: 20.0% in the RS group and 19.2% in the S group.
Table 4.
Adverse Events.
| Variable | Group RS (n=15) | Group S (n=47) | p value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| All grades (%) | Grade ≥3 (%) | All grades (%) | Grade ≥3 (%) | ||||||
| Overall | 13 | (93.3%) | 3 | (20.0%) | 43 | (91.5%) | 9 | (19.2%) | 0.82 |
| Dermatological | |||||||||
| Hand-foot syndrome | 6 | (40.0%) | 0 | (0%) | 28 | (59.6%) | 2 | (4.3%) | 0.24 |
| Other skin reaction | 10 | (66.7%) | 0 | (0%) | 13 | (27.7%) | 0 | (0%) | 0.01 |
| Hematological | |||||||||
| Leukopenia | 6 | (40.0%) | 0 | (0%) | 5 | (10.6%) | 0 | (0%) | 0.02 |
| Anemia | 3 | (20.0%) | 0 | (0%) | 4 | (8.9%) | 0 | (0%) | 0.35 |
| Thrombocytopenia | 11 | (73.3%) | 0 | (0%) | 17 | (36.2%) | 0 | (0%) | 0.02 |
| Transaminase elevation | 3 | (20.0%) | 0 | (0%) | 18 | (38.3%) | 3 | (6.4%) | 0.23 |
| Amylase elevation | 3 | (20.0%) | 0 | (0%) | 11 | (23.4%) | 0 | (0%) | 1.00 |
| Gastrointestinal | |||||||||
| Anorexia | 4 | (26.7%) | 1 | (6.7%) | 25 | (53.2%) | 1 | (2.2%) | 0.08 |
| Fatigue | 6 | (40.0%) | 1 | (6.7%) | 27 | (57.5%) | 1 | (2.2%) | 0.24 |
| Diarrhea | 4 | (26.7%) | 0 | (0%) | 24 | (51.1%) | 1 | (2.2%) | 0.14 |
| Gastrointestinal bleeding | 1 | (6.7%) | 1 | (6.7%) | 1 | (2.2%) | 1 | (2.2%) | 0.38 |
Discussion
This study investigated the efficacy of the combination of radiotherapy as locoregional therapy and sorafenib therapy for advanced HCC patients with EHS or MVI. Only a few studies (14,16) on the combination therapy of radiotherapy and sorafenib therapy have been published, and as such, the safety of this combination therapy was not established. In our study, the rates of thrombocytopenia, leukopenia, and skin reactions, including pigmentation, significantly increased due to the combination therapy. In a randomized trial of sorafenib monotherapy, the incidence of overall thrombocytopenia was 46%, and that of grade 3-4 thrombocytopenia was 4% (5). In trials on radiotherapy for hepatic lesions combined with systemic or regional chemotherapy, the incidence of overall and grade 3-4 thrombocytopenia was 18-49% and 6-12%, respectively (19-21). In our study, the overall incidence of thrombocytopenia was 73.3%, and that of grade 3-4 thrombocytopenia was 0%. The increased incidence rate in our study might be due to the concurrent administration of sorafenib and radiotherapy; however, none of our patients showed grade 3-4 thrombocytopenia, due to a reduced starting dose of sorafenib. With regard to the other adverse events, the rate of grade 3-4 adverse events was similar between the two groups. In group RS, one patient experienced gastrointestinal bleeding due to gastric antral vascular ectasia. However, this event was not an acute toxic event caused by radiotherapy because its onset was 5 months after the completion of radiotherapy. The overall incidence of adverse effects due to the combination therapy was within the acceptable range, although the reduced starting dose of sorafenib may have affected the grade of the adverse events in our study.
Sorafenib is an established therapy for advanced HCC with EHS or MVI. Although it improves the OS, it does not show an objective response (5,6). The efficacy of radiotherapy for lymph node metastasis (8-10) for advanced HCC has shown promising outcomes, with an objective response rate of 66-100% for metastatic lymph nodes and a median OS of 7-13 months (9,10,22-24). However, the combination of radiotherapy and sorafenib therapy showed promising outcomes in the present study, with a median OS of 31.3 months and median PFS for the EHS category of 17.3 months in patients with only EHS without MVI. In contrast, radiotherapy resulted in a median OS of 5.9-13.7 months in patients with advanced HCC with MVI (12,13,25). In our study, the combination of radiotherapy and sorafenib therapy resulted in a median OS of 26.2 months and a median PFS for the MVI category of 33.5 months, although there were only 4 patients with MVI. A large-scale study is required to confirm the efficacy of this combination therapy for MVI or EHS in the future.
However, the majority of patients with advanced HCC with EHS or MVI have concomitant intrahepatic tumors. A cohort study (26) of 34 patients with EHS reported that the major cause of death was intrahepatic tumor progression, and it was important to control intrahepatic tumors, as the tumor status is significantly associated with the OS in patients with advanced HCC (8,10,26). However, while controlling locoregional metastatic lesions did help improve the survival (9,15,23), whether controlling intrahepatic lesions or extrahepatic lesions contributed more to the survival is unclear.
The present study showed that the combination of radiotherapy and sorafenib therapy, even with a low dose of sorafenib, had good efficacy in the long-term. The strong ability of radiotherapy to control locoregional lesions is remarkable; however, its effects are temporary and limited. Therefore, the combination of sorafenib and radiotherapy may sustain the locoregional control for a long period. Furthermore, locoregional control for a long period can improve the OS of patients with advanced HCC with EHS or MVI. The above-mentioned findings are supported by the present study, which showed that the median OS of 31.2 months in group RS was much longer than that of 12.1 months in group S, despite the ratio of concomitant intrahepatic lesions not differing significantly between the two groups.
Despite our important findings, this study has several limitations. First, we did not compare the combination therapy to radiation monotherapy. The present study showed that the median OS in the RS group was 31.2 months. However, previous studies (9,10,12,13,22-25) have reported a median OS of only 5.9-13.7 months in patients with advanced HCC with EHS or MVI treated with radiation monotherapy. Although these results cannot be compared, the combination therapy is capable of improving the survival compared to radiation monotherapy. Second, the treatment schedule of sorafenib was sequential or concurrent in our study. In vitro and in vivo studies (27) have reported that a sequential schedule showed greater antitumor activity than concurrent treatment, but the optimum schedule of sequential treatment remains unclear. As most patients had concomitant intrahepatic tumors in the present study and sorafenib is currently recognized as the standard therapy for advanced HCC patients, almost all patients were first treated with sorafenib, followed by radiotherapy. Further prospective studies should compare these schedules to determine the more effective one. Third, the subtle differences in the characteristics between the RS and S groups may reflect an imbalance in the survival. Most factors were less favorable in group S, including fewer Child-Pugh A5 patients, a higher percentage of concomitant intrahepatic diseases, and high AFP levels (Table 1). A Japanese large-scale, multicenter observational study (28) reported that the median OS was 15.1 (95% confidence interval: 13.5-17.3) months in Child-Pugh A5 patients with advanced HCC treated with sorafenib. In our study, the median OS in group S (12.1 months) was similar, whereas the median OS in group RS was 31.2 months; owing to the longer median OS, group RS may have had a survival advantage. Fourth, the study design was retrospective and non-randomized in nature, and the selection of combination therapy by the attending physicians involved some bias. Finally, the size of the study cohort was small. Therefore, further prospective studies with a larger number of subjects are required to confirm our findings.
Conclusion
The control of EHS or MVI, even if intrahepatic lesions coexist, may be necessary for improving the survival of patients with advanced HCC. The combination of sorafenib therapy and radiotherapy has good efficacy and safety and may improve the survival in patients with advanced-stage HCC. As this study was a retrospective, single-institute, and small-sized study, a controlled clinical trial is required to confirm our findings.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Parkin DM. Global cancer statistics in the year 2000. The Lancet Oncology 2: 533-543, 2001. [DOI] [PubMed] [Google Scholar]
- 2. El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 132: 2557-2576, 2007. [DOI] [PubMed] [Google Scholar]
- 3. Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 362: 1907-1917, 2003. [DOI] [PubMed] [Google Scholar]
- 4. Wilhelm SM, Carter C, Tang L, et al. . BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 64: 7099-7109, 2004. [DOI] [PubMed] [Google Scholar]
- 5. Llovet JM, Ricci S, Mazzaferro V, et al. . Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359: 378-390, 2008. [DOI] [PubMed] [Google Scholar]
- 6. Cheng AL, Kang YK, Chen Z, et al. . Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10: 25-34, 2009. [DOI] [PubMed] [Google Scholar]
- 7. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 53: 1020-1022, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park YJ, Lim DH, Paik SW, et al. . Radiation therapy for abdominal lymph node metastasis from hepatocellular carcinoma. J Gastroenterol 41: 1099-1106, 2006. [DOI] [PubMed] [Google Scholar]
- 9. Zeng ZC, Tang ZY, Fan J, et al. . Consideration of role of radiotherapy for lymph node metastases in patients with HCC: retrospective analysis for prognostic factors from 125 patients. Int J Radiat Oncol Biol Phys 63: 1067-1076, 2005. [DOI] [PubMed] [Google Scholar]
- 10. Chen YX, Zeng ZC, Fan J, et al. . Defining prognostic factors of survival after external beam radiotherapy treatment of hepatocellular carcinoma with lymph node metastases. Clin Transl Oncol 15: 732-740, 2013. [DOI] [PubMed] [Google Scholar]
- 11. Minagawa M, Makuuchi M. Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J Gastroenterol 12: 7561-7567, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xi M, Zhang L, Zhao L, et al. . Effectiveness of stereotactic body radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombosis. PLoS One 8: e63864, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tanaka Y, Nakazawa T, Komori S, et al. . Radiotherapy for patients with unresectable advanced hepatocellular carcinoma with invasion to intrahepatic large vessels: efficacy and outcomes. J Gastroenterol Hepatol 29: 352-357, 2014. [DOI] [PubMed] [Google Scholar]
- 14. Cha J, Seong J, Lee IJ, Kim JW, Han KH. Feasibility of sorafenib combined with local radiotherapy in advanced hepatocellular carcinoma. Yonsei Med J 54: 1178-1185, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee DY, Park JW, Kim TH, et al. . Prognostic indicators for radiotherapy of abdominal lymph node metastases from hepatocellular carcinoma. Strahlenther Onkol 191: 835-844, 2015. [DOI] [PubMed] [Google Scholar]
- 16. Zhao JD, Liu J, Ren ZG, et al. . Maintenance of Sorafenib following combined therapy of three-dimensional conformal radiation therapy/intensity-modulated radiation therapy and transcatheter arterial chemoembolization in patients with locally advanced hepatocellular carcinoma: a phase I/II study. Radiat Oncol 5: 12, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. European Association For The Study Of The L, European Organisation For Research And Treatment Of Cancer.. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 56: 908-943, 2012. [DOI] [PubMed] [Google Scholar]
- 18. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 30: 52-60, 2010. [DOI] [PubMed] [Google Scholar]
- 19. Stillwagon GB, Order SE, Guse C, et al. . 194 hepatocellular cancers treated by radiation and chemotherapy combinations: toxicity and response: a Radiation Therapy Oncology Group Study. Int J Radiat Oncol Biol Phys 17: 1223-1229, 1989. [DOI] [PubMed] [Google Scholar]
- 20. Robertson JM, McGinn CJ, Walker S, et al. . A phase I trial of hepatic arterial bromodeoxyuridine and conformal radiation therapy for patients with primary hepatobiliary cancers or colorectal liver metastases. Int J Radiat Oncol Biol Phys 39: 1087-1092, 1997. [DOI] [PubMed] [Google Scholar]
- 21. Mohiuddin M, Chen E, Ahmad N. Combined liver radiation and chemotherapy for palliation of hepatic metastases from colorectal cancer. J Clin Oncol 14: 722-728, 1996. [DOI] [PubMed] [Google Scholar]
- 22. Yoon SM, Kim JH, Choi EK, et al. . Radioresponse of hepatocellular carcinoma-treatment of lymph node metastasis. Cancer Research and Treatment 36: 79-84, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim K, Chie EK, Kim W, et al. . Absence of symptom and intact liver function are positive prognosticators for patients undergoing radiotherapy for lymph node metastasis from hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 78: 729-734, 2010. [DOI] [PubMed] [Google Scholar]
- 24. Yamashita H, Nakagawa K, Shiraishi K, et al. . Radiotherapy for lymph node metastases in patients with hepatocellular carcinoma: retrospective study. J Gastroenterol Hepatol 22: 523-527, 2007. [DOI] [PubMed] [Google Scholar]
- 25. Nakazawa T, Hidaka H, Shibuya A, et al. . Overall survival in response to sorafenib versus radiotherapy in unresectable hepatocellular carcinoma with major portal vein tumor thrombosis: propensity score analysis. BMC Gastroenterol 14: 84, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Uchino K, Tateishi R, Shiina S, et al. . Hepatocellular carcinoma with extrahepatic metastasis: clinical features and prognostic factors. Cancer 117: 4475-4483, 2011. [DOI] [PubMed] [Google Scholar]
- 27. Plastaras JP, Kim SH, Liu YY, et al. . Cell cycle dependent and schedule-dependent antitumor effects of sorafenib combined with radiation. Cancer Res 67: 9443-9454, 2007. [DOI] [PubMed] [Google Scholar]
- 28. Kaneko S, Ikeda K, Matsuzaki Y, et al. . Safety and effectiveness of sorafenib in Japanese patients with hepatocellular carcinoma in daily medical practice: interim analysis of a prospective postmarketing all-patient surveillance study. J Gastroenterol 51: 1011-1021, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]