Abstract
The patient was an 81-year-old man who was found to have bacteremia due to Raoultella planticola, which might have entered the circulation through the bile duct during the passing of a gallbladder stone. In the present case, we screened for malignancies because most cases of R. planticola bacteremia occur after trauma, invasive procedures, or in patients with malignancy (70.6%). Early gastric cancer was detected. Although the association between R. planticola bacteremia and malignancy remains speculative in the present case, it may be useful to scrutinize similar cases involving low-virulence bacteremia for possible malignancies or immune conditions.
Keywords: bacteremia, gastric cancer, malignancy, Raoultella planticola
Introduction
Raoultella planticola is a gram-negative rod, aerobic, non-motile, and capsulated bacterium that was first described as Klebsiella planticola in 1981 (1,2). R. planticola is included in the Enterobacteriaceae family and has a histidine decarboxylase enzyme that produces histamine from histidine; thus, it can cause histamine fish poisoning (3). In 2001, it was reclassified as R. planticola based on a 16S rRNA and rpoB gene analysis (2). R. planticola was initially identified as an environmental bacterium of aquatic, botanic, and soil systems (1,4). R. planticola is generally harmless and rarely causes infection in humans. It colonizes 9-18% of humans, mainly in the urine, feces, and sputum (5,6). Two cases of infection by R. planticola were first reported in 1984 (7). Since then, cases of R. planticola infection have been reported in humans with trauma, malignancy, and gastroenteritis after consuming poorly prepared fish and after invasive medical examinations (5,8-10). Although both immunocompetent and immunocompromised hosts can develop R. planticola bacteremia, 82.4% of patients are immunocompromised.
We herein report a case of R. planticola bacteremia that seemed to be a complication of gallbladder stones and bile duct damage. Because of the rarity of R. planticola bacteremia in immunocompetent patients, we screened for possible malignancies and detected early gastric cancer.
Case Report
The patient was an 81-year-old Japanese who presented to our hospital with chills, anorexia, and fatigue that had persisted for several days. He also described intermittent and piercing abdominal pain. He had a history of coronary spastic angina, for which he had been taking diltiazem.
A physical examination at the first visit revealed the following findings: blood pressure, 139/61 mmHg; pulse rate, 55 beats per minute; body temperature, 38.1℃; respiration rate, 24 per minute; and percutaneous oxygen saturation, 95% under room air. The patient's consciousness was clear. The abdominal pain had already subsided and he did not have any abdominal tenderness and his system review was unremarkable. Routine laboratory tests were performed because of his advanced age, and due to the presence of fever, and tachypnea. Routine laboratory tests revealed a decreased platelet count (9.1×104/μL) and elevated levels of C-reactive protein (26.3 mg/dL), aspartate transaminase (233 U/L), alanine transaminase (155 U/L), lactate dehydrogenase (477 U/L), γ-glutamyltranspeptidase (127 U/L), creatinine kinase (3,278 U/L), and fibrin degradation product (13.7 μg/dL) (Table 1). Abdominal ultrasonography and plain CT of the chest, abdomen, and pelvis showed two stones of 5 mm and 8 mm in diameter in the gallbladder. The patient was admitted to our hospital based on the suspicion of a bacterial infection of unknown nature and rhabdomyolysis. Antibiotic therapy was started empirically with intravenous ampicillin-sulbactam (4.5 g daily) after drawing two sets of blood specimens for bacterial culturing. On the third hospital day, two sets of blood cultures were found to be positive for R. planticola. The bacterium was susceptible to ampicillin-sulbactam, cefazolin, ceftriaxone, and levofloxacin, but not ampicillin or amoxicillin (Table 2). Although the entry focus of R. planticola was unknown, his constitutional symptoms and laboratory data, including his liver function, improved after a total of 14 days of antibiotic therapy (initially with ampicillin-sulbactam, then with ceftriaxone). He also recovered from rhabdomyolysis without aftereffects with fluid replacement alone, and his creatine phosphokinase (CK) level returned to 258 U/L (within the normal range) on the 4th hospital day. He was discharged on the 15th hospital day.
Table 1.
Laboratory Data on Admission.
| Leukocytes (×103/µL) | 7.0 (3.7 - 7.0) | AST (U/L) | 233 (13 - 33) | |
| Neutrophils (%) | 85.9 (41.6 - 68.2) | ALT (U/L) | 155 (8 - 42) | |
| Eosinophils (%) | 0 (0.1 - 4.2) | LDH (U/L) | 477 (119 - 229) | |
| Basophils (%) | 0.4 (0 - 1.0) | γ-GT (U/L) | 127 (11 - 58) | |
| Monocytes (%) | 8.4 (4.9 - 9.7) | ALP (U/L) | 303 (115 - 359) | |
| Lymphocytes (%) | 5.2 (23.1 - 44.7) | T. Bil (mg/dL) | 1.0 (0.2 - 1.2) | |
| Hemoglobin (g/dL) | 14.6 (14.1 - 17.0) | BUN (mg/dL) | 34 (8 - 22) | |
| Platelets (×104/µL) | 9.1 (15.9 - 30.0) | Cr (mg/dL) | 1.2 (0.6 - 1.1) | |
| CRP (mg/dL) | 26.3 (<0.2) | FDP (μg/dL) | 13.7 (<5.0) | |
| CK (U/L) | 3,278 (62 - 287) | PT-INR | 1.05 |
CRP: C-reactive protein, CK: creatine phosphokinase, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, γ-GT: γ-glutamyltranspeptidase, ALP: alkaline phosphatase, T. Bil: total bilirubin, BUN: blood urea nitrogen, Cr: creatinine, FDP: fibrin degradation products, PT-INR: prothrombin time-international normalized ratio
Table 2.
Susceptibility of R. planticola in the Present Case.
| Agent | Susceptibility | MIC (µg/mL) |
|---|---|---|
| Amoxicillin | R | >16 |
| Ampicillin | R | >16 |
| Amoxicillin/clavulanate | S | ≤8 |
| Ampicillin/sulbactam | S | ≤8 |
| Piperacillin/tazobactam | S | ≤16 |
| Cefazolin | S | ≤2 |
| Ceftazidime | S | ≤4 |
| Cefmetazole | S | ≤16 |
| Ceftriaxone | S | ≤1 |
| Cefepime | S | ≤2 |
| Imipenem | S | ≤0.5 |
| Meropenem | S | ≤0.5 |
| Gentamicin | S | ≤4 |
| Minocycline | S | ≤4 |
| Ciprofloxacin | S | ≤0.06 |
| Levofloxacin | S | ≤0.12 |
| Trimethoprim/sulfamethoxazole | S | ≤40 |
R: resistant, S: susceptible, MIC: minimum inhibitory concentration
Upper gastrointestinal endoscopy was performed for screening purposes, because most patients with R. planticola bacteremia are either immunocompromised or cancer-bearing. An ulcerative lesion was found at the lesser curvature of the upper gastric body (Figure), and a histological examination showed well-differentiated tubular adenocarcinoma. A biopsy of the ulcer showed no sign of Helicobacter pylori infection, and the specimen was negative for IgG antibody to H. pylori. He was referred to another hospital that specialized in gastroenterology for further examinations and treatment.
Figure.
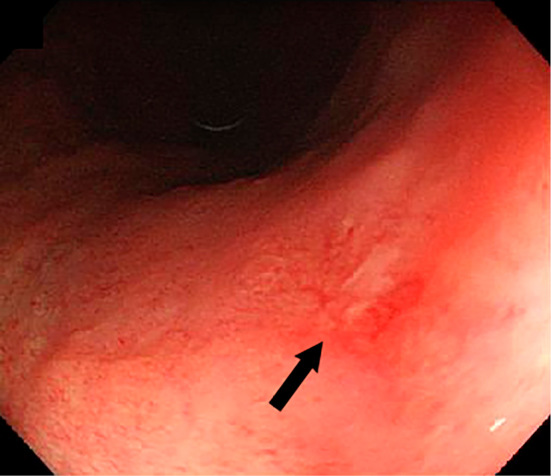
Upper gastrointestinal endoscopy showed an ulcerative lesion in the upper gastric body of the lesser curvature (arrow).
Discussion
R. planticola is a type of commensal bacteria. It is rarely associated with serious infections in humans. In recent years, however, the number of R. planticola infections has been increasing. The incidence of R. planticola infection might have previously been underestimated due to the difficulty in isolating the bacterium and confusion with other bacteria, including Klebsiella spp. (7).
In the present case, R. planticola was detected in the blood, but the focus of bacterial entry was unknown. The abdominal pain, elevated liver enzyme levels, and the presence of gallbladder stones indicated the passage of gallbladder stones through the bile duct, and retrograde infection during this process was a possibility; the gastrointestinal tract is the site of R. planticola colonization and no other focus of infection was found in the present case.
We only found 34 cases of R. planticola bacteremia in our review of the literature (Table 3). The median patient age was 64 years (range: 11 months to 83 years) and the ratio of males was 59.4%. Seven of 34 patients (20.6%) died of R. planticola bacteremia. Twenty-four of 34 (70.6%) patients also had a malignancy. The malignancies included hematological malignancies (n=7, 29.2%), biliary tract neoplasms (n=7, 29.2%), pancreatic neoplasms (n=4, 16.7%), and others (n=6, 25.0%). Twenty of 24 patients (83.3%) with malignancies were treated with chemotherapy or stem cell transplantation (11-15) before the development of bacteremia. Thus, an immunocompromised state - due to either a malignancy itself or the associated chemotherapy - appears to be associated with the development of R. planticola bacteremia. Eight of 34 (23.5%) patients received invasive medical procedures such as endoscopic retrograde cholangiopancreatography, central venous catheterization, and cardiovascular surgical procedures (6,7,15-17). It is noteworthy that 14 of 34 (41.2%) patients had a malignancy or a history of invasive medical procedures to the hepatobiliary system or pancreas, indicating that the hepatobiliary system or pancreas is one of the foci of R. planticola bacteremia.
Table 3.
Reported Cases of R. planticola Bacteremia.
| Reference | Age / Sex | Comorbidity | Invasive procedures |
Antibiotics | Outcome |
|---|---|---|---|---|---|
| 7 | 69 / F | Mitral stenosis | Mitral valve replacement | Tobramycin and cefotaxime | Recovered |
| 6 | 57 / N/A | N/A | Post-CABG | Ceftriaxone | Recovered |
| 11 | 83 / F | N/A | N/A | Moxifloxacin, ceftriaxone, azithromycin, and meropenem | Died |
| 11 | 64 / M* | B cell lymphoblastic lymphoma | N/A | Doxycycline | Died |
| 16 | 65 / M | Advanced apocrine adenocarcinoma | ERCP | Cefoperazone / sulbactam, meropenem, and piperacillin / tazobactam | Recovered |
| 17 | 59 / M | Pancreatic carcinoma | ERCP | Piperacillin / tazobactam | Recovered |
| 24 | 75 / M | Pancreatic carcinoma | N/A | Cefotaxime and metronidazole | Died |
| 10 | 63 / M | Hypercholesterolemia, BPH, and Posterior pituitary adenoma | N/A | Piperacillin / tazobactam and Cefotaxime | Recovered |
| 12 | 70 / M* | Pancreatic adenocarcinoma, COPD, and Bronchiectasis | N/A | Ciprofloxacin and metronidazole | Recovered |
| 13 | 57 / M* | Non-small-cell lung cancer with multiorgan metastasis | N/A | Levofloxacin, gentamicin, and ceftazidime | Recovered |
| 14 | 56 / F* | Non-small-cell lung cancer with liver metastases | N/A | Ceftriaxone and metronidazole | Recovered |
| 15 | 51 / F* | Multiple myeloma | N/A | Ciprofloxacin | Recovered |
| 15 | 69 / F* | Cervical cancer | N/A | Ceftriaxone and ciprofloxacin | Recovered |
| 15 | 64 / M* | Cholangiocarcinoma | N/A | Piperacillin / tazobactam | Recovered |
| 15 | 64 / M* | Acute myeloid leukemia | Central line | Cefepime | Recovered |
| 15 | 59 / M | AMI, ROSC after cardiac arrest | Central line | Vancomycin and imipenem | Died |
| 15 | 66 / F* | Gallbladder adenocarcinoma | N/A | Piperacillin / tazobactam | Recovered |
| 15 | 81 / M* | Cholangiocarcinoma | N/A | Piperacillin / tazobactam and levofloxacin | Recovered |
| 15 | 72 / M | Hepatocellular carcinoma | N/A | No treatment | Died |
| 15 | 59 / M* | Multiple myeloma | N/A | Cefepime and metronidazole | Recovered |
| 15 | 54 / F* | Cervical cancer | N/A | Meropenem and tobramycin | Died |
| 15 | 69 / F | Diabetes mellitus | N/A | Ciprofloxacin | Recovered |
| 15 | 60 / F* | Diffuse large B cell lymphoma | N/A | Vancomycin and cefepime | Recovered |
| 15 | 75 / F* | Gallbladder adenocarcinoma | N/A | Ceftriaxone and metronidazole | Recovered |
| 15 | 78 / F* | Cholangiocarcinoma | N/A | Ceftriaxone and metronidazole | Recovered |
| 15 | 53 / F* | Gallbladder adenocarcinoma | N/A | Ceftriaxone and metronidazole | Recovered |
| 15 | 65 / M* | Pancreatic adenocarcinoma | N/A | Ceftriaxone and metronidazole | Recovered |
| 15 | 69 / F | Nonspecific | N/A | Ceftriaxone and metronidazole | Recovered |
| 15 | 18 / M* | B cell lymphoblastic lymphoma | Central line | Cefepime and teicoplanin | Recovered |
| 15 | 75 / M* | Cholangiocarcinoma | N/A | Piperacillin / tazobactam | Recovered |
| 15 | 21 / M* | Acute myeloid leukemia | Central line | Meropenem and cefepime | Recovered |
| 25 | 11 month / N/A | N/A | N/A | N/A | N/A |
| 9 | 52 / M | Chronic pancreatitis, HT, and CRD | N/A | N/A | Died |
| 26 | 62 / M | DM, HT, and BPH | N/A | Piperacillin / tazobactam, ceftriaxone, and ciprofloxacin | Recovered |
| Our case | 81 / M | Coronary spastic angina and gastric carcinoma | None | Ampicillin / sulbactam and ceftriaxone | Recovered |
* The patient was treated with chemotherapy or stem cell transplantation.
M: male, F: female, N/A: not available, CABG: coronary artery bypass grafting, ERCP: endoscopic retrograde cholangiopancreatography, BPH: benign prostatic hypertrophy, COPD: chronic obstructive pulmonary disease, AMI: acute myocardial infarction, ROSC: return of spontaneous circulation, HT: hypertension, CRD: chronic renal disease, DM: diabetes mellitus
R. planticola is usually susceptible to most antibiotics except ampicillin. However, recently, R. planticola with resistance to carbapenems or with extended spectrum β lactamase has been reported (18,19). In two of the cases in Table 3, R. planticola was resistant to carbapenems (11,13). In one of these two cases, R. planticola was susceptible to gentamicin, levofloxacin, and tetracycline (11); in the other, it was susceptible to fluoroquinolone, aminoglycoside, and colistin (13). Based on these findings, aminoglycoside or fluoroquinolone may appropriate choices of antibiotics for carbapenem-resistant R. planticola.
Some bacteria are considered to be related to malignancy. For example, Streptococcus gallolyticus subsp. gallolyticus (SGG), which was formerly named Streptococcus bovis biotype I, and Clostridium septicum bacteremia are associated with colorectal malignancy (20). In addition to colonizing colorectal neoplasms and invading the blood from the damaged mucosa, SGG may also actually cause colorectal malignancies. On the other hand, C. septicum bacteremia occurs through mucosal damage caused by carcinoma (21-23). Although the cause-and-effect relationship between R. planticola bacteremia and malignancy is unknown, the literature suggests that R. planticola bacteremia occurs in patients who are immunocompromised as a result of malignancy. We need to accumulate additional cases of R. planticola bacteremia to clarify the relationship between R. planticola and early-stage cancer.
It is intriguing to consider the cause-and-effect relationship between R. planticola bacteremia and early gastric cancer in the present case. Although the association remains elusive, the fact that most patients with R. planticola bacteremia are immunocompromised or cancer-bearing led us to screen for malignancies; the patient happened to have gastric cancer without any symptoms. Thus, when we encounter such patients, it may be worthwhile to screen for malignancies.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Susan TB, Ramon JS, Don JB. Klebsiella planticola sp. nov.: A new species of enterobacteriaceae found primarily in nonclinical environments. Curr Microbiol 6: 105-109, 1981. [Google Scholar]
- 2. Drancourt M, Bollet C, Carta A, Rousselier P. Phylogenetic analyses of Klebsiella species delineate Klebsiella and Raoultella gen. nov., with description of Raoultella ornithinolytica comb. nov., Raoultella terrigena comb. nov. and Raoultella planticola comb. nov. Int J Syst Evol Microbiol 51: 925-932, 2001. [DOI] [PubMed] [Google Scholar]
- 3. Kanki M, Yoda T, Tsukamoto T, Shibata T. Klebsiella pneumoniae produces no histamine: Raoultella planticola and Raoultella ornithinolytica strains are histamine producers. Appl Environ Microbiol 68: 3462-3466, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferragut C, Izard D, Gavini F, Kersters K, Deley J, Leclerc H. Klebsiella trevisanii: a new species from water and soil. Int J Syst Bacteriol 33: 133-142, 1983. [Google Scholar]
- 5. Ershadi A, Weiss E, Verduzco E, Chia D, Sadigh M. Emerging pathogen: a case and review of Raoultella planticola. Infection 42: 1043-1046, 2014. [DOI] [PubMed] [Google Scholar]
- 6. Freney J, Gavini F, Alexandre H, et al. . Nosocomial infection and colonization by Klebsiella trevisanii. J Clin Microbiol 23: 948-950, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Freney J, Fleurette J, Gruer LD, Desmonceaux M, Gavini F, Leclerc H. Klebsiella trevisanii colonisation and septicaemia. Lancet 1: 909, 1984. [DOI] [PubMed] [Google Scholar]
- 8. Kim SW, Kim JE, Hong YA, Ko GJ, Pyo HJ, Kwon YJ. Raoultella planticola peritonitis in a patient on continuous ambulatory peritoneal dialysis. Infection 43: 771-775, 2015. [DOI] [PubMed] [Google Scholar]
- 9. de Campos FP, Guimarães TB, Lovisolo SM. Fatal pancreatic pseudocyst co-infected by Raoultella planticola: an emerging pathogen. Autops Case Rep 6: 27-31, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Puerta-Fernandez S, Miralles-Linares F, Sanchez-Simonet MV, Bernal-Lopez MR, Gomez-Huelgas R. Raoultella planticola bacteraemia secondary to gastroenteritis. Clin Microbiol Infect 19: E236-E237, 2013. [DOI] [PubMed] [Google Scholar]
- 11. Castanheira M, Deshpande LM, DiPersio JR, Kang J, Weinstein MP, Jones RN. First descriptions of blaKPC in Raoultella spp. (R. planticola and R. ornithinolytica): report from the SENTRY Antimicrobial Surveillance Program. J Clin Microbiol 47: 4129-4130, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Salmaggi C, Ancona F, Olivetti J, Pagliula G, Ramirez GA. Raoultella planticola-associated cholangitis and sepsis: a case report and literature review. Q J Med 107: 911-913, 2014. [DOI] [PubMed] [Google Scholar]
- 13. Tseng SP, Wang JT, Liang CY, Lee PS, Chen YC, Lu PL. First report of blaIMP-8 in Raoultella planticola. Antimicrob Agents Chemother 58: 593-595, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lam PW, Salit IE. Raoultella planticola bacteremia following consumption of seafood. Can J Infect Dis Med Microbiol 25: e83-e84, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chun S, Yun JW, Huh HJ, Lee NY. Low virulence? Clinical characteristics of Raoultella planticola bacteremia. Infection 42: 899-904, 2014. [DOI] [PubMed] [Google Scholar]
- 16. Yokota K, Gomi H, Miura Y, Sugano K, Morisawa Y. Cholangitis with septic shock caused by Raoultella planticola. J Med Microbiol 61: 446-449, 2012. [DOI] [PubMed] [Google Scholar]
- 17. Hu AY, Leslie KA, Baskette J, Elsayed S. Raoultella planticola bacteraemia. J Med Microbiol 61: 1488-1489, 2012. [DOI] [PubMed] [Google Scholar]
- 18. Demiray T, Koroglu M, Ozbek A, Altindis M. A rare cause of infection, Raoultella planticola: emerging threat and new reservoir for carbapenem resistance. Infection 44: 713-717, 2016. [DOI] [PubMed] [Google Scholar]
- 19. Cho YJ, Jung EJ, Seong JS, et al. . A case of pneumonia caused by Raoultella planticola. Tuberc Respir Dis 79: 42-45, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schlegel L, Grimont F, Ageron E, Grimont PA, Bouvet A. Reappraisal of the taxonomy of the Streptococcus bovis/Streptococcus equinus complex and related species: description of Streptococcus gallolyticus subsp. gallolyticus subsp. nov., S. gallolyticus subsp. macedonicus subsp. nov. and S. gallolyticus subsp. pasteurianus subsp. nov. Int J Syst Evol Microbiol 53: 631-645, 2003. [DOI] [PubMed] [Google Scholar]
- 21. Corredoira-Sánchez J, García-Garrote F, Rabuñal R, et al. . Association between bacteremia due to Streptococcus gallolyticus subsp. gallolyticus (Streptococcus bovis I) and colorectal neoplasia: a case-control study. Clin Infect Dis 55: 491-496, 2012. [DOI] [PubMed] [Google Scholar]
- 22. Corredoira J, García-País MJ, Coira A, et al. . Differences between endocarditis caused by Streptococcus bovis and Enterococcus spp. and their association with colorectal cancer. Eur J Clin Microbiol Infect Dis 34: 1657-1665, 2015. [DOI] [PubMed] [Google Scholar]
- 23. Corredoira J, Grau I, Garcia-Rodriguez JF, et al. . Colorectal neoplasm in cases of Clostridium septicum and Streptococcus gallolyticus subsp. gallolyticus bacteraemia. Eur J Intern Med 41: 68-73, 2017. [DOI] [PubMed] [Google Scholar]
- 24. Lee JH, Choi WS, Kang SH, et al. . A case of severe cholangitis caused by Raoultella planticola in a patient with pancreatic cancer. Infect Chemother 44: 210-212, 2012. [Google Scholar]
- 25. Gözmen S, Şükran Gözmen K, Apa H, et al. . Secondary bacteremia in rotavirus gastroenteritis. Pediatr Infect Dis J 33: 775-777, 2014. [DOI] [PubMed] [Google Scholar]
- 26. Sitaula S, Shahrrava A, Al Zoubi M, Malow J. The first case report of Raoultella planticola liver abscess. IDCases 5: 69-71, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]


